IL-17 promotes collagen deposition by cancer-associated fibroblasts and enhances immune exclusion of tumors.
Abstract
Excessive collagen deposition by fibroblasts surrounding some tumors has seriously limited the efficacy of checkpoint inhibitor therapies. Chen et al. (2022. J. Exp. Med. https://doi.org/10.1084/jem.20210693) show that IL-17 promotes collagen deposition by cancer-associated fibroblasts, enhancing immune exclusion of tumors, and that targeting IL-17–triggered HIF1α expression can reverse matrix mediated immune exclusion.
Immune-excluded tumors are characteristically surrounded by a “wall” of cancer-associated fibroblasts (CAFs) producing collagen and other extracellular matrix (ECM) proteins. Immune cells including T cells are found in this fibrotic wall but do not enter the tumor bed, even after they are activated by administration of checkpoint inhibitors such as anti–PD-L1. Hence, immune exclusion represents a significant hurdle to therapies that seek to harness the immune system, and these types of tumors continue to carry a poor prognosis.

Insights from Mandy J. McGeachy.
Increased cancer risk is an important comorbidity in people with inflammatory bowel disease and other chronic inflammatory disease. IL-17 has a long-established role in the development of spontaneous tumors in murine models of carcinoma in skin and gut. This is attributed to the pro-inflammatory functions of IL-17, as inflammation helps to establish the supporting tumor stroma. More recent studies have highlighted the pro-proliferative effects of IL-17 on epithelial progenitor cells in wound healing after injury in gut and skin, and that IL-17–enhanced proliferation of stem cells increases the risk of tumorigenesis in the presence of carcinogens.
In humans, high intratumoral IL-17 correlates with poor prognosis and resistance to therapy in a number of cancers. Prior studies have indicated that IL-17 enhances PD-L1 expression in a model of colorectal cancer (Liu et al., 2021), and that IL-17–recruited neutrophils exclude tumor infiltration of T cells following checkpoint blockade through NETosis (Zhang et al., 2020). The study by Chen et al. (2022) in this issue of JEM shows an additional contribution of IL-17 to tumor immune exclusion by acting on CAFs to increase ECM deposition. There have been indications that IL-17 contributes to fibrotic disease and can enhance pro-fibrotic phenotypes in synergy with TGFβ (Majumder and McGeachy, 2021). In vitro studies aiming to determine mechanisms by which cytokines target fibroblasts are not always satisfying due to limitations of two-dimensional culture of a single cell type.
In the current study by Chen et al. (2022), they employed a temporal deletion of IL-17 receptor expression in a key target of IL-17 signaling in vivo: fibroblasts. In spontaneous and transferred models of skin carcinoma, they blocked the capacity for fibroblasts to respond to IL-17 by tamoxifen-induced conditional deletion of the IL-17RC subunit of the IL-17 receptor. Fibroblast-specific deletion of the IL-17 receptor IL-17RC (shared by IL-17A and IL-17F) after initiation of skin carcinoma led to reduced proliferation and numbers of CAFS, and reduced collagen deposition. Excitingly, this reduction in CAFs and ECM rendered the tumors susceptible to anti–PD-L1 by increasing entry of activated T cells into the tumor.
In both murine and human CAFs, the authors show that IL-17 enhances the program for collagen deposition through induction of procollagen–proline dioxygenase (P4H) enzymes that facilitate the proper folding and secretion of pro-collagen, and lysl oxidase (LOX) to crosslink pro-collagen into collagen fibers. The genes encoding P4Hs and LOX are transcriptional targets of HIF1α, and the authors confirmed that HIF1α is required for IL-17–enhanced collagen deposition in both mouse and human CAFS. Through a series of elegant studies, they demonstrate that rather than inducing Hif1a gene expression, IL-17 promotes the translation of HIF1α protein through Act1 binding to Hif1a mRNA transcripts. In fact, this is very much on par for IL-17 signaling, which is increasingly found to reinforce and enhance its own signaling outcomes through modulation of RNA-binding proteins including Act1, Arid5a, and Regnase to regulate expression of transcription factors and co-activators (Li et al., 2019).
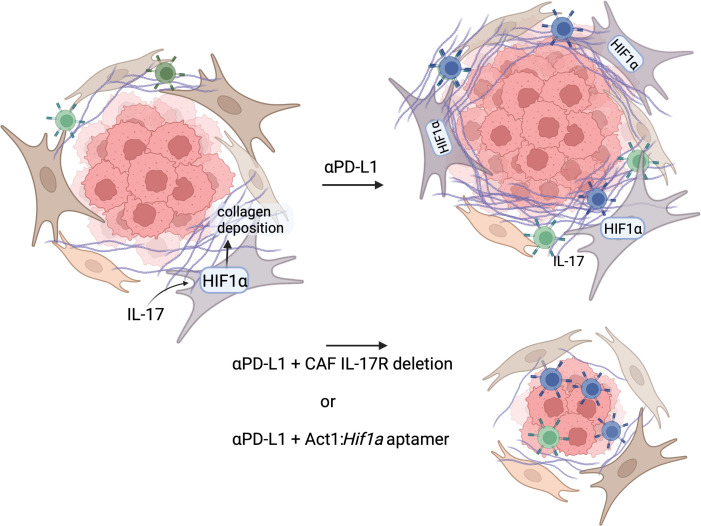
IL-17 enhances collagen deposition through HIF1α expression in CAFs. Checkpoint inhibitor therapy (anti–PD-L1) in mice with established tumors activates T cells and increases IL-17 production, leading to increased collagen deposition and inhibition of activated CD8+ T cell access to the tumor, which continues to grow. Deletion of IL-17 receptor on CAFs or blockade of IL-17 induction of HIF1a with an Act1:Hif1a aptamer reduces collagen deposition around the established tumor to allow anti–PD-L1 activated T cells to enter and restrict tumor growth. Figure created with Biorender.com.
Antibodies targeting IL-17 and IL-17 receptor are already in clinical use for autoimmune diseases including psoriasis. They have a good safety profile and have been used off-label to reduce autoimmune adverse immune-related events that arise in some patients treated with checkpoint blockade. So, one therapeutic implication of this study would be to incorporate existing IL-17–neutralizing therapies with checkpoint inhibitors, supporting previous studies suggesting this add-on. However, IL-17 blockade does lead to a small increase in the risk of oral infections with Candida species and new onset of inflammatory bowel disease in some patients. These side effects correspond to well-established roles for IL-17 in combating fungal infections in mouse models, particularly oropharyngeal candidiasis, and in maintaining gut health (Majumder and McGeachy, 2021).
The approach employed by Chen et al. (2022) was to more precisely target just one facet of IL-17 signaling by interrupting the binding of Act1 to Hif1a mRNA. Aptamers are RNA oligomers designed to mimic the RNA target sequence of an RNA-binding protein, in this case the stem-loop structure that forms the Act1 SEFIR binding element (SBE) on Hif1a mRNA transcripts. The aptamer approach was previously employed by this group to target Act1 binding to the SBE at the 3′UTR of IL-17–induced inflammatory mediators Cxcl1, Tnf, and Csf2 (Herjan et al., 2018). In that study, Act1 was found to increase the stability of pro-inflammatory mRNA transcripts downstream of IL-17 signaling, and inflammatory skin disease could be blocked with SBE aptamer. Here, Hif1a was found to have a unique SBE that Act1 binds to enhance translation. Excitingly, the Hif1a-SBE aptamer did not block inflammatory chemokines and cytokines or metabolic gene expression. It is therefore predicted that the antimicrobial outcomes of IL-17 signaling and proliferation important for wound healing will be unaffected. Of course, it would be important to confirm this premise in cell type–specific hypoxia assays and fungal infection models if this approach were to move forward to pre-clinical stages.
IL-17 has been associated with fibrotic disease in chronic infection and in idiopathic fibrosis, where anti-inflammatory approaches have not been useful. One could therefore speculate that this aptamer approach to block the pro-fibrotic component of IL-17 signaling could have uses beyond cancer. On the other hand, there are anecdotal reports of impaired wound healing in patients receiving IL-17–blocking biologic therapy for psoriasis, and so aptamers designed to target the inflammatory process while sparing proliferation or matrix production could reduce side effects in autoimmune therapy.
The therapeutic potential raised by the precision approach to targeting RNA-binding protein interactions with specific transcripts is intriguing and vast. Blocking transcription factors such as HIF1α could have broad side effects that reduce their long-term usefulness. However, being able to block a key biological process in a context-dependent way through RNA-binding protein targets suggests a new generation of therapeutics. Act1 is only used by a limited number of receptors, principally IL-17 family cytokines, so the effects should be quite narrowly targeted to IL-17–induced HIF1α induction while sparing HIF1α stabilization when it is induced in a beneficial way by other signals including local hypoxia. The current study highlights the importance of understanding post-transcriptional regulation of gene expression by RNA-binding proteins as an untapped reservoir for therapeutic tuning of immune responses.
References
- Chen, X., et al. 2022. J. Exp. Med. 10.1084/jem.20210693 [DOI] [Google Scholar]
- Herjan, T., et al. 2018. Nat. Immunol. 10.1038/s41590-018-0071-9 [DOI] [Google Scholar]
- Li, X., et al. 2019. Nat. Immunol. 10.1038/s41590-019-0514-y [DOI] [Google Scholar]
- Liu, C., et al. 2021. J. Immunother. Cancer. 10.1136/jitc-2020-001895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder, S., and McGeachy M.J.. 2021. Annu. Rev. Immunol. 10.1146/annurev-immunol-101819-092536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y., et al. 2020. J. Exp. Med. 10.1084/jem.20190354 [DOI] [Google Scholar]