This randomized clinical trial assesses whether adding denosumab to anthracycline/taxane-containing neoadjuvant chemotherapy increases the pathological complete response rate and which schedule is more effective.
Key Points
Question
Does the addition of denosumab to anthracycline/taxane-containing neoadjuvant chemotherapy increase pathological complete response (pCR) rate, and which nab-paclitaxel schedule is more effective in the neoadjuvant setting?
Findings
In this randomized clinical trial of 780 patients with breast cancer, the pCR rate was 41% with denosumab vs 43% without. Despite greater toxicity, nab-paclitaxel at a dosage of 125 mg/m2 weekly resulted in a significantly higher pCR rate of 45% vs 39% with a dosage of 125 mg/m2 days 1 and 8 every 3 weeks.
Meaning
Denosumab added to an anthracycline/taxane-based neoadjuvant chemotherapy did not improve pCR rates, while nab-paclitaxel weekly was more effective than the every-3-weeks regimen as it significantly increased the pCR rates, especially in triple-negative breast cancer.
Abstract
Importance
Adjuvant denosumab might improve disease-free survival in hormone receptor (HR)–positive primary breast cancer (BC). The optimal neoadjuvant nab-paclitaxel schedule in terms of efficacy and safety is unclear.
Objective
To determine whether adding denosumab to anthracycline/taxane-containing neoadjuvant chemotherapy (NACT) increases the pathological complete response (pCR) rate and which nab-paclitaxel schedule is more effective in the NACT setting.
Design, Setting, and Participants
The GeparX was a multicenter, prospective, open-label, phase 2b, 2 × 2 randomized clinical trial conducted by GBG and AGO-B at 38 German sites between February 2017 and March 2019. The analysis data set was locked September 4, 2020; analysis was completed November 13, 2020. Patients had unilateral or bilateral primary BC, stage cT2-cT4a-d or cT1c, with either clinically node-positive or pathologically node-positive or HR-negative disease, or Ki-67 proliferation index greater than 20%, or ERBB2 (formerly HER2)–positive BC.
Interventions
Patients were randomized to receive or not receive denosumab, 120 mg subcutaneously every 4 weeks for 6 cycles, and either nab-paclitaxel, 125 mg/m2 weekly for 12 weeks or days 1 and 8 every 3 weeks for 4 cycles (8 doses), followed by 4 cycles of epirubicin/cyclophosphamide, 90/600 mg/m2 (every 2 weeks or every 3 weeks). Carboplatin was given in triple-negative BC (TNBC), and trastuzumab biosimilar ABP980 plus pertuzumab was given in ERBB2-positive BC (ERBB2-positive substudy).
Main Outcomes and Measures
The primary outcome was pCR rates between arms for each randomization.
Results
A total of 780 female (n = 779) and male (n = 1) patients (median [range] age, 49.0 [22-80] years) were randomized to the 4 treatment groups. The pCR (ypT0 ypN0) rate was 41.0% (90% CI, 37%-45%) with denosumab vs 42.8% (90% CI, 39%-47%) (P = .58) without denosumab, irrespective of BC subtype. Nab-paclitaxel weekly resulted in a significantly (significance level of α = .10) higher pCR rate of 44.9% (90% CI, 41%-49%) vs 39.0% (90% CI, 35%-43%) (P = .06) with nab-paclitaxel days 1 and 8 every 3 weeks. The pCR rates for nab-paclitaxel schedules in subgroups were only significantly different for TNBC (60.4% vs 50.0%; P = .06). Grade 3 to 4 toxic effects did not differ with or without denosumab. Nonhematologic toxic effects of grade 3 to 4 were higher with nab-paclitaxel weekly (33.7% vs 24.1%; P = .004).
Conclusions and Relevance
In this randomized clinical trial, denosumab added to anthracycline/taxane-based NACT did not improve pCR rates. Nab-paclitaxel at a dosage of 125 mg/m2 weekly significantly increased the pCR rate compared with the days 1 and 8, every-3-weeks schedule overall and in TNBC, but generated higher toxicity.
Trial Registration
ClinicalTrials.gov Identifier: NCT02682693
Introduction
Receptor activator of nuclear factor κB (RANK) and its ligand (RANKL) have a regulatory function in bone metabolism, the immune system, and development of lactating breast epithelial cells.1 Both are involved in tumorigenesis and have regenerative potential in mammary stem cell development.2 The RANKL inhibitor denosumab is approved for the treatment of osteoporosis and prevention of skeletal-related events in patients with bone metastases.3 Previous studies using denosumab or bisphosphonates as adjuvant therapy demonstrated reduced rates of bone metastases and improved breast cancer (BC) survival, alongside a prevention of treatment-induced bone loss and a reduction of fractures.4,5,6,7,8 To our knowledge, there are no data on the use of denosumab in the neoadjuvant setting, where the pathological complete response (pCR) rate can be indicative of long-term survival.9,10
The GeparSepto study showed a significantly higher pCR11 in triple-negative BC (TNBC) and improved disease-free survival (DFS)12 (TNBC and hormone receptor–positive BC) using weekly nab-paclitaxel compared with weekly solvent-based paclitaxel. However, this could not be confirmed in the Evaluating Treatment With Neoadjuvant Abraxane (ETNA) study,13,14 which used a different nab-paclitaxel schedule. The ADAPT study demonstrated that nab-paclitaxel administered days 1 and 8 every 3 weeks in combination with carboplatin in TNBC is feasible, leading to a pCR rate of 45.9% (95% CI, 0.38-0.54) after 12 weeks of treatment.15
It still remains unclear which regimen is preferred for nab-paclitaxel in the early setting balancing efficacy and toxicity, especially in combination with carboplatin. The GeparX trial used a 2 × 2 factorial design to investigate whether the addition of denosumab increases the pCR rate and which is the optimal schedule of nab-paclitaxel in patients with early BC.
Methods
Patient Selection and Study Design
GeparX was a multicenter, prospective, 2 × 2 randomized, open-label phase 2b study conducted by the German Breast Group (GBG) in collaboration with the Breast Study Group of the Arbeitsgemeinschaft für Gynäkologische Onkologie (AGO-B) at 38 German sites. The study protocol (Supplement 1; statistical analysis plan in Supplement 2) was approved by the ethics committees/institutional review boards and relevant health authorities. This report followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Patients with unilateral or bilateral primary BC (palpable size of ≥2 cm or a sonographic size of ≥1 cm) were eligible following written informed consent. Patients had to have stage cT2 to cT4a-d or cT1c with either clinically node-positive or pathologically node-positive (by biopsy; sentinel node biopsy was not recommended) or estrogen receptor (ER)–negative/progesterone receptor (PR)–negative or Ki-67 proliferation index greater than 20% or ERBB2 (formerly HER2)–positive BC. The ER, PR, and ERBB2 status was centrally assessed; ER negative was defined as less than 1% stained cells, PR negative as less than 10% stained cells, and ERBB2 positive as immunohistochemistry 3+ in greater than 10% of immunoreactive cells or fluorescence in situ hybridization or equivalent test ratio 2.0 or greater. Central pathology also included assessment of Ki-67, stromal tumor-infiltrating lymphocytes (sTILs), and RANK/RANKL status. Lymphocyte-predominant BC (LPBC) was defined as sTILs greater than 50%. With amendment 1, the open-label nonrandomized ERBB2-positive substudy investigating the safety of the trastuzumab biosimilar ABP 980 in combination with pertuzumab was implemented.
Patients were first randomized centrally in a 1:1 ratio to either denosumab or no denosumab in addition to neoadjuvant chemotherapy (NACT), stratified by LPBC, BC subtype, and epirubicin and cyclophosphamide (EC) schedule. Secondarily, patients were randomized 1:1 to nab-paclitaxel weekly or nab-paclitaxel days 1 and 8 every 3 weeks both followed by EC (Figure 1). The first randomization (denosumab yes vs no) was an additional stratification factor for the second randomization (nab-paclitaxel schedule). Further eligibility criteria and randomization details are provided in the eAppendix in Supplement 3.
Figure 1. GeparX Study Design.
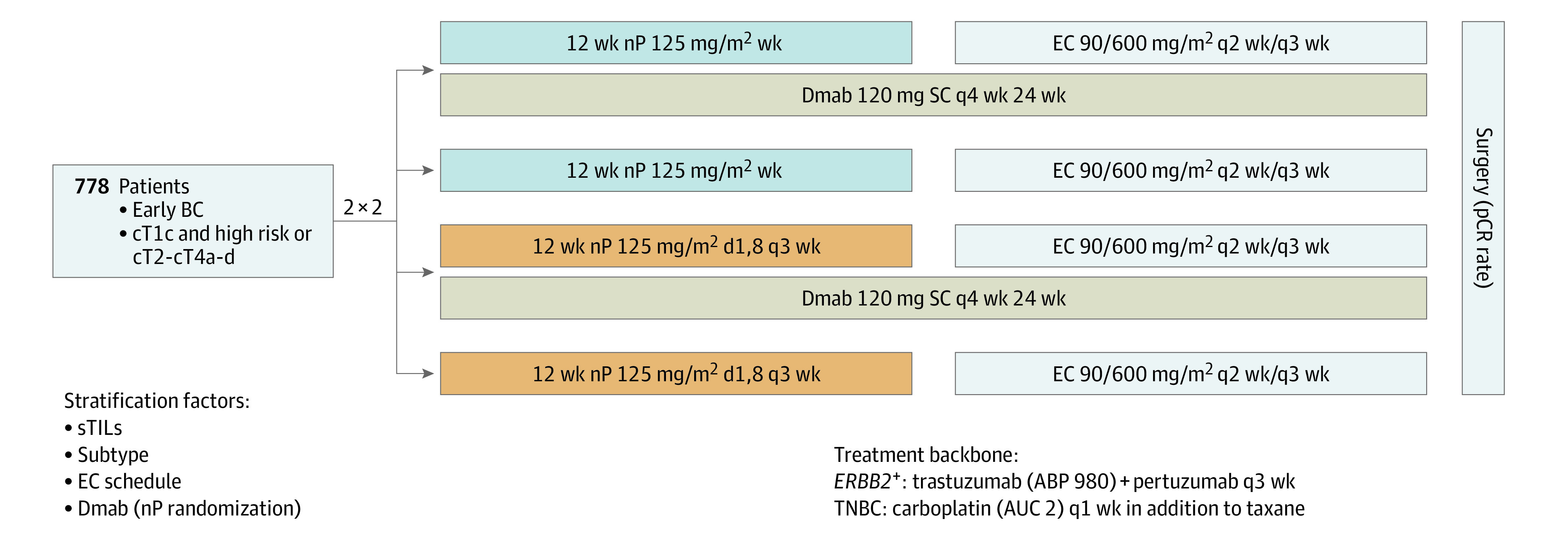
AUC indicates area under the curve; BC, breast cancer; Dmab, denosumab; EC, epirubicin and cyclophosphamide; nP, nab-paclitaxel; pCR, pathological complete response; SC, subcutaneously; sTILs, stromal tumor-infiltrating lymphocytes; TNBC, triple-negative BC; wk, weekly.
Treatment
Denosumab, 120 mg subcutaneously, was given every 4 weeks for 6 cycles. Nab-paclitaxel, 125 mg/m2, was given weekly for 12 weeks or days 1 and 8 every 3 weeks for 4 cycles (8 doses) both followed by EC, 90/600 mg/m2 every 2 weeks or 3 weeks, according to investigator’s choice.
A total of 150 patients with ERBB2-positive disease received trastuzumab biosimilar ABP 980, 6 mg/kg (loading dose, 8 mg/kg), and pertuzumab, 420 mg (840 mg), every 3 weeks for 8 cycles simultaneously to nab-paclitaxel and EC. Carboplatin, area under the curve 2, was given weekly for 12 weeks in addition to both nab-paclitaxel regimens in patients with TNBC.
Study End Points
The coprimary objectives were to compare pCR rates of neoadjuvant treatment with vs without denosumab and with weekly nab-paclitaxel vs days 1 and 8 every-3-weeks nab-paclitaxel.
The primary end point pCR was defined as no microscopic evidence of residual viable invasive or noninvasive tumor cells in any resected specimens of the breast and axillary nodes (ypT0 ypN0). The pCR was centrally evaluated based on local histopathology reports. Secondary end points included the pCR rate according to other definitions (ypT0/Tis ypN0, ypT0 ypN0/+, ypT0/is ypN0/+, ypT[any] ypN0) and in stratified subgroups. Additional secondary end points were breast conservation rate, tolerability, and treatment adherence, including frequency of discontinuations, dose delays, and dose reductions. Toxicity reported as adverse events (AEs) was based on the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. Functional Assessment of Cancer Therapy–Taxane (FACT-Taxane) was used to assess patient-reported outcomes (PROs). Detailed definitions of end points are provided in the eAppendix in Supplement 3.
Statistical Analysis
Sample size calculation was based on the primary end point pCR comprising both randomizations. Assuming an 11% increase in pCR by denosumab (35% to 46%) and a 9% increase by using weekly nab-paclitaxel (36% to 45%) without interaction between treatments, recruitment of 778 patients was planned to have 92% power to the 2-sided significance level α = .10 for the denosumab question and 80% power to the 2-sided significance level α = .10 for the nab-paclitaxel question. Coprimary objectives were tested according to the improved Bonferroni procedure16 comparing the smaller of the 2 P values with α = .10 and the larger (if the first one is significant) with α = .20 to keep the overall significance level of the study of α = .20. The significance level for all further analyses was set to a 2-sided α = .05. All randomized patients were included in the intent-to-treat population; patients who did not receive surgery were counted as no pCR.
The pCR end points and breast conservation were summarized as number and percentage of patients for each treatment group and compared between groups using the 2-sided Cochran-Mantel-Haenszel χ2 test stratified by the stratification factors. Two-sided 90% (only for primary and secondary pCR definitions) and 95% CIs were calculated according to Pearson and Clopper,17 and odds ratios (ORs) between treatment groups from univariate logistic regression were reported for all of them. Subgroup analyses of the primary end point were performed for stratification factors; interaction between treatment and subgroup was assessed using Breslow-Day test for binary subgroups and Wald test for interaction term for biological subtype. Multivariable logistic regression analysis adjusted for stratification factors and predefined covariates. The incidence of treatment discontinuations and modifications was compared using Fisher exact test. Further details on statistical analysis and software are provided in the eAppendix in Supplement 3.
Results
Baseline
Between February 2017 and March 2019, 1017 female and male patients were screened for eligibility, and 780 female (n = 779) and male (n = 1) patients were randomized to denosumab (n = 390) or no denosumab (n = 390) (median [range] age at study entry, 49.0 [22-80] years). Subsequently, patients were randomized to nab-paclitaxel weekly (n = 390) or to nab-paclitaxel days 1 and 8 every 3 weeks (n = 390) (Figure 2). Baseline patient and tumor characteristics were well balanced between the treatment arms (eTable 1 in Supplement 3). However, there was an imbalance between the 4 treatment groups regarding stratified BC subtypes (eTable 2 in Supplement 3). A total of 435 (56.3%) patients had cT2 tumors, 46 (6.0%) had cT3/T4 tumors, and 298 (38.3%) had clinically node-positive tumors. A total of 519 (66.5%) patients had G3 tumors; 648 (83.1%) tumors showed Ki-67 proliferation index greater than 20%; and 62 (7.9%) tumors had sTILs greater than 50%. A total of 153 patients (19.6%) had ERBB2-positive disease; 310 (39.7%) had ERBB2-negative/HR-positive disease; and 317 (40.6%) had TNBC.
Figure 2. CONSORT Diagram of the GeparX Study.
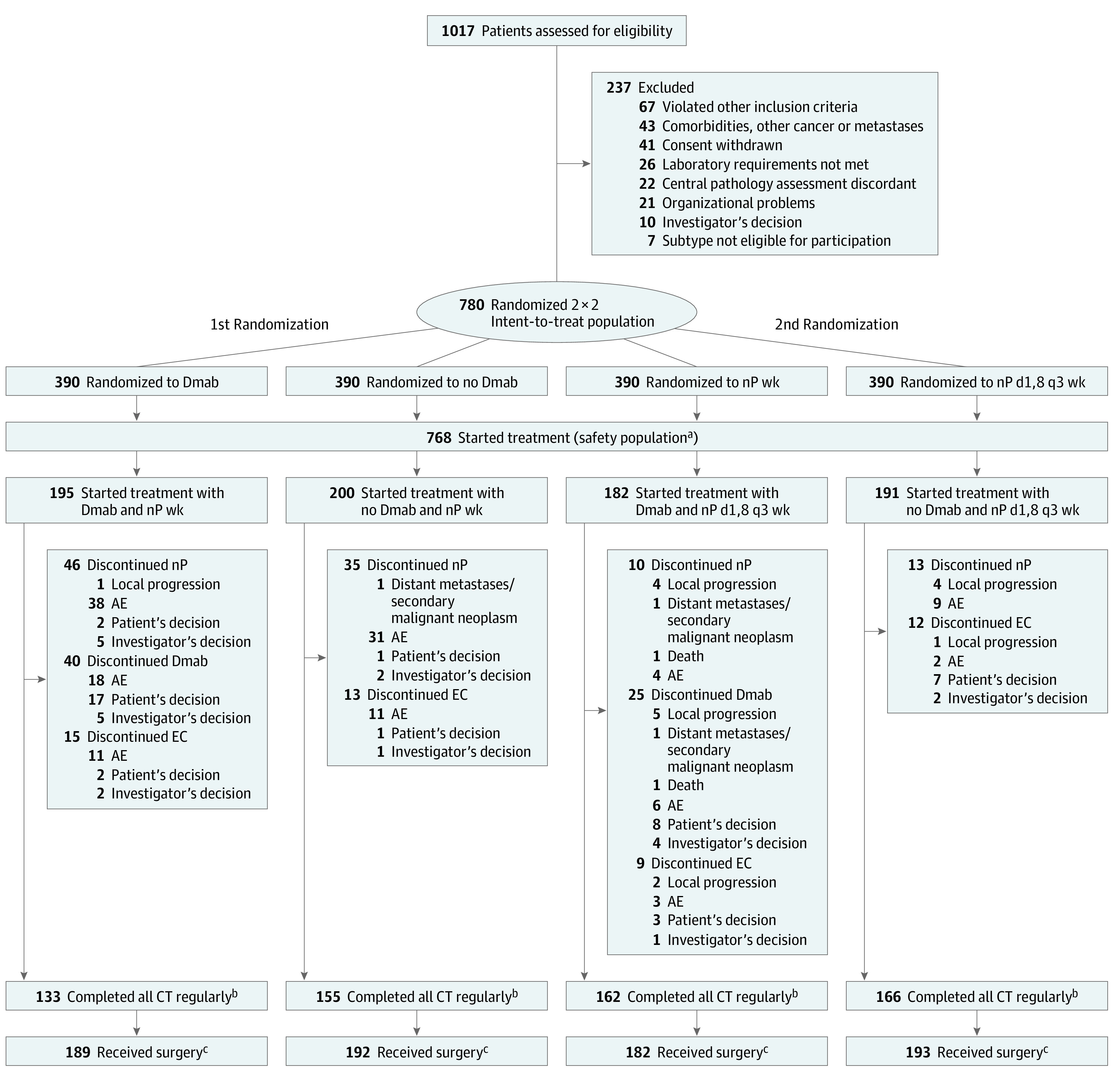
AE indicates adverse event; CT, chemotherapy; Dmab, denosumab; EC, epirubicin and cyclophosphamide; nP, nab-paclitaxel; wk, weekly.
aThree patients did not start Dmab and were analyzed for safety and adherence in no Dmab arm; in 7 patients of nP d1,8 q3 wk arm, there was no pause at day 15 in at least 2 cycles, and they were analyzed for safety and adherence in the nP weekly arm.
bIncludes patients who completed both nP and EC.
cOf patients “as randomized.”
Efficacy
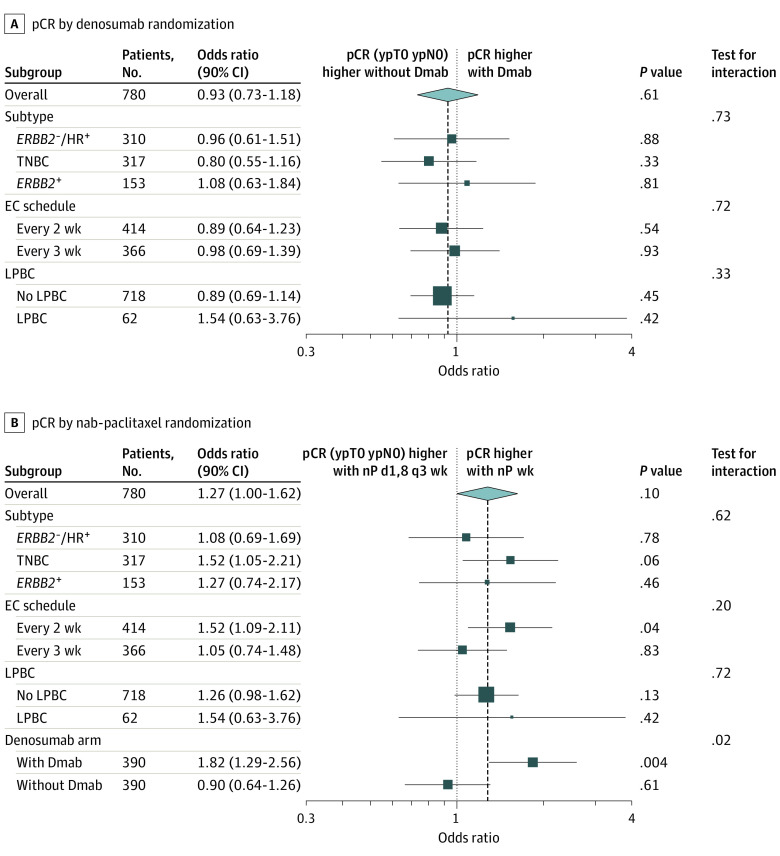
A total of 756 of 780 (96.9%) patients underwent surgery. The pCR (ypT0 ypN0) rate with denosumab was 41% (90% CI, 37%-45%) vs 43% (90% CI, 39%-47%; P = .58) without denosumab, irrespective of BC subtype (Figure 3A, Table). Nab-paclitaxel weekly showed a significantly (to the significance level of α = .10) higher pCR (ypT0 ypN0) rate compared with nab-paclitaxel days 1 and 8 every 3 weeks (nab-paclitaxel weekly: 44.9% [90% CI, 41%-49%] vs nab-paclitaxel days 1 and 8 every 3 weeks: 39.0% [90% CI, 35%-43%]; P = .06; OR, 1.27; 90% CI, 1.00-1.62; P = .09). The pCR rate with nab-paclitaxel weekly was also increased in the stratified subgroups of TNBC (60.4 [90% CI, 54.0%-66.8%] vs 50.0% [90% CI, 43.5%-56.5%]; P = .06), EC every 2 weeks (46.9% [90% CI, 41.2%-52.6%] vs 36.7% [90% CI, 31.2%-42.2%]; P = .04), and denosumab (48.2% [90% CI, 42.3%-54.1%] vs 33.8% [90% CI, 28.3%-39.4%]; P = .03), but not in other subgroups (Figure 3B, Table). Multivariable logistic regression analysis confirmed results of univariate analysis: treatment with denosumab was not associated with a higher pCR rate (OR, denosumab vs no denosumab: 0.92; 90% CI, 0.71-1.20; 95% CI, 0.67-1.27; P = .61), but nab-paclitaxel schedule was (OR, nab-paclitaxel weekly vs nab-paclitaxel days 1 and 8 every 3 weeks: 1.36; 90% CI, 1.04-1.78; 95% CI, 0.99-1.87; P = .06) (eTable 3 in Supplement 3).
Figure 3. Forest Plot of Univariate Logistic Regression for pCR (ypT0 ypN0) in Subgroups for the Denosumab Randomization and the nab-Paclitaxel Randomization.
Dmab indicates denosumab; EC, epirubicin and cyclophosphamide; HR, hormone receptor; LPBC, lymphocyte-predominant breast cancer; nP, nab-paclitaxel; pCR, pathological complete response; TNBC, triple-negative breast cancer; wk, weekly.
Table. Comparison of Short-term Efficacy End Point pCR (ypT0 ypN0) Overall and in Predefined Subgroups.
| pCRa | No. (%) [90% CI] | P value, Dmab stratifiedb | No. (%) [90% CI] | P value, nP stratifiedc | Overall No. (%) | ||
|---|---|---|---|---|---|---|---|
| Dmab | No Dmab | nP wk | nP d1,8 q3 wk | ||||
| ypT0 ypN0 (primary definition) | |||||||
| No. | 390 | 390 | 390 | 390 | 780 | ||
| Yes | 160 (41.0) [36.9-45.1] | 167 (42.8) [38.7-46.9] | .58 | 175 (44.9) [40.7-49.0] | 152 (39.0) [34.9-43.0] | .06 | 327 (41.9) |
| ypT0 ypN0 in predefined subgroups | |||||||
| ERBB2–/HR+ | |||||||
| No. | 153 | 157 | 155 | 155 | 310 | ||
| Yes | 33 (21.6) [16.1-27.0] | 35 (22.3) [16.8-27.8] | .96 | 35 (22.6) [17.1-28.1] | 33 (21.3) [15.9-26.7] | .91 | 68 (21.9) |
| TNBC | |||||||
| No. | 160 | 157 | 159 | 158 | 317 | ||
| Yes | 84 (52.5) [46.0-59.0] | 91 (58.0) [51.5-64.4] | .31 | 96 (60.4) [54.0-66.8] | 79 (50.0) [43.5-56.5] | .06 | 175 (55.2) |
| ERBB2 + | |||||||
| No. | 77 | 76 | 76 | 77 | 153 | ||
| Yes | 43 (55.8) [46.5-65.2] | 41 (53.9) [44.5-63.4] | .82 | 44 (57.9) [48.6-67.2] | 40 (51.9) [42.6-61.3] | .29 | 84 (54.9) |
| No LPBC | |||||||
| No. | 359 | 359 | 359 | 359 | 718 | ||
| Yes | 138 (38.4) [34.2-42.7] | 148 (41.2) [37.0-45.5] | .45 | 153 (42.6) [38.3-46.9] | 133 (37.0) [32.9-41.2] | .13 | 286 (39.8) |
| LPBC | |||||||
| No. | 31 | 31 | 31 | 31 | 62 | ||
| Yes | 22 (71.0) [57.6-84.4] | 19 (61.3) [46.9-75.7] | .53 | 22 (71.0) [57.6-84.4] | 19 (61.3) [46.9-75.7] | .14 | 41 (66.1) |
| EC every 2 wk | |||||||
| No. | 206 | 208 | 207 | 207 | 414 | ||
| Yes | 83 (40.3) [34.7-45.9] | 90 (43.3) [37.6-48.9] | .61 | 97 (46.9) [41.2-52.6] | 76 (36.7) [31.2-42.2] | .04 | 173 (41.8) |
| EC every 3 wk | |||||||
| No. | 184 | 192 | 183 | 183 | 366 | ||
| Yes | 77 (41.8) [35.9-47.8] | 77 (42.3) [36.3-48.3] | .79 | 78 (42.6) [36.6-48.6] | 76 (41.5) [35.5-47.5] | .60 | 154 (42.1) |
| With Dmab | |||||||
| No. | NA | NA | 195 | 195 | 390 | ||
| Yes | NA | NA | NA | 94 (48.2) [42.3-54.1] | 66 (33.8) [28.3-39.4] | .03 | 160 (41.0) |
| Without Dmab | |||||||
| No. | NA | NA | 195 | 195 | 390 | ||
| Yes | NA | NA | NA | 81 (41.5) [35.7-47.3] | 86 (44.1) [38.3-50.0] | .67 | 167 (42.8) |
Abbreviations: Dmab, denosumab; EC, epirubicin and cyclophosphamide; d1,8 q3 wk, days 1 and 8 every 3 wk; HR, hormone receptor; LPBC, lymphocyte-predominant breast cancer; NA, not applicable; nP, nab-paclitaxel; pCR, pathological complete response; TNBC, triple-negative breast cancer; wk, weekly.
Primary end point significant to the .10 level; significance level for all further analyses is set to a 2-sided α = .05.
Stratified test, primary analysis (stratified by 3 factors).
Stratified test, primary analysis (stratified by 4 factors including denosumab arm).
Similarly, for all other pCR definitions, there was no significant difference between denosumab arms, while nab-paclitaxel weekly led to significantly higher pCR rates compared with nab-paclitaxel days 1 and 8 every 3 weeks (with α = .05) when pCR was defined as ypT0/is ypN0 (nab-paclitaxel weekly: 50.5%, nab-paclitaxel days 1 and 8 every 3 weeks: 43.8%; P = .04) or ypT0/is, ypN0/+ (nab-paclitaxel weekly: 54.9% vs nab-paclitaxel days 1 and 8 every 3 weeks: 47.2%; P = .02) (eTable 4 in Supplement 3).
There was no significant difference in breast-conserving surgery rate between denosumab (70.4% in denosumab vs 76.1% in no denosumab; P = .08) or nab-paclitaxel arms (74.5% in nab-paclitaxel weekly vs 72.1% in nab-paclitaxel days 1 and 8 every 3 weeks; P = .44).
Adherence and Safety
Patients were analyzed for adherence and safety based on the treatment received. Three patients did not start denosumab and were analyzed for safety and adherence in the no denosumab arm. Seven patients in the nab-paclitaxel days 1 and 8 every-3-weeks arm did not pause at day 15 in 2 or more cycles and were therefore analyzed for safety and adherence in the nab-paclitaxel weekly arm. Details are given in Figure 2 and in eTable 5 in Supplement 3.
Denosumab did not affect chemotherapy discontinuation, delays, and dose reductions. Significantly more patients receiving nab-paclitaxel weekly discontinued nab-paclitaxel than patients in the days 1 and 8 every-3-weeks regimen (20.5% vs 6.2%; P < .001). Treatment delays (76.2% vs 56.0%; P < .001) and dose reductions (26.1% vs 11.5%; P < .001) of nab-paclitaxel, as well as dose reductions of EC (23.6% vs 15.7%; P = .009) were all significantly more common in the nab-paclitaxel weekly arm (eTable 5 in Supplement 3).
There were no significant differences of any-grade and grade 3 to 4 hematologic or nonhematologic AEs between the denosumab arms. Overall, any-grade and grade 3 to 4 hematologic AEs did not differ between nab-paclitaxel arms, while grade 3 to 4 nonhematologic AEs were more frequent with nab-paclitaxel weekly compared with nab-paclitaxel days 1 and 8 every 3 weeks: 133 (33.7%) vs 90 (24.1%) (P = .004), respectively. With regard to any-grade nonhematologic AEs, nab-paclitaxel weekly was associated with significantly higher frequencies of increased bilirubin and increased aspartate aminotransferase, fatigue, decreased appetite, diarrhea, arthralgia, epistaxis, palmar-plantar erythrodysesthesia syndrome, and pneumonia. In addition, a relevant higher frequency of peripheral sensory neuropathy (PNP) of any grade and grade 3 to 4 was found in the nab-paclitaxel weekly arm (any grade: 74.9% with nab-paclitaxel weekly vs 46.9% with nab-paclitaxel days 1 and 8 every 3 weeks ; P < .001; grade 3-4: 5.3% with nab-paclitaxel weekly vs 1.1% with nab-paclitaxel days 1 and 8 every 3 weeks; P < .001) (eTable 6 in Supplement 3). In 6 of 21 patients in the nab-paclitaxel weekly arm and in 1 of 4 patients in the nab-paclitaxel days 1 and 8 every-3-weeks arm, grade 3 to 4 PNP resolved to grade 1 or less before surgery. In the subgroup of patients treated with additional carboplatin, the frequency of any-grade PNP was 75.4% with nab-paclitaxel weekly vs 50.3% with nab-paclitaxel days 1 and 8 every 3 weeks (P < .001); frequency of high-grade PNP was 6.0% vs 0.7% (P = .01). The PNP frequencies in patients treated without carboplatin were similar (any grade: 74.6% vs 44.7%; P < .001; grade 3-4: 4.8% vs 1.3%; P = .05). Interaction tests for nab-paclitaxel carboplatin were negative.
Overall, 218 (28.4%) patients reported at least 1 serious AE (SAE), and 29 (3.8%) reported at least 1 AE of special interest (eTable 5 in Supplement 3). The rate of patients with at least 1 SAE was higher with nab-paclitaxel weekly than nab-paclitaxel days 1 and 8 every 3 weeks (31.9% vs 24.7%; P = .03) irrespective of the addition of denosumab. There was 1 death during study treatment, which occurred in a patient with an ERBB2-positive tumor treated with denosumab and nab-paclitaxel days 1 and 8 every 3 weeks (plus ABP 980 and pertuzumab) owing to an unknown reason in cycle 4.
More than 70% of patients completed questionnaires on PROs throughout the trial. Denosumab did not change the quality-of-life (QOL) scores at any time point (eFigure 1 in Supplement 3). Patients receiving nab-paclitaxel weekly reported significantly lower mean scores of physical/functional well-being, additional concerns, and FACT-Taxane total score (P < .001) compared with patients receiving nab-paclitaxel days 1 and 8 every 3 weeks (supporting data in eFigure 2 in Supplement 3). The decreased well-being with nab-paclitaxel weekly partly persisted 90 days postsurgery. The mean scores of additional concerns and FACT-Taxane total score significantly differed, favoring nab-paclitaxel days 1 and 8 every 3 weeks in all postbaseline assessments (P < .001) (supporting data in eFigure 2 in Supplement 3). Social/family and emotional aspects were not affected by the regimen.
Discussion
The GeparX trial demonstrated that weekly nab-paclitaxel, 125 mg/m2, resulted in a significantly higher pCR rate than nab-paclitaxel days 1 and 8 every 3 weeks, although more patients discontinued the chemotherapy. However, the addition of denosumab did not increase the pCR rate. To our knowledge, there is currently no other evidence on short-term efficacy of denosumab in the neoadjuvant setting. Most adjuvant studies with denosumab have primarily focused on bone health–related outcomes.6,18 The randomized, double-blind, placebo-controlled D-CARE study failed to show an improvement of bone-metastases–free survival and DFS after (neo)adjuvant denosumab, despite promising preclinical data.19 Conversely, the double-blind, placebo-controlled ABCSG-18 study including postmenopausal patients randomized to receive adjuvant therapy with an aromatase inhibitor with or without denosumab showed a significantly improved DFS, a secondary end point, for denosumab vs placebo.6 While D-CARE19 and GeparX included patients irrespective of the histologic tumor subtype, the ABCSG-18 study included only patients with ERBB2-negative/HR-positive BC. However, subgroup analyses of GeparX did not show differential effects on pCR by biological subtypes. While there might be no effect on pCR, a long-term effect cannot be excluded, and patients will be followed up for DFS and overall survival. A more comprehensive analysis in patients with RANK expression in GeparX revealed that high RANK expression was associated with significantly higher pCR rates, an effect that was pronounced in patients with ERBB2-negative/HR-positive BC, while there was no clinical benefit of denosumab regarding RANK expression.20 According to preclinical data,21 RANK expression might be one of the reasons for stemness and endocrine resistance in HR-positive BC. In human BC cell lines, RANK and ERBB2 receptors dimerize. This dimerization and cell proliferation can be impaired with denosumab, trastuzumab, and pertuzumab treatment.22 Preclinically, bisphosphonates also demonstrate anticancer activity,23,24,25,26 but adding bisphosphonates to NACT was not found to be associated with increased pCR rates.27
Neoadjuvant nab-paclitaxel, 125 mg/m2, given weekly followed by EC resulted in a significantly higher pCR rate than when given days 1 and 8 every 3 weeks (45% vs 39%; P < .10). In TNBC, optimized NACT with nab-paclitaxel, 125 mg/m2, weekly plus carboplatin followed by EC achieved a pCR rate of 60% vs 50% with the days 1 and 8 every-3-weeks regimen plus weekly carboplatin. This pCR rate is comparable with pCR rates seen with checkpoint inhibitors combined with chemotherapy28,29,30 and raises the question of which chemotherapy drugs and schedules are warranted in different TNBC molecular subtypes.31
The ETNA study13 comparing nab-paclitaxel vs solvent-based paclitaxel both given in 3 out of 4 weeks followed by anthracyclines in ERBB2-negative BC did not show a significant difference between the 2 taxanes, and the pCR rates were lower in both subgroups. Median age was comparable to that in GeparX, but in the ETNA study,13 about 70% of all patients had clinical T2 (<1% T1) cancer, and about 50% had clinically involved axillary lymph nodes and locally advanced BC in 24%. The ADAPT study15 using nab-paclitaxel days 1 and 8 every 3 weeks in combination with carboplatin for 12 weeks only demonstrated a pCR rate of 45%. Because it used 2 experimental arms as part of neoadjuvant therapy, a selection of the patients cannot be excluded. Another explanation for the higher pCR rate in TNBC in GeparX might be the higher dose and higher dose intensity achieved with the weekly regimen. Previously, dose-intensified chemotherapy was shown to be more effective than conventional-dosed treatment in all subgroups.32 However, the absolute differences in pCR rates between both schedules in GeparX were small with 6% overall, 6% in ERBB2-positive BC, 1.3% in HR-positive BC (both were nonsignificant), and 10.4% in TNBC. Long-term follow-up needs to be completed to see whether the pCR difference translates into a survival difference as in the GeparSepto study.12
Weekly nab-paclitaxel was associated with higher frequencies of nonhematologic AEs, which are well known and mostly well manageable. Any-grade and grade 3 to 4 PNP was a significant and clinically relevant AE of weekly nab-paclitaxel, as previously shown in GeparSepto.11 There, grade 3 to 4 PNP was more frequent after weekly nab-paclitaxel, 125 mg/m2, vs solvent-based paclitaxel, 80 mg/m2 (8.3 vs 2.7%), but resolved to grade 1 or less in 68.7%.11 In GeparX, grade 3 to 4 PNP resolution to grade 1 or less before surgery was reported in about 30% of patients for weekly nab-paclitaxel and in 25% of patients for nab-paclitaxel days 1 and 8 every 3 weeks. The higher pCR rates with the weekly regimen have to be weighed against a potential higher risk of AEs. In particular, PNP may lead to impaired QOL, persistent limiting of activities of daily living, and excess health care costs during and after completion of chemotherapy.33 We do not have long-term data regarding PNP resolution after surgery. However, PROs revealed a decreased QOL during treatment with weekly nab-paclitaxel, only partly recovering postsurgery.
Strengths and Limitations
To our knowledge, this is the first study investigating denosumab as part of NACT offering a head-to-head comparison of 2 different nab-paclitaxel regimens in early BC with addition of carboplatin in TNBC. The data in metastatic BC are comparing different nab-paclitaxel regimens with docetaxel.34 The study has several strengths and limitations. The sample size was adequate for a phase 2, 2 × 2 factorial design and comparable with other trials. While sample size was planned under the assumption of no interaction between denosumab and nab-paclitaxel, baseline characteristics according to the 2 randomized treatments (4 groups) revealed an imbalance in the biological subtypes, presumably leading to an artifactual significant test for interaction between treatment arms. Overall, results are suitable for guiding research; however, nab-paclitaxel is approved only for the treatment of metastatic BC and, despite reassuring data, cannot currently be considered as a standard neoadjuvant treatment of BC.
Conclusions
In this randomized clinical trial, findings demonstrated that the weekly application of nab-paclitaxel was more effective than administration on days 1 and 8 every 3 weeks, even in combination with carboplatin in TNBC, despite greater toxicity. The addition of carboplatin in TNBC resulted in a noteworthy pCR rate of 60%. Results did not demonstrate that the addition of denosumab to an anthracycline/taxane-based chemotherapy increased pCR rate in early BC. Long-term follow-up is ongoing.
Trial Protocol
Statistical Analysis Plan
eAppendix.
eTable 1. Patient and tumor baseline characteristics (ITT population)
eTable 2. Tumor baseline stratification parameters in 4 groups as randomized (ITT population)
eTable 3. Multivariable logistic regression analysis adjusted for primary endpoint pCR (ypT0 ypN0)
eTable 4. Comparison of short-term efficacy according to different pCR endpoints
eTable 5. Adherence to treatment
eTable 6. Hematological and non-hematological adverse events
eFigure 1. Patient-reported outcomes denosumab
eFigure 2. Patient-reported outcomes nab-paclitaxel
GBG and AGO-B Principal Investigators
Data Sharing Statement
References
- 1.Ono T, Hayashi M, Sasaki F, Nakashima T. RANKL biology: bone metabolism, the immune system, and beyond. Inflamm Regen. 2020;40:2. doi: 10.1186/s41232-019-0111-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sisay M, Mengistu G, Edessa D. The RANK/RANKL/OPG system in tumorigenesis and metastasis of cancer stem cell: potential targets for anticancer therapy. Onco Targets Ther. 2017;10:3801-3810. doi: 10.2147/OTT.S135867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iranikhah M, Wilborn TW, Wensel TM, Ferrell JB. Denosumab for the prevention of skeletal-related events in patients with bone metastasis from solid tumor. Pharmacotherapy. 2012;32(3):274-284. doi: 10.1002/j.1875-9114.2011.01092.x [DOI] [PubMed] [Google Scholar]
- 4.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) . Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet. 2015;386(10001):1353-1361. [DOI] [PubMed] [Google Scholar]
- 5.Gnant M, Pfeiler G, Dubsky PC, et al. ; Austrian Breast and Colorectal Cancer Study Group . Adjuvant denosumab in breast cancer (ABCSG-18): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2015;386(9992):433-443. doi: 10.1016/S0140-6736(15)60995-3 [DOI] [PubMed] [Google Scholar]
- 6.Gnant M, Pfeiler G, Steger GG, et al. ; Austrian Breast and Colorectal Cancer Study Group . Adjuvant denosumab in postmenopausal patients with hormone receptor-positive breast cancer (ABCSG-18): disease-free survival results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(3):339-351. doi: 10.1016/S1470-2045(18)30862-3 [DOI] [PubMed] [Google Scholar]
- 7.Coleman R, de Boer R, Eidtmann H, et al. Zoledronic acid (zoledronate) for postmenopausal women with early breast cancer receiving adjuvant letrozole (ZO-FAST study): final 60-month results. Ann Oncol. 2013;24(2):398-405. doi: 10.1093/annonc/mds277 [DOI] [PubMed] [Google Scholar]
- 8.Gnant M, Mlineritsch B, Luschin-Ebengreuth G, et al. ; Austrian Breast and Colorectal Cancer Study Group (ABCSG) . Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 5-year follow-up of the ABCSG-12 bone-mineral density substudy. Lancet Oncol. 2008;9(9):840-849. doi: 10.1016/S1470-2045(08)70204-3 [DOI] [PubMed] [Google Scholar]
- 9.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164-172. doi: 10.1016/S0140-6736(13)62422-8 [DOI] [PubMed] [Google Scholar]
- 10.von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30(15):1796-1804. doi: 10.1200/JCO.2011.38.8595 [DOI] [PubMed] [Google Scholar]
- 11.Untch M, Jackisch C, Schneeweiss A, et al. ; German Breast Group (GBG); Arbeitsgemeinschaft Gynäkologische Onkologie—Breast (AGO-B) Investigators . Nab-paclitaxel versus solvent-based paclitaxel in neoadjuvant chemotherapy for early breast cancer (GeparSepto-GBG 69): a randomised, phase 3 trial. Lancet Oncol. 2016;17(3):345-356. doi: 10.1016/S1470-2045(15)00542-2 [DOI] [PubMed] [Google Scholar]
- 12.Untch M, Jackisch C, Schneeweiss A, et al. Nab-paclitaxel improves disease-free survival in early breast cancer: GBG 69-GeparSepto. J Clin Oncol. 2019;37(25):2226-2234. doi: 10.1200/JCO.18.01842 [DOI] [PubMed] [Google Scholar]
- 13.Gianni L, Mansutti M, Anton A, et al. Comparing neoadjuvant nab-paclitaxel vs paclitaxel both followed by anthracycline regimens in women with ERBB2/HER2-negative breast cancer—the Evaluating Treatment With Neoadjuvant Abraxane (ETNA) trial: a randomized phase 3 clinical trial. JAMA Oncol. 2018;4(3):302-308. doi: 10.1001/jamaoncol.2017.4612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gianni L, Mansutti M, Anton A, et al. Event-free survival analysis of the prospectively randomized phase III ETNA study with neoadjuvant nab-paclitaxel (nab-P) versus paclitaxel (P) followed by anthracycline regimens in women with HER2-negative high-risk breast cancer. Abstract. J Clin Oncol. 2019;37(15)(suppl):515. doi: 10.1200/JCO.2019.37.15_suppl.515 [DOI] [Google Scholar]
- 15.Gluz O, Nitz U, Liedtke C, et al. Comparison of neoadjuvant nab-paclitaxel+carboplatin vs nab-paclitaxel+gemcitabine in triple-negative breast cancer: randomized WSG-ADAPT-TN trial results. J Natl Cancer Inst. 2018;110(6):628-637. doi: 10.1093/jnci/djx258 [DOI] [PubMed] [Google Scholar]
- 16.Simes RJ. An improved Bonferroni procedure for multiple tests of significance. Biometrika. 1986;73(3):751-754. doi: 10.1093/biomet/73.3.751 [DOI] [Google Scholar]
- 17.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26(4):404-413. doi: 10.1093/biomet/26.4.404 [DOI] [Google Scholar]
- 18.Ellis GK, Bone HG, Chlebowski R, et al. Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J Clin Oncol. 2008;26(30):4875-4882. doi: 10.1200/JCO.2008.16.3832 [DOI] [PubMed] [Google Scholar]
- 19.Coleman R, Finkelstein DM, Barrios C, et al. Adjuvant denosumab in early breast cancer (D-CARE): an international, multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2020;21(1):60-72. doi: 10.1016/S1470-2045(19)30687-4 [DOI] [PubMed] [Google Scholar]
- 20.Link T, Blohmer JU, Just M, et al. GeparX: denosumab (Dmab) as add-on to different regimen of nab-paclitaxel (nP)-anthracycline based neoadjuvant chemotherapy (NACT) in early breast cancer (BC): subgroup analyses by RANK expression and HR status. Abstract 168MO. Ann Oncol. 2020;31(suppl 4):S308-S309. doi: 10.1016/j.annonc.2020.08.290 [DOI] [Google Scholar]
- 21.Gomes I, de Almeida BP, Dâmaso S, et al. Expression of receptor activator of NFkB (RANK) drives stemness and resistance to therapy in ER+HER2- breast cancer. Oncotarget. 2020;11(19):1714-1728. doi: 10.18632/oncotarget.27576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zoi I, Karamouzis MV, Xingi E, et al. Combining RANK/RANKL and ERBB-2 targeting as a novel strategy in ERBB-2-positive breast carcinomas. Breast Cancer Res. 2019;21(1):132. doi: 10.1186/s13058-019-1226-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gnant M. The evolving role of zoledronic acid in early breast cancer. Onco Targets Ther. 2009;2:95-104. doi: 10.2147/OTT.S4082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gnant M. Bisphosphonates in the prevention of disease recurrence: current results and ongoing trials. Curr Cancer Drug Targets. 2009;9(7):824-833. doi: 10.2174/156800909789760267 [DOI] [PubMed] [Google Scholar]
- 25.Winter MC, Holen I, Coleman RE. Exploring the anti-tumour activity of bisphosphonates in early breast cancer. Cancer Treat Rev. 2008;34(5):453-475. doi: 10.1016/j.ctrv.2008.02.004 [DOI] [PubMed] [Google Scholar]
- 26.Neville-Webbe HL, Coleman RE, Holen I. Combined effects of the bisphosphonate, zoledronic acid and the aromatase inhibitor letrozole on breast cancer cells in vitro: evidence of synergistic interaction. Br J Cancer. 2010;102(6):1010-1017. doi: 10.1038/sj.bjc.6605579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chavez-Macgregor M, Brown E, Lei X, et al. Bisphosphonates and pathologic complete response to taxane- and anthracycline-based neoadjuvant chemotherapy in patients with breast cancer. Cancer. 2012;118(2):326-332. doi: 10.1002/cncr.26144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmid P, Cortes J, Pusztai L, et al. ; KEYNOTE-522 Investigators . Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382(9):810-821. doi: 10.1056/NEJMoa1910549 [DOI] [PubMed] [Google Scholar]
- 29.Mittendorf EA, Zhang H, Barrios CH, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet. 2020;396(10257):1090-1100. doi: 10.1016/S0140-6736(20)31953-X [DOI] [PubMed] [Google Scholar]
- 30.Schmid P, Salgado R, Park YH, et al. Pembrolizumab plus chemotherapy as neoadjuvant treatment of high-risk, early-stage triple-negative breast cancer: results from the phase 1b open-label, multicohort KEYNOTE-173 study. Ann Oncol. 2020;31(5):569-581. doi: 10.1016/j.annonc.2020.01.072 [DOI] [PubMed] [Google Scholar]
- 31.Wang DY, Jiang Z, Ben-David Y, Woodgett JR, Zacksenhaus E. Molecular stratification within triple-negative breast cancer subtypes. Sci Rep. 2019;9(1):19107. doi: 10.1038/s41598-019-55710-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) . Increasing the dose intensity of chemotherapy by more frequent administration or sequential scheduling: a patient-level meta-analysis of 37 298 women with early breast cancer in 26 randomised trials. Lancet. 2019;393(10179):1440-1452. doi: 10.1016/S0140-6736(18)33137-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mustafa Ali M, Moeller M, Rybicki L, Moore HCF. Long-term peripheral neuropathy symptoms in breast cancer survivors. Breast Cancer Res Treat. 2017;166(2):519-526. doi: 10.1007/s10549-017-4437-8 [DOI] [PubMed] [Google Scholar]
- 34.Gradishar WJ, Krasnojon D, Cheporov S, et al. Significantly longer progression-free survival with nab-paclitaxel compared with docetaxel as first-line therapy for metastatic breast cancer. J Clin Oncol. 2009;27(22):3611-3619. doi: 10.1200/JCO.2008.18.5397 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eAppendix.
eTable 1. Patient and tumor baseline characteristics (ITT population)
eTable 2. Tumor baseline stratification parameters in 4 groups as randomized (ITT population)
eTable 3. Multivariable logistic regression analysis adjusted for primary endpoint pCR (ypT0 ypN0)
eTable 4. Comparison of short-term efficacy according to different pCR endpoints
eTable 5. Adherence to treatment
eTable 6. Hematological and non-hematological adverse events
eFigure 1. Patient-reported outcomes denosumab
eFigure 2. Patient-reported outcomes nab-paclitaxel
GBG and AGO-B Principal Investigators
Data Sharing Statement