Abstract
Study Design:
Systematic review and meta-analysis.
Objectives:
We performed this meta-analysis to evaluate whether intradiscal Platelet Rich Plasma(PRP) injection has any beneficial role in the management of lumbar disc disease.
Methods:
We conducted independent and duplicate electronic database searches including PubMed, Embase, and Cochrane Library till September 2020 for studies investigating the role of intradiscal PRP in the management of lumbar disc disease. The analysis was performed in the R platform using OpenMeta[Analyst] software.
Results:
13 studies including 2 RCTs, 5 prospective, and 6 retrospective studies involving 319 patients were included in the meta-analysis. A single-arm meta-analysis of the included studies showed a beneficial effect of the intervention in terms of pain relief outcomes like VAS score (p < 0.001), pain component of SF-36 (p = 0.003) while such improvement was not seen in functional outcome measures like ODI score (p = 0.071), the physical component of SF-36 (p = 0.130) with significant heterogeneity noted among the included studies. No structural improvement in magnetic resonance imaging was observed (p = 0.106). No additional procedure-related adverse events were noted in the included studies (p = 0.662).
Conclusion:
There is a paucity of high-quality studies to give conclusive evidence on the benefits of intradiscal PRP for lumbar disc disease. Although intradiscal PRP injection has shown some beneficial effect in controlling pain for lumbar disc disease, we could not find structural or functional improvement from the included studies. Hence, we recommend large double-blind double-arm randomized controlled studies to analyze the benefits of the intervention being analyzed.
Keywords: PRP, platelet-rich plasma, lumbar disc disease, regenerative orthopaedics, intradiscal injection
Introduction
Chronic pain in lower back falls under one of the most common etiology of prime importance in adulthood resulting in physical disability and overall cast a high impact on economic, social, and health aspects. Out of all the causes of low backache, age-related intervertebral disc degeneration pose major morbidity and affects the functional quality of life of individuals.1,2 Biochemically, the degenerated intervertebral disc is characterized by extracellular matrix degradation, loss of proteoglycan and water in nucleus pulposus and proteoglycan, fibronectin, and collagen disruption in annulus fibrosus, which may further lead to disruption of the internal architecture of the disc, tears, cracking and fissuring.3,4 These changes lead to the generation of dull aching pain around the particular vertebral level. 5 Though the intervertebral disc possess limited healing potential, there is increased expression of proinflammatory cytokines and disarticulation of internal disc architecture which forms the pathogenesis of discogenic low backache.5,6
Due to the inherent avascular nature and limited regeneration capacity of the intervertebral disc, the global researchers traced out the path of biological regeneration. The concept of “Orthobiologics” has paved a way for degenerative diseases of the spine with a potential for regeneration. Of all orthobiologics, Platelet Rich Plasma (PRP) has proven the potentials of regeneration of the degenerated disc. 7 PRP consists of a supra-normal concentration of autologous platelets in a small amount of plasma. They remain a potential source of growth factor that are essential for wound healing and synthesis of the extracellular matrix. 8
PRP has shown to possess the potentiality of reverting pathophysiology of the degenerated disc whereby noted to alter cytokines release at molecular level in a way that the anti-inflammatory cytokines are upregulated and pro-inflammatory cytokines in contrary are downregulated respectively. 9 PRP being an autologous product, the risk of immune reactions are negligible. 10 Since PRP has anti-inflammatory and anti-microbial properties, the risk of infection in the post-injection period for disc regeneration is less.11,12 Wang et al. 13 reviewed the role of PRP in degenerative disc disease among various tissue models and established their benefits at the cellular level. Khalaf et al. 14 established the restoration of mechanical properties of the denatured disc with a significant increase in glycosaminoglycan content compared to the controls. Hence, PRP therapy seems promising to quiesce the inflammatory cascade and to restore the structural integrity of the degenerated anatomy.
To harness the potential of PRP in clinical practice toward disc regeneration various studies were undertaken. But only a few of the human trials have established their beneficial effects on human subjects 15 and there is a need for a comprehensive systematic review to summarize the available evidence for evaluating their use in routine clinical practice. We conducted the present meta-analysis for analyzing it comprehensively whether intradiscal injection of PRP has any beneficial role in managing the lumbar disc disease from the available literature.
Methods
We present herewith a meta-analysis study which was being performed by duly cohering the guidelines of the Back Review Group of Cochrane Collaboration 16 and aim to report the same based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). 17
Search Strategy
Two reviewers conducted an independent literature search for studies evaluating the role of intradiscal application of PRP for managing degenerative disease of the lumbar disc. Electronic database search was conducted in PubMed, Web of Science, Embase, and the Cochrane Library until September 2020. Our search was neither restricted to any particular language nor confined to a specific time period. Keywords used for the search were as follows: “spine,” “platelet,” “disc,” “lumbar disc herniation,” “PRP,” “platelet-rich plasma” together with Boolean operators such as “AND,” “OR” and “NOT.” The search strategy used for PubMed database has been given in Supplementary File 1. We also searched the reference list of the selected articles to identify studies not identified in the primary search. We included and analyzed all the studies meeting the inclusion criteria. Any discrepancy between the reviewers was resolved through discussion until a consensus was obtained. A PRISMA flow diagram for study selection into the analysis has been depicted for the same as Figure 1.
Figure 1.
PRISMA flow diagram of the included studies. PRP, platelet-rich plasma; #, list of excluded studies are given in Supplementary File 2.
Inclusion Criteria
Population Patients with lumbar disc disease.
Intervention Intradiscal injection of PRP
Comparator Placebo
Outcomes Visual Analog Scale (VAS) score, Oswestry Disability Index (ODI), Magnetic Resonance Imaging (MRI) signal density changes, Short Form Health Survey Questionnaire (SF-36), and complication rate
Time frame minimum 6 months follow-up
Study Design RCTs, prospective studies and retrospective cohort studies.
If sufficient placebo-controlled studies are not available, we considered having a single-arm meta-analysis being done to evaluate the beneficial effect of the intervention compared to the pre-operative state of the patient.
Exclusion Criteria
All those studies on PRP injection in regions other than the affected disc such as epidural or intra-muscular regions for lumbar disc disease were excluded. We also excluded anatomical studies and studies involving animal models to evaluate the intervention being analyzed.
Data Extraction
Data was extracted from the articles included in analysis by 2 independent reviewers respectively. Notably, the data extracted from the studies was as follows:
Study characteristics: Author, year of publication, nature of study, number of patients enrolled, and duration of follow-up
Baseline characteristics: mean age, gender proportions, pre-operative pain, and functional scores
Primary Outcome: Pain relief as measured by VAS score at final follow-up
Secondary Outcomes: Functional improvement as measured by ODI score, SF-36 score at final follow-up
Other Outcomes: MRI signal change and reported complications
Risk of Bias and Quality Assessment
Two reviewers independently assessed the methodological quality of the included studies using The Cochrane Collaboration’s RoB 2 tool for Randomised Controlled Trials(RCTs) 18 and the ROBINS-I tool for non-randomized studies which has 5 and 7 domains of bias assessment respectively. 19
Statistical Analysis
We performed this meta-analysis using R platform with OpenMeta[Analyst]. 20 Outcomes with continuous variables were analyzed using Weighted Mean Difference (WMD) with 95% Confidence Intervals (CI) while for outcomes with dichotomous variable outcomes Odds Ratio(OR) with 95% CI was used. I2 test was used for heterogeneity assessment. 21 We used fixed-effects model for analysis if I2 < 50% and p > 0.1, otherwise, a random-effects model was employed. In case of heterogeneity in the outcomes analyzed, sensitivity analysis was performed. Funnel plot and Egger Regression test was used for publication bias assessment.
Results
Search Results
Comprehensive search at electronic database generated 1544 articles and the aforementioned were subjected to initial screen for removing duplicate article. Afterward the screening process, it resulted in 1227 articles. Upon title and abstract screening of the resultant articles, notably we excluded 1204 articles. Therefore, 23 articles qualified for reviewing the full-text. On full-text review by both the reviewers 11 of them were excluded. A list of excluded articles was given in Supplementary File 2. Finally, 12 studies including 2 RCTs15,22, 5 prospective studies23-27, and 5 retrospective studies28-32 having a total of 317 patients were included in our analysis. At a glance, PRISMA flow diagram of study selection has been depicted as Figure 1 and general characteristics of the studies included in our meta-analysis have been elucidated in Table 1.
Table 1.
General Characteristics of the Included Studies.
| Sl. No |
Author |
Year |
Study Design |
Sample Size |
Diagnostic Discography |
PRP Type | PRP Preparation Method | Activator |
Volume of whole blood used | Injectate Volume | Mean Follow-up period (months) | Outcome Measures |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | YA Tuakli-Wosornu et al. | 2016 | Randomised controlled trial | 43 | Yes | L-PRP | SmartPrep (Harvest) | No | 30 ml | 1-2 ml | 12 | VAS, FRI, SF-36, NASS |
| 2 | J Cheng et al. | 2019 | Randomized controlled trial | 21 | Yes | L-PRP | Harvest centrifuge | NA | NA | 3-4 ml | 78.84 | VAS, SF-36 |
| 3 | C Kristin et al. | 2017 | Prospective study | 15 | No | L-PRP +SVF | NA | No | 30ml | 1 ml | 6 | VAS, ODI SF-12, PPI, BDI, DPQ |
| 4 | D Jain et al. | 2020 | Prospective study | 25 | Yes | L-PRP | PRP Plus - 2 spin technique |
CaCl2 | 18ml | 1-2 ml | 6 | VAS, ODI |
| 5 | D Levi et al. | 2015 | Prospective study | 22 | Partial | L-PRP | SmartPrep (Harvest) | No | 30/60ml | 1.5 ml | 6 | VAS, ODI |
| 6 | K Akeda et al. | 2017 | Prospective study | 14 | Yes | P-PRP releasate | Buffy coat method | CaCl2 + autologous serum | 200ml | 2 ml | 10 | VAS, RDQ |
| 7 | M Monfett et al. | 2016 | Prospective study | 29 | Yes | L-PRP | SmartPrep (Harvest) | No | 30ml | 1-2 ml | 12 | VAS, FRI, SF-36, NASS |
| 8 | F Kirchner et al. | 2016 | Retrospective cohort study | 86 | Yes | PRGF | PRGF system IV (BTI-Biotechnology Institute, Vitoria, Álava, Spain) | CaCl2 | 9ml | 4 ml | 6 | VAS |
| 9 | A Navani et al. | 2015 | Retrospective cohort study | 6 | Yes | L-PRP | Pure-PRP system (EmCyte) | No | 60ml | 1.5-3 ml | 6 | VAS, SF-36 |
| 10 | A Navani et al. | 2018 | Retrospective cohort study | 20 | Yes | L-PRP | Pure-PRP system (EmCyte) | No | 60ml | 1-2 ml | 18 | VAS, SF-36 |
| 11 | M Bodor et al. | 2014 | Retrospective cohort study | 35 | Yes | NA | NA | NA | 9ml | 1.25-2 ml | 10 | ODI |
| 12 | GE Lutz | 2017 | Case report | 1 | Yes | L-PRP | Arteriocyte (Magellan) |
NA | NA | 1.5 ml | 12 | MRI |
ACP – autologous conditioned plasma; BDI – Beck Depression Inventory; DPQ – Dallas Pain Questionnaire; FRI – Functional Rating Index; L-PRP – Leukocyte- and Platelet Rich Plasma; MRI – Magnetic Resonance Imaging; NA – Not available; NASS – North American Spine Society outcome questionnaire; NRS – Numeric Rating Scale; ODI – Oswestry Disability Index; PPI – present pain intensity; P-PRP – Leukocyte-poor PRP; PRGF – plasma rich in growth factors; PRP – platelet-rich plasma; RDQ – Roland-Morris Disability Questionnaire; SF – Short Form.
Of the 12 included studies, 10 studies enrolled patients with refractory low back pain with concordant changes in MRI and localized the problematic level with positive discography findings intraoperatively before the intervention is being applied. Whereas C Kristin et al. 23 in their study did not use discography to localize the level of pathology and included patients who had degenerative disc disease with predominant discogenic back pain based on clinical and radiological parameters and who were refractory to conservative care for 6 months. In case of multilevel involvement they applied the intervention to all the degenerated levels. Similarly, D Levi et al. 25 enrolled patients who were either diagnosed to have discogenic back pain based on positive discography or clinic-radiological parameters. All the studies excluded patients with non-discogenic source of back including spinal canal stenosis and instability.
Since the control group for the intervention was available in only one of the RCTs 15 included we performed a single-arm meta-analysis of the effects of intervention including all the 12 studies. Since the included studies were of various study designs, we hereby present the results in a stratified manner based on the nature of their study design for each outcome analyzed.
Quality Assessment
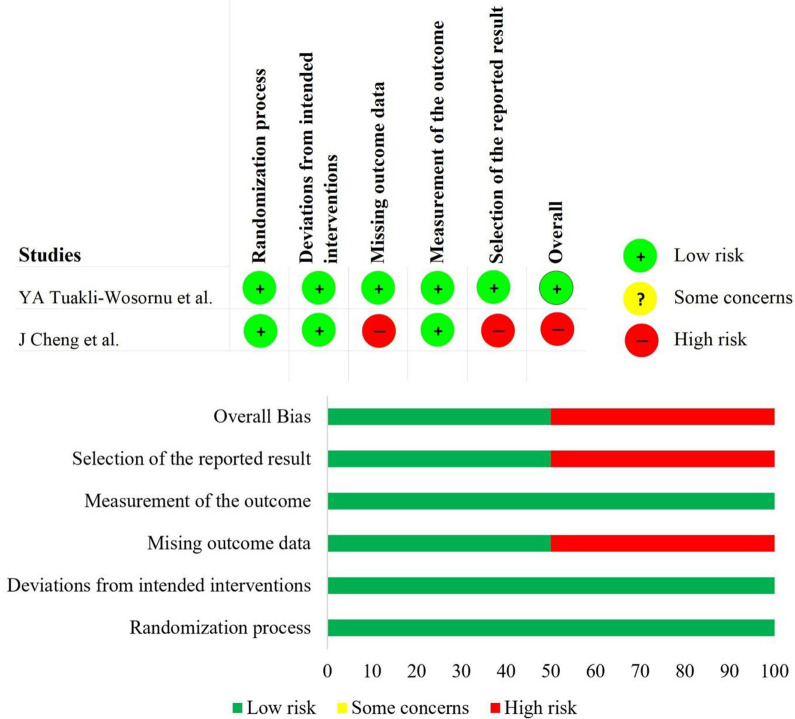
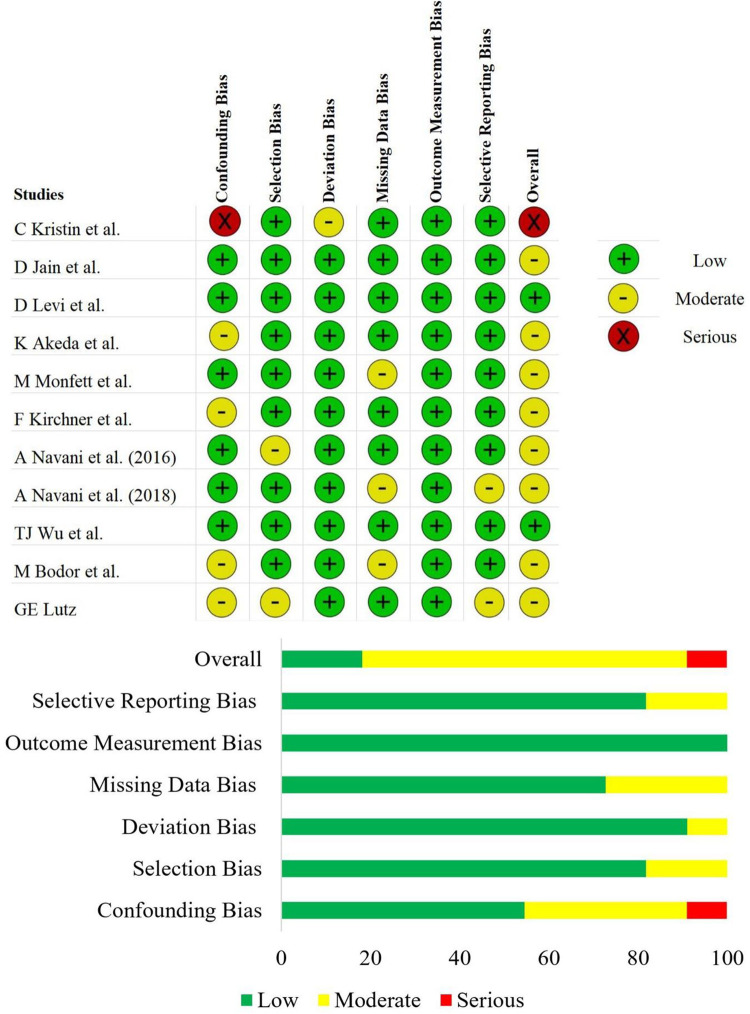
The methodological quality and the risk of bias of the included prospective randomized, prospective non-randomized, and retrospective studies were given in Figure 2 and Figure 3 respectively. Since the studies included were of single-arm design, we excluded the intervention classification bias domain from the ROBINS-I tool. Although the risk of bias was high in one of the included RCTs 22 due to selective reporting of the analysis of the treatment arm, we included them into our analysis since we performed only single-arm meta-analysis. All the studies being subjected to our aimed analysis did not warrant exclusion with regard to their methodological quality.
Figure 2.
Risk of bias evaluation of the RCTs using the ROB 2 tool.
Figure 3.
Risk of Bias evaluation of the non-randomized studies using the ROBINS-I tool.
Primary Outcome
VAS Score
2 RCTs,15,22 5 prospective23-27, and 3 retrospective28-30 studies involving 281 patients reported the outcome of the intervention based on VAS Score at the final follow-up. The mean VAS score pre-intervention was 6.98 which reduced to 2.65 at the final follow-up post-intervention. Hence a 4.33 scale reduction of VAS was noted. Significant heterogeneity was noted among the included studies (I2 = 97.84%, p < 0.001) Hence, a random-effects model was used for analysis. On stratifying the analysis based on the level of evidence of the studies, we found that VAS score in RCTs were significantly reduced (WMD = 3.644, 95% CI [0.626, 6.663], p < 0.001). Interestingly, we noted similar results upon analyzing the prospective (WMD = 3.483, 95% CI [1.816, 5.149], p < 0.001) and retrospective studies (WMD = 5.535, 95% CI [2.532, 5.734], p < 0.001) respectively as shown in Figure 4.
Figure 4.
Forest plot of the included RCTs, non-randomized prospective, and retrospective studies comparing VAS score between the baseline and final follow-up.
To further analyze the effect of the intervention on the pain relief, we analyzed the results of the included studies based on their mean scores categorized based on the follow-up period found a steady maintenance of pain scores as shown in Figure 5.
Figure 5.

Analysis of VAS score reduction post-intervention across varied follow-up period among the included studies.
Secondary Outcome
ODI Score at Final Follow-Up
3 prospective studies23-25 and 1 retrospective study 32 involving 97 patients reported ODI scores at the final follow-up. The mean ODI score in the included patients pre-intervention was 35.35 which reduced to 22.25 at the final follow-up post-intervention. Hence a mean reduction of 13.1 in the ODI score was noted. We noted significant heterogeneity among the studies included under this meta-analysis (I2 = 96.63%, p < 0.001) Hence, analysis was done by random-effects model. On stratifying the analysis based on the level of evidence of the studies, no significant reduction in the ODI score was noted among prospective studies (WMD = 9.485, 95% CI [-0.801, 19.771], p = 0.071). However, a significant difference was noted in analyzing the retrospective study (WMD = 13.014, 95% CI [2.795, 23.232], p = 0.013) as shown in Figure 6.
Figure 6.
Forest plot of the included prospective and retrospective studies comparing ODI score between the baseline and final follow-up.
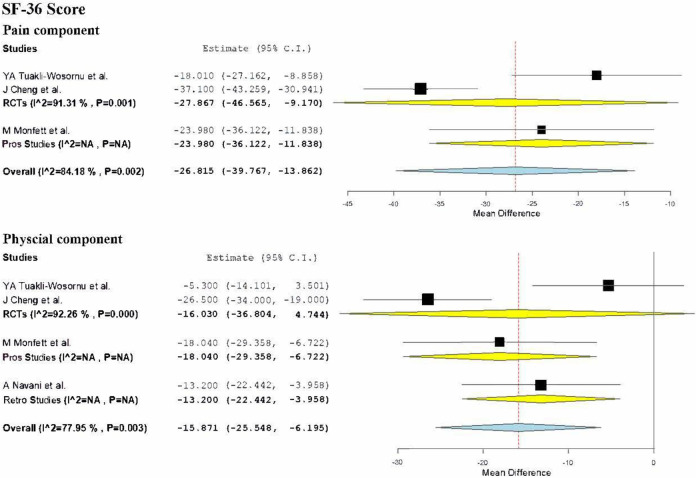
SF-36 Score at Final Follow-Up
SF-36 Pain Score
2 RCTs15,22 involving 64 patients compared the scores of the pain component of the SF-36 health questionnaire. The mean pain score of the included patients pre-intervention was 43.49 which improved to 70.84 at the final follow-up post-intervention. Hence a mean improvement of 27.35 in the pain component of the SF-36 score was noted. We noted a significant heterogeneity among the studies included for analysis (I2 = 91.3%, p < 0.001) Hence, we used random-effects model for analysis. On analysis, a significant improvement in the pain component of SF-36 score was noted (WMD = -27.867, 95% CI [-46.565, -9.170], p = 0.003) as shown in Figure 7.
Figure 7.
Forest plot of the included RCTs, non-randomized prospective, and retrospective studies comparing pain and physical component of SF-36 health questionnaire between the baseline and final follow-up.
SF-36 Physical Score
2 RCTs15,22 and one retrospective study 28 involving 70 patients compared the scores of the physical component of the SF-36 health questionnaire. The mean physical component score of the included patients pre-intervention was 49.2 which improved to 64.2 at the final follow-up post-intervention. Hence a mean improvement of 15 in the physical component of the SF-36 score was noted. Significant heterogeneity was noted among the studies included for analysis (I2 = 85.23%, p < 0.001) Hence, we used random-effects model. On stratifying the analysis based on the level of evidence of the studies, no significant reduction in the ODI score was noted among RCTs (WMD = -16.030, 95% CI [-36.804, 4.744], p = 0.130). However, significant difference was noted on making the analysis including the retrospective study (WMD = -15.180, 95% CI [-27.935, -2.425], p = 0.02) as shown in Figure 7.
Other Outcomes
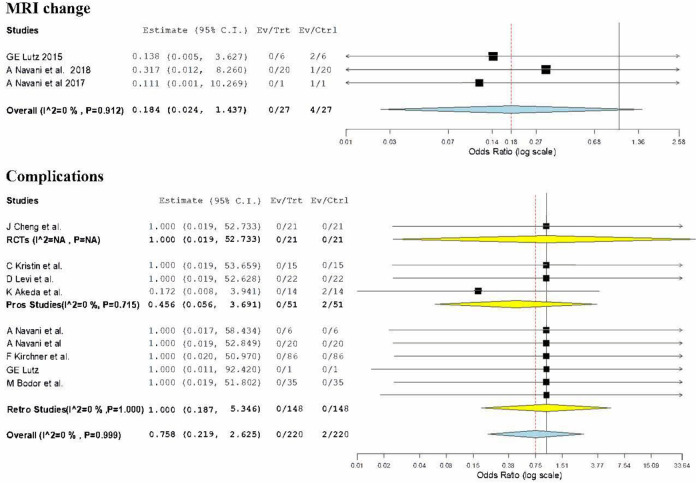
MRI Signal Change
3 retrospective studies28,29,31 involving 27 patients reported the post-operative signal changes in the MRI at the final follow-up. Of the 27 cases, 4 cases had signal changes in the MRI. On analysis no significance difference was noted compared to the pre-operative state (OR = 0.184, 95% CI [0.024, 1.437], p = 0.106) as shown in Figure 8. To take a note, we did not find any significant heterogeneity between individual studies being hereby analyzed (I2 = 0%, p = 0.912).
Figure 8.
Forest plot of the included RCTs, non-randomized prospective, and retrospective studies comparing the complications and reported MRI changes at final follow-up.
Complication Rate
9 studies including 1 RCT 22 , 3 prospective studies23,25,26, and 6 retrospective studies28-32 involving 220 patients reported the complications noted in their study until final follow-up. Of the 220 cases, only 2 cases had a minor complication in the form of transient sensory disturbance which recovered within 7 days of onset. On analysis no significance difference was noted compared to the pre-operative state (OR = 0.758, 95% CI [0.219, 2.625], p = 0.662) as shown in Figure 8. There was no significant heterogeneity between individual studies included in the analysis (I2 = 0%, p = 0.999).
Sensitivity Analysis
Importantly, we performed a sensitivity analysis while analyzing each respective subsets. Results such as VAS score, SF-36 pain component score, MRI signal change, and complications were not significantly altered by sequentially omitting each study in the meta-analysis. However, the significance of the results of the ODI and SF-36 physical component scores were altered on omitting certain studies from the analysis. Hence based on the sensitivity analysis their results were stratified based on the study design and only the results of prospective studies with results on ODI score and SF-36 physical component were taken into further consideration as shown in Figure 5. On the other hand, results maintained their consistency upon reanalysis by changing to random-effects model.
Publication Bias Analysis
Evaluation of the publication bias of the included studies was done using the Funnel plot and Egger Regression test based on the VAS score reported. We hereby mention that we didn’t note any evidence of significant publication bias by the Egger Regression test (p = 0.578) and a symmetric distribution of the studies on both the sides of the plot within the 95% CI implying minimal publication bias as demonstrated by funnel plot in Figure 9.
Figure 9.

Funnel plot for VAS score of the included studies.
Discussion
Autologous PRP is a cocktail of various growth factors with an inherent potential to promote nucleus pulposus differentiation and regeneration.33,34 Due to the enormous availability of growth factor pockets in autologous PRP, they tend to induce the paracrine factors and local mechanisms in the degenerated discs and proceed toward disc regeneration.35,36 With the availability of phenotypically stable cells in the disc, it is possible to regenerate the early degenerated discs with a variety of cytokines derived from autologous PRP. 37 The bio-active micromolecules produce supra-physiological effects in the microenvironment and stabilizes the extracellular matrix which nurtures locally available stem cells to regenerate. 10 The administration of micromolecules under fluoroscopic guidance carries less risk comparing with spinal surgeries and minimizes the risk to spinal injury to the maximum possible extent.
Evidences of both in vivo and in vitro clinical trials have proved the efficacy on the usage of multiple growth factors to induce the disc regeneration with positive results.38-40 Regenerative medicine experts exhibited the proliferation of annulus fibrosus cells after 4 days of TGF-β1 exposure. 41 Hayes et al. 38 revealed the stimulation of sulfated GAG and type I & II collagen synthesis by annulus cells when exposed to TGF-β1 and IGF-1. Administration of IGF-1 and PDGF reduces the percentage of apoptotic annulus fibrosus cells. 41 Chen et al. 42 postulated that PRP promoted the regeneration of nucleus pulposus cells and resulted in significantly accumulation of matrix and raised levels of mRNAs responsible for chondrogenesis, moreover, a significant rise in the disc height index was observed in the PRP regeneration groups. Due to the unstable release of growth factors from the injected PRP, various researchers have introduced the usage of gelatin hydrogel microspheres along with PRP and shown the results of retardation of disc degeneration and regeneration of disc cells. 10 In vivo studies, PRP has shown the improvement of disc height and disc hydration. 43 Thus, platelets serve as a biological sponge as they can absorb, store and transfer micromolecules that regulate disc regeneration. 44
Main Finding
We made a comprehensive and systematic reviewe of all the available literature on intradiscal injection of PRP for lumbar disc disease and found that
Pain relief was noted in patients undergoing an intradiscal injection of PRP based on outcomes like VAS score (p < 0.001) and pain component of the SF-36 questionnaire (p = 0.003) although significant heterogeneity was noted among the included studies.
No functional improvement based on the ODI score (p = 0.071), the physical component of the SF-36 questionnaire (p = 0.130), or structural improvement based on MRI signal changes (p = 0.106) were observed in patients undergoing intradiscal injection of PRP.
No procedure-related additional adverse events were noted among the included studies (p = 0.662)
Comparison With Other Meta-Analyses
J Sanapati et al. 45 assessed the effectiveness of PRP injection for managing pain in lower back wherein their meta-analysis including one RCT and 5 observational studies. They also found a significant reduction in low back pain similar to our results, however, their analysis was limited by the number of included studies, and their results were not stratified accordingly to the design of the studies involved.
T Hirase et al. 46 in their critical review analyzing the role of intradiscal PRP injection for managing degenerative disease involving lumbar disc included one RCT and 4 case series for analysis. Owing to the limited number of included studies, they were able to conclude only on the improvement in pain compared to the baseline and not on the structural or functional components as in our study.
On comparing the results of our meta-analysis with that of the only double-blinded double-arm randomized controlled trial on intradiscal PRP by YA Tuakli-Wosornu 15 they noted a significant improvement in pain and patient satisfaction. On contrary to our finding they founded a significant improvisation in functional improvement based on the SF-36 health survey in both pain and physical component, Functional Rating Index and modified North American Spine Society outcome questionnaire during their short-term follow-up of 8 weeks compared to the controls which need further validation by long term studies.
Apart from these 2 systematic reviews, other reviews 34,35,47-50 involving the intradiscal injection of PRP for lumbar degenerative disc disease were not systematic or comprehensive enough to give an overall summary of evidence on the subject on varied outcomes as analyzed in our study.
Directions for Future
Although studies using PRP for low back pain have shown that the intervention was effective in managing the back pain, only one double-blinded double-arm study with a limited patient number without specific characterization of the PRP preparation demonstrated some positive results in limited outcome measures. Moreover, only a few studies have evaluated and documented successful radiological changes in disc post-intervention. Clinical evidence that PRP particularly induced tissue repair inside the degenerated discs has not been confirmed yet. Hence future studies are needed to establish the definite role of PRP in the management of degenerated disc diseases.
Although the results of the studies analyzed were promising we are awaiting the results of the completed trials on this intervention to further strengthen the evidence on the intervention. 51 Future research on defining the ideal patient characteristics which have the maximum potential for response and the effects of various methods of preparation of PRP such as leukocyte rich- or poor-PRP, with/without activation, either the platelets as a whole or their releasate which provides maximum beneficial role needs to be analyzed in detail in future.
Limitations
The limitation of the current meta-analysis was that it involved single-arm trials which need further validation by large double-blinded double-arm randomized controlled trials. We also had significant heterogeneity among the outcomes measures between the studies included. The practical limitation of the included studies involves the utilization of devices from multiple manufacturers to prepare the PRP used among the studies analyzed.
Conclusion
There is a paucity of high-quality studies to give conclusive evidence on the benefits of intradiscal PRP for lumbar disc disease. Although intradiscal PRP injection has shown some beneficial effect in controlling pain for lumbar disc disease, we could not find structural or functional improvement from the included studies. Hence, we recommend large double-blind double-arm randomized controlled studies to analyze the benefits of the intervention being analyzed.
Supplemental Material
Supplemental Material, sj-docx-1-gsj-10.1177_2192568221998367 for Does the Intradiscal Injection of Platelet Rich Plasma Have Any Beneficial Role in the Management of Lumbar Disc Disease? by Sathish Muthu, Madhan Jeyaraman, Girinivasan Chellamuthu, Naveen Jeyaraman, Rashmi Jain and Manish Khanna in Global Spine Journal
Supplemental Material, sj-docx-2-gsj-10.1177_2192568221998367 for Does the Intradiscal Injection of Platelet Rich Plasma Have Any Beneficial Role in the Management of Lumbar Disc Disease? by Sathish Muthu, Madhan Jeyaraman, Girinivasan Chellamuthu, Naveen Jeyaraman, Rashmi Jain and Manish Khanna in Global Spine Journal
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Sathish Muthu, MS Ortho  https://orcid.org/0000-0002-7143-4354
https://orcid.org/0000-0002-7143-4354
Madhan Jeyaraman, MS Ortho  https://orcid.org/0000-0002-9045-9493
https://orcid.org/0000-0002-9045-9493
Girinivasan Chellamuthu, MS Ortho  https://orcid.org/0000-0001-5800-714X
https://orcid.org/0000-0001-5800-714X
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Vo NV, Hartman RA, Patil PR, et al. Molecular mechanisms of biological aging in intervertebral discs. J Orthop Res Off Publ Orthop Res Soc. 2016;34(8):1289–1306. doi:10.1002/jor.23195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taher F, Essig D, Lebl DR, et al. Lumbar degenerative disc disease: current and future concepts of diagnosis and management. Adv Orthop. 2012;2012:970752. doi:10.1155/2012/970752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freemont AJ. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatol Oxf Engl. 2009;48(1):5–10. doi:10.1093/rheumatology/ken396 [DOI] [PubMed] [Google Scholar]
- 4.Videman T, Nurminen M. The occurrence of anular tears and their relation to lifetime back pain history: a cadaveric study using barium sulfate discography. Spine. 2004;29(23):2668–2676. doi:10.1097/01.brs.0000146461.27105.2b [DOI] [PubMed] [Google Scholar]
- 5.García-Cosamalón J, del Valle ME, Calavia MG, et al. Intervertebral disc, sensory nerves and neurotrophins: who is who in discogenic pain? J Anat. 2010;217(1):1–15. doi:10.1111/j.1469-7580.2010.01227.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng B, Hao J, Hou S, et al. Possible pathogenesis of painful intervertebral disc degeneration. Spine. 2006;31(5):560–566. doi:10.1097/01.brs.0000201324.45537.46 [DOI] [PubMed] [Google Scholar]
- 7.Wang SZ, Chang Q, Lu J, Wang C. Growth factors and platelet-rich plasma: promising biological strategies for early intervertebral disc degeneration. Int Orthop. 2015;39(5):927–934. [DOI] [PubMed] [Google Scholar]
- 8.Masuda K. Biological repair of the degenerated intervertebral disc by the injection of growth factors. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2008;17 Suppl 4:441–451. doi:10.1007/s00586-008-0749-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tavakoli J, Diwan AD, Tipper JL. Advanced strategies for the regeneration of lumbar disc annulus fibrosus. Int J Mol Sci. 2020;21(14). doi:10.3390/ijms21144889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagae M, Ikeda T, Mikami Y, et al. Intervertebral disc regeneration using platelet-rich plasma and biodegradable gelatin hydrogel microspheres. Tissue Eng. 2007;13(1):147–158. doi:10.1089/ten.2006.0042 [DOI] [PubMed] [Google Scholar]
- 11.Bielecki TM, Gazdzik TS, Arendt J, Szczepanski T, Król W, Wielkoszynski T. Antibacterial effect of autologous platelet gel enriched with growth factors and other active substances: an in vitro study. J Bone Joint Surg Br. 2007;89(3):417–420. doi:10.1302/0301-620X.89B3.18491 [DOI] [PubMed] [Google Scholar]
- 12.Fabbro MD, Bortolin M, Taschieri S, Ceci C, Weinstein RL. Antimicrobial properties of platelet-rich preparations. A systematic review of the current pre-clinical evidence. Platelets. 2016;27(4):276–285. doi:10.3109/09537104.2015.1116686 [DOI] [PubMed] [Google Scholar]
- 13.Wang H-L, Avila G.Platelet Rich Plasma: Myth or Reality? Eur J Dent. 2007;1(4):192–194. [PMC free article] [PubMed] [Google Scholar]
- 14.Khalaf K, Nikkhoo M, Ya-Wen Kuo, et al. Recovering the mechanical properties of denatured intervertebral discs through Platelet-Rich Plasma therapy. Conf Proc Annu Int Conf IEEE Eng Med Biol Soc IEEE Eng Med Biol Soc Annu Conf. 2015;2015:933–936. [DOI] [PubMed] [Google Scholar]
- 15.Tuakli-Wosornu Y, Terry A, Boachie-Adjei K, et al. Lumbar intradiskal platelet-rich plasma (PRP) injections: a prospective, double-blind, randomized controlled study. PM&R. 2016;8(1):1–10. [DOI] [PubMed] [Google Scholar]
- 16.Furlan AD, Malmivaara A, Chou R, et al. 2015 updated method guideline for systematic reviews in the Cochrane back and neck group. Spine. 2015;40(21):1660–1673. doi:10.1097/BRS.0000000000001061 [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA statement. PLOS Med. 2009;6(7):e10000.97. doi:10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi:10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 19.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355. doi:10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Closing the Gap between Methodologists and End-Users: R as a Computational Back-End | Wallace | Journal of Statistical Software. Accessed September 23, 2020. https://www.jstatsoft.org/article/view/v049i05
- 21.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng J, Santiago K, Nguyen J, et al. Treatment of symptomatic degenerative intervertebral discs with autologous platelet-rich plasma: follow-up at 5-9 years. Regen Med. 2019;14(9):831–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kristin C, Robert S, Michelle P, Kristin C, Robert S, Michelle P. Effects of the intradiscal implantation of stromal vascular fraction plus platelet rich plasma in patients with degenerative disc disease. J Transl Med. 2017;15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain D, Goyal T, Verma N, Paswan AK, Dubey RK. Intradiscal platelet-rich plasma injection for discogenic low back pain and correlation with platelet concentration: a prospective clinical trial. Pain Med Malden Mass. Published online 2020. https://pubmed.ncbi.nlm.nih.gov/32869064/ [DOI] [PubMed]
- 25.Levi D, Horn S, Tyszko S, et al. Intradiscal platelet-rich plasma injection for chronic discogenic low back pain: preliminary results from a prospective trial. PAIN Med. 2016;17(6):1010–1022. [DOI] [PubMed] [Google Scholar]
- 26.Akeda K, Ohishi K, Masuda K, et al. Intradiscal injection of autologous platelet- rich plasma releasate to treat discogenic low back pain: a preliminary clinical trial. ASIAN SPINE J. 2017;11(3):380–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monfett M, Harrison J, Boachie-Adjei K, Lutz G. Intradiscal platelet-rich plasma (PRP) injections for discogenic low back pain: an update. Int Orthop. 2016;40(6):1321–1328. doi:10.1007/s00264-016-3178-3 [DOI] [PubMed] [Google Scholar]
- 28.Navani A, Hames A.Platelet-rich plasma injections for lumbar discogenic pain: a preliminary assessment of structural and functional changes. Tech Reg Anesth Pain Manag. 2015;19(1):38–44. doi:10.1053/j.trap.2016.09.007 [Google Scholar]
- 29.Navani A, Ambach MA, Navani R, Wei J. Biologics for Lumbar Discogenic Pain: 18 month follow-up for safety and efficacy. 2018;2(3):8. [Google Scholar]
- 30.Kirchner F, Anitua E. Intradiscal and intra-articular facet infiltrations with plasma rich in growth factors reduce pain in patients with chronic low back pain. J Craniovertebral Junction Spine. 2016;7(4):250–256. doi:10.4103/0974-8237.193260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lutz GE. Increased nuclear T2 signal intensity and improved function and pain in a patient one year after an intradiscal platelet-rich plasma injection. Pain Med Malden Mass. 2017;18(6):1197–1199. doi:10.1093/pm/pnw299 [DOI] [PubMed] [Google Scholar]
- 32.Bodor M, Toy A, Aufiero D. Disc regeneration with platelets and growth factors. In: Lana JFSD, Andrade Santana MH, Dias Belangero W, Malheiros Luzo AC, eds. Platelet-Rich Plasma: Regenerative Medicine: Sports Medicine, Orthopedic, and Recovery of Musculoskeletal Injuries. Lecture Notes in Bioengineering. Springer; 2014:265–279. doi:10.1007/978-3-642-40117-6_14 [Google Scholar]
- 33.Akeda K, An HS, Pichika R, et al. Platelet-rich plasma (PRP) stimulates the extracellular matrix metabolism of porcine nucleus pulposus and anulus fibrosus cells cultured in alginate beads. Spine. doi:10.1097/01.brs.0000214942.78119.24 [DOI] [PubMed] [Google Scholar]
- 34.Basso M, Cavagnaro L, Zanirato A, et al. What is the clinical evidence on regenerative medicine in intervertebral disc degeneration? Musculoskelet Surg. 2017;101(2):93–104. [DOI] [PubMed] [Google Scholar]
- 35.Mohammed S, Yu J. Platelet-rich plasma injections: an emerging therapy for chronic discogenic low back pain. J Spine Surg Hong Kong. 2018;4(1):115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang S, Rui Y, Tan Q, et al. Enhancing intervertebral disc repair and regeneration through biology: platelet-rich plasma as an alternative strategy. ARTHRITIS Res Ther. 2013;15(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masuda K, Oegema TR, Jr, An HS. Growth factors and treatment of intervertebral disc degeneration. Spine. doi:10.1097/01.brs.0000146048.14946.af [DOI] [PubMed] [Google Scholar]
- 38.Hayes AJ, Ralphs JR. The response of foetal annulus fibrosus cells to growth factors: modulation of matrix synthesis by TGF-β1 and IGF-1. Histochem Cell Biol. 2011;136(2):163–175. doi:10.1007/s00418-011-0835-x [DOI] [PubMed] [Google Scholar]
- 39.Pratsinis H, Kletsas D. PDGF, bFGF and IGF-I stimulate the proliferation of intervertebral disc cells in vitro via the activation of the ERK and Akt signaling pathways. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2007;16(11):1858–1866. doi:10.1007/s00586-007-0408-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujita N, Imai J-I, Suzuki T, et al. Vascular endothelial growth factor-A is a survival factor for nucleus pulposus cells in the intervertebral disc. Biochem Biophys Res Commun. doi:10.1016/j.bbrc.2008.05.044 [DOI] [PubMed] [Google Scholar]
- 41.Gruber HE, Fisher EC, Desai B, Stasky AA, Hoelscher G, Hanley EN. Human intervertebral disc cells from the annulus: three-dimensional culture in agarose or alginate and responsiveness to TGF-beta1. Exp Cell Res. 1997;235(1):13–21. doi:10.1006/excr.1997.3647 [DOI] [PubMed] [Google Scholar]
- 42.Chen W-H, Liu H-Y, Lo W-C, et al. Intervertebral disc regeneration in an ex vivo culture system using mesenchymal stem cells and platelet-rich plasma. Biomaterials. 2009;30(29):5523–5533. doi:10.1016/j.biomaterials.2009.07.019 [DOI] [PubMed] [Google Scholar]
- 43.Sawamura K, Ikeda T, Nagae M, et al. Characterization of in vivo effects of platelet-rich plasma and biodegradable gelatin hydrogel microspheres on degenerated intervertebral discs. Tissue Eng Part A. 2009;15(12):3719–3727. doi:10.1089/ten.TEA.2008.0697 [DOI] [PubMed] [Google Scholar]
- 44.Leslie M. Cell biology. Beyond clotting: the powers of platelets. Science. 2010;328(5978):562–564. doi:10.1126/science.328.5978.562 [DOI] [PubMed] [Google Scholar]
- 45.Sanapati J, Manchikanti L, Atluri S, et al. Do regenerative medicine therapies provide long-term relief in chronic low back pain: a systematic review and metaanalysis. Pain Physician. 2018;21(6):515–540. [PubMed] [Google Scholar]
- 46.Hirase T, Jack Ii RA, Sochacki KR, Harris JD, Weiner BK. Systemic review: is an intradiscal injection of platelet-rich plasma for lumbar disc degeneration effective? Cureus. 2020;12(6):e8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anitua E, Padilla S. Biologic therapies to enhance intervertebral disc repair. Regen Med. 2018;13(1):55–72. [DOI] [PubMed] [Google Scholar]
- 48.Tendulkar G, Chen T, Ehnert S, et al. Intervertebral disc nucleus repair: hype or hope? Int J Mol Sci. 2019;20(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Urits I, Viswanath O, Galasso A, et al. Platelet-rich plasma for the treatment of low back pain: a comprehensive review. Curr Pain Headache Rep. 2019;23(7). [DOI] [PubMed] [Google Scholar]
- 50.Akeda K, Yamada J, Linn ET, Sudo A, Masuda K. Platelet-rich plasma in the management of chronic low back pain: a critical review. J Pain Res. 2019;12:753–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Platelet-rich Plasma for Low Back Pain – Full Text View – ClinicalTrials.gov. Accessed September 26, 2020. https://clinicaltrials.gov/ct2/show/NCT03197415
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-docx-1-gsj-10.1177_2192568221998367 for Does the Intradiscal Injection of Platelet Rich Plasma Have Any Beneficial Role in the Management of Lumbar Disc Disease? by Sathish Muthu, Madhan Jeyaraman, Girinivasan Chellamuthu, Naveen Jeyaraman, Rashmi Jain and Manish Khanna in Global Spine Journal
Supplemental Material, sj-docx-2-gsj-10.1177_2192568221998367 for Does the Intradiscal Injection of Platelet Rich Plasma Have Any Beneficial Role in the Management of Lumbar Disc Disease? by Sathish Muthu, Madhan Jeyaraman, Girinivasan Chellamuthu, Naveen Jeyaraman, Rashmi Jain and Manish Khanna in Global Spine Journal