Abstract
Introduction
Neuromyelitis Optica (NMO; Devic syndrome,1894) is a CNS demyelinating syndrome. Significant proportion of neuromyelitis optica spectrum disorder is associated with Anti AQ4 Ab. The revised diagnostic criteria for neuromyelitis optica spectrum disorder (2015) has been proposed on the basis of Anti AQ4 Ab status. Most of cases reported has been found in females. It presents with multiple remissions. Common features of acute myelitis and optic neuritis seems to be the usual presentation.
Case presentation
Herein we report a case of a 35-year-old male with longitudinally extending transverse myelitis and Optic Neuritis with confirmation of Anti AQ4 Ab negative status with presentation of bilateral below knee weakness and incontinence of bowel and bladder. It was confirmed by Magnetic Resonance Imaging.
Clinical discussion
Seronegative neuromyelitis optica spectrum disorder recently classified by 2015 diagnostic criteria associated with strict clinical presentations neuroimaging findings and exclusions of differentials. It presents with a poor prognosis particularly in relapsing course.
Conclusion
We report a case of seronegative neuromyelitis optica spectrum disorder. The prognosis of relapsing course is poor. Early diagnosis and immunomodulators are required to decrease chances of recurrence. Further development of diagnostic modalities in seronegative neuromyelitis optica spectrum disorder is required.
Keywords: Neuromyelitis optica, Seronegative NMOSD, Acute myelitis, Optic neuritis, MOG antibody Negative
Highlights
-
•
Neuromyelitis optica is a CNS demyelinating syndrome characterized clinically by optic neuritis (ON) and acute myelitis.
-
•
The discovery of a specific marker autoantibody NMO-IgG that binds at or near the blood brain barrier has helped in distinction of NMOSD from its differential diagnosis.
-
•
NMOSD with AQP4-IgG include clinical syndromes or MRI findings related to optic nerve, spinal cord, area postrema, other brainstem, diencephalic, or cerebral presentations.
-
•
The mainstay of treatment for NMOSD includes immunosuppressive therapy for both acute attacks and prevention of recurrence and remissions.
1. Introduction
Neuromyelitis Optica (NMO) is a Central Nervous System demyelinating syndrome. The characterized clinical feature of NMO involves Optic Neuritis (ON) and acute myelitis [1]. Previously NMO had been thought as a variant of Multiple Sclerosis (MS). However, multiple clinical, laboratory, immunological and pathological differences between NMO and MS has been found [2]. The discovery of a specific marker autoantibody of Neuromyelitis Optica, Neuromyelitis Optica-Immunogloulin G (NMO-IgG) has further helped in distinction of Neuromyelitis Optica Spectrum Disorder (NMOSD) from its differential diagnosis [1,3]. The new nomenclature defines the unifying term Neuromyelitis Optica spectrum disorders (NMOSD). This is stratified further by serologic testing NMOSD with or without Aquaporin 4-Immunoglobulin G (AQP4-IgG). The core clinical characteristics required for patients with NMOSD with AQP4-IgG include clinical syndromes or Magnetic Resonance Imaging (MRI) findings related to optic nerve, spinal cord, area postrema, other brainstem, diencephalic, or cerebral presentations. More stringent clinical criteria, with additional neuroimaging findings, are required for diagnosis of NMOSD without AQP4-IgG or when serologic testing is unavailable [4]. We present a case of 35-year-old male Seronegative NMO with Myelin Oligodendrocytes Glycoprotein antibody (MOG ab) negative.
2. Case presentation
A 35 years old Male, without any known comorbidities presented to the Emergency Department with chief complaint of bilateral lower limb weakness along with bowel and bladder incontinence for 2 days. Patient gave past history of tuberculosis for which he had completed Anti-Tubercular Therapy (ATT) according to the national guideline. According to the patient the weakness was present below both knee joints and patient had difficulty in walking, later progressing to patient being unable to walk. It was associated with bowel and bladder incontinence.
On general examination there were no significant findings. We performed detail neurological examination which on motor examination showed power of 0/5 on medical research council (MRC) grading below the bilateral knee joints with upgoing plantars. On examination of reflex and tone, exaggerated response at bilateral lower limb (knee and ankle) was observed with both patellar and ankle clonus was present along with flaccid tone. On sensory examination crude touch, pain, temperature and joint proprioception was reduced below the knee level. So, our first impression by the history and clinical examination was found to be Paraplegia with sensory abnormality involving at level of Lumbar spine 4–5 level. So, we had differential diagnosis of compressive myelopathy, Potts spine, posterior cord infraction and conus medullaris.
Then, we sent the baseline investigations during admission as shown in Table 1.
Table 1.
Baseline investigations at the time of admission.
| Test name | Results | Reference Value |
|---|---|---|
| Hemoglobin | 15.2 | M:13–17 gm/dl |
| F: 12–15 gm/dl | ||
| Platelet | 310000 | 150000- 400000 cells/cu.mm |
| Total Leukocyte Count | 14000 | 4000- 11000 cells/cu.mm |
| Differential Leukocyte Count | ||
| Neutrophils | 82 | 40–80% |
| Lymphocytes | 13 | 20–40% |
| Monocyte | 04 | 2–10% |
| Eosinophil | 01 | 1–6% |
| Basophils | – | <1–2% |
| Glucose (Random) | 89 | <140mg/dl |
| Urea | 36 | 13–43 mg/dl |
| Creatinine | 1.0 | Male: 0.9–1.3 mg/dl |
| Female: 0.6–1.1 mg/dl | ||
| Sodium | 142 | 136–145meq/l |
| Potassium | 4.4 | 3.5–5.1 meq/l |
| Amylase | 56 | Up to 90 u/l |
| Total Bilirubin | 0.7 | 0.3–1.2 mg/dl |
| Direct Bilirubin | 0.3 | <0.2 mg/dl |
| Alkaline Phosphate | 116 | Up to 46 U/l |
| SGPT | 33 | Up to 46 U/l |
| CRP | 240.43 | Up to 10mg/l |
| Vitamin B12 | >1500 | 239–931 |
| TSH | 2.25 | 0.4–4.2mIU/L |
| FT3 | 3.52 | 0.8–2.7ng/dl |
| FT4 | 1.63 | 2.6–4.8pg/ml |
We sent the lumbar puncture next day which revealed increase total protein and leucocytes with a predominance of lymphocytes as illustrated in Table 2.
Table 2.
Lumbar puncture with increase total protein and leucocytes with a predominance of lymphocytes.
| Parameters | Values | Normal Values |
|---|---|---|
| Total Leukocyte count | 30 cells/cu.mm. Predominantly Lymphocytes | <5 |
| Glucose | 47.5mg/dl | 50–80 |
| Protein | 177.2mg/dl | 15–45 |
| Adenosine Deaminase Test (ADA) | 2.3 U/L | 0–9 |
| Culture, Gram staining, Ziehl Neelsen (ZN) staining | Negative |
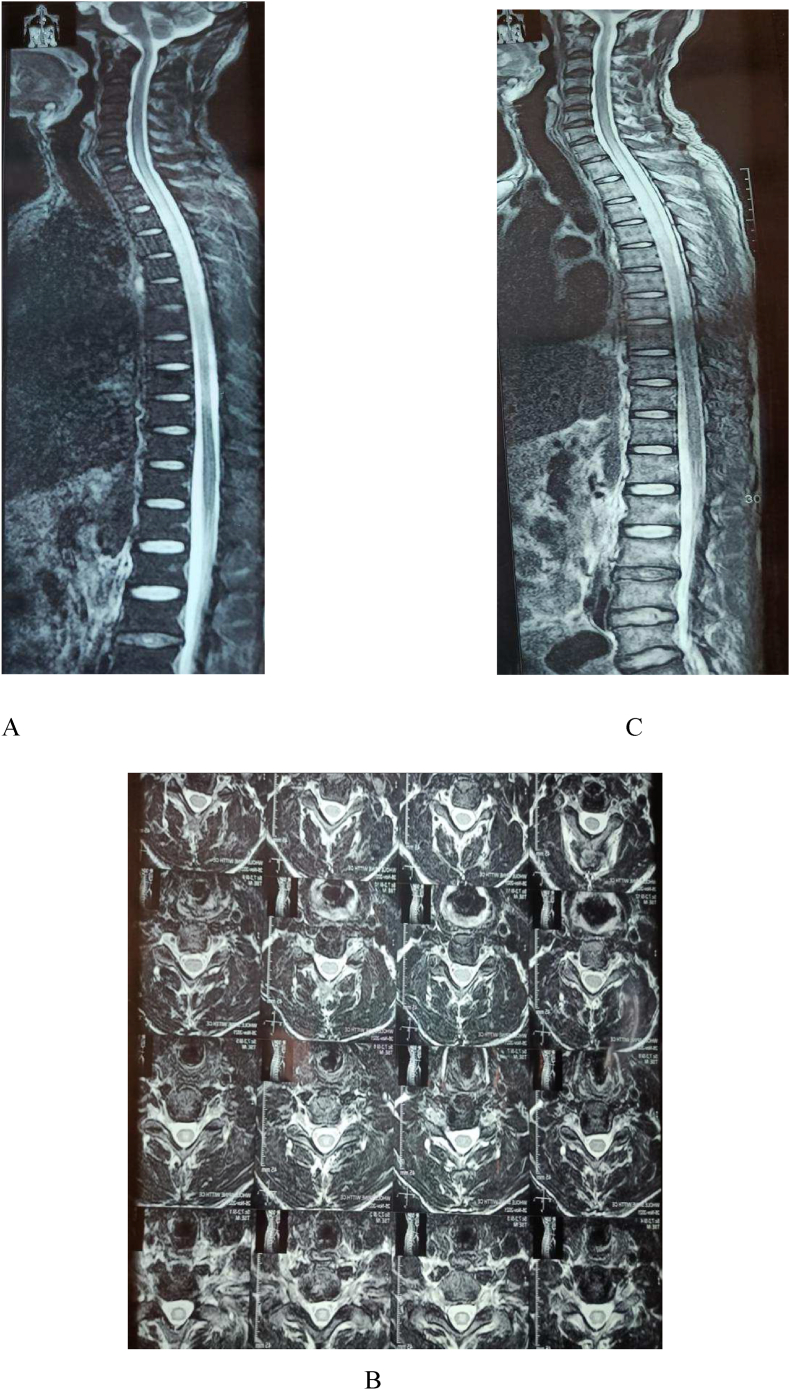
MRI was done on the same day where Post Gadolinium scan showed findings of abnormal high T2W and STIR signal changes of the spinal cord from the level of Cervical 6 till level of Thoracic 8 level as shown in Fig. 1. He was then treated in the line of Longitudinally Extending Transverse Myelitis (LETM). Initially, he was treated with Azathioprine (75 mg per oral twice a day) along with Methylprednisolone (intravenous 1 gm per day) for 5-day course and switched to oral steroid (1 mg/kg/day) for a week.
Fig. 1.
MRI Post Gadolinium showing abnormal high T2W and STIR signal changes of the spinal cord from the level of Cervical 6 till level of Thoracic 8 level (Note T2W Vertical “A”, T2W Axial “B”,STIR Vertical “C”).
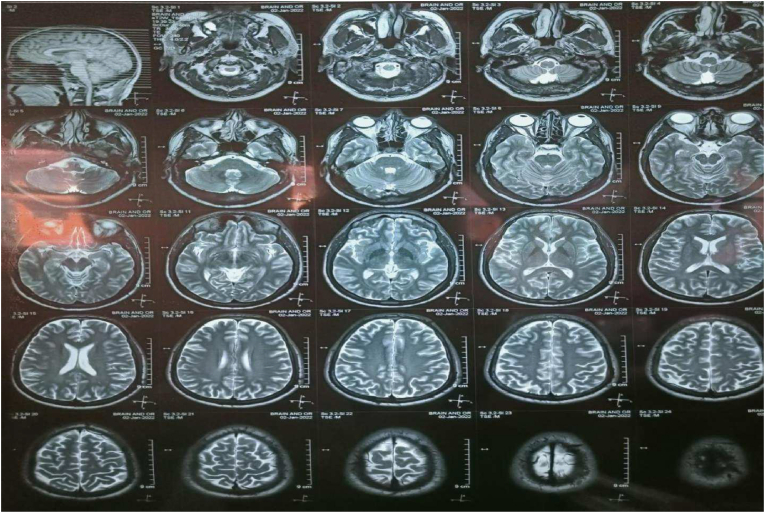
During the hospital stay patient experienced blurring of vision on his left eye for which MRI of orbit and head was done. It revealed normal MRI brain and increase T2 and FLAIR signal intensity in the left optic nerve adherent to optic nerve sheath in mid part as shown in Fig. 2. This was suggestive of Left Optic Neuritis. He was diagnosed as ON of the left eye on basis of clinical findings and MRI imaging. Patient was started on Methyl prednisolone (1 gm per day) for 3 days and then oral steroid (1mg/kg/day) trial for 11 days was given. Lumbar puncture was again performed for Oligoclonal bands, Anti Aquaporin 4 Antibody (Anti AQ4 Ab) and Anti Myelin Oligodendrocytes Glycoprotein Antibody (Anti MOG Ab) to rule out NMOSD, Myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD) and Multiple Sclerosis. All test were negative which ruled out multiple sclerosis as shown in Table 3.
Fig. 2.
MRI of Brain and Orbit with normal MRI brain and increase T2 signal intensity in the left optic nerve adherent to optic nerve sheath in mid part.
Table 3.
Parameters for differentiation of NMOSD, Myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD) and Multiple Sclerosis.
| Parameters | Status |
|---|---|
| Oligoclonal Bands IgG, Cerebrospinal Fluid(CSF) | Not seen |
| Anti AQ4 Ab | Negative |
| Anti MOG Ab | Negative |
After all primary investigations a final diagnosis of Seronegative Neuromyelitis Optic Spectrum Disorder (NMO-SD) was made referencing the 2015 diagnostic criteria [4]. He was maintained on a therapy of Azathioprine (75 mg twice a day) along with oral steroid (1 mg/kg/day) and was planned to start Rituximab.And for this Interferon Gamma Release Assay (IGRA) test and High-Resolution Computed Tomography (HRCT) of chest was done to rule out latent TB.
The patient was discharged on Azathioprine (75 mg twice a day) and tapering oral steroid (60 mg/day) with continuous physiotherapy recommendations. He was again admitted after two weeks due to relapse and lack of improvement. The Rituximab was then started on two doses of 1 gm in every two weeks with continuation in every six months. During hospital stay, although the patient accepted treatment, there was no significant improvement in his clinical status and performance. He was discharged with continuous physiotherapy and palliative care recommendations.
3. Discussion
The prevalence of NMOSD ranges from∼0.5–4/100,000, with highest incidence of up to 10/100,000 in Blacks [5]. NMOSD has a significant female predisposition with male to female ratio 10–9:1, however the difference seems to be absent in children [[5], [6], [7]]. The mean age of incidence of NMOSD is 32–45 years, but children and the elderly account for 18% to one fourth of cases [7,8].
AQP4- IgG is present in up to 70%–90% of NMOSD patients and is highly specific for the disease [9]. In a recently conducted study 42% of Seronegative NMOSD individuals tested positive for Anti MOG ab whereas, none of the Seropositive NMOSD individuals showing Anti MOG ab [10].
There have been multiple revisions for Diagnostic criteria for NMO [1,2,4,11]. The newly proposed revised diagnostic criteria for NMOSD (2015) have been proposed in regards to seropositivity status of patients with more rigid clinical and imaging characteristics required for the successful diagnosis of NMOSD in the absence of AQP4-Ab positive status [4].Our patient has fulfilled core clinical characteristic of optic neuritis, left side and acute myelitis with LETM with characteristic MRI findings along with AQP4 antibody negative status.
Besides the utilization of clinical features, MRI and serum antibody marker in the diagnosis of NMOSD other modalities such as Optic coherence tomography (OCT), Visual Evoked Potential (VEP) and Cerebrospinal Fluid (CSF). OCT shows more markedly decreased RNFL thickness and macular volume in NMO/NMOSD than in MS. For the clinical manifestation of ON, VEP has been mainly employed to show subclinical involvement of the optic nerve. In NMO/NMOSD, patients usually demonstrate absence of response along with decreased amplitude associated with normal latency, giving a picture of more severe axonal damage. Pleocytosis (>50 cells/mm3) with neutrophils in the CSF is found in some NMO/NMOSD patients during acute attacks. Oligoclonal IgG bands are only present in about 10%–30% of these patients, in contrast to about 90% in MS patients. A useful biomarker of astrocytic destruction in NMO/NMOSD is the level of glial fibrillary acidic protein (GFAP) in the CSF which is markedly raised in acute attacks [12].
The mainstay of treatment for NMOSD includes immunosuppressive therapy for both acute attacks and prevention of recurrence and remissions. For the treatment of acute attacks high dose corticosteroids with typical starting dose 1000 mg of Methylprednisolone intravenously for 5 days, followed by an oral steroid taper for 2–8 weeks is seen as first line therapy. The proposed reason of use in acute NMOSD is for the reduction of edema and secondary inflammation in the lesion. According to the response and severely inflamed attacks additional steroid doses may be indicated. Plasma exchange has been suggested as a rescue treatment for patients with severe symptoms not responding to steroids [13].
For the prevention of relapses immunomodulators such as mycophenolate mofetil and rituximab are the most common used therapies with comparative Absolute Relapse Rate (ARR) and Expanded Disability Status Scale (EDSS) scores. Azathioprine, mitoxantrone, and methotrexate are some alternatives for reduction of relapses however, they demonstrate reduction in ARR and stabilization of EDSS but with higher relapse rates and exposure to greater risk of treatment toxicities. Usually, these agents are used along with supplementation with corticosteroids [6,13]. Newer therapies for the management of NMOSD available are Eculizumab, natalizumab, tocilizumab, inebilizumab. They have shown reduction in relapse rates [9].
The course on NMO can be monophasic or relapsing. It is associated with poor prognosis. Specially, relapsing courses are associated with poor prognosis with stepwise increment in disability during myelitis. Significant, mortality is associated with respiratory failure [11].
The limitation in our study was that we could not do adequate follow up with the patient after he was discharged for the second time.
4. Conclusion
To date, there is no reported case of NMOSD in Nepal. The finding of LETM is considered a classic presentation of NMOSD. Although there have been few reported cases of LETM attributed to NMOSD with anti-AQP4 antibody-positive status in Nepal, no diagnosis of Seronegative NMOSD has been made. As such, increased scrutiny is the key in the detection of NMOSD with seronegative status per the newly revised criteria. It becomes increasingly crucial as NMOSD is associated with poor prognosis when compared to its differential diagnosis. The relapsing course of the disease, in particular, increases the disability and mortality among people affected by this condition. As such, prompt diagnosis and management to reduce relapse is necessary. Thus, further understanding of seronegative NMOSD is required.
Author agreement statement
We the undersigned declare that this manuscript is original, has not been published before and is not currently being considered for publication elsewhere.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We understand that the Corresponding Author is the sole contact for the Editorial process. He/she is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs.
Ethical approval
This is a case report, therefore, it did not require ethical approval from ethics committee.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editor-in-chief of this journal on request.
Funding
The study did not receive any grant from funding agencies in the public, commercial or not-for-profit sectors.
Authors contribution
BK: Led data collection, contributed to writing the case information and discussion.
AMB: Contributed to the process of original draft preparation and introduction, edited the rough draft into the final manuscript.
BD: Contributed in conceptualization, literature review, supervision and manuscript editing.
AA: Revised it critically for important intellectual content, contributed to review and editing.
AK: The resident physician, who helped in the diagnosis and helped in the discussion section.
RP: The neurologist, who diagnosed the case, supervised the process of manuscript writing.
All the authors read and approved the final manuscript.
Guarantor
Ayush Mohan Bhattarai, Shree Birendra Hospital, 44600 Kathmandu, Nepal. Email: ayushbhattarai77@gmail.com, Phone: +977–9862438437.
Registration of research studies
Not applicable.
Declaration of interest statement
The authors report no conflicts of interest.
Provenance and peer review
Not commissioned, externally peer reviewed.
Acknowledgment
None.
References
- 1.Wingerchuk D., Lennon V., Pittock S., Lucchinetti C., Weinshenker B. Revised diagnostic criteria for neuromyelitis optica. Neurology [Internet] 2006. https://n.neurology.org/content/neurology/66/10/1485.full.pdf Crossref Pubmed Available from: 66, 10, 1485-1489. [DOI] [PubMed]
- 2.Wingerchuk D.M., Lennon V.A., Lucchinetti C.F., Pittock S.J., Weinshenker B.G. The spectrum of neuromyelitis optica. Lancet Neurol. 2007 Sep 1;6(9):805–815. doi: 10.1016/S1474-4422(07)70216-8. (Crossref Pubmed) [DOI] [PubMed] [Google Scholar]
- 3.Lennon V.A., Wingerchuk D.M., Kryzer T.J., Pittock S.J., Lucchinetti C.F., Fujihara K., Nakashima I., Weinshenker B.G. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004 Dec 11;364(9451):2106–2112. doi: 10.1016/S0140-6736(04)17551-X. (Crossref Pubmed) [DOI] [PubMed] [Google Scholar]
- 4.Wingerchuk D., Banwell B., Bennett J., Cabre P., Carroll W., Chitnis T., et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology [Internet] 2015. cited, Available from: 85, 2, 177-189. [DOI] [PMC free article] [PubMed]
- 5.Hor J.Y., Asgari N., Nakashima I., Broadley S.A., Leite M.I., Kissani N., Jacob A., Marignier R., Weinshenker B.G., Paul F., Pittock S.J. Epidemiology of neuromyelitis optica spectrum disorder and its prevalence and incidence worldwide. Front. Neurol. 2020 Jun 26;11:501. doi: 10.3389/fneur.2020.00501. (Crossref Pubmed) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borisow N., Mori M., Kuwabara S., Scheel M., Paul F. Diagnosis and treatment of NMO spectrum disorder and MOG-encephalomyelitis. Front. Neurol. 2018:888. doi: 10.3389/fneur.2018.00888. Crossref Pubmed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lana-Peixoto M.A., Talim N. Neuromyelitis optica spectrum disorder and anti-MOG syndromes. Biomedicines. 2019 Jun;7(2):4. doi: 10.3390/biomedicines7020042. Crossref Pubmed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papp V., Magyari M., Aktas O., Berger T., Broadley S.A., Cabre P., Jacob A., Kira J.I., Leite M.I., Marignier R., Miyamoto K. Worldwide incidence and prevalence of neuromyelitis optica: a systematic review. Neurology. 2021 Jan 12;96(2):59–77. doi: 10.1212/WNL.0000000000011153. (Crossref Pubmed) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fiala C., Rotstein D., Pasic M.D. Pathobiology, diagnosis, and current biomarkers in neuromyelitis optica spectrum disorders. J. Appl.Labo. Med. 2022 Jan;7(1):305–310. doi: 10.1093/jalm/jfab150. Crossref Pubmed. [DOI] [PubMed] [Google Scholar]
- 10.Hamid S.H., Whittam D., Mutch K., Linaker S., Solomon T., Das K., Bhojak M., Jacob A. What proportion of AQP4-IgG-negative NMO spectrum disorder patients are MOG-IgG positive? A cross sectional study of 132 patients. J. Neurol. 2017 Oct;264(10):2088–2094. doi: 10.1007/s00415-017-8596-7. Crossref Pubmed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wingerchuk D.M., Hogancamp W.F., O’brien P.C., Weinshenker B.G. The clinical course of neuromyelitis optica (Devic's syndrome) Neurology. 1999 Sep 1;53(5):1107–1110. doi: 10.1212/wnl.53.5.1107. (Crossref Pubmed) [DOI] [PubMed] [Google Scholar]
- 12.Sato D.K., Lana‐Peixoto M.A., Fujihara K., De Seze J. Clinical spectrum and treatment of neuromyelitis optica spectrum disorders: evolution and current status. Brain Pathol. 2013 Nov;23(6):647–660. doi: 10.1111/bpa.12087. Crossref Pubmed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sherman E., Han M.H. Acute and chronic management of neuromyelitis optica spectrum disorder. Curr. Treat. Options Neurol. 2015 Nov;17(11):1–4. doi: 10.1007/s11940-015-0378-x. (Crossref Pubmed) [DOI] [PMC free article] [PubMed] [Google Scholar]