Abstract
Aims
Achieving purchase in native glenoid bone is essential for the stability of the glenoid baseplate when bone graft is used to address bone loss in both primary and revision shoulder arthroplasty procedures. The aim of this study is to assess the required depth of the baseplate peg in native bone when bone graft is used to result in satisfactory integration.
Patients and methods
The CT scans of patients who underwent either primary or revision arthroplasty procedures with bone graft using the SMR Axioma Trabecular Titanium (TT) Metal Backed glenoid system were assessed. We measured the depth of the glenoid peg in native glenoid bone. Measurements were taken by two authors separately.
Results
The scans of 53 patients (mean age 68 years) with a minimum follow-up of two years were reviewed. Implants included 12 anatomical and 41 reverse geometry prostheses. There were 17 primaries and 36 revisions: hemiarthroplasties (20) total (14) and reverse (2) implants. Bone grafts were from humeral head (15), iliac crest (34) and allograft (4). The mean depths were 8.8 mm (first assessor) and 9.10 mm (second assessor). The glenoid peg violated the glenoid vault in 32 patients and this did not adversely affect the outcome. There were three failures of implants all of which were aseptic failures and had peg penetration of less than 6 mm.
Conclusions
The mean depth of glenoid peg in native bone was 9 mm (variation between 0.2 and 0.52 mm at 95% confidence interval). Aseptic loosening was seen with peg penetration less than 6 mm in native bone. Glenoid vault violation was not associated with loosening.
Keywords: Glenoid peg depth, native glenoid, glenoid bone graft, primary shoulder arthroplasty, revision shoulder arthroplasty
Introduction
As the demand for shoulder arthroplasty increases, 1 the subsequent increase in revision surgery will pose a surgical challenge. Glenoid bone loss is mainly encountered during revision surgery but also in primary procedures,2,3 when there is a significant glenoid wear or dysplasia (Walch Type B and C glenoids). 4 Glenoid bone loss can be described as contained or uncontained depending on the nature of the lost bone and remaining vault. Contained bone loss can be dealt with by impaction bone grafting techniques. 5 Uncontained bone loss requires reconstruction with structural grafts. These can be harvested from the humeral head during primary procedures, while iliac crest autograft and various types of allograft are required in revision procedures.6–8
Bone graft needs to integrate to native bone to be successful. 9 Current techniques have developed to harvest grafts using the baseplate and then implant the baseplate/graft construct en masse onto the prepared native scapula. 10 The central baseplate peg is longer than the graft and therefore inserts into the native vault. The prerequisite for successful reconstruction is to achieve sufficient stability and compression of the baseplate peg by placing screws within the native glenoid bone at the time of implantation. 11 Implants with longer peg lengths have therefore been designed to facilitate bypassing the bone graft and purchase into native scapula. Boileau et al. used a baseplate peg length of 25 mm with graft thickness varying from 7 to 10 mm implying a penetration of 15 to 18 mm in native bone. 12
Whilst previous series have highlighted the importance of peg insertion into native scapula,11,13 to date no study to our knowledge has specifically assessed the required depth of penetration. The purpose of this study is to assess the required minimum depth of glenoid peg in native bone in order to achieve initial stability and later bone integration of the glenoid implant when used with bone graft.
Patients and methods
We retrospectively reviewed a consecutive series of patients who underwent primary or revision shoulder arthroplasty with bone graft to address severe glenoid bone loss with a minimum of two years follow-up. Surgeries were performed between May 2013 and November 2015.
All patients had preoperative assessment with computer tomography scan (CT scan) to evaluate the amount of glenoid bone loss and to help with the operative planning. The axial CT images that showed the tip of coracoid were used to identify the glenoid deficit as described by Friedman et al. 14 For primary procedures, and for revisions of hemiarthroplasty, the amount of glenoid wear was evaluated using the Walch classification. 4 For revision procedures, the glenoid bone loss was quantified according to the Antuna classification. 15
The glenoid implant used in all patients was the Axioma SMR Trabecular Titanium (TT) Metal Backed (Lima Corporate, Villanova di San Daniele del Friuli, Italy; Figure 1). The SMR Axioma builds upon the SMR system with a modular metal glenoid base plate with TT stems of varying lengths. The stem consists of a cylindrical TT coated portion and a tapered bullet nose. The TT has been shown to be both osteoinductive and oseteoconductive providing graft incorporation and stimulating bone in-growth.16,17
Figure 1.
Axioma SMR Trabecular Titanium (TT) Metal Backed implant.
Surgical procedure
All procedures were performed by three shoulder surgeons with more than five years of experience in complex revision shoulder arthroplasty at a tertiary upper limb unit. The surgical approach used was deltopectoral. Bone grafts were harvested from the humeral head, if suitable, in primary procedures and from either the ilium or from femoral head allograft in revision procedures. The Axioma system has specifically designed barrel reamers and cutting guides to perform accurate graft harvesting from both the iliac crest and humeral head. For humeral grafting, we used a modification of the technique initially described by Boileau et al. 12 A guidewire is placed perpendicular to the apex of the humeral head. The standard baseplate reamer is used and followed by a cannulated drill for the central peg. The baseplate is inserted directly onto the humeral head and the barrel reamers are used to harvest a graft of the desired depth. The graft can be further contoured by hand to fit the defect. For iliac crest autograft, we used a modification of the technique described by Norris et al. 7 Following one complication of iliac bone fracture in our series after harvesting from the superior iliac crest, we changed our approach to harvest graft from the lateral ileum. In this manner, a circular bone graft can be harvested of the inner and outer tables whilst preserving, in most cases, the structural integrity of the brim. A long peg baseplate is directly implanted on the side of the ileum and the bone harvested with the same barrel reamer, the inner table cortex is removed and finally contouring of the graft to match the defect. The graft-glenoid baseplate construct is then implanted into the native glenoid with the addition of one superior and one inferior 6.5 mm compression screws.
CT scan measurements
All patients had a postoperative CT scan to assess bone integration, at a minimum of three months post-surgery and then at regular intervals of six months until bone integration or failure were established. The depth of the baseplate peg penetration into the native glenoid bone was measured using the measurement tool on the picture archiving and communication system (Figure 2). The metal artefact reduction scan (MARS) was used to do the measurements. The CT image that best demonstrated the maximal depth in native glenoid was selected by correlating the coronal and axial images. Two authors agreed on the selected CT image for each patient and documented the serial number to ensure that measurements by each author were made on same CT image. Each author made the measurements independently from the other.
Figure 3.
Bland Altman plot comparing the two measurements done by the two authors.
Figure 2.
Coronal CT scan showing bone graft (outlined in yellow), the interphase between the graft and the native glenoid (yellow arrow) and the depth of the TT stem in native glenoid (distance between the two green lines).
Data analysis
Each author made the two measurements for each patient on two separate occasions. Intra-observer reliability was calculated by calculating the mean and standard deviation of the two measurements. The coefficient of variation (CV) for the two measurements made by same author for each patient was calculated. The average of the individual CVs is reported as the intra-observer CV. A value of less than 10% is considered satisfactory.
The intraclass correlation coefficient (ICC) using a two-way mixed model on absolute agreement was used to assess measurement reliability between the two authors. The average ICC of multiple measurements may range from 0 to 1, with a higher value indicating better reliability. ICC < 0.40 is considered as poor, 0.40–0.59 as fair, 0.60–0.74 as good and 0.75–1.00 as excellent. Statistical analyses were performed using SPSS 13.0 software (SPSS Inc., Chicago, IL).
Using the 95% confidence interval data were also plotted using the Bland Altman chart to assess the agreement between the two assessors.
Failure was defined as the lack of peg integration and graft incorporation using the classification by Granville-Chapman et al. 18 along with the need for revision surgery. In practice all of these occurred together in all ‘failed cases’.
Results
Patient demographics
Patients’ demographics are summarised in Table 1. There were 17 primary procedures consisting of 9 total anatomical shoulder replacement (ATSR) and 8 reverse shoulder arthroplasty (RSA) procedures. There were 36 revision procedures which details are given in Table 1. One patient was revised to ATSR and the remaining 35 patients revised to RSA. All revision pro- cedures were single stage, and none was performed for infection. Indications for surgery consisted of glenoid erosion in 20 patients with hemiarthroplasty and aseptic loosening of glenoid implant in 14 patients with ATSRs and 2 patients with RSAs. The types of glenoid erosion and wear encountered in primary and revision procedures are shown in Table 2. Bone grafts consisted of iliac crest autografts in 32 patients and femoral head allografts in 4 patients. The decision to use allograft was made when the risks from iliac crest harvesting were felt significant particularly in osteoporotic bones.
Table 1.
Patients demographics and details of procedures.
| Number of CT scans/ number of patients | 53/53 |
|---|---|
| Female/Male | 30/23 |
| Mean age (range) | 68 (30–88 years) |
| Laterality: right/left | 38/15 |
| Type of implant | |
| RSA | 43 |
| TSR | 10 |
| Type of procedure | |
| Primary | 17 |
| Revision | 36 |
| Revised implants | |
| Hemiarthroplasty | 20 |
| TSR | 14 |
| RSA | 2 |
| Types of bone grafts (in primary procedures) | 2 |
| Humeral autograft | 15 |
| Iliac crest autograft | 2 |
| Types of bone grafts (in revision procedures) | |
| Iliac crest autograft | 32 |
| Femoral head allograft | 4 |
RSA: reverse shoulder arthroplasty; TSR: total shoulder arthroplasty.
Measurements of peg depth
CT scans were available at regular intervals following surgery with a minimum follow-up of two years at the final review. The mean of depth of native glenoid penetration obtained by the first assessor was 8.8 mm (standard deviation (SD): 2.16, range: 4 -15mm). The mean by the second assessor was 9.10 (SD: 1.7, range 3.4 -15 mm). The average of the individual CV for the measurements made by each author was 5.6% for first author and 6.2% for second author indicating good intra-observer reliability. The `ICC' was 0.808 and this indicated excellent agreement between the two authors. As shown in Figure 3, Bland Altman plot demonstrates how small the differences in measurements were between the two assessors.
Failed implants
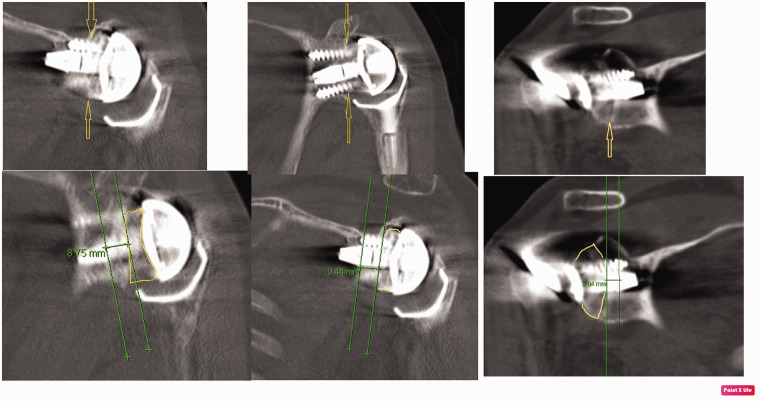
There were three failures of glenoid implants: one failure in patient with ATSR, two failures in patients with RSAs. The grafts used in the failed cases consisted of humeral head (1 patient) graft and iliac crest autograft (2 patients). One patient had significant glenoid wear from severe OA and underwent RSA with humeral head autograft. A second patient had loosening of glenoid implant and underwent revision to ATSR using allograft. A third patient had glenoid wear from hemiarthroplasty and revised to RSA using allograft. All failed cases had peg penetration of less than 6 mm in native glenoid bone (examples are given in Figure 4). Tissue biopsies harvested at the time of revision surgeries of these cases confirmed that failure of the glenoid component was not caused by infection. At two-year follow-up the remaining 50 arthroplasties showed peg integration and graft incorporation with no signs of progressive loosening.
Figure 4.
Case 1 – 1(a): Preoperative x-ray demonstrating severe glenoid wear. 1(b): Immediate postoperative radiograph. 1(c): Measurements of peg penetration at six weeks. 1(d): Aseptic failure of baseplate on CT scan at three months. Case 2 – 2(a): Preoperative x-ray demonstrating glenoid loosening. 2(b): Immediate postoperative radiograph. 2(c): Measurements of peg penetration at six weeks. 2(d): Aseptic failure of baseplate on CT scan at three months.
The tapered portion of the stem was designed to facilitate greater penetration into the apex of the vault. It is not TT coated and we found that the lack of bone integration on this part of the peg was not associated with implant loosening (Figure 5).
Figure 5.
Coronal MARS CT scans showing no bone integration at the tapered part of the glenoid peg and this was not associated with failure.
The tip of the glenoid peg was found to penetrate the cortex of the glenoid vault in 32 patients (uncontained pegs) while the peg remained within the vault in 21 patients (contained pegs; Figure 6). Penetration was through the anterior cortex in 29 patients and through the posterior cortex in 3 patients. None of these patients exhibited loosening of the glenoid components.
Figure 6.
(a) Axillary radiograph and (b) axial CT scan showing the tapered part of the glenoid stem penetrating the anterior wall (uncontained peg). (c) Axial CT scan showing the tapered part of the glenoid stem lying within the vault (contained peg).
Discussion
The principal of surgery when using bone graft to reconstruct the glenoid is to achieve maximal stability to allow bone integration. The key aspects of this are graft quality, adequate compression and stability of the glenoid peg within the native scapula. Whilst these principals are universally acknowledged there is currently no scientific consensus regarding the minimally required glenoid peg depth within native bone obtain initial stability of the construct at the time of implantation.
In his original article describing bony-increased offset RSA (BIO-RSA), Boileau et al. 12 used a baseplate with a 25 mm-long central peg for primary fixation of bone graft into native glenoid vault. The graft thickness in this study varied between 7 and 10 mm which then leaves a remaining peg length between 15 and 18 mm into native glenoid.
In a recent article by Seidl et al. 19 on the management of glenoid bone defects, they recommended using longer glenoid pegs (30 to 35 mm) for severe defects or when the thickness of bone graft used is larger than 15 mm, again referring to a remaining peg length in native bone between 15 and 20 mm.
In a study by Werner et al. 20 using RSA for neglected anterior shoulder dislocation, they aimed to achieve 10 mm in native bone by adjusting the version while preparing for the central peg (10 to 15 of anteversion). Depth measurement was performed to confirm a minimum anchorage length of 10 mm for the central peg. One failure in their series of 32 patients was due to aseptic loosening at 12 months which they related to an anchorage length of less than 10 mm.
Similarly, Norris et al. 7 used a custom-made long post baseplate (25 to 30 mm) in revision surgery for RSA procedures using tricortical iliac crest bone grafts to address glenoid defects. They believed that such a length of the post was enough to penetrate through the bone graft to reach the native scapula. However, they did not analyse the achieved depth of the peg in native bone following impaction and implantation nor did they determine the average thickness of the bone graft used.
In our study, a peg penetration of a minimum 6 mm in native glenoid was associated with successful initial implant stability and subsequent bone integration. It should be noted that the thickness of bone graft may change when the baseplate is implanted by impaction and compression which would lead to more peg penetration in native glenoid. This success could also be attributed to the implant used in our series as the TT has previously shown osteoinductive and oseteoconductive properties.16,17
In our study, glenoid vault penetration by the glenoid peg was a common finding and we believe this conferred additional stability at the time of implantation. In a study by Hsu et al. 21 on patients undergoing primary total shoulder arthroplasty, they had a subset of patients in whom the central pegs or peripheral pegs violating the medial cortex of the glenoid vault and this occurred anteriorly. They found that this is not associated with aseptic loosening of the glenoid component. In another study, Flynn et al. 22 evaluated the glenoid vault perforation by glenoid pegs and found that this was not associated with implant loosening or increased risk of revision surgery. In our study, 34 patients (60%) had perforation of the glenoid vault and this was not associated with implant loosening at a minimum of two years follow-up.
While previous studies have investigated the effects of different peg lengths on implant stability, none so far have evaluated the effect of peg diameter on outcome. The diameter of the glenoid peg used in this study was marginally larger than that of other designs (used diameter in this study varied between 12–13.7 mm at its cylindrical largest part compare to 8–12.5 mm of various available designs). However, it would be interesting to know through future studies whether peg diameter can influence stability of the glenoid implant.
We acknowledge that our study is limited by the fact it is a retrospective case review (level IV evidence) and the variety of primary and revision cases and differing bone grafts used. However, these are relatively uncommon cases and we present a large series detailing our experience of these challenging operations which we believe will be of benefit to other arthroplasty surgeons.
Conclusion
The SMR Axioma TT baseplate provides reliable integration of structural bone grafts when used to reconstruct glenoid deficiency. On the basis of the findings of this study we would recommend a minimum of 6 mm of cylindrical TT stem be implanted in the native scapula to achieve sufficient initial stability and subsequent union.
Table 2.
Type of glenoid erosion.
| Primary procedures | 17 |
| Walch A2 | 1 |
| Walch B2 | 4 |
| Walch B3 | 4 |
| Walch C | 7 |
| Walch D | 1 |
| Revision of hemiarthroplasty | 20 |
| Walch A2 | 6 |
| Walch B2 | 6 |
| Walch B3 | 5 |
| Walch C | 3 |
| Revision of TSR | 14 |
| Severe central a | 3 |
| Severe peripheral a | 4 |
| Moderate combined a | 3 |
| Severe combined a | 4 |
| Revision of RSA | 2 |
| Severe combined | 2 |
TSR: total shoulder replacement; RSA: reverse shoulder arthroplasty.
aAntuna classification.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Basel Balbisi https://orcid.org/0000-0002-1413-8268
References
- 1.Day JS, Lau E, Ong KL, et al. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg 2010; 19: 1115–1120. [DOI] [PubMed] [Google Scholar]
- 2.Klein SM, Dunning P, Mulieri P, et al. Effects of acquired glenoid bone defects on surgical technique and clinical outcomes in reverse shoulder arthroplasty. J Bone Joint Surg Am 2010; 92-A: 1144–1154. [DOI] [PubMed] [Google Scholar]
- 3.Gunther SB, Lynch TL. Total shoulder replacement surgery with custom glenoid implants for severe bone deficiency. J Shoulder Elbow Surg 2012; 21: 675–684. [DOI] [PubMed] [Google Scholar]
- 4.Walch G, Badet R, Boulahia A, et al. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty 1999; 14: 756–760. [DOI] [PubMed] [Google Scholar]
- 5.Page RS, Haines JF, Trail I. Impaction bone grafting of the glenoid in revision shoulder arthroplasty: classification, technical description and early results. Shoulder Elbow 2009; 1: 81–88. [Google Scholar]
- 6.Bateman E, Donald SM. Reconstruction of massive uncontained glenoid defects using a combined autograft-allograft construct with reverse shoulder arthroplasty: preliminary results. J Shoulder Elbow Surg 2012; 21: 925–937. [DOI] [PubMed] [Google Scholar]
- 7.Norris TR, Kelly JD, Humphrey CS. Management of glenoid bone defects in revision shoulder arthroplasty: a new application of the reverse total shoulder prosthesis. Tech Shoulder Elbow Surg 2007; 8: 37–46. [Google Scholar]
- 8.Wagner E, Houdek MT, Griffith T, et al. Glenoid bone-grafting in revision to a reverse total shoulder arthroplasty. J Bone Joint Surg Am 2015; 97: 1653–1660. [DOI] [PubMed] [Google Scholar]
- 9.Malhas AM, Granville-Chapman J, Robinson PM, et al. Reconstruction of the glenoid using autologous bone-graft and the SMR Axioma TT metal-backed prosthesis: the first 45 sequential cases at a minimum of two years’ follow-up. Bone Joint J 2018; 100-B: 1609–1617. [DOI] [PubMed] [Google Scholar]
- 10.Malhas A, Rashid A, Copas D, et al. Glenoid bone loss in primary and revision shoulder arthroplasty. Shoulder Elbow 2016; 8: 229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Königshausen M, Jettkant B, Sverdlova N, et al. Influence of different peg length in glenoid bone loss: a biomechanical analysis regarding primary stability of the glenoid baseplate in reverse shoulder arthroplasty. Technol Health Care 2015; 23: 855–869. [DOI] [PubMed] [Google Scholar]
- 12.Boileau P, Moineau G, Roussanne Y, et al. Bony increased-offset reversed shoulder arthroplasty minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res 2011; 469: 2558–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seebauer L, Ekelund AL. Management of glenoid bone loss in primary and revision reverse total shoulder arthroplasty. Obere Extremität 2017; 12: 6–15. [Google Scholar]
- 14.Friedman RJ, Hawthorne KB, Genez BM. The use of computerized tomography in the measurement of glenoid version. J Bone Joint Surg Am 1992; 74: 1032–1037. [PubMed] [Google Scholar]
- 15.Antuna SA, Sperling JW, Cofield RH, et al. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg 2001; 10: 217–224. [DOI] [PubMed] [Google Scholar]
- 16.Asti A, Gastaldi G, Dorati R, et al. Stem cells grown in osteogenic medium on PLGA, PLGA/HA, and titanium scaffolds for surgical applications. Bioinorg Chem Appl 2010; 6: 831031–831031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devine D, Arens D, Burelli S, et al. In vivo evaluation of the osteointegration of new highly porous titanium™. J Bone Joint Surg Br 2012; 94-B(Suppl XXXVII): 201. [Google Scholar]
- 18.Granville-Chapman J, Copas D, Robinson S, et al. The first series of SMR Axioma TT®: early experiences with an innovative trabecular titanium implant for complex glenoid reconstruction. Shoulder Elbow 2015; 7: 309–332. [Google Scholar]
- 19.Seidl AJ, Williams GR, Boileau P. Challenges in reverse shoulder arthroplasty: addressing glenoid bone loss. Orthopedics 2016; 39: 14–23. [DOI] [PubMed] [Google Scholar]
- 20.Werner BS, Böhm D, Abdelkawi Gohlke F. Glenoid bone grafting in reverse shoulder arthroplasty for long-standing anterior shoulder dislocation. J Shoulder Elbow Surg 2014; 23: 1655–1651. [DOI] [PubMed] [Google Scholar]
- 21.Hsu JE, Namdari S, Baron M, et al. Glenoid perforation with pegged components during total shoulder arthroplasty. Orthopedics 2014; 37: e587–591. [DOI] [PubMed] [Google Scholar]
- 22. Flynn JN, Wijeratna M, Evans M, et al. Glenoid vault perforation in total shoulder arthroplasty: do we need computer guidance? Shoulder Elbow. Epub ahead of print 2019. [DOI] [PMC free article] [PubMed]