Abstract
Study Design:
Prospective cohort study.
Objectives:
Management of osteoporotic vertebral compression fracture (OVCF) remains an unsolved problem for a spine surgeon. We hypothesize that instability at the fracture site rather than neural compression is the main factor leading to a neurological deficit in patients with OVCF.
Methods:
In this study, the prospective data of patients with osteoporotic fractures with incomplete neurological deficits from January 2015 to December 2017 was analyzed in those who underwent posterior instrumented fusion without neural decompression.
Results:
A total of 61 patients received posterior indirect decompression via ligamentotaxis and stabilization only. Of these 17 patients had polymethylmethacrylate (PMMA) augmented screws and in 44 patients no PMMA augmentation was done. The mean preoperative kyphosis was 27.12° ± 9.63°, there was an improvement of 13.5° ± 6.87° in the immediate postoperative period and at the final follow-up, kyphosis was 13.7° ± 7.29° with a loss of correction by 2.85° ± 3.7°. The height restoration at the final follow-up was 45.4% ± 18.29%. In all patients, back pain was relieved, and neurological improvement was obtained by at least 1 American Spinal Injury Association Impairment Scale in all except 3 patients.
Conclusion:
We propose that neural decompression of the spinal cord is not always necessary for the treatment of neurological impairment in patients with osteoporotic vertebral collapse with dynamic mobility. Dynamic magnetic resonance imaging is a valuable tool to make an accurate diagnosis and determine precise surgical plan and improving the surgical strategy of OVCF.
Keywords: osteoporosis, vertebral compression fracture, thoracolumbar, neurological deficit
Introduction
Osteoporotic vertebral compression fracture (OVCF) remains an enigma for a spine surgeon. The ability of the osteoporotic fragile bone to optimally hold spinal implants is doubtful. Hence, the question of how to improve surgery for osteoporotic vertebral collapse accompanied by paraparesis remains unanswered and is a topic of discussion even today. Previously published reports have shown that incomplete neurological deficits following a vertebral collapse in the osteoporotic thoracolumbar spine is usually due to neural compression by retropulsed bone fragments in the spinal canal, progression of kyphotic deformity and instability at the fracture site. Most of the authors have stressed the importance of neural decompression, which is achieved by either an anterior or posterior approach or by a combined anterior plus posterior approach.1-6
Previously published literature reports that fractured vertebrae gradually undergoes remodeling and normal anatomy of the spinal canal is regained with time.7,8 Hence the role of neural decompression to increase the area available for neural elements within the spinal canal is debatable. We evaluated patients presenting with OVCF with dynamic Magnetic resonance imaging (MRI) and hypothesized that instability at the fracture site rather than neural compression is the main factor leading to neurological deficits in patients with osteoporotic thoracolumbar vertebral collapse and have performed posterior instrumented fusion without neural decompression. Through this study, we aim to analyze functional outcomes of treating osteoporotic thoracolumbar fractures with incomplete neurological deficits by posterior instrumentation without neural decompression.
Materials and Methods
This prospective study was conducted in the department of spine services at a tertiary care spine institute. Approval from the institutional review board and institutional ethics committee was taken before commencement (ISIC/RP/2015/014). Data of patients with osteoporotic fractures with neurological deficits from January 2015 to December 2017 was analyzed. Surgical indication for this study was vertebral collapse or compression in the thoracolumbar spine with neurologic deficit with or without bowel and bladder involvement and with compression over the spinal cord on MRI. Every patient had body collapse at one or more vertebral body level.
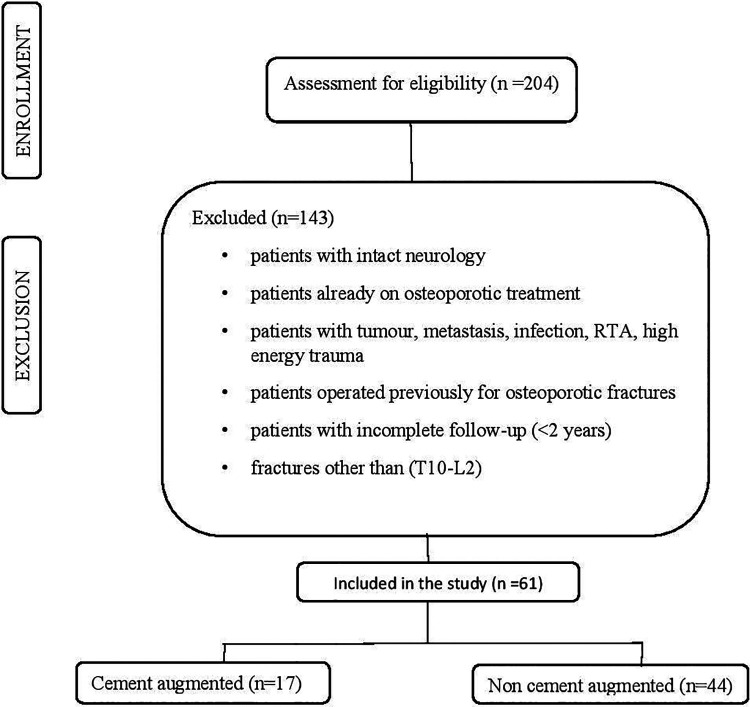
Consecutive patients with confirmed osteoporosis with BMD (bone mineral density) <2.5, 4 neurological deficits with or without bowel and bladder involvement previously not on antiosteoporotic treatment who underwent posterior only approach with fixation without neural decompression with a minimum follow-up duration of 2 years were included in the study. Dual-energy X-ray absorptiometry (DEXA) was used to measure BMD at the lumbar spine (L1-L4), hip and forearm (3-site DEXA scan) and a T-score ≤2.5 SD at the femoral neck as defined by the World Health Organization was one of the prerequisites for inclusion in the study. Patients with fractures caused by any diagnosis other than osteoporosis (metastasis, tumor, infection, etc), those who have sustained high energy injury, those who were operated previously for osteoporotic fractures, or those with incomplete records were excluded (Figure 1). Demographic data of all the patients, including age, sex, comorbidities, and medications that can lead to secondary osteoporosis, the number of vertebrae fractured, and levels involved were collected. Postoperatively, the antiosteoporotic treatment taken by all patients and the incidence of new-onset fractures were also taken into consideration during follow-up. Information regarding surgical technique, including levels of fixation, mode of stabilization, use of cement augmentation for the fractured vertebral body, use of cement augmentation for pedicle screw insertion was assessed and a case report form was prepared. Instrumentation related complications like screw cut out, implant loosening, rod breakage, and also adjacent segment fractures/degeneration were assessed. Pre- and postoperative neurologic status and neurologic status at the final follow-up were assessed using International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI). 9
Figure 1.
Flowchart: selection and inclusion of patient data set.
Radiological Examination
Preoperative plain radiographs, DEXA scan, and MRI were taken for all patients (Model: 1.5 Tesla Magnetom Avanto, SIEMENS Equip ID: 1 007 694 794). All the patients were evaluated by dynamic MRI (dMRI) (flexion and extension protocol) under supervision as shown in Figure 2 to establish the dynamic nature of spinal cord compression (Figure 3). In all patients, MRI showed the compression of neural tissues by a protruding bony fragment to a variable extent on dynamic imaging. MRI can identify vertebral body edema, which may be unrecognized in conventional radiographs, hence it proves to be a sensitive tool. It is also useful in patients with chronic persistent pain and will show a typical fluid signal within the vertebral body diagnostic of pseudoarthrosis. The presence or absence of spinal canal compression was also assessed in flexion, neutral and extension positions of MRI and compared. In most patients, short tau inversion recovery (STIR) sequences are sufficient. Plain radiographs were reviewed before surgery, immediately after surgery, and at 3- to 6-month intervals after surgery up to the latest follow-up. The local kyphosis angle was measured as the angle between the lower endplate of the uninvolved vertebrae above the fractured level, and the upper endplate of the uninvolved vertebra below the fractured level. Restoration of kyphotic angle (difference in preoperative and postoperative angle of the focal kyphosis) were measured along with loss of vertebral height at the end of 1 year follow-up. Vertebral height restoration on lateral plain radiographs as described by Leiberman et al 10 as percentage restored in the preoperative period and immediate postoperative period and follow-up as described in Figure 4. The neurological deficit in patients with compression on dynamic MRI and those with compression on static MRI was compared using ASIA (American Spinal Injury Association) motor score. The recovery rate of ASIA score is defined as [ASIA score at time of latest follow-up – Initial ASIA score]/50 − Initial ASIA score as described by Zhang et al. 11 Cement leak, recollapse, and adjacent segment fracture were noted. In the majority of cases, the degree of posterior cortex lesion can be evaluated sufficiently by MRI and conventional radiographs.
Figure 2.
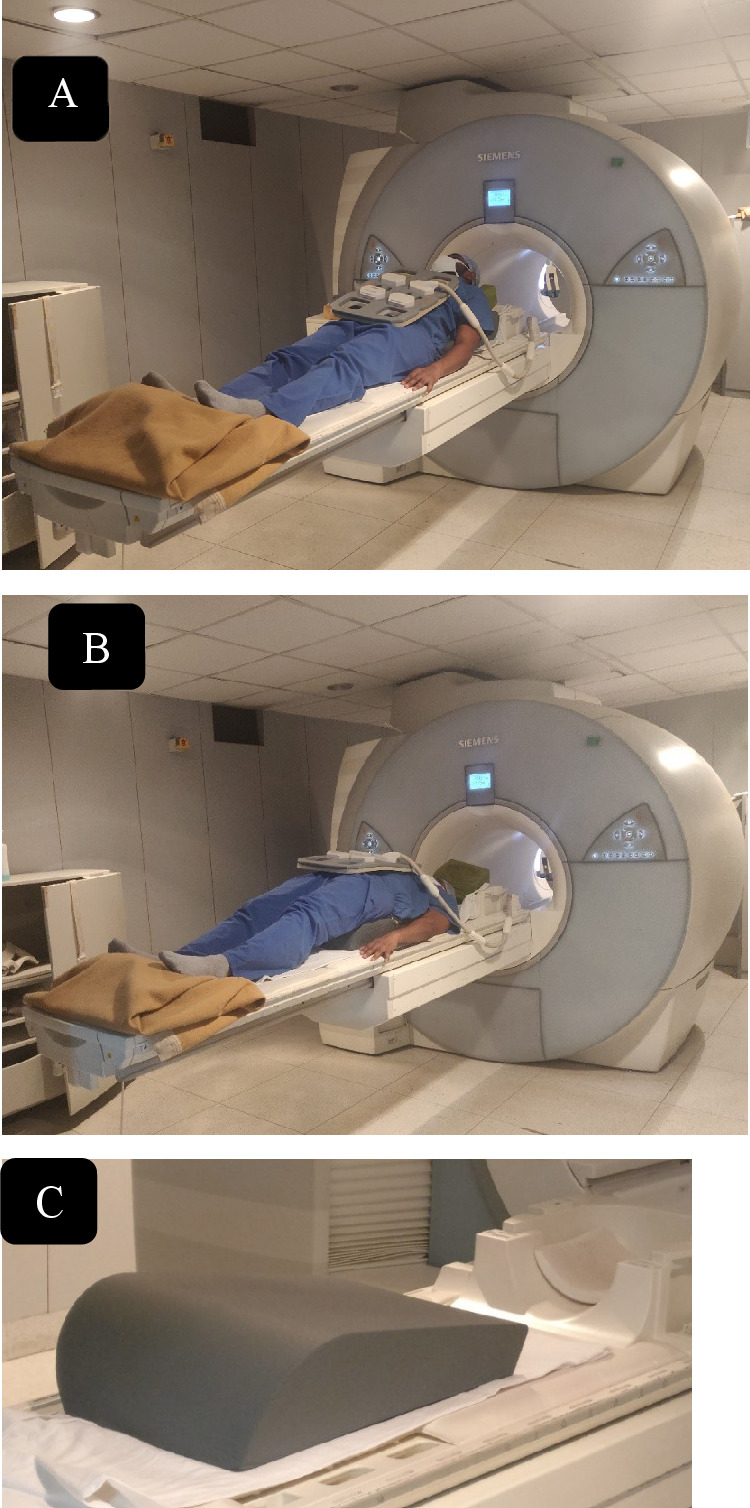
Representative image showing flexion (A) and extension (B) protocol of dynamic magnetic resonance imaging (MRI) of thoracolumbar spine. (C) Wedge-shaped bolster used for positioning in dynamic MRI.
Figure 3.
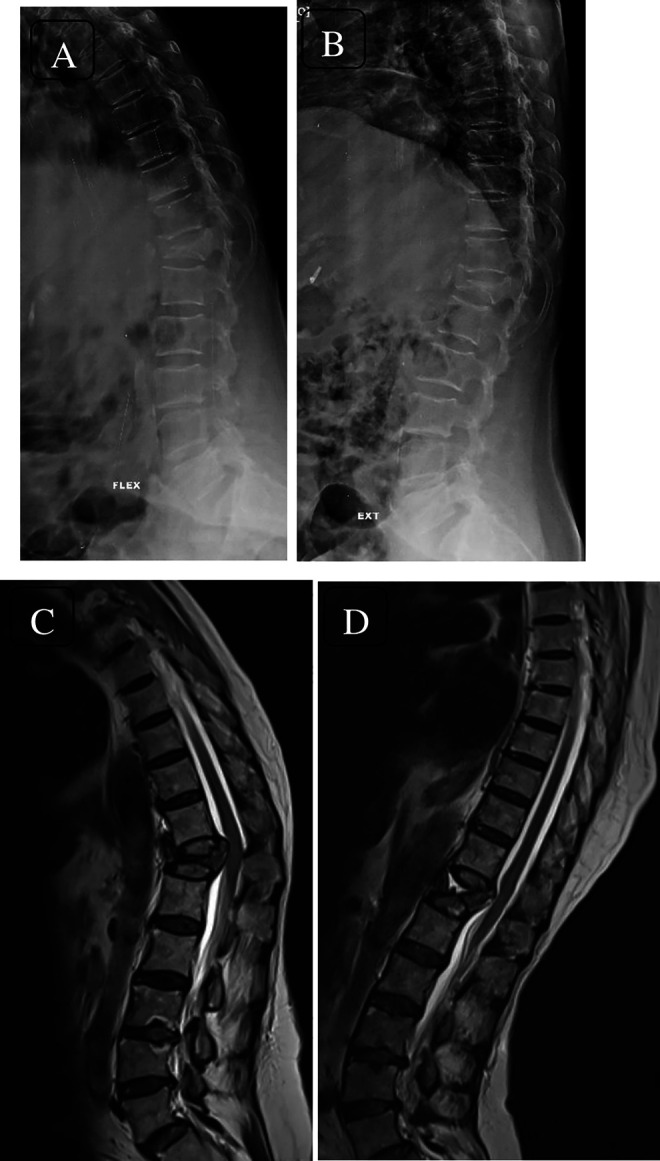
Dynamic lateral radiographs (flexion—A; extension—B) showing osteoporotic fracture of T12 vertebra with dynamic spinal cord compression seen on dynamic magnetic resonance imaging (flexion MRI—C; extension MRI—D). Spinal compression due to bony protrusion into the canal on flexion and restoration of cerebrospinal fluid column in extension can be appreciated.
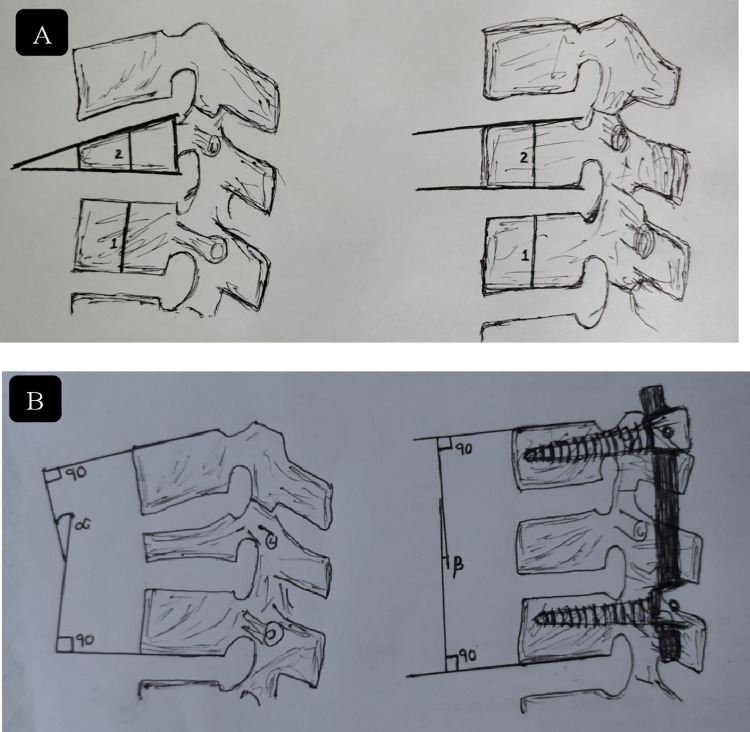
Figure 4.
(A) Method of Leiberman: % height improvement = postkyphoplasty % (2/1 × 100) – prekyphoplasty % value (2/1 × 100) ÷ 100% − prekyphoplasty % value. (B) Cobbs method for measuring focal kyphosis angle, angle between superior end plate of upper normal vertebra and inferior end plate of lower normal vertebra.
Surgical Technique
All patients were operated in a prone position under general anesthesia. A standard posterior midline approach was taken. Pedicle screws were inserted by freehand technique under fluoroscopic guidance. The decision of whether to augment the screw with cement or not was taken by the operating surgeon depending on the subjective assessment of the bone quality as per insertional torque. Posterior instrumentation without neural decompression was done in all cases. We did not perform laminectomy in any patient included in this series. Vertebroplasty augmented pedicle screws were inserted in select cases (Figure 5). Vertebroplasty was performed in patients in whom vertebral cleft was evident on postural reduction and fracture segment screw was not inserted. The reduction of the retropulsed fragment was attempted by positioning and intraoperative ligamentotaxis only, using minimal force to fix the connecting rods in-situ to position obtained. There was no attempt to actively correct either kyphosis or vertebral height by applying force to the implant or manual corrective adjustment. The preoperative dynamic MRI revealed amount of passive correction of the alignment of the thoracolumbar spine, which was evident on positioning the patient prone intraoperatively. The rod was contoured to suit the regional spinal alignment and fixed in situ as per the postural reduction. This is especially important in an osteoporotic spine where applying reduction force on pedicle screws may cause loosening. The closure was done in layers with a negative suction drain.
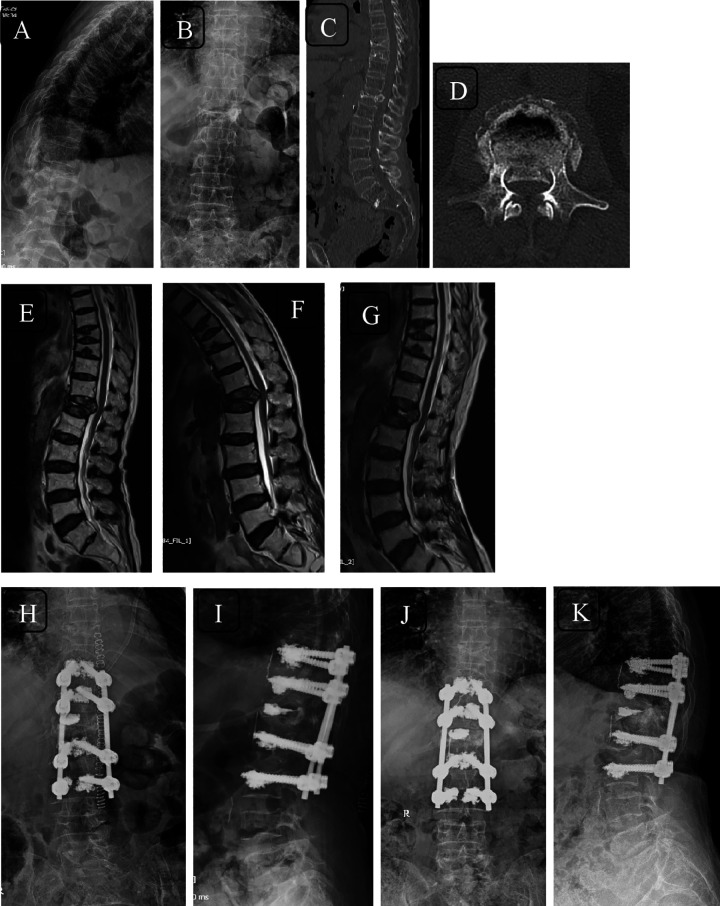
Figure 5.
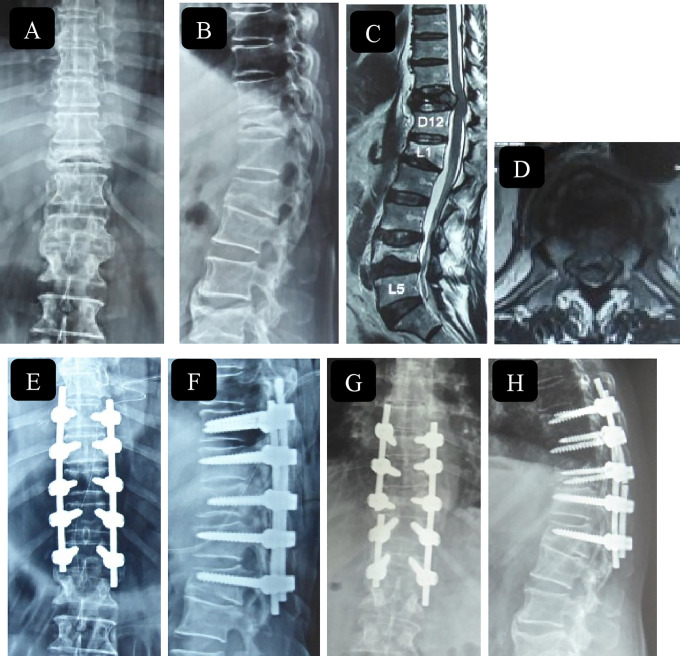
Radiographs (A, B) and computed tomography (CT) scans (sagittal—C; axial—D) of a 61-year-old woman with fracture T12 with incomplete neurology with canal compromise evident on CT and neutral magnetic resonance imaging (MRI) (E). The dynamic nature of compression on spinal cord can be appreciated on flexion MRI (F) and canal clearance on extension MRI (G). Immediate postoperative images (H, I) and 2-year follow-up images (J, K) showing cement augmented long segment fixation.
Patients were mobilized on the second postoperative day as per pain tolerance. Drain removal was done when output fell below 50 mL. The follow-up was done at 2 weeks, 3 months, 6 months, 1 year, and 2 years. Postsurgery antiosteoporotic treatment was started in all patients.
Statistical Analysis
SPSS software version 25 (IBM SPSS, IBM Corp, 2017) was used for statistical analysis. The results were represented in terms of mean and standard deviation. A P value of less than .05 was considered statistically significant. This study compares pre- and postoperative data for kyphosis angle and vertebral height restoration. We used t test to compare the results of preoperative kyphotic angle, immediate postoperative kyphosis, and follow-up period. McNemar test was used to compare spinal cord compression on flexion and extension MRI. Correlation between preoperative kyphosis and percentage change in height restoration was done using Pearson’s correlation (R = 11.92%, P < .05). Nonparametric statistical analysis (ie, median values for 2-group comparison and Fisher’s test for categorical variables) was performed considering the relatively small sample size.
Results
In this study, 63 patients received posterior indirect decompression via ligamentotaxis and stabilization only. Two patients were excluded as they required revision surgery due to implant failure. Of these, 21 were males and 40 were females. The mean age was 70 years (age ranged from 41 to 87 years). The follow-up period ranged from 2 to 4 years. Only patients with a minimum follow-up period of 2 years were included. All patients had a T score <2.5. The mean T score in our series was −2.8 (range −2.5 to −5.8).
No obvious history of injury was given by 38 patients and they reported spontaneous onset of low back pain progressive in nature. Twenty patients had a trivial fall and 3 patients reported a fall from height. The age at injury (acute/subacute/chronic) could not be established based on the available history as majority of the patients did not have any significant trauma prior to onset of symptoms. Seven patients had fractures at more than 1 vertebral level. The distribution of fracture levels showed maximum fractures at T12 vertebra followed by L1 vertebra, T11 vertebra, L2 vertebra, and T10 vertebra. Except for 3 patients all the patients had comorbidities owing to the age or preexisting diseases. Nearly half the patients had coexisting hypertension and type 2 diabetes mellitus (n = 29). Hypertension was present in 36 patients and 7 patients had hypothyroidism. All the patients had low back pain localizing to the thoraco-lumbar region and low-lumbar region, and weakness in lower limbs with difficulty in walking. Bowel and bladder involvement (incontinence, urgency, frequency, hesitation) was present in 22 patients (Table 1). In 6 patients, the most likely cause for osteoporosis was steroid intake. The patients had coexisting diseases requiring long-term administration of steroids.
Table 1.
Demographic Data and Statistical Analysis of the Patient Population.
| Variable | Improved | Not Improved | P |
|---|---|---|---|
| Gender (n = 61) | .852 | ||
| Male (n = 21) | 20 | 1 | |
| Female (n = 40) | 38 | 2 | |
| BBI involvement (n = 22) | 18 | 4 | <.000* |
| Functional status | <.000* | ||
| Walker (n = 22) | 22 | 0 | |
| No walker(n = 39) | 36 | 3 | |
| Trauma (n = 23) | .564 | ||
| Fall from minor height | 3 | 1 | |
| Trivial fall | 19 | 1 | |
| Vertebral level | .473 | ||
| T10 (n = 2) | 2 | — | |
| T11 (n = 9) | 9 | — | |
| T12 (n = 23) | 22 | 1 | |
| L1 (n = 20) | 18 | 2 | |
| L2 (n = 7) | 7 | — | |
| Steroid (n = 6) | 5 | 1 | .753 |
| Levels fixation | .555 | ||
| Long (n = 38) | 36 | 2 | |
| Short (n =23) | 22 | 1 | |
| Cement augmentation | .821 | ||
| Long-segment PMMA | 11 | 1 | |
| Short-segment PMMA | 6 | 1 |
Abbreviations: BBI, bowel and bladder involvement; T, thoracic; L, lumbar; PMMA, polymethylmethacrylate.
The mean preoperative kyphosis was 27.12° ± 9.63° (range 9° to 50°) as measured from the upper endplate of the upper normal vertebra and lower end plate of the lower normal vertebra using Cobb’s method. There was an improvement of an average of 13.5° ± 6.87° (range 0° to 32°) in the immediate postoperative period (13.7° ±7.29°) (range 2° to 44°) and at the final follow-up kyphosis was (16.67° ± 6.87°) (range 11° to 29°) with a loss of correction by 2.85° ± 3.7° when compared with immediate postoperative period. Thus, the mean change in the local kyphosis angle in preoperative and immediate postoperative demonstrates the marked instability at the collapsed vertebrae. Spinal canal compression anteriorly was majorly due to bone fragments was shown on MRI. The height restoration at the final follow-up was 45.4% ± 18.29% with a range of 0% to 78%.
Seventeen patients received polymethylmethacrylate (PMMA) augmentation screws (long-segment fixation n = 12, short-segment fixation n = 5) and the remaining 44 patients did not need PMMA augmentation (long-segment fixation n = 26, short-segment fixation n = 18). Vertebroplasty was performed in 5 patients of long-segment fixation (n = 38) and 8 patients with short-segment fixation (n = 23) in whom vertebral cleft was evident on postural reduction and fracture segment screw was not inserted. A total of 22 patients improved from AIS (ASIA Impairment Scale) grade D to E, 25 from grade C to E, 7 from grade C to D, and 4 patients improved from grade B to D (Table 2). In 2 patients with grade B and 1 with grade C, there was no change in neurological status. Using Fisher’s exact test, the correlation between long- or short-segment fixation and improvement in neurology was done, which was found to be not significant as seen in Table 1.
Table 2.
Preoperative and Postoperative Neurological Status at Follow-up.
| ASIA Impairment Scale grade | Patients, n | Postoperative B | Postoperative C | Postoperative D | Postoperative E |
|---|---|---|---|---|---|
| Preoperative | |||||
| B | 6 | 2 | 0 | 4 | 0 |
| C | 33 | 0 | 1 | 7 | 25 |
| D | 22 | 0 | 0 | 0 | 22 |
| Total | 61 | 2 | 1 | 11 | 47 |
Abbreviation: ASIA, American Spinal Injury Association.
In the present study, all the patients showed spinal cord compression on flexion MRI, which was not relieved on extension protocol MRI as evident by restoration of spinal alignment and cerebrospinal fluid (CSF) column. A total of 65.56% (40/61) patients did not show any significant compression on neutral MRI. Based on McNemar test, the prevalence of compression was higher in flexion position as compared with extension position. (P < .001). The mean ASIA motor score in patients with compression on dynamic MRI improved from 23.7 ± 4.3 to 39.0 ± 6.1. The mean ASIA motor score in patients with compression on static MRI improved from 21.2 ± 3.4 to 36.8 ± 7.0. The difference in neurological recovery between these 2 groups was not statistically significant.
There were 2 episodes of wound dehiscence, which healed by secondary suturing and 2 cases of screw pull out, 1 in the cement augmented group (Figure 6). These cases were managed by adding another fixation level and were excluded from the study. Rod breakage was seen in 1 case with no subsequent new-onset neurological deficits (Figure 7). Subsequent adjacent vertebral compression fractures developed in 19 patients after surgery at latest follow-up, which were managed nonoperatively. No patients complained of residual back pain at the final follow-up.
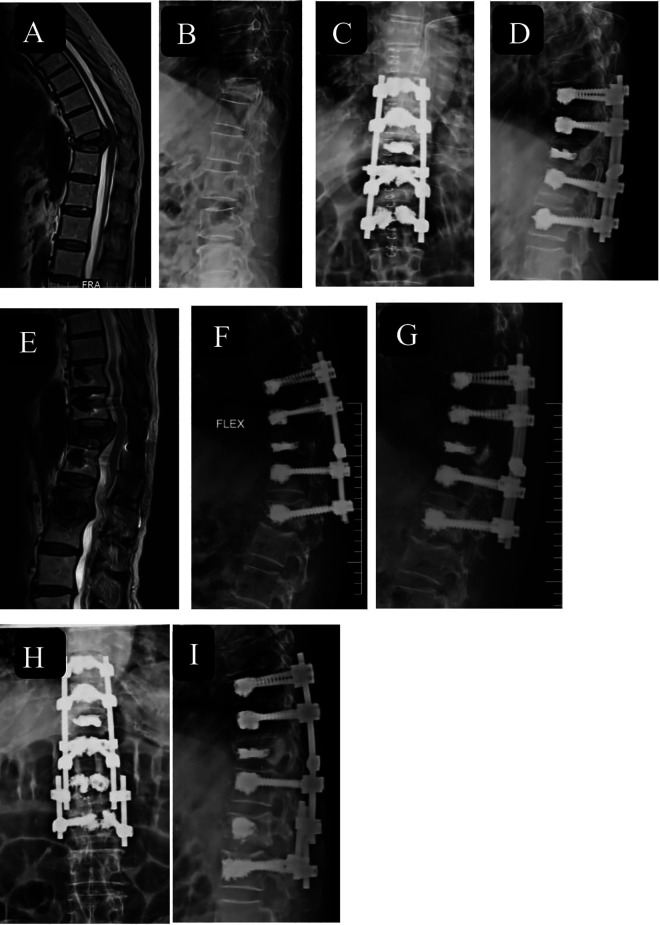
Figure 6.
An 80-year-old man with flexion magnetic resonance imaging (MRI) (A) and X-ray lateral (B) image showing osteoporotic vertebral compression fracture (OVCF) T11 vertebra with retropulsion of bone fragment and dynamic cord compression with American Spinal Injury Association (ASIA) grade C neurology. Immediate postoperative X-rays (C, D) showing cement augmented long-segment fixation of D9-L1 and vertebroplasty of fracture D11 vertebra with postoperative MRI image (E) showing adequate cord decompression. Failure of instrumentation at distal end at 1-year follow-up (F, G) and extension of construct up to L2 using dominoes (H, I).
Figure 7.
A 65-year-old man with American Spinal Injury Association (ASIA) grade C neurology with D11 vertebral fracture with intravertebral cleft seen on X-ray (A, B) and magnetic resonance imaging (MRI), sagittal (C) and axial (D) section with cord compression due to repulsion of bone fragments and old healed osteoporotic fracture L1 and L4. Immediate postoperative images showing long-segment fixation from D9 to L1. At latest follow-up at 2 years, the patient had rod breakage with recollapse of D11 vertebral segment with loss of correction. Patient improved neurologically to ASIA grade E.
Discussion
Osteoporosis is a skeletal disease with the characteristic feature of low bone mass leading to a micro architectural deterioration of bone. 12 This increases the risk of fracture even with a trivial injury. The clinical and radiologic appearances of fractures in the osteoporotic spine are different from those of a healthy spine. OVCFs are increasingly being recognized as an important health care issue because these fractures can result in significant morbidity and even potential mortality. The burden of OVCF is going to increase drastically given the demographic changes leading to an aging society with an increased life span. 13 Most of these fractures may heal, but 15% to 35% may lead to adverse outcomes. 12 These include chronic pain, poor chest function, decreased appetite, kyphotic deformity, fatigue, and neurological deficit with its resultant immobility. Also, prolonged bed rest while the fracture heals may be associated with problems of recumbency like a pressure sore. The lower thoracic spine and thoracolumbar junction are the regions highly susceptible to vertebral fractures. These areas account for 60% of such lesions. 13
Benign osteoporotic compression fractures without any neurologic deficits are stable by nature. They involve only the anterior column of the vertebral body. These injuries usually result from axial compression load without shear, translational, or rotational force. Therefore, nonoperative treatment in form of brace wear, analgesics, mobilization as per the pain protocol along with medical management of osteoporosis is considered as the initial treatment of choice. 14 Incomplete neurological deficits following a vertebral collapse in the osteoporotic thoracolumbar spine can be caused by neural compression due to retropulsed bone fragments in the spinal canal, progression of kyphosis, and instability at the fracture site. 15 The neurologic deficit can range from acute paraplegia (usually after an acute crush fracture) to delayed onset of insidious paraparesis. The latter phenomenon is usually caused by the delayed vertebral collapse and progressive kyphotic deformity. Patients with unstable fractures, chronic pseudoarthrosis, neurological deficit, and vertebral deformities usually need spinal instrumentation. 16
Dynamic MRI is a valuable adaptation of conventional MRI to obtain load-bearing images in flexion–extension positions. Additional information inaccessible on routine supine MRI can be demonstrated noninvasively using dMRI. Various pathological features, like compression of dural sac, nerve root, dynamic mobility of bony protrusion into the canal, buckling of the ligamentum flavum, and/or decrease in foramen size may be detected. Also, traditional MRI may underestimate the severity of pathologic fractures in the dorsolumbar junction. In all, 40/61 patients did not reveal the any significant compression on neutral MRI. All of them showed significant retropulsion and compression of spinal cord in flexion MRI, which was relieved in extension MRI with restoration of CSF column and spinal alignment. Hence dMRI in our study helped us in making an accurate diagnosis and determining the precise surgical plan allowing better understanding of the true nature of the pathology dynamic vertebral motion and improving the surgical strategy of OVCF.17,18
The surgical modalities for OVCF with neurological deficits include anterior, posterior, or combined approaches. Many surgical procedures have been reported successfully effecting neurological improvement in patients with neurological deficits due to osteoporotic vertebral collapse. Kaneda et al 4 and Uchida et al 19 have reported the significance of anterior decompression and reconstruction with the use of anterior instrumentation. However, implant-related complications and pseudoarthrosis occurred in some patients in their series because of poor bone quality. Advantages of anterior surgery with a vertebral spacer include direct resection of the retropulsed bony fragment under visualization and the reconstruction of the stable anterior spinal column, which carries the majority of the axial load (80% in the lumbar spine as compared with 20% carried by posterior column). However, this approach is morbid and is associated with pulmonary, urologic, intra-abdominal, and retroperitoneal complications especially in elderly individuals who may have several cardiopulmonary abnormalities. Kanayama et al 20 reported that 19% of patients with OVCF undergoing anterior instrumentation surgery also required additional posterior reinforcement surgery. A combined anterior and posterior approach has a good fusion rate with a kyphosis angle correction over the short- and long-term period. However, this procedure becomes morbid as the operative time is longer and the blood loss higher. Therefore, the development of a one-stage effective treatment is required for OVCF with neurological deficits. 20
Posterolateral decompression and posterior reconstruction using the posterior egg-shell procedure was performed and reported by Kim et al 21 and Shikata et al. 22 Recent literature also reports posterior closing wedge osteotomy including posterior spinal shortening that has been performed for both neural decompression and correction of the kyphotic deformity.23,24 Although posterior procedures offer better kyphosis correction compared with anterior procedures, they carry the risk of neural tissue damage, such as dural tear and spinal cord kinking due to the shortening of the spinal column. Also, the strength of the implant fixation in osteoporotic bone is not sufficient for the reconstruction of a spine with marked instability. Decompressive surgeries in the form of laminectomy and resection of all posterolateral components including the pedicle may further destabilize the spinal column in this already unstable condition.
A single-stage posterior instrumentation technique with vertebroplasty has been studied for management of OVCF. Augmenting anterior column with vertebroplasty provides a more rigid fixation as compared with stand-alone posterior stabilization allowing anterior column support.25,26 Previously published literature on outcome analysis after anterior/posterior surgical approaches were based on heterogeneous groups of patients with regard to the type of vertebral collapse, number and level of collapsed vertebral body, degree of osteoporosis, and neurological status.
Recently Guo et al, 27 in a single center retrospective analysis of 56 patients with OVCF leading to neurological deficits, used a novel surgical strategy using extension CT, MRI, and radiographs. They divided patients into 4 groups and treated them with vertebroplasty/kyphoplasty, vertebroplasty/kyphoplasty with posterior fixation and posterolateral fusion, and additional laminectomy/osteotomy, respectively. They found satisfactory mid- and long-term clinical and radiological outcomes. 27
We believe that the reason for delayed neurological deficits following a vertebral collapse in the osteoporotic spine is the dynamic instability causing repeated micro trauma at the fracture site rather than static mechanical compression of spinal cord by the retropulsed bone fragments. The same was established in our cases using dMRI. The difference in neurological recovery of patients with compression on dynamic MRI and those with compression on static MRI when compared with using ASIA motor score was not statistically significant. The simple posterior fixation without neural decompression is not only technically easier but also much safer. The risks of the anterior approach and potential damage to neural tissue from the decompressive procedure can be avoided. The surgical time, blood loss and operative morbidity are reduced. Also, the posterior elements of the spine that are vital for maintaining spinal stability in this condition are preserved and adequate space is available for bone grafting on the lamina of the affected vertebra. In our series, 61 patients underwent nondecompressive procedures. They showed improvement in neurology and complication rate with regards to implant failure was also less in these patients. Ataka et al 15 reported that posterior stabilization without neural decompression provides significant neurological improvement and relief of back pain without severe complications. They concluded that OVCFs with neurological deficits are mainly caused by the instability of the fractured vertebra rather than by neural compression and that neural decompression is not necessary for the treatment of OVCF with neurological deficits with dynamic mobility. 15 However this hypothesis of dynamic instability causing neurodeficits was not supported by any imaging modality. In our study, the dMRI confirmed the hypothesis of dynamic instability leading to neurological deficits.
Only posterior fixation carries a risk of implant failure. Increasing space between trabeculae in cancellous bone in the osteoporotic spine limits the mechanical grip of screws and compromises integration at the bone metal interface level, encouraging osteolysis all around the pedicle screw and the implant loosening driving to the so-called pull-out phenomenon. 28 Patients with osteoporosis have decreased pull-out strength and insertional torque, which is related to pedicle screw loosening. Many strategies have been described in the literature to improve pedicle screws’ stability. The use of screw with bigger diameters is one method to improve pullout strength. However, it is not always possible to insert big screws for anatomical reasons, without increasing vascular or organic lesion risk. 29 Moreover, the use of screws with larger diameter increases the risk of pedicle fractures. Zindrick et al 30 demonstrated screws anchored to anterior vertebral body cortex have 30% increase in pullout strength. However, the integration between bone surface and screw cannot be reached immediately. The use of PMMA to fill the space and stabilize the implants is practiced in orthopedic surgery for more than 10 years. PMMA could be used to increase the strength of the fixation of pedicle screws in case of reduction of bone quality in osteoporosis-affected patients and permit immediate screw fixation to the cancellous bone. Numerous studies demonstrate that PMMA augmentation can increase the screws’ pull-out strength in osteoporotic and normal vertebrae. Augmented PMMA pedicle screws can improve the implant’s primary stability and its resistance to fatigue increasing the resistance to axial forces that drive to pull out. 28 The use of PMMA has multiple advantages: its immediate availability, it is cheap and it is an easy and fast preparation technique. PMMA permits immediate screw fixation to the cancellous bone that is not permitted by other materials (calcium sulfate, calcium phosphate). To prevent cement leakage, accurate palpation of the pedicle and the vertebral body’s anterior cortex is important, before and after the screw insertion. The optimal cement quantity to inject in the vertebral body is not known in the literature. Theoretically, a higher screw’s fixation strength can be obtained with higher cement quantity; it is very important to find the right compromise to avoid cement leakage. In our series, 17 patients received cement-augmented screws for fixation when insertional torque was found to be very less. When compared to the rest of the patients only 1 patient with cement augmented fixation had a failure due to screw pull-out and compression fracture at the distal level of instrumentation. It was revised using cemented augmented fixation up to the next level.
Also, osteoporosis is essentially a medical disorder and treating the underlying pathology is of utmost importance. All patients in our series were given antiosteoporotic medications (bisphosphonates/teriparatide) following surgery.
Our study is not without limitations. This is not a randomized controlled trial or carefully matched cohort study. Our study population was relatively heterogeneous as there was a lack of a control group and continuous BMD monitoring. However, it is the largest series of OVCF with neurological deficit managed by fixation only without neural decompression using dMRI to confirm dynamic cord compression. Also, not all OVCFs with neurological deficits can be managed by fixation only, few patients may require decompression and circumferential fusion. Limited sample size restricted our ability to separately analyze PMMA- versus non-PMMA-augmented fixation. A prospective multicentric randomized controlled trial is needed to further validate our findings.
Conclusion
Our results indicate that posterior instrumented fusion sans neural decompression leads to neurological improvement and relief from back pain without major complications. We propose that neural decompression of the spinal cord is not always necessary for the treatment of neurological impairment in patients with osteoporotic vertebral collapse with dynamic mobility. Dynamic MRI is a valuable tool to make an accurate diagnosis and determine precise surgical plan and improving the surgical strategy of osteoporotic vertebral compression fractures.
Supplemental Material
Supplemental Material, Abbreviations for Posterior Stabilization Without Neural Decompression in Osteoporotic Thoracolumbar Fractures With Dynamic Cord Compression Causing Incomplete Neurological Deficits by Abhinandan Reddy Mallepally, Nandan Marathe, Gururaj Sangondimath, Kalidutta Das and Harvinder Singh Chhabra in Global Spine Journal
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Abhinandan Reddy Mallepally, MD, DNB  https://orcid.org/0000-0001-8153-7499
https://orcid.org/0000-0001-8153-7499
Nandan Marathe, MD  https://orcid.org/0000-0002-8939-2690
https://orcid.org/0000-0002-8939-2690
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Arciero RA, Leung KY, Pierce JH. Spontaneous unstable burst fracture of the thoracolumbar spine in osteoporosis. a report of two cases. Spine (Phila Pa 1976). 1989;14:114–117. doi:10.1097/00007632-198901000-00024 [DOI] [PubMed] [Google Scholar]
- 2.Tanaka S, Kubota M, Fujimoto Y, Hayashi J, Nishikawa K. Conus medullaris syndrome secondary to an L1 burst fracture in osteoporosis: a case report. Spine (Phila Pa 1976). 1993;18:2131–2134. doi:10.1097/00007632-199310001-00034 [DOI] [PubMed] [Google Scholar]
- 3.Chang KW, Chen YY, Lin CC, Hsu HL, Pai KC. Apical lordosating osteotomy and minimal segment fixation for the treatment of thoracic or thoracolumbar osteoporotic kyphosis. Spine (Phila Pa 1976). 2005;30:1674–1681. doi:10.1097/01.brs.0000170450.77554.bc [DOI] [PubMed] [Google Scholar]
- 4.Kaneda K, Asano S, Hashimoto T, Satoh S, Fujiya M. The treatment of osteoporotic-posttraumatic vertebral collapse using the kaneda device and a bioactive ceramic vertebral prosthesis. Spine (Phila Pa 1976). 1992;17(8 suppl):S295–S303. doi:10.1097/00007632-199208001-00015 [DOI] [PubMed] [Google Scholar]
- 5.Mochida J, Toh E, Chiba M, Nishimura K. Treatment of osteoporotic late collapse of a vertebral body of thoracic and lumbar spine. J Spinal Disord. 2001;14:393–398. doi:10.1097/00002517-200110000-00004 [DOI] [PubMed] [Google Scholar]
- 6.Sutherland CJ, Miller F, Wang GJ. Early progressive kyphosis following compression fractures. Two case reports from a series of “stable” thoracolumbar compression fractures. Clin Orthop Relat Res. 1983;173:216–220. [PubMed] [Google Scholar]
- 7.Fidler MW.Remodelling of the spinal canal after burst fracture. A prospective study of two cases. J Bone Joint Surg Br. 1988;70:730–732. doi:10.1302/0301-620x.70b5.3192569 [DOI] [PubMed] [Google Scholar]
- 8.Dai LY. Remodeling of the spinal canal after thoracolumbar burst fractures. Clin Orthop Relat Res. 2001;(382):119–123. doi:10.1097/00003086-200101000-00018 [DOI] [PubMed] [Google Scholar]
- 9.ASIA and ISCoS International Standards Committee. The 2019 revision of the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI)—What’s new? Spinal Cord. 2019;57:815–817. [DOI] [PubMed] [Google Scholar]
- 10.Lieberman IH, Dudeney S, Reinhardt MK, Bell G. Initial outcome and efficacy of “kyphoplasty” in the treatment of painful osteoporotic vertebral compression fractures. Spine (Phila Pa 1976). 2001;26:1631–1638. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Z, Chen G, Sun J, et al. Posterior indirect reduction and pedicle screw fixation without laminectomy for Denis type B thoracolumbar burst fractures with incomplete neurologic deficit. J Orthop Surg Res. 2015;10:85. doi:10.1186/s13018-015-0227-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajasekaran S, Kanna RM, Schnake KJ, et al. Osteoporotic thoracolumbar fractures—how are they different? Classification and treatment algorithm. J Orthop Trauma. 2017;31(suppl 4):S49–S56. doi:10.1097/BOT.0000000000000949 [DOI] [PubMed] [Google Scholar]
- 13.NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785–795.11176917 [Google Scholar]
- 14.Kim HJ, Yi JM, Cho HG, et al. Comparative study of the treatment outcomes of osteoporotic compression fractures without neurologic injury using a rigid brace, a soft brace, and no brace: a prospective randomized controlled non-inferiority trial. J Bone Joint Surg Am. 2014;96:1959–1966. doi:10.2106/JBJS.N.00187 [DOI] [PubMed] [Google Scholar]
- 15.Ataka H, Tanno T, Yamazaki M. Posterior instrumented fusion without neural decompression for incomplete neurological deficits following vertebral collapse in the osteoporotic thoracolumbar spine. Eur Spine J. 2009;18:69–76. doi:10.1007/s00586-008-0821-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho Y. Corpectomy and circumferential fusion for advanced thoracolumbar Kümmell’s disease. Musculoskelet Surg. 2017;101:269–274. doi:10.1007/s12306-017-0480-1 [DOI] [PubMed] [Google Scholar]
- 17.Kanno H, Ozawa H, Koizumi Y, et al. Changes in lumbar spondylolisthesis on axial-loaded MRI: do they reproduce the positional changes in the degree of olisthesis observed on X-ray images in the standing position? Spine J. 2015;15:1255-1262. doi:10.1016/j.spinee.2015.02.016 [DOI] [PubMed] [Google Scholar]
- 18.Lao L, Daubs MD, Takahashi S, et al. Kinetic magnetic resonance imaging analysis of lumbar segmental motion at levels adjacent to disc herniation. Eur Spine J. 2016;25:222-229. doi:10.1007/s00586-015-3977-z [DOI] [PubMed] [Google Scholar]
- 19.Uchida K, Kobayashi S, Nakajima H, et al. Anterior expandable strut cage replacement for osteoporotic thoracolumbar vertebral collapse. J Neurosurg Spine. 2006;4:454–462. doi:10.3171/spi.2006.4.6.454 [DOI] [PubMed] [Google Scholar]
- 20.Kanayama M, Ishida T, Hashimoto T, et al. Role of major spine surgery using kaneda anterior instrumentation for osteoporotic vertebral collapse. J Spinal Disord Tech. 2010;23:53–56. doi:10.1097/BSD.0b013e318193e3a5 [DOI] [PubMed] [Google Scholar]
- 21.Kim KT, Suk KS, Kim JM, Lee SH. Delayed vertebral collapse with neurological deficits secondary to osteoporosis. Int Orthop. 2003;27:65–69. doi:10.1007/s00264-002-0418-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shikata J, Yamamuro T, Iida H, Shimizu K, Yoshikawa J. Surgical treatment for paraplegia resulting from vertebral fractures in senile osteoporosis. Spine (Phila Pa 1976). 1990;15:485–489. doi:10.1097/00007632-199006000-00010 [DOI] [PubMed] [Google Scholar]
- 23.Suk SI, Kim JH, Lee SM, Chung ER, Lee JH. Anterior-posterior surgery versus posterior closing wedge osteotomy in posttraumatic kyphosis with neurologic compromised osteoporotic fracture. Spine (Phila Pa 1976). 2003;28:2170–2175. doi:10.1097/01.BRS.0000090889.45158.5A [DOI] [PubMed] [Google Scholar]
- 24.Saita K, Hoshino Y, Kikkawa I, Nakamura H. Posterior spinal shortening for paraplegia after vertebral collapse caused by osteoporosis. Spine (Phila Pa 1976). 2000;25:2832–2835. doi:10.1097/00007632-200011010-00018 [DOI] [PubMed] [Google Scholar]
- 25.Matsuyama Y, Goto M, Yoshihara H, et al. Vertebral reconstruction with biodegradable calcium phosphate cement in the treatment of osteoporotic vertebral compression fracture using instrumentation. J Spinal Disord Tech. 2004;17:291-296. doi:10.1097/01.bsd.0000097253.54459.a6 [DOI] [PubMed] [Google Scholar]
- 26.Nakashima H, Imagama S, Yukawa Y, et al. Comparative study of 2 surgical procedures for osteoporotic delayed vertebral collapse: anterior and posterior combined surgery versus posterior spinal fusion with vertebroplasty. Spine (Phila Pa 1976). 2015;40:E120-E126. doi:10.1097/BRS.0000000000000661 [DOI] [PubMed] [Google Scholar]
- 27.Guo DQ, Yu M, Zhang SC, et al. Novel surgical strategy for treating osteoporotic vertebral fractures with cord compression. Orthop Surg. 2019;11:1082-1092. doi:10.1111/os.12558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Girardo M, Cinnella P, Gargiulo G, Viglierchio P, Rava A, Aleotti S. Surgical treatment of osteoporotic thoraco-lumbar compressive fractures: the use of pedicle screw with augmentation PMMA. Eur Spine J. 2017;26:546–551. doi:10.1007/s00586-017-5037-3 [DOI] [PubMed] [Google Scholar]
- 29.Wittenberg RH, Lee KS, Shea M, White AA, 3rd, Hayes WC. Effect of screw diameter, insertion technique, and bone cement augmentation of pedicular screw fixation strength. Clin Orthop Relat Res. 1993;(296):278–287. [PubMed] [Google Scholar]
- 30.Zindrick MR, Wiltse LL, Widell EH, et al. A biomechanical study of intrapeduncular screw fixation in the lumbosacral spine. Clin Orthop Relat Res. 1986;203:99–112. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Abbreviations for Posterior Stabilization Without Neural Decompression in Osteoporotic Thoracolumbar Fractures With Dynamic Cord Compression Causing Incomplete Neurological Deficits by Abhinandan Reddy Mallepally, Nandan Marathe, Gururaj Sangondimath, Kalidutta Das and Harvinder Singh Chhabra in Global Spine Journal