B-cell depleting therapy is a mainstay to treat autoimmune rheumatic diseases characterised by autoantibody production. While B cells depletion effectively turns down autoimmune inflammation, it also hampers the development of protective immunity after infections and vaccination. The latter has become of particular concern, as treatment with anti-CD20 antibody rituximab is associated with more severe courses of COVID-191 and abolished or severely impaired humoral responses to SARS-CoV-2 vaccination.2
While newly initiated B-cell depletion is usually preceded by SARS-CoV-2 vaccination, several situations leave patients without protective immunity during treatment. These include (1) pre-existing B-cell depletion before vaccination was available, (2) vaccination refusal and (3) loss of vaccination response during treatment. In addition, B-cell depletion is often long-lasting and needs to be repeated to prevent potentially life-threatening flares. Therefore, strategies need to be developed providing anti-SARS-CoV-2 immunity in such patients. Several neutralising monoclonal antibodies (mAbs) against spike S1 protein of SARS-CoV-2 have been developed. Casirivimab and imdevimab are two recombinant IgG1 mAbs that bind the receptor-binding domain of the SARS-CoV-2 spike protein and prevent its binding to the angiotensin-converting enzyme 2 at low concentrations (31pM). Use of casirivimab/imdevimab combination has been shown to reduce hospitalisations and deaths from COVID-19.3
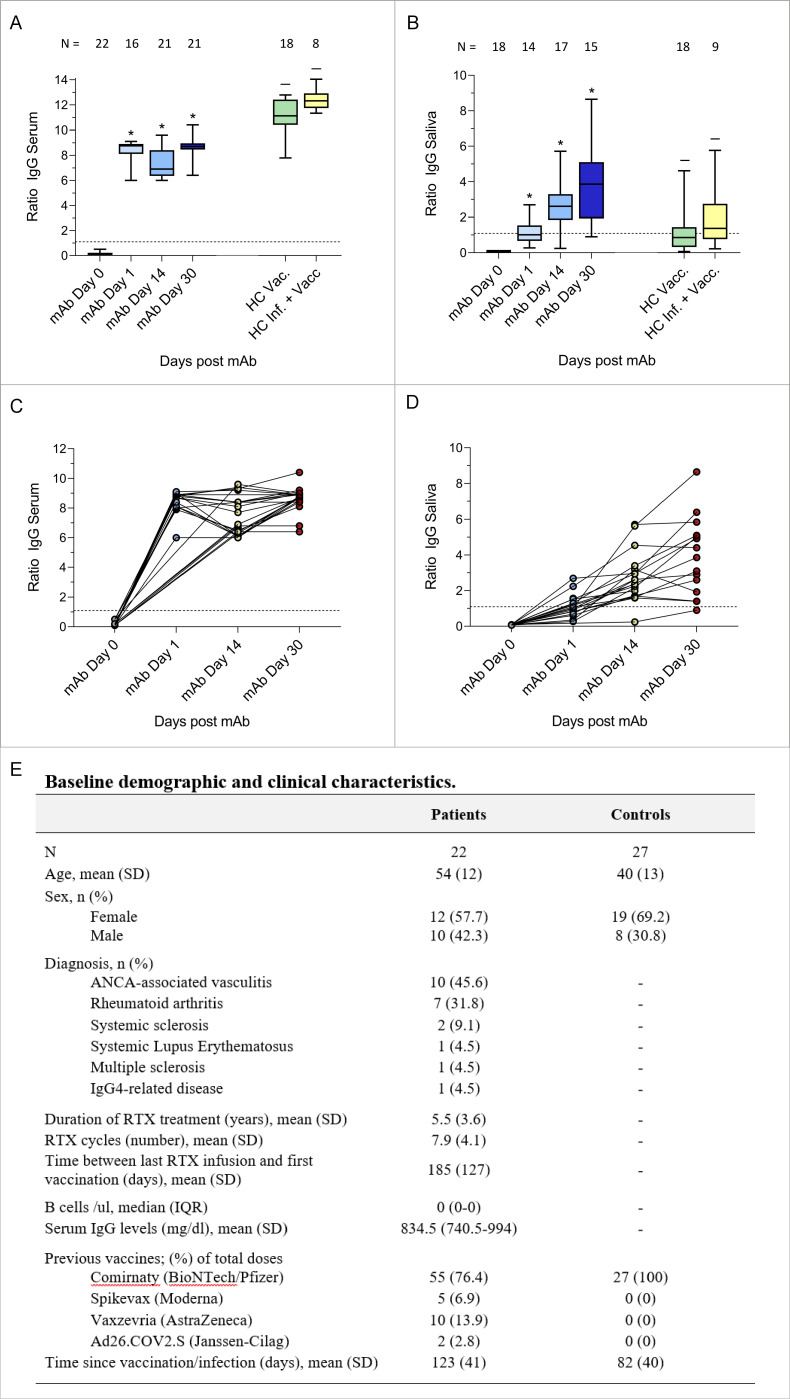
We analysed 22 patients with autoimmune rheumatic diseases who received pre-exposure prophylaxis (PrEP) with a single subcutaneous injection of casirivimab/imdevimab (600 mg/600 mg) in December 2021 due to lack of anti-SARS-CoV-2 IgG at least 21 days after three SARS-CoV-2 vaccinations. Patients’ characteristics are shown in figure 1. We obtained 92 serum and 75 saliva samples at 4 consecutive timepoints. Serum and salivary anti-SARS-CoV-2 Spike IgG were quantified by ELISA (Euroimmun, Lübeck, Germany) at baseline and 1, 14 and 30 days after administration. IgG levels are given as antibody ratios by dividing the optical density of the sample by that of the calibrator. Serum anti-SARS-CoV-2 IgG, which were absent at baseline (mean ratio±SD 0.2±0.1; cut-off for positive>1.1), reached maximal concentrations on day 1 (8.4±0.8) after administration and remained stable on day 14 (7.5±1.3) and 30 (8.6±0.8). Anti-SARS-CoV-2 IgG serum levels 30 days after PrEP were significantly lower (p<0.001) than after vaccination of healthy individuals (11.1±1.4) or after infection and vaccination (12.4±0.9). Furthermore, anti-SARS-CoV-2 IgG were also found in saliva on day 1 (1.2±0.7), with further increases on day 14 (2.8±1.4) and 30 (3.9±2.2). Salivary anti-SARS-CoV-2 IgG at day 30 were significantly higher (p<0.05) than in vaccinated (1.4±1.4) or infected and vaccinated controls (1.9±1.7). No side effects were reported. Five patients (19.2%) had a close contact with a person infected with SARS-CoV-2, after which all but one remained PCR negative. The fifth patient turned PCR positive and developed mild fever and cough.
Figure 1.
Box and whiskers (top) and spaghetti plots (bottom) of the levels of serum (A, C) and salivary (B, D) anti-SARS-CoV-2 spike IgG antibodies before and after 1 day, 14 dayS and 30 days from casirivimab/imdevimab treatment. Serum and salivary anti-SARS-CoV-2 spike IgG antibody concentrations in healthy controls after SARS-CoV-2 vaccination (HC Vacc.), and after SARS-CoV-2 infection and vaccination (HC Inf.+Vacc.) are shown for comparison. Healthy controls were recruited from the general population and from healthcare workers. The number of subjects analysed for each timepoint is indicated in the figure. The dashed horizontal line represents cut-off values of 1.1 (anti-SARS-CoV-2 IgG negative/positive). *=p <0.0001 compared with day 0. –=p <0.0001 (A) and p<0.05 (B) compared with day 30 after pre-exposure prophylaxis. Baseline demographic and clinical characteristics of the patients and of the controls are reported in (E). ANCA, antineutrophil cytoplasmic antibodies; HC, healthy controls; mAb, monoclonal antibodies; RTX, rituximab.
These data show that PrEP with casirivimab/imdevimab is safe and provides a fast induction of anti-SARS-CoV-2 humoral immunity in B-cell depleted patients failing previous vaccination. Furthermore, substantial levels of casirivimab/imdevimab were found in saliva, exceeding the levels of anti-SARS-CoV-2-specific IgG observed in healthy controls after infection or vaccination4. Thus, mAbs also seem to offer mucosal protection from SARS-CoV-2, comparable to active immunisation. This approach provides a proof of concept for the utility of anti-SARS-CoV-2 PrEP in autoimmune rheumatic disease, that is, to protect vulnerable patients against severe COVID-19.
Notably, these data were obtained during the wave of the SARS-CoV-2 Delta variant in Germany, for which casirivimab/imdevimab is highly effective. For the Omicron variants casirivimab and imdevimab are less effective, although imdevimab retained some in vitro efficacy against the BA.2 subvariant.5 Recently, the long-acting tixagevimab/cilgavimab has been approved as PrEP for all Omicron variants.6 Whether tixagevimab/cilgavimab reaches similar serum and saliva concentrations in B-cell depleted patients with autoimmune rheumatic diseases remains to be determined.
Footnotes
Contributors: FF, KS, AK, DS, GS and TH were involved in study design. Sample collection was done by DB, LV-M, FH, KT, KM, BM, AK, DS and GS. Experiments and data analysis were performed by FF, KS, KT and TH. FF, KS, GS and TH were responsible for the figure. Data interpretation was done by FF, KS, DB, LV-M, KT, AK, DS, GS and TH. Writing of the manuscript was done by FF, KS, AK, DS, GS and TH. GS and TH contributed equally and share senior coauthorship.
Funding: The study was supported by the Deutsche Forschungsgemeinschaft (DFG-FOR2886, PANDORA and the CRC1181 Checkpoints for Resolution of Inflammation). Additional funding was received from the Bundesministerium für Bildung und Forschung (BMBF; project MASCARA), the Bayerisches Staatsministerium für Wissenschaft und Kunst, the ERC Synergy grant 4D Nanoscope, the IMI funded projects RTCure and HIPPOCRATES, the Emerging Fields Initiative MIRACLE of the Friedrich-Alexander-Universität Erlangen-Nürnberg, the Schreiber Stiftung, the Else Kröner-Memorial Scholarship (DS, no. 2019_EKMS.27), the H.W. & J. Hector Stiftung (project M2102).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Ethics Committee of the Friedrich-Alexander-Universität Erlangen-Nürnberg. Ethical approval number 157_20 B. Participants gave informed consent to participate in the study before taking part.
References
- 1.Andersen KM, Bates BA, Rashidi ES, et al. Long-Term use of immunosuppressive medicines and in-hospital COVID-19 outcomes: a retrospective cohort study using data from the National COVID cohort collaborative. Lancet Rheumatol 2022;4:e33–41. 10.1016/S2665-9913(21)00325-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simon D, Tascilar K, Schmidt K. Humoral and cellular immune responses to SARS–CoV-2 infection and vaccination in autoimmune disease patients with B-cell depletion. Arthritis Rheumatol 2022;75:33–7. 10.1002/art.41914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinreich DM, Sivapalasingam S, Norton T, et al. REGEN-COV antibody combination and outcomes in outpatients with Covid-19. N Engl J Med 2021;385:e81. 10.1056/NEJMoa2108163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ketas TJ, Chaturbhuj D, Portillo VMC, et al. Antibody responses to SARS-CoV-2 mRNA vaccines are detectable in saliva. Pathog Immun 2021;6:116–34. 10.20411/pai.v6i1.441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takashita E, Kinoshita N, Yamayoshi S, et al. Efficacy of antiviral agents against the SARS-CoV-2 omicron Subvariant BA.2. N Engl J Med 2022;386:1475–7. 10.1056/NEJMc2201933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tixagevimab and Cilgavimab (Evusheld) for pre-exposure prophylaxis of COVID-19. JAMA 2022;327:384–5. 10.1001/jama.2021.24931 [DOI] [PubMed] [Google Scholar]