Abstract
Sucrose and ectoine (1,4,5,6-tetrahydro-2-methyl-4-pyrimidine carboxylic acid) are very unusual osmoprotectants for Sinorhizobium meliloti because these compounds, unlike other bacterial osmoprotectants, do not accumulate as cytosolic osmolytes in salt-stressed S. meliloti cells. Here, we show that, in fact, sucrose and ectoine belong to a new family of nonaccumulated sinorhizobial osmoprotectants which also comprises the following six disaccharides: trehalose, maltose, cellobiose, gentiobiose, turanose, and palatinose. Also, several of these disaccharides were very effective exogenous osmoprotectants for strains of Rhizobium leguminosarum biovars phaseoli and trifolii. Sucrose and trehalose are synthesized as endogenous osmolytes in various bacteria, but the other five disaccharides had never been implicated before in osmoregulation in any organism. All of the disaccharides that acted as powerful osmoprotectants in S. meliloti and R. leguminosarum also acted as very effective competitors of [14C]sucrose uptake in salt-stressed cultures of these bacteria. Conversely, disaccharides that were not osmoprotective for S. meliloti and R. leguminosarum did not inhibit sucrose uptake in these bacteria. Hence, disaccharide osmoprotectants apparently shared the same uptake routes in these bacteria. Natural-abundance 13C nuclear magnetic resonance spectroscopy and quantification of cytosolic solutes demonstrated that the novel disaccharide osmoprotectants were not accumulated to osmotically significant levels in salt-stressed S. meliloti cells; rather, these compounds, like sucrose and ectoine, were catabolized during early exponential growth, and contributed indirectly to enhance the cytosolic levels of two endogenously synthesized osmolytes, glutamate and the dipeptide N-acetylglutaminylglutamine amide. The ecological implication of the use of these disaccharides as osmoprotectants is discussed.
Turgor adjustment in bacteria exposed to hyperosmotic environments (e.g., seawater, dry soils, etc.) is generally achieved by the accumulation of very large amounts of a few organic solutes and potassium ions. The amassing of these compounds counteracts cell dehydration and thus contributes to maintain an outwardly oriented cytoplasmic pressure, which is the driving force for cell growth (9, 14, 31). Organic osmolytes are often termed compatible solutes because very high cytosolic concentrations of these compounds are not deleterious to essential biochemical and metabolic functions in the cells (5, 9, 62). Bacterial osmolytes are accumulated either by uptake from the environment (exogenous osmolytes) or by de novo biosynthesis (endogenous osmolytes). Several organic osmolytes found in the environment also function as so-called bacterial osmoprotectants or osmoprotective compounds. These operational definitions usually refer to exogenous solutes that strongly stimulate bacterial growth in hyperosmotic environments (9, 27, 31). For example, glycine betaine (N,N,N-trimethylglycine) and 3-dimethylsulfoniopropionate (DMSP) are common algal and plant osmolytes (46, 49) that function as exogenous osmoprotectants in numerous bacterial species, including the model organisms Escherichia coli and Bacillus subtilis (9, 14, 18, 27, 43). Likewise, proline and ectoine (1,4,5,6-tetrahydro-2-methyl-4-pyrimidine carboxylic acid) also function as powerful osmoprotectants for many bacterial species (1, 9, 14, 24). Glycine betaine, proline, DMSP, and ectoine are highly effective osmoprotectants because many bacteria can rapidly accumulate large amounts of these compounds via specific osmoporters that are either induced or activated in hyperosmotic environments (8, 9, 18, 19, 23, 27).
Prominent endogenous osmolytes synthesized by bacteria exposed to hyperosmotic environments include a few amino and imino acids (e.g., glutamate, proline, and ectoine), the polyol glucosylglycerol, and two disaccharides, trehalose and sucrose, which are common endogenous osmolytes in cyanobacteria as well as sulfur bacteria (9, 14, 36, 48, 59). Trehalose is also involved in osmotic-shock tolerance and as a desiccation protectant in yeast and fungi, which also accumulate polyol osmolytes such as arabinitol, erythritol, and mannitol (4). Trehalose accumulation by de novo biosynthesis is also a common response to desiccation stress in bacteria (33, 44), and sucrose accumulates in response to harsh water stress in desiccation-tolerant plants (3). Curiously, however, the osmoprotective activity of exogenously added sugars and polyols has rarely been investigated in bacteria (29, 38, 45), because these compounds, unlike betaines (9, 49), are commonly used as growth substrates by microorganisms (10, 40). For example, salt-stressed E. coli and Erwinia chrysanthemi do not use trehalose as an exogenous osmoprotectant; nonetheless, these bacteria accumulate trehalose as an endogenous osmolyte (29, 45). In fact, stressed E. coli cells hydrolyze trehalose into glucose, which they use both as a growth substrate and for the synthesis of accumulated trehalose (55). Nevertheless, exogenous sucrose accumulates as a cytosolic osmolyte in salt-stressed cultures of Natronococcus occultus ATCC 43101, as well as in a Synechocystis sp. strain that cannot synthesize the cyanobacterial osmolyte glucosylglycerol (11, 38). In contrast, the root nodule bacterium Sinorhizobium meliloti uses sucrose as both a powerful osmoprotectant (10, 20, 54) and a carbon and energy source. Sucrose is not a conventional osmoprotectant for S. meliloti; its behavior resembles that of ectoine in salt-stressed cultures of S. meliloti (56). Specifically, sucrose and ectoine neither accumulate as cytosolic osmolytes nor act as immediate precursors to accumulated osmolytes in S. meliloti cells. Thus, sucrose and ectoine, unlike glycine betaine, proline betaine, and DMSP (2, 16, 43, 57), do not directly contribute to turgor adjustment in stressed S. meliloti cells. So far, this mechanism of osmoprotection (growth stimulation without accumulation of the supplied osmoprotectant) has not been described in bacteria other than S. meliloti. Interestingly, salt-stressed cultures of S. meliloti also accumulate trehalose as an endogenous osmolyte during the late exponential and stationary phases of their growth cycles (51, 56); however, it is not clear if exogenous trehalose can itself act as an exogenous osmoprotectant in S. meliloti as well as in several other rhizobia (13, 52). These observations led us to examine whether sugars and other compounds which are structurally related to sucrose and trehalose could possibly be used as exogenous osmoprotectants by S. meliloti and several other bacteria. Here, we report the identification of novel disaccharide osmoprotectants for S. meliloti, as well as Rhizobium leguminosarum strains. We have also examined the structural and physiological bases for the difference in biological activity between osmoprotective and nonosmoprotective sugars in S. meliloti, as well as in other Rhizobiaceae.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
The bacterial strains used in this study are listed in Table 1. Rhizobial strains and “Arthrobacter aureus” C70 were grown aerobically at 30°C. Other bacterial species were grown at 37°C. Bacterial growth was monitored spectrophotometrically by measuring the optical densities of cell suspensions at 570 nm (OD570). Inocula were cultured to an OD570 of 1.5 in mannitol-salts-yeast extract for rhizobia (41) and in Luria-Bertani medium (39) for E. coli MC4100, B. subtilis JH642, Pseudomonas aeruginosa ATCC 27853, and “A. aureus” C70. Cells from these precultures were harvested by centrifugation (6,000 × g for 10 min). Cells were then inoculated at an OD570 of 0.1 in the appropriate defined S medium, which consisted of the mineral base of the minimal media used to grow the cultures. Unless indicated otherwise, S. meliloti cultures were grown in lactate-aspartate-salts (LAS) medium containing d,l-lactate and l-aspartate, each at a final concentration of 10 mM. Other rhizobial strains were grown in the same minimal medium, except that mannitol (10 mM) was substituted for d,l-lactate as the source of carbon and energy. The minimal medium for E. coli MC4100, P. aeruginosa ATCC 27853, and “A. aureus” C70 was M63 medium (39) containing 10 mM glucose and 10 mM ammonium sulfate as the carbon and nitrogen sources, respectively. The minimal medium for B. subtilis was Spizizen’s minimal medium with 0.5% (wt/vol) glucose as the carbon source; this medium was supplemented with l-tryptophan (20 mg/liter) and l-phenylalanine (18 mg/liter) and a solution of trace elements (21). Compounds supplied as putative osmoprotectants were introduced into the growth medium at a concentration of 1 mM. Glycine betaine, sugars, and sugar-related compounds were of the highest chemical grade available (Sigma, Chimie, St. Quentin Fallavier, France). Ectoine was purified from salt-stressed cultures of Brevibacterium linens CNRZ 211 as described previously (1). Stock solutions of all of the organic compounds used in this study were sterilized by filtration. The osmolality of the growth media was usually increased by the addition of high concentrations of either NaCl or mannitol. The protein contents of the cultures were determined by the method of Lowry et al. (35), using bovine serum albumin as the standard. Growth experiments were replicated at least three times with less than 10% standard deviations.
TABLE 1.
Bacterial strains used in this study
| Strain | Origin or reference |
|---|---|
| Sinorhizobium meliloti | |
| 102F34 | 12 |
| M5N1 | B. Courtois |
| 444 | B. Courtois |
| 2009 | B. Courtois |
| SU47 | B. Courtois |
| Sinorhizobium fredii USDA 205T | |
| Rhizobium leguminosarum | |
| bv. phaseoli | |
| p15S | B. Courtois |
| p12S | B. Courtois |
| H132 | N. Amarger |
| bv. trifolii | |
| T22 | B. Courtois |
| T8S | B. Courtois |
| T17S | B. Courtois |
| bv. viciae ATCC 10006 | |
| Mesorhizobium huakuii CCBAU 2609T | |
| Bradyrhizobium japonicum USDA 110spc4 | |
| Escherichia coli MC4100 | 7 |
| Pseudomonas aeruginosa ATCC 27853 | |
| Bacillus subtilis JH642 | 25 |
| “Arthrobacter aureus” C70 | 30 |
Uptake assays.
Rhizobial cultures were grown in minimal medium containing 0.5 mM sucrose, in the presence or the absence of a high concentration of NaCl. Cells were washed twice and concentrated to an OD570 of 1 in isotonic S medium without sucrose. Uptake experiments were performed by filtration of bacterial cells on GF/F glass microfiber filters (Whatman, Springfield, England). [U-14C]sucrose (23.3 GBq/mmol; Amersham, Les Ulis, France) was used at a final concentration of 0.5 mM in 400 μl of bacterial suspension. Subsamples of this suspension (50 μl) were filtered 1, 2, 3, and 4 min after the addition of [14C]sucrose to ensure that [14C]sucrose uptake was a linear function of time. Then, cells were washed twice with isotonic S medium, and the radioactivity retained by the filters was determined by liquid scintillation counting. Results of uptake experiments presented in this paper are the means of triplicate assays with standard deviations lower than 10%.
NMR spectroscopy and osmolyte assays.
S. meliloti cultures used for the identification of osmolytes by nuclear magnetic resonance (NMR) spectroscopy were grown in LAS medium containing 0.5 M NaCl, with or without a 1 mM concentration of a saccharide. Each NMR sample was prepared from about 2 × 1012 cells, which were harvested by centrifugation (6,000 × g for 10 min) at different stages of growth, as specified in the text below. Unincorporated growth substrates and saccharides were removed by washing the cells twice in carbon-free S medium containing 0.5 M NaCl. The cells were then extracted twice by magnetic stirring for 30 min in 80% (vol/vol) ethanol/water. After centrifugation (8,000 × g for 15 min), the pooled supernatants (containing cytosolic osmolytes) were evaporated to dryness, redissolved in 1 ml of 20% deuterated water (D2O), and analyzed by natural-abundance 13C NMR spectroscopy (56).
The dipeptide osmolyte N-acetylglutaminylglutamine amide (NAGGN) was purified from ethanolic cell extracts by ion exchange chromatography, and it was chemically converted into glutamate as described previously (20). NAGGN-derived glutamate and glutamate in cell extracts were quantified spectrophotometrically at 340 nm by measuring NADH2 produced by the deamination of glutamate by bovine glutamate dehydrogenase (EC 1.4.1.3; Sigma Chimie). Cytosolic trehalose was first converted into glucose by porcine kidney trehalase (EC 3.2.1.28; Sigma Chimie), and trehalose-derived glucose was quantified by using a commercial glucose oxidase-peroxidase assay (Trinder; Sigma Chimie).
Saccharides remaining in the growth media after harvesting cells (12,000 × g for 5 min) grown in the presence of these compounds were quantified by the anthrone method (26). Briefly, 250 μl of fuming HCl (11.6 M) and 25 μl of formic acid (23.4 M) were added to 250 μl of culture medium (supernatant). Then, 2 ml of anthrone reagent (1 mM in H2SO4 [14.4 M]; Sigma Chimie) were added, and acid hydrolysis of the sugars was performed at 100°C for 12 min. It was verified that anthrone reacted with neither the components of the growth media nor the compounds released by the bacteria.
RESULTS
Screening of disaccharide osmoprotectants for S. meliloti.
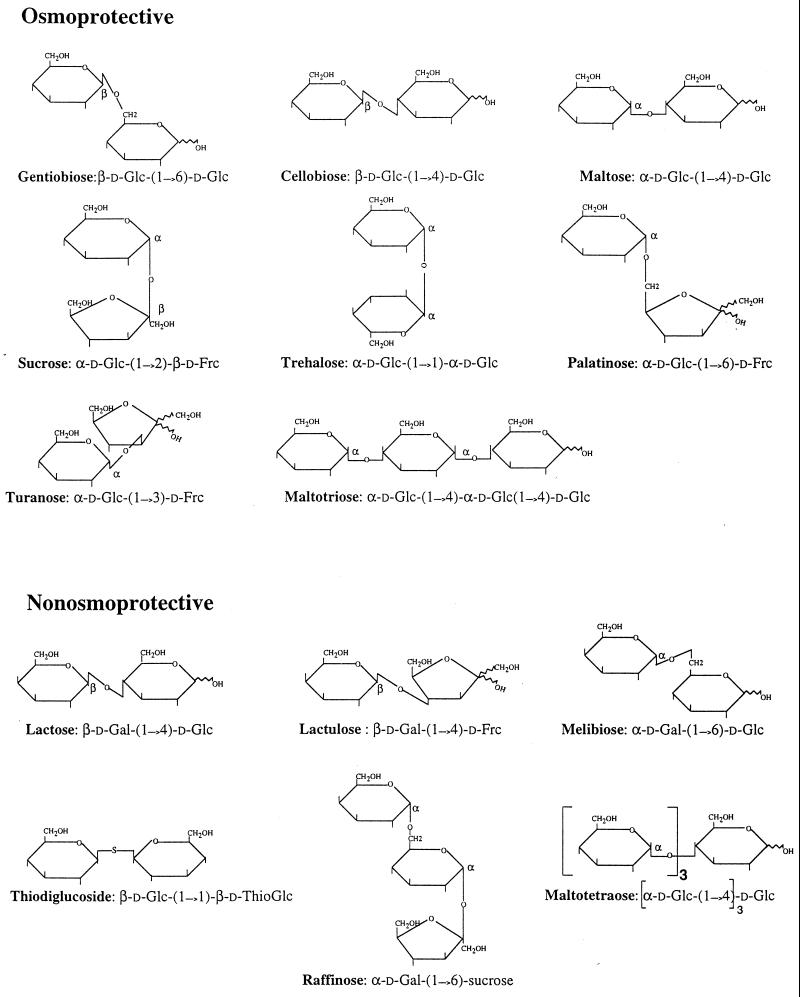
The unusual osmoprotection of S. meliloti by exogenous sucrose (20) raised the question of whether the osmoprotective activity of this common disaccharide was linked to its chemical structure. To address this question, S. meliloti 102F34 was grown at inhibitory osmolalities in LAS medium containing either 0.5 M NaCl or 0.8 M mannitol, and 1 mM of a sugar or a sugar analog, which were supplemented as potential osmoprotectants. Sucrose (20), glycine betaine (2), and ectoine (56) were used as positive controls of osmoprotection (Table 2). All of the monosaccharides (ribose, arabinose, xylose, glucose, fructose, galactose, mannose, rhamnose, and fucose), the polyols (arabinitol, mannitol, myo-inositol, sorbitol, and dulcitol), the sugar-related acids (glucuronate and galacturonate) and the C4-dicarboxylic acids (succinate and malate) assayed in this study failed to stimulate the growth of salt-stressed cultures of S. meliloti 102F34 (data not shown). Disaccharides were also tested for a potential osmoprotective activity in LAS medium. In addition to sucrose, six other disaccharides (trehalose, maltose, cellobiose, turanose, gentiobiose, and palatinose [Fig. 1]) also stimulated the growth of salt-stressed and mannitol-stressed cells of S. meliloti. Interestingly, the latter six disaccharides were as effective as sucrose, glycine betaine, and ectoine, which are the most powerful sinorhizobial osmoprotectants known to date (2, 20, 56). Indeed, all of these compounds restored the maximal growth yields of the stressed cultures to the unstressed level and significantly reduced the doubling time of NaCl-stressed cultures from 20 h to about 7 to 8.5 h, and they decreased the generation time of mannitol-stressed cells from 18 h to 8 to 9 h (Table 2). Similar levels of growth stimulation were also conferred by the same compounds to salt-stressed cultures grown in LAS containing either 0.5 M KCl or 0.4 M K2SO4 (data not shown). Thus, trehalose, maltose, cellobiose, turanose, gentiobiose, and palatinose can actually be considered osmoprotectants for S. meliloti 102F34 because these disaccharides, like sucrose (20), glycine betaine (2), and ectoine (56), relieved the inhibitory effects of high concentrations of electrolytes (NaCl, KCl, and K2SO4) and a nonelectrolyte (mannitol). Moreover, in agreement with this interpretation, we observed that high concentrations of each beneficial disaccharide (0.8 to 0.9 M), like submolar concentrations of glycine betaine (20), had little effect on sinorhizobial growth when these sugars were used as nonionic solutes to increase the osmotic strength of LAS medium (data not shown). In contrast, we observed that submolar concentrations (0.8 to 0.9 M) of lactose, lactulose, and melibiose strongly inhibited the growth of S. meliloti 102F34 in LAS medium, i.e., caused a 60 to 75% decrease in growth rate and a ca. 55% reduction in the final cell yield. Moreover, as expected from these data, no protection against salt and mannitol stresses was observed with a concentration of 1 mM of either lactose, lactulose, or melibiose (Table 2). Likewise, the synthetic disaccharide thiodiglucoside [β-d-Glc-(1→6)-β-d-Thio-Glc] and the trisaccharide raffinose [α-d-Gal-(1→6)-sucrose] were not osmoprotective for S. meliloti. Lastly, maltotriose and maltotetraose contain, respectively, three and four glucosyl residues that are linked together by α(1→4) glycosidic bonds (Fig. 1). Maltotriose was slightly less osmoprotective than maltose. Indeed, the trisaccharide and the disaccharide reduced the doubling times of stressed cultures of strain 102F34 from 18 to 20 h to about 11 and 8.5 h, respectively. Meanwhile, maltotetraose showed no detectable osmoprotective activity (Table 2).
TABLE 2.
Comparative effects of glycine betaine, ectoine, and various exogenous polysaccharides on the growth of S. meliloti 102F34 at high osmolaritiesa
| Putative osmoprotectant added | Growth parametersb in LAS with:
|
|||||
|---|---|---|---|---|---|---|
| No osmoticum
|
0.5 M NaCl
|
0.8 M Mannitolc
|
||||
| DT (h) | ODmax | DT (h) | ODmax | DT (h) | ODmax | |
| None | 4.0 | 2.0 | 20.0 | 1.0 | 18.0 | 0.9 |
| Sucrose | 4.0 | 2.0 | 8.0 | 2.0 | 8.0 | 1.8 |
| Glycine betaine | 3.9 | 2.0 | 8.0 | 2.0 | 8.0 | 2.1 |
| Ectoine | 4.0 | 1.9 | 7.5 | 1.8 | 9.0 | 1.8 |
| Trehalose | 3.9 | 2.1 | 8.0 | 2.0 | 8.5 | 2.1 |
| Maltose | 4.0 | 2.0 | 8.5 | 2.0 | 8.5 | 1.9 |
| Cellobiose | 4.2 | 1.9 | 8.0 | 1.9 | 9.3 | 1.8 |
| Turanose | 4.1 | 2.0 | 8.0 | 2.0 | 9.0 | 1.9 |
| Gentiobiose | 4.0 | 2.0 | 7.0 | 1.9 | 8.5 | 2.1 |
| Palatinose | 4.1 | 1.9 | 7.0 | 1.9 | 8.5 | 1.8 |
| Lactosed | 4.0 | 2.0 | 20.0 | 1.0 | 18.0 | 0.9 |
| Thiodiglucoside | 3.9 | 2.0 | 20.2 | 1.0 | 18.0 | 0.9 |
| Raffinose | 3.9 | 2.0 | 20.5 | 1.1 | 18.0 | 0.9 |
| Maltotriose | 3.9 | 1.8 | 11.0 | 2.0 | 10.5 | 1.9 |
| Maltotetraose | 4.0 | 1.9 | 20.0 | 1.1 | 18.0 | 0.9 |
Cultures were grown in LAS without or with an osmoticum (0.5 M NaCl or 0.8 M mannitol) in the presence of 1 mM of the indicated putative osmoprotectant.
Growth parameters are expressed as the doubling time (DT) (in hours per generation) and the maximal optical density (ODmax), which was measured at 570 nm at stationary phase.
LAS with 0.8 M mannitol is iso-osmotic with LAS plus 0.5 M NaCl [980 mosm/kg H2O (20)].
Similar results were obtained with 1 mM melibiose and lactulose.
FIG. 1.
Chemical structures of the disaccharides and related sugars used in this study. The denominations Osmoprotective and Nonosmoprotective refer, respectively, to sugars that alleviated and did not alleviate osmotic stress in S. meliloti 102F34 (Table 2).
The possibility that a link may occur between the osmoprotective capacity for S. meliloti of some sugars and the ability to catabolize these compounds was also investigated. Thus, 10 mM mono- and disaccharides, polyols, and C4-dicarboxylic acids were introduced as carbon source in aspartate-S medium in the presence and absence of 0.5 M NaCl. All of the supplied sugars except glucuronate, galacturonate, and maltotetraose supported the growth of S. meliloti in media of low and high osmotic strength, independent of whether they were or were not osmoprotectants (data not shown). These observations demonstrated the absence of a correlation between the utilization of a sugar as a carbon source and its osmoprotective ability. Nevertheless, thiodiglucoside was actively catabolized by S. meliloti only in the medium of low osmolarity.
Disaccharide osmoprotectants share the same uptake pathway.
We sought to determine whether the contrasting biological activities of disaccharides in S. meliloti 102F34 (presence or absence of osmoprotective activity) were linked to differences in transport activities. Therefore, [14C]sucrose uptake was investigated, and competition assays were performed to evaluate the ability of other sugars to act as competitors of [14C]sucrose uptake. Cultures of strain 102F34 were grown in LAS medium containing 0.5 mM sucrose, with or without 0.5 M NaCl. Cells were harvested in mid-exponential phase and assayed for [14C]sucrose uptake (0.5 mM) in the presence of a 10-fold excess of an unlabeled sucrose analog. Maltose was about as inhibitory as unlabeled sucrose itself, i.e., maltose virtually prevented the uptake of [14C]sucrose by S. meliloti cells grown at low and high osmolarities. Moreover, three other disaccharide osmoprotectants (trehalose, cellobiose, and turanose) caused more than 75% inhibition of [14C]sucrose uptake in these cells (Table 3). Gentiobiose and thiodiglucoside were significantly more inhibitory of [14C]sucrose uptake in stressed cells (72 and 80% inhibition, respectively) than in unstressed cells (55 and 45% inhibition, respectively). In contrast, maltotetraose was a stronger inhibitor of [14C]sucrose uptake in unstressed cells (80% inhibition) than in stressed cells (37% inhibition). Moreover, the ability of maltose, maltotriose, and maltotetraose to inhibit [14C]sucrose uptake in stressed cells (84, 52, and 37% inhibition, respectively) was inversely proportional to the number of glucosyl monomers composing these sugars. This observation was consistent with the facts that (i) maltose, on the one hand, was more osmoprotective than maltotriose and that (ii) maltotetraose, on the other hand, was not an osmoprotectant for S. meliloti 102F34 (Table 2). Lastly, we observed that lactose, lactulose, and melibiose (which also lacked osmoprotective activity for strain 102F34 [Table 2]) were unable to inhibit the uptake of [14C]sucrose (less than 10% inhibition), even when these disaccharides were supplied at a 100-fold molar excess over [14C]sucrose (Table 3).
TABLE 3.
Effects of structural analogs of sucrose on the uptake of [14C]sucrose by S. meliloti 102F34
| Growth conditiona | % Inhibition of sucrose uptake by unlabeled analogb
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sucrose | Maltose | Trehalose | Cellobiose | Turanose | Gentiobiose | Maltotriose | Maltotetraose | Thiodiglucoside | Lactosec | |
| Low osmolarity | 95 | 96 | 78 | 76 | 75 | 55 | 89 | 80 | 45 | 2 |
| High osmolarity | 95 | 84 | 85 | 85 | 87 | 72 | 52 | 37 | 80 | 10 |
Cells were grown to mid-exponential phase in LAS medium containing 0.5 mM sucrose, in the absence (low osmolarity) or the presence (high osmolarity) of 0.5 M NaCl.
Initial rates of sucrose uptake were measured in fresh growth medium containing 0.5 mM [14C]sucrose (23.3 GBq mmol−1) and 5 mM of the indicated unlabeled sucrose analog. The results are expressed as percentages of reduction compared with uninhibited transport rates, which were 30 ± 1.5 nmol min−1 mg of protein−1 for both unstressed and stressed cells.
Lactose was used at 50 mM, and similar results were obtained with a 100-fold excess of either lactulose or melibiose.
Together, the above data indicate that, except for thiodiglucoside (see discussion below), all of the sugars that were strong inhibitors of [14C]sucrose uptake by salt-stressed S. meliloti cells (e.g., sucrose itself, maltose, trehalose, cellobiose, turanose, gentiobiose, and maltotriose [Table 3]) also acted as powerful sinorhizobial osmoprotectants (Table 2). In contrast, disaccharides that failed to inhibit [14C]sucrose uptake in salt-stressed cells (lactose, lactulose, and melibiose [Table 3]) were not osmoprotective for S. meliloti 102F34 (Table 2).
Osmolyte composition of stressed cells of S. meliloti grown in the presence of disaccharide osmoprotectants.
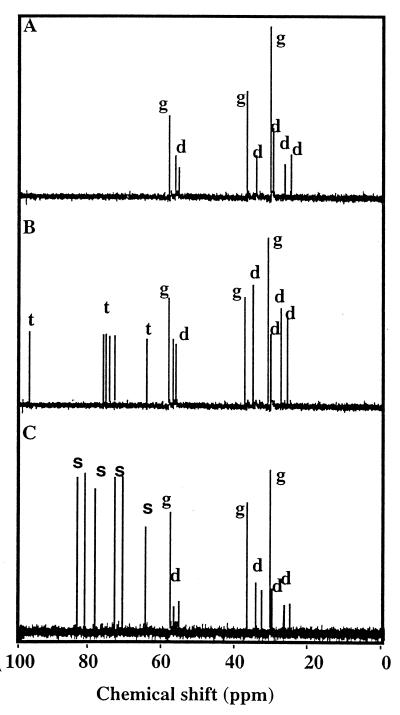
Natural-abundance 13C NMR spectroscopy allows the detection of all of the organic solutes that accumulate to osmotically significant levels and thus contribute to turgor adjustment in microorganisms (4, 47, 51). Therefore, this technique was used to identify the organic osmolytes that were accumulated by salt-stressed cultures (0.5 M NaCl) of S. meliloti 102F34 grown in the presence of disaccharide osmoprotectants. Cultures were harvested at different stages of their growth cycles, because the accumulation of bacterial osmolytes is sometimes growth phase dependent (22, 52, 55, 57). The spectra of ethanolic extracts from stressed cells grown in the presence of either trehalose, maltose, cellobiose, turanose, gentiobiose, or palatinose (1 mM) were always very similar to each other at any given stage of the growth cycles of the cultures. Moreover, the spectra from cultures grown with any one of the disaccharide osmoprotectants were always similar to the spectra from control cells which were grown without osmoprotectant and were harvested at similar stages of growth. Specifically, stressed S. meliloti cells, grown with or without a disaccharide osmoprotectant, accumulated only osmolytes that were synthesized de novo: glutamate and the dipeptide NAGGN during the early and mid-exponential phases of growth (Fig. 2A), and glutamate, NAGGN, and trehalose in the late exponential and stationary phases (Fig. 2B). Peaks from the supplied disaccharides were never detected in any extract at any stage of growth. This indicates that the six novel disaccharide osmoprotectants were not accumulated as cytosolic osmolytes by stressed cells of S. meliloti 102F34. Therefore, the nonaccumulated osmoprotectants were quantified in the growth media of cells harvested in late exponential phase. Interestingly, the levels of disaccharide osmoprotectants remaining in the growth medium were all very low and represented less than 9% of the initial amount of exogenously supplied trehalose, maltose, cellobiose, gentiobiose, turanose, and palatinose. Therefore, these osmoprotectants were largely catabolized by stressed cultures of S. meliloti.
FIG. 2.
Representative 13C NMR spectra from salt-stressed cultures (0.5 M NaCl) of S. meliloti 102F34 grown to early (A) and late (B) exponential phases in LAS medium without or with one of the following osmoprotectants (1 mM): trehalose, maltose, cellobiose, turanose, gentiobiose, or palatinose. (C) Spectrum from a stressed culture grown to late exponential phase in LAS with 0.5 M NaCl plus 1 mM thiodiglucoside. All of the spectra were obtained from a defined amount of cells (about 2 × 1012 CFU). Sample preparation and NMR analysis were performed as previously described (56). Resonances from endogenously synthesized glutamate (g), the dipeptide NAGGN (d), and trehalose (t), as well as exogenously supplied thiodiglucoside (s), are indicated for points at which these compounds were accumulated as cytosolic osmolytes.
NMR spectra were also obtained from salt-stressed cells which were grown with 1 mM thiodiglucoside. Surprisingly, peaks from thiodiglucoside dominated the spectra, even when cells were harvested in late exponential phase of growth (Fig. 2C). These cells also accumulated high levels of glutamate as well as lower levels of NAGGN; however, they did not accumulate detectable levels of trehalose. In other words, these data demonstrate that (i) thiodiglucoside was accumulated as a dominant cytosolic solute in stressed cells of S. meliloti, although it did not enhance sinorhizobial growth at high osmolarity (Table 2); and (ii) the synthetic disaccharide suppressed the accumulation of endogenously synthesized trehalose.
Because disaccharide osmoprotectants had no apparent effects on the qualitative osmolyte composition of stressed cultures of S. meliloti (Fig. 2A and B), we investigated the possibility that the nonaccumulated osmoprotectants could affect the levels of endogenously synthesized osmolytes, i.e., glutamate, NAGGN, and trehalose levels (51). Cultures were grown in LAS with 0.5 M NaCl in the absence or presence of either sucrose, trehalose, maltose, or cellobiose (1 mM). Endogenous osmolytes were quantified periodically during the growth cycles of the cultures. Interestingly, the profiles of glutamate, NAGGN, and trehalose accumulation showed individual patterns of variation that were very similar in all of the cultures grown with a disaccharide osmoprotectant. The data from cells harvested in the early and late exponential phases are summarized in Table 4. Briefly, glutamate levels in control cells grown without osmoprotectant decreased from 650 nmol mg of protein−1 in early exponential phase to 510 nmol mg of protein−1 in late exponential phase. In contrast, glutamate levels in cells grown in the presence of a disaccharide osmoprotectant increased from about 700 to ca. 1,200 nmol mg of protein−1 during exponential growth (Table 4). Glutamate levels in these cells then decreased steadily as the cultures entered into the stationary phase of growth (data not shown). Ultimately, the levels of cytosolic glutamate at stationary phase were similar in stressed cells grown with or without a disaccharide osmoprotectant (about 400 nmol mg of protein−1).
TABLE 4.
Effects of disaccharide osmoprotectants on the osmolyte compositions of salt-stressed cultures of S. meliloti 102F34
| Disac-charide addeda | Amount of accumulated osmolytes (nmol mg of protein−1) in cells at:
|
|||||
|---|---|---|---|---|---|---|
| Early exponential phaseb
|
Late exponential phaseb
|
|||||
| Glutamate | NAGGN | Trehalose | Glutamate | NAGGNc | Trehalosec | |
| None | 650 | 150 | 50 | 510 | 510 | 150 |
| Sucrose | 700 | 190 | 65 | 1,160 | 670 | 130 |
| Trehalose | 710 | 180 | 55 | 1,190 | 650 | 130 |
| Maltose | 690 | 190 | 60 | 1,220 | 690 | 130 |
| Cellobiose | 710 | 180 | 55 | 1,170 | 620 | 130 |
Cultures were grown in LAS with 0.5 M NaCl plus the indicated disaccharide (1 mM).
The patterns of glutamate, NAGGN, and trehalose accumulation during the growth cycles of stressed cultures grown with trehalose, maltose, or cellobiose were very similar to those observed during growth of stressed cells cultured in the presence of either sucrose (20) or ectoine (56). Osmolyte levels in cells at early and late exponential phase of growth were determined after 5 to 8 h and 24 to 30 h of culture (corresponding to their maximal level), respectively. Data are means of triplicate assays with less than 10% standard deviations.
Similar levels of NAGGN and trehalose were found in cells at stationary phase, while glutamate contents were lower.
The basal levels of accumulated NAGGN at the beginning of the exponential phase were approximately 30% higher in stressed cells grown with either sucrose, trehalose, maltose, or cellobiose (about 180 nmol mg of protein−1) than in control cells cultured without a disaccharide osmoprotectant (145 nmol mg of protein−1). Then, during the exponential phase, NAGGN levels increased about 3.5-fold in all of the cultures. Consequently, cytosolic NAGGN at the end of the exponential phase reached 510 nmol mg of protein−1 in control cells and 620 to 700 nmol mg of protein−1 in stressed cells grown with a disaccharide osmoprotectant (Table 4). These NAGGN levels were maintained in stationary-phase cells.
The profiles of trehalose accumulation in stressed cultures of S. meliloti 102F34 grown without or with a disaccharide osmoprotectant were very similar: trehalose levels at the beginning of the exponential phase were very low in all of the cultures (50 to 65 nmol mg of protein−1) and increased steadily to reach about 130 nmol mg of protein−1 in late exponential phase (Table 4). These trehalose levels were maintained in stationary-phase cells in all of the cultures. In summary, exogenously added disaccharide osmoprotectants, including trehalose itself, did not modify trehalose levels in stressed S. meliloti 102F34 cells, at any stage of their growth cycles.
Effect of four disaccharides on stressed cultures of other bacterial species.
The potential osmoprotective activity of sucrose, trehalose, maltose, and cellobiose was also evaluated in a series of other rhizobial and bacterial species. In this experiment, mannitol (10 mM) was substituted for lactate as the carbon source for members of Rhizobiaceae, because lactate was not an adequate growth substrate for several of these bacteria (data not shown). In addition to S. meliloti 102F34, the four disaccharides also stimulated the growth of salt-stressed cultures of S. meliloti SU47, M5N1, 444, and 2009; the growth rates of these strains were stimulated two- to threefold, and their final cell yields (maximal ODs at stationary phase) were restored to unstressed levels (Table 5). Likewise, sucrose, trehalose, maltose, and cellobiose also stimulated about twofold the growth rates of salt-stressed cultures of R. leguminosarum bv. phaseoli strains H132, p15S, and p12S; also, the four disaccharides restored the final densities of these cultures to unstressed levels, or slightly below unstressed levels. The responses of salt-stressed cultures of strains of R. leguminosarum bv. trifolii to the four disaccharides were not homogeneous; strain T22 also used sucrose, trehalose, maltose, and cellobiose as alleviators of salt stress, but strain T8S was not protected by maltose, while strain T17S did not respond to maltose and cellobiose, although this strain was protected by sucrose and trehalose (Table 5). Also, we observed that neither sucrose, nor the other three disaccharides, alleviated growth inhibition by high salt concentrations in the following bacterial species and strains: Mesorhizobium huakuii CCBAU 2609T, Sinorhizobium fredii USDA 205T, Bradyrhizobium japonicum USDA 110spc4, R. leguminosarum bv. viciae ATCC 10006, E. coli MC4100, B. subtilis JH642, P. aeruginosa ATCC 27853, and “A. aureus” C70 (data not shown).
TABLE 5.
Effects of exogenous disaccharides on the growth of salt-stressed cultures of several rhizobiaa
| Rhizobial strain | Growth parameters of the indicated strains in minimal mediumb
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Without NaCl
|
With NaClc
|
|||||||||||
| Without disaccharide
|
With a disaccharided
|
|||||||||||
| Sucrose
|
Trehalose
|
Maltose
|
Cellobiose
|
|||||||||
| DT (h) | ODmax | DT (h) | ODmax | DT (h) | ODmax | DT (h) | ODmax | DT (h) | ODmax | DT (h) | ODmax | |
| S. meliloti | ||||||||||||
| 102F34 | 4 | 3.1 | 18 | 0.9 | 8 | 2.9 | 7 | 3.0 | 6 | 2.8 | 7 | 2.9 |
| SU47 | 2 | 3.0 | 9 | 1.5 | 3 | 3.1 | 3 | 3.1 | 3 | 3.0 | 3 | 3.1 |
| M5N1 | 7 | 2.7 | 17 | 0.7 | 7 | 3.1 | 7 | 3.1 | 7 | 3.0 | 7 | 3.1 |
| 444 | 4 | 2.5 | 12 | 0.8 | 6 | 2.5 | 6 | 2.5 | 6 | 2.6 | 6 | 2.6 |
| 2009 | 4 | 1.5 | 12 | 0.4 | 6 | 1.4 | 6 | 1.4 | 6 | 1.5 | 6 | 1.6 |
| R. leguminosarum | ||||||||||||
| bv. phaseoli | ||||||||||||
| H132 | 9 | 1.1 | 20 | 0.5 | 11 | 1.1 | 11 | 1.0 | 11 | 1.0 | 11 | 1.0 |
| p15S | 4 | 2.8 | 17 | 1.3 | 8 | 2.8 | 8 | 2.8 | 8 | 2.4 | 8 | 1.9 |
| p12S | 6 | 3.9 | 15 | 1.3 | 7 | 3.2 | 7 | 3.3 | 7 | 3.3 | 7 | 3.1 |
| bv. trifolii | ||||||||||||
| T22 | 4 | 2.1 | 10 | 0.9 | 4 | 1.6 | 4 | 2.2 | 4 | 1.6 | 4 | 1.6 |
| T8S | 5 | 2.6 | 31 | 0.6 | 14 | 2.6 | 14 | 1.7 | 31 | 0.6 | 14 | 1.6 |
| T17S | 4 | 2.5 | 20 | 1.1 | 8 | 1.5 | 8 | 1.6 | 20 | 1.0 | 20 | 1.0 |
Cultures were grown in mannitol-aspartate minimal medium.
See footnote b in Table 2.
NaCl concentrations in the growth medium were 0.5 M for S. meliloti; 0.3, 0.5, and 0.6 M for R. leguminosarum bv. phaseoli strains H132, p12S, and p15S, respectively; and 0.7 M for R. leguminosarum bv. trifolii.
Disaccharides provided as putative osmoprotectants were supplied at a concentration of 1 mM.
As observed above in S. meliloti 102F34 (Table 3) competition studies revealed that the uptake of [14C]sucrose was virtually abolished (≥88% inhibition) by unlabeled sucrose, maltose, trehalose, and cellobiose in four other strains of S. meliloti, as well as in three strains of R. leguminosarum bv. phaseoli (Table 6). Moreover, in addition to unlabeled sucrose itself, maximal percentages of inhibition of [14C]sucrose uptake (≥96% reduction) were also observed with trehalose and maltose in R. leguminosarum bv. trifolii strain T22, as well as with trehalose in R. leguminosarum bv. trifolii strain T8S. Cellobiose was slightly less inhibitory (68% inhibition) in these two strains, and trehalose caused 64% inhibition of [14C]sucrose uptake in R. leguminosarum bv. trifolii strain T17S. In contrast, maltose and cellobiose caused only ca. 20% inhibition of [14C]sucrose transport in the latter strain. Moreover, maltose was also a weak inhibitor of sucrose uptake in R. leguminosarum bv. trifolii T8S (Table 6). Collectively, these data were consistent with the results of the osmoprotection bioassays presented in Table 5. They indicated that sucrose uptake by three species of rhizobia was strongly inhibited (64 to 98%) by disaccharides that acted as alleviators of salt stress in these strains. In contrast, disaccharides that did not stimulate rhizobial growth at high salinity were very weak inhibitors of [14C]sucrose uptake (e.g., maltose in R. leguminosarum bv. trifolii T8S and T17S, as well as cellobiose in the latter strain). Nevertheless, S. meliloti and R. leguminosarum bv. trifolii and phaseoli strains were all able to use sucrose, trehalose, maltose, and cellobiose as carbon and energy source in media of low osmolarity. Lastly, in agreement with this interpretation, all of the rhizobial strains that did not use sucrose, trehalose, cellobiose, and maltose as exogenous osmoprotectants (i.e., M. huakuii CCBAU 2609T, S. fredii USDA 205T, B. japonicum USDA 110spc4 and R. leguminosarum bv. viciae ATCC 10006) showed very low or no detectable levels of [14C]sucrose uptake activity at high salinities (data not shown). Beyond them, only M. huakuii CCBAU 2609T and B. japonicum USDA 110spc4 were unable to use these compounds as carbon and energy source.
TABLE 6.
Effect of unlabeled disaccharides on the uptake of [14C]sucrose by salt-stressed cultures of various rhizobial strainsa
| Strain | % Inhibition of sucrose uptake by unlabeled disaccharidesb
|
|||
|---|---|---|---|---|
| Sucrose | Trehalose | Maltose | Cellobiose | |
| S. meliloti 444c | 95c | 90c | 91c | 92c |
| R. leguminosarum | ||||
| bv. phaseoli H132d | 96d | 91d | 90d | 88d |
| bv. trifolii | ||||
| T22 | 96 | 98 | 98 | 68 |
| T8S | 97 | 96 | 27 | 68 |
| T17S | 98 | 64 | 20 | 19 |
Cultures were grown for 24 h in mannitol-aspartate minimal medium containing 1 mM sucrose plus NaCl at the following concentrations: S. meliloti 444, 0.5 M; R. leguminosarum bv. phaseoli H132, 0.3 M; R. leguminosarum bv. trifolii strains, 0.7 M.
Percentages of inhibition of sucrose uptake were measured after a 5-min incubation in the presence of 0.5 mM [14C]sucrose (23.3 GBq/mmol) plus 25 mM of the indicated unlabeled analog. Uninhibited transport rates were 28 nmol mg of protein−1 for both S. meliloti 444 and R. leguminosarum bv. phaseoli H132, and 17, 27, and 11 nmol mg of protein−1 for R. leguminosarum bv. trifolii strains T22, T8S, and T17S, respectively.
Similar percentages of inhibition were observed in S. meliloti SU47, M5N1, and 2009.
Similar percentages of inhibition were observed in R. leguminosarum bv. phaseoli p12S and p15S.
DISCUSSION
The possibility that sugars may act as exogenous osmoprotectants for bacteria has rarely been investigated (29, 38, 45). Recently, we showed that sucrose, like ectoine (56), is a very effective but unusual osmoprotectant for S. meliloti strains (20). Here, we show that sucrose and ectoine belong to a broader class of nonaccumulated osmoprotectants for S. meliloti. The new sinorhizobial osmoprotectants are six disaccharides (trehalose, maltose, cellobiose, gentiobiose, turanose, and palatinose), and a trisaccharide, maltotriose (Fig. 1; Table 1). Moreover, sucrose, trehalose, cellobiose, and maltose also act as powerful osmoprotectants for several strains of R. leguminosarum bv. trifolii and phaseoli (Table 5). Except for sucrose and trehalose (see discussion below), prior to this study, the other five disaccharides and maltotriose have never been shown to participate in osmoregulation. Structurally, the disaccharide osmoprotectants contain either two glucosyl residues (trehalose, maltose, cellobiose, and gentiobiose), or a glucosyl residue linked to a fructosyl residue (sucrose, palatinose, and turanose) (Fig. 1). In contrast, disaccharides that contain a galactosyl residue linked to glucose (lactose and melibiose) or fructose (lactulose) all lack osmoprotective activity for strains of S. meliloti, as well as other rhizobia. Similarly, raffinose is a trisaccharide that contains a galactosyl residue linked to sucrose by an α(1→6) glycosidic bond (Fig. 1). Interestingly, raffinose, like galactosyl-containing disaccharides, showed no osmoprotective activity for S. meliloti 102F34. Hence, we infer that the presence of a galactosyl residue in a disaccharide, as well as the addition of a galactosyl residue to sucrose, apparently prevents these compounds from acting as osmoprotectants for S. meliloti. Moreover, we observed that maltose [α-d-Glc-(1→4)-α-d-Glc] is more osmoprotective than maltotriose [α-d-Glc-(1→4)-α-d-Glc-(1→4)-α-d-Glc] but that maltotetraose [α-d-Glc-(1→4)-α-d-Glc-(1→4)-α-d-Glc-(1→4)-α-d-Glc] and glucose are not osmoprotective for S. meliloti (Table 1). This indicates that at least two, but no more than three, glucose residues are required in an oligosaccharide for it to effect osmoprotection in S. meliloti.
Compared to the above-mentioned structural features, other structural and chemical properties of disaccharides do not seem to interfere in the determination of their putative osmoprotective activity. For example, the presence or the absence of a reductive function is not required, since both reducing (maltose, cellobiose, gentiobiose, turanose, and palatinose) and nonreducing disaccharides (trehalose and sucrose) are equally osmoprotective for S. meliloti 102F34 (Fig. 1; Table 2). Likewise, the osmoprotective activity of a disaccharide is apparently not determined by the anomerism of the constituting hexoses [i.e., by the position (α or β) of the oxygen atom that is linked to either the C-1 atom of the glucose residue(s) or the C-2 atom of the fructose residue, which compose the disaccharide osmoprotectants (Fig. 1)]. This inference is drawn from the following observations. (i) The anomeric form of the glucosyl residue (C-1 atom), which is found in all of the disaccharide osmoprotectant (Fig. 1), is either the α-form (in maltose, sucrose, trehalose, palatinose, turanose, and maltotriose) or the β-form (in cellobiose and gentiobiose). (ii) The anomeric carbon (C-1 atom) of the second glucose residue in gentiobiose, cellobiose, and maltose harbors a free hydroxyl group; hence, both the α- and β-anomers of these disaccharides coexist in solution (37). (iii) Sucrose contains a β-fructose residue (C-2 atom), while both α- and β-fructose anomers coexist in turanose and palatinose (Fig. 1). In summary, the minimal structural requirements that are common to disaccharide osmoprotectants analyzed in this work are the absence of a galactosyl residue and the presence of either two glucose residues or a glucosyl residue linked to fructose.
All of the disaccharides that act as osmoprotectants for strains of S. meliloti (Table 2), as well as R. leguminosarum bv. phaseoli and trifolii (Table 5), are very strong inhibitors of [14C]sucrose uptake in these strains (Tables 3 and 6). Conversely, except for thiodiglucoside in S. meliloti 102F34 (see discussion below), disaccharides that are not osmoprotective for stressed cultures of S. meliloti and R. leguminosarum do not inhibit [14C]sucrose uptake in these strains. Thus, disaccharide osmoprotectants are apparently taken up via sucrose-disaccharide porters that cannot mediate the uptake of disaccharides that are not osmoprotective for these strains, such as galactose-containing disaccharides (lactose, lactulose, and melibiose) in S. meliloti strains. Our data are consistent with a report by Glenn and Dilworth (15) which shows that two types of disaccharide transporters coexist in S. meliloti WU60, Rhizobium sp. NGR 236, Rhizobium bv. trifolii WU 420, and R. leguminosarum WU 163 and W 235, namely, lactose porters on the one hand, and, on the other hand, transporters that mediate the uptake of sucrose, maltose, and trehalose but do not take up lactose.
Thiodiglucoside, unlike other disaccharide inhibitors of sucrose uptake (Table 3), is not osmoprotective for S. meliloti 102F34 (Table 2). Moreover, the fate of thiodiglucoside in salt-stressed 102F34 cells is strikingly different from the fate of disaccharide osmoprotectants and ectoine: thiodiglucoside accumulates at high cytosolic levels (Fig. 2C), whereas exogenously supplied trehalose, maltose, cellobiose, gentiobiose, turanose, and palatinose (Fig. 2A and B), like sucrose (20) and ectoine (56), are not accumulated but rather are catabolized by stressed S. meliloti cells. Moreover, we observed that thiodiglucoside, unlike disaccharide osmoprotectants (10 mM), cannot be used as a sole source of carbon and energy by salt-stressed cells of S. meliloti 102F34 (data not shown). These observations suggest that the catabolism of thiodiglucoside might be necessary for it to effect osmoprotection in S. meliloti. Alternatively, the accumulation of thiodiglucoside in stressed cells might not be sufficient to elicit osmoprotection of S. meliloti by this compound.
The data presented in this paper also establish that there are two different pathways for the utilization of exogenous osmoprotectants in S. meliloti. In a first pathway, the supplied osmoprotectant (i.e., betaines and DMSP) accumulates to high levels in the cytoplasm of the stressed cells (2, 16, 43, 57). The accumulated osmoprotectants contribute to the recovery of cell turgor, which is the driving force behind cell growth and division (9, 14, 31). This mode of osmoprotection (accumulation of so-called compatible solutes) is universal in bacteria and in higher plants and animal cells (6, 9, 14, 27, 49, 53). In a second pathway, the exogenous osmoprotectant (ectoine or a disaccharide) is never accumulated to osmotically significant levels in the cytoplasm of stressed S. meliloti cells (20, 56) (Fig. 2). The mechanism of sinorhizobial osmoprotection by nonaccumulated osmoprotectants is intriguing because ectoine and disaccharide osmoprotectants do not directly contribute to turgor adjustment in stressed cells; however, ectoine (56), sucrose (20), and the novel disaccharide osmoprotectants (Table 4) indirectly contribute to cell turgor by eliciting a sharp rise in glutamate and NAGGN levels during the exponential phase of growth. These compounds might play a central role in osmoadaptation in S. meliloti.
Disaccharide osmoprotectants should be of prime environmental interest for rhizobia, because several of these compounds are naturally present in the soil and the rhizosphere. For example, sucrose is a photosynthetic product which is abundant in root exudates (40, 42). Hence, sucrose should be a readily available osmoprotectant for rhizobia experiencing salt and water stresses in the rhizosphere. Plant polysaccharides are also natural sources of disaccharide osmoprotectants. For example, cellulose and starch contain multiple units of cellobiose and maltose, respectively, which are released into the soil by extracellular enzymes produced by fungi and bacteria that degrade decaying plant materials (32, 58). Hence, it is particularly interesting that plant-derived disaccharides are highly osmoprotective for plant-beneficial bacteria such as S. meliloti and R. leguminosarum, but are not osmoprotective for other soil bacteria such as B. subtilis, “A. aureus,” and P. aeruginosa. In fact, soil and rhizosphere microorganisms are subjected to frequent changes in the osmolalities of their environments, due to the succession of drought and rain periods. The availability of osmoprotectants such as betaines is probably limiting in the soil because betaines are released only by germinating leguminous seeds and by primary betaine producers (plants and bacteria) subjected to natural decay or sudden osmotic downshocks (9, 42, 49, 60). Moreover, betaines are rapidly degraded by unstressed rhizosphere bacteria, such as S. meliloti, Pseudomonas spp., and Arthrobacter spp. (2, 17, 34, 50, 61), and this probably reduces their availability in the soil. In contrast, high contents of organic matter and the presence of extracellular enzymes produced by cellulolytic and amylolytic fungi and bacteria ensure a rather low but continuous production of assimilable carbohydrates, which sustain microbial growth in the soil (32, 58). Hence, the availability of disaccharides such as sucrose, cellulose, and maltose in the rhizosphere may ultimately confer a selective advantage to rhizobia over other bacterial species in salinity-affected soils. Lastly, it is noteworthy that sucrose, trehalose, and maltose added to an alfalfa seed coating formulation increase severalfold the survival rate of S. meliloti cells exposed to desiccation stress in vitro (28). Future studies will help to assess whether disaccharide osmoprotectants can also enhance the proliferation and survival of S. meliloti and other rhizobia in response to desiccation and drought stresses in the soil.
ACKNOWLEDGMENTS
This research was supported by grants from the Direction de la Recherche et des Etudes Doctorales and by the Centre National de la Recherche Scientifique.
We acknowledge E. Bremer, B. Courtois, N. Amarger, P. Y. Donnio, H. Hennecke, and P. van Berkum for kindly providing bacterial strains. We are grateful to J.-A. Pocard for language improvement; J. Hamelin for 13C NMR analysis; T. Bernard, M. Jebbar, and D. Plusquellec for helpful discussions; and M. Huguet and C. Monnier for technical assistance.
REFERENCES
- 1.Bernard T, Jebbar M, Rassouli Y, Himdi-Kabbab S, Hamelin J, Blanco C. Ectoine accumulation and osmotic regulation in Brevibacterium linens. J Gen Microbiol. 1993;139:129–138. [Google Scholar]
- 2.Bernard T, Pocard J-A, Perroud B, Le Rudulier D. Variations in the response of salt-stressed Rhizobium strains to betaines. Arch Microbiol. 1986;143:359–364. [Google Scholar]
- 3.Bianchi G, Gamba A, Limiroli R, Pozzi N, Elster R, Salamini F, Bartels D. The unusual sugar composition in leaves of the resurrection plant Myrothamnus flabellifolia. Physiol Plant. 1993;87:223–226. [Google Scholar]
- 4.Blomberg A, Adler N. Physiology of osmotolerance in fungi. Adv Microb Physiol. 1992;33:145–212. doi: 10.1016/s0065-2911(08)60217-9. [DOI] [PubMed] [Google Scholar]
- 5.Brown A D. Microbial water stress. Bacteriol Rev. 1976;40:803–846. doi: 10.1128/br.40.4.803-846.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burg M B, Kwon E D, Kultz D. Regulation of gene expression by hypertonicity. Annu Rev Physiol. 1997;59:437–455. doi: 10.1146/annurev.physiol.59.1.437. [DOI] [PubMed] [Google Scholar]
- 7.Casadaban M J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and mu. J Mol Biol. 1976;104:541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- 8.Csonka L N, Epstein W. Osmoregulation. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1996. pp. 1210–1223. [Google Scholar]
- 9.Csonka L N, Hanson A D. Prokaryotic osmoregulation: genetics and physiology. Annu Rev Microbiol. 1991;45:569–606. doi: 10.1146/annurev.mi.45.100191.003033. [DOI] [PubMed] [Google Scholar]
- 10.De Lajudie P, Willems A, Pot B, Dewettinck D, Maestrojuan G, Neyra M, Collins M D, Dreyfus B, Kersters K, Gillis M. Polyphasic taxonomy of rhizobia. Emendation of the genus Sinorhizobium and description of Sinorhizobium meliloti comb. nov., Sinorhizobium saheli sp. nov., and Sinorhizobium teranga sp. nov. Int J Syst Bacteriol. 1995;44:3443–3453. [Google Scholar]
- 11.Desmarais D, Jablonski P E, Fedarko N S, Roberts M F. 2-Sulfotrehalose, a novel osmolyte in haloalkaliphilic archaea. J Bacteriol. 1997;179:3146–3153. doi: 10.1128/jb.179.10.3146-3153.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ditta G, Virts E, Palomares A, Kim C-H. The nifA gene of Rhizobium meliloti is oxygen regulated. J Bacteriol. 1987;169:3217–3223. doi: 10.1128/jb.169.7.3217-3223.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elsheikh E A E, Wood M. Rhizobia and bradyrhizobia under salt stress: possible role of trehalose in osmoregulation. Lett Appl Microbiol. 1990;10:127–129. [Google Scholar]
- 14.Galinski E A. Osmoadaptation in bacteria. Adv Microbiol Physiol. 1995;37:273–328. [PubMed] [Google Scholar]
- 15.Glenn A R, Dilworth M J. The uptake and hydrolysis of disaccharides by fast- and slow-growing species of Rhizobium. Arch Microbiol. 1981;129:233–239. [Google Scholar]
- 16.Gloux K, Le Rudulier D. Enhancement of salt tolerance by proline betaine in Rhizobium meliloti. Plant Physiol. 1986;80(Suppl.):134. [Google Scholar]
- 17.Gloux K, Le Rudulier D. Transport and catabolism of proline betaine in salt-stressed Rhizobium meliloti. Arch Microbiol. 1989;151:143–148. [Google Scholar]
- 18.Gouesbet G, Jebbar M, Talibart R, Bernard T, Blanco C. Pipecolic acid is an osmoprotectant for Escherichia coli taken up by the general osmoporters ProU and ProP. Microbiology. 1994;140:2415–2422. doi: 10.1099/13500872-140-9-2415. [DOI] [PubMed] [Google Scholar]
- 19.Gouesbet G, Trautwetter A, Bonnassie S, Wu L F, Blanco C. Characterization of the Erwinia chrysanthemi osmoprotectant transporter gene ousA. J Bacteriol. 1996;178:447–455. doi: 10.1128/jb.178.2.447-455.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gouffi K, Pichereau V, Rolland J-P, Thomas D, Bernard T, Blanco C. Sucrose is a nonaccumulated osmoprotectant in Sinorhizobium meliloti. J Bacteriol. 1998;180:5044–5051. doi: 10.1128/jb.180.19.5044-5051.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardwood C R, Archibald A R. Growth, maintenance and general techniques. In: Hardwood R C, Cutting S M, editors. Molecular biological methods for Bacillus. Chichester, United Kingdom: John Wiley & Sons; 1990. pp. 1–26. [Google Scholar]
- 22.Hengge-Aronis R, Klein W, Lange R, Rimmele M, Boos W. Trehalose synthesis genes are controlled by the putative sigma factor encoded by rpoS and are involved in stationary-phase thermotolerance in Escherichia coli. J Bacteriol. 1991;173:7918–7924. doi: 10.1128/jb.173.24.7918-7924.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jebbar M, Gouesbet G, Himdi-Kabbab S, Blanco C, Bernard T. Osmotic adaptation in Brevibacterium linens: differential effect of proline and glycine betaine on cytoplasmic osmolyte pool. Arch Microbiol. 1995;163:380–386. [Google Scholar]
- 24.Jebbar M, Talibart R, Gloux K, Bernard T, Blanco C. Osmoprotection of Escherichia coli by ectoine: uptake and accumulation characteristics. J Bacteriol. 1992;174:5027–5035. doi: 10.1128/jb.174.15.5027-5035.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jebbar M, von Blohn C, Bremer E. Ectoine functions as an osmoprotectant in Bacillus subtilis and is accumulated via the ABC-transport system OpuC. FEMS Microbiol Lett. 1997;154:325–330. [Google Scholar]
- 26.Jermyn M A. Increasing the sensitivity of the anthrone method for carbohydrate. Anal Biochem. 1975;68:332–335. doi: 10.1016/0003-2697(75)90713-7. [DOI] [PubMed] [Google Scholar]
- 27.Kempf B, Bremer E. Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments. Arch Microbiol. 1998;170:319–330. doi: 10.1007/s002030050649. [DOI] [PubMed] [Google Scholar]
- 28.Lambert A, Le Rudulier D, Gouzou L, Vergneau J P, Bazin M. Abstracts of the 11th International Congress on Nitrogen Fixation. Paris, France: Institut Pasteur; 1997. Alfalfa seed coating with Sinorhizobium meliloti and desiccation stress tolerance, abstr. 11.30; p. 126. [Google Scholar]
- 29.Larsen P I, Sydnes L K, Landfald B, Strøm A R. Osmoregulation in Escherichia coli by accumulation of organic osmolytes: betaines, glutamic acid, and trehalose. Arch Microbiol. 1987;147:1–7. doi: 10.1007/BF00492896. [DOI] [PubMed] [Google Scholar]
- 30.Le Marrec C, Michotey V, Blanco C, Trautwetter A. φAAU2, a temperate bacteriophage specific for “Arthrobacter aureus”, whose integrative functions work in other corynebacteria. Microbiology. 1994;140:3071–3077. [Google Scholar]
- 31.Le Rudulier D, Strøm A R, Dandekar A M, Smith L T, Valentine R C. Molecular biology of osmoregulation. Science. 1984;224:1064–1068. doi: 10.1126/science.224.4653.1064. [DOI] [PubMed] [Google Scholar]
- 32.Leschine S B. Cellulose degradation in anaerobic environments. Annu Rev Microbiol. 1995;49:399–426. doi: 10.1146/annurev.mi.49.100195.002151. [DOI] [PubMed] [Google Scholar]
- 33.Leslie S B, Israeli E, Lighthart B, Crowe J H, Crowe L M. Trehalose and sucrose protect both membranes and proteins in intact bacteria during drying. Appl Environ Microbiol. 1995;61:3592–3597. doi: 10.1128/aem.61.10.3592-3597.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levering P R, van Dijken J P, Veenhuis M, Harder W. Arthrobacter P1, a fast growing versatile methylotroph with amine oxidase as a key enzyme in the metabolism of methylated amines. Arch Microbiol. 1981;129:72–80. doi: 10.1007/BF00417184. [DOI] [PubMed] [Google Scholar]
- 35.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 36.Mackay M A, Norton R S, Borowitzka L J. Organic osmoregulatory solutes in cyanobacteria. J Gen Microbiol. 1984;130:2177–2191. [Google Scholar]
- 37.McNaught A D. Nomenclature of carbohydrates. Pure Appl Chem. 1996;68:1919–2008. [Google Scholar]
- 38.Mikkat S, Effmert U, Hagemann M. Uptake and use of the osmoprotective compounds trehalose, glucosylglycerol, and sucrose by the cyanobacterium Synechocystis sp. PCC6803. Arch Microbiol. 1997;167:112–118. [PubMed] [Google Scholar]
- 39.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 40.Miller K J, Wood J M. Osmoadaptation by rhizosphere bacteria. Annu Rev Microbiol. 1996;50:101–136. doi: 10.1146/annurev.micro.50.1.101. [DOI] [PubMed] [Google Scholar]
- 41.O’Gara F, Shanmugam K T. Regulation of nitrogen fixation by rhizobia: export of fixed N2 as NH4+ Biochim Biophys Acta. 1976;437:313–321. doi: 10.1016/0304-4165(76)90001-5. [DOI] [PubMed] [Google Scholar]
- 42.Phillips D A, Streit W R. Applying plant-microbe signalling concepts to alfalfa: roles for secondary metabolites. In: McKersie B D, Brown D C W, editors. Biotechnology and the improvement of forage legumes. Wallingford, England: CAB International; 1997. pp. 319–412. [Google Scholar]
- 43.Pichereau V, Pocard J-A, Hamelin J, Blanco C, Bernard T. Differential effects of dimethylsulfoniopropionate, dimethylsulfonioacetate, and other S-methylated compounds on the growth of Sinorhizobium meliloti at low and high osmolarities. Appl Environ Microbiol. 1998;64:1420–1429. doi: 10.1128/aem.64.4.1420-1429.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Potts M. Desiccation tolerance of prokaryotes. Microbiol Rev. 1994;58:755–805. doi: 10.1128/mr.58.4.755-805.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prior B A, Hewitt E, Brandt E V, Clarke A, Mildenhall J P. Growth, pectate lyase production and solute accumulation by Erwinia chrysanthemi under osmotic stress: effect of osmoprotectants. J Appl Bacteriol. 1994;77:433–439. [Google Scholar]
- 46.Reed R H. Measurement and osmotic significance of β-dimethylsulphoniopropionate in marine macroalgae. Mar Biol Lett. 1983;34:173–181. [Google Scholar]
- 47.Reed R H. Osmotic adjustment: organic solutes. Methods Enzymol. 1988;167:528–534. [Google Scholar]
- 48.Reed R H, Stewart W D P. Osmotic adjustment and organic solute accumulation in unicellular cyanobacteria from freshwater and marine habitats. Mar Biol. 1985;88:1–9. [Google Scholar]
- 49.Rhodes D, Hanson A D. Quaternary ammonium and tertiary sulfonium compounds in higher plants. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:357–384. [Google Scholar]
- 50.Sage A E, Vasil M L. Osmoprotectant-dependent expression of plcH, encoding the hemolytic phospholipase C, is subject to novel catabolite repression control in Pseudomonas aeruginosa PAO1. J Bacteriol. 1997;179:4874–4881. doi: 10.1128/jb.179.15.4874-4881.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith L T, Smith G M. An osmoregulated dipeptide in stressed Rhizobium meliloti. J Bacteriol. 1989;171:4714–4717. doi: 10.1128/jb.171.9.4714-4717.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith L T, Smith G M, D’Souza M R, Pocard J-A, Le Rudulier D, Madkour M A. Osmoregulation in Rhizobium meliloti: mechanism and control by other environmental signals. J Exp Zool. 1994;268:162–165. [Google Scholar]
- 53.Smith L T, Smith G M, Madkour M A. Osmoregulation in Agrobacterium tumefaciens: accumulation of a novel disaccharide is controlled by osmotic strength and glycine betaine. J Bacteriol. 1990;172:6849–6855. doi: 10.1128/jb.172.12.6849-6855.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stowers M D. Carbon metabolism in Rhizobium species. Annu Rev Microbiol. 1985;39:89–108. doi: 10.1146/annurev.mi.39.100185.000513. [DOI] [PubMed] [Google Scholar]
- 55.Strøm A R, Kaasen I. Trehalose metabolism in Escherichia coli: stress protection and stress regulation of gene expression. Mol Microbiol. 1993;8:205–210. doi: 10.1111/j.1365-2958.1993.tb01564.x. [DOI] [PubMed] [Google Scholar]
- 56.Talibart R, Jebbar M, Gouesbet G, Himdi-Kabbab S, Wroblewski H, Blanco C, Bernard T. Osmoadaptation in rhizobia: ectoine-induced salt tolerance. J Bacteriol. 1994;176:5210–5217. doi: 10.1128/jb.176.17.5210-5217.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Talibart R, Jebbar M, Gouffi K, Pichereau V, Gouesbet G, Blanco C, Bernard T, Pocard J-A. Transient accumulation of glycine betaine and dynamics of endogenous osmolytes in salt-stressed cultures of Sinorhizobium meliloti. Appl Environ Microbiol. 1997;63:4657–4663. doi: 10.1128/aem.63.12.4657-4663.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Warren R A J. Microbial hydrolysis of polysaccharides. Annu Rev Microbiol. 1996;50:183–212. doi: 10.1146/annurev.micro.50.1.183. [DOI] [PubMed] [Google Scholar]
- 59.Welsh D T, Herbert R A. Identification of organic solutes accumulated by purple and green sulphur bacteria during osmotic stress using natural abundance 13C nuclear magnetic resonance spectroscopy. FEMS Microbiol Ecol. 1993;13:145–149. [Google Scholar]
- 60.Wood K V, Stringham K J, Smith D L, Volenec J J, Hendershot K L, Jackson K A, Rich P J, Yang W J, Rhodes D. Betaines of alfalfa. Characterization by fast atom bombardment and desorption chemical ionization mass spectrometry. Plant Physiol. 1991;96:892–897. doi: 10.1104/pp.96.3.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wyn Jones R G, Rippin A J, Storey R. Metabolism of choline in the rhizosphere and its possible influence on plant growth. Pestic Sci. 1973;4:375–383. [Google Scholar]
- 62.Yancey P H, Clark M E, Hand S C, Bowlus R D, Somero G N. Living with water stress: evolution of osmolyte systems. Science. 1982;217:1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]