Introduction
Noonan-like syndrome with loose anagen hair (NS/LAH) is a newly described rare disorder caused by a germline mutation in SHOC2, which modulates the RAS/MAPK signaling pathway. NS/LAH has features overlapping with other RASopathies, genetic conditions caused by dysregulation in RAS/MAPK signaling, including structural cardiac defects, macrocephaly, central nervous system anomalies, and short stature with the addition of loose anagen hair and atopic dermatitis.1
Tumor predisposition disorders are well described in RAS/MAPK syndromes. However, few malignancies have been reported in patients with NS/LAH. Here, we report the first case of cutaneous T-cell lymphoma (CTCL) in a 25-year-old patient with confirmed NS/LAH with SHOC2 mutation.
Case report
A premature male infant with distinctive facial features and pulmonary stenosis was first evaluated by medical genetics at the age of 3.5 months. Facial features included ptosis, upslanting epicanthic folds; small, low set and posteriorly rotated ears; and short broad neck with low posterior hairline. Sparse, slow-growing hair and xeroderma were present. Short stature, poor weight gain, developmental delay, and vision impairment were noted. Hydrocephalus and epilepsy later developed. Family history was significant for atopy but negative for dermatologic and internal malignancies. Initial genetics evaluation including high-resolution chromosome analysis and fluorescence in-situ hybridization for 22q11.2 was unremarkable. Noonan syndrome was considered later in childhood; however, gene sequencing of PTPN11 was normal. As a teenager, sequencing of the SHOC2 gene revealed a c.4A>G p.S2G mutation, consistent with NS/LAH.
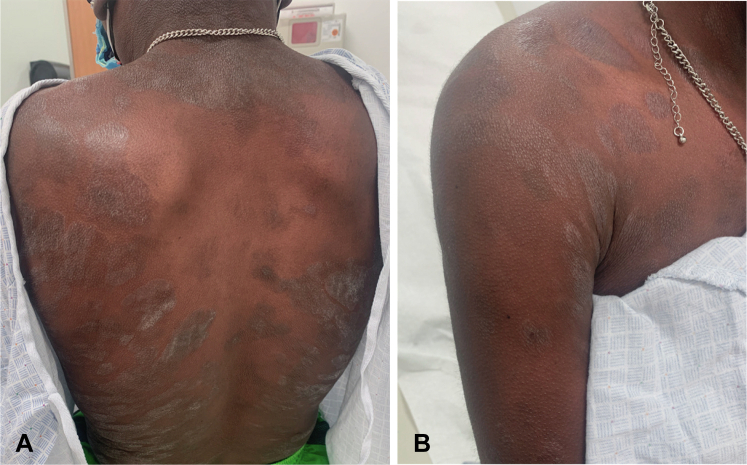
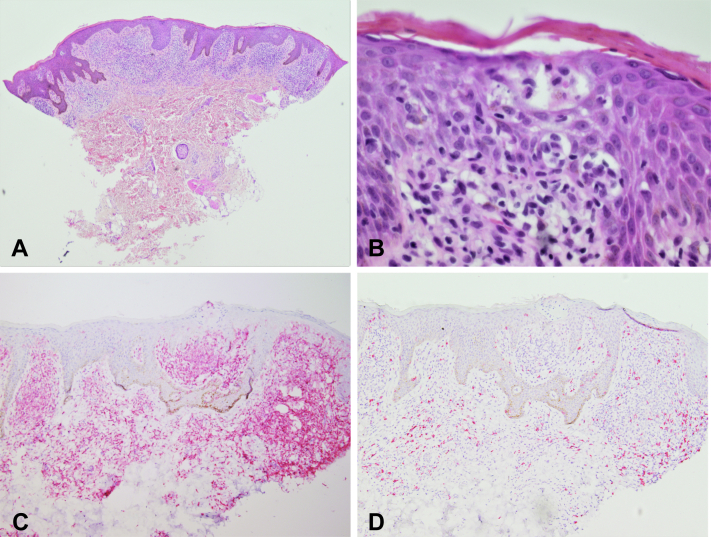
At the age of 25, the patient presented with a several-year history of a progressive pruritic eruption, recently treated as atopic dermatitis with dupilumab. On examination, he had hyperpigmented patches and lichenified and scaling plaques involving the face, trunk, and extremities, encompassing more than 60% of the total body surface area (Fig 1). Associated lymphadenopathy was absent. A punch biopsy revealed an atypical band-like infiltrate filling the papillary dermis with single-cell epidermotropism and areas of tagging the dermal epidermal junction by hyperchromatic CD4+ T-lymphocytes. Pautrier microabscesses were present. The CD4:CD8 ratio was greatly increased, at least 4:1, in the atypical infiltrate. Epidermal spongiosis was absent (Fig 2). Immunohistochemical staining for CD7 demonstrated diminished staining in a subset population. T-cell receptor gene rearrangement polymerase chain reaction was negative for clone; however, clinical and histopathologic features were consistent with CTCL. Staging was consistent with T2bN0B0M0. Treatment with narrowband ultraviolet B phototherapy and triamcinolone ointment has provided relief in pruritus and improvement in skin lesions.
Fig 1.
Clinical images. A, B, Hyperpigmented patches and well-demarcated hyperkeratotic plaques with secondary lichenification involving the trunk.
Fig 2.
Histopathology. A, Epidermal hyperplasia and a dense dermal population of atypical lymphocytes with focal epidermotropism. B, Epidermotropism and Pautrier microabscesses at higher power. C, CD4 immunostain highlighting most of the atypical lymphoid infiltrate. D, CD8 immunostain highlighting few reactive T-lymphocytes (hematoxylin-eosin stain; original magnification: A, ×40; B, ×600; C, D, ×100).
Discussion
The family of RAS/MAPK syndromes is a group of autosomal dominant genetic disorders caused by germline mutations involving the RAS/MAPK signaling pathway. NS/LAH was first described by Mazzanti et al in 2003.2 A missense mutation of the SHOC2 gene (c.4A>G, p.S2G), discovered in 2009, causes NS/LAH.3 SHOC2 is a scaffold protein that tethers RAS, RAF-1, and protein phosphatase 1c, thereby promoting MAPK signaling. Mutations in the SHOC2 gene, as seen in NS/LAH, enhance MAPK activation.3
NS/LAH is characterized by several similar characteristics to Noonan syndrome and other related RASopathies, such as Costello syndrome and cardiofaciocutaneous syndrome, including distinctive craniofacial features (macrocephaly, high forehead, hypertelorism, posteriorly rotated ears), structural heart defects (pulmonic stenosis, atrial septal defect, mitral valve anomaly, hypertrophic cardiomyopathy), reduced growth (growth hormone deficiency), mild cognitive defects, behavioral issues, and central nervous system anomalies. Additionally, patients with NS/LAH have ectodermal abnormalities including sparse, thin loose anagen hair, skin hyperpigmentation, palmar/plantar wrinkling, hyperelastic skin, atopic dermatitis, and ichthyosis.1,3, 4, 5, 6 Activation of the H-Ras protein in the RAS/MAPK pathway results in accumulation of immune cells in the mouse epidermis. The SHOC2 gene affects proliferation, survival, and differentiation of epithelial stem cell-derived cells in hair follicles; however, the relationship between SHOC2 mutations and the other cutaneous pathologies seen specifically in NS/LAH remains unclear.7
Increased signaling through RAS/MAPK contributes to oncogenesis, and solid tumors and hematologic malignancies are described in many of the RASopathies.8 Patients with Costello syndrome can develop rhabdomyosarcoma, ganglioneuroblastoma, and bladder carcinoma. Juvenile myelomonocytic leukemia and other leukemias are seen in patients with Noonan syndrome. Acute lymphoblastic leukemia and non-Hodgkin lymphoma have been reported in cardiofaciocutaneous syndrome.1 SHOC2 gene mutations were not identified in DNA samples from 82 leukemia patients1 or in 266 RASopathy-associated childhood cancers (neuroblastoma, brain tumors, rhabdomyosarcoma, acute leukemia).8 However, 2 cases of neuroblastoma and 1 case of myelofibrosis have been reported in patients with NS/LAH.5,8,9 As demonstrated by Kießling et al, RAS mutations are a rare occurrence in cases of CTCL, with oncogenic mutations in 4 of 90 biopsy specimens. Unlike our case presentation, these mutations were exclusively found in stage 4 disease.10
Conclusion
The family of disorders known as the RASopathies is known to be associated with specific cancer predispositions. Unfortunately, patients diagnosed with NS/LAH are not spared, as there are case reports of patients developing neuroblastoma, myelofibrosis, and now a case of CTCL. To date, because of the limited number of reported cases of patients with NS/LAH, the full phenotype of the disorder and impact of SHOC2 protein dysregulation is yet to be determined. While a definitive relationship has not been found, CTCL progression in patients on dupilumab has been reported, and further studies are required for risk assessment.11
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Komatsuzaki S., Aoki Y., Niihori T., et al. Mutation analysis of the SHOC2 gene in Noonan-like syndrome and in hematologic malignancies. J Hum Genet. 2010;55(12):801–809. doi: 10.1038/jhg.2010.116. [DOI] [PubMed] [Google Scholar]
- 2.Mazzanti L., Cacciari E., Cicognani A., Bergamaschi R., Scarano E., Forabosco A. Noonan-like syndrome with loose anagen hair: a new syndrome? Am J Med Genet A. 2003;118A(3):279–286. doi: 10.1002/ajmg.a.10923. [DOI] [PubMed] [Google Scholar]
- 3.Cordeddu V., Di Schiavi E., Pennacchio L.A., et al. Mutation of SHOC2 promotes aberrant protein N-myristoylation and causes Noonan-like syndrome with loose anagen hair. Nat Genet. 2009;41(9):1022–1026. doi: 10.1038/ng.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capalbo D., Scala M.G., Melis D., et al. Clinical heterogeneity in two patients with Noonan-like Syndrome associated with the same SHOC2 mutation. Ital J Pediatr. 2012;38:48. doi: 10.1186/1824-7288-38-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gripp K.W., Zand D.J., Demmer L., et al. Expanding the SHOC2 mutation associated phenotype of Noonan syndrome with loose anagen hair: structural brain anomalies and myelofibrosis. Am J Med Genet A. 2013;161A(10):2420–2430. doi: 10.1002/ajmg.a.36098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baldassarre G., Mussa A., Banaudi E., et al. Phenotypic variability associated with the invariant SHOC2 c.4A>G (p.Ser2Gly) missense mutation. Am J Med Genet A. 2014;164A(12):3120–3125. doi: 10.1002/ajmg.a.36697. [DOI] [PubMed] [Google Scholar]
- 7.Okazaki T., Saito Y., Sugita K., et al. Recurrent erythema nodosum in a child with a SHOC2 gene mutation. Yonago Acta Med. 2019;62(1):159–162. doi: 10.33160/yam.2019.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garavelli L., Cordeddu V., Errico S., et al. Noonan syndrome-like disorder with loose anagen hair: a second case with neuroblastoma. Am J Med Genet A. 2015;167A(8):1902–1907. doi: 10.1002/ajmg.a.37082. [DOI] [PubMed] [Google Scholar]
- 9.Ekvall S., Hagenäs L., Allanson J., Annerén G., Bondeson M.L. Co-occurring SHOC2 and PTPN11 mutations in a patient with severe/complex Noonan syndrome-like phenotype. Am J Med Genet A. 2011;155A(6):1217–1224. doi: 10.1002/ajmg.a.33987. [DOI] [PubMed] [Google Scholar]
- 10.Kiessling M.K., Oberholzer P.A., Mondal C., et al. High-throughput mutation profiling of CTCL samples reveals KRAS and NRAS mutations sensitizing tumors toward inhibition of the RAS/RAF/MEK signaling cascade. Blood. 2011;117(8):2433–2440. doi: 10.1182/blood-2010-09-305128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espinosa M.L., Nguyen M.T., Aguirre A.S., et al. Progression of cutaneous T-cell lymphoma after dupilumab: case review of 7 patients. J Am Acad Dermatol. 2020;83(1):197–199. doi: 10.1016/j.jaad.2020.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]