This cohort study investigates the association of diabetes medication with open-angle glaucoma, age-related macular degeneration, and cataract in the Rotterdam Study.
Key Points
Question
What is the association between diabetes medication and the common eye diseases open-angle glaucoma (OAG), age-related macular degeneration (AMD), and cataract in a population of European ethnicity?
Findings
In this cohort study of 11 260 participants, treatment with metformin was associated with a lower risk of OAG whereas other diabetes medication was associated with a lower risk of AMD. No association was found between diabetes medication and cataract.
Meaning
The finding that diabetes medication was associated with a reduced risk of OAG and AMD calls for clinical trials to confirm causality.
Abstract
Importance
Recent studies suggest that the diabetes drug metformin has a protective effect on open-angle glaucoma (OAG) and age-related macular degeneration (AMD). However, studies have not addressed the critical issue of confounding by indication, and associations have not been evaluated in a large prospective cohort.
Objective
To determine the association between diabetes medication and the common eye diseases OAG, AMD, and cataract and to evaluate their cumulative lifetime risks in a large cohort study.
Design, Setting, and Participants
This cohort study included participants from 3 independent cohorts from the prospective, population-based Rotterdam Study between April 23, 1990, and June 25, 2014. Participants were monitored for incident eye diseases (OAG, AMD, cataract) and had baseline measurements of serum glucose. Data on diabetes medication use and data from ophthalmologic examinations were gathered.
Exposures
Type 2 diabetes (T2D) and the diabetes medications metformin, insulin, and sulfonylurea derivatives.
Main Outcomes and Measures
Diagnosis and cumulative lifetime risk of OAG, AMD, and cataract.
Results
This study included 11 260 participants (mean [SD] age, 65.1 [9.8]; 6610 women [58.7%]). T2D was diagnosed in 2406 participants (28.4%), OAG was diagnosed in 324 of 7394 participants (4.4%), AMD was diagnosed in 1935 of 10 993 participants (17.6%), and cataract was diagnosed in 4203 of 11 260 participants (37.3%). Untreated T2D was associated with a higher risk of OAG (odds ratio [OR], 1.50; 95% CI, 1.06-2.13; P = .02), AMD (OR, 1.35; 95% CI, 1.11-1.64; P = .003), and cataract (OR, 1.63; 95% CI, 1.39-1.92; P < .001). T2D treated with metformin was associated with a lower risk of OAG (OR, 0.18; 95% CI, 0.08-0.41; P < .001). Other diabetes medication (ie, insulin, sulfonylurea derivates) was associated with a lower risk of AMD (combined OR, 0.32; 95% CI, 0.18 to 0.55; P < .001). The cumulative lifetime risk of OAG was lower for individuals taking metformin (1.5%; 95% CI, 0.01%-3.1%) than for individuals without T2D (7.2%; 95% CI, 5.7%-8.7%); the lifetime risk of AMD was lower for individuals taking other diabetes medication (17.0%; 95% CI, 5.8%-26.8% vs 33.1%; 95% CI, 30.6%-35.6%).
Conclusions and Relevance
Results of this cohort study suggest that, although diabetes was clearly associated with cataract, diabetes medication was not. Treatment with metformin was associated with a lower risk of OAG, and other diabetes medication was associated with a lower risk of AMD. Proof of benefit would require interventional clinical trials.
Introduction
Open-angle glaucoma (OAG), age-related macular degeneration (AMD), and cataract are the leading causes of global blindness.1 Together, these diseases make up approximately 65% of blindness in people older than 50 years worldwide. With the global increase in life expectancy, prevalence numbers for OAG and AMD are expected to double over the next 2 decades.2 Although cataracts can be surgically removed, treatments for OAG and AMD are still insufficient, and blindness attributable to these diseases is irreversible.
Recent studies have suggested a protective effect of metformin on OAG and AMD. Metformin is a well-known insulin-sensitizing drug and first-line treatment for type 2 diabetes (T2D). The mechanism for protection appears independent of its glucose-lowering effects.3,4,5,6 Metformin is an activator of adenosine monophosphate–activated kinase, which induces downstream inhibition of mammalian target of rapamycin complex 1 and nuclear factor kB (NFκB). The transcription factor NFκB is known to play a key role in neurodegenerative diseases.7 Although study results have been promising, the ability to make a true causal inference for metformin in the protection against OAG or AMD has been hampered owing to cross-sectional study designs and the noninclusion of T2D as an indication. Hence, confounding by indication may have distorted these important findings.
The aim of this study was to determine the association between metformin and other diabetes medication (ie, insulin, sulfonylurea derivatives) with OAG, AMD, and cataract in the large, prospective, population-based Rotterdam Study. We also examined the association between untreated T2D with these eye diseases to investigate potential comorbidity. The longitudinal study design allowed us to determine T2D and medication use before the onset of eye disorders and provided the potential to study their cumulative lifetime risks.
Methods
Ethics Statement
The Rotterdam Study has been approved by the medical ethics committee of the Erasmus University Medical Center and by the Dutch Ministry of Health, Welfare, and Sport. The Rotterdam Study has been entered into the Netherlands National Trial Register and into the World Health Organization International Clinical Trials Registry Platform. All participants provided written informed consent (following the declaration of Helsinki) to participate in the study and to have their information obtained from their treating physicians. The participants did not receive any compensation for their participation in the study. The study followed Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Study Population
The study population was derived from 3 independent cohorts from the population-based Rotterdam Study, an epidemiologic study designed to assess the determinants of age-related diseases in the middle-aged and older adult population (45 years or older). Enrollment took place from April 23, 1990, to June 25, 2014, with response rates varying between 65% and 78%. Participants underwent extensive examinations at baseline and 5-year follow-up visits.8 Further details are described in the eMethods in the Supplement. The Rotterdam Study had 14 926 participants. We included the 11 260 participants who had undergone ophthalmologic examinations and had measurements of serum glucose at baseline, continuous monitoring of medications, and at least 1 follow-up visit (eTable 1 in the Supplement). Follow-up duration was calculated from baseline until the last visit with reliable ophthalmic examination or the first visit with the eye disease under study. As ethnicity was homogenous in our study population (98.0% European ancestry), we did not include it as a risk factor (eTable 1 in the Supplement).
Ophthalmic Assessment
The eye examinations included visual acuity testing, Goldmann applanation tonometry (Haag-Streit AG), visual field testing (24-2 Swedish Interactive Thresholding Algorithm standard test, Humphrey field analyzer [HFA], model HFA II 740; [Carl Zeiss]), color fundus photography centered on the macula and optic disc (Topcon TRV-50VT and TRC 50EX [Topcon Optical Company] for the first 3 Rotterdam Study [cohort I] visits and Sony DXC-950P digital camera [Sony Corporation] for the remaining visits), and optical coherence tomography (Topcon Optical Company).
OAG was defined as glaucomatous visual field loss in at least 1 eye with reproducibility of the defect, independent of the intraocular pressure (IOP).9 All other possible causes of field loss were excluded. OAG cases had an open anterior chamber angle and no history or signs of secondary glaucoma. For the IOP (the main risk factor for OAG), 3 measurements were taken from each eye, of which the median value was recorded. For OAG cases, we used the measurement of the affected eye. If both eyes were affected or unaffected, a random eye was selected.
AMD was defined as the presence of intermediate AMD (ie, soft drusen ≥125 μm and/or reticular drusen with or without pigment changes) or late AMD (neovascular macular degeneration or geographic atrophy) according to the Rotterdam Study classification10 on color fundus photographs; AMD features were validated on optical coherence tomography.
Cataract was defined as the evidence of lens opacities and best-corrected visual acuity below 1.0 (20/20), a diagnosis of cataract by an ophthalmologist based on slitlamp examination, or the presence of pseudophakia. The severity or type of cataract was not determined. Participants with aphakia were excluded.
Medication Data
Medication data were obtained from 7 fully automated pharmacies using a centralized computer network in the study district from January 1, 1991, onward. This included the product name, anatomical therapeutic chemical code, the number of dispensed tablets, duration of treatment, average daily dose, and the date of the first dispensed prescription. Participants were considered to be taking metformin if they were diagnosed with T2D and the start date of metformin was before the baseline date (for prevalent cases) or before the date of the last follow-up visit (for incident cases). Metformin therapy was defined as either monotherapy or combination therapy with other diabetes medication. We calculated the duration of metformin therapy from the first prescription date until the date closest to the diagnosis of the eye disease or last eye examination. In case the diagnosis was after the last prescription date of metformin, we used the last prescription date to calculate the duration of metformin therapy. The cumulative dose was calculated by multiplying the mean daily dose with the total number of days of treatment and converted to a cumulative dose in a 1-year time window. Therapy with other diabetes medications included insulin or sulfonylurea derivatives; this could be either monotherapy or a combination of both. Statin and antihypertensive medication were only assessed at baseline.
Definition of T2D
At baseline and follow-up, T2D was diagnosed based on general practitioners’ records, hospital discharge letters, use of blood glucose–lowering medication, or serum glucose measurements (fasting >7.0 mmol/L or nonfasting >11.1 mmol/L; to convert to milligrams per deciliter, divide by 0.0555) collected at center visits.11 All diagnoses of T2D were adjudicated by 2 physicians, and in case of any disagreement, adjudication was applied by an endocrinologist.
Statistical Analysis
Statistical analyses were performed from March 1 to March 31, 2021, using SPSS, version 25.0 (SPSS Inc), and R, version 3.6.1 (R Project). We considered a 2-sided P value < .05 as statistically significant. Differences in baseline characteristics were evaluated using χ2 tests and independent-samples t tests. For cross-sectional analyses, prevalent and incident cases were combined for each disease. For incidence analyses, individuals without any follow-up or with prevalent disease were excluded. To address confounding by indication, we determined the association of T2D with eye diseases. Next, we assessed the association of various diabetes medications with eye diseases in participants with T2D. To do so, we performed multivariable logistic regression analyses to calculate odds ratios (ORs) with corresponding 95% CIs. The reference category was untreated participants with T2D, unless stated otherwise. Models were adjusted for age, sex, body mass index, treatment with statins, and treatment with antihypertensive medications. The models for diabetes medications were additionally adjusted for fasting serum glucose. To study whether the association of metformin with OAG was mediated by IOP, we performed logistic regression analysis with and without adjustment for IOP. The association of metformin with IOP was also analyzed using multivariable linear regression with IOP at the last follow-up visit as the dependent variable, adjusting for covariates as described. To assess whether the association of metformin with OAG, AMD, and cataract was dose-dependent, we performed a trend test, with years of metformin use and cumulative dose during a 1-year time period as continuous variables. Cumulative lifetime risks of incident eye diseases were calculated for diabetes treatments up to age 85 years using the R packages survival and survminer.
Results
Baseline Characteristics
The baseline characteristics of the study participants, stratified according to the diagnosis of eye disease, are presented in the Table. This study included 11 260 participants (mean [SD] age, 65.1 [9.8]; 6610 women [58.7%]; 4650 men [41.3%]). Individuals with T2D who received treatment and had concomitant eye disease were significantly older than those without eye disease (mean [SD] age: OAG, 67.6 [7.4] years vs 63.5 [7.3] years; AMD, 66.5 [9.0] years vs 63.5 [7.5] years; cataract, 67.6 [8.5] years vs 61.2 [6.5] years). Cataract was more frequently present in women than in men (no diabetes, 2153 [63.8%] vs 1219 [36.2%]; untreated diabetes, 215 [58.3%] vs 154 [41.7%]; treated diabetes, 240 [51.9%] vs 222 [48.1%]). Baseline characteristics for participants who underwent ophthalmic examination and entered the analyses did not appear to differ from those excluded from analyses owing to missing eye data (eTable 1 in the Supplement).
Table. Baseline Characteristics of Study Participants.
| Characteristic | Open-angle glaucoma | Cataract | Age-related macular degeneration | |||
|---|---|---|---|---|---|---|
| Absent (n = 7070) | Present (n = 324) | Absent (n = 7057) | Present (n = 4203) | Absent (n = 9058) | Present (n = 1935) | |
| No diabetes, No. (%) | 5697 (80.6) | 264 (81.5) | 6178 (87.5) | 3372 (80.2) | 7787 (86.0) | 1667 (86.1) |
| Age, mean (SD), y | 62.0 (7.9) | 68.7 (8.8) | 64.8 (11.2) | 68.5 (8.5) | 64.2 (9.7) | 69.9 (10.2) |
| Female sex, No. (%) | 3421 (60.0) | 130 (49.2) | 3631 (58.8) | 2153 (63.8) | 4644 (59.6) | 992 (59.5) |
| Male sex, No. (%) | 2276 (40.0) | 134 (50.8) | 2547 (41.2) | 1219 (36.2) | 3143 (40.4) | 675 (40.5) |
| BMI, mean (SD)a | 26.3 (3.7) | 25.9 (3.4) | 26.5 (3.9) | 26.3 (3.7) | 26.4 (3.9) | 26.1 (3.6) |
| Antihypertensives | 1981 (34.8) | 55 (21) | 1897 (30.7) | 757 (22.4) | 2289 (29.4) | 291 (17.5) |
| Statins | 287 (5.0) | 9 (3) | 275 (4.5) | 123 (3.6) | 330 (4.2) | 53 (3) |
| Smoking, current, No. (%) | 1275 (22.4) | 46 (17) | 1579 (25.6) | 645 (19.1) | 1873 (24.1) | 359 (21.5) |
| Diabetes, untreated, No. (%) | 577 (8.2) | 42 (13) | 440 (6.2) | 369 (8.8) | 594 (6.6) | 157 (8.1) |
| Age, mean (SD), y | 63.8 (7.2) | 67.5 (7.3) | 63.9 (8.3) | 68.7 (8.2) | 64.5 (7.6) | 68.4 (8.0) |
| Female sex, No. (%) | 308 (53.4) | 19 (45.2) | 202 (45.9) | 215 (58.3) | 315 (53.0) | 85 (54) |
| Male sex, No. (%) | 269 (46.6) | 23 (54.8) | 238 (54.1) | 154 (41.7) | 279 (47.0) | 72 (45.9) |
| BMI, mean (SD) | 28.1 (4.1) | 27.9 (3.2) | 28.7 (4.2) | 27.9 (3.8) | 28.5 (4.2) | 27.9 (4.0) |
| Antihypertensives | 201 (34.8) | 13 (31) | 168 (38.2) | 92 (25) | 201 (33.8) | 30 (19) |
| Statins | 51 (9) | 5 (12) | 43 (10) | 28 (8) | 52 (9) | 7 (4) |
| Smoking, current, No. (%) | 126 (21.8) | 11 (26) | 118 (26.8) | 70 (19) | 135 (22.7) | 31 (20) |
| Diabetes, treated, No. (%) | 796 (11.3) | 18 (6) | 439 (6.2) | 462 (11.0) | 677 (7.5) | 111 (5.7) |
| Age, mean (SD), y | 63.5 (7.3) | 67.6 (7.4) | 61.2 (6.5) | 67.6 (8.5) | 63.5 (7.5) | 66.5 (9.0) |
| Female sex, No. (%) | 392 (49.2) | 8 (44.0) | 191 (43.5) | 240 (51.9) | 318 (47.0) | 62 (56) |
| Male sex, No. (%) | 404 (50.8) | 10 (55.0) | 248 (56.5) | 222 (48.1) | 359 (53.0) | 49 (44.0) |
| BMI, mean (SD) | 29.6 (4.8) | 26.2 (3.0) | 30.3 (4.9) | 29.3 (4.7) | 29.6 (4.8) | 28.9 (4.7) |
| Antihypertensives | 371 (46.6) | 8 (44) | 265 (60.4) | 175 (37.9) | 306 (45.2) | 47 (42) |
| Statins | 146 (18.3) | 5 (28) | 119 (27.1) | 61 (13) | 127 (18.8) | 23 (21) |
| Smoking, current, No. (%) | 180 (22.6) | 3 (17) | 110 (25.1) | 94 (20) | 161 (23.8) | 26 (23) |
| Metforminb | 618 (77.6) | 8 (44) | 359 (81.8) | 295 (63.9) | 531 (78.4) | 86 (77) |
| Other diabetes medicationb,c | 178 (22.4) | 10 (56) | 80 (18) | 167 (36.1) | 146 (21.6) | 25 (23) |
Abbreviation: BMI, body mass index.
Calculated as weight in kilograms divided by height in meters squared.
Untreated participants used as control group.
Diabetes medication for each group: open-angle glaucoma (130 of 188 [69.1%] sulfonylurea; 46 of 188 [24.5%] insulin; 12 of 188 [6.4%] combination); age-related macular degeneration (125 of 171 [73.1%] sulfonylurea; 39 of 171 [22.8%] insulin; 7 of 171 [4.1%] combination); cataract (176 of 247 [71.3%] sulfonylurea; 57 of 247 [23.1%] insulin; 14 of 247 [5.7%] combination).
Overall, T2D was diagnosed in 2406 participants (28.4%). Metformin had been prescribed to 1388 of 2406 patients (57.7%) with T2D; 501 of 2406 (20.8%) received other diabetes medication, and 517 of 2406 (21.5%) received lifestyle advice only.
OAG was diagnosed in 324 of 7394 participants (4.4%), in 42 of 619 participants (7%) with untreated T2D, and in 8 of 626 participants (1%) with T2D who were treated with metformin. AMD was diagnosed in 1935 of 10 993 participants (17.6%), in 150 of 751 participants (20.0%) with untreated T2D, and in 86 of 617 participants (14%) with T2D who were treated with metformin. Cataract was diagnosed in 4203 of 11 260 participants (37.3%), in 369 of 809 participants (45.6%) with untreated T2D, and in 295 of 654 participants (45.1%) with T2D who were treated with metformin.
Diabetes and Eye Diseases
We first assessed the association between diabetes and eye diseases. We observed a positive association between T2D and disease risk for OAG (OR, 1.50; 95% CI, 1.06-2.13; P = .02), AMD (OR, 1.35; 95% CI, 1.11-1.64, P = .003), and cataract (OR, 1.63; 95% CI, 1.39-1.92; P < .001).
Diabetes Medication and Eye Diseases
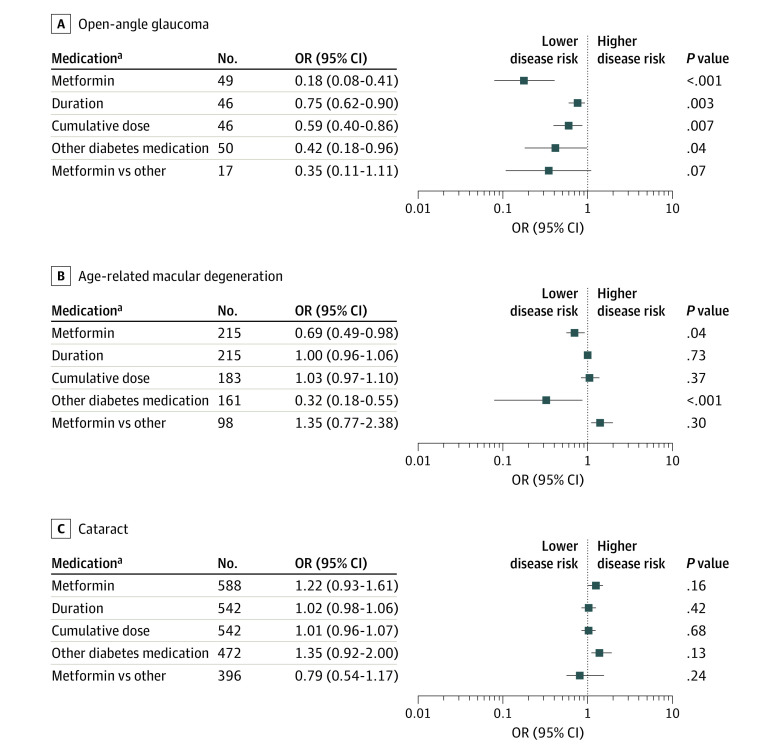
We subsequently investigated diabetes medication and eye diseases. With respect to OAG, metformin treatment was associated with a lower disease risk (OR, 0.18; 95% CI, 0.08-0.41; P < .001) (Figure 1) compared with no treatment with diabetes medication. Association was similar albeit nonsignificant (OR, 0.35; 95% CI, 0.11-1.11; P = .07) when compared to other diabetes medication. Moreover, 1-year-longer treatment with and a 1-mg-per-day-higher cumulative dose of metformin were associated with a lower OAG risk (OR, 0.75; 95% CI, 0.62-0.90, P = .003; OR, 0.59; 95% CI, 0.40-0.86, P = .007, respectively). Adjustment of previous analyses for IOP did not change the risk estimates (eTable 2 in the Supplement). We observed no association between metformin treatment and IOP (β = 0.174; 95% CI, −0.307 to 0.656; P = .48) (eTable 2 in the Supplement). For participants with T2D, treatment with other diabetes medication was associated with a lower OAG risk (OR, 0.42; 95% CI, 0.18-0.96; P = .04), than no treatment with diabetes medication.
Figure 1. Association Between Diabetes Medication and Eye Diseases .
Data presented as odds ratios (ORs) with 95% CIs for open-angle glaucoma (A), age-related macular degeneration (B), and cataract (C). All analyses are adjusted for age, sex, body mass index, use of antihypertensives, use of statins, and fasting serum glucose. The reference category were participants with diabetes who were not receiving treatment, unless otherwise stated.
aOnly participants with diabetes.
Metformin was associated with a lower risk of AMD (OR, 0.69; 95% CI, 0.49-0.98; P < .04) (Figure 1). Prolonged treatment (per year) and a higher cumulative dose (per milligram per day) did not decrease the risk further (OR, 1.00; 95% CI, 0.96-1.06; P = .73; OR, 1.03; 95% CI, 0.97-1.10; P = .37, respectively). Other diabetes medication was also associated with a lower AMD risk (OR, 0.32; 95% CI, 0.18-0.55; P < .001). Of these, both sulfonylurea and insulin contributed to this association (eTable 3 in the Supplement). The association of metformin was not significantly different from that of other diabetes medication (OR, 1.35; 95% CI, 0.77-2.38; P = .30). None of the diabetes medication was associated with the risk of cataract (Figure 1).
Lifetime Risk of Eye Diseases
Over a mean follow-up period of 9.9 (range, 1.0-24.5) years for OAG, 8.2 (range, 1.1-21.7) years for AMD, and 7.9 (range, 1.3-21.5) years for cataract, a total of 4191 participants developed incident eye diseases (OAG, 182 [4.3%]; AMD, 869 [20.7%]; cataract, 3140 [74.9%]). Of participants with incident eye diseases, 285 received metformin (OAG, 5 [1.8%]; AMD, 52 [18.2%]; cataract, 228 [80.0%]), and 145 received other diabetes medication (OAG, 8 [5.5%]; AMD, 10 [6.9%]; cataract, 127 [87.6%]). Figure 2 shows the cumulative incidence of OAG, AMD, and cataract among participants treated with metformin, participants treated with other diabetes medication, participants with untreated T2D, and participants without diabetes. The lifetime risk, or cumulative incidence at age 85 years, of OAG for participants taking metformin was lower (1.5%; 95% CI, 0.01%-3.1%) than that of participants with untreated diabetes (10.1%; 95% CI, 5.2%-14.9%) and participants taking other diabetes medication (8.0%; 95% CI, 0.9%-14.6%). The risk of OAG in individuals without diabetes was 7.2% (95% CI, 5.7%-8.7%).
Figure 2. Cumulative Incidence of Eye Diseases as a Function of Age.
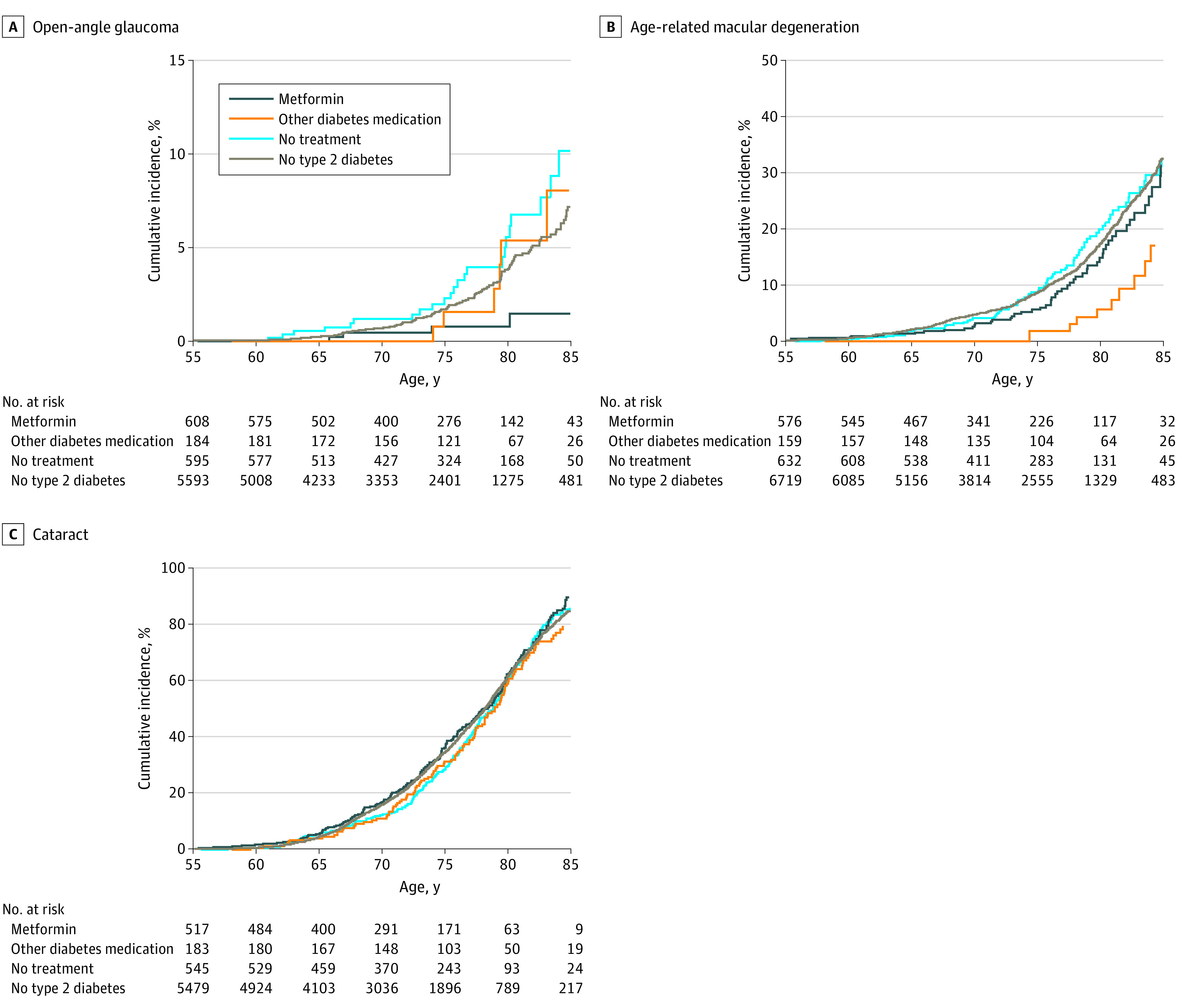
Kaplan-Meier curves showing cumulative incidence of open-angle glaucoma (A), age-related macular degeneration (B), and cataract (C) of participants with diabetes treated with metformin and those treated with other diabetes medications, untreated participants with diabetes, and individuals without type 2 diabetes.
The lifetime risk of AMD for participants taking metformin (31.5%; 95% CI, 21.4%-40.2%) was similar to that of participants with untreated diabetes (32.4%; 95% CI, 24.5%-39.6%) and participants without diabetes (33.1%; 95% CI, 30.6%-35.6%). Participants taking other diabetes medication (17.0%; 95% CI, 5.8%-26.8%) had the lowest lifetime risk of AMD, lower than that of individuals without diabetes. Of the diabetes medication, only sulfonylurea treatment had a significantly lower lifetime risk (11.5%; 95% CI, 0.25%-20.0%; P < .001) than the population of individuals without diabetes (eFigure in the Supplement).
For cataract, the lifetime risk did not differ between participants treated with metformin (90.5%; 95% CI, 83.7%-94.4%), other diabetes medication (79.2%; 95% CI, 70.1%-85.5%), untreated participants with diabetes (85.8%; 95% CI, 80.2%-89.9%), or individuals without diabetes (85.1%; 95% CI, 83.3%-86.6%).
Discussion
In this prospective, population-based, cohort study, we found that metformin had the strongest inverse association with OAG followed by AMD but was not associated with cataract. Other diabetes medication, including insulin and sulfonylurea, showed a similar association, but appeared more protective against AMD. Our findings accentuate the potential role of diabetes medication in the pathogenesis of OAG and AMD.
The association between T2D and OAG had been investigated previously but had yielded inconsistent results.12,13,14 We found a significant association between T2D and the risk of OAG. Two earlier reports found a lower risk of OAG with metformin treatment.15,16 Our data confirmed this association and addressed confounding by indication. Metformin treatment was not associated with IOP, which suggests that other mechanisms are involved. Moreover, we found that prolonged treatment with and higher cumulative dose of metformin were associated with a lower OAG risk.
A number of studies have listed T2D as a potential risk factor for AMD, although results were inconsistent for this disease as well.17 In this large, population-based study, we showed that untreated T2D was associated with a greater than 30% higher risk of AMD. With respect to diabetes medication, several studies found that metformin had a protective effect in individuals at risk of AMD, but not for other forms of diabetes medication.18 We not only found that metformin was associated with a lower risk of AMD, but we also observed lower frequencies of AMD for those taking other diabetes medication. Our incidence analyses suggest that metformin treatment was mainly associated with a later onset of AMD but does not alter lifetime risk. Interestingly, those treated with other diabetes medication did show lower lifetime risks of AMD, most prominently for sulfonylurea.
T2D and its association with cataract has been known for some time.19 The reported prevalence of cataract is 2 to 4 times higher in patients with T2D,20 similar to our findings. Few studies investigated diabetes medications and cataract but, in concordance with us, did not find clear associations.21
Biological studies have demonstrated that metformin affects multiple pathways that may affect the aging process. Metformin acts directly and indirectly on several targets, including adenosine monophosphate–activated kinase, mammalian target of rapamycin, and sirtuin 1, affecting survival, stress defense, autophagy, oxidative stress, and protein synthesis. As a result, inflammatory reactions are inhibited.3,4,5,6 Oxidative processes and inflammation play a key role in the pathogenesis of both OAG and AMD.22,23 Sulfonylurea played a more prominent role than metformin in AMD. This class of diabetes medications was also shown to have anti-inflammatory effects, but through inhibiting interleukin 1β in the nucleotide-binding and oligomerization domain–like receptor family protein inflammasome pathway.24,25 These effects are intriguing and require functional studies on disease-related pathways.
Strengths and Limitations
The strengths of this study included its prospective population-based design, the large study sample, the access to extensive automated prescription records, prospective ascertainment of multiple eye diseases, and sensitivity analyses using the Bradford Hill criteria. Importantly, we addressed confounding by indication by assessing the association between eye diseases and T2D in persons without medication. We found opposite outcomes for indication and medication, which makes independent associations more likely. To reduce the potential association of other confounders, we adjusted our models for statin use and antihypertensive medications, of which the treatment is associated with severity of T2D. We also considered risk factors, such as smoking, ethnicity, and education level. Additional adjustment for smoking did not change the results of any of our analyses. Prior work did not show a significant association between education level and OAG26 or AMD.27 Reversed causality in our study is not likely, as participants who were treated with diabetes medication after the development of the eye diseases were excluded. Collider bias is unlikely as we found no differences in the determinants under study in those with or without eye data. We reviewed our findings against the Bradford Hill viewpoints.28 Seven of 9 criteria were met, ie, strength of association, specificity, temporality, plausibility, coherence, biological gradient, and analogy. Although this favors a causal association, external replication and functional proof are still needed.
Our study had limitations as well. The possibility of residual confounding cannot be excluded. Although the use of a population-based design yielded highly representative participants for the population, it limited the number of participants with OAG,9 especially those taking metformin. However, by performing multivariable logistic regression analyses, we were able to include both prevalent and incident cases. To have visual representation of disease progression, we did calculate cumulative lifetime risks for the incident eye diseases. Moreover, compliance of prescribed medication was not guaranteed, which may have led to an overestimation of medication use. Nevertheless, this is likely nondifferential misclassification that causes a bias toward the null hypothesis, underestimating the true association.
Conclusions
Results of this cohort study suggest that diabetes medication was associated with a reduced risk of OAG and AMD; however, no association was found between treatment with diabetes medication and cataract. Metformin was associated with a lower risk of OAG, and treatment with other diabetes medication was associated with a lower risk of AMD. The magnitude of these associations warrants interventional clinical trials.
eMethods. Rotterdam Study I/II/III Description
eTable 1. Baseline Characteristics of Participants Without and With Eye Examination
eTable 2. Associations of Metformin and Intraocular Pressure
eTable 3. Associations of Separate Diabetes Medications and Age-Related Macular Degeneration
eFigure. Cumulative Lifetime Risk of Development of Age-Related Macular Degeneration
References
- 1.GBD 2019 Blindness and Vision Impairment Collaborators; Vision Loss Expert Group of the Global Burden of Disease Study . Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9(2):e144-e160. doi: 10.1016/S2214-109X(20)30489-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tham Y-C, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081-2090. doi: 10.1016/j.ophtha.2014.05.013 [DOI] [PubMed] [Google Scholar]
- 3.Howell JJ, Hellberg K, Turner M, et al. Metformin inhibits hepatic mTORC1 signaling via dose-dependent mechanisms involving AMPK and the TSC complex. Cell Metab. 2017;25(2):463-471. doi: 10.1016/j.cmet.2016.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prattichizzo F, Giuliani A, Mensà E, et al. Pleiotropic effects of metformin: shaping the microbiome to manage type 2 diabetes and postpone ageing. Ageing Res Rev. 2018;48:87-98. doi: 10.1016/j.arr.2018.10.003 [DOI] [PubMed] [Google Scholar]
- 5.Saisho Y. Metformin and inflammation: its potential beyond glucose-lowering effect. Endocr Metab Immune Disord Drug Targets. 2015;15(3):196-205. doi: 10.2174/1871530315666150316124019 [DOI] [PubMed] [Google Scholar]
- 6.Zheng Z, Chen H, Li J, et al. Sirtuin 1-mediated cellular metabolic memory of high glucose via the LKB1/AMPK/ROS pathway and therapeutic effects of metformin. Diabetes. 2012;61(1):217-228. doi: 10.2337/db11-0416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sivandzade F, Prasad S, Bhalerao A, Cucullo L. NRF2 and NF-қB interplay in cerebrovascular and neurodegenerative disorders: molecular mechanisms and possible therapeutic approaches. Redox Biol. 2019;21:101059. doi: 10.1016/j.redox.2018.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikram MA, Brusselle GGO, Murad SD, et al. The Rotterdam Study: 2018 update on objectives, design, and main results. Eur J Epidemiol. 2017;32(9):807-850. doi: 10.1007/s10654-017-0321-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Springelkamp H, Wolfs RC, Ramdas WD, et al. Incidence of glaucomatous visual field loss after 2 decades of follow-up: the Rotterdam Study. Eur J Epidemiol. 2017;32(8):691-699. doi: 10.1007/s10654-017-0270-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thee EF, Meester-Smoor MA, Luttikhuizen DT, et al. ; EyeNED Reading Center . Performance of classification systems for age-related macular degeneration in the Rotterdam Study. Transl Vis Sci Technol. 2020;9(2):26-26. doi: 10.1167/tvst.9.2.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmadizar F, Ochoa-Rosales C, Glisic M, Franco OH, Muka T, Stricker BH. Associations of statin use with glycaemic traits and incident type 2 diabetes. Br J Clin Pharmacol. 2019;85(5):993-1002. doi: 10.1111/bcp.13898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laville V, Kang JH, Cousins CC, et al. ; UK Biobank; International Glaucoma Genetics Consortium; NEIGHBORHOOD Consortium . Genetic correlations between diabetes and glaucoma: an analysis of continuous and dichotomous phenotypes. Am J Ophthalmol. 2019;206:245-255. doi: 10.1016/j.ajo.2019.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Voogd S, Ikram MK, Wolfs RC, et al. Is diabetes mellitus a risk factor for open-angle glaucoma? the Rotterdam Study. Ophthalmology. 2006;113(10):1827-1831. doi: 10.1016/j.ophtha.2006.03.063 [DOI] [PubMed] [Google Scholar]
- 14.Lavaju P, Shah S, Sharma S, Maskey R. Diabetes mellitus and the risk of primary open-angle glaucoma. Nepal J Ophthalmol. 2017;9(18):17-23. doi: 10.3126/nepjoph.v9i1.17526 [DOI] [PubMed] [Google Scholar]
- 15.Lin HC, Stein JD, Nan B, et al. Association of geroprotective effects of metformin and risk of open-angle glaucoma in persons with diabetes mellitus. JAMA Ophthalmol. 2015;133(8):915-923. doi: 10.1001/jamaophthalmol.2015.1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maleškić S, Kusturica J, Gušić E, et al. Metformin use associated with protective effects for ocular complications in patients with type 2 diabetes—observational study. Acta Med Acad. 2017;46(2):116-123. doi: 10.5644/ama2006-124.196 [DOI] [PubMed] [Google Scholar]
- 17.Chen X, Rong SS, Xu Q, et al. Diabetes mellitus and risk of age-related macular degeneration: a systematic review and meta-analysis. PLoS One. 2014;9(9):e108196. doi: 10.1371/journal.pone.0108196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romdhoniyyah DF, Harding SP, Cheyne CP, Beare NAV. Metformin—a potential role in age-related macular degeneration: a systematic review and meta-analysis. Ophthalmol Ther. 2021;10(2):245-260. doi: 10.1007/s40123-021-00344-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiziltoprak H, Tekin K, Inanc M, Goker YS. Cataract in diabetes mellitus. World J Diabetes. 2019;10(3):140-153. doi: 10.4239/wjd.v10.i3.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein BE, Klein R, Moss SE. Prevalence of cataracts in a population-based study of persons with diabetes mellitus. Ophthalmology. 1985;92(9):1191-1196. doi: 10.1016/S0161-6420(85)33877-0 [DOI] [PubMed] [Google Scholar]
- 21.Becker C, Schneider C, Aballéa S, et al. Cataract in patients with diabetes mellitus—incidence rates in the UK and risk factors. Eye (Lond). 2018;32(6):1028-1035. doi: 10.1038/s41433-017-0003-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cameron AR, Morrison VL, Levin D, et al. Anti-inflammatory effects of metformin irrespective of diabetes status. Circ Res. 2016;119(5):652-665. doi: 10.1161/CIRCRESAHA.116.308445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Datta S, Cano M, Ebrahimi K, Wang L, Handa JT. The impact of oxidative stress and inflammation on RPE degeneration in nonneovascular AMD. Prog Retin Eye Res. 2017;60:201-218. doi: 10.1016/j.preteyeres.2017.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawahara Y, Kaneko T, Yoshinaga Y, et al. Effects of sulfonylureas on periodontopathic bacteria-induced inflammation. J Dent Res. 2020;99(7):830-838. doi: 10.1177/0022034520913250 [DOI] [PubMed] [Google Scholar]
- 25.Coll RC, Robertson AA, Chae JJ, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21(3):248-255. doi: 10.1038/nm.3806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramdas WD, Wolfs RC, Hofman A, de Jong PT, Vingerling JR, Jansonius NM. Lifestyle and risk of developing open-angle glaucoma: the Rotterdam Study. Arch Ophthalmol. 2011;129(6):767-772. doi: 10.1001/archophthalmol.2010.373 [DOI] [PubMed] [Google Scholar]
- 27.de Koning-Backus APM, Buitendijk GHS, Kiefte-de Jong JC, et al. Intake of vegetables, fruit, and fish is beneficial for age-related macular degeneration. Am J Ophthalmol. 2019;198:70-79. doi: 10.1016/j.ajo.2018.09.036 [DOI] [PubMed] [Google Scholar]
- 28.Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58(5):295-300. doi: 10.1177/003591576505800503 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Rotterdam Study I/II/III Description
eTable 1. Baseline Characteristics of Participants Without and With Eye Examination
eTable 2. Associations of Metformin and Intraocular Pressure
eTable 3. Associations of Separate Diabetes Medications and Age-Related Macular Degeneration
eFigure. Cumulative Lifetime Risk of Development of Age-Related Macular Degeneration