The limited understanding of itch mechanisms hinders development of antipruritic treatments. Accumulating evidence suggests that transient receptor potential (TRP) ion channels are potential therapeutic targets for itch (1–3). Compared with extensive studies on transient receptor potential cation channel subfamily V member 1 (TRPV1) and transient receptor potential cation channel subfamily A member 1 (TRPA1) in itch, transient receptor potential cation channel subfamily V member 4 (TRPV4) has received little research attention until recently. TRPV4, a multimodally activated non-selective cation channel, has been detected in sensory neurones of dorsal root ganglion (DRG) and trigeminal ganglion (TG) and skin cells (4–6), which are involved in itch sensation and development. Using Trpv4 knockout (KO) mice, several groups have reported that TRPV4 is required for pruritogen-evoked acute itch (7–9). To determine cellular sites of action of Trpv4 in itch, keratinocyte-Trpv4 conditional KO (cKO) mice were studied. It was found that deletion of Trpv4 significantly reduced acute itch evoked by histaminergic (histamine and 48/80), but not non-histaminergic (chloroquine, CQ), pruritogens (8). In addition, we and others have found previously that TRPV4 in skin keratinocytes and macrophages also critically mediates chronic itch (4, 5). Although these studies strongly suggest that TRPV4 in skin cells is essential for itch, whether neuronal-TRPV4 directly contributes to acute and chronic itch remains largely elusive. This study addressed this question using sensory neurone-Trpv4 cKO mice.
MATERIALS AND METHODS (See Appendix S1)
RESULTS
For acute itch, it was found that cKO of Trpv4 in sensory neurones significantly attenuated scratching behaviours evoked by histamine, 48/40 and 5-HT, but not SLIGRL and CQ, in male mice (Fig. 1A). The findings in females are consistent with those in males, except that CQ-induced itch was reduced (Fig. 1B).
Fig. 1.
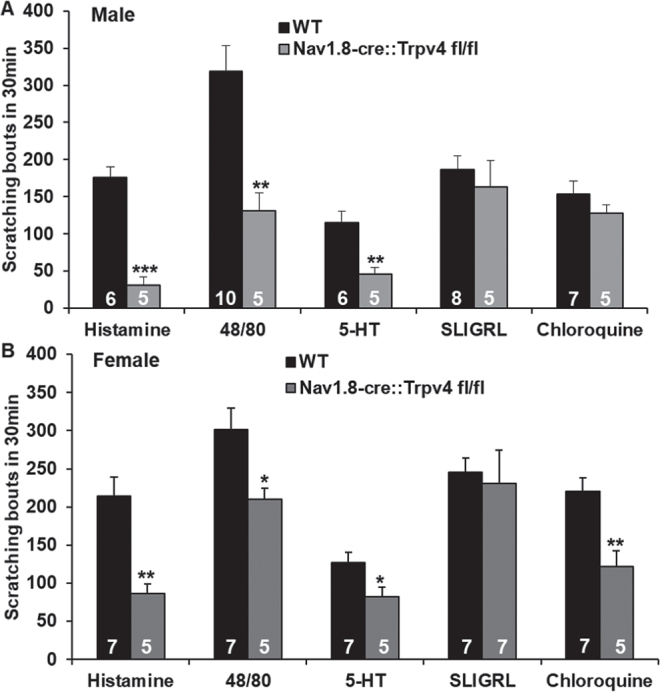
Effect of sensory neurone-transient receptor potential cation channel subfamily V member 4 (TRPV4) on acute itch induced by pruritogens evoked acute scratching behaviours that were significantly attenuated in Trpv4 conditional knockout (cKO) vs wild-type mice (WTs) in both males (A) and females (B). Histamine, compound 48/80, and 5-hydroxtryptamine (5-HT), but not Ser-Leu-Ile-Gly-Arg-Leu-NH2 (SLIGRL), evoked acute scratching behaviours that were significantly attenuated in Trpv4 conditional knockout (cKO) vs wild-type mice (WTs) in both sexes. Chloroquine (CQ)-induced itch was significantly reduced in female, but not male, Trpv4 cKO mice. *p < 0.05, **p < 0.01, and ***p < 0.001 vs WT, 2-tail t-test. Group size is indicated on the bars.
In the dry skin chronic itch model, it was found that scratching behaviours were significantly reduced in Trpv4 cKO mice on days 7 and 9 after AEW treatment (Fig. 2A–B) in both sexes. In contrast, in the allergic contact dermatitis chronic itch model, Trpv4 cKO mice did not display a significant reduction in scratching on days 12 and 14 after DNFB treatment in either sex (Fig. 2C–D).
Fig. 2.
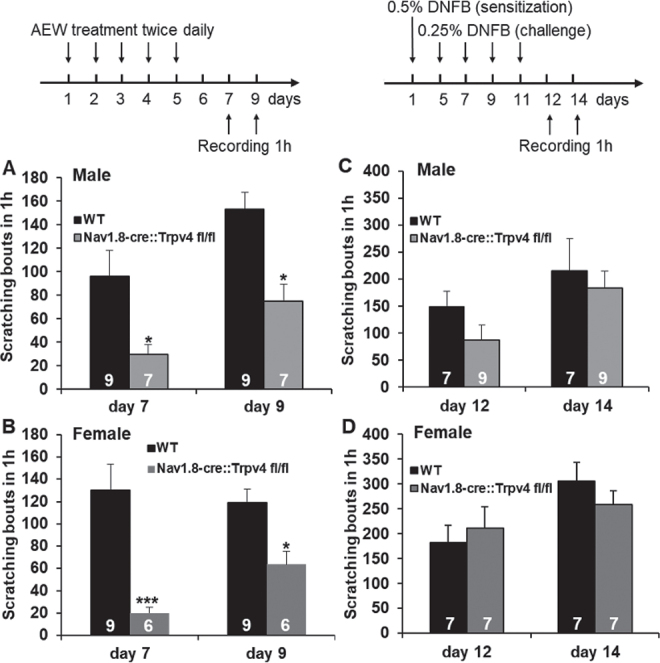
Effect of sensory neurone-transient receptor potential cation channel subfamily V member 4 (TRPV4) on chronic itch associated with dry skin and with allergic contact dermatitis. Dry skin (A, B: acetone and diethyl-ether (1:1) following water (AEW) model), but not allergic contact dermatitis (C, D: 1-fluoro-2, 4-dinitrobenzene (DNFB) model), induced scratching behaviours that were significantly attenuated in Trpv4 conditional knockout (cKO) vs wild-type (WT) mice in both sexes. *p < 0.05, 2-way analysis of variance (ANOVA) with Bonferroni post-hoc test. Group size is indicated on the bars.
DISCUSSION
This study determined the specific contribution of neuronal-TRPV4 to acute and chronic itch behaviours in both male and female mice. The results showed that TRPV4 in sensory neurones contributes to scratching behaviour evoked by histamine and 48/80 (histaminergic pruritogen) and 5-HT (non-histaminergic), but not SLIGRL and CQ (non-histaminergic) in males. The findings between females and males are consistent, except that TRPV4 has a sexually dimorphic effect on CQ-induced itch. Moreover, it was observed that TRPV4 in sensory neurones is required for chronic itch associated with dry skin, but not with allergic contact dermatitis.
Several studies have demonstrated that TRPV4 is involved in pruritogen-evoked acute itch, although the results across laboratories are inconsistent. Akiyama et al. reported that Trpv4 KO mice display reduced scratching behaviour in response to 5-HT, but not to histamine and SLIGRL (PAR-2 agonist). Interestingly, they found that CQ-induced itch is increased in Trpv4 KO mice (7). In contrast, Kim et al. showed that both histamine- and CQ-induced itch is attenuated in mice lacking Trpv4 (9). Our group found that histamine-induced itch is decreased, but CQ-induced itch remained unchanged in Trpv4 KO mice (8). Although these inconsistent findings imply that TRPV4 plays a role in regulating acute itch, the crucial cellular sites of action of TRPV4 are unknown. The current study showed that selective deletion of Trpv4 in sensory neurones significantly attenuated histamine-induced scratching behaviour, which is in agreement with Kim et al.’s study (9) and our previous study (8), but in contrast to Akiyama’s finding (7) using Trpv4 KO mice. In support of Akiyama’s report, we observed that cKO of neuronal-Trpv4 significantly reduced 5-HT, but not SLIGRL-evoked itch. Although CQ-induced itch has been reported as increased (7), decreased (9) or unchanged (8) in Trpv4 KO mice, we found that it is significantly reduced in sensory neurone-Trpv4 cKO female mice, but not in males. The reason why TRPV4 in sensory neurones shows a sexually dimorphic effect on chloroquine-induced itch requires further clarification. Together, the current data suggest that sensory neurones in DRG are an important locale where TRPV4 differentially regulates various forms of acute itch. In addition, the inconsistencies in certain types of acute itch between Trpv4 KO and sensory neuron-Trpv4 cKO mice point to the possible contributions of TRPV4 to itch in other cell lineages, such as skin macrophages, mast cells, and endothelial cells (2).
Using AEW-induced dry skin and DNFB-induced allergic contact dermatitis mouse models, we subsequently elucidated the specific contribution of neuronal-TRPV4 to chronic itch. Luo et al. recently reported that itch associated with both dry skin and with allergic contact dermatitis is attenuated in Trpv4 KO mice (5). They further found that dry skin-associated itch is reduced in keratinocyte, but not macrophage-Trpv4 cKO mice and allergic contact dermatitis-associated itch is reduced in macrophage, but not keratinocyte-Trpv4 cKOs. The current study found that Trpv4 deficiency in sensory neurones leads to a reduction in dry skin, but not contact allergic dermatitis-associated itch. Together, these results imply that both skin keratinocytes and sensory neurones contribute to dry skin-induced itch. However, allergic contact dermatitis-associated itch might rely on TRPV4 in skin macrophages, but not sensory neurones. It is notable that TRPV4 expression in DRG neurones was significantly increased after dry skin or contact allergic dermatitis in both sexes (Fig. S1). Increased expression of TRPV4 may lead to a sensitization of sensory neurones, which potentially contributes to the ongoing itch in dry skin. However, itch associated with dry skin and with allergic contact dermatitis are certainly not mono-factorial. Most likely, they involve multiple cells, mediators, and neural circuits, which function in a co-contributory manner for chronic itch modulation. Further studies, such as whether sensory neurone-TRPV4 differentially communicates with itch-relevant mediators released from skin cells or interacts with other itch-relevant ion channels or receptors in neurones between these 2 models, might be helpful for elucidating the underlying mechanisms of how sensory neurone-TRPV4 contributes to itch associated with dry skin, but not with contact allergic dermatitis under the condition that its expression is upregulated in both models.
In conclusion, this study provides initial information regarding the specific contribution of neuronal-TRPV4 to acute and chronic itch behaviours. The results suggest that TRPV4 in sensory neurones is essential for certain types of acute and chronic itch, and implies that different forms of itch may rely on different cellular sites of action of TRPV4.
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health Grant R01DE027454 to YC.
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Kittaka H, Tominaga M. The molecular and cellular mechanisms of itch and the involvement of TRP channels in the peripheral sensory nervous system and skin. Allergol Int 2017; 66: 22–30. [DOI] [PubMed] [Google Scholar]
- 2.Moore C, Gupta R, Jordt SE, Chen Y, Liedtke WB. Regulation of pain and itch by TRP channels. Neurosci Bull 2018; 34: 120–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun S, Dong X. Trp channels and itch. Semin Immunopathol 2016; 38: 293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y, Wang ZL, Yeo M, Zhang QJ, López-Romero AE, Ding HP, et al. Epithelia-sensory neuron cross talk underlies cholestatic itch induced by lysophosphatidylcholine. Gastroenterology 2021; 161: 301–317.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo J, Feng J, Yu G, Yang P, Mack MR, Du J, et al. Transient receptor potential vanilloid 4-expressing macrophages and keratinocytes contribute differentially to allergic and nonallergic chronic itch. J Allergy Clin Immunol 2018; 141: 608–619.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mascarenhas NL, Wang Z, Chang YL, Di Nardo A. TRPV4 mediates mast cell activation in cathelicidin-induced rosacea inflammation. J Invest Dermatol 2017; 137: 972–975. [DOI] [PubMed] [Google Scholar]
- 7.Akiyama T, Ivanov M, Nagamine M, Davoodi A, Carstens MI, Ikoma A, et al. Involvement of TRPV4 in serotonin-evoked scratching. J Invest Dermatol 2016; 136: 154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, Fang Q, Wang Z, Zhang JY, MacLeod AS, Hall RP, et al. Transient receptor potential vanilloid 4 ion channel functions as a pruriceptor in epidermal keratinocytes to evoke histaminergic itch. J Biol Chem 2016; 291: 10252–10262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim S, Barry DM, Liu XY, Yin S, Munanairi A, Meng QT, et al. Facilitation of TRPV4 by TRPV1 is required for itch transmission in some sensory neuron populations. Sci Signal 2016; 9: ra71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyamoto T, Nojima H, Shinkado T, Nakahashi T, Kuraishi Y. Itch-associated response induced by experimental dry skin in mice. Jpn J Pharmacol 2002; 88: 285–292. [DOI] [PubMed] [Google Scholar]
- 11.Liu T, Han Q, Chen G, Huang Y, Zhao LX, Berta T, et al. Toll-like receptor 4 contributes to chronic itch, alloknesis, and spinal astrocyte activation in male mice. Pain 2016; 157: 806–817. [DOI] [PMC free article] [PubMed] [Google Scholar]


