Abstract
Study Design
Systemic review and meta-analysis.
Objective
To review and establish the effect of tobacco smoking on risk of nonunion following spinal fusion.
Methods
A systematic search of Medline, Embase, Cochrane Central Register of Controlled Trials, and the Cochrane Database of Systematic Reviews from inception to December 31, 2020, was conducted. Cohort studies directly comparing smokers with nonsmokers that provided the number of nonunions and fused segments were included. Following data extraction, the risk of bias was assessed using the Quality in Prognosis Studies Tool, and the strength of evidence for nonunion was evaluated using the GRADE working group criteria. All data analysis was performed in Review Manager 5, and a random effects model was used.
Results
Twenty studies assessing 3009 participants, which included 1117 (37%) smokers, met inclusion criteria. Pooled analysis found that smoking was associated with increased risk of nonunion compared to not smoking ≥1 year following spine surgery (RR 1.91, 95% CI 1.56 to 2.35). Smoking was significantly associated with increased nonunion in those receiving either allograft (RR 1.39, 95% CI 1.12 to 1.73) or autograft (RR 2.04, 95% CI 1.54 to 2.72). Both multilevel and single level fusions carried increased risk of nonunion in smokers (RR 2.30, 95% CI 1.64 to 3.23; RR 1.79, 95% CI 1.12 to 2.86, respectively).
Conclusion
Smoking status carried a global risk of nonunion for spinal fusion procedures regardless of follow-up time, location, number of segments fused, or grafting material. Further comparative studies with robust methodology are necessary to establish treatment guidelines tailored to smokers.
Keywords: smoking, nicotine, tobacco, fusion, nonunion, pseudarthrosis, meta-analysis, systematic review
Background
Tobacco use remains a global public health problem in the 21st century. In the United States alone, cigarette smoking remains the leading cause of preventable disease, disability, and death, accounting for nearly 500,000 annual deaths or 1 in 5 of all deaths. 1 Health policy strategies and pharmacologic interventions have demonstrated only partial effectiveness in mitigating rates of tobacco use.2,3 The wide-ranging negative impact of smoking on outcomes after surgical procedures in diverse disciplines is well documented, significantly affecting rates of infection, intraoperative and postoperative medical complications, and long-term outcomes.4-8
At the same time, the preceding decade has seen a substantial rise in both the rates of spine surgery as well as its associated health care costs. An analysis of the National Inpatient Sample between 2004 and 2015 found a 62.3% increase in rates of lumbar fusion, coinciding with a 177% increase in aggregate hospital costs. 9 It has been estimated that smokers comprise a noteworthy 24.0% and 31.8% of this growing population of surgical spine patients, which is higher than the national average of 14.0%.5,10 One of the relevant sequelae adversely affecting long-term outcomes after spinal fusion is the development of nonunion, or pseudarthrosis; this clinical entity can result in disabling pain, disability, and compromised function, frequently requiring reoperation. 11
While the mechanism by which nicotine affects bone health and healing is not yet fully understood, nicotine has been shown to impair gene expression of a number of osteogenic growth factors in addition to affecting vascularization of bone. 12 In addition, a recent Systematic Review identified a number of studies suggesting smokers are more likely to experience pseudarthrosis following cervical or lumbar surgery. 13 However, to our knowledge, there is no formal Meta-Analysis of the existing literature seeking to quantify the effect of smoking on the development of nonunion following spine fusion surgery. Therefore, we conducted this Systematic Review with Meta-Analysis to determine if smoking tobacco resulted in increased risk of nonunion after cervical or thoracolumbar fusion surgery with formal calculation of relative risk.
Methods
Protocol and Registration
This work was registered with PROSPERPO (registration number CRD42021231462). 14 We conducted this study following the framework outlined by the Cochrane Prognosis Methods Group15,16 as well as the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. 17
Identification of Studies
We searched MEDLINE (PubMed), EMBASE (Ovid), Cochrane Central Register of Controlled Trials (CENTRAL), and Cochrane Database of Systematic Reviews (CDSR) from inception to December 31, 2020. The search strategies are included in Supplemental Table 1 in the supplemental material. We reviewed reference lists of included studies and systematic reviews for additional articles. The search was restricted to articles published in English.
Assessment of Eligibility
Two review authors (RSN and JRD) independently screened titles and abstracts to identify articles for full text review. Any citation deemed appropriate for inclusion by at least one of the reviewers was retrieved. Each full-text article was independently reviewed for eligibility by the same 2 team members. Any disagreements were resolved by consensus.
Pre-established criteria were used to determine eligibility for inclusion and exclusion of full text articles based on PECOT (patient population of interest, exposure, comparator, outcome, and timing) (Table 1).
Table 1.
Inclusion/Exclusion Criteria.
| PECOT | Inclusion | Exclusion |
|---|---|---|
| Population of interest | Adult (≥18 years) undergoing spinal fusion (single-level or multilevel; cervical or thoracolumbar) | Cancer, infection, and trauma |
| Exposure | Persons smoking tobacco (current, defined as smoking within 1 year prior to surgery) | Smokeless tobacco |
| Comparison | Persons not smoking tobacco (non-smokers) | |
| Outcomes | Risk of nonunion | Delayed union |
| Time | Nonunion at ≥1 year | Included patients with follow-up <1 year |
We included only cohort studies that directly compared smokers with nonsmokers and provided the absolute number of nonunions (numerator) and the population or number of fused segments at risk (denominator). We excluded prognostic studies that looked at several risk factors for nonunion that included smoking in a multivariate analysis but did not provide numerator and denominator data. Additionally, we excluded patients that received fusion with recombinant human bone morphogenetic protein-2 (rh-BMP2) as this would be a confounding factor to the rate of fusion.
Data Abstraction and Data Management
Two review authors extracted data from each study into a spreadsheet (Microsoft Excel). Data included author last name, publication year, study design, country, sample size, population characteristics, data source, location and levels of fusion, surgical approach, follow-up time, graft material, definition of fusion, and results.
Assessment of Methodological Quality of Individual Studies
We assessed the risk of bias from these non-randomized studies using the Quality in Prognosis Studies (QUIPS) tool. 18 QUIPS evaluates 6 domains: study participation, study attrition, prognostic factor measurement, outcome measurement, study confounding, and statistical analysis and reporting. Studies were judged as “good quality” when the majority of criteria was met (little or no risk of bias); “fair quality” if most criteria was met (some flaws in the study with an associated risk of bias); and “poor quality” if either most criteria were not met, or if significant flaws relating to key aspects of study design were present. 19 Two team members independently assessed risk of bias and quality, and disagreements were resolved through discussion.
Data Synthesis
We performed meta-analyses on the cumulative proportion of nonunion defined as the number of participants with one or more unfused segments. The data were pooled via the Mantel-Haenszel method using a random effects model. We calculated risk ratios (RRs), and since we were interested in assessing the potential excess risk of nonunion associated with smoking, we calculated the risk difference (RD) and 95% confidence interval. All data analysis and presentation were performed using Review Manager 5. We inspected heterogeneity by examining the forest plots and quantified the heterogeneity using the I2 statistic from the Chi-squared test for heterogeneity (I2 < 40, low heterogeneity; I2 ≥ 75% considerable heterogeneity). We conducted stratified analyses to investigate whether effects varied by surgical location (cervical or thoracolumbar), number of segments fused (single or multiple), or graft material used (autograft or allograft). Some studies evaluated fusion status after 1 to 2 years, some after ≥2 years. In order to account for this disparity of enrollment definition, we further conducted a stratified analysis to investigate if the cumulative proportion of nonunion varied by follow-up period. The risk of publication bias was examined using a funnel plot with the study size on the y-axis. We did sensitivity analyses to investigate whether study quality influenced effect estimates by including studies that were deemed fair or good quality.
We evaluated the strength of evidence (our confidence in the estimate of the pooled effect) for nonunion using the GRADE working group criteria for the assessment of evidence about prognostic factors. 20 To ensure consistency and validity of the evaluation, the quality of evidence was reviewed by 2 investigators prior to assigning a final grade. According to GRADE, a body of observational evidence for questions of prognosis begins as high certainty in the evidence. The evidence can be downgraded due to risk of bias, imprecision, inconsistency, indirectness, and publication bias. Rating up also applies when estimates of associations between prognostic factors and outcome are very strong. The strength of evidence was assigned an overall grade of high, moderate, low, or very low by evaluating and weighing the combined results of the above domains (Table 2).
Table 2.
Strength of Evidence Definition.
| Strength Level | Definition |
|---|---|
| High | We are very confident that the variation in risk associated with the prognostic factor (probability of future events in those with/without the prognostic factor) lies close to that of the estimate |
| Moderate | We are moderately confident that the variation in risk associated with the prognostic factor (probability of future events in those with/without the prognostic factor) is likely to be close to the estimate, but there is a possibility that it is substantially different |
| Low | Our certainty in the estimate is limited: The variation in risk associated with the prognostic factor (probability of future events in those with/without the prognostic factor) may be substantially different from the estimate |
| Very low | We have very little certainty in the estimate: The variation in risk associated with the prognostic factor (probability of future events in those with/without the prognostic factor) is likely to be substantially different from the estimate |
Great scrutiny was taken by the present authors to evaluate each study’s risk of bias and codify results in a consistent manner. However, definition of nonunion between studies varied based on the method of postoperative assessment (Table 3). Many studies dichotomized results as fused vs not fused, while a select few separated results into 3 categories: solid or definite fusion, uncertain, and definite nonunion. In those cases, we collapsed the uncertain category with the nonunion category for our synthesis. When evaluating the risk of bias in outcome assessment of fusion, CT or flexion/extension radiographic evidence was considered a lower risk of bias than static radiographs alone. Similarly, we determined risk of bias in smoking status based on if participants were categorized in a consistent setting and with explicit inclusion/exclusion criteria.
Table 3.
Characteristics of Included Studies.
| Author (Year) | Country | n (S vs NS) Mean Age % Males |
Spine Region Levels Fused Approach |
Graft Material | Nonunion Definition | Follow-Up (Months) | Study Quality |
|---|---|---|---|---|---|---|---|
| An 1995 | USA | 34 vs 43 47 years NR |
Cervical 1 3 levels (1 level = 49%) anterior |
Autograft (49%) allograft+DBM (51%) | Clear radiographic cleft and evidence of motion on flexion–extension views, or partial or complete radiolucent line or cleft extending along 1 or both surfaces of the graft. | Mean 18 (range, 12-33) | Fair |
| Andersen 2009 | Denmark | 28 vs 56 70 years NR |
Thoracolumbar 1 4 levels (1 level = 29%) posterior |
Allograft | Only unilateral facet joint fusion, questionable bilateral facet fusion, clear or possible presence of a cleft in the bony bridge, or resorption of most of the fusion mass. | 24 | Poor |
| Andersen 2001 | Denmark | 215 vs 181 46 years 40% |
Thoracolumbar 1 4 levels (1 level = 55%) posterior |
NR | No evidence of continuous trabecular intertransverse bony bridge on at least 1 side or suboptimal quality of fusion, including fusion mass hidden behind instrumentation. | 24 | Fair |
| Bose 2001 | USA | 46 vs 60 50 years 44% |
Cervical 2-4 levels anterior |
Autograft (85%) allograft (15%) | Lack of trabecular bony bridging across the disk space, motion on flexion–extension views. | 12 | Poor |
| Brown 1986 | USA | 50 vs 50 38 years 50% |
Thoracolumbar 2 levels posterior |
NR | NR | 12-24 | Fair |
| Deguchi 1998 | USA | 36 vs 37 38 years (median) 63% |
Thoracolumbar 1,2 levels (1 level = 52%) posterior |
Autograft | No evidence of bridging bone between transverse processes with trabeculation confluent across fusion mass on AP, oblique radiographs, and flexion–extension ≥2° of motion. | Mean 46 (range, 12-89) | Poor |
| Emery 1997 | USA | 3 vs 13 59 years 38% |
Cervical 3 levels anterior |
Autograft | No evidence of bridging of disc space with trabecular bone and >1 mm difference between tips of spinous processes on flexion–extension. | Mean 37 (range, 23-75) | Fair |
| Glassman 2007 | USA | 42 vs 106 51 years 47% |
Thoracolumbar 1 level posterior |
Autograft | No evidence of bilateral bridging trabecular bone on plain radiographs with ≥3° of translation and ≥5° of angulation on flexion–extension. CT scans as secondary measure when bridging trabecular bone not observed on plain radiographs. | 12 ≥2 years | Fair |
| Goldberg 2002 | USA | 30 vs 50 45 years 54% |
Cervical 1 3 levels (1 level = 71%) anterior |
Autograft (84%) allograft (16%) | Motion on flexion–extension views, or a visible cleft at one or both graft–end plate surfaces. | Mean 48 (range, 24-84) | Poor |
| Groff 2003 | USA | 55 vs 89 49 years 83% |
Cervical 1 4 levels (1 level = 67%) anterior |
Autograft (40%) allograft (60%) | Slight lucency or not fused without evidence of full incorporation. Flexion–extension radiographs and bone scans obtained to aid in determining pseudarthrosis. | Mean 34 (range, 24-?) | Poor |
| Hanley 1989 | USA | 27 vs 23 37 years 72% |
Thoracolumbar 2 levels posterior |
Autograft | NR | Mean 40 (range, 25-73) | Poor |
| Hermann 2016 | Germany | 16 vs 34 48 vs 58 years 46% |
Thoracolumbar 1 level |
Allograft (allograft added in a few cases) | Ongoing remodeling, uncertain fusion, or clear pseudarthrosis. | 12 | Poor |
| Hilibrand 2001 | USA | 55 vs 135 NR NR |
Cervical 1 4 levels (1 level = 8%) anterior |
Allograft | >1 mm change interspinous distance between flex/ext radiographs, absent continuous trabeculation, or intervertebral lucency. | Mean 68 (range, 24-183) | Poor |
| Lau 2014 | USA | 40 vs 120 53 years 58% |
Cervical 1 3 levels (1 level = 74%) anterior (74%), ant+post (27%) |
Allograft | Presence of radiolucent lines/area across the fusion site or around any screw sites, or absence of bridging trabeculae across fusion site, or presence of motion between the spinous processes on flexion–extension, or presence of motion between vertebral bodies on flexion–extension. | 12 | Fair |
| Luszczyk 2013 | USA | 156 vs 417 NR NR |
Cervical 1 level anterior |
Allograft | Lucency visualized between graft and vertebral endplate or when motion detected at the operative segment. | ≥24 | Fair |
| Martin 1999 | USA | 75 vs 214 NR 56% |
Cervical 1 3 levels (1 level = 93%) anterior |
Allograft | Any lucency at graft-vertebral body interface. If multiple levels, a pseudoarthrosis at any level counted as nonunion. | Mean 33 (range, 24-51) | Good |
| Suchomel 2004 | Czech Republic | 48 vs 31 47.8 years 62.1% |
Cervical 1,2 levels (1 level % NR) anterior |
Autograft (67%) allograft (33%) | <50% trabecular bridging between VBs and bone graft on radiographs, or >2 mm los of height, or >5° kyphotic angle, or any migration of bone graft into adjacent endplate. | 12 and 24 | Good |
| Urrutia 2013 | Chile | 9 vs 38 46.5 years 23% |
Thoracolumbar 1 3+ levels (1 level = 80%) posterior |
Autograft (100%) allograft (100%) | For PLF, no evidence of trabeculae crossing the graft-transverse process interface or cortication of the graft. For interbody fusion, no evidence of trabeculae crossing the graft-vertebral body interface on both sides of the graft either in plain radiographs or in CT scan. | 12 | Fair |
| Zdeblick 1993 | USA | 45 vs 79 48 years NR |
Thoracolumbar mean 1.6 levels/patient posterior |
Autograft | No evidence of bridging bone between transverse processes with trabeculation confluent across fusion mass on AP, oblique radiographs, and flexion–extension ≥2° of motion. | 12 | Fair |
Abbreviations: NR, not reported; NS, nonsmoking, S, smoking.
Results
Study Selection
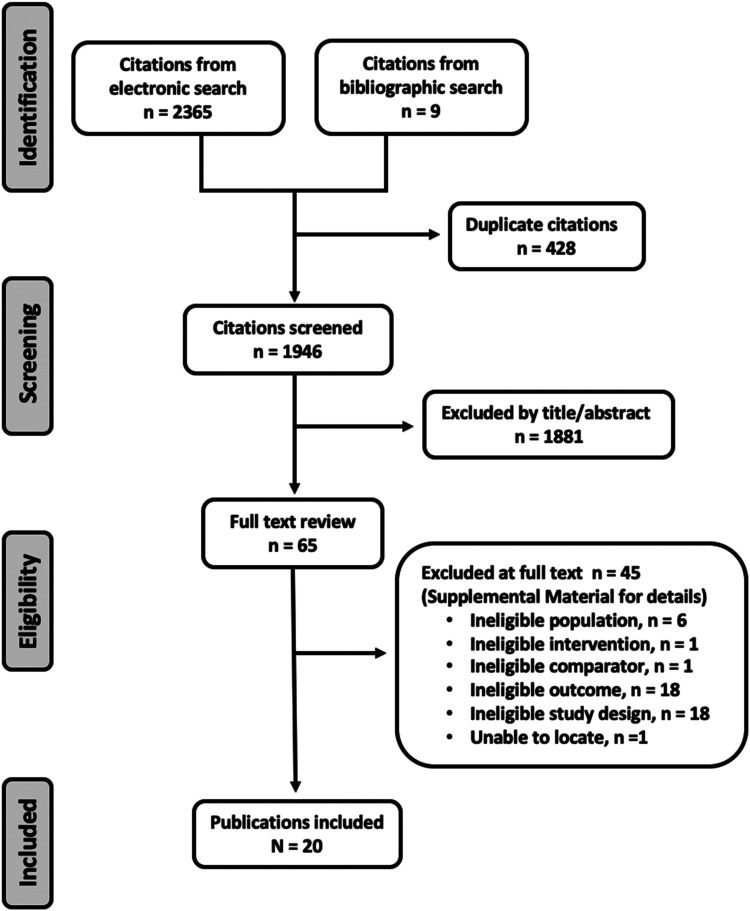
Our search identified 2374 citations. We screened 1946 titles/abstracts after removing 428 duplicates, and we evaluated 65 full texts. Twenty studies21-40 assessing 3009 participants met inclusion criteria (Figure 1). A list of studies excluded at full text is found in Supplemental Table S2 in the supplemental material.
Figure 1.
Search results.
Study Characteristics
Characteristics of the included studies are described in Table 3 21-32,34-37,40 with Germany 33 Czech Republic38,39 all contributing 1 study each.
Of the 3009 participants, 1117 (37%) were smokers and 1892 (63%) were nonsmokers. The mean age for the study population was 48 years, and 54% were males. Seven studies provided nonunion risks at a minimum of 12 month follow-up,21,24,25,33,35,39,40 11 at a minimum of 24 month follow-up,22,23,26,27,29-32,34,36,37 and 2 at both 12 and 24 month follow-up.28,38 Eleven studies assessed the effect of smoking on fusion in the cervical spine21,24,25,27,30,31,34-38 and 9 in the thoracolumbar spine.22,23,26,28,29,32,33,39,40 Single-level fusion was performed in 63% of participants. The date of study publication ranged 30 years (1986-2016). Graft material used in fusion surgery and the definition of nonunion varied among studies and are summarized in Table 3.
Study Quality
Two studies were judged as good-,37,38 9 fair-,21,22,25,27,28,35,36,39,40 and 9 poor-quality23,24,26,29-34 studies. The concerns about bias in the studies were primarily related to study participation (eg, concerns about sampling/recruiting), lack of control for confounding, and high study attrition. Individual study quality is summarized in Supplemental Table 3.
Nonunion
Smoking was associated with increased risk of nonunion compared with no smoking ≥1 year following spine surgery (19 studies, pooled risk ratio (RR) 1.91, 95% CI 1.56 to 2.35, I2 = 27%) Figure 2 (Strength of Evidence, Moderate, Supplemental Table S4). The absolute RD (excess risk) for nonunion associated with smoking was .13 and the number needed to treat (NNT) for an additional nonunion of 8 (95% CI 6 to 13). This association was seen both in the cervical spine (10 studies,21,24,25,27,30,31,34-37 pooled RR 2.03, 95% CI 1.46 to 2.81, I2 =36%) and the lumbar spine (9 studies,22,23,26,28,29,32,33,39,40 pooled RR 1.78, 95% CI 1.37 to 2.31, I2 = 16). The RD for cervical and thoracolumbar fusion was .14 and .11, respectively. This relationship held true whether the follow-up was 12-23 months or ≥24 months (Table 4), or when 9 poor-quality trials were excluded (10 studies, RR 1.74, 95% CI 1.37 to 2.21, I2 = 0%) (Supplemental Material, Supplemental Figure S1).
Figure 2.
Forest plot depicting risk ratio of nonunion for smokers vs nonsmokers stratified by cervical and thoracolumbar (TL) fusion.
Table 4.
Nonunion (%) Stratifying on Length of Follow-Up for All, Cervical and Thoracolumbar Fusions.
| Length of Follow-Up | Smoking % (n/N) | Nonsmoking % (n/N) | Pooled RR (95% CI) (M-H) |
|---|---|---|---|
| All | 22.4% (241/1077) | 11.5% (225/1959) | 1.88 (1.55, 2.29) |
| 12-23 mo | 26.7% (70/262) | 11.5% (59/512) | 2.00 (1.46, 2.74) |
| ≥24 mo | 21.0% (171/815) | 11.5% (166/1447) | 1.86 (1.44, 2.40) |
| Cervical | 22.4% (112/501) | 10.3% (121/1178) | 2.03 (1.46, 2.81) |
| 12-23 mo | 26.5% (41/155) | 9.2% (24/260) | 2.37 (1.22, 4.60) |
| ≥24 mo | 20.5% (71/346) | 10.6% (97/918) | 1.92 (1.27, 2.89) |
| Thoracolumbar | 22.4% (129/576) | 13.3% (104/781) | 1.69 (1.36, 2.10) |
| 12-23 mo | 27.1% (29/107) | 13.9% (35/252) | 1.82 (1.18, 2.80) |
| ≥24 mo | 21.3% (100/469) | 13.0% (69/529) | 1.68 (1.33, 2.13) |
Smoking was significantly associated with increased nonunion in single-level (4 studies,28,33,36,37 pooled RR 1.79, 95% CI 1.12 to 2.86, I2 = 34%) or multilevel fusions (7 studies,24,25,27,29,32,34,37 pooled RR 2.30, 95% CI 1.64 to 3.23, I2 = 2%) (Figure 3 and Table 5). Likewise, smoking was significantly associated with increased nonunion in those receiving either allograft (6 studies,21,22,28,35-37 pooled RR 1.39, 95% CI 1.12 to 1.73, I2 = 0%) or autograft (8 studies,21,26-29,32,34,40 pooled RR 2.04, 95% CI 1.54 to 2.72, I2 = 0%) (Figure 4 and Table 6). The association remained in all subgroups when poor studies were removed in the sensitivity analyses (Supplemental Material, Supplemental Figures S2 and S3).
Figure 3.
Level (2 subgroups): Forest plot depicting risk ratio of nonunion for smokers vs nonsmokers stratified by single- and multilevel fusion.
Table 5.
Nonunion (%) Stratifying on Single- vs Multilevel Fusions in the Cervical and Thoracolumbar Spine.
| No. of Studies | Smoking % (n/N) | Nonsmoking % (n/N) | Pooled RR (95% CI) (M-H) | Excess Risk Associated with Smoking (95% CI) | |
|---|---|---|---|---|---|
| All | 19.7% (113/574) | 9.6% (112/1162) | 2.04 (1.55, 2.69) | 13% (5% to 21%) | |
| 1 level | 428,33,36,37 | 14.1% (40/283) | 8.5% (64/753) | 1.79 (1.12, 2.86) | 8% (−1% to 16%) |
| 2+ levels | 724,25,27,29,32,34,37 | 25.1% (73/291) | 11.7% (48/409) | 2.30 (1.64, 3.23) | 18% (3% to 32%) |
| Cervical | 19.0% (70/369) | 10.3% (90/872) | 1.81 (1.25, 2.61) | 11% (1% to 21%) | |
| 1 level | 236,37 | 11.0% (25/227) | 8.3% (51/615) | 1.38 (0.78, 2.44) | 3% (−4% to 9%) |
| 2+ levels | 524,25,27,34,37 | 31.7% (45/142) | 15.2% (39/257) | 2.17 (1.41, 3.34) | 20% (−9% to 49%) |
| Thoracolumbar | 21.0% (43/205) | 7.6% (22/290) | 2.75 (1.71, 4.42) | 15% (6% to 23%) | |
| 1 level | 228,33 | 26.8% (15/56) | 9.4% (13/138) | 2.68 (1.42, 5.07) | 20% (−7% to 46%) |
| 2+ levels | 229,32 | 18.8% (28/149) | 5.9% (9/152) | 2.86 (1.35, 6.06) | 14% (4% to 24%) |
Figure 4.
Forest plot depicting risk ratio of nonunion for smokers vs nonsmokers stratified by graft type.
Table 6.
Nonunion (%) Stratifying on Graft Type for Cervical and Thoracolumbar Fusions.
| No. of Studies | Smoking % (n/N) | Nonsmoking % (n/N) | Pooled RR (95% CI) (M-H) | Excess Risk Associated with Smoking (95% CI) | |
|---|---|---|---|---|---|
| All | 23.3% (155/665) | 13.0% (182/1405) | 1.60 (1.35, 1.91) | 10% (6% to 15%) | |
| Autograft | 821,26-29,32,34,40 | 24.4% (86/352) | 14.0% (68/487) | 2.04 (1.54, 2.72) | 14% (9% to 20%) |
| Allograft | 621,22,28,35-37 | 20.3% (69/340) | 12.4% (114/918) | 1.39 (1.12, 1.73) | 6% (0 to 11%) |
| Cervical | 20.0% (70/350) | 11.2% (104/929) | 1.70 (1.32, 2.20) | 9% (1% to 16%) | |
| Autograft | 321,27,34 | 38.7% (29/75) | 20.1% (34/169) | 2.02 (1.39, 2.95) | 19% (7% to 32%) |
| Allograft | 421,35-37 | 14.9% (41/275) | 9.2% (70/760) | 1.47 (1.03, 2.09) | 4% (−1% to 10%) |
| Thoracolumbar | 27.0% (85/315) | 16.4% (78/476) | 1.69 (1.18, 2.40) | 12% (7% to 18%) | |
| Autograft | 526,28,29,32,40 | 22.8% (57/250) | 10.7% (34/318) | 2.08 (1.34, 3.23) | 13% (7% to 19%) |
| Allograft | 222,28 | 43.1% (28/65) | 27.8% (44/158) | 1.35 (1.02, 1.78) | 11% (−3% to 24%) |
Discussion
Understanding the long-term outcomes following spinal fusion, particularly in nicotine users, is becoming increasingly important given that cigarette smoking remains the leading cause of preventable disease in our times and might play a considerable adverse role in spinal fusion surgery. The conjunction of nicotine exposure with the significant increase in the rates of spine surgery seen over the preceding decade creates a possibly adversarial confounding interaction, as smokers comprise a remarkable 24.0% to 31.8% of the expanding population of surgical spine patients. While the precise mechanism by which nicotine affects bone health and healing has not yet been clearly reconstructed, nicotine use has been shown to impair gene expression affecting osteogenic growth factors as well as bone vascularization, which ultimately can result in postoperative complications, with nonunion or pseudarthrosis for fusion surgery presenting an undesirable outcome for this population. Pseudarthrosis following spine fusion surgery can consequently result in intractable pain, disability, and the frequent need for protracted use of health care resources such as more involved imaging follow-up, increased nonsurgical management efforts as well as repeat surgeries with further escalating indirect cost accruements surrounding disability and general claims. In our survey of the pertinent literature, we were surprised to find no formal risk calculations for the interrelationship of smoking and nonunion risk after spine fusion surgery. To help answer this question, we therefore conducted this Systematic Review in which we identified a number of studies that allowed us to examine the interrelation of a higher likelihood of nonunion following spinal fusion surgery and to further explore differences of nonunion occurrence following either cervical or thoracolumbar fusion surgery.
Achieving a solid fusion is considered the gold-standard end result of spinal arthrodesis surgeries indicated for the management of a number of pathologies unresponsive to conservative management. Despite best efforts, some patients fail to achieve radiographic evidence of osseous union through the passage of expected bone healing time on postsurgical follow-up; the potential mechanisms and adjuvant predisposing factors for this complication have been the subject of numerous published studies. 41 Although smoking has been implicated as a predictor of pseudarthrosis, the characteristics of smaller, individual studies precludes precise quantification of the conferred risk. This in turn minimizes our ability as clinicians to better estimate the degree of potential clinical benefits that are at stake when we try to engage patients’ preoperative optimization processes. It further prevents more precise risk calculations for long term complications estimations and interpretations of patient reported outcomes reports. To help provide more foundational objectifiable numbers to this end, we conducted a formal meta-analysis comparing active smokers to nonsmokers using generally accepted operational definitions for this life-style choice in patients who received spinal fusion surgery. We consistently demonstrated that smoking is a substantial risk factor for nonunion, a finding that persisted even after stratification by length of follow-up intervals, the number of spine levels, region of spine, and types of graft material involved in the intended spine fusion surgery.
The results of this meta-analysis add to the growing body of literature suggesting that smoking is a modifiable risk factor for nonunion following surgery for spinal fusion. While not assessed herein, smoking cessation by patients at various stages of treatment may reduce poor outcomes. A previous retrospective analysis by Glassman et al. indeed concluded that postoperative smoking cessation was associated with decreased risk of nonunion at follow-up. 42
Length of Follow-Up
One question of fusion as outcome is the persistent question of constitutes an adequate follow-up time for bone fusion. A definitive time period has yet to be established, but mostly revolves around 1-2 years. 43 One underlying problem is that osseous homeostasis is a continuous process, in which bone is constantly being resorbed by osteoclasts and reformed by osteoblasts (“bone turnover”). 44 Spinal fusion, therefore, is a dynamic process that may occur over several months to years, an especially unclear timeline in the case of the spinal column. Although several guidelines and definitions exist regarding the radiographic assessment of fusion status, specific evidence-based recommendations for the timing of radiographic assessment have not been established. 45 It has become common within the surgical literature to assign a diagnosis of pseudarthrosis after at least 1 year has elapsed since the index procedure, presumably to permit sufficient opportunity for the bone healing process to conclude. 43 However, several studies have demonstrated disparities in the average time until diagnosis of pseudarthrosis using plain radiographs, ranging as high as 2 to even 3.5 years.46,47 Tokuhashi et al 48 found that in 48 patients without evidence of union 2 years following lumbar fusion, 30% eventually went on to show new bone union without reoperation, leading to the concept of a “stable nonunion” as an in-between alternative to a “solid fusion” and an “unstable nonunion.” 48
Given the absence of clearly established radiographic and timeline definitions for spinal fusions following surgery, we analyzed studies within categories of follow-up ranging 12-24 and ≥24 months. In our meta-analysis, smoking was associated with increased nonunion risk regardless of follow-up time category in both the cervical and thoracolumbar spine assessed distinctly as separate spinal column regions. Within each spinal segment, 95% confidence intervals for nonunion risk in smokers compared to nonsmokers at 12-24 and ≥24 month categories overlapped, suggesting that the impact of smoking on nonunion rates was similar regardless of the follow-up time. However, the absence of broadly accepted “optimal” timeframes during which nonunion should be diagnosed, should any such time point actually exist, represents an area for future studies to standardize criteria for both clinical and research applications.
Spinal Segment and Levels
Stratification of studies by location and number of segments fused revealed several key associations; overall pooled risk ratios demonstrated that smokers were statistically significantly more likely than nonsmokers to experience nonunion for all major variables including cervical, thoracolumbar, single level, and multilevel procedures. Heterogeneity was largest in the analysis of cervical procedures (I2 = 38%) although likely of minimal significance. 49 Within subgroups, the only population that did not display a significant association was single-level cervical fusion procedures (RR 1.38, 95% CI 0.78-2.44). Luszczyk et al. studied 573 patients who underwent single-level anterior cervical fusion with allograft and found no significant association between smoking and nonunion rates. 36 The authors attributed their findings to the effect of rigid plate fixation, and further speculated that for single-level cervical pathologies, the deleterious effect of smoking on bone growth may not substantially alter fusion rates. Conversely, smoking was associated with increased risk relative to nonsmokers in individuals undergoing multilevel cervical fusion. Multilevel cervical fusion itself is a known risk factor for pseudarthrosis and other complications, particularly in anterior approaches involving 3 or 4 levels.50-52 The relationship between smoking and nonunion in multilevel posterior cervical fusion, however, is controversial, with one study finding no difference in fusion rates between smokers and nonsmokers. 53 In the thoracolumbar spine, the relationship between smoking and pseudarthrosis is consistent with a multitude of prior studies reporting increased rates of complications in this population.54,55 Interestingly, recent retrospective analysis of 128 patients undergoing thoracolumbar fusion due to presumed aseptic pseudarthrosis found positive intraoperative culture rates of 10%. 56 Underlying occult infections, however, were not associated with smoking status or number of fused levels, among other parameters. Although the study design of the present meta-analysis excluded infectious causes of pseudarthrosis from analysis, it is likely that at least some of these studies included patients with underlying occult infection, as protocols for intraoperative culture were not uniformly reported. Nevertheless, the relative rarity of these occult infections (10% prevalence) suggests that this mechanism of nonunion is rare in the studies included in the present analysis, and that smoking may negatively impact fusion rates by other means, such as impairment of normal bone turnover and cellular differentiation.57,58
Graft Material
Smoking was associated with increased risk for nonunion in studies utilizing autograft, as well as in those utilizing allograft. Studies reporting autograft had little heterogeneity in the risk of nonunion by smoking status (I2 = 0), although approximately half of the studies were of poor-quality evidence. Interestingly, while allograft studies demonstrated minimal heterogeneity with regard to the effect of smoking on nonunion rates (I2 = 0), the individual studies were largely unable to demonstrate a statistically significant relative risk in smokers (with the sole exception of Andersen et al. with 95% CI 1.00-1.79)). 22 Taken together, these suggest that the effect of smoking on fusion rates in the allograft population was uncovered by the increased statistical power afforded by the present meta-analysis.
Regulation of bone is governed by the constant balance between resorption and replacement maintained by cells, secondary messengers, and the local environment. 44 Smoking is thought to have depressing effects on bone metabolism and cell differentiation, which in animal models, has been shown to generate osteopenia. 58 Similarly, in observational studies involving humans, smoking has been shown to be negatively correlated with critical bone turnover markers such as osteoprotegerin, suggesting that disruption of such cellular signaling processes may be in fact the underlying patho-mechanism by which smoking results nonunion following spine surgery. 59 Autologous bone graft is usually reported to be the “gold standard” material for surgical fusion surgery due to the presence of numerous elements critical for promoting bone formation, including osteoprogenitor cells, matrix, and a number of bone morphogenetic proteins. 60 A meta-analysis studying rates of pseudarthrosis in anterior cervical fusion reported mean rates of pseudarthrosis of 4.8% in allograft studies compared to 0.9% in autograft studies; however, the calculation of relative risk for nonunion based on graft material was not possible due to the paucity of studies directly comparing autograft fusion to allograft fusion. 61 The present study, in contrast, found that the use of autograft provided no additional benefit to allograft in terms of bony fusion in smokers. In fact, our findings may suggest the possibility that autograft may demonstrate diminished fusion rates in comparison to allograft (test for subgroup differences, P = .04). It may be that the same deleterious effects of smoking on bone growth make autologous grafts inherently more susceptible to nonunion when compared to allograft. Various graft materials were utilized by the studies included in the present analysis. Further studies may establish a distinctive fusion benefit to smokers from certain autograft procurement locations, such as iliac crest or fibula.
Strengths and Limitations
There are several advantages and limitations to the present study design. To begin, this is the only meta-analysis to specifically calculate the effect size of smoking on the risk of nonunion following spinal fusion, allowing us to quantify the excess risk associated with this modifiable risk factor. Additionally, by performing a comprehensive literature review, we were able to appreciate differences in nonunion risks for specific subpopulations that smaller, individual studies may be underpowered to detect. For example, we were able to stratify individuals by both number of levels and spinal segments operated upon. Furthermore, detailed appraisal of study/evidence quality and heterogeneity revealed little inherent variation between studies, and the primary findings of our analysis were supported by sensitivity analyses that excluded poor-quality data.
A challenge of analyzing the impact of smoking on surgical outcomes is that the included studies consist primarily of retrospective case series, which limits the level of evidence contributed by each. Additionally, studies lacked a uniform radiographic protocol for diagnosing nonunion, with some studies not presenting the definition utilized at all. Although we were able to categorize studies into several clinically relevant subgroups such as graft type and spinal segment, the relatively small number of studies directed at posterior cervical fusion and anterior thoracolumbar fusion prevented stratification by surgical approach. As the present study excluded patients receiving rh-BMP2 in order to minimize the presence of confounders, we cannot address its potential beneficial effects.
Several relevant recommendations related to smoking cessation are unable to be established by the present study; namely, the present data do not allow us to define a preferred time of cessation prior to elective fusion surgery, although much earlier rather than later cessation likely is preferable for any elective fusion surgery. Similarly, dose reduction and use of alternate nicotine delivery modalities—for example, patches, gums, and “vaping” are not clearly amenable for differentiation at this juncture, but the globally deleterious effect of nicotine use would make complete nicotine use cessation for an earliest yet to be determined time point prior to elective time point preferable. For urgent and emergent spine fusion surgery, immediate post-nicotine cessation remains a practical and reasonable request. Overall, from an outcomes determination standpoint, it seems clear that smokers should continue to be assessed separately from nonsmokers due to a clearly different nonunion risk profile.
Conclusion
Tobacco smoking status carries a global risk of nonunion for spinal fusion procedures regardless of follow-up time, location, number of segments fused, or grafting material. Use of autograft did not carry a reduced risk of nonunion compared to allograft in smokers. Further retrospective studies with comprehensive methodology in addition to randomized control trials are necessary to establish a more extensive risk profile of smoking and spinal fusion. Efforts should promote establishment of treatment guidelines tailored to smokers.
Supplemental Material
Supplemental Material, sj-pdf-1-gsj-10.1177_21925682211046899 for The Risk of Nonunion in Smokers Revisited: A Systematic Review and Meta-Analysis by Ravi S. Nunna, Philip B. Ostrov, Darius Ansari, Joseph R. Dettori, Periklis Godolias, Elias Elias, Angela Tran, Rod J. Oskouian, Robert Hart, Amir Abdul-Jabbar, Keith L. Jackson, John G.Devine, Ankit I. Mehta, Owoicho Adogwa and Jens R. Chapman in Global Spine Journal
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Ravi S. Nunna https://orcid.org/0000-0003-2516-5445
Philip B. Ostrov https://orcid.org/0000-0002-5121-6587
Joseph R. Dettori https://orcid.org/0000-0002-0216-8363
Elias Elias https://orcid.org/0000-0003-0339-3666
Amir Abdul-Jabbar https://orcid.org/0000-0001-6613-3111
Keith L. Jackson https://orcid.org/0000-0002-3883-8760
John G. Devine https://orcid.org/0000-0002-8958-2996
Ankit I. Mehta https://orcid.org/0000-0001-6931-6095
Owoicho Adogwa https://orcid.org/0000-0001-9403-2799
References
- 1.U.S. Department of Health and Human Services . The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. Accessed January 30, 2019. [Google Scholar]
- 2.Jha P, Peto R. Global effects of smoking, of quitting, and of taxing tobacco. N Engl J Med. 2014;370:60-68. [DOI] [PubMed] [Google Scholar]
- 3.Kim SS, Chen W, Kolodziej M, Wang X, Wang VJ, Ziedonis D. A systematic review of smoking cessation intervention studies in China. Nicotine Tob Res. 2012;14:891-899. [DOI] [PubMed] [Google Scholar]
- 4.Beahrs TR, Reagan J, Bettin CC, Grear BJ, Murphy GA, Richardson DR. Smoking effects in foot and ankle surgery: an evidence-based Review. Foot Ankle Int. 2019;40:1226-1232. [DOI] [PubMed] [Google Scholar]
- 5.Durand WM, DePasse JM, Bokshan SL, Eltorai AEM, Daniels AH. Tobacco use and complications following spinal fusion: a comparison of the national surgical quality improvement program and national inpatient sample datasets. World Neurosurgery. 2019;123:e393-e407. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Merchan EC. The importance of smoking in orthopedic surgery. Hosp Pract. 1995;46:175-182. [DOI] [PubMed] [Google Scholar]
- 7.Spangler EL, Goodney PP. Smoking cessation strategies in vascular surgery. Semin Vasc Surg. 2015;28:80-85. [DOI] [PubMed] [Google Scholar]
- 8.Fisahn C, Jeyamohan S, Norvell DC, Tubbs RS, Moisi M, Chapman JR, et al. Association between allogeneic blood transfusion and postoperative infection in major spine surgery. Clinical Spine Surgery: A Spine Publication. 2017;30:E988-E992. [DOI] [PubMed] [Google Scholar]
- 9.Martin BI, Mirza SK, Spina N, Spiker WR, Lawrence B, Brodke DS. Trends in lumbar fusion procedure rates and associated hospital costs for degenerative spinal diseases in the United States, 2004 to 2015. Spine. 1976;44:369-376. [DOI] [PubMed] [Google Scholar]
- 10.Cornelius ME, Wang TW, Jamal A, Loretan CG, Neff LJ. Tobacco product use among adults - United States, 2019. MMWR. Morbidity and Mortality Weekly Report. 2020;69:1736-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raizman NM, O'Brien JR, Poehling-Monaghan KL, Yu WD. Pseudarthrosis of the spine. J Am Acad Orthop Surg. 2009;17:494-503. [DOI] [PubMed] [Google Scholar]
- 12.Daffner SD, Waugh S, Norman TL, Mukherjee N, France JC. Nicotine increases osteoblast activity of induced bone marrow stromal cells in a dose-dependent manner: an in vitro cell culture experiment. Global Spine J. 2012;2:153-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson KL, Devine JG. The effects of smoking and smoking cessation on spine surgery: a systematic review of the literature. Global Spine J. 2016;6:695-701. doi: 10.1055/s-0036-1571285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nunna R, Dettori JR, Frieler S, et al. The risk of non-Union after spinal fusion in smokers: a systematic review and meta-analysis. PROSPERO 2021 CRD42021231462 Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021231462 https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021231462.
- 15.Hemingway H, Croft P, Perel P, Hayden JA, Abrams K, Timmis A, et al. Prognosis research strategy (PROGRESS) 1: a framework for researching clinical outcomes. BMJ. 2013;346:e5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moons KG, Hooft L, Williams K, Hayden JA, Damen JA, Riley RD. Implementing systematic reviews of prognosis studies in Cochrane. Cochrane Database Syst Rev. 2018;10:ED000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1-e34. [DOI] [PubMed] [Google Scholar]
- 18.Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158:280-286. [DOI] [PubMed] [Google Scholar]
- 19.Scottish Intercollegiate Guidelines Network (SIGN). Scottish Intercollegiate Guidelines Network: Rating a Quality of Cohort Studies. Methodology Checklist 3: Cohort Studies. https://www.sign.ac.uk/what-we-do/methodology/checklists/. Accessed January 14, 2021. [Google Scholar]
- 20.Foroutan F, Guyatt G, Zuk V, Vandvik PO, Alba AC, Mustafa R, et al. GRADE guidelines 28: use of GRADE for the assessment of evidence about prognostic factors: rating certainty in identification of groups of patients with different absolute risks. J Clin Epidemiol. 2020;121:62-70. [DOI] [PubMed] [Google Scholar]
- 21.An HS, Simpson JM, Glover JM, Stephany J. Comparison between allograft plus demineralized bone matrix versus autograft in anterior cervical fusion. a prospective multicenter study. Spine. 1976;20:2211-2216. [PubMed] [Google Scholar]
- 22.Andersen T, Christensen FB, Egund N, Ernst C, Fruensgaard S, Østergaard J, et al. The effect of electrical stimulation on lumbar spinal fusion in older patients: a randomized, controlled, multi-center trial: part 2: fusion rates. Spine. 1976;34:2248-2253. [DOI] [PubMed] [Google Scholar]
- 23.Andersen T, Christensen FB, Laursen M, Høy K, Hansen ES, Bünger C. Smoking as a predictor of negative outcome in lumbar spinal fusion. Spine. 1976;26:2623-2628. [DOI] [PubMed] [Google Scholar]
- 24.Bose B. Anterior cervical instrumentation enhances fusion rates in multilevel reconstruction in smokers. J Spinal Disord. 2001;14:3-9. [DOI] [PubMed] [Google Scholar]
- 25.Brown CW, Orme TJ, Richardson HD. The rate of pseudarthrosis (surgical nonunion) in patients who are smokers and patients who are nonsmokers: a comparison study. Spine. 1976;11:942-943. [DOI] [PubMed] [Google Scholar]
- 26.Deguchi M, Rapoff AJ, Zdeblick TA. Posterolateral fusion for isthmic spondylolisthesis in adults. J Spinal Disord. 1998;11:459-464. [PubMed] [Google Scholar]
- 27.Emery SE, Fisher JR, Bohlman HH. Three-level anterior cervical discectomy and fusion: radiographic and clinical results. Spine. 1976;22:2622-2625. discussion 2625. [DOI] [PubMed] [Google Scholar]
- 28.Glassman SD, Dimar JR, 3rd, Burkus K, Hardacker JW, Pryor PW, Boden SD, et al. The efficacy of rhBMP-2 for posterolateral lumbar fusion in smokers. Spine. 1976;32:1693-1698. [DOI] [PubMed] [Google Scholar]
- 29.Glassman SD, Rose SM, Dimar JR, Puno RM, Campbell MJ, Johnson JR. The effect of postoperative nonsteroidal anti-inflammatory drug administration on spinal fusion. Spine. 1976;23:834-838. [DOI] [PubMed] [Google Scholar]
- 30.Goldberg EJ, Singh K, Van U, Garretson R, An HS. Comparing outcomes of anterior cervical discectomy and fusion in workman's versus non-workman's compensation population. Spine J. 2002;2:408-414. [DOI] [PubMed] [Google Scholar]
- 31.Groff MW, Sriharan S, Lee SM, Maiman DJ. Partial corpectomy for cervical spondylosis. Spine. 1976;28:14-20. [DOI] [PubMed] [Google Scholar]
- 32.Hanley EN, Jr., Levy JA. Surgical treatment of isthmic lumbosacral spondylolisthesis. analysis of variables influencing results. Spine. 1976;14:48-50. [DOI] [PubMed] [Google Scholar]
- 33.Hermann PC, Webler M, Bornemann R, Jansen TR, Rommelspacher Y, Sander K, et al. Influence of smoking on spinal fusion after spondylodesis surgery: a comparative clinical study. Technol Health Care. 2016;24:737-744. [DOI] [PubMed] [Google Scholar]
- 34.Hilibrand AS, Fye MA, Emery SE, Palumbo MA, Bohlman HH. Impact of smoking on the outcome of anterior cervical arthrodesis with interbody or strut-grafting. J Bone Jt Surg Am Vol. 2001;83:668-673. [DOI] [PubMed] [Google Scholar]
- 35.Lau D, Chou D, Ziewacz JE, Mummaneni PV. The effects of smoking on perioperative outcomes and pseudarthrosis following anterior cervical corpectomy. J Neurosurg Spine. 2014;21:547-558. [DOI] [PubMed] [Google Scholar]
- 36.Luszczyk M, Smith JS, Fischgrund JS, Ludwig SC, Sasso RC, Shaffrey CI, et al. Does smoking have an impact on fusion rate in single-level anterior cervical discectomy and fusion with allograft and rigid plate fixation? J Neurosurg Spine. 2013;19:527-531. [DOI] [PubMed] [Google Scholar]
- 37.Martin GJ, Jr., Haid RW, Jr., MacMillan M, Rodts GE, Berkman R. Anterior cervical discectomy with freeze-dried fibula allograft. Overview of 317 cases and literature review. Spine. 1976;24:852-859. discussion 858-859. [DOI] [PubMed] [Google Scholar]
- 38.Suchomel P, Barsa P, Buchvald P, Svobodnik A, Vanickova E. Autologous versus allogenic bone grafts in instrumented anterior cervical discectomy and fusion: a prospective study with respect to bone union pattern. Eur Spine J. 2004;13:510-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Urrutia J, Molina M. Fresh-frozen femoral head allograft as lumbar interbody graft material allows high fusion rate without subsidence. J Orthop Traumatol: Surgery & Research. 2013;99:413-418. [DOI] [PubMed] [Google Scholar]
- 40.Zdeblick TA. A prospective, randomized study of lumbar fusion. Spine. 1993;18:983-991. [DOI] [PubMed] [Google Scholar]
- 41.Abdu WA, Sacks OA, Tosteson ANA, Zhao W, Tosteson TD, Morgan TS, et al. Long-term results of surgery compared with nonoperative treatment for lumbar degenerative spondylolisthesis in the spine patient outcomes research trial (SPORT). Spine. 2018;43:1619-1630. doi: 10.1097/BRS.0000000000002682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glassman SD, Anagnost SC, Parker A, Burke D, Johnson JR, Dimar JR. The effect of cigarette smoking and smoking cessation on spinal fusion. Spine. 1976;25:2608-2615. [DOI] [PubMed] [Google Scholar]
- 43.Chun DS, Baker KC, Hsu WK. Lumbar pseudarthrosis: a review of current diagnosis and treatment. Neurosurg Focus. 2015;39:E10. doi: 10.3171/2015.7.Focus15292. [DOI] [PubMed] [Google Scholar]
- 44.Tanaka Y, Nakayamada S, Okada Y. Osteoblasts and osteoclasts in bone remodeling and inflammation. Curr Drug Targets - Inflamm Allergy. 2005;4:325-328. doi: 10.2174/1568010054022015. [DOI] [PubMed] [Google Scholar]
- 45.Choudhri TF, Mummaneni PV, Dhall SS, Eck JC, Groff MW, Ghogawala Z, et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 4: radiographic assessment of fusion status. J Neurosurg Spine. 2014;21:23-30. doi: 10.3171/2014.4.Spine14267. [DOI] [PubMed] [Google Scholar]
- 46.Ahn DK, Lee S, Moon SH, Kim DG, Hong SW, Shin WS. Bulb syringe and pulsed irrigation. Clinical Spine Surgery: A Spine Publication. 2016;29:34-37. doi: 10.1097/BSD.0000000000000068. [DOI] [PubMed] [Google Scholar]
- 47.Dickson DD, Lenke LG, Bridwell KH, Koester LA. Risk factors for and assessment of symptomatic pseudarthrosis after lumbar pedicle subtraction osteotomy in adult spinal deformity. Spine. 2014;39:1190-1195. doi: 10.1097/brs.0000000000000380.2014 [DOI] [PubMed] [Google Scholar]
- 48.Tokuhashi Y, Ajiro Y, Umezawa N. Follow-up of patients with delayed union after posterior fusion with pedicle screw fixation. Spine. 2008;33:786-791. doi: 10.1097/BRS.0b013e31816956f7. [DOI] [PubMed] [Google Scholar]
- 49.Dettori JR, Norvell DC, Chapman JR. Seeing the forest by looking at the trees: how to interpret a meta-analysis forest plot. Global Spine J. 2021;11:614-616. doi: 10.1177/21925682211003889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hofler RC, Swong K, Martin B, Wemhoff M, Jones GA. Risk of pseudoarthrosis after spinal fusion: analysis from the healthcare cost and utilization project. World Neurosurgery. 2018;120:e194-e202. [DOI] [PubMed] [Google Scholar]
- 51.Wewel JT, Kasliwal MK, Adogwa O, Deutsch H, O'Toole JE, Traynelis VC. Fusion rate following three- and four-level ACDF using allograft and segmental instrumentation: a radiographic study. J Clin Neurosci. 2019;62:142-146. doi: 10.1016/j.jocn.2018.11.040. [DOI] [PubMed] [Google Scholar]
- 52.Leven D, Cho SK. Pseudarthrosis of the cervical spine: risk factors, diagnosis and management. Asian Spine Journal. 2016;10:776-786. doi: 10.4184/asj.2016.10.4.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eubanks JD, Thorpe SW, Cheruvu VK, Braly BA, Kang JD. Does smoking influence fusion rates in posterior cervical arthrodesis with lateral mass instrumentation? Clin Orthop Relat Res. 2011;469:696-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manzur M, Virk SS, Jivanelli B, Vaishnav AS, McAnany SJ, Albert TJ, et al. The rate of fusion for stand-alone anterior lumbar interbody fusion: a systematic review. Spine J. 2019;19:1294-1301. [DOI] [PubMed] [Google Scholar]
- 55.Zhuang T, Ku S, Shapiro LM, Hu SS, Cabell A, Kamal RN. A Cost-Effectiveness analysis of smoking-cessation interventions prior to posterolateral lumbar fusion. J Bone Joint Surg. 2020;102:2032-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burkhard MD, Loretz R, Uçkay I, et al. Occult infection in pseudarthrosis revision after spinal fusion. Spine J. 2021;21:370-376. [DOI] [PubMed] [Google Scholar]
- 57.Gao S-g., Li K-h., Xu M, Jiang W, Shen H, Luo W, et al. Bone turnover in passive smoking female rat: relationships to change in bone mineral density. BMC Muscoskel Disord. 2011;12:131. doi: 10.1186/1471-2474-12-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ko CH, Chan RLY, Siu WS, Shum WT, Leung PC, Zhang L, et al. Deteriorating effect on bone metabolism and microstructure by passive cigarette smoking through dual actions on osteoblast and osteoclast. Calcif Tissue Int. 2015;96:389-400. doi: 10.1007/s00223-015-9966-8. [DOI] [PubMed] [Google Scholar]
- 59.Jorde R, Stunes AK, Kubiak J, Grimnes G, Thorsby PM, Syversen U. Smoking and other determinants of bone turnover. PLoS One. 2019;14:e0225539. doi: 10.1371/journal.pone.0225539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Medical Advisory Secretariat . Osteogenic protein-1 for long bone nonunion: an evidence-based analysis. Ontario health technology assessment series. 2005;5:1-57. [PMC free article] [PubMed] [Google Scholar]
- 61.Shriver MF, Lewis DJ, Kshettry VR, Rosenbaum BP, Benzel EC, Mroz TE. Pseudoarthrosis rates in anterior cervical discectomy and fusion: a meta-analysis. Spine J. 2015;15:2016-2027. doi: 10.1016/j.spinee.2015.05.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-gsj-10.1177_21925682211046899 for The Risk of Nonunion in Smokers Revisited: A Systematic Review and Meta-Analysis by Ravi S. Nunna, Philip B. Ostrov, Darius Ansari, Joseph R. Dettori, Periklis Godolias, Elias Elias, Angela Tran, Rod J. Oskouian, Robert Hart, Amir Abdul-Jabbar, Keith L. Jackson, John G.Devine, Ankit I. Mehta, Owoicho Adogwa and Jens R. Chapman in Global Spine Journal