Abstract
Lung cancer is the leading cause of cancer death in Europe. Screening by means of low-dose computed tomography (LDCT) can shift detection to an earlier stage and reduce lung cancer mortality in high-risk individuals. However, to date, Poland, Croatia, Italy, and Romania are the only European countries to commit to large-scale implementation of targeted LDCT screening. Using a health systems approach, this article evaluates key factors needed to enable the successful implementation of screening programs across Europe. Recent literature on LDCT screening was reviewed for 10 countries (Belgium, Croatia, France, Germany, Italy, the Netherlands, Poland, Spain, Sweden, and United Kingdom) and complemented by 17 semistructured interviews with local experts. Research findings were mapped against a health systems framework adapted for lung cancer screening. The European policy landscape is highly variable, but potential barriers to implementation are similar across countries and consistent with those reported for other cancer screening programs. While consistent quality and safety of screening must be ensured across all screening centers, system factors are also important. These include appropriate data infrastructure, targeted recruitment methods that ensure equity in participation, sufficient capacity and workforce training, full integration of screening with multidisciplinary care pathways, and smoking cessation programs. Stigma and underlying perceptions of lung cancer as a self-inflicted condition are also important considerations. Building on decades of implementation research, governments now have a unique opportunity to establish effective, efficient, and equitable lung cancer screening programs adapted to their health systems, curbing the impact of lung cancer on their populations.
Keywords: Computed tomography, Early detection, Lung cancer, Policy, Screening
Introduction
Lung cancer is the leading cause of cancer deaths in Europe.1 It is also the most expensive of all cancers, accounting for nearly a quarter of productivity losses because of cancer.2 Given that a large proportion of cases are detected at an advanced stage when prognosis is poor, early detection is recognized as the most promising tool to reduce mortality from lung cancer.3 Cumulative evidence from randomized controlled trials found that targeted screening of former and current heavy smokers through low-dose computed tomography (LDCT) results in a substantial shift to an earlier stage of detection and reduction in mortality.3, 4, 5 However, the global momentum for implementation of population-wide lung cancer screening programs has been generally slow. In Europe, Croatia, Poland, Italy, and Romania are the only countries to have formally committed to setting up nationwide, organized LDCT screening programs that target high-risk individuals,6, 7, 8, 9 with other countries remaining cautious about investing in lung cancer screening.
As has been seen with other forms of cancer screening, translating findings from clinical trials into real-world, large-scale screening programs is invariably complex. Ensuring consistent quality across all participant screening centers is essential to minimizing the risks of screening and optimizing its benefits. In addition, health system factors external to the screening process itself—such as system governance, workforce capacity, quality and interoperability of data systems, and integration of screening into other health service delivery—significantly impacts the ability to screen programs to meet their stated objectives.10, 11, 12 Taking a health systems approach to planning for screening programs can, thus, help determine what interplay of services, organizations, people, technology, and information is needed to foster successful implementation.
Health systems thinking has grown extensively over recent years.13 It has been previously applied to assessing barriers to uptake of breast, colorectal, and cervical cancer screening programs across Europe,14 and ensuring the sustainability of lung cancer screening programs.15 The challenges specific to implementing LDCT screening programs have been amply described in the literature.16, 17, 18, 19, 20 Building on this research, we conducted an analysis to understand the current policy landscape for lung cancer screening in 10 European countries and identify key considerations related to implementation. This article presents a synthesis of our findings.
Materials and Methods
A structured review of peer-reviewed and gray literature was conducted to inform a policy landscape analysis of lung cancer screening in 10 countries (Belgium, Croatia, France, Germany, Italy, the Netherlands, Poland, Spain, Sweden, and the United Kingdom). A common structured search strategy was used across all countries to identify relevant publications published from January 2015 to July 2021 describing local clinical trials and feasibility studies, pilot studies, implementation research, and expert commentary on lung cancer screening. Country-specific gray literature sources, including policy reports, position papers, and advocacy materials, were also searched for contextual information. Searches were conducted in English, Spanish, French, German, and Italian. For Sweden, the Netherlands, Croatia, and Poland, searches were conducted in English, but key sources were identified in their respective local languages and translated into English.
Literature search findings were used to identify relevant lung cancer screening experts for interview. A total of 17 semistructured interviews were conducted with local experts to discuss the current policy landscape surrounding lung cancer screening. Interviewees were not remunerated for their participation. Findings from interviews were merged with those from the literature to strengthen initial findings from desk research and identify common themes. These were then mapped against the WHO Health Systems Framework.13 No patients were involved in this study thus no informed consent was required.
Results
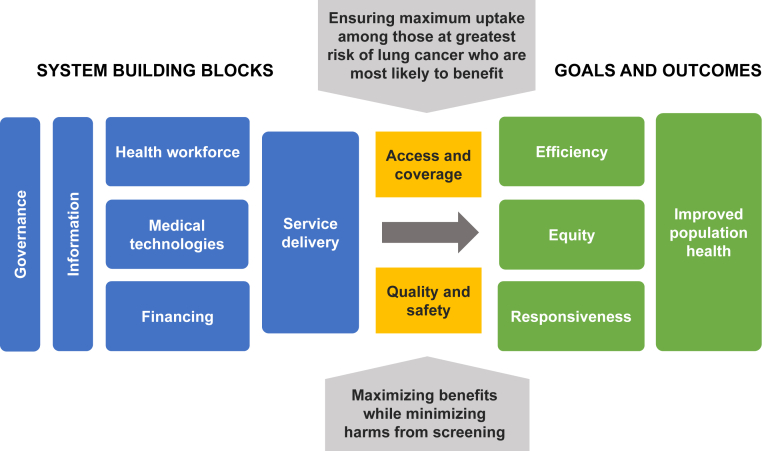
The policy landscape on lung cancer screening is highly variable among the 10 countries studied. The governments of Poland, Croatia, and Italy have committed to implementing nationwide organized lung cancer screening programs for high-risk individuals. The United Kingdom, Sweden, and Germany are at advanced stages of exploring the feasibility of large-scale implementation, whereas France, Belgium, the Netherlands, and Spain are more hesitant in their approach (Table 1). Regardless of where countries are on the road to implementation, our research suggests they are all exploring similar issues. Many relate to core health system functions, especially governance, workforce and technical capacity, service delivery, technology, information, and data systems. Establishing the economic rationale for investing in large-scale LDCT screening programs is also an important concern, especially as governments are still grappling with the impact of the coronavirus disease 2019 (COVID-19) pandemic. Other issues concern the ability of LDCT screening programs to achieve sufficient coverage and access and consistent quality and safety. Finally, as a public health investment, LDCT programs must also improve population health and achieve equity, responsiveness, and efficiency of resource use, as defined by the WHO Health Systems Framework (Fig. 1).13
Table 1.
The Position of 10 Countries Along the Policy Development Process for Lung Cancer Screening in Europe (as of February 2022)
| Stage of policy development | Belgium | Croatia | France | Germany | Italy | Spain | Poland | Sweden | The Netherlands | United Kingdom |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Recent assessment of evidence (since 2019) | No | No | Yes | Yes | Yes | No | Yes | Yes | Yes | Ongoing |
| 2. Economic evaluation to determine cost-effectiveness | Ongoing | No | No | Yes | Yes | No | Yes | Yes | No | Ongoing |
| 3. Local pilot/feasibility studies | Awaiting funding/approval | No | Ongoing | Ongoing | Ongoing | Small independent studies | Ongoing | Ongoing | Ongoing | Ongoing |
| 4. Commitment to program setup | No | Yes, program ongoing | No | Yes | Yes | No | Yes, program ongoing | In current discussion | No | In current discussion |
| 5. Development of a national screening protocol | Taskforce currently working on this | Yes | No | No | In progress | No | Yes | In progress | Taskforce currently working on this | No |
| 6. Organizational setup and implementation of national program | No | Yes | No | No | In progress | No | Yes | No | No | No |
Figure 1.
The WHO Health Systems Framework adapted for lung cancer screening programs.
Governance
The policy and regulatory process governing the decision to implement screening programs is well defined in all countries studied. All countries use Wilson and Jungner’s original criteria,21 or slight variations thereof, for assessing the merits of screening programs. Decisions regarding implementation take place at the national level in all countries except Belgium, in which each region considers implementation independently.22 In other countries, even when decisions are nationally led, the implementation and organization of screening programs are often regionally led, with monitoring of data on coverage, quality, and performance centralized at the national level.
The role of pilot studies in guiding implementation decisions varies by country. Several pilots are currently underway in France,23, 24, 25, 26 each with its own model of funding sources, combining the private sector, local charity, and government funds. It is unclear, however, how the findings from these studies will be built into national policy decisions around implementation—especially as the government recently expressed its commitment to begin exploring pilot implementation.27 In Belgium, a multidisciplinary task force in the Flanders region is submitting an application to the regional health authorities and plans to run a pilot project.22 In Italy and Sweden, pilots are endorsed by the national government and built into the screening implementation strategy.8,28 In Croatia, the decision was made to adopt a national program on the basis of international studies, without any local pilots. In the United Kingdom, the rollout of “Targeted Lung Health Check” pilots has been ongoing across England since 2019,29,30 and it is assumed that this protocol, adapted in some way, will be adopted by the U.K. National Screening Committee should it decide in favor of LDCT screening.31
Leadership for screening has been multidisciplinary in Europe, with radiologists, thoracic surgeons, and pulmonologists having issued joint position papers on LDCT screening at the European Union (EU) level and in several countries.25,32, 33, 34, 35 In Spain, the debate over LDCT screening is somewhat polarized, with most clinicians in favor of implementation and some members of the epidemiologic community against it.36,37 Lung cancer patient organizations have been actively involved in policy discussions about LDCT screening at the EU level38,39; their engagement at the national level is variable, often reflecting limited capacity.
A consistent theme across all countries is the importance of engaging family physicians and general practitioners (GPs) to help recruit high-risk individuals who could benefit from screening. Yet securing GP engagement remains a significant challenge in many countries,32 and providing training to GPs on lung cancer screening is a recognized priority.40,41 In the Netherlands and Belgium, experts suggested one reason behind GPs’ reluctance to endorse lung cancer screening is that they fear it will create additional pressures on their already limited capacity. In France, earlier pilot studies in the Somme region found that involving GPs in the follow-up of their patients after screening helped secure their buy-in; therefore, this role was built into the protocol of later pilots.26 In Italy, the national pilot studies are formally endorsed by the national society for GPs, and all participating screening centers offer specific training for GPs. In Poland, each regional screening center collaborates with approximately 40 primary care centers,7 involving a total of 600 primary care centers nationwide. These are provided with leaflets and accessible educational materials for potential screening candidates. GPs also play a central role in identifying, following up, and monitoring participants in the Croatian program, but equitable access to primary health care remains an ongoing challenge across the country.42
Workforce Capacity
From expert interviews, concerns on human and technical capacity to deliver large-scale LDCT screening seem to vary by country. This is a particular concern in the United Kingdom, where radiology capacity is already failing to meet demand.43 All countries recognize the importance of investing in dedicated training for radiologists, robust quality control programs, and standardized protocols for nodule interpretation and management.32 The potential for computer-aided detection tools, such as those using artificial intelligence to support radiologists in reading computed tomography (CT) scans, improve the accuracy of interpretation, and alleviate pressures on existing capacity, is also being explored.16
A joint position statement by the European Respiratory Society and the European Society of Radiology recommends centralizing screening in multidisciplinary accredited centers of excellence to ensure consistent quality of screening and interpretation.32 In practice, however, countries must balance the need to optimize quality with ensuring ease of access to screening across the country, leading to different organizational models. Poland has privileged a centralized approach for its National Program of Early Lung Cancer Detection7; one leading center is appointed by the Ministry of Health in each region and works with two to four selected screening centers that perform CT scans. All diagnosis and care decisions are centralized within a multidisciplinary team at the leading center.33,44 Italy is also organizing screening in 18 centers of excellence.8 In contrast, Germany, which has a large proportion of private sector and ambulatory-based radiologists, is likely to adopt a decentralized approach, with screening offered either in community- or hospital-based radiology clinics. All participating centers would be centrally accredited, with interpretation and follow-up centralized in specialist lung cancer centers.34 France is likely to follow a similar model, mirroring its delivery of breast cancer screening. The Targeted Lung Health Check model in England offers screening in community settings to improve outreach to high-risk individuals who live in the most deprived neighborhoods, but also at some fixed sites at hospitals where access is not a significant issue.29 All screening centers are connected remotely to centralized specialist multidisciplinary teams.
Service Delivery
Lung cancer screening is not an isolated intervention, and its success hinges, in part, on the quality of existing lung cancer pathways and their ability to accommodate an increased volume of cases detected through screening. In particular, surgical capacity is likely to require scaling up, as it is the primary treatment modality for early-stage lung cancer, the numbers of which are expected to rise with LDCT screening.6,45 Addressing deficits in existing lung cancer pathways, particularly in terms of rapid referral pathways for patients with suspected lung cancer and provision of care by a multidisciplinary team, is also essential. In Croatia, for example, a priority waiting list was introduced in 2017 to accelerate access to specialist care once a referral from the GP has been made.42 In the United Kingdom, a 23% difference in one-year net survival rates for lung cancer has been reported among regions, greater than for other common cancers,46 and it has been suggested that urgent action should be taken to address deficiencies in staffing provision for lung cancer care.47
Integration With Smoking Cessation Programs
Integration of LDCT screening with smoking cessation programs is essential to maximize the impact of screening.32 One of the prerequisites for screening on the basis of Wilson and Jungner's criteria is that all primary prevention efforts have been taken before offering secondary prevention (risk reduction) strategies.21 Operationalizing this integration in practice, however, can be challenging. Both at the EU level and in many countries, policy and advocacy platforms for antitobacco efforts and lung cancer screening have traditionally been disjointed, although recent efforts from several professional and patient organizations have helped to portray them as complementary approaches to reduce the impact of smoking on our societies.48,49 The unclear positioning of smoking cessation services within some health systems may also hinder their delivery as a complement to screening. In Germany, until recently, social health insurance providers were prevented by law from paying for smoking cessation medication, which was classified as a “lifestyle” drug instead of a treatment for addiction.50 The limited capacity of GPs to provide smoking cessation advice was raised as an issue by experts in Poland, the Netherlands, and Belgium. In response, Belgium created the role of tobaccologists,22 which include medical doctors, psychologists, pharmacists, and other health professionals, who are trained and certified in providing smoking cessation advice. Another issue in some countries is the low uptake of smoking cessation programs. In Italy, for example, the 2019 National Prevention Plan suggests that only 2% to 4% of people who quit smoking had used state-funded smoking cessation centers.51
Information and Data Management Systems
Information plays a central role in the operationalization of organized screening programs. Sophisticated data management systems are needed to bring together all data elements, including regularly-updated recruitment databases, screening results, image banks, and follow-up data, to allow ongoing evaluation and monitoring of the program and its impact.
Programs also need to have a proper data infrastructure to ensure the recruitment of eligible populations at successive screening rounds. Identifying the eligible population for targeted LDCT screening is infinitely more complex than for breast, cervical, or colorectal screening, in which eligibility is solely on the basis of age and sex. Detailed individual data on smoking history are needed, yet most countries lack a population-wide database that contains this information.19 One exception is the United Kingdom, where smoking status is recorded in electronic medical records by GPs. However, these data are not always entirely reliable.19 Most often, a multistep approach is needed to identify those eligible for LDCT screening, involving a combination of centralized recruitment, individual outreach by GPs, and online questionnaires that capture other data to refine candidate selection.16,52 In Germany, the German Radiological Society and the German Respiratory Society recommend that initial invitations to screening should be issued by pulmonologists rather than GPs.34
All countries are also exploring the use of existing personalized risk models to expand recruitment to people who are at high-risk for lung cancer owing to other factors, such as race and ethnicity, socioeconomic status, family history, and the presence of comorbidities.32 These individuals may otherwise be excluded if eligibility is defined solely on smoking status and age.20,53 Many ongoing pilot studies include existing risk models to make sure they are adapted to their population’s epidemiologic and demographic characteristics.
Access, Coverage, Responsiveness, and Equity
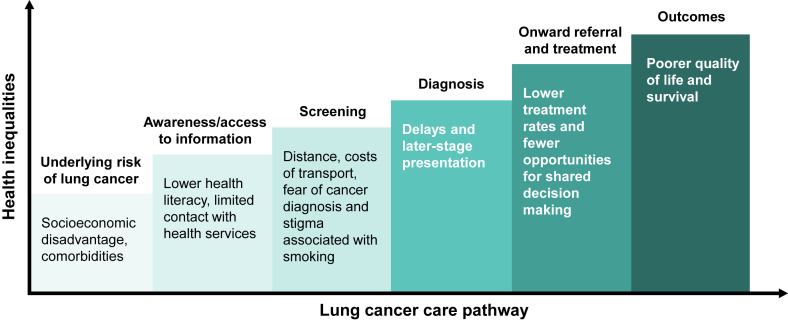
One of the most typically cited challenges by experts interviewed for this work was securing high attendance from vulnerable groups, particularly those from socially disadvantaged backgrounds. These individuals typically have the highest risk of lung cancer but are the least likely to participate in screening.54 They also experience cumulative inequalities along the lung cancer pathway (Fig. 2).10,55 Experts emphasized the importance of developing targeted approaches to secure attendance from these vulnerable populations and ensure that organized screening programs do not inadvertently exacerbate existing inequalities.
Figure 2.
Cumulative inequalities along the lung cancer care pathway.
Stigma was recognized as being a pervasive barrier to attendance by many of the experts interviewed. Stigma toward smoking, coupled with fear of a diagnosis of lung cancer, can act as a significant psychological barrier to attending screening, particularly among vulnerable groups.19,54 The Targeted Lung Health Check model adopted in England addresses this by presenting itself as a “wellness” service, as opposed to a cancer screening service.31 Information provided to the public deliberately avoids reference to either lung cancer or smoking and participants are offered comprehensive psychological counseling to help them overcome any feelings of stigma or fear during screening.29 This approach has proven very successful in recruiting people from vulnerable groups,30 and its application is currently being explored in Sweden and elsewhere in Europe.
Localized qualitative research can help identify context-specific barriers to screening and develop appropriate outreach approaches targeted at specific high-risk groups.10,56 For example, population surveys in France found that respondents overestimated the five-year survival rate from lung cancer, yet underestimated the potential for cure by surgery when performed at an early stage.57 Local research also uncovered different perceptions of lung cancer risk depending on smoking status: current smokers were generally more aware of the risks of lung cancer and more likely to participate in screening, whereas former smokers were susceptible to underestimating risk and declining participation.57,58 Interestingly, these findings contrast with those from the U.K. Lung Screening trial and the Dutch-Belgian randomised controlled lung cancer CT screening trial, in which current smokers were less likely to participate than former smokers.19,54
Targeted approaches may also be helpful for women. In most countries, the incidence of, and mortality from, lung cancer is rising in women, whereas they are slowly declining or stable in men.59 There is also evidence that LDCT screening results in a greater reduction in lung cancer mortality in women than in men.4 Drawing on these findings, the Stockholm region in Sweden is exploring the integration of LDCT screening into existing breast cancer screening programs.60
Minimizing Harms and Optimizing Benefits of Screening
A core consideration in all countries is how to achieve an acceptable balance of benefits and harms from screening while minimizing the risk of unnecessary exposure to radiation and false-positive findings resulting in unnecessary procedures. Adoption of the most up-to-date protocols for nodule interpretation and clinical workup in combination with systematic quality assurance is key,52,61 as is finding the screening frequency that balances specificity and sensitivity. There is currently no consensus in the literature on whether annual or biennial screening is preferred,62 and both are being considered across the 10 countries studied. An area of active research is whether screening intervals can be personalized according to baseline risk, measured by baseline CT scan, biomarkers, and other factors. The feasibility of this risk-based approach, including potential gender-based protocols, is being explored in the EU-funded towards INdividually tailored INvitations, screening INtervals, and INtegrated co-morbidity reducing strategies in lung cancer screening trial currently underway in five countries.63
Discussion
Our analysis confirms that many systemic factors need to align to ensure the success of LDCT screening programs (Table 2). Screening programs are not just about optimizing the quality and safety of the screening event itself. They need to ensure screening is built into efficient, well-coordinated programs, supported by appropriate data infrastructure that allows accurate monitoring of the outcomes throughout the screening pathway. Programs should address potential inequalities in participation, be supported by sufficient capacity and workforce training, and be fully integrated with multidisciplinary care pathways and smoking cessation interventions.16,52
Table 2.
Key Considerations for Successful Implementation of Lung Cancer Screening Programs
| System building blocks | Governance |
|
| Information |
|
|
| Health workforce |
|
|
| Medical technologies |
|
|
| Service delivery |
|
|
| System goals | Access, coverage, equity, and responsiveness |
|
| Quality and safety |
|
|
| Efficiency |
|
|
| Population health |
|
AI, artificial intelligence; CT, computed tomography.
Reliable population-level information is needed to identify those at the highest risk of lung cancer who are most likely to benefit from screening. Culturally sensitive messaging and shared decision-making tools, ideally co-designed with target populations, are fundamental to overcoming informational and psychological barriers to screening and conveying an accurate account of risks and benefits to eligible participants.10,64 Awareness campaigns from patient organizations, professional societies, and antitobacco groups can help shift attitudes toward lung cancer and smoking, in particular, by reducing stigma and fatalistic attitudes around lung cancer. These campaigns can also serve to galvanize public and political support for LDCT screening.
Equity is an essential dimension of any LDCT screening program. People from socially disadvantaged populations are at the highest risk of lung cancer, presenting late with symptoms, and experiencing poor survival as a result.55 It is broadly recognized that targeted outreach approaches are needed to secure attendance from these high-risk groups. However, there is little consensus as to what constitutes an optimal approach. Some interventions, such as individual outreach by GPs to high-risk individuals, may not be economically viable.31,65 One possible approach that merits exploring is the role that other community providers (for instance, pharmacists) can play in engaging high-risk individuals who may not be in regular contact with primary care services.66
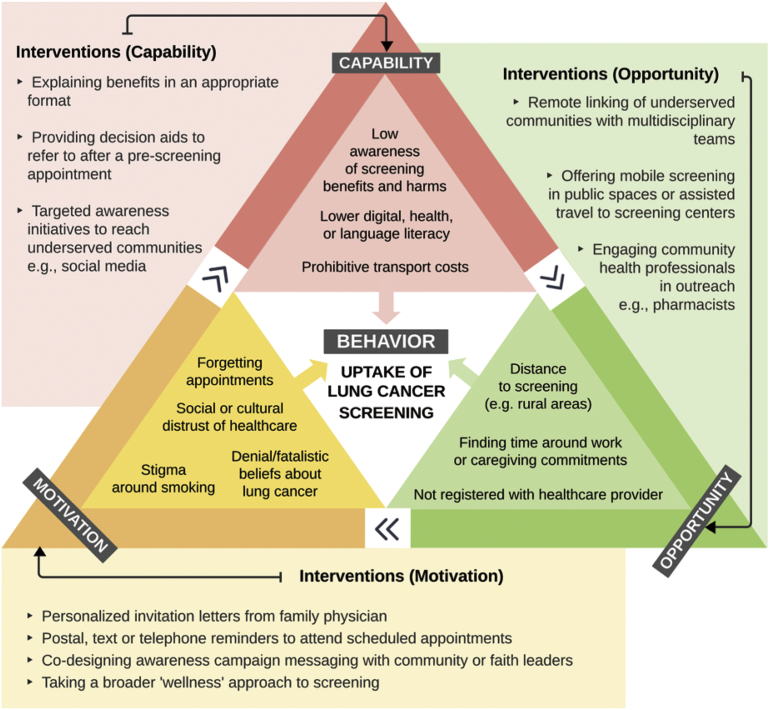
A helpful framework that can be applied to determine the most appropriate approaches to address barriers to lung cancer screening attendance is the Capability, Opportunity, Motivation, and Behavior model.56 The model maps the range of physical, psychological, and social barriers that impact an individual’s capability, opportunity, and motivation to engage in lung cancer screening.54 Drawing on lessons learned from other cancer screening programs and expert interviews conducted during this research,64,67 potential interventions to address specific barriers to lung cancer screening are mapped against the Capability, Opportunity, Motivation, and Behavior model in Figure 3.
Figure 3.
Possible approaches to address barriers to lung cancer screening. Possible approaches to counter each identified barrier are suggested outside the triangle. Please note that some identified barriers could fall across several areas.
One question that was being asked in all countries pertained to the economic feasibility of LDCT screening. Experts suggested their governments have traditionally been cautious in investing in another large-scale screening program, and their hesitancy may be exacerbated by budgetary pressures brought on by the COVID-19 pandemic. Yet economic evaluations conducted internationally and in many of the 10 countries studied suggest that high-quality targeted LDCT screening is likely to be within acceptable thresholds for cost effectiveness.28,68, 69, 70, 71, 72 A recent analysis in Italy suggests that the cost-effectiveness ratio for LDCT screening compares favorably with those for colorectal, breast, and cervical cancer screening.73 Studies also suggest that LDCT screening is more efficient than colorectal, breast, and cervical cancer screening, requiring fewer people to be screened to prevent one cancer death (Table 3).5,6,74,75
Table 3.
Reported Efficiency of Lung Cancer Screening Compared With Other Cancer Screening Programs
| Cancer | Screening method | Number needed to screen to avoid one cancer deatha | Reported 95% CIs |
|---|---|---|---|
| Colorectal cancerb | gFOBT Flexible sigmoidoscopy |
377–515 864 |
377 (249 to 887) 515 (373 to 867) 864 (672 to 1266) |
| Breast cancerc | Mammography | 645–1724 | 645 (441 to 1389) 1724 (1176 to 3704) |
| Lung cancerd | LDCT | 130–320 | .. |
CIs, confidence intervals; gFOBT, guaiac fecal occult blood testing; LDCT, low-dose computed tomography.
Will vary by screening interval selected.
Estimates for colorectal cancer vary by screening test offered: the gFOBT lower estimate reported is 377 (95% CI: 249, 887) and the upper estimate is 515 (95% CI: 373, 867). For flexible sigmoidoscopy, the estimate is 864 (95% CI: 672–1266).74
Mammography compared with usual care: lowest estimate is for women aged 70 to 74 years (95% CI: 441, 1389), the highest estimate is 1724 for women aged 40 to 49 years (95% CI: 1176, 3704).75
Personalization of LDCT screening is likely to play an important role in optimizing the balance of benefits and risks, improving the efficiency of resource use, and helping screening programs maximize their impact in shifting the detection of lung cancer to an earlier stage.53 At the same time, tailoring screening minimizes the risk of unnecessary explorations and repeat scans for individuals who present with lower risk.76 Several studies are exploring the role that biomarkers, such as liquid biopsies, can play to determine the baseline risk of people attending the screening and individualize ensuing protocols. However, the precise role they can play remains to be elucidated.77
Another important consideration is that LDCT screening may offer the opportunity to detect other common conditions—namely chronic obstructive pulmonary disease and cardiovascular disease78—and potentially act as a catalyst for some people to quit smoking and adopt healthier behaviors.33 Thus, its impact could extend beyond lung cancer to reducing the burden of other common noncommunicable diseases as well. Clear management protocols would be required to guide individuals to appropriate care pathways on the basis of LDCT findings, as is the case with incidental nodule management protocols.52 This would involve a shift away from indication-specific care pathways to a multidisciplinary, multidisease approach, and would require close coordination among pulmonologists, cardiologists, and other health professionals,52 supported by a comprehensive electronic medical record system that bridges different care settings.11
In conclusion, many of the challenges identified in our research are consistent with previous findings in the literature.16, 17, 18, 19 There is a wealth of ongoing feasibility research in Europe and elsewhere that can help identify sustainable solutions most suited to each national context. It is also worth bearing in mind that similar challenges existed for the implementation of other cancer screening programs.11,67
Ultimately, the decision to invest in large-scale LDCT screening programs will be a matter of political will and judgment. Given the maturity of the evidence supporting LDCT screening,3 it is disappointing that the pace of implementation around Europe has been so slow. The pressures of the COVID-19 pandemic have undoubtedly contributed to this slow pace, but this is not the only factor. Underlying perceptions of lung cancer as a self-inflicted condition, stigma toward people who smoke,38 and possible reluctance by governments to commit to long-term investment in a large-scale prevention program, are all possible contributing factors.
With lung cancer outcomes badly hit by the COVID-19 pandemic, shifting the tide on lung cancer takes on renewed urgency. Early detection is the most effective way to transform lung cancer from a fatal to a treatable condition. Building on decades of research, governments now have a unique opportunity to create the most locally appropriate, effective, efficient, and equitable lung cancer screening programs within their health systems.
Acknowledgments
The initial research and drafting of this article were conducted by The Health Policy Partnership with financial support from AstraZeneca, who were also invited to comment on the article. None of the other co-authors received any compensation for their contributions to this article.
Footnotes
Disclosure: Dr. Wait is employed by The Health Policy Partnership and reported receiving funding through institution from AstraZeneca to conduct this research and for the initial drafting of the article; The Health Policy Partnership has also been commissioned to conduct non–product-related, nonpromotional policy work over the past 36 months for Amgen, AstraZeneca, Bristol Myers Squibb (BMS), Johnson & Johnson, Novartis, Advanced Accelerator Applications, Roche Diagnostics, Sanofi, GE Healthcare, Elekta, Vifor Pharma, UCB, Pfizer, Boehringer Ingelheim, and Philips. Dr. Alvarez-Rosete served as consultant researcher of The Health Policy Partnership. Dr. Osama and Ms. Bancroft are also employed by The Health Policy Partnership. Professor Garrido reports receiving a grant/contract from AstraZeneca; honoraria and meeting support from AstraZeneca, Boehringer Ingelheim, BMS, Janssen, MSD, Medscape, Novartis, Pfizer, Roche, Takeda, and Touch Time; and had advisory board participation for Abbvie, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, GlaxoSmithKline, Janssen, Lilly, MSD, Novartis, Pfizer, Roche, Takeda. Professor Adamek reports advisory board participation for Medtronic; received honoraria from Roche; and has stock options with F.O.R.E. Therapeutics (formerly NovellusDx). Professor van Meerbeeck had advisory board participation and received honoraria from Sanofi-Regeneron. Dr. Leleu reports receiving contract/grant, advisory board fees, meeting support, and honoraria from AstraZeneca. Ms. Hallersjö Hult reports receiving honoraria from AstraZeneca and is a board member of All.Can Sweden. Professor Couraud reports participating in the advisory board and receiving honoraria, meeting support, and grant/contract from Roche and AstraZeneca. Professor Baldwin reports receiving honoraria for speaking engagements with MSD, BMS, AstraZeneca, and Roche; and served as an advisor to the UK National Screening Committee in a personal capacity. The remaining authors declare no conflict of interest.
Cite this article as: Wait S, Alvarez-Rosete A, Osama T, et al. Implementing lung cancer screening in Europe: taking a systems approach. JTO Clin Res Rep 2020;3:100329.
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Luengo-Fernandez R., Leal J., Gray A., Sullivan R. Economic burden of cancer across the European Union: a population-based cost analysis. Lancet Oncol. 2013;14:1165–1174. doi: 10.1016/S1470-2045(13)70442-X. [DOI] [PubMed] [Google Scholar]
- 3.Field J.K., Vulkan D., Davies M.P.A., et al. Lung cancer mortality reduction by LDCT screening: UKLS randomised trial results and international meta-analysis. Lancet Reg Health Eur. 2021;10:100179. doi: 10.1016/j.lanepe.2021.100179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Koning H.J., van der Aalst C.M., de Jong P.A., et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382:503–513. doi: 10.1056/NEJMoa1911793. [DOI] [PubMed] [Google Scholar]
- 5.National Lung Screening Trial Research Team. Aberle D.R., Adams A.M., Adams A.M., et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Meerbeeck J.P., Franck C. Lung cancer screening in Europe: Where are we in 2021? Transl Lung Cancer Res. 2021;10:2407–2417. doi: 10.21037/tlcr-20-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rzyman W., Szurowska E., Adamek M. Implementation of lung cancer screening at the national level: polish example. Transl Lung Cancer Res. 2019;8(suppl 1):S95–S105. doi: 10.21037/tlcr.2019.03.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ministero della Salute (AO1) 0020222-08/11/2021-Gab-GAB-P. Rome: Ministry of Health. Appendix 1. www.sanita24.ilsole24ore.com/pdf2010/Editrice/ILSOLE24ORE/QUOTIDIANO_SANITA/Online/_Oggetti_Correlati/Documenti/2021/11/10/decreto.pdf?uuid=AElVmnv
- 9.Ministerul Sănătății Bucharest: Ministry of Health. National Cancer Control Plan. http://www.ms.ro/wp-content/uploads/2022/01/Plan-National-de-Combatere-a-Cancerului.pdf
- 10.Vaccarella S., Lortet-Tieulent J., Saracci R., et al. Reducing social inequalities in cancer: evidence and priorities for research. Lyon: International Agency for Research on Cancer. IARC Sci Publ. 2019;168. https://www.iarc.who.int/news-events/reducing-social-inequalities-in-cancer-evidence-and-priorities-for-research/ [PubMed]
- 11.Anttila A., Lönnberg S., Ponti A., et al. Towards better implementation of cancer screening in Europe through improved monitoring and evaluation and greater engagement of cancer registries. Eur J Cancer. 2015;51:241–251. doi: 10.1016/j.ejca.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 12.WHO 12 February. Copenhagen: World Health Organization regional Office for Europe. https://www.euro.who.int/en/media-centre/events/events/2020/02/who-european-conference-on-screening/who-european-conference-on-screening.-meeting-report
- 13.WHO Monitoring the building blocks of health systems: a handbook of indicators and their measurement strategies. Geneva: WHO. https://www.who.int/workforcealliance/knowledge/toolkit/26/en/ Accessed February 7, 2022.
- 14.EU. TOPIA TOwards imProved screening for breast, cervical, and colorectal cancer In All of EUrope. https://eu-topia.org/
- 15.The Health Policy Partnership Lung cancer screening: building resilience and sustainability of healthcare. systems. https://www.healthpolicypartnership.com/project/health-system-sustainability-and-resilience/
- 16.Oudkerk M., Liu S., Heuvelmans M.A., Walter J.E., Field J.K. Lung cancer LDCT screening and mortality reduction - evidence, pitfalls and future perspectives. Nat Rev Clin Oncol. 2021;18:135–151. doi: 10.1038/s41571-020-00432-6. [DOI] [PubMed] [Google Scholar]
- 17.van der Aalst C.M., Ten Haaf K., de Koning H.J. Implementation of lung cancer screening: what are the main issues? Transl Lung Cancer Res. 2021;10:1050–1063. doi: 10.21037/tlcr-20-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sands J., Tammemägi M.C., Couraud S., et al. Lung screening benefits and challenges: a review of the data and outline for implementation. J Thorac Oncol. 2021;16:37–53. doi: 10.1016/j.jtho.2020.10.127. [DOI] [PubMed] [Google Scholar]
- 19.Rankin N.M., McWilliams A., Marshall H.M. Lung cancer screening implementation: complexities and priorities. Respirology. 2020;25(suppl 2):5–23. doi: 10.1111/resp.13963. [DOI] [PubMed] [Google Scholar]
- 20.Lam S., Tammemagi M. Contemporary issues in the implementation of lung cancer screening. Eur Respir Rev. 2021:30. doi: 10.1183/16000617.0288-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson J., Jungner G. Principles and practice of screening for disease. Geneva: World Health Organization. Public Health Pap. 1968;34. https://www.who.int/ionizing_radiation/medical_radiation_exposure/munich-WHO-1968-Screening-Disease.pdf
- 22.Ocak S., Tournoy K., Berghmans T., et al. Lung cancer in Belgium. J Thorac Oncol. 2021;16:1610–1621. doi: 10.1016/j.jtho.2021.07.022. [DOI] [PubMed] [Google Scholar]
- 23.US National Library of Medicine Epidemiological study to assess the prevalence of lung cancer (PREVALUNG). Clin Trials.gov identifier NCT03976804. https://clinicaltrials.gov/ct2/show/NCT03976804
- 24.CRCDC-CORSE Project ACAPULCO. https://www.acapulco.crcdc-corse.fr/projet-acapulco
- 25.Couraud S., Ferretti G., Milleron B., et al. Recommendations of French specialists on screening for lung cancer. Rev Mal Respir. 2021;38:310–325. doi: 10.1016/j.rmr.2021.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Leleu O., Basille D., Auquier M., et al. Lung cancer screening by low-dose CT scan: baseline results of a French prospective study. Clin Lung Cancer. 2020;21:145–152. doi: 10.1016/j.cllc.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 27.Haute Autorité de Santé Screening for bronchopulmonary cancer by low-dose chest CT scan without injection: update of the 2016 opinion. Orientation report. https://www.has-sante.fr/upload/docs/application/pdf/2022-02/rapport_dorientation_depistage_du_cancer_bronchopulmonaire_par_scanner_thoracique_faible_dose_sans_injection_actualisation_d.pdf
- 28.Andersson E., Wilking N., Fridhammar A., Lindgren P. Lung cancer in Sweden – an analysis of the burden of disease and the value of previous detection. Lund: Institutet för Hälso- och Sjukvårdsekonomi. https://ihe.se/publicering/sjukdomsborda-lungcancer/
- 29.NHS England Targeted screening for lung cancer with Low Radiation Dose Computed Tomography: Standard Protocol Prepared for the Targeted Lung Health Checks Programme. https://www.england.nhs.uk/publication/targeted-screening-for-lung-cancer/
- 30.Ghimire B., Maroni R., Vulkan D., et al. Evaluation of a health service adopting proactive approach to reduce high risk of lung cancer: the Liverpool Healthy Lung Programme. Lung Cancer. 2019;134:66–71. doi: 10.1016/j.lungcan.2019.05.026. [DOI] [PubMed] [Google Scholar]
- 31.Moffat J., Hiom S., Kumar H.S., Baldwin D.R. Lung cancer screening – gaining consensus on next steps. Proceedings of a Closed Workshop in the UK. Lung Cancer. 2018;125:121–127. doi: 10.1016/j.lungcan.2018.07.029. [DOI] [PubMed] [Google Scholar]
- 32.Kauczor H.U., Baird A.M., Blum T.G., et al. ESR/ERS statement paper on lung cancer screening. Eur Radiol. 2020;30:3277–3294. doi: 10.1007/s00330-020-06727-7. [DOI] [PubMed] [Google Scholar]
- 33.Rzyman W., Didkowska J., Dziedzic R., et al. Consensus statement on a screening programme for the detection of early lung cancer in Poland. Adv Respir Med. 2018;86:53–74. doi: 10.5603/ARM.2018.0009. [DOI] [PubMed] [Google Scholar]
- 34.Wormanns D., Kauczor H.U., Antoch G., et al. Joint statement of the German radiological society and the German respiratory society on a quality-assured early detection Program for Lung Cancer With Low-Dose CT. RoFo. 2019;191:993–997. doi: 10.1055/a-0998-4399. [DOI] [PubMed] [Google Scholar]
- 35.United Kingdom lung cancer coalition Access Matters: achieving universal access to optimal lung cancer care in the UK. London: UKLCC. https://www.uklcc.org.uk/our-reports/
- 36.Ruano-Ravina A., Fernández-Villar A., Provencio-Pulla M. Cons: lung cancer screening with low-dose computed tomography. Gac Sanit. 2016;30:383–385. doi: 10.1016/j.gaceta.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 37.Garrido P., Sánchez M., Belda Sanchis J., et al. Reflections on the implementation of low-dose computed tomography screening in individuals at high risk of lung cancer in Spain. Arch Bronconeumol. 2017;53:568–573. doi: 10.1016/j.arbres.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 38.Global lung cancer coalition Insights from the Global Lung Cancer Coalition’s 2021 patient experience survey. Summary report. https://www.lungcancercoalition.org/surveys/2021-patient-experience-survey/2021
- 39.Lung cancer Europe Position paper. Bern: LuCE. Disparities and Challenges in Access to Lung Cancer Diagnostics and Treatment Across Europe. https://www.lungcancereurope.eu/2020/02/04/lung-cancer-europe-2020-position-paper-disparities-and-challenges-in-access-to-lung-cancer-diagnosis-and-treatment-across-europe/
- 40.Garnier C., Frauenfelder T., Puhan M., Kaufmann C., Laubereau B. Feasibility study on an LDCT lung cancer screening program in Switzerland. Project reference P19-64, part 1: Foundations. Lausanne, Zurich and Lucerne. https://www.lungenliga.ch/fileadmin/user_upload/LLS/01_MetaNavigation/04_Fachpersonen/Research_Fund/2019/EXTERNAL_USE_Part_1_Foundations_LDCTscreening.pdf Accessed May 9, 2022.
- 41.Harris M., Thulesius H., Neves A.L., et al. How European primary care practitioners think the timeliness of cancer diagnosis can be improved: a thematic analysis. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2019-030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.OECD/European Observatory on Health Systems and Policies . OECD Publishing; Paris: 2021. Croatia: Country Health Profile 2019. State of Health in the EU.https://www.oecd.org/publications/croatia-country-health-profile-2021-717e5510-en.htm Accessed May 9, 2022. [Google Scholar]
- 43.British Society of Thoracic Imaging The Royal College of Radiologists. Considerations to ensure optimum roll-out of targeted lung cancer screening over the next five years. London: RCR. https://www.rcr.ac.uk/posts/considerations-ensure-optimum-roll-out-targeted-lung-cancer-screening-over-next-five-years
- 44.Ministerstwo zdrowia Rzeczypospolitej Polskiej National Program of Early Lung Cancer Detection (WWRP) using Low Dose Computed Tomography (NDTK) - a combination of secondary and primary prevention in order to improve awareness of lung cancer among the public and health care personnel]. Appendix No. 17. Warsaw: Ministry of Health. https://www.funduszeeuropejskie.gov.pl/media/72320/Zalacznik_17_Ogolnopolski_Program_WWRP.pdf
- 45.Ng C., Maier H., Augustin F. Lung cancer screening—the surgeon’s perspective [Memo] Mag Eur Oncol. 2019;12:171–174. [Google Scholar]
- 46.Office for National Statistics Public Health England. Index of Cancer Survival for Clinical Commissioning Groups in England: Adults Diagnosed Between 2001 and 2016 and Followed up to 2017. Newport: ONS Statistical [Bulletin] https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/indexofcancersurvivalforclinicalcommissioninggroupsinengland/adultsdiagnosed2001to2016andfollowedupto2017
- 47.Royal College of Physicians National Lung Cancer Audit: organisational audit report 2019. London: RCP. https://www.rcplondon.ac.uk/projects/outputs/organisational-audit-report-2019
- 48.Longkanker Nederland 21st National Lung Cancer Symposium - screening for lung cancer. https://www.longkankernederland.nl/nieuws/21ste-nationale-longkanker-symposium-screening-naar-longkanker
- 49.Roy Castle Lung Cancer Foundation Campaigns: look after your lungs. https://roycastle.org/campaigns/look-after-your-lungs/
- 50.Gohlke H. Smoking Cessation – an update. Dtsch Z Sportmed. 2017;68:281–286. [Google Scholar]
- 51.Direzione generale della prevenzione sanitaria Rome: Ministero della Salute. [National Prevention Plan; 2020–2025] https://www.salute.gov.it/imgs/C_17_notizie_5029_0_file.pdf
- 52.Field J.K., de Koning H., Oudkerk M., et al. Implementation of lung cancer screening in Europe: challenges and potential solutions: summary of a multidisciplinary roundtable discussion. ESMO Open. 2019;4 doi: 10.1136/esmoopen-2019-000577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ten Haaf K., van der Aalst C.M., de Koning H.J., Kaaks R., Tammemägi M.C. Personalising lung cancer screening: an overview of risk-stratification opportunities and challenges. Int J Cancer. 2021;149:250–263. doi: 10.1002/ijc.33578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ali N., Lifford K.J., Carter B., et al. Barriers to uptake among high-risk individuals declining participation in lung cancer screening: a mixed methods analysis of the UK Lung Cancer Screening (UKLS) trial. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2015-008254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peake M.D. Deprivation, distance, and death in lung cancer. Thorax. 2015;70:108–109. doi: 10.1136/thoraxjnl-2014-206153. [DOI] [PubMed] [Google Scholar]
- 56.Baldwin D.R., Brain K., Quaife S. Participation in lung cancer screening. Transl Lung Cancer Res. 2021;10:1091–1098. doi: 10.21037/tlcr-20-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mazieres J., Pujol J., Kalampalikis N., et al. Perception of lung cancer among the general population and comparison with other cancers. J Thorac Oncol. 2015;10:420–425. doi: 10.1097/JTO.0000000000000433. [DOI] [PubMed] [Google Scholar]
- 58.Couraud S., Greillier L., Brignoli-Guibaudet L., et al. Current and former smokers: who wants to be screened? Clin Lung Cancer. 2018;19:493–501. doi: 10.1016/j.cllc.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 59.WHO European tobacco use: trends report 2019. Copenhagen: World Health Organization Regional Office for Europe. https://www.euro.who.int/en/health-topics/disease-prevention/tobacco/publications/2019/european-tobacco-use-trends-report-2019-2019
- 60.Regionala cancercentrum I Samverkan. [Prevention and early detection: Stockholm Gotland]. 2020. https://cancercentrum.se/samverkan/vara-uppdrag/prevention-och-tidig-upptackt/. Accessed May 9, 2022.
- 61.Oudkerk M., Devaraj A., Vliegenthart R., et al. European position statement on lung cancer screening. Lancet Oncol. 2017;18:e754–e766. doi: 10.1016/S1470-2045(17)30861-6. [DOI] [PubMed] [Google Scholar]
- 62.Pastorino U., Silva M., Sestini S., et al. Prolonged lung cancer screening reduced 10-year mortality in the MILD trial: new confirmation of lung cancer screening efficacy. Ann Oncol. 2019;30:1672. doi: 10.1093/annonc/mdz169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.European Commission Cordis. 4-IN THE LUNG RUN: towards INdividually tailored INvitations, screening INtervals, and INtegrated co-morbidity reducing strategies in lung cancer screening. https://cordis.europa.eu/project/id/848294
- 64.National Institute of Public Health Slovenia (NIJZ) WP5 Cancer screening webinar: summary report. New Openings Cancer Screen Eur. 2021;2. Llubljana. Innovative partnership for action against cancer joint action (iPAAC JA) https://www.ipaac.eu/news-detail/en/51-wp5-cancer-screening-webinar/
- 65.Veronesi G., Colombo P., Novellis P., et al. Pilot study on use of home telephoning to identify and recruit high-risk individuals for lung cancer screening. Lung Cancer. 2017;105:39–41. doi: 10.1016/j.lungcan.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 66.Royal Pharmaceutical Society Utilising community pharmacists to support people with cancer. London: RCP. https://www.rpharms.com/Portals/0/RPS%20document%20library/Open%20access/Policy/00207%20001a%202001%20Cancer%20Paper%20WEB.pdf
- 67.Duffy S.W., Myles J.P., Maroni R., Mohammad A. Rapid review of evaluation of interventions to improve participation in cancer screening services. J Med Screen. 2017;24:127–145. doi: 10.1177/0969141316664757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ten Haaf K., Tammemägi M.C., Bondy S.J., et al. Performance and cost-effectiveness of computed tomography lung cancer screening scenarios in a population-based setting: a microsimulation modeling analysis in Ontario, Canada. PLoS Med. 2017;14 doi: 10.1371/journal.pmed.1002225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Black W.C., Gareen I.F., Soneji S.S., et al. Cost-effectiveness of CT screening in the National Lung Screening Trial. N Engl J Med. 2014;371:1793–1802. doi: 10.1056/NEJMoa1312547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cressman S., Peacock S.J., Tammemägi M.C., et al. The cost-effectiveness of high-risk lung cancer screening and drivers of program efficiency. J Thorac Oncol. 2017;12:1210–1222. doi: 10.1016/j.jtho.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 71.Griffin E., Hyde C., Long L., et al. Lung cancer screening by low-dose computed tomography: a cost-effectiveness analysis of alternative programmes in the UK using a newly developed natural history-based economic model. Diagn Progn Res. 2020;4:20. doi: 10.1186/s41512-020-00087-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tomonaga Y., Ten Haaf K., Frauenfelder T., et al. Cost-effectiveness of low-dose CT screening for lung cancer in a European country with high prevalence of smoking - a modelling study. Lung Cancer. 2018;121:61–69. doi: 10.1016/j.lungcan.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 73.Veronesi G. ES21.02 Barriers to implementing screening. Online: World Conference on Lung Cancer, 14 September 2021. International Association for the Study of Lung Cancer.
- 74.Fitzpatrick-Lewis D., Ali M.U., Warren R., Kenny M., Sherifali D., Raina P. Screening for colorectal cancer: a systematic review and meta-analysis. Clin Colorectal Cancer. 2016;15:298–313. doi: 10.1016/j.clcc.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 75.Klarenbach S., Sims-Jones N., Lewin G., et al. Recommendations on screening for breast cancer in women aged 40–74 years who are not at increased risk for breast cancer. CMAJ. 2018;190 doi: 10.1503/cmaj.180463. e1441–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Novellis P., Cominesi S.R., Rossetti F., Mondoni M., Gregorc V., Veronesi G. Lung cancer screening: who pays? Who receives? The European perspectives. Transl Lung Cancer Res. 2021;10:2395–2406. doi: 10.21037/tlcr-20-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Baldwin D.R., Callister M.E., Crosbie P.A., et al. Biomarkers in lung cancer screening: the importance of study design. Eur Respir J. 2021;57(57):2004367. doi: 10.1183/13993003.04367-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Heuvelmans M.A., Vonder M., Rook M., et al. Screening for early lung cancer, chronic obstructive pulmonary disease, and cardiovascular disease (the Big-3) using low-dose chest computed tomography: current evidence and technical considerations. J Thorac Imaging. 2019;34:160–169. doi: 10.1097/RTI.0000000000000379. [DOI] [PubMed] [Google Scholar]