Abstract
Introduction
Paraneoplastic autoimmune diseases (ADs) are a hallmark of thymic epithelial tumors (TETs) and affect treatment management in patients with advanced-stage tumors, yet the risk factors for development of AD in advanced TET remain poorly understood.
Methods
All patients with advanced TET treated at Stanford University between 2006 and 2020 were included. Charts were retrospectively reviewed for the presence of AD, demographic information, and treatment history. Next-generation sequencing was performed on available TET tissue. Multivariate regression was used to evaluate variables associated with AD.
Results
A total of 48 patients were included in the analysis with a median follow-up of 5.4 years. One-third (n = 16, 33%) were diagnosed with having ADs, with 28 distinct ADs identified. The only significant difference observed in the AD cohort compared with the non-AD cohort was a higher proportion of thymoma histotype (81% versus 47%, p = 0.013). The most common AD events were myasthenia gravis (n = 7, 44%) followed by pure red cell aplasia (n = 5, 31%). In the multivariate models, there were no independent factors associated with AD, either at TET diagnosis or subsequent to TET diagnosis. Genomic data were available on 18 patients, and there were no overlapping mutations identified in the nine patients with AD.
Conclusions
ADs are common in patients with advanced TETs. Prior total thymectomy does not affect the development of subsequent AD. Patients who developed AD other than myasthenia gravis were more likely to do so several years after TET diagnosis. Additional work, including multiomic analyses, is needed to develop predictive markers for AD in advanced TET.
Keywords: Thymoma, Thymic carcinoma, Autoimmune disease, Paraneoplastic syndrome, Thymic epithelial tumor
Introduction
Thymic epithelial tumors (TETs), particularly thymomas, are associated with paraneoplastic autoimmune diseases (ADs).1,2 Myasthenia gravis (MG) is the most common AD observed in patients with TET, with 30% to 44% of patients with thymoma developing MG either at the time of diagnosis or subsequently thereafter.3,4 Other described paraneoplastic ADs include cytopenias such as pure red cell aplasia and hypogammaglobulinemia, systemic lupus erythematous, and polymyositis.5,6
Although the pathophysiology of AD in TET is poorly understood, it is postulated to be related to dysregulation of the normal immune function of the thymus. In normal thymic epithelial tissue, immature T-cell progenitors undergo positive and negative selection to prevent reaction with self-antigens.7,8 In thymomas, the risk of AD is increased owing to abnormal thymic architecture combined with additional pathologic features that affect positive and negative selection of T-cells. The neoplastic epithelial cells minimally express major histocompatibility complex class II, which plays a vital function in T-cell positive selection. The autoimmune regulator protein, which is an important factor for T-cell negative selection, is absent in more than 95% of thymomas.8,9 In contrast, ADs are rare in thymic carcinoma (TC), and as a result, there is a paucity of data on the potential mechanisms leading to AD in this subgroup.8
Prior studies describing AD in TET were primarily in patients with early stage disease.3,5,6 A previous retrospective study by Padda et al.5 of patients in the International Thymic Malignancy Interest Group retrospective database found improved recurrence-free survival and overall survival (OS) in patients with TET who had concurrent AD compared with those without AD.5 Similarly, Filosso et al.10 reported improved OS in patients with TET and MG compared with those without MG. Nevertheless, AD was associated with earlier stage TET in both studies, which may suggest that the reported favorable prognosis is a result of lead-time bias rather than favorable TET tumor biology. Additional research is needed to better understand the potential impact of AD on prognosis.5,10
Little is known regarding the characteristics and prognostic features of AD in patients with advanced-stage TET. Here, we retrospectively reviewed all patients with advanced TET treated at our institution to determine the demographic, clinical, molecular, and treatment characteristics of patients who develop AD.
Materials and Methods
Database and Study Cohort
We performed a retrospective cohort study of patients with advanced-stage or recurrent TET treated between 2006 and 2017 using the Stanford Cancer Institute Research Database (SCIRDB).11 The SCIRDB identified patients for potential inclusion in the cohort by means of programmed searches of the electronic medical record (EMR) which included International Classification of Diseases (ICD) Ninth and Tenth Revision codes that matched TET diagnoses. Patient eligibility for this cohort study was subsequently confirmed manually in the EMR. Patients were additionally included in the cohort who were identified by manual searches of the medical thoracic oncology clinics between 2017 and 2020. Patients were excluded if they (1) did not have a pathologic diagnosis of TET and (2) did not have imaging or pathologic diagnosis confirming advanced-stage or recurrent TET. The Stanford University Institutional Review Board approved this study, including web-based informed consent for patients who participated in the molecular analysis and waiver of consent granted for retrospective data analysis.
Outcomes and Measurements
There were 2 members of the research team (SS, JH) who abstracted the following data from the EMR: demographic characteristics, including age at diagnosis, race, and sex; clinical characteristics, including pathologic classification, stage, presence of AD at TET diagnosis, and AD onset after TET diagnosis; and treatment characteristics, including total thymectomy, radiation therapy, and systemic treatments for TET and AD. Pathologic diagnosis was classified using the 2004 or 2015 WHO criteria depending on the year of diagnosis.12,13 We reclassified cases diagnosed before 2004 per the 2004 WHO criteria. Stage was described by Masaoka-Koga classification with International Thymic Malignancy Interest Group clarifications.14 Date of TET diagnosis was the date of initial pathologic confirmation; date of advanced or recurrent disease, whether at initial diagnosis or recurrence, was defined as the date of radiologic or pathologic confirmation of advanced or recurrent disease; and date of AD onset was the date of initial clinician note in the EMR documenting the AD diagnosis. For patients with two or more ADs, the unique events were evaluated separately to determine onset of AD before or at the time of TET diagnosis versus after TET diagnosis. We determined TET and AD-specific treatments by review of clinician documentation for indication. A subgroup of patients provided written informed consent as part of a Stanford University Institutional Review Board–approved protocol for next-generation sequencing (NGS) on tumor specimens. Targeted NGS was performed by means of The Solid Tumor Actionable Mutation Panel (STAMP) covering 130 genes,15 FoundationOne covering 324 genes,16 or NCI-Match NGS Assay covering 323 genes.17 Pathogenic versus variant of unknown significance status was determined by independent pathologist (MO) review by means of evaluation of functional data published in literature, previously published expert panel review,18 and search of ClinVar, Catalogue of Somatic Mutations in Cancer, OncoKB, and Jax Clinical Knowledgebase databases.
Statistical Analysis
All analyses were performed by means of STATA 16 (StataCorp 2019, College Station, TX). The baseline demographic, clinical, NGS, and treatment characteristics were summarized using descriptive statistics. The continuous variables were summarized as means with SD or medians with range, and the categorical variables were summarized as frequencies with relative percentages. Student’s two-sided t test and Pearson’s chi-square test were used to compare continuous and categorical variables, respectively. To evaluate the association of demographic and clinical factors with the presence of AD at time of TET diagnosis, we used multivariate logistic regression. To determine the association of demographic, clinical, and treatment factors with the onset of AD after TET diagnosis, we used Cox proportional hazards regression, adjusting for covariates. Patients with AD before TET diagnosis and subsequent new AD after TET diagnosis were included in both the multivariate logistic and Cox regression analyses. The full list of variables included in the analyses is listed in Supplementary Tables 1 and 2, respectively. Patients with missing data were excluded from the multivariate analyses. The p value less than 0.05 was defined as statistically significant.
Results
Overall Cohort Characteristics
The analysis cohort consisted of 48 patients (Table 1). The mean age at time of TET diagnosis was 54 years (SD = 15), and 30 patients (63%) were males. Type B thymoma (n = 18, 38%) and TC (n = 18, 38%) were the most common TET subtypes, although definitive subtype classification was not always possible given 75% were samples obtained by core biopsy. Most patients were diagnosed with advanced or recurrent disease at the time of TET diagnosis (n = 30, 63%). One-third of the patients (n = 16, 33%) had AD either at TET diagnosis or developed AD subsequently.
Table 1.
Demographic, Clinical, and Treatment Characteristics
| Characteristic | N (%) |
p Value | ||
|---|---|---|---|---|
| Total | AD | No AD | ||
| N | 48 | 16 | 32 | |
| Mean age at diagnosis (y) (SD) | 54 (15) | 51 (17) | 55 (14) | 0.37 |
| Male sex | 30 (63) | 8 (50) | 22 (69) | 0.21 |
| Race | ||||
| Black | 0 (0) | 0 (0) | 0 (0) | 0.46 |
| Native American or Alaskan Native | 1 (2.1) | 0 (0) | 1 (3.1) | |
| Asian | 22 (46) | 10 (63) | 12 (38) | |
| White | 18 (38) | 5 (31) | 13 (41) | |
| Native Hawaiian or Other Pacific Islander | 2 (4.2) | 0 | 2 (6.3) | |
| Other | 5 (10) | 1 (6.3) | 4 (13) | |
| WHO classification | ||||
| Thymoma | 28 (58) | 13 (81) | 15 (47) | 0.01 |
| Thymic carcinoma | 18 (38) | 2 (13) | 16 (50) | |
| Not defined | 2 (4) | 1 (6) | 1 (3) | |
| Masaoka-Koga stage at diagnosis | ||||
| I | 2 (4.2) | 1 (6.3) | 1 (3.2) | 0.81 |
| II | 2 (4.2) | 1 (6.3) | 1 (3.2) | |
| III | 9 (19) | 3 (19) | 6 (19) | |
| IV | 30 (63) | 8 (50) | 22 (69) | |
| Not defined | 5 (10) | 3 (19) | 2 (6.3) | |
| Total thymectomy | 29 (60) | 10 (63) | 19 (59) | 0.84 |
| Radiation therapya | 36 (75) | 11 (69) | 25 (78) | 0.48 |
| Systemic treatment for thymic neoplasm | 46 (96) | 15 (94) | 31 (97) | 0.61 |
| Median number of systemic treatments (range) | 3.0 (0–8) | 3.0 (0–5) | 3.0 (0–8) | 0.83 |
Note: Data are expressed as number and percentage, n (%) except where otherwise stated. Bold values indicate statistical significant.
AD, autoimmune disease.
Indications for radiation therapy included the following: adjuvant, definitive, oligometastatic, and palliative.
Most patients received systemic medical treatment for TET (n = 46, 96%), along with 29 patients (60%) also undergoing total thymectomy and 36 patients (75%) undergoing radiation either postoperative or for a variety of other indications. Of the 13 patients who presented with stages I to III disease, 11 patients (85%) underwent total thymectomy and 11 patients (85%) underwent radiation therapy. Those with stage IV disease underwent these local treatments less frequently: 13 patients (43%) and 20 patients (67%) underwent total thymectomy and radiation, respectively. Patients received a median of 3 (range: 0–8) systemic medical treatments. The most common regimen was cyclophosphamide, doxorubicin, and cisplatin (n = 27, 56%), followed by carboplatin and paclitaxel (n = 24, 50%). One patient with thymoma received immunotherapy in the fourth-line line setting as part of a clinical trial and did not have a diagnosis of or develop AD. The median length of follow-up for the cohort was 5.4 years.
Molecular Abnormalities
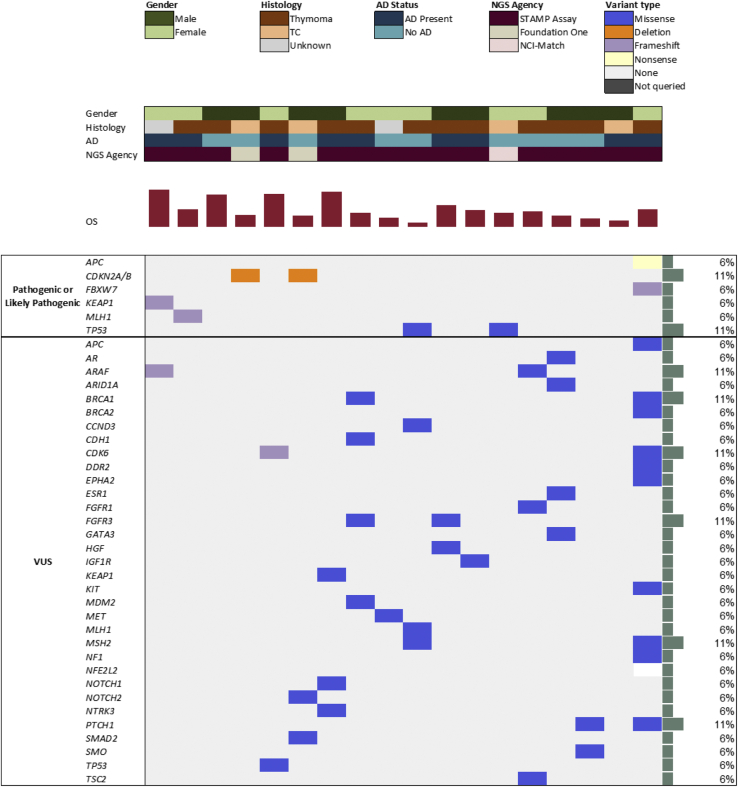
A total of 18 patients (38%) had NGS performed with the distribution of gene mutations and aberrations illustrated in Figure 1. Half of the patients with NGS data had AD (n = 9, 50%). There was one patient with AD and thymoma with pathogenic or likely pathogenic mutations in two genes: APC and FBXW7. Two additional patients with AD had a pathogenic mutation in KEAP1 (TET pathology unknown) and MLH1 (thymoma), respectively. Of the patients without AD, there were two with pathogenic mutations in TP53 (one TC and one thymoma) and two with pathogenic mutations in CDKN2A/B (both with TC).
Figure 1.
OncoPrint of genomic information using targeted NGS panel STAMP (n = 15), Foundation One (n = 2), and NCI-Match (n = 1). Pathogenic or likely pathogenic mutations are reported separately from VUS. AD, autoimmune disease; NCI, National Cancer Institute; NGS, next-generation sequencing; OS, overall survival; STAMP, Solid Tumor Actionable Mutation Panel; TC, thymic carcinoma; VUS, variant of unknown significance.
Paraneoplastic Disease and AD Characteristics
There were 28 ADs among 16 patients (Table 2). There was a median of one AD event per patient and a range of one to six AD events per patient (Table 2). Nine deaths occurred in the 16 patients with AD (56%) during a median follow-up time since TET diagnosis of 100 months. There were six patients (38%) with first onset of AD before TET diagnosis and all patients had thymoma. Of the six patients with AD before TET diagnosis, four were diagnosed with MG only, one was diagnosed with autoimmune neuropathy, and one was diagnosed with MG, pemphigus vulgaris, and dermatomyositis. There were 10 patients who had first onset of AD after TET diagnosis with a median time to onset of 65 months (interquartile range: 32–164 mo), and this included two patients with TC. The total number of AD per patient was not significantly different between those who had initial AD onset before or at onset of TET diagnosis and those who had AD onset after TET diagnosis (p = 0.13).
Table 2.
AD Onset and Characteristics
| Patient | AD (n) | AD Events | WHO Classificationa | TET Diagnosis to First AD (mo)b | AD Treatments |
|---|---|---|---|---|---|
| 1 | 6 | CASPR2 encephalitis; Good syndrome; enteropathy; minimal change disease; MG; Sweet syndrome | Type B | N/A | Azathioprine, cyclophosphamide, cyclosporine, daratumumab, everolimus, IVIG, plasmapheresis, rituximab, steroids, tacrolimus |
| 2 | 1 | MG | Type B | N/A | IVIG, mycophenolate, pyridostigmine, steroids |
| 3 | 1 | MG | Type B | 84 | None |
| 4 | 2 | Good syndrome; MG | Type B | N/A | Azathioprine, pyridostigmine, plasmapheresis |
| 5 | 1 | Neuropathy | Type B | N/A | IVIG, steroids |
| 6 | 1 | MG | Type B | N/A | Pyridostigmine |
| 7 | 3 | Amegakaryocytic thrombocytopenia; arthritis; PRCA | Type B | 160 | Steroids |
| 8 | 1 | MG | Type AB | 1 | None |
| 9 | 1 | PRCA | Type AB | 39 | Steroids |
| 10 | 1 | PRCA | Thymoma, NOS | 172 | Steroids |
| 11 | 2 | Enteropathy, neutropenia | Thymoma, NOS | 47 | Everolimus, octreotide, steroids |
| 12 | 1 | PRCA | Thymoma, NOS | 168 | None |
| 13 | 3 | Dermatomyositis, pemphigus vulgaris, MG | Thymoma, NOS | N/A | Mycophenolate, pyridostigmine, steroids |
| 14 | 1 | PRCA | TC | 30 | Cyclosporine, IVIG, steroids |
| 15 | 1 | ITP | TC | 30 | Steroids |
| 16 | 2 | Arthritis; Good syndrome | Thymic neoplasm | 184 | IVIG, methotrexate, steroids |
AD, autoimmune disease; ITP, immune thrombocytopenic purpura; IVIG, intravenous immune globulin; MG, myasthenia gravis; N/A, not applicable; NOS, not otherwise specified; PRCA, pure red cell aplasia; TC, thymic carcinoma; TET, thymic epithelial tumor.
Thymoma listed as type A, type B, or type AB.
Cases where AD preceded TET diagnosis, including those with multiple ADs if at least one AD occurred before TET diagnosis, were designated as N/A.
The most common AD was MG in seven patients (44%), followed by pure red cell aplasia in five patients (31%) and Good syndrome (thymoma-associated hypogammaglobulinemia and immunodeficiency) in three patients (19%). MG occurred before TET diagnosis in five of seven (71%) cases. Other ADs before TET diagnosis included one patient with neuropathy and one patient with both dermatomyositis and pemphigus vulgaris. All other ADs, including all autoimmune cytopenias (Good syndrome, amegakaryocytic thrombocytopenia, pure red cell aplasia, neutropenia, and immune thrombocytopenic purpura) occurred after TET diagnosis.
Treatment Course of TET-Associated AD
Patients with AD received a median of 2.5 AD-directed treatments (interquartile range: 1–3). Acknowledging the different types and numbers of AD, we noted a trend for a higher median number of AD treatments for those who had AD onset after TET diagnosis versus those who had AD onset before TET diagnosis (4 versus 3, respectively, p = 0.058). In total, patients received 15 unique AD-directed treatments (Table 2). The most common AD-directed treatment for MG was pyridostigmine (n = 4 of 7; 57%), whereas two patients with MG did not require AD-directed treatments beyond treatment of the TET (29%). Of the five patients with pure red cell aplasia, three patients received systemic steroid monotherapy (60%); one patient received combination cyclosporine, intravenous immunoglobulin, and systemic steroid; and another patient received supportive transfusions alone.
Factors Associated With AD
There were no demographic, clinical, or treatment characteristics associated with AD except for an increased proportion of patients with thymoma histotype (81% versus 47%, p = 0.013; Table 1). After adjusting for covariates, demographic and clinical factors were not associated with increased odds of AD at the time of TET diagnosis (Supplementary Table 1) or development of AD after TET diagnosis (Supplementary Table 2). Treatment variables of total thymectomy and radiation therapy were also not associated with development of AD after TET diagnosis.
Discussion
In this single-institution retrospective cohort study, our goal was to analyze factors associated with AD in patients with advanced TET along with describing the onset, chronology, and characteristics of these ADs. The types of AD observed varied depending on whether the AD onset occurred before or after TET diagnosis. Patients who developed AD before TET diagnosis most often had MG, whereas patients who developed AD after TET diagnosis (often several years after) had AD other than MG, particularly autoimmune cytopenias and Good syndrome. There was a higher proportion of thymomas observed in the patients who had AD, but thymoma histotype was not independently associated with AD. There were no independent demographic and clinical factors associated with AD at or after TET diagnosis. A molecular analysis was performed on a subset of 18 patients, and there was no overlap in pathogenic or likely pathogenic mutations in those with AD, although there was only a total of seven tumors with pathogenic mutations observed.
Similar to prior findings in early stage disease,5,19 one-third of our cohort with advanced TET had an associated paraneoplastic AD. Of the patients diagnosed with having AD before a diagnosis of TET, most had neurologic phenomena (MG, neuropathy) with dermatologic (pemphigus vulgaris, dermatomyositis) phenomena observed in one patient. Although prior studies revealed the trend for MG onset before TET diagnosis, this has not been described for dermatologic phenomena and needs further study.6,20 For patients who developed AD after a TET diagnosis, the timeline to onset of these AD in our cohort was substantially longer than previous reports.6 We speculate that this, in part, may reflect a delay in AD diagnosis for patients with autoimmune cytopenias or recurrent infections associated with Good syndrome, which could initially be attributed to myelosuppressive chemotherapy treatment.
In agreement with previously reported literature, we identified AD more frequently in patients with thymoma compared with those with TC.5,10 Interestingly, two of the TC patients with AD had an autoimmune cytopenia develop 30 months after diagnosis: pure red cell aplasia (PRCA) and immune thrombocytopenic purpura, respectively. Although rare to develop AD in TC, prior studies reported patients with TC and concurrent PRCA, Sjogren syndrome, and MG.21, 22, 23 Although we did not identify age or sex to be associated with AD, either before or after TET diagnosis, other retrospective studies conducted in patients with primarily early stage TET found that patients who had a TET-associated AD were more likely to be female and younger.5,6,10 The association of age and sex with AD in patients with TET may vary based on the specific AD diagnosis. For example, in some studies, men and women have been reported to be equally affected by thymoma-associated MG, but women are more likely to develop PRCA than men.6,24 Although we did not observe these demographic associations in our study, this could represent an inherent difference between patients who have early stage versus advanced TET, or could reflect a limitation in our sample size and inability to perform independent comparisons of unique AD diagnoses.
In our study, treatment characteristics, including total thymectomy, radiotherapy in various settings, or number of systemic therapies, did not significantly affect the risk of developing AD after a TET diagnosis. Treatment with immunotherapy has been extensively associated with the development of AD in patients with both thymoma and TC.25,26 This was recently confirmed in a phase 2 clinical trial of pembrolizumab in relapsed and refractory TET in which 71% of patients with thymoma and 15% of patients with TC experienced grade 3 to 4 immune-related adverse events and 24% of patients discontinued treatment owing to immune-related adverse events.27 Prior case reports and series have revealed that systemic treatment for TET can also treat the AD—sunitinib for autoimmune neutropenia, platinum-based chemotherapy for autoimmune hepatitis, and everolimus for enteropathy and PRCA.28, 29, 30 Nevertheless, we did not identify larger scale studies that evaluate the impact of anticancer systemic therapy on outcomes of AD, as one may hypothesize that tumor response correlates with improvement of AD. This lack of data may be because most published literature evaluates patients with early stage TET where systemic therapy is generally not used. Nevertheless, these data are important to help guide management of patients with TETs and AD.
Although we did not identify a substantial association between total thymectomy and development of a subsequent AD, a retrospective study conducted in patients who underwent thymectomy for diverse reasons (MG, TET, benign conditions, etc.) found that patients were more likely to subsequently develop AD if they underwent thymectomy compared with those without thymectomy.31 This is particularly interesting because thymectomy is part of the treatment for nonthymomatous MG but may be associated with development of other unique AD in the future, or could reflect selection bias of this treatment as patients with initial AD requiring thymectomy may have a greater propensity for future AD. Several case reports have even described the appearance or worsening of systemic lupus erythematous after thymectomy.32, 33, 34 In our cohort, there were five patients who underwent total thymectomy for thymoma-associated MG, four of whom had stages I to III TET where the total thymectomy was part of the initial definitive TET treatment. Two of the five patients who underwent total thymectomy, both with initial diagnosis of early stage TET, developed a subsequent new AD years after initial thymectomy. It is also possible that the presence of a thymic abnormality, particularly malignancy, may disrupt development of the immune repertoire in an irreversible way, and therefore, total thymectomy is not effective in mitigating this impact.6
We also conducted tissue NGS to evaluate whether genetic differences exist in patients with TET stratified by AD status. Previous large-scale genomic sequencing efforts detected molecular abnormalities in TET, including mutations in TP53, GTF2I, c-KIT, BCL2, EGFR, HRAS, and NRAS.35, 36, 37, 38 Consistent with CDKN2A/B and TP53 mutations previously reported in TC, we also identified these mutations more frequently in patients with TC than thymoma.39 Ardeshir-Larijani et al.40 recently presented the genomic characterization of a cohort with advanced TET and identified frequent TP53 and CDKN2A/B mutations, with TP53 mutations frequent in both thymoma and TC (affecting approximately one-third) and cell cycle alterations more frequent in TC than thymoma. This group also identified pathogenic PIK3CA and NF1 mutations, which were not observed in our cohort, and did not evaluate molecular associations with AD status.40 This difference in genomic findings could be because Ardeshir-Larijani et al.40 conducted NGS on advanced TET specimens (i.e., recurrent or metastatic sites), whereas we conducted NGS on TET specimens at various time points in the clinical course (i.e., some from the initial diagnosis primary site and others from recurrent or metastatic sites). A study in mostly early stage TETs found increased aneuploidy in patients with concurrent thymoma and MG, but no association between MG and mutations in single genes.35 Here, we identified nonoverlapping pathogenic mutations in four genes—KEAP1, MLH1, APC, and FBXW7—across three of nine patients with AD; none of these mutations were detected in patients without AD. Although chromosomal aneuploidy has been implicated in paraneoplastic autoimmunity of TETs in The Cancer Genome Atlas study,35 we could not identify any existing literature describing if and how genomic alterations affect AD in TET, and further studies are necessary to elucidate the potential role of KEAP1, MLH1, APC, and FBXW7 on development of AD in patients with TETs.
These data have limitations due to their observational and retrospective nature. Our cohort originates from a single institution. NGS data could not be performed on all patients, were not completed with a single assay, and were not conducted at the same time point in clinical course.
To the best of our knowledge, we provide the first description of patients with advanced TET and the characteristics of their associated AD. Given we did not identify any factors associated with developing AD after an advanced TET diagnosis, clinicians must maintain a high index of suspicion for AD years after TET diagnosis given the latency from TET diagnosis to AD diagnosis and should be aware that multiple ADs can develop in a single patient throughout their TET disease course.
CRediT Authorship Contribution Statement
Surbhi Singhal: Conceptualization, Methodology, Formal analysis, Writing - original draft.
Jessica Hellyer: Conceptualization, Methodology, Data acquisition, Writing - original draft.
Madhu M. Ouseph: Data acquisition, Writing - review & editing.
Heather A. Wakelee: Conceptualization, Funding acquisition, Writing - review & editing.
Sukhmani K. Padda: Supervision, Writing - original draft.
Acknowledgments
This work was supported by a National Cancer Institute Cancer Center Support Grant (P30CA124435). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute. The authors acknowledge the Stanford Cancer Institute Research Database.
Footnotes
Disclosure: The authors declare no conflict of interest.
Cite this article as: Singhal S, Hellyer J, Ouseph MM, et al. Autoimmune disease in patients with advanced thymic epithelial tumors. JTO Clin Res Rep. 2022;3:100323.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2022.100323.
Supplementary Data
References
- 1.Evoli A., Lancaster E. Paraneoplastic disorders in thymoma patients. J Thorac Oncol. 2014;9(suppl 2):S143–S147. doi: 10.1097/JTO.0000000000000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffacker V., Schult A., Tiesinga J.J., et al. Thymomas alter the T-cell subset composition in the blood: a potential mechanism for thymoma-associated autoimmune disease. Blood. 2000;96:3872–3879. [PubMed] [Google Scholar]
- 3.Souadjian J.V., Enriquez P., Silverstein M.N., Pépin J.M. The spectrum of diseases associated with thymoma: coincidence or syndrome? Arch Intern Med. 1974;134:374–379. [PubMed] [Google Scholar]
- 4.Gilhus N.E., Verschuuren J.J. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol. 2015;14:1023–1036. doi: 10.1016/S1474-4422(15)00145-3. [DOI] [PubMed] [Google Scholar]
- 5.Padda S.K., Yao X., Antonicelli A., et al. Paraneoplastic Syndromes and thymic malignancies: an examination of the International Thymic Malignancy Interest Group Retrospective Database. J Thorac Oncol. 2018;13:436–446. doi: 10.1016/j.jtho.2017.11.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernard C., Frih H., Pasquet F., et al. Thymoma associated with autoimmune diseases: 85 cases and literature review. Autoimmun Rev. 2016;15:82–92. doi: 10.1016/j.autrev.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Klein L., Kyewski B., Allen P.M., Hogquist K.A. Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see) Nat Rev Immunol. 2014;14:377–391. doi: 10.1038/nri3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weksler B., Lu B. Alterations of the immune system in thymic malignancies. J Thorac Oncol. 2014;9(suppl 2):S137–S142. doi: 10.1097/JTO.0000000000000299. [DOI] [PubMed] [Google Scholar]
- 9.Ströbel P., Murumägi A., Klein R., et al. Deficiency of the autoimmune regulator AIRE in thymomas is insufficient to elicit autoimmune polyendocrinopathy syndrome type 1 (APS-1) J Pathol. 2007;211:563–571. doi: 10.1002/path.2141. [DOI] [PubMed] [Google Scholar]
- 10.Filosso P.L., Evangelista A., Ruffini E., et al. Does myasthenia gravis influence overall survival and cumulative incidence of recurrence in thymoma patients? A retrospective clinicopathological multicentre analysis on 797 patients. Lung Cancer. 2015;88:338–343. doi: 10.1016/j.lungcan.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Stanford Medicine Research Informatics Center. SCIRDB. https://med.stanford.edu/ric/coordination/SCIRDB.html
- 12.Travis WD, World Health Organization, International Agency for Research on Cancer . IARC Press; Lyon, France: 2004. International Association for the Study of Lung Cancer, International Academy of Pathology. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus, and Heart. [Google Scholar]
- 13.Marx A., Chan J.K.C., Coindre J.M., et al. The 2015 World Health Organization classification of tumors of the thymus: continuity and changes. J Thorac Oncol. 2015;10:1383–1395. doi: 10.1097/JTO.0000000000000654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Detterbeck F.C., Nicholson A.G., Kondo K., Van Schil P., Moran C. The Masaoka-Koga stage classification for thymic malignancies: clarification and definition of terms. J Thorac Oncol. 2011;6(suppl 3):S1710–S1716. doi: 10.1097/JTO.0b013e31821e8cff. [DOI] [PubMed] [Google Scholar]
- 15.Newman A.M., Bratman S.V., To J., et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat Med. 2014;20:548–554. doi: 10.1038/nm.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foundation Medicine Inc. FoundationOne®CDx technical information. https://info.foundationmedicine.com/hubfs/FMI%20Labels/FoundationOne_CDx_Label_Technical_Info.pdf
- 17.Lih C.J., Harrington R.D., Sims D.J., et al. Analytical validation of the next-generation sequencing assay for a nationwide signal-finding clinical trial: molecular analysis for therapy choice clinical trial. J Mol Diagn. 2017;19:313–327. doi: 10.1016/j.jmoldx.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gelb B.D., Cavé H., Dillon M.W., et al. ClinGen’s RASopathy Expert Panel consensus methods for variant interpretation. Genet Med. 2018;20:1334–1345. doi: 10.1038/gim.2018.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kondo K., Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from. Japan Ann Thorac Surg. 2003;76:878–884. doi: 10.1016/s0003-4975(03)00555-1. [DOI] [PubMed] [Google Scholar]
- 20.De Perrot M., Liu J., Bril V., McRae K., Bezjak A., Keshavjee S.H. Prognostic significance of thymomas in patients with myasthenia gravis. Ann Thorac Surg. 2002;74:1658–1662. doi: 10.1016/s0003-4975(02)04083-3. [DOI] [PubMed] [Google Scholar]
- 21.Levy Y., Afek A., Sherer Y., et al. Malignant thymoma associated with autoimmune diseases: a retrospective study and review of the literature. Semin Arthritis Rheum. 1998;28:73–79. doi: 10.1016/s0049-0172(98)80039-5. [DOI] [PubMed] [Google Scholar]
- 22.Haurt F.P., Wee A., Chan H.L., Tan Y.O. Primary thymic carcinoma and its association with dermatomyositis and pure red cell aplasia. Int J Dermatol. 1992;31:426–428. doi: 10.1111/j.1365-4362.1992.tb02675.x. [DOI] [PubMed] [Google Scholar]
- 23.DiMario F.J., Lisak R.P., Kornstein M.J., Brooks J.J. Myasthenia gravis and primary squamous cell carcinoma of the thymus: a case report. Neurology. 1988;38:580–582. doi: 10.1212/wnl.38.4.580. [DOI] [PubMed] [Google Scholar]
- 24.Murakawa T., Nakajima J., Sato H., Tanaka M., Takamoto S., Fukayama M. Thymoma associated with pure red-cell aplasia: clinical features and prognosis. Asian Cardiovasc Thorac Ann. 2002;10:150–154. doi: 10.1177/021849230201000213. [DOI] [PubMed] [Google Scholar]
- 25.Giaccone G., Kim C., Thompson J., et al. Pembrolizumab in patients with thymic carcinoma: a single-arm, single-centre, phase 2 study. Lancet Oncol. 2018;19:347–355. doi: 10.1016/S1470-2045(18)30062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rajan A., Heery C.R., Thomas A., et al. Efficacy and tolerability of anti-programmed death-ligand 1 (PD-L1) antibody (avelumab) treatment in advanced thymoma. J Immunother Cancer. 2019;7:269. doi: 10.1186/s40425-019-0723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho J., Kim H.S., Ku B.M., et al. Pembrolizumab for patients with refractory or relapsed thymic epithelial tumor: an open-label phase II trial. J Clin Oncol. 2019;37:2162–2170. doi: 10.1200/JCO.2017.77.3184. [DOI] [PubMed] [Google Scholar]
- 28.Becker H., Auman K., Claus R., von Bubnoff N., Sachs U.J., Waller C.F. Sunitinib in the treatment of thymoma and associated autoimmune neutropenia. JCO Precis Oncol. 2017;1:1–7. doi: 10.1200/PO.17.00095. [DOI] [PubMed] [Google Scholar]
- 29.Mejri N., Chabchoub I., Gargouri I., et al. Effect of chemotherapy on autoimmune hepatitis in thymoma: a case report and literature review. Cancer Biol Med. 2013;10:169–173. doi: 10.7497/j.issn.2095-3941.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hellyer J.A., Ouseph M.M., Padda S.K., Wakelee H.A. Everolimus in the treatment of metastatic thymic epithelial tumors. Lung Cancer. 2020;149:97–102. doi: 10.1016/j.lungcan.2020.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Lin T.M., Chang Y.S., Hou T.Y., et al. Risk of incident autoimmune diseases in patients with thymectomy. Ann Clin Transl Neurol. 2020;7:1072–1082. doi: 10.1002/acn3.51055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park M.J., Kim Y.A., Lee S.S., Kim B.C., Kim M.K., Cho K.H. Appearance of systemic lupus erythematosus in patients with myasthenia gravis following thymectomy: two case reports. J Korean Med Sci. 2004;19:134–136. doi: 10.3346/jkms.2004.19.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mevorach D., Perrot S., Laoussadi S., et al. Appearance of systemic lupus erythematosus after thymectomy: four case reports and review of the literature. Lupus. 1995;4:33–37. doi: 10.1177/096120339500400108. [DOI] [PubMed] [Google Scholar]
- 34.Boonen A., Rennenberg R., Van Der Linden S. Thymoma-associated systematic lupus erythematosus, exacerbating after thymectomy. A case report and review of the literature [2] Rheumatology. 2000;39:1044–1046. doi: 10.1093/rheumatology/39.9.1044. [DOI] [PubMed] [Google Scholar]
- 35.Radovich M., Pickering C.R., Felau I., et al. The integrated genomic landscape of thymic epithelial tumors. Cancer Cell. 2018;33:244–258.e10. doi: 10.1016/j.ccell.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rajan A., Girard N., Marx A. State of the art of genetic alterations in thymic epithelial tumors. J Thorac Oncol. 2014;9(suppl 2):S131–S136. doi: 10.1097/JTO.0000000000000298. [DOI] [PubMed] [Google Scholar]
- 37.Kelly R.J., Petrini I., Rajan A., Wang Y., Giaccone G. Thymic malignancies: from clinical management to targeted therapies. J Clin Oncol. 2011;29:4820–4827. doi: 10.1200/JCO.2011.36.0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Padda S.K., Gökmen-Polar Y., Hellyer J.A., et al. Genomic clustering analysis identifies molecular subtypes of thymic epithelial tumors independent of World Health Organization histologic type. Oncotarget. 2021;12:1178–1186. doi: 10.18632/oncotarget.27978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Enkner F., Pichlhöfer B., Zaharie A.T., et al. Molecular profiling of thymoma and thymic carcinoma: genetic differences and potential novel therapeutic targets. Pathol Oncol Res. 2017;23:551–564. doi: 10.1007/s12253-016-0144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ardeshir-Larijani F., Radovich M., Schneider B.P., Loehrer P.J. Clinicogenomic characterization of metastatic thymic epithelial tumors. J Clin Oncol. 2021;39(suppl 15) doi: 10.1200/PO.22.00465. 8579–8579. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.