Abstract
Background
COVID‐19 illness is highly variable, ranging from infection with no symptoms through to pneumonia and life‐threatening consequences. Symptoms such as fever, cough, or loss of sense of smell (anosmia) or taste (ageusia), can help flag early on if the disease is present. Such information could be used either to rule out COVID‐19 disease, or to identify people who need to go for COVID‐19 diagnostic tests. This is the second update of this review, which was first published in 2020.
Objectives
To assess the diagnostic accuracy of signs and symptoms to determine if a person presenting in primary care or to hospital outpatient settings, such as the emergency department or dedicated COVID‐19 clinics, has COVID‐19.
Search methods
We undertook electronic searches up to 10 June 2021 in the University of Bern living search database. In addition, we checked repositories of COVID‐19 publications. We used artificial intelligence text analysis to conduct an initial classification of documents. We did not apply any language restrictions.
Selection criteria
Studies were eligible if they included people with clinically suspected COVID‐19, or recruited known cases with COVID‐19 and also controls without COVID‐19 from a single‐gate cohort. Studies were eligible when they recruited people presenting to primary care or hospital outpatient settings. Studies that included people who contracted SARS‐CoV‐2 infection while admitted to hospital were not eligible. The minimum eligible sample size of studies was 10 participants. All signs and symptoms were eligible for this review, including individual signs and symptoms or combinations. We accepted a range of reference standards.
Data collection and analysis
Pairs of review authors independently selected all studies, at both title and abstract, and full‐text stage. They resolved any disagreements by discussion with a third review author. Two review authors independently extracted data and assessed risk of bias using the QUADAS‐2 checklist, and resolved disagreements by discussion with a third review author. Analyses were restricted to prospective studies only. We presented sensitivity and specificity in paired forest plots, in receiver operating characteristic (ROC) space and in dumbbell plots. We estimated summary parameters using a bivariate random‐effects meta‐analysis whenever five or more primary prospective studies were available, and whenever heterogeneity across studies was deemed acceptable.
Main results
We identified 90 studies; for this update we focused on the results of 42 prospective studies with 52,608 participants. Prevalence of COVID‐19 disease varied from 3.7% to 60.6% with a median of 27.4%. Thirty‐five studies were set in emergency departments or outpatient test centres (46,878 participants), three in primary care settings (1230 participants), two in a mixed population of in‐ and outpatients in a paediatric hospital setting (493 participants), and two overlapping studies in nursing homes (4007 participants). The studies did not clearly distinguish mild COVID‐19 disease from COVID‐19 pneumonia, so we present the results for both conditions together.
Twelve studies had a high risk of bias for selection of participants because they used a high level of preselection to decide whether reverse transcription polymerase chain reaction (RT‐PCR) testing was needed, or because they enrolled a non‐consecutive sample, or because they excluded individuals while they were part of the study base. We rated 36 of the 42 studies as high risk of bias for the index tests because there was little or no detail on how, by whom and when, the symptoms were measured. For most studies, eligibility for testing was dependent on the local case definition and testing criteria that were in effect at the time of the study, meaning most people who were included in studies had already been referred to health services based on the symptoms that we are evaluating in this review.
The applicability of the results of this review iteration improved in comparison with the previous reviews. This version has more studies of people presenting to ambulatory settings, which is where the majority of assessments for COVID‐19 take place. Only three studies presented any data on children separately, and only one focused specifically on older adults.
We found data on 96 symptoms or combinations of signs and symptoms. Evidence on individual signs as diagnostic tests was rarely reported, so this review reports mainly on the diagnostic value of symptoms. Results were highly variable across studies. Most had very low sensitivity and high specificity. RT‐PCR was the most often used reference standard (40/42 studies).
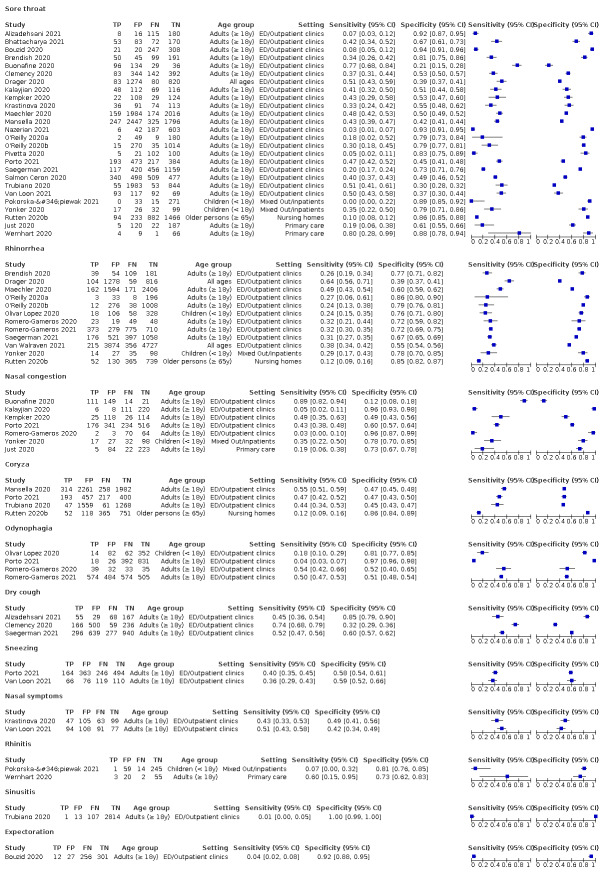
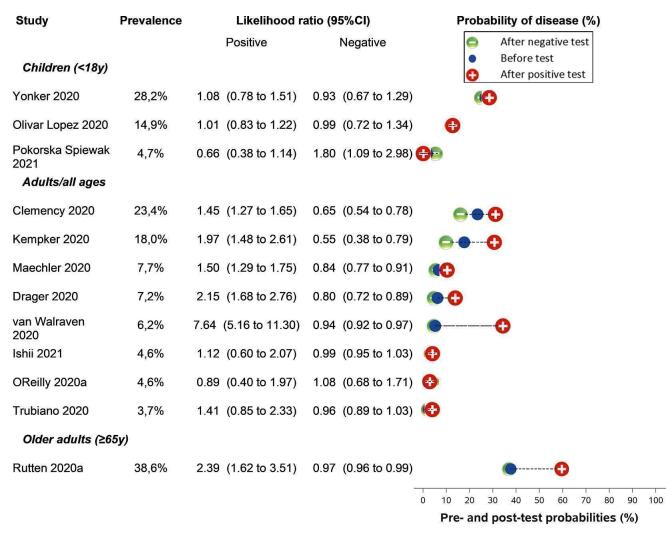
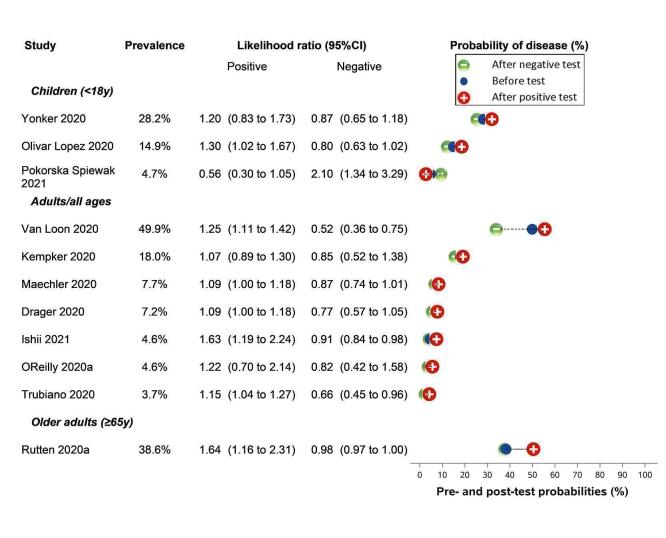
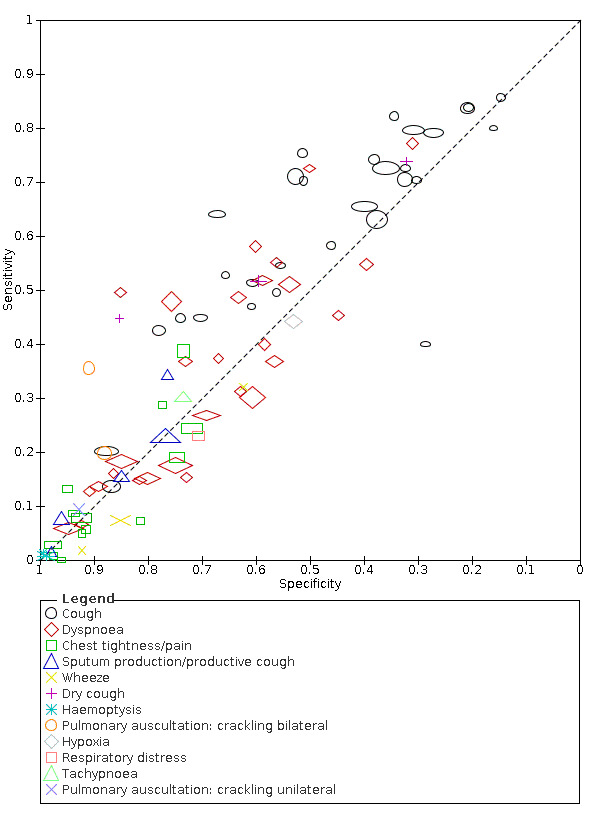
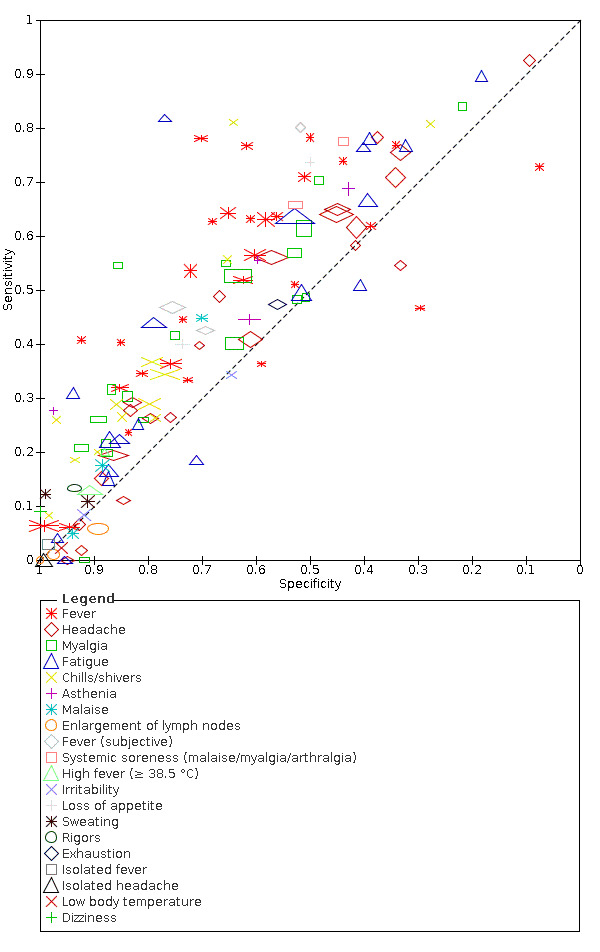
Only cough (11 studies) had a summary sensitivity above 50% (62.4%, 95% CI 50.6% to 72.9%)); its specificity was low (45.4%, 95% CI 33.5% to 57.9%)). Presence of fever had a sensitivity of 37.6% (95% CI 23.4% to 54.3%) and a specificity of 75.2% (95% CI 56.3% to 87.8%). The summary positive likelihood ratio of cough was 1.14 (95% CI 1.04 to 1.25) and that of fever 1.52 (95% CI 1.10 to 2.10). Sore throat had a summary positive likelihood ratio of 0.814 (95% CI 0.714 to 0.929), which means that its presence increases the probability of having an infectious disease other than COVID‐19.
Dyspnoea (12 studies) and fatigue (8 studies) had a sensitivity of 23.3% (95% CI 16.4% to 31.9%) and 40.2% (95% CI 19.4% to 65.1%) respectively. Their specificity was 75.7% (95% CI 65.2% to 83.9%) and 73.6% (95% CI 48.4% to 89.3%). The summary positive likelihood ratio of dyspnoea was 0.96 (95% CI 0.83 to 1.11) and that of fatigue 1.52 (95% CI 1.21 to 1.91), which means that the presence of fatigue slightly increases the probability of having COVID‐19.
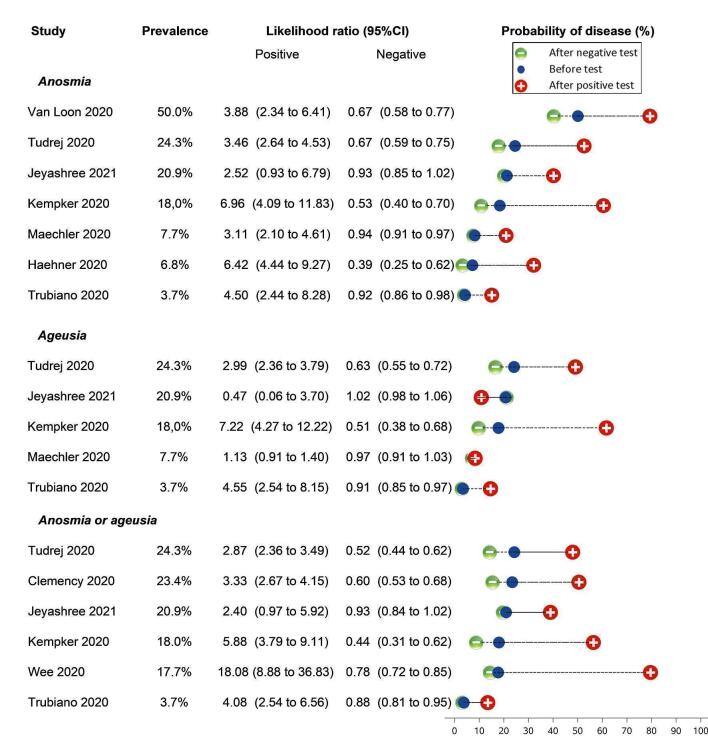
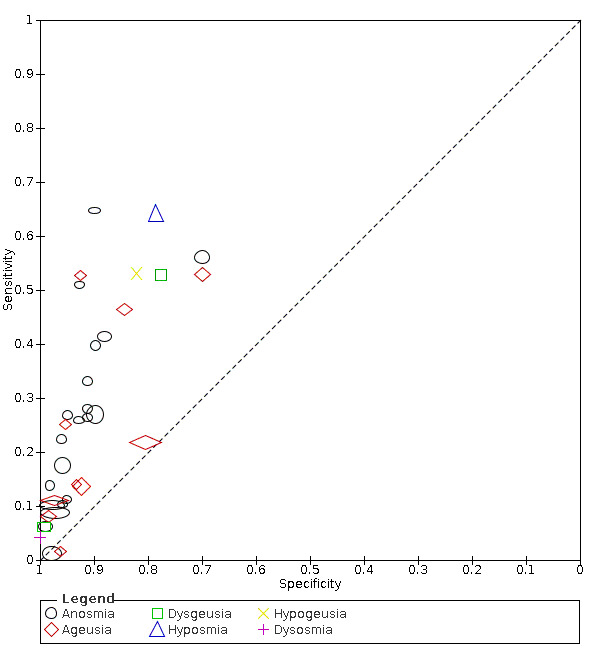
Anosmia alone (7 studies), ageusia alone (5 studies), and anosmia or ageusia (6 studies) had summary sensitivities below 50% but summary specificities over 90%. Anosmia had a summary sensitivity of 26.4% (95% CI 13.8% to 44.6%) and a specificity of 94.2% (95% CI 90.6% to 96.5%). Ageusia had a summary sensitivity of 23.2% (95% CI 10.6% to 43.3%) and a specificity of 92.6% (95% CI 83.1% to 97.0%). Anosmia or ageusia had a summary sensitivity of 39.2% (95% CI 26.5% to 53.6%) and a specificity of 92.1% (95% CI 84.5% to 96.2%). The summary positive likelihood ratios of anosmia alone and anosmia or ageusia were 4.55 (95% CI 3.46 to 5.97) and 4.99 (95% CI 3.22 to 7.75) respectively, which is just below our arbitrary definition of a 'red flag', that is, a positive likelihood ratio of at least 5. The summary positive likelihood ratio of ageusia alone was 3.14 (95% CI 1.79 to 5.51).
Twenty‐four studies assessed combinations of different signs and symptoms, mostly combining olfactory symptoms. By combining symptoms with other information such as contact or travel history, age, gender, and a local recent case detection rate, some multivariable prediction scores reached a sensitivity as high as 90%.
Authors' conclusions
Most individual symptoms included in this review have poor diagnostic accuracy. Neither absence nor presence of symptoms are accurate enough to rule in or rule out the disease. The presence of anosmia or ageusia may be useful as a red flag for the presence of COVID‐19. The presence of cough also supports further testing. There is currently no evidence to support further testing with PCR in any individuals presenting only with upper respiratory symptoms such as sore throat, coryza or rhinorrhoea.
Combinations of symptoms with other readily available information such as contact or travel history, or the local recent case detection rate may prove more useful and should be further investigated in an unselected population presenting to primary care or hospital outpatient settings.
The diagnostic accuracy of symptoms for COVID‐19 is moderate to low and any testing strategy using symptoms as selection mechanism will result in both large numbers of missed cases and large numbers of people requiring testing. Which one of these is minimised, is determined by the goal of COVID‐19 testing strategies, that is, controlling the epidemic by isolating every possible case versus identifying those with clinically important disease so that they can be monitored or treated to optimise their prognosis. The former will require a testing strategy that uses very few symptoms as entry criterion for testing, the latter could focus on more specific symptoms such as fever and anosmia.
Plain language summary
How accurate are symptoms and medical examination to diagnose COVID‐19?
Key messages
‐ The results suggest that a single symptom included in this review cannot accurately diagnose COVID‐19.
‐ Loss of sense of taste or smell could be a 'red flag' for the presence of COVID‐19. Cough or fever might be useful to identify people who might have COVID‐19. These symptoms might be useful to prompt further testing when they are present.
‐ We need more research to investigate combinations of symptoms and signs with other information such as recent contact or travel history, or vaccination status, and in children, and adults aged 65 years and over.
What are symptoms or signs of COVID‐19?
Symptoms are experienced by patients. COVID‐19 symptoms include cough, sore throat, high temperature, diarrhoea, headache, muscle or joint pain, fatigue, and loss of sense of smell and taste.
Signs are measured by healthcare workers during clinical examination. They include lung sounds, blood pressure, blood oxygen level and heart rate.
Symptoms and signs of COVID‐19 might be important to help people know whether they and the people they come into contact with should isolate at home, undergo testing with a rapid lateral flow test or PCR (laboratory‐based) test, or be hospitalised.
What did we want to find out?
Symptoms and signs of COVID‐19 are varied and may indicate other diseases, not just COVID‐19. We wanted to know how accurate diagnosis of COVID‐19 is, based on symptoms and signs from medical examination. We were interested in people with suspected COVID‐19, who go to their doctor, outpatient test centres or hospital.
What did we do?
We searched for studies that assessed the accuracy of symptoms and signs to diagnose COVID‐19. Studies had to be conducted in general practice, outpatient test centres or hospital outpatient settings only. We only included studies of people in hospital if signs and symptoms were recorded when they were admitted to the hospital, for example through the emergency department.
What did we find?
We focused on 42 studies with 52,608 participants in this review. The studies assessed 96 separate or combined symptoms and signs. Thirty‐five studies were conducted in emergency departments or outpatient COVID‐19 test centres (46,878 participants), 3 studies in general practice (1230 participants), 2 studies in children’s hospitals (493 in‐ and outpatients), and 2 studies in nursing homes (4007 participants). The studies were conducted in 18 different countries around the world. Twenty‐three studies were conducted in Europe, 8 in North‐America, 5 in Asia, and 3 in South‐America and 3 in Australia. We didn’t find any studies conducted in Africa. Three focused specifically on children, and only 1 focused on adults aged 65 years and over.
Most studies did not clearly distinguish between mild and severe COVID‐19, so we present the results for mild, moderate and severe disease together.
Few studies reported individual signs as diagnostic tests, so we focus mainly on the diagnostic value of symptoms. The most frequently reported symptoms were cough, fever, shortness of breath and sore throat.
According to the studies in our review, in a group of 1000 people with suspected COVID‐19 of whom 270 (27%) would actually have COVID‐19, around 567 people would have a cough. Of these 567, 168 would actually have COVID‐19. Of the 433 who do not have a cough, 102 would have COVID‐19. In the same 1000 people, around 283 people would have a fever. Of these 283, 102 would actually have COVID‐19. Of the 717 people without fever, 168 would have COVID‐19.
Someone who has lost their sense of smell or taste is five times more likely to have COVID‐19 than someone who hasn’t.
Other symptoms, such as a sore throat or runny nose, are more likely to indicate the presence of an infectious disease other than COVID‐19. In the same 1000 people as described above, around 362 people would have a sore throat. Of these, only 84 would actually have COVID‐19. Of the 638 patients without sore throat, 186 would have COVID‐19. We found similar figures for having a runny nose.
What are the limitations of the evidence?
The results of this updated review are more reliable than those in previous versions as we included more high‐quality studies. However, the accuracy of individual symptoms varied across studies and the diagnostic value of symptoms such as fever, cough or other respiratory symptoms might still be overestimated, as most studies deliberately included participants because they had these symptoms.
The results do not clearly differentiate between people with mild, moderate or severe COVID‐19. Only a few studies investigated the symptom‐based diagnosis of COVID‐19 in children or older adults.
How up to date is this review?
This review updates our previous review. The evidence is up to date to June 2021.
Summary of findings
Summary of findings 1. Symptoms to determine if a patient presenting in primary care or hospital outpatient setting has COVID‐19.
| Symptoms to determine if a patient presenting in primary care or hospital outpatient setting has COVID‐19 | |||||
|
Patient or population: people with COVID‐19 symptoms Setting: primary care or hospital outpatient departments Index test(s): symptoms of COVID‐19 Target condition: SARS‐CoV‐2 infection (symptomatic of any severity); mild or moderate COVID‐19; severe or critical COVID‐19 Reference standard: RT‐PCR Top 10 of most reported symptoms to determine if a patient presenting in primary care or hospital outpatient setting has COVID‐19 (prospective cross‐sectional studies only). We estimated pooled sensitivity and specificity only for prospective studies with a low risk of bias rating for participant selection. | |||||
| Symptom | Setting | Number of studies/number of participants | Sensitivity (ranges) | Specificity (ranges) |
Strength of evidence Number of studies with high risk of bias per QUADAS‐2 domain: participant selection/index test/reference standard/flow and timing |
| Cough | Primary care | 2/414 | 70% to 80% | 16% to 30% | 2/1/0/0 |
| Outpatient clinics/ED | 25/32,756 | 14% to 86% | 15% to 88% | 7/20/1/1 | |
| Mixed: paediatric hospital inpatients/ outpatients | 2/493 | 40% to 47% | 29% to 61% | 0/2/0/0 | |
| Nursing homes | 1/3764 | 63% | 38% | 0/1/0/0 | |
|
All settings (only prospective studies with low risk of bias for participant selection) |
11/18,702 |
Summary estimate: 62% (95% CI 51% to 73%) |
Summary estimate: 45% (95% CI 34% to 58%) |
||
| Fever | Primary care | 1/334 | 33% | 73% | 1/1/0/0 |
| Outpatient clinics/ED | 25/40,278 | 6% to 78% | 8% to 99% | 6/21/1/1 | |
| Mixed: paediatric hospital inpatients/ outpatients | 2/493 | 47% to 51% | 30% to 53% | 0/2/0/0 | |
| Nursing homes | 1/3771 | 63% | 58% | 0/1/0/0 | |
|
All settings (only prospective studies with low risk of bias for participant selection) |
12/28,495 |
Summary estimate: 38% (95% CI 23% to 54%) |
Summary estimate: 75% (95% CI 56% to 88%) |
||
| Dyspnoea | Primary care | 1/334 | 15% | 82% | 1/1/0/0 |
| Outpatient clinics/ED | 24/30,809 | 6% to 77% | 31% to 95% | 7/21/1/1 | |
| Mixed: paediatric hospital inpatients/ outpatients | 2/493 | 7% to 16% | 86% to 92% | 0/2/0/0 | |
| Nursing homes | 1/3622 | 30% | 61% | 0/1/0/0 | |
|
All settings (only prospective studies with low risk of bias for participant selection) |
12/19,545 |
Summary estimate: 23% (95% CI 16% to 32%) |
Summary estimate: 76% (95% CI 65% to 84%) |
||
| Sore throat | Primary care | 2/414 | 19% to 80% | 61% to 88% | 2/1/0/0 |
| Outpatient clinics/ED | 21/26,470 | 3% to 77% | 21% to 94% | 8/19/1/1 | |
| Mixed: paediatric hospital inpatients/ outpatients | 2/493 | 0% to 35% | 79% to 89% | 0/2/0/0 | |
| Nursing homes | 1/2675 | 10% | 86% | 0/1/0/0 | |
|
All settings (only prospective studies with low risk of bias for participant selection) |
10/14,548 |
Summary estimate: 31% (95% CI 20% to 45%) |
Summary estimate: 62% (95% CI 47% to 75%) |
||
| Headache | Primary care | 2/414 | 11% to 40% | 71% to 85% | 2/1/0/0 |
| Outpatient clinics/ED | 19/26,135 | 2% to 93% | 9% to 93% | 10/16/1/1 | |
| Mixed: paediatric hospital inpatients/ outpatients | 2/493 | 0% to 27% | 76% to 95% | 0/2/0/0 | |
| Nursing homes | ‐ | ‐ | ‐ | ‐ | |
|
All settings (only prospective studies with low risk of bias for participant selection) |
7/10899 |
Summary estimate: 36% (95% CI 17% to 60%) |
Summary estimate: 73% (95% CI 53% to 86%) |
||
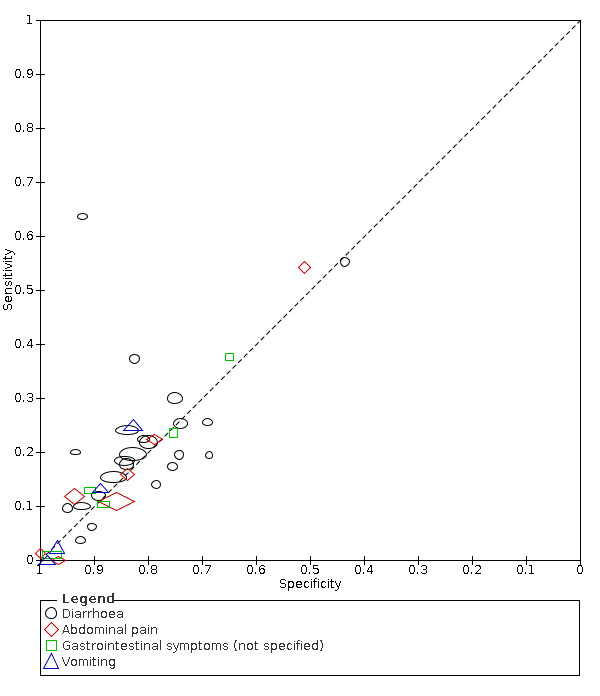
| Diarrhoea | Primary care | 1/334 | 4% | 93% | 1/1/0/0 |
| Outpatient clinics/ED | 19/24042 | 10% to 64% | 44% to 95% | 6/17/1/1 | |
| Mixed: paediatric hospital inpatients/ outpatients | 2/493 | 6% to 20% | 90% to 93% | 0/2/0/0 | |
| Nursing homes | 1/1286 | 18% | 84% | 0/1/0/0 | |
|
All settings (only prospective studies with low risk of bias for participant selection) |
11/13,669 |
Summary estimate: 19% (95% CI 16% to 22%) |
Summary estimate: 84% (95% CI 79% to 88%) |
||
| Myalgia | Primary care | 1/334 | 26% | 81% | 1/1/0/0 |
| Outpatient clinics/ED | 17/16,106 | 20% to 84% | 22% to 92% | 9/15/0/0 | |
| Mixed: paediatric hospital inpatients/ outpatients | 1/319 | 0% | 92% | 0/1/0/0 | |
| Nursing homes | ‐ | ‐ | ‐ | ‐ | |
|
All settings (only prospective studies with low risk of bias for participant selection) |
6/2684 |
Summary estimate: 38% (95% CI 21% to 58%) |
Summary estimate: 75% (95% CI 58% to 87%) |
||
| Anosmia | Primary care | 2/1150 | 26% to 41% | 88% to 93% | 1/2/0/0 |
| Outpatient clinics/ED | 18/18,958 | 1% to 65% | 70% to 99% | 9/15/1/2 | |
| Mixed: paediatric hospital inpatients/ outpatients | ‐ | ‐ | ‐ | ‐ | |
| Nursing homes | ‐ | ‐ | ‐ | ‐ | |
|
All settings (only prospective studies with low risk of bias for participant selection) |
7/9456 |
Summary estimate: 26% (95% CI 14% to 45%) |
Summary estimate: 94% (95% CI 91% to 97%) |
||
| Fatigue | Primary care | 1/334 | 19% | 71% | 1/1/0/0 |
| Outpatient clinics/ED | 15/12,369 | 15% to 90% | 18% to 94% | 6/14//1/1 | |
| Mixed: paediatric hospital inpatients/ outpatients | 2/493 | 0% to 4% | 95% to 97% | 0/2/0/0 | |
| Nursing homes | 1/1286 | 22% | 87% | 0/1/0/0 | |
|
All settings (only prospective studies with low risk of bias for participant selection) |
8/7967 |
Summary estimate: 40% (95% CI 19% to 65%) |
Summary estimate: 74% (95% CI 48% to 89%) |
||
| Chills/shivers | Primary care | 2/414 | 19% to 20% | 89% to 93% | 2/1/0/0 |
| Outpatient clinics/ED | 10/21,980 | 26% to 81% | 28% to 97% | 4/10/0/0 | |
| Mixed: paediatric hospital inpatients/ outpatients | 1/174 | 8% | 98% | 0/1/0/0 | |
| Nursing homes | ‐ | ‐ | ‐ | ‐ | |
|
All settings (only prospective studies with low risk of bias for participant selection) |
5/14,472 |
Summary estimate: 25% (95% CI 15% to 39%) |
Summary estimate: 85% (95% CI 72% to 93%) |
||
| CI: confidence interval; ED: emergency department; RT‐PCR: reverse transcription polymerase chain reaction | |||||
Background
The severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) virus and resulting COVID‐19 pandemic present important diagnostic evaluation challenges. These range from, on the one hand, understanding the value of signs and symptoms in predicting possible infection, assessing whether existing biochemical and imaging tests can identify infection and recognise patients needing critical care, and on the other hand, evaluating whether new diagnostic tests can allow accurate rapid and point‐of‐care testing. Also, the diagnostic aims are diverse, including identifying current infection, ruling out infection, identifying people in need of care escalation, or testing for past infection and immunity.
This review is the second update of a review summarising evidence of the diagnostic accuracy of presenting clinical signs and symptoms for COVID‐19. This review is part of a suite of reviews on the diagnosis of SARS‐CoV‐2 infection and COVID‐19 disease, exploring the accuracy of antibody tests (Deeks 2020a), routine laboratory testing (Stegeman 2020), rapid point‐of‐care tests (Dinnes 2021) and thoracic imaging tests (Islam 2021).
Target condition being diagnosed
The key target conditions for this suite of reviews are current SARS‐CoV‐2 infection, current COVID‐19, and past SARS‐CoV‐2 infection.
For current infection, the severity of the disease is of importance. SARS‐CoV‐2 infection can be asymptomatic (no symptoms); mild or moderate (symptoms such as fever, cough, loss of smell (anosmia) or taste (ageusia), aches, lethargy but without difficulty breathing at rest); severe (symptoms with breathlessness and increased respiratory rate indicative of pneumonia and oxygen need); or critical (requiring intensive support due to severe acute respiratory syndrome (SARS) or acute respiratory distress syndrome (ARDS), shock or other organ dysfunction (NIH 2021). People with COVID‐19 pneumonia (severe or critical disease) require different patient management, which makes it important to distinguish between them and mild or moderate disease.
Thus, there are three target conditions for current infection:
SARS‐CoV‐2 infection (asymptomatic or symptomatic of any severity);
mild or moderate COVID‐19 disease;
COVID‐19 pneumonia (severe or critical).
Here we summarise the evidence on signs and symptoms; as a result asymptomatic SARS‐CoV‐2 and past SARS‐CoV‐2 infection are out of scope for this review.
Index test(s)
Signs and symptoms
Signs and symptoms are used in the initial diagnosis of suspected COVID‐19 disease, and to identify people with COVID‐19 pneumonia. Symptoms are what are experienced by patients, for example cough or nausea. Signs are obtained by clinical examination. Signs of COVID‐19 examined in this review include lung sounds, blood pressure, blood oxygen level and heart rate.
Key symptoms that have been associated with mild to moderate COVID‐19 disease include: troublesome dry cough (for example, coughing more than usual over a one‐hour period, or three or more coughing episodes in 24 hours), fever at examination greater than 37.8 °C, diarrhoea, headache, breathlessness on light exertion, muscle pain, fatigue, and loss of sense of smell and taste (Struyf 2021). Signs and symptoms indicating possible pneumonia (severe or critical disease) include breathlessness at rest, loss of appetite, confusion, pain or pressure in the chest, and temperature above 38 °C.
Clinical pathway
Important in the context of COVID‐19 is that the pathway is multifaceted because it is designed to care for the diseased individual and to protect the community from further spread. Decisions about patient and isolation pathways for COVID‐19 vary according to health services and settings, available resources, and stages of the epidemic. They will change over time if and when effective treatments are identified and populations are increasingly vaccinated. The decision points between these pathways vary, but all include points at which knowledge of the accuracy of diagnostic information is needed to inform rational decision making.
Prior test(s)
Prior testing will depend on whether people are being investigated for SARS‐CoV‐2 infection, mild COVID‐19 or COVID‐19 pneumonia. In this review on signs and symptoms, no prior tests are required because signs and symptoms are used in the initial diagnosis of suspected SARS‐CoV‐2 infection, and in identifying people with mild COVID‐19 or COVID‐19 pneumonia.
Role of index test(s)
Signs and symptoms are used as triage tests, that is, to rule out SARS‐CoV‐2 infection or COVID‐19 disease, but also to identify people with possible COVID‐19 who may require further testing, care escalation or isolation.
Alternative test(s)
We are producing a suite of Cochrane 'living systematic reviews', which will summarise evidence on the clinical accuracy of different tests and diagnostic features, grouped according to the present research questions and settings in the diagnosis of SARS‐CoV‐2 infection and COVID‐19. Summary estimates of accuracy from these reviews will help inform diagnostic, screening, isolation, and patient‐management decisions.
New tests are being developed and evidence is emerging at an unprecedented rate during the COVID‐19 pandemic. We will aim to update these reviews as often as is feasible to ensure that they provide the most up‐to‐date evidence about test accuracy.
These reviews are being produced rapidly to assist in providing a central resource of evidence to assist in the COVID‐19 pandemic, summarising available evidence on the accuracy of the tests and presenting characteristics.
Other Cochrane diagnostic test accuracy (DTA) reviews in the suite of reviews are addressing the following tests.
Chest imaging (computed tomography (CT), chest X‐ray and ultrasound (Islam 2021)
Routine laboratory testing, such as for C‐reactive protein (CRP) and procalcitonin (PCT) (Stegeman 2020)
Antibody tests (Deeks 2020a)
Laboratory‐independent point‐of‐care and near‐patient molecular and antigen tests (Dinnes 2021)
Molecular laboratory tests (in preparation)
Rationale
It is essential to understand the accuracy of diagnostic features and tests to identify the best way they can be used in different settings to develop effective diagnostic and management pathways. For example, the absence of a highly sensitive sign or symptom is good for ruling out COVID‐19, while the presence of a sign or symptom with high specificity is good for ruling in COVID‐19 ('red flag').
Objectives
To assess the diagnostic accuracy of signs and symptoms to determine if a person presenting in primary care or to hospital outpatient settings, such as the emergency department or dedicated COVID‐19 clinics, has COVID‐19.
Secondary objectives
Where data are available, we investigated diagnostic accuracy (either by stratified analysis or meta‐regression) according to:
days since symptom onset;
population (children, adults, older adults ≥ 65 years);
reference standard;
study design;
setting; and
risk of bias in participant selection (as scored using QUADAS‐2)
Objectives of future updates of this review
This review will no longer be updated in its current form. Objectives of any future updates of this review are:
to look at a broader approach involving a combination of signs and symptoms with other easy‐to‐obtain information, for example, point‐of‐care test results;
to perform the listed stratified analyses;
to explore seasonality;
to investigate people who require respiratory support or intensive care.
Summary of previous review
In the first update of our review, we found 44 relevant studies with 26,884 participants. Prevalence of COVID‐19 disease varied from 3% to 71% with a median of 21%. There were three studies from primary care settings (1824 participants), nine studies from outpatient testing centres (10,717 participants), 12 studies performed in hospital outpatient wards (5061 participants), seven studies in hospitalised patients (1048 participants), 10 studies in the emergency department (3173 participants), and three studies in which the setting was not specified (5061 participants). The studies did not clearly distinguish mild COVID‐19 disease from COVID‐19 pneumonia, so we presented the results for both conditions together.
Fifteen studies had a high risk of bias for selection of participants because inclusion in the studies depended on the applicable testing and referral protocols, which included many of the signs and symptoms under study in the review. Five studies only included participants with pneumonia on imaging, suggesting that this is a highly selected population. In an additional 12 studies, we were unable to assess the risk for selection bias. This makes it very difficult to judge the validity of the diagnostic accuracy of the signs and symptoms from these included studies.
None of the studies presented any data on children separately, and only one focused specifically on older adults.
We found data on 84 signs and symptoms. Results were highly variable across studies. Most had very low sensitivity and high specificity. Only cough (25 studies) and fever (7 studies) had a summary sensitivity of at least 50% but specificities were moderate to low. Cough had a sensitivity of 67.4% (95% CI 59.8% to 74.1%) and specificity of 35.0% (95% CI 28.7% to 41.9%). Fever had a sensitivity of 53.8% (95% CI 35.0% to 71.7%) and a specificity 67.4% (95% CI 53.3% to 78.9%). The summary positive likelihood ratio of cough was only 1.04 (95% CI 0.97 to 1.11) and that of fever 1.65 (95% CI 1.41 to 1.93).
Anosmia alone (10 studies), ageusia alone (5 studies), and anosmia or ageusia (6 studies) had sensitivities below 50% but specificities over 85%. Anosmia had a summary sensitivity of 30.5% (95% CI 19.4% to 44.4%) and a specificity of 92.7% (95% CI 87.1% to 96.0%). Ageusia had a summary sensitivity of 29.4% (95% CI 15.1% to 49.5%) and a specificity of 89.0% (95% CI 77.6% to 94.9%). Anosmia or ageusia had a summary sensitivity of 41.0% (95% CI 27.0% to 56.6%) and a specificity of 90.5% (95% CI 81.2% to 95.4%). The summary positive likelihood ratios of anosmia alone and anosmia or ageusia were 4.16 (95% CI 3.10 to 5.60) and 4.31 (95% CI 3.00 to 6.18) respectively, which is just below our arbitrary definition of a red flag, that is, a positive likelihood ratio of at least 5. The summary positive likelihood ratio of ageusia alone was 2.67 (95% CI 1.96 to 3.64).
Only two studies assessed combinations of different signs and symptoms, mostly combining both fever and cough. These combinations had a specificity above 80%, but at the cost of very low sensitivity (< 30%).
We concluded that the majority of individual signs and symptoms included in the review appear to have very poor diagnostic accuracy, although this should be interpreted in the context of selection bias and heterogeneity between studies. Based on the available data, neither absence nor presence of signs or symptoms are accurate enough to rule in or rule out disease. The presence of anosmia or ageusia may be useful as a red flag for the presence of COVID‐19. The presence of fever or cough may, given their high sensitivities, be useful as a triage tool for further testing.
New evidence since previous review
We found more studies on symptoms in people with suspected COVID‐19 that used prospective data collection, allowing for more reliable estimation of measures of diagnostic accuracy. Moreover, this update contains new studies on the diagnostic value of 29 different combinations of signs and symptoms.
Limitations of previous review
The main weakness of the initial review and of the first update was the high risk of selection bias, with many studies including patients who had already been admitted to hospital or who had presented to hospital settings with the intent to hospitalise.
The lack of data on combinations of signs and symptoms was an important evidence gap. Only two studies presented data on such combinations. The few composite signs and symptoms that were presented in those studies had little added diagnostic value compared to single tests.
Methods
Criteria for considering studies for this review
Types of studies
We included published studies of all designs that produce estimates of sensitivity and specificity or provide data from which estimates can be computed. As of this update, we no longer included preprints. If no published version of previously included preprints could be found, we excluded these preprints.
As of this review update, we only included single‐gate, cross‐sectional designs (studies that recruit from a patient pathway before disease status has been ascertained). We included both studies that used retrospective data collection and studies that used prospective data collection, but the main findings of this review will be based on the prospective studies only, as retrospective studies tend to overestimate the diagnostic accuracy of the index tests (Rutjes 2006).
Studies had to have a minimum sample size of 10 participants.
Participants
Studies recruiting people presenting with a clinical suspicion of SARS‐CoV‐2 infection, based on a symptomatic presentation, were eligible. At least 50% of the study population had to present with COVID‐19‐compatible symptoms.
Index tests
All signs and symptoms, including:
signs such as oxygen saturation, measured by oximetry and blood pressure;
symptoms, such as fever or cough.
Target conditions
To be eligible, studies had to identify at least one of:
mild or moderate COVID‐19;
severe or critical COVID‐19 (including COVID‐19 pneumonia).
Asymptomatic infection with SARS‐CoV‐2 is out of scope for this review, considering it is by definition not possible to detect this based on signs and symptoms.
Reference standards
We anticipated that studies would use a range of reference standards. Although reverse transcription polymerase chain reaction (RT‐PCR) is considered the best available test, due to rapidly evolving knowledge about the target conditions, multiple reference standards on their own as well as in combination have emerged.
We expected to encounter cases defined by:
RT‐PCR alone;
RT‐PCR, clinical expertise, and imaging (for example, CT thorax);
repeated RT‐PCR several days apart or from different samples;
plaque reduction neutralisation test (PRNT) or enzyme‐linked immunosorbent assay (ELISA) tests;
information available at a subsequent time point;
World Health Organization (WHO) and other case definitions (see Appendix 1).
This list is not exhaustive, and we recorded all reference standards encountered.
Search methods for identification of studies
The final search date for this version of the review is 10 June 2021.
Electronic searches
For this updated review, we used the University of Bern living search database as our primary register. This registry searches PubMed, Embase and preprint archives (medRxiv and bioRxiv) daily for COVID‐19 research. The strategies to build the database can be found on the ISPM web site are described here ispmbern.github.io/COVID-19/ and in Appendix 2.
Due to the increased volume of literature a specific classifier was built for the review topic in Eppi reviewer. In brief, manual annotations of references on in‐ or exclusion from repeated retrieval dates from the previous versions of the review were partially used as training data and the remaining for validation and threshold for optimal recall determination. All references from the University of Bern living search database from 15 July 2020 till 10 June 2021 were run against the classifier and references labelled as potentially relevant were screened manually. See Appendix 3.
Searching other resources
We also checked our search results against two additional repositories of COVID‐19 publications including:
the Evidence for Policy and Practice Information and Co‐ordinating Centre (EPPI‐Centre) 'COVID‐19: Living map of the evidence' (eppi.ioe.ac.uk/COVID19_MAP/covid_map_v4.html);
the Norwegian Institute of Public Health 'NIPH systematic and living map on COVID‐19 evidence' (www.nornesk.no/forskningskart/NIPH_diagnosisMap.html).
Both of these repositories allow their contents to be filtered according to studies potentially relating to diagnosis, and both have agreed to provide us with updates of new diagnosis studies added. For this iteration of the review, we examined all diagnosis studies from both sources up to 10 June 2021.
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
Pairs of review authors independently screened studies. We resolved disagreements by discussion with a third, experienced review author for initial title and abstract screening, and through discussion between three review authors for eligibility assessments.
Data extraction and management
Pairs of review authors independently performed data extraction. We resolved disagreements by discussion between three review authors.
We contacted study authors where we needed to clarify details or obtain missing information.
Assessment of methodological quality
Pairs of review authors independently assessed risk of bias and applicability concerns using the QUADAS‐2 (Quality Assessment tool for Diagnostic Accuracy Studies) checklist, which was common to the suite of reviews but tailored to each particular review (Whiting 2011; Table 2). For this review, we excluded the questions on the nature of the samples as these were not relevant, and we added a question on who assessed the signs. We resolved disagreements by discussion between three review authors.
1. QUADAS‐2 checklist.
| Index test(s) | Signs and symptoms |
| Patients (setting, intended use of index test, presentation, prior testing) | Primary care, hospital outpatient settings including emergency departments Inpatients presenting with suspected COVID‐19 No prior testing Signs and symptoms often used for triage or referral |
| Reference standard and target condition | The focus will be on the diagnosis of COVID‐19 disease and COVID‐19 pneumonia. For this review, the focus will not be on prognosis. |
| Participant selection | |
| Was a consecutive or random sample of patients enrolled? | This will be similar for all index tests, target conditions, and populations. YES: if a study explicitly stated that all participants within a certain time frame were included; that this was done consecutively; or that a random selection was done. NO: if it was clear that a different selection procedure was employed; for example, selection based on clinician's preference, or based on institutions. UNCLEAR: if the selection procedure was not clear or not reported. |
| Was a case‐control design avoided? | This will be similar for all index tests, target conditions, and populations. YES: if a study explicitly stated that all participants came from the same group of (suspected) patients. NO: if it was clear that a different selection procedure was employed for the participants depending on their COVID‐19 (pneumonia) status or SARS‐CoV‐2 infection status. UNCLEAR: if the selection procedure was not clear or not reported. |
| Did the study avoid inappropriate exclusions? | Studies may have excluded participants, or selected participants in such a way that they avoided including those who were difficult to diagnose or likely to be borderline. Although the inclusion and exclusion criteria will be different for the different index tests, inappropriate exclusions and inclusions will be similar for all index tests: for example, only elderly patients excluded, or children (as sampling may be more difficult). This needs to be addressed on a case‐by‐case basis. YES: if a high proportion of eligible patients was included without clear selection. NO: if a high proportion of eligible patients was excluded without providing a reason; if, in a retrospective study, participants without index test or reference standard results were excluded; if exclusion was based on severity assessment post‐factum or comorbidities (cardiovascular disease, diabetes, immunosuppression). UNCLEAR: if the exclusion criteria were not reported. |
| Did the study avoid inappropriate inclusions? | YES: if samples included were likely to be representative of the spectrum of disease. NO: if the study oversampled patients with particular characteristics likely to affect estimates of accuracy. UNCLEAR: if the exclusion criteria were not reported. |
| Could the selection of patients have introduced bias? | HIGH: if one or more signalling questions were answered with NO, as any deviation from the selection process may lead to bias. LOW: if all signalling questions were answered with YES. UNCLEAR: all other instances. |
| Is there concern that the included patients do not match the review question? | HIGH: if accuracy of signs and symptoms were assessed in a case‐control design, or in an already highly selected group of participants, or the study was able to only estimate sensitivity or specificity. LOW: any situation where signs and symptoms were the first assessment/test to be done on the included participants. UNCLEAR: if a description about the participants was lacking. |
| Index tests | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | This will be similar for all index tests, target conditions, and populations. YES: if blinding was explicitly stated or index test was recorded before the results from the reference standard were available. NO: if it was explicitly stated that the index test results were interpreted with knowledge of the results of the reference standard. UNCLEAR: if blinding was unclearly reported. |
| If a threshold was used, was it prespecified? | This will be similar for all index tests, target conditions, and populations. YES: if the test was dichotomous by nature, or if the threshold was stated in the methods section, or if authors stated that the threshold as recommended by the manufacturer was used. NO: if a receiver operating characteristic curve was drawn or multiple threshold reported in the results section; and the final result was based on one of these thresholds; if fever was not defined beforehand. UNCLEAR: if threshold selection was not clearly reported. |
| Could the conduct or interpretation of the index test have introduced bias? | HIGH: if one or more signalling questions were answered with NO, as even in a laboratory situation knowledge of the reference standard may lead to bias. LOW: if all signalling questions were answered with YES. UNCLEAR: all other instances. |
| Is there concern that the index test, its conduct, or interpretation differ from the review question? | This will probably be answered 'LOW' in all cases except when assessments were made in a different setting, or using personnel not available in practice. |
| Reference standard | |
| Is the reference standard likely to correctly classify the target condition? | We will define acceptable reference standards using a consensus process once the list of reference standards that have been used has been obtained from the eligible studies. For severe pneumonia, we will consider how well processes adhered to the WHO case definition in Appendix 1 . |
| Were the reference standard results interpreted without knowledge of the results of the index test? | YES: if it was explicitly stated that the reference standard results were interpreted without knowledge of the results of the index test, or if the result of the index test was obtained after the reference standard. NO: if it was explicitly stated that the reference standard results were interpreted with knowledge of the results of the index test or if the index test was used to make the final diagnosis. UNCLEAR: if blinding was unclearly reported. |
| Did the definition of the reference standard incorporate results from the index test(s)? | YES: if results from the index test were a component of the reference standard definition. NO: if the reference standard did not incorporate the index standard test. UNCLEAR: if it was unclear whether the results of the index test formed part of the reference standard. |
| Could the conduct or interpretation of the reference standard have introduced bias? | HIGH: if one or more signalling questions were answered with NO. LOW: if all signalling questions were answered with YES. UNCLEAR: all other instances. |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | HIGH: if the target condition was COVID‐19 pneumonia, but only RT‐PCR was used; if alternative diagnosis was highly likely and not excluded (will happen in paediatric cases, where exclusion of other respiratory pathogens is also necessary); if tests used to follow up viral load in known test‐positives. LOW: if above situations were not present. UNCLEAR: if intention for testing was not reported in the study. |
| Flow and timing | |
| Was there an appropriate interval between index test(s) and reference standard? | YES: this will be similar for all index tests, populations for the current infection target conditions: as the situation of a patient, including clinical presentation and disease progress, evolves rapidly and new/ongoing exposure can result in case status change, an appropriate time interval will be within 24 h. NO: if there was more than 24 h between the index test and the reference standard or if participants were otherwise reported to be assessed with the index versus reference standard test at moments of different severity. UNCLEAR: if the time interval was not reported. |
| Did all patients receive a reference standard? | YES: if all participants received a reference standard (clearly no partial verification). NO: if only (part of) the index test‐positives or index test‐negatives received the complete reference standard. UNCLEAR: if it was not reported. |
| Did all patients receive the same reference standard? | YES: if all participants received the same reference standard (clearly no differential verification). NO: if (part of) the index test‐positives or index test‐negatives received a different reference standard. UNCLEAR: if it was not reported. |
| Were all patients included in the analysis? | YES: if all included participants were included in the analyses. NO: if after the inclusion/exclusion process, participants were removed from the analyses for different reasons: no reference standard done, no index test done, intermediate results of both index test or reference standard, indeterminate results of both index test or reference standard, samples unusable. UNCLEAR: if this was not clear from the reported numbers. |
| Could the patient flow have introduced bias? | HIGH: if one or more signalling questions were answered with NO. LOW: if all signalling questions were answered with YES. UNCLEAR: all other instances. |
| ICU: intensive care unit; RT‐PCR: reverse transcription polymerase chain reaction; SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2; WHO: World Health Organization | |
Statistical analysis and data synthesis
We presented results of estimated sensitivity and specificity using paired forest plots in Review Manager 2020, and tables as appropriate.
We considered tests to be useful in ruling out a serious infection in ambulatory care if their negative likelihood ratio (LR‐) was lower than 0.20; conversely, we considered diagnostic tests useful as red flags for infections when their positive likelihood ratio (LR+) was 5.0 or higher (Jaeschke 1994; Van den Bruel 2010).
We disaggregated data by study design, reporting results from prospective studies separately from studies that used a retrospective design, which we assessed as prone to high risk of bias (Rutjes 2006). We focused on the results of prospective studies in this 2022 update. When interpreting the results, we made sure that the limitations of different study designs were carefully considered, using quality assessment and analysis.
We estimated summary sensitivity and specificity using a bivariate random‐effects meta‐analysis (Macaskill 2013). We undertook meta‐analyses using the lme4 package (R 2020), implemented in MetaDTA (crsu.shinyapps.io/dta_ma/). We based the decision to pool data on the following criteria: clinically acceptable heterogeneity on visual inspection of the forest and ROC plots, the availability of at least five studies, and low risk of bias for participant selection.
Investigations of heterogeneity
Sources of heterogeneity that we investigated if adequate data were available are listed in the Secondary objectives, either using stratification (where we believed it was inappropriate to combine studies) or through meta‐regression models.
In this version of the review, we have stratified by population (age group) and care setting.
Sensitivity analyses
We aimed to undertake sensitivity analyses considering the impact of unpublished studies, but this was not possible in this version of the review due to the small number of studies in each meta‐analysis.
Assessment of reporting bias
We aimed to publish lists of studies that we know exist but for which we have not managed to locate reports, and request information to include in updates of these reviews. However, at the time of writing this version of the review, we are unaware of unpublished studies.
Summary of findings
We have listed our key findings in Table 1 to determine the strength of evidence for each test and findings, and to highlight important gaps in the evidence.
Updating
As we will explain in the discussion, this review will no longer be updated in its current form. Resources allowing, we will consider updating this review when sufficient studies of high methodological quality become available examining the combination of signs and symptoms with other, easy‐to‐obtain information such as demographics, point‐of‐care test results, prior exposure to an infected person, and recent case detection rate. Another important outcome would be to investigate whether tests exist that identify people requiring respiratory support (SARS or ARDS) or intensive care.
Results
Results of the search
The first selection resulted in 23,683 potentially eligible articles. This included the 658 articles that we screened in our initial review and 7394 we screened in the first review update. After screening 15,631 articles on title and abstract for this update, we excluded 15,348 articles, leaving 283 full‐text articles to be assessed. We included 90 studies in this version of the review, 28 of which were included in the previous reviews. We excluded 16 studies from the previous review versions from this review because they were either preprints of which no published version was available at the time of our final search (n = 7), or because they were case‐control studies (multi‐gate designs, n = 9); see Characteristics of excluded studies. The reasons for excluding 221 articles are listed in the flow chart (Figure 1; Moher 2009); reasons for excluding a selected number of studies (n = 143) that Cochrane readers might reasonably expect to find are also listed in Characteristics of excluded studies.
1.
PRISMA flowchart
The participants in Zimmerman 2020 and Rutten 2020a were a subset of those included in Chung 2021 and Rutten 2020b, respectively. We included all four studies in this review, but we used only the more complete data from Chung 2021 and Rutten 2020b. We determined the most appropriate data set in consultation with both study authors.
A summary of the main study characteristics of the prospective studies can be found in Table 3.
2. Study characteristics (cross‐sectional prospective studies only).
| Study ID | Target condition | Sample size | Prevalence | Country | Setting | Population | Reference standard |
| Alizadehsani 2021 | COVID‐19 pneumonia | 319 | 39% | Iran | ED | All patients referred to the imaging department on suspicion of COVID‐19 (with flu‐like symptoms) | Thin‐slice high‐resolution multi‐slice spiral CT scan in a supine position, and high‐resolution CT images |
| Bhattacharya 2021 | COVID‐19 | 378 | 33% | India | ED | Patients who were suspected of COVID‐19. From 1066 suspected patients who were tested during this period, 384 patients were enrolled in the study based on the availability of informed consent and successful telephonic communication. Suspicion based on the testing advisory developed by the Indian Council of Medical Research (ICMR), Version 5, dated May 18, 2020. "ILI symptoms", defined as acute respiratory infection with fever ≥ 38 °C AND cough | RT‐PCR for SARS‐CoV‐2 (nasal + throat swab) |
| Bouzid 2020 | COVID‐19 | 596 | 45% | France | ED | All consecutive patients presenting with an influenza‐like illness (ILI: fever with a temperature > 38.5°C, malaise, headache, and myalgia; and 1 respiratory symptom (cough, sore throat, and dyspnoea)) and admitted to the hospital through the ED | Either with QIAstat‐Dx Respiratory SARS‐CoV‐2 Panel or with a combination of the RT‐PCR RealStar SARS‐CoV‐2 Kit RUO and rapid multiplex PCR FilmArray RP2; specimen not specified |
| Brendish 2020 | COVID‐19 | 1054 | 33% | UK | ED | All consecutive adults presenting with an acute respiratory illness or otherwise clinically suspected of having COVID‐19 | Either laboratory RT‐PCR or MPOCT (QIAGEN) for SARS‐CoV‐2 (nasopharyngeal swab) |
| Buonafine 2020 | COVID‐19 | 295 | 42% | Brazil | Outpatient setting | HCW with self‐reported fever or any of the following: acute respiratory symptoms (cough, nasal congestion, sore throat, shortness of breath), loss or changed sense of smell or taste, ocular symptoms, headache, arthralgia, myalgia, fatigue, diarrhoea, nausea, and vomiting | RT‐PCR for SARS‐CoV‐2 (nasopharyngeal and oropharyngeal swab) |
| Clemency 2020 | COVID‐19 | 961 | 23% | USA | Outpatient setting | HCWs triaged by phone, tested at drive‐through site | RT‐PCR on nasopharyngeal or oropharyngeal swabs |
| Drager 2020 | COVID‐19 | 2257 | 7% | Germany | Outpatient setting | All patients presenting themselves at the outpatient clinic: patients with symptoms (not further specified) + high‐risk contacts or returning from a high‐risk area were tested for SARS‐CoV‐2 | Not specified (throat swab) |
| Fink 2021 | COVID‐19/ COVID‐19 pneumonia | 219 | 33% | Germany | ED | Patients who presented at ED with signs of a respiratory infection suspicious for COVID‐19 and received radiological imaging as well as RT‐PCR for SARS‐CoV‐2 | RT‐PCR for SARS‐CoV‐2 (nasopharyngeal and oropharyngeal swab) |
| Gilbert 2020 | COVID‐19 | 598 | 29% | Belgium | Outpatient setting | Suspected patients sent to testing centres close to ED | RT‐PCR on nasopharyngeal swabs |
| Haehner 2020 | COVID‐19 | 500 | 7% | Germany | Outpatient setting | Patients presenting with symptoms of a common cold to a corona testing centre | RT‐PCR on throat swabs |
| Ishii 2021 | COVID‐19 | 3540 | 5% | Japan | Outpatient setting | All consecutive participants who underwent drive‐through nasopharyngeal swab testing at an outpatient clinic. Reason for testing: upon request of the participant or participants who had been confirmed to have contacted COVID‐19 patients based on contact tracing. No clinical suspicion needed per se, but 54% of individuals were symptomatic, suggestive of COVID‐19 | RT‐PCR, nasopharyngeal swab |
| Jeyashree 2021 | COVID‐19 | 277 | 21% | India | Outpatient setting | All consecutive adults who visited COVID‐19 testing centres in Chennai city in Southern India | RT‐PCR, nasopharyngeal swab |
| Just 2020 | COVID‐19 | 374 | 11% | Germany | Primary care | Convenience sample of patients who were tested in GPs' practices | RT‐PCR, samples not specified |
| Kalayjian 2020 | COVID‐19 | 345 | 34% | USA | Outpatient setting | Clients entering the health centre (walk‐in clinic) were screened for symptoms and triaged to the COVID‐19 clinic. Testing was performed for patients with a documented or subjective fever within the past 72 h. | Labcorp’s nucleic‐acid amplification, threshold not specified, nasopharyngeal swabs |
| Kempker 2020 | COVID‐19 | 283 | 18% | USA | Outpatient setting | HCWs with a viral‐like illness, triaged to the employee health services staff for a virtual clinical assessment and then scheduled for SARS‐CoV‐2 testing | RT‐PCR, nasopharyngeal swab |
| Krastinova 2020 | COVID‐19 | 314 | 110 | France | Outpatient setting | Symptomatic HCWs, defined as the presence of fever and/or respiratory symptoms | RT‐PCR, nasopharyngeal swabs |
| Leal 2020 | COVID‐19 | 1583 | 28% | Brazil | Outpatient setting | Patients meeting the suspected COVID‐19 case definition (tested after initial screening questionnaire) | RT‐PCR, samples not specified |
| Maechler 2020 | COVID‐19 | 4333 | 8% | Germany | Outpatient setting | Until 24 March 2020: symptomatic patients with high‐risk contacts or return from high‐risk area. From 24 March: also symptomatic people with risk factors and if the test capacity allowed also only symptomatic patients. Plus 2 subgroups of high‐risk patients in a nightclub and Charité employees | SARS‐CoV‐2 RT‐PCR test (combined oro‐ and nasopharyngeal swab) |
| Mansella 2020 | COVID‐19 | 4815 | 12% | Switzerland | Outpatient setting | All patients presenting at the test centre with respiratory symptoms (such as shortness of breath), other flu‐like symptoms (fever, sore throat, cough) and self‐reported exposure to COVID‐19 | RT‐PCR, 2 swabs from naso‐ and oropharyngeal sites combined into 1 |
| Martin‐Sanz 2020 | COVID‐19 | 355 | 61% | Spain | Outpatient setting | HCWs with suspicion of COVID‐19 infection. Suspicion of COVID‐19 was determined by the presence of either cough, fever (> 37.5 °C), headache, or breathlessness, regardless of contact with a COVID‐19 patient | SARS‐CoV‐2 next‐generation sequencing or real‐time (RT)‐PCR methods (nasal‐ and pharyngeal swabs) |
| Nazerian 2021 | COVID‐19 | 838 | 23% | Italy | ED | Patients with suspected COVID‐19 were prospectively enrolled in 2 EDs | RT‐PCR, positive result within 5 days after ED presentation, or suggestive symptoms plus chest imaging (showing acute interstitial lung disease in the absence of an alternative diagnosis), or panel adjudication (for 3 cases without a positive PCR) |
| Olivar Lopez 2020 | COVID‐19 | 510 | 15% | Mexico | ED | All patients < 18 years who presented with a clinical picture compatible with COVID‐19 (= fever, respiratory symptoms or general malaise) at the ED of a COVID paediatric reference hospital | RT‐PCR, nasopharyngeal swabs |
| O'Reilly 2020a | COVID‐19 | 240 | 5% | Australia | ED | Patients who meet the testing criteria for COVID‐19 and who present at the ED | RT‐PCR, sample not specified |
| O'Reilly 2020b | COVID‐19 | 1334 | 4% | Australia | ED | All adult patients who met criteria for "suspected COVID‐19" and underwent testing for SARS‐CoV‐2 were eligible for inclusion. Testing criteria guided by various health jurisdictions and evolved throughout the project | SARS‐CoV‐2 RT‐PCR test (nasopharyngeal swab) |
| Peyrony 2020 | COVID‐19 | 391 | 58% | France | ED | Patients tested at ED, decision to test based on clinician’s discretion | RT‐PCR on nasal swabs |
| Pivetta 2020 | COVID‐19 | 228 | 47% | Italy | ED | All adults (≥18 years) who screened positive for acute symptoms associated with SARS‐CoV‐2 infection at triage (= fever, dyspnoea, new or worsening cough, sore throat, diarrhoea, ageusia, anosmia and asthenia) | RT‐PCR (nasopharyngeal swabs), and in some cases other information including clinical, lab, imaging |
| Pokorska‐Śpiewak 2021 | COVID‐19 | 319 | 5% | Poland | Mixed in/outpatient paediatric setting | All consecutive paediatric patients referred to a tertiary healthcare department (referral based on clinical symptoms (WHO definition) of the disease or positive epidemiological history (international travel or contact with infected person) | RT‐PCR (nasopharyngeal swabs) |
| Porto 2021 | COVID‐19 | 1297 | 32% | Brazil | Outpatient setting | All patients presenting at the Piquet Carneiro Polyclinic, test indication not specified, but high proportion of symptomatic individuals in recruited population | RT‐PCR (nasopharyngeal swabs) |
| Romero‐Gameros 2020 | COVID‐19 | 139 | 52% | Mexico | ED | Patients who sought a respiratory triage assessment at ED of tertiary care hospital due to COVID‐19 suspicion | RT‐PCR (nasopharyngeal swabs) |
| Romero‐Gameros 2021 | COVID‐19 | 2137 | 54% | Mexico | ED | Adults > 17 years, with high clinical probability of SARS‐CoV‐2 and confirmatory RT‐PCR available | RT‐PCR (nasopharyngeal swabs) |
| Rutten 2020a | COVID‐19 | 1969 | 44% | The Netherlands | Nursing home | Patients with at least 2 of the following symptoms: fever/feverish feeling, cough and shortness of breath ‐ later on (from 10 April 2020) patients with atypical symptoms were added | RT‐PCR test (specimen not specified) |
| Rutten 2020b | COVID‐19 | 4007 | 38% | The Netherlands | Nursing home | All nursing home residents with a clinical suspicion of COVID‐19 based on the physician's assessment and for whom they had the result of the RT‐PCR | RT‐PCR test (specimen not specified) |
| Saegerman 2021 | COVID‐19 | 2152 | 27% | Belgium | ED | All suspected patients directed to the triage centres of 2 university hospital EDs (no definition of 'suspected') | RT‐PCR test (specimen not specified) |
| Salmon Ceron 2020 | COVID‐19 | 1824 | 47% | France | Outpatient setting | Patients suspected of SARS‐CoV‐2 infection, tested at screening centre | RT‐PCR test (nasopharyngeal swabs) |
| Trubiano 2020 | COVID‐19 | 2935 | 4% | Australia | Outpatient setting | Patients presenting at a COVID‐19 rapid assessment screening clinic, meeting DHHS screening criteriaa | RT‐PCR test (nasopharyngeal swabs) |
| Tudrej 2020 | COVID‐19 | 816 | 24% | France | Primary care/ outpatient setting | Patients referred by GPs for PCR testing at lab | RT‐PCR test (nasopharyngeal swabs) |
| Van Loon 2021 | COVID‐19 | 373 | 50% | Belgium | Outpatient setting | All hospital HCWs self‐reporting mild symptoms of an acute upper or lower respiratory tract infection were tested in a large non‐academic hospital | RT‐PCR test (nasopharyngeal swab) |
| Van Walraven 2021 | COVID‐19 | 9172 | 6% | Canada | Outpatient setting | Presence of symptoms including rhinorrhoea; fever symptoms including rigor, chills, perceived fever, or documented fever at home or at the screening clinic; cough; and shortness of breath. Any infection risk factor including close contact with a person with known or presumed COVID‐19 disease or recent travel outside of Canada. In the absence of these indications, HCWs were included if they had symptoms of sore throat, sputum production, or rhinorrhoea. | RT‐PCR test (nasopharyngeal and throat swabs) |
| Villerabel 2021 | COVID‐19 | 809 | 7% | France | Outpatient setting | All HCWs and adult patients presenting themselves at the COVID‐19 screening facility of the university hospital of Montpellier | RT‐PCR test (nasopharyngeal swabs) |
| Wee 2020 | COVID‐19 | 870 | 18% | Singapore | ED | Patients presenting with respiratory symptoms or travel history | RT‐PCR test (nasopharyngeal swabs) |
| Wernhart 2020 | COVID‐19 | 80 | 6% | Germany | Primary care | All patients with respiratory symptoms reporting to 3 rural GP offices in North Rhine‐Westphalia, Germany | RT‐PCR test (nasopharyngeal swabs) |
| Yonker 2020 | COVID‐19 | 174 | 28% | USA | Mixed in/outpatient paediatric setting | Paediatric patients ≤ 22 years of age; symptoms concerning for COVID‐19 or admitted for acute symptoms related to COVID‐19 or multisystem inflammatory syndrome in children | RT‐PCR test (nasopharyngeal or oropharyngeal swabs) |
| CT: computed tomography; ED: emergency department; GP: general practitioner; HCW: healthcare worker; MPOCT: molecular point‐of‐care test; RT‐PCR: reverse transcription polymerase chain reaction; WHO: World Health Organization | |||||||
aDHHS (Victorian Department of Health and Human Services) criteria for SARS‐CoV‐2 testing: fever OR chills in the absence of an alternative diagnosis that explains the clinical presentation OR acute respiratory infection symptoms (e.g. cough, sore throat, shortness of breath, runny nose, loss of smell or loss of taste)
Methodological quality of included studies
The results of the quality assessment for all 90 included studies are summarised in Figure 2 and Figure 3. Of the 90 single‐gate studies included in this review, 42 studies collected their data prospectively. Only one of the 48 retrospective studies applied a nested case‐control design (Tordjman 2020).
2.
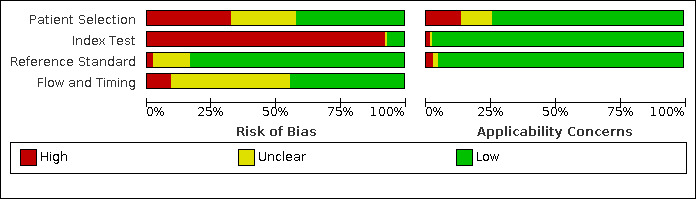
Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies
3.
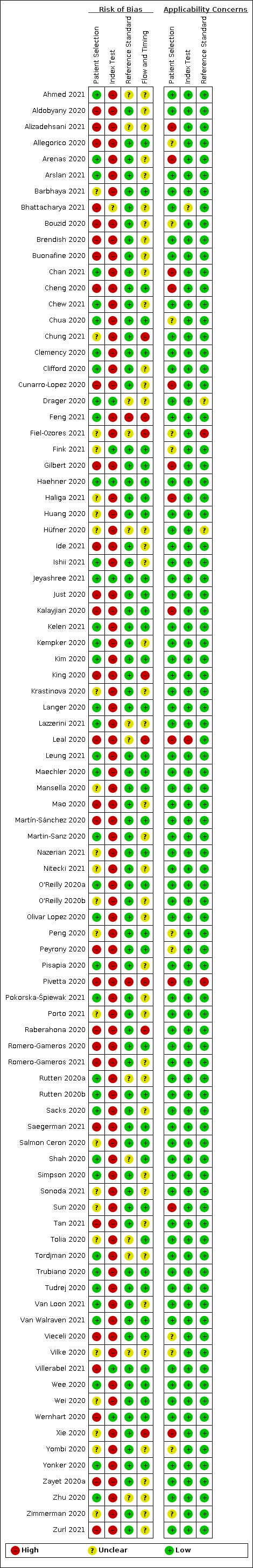
Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study
In the next section, we discuss the quality assessment of the 42 prospective studies only (Alizadehsani 2021; Bhattacharya 2021; Bouzid 2020; Brendish 2020; Buonafine 2020; Clemency 2020; Drager 2020; Fink 2021; Gilbert 2020; Haehner 2020; Ishii 2021; Jeyashree 2021; Just 2020; Kalayjian 2020; Kempker 2020; Krastinova 2020; Leal 2020; Maechler 2020; Mansella 2020; Martin‐Sanz 2020; Nazerian 2021; O'Reilly 2020a; O'Reilly 2020b; Olivar Lopez 2020; Peyrony 2020; Pivetta 2020; Pokorska‐Śpiewak 2021; Porto 2021; Romero‐Gameros 2020; Romero‐Gameros 2021; Rutten 2020a; Rutten 2020b; Saegerman 2021; Salmon Ceron 2020; Trubiano 2020; Tudrej 2020; Van Loon 2021; Van Walraven 2021; Villerabel 2021; Wee 2020; Wernhart 2020; Yonker 2020).
Participant selection
Participant selection was at high risk of bias in 12 out of 42 prospective studies. In seven studies (Alizadehsani 2021; Bhattacharya 2021; Brendish 2020; Buonafine 2020; Kalayjian 2020; Peyrony 2020; Romero‐Gameros 2021), this was because a high level of preselection was used to decide whether RT‐PCR testing was needed. For example, in Alizadehsani 2021, only patients with flu‐like symptoms who were referred to the imaging department were included, leading to a preselection of individuals who are more likely to be infected with the SARS‐CoV‐2 virus and thus to a higher disease prevalence (38.5% in this example). Three studies (Bhattacharya 2021; Gilbert 2020; Just 2020), did not select a consecutive or random sample. Six studies (Leal 2020; Pivetta 2020; Romero‐Gameros 2020; Saegerman 2021; Villerabel 2021; Wernhart 2020), excluded individuals while they were part of the study base.
For most studies, testing was dependent on the local case definition and testing criteria that was in effect at the time of the study, meaning all patients who were included in studies had already gone through a referral or selection filter.
Index tests
We rated all studies except seven (Bhattacharya 2021; Drager 2020; Fink 2021; Haehner 2020; Jeyashree 2021; Villerabel 2021; Wernhart 2020), as high risk of bias for the index tests because there was little to no detail on how, and by whom and when, the signs and symptoms were measured. However, concerns that the index tests, their performance or interpretation deviated from the research question were rated as low in all but one study (Leal 2020), where symptoms were ascertained via telephone assessment by a medical student. Olfactory symptoms were collected in different ways: interviews by telephone or in person using standardised questionnaires, online surveys, self‐reporting at presentation, or systematic assessment by staff at enrolment without standardisation. The standardised questionnaires themselves are rarely reported, and are often newly developed by each research team.
Reference standard
We rated one study (Pivetta 2020), high risk of bias concerning the reference standard. They used either an RT‐PCR or other information including clinical, lab data or imaging. All other studies used RT‐PCR or CT scans, depending on the target condition, and we rated them low risk of bias, although some studies provided little detail on blinding. This lack of reporting of blinding of the reference standard did not result in a high risk of bias rating in studies with SARS‐CoV‐2 infection as the target condition, as we assumed that a lab‐based RT‐PCR result is not influenced by the index test results. Only one study (Alizadehsani 2021), was at unclear risk of bias because it was unclear whether the radiologist interpreting the CT scans was blinded to the index test results.
Flow and timing
Patient flow was unclear in 17 studies (Alizadehsani 2021; Bhattacharya 2021; Bouzid 2020; Brendish 2020; Buonafine 2020; Drager 2020; Ishii 2021; Kempker 2020; Krastinova 2020; Martin‐Sanz 2020; O'Reilly 2020b; Olivar Lopez 2020; Pokorska‐Śpiewak 2021; Porto 2021; Romero‐Gameros 2021; Rutten 2020a; Van Loon 2021), either because the timing of recording signs and symptoms and conduct of the reference standard was unclear, or because some patients received a second or third reference standard at unclear time points during hospital admission, or because participant records were deleted when containing missing data. We rated two studies (Leal 2020; Pivetta 2020), high risk of bias concerning patient flow as not all participants received the same reference standard.
Overall ratings
In summary, we rated 36 of the 42 studies as high risk of bias for the index tests because there was little or no detail on how, by whom and when, the signs and symptoms were measured. Participant selection had a high risk of bias in 12 of the 42 studies. Risk of bias was most often rated low with regard to the implementation of the reference standard, and unclear with regard to flow and timing. However, the applicability of the study findings to our review question did not often give rise to substantial concerns.
Findings
Findings: prospective studies
The main characteristics of all 42 prospective included studies are listed in Table 3.
Setting
Thirty‐five studies were set in emergency departments or outpatient test centres (Alizadehsani 2021; Bhattacharya 2021; Bouzid 2020; Brendish 2020; Buonafine 2020; Clemency 2020; Drager 2020; Fink 2021; Gilbert 2020; Haehner 2020; Ishii 2021; Jeyashree 2021; Kalayjian 2020; Kempker 2020; Krastinova 2020; Leal 2020; Maechler 2020; Mansella 2020; Martin‐Sanz 2020; Nazerian 2021; O'Reilly 2020a; O'Reilly 2020b; Olivar Lopez 2020; Peyrony 2020; Pivetta 2020; Porto 2021; Romero‐Gameros 2020; Romero‐Gameros 2021; Saegerman 2021; Salmon Ceron 2020; Trubiano 2020; Van Loon 2021; Van Walraven 2021; Villerabel 2021; Wee 2020), three studies in primary care settings (Just 2020; Tudrej 2020; Wernhart 2020), two studies in a mixed population of in‐ and outpatients in a hospital setting (Pokorska‐Śpiewak 2021; Yonker 2020), and two overlapping studies in nursing homes (Rutten 2020a; Rutten 2020b).
Target conditions
Only one study assessed accuracy of signs and symptoms for the diagnosis of COVID‐19 pneumonia (Alizadehsani 2021), the remaining studies had SARS‐CoV‐2 infection as the target condition. The distinction between these two target conditions was not always very clear though, and a degree of overlap is to be assumed and we therefore present the results for both conditions together. All but two studies (Alizadehsani 2021; Drager 2020) used RT‐PCR testing as reference standard, with some variation in the samples that were used. Alizadehsani 2021 used CT scanning for the diagnosis of COVID‐19 pneumonia. Drager 2020 did not specify the reference standard.
General results
There were 52,608 participants in all 42 prospective studies, the median number of participants was 553. Prevalence varied from 3.7% to 60.6% with a median of 27.4%.
We found data on 96 symptoms, which fall into seven different categories,that is, systemic signs and symptoms, upper respiratory, lower respiratory, olfactory, gastro‐intestinal, cardiovascular and multivariable combinations of signs or symptoms. Evidence on individual signs as diagnostic tests was rarely reported, so this review reports mainly on the diagnostic value of symptoms. Results for the prospective cross‐sectional studies are presented in forest plots (Figure 4; Figure 5; Figure 6; Figure 7; Figure 8; Figure 9; Figure 10), and are plotted in ROC (receiver operating characteristic) space (Figure 11; Figure 12; Figure 13; Figure 14; Figure 15; Figure 16; Figure 17; Figure 18; Figure 19).
4.
Forest plot of upper respiratory tract symptoms
5.
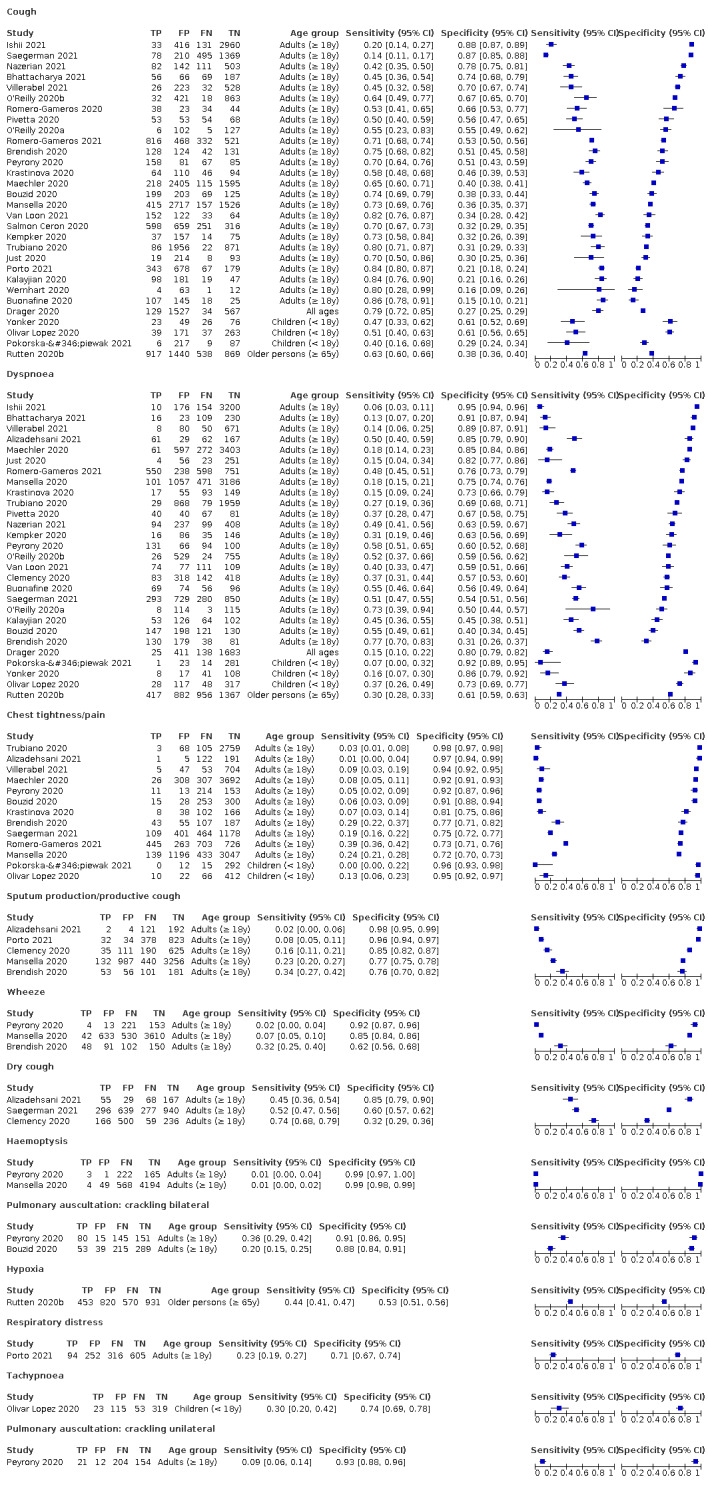
Forest plot of lower respiratory tract symptoms
6.

Forest plot of systemic signs and symptoms
7.
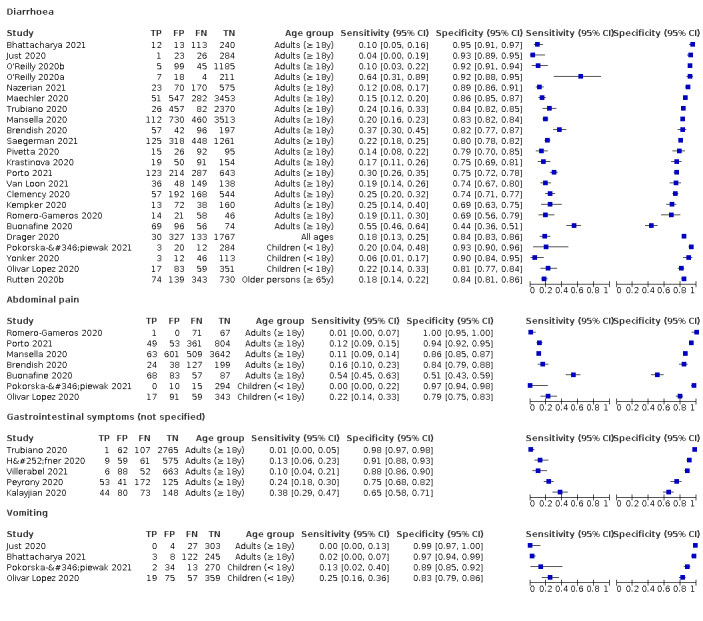
Forest plot of gastrointestinal symptoms
8.

Forest plot of cardiovascular symptoms (palpitations)
9.
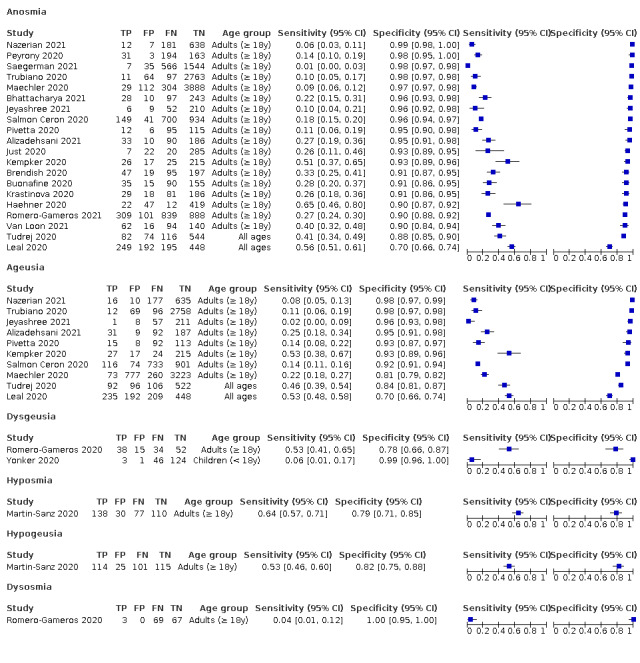
Forest plot of olfactory symptoms
10.
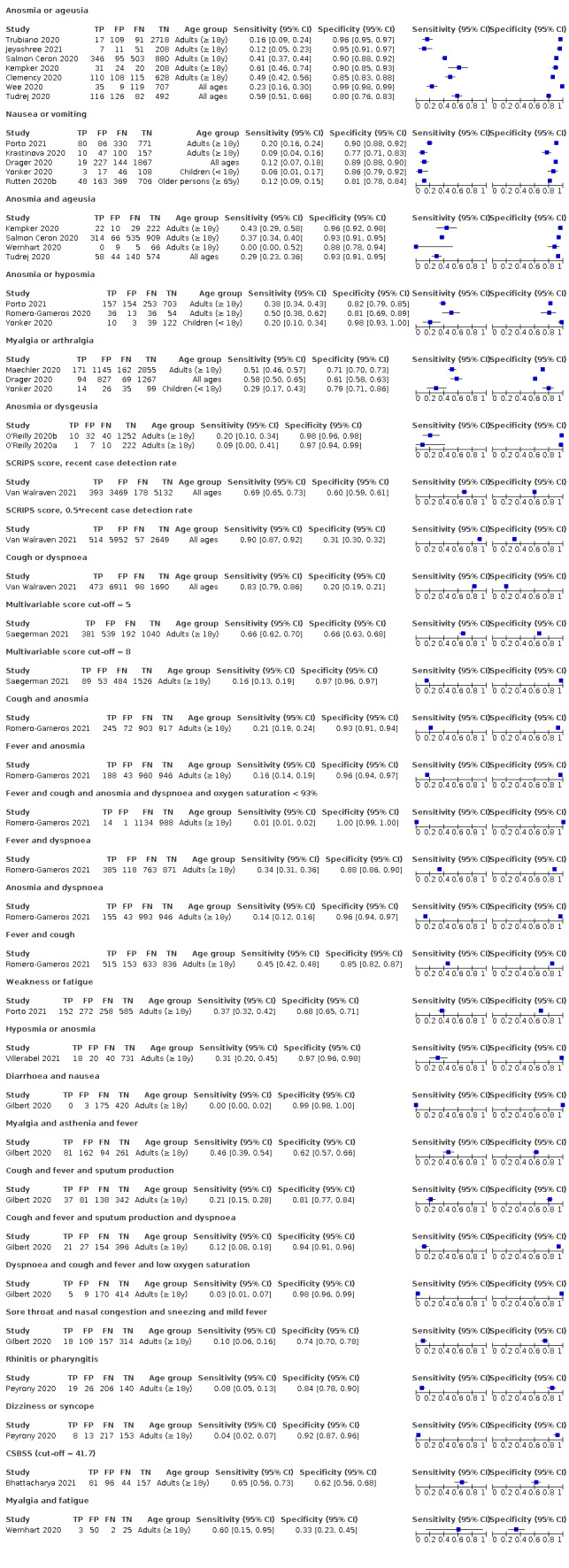
Forest plot of multivariable combinations of signs and symptoms
11.
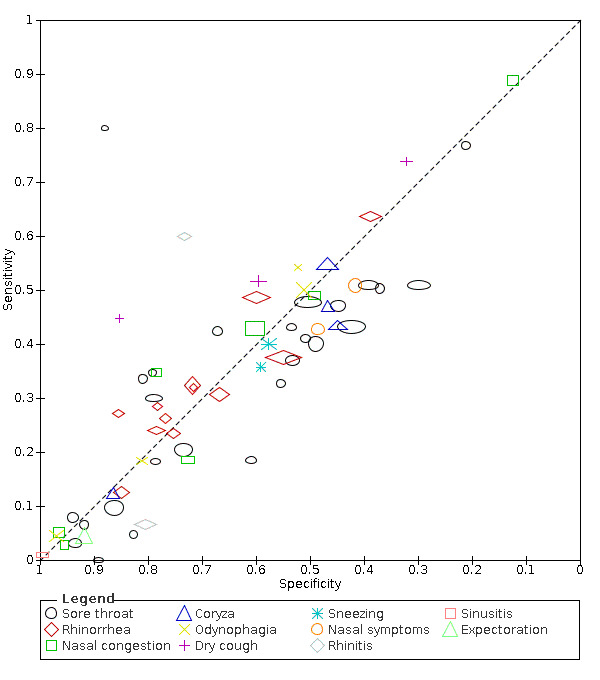
Summary ROC plot of upper respiratory tract symptoms. The study points (symbols) were scaled according to the sample size
12.
Summary ROC plot of lower respiratory tract symptoms. The study points (symbols) were scaled according to the sample size
13.
Summary ROC plot of systemic signs and symptoms. The study points (symbols) were scaled according to the sample size
14.
Summary ROC plot of gastrointestinal symptoms. The study points (symbols) were scaled according to the sample size
15.
Summary ROC plot of olfactory symptoms. The study points (symbols) were scaled according to the sample size
16.
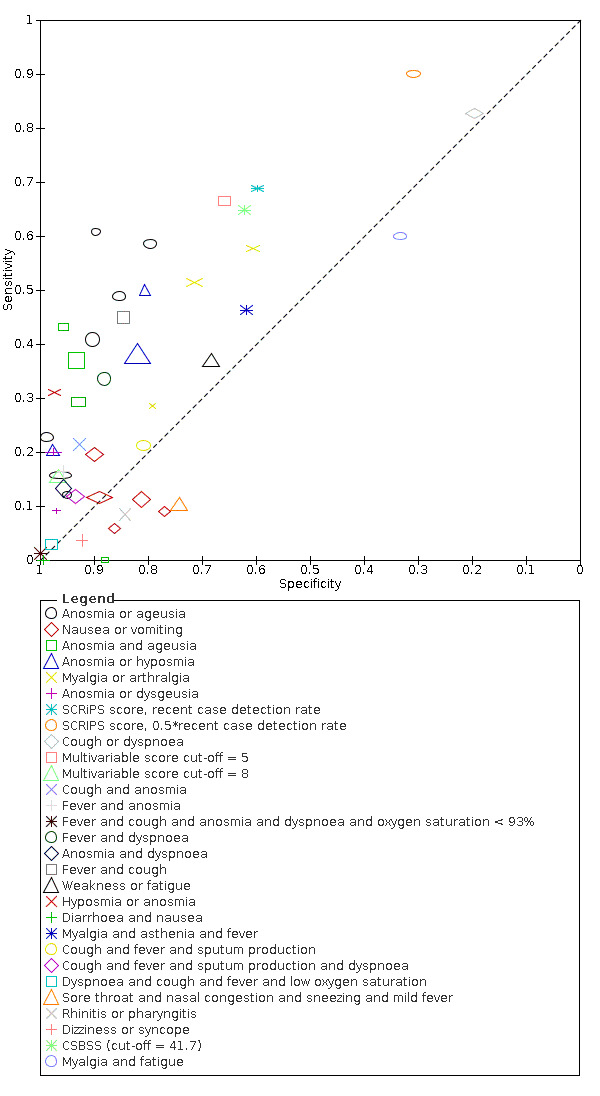
Summary ROC plot of multivariable combinations of signs and symptoms. The study points (symbols) were scaled according to the sample size
17.
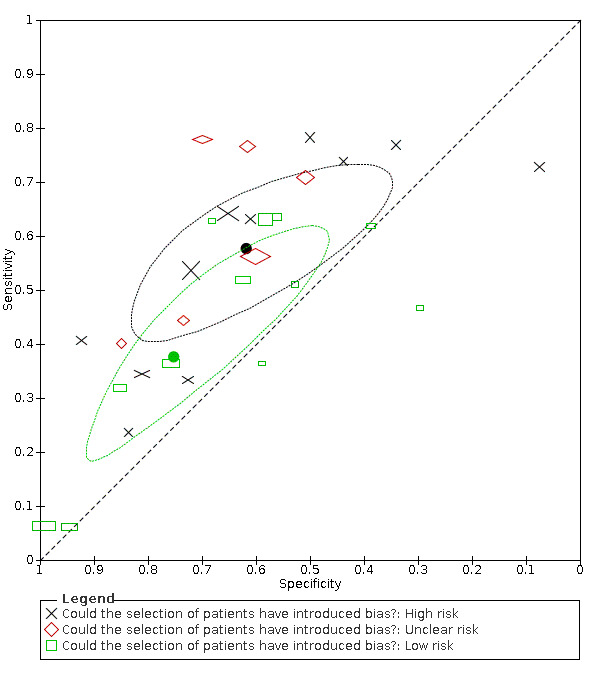
Summary ROC plot of fever by risk of bias concerning participant selection. Summary points and their 95% confidence regions are shown for high and low risk of bias only. The study points (symbols) were scaled according to the sample size
18.
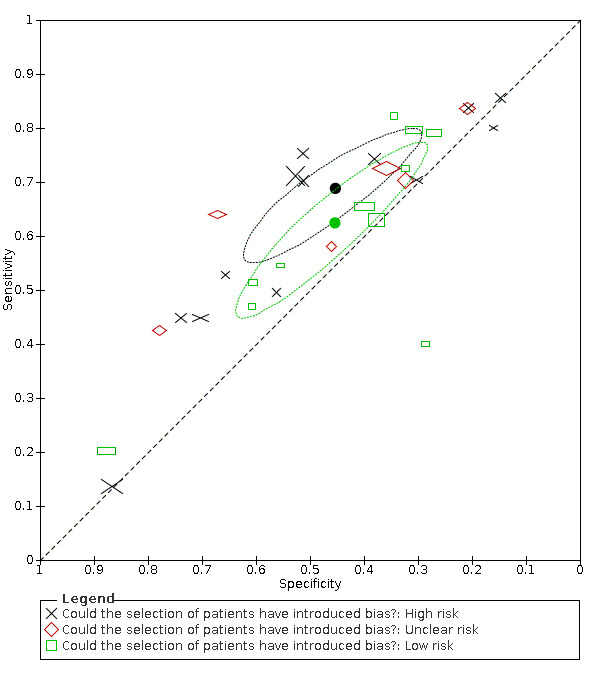
Summary ROC plot of cough by risk of bias concerning participant selection. Summary points and their 95% confidence regions are shown for high and low risk of bias only. The study points (symbols) were scaled according to the sample size
19.
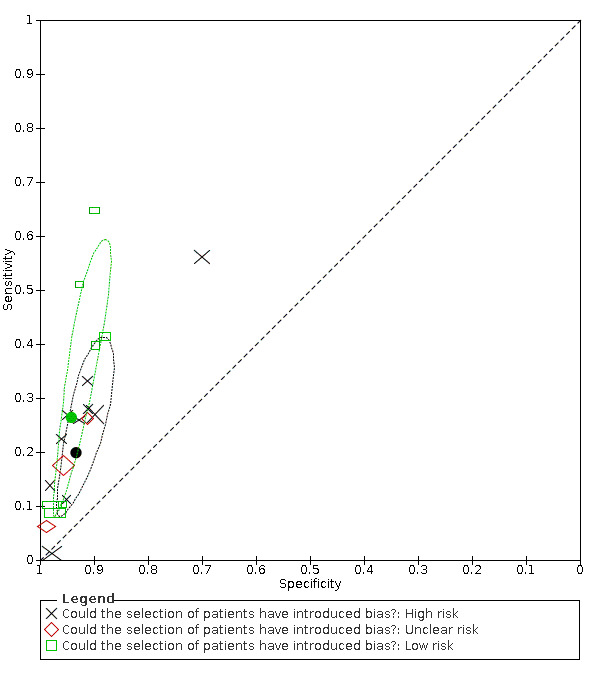
Summary ROC plot of anosmia by risk of bias concerning participant selection. Summary points and their 95% confidence regions are shown for high and low risk of bias only. The study points (symbols) were scaled according to the sample size
Summary results
We conducted meta‐analyses for 13 symptoms at presentation (fever, dyspnoea, cough, diarrhoea, sore throat, fatigue, rhinorrhoea, headache, anosmia, anosmia or ageusia, ageusia, myalgia, chills/shivers). The ranges and summary estimates of the sensitivity and specificity of the 13 index tests are listed below, ordered by decreasing number of studies included. Summary estimates of test accuracy are listed in additional Table 4. They are based on bivariate meta‐analyses of prospective studies with low risk of bias for participant selection.
3. Summary estimates of test accuracy for selected index tests, including 95% confidence intervals (bivariate meta‐analysis of prospective studies with low risk of bias for participant selection).
| Index test | Number of studies |
Number of COVID‐19 positives/ Total number of participants (%) |
Summary sensitivity % (95% CI) |
Summary specificity % (95% CI) |
Summary LR+ (95% CI) |
Summary LR‐ (95% CI) |
| Fever | 12 | 3221/28,495 (11.3) | 37.6 (23.4 to 54.3) |
75.2 (56.3 to 87.8) |
1.520 (1.099 to 2.101) |
0.829 (0.740 to 0.928) |
| Dyspnoea | 12 | 2753/19,545 (14.1) | 23.3 (16.4 to 31.9) |
75.7 (65.2 to 83.9) |
0.959 (0.830 to 1.107) |
1.013 (0.966 to 1.063) |
| Cough | 11 | 2586/18,702 (13.8) | 62.4 (50.6 to 72.9) |
45.4 (33.5 to 57.9) |
1.143 (1.043 to 1.253) |
0.828 (0.738 to 0.928) |
| Diarrhoea | 11 | 1633/13,669 (11.9) | 18.5 (15.7 to 21.6) |
84.1 (79.4 to 87.9) |
1.167 (0.967 to 1.408) |
0.969 (0.935 to 1.003) |
| Sore throat | 10 | 2116/14,548 (14.5) | 31.0 (20.2 to 44.5) |
61.9 (46.7 to 75.0) |
0.814 (0.714 to 0.929) |
1.114 (1.021 to 1.216) |
| Fatigue | 8 | 1286/7967 (16.1) | 40.2 (19.4 to 65.1) |
73.6 (48.4 to 89.3) |
1.522 (1.213 to 1.909) |
0.813 (0.709 to 0.932) |
| Rhinorrhoea | 7 | 1620/17,972 (9.0) | 30.3 (18.7 to 45.1) |
70.0 (56.8 to 80.6) |
1.011 (0.848 to 1.205) |
0.985 (0.922 to 1.074) |
| Headache | 7 | 929/10,899 (8.5) | 35.8 (17.2 to 60.0) |
73.0 (53.4 to 86.4) |
1.325 (1.161 to 1.513) |
0.879 (0.767 to 1.008) |
| Anosmia | 7 | 938/9456 (9.9) | 26.4 (13.8 to 44.6) |
94.2 (90.6 to 96.5) |
4.546 (3.461 to 5.972) |
0.781 (0.648 to 0.942) |
| Anosmia or ageusia | 6 | 794/6142 (12.9) | 39.2 (26.5 to 53.6) |
92.1 (84.5 to 96.2) |
4.992 (3.215 to 7.751) |
0.659 (0.551 to 0.790) |
| Myalgia | 6 | 563/2684 (21.0) | 37.5 (20.6 to 58.1) |
75.4 (58.4 to 87.0) |
1.525 (1.207 to 1.926) |
0.829 (0.708 to 0.970) |
| Chills/shivers | 5 | 1080/14,472 (7.5) | 25.3 (15.1 to 39.3) |
85.0 (72.1 to 92.6) |
1.691 (1.231 to 2.323) |
0.878 (0.812 to 0.950) |
| Ageusia | 5 | 748/8644 (8.7) | 23.2 (10.6 to 43.3) |
92.6 (83.1 to 97.0) |
3.137 (1.786 to 5.510) |
0.830 (0.701 to 0.982) |
| CI: confidence interval; LR+: positive likelihood ratio; LR‐: negative likelihood ratio | ||||||
Fever, 12 studies, 28,495 participants: sensitivity range 6% to 64% (summary 37.6%, 95% CI 23.4% to 54.3%); specificity range 30% to 99% (summary 75.2%, 95% CI 56.3% to 87.8%)
Dyspnoea, 12 studies, 19,545 participants: sensitivity range 6% to 73% (summary 23.3%, 95% CI 16.4% to 31.9%); specificity range 50% to 95% (summary 75.7%, 95% CI 65.2% to 83.9%)
Cough, 11 studies, 18,702 participants: sensitivity range 20% to 82% (summary 62.4%, 95% CI 50.6% to 72.9%); specificity range 27% to 88% (summary 45.4%, 95% CI 33.5% to 57.9%)
Diarrhoea, 11 studies, 13,669 participants: sensitivity range 6% to 64% (summary 18.5%, 95% CI 15.7% to 21.6%); specificity range 69% to 93% (summary 84.1%, 95% CI 79.4% to 87.9%)
Sore throat, 10 studies, 14,548 participants: sensitivity range 0% to 51% (summary 31.0%, 95% CI 20.2% to 44.5%); specificity range 30% to 89% (summary 61.9%, 95% CI 46.7% to 75.0%)
Fatigue, eight studies, 7967 participants: sensitivity range 0% to 82% (summary 40.2%, 95% CI 19.4% to 65.1%); specificity range 32% to 97% (summary 73.6%, 95% CI 48.4% to 89.3%)
Rhinorrhoea, seven studies, 17,972 participants: sensitivity range 12% to 64% (summary 30.3%, 95% CI 18.7% to 45.1%); specificity range 39% to 86% (summary 70.0%, 95% CI 56.8% to 80.6%)
Headache, seven studies, 10,899 participants: sensitivity range 0% to 78% (summary 35.8%, 95% CI 17.2% to 60.0%); specificity range 38% to 95% (summary 73.0%, 95% CI 53.4% to 86.4%)
Anosmia, seven studies, 9456 participants: sensitivity range 9% to 65% (summary 26.4%, 95% CI 13.8% to 44.6%); specificity range 88% to 98% (summary 94.2%, 95% CI 90.6% to 96.5%)
Anosmia or ageusia, six studies, 6142 participants: sensitivity range 12% to 61% (summary 39.2%, 95% CI 26.5% to 53.6%); specificity range 80% to 99% (summary 92.1%, 95% CI 84.5% to 96.2%)
Myalgia, six studies, 2684 participants: sensitivity range 0% to 70% (summary 37.5%, 95% CI 20.6% to 58.1%); specificity range 48% to 92% (summary 75.4%, 95% CI 58.4% to 87.0%)
Chills/shivers, five studies, 14,472 participants: sensitivity range 8% to 81% (summary 25.3%, 95% CI 15.1% to 39.3%); specificity range 64% to 98% (summary 85.0%, 95% CI 72.1% to 92.6%)
Ageusia, five studies, 8644 participants: sensitivity range 2% to 53% (summary 23.2%, 95% CI 10.6% to 43.3%); specificity range 81% to 98% (summary 92.6%, 95% CI 83.1% to 97.0%)
Cough was the only index test with a summary sensitivity above 50%. Its summary specificity was 45.4% (Table 4). Summary specificity was above 90% for anosmia, ageusia and for the presence of anosmia or ageusia (Table 4). However, their summary sensitivity was low (maximum 39.2% for anosmia or ageusia).
The summary positive likelihood ratios (LRs+) of anosmia as a single test or in combination with ageusia ('anosmia or ageusia') was just below our predefined cut‐off of 5 for a useful red flag (4.55, 95% CI 3.46 to 5.97) and 4.99, 95% CI 3.22 to 7.75) respectively). The summary negative likelihood ratios (LRs‐) were too high to make any of the reported tests useful for ruling out COVID‐19. In other words, the absence of the above‐mentioned symptoms or signs does not necessarily imply the absence of COVID‐19.
Combinations of signs and symptoms
Twenty‐four studies assessed combinations of different signs and symptoms (Figure 10; Figure 16). In total, 29 different combinations were assessed, of which only six were assessed by more than one study.
Three multivariable prediction scores were reported (Bhattacharya 2021; Saegerman 2021; Van Walraven 2021). Bhattacharya 2021 (378 participants) used a combination of only signs and symptoms (Clinical Symptom‐Based Scoring System (CSBSS) based on body temperature, cough, headache, myalgia and anosmia), leading to a sensitivity of 64.8% (95% CI 55.8% to 73.1%) and a specificity of 62.1% (95% CI 55.8% to 68.1%) at the proposed cut‐off of 41.7 points. Reducing the cut‐off to 10 points led to a sensitivity of 75.0% and a specificity of 42.6%. The Area Under the ROC Curve (AUC) was 0.69 (95% CI 0.62 to 0.76) for the validation dataset.
The other two multivariable prediction score studies combined signs and symptoms with other information: Saegerman 2021 (2152 participants) combined age (> 56.5 years) with the presence of chest pain, sore throat, dry cough or fever, leading to a sensitivity of 66.5% (95% CI 62.5% to 70.4%) and a specificity of 65.9% (95% CI 63.5% to 68.2%) at the lowest cut‐off. The AUC of this score was 0.71 (95% CI 0.69 to 0.73). Van Walraven 2021 (9172 participants) developed a score, based on a combination of gender, being a healthcare worker, recent contact with a COVID‐19 case, recent travel, the recent local case detection rate, the presence of rhinorrhoea, cough or dyspnoea, and a combination of age and the presence of fever (SARS‐CoV‐2 Risk Prediction Score (SCRiPS)). The recent local case detection rate was calculated as the proportion of tests from the testing clinic in the previous three days that were SARS‐CoV‐2‐positive. The SCRiPS score using 0.5 times the recent local case detection rate demonstrated the highest sensitivity of all combinations (90.0%; 95% CI 87.3% to 92.4%) at the cost of lower specificity (30.8%, 95% CI 29.8% to 31.8%). The second highest sensitivity was reported by the same study (Van Walraven 2021), using only the presence of cough or dyspnoea. This combination led to a sensitivity of 82.8% (95% CI 79.5% to 85.8%) at a specificity of 19.6% (95% CI 18.8% to 20.5%).
In addition to anosmia or ageusia (see pooled results), some other paired combinations of symptoms were investigated (Figure 10; Figure 16). The following combinations showed a sensitivity of more than 50% in at least one study.
Anosmia or hyposmia (3 studies): sensitivity from 20% to 50% and specificity from 81% to 98%
Myalgia or arthralgia (3 studies): sensitivity from 29% to 58% and specificity from 61% to 79%
Cough or dyspnoea (1 study): sensitivity of 83%, specificity of 20%
Myalgia and fatigue (1 study): sensitivity of 60%, specificity of 33%
Positivity rates
Positivity rates (presence of the symptom in the study population) of signs and symptoms depend on prevalence of COVID‐19 and population characteristics, especially preselection. As a result, positivity rates were highly variable. In studies with prevalence less than 10%, suggesting little preselection had taken place, positivity rates for fever were between 1.2% and 69.3% (24.7% average), for cough between 12.7% and 83.7% (54.9% average), for anosmia between 2.5% and 13.8% (7.1% average), for ageusia (2 studies) between 2.8% and 19.6% (11.2% average), and for anosmia or ageusia (1 study) 4.3%.
Stratified analyses
Stratification by age group
Three prospective studies were performed specifically in children (Olivar Lopez 2020; Pokorska‐Śpiewak 2021; Yonker 2020), and two overlapping studies in older people living in a nursing home (Rutten 2020a; Rutten 2020b). All other studies were either performed in adults or in individuals of all ages. Meta‐analysis of data in children or older adults was impossible due to the limited number of studies in these age groups. We therefore limited ourselves to descriptive analyses. All forest plots (Figure 4; Figure 5; Figure 6; Figure 7; Figure 8; Figure 9; Figure 10) and Dumbbell plots (Figure 20; Figure 21; Figure 22) are ordered by age group (adults, all ages, children, older adults (≥ 65 years)).
20.
Dumbbell plot: olfactory symptoms
21.
Dumbbell: plot fever. Ordered by age group ‐ children (< 18 years), adults/all ages, older adults (≥ 65 years)
22.
Dumbbell plot: cough. Ordered by age group ‐ children (< 18 years), adults/all ages, older adults (≥ 65 years)
In children, compared to the adult population, the differences in diagnostic accuracy depended strongly on the type of symptom. For example, the sensitivity of fever in children ranged from 47% to 62%, which is higher than the overall summary result for all ages (29%). The specificity of fever in children ranged from 30% to 53%, which is lower than the overall summary result (82.2%). In contrast, we observed lower sensitivity and higher specificity for fatigue, headache and myalgia in children than in the population as a whole.
The results for sore throat, rhinorrhoea, cough, dyspnoea and diarrhoea were comparable with the overall population. Olfactory symptoms were rarely investigated in children. One study (Yonker 2020), investigated the diagnostic value of dysgeusia, observing similar accuracy to in the adult population.
In older adults living in a nursing home, most symptoms showed sensitivities and specificities that were comparable to the overall summary results (Rutten 2020b). The sensitivity of fever appeared to be higher in older people, but this may be due to a strong preselection based on the presence of fever.
Stratification according to risk of bias in patient selection
The summary estimates of fever, cough and anosmia from prospective studies that had either a low or a high risk of bias concerning participant selection are presented in ROC curves for comparison (Figure 17; Figure 18; Figure 19). We observed the largest difference between both summary estimates for fever. The differences between the summary estimates between low and high risk of bias concerning participant selection were less pronounced for cough and for anosmia.
Stratification by care setting
Stratification according to setting did not explain the observed heterogeneity in diagnostic accuracy.
Additional analyses
To further illustrate the ability of a test to either rule in or rule out COVID‐19, we constructed dumbbell plots showing pre‐ and post‐test probabilities for anosmia, anosmia or ageusia, fever and cough by age group in each prospective cross‐sectional study with a low risk of bias rating for selection of participants (Haehner 2020; Jeyashree 2021; Kempker 2020; Maechler 2020; Trubiano 2020; Tudrej 2020; Van Loon 2021; see Figure 20; Figure 21; Figure 22). For each test, we have plotted the pre‐test probability, which is the prevalence of COVID‐19 disease in the study (blue dot). The probability of having COVID‐19 disease after testing (post‐test probability) then changes depending on a positive test result (red dot marked +) or a negative test result (green dot marked ‐). The plot shows that the presence of anosmia, for example, increased the probability of COVID‐19 in all seven studies. Its absence slightly decreased the probability of COVID‐19 in three studies (Kempker 2020; Tudrej 2020; Van Loon 2021), and in the four other studies there was not much difference between pre‐ and post‐test probability (Haehner 2020; Jeyashree 2021; Maechler 2020; Trubiano 2020).
Findings: retrospective studies
Results of retrospective studies are presented in forest plots (Figure 23; Figure 24; Figure 25; Figure 26; Figure 27; Figure 28; Figure 29).
23.

Forest plot of upper respiratory signs/symptoms (retrospective data collection)
24.
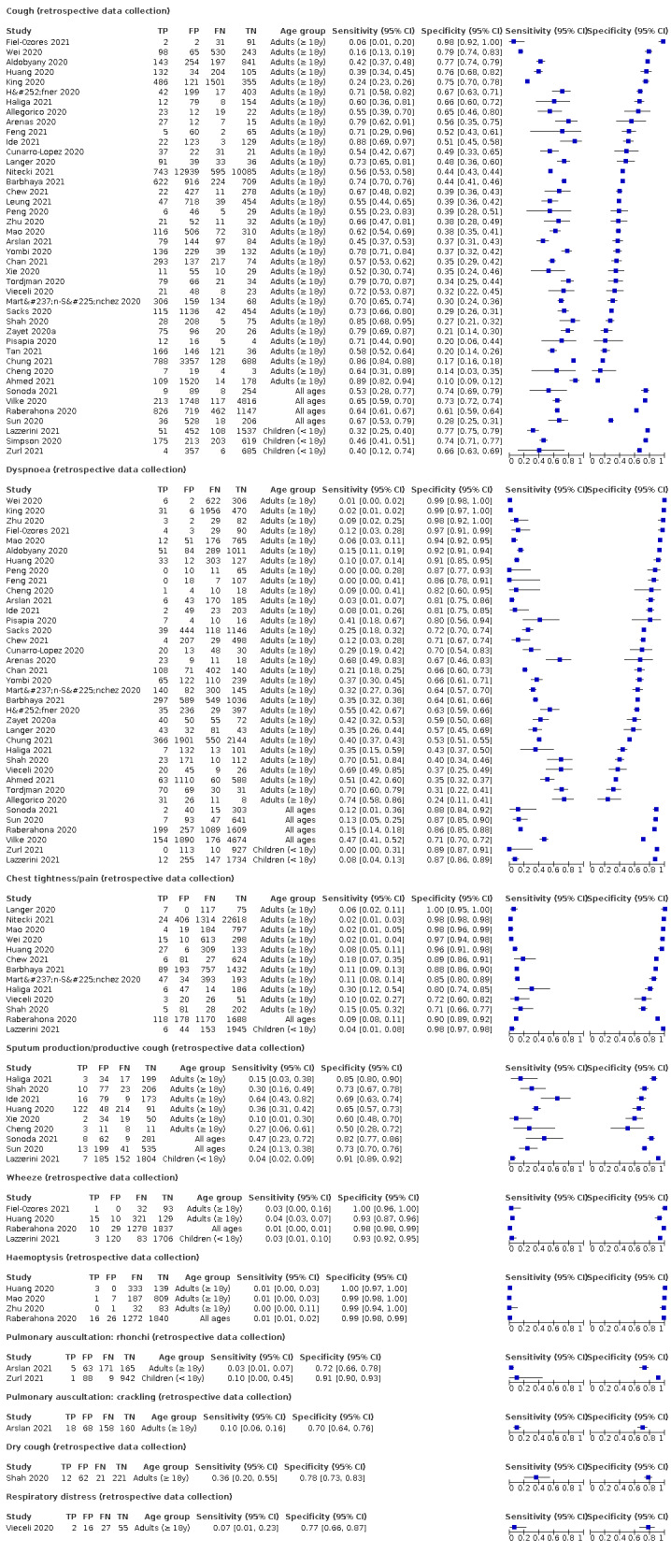
Forest plot of lower respiratory signs/symptoms (retrospective data collection)
25.
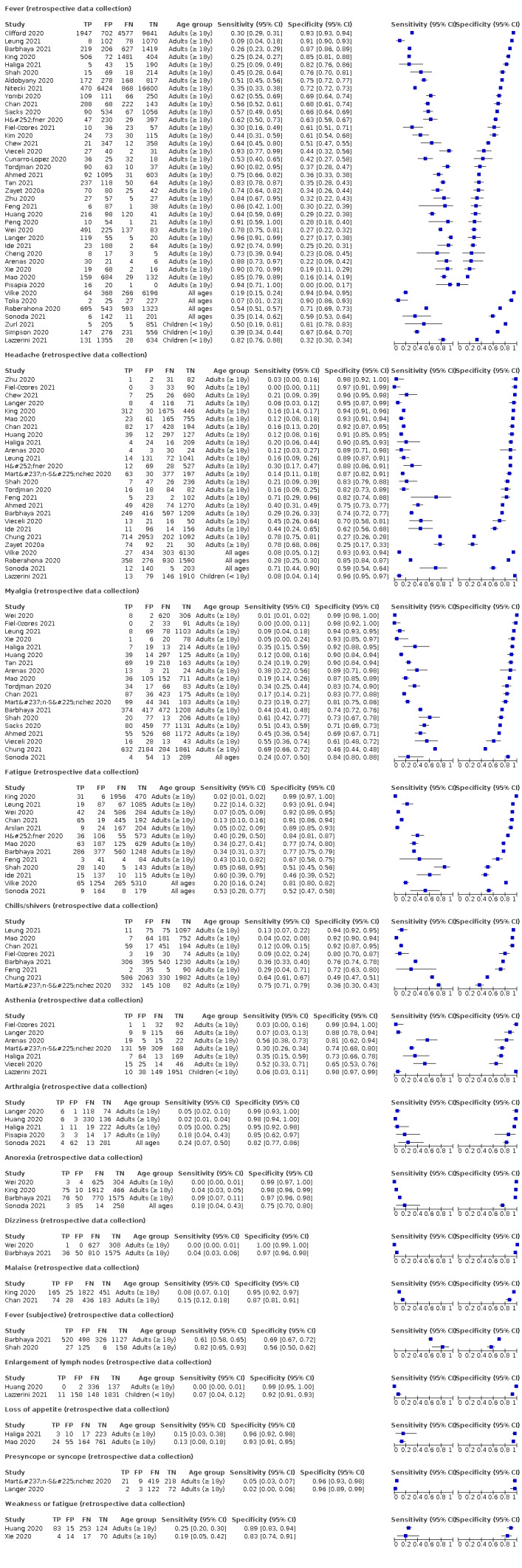
Forest plot of systemic signs/symptoms (retrospective data collection)
26.
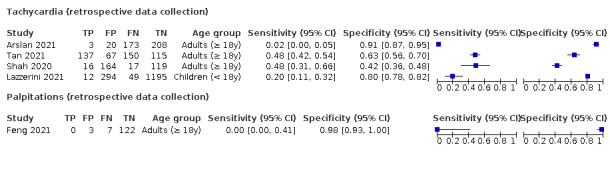
Forest plot of cardiovascular signs/symptoms (retrospective data collection)
27.
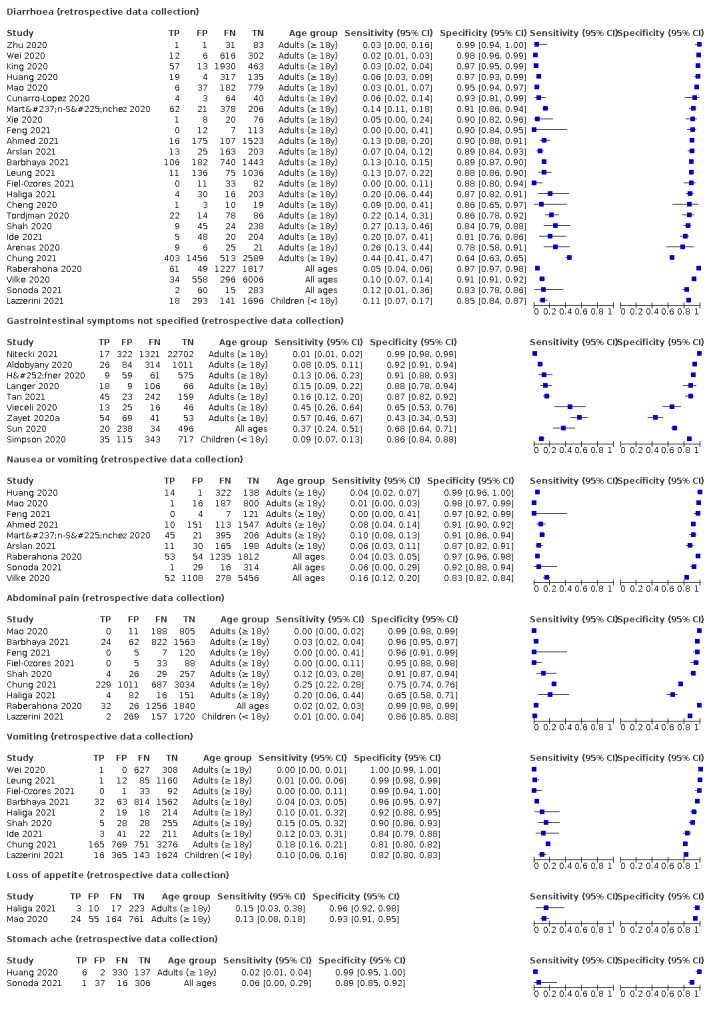
Forest plot of gastrointestinal signs/symptoms (retrospective data collection)
28.
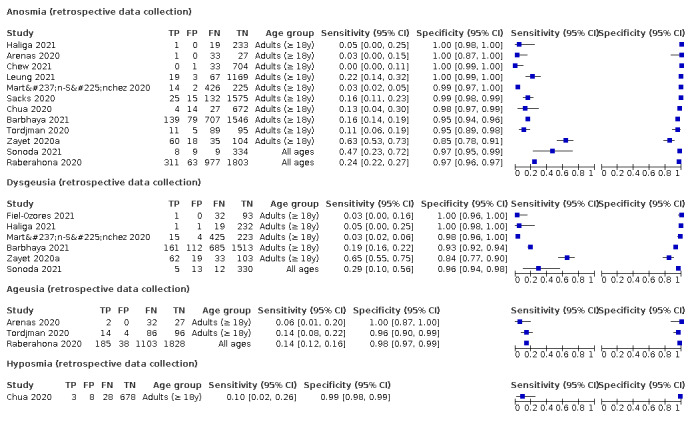
Forest plot of olfactory signs/symptoms (retrospective data collection)
29.
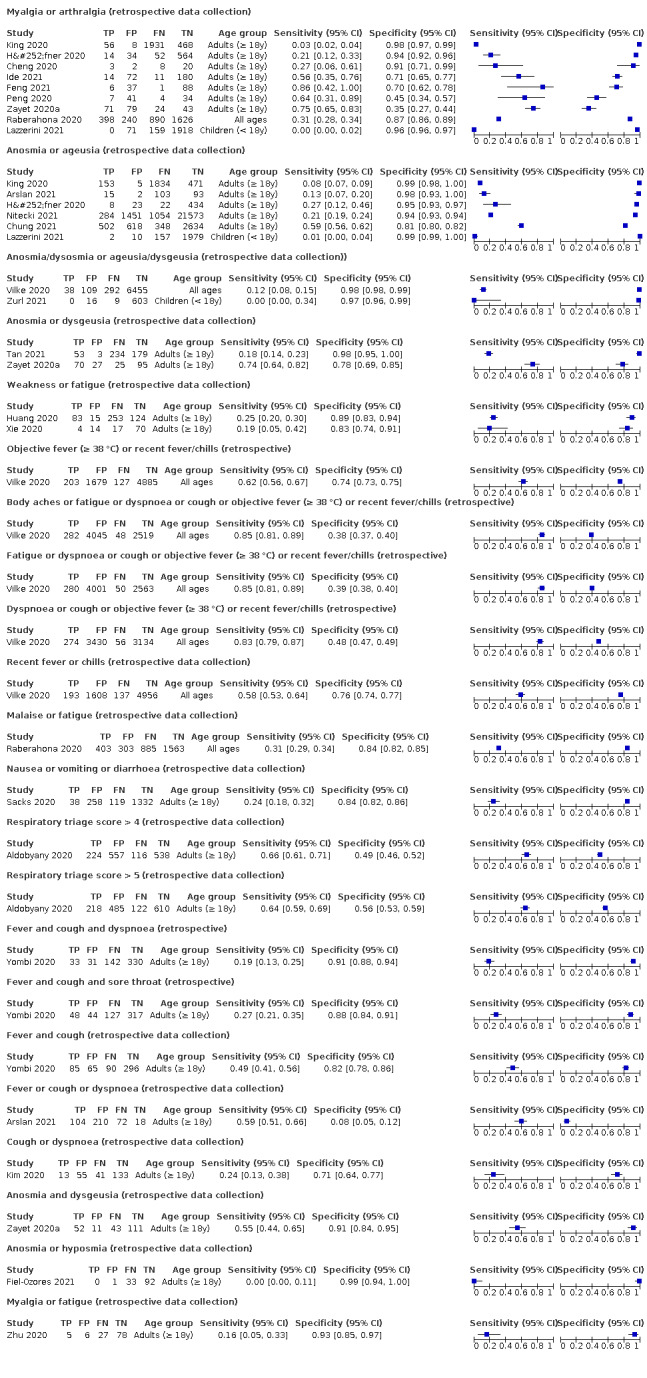
Forest plot of combinations of signs/symptoms (retrospective data collection)
Discussion
Summary of main results
This is the second update of a living systematic review of signs and symptoms for the diagnosis of COVID‐19 in an outpatient setting. For the first time, this update provided a large number of cross‐sectional studies using prospective data collection, leading to results that are more reliable than those presented in previous versions of this review. Nevertheless, considerable heterogeneity between study results remains a concern. Our main conclusion remains unchanged: most individual symptoms appear to have poor diagnostic accuracy. Neither absence nor presence of symptoms are accurate enough to rule in or rule out the disease. The presence of anosmia or ageusia may be useful as a red flag for the presence of COVID‐19. Some combinations of signs and symptoms may be useful as a tool to triage patients for further testing.
This review update identified more studies on combinations of signs and symptoms, but only six out of 29 different combinations were assessed by more than one study. Some combinations may be useful as a triage tool. For example, in a cohort with a disease prevalence (pre‐test probability) of 5%, the presence of either anosmia or ageusia would increase the post‐test probability of the presence of COVID‐19 to 21%.
The combination of signs and symptoms with other readily available information may prove useful in safely ruling out COVID‐19. The multivariable prediction score with the highest sensitivity (90.0%) combined gender, being a healthcare worker, recent contact with a COVID‐19 case, recent travel, 0.5 times the recent local case detection rate, the presence of rhinorrhoea, cough or dyspnoea, and a combination of age and the presence of fever (SCRiPS Score, Van Walraven 2021). This type of score may be especially useful to safely rule out COVID‐19, because it achieves a sensitivity that cannot be obtained by combining symptoms alone. For example, using this score, a 35‐year old female healthcare worker without chest symptoms but with fever and rhinorrhoea, who did not have contact with a COVID‐19 case recently and who did not travel recently, would have a 1% chance of having COVID‐19 if the local recent case detection rate was 5%. An 85‐year old man with fever, without any other symptom, who did not travel recently but who did have a recent contact with a COVID‐19 case, would have a 46% chance of having COVID‐19 at the same local recent case detection rate of 5%. Of course, such a score should ideally be validated externally in other populations before being applied in practice.
The presence of upper respiratory symptoms such as a sore throat, rhinorrhoea or coryza, increases the probability of having an infectious disease other than COVID‐19. In a hypothetical cohort of 1000 individuals, at a COVID‐19 prevalence of 5%, 16 people with a sore throat would have COVID‐19 and there would be 362 people with a sore throat without COVID‐19 (false positives). Not using sore throat as an indication to test wouldn't necessarily lead to 16 people with COVID‐19 being missed, as some of those individuals would present with other symptoms that would prompt further testing. We found similar figures for rhinorrhoea and coryza. There is currently no evidence to support further testing in all individuals presenting only with upper respiratory symptoms.
Selection bias is present when selective and non‐random inclusion and exclusion of patients apply and the resulting association between exposure and outcome (here the accuracy of the test) differs in the selected study population compared to the eligible study population (Rutjes 2006). For the diagnosis of COVID‐19, rapidly and constantly changing and widely variable test criteria have influenced who was referred for testing and who was not, both for the presence of infection and disease. Selection in the study of only a fraction of the eligible patients can result in a biased estimate of the true accuracy of the index test when measured against the reference standard and true disease status. Griffith 2020 have reported on the problematic presence of collider stratification bias in the published studies on COVID‐19. Appropriate sampling strategies need to be applied to avoid conclusions of spurious relationships, more specific in our case the biased accuracy estimates of signs and symptoms for the diagnosis of both SARS‐CoV‐2 infection and COVID‐19 disease. Selection of patients based on the presence of specific preset symptoms, such as fever and cough, leads to biased associations between these symptoms and to disease and diagnostic accuracy estimates that differ from their true values. The example of collider bias for cough is illustrated in Figure 30. Grouping studies by diagnostic criteria for selection might clarify this issue, but studies do not clearly describe them, with study authors referring to the guidelines in general that were applicable at the time.
30.
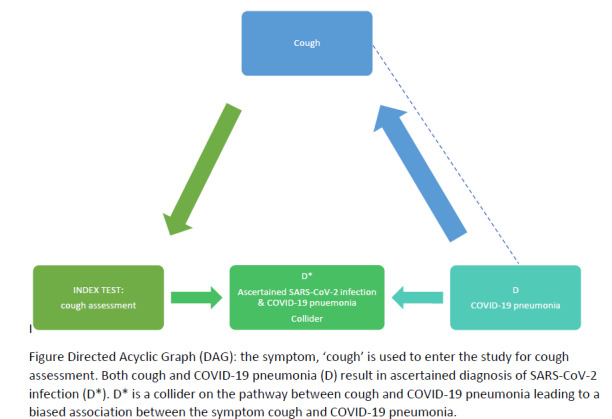
Collider bias
Another form of selection bias is spectrum bias, where the patients included in the studies do not reflect the patient spectrum the index test will be applied to. The inclusion of hospitalised patients can lead to such a bias, when the distribution of signs and symptoms differ in these patients and assessment with the reference standard is differential. In addition, the distribution and severity of alternative diagnoses may be different in hospitalised populations than in patients presenting to ambulatory care settings. By focusing on prospective studies, spectrum bias was minimised in this review update, as all but two studies, that were performed in a mix of in‐ and outpatients from paediatric hospitals (Pokorska‐Śpiewak 2021; Yonker 2020), were conducted in an outpatient setting.
The observed better sensitivity for cough (and lower specificity) compared to other index tests is unsurprising considering cough was a key feature of COVID‐19 that was used in selecting patients for further testing in included studies. As a result, a large proportion of patients in these studies would have a cough, both cases and non‐cases. The same applies to fever, for which we observed a large heterogeneity between studies. Some of this heterogeneity was reduced by selecting only studies with a low risk of bias in participant selection for the analyses, but a residual selection bias remained due to preselection based on the presence of fever. The way fever was determined (measured at presentation or self‐reported by the patient) and the use of different cut‐off levels for fever also contributed to this residual heterogeneity.
Strengths and weaknesses of the review
Strengths of our review are the systematic and broad search performed to include all possible studies, to gather the largest number of peer‐reviewed studies available at this point. Exclusion of retrospective studies, the largest number of the published cohorts of patients with COVID‐19, from the data analyses limits the available data but improves the quality of the evidence.
The greatest weakness of the review is the risk of selection bias, as discussed above, with many studies selecting their participants based on the presence of fever or respiratory symptoms. Since individuals may or may not be tested on the basis of symptomatic presentation, this limitation is difficult to avoid completely.
Although we included all studies published until June 2021, all studies reported data from 2020 and over 90% of these data were collected in the first half of 2020. Consequently, no studies reported signs or symptoms of specific viral variants.
We need to assess multiple variables for their possible confounding effect on the summary estimates. Possible confounders include the presence of other respiratory pathogens (seasonality), the phase of the epidemic, exposure to high‐ versus low‐prevalence setting, high or low exposure risk, comorbidity of the participants, or time since infection. Seasonality may influence specificity, because alternative diagnoses such as influenza or other respiratory viruses are more prevalent in winter, leading to more non‐COVID‐19 patients displaying symptoms such as cough or fever, decreasing specificity. In this version of the review, most studies were conducted during the winter or early spring seasons, suggesting this may still have been at play. However, social distancing policies have shortened this year's influenza season in several countries (who.int/influenza/surveillance_monitoring/updates), which may have led to higher specificity for signs and symptoms than what we may expect in the next influenza season. In future updates of the review, we will explore seasonality effects if data allow. As for time since onset, given that the moment of infection is more likely than not an unrecognisable and unmeasurable variable, time since onset of symptoms can be used as a proxy. Reporting of studies, with presentation of the 2x2 table stratified by time since onset of disease, is informative and might have the potential to increase accuracy of the signs and symptoms and their diagnostic differential potential.
Applicability of findings to the review question
In comparison with previous updates, in which many studies included patients who had already been admitted to hospital or who presented to hospital settings with the intent to hospitalise, selection bias was reduced by focusing on prospective studies only. These studies were primarily conducted in outpatient settings, improving the applicability of our findings in comparison with previous review versions.
This review identified only three studies in children. The differences in diagnostic accuracy with adults depended on the type of symptom. The sensitivity of fever in children was higher than the summary estimate across all ages and its specificity was lower. Fever was much more common in children than in adults (higher positivity rate), but often indicated the presence of an infectious disease other than COVID‐19 (high proportion of false positives). In contrast, we observed lower sensitivity and higher specificity for fatigue, headache and myalgia in children than in the population as a whole. Olfactory symptoms were investigated by very few studies in children.
Children have been disproportionally underrepresented in studies concerning the diagnosis of SARS‐CoV‐2 infection. Their absence seems related to the general mild presentation of the disease in the paediatric population and even more frequently the complete asymptomatic course. The full scope of disease presentation in children is however not known. It is important to identify signs and symptoms that can be used to clinically assess children with suspected SARS‐CoV‐2 infection, especially because non‐specific presentations and fever without a source are already common in this age group. Children present as a heterogeneous group, having separate data for neonates, young infants, toddlers, school‐aged children and adolescents is of value. Misclassification of children both at their presentation to the healthcare system and in the short term, where children will be asked to remain in quarantine when they present with predefined, but not yet evidence‐based symptoms needs to be avoided to decrease the possible damage done to children’s health.
Another important patient group that has been neglected in the literature is older adults. They are most at risk of a negative outcome of SARS‐CoV‐2 infection, especially mortality but also intensive care support. In this version of the review, only two overlapping studies (1 dataset) focused on adults aged 65 years or older. All other studies included adults of all ages and did not present results separately for the older age groups. The lack of a solid evidence base for the diagnosis of COVID‐19 in older adults adds to the difficulty in diagnosing serious infections in this age group, in whom other serious infections such as bacterial pneumonia or urinary sepsis also tend to lead to non‐specific presentations.
Studies specifically focusing on older adults or children may also enable us to estimate any difference in the diagnostic accuracy of signs and symptoms within these age groups. Given the distinct biological characteristics of children versus younger and versus older adults, these accuracy estimates are likely to be different in different age groups. The current presentation of overall summary estimates may therefore prove too simplistic.
Authors' conclusions
Implications for practice.
The presence of some signs and symptoms may increase the probability of COVID‐19 to an extent that is clinically relevant. Within the context of selection bias in most of the studies in this review, the presence of fever, cough, or anosmia/ageusia should be a reason for further testing for COVID‐19.
Other symptoms, such as sore throat, rhinorrhoea or coryza, increase the probability of COVID‐19 to a much lesser extent. Selecting patients for reverse transcription polymerase chain reaction (RT‐PCR) testing based on these minor symptoms will result in large numbers of people who need to be tested and low positivity rates, making such testing strategies more cumbersome, expensive and less efficient.
However, if the main goal of a country's testing strategy remains to detect as many SARS‐CoV‐2‐positive individuals as possible, denying further testing to people based on the absence of major (and even minor) symptoms will result in many missed cases.
In short, the diagnostic accuracy of symptoms for COVID‐19 is moderate to low and any testing strategy using symptoms as selection mechanism will result in both large numbers of missed cases and large numbers of people requiring testing. Which one of these is minimised, is determined by the goal of COVID‐19 testing strategies, that is, controlling the epidemic by isolating every possible case versus identifying those with clinically important disease so that they can be monitored or treated, or both, to optimise their prognosis. The former will require a testing strategy that uses very few symptoms as entry criterion for testing, the latter could focus on more specific symptoms such as fever and anosmia.
Implications for research.
Our review update reflects the need for a broader diagnostic approach than looking at signs or symptoms alone. Combinations with other, easy‐to‐obtain information such as patient demographics, the local case detection rate, recent contact with a SARS‐CoV‐2‐positive patient, travel history of the patient, point‐of‐care test results, vaccination status, and others, may help safely rule out COVID‐19 and should be investigated further. Vaccination leads to a reduced rate of asymptomatic infections (Tande 2022). Whether it also alters the clinical presentation of symptomatic COVID‐19 patients is unclear at this time. New variants of the virus might also change the clinical presentation. Given the speed with which these variants emerge, it is difficult to provide up‐to‐date information from a systematic review regarding the diagnostic value of symptoms for COVID‐19.
This review will therefore no longer be updated in its current form. Resources allowing, we will consider updating this review when sufficient studies of high methodological quality examining the combination of signs and symptoms with other clinically relevant information such as contact or travel history, or the local recent case detection rate become available. Another important outcome for future updates would be to investigate whether tests exist that identify people requiring respiratory support (SARS or ARDS) or intensive care.
Studies should report the definition of signs and symptoms more clearly, how they were measured, by whom and when. The measurement of key symptoms such as anosmia and ageusia could benefit from standardisation, including the severity and nature of the loss of smell or taste. Yet such standardisation should not be overly complicated as signs and symptoms will typically be used by frontline clinicians who will incorporate these in their more holistic assessment of the patient, which includes more than just diagnosis of COVID‐19.
Future studies should also include a broader spectrum of patients with studies in the primary healthcare setting to properly evaluate the diagnostic accuracy of signs and symptoms in this setting. Studies aimed at screening for SARS‐CoV‐2 infections should also be included, as an increased need for screening is to be expected with the relaxation of quarantine measures. Finally, data are needed on specific patient groups with comorbidities with a higher risk of complications or serious illness and a greater impact of missing early diagnosis of SARS‐CoV‐2 infection, and the paediatric and elderly population should be added.
We would like to recommend that authors adhere to the STARD guidelines when reporting new studies on this topic (Bossuyt 2015).
What's new
| Date | Event | Description |
|---|---|---|
| 15 February 2022 | New citation required and conclusions have changed | Findings from 42 prospective studies involving 52,608 participants included in this second update version indicate that there is currently no evidence to support further testing in all individuals presenting only with upper respiratory symptoms such as sore throat, coryza or rhinorrhoea. Based on currently available data, neither absence nor presence of a single sign or symptom are accurate enough to rule in or rule out COVID‐19 disease. |
| 16 November 2021 | New search has been performed | Review updated with search until 10 June 2021. |
History
Review first published: Issue 7, 2020
| Date | Event | Description |
|---|---|---|
| 4 March 2021 | Amended | Corrected peer reviewer's name in Acknowledgements section |
| 11 February 2021 | New citation required and conclusions have changed | Review updated: We retrieved 28 more studies on signs and symptoms in suspected COVID‐19 patients, allowing pooling of the data for some features and estimation of summary measures of diagnostic accuracy. Moreover, this update contains new studies on the diagnostic value of olfactory symptoms, and includes a limited number of studies on combinations of symptoms. |
| 8 December 2020 | New search has been performed | Review updated |
| 7 July 2020 | Amended | Resolution of two figures improved |
Acknowledgements
Members of the Cochrane COVID‐19 Diagnostic Test Accuracy Review Group include the following.
The project team (Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Leeflang MMG, Spijker R, Hooft L, Van den Bruel A, McInnes MDF, Emperador D, Dittrich S)
-
The systematic review teams for each review:
molecular, antigen, and antibody tests (Adriano A, Beese S, Dretzke J, Ferrante di Ruffano L, Harris I, Price M, Taylor‐Phillips S);
signs and symptoms (Struyf T, Domen J, Horn S, Lannoy V, Wickramasinghe D, Janssens S, Tans A);
routine laboratory markers (Yang B, Langendam M, Ochodo E, Guleid F, Holtman G, Verbakel J, Wang J, Stegeman I);
imaging tests (Salameh JP, McGrath TA, van der Pol CB, Frank RA, Prager R, Hare SS, Dennie C, Jenniskens K, Korevaar DA, Cohen JF, Van de Wijgert J, Damen JAAG, Wang J).
The wider team of systematic reviewers from University of Birmingham, UK who assisted with title and abstract screening across the entire suite of reviews for the diagnosis of COVID‐19 (Agarwal R, Baldwin S, Berhane S, Herd C, Kristunas C, Quinn L, Scholefield B): see Deeks 2020a; Dinnes 2021; Islam 2021; Stegeman 2020).
Jonathan Deeks is a UK National Institute for Health Research (NIHR) Senior Investigator Emeritus. Yemisi Takwoingi is supported by a NIHR Postdoctoral Fellowship. Jonathan Deeks, Jacqueline Dinnes, Yemisi Takwoingi, Clare Davenport and Malcolm Price are supported by the NIHR Birmingham Biomedical Research Centre. This paper presents independent research supported by the NIHR Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
We thank Dr Jane Cunningham (World Health Organization) for participation in technical discussions and comments on the review.
Cochrane Infectious Diseases supported the review authors in the development of this second update. The Cochrane Infectious Diseases' editorial base is funded by UK aid from the UK government for the benefit of low‐ and middle‐income countries (project number 300342‐104). The views expressed do not necessarily reflect the UK government’s official policies.
The following people conducted the editorial process for this review update.
Sign‐off Editor (final editorial decision): Michael Brown, Michigan State University College of Human Medicine, USA
Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the update): Joey Kwong, Cochrane Evidence Production & Methods Directorate
Editorial Assistant (conducted editorial policy checks and supported editorial team): Leticia Rodrigues, Cochrane Evidence Production & Methods Directorate
Copy Editor (copy‐editing and production): Denise Mitchell, Cochrane Copy Edit Support
Peer‐reviewers (provided comments and recommended an editorial decision): Brendan Delaney, Imperial College London (clinical review); Lao‐Tzu Allan‐Blitz, Division Global Health Equity: Department of Medicine, Brigham and Women's Hospital Department of Pediatrics, Boston Children's Hospital (clinical review); Paul Garner, Liverpool School of Tropical Medicine (clinical review)*; Jenny Negus (consumer review); Lynda Ware, Cochrane UK (summary versions review); Gianni Virgili and Tanya Walsh, Cochrane Diagnostic Test Accuracy Reviews Editorial Team (methods review); Robin Featherstone, Cochrane Evidence Production & Methods Directorate (search review). *Paul Garner is a member of Cochrane Infectious Diseases and provided peer‐review comments on this update, but was not otherwise involved in the editorial process or decision making. One additional peer reviewer provided clinical peer review but chose not to be publicly acknowledged.
Appendices
Appendix 1. World Health Organization case definitions
Severe pneumonia
Adolescent or adult: fever or suspected respiratory infection, plus one of the following: respiratory rate higher than 30 breaths per minute; severe respiratory distress; or oxygen saturation (SpO2) 93% or less on room air. Child with cough or difficulty in breathing, plus at least one of the following: central cyanosis or SpO2 less than 90%; severe respiratory distress (for example, grunting, very severe chest indrawing); signs of pneumonia with a general danger sign: inability to breastfeed or drink, lethargy or unconsciousness, or convulsions.
Other signs of pneumonia may be present: chest indrawing, fast breathing (in breaths per minute): aged under 2 months: 60 or higher; aged 2 to 11 months: 50 or higher; aged 1 to 5 years: 40 or higher. While the diagnosis is made on clinical grounds; chest imaging may identify or exclude some pulmonary complications.
Acute respiratory distress syndrome (ARDS)
Onset within one week of a known clinical insult or new or worsening respiratory symptoms.
Chest imaging (that is, X‐ray, computed tomography (CT) scan, or lung ultrasound): bilateral opacities, not fully explained by volume overload, lobar or lung collapse, or nodules.
Origin of pulmonary infiltrates: respiratory failure not fully explained by cardiac failure or fluid overload. Need objective assessment (for example, echocardiography) to exclude hydrostatic cause of infiltrates/oedema if no risk factor present.
Oxygenation impairment in adults:
mild ARDS: 200 mmHg less than ratio of arterial oxygen partial pressure/fractional inspired oxygen (PaO2/FiO2) 300 mmHg or less (with positive end‐expiratory pressure (PEEP) or continuous positive airway pressure (CPAP) 5 cmH2 O, or more, or non‐ventilated);
moderate ARDS: 100 mmHg < PaO2/FiO2 ≤ 200 mmHg (with PEEP ≥ 5 cmH2 O, or non‐ventilated);
severe ARDS: PaO2/FiO 2 ≤ 100 mmHg (with PEEP ≥ 5 cm H2O, or non‐ventilated);
when PaO2 is not available, SpO2/FiO2 ≤ 315 mmHg suggests ARDS (including in non‐ventilated patients).
Oxygenation impairment in children: note OI = Oxygenation Index and OSI = Oxygenation Index using SpO2 . Use PaO2‐based metric when available. If PaO2 not available, wean FiO2 to maintain SpO2 ≤ 97% to calculate OSI or SpO2/FiO2 ratio:
bilevel (non‐invasive ventilation or CPAP) ≥ 5 cm H2O via full‐face mask: PaO2/FiO2 ≤ 300 mmHg or SpO2/FiO2 ≤ 264;
mild ARDS (invasively ventilated): 4 ≤ OI < 8 or 5 ≤ OSI < 7.5;
moderate ARDS (invasively ventilated): 8 ≤ OI < 16 or 7.5 ≤ OSI < 12.3;
severe ARDS (invasively ventilated): OI ≥ 16 or OSI ≥ 12.3.
Appendix 2. University of Bern living search database
From 30 October 2020
Embase:
(exp SARS‐related coronavirus/ or severe acute respiratory syndrome/ or coronavirus disease 2019/ or (coronavir* or corona virus* or HCoV* or ncov* or 2019 cov or covid or covid19 or sars‐cov* or sarscov* or sars‐coronavirus* or Severe Acute Respiratory Syndrome Coronavirus* or nCoV).mp.) and 20191101:20301231.(dc).
MEDLINE:
("severe acute respiratory syndrome coronavirus 2"[Supplementary Concept] OR "COVID‐19" [Supplementary Concept] OR "coronavirus" OR "corona virus" OR "HCoV" OR "nCoV" OR "2019 CoV" OR "covid" OR "covid19" OR "Severe Acute Respiratory Syndrome Coronavirus 2" OR "SARS‐CoV2" OR "SARS‐CoV 2" OR "SARS Coronavirus 2") AND (2019/11/01:3000/12/31[PDAT])
Appendix 3. Search classification model
We needed a more efficient approach to keep up with the rapidly increasing volume of COVID‐19 literature. A classification model for this specific review was built with the model‐building function within Eppi Reviewer, which uses the standard SGCClassifier in Scikit‐learn on word trigrams. As outputs, new documents receive a percentage (from the predict_proba function) where scores close to 100 indicate a high probability of belonging to the class ‘relevant document’ and scores close to 0 indicate a low probability of belonging to the class ‘relevant document’. We used three iterations of manual screening (title and abstract screening, followed by full‐text review) to build and test classifiers. The final included studies were used as relevant documents, while the remainder of the COVID‐19 studies were used as irrelevant documents. The classifier was trained on the first round of selected articles, and tested and retrained on the second round of selected articles. Testing on the second round of selected articles revealed poor positive predictive value but 100% sensitivity at a cut‐off of 10. The poor positive predictive value is mainly due to the broad scope of our topic (any signs or symptoms associated with COVID‐19)), poor reporting in abstracts, and a small set of included documents.
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
1. Test.
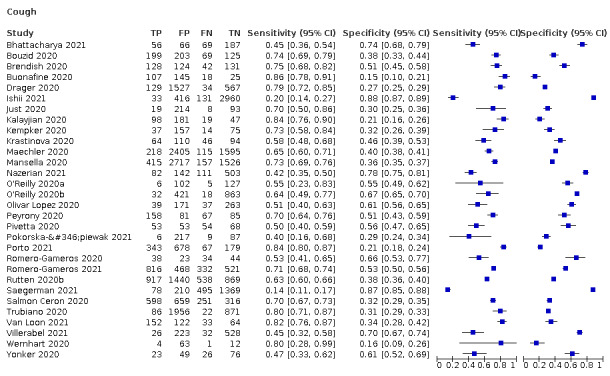
Cough
2. Test.
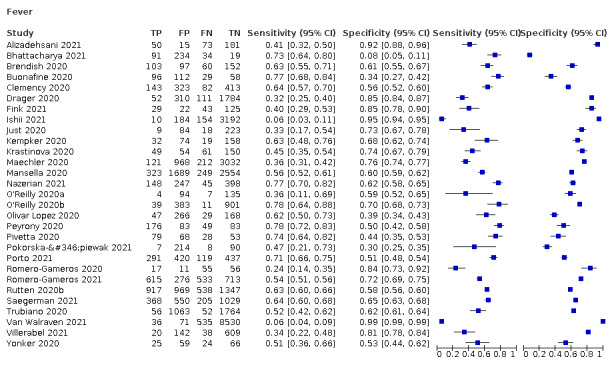
Fever
3. Test.
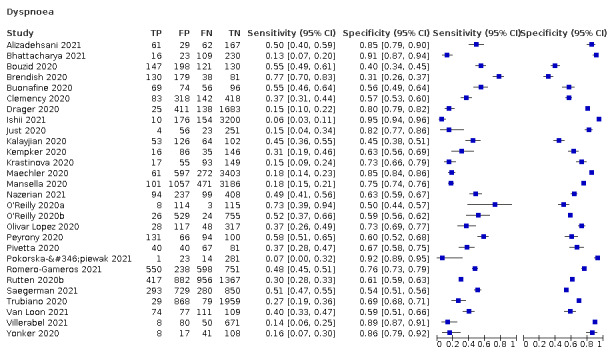
Dyspnoea
4. Test.
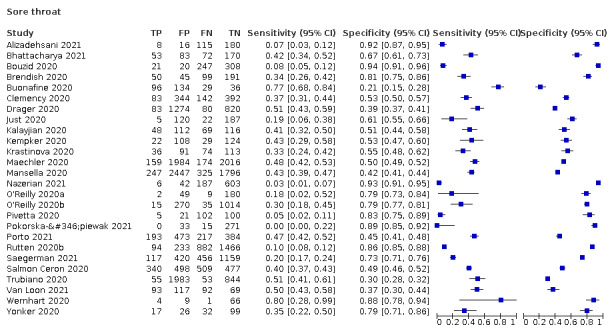
Sore throat
5. Test.
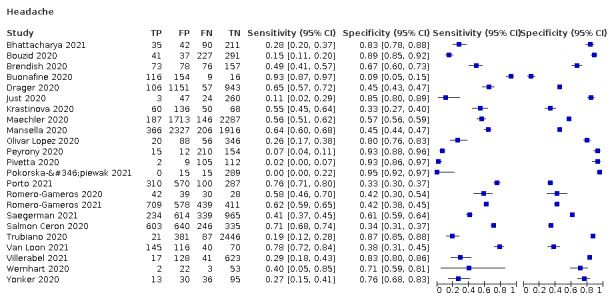
Headache
6. Test.
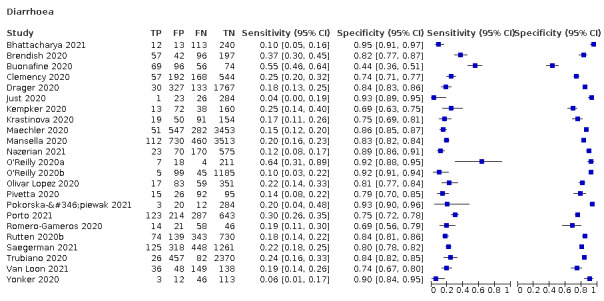
Diarrhoea
7. Test.
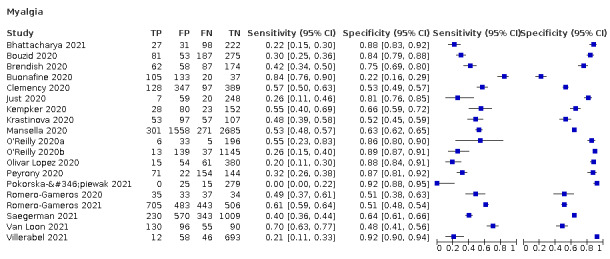
Myalgia
8. Test.
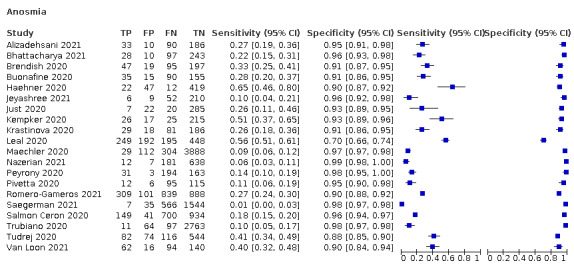
Anosmia
9. Test.
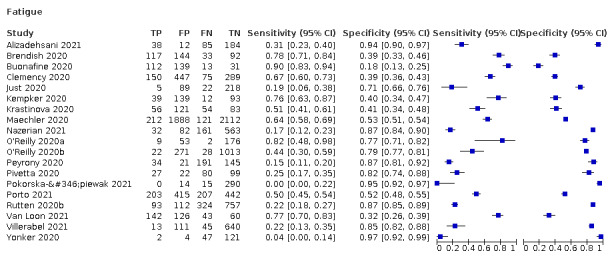
Fatigue
10. Test.
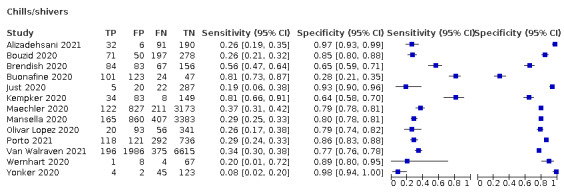
Chills/shivers
11. Test.
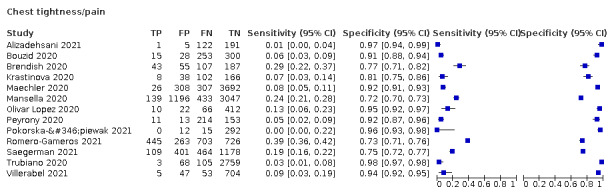
Chest tightness/pain
12. Test.
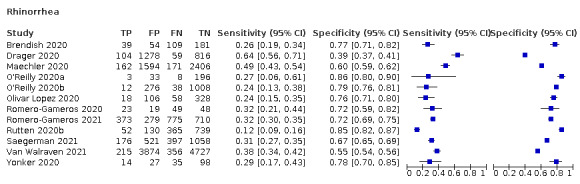
Rhinorrhea
13. Test.
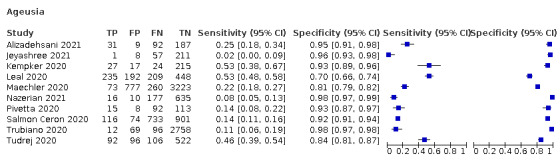
Ageusia
14. Test.
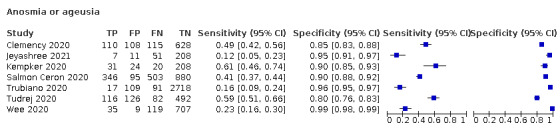
Anosmia or ageusia
15. Test.
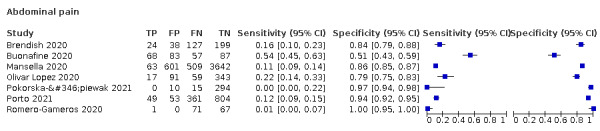
Abdominal pain
16. Test.
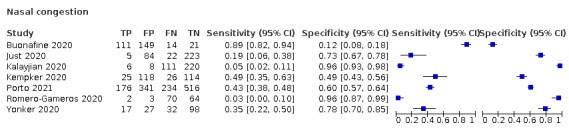
Nasal congestion
17. Test.
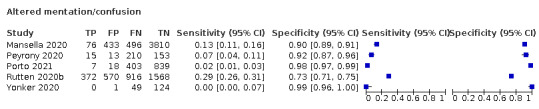
Altered mentation/confusion
18. Test.
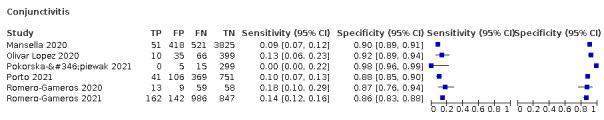
Conjunctivitis
19. Test.
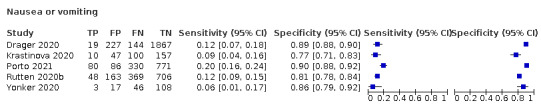
Nausea or vomiting
20. Test.
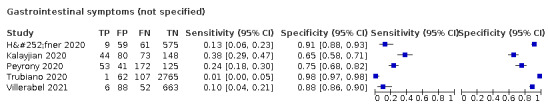
Gastrointestinal symptoms (not specified)
21. Test.
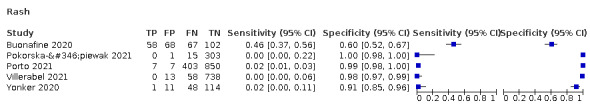
Rash
22. Test.
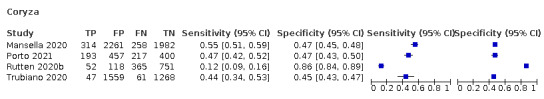
Coryza
23. Test.
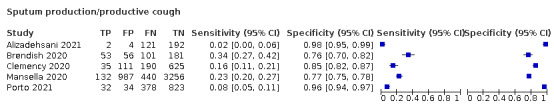
Sputum production/productive cough
24. Test.
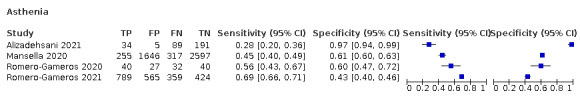
Asthenia
25. Test.
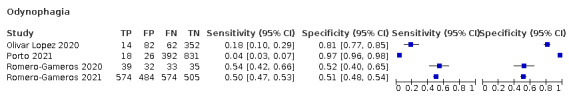
Odynophagia
26. Test.
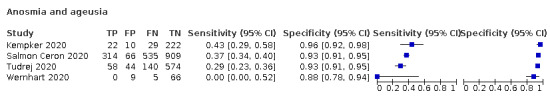
Anosmia and ageusia
27. Test.
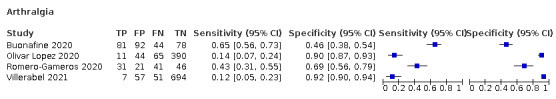
Arthralgia
28. Test.

Vomiting
29. Test.

Wheeze
30. Test.

Nausea
31. Test.

Dry cough
32. Test.

Malaise
33. Test.

Enlargement of lymph nodes
34. Test.
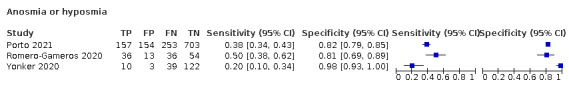
Anosmia or hyposmia
35. Test.
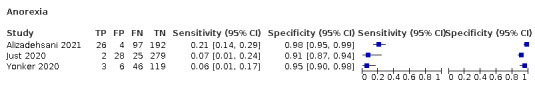
Anorexia
36. Test.
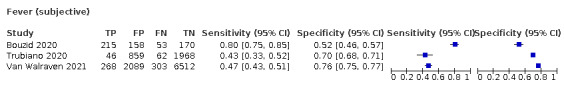
Fever (subjective)
37. Test.

Haemoptysis
38. Test.

Earache
39. Test.
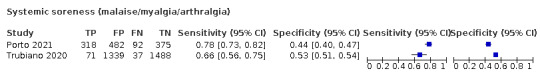
Systemic soreness (malaise/myalgia/arthralgia)
40. Test.

High fever (≥ 38.5 °C)
41. Test.
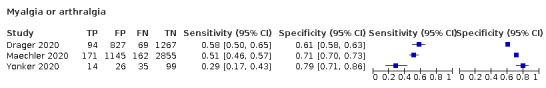
Myalgia or arthralgia
42. Test.
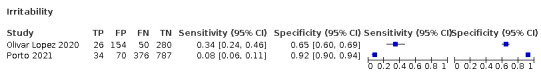
Irritability
43. Test.
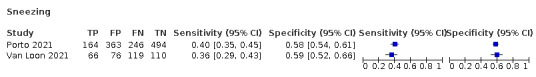
Sneezing
44. Test.

Anosmia or dysgeusia
45. Test.
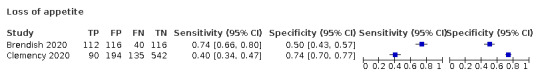
Loss of appetite
46. Test.

Pulmonary auscultation: crackling bilateral
47. Test.
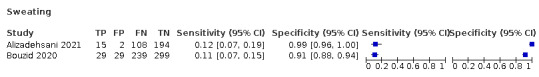
Sweating
48. Test.

Nasal symptoms
49. Test.

Rhinitis
50. Test.

Dysgeusia
51. Test.

SCRiPS score, recent case detection rate
52. Test.

SCRIPS score, 0.5*recent case detection rate
53. Test.

Rigors
54. Test.

Cough or dyspnoea
55. Test.

Dysuria
56. Test.

Seizure
57. Test.

Exanthema
58. Test.

Exhaustion
59. Test.

Sinusitis
60. Test.

Hypoxia
61. Test.

Multivariable score cut‐off = 5
62. Test.

Multivariable score cut‐off = 8
63. Test.

Cough and anosmia
64. Test.

Fever and anosmia
65. Test.

Fever and cough and anosmia and dyspnoea and oxygen saturation < 93%
66. Test.

Fever and dyspnoea
67. Test.

Anosmia and dyspnoea
68. Test.

Fever and cough
69. Test.
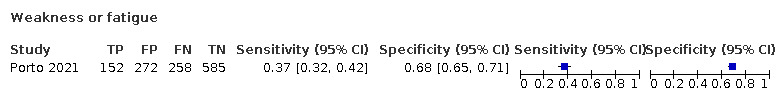
Weakness or fatigue
70. Test.

Palpitations
71. Test.

Anxiety
72. Test.

Respiratory distress
73. Test.

Hyposmia or anosmia
74. Test.

Diarrhoea and nausea
75. Test.

Isolated fever
76. Test.

Myalgia and asthenia and fever
77. Test.

Cough and fever and sputum production
78. Test.

Cough and fever and sputum production and dyspnoea
79. Test.

Isolated headache
80. Test.

Dyspnoea and cough and fever and low oxygen saturation
81. Test.

Sore throat and nasal congestion and sneezing and mild fever
82. Test.

Low body temperature
83. Test.

Expectoration
84. Test.

Tachypnoea
85. Test.

Cyanosis
86. Test.

Skin lesions
87. Test.

Rhinitis or pharyngitis
88. Test.

Dizziness or syncope
89. Test.

Pulmonary auscultation: crackling unilateral
90. Test.

CSBSS (cut‐off = 41.7)
91. Test.

Hyposmia
92. Test.

Hypogeusia
93. Test.

Dizziness
94. Test.

Change to chronic cough
95. Test.

Dysosmia
96. Test.

Myalgia and fatigue
97. Test.
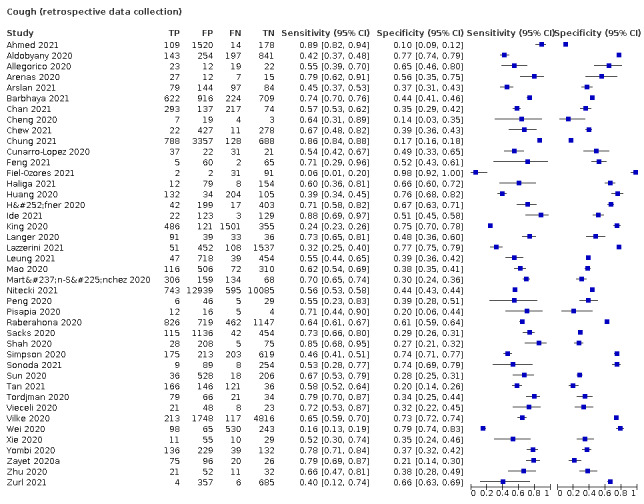
Cough (retrospective data collection)
98. Test.
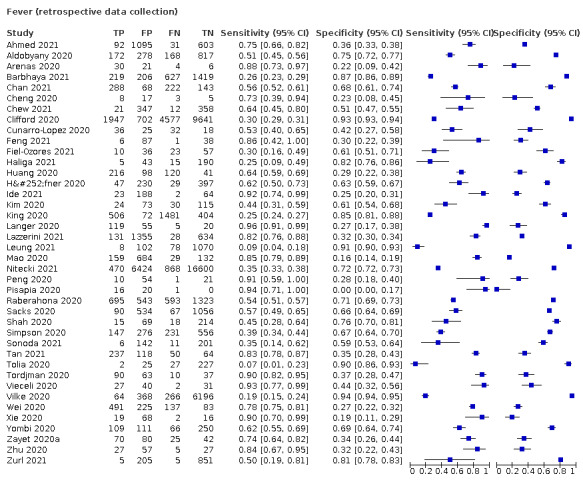
Fever (retrospective data collection)
99. Test.
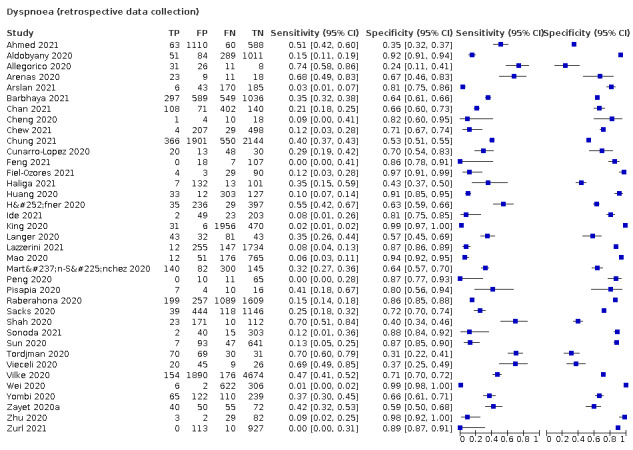
Dyspnoea (retrospective data collection)
TST-100. Test.
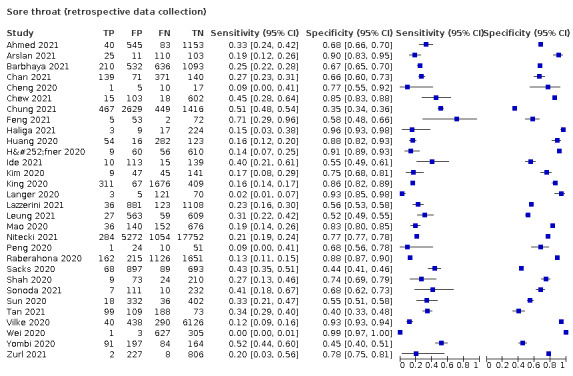
Sore throat (retrospective data collection)
TST-101. Test.
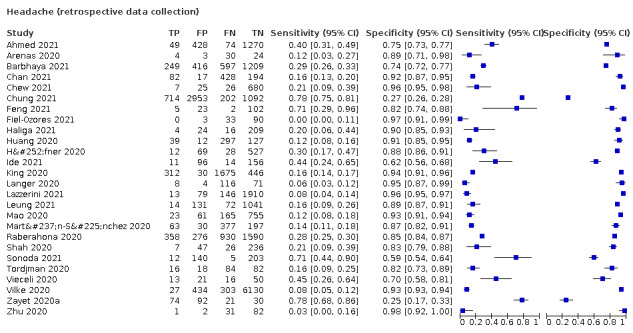
Headache (retrospective data collection)
TST-102. Test.
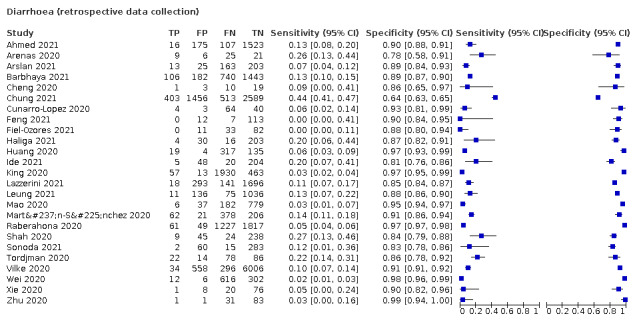
Diarrhoea (retrospective data collection)
TST-103. Test.
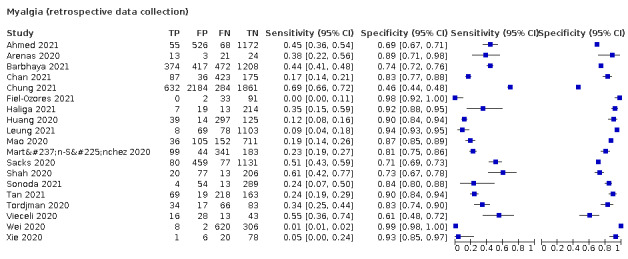
Myalgia (retrospective data collection)
TST-104. Test.
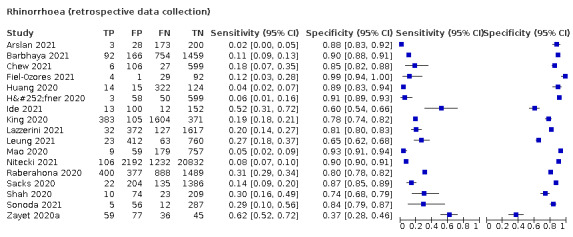
Rhinorrhoea (retrospective data collection)
TST-105. Test.
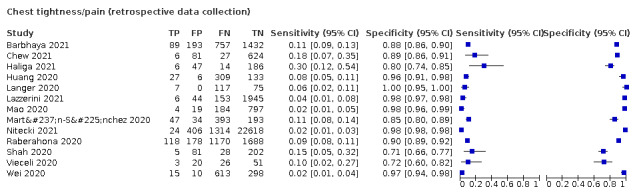
Chest tightness/pain (retrospective data collection)
TST-106. Test.
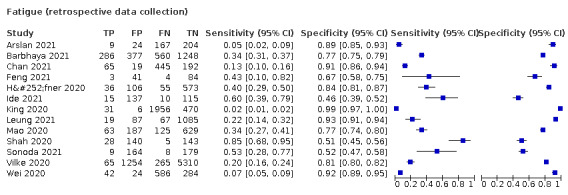
Fatigue (retrospective data collection)
TST-107. Test.
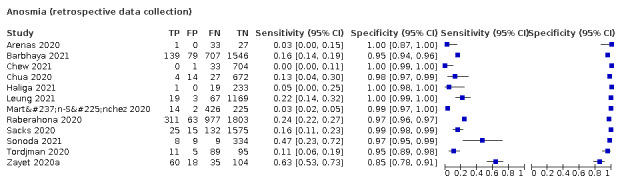
Anosmia (retrospective data collection)
TST-108. Test.
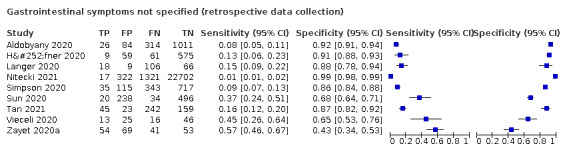
Gastrointestinal symptoms not specified (retrospective data collection)
TST-109. Test.
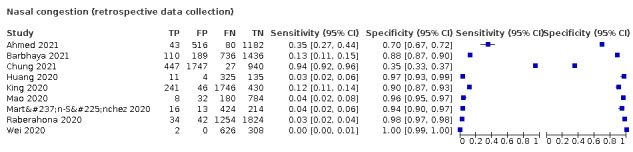
Nasal congestion (retrospective data collection)
TST-110. Test.
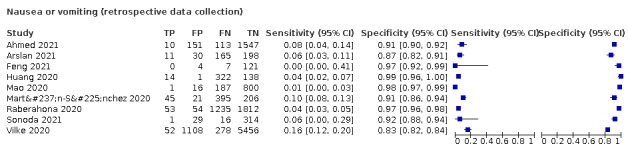
Nausea or vomiting (retrospective data collection)
TST-111. Test.
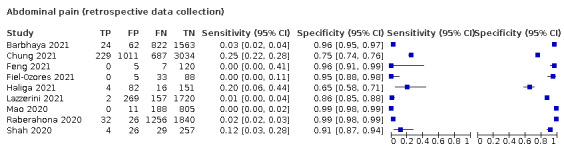
Abdominal pain (retrospective data collection)
TST-112. Test.
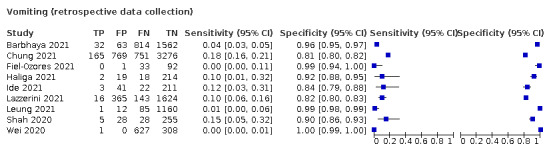
Vomiting (retrospective data collection)
TST-113. Test.
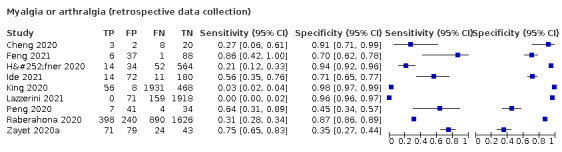
Myalgia or arthralgia (retrospective data collection)
TST-114. Test.
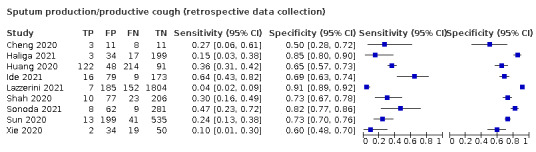
Sputum production/productive cough (retrospective data collection)
TST-115. Test.
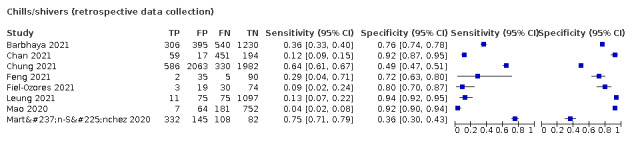
Chills/shivers (retrospective data collection)
TST-116. Test.
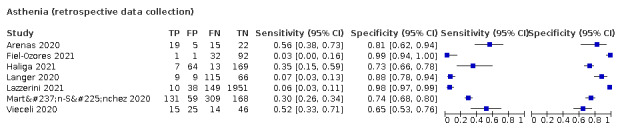
Asthenia (retrospective data collection)
TST-117. Test.
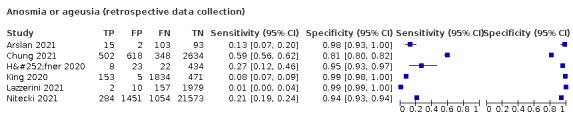
Anosmia or ageusia (retrospective data collection)
TST-118. Test.
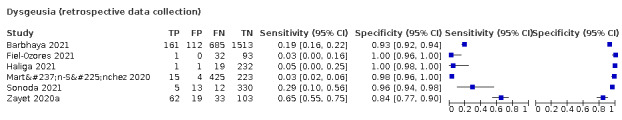
Dysgeusia (retrospective data collection)
TST-119. Test.
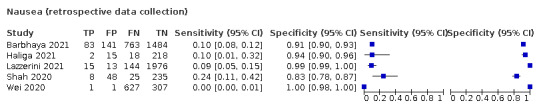
Nausea (retrospective data collection)
TST-120. Test.
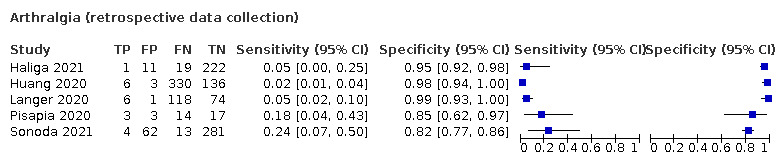
Arthralgia (retrospective data collection)
TST-121. Test.
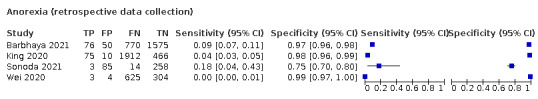
Anorexia (retrospective data collection)
TST-122. Test.

Wheeze (retrospective data collection)
TST-123. Test.

Haemoptysis (retrospective data collection)
TST-124. Test.
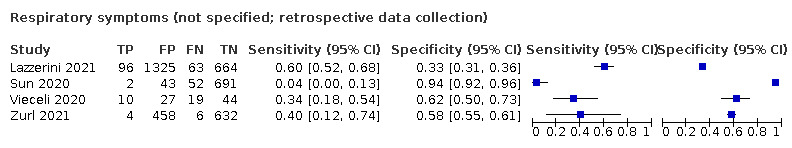
Respiratory symptoms (not specified; retrospective data collection)
TST-125. Test.

Skin lesions (retrospective data collection)
TST-126. Test.
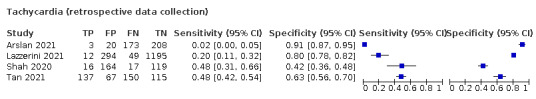
Tachycardia (retrospective data collection)
TST-127. Test.
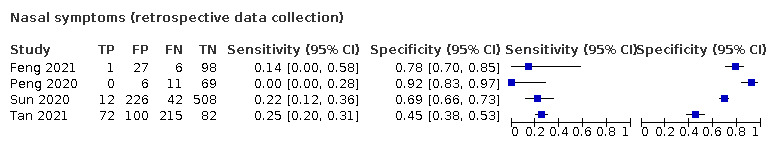
Nasal symptoms (retrospective data collection)
TST-128. Test.
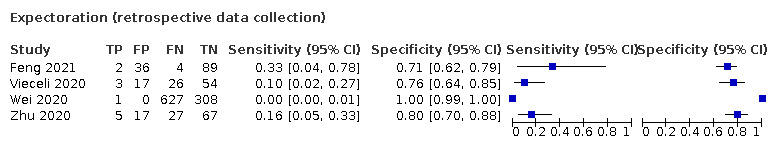
Expectoration (retrospective data collection)
TST-129. Test.
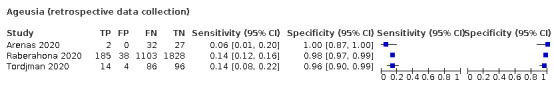
Ageusia (retrospective data collection)
TST-130. Test.
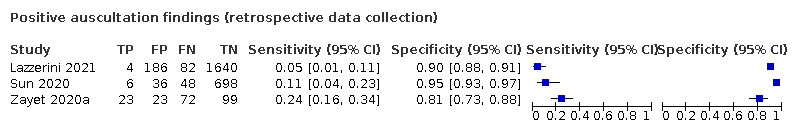
Positive auscultation findings (retrospective data collection)
TST-131. Test.

Tachypnea (retrospective data collection)
TST-132. Test.
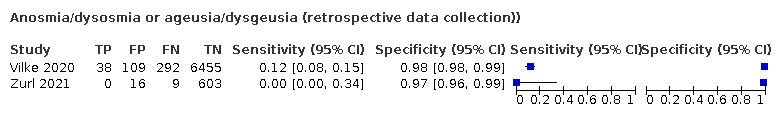
Anosmia/dysosmia or ageusia/dysgeusia (retrospective data collection))
TST-133. Test.

Earache (retrospective data collection)
TST-134. Test.

Sneezing (retrospective data collection)
TST-135. Test.

Dizziness (retrospective data collection)
TST-136. Test.

Malaise (retrospective data collection)
TST-137. Test.
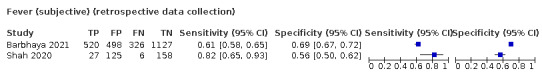
Fever (subjective) (retrospective data collection)
TST-138. Test.

Enlargement of lymph nodes (retrospective data collection)
TST-139. Test.

Conjunctivitis (retrospective data collection)
TST-140. Test.

Hypoxia (retrospective data collection)
TST-141. Test.

Pulmonary auscultation: rhonchi (retrospective data collection)
TST-142. Test.

Loss of appetite (retrospective data collection)
TST-143. Test.

Altered mentation/confusion (retrospective data collection)
TST-144. Test.

Presyncope or syncope (retrospective data collection)
TST-145. Test.

Stomach ache (retrospective data collection)
TST-146. Test.

Odynophagia (retrospective data collection)
TST-147. Test.
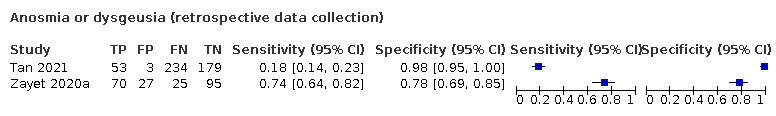
Anosmia or dysgeusia (retrospective data collection)
TST-148. Test.

Weakness or fatigue (retrospective data collection)
TST-149. Test.

Objective fever (≥ 38 °C) or recent fever/chills (retrospective)
TST-150. Test.

Body aches or fatigue or dyspnoea or cough or objective fever (≥ 38 °C) or recent fever/chills (retrospective)
TST-151. Test.

Fatigue or dyspnoea or cough or objective fever (≥ 38 °C) or recent fever/chills (retrospective)
TST-152. Test.

Dyspnoea or cough or objective fever (≥ 38 °C) or recent fever/chills (retrospective)
TST-153. Test.

Cough or objective fever (≥ 38 °C) or recent fever/chills (retrospective)
TST-154. Test.

Recent fever or chills (retrospective data collection)
TST-155. Test.

Sinusitis (retrospective data collection)
TST-156. Test.

Systemic soreness (malaise/myalgia/arthralgia) (retrospective)
TST-157. Test.

Malaise or fatigue (retrospective data collection)
TST-158. Test.

Lethargy (retrospective data collection)
TST-159. Test.

Nausea or vomiting or diarrhoea (retrospective data collection)
TST-160. Test.

Respiratory triage score > 4 (retrospective data collection)
TST-161. Test.

Respiratory triage score > 5 (retrospective data collection)
TST-162. Test.

Lower respiratory tract symptoms (retrospective data collection)
TST-163. Test.

Neurologic symptoms (not specified; retrospective data collection)
TST-164. Test.

Upper respiratory tract symptoms (retrospective data collection)
TST-165. Test.

Laryngitis/hoarseness/stridor (retrospective data collection)
TST-166. Test.

High fever (≥ 38.5 °C) (retrospective data collection)
TST-167. Test.

Abdominal distention (retrospective data collection)
TST-168. Test.

Aversion to cold (retrospective data collection)
TST-169. Test.

Xerostomia (retrospective data collection)
TST-170. Test.

Hypersomnia (retrospective data collection)
TST-171. Test.

Hyposmia (retrospective data collection)
TST-172. Test.

Fever and cough and dyspnoea (retrospective)
TST-173. Test.

Fever and cough and sore throat (retrospective)
TST-174. Test.

Fever and cough (retrospective data collection)
TST-175. Test.

Unconsciousness (retrospective data collection)
TST-176. Test.

Rash (retrospective data collection)
TST-177. Test.

Fever or cough or dyspnoea (retrospective data collection)
TST-178. Test.

Pulmonary auscultation: crackling (retrospective data collection)
TST-179. Test.

Dysphonia (retrospective data collection)
TST-180. Test.

Dry cough (retrospective data collection)
TST-181. Test.

History of fever at home (retrospective data collection)
TST-182. Test.

Cough or dyspnoea (retrospective data collection)
TST-183. Test.

Anosmia and dysgeusia (retrospective data collection)
TST-184. Test.

Palpitations (retrospective data collection)
TST-185. Test.
Anosmia or hyposmia (retrospective data collection)
TST-186. Test.

Myalgia or fatigue (retrospective data collection)
TST-187. Test.

Respiratory distress (retrospective data collection)
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahmed 2021.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to compare demographic characteristics of patients who received positive and negative test results for SARS‐CoV‐ 2 in a population with higher testing rates than among previously published cohorts Design: multicenter cross‐sectional cohort study, retrospective data collection Recruitment: all patients tested for SARSCoV‐2 in the UHealth system during the study period were eligible for this cohort study. For a random subset of patients tested during 10‐31 March 2020, symptom data were manually extracted from medical records from 24 h before and 24 h after SARS‐CoV‐2 testing. Medical records were selected to include at least 20% of patients tested on each day, for a total of 1821 patients. Sample size: n = 1821 (123 cases) 2021 Inclusion criteria: all patients tested for SARS‐CoV‐2 in the UHealth system during the study period. Test eligibility required at least 1 of the following symptoms ‐ cough, fever, shortness of breath, or a high risk of exposure given recent travel or contact with a person who tested positive. Exclusion criteria: none specified |
||
| Patient characteristics and setting |
Facility cases: positive SARS‐CoV‐2 test. Primarily outpatient settings Facility controls: negative SARS‐CoV‐2 test. Primarily outpatient settings Country: Utah, USA Dates: 10 March 2020‐31 March 2020 Symptoms and severity: not specified. Primarily mild to moderate severity Demographics: median age cases: 38.2 years controls: 39.6 years. Gender: % female cases: 45.8%, controls: 56.7% (entire cohort) Exposure history: previous exposure: 56% of cases, 28% of controls; travel: 45% of cases, 25% of controls |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Time interval not specified | ||
| Comparative | |||
| Notes | Funding: supported by the National Institute of Allergy and Infectious Diseases (R01 AI135114 to D.T.L.) and the National Heart, Lung, and Blood Institute (K08 HL13650 to R.U.S.) of the National Institutes of Health; and the Centers for Disease Control and Prevention (5U01CK000555‐02 to M.H.S. and 5U01CK000538‐03 to M.H.S. and L.T.K.) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Aldobyany 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to determine the diagnostic performance characteristics of the COVID‐19 respiratory triage score Design: cross‐sectional cohort study, retrospective data collection Recruitment: all participants presenting to a tertiary hospital (presenting to ED or clinic) and tested for COVID‐19: participants either self‐presenting due to symptoms (> 50%), or active screening of contacts to COVID‐19 patients or recently travelled (mostly asymptomatic group) Sample size: n = 1435 (340 cases) Inclusion criteria: all participants presented to King Abdullah Medical City and tested for COVID‐19 Exclusion criteria: participants who did not have a documented COVID‐19 respiratory triage score, or presented prior to 2 April 2020 |
||
| Patient characteristics and setting |
Facility cases: positive RT‐PCR for SARS‐CoV‐2 Facility controls: negative RT‐PCR for SARS‐CoV‐2 Country: Saudi‐Arabia Dates: 02 April 2020‐12 September 2020 (date of submission article) Symptoms and severity: mostly asymptomatic, mild or moderate severity Demographics: age: controls mean 39.3 years, cases mean 38.7 years M%/F%: cases 68.5/31.5, controls 55.4/44.6 Exposure history: not specified, > 70% of participants were HCW |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Timing not specified | ||
| Comparative | |||
| Notes | Setting unclear Funding: none declared |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Alizadehsani 2021.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of COVID‐19 pneumonia; to identify risk factors for disease and mortality Design: cross‐sectional cohort, prospective data collection Recruitment: all patients referred to the imaging department through the ED on suspicion of COVID‐19 (with flu‐like symptoms) Sample size: n = 319 (123 cases) Inclusion criteria: patients with flu‐like symptoms referred to the imaging department Exclusion criteria: none specified |
||
| Patient characteristics and setting |
Facility cases: patients with positive findings on lung CT Facility controls: patients with negative lung CT Country: Iran Dates: 01 March 2020‐08 April 2020 Symptoms and severity: not specified. 1/3 had COVID‐19 pneumonia Demographics: mean age cases: 52.0 years controls: 44.1 years M%/F%: cases 40.8/59.2, controls 50.4/49.6 Exposure history: travelling in past 3 months: cases 4.9%, controls 3.1% |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Timing not specified | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Allegorico 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to explore the value of lung ultrasonography to predict RT‐PCR test results Design: cross‐sectional cohort study, retrospective data collection Recruitment: consecutive ED patients were included if they had either fever (body temperature > 37.5 °C) and/or history of cough and/or dyspnoea within the previous 48 h as assessed on day 1 Sample size: n = 79 (42 cases) Inclusion criteria: all patients with fever (body temperature > 37.5 °C measured using infrared thermometer) and of cough and/or dyspnoea Exclusion criteria: patients with missing clinical, biochemistry and radiological data (thoracic ultrasound, CT scan) |
||
| Patient characteristics and setting |
Facility cases: positive RT‐PCR for SARS‐CoV‐2 Facility controls: negative RT‐PCR for SARS‐CoV‐2 Country: Italy Dates: 01 March 2020‐30 April 2020 Symptoms and severity: not specified. 75% of all participants had dyspnoea Demographics: median age cases 68.5 years, controls 67.5 years M%/F%: cases 69.0/31.0, controls 67.6/32.4 Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Index tests and RS both taken on day 1 | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Arenas 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to describe the clinical features of kidney transplant (KT) and maintenance haemodialysis (MHD) patients, comparing confirmed and suspected non‐confirmed COVID‐19 cases Design: cross‐sectional multicenter cohort study, retrospective data collection Recruitment: all KT recipients and MHD patients who were studied in the Hospital del Mar for suspected COVID‐19 infection Sample size: n = 61 (34 cases) Inclusion criteria: any patients admitted in that 40‐day period with COVID‐19‐compatible signs, fever was the main symptom leading to suspicion of COVID‐19 and testing Exclusion criteria: none specified |
||
| Patient characteristics and setting |
Definition cases: all KT and MHD patients admitted with a single positive RT‐PCR test for SARS‐CoV‐2 (1 case with first a negative and later on a positive test) Definition controls: negative RT‐PCR test for SARS‐CoV‐2 (17/27 got a consecutive negative test) Country: Spain Dates: 12 March 2020‐21 April 2020 Symptoms and severity: inclusion based on potential COVID‐symptoms 30/34 cases pneumonia, 5/27 controls had pneumonia Demographics: age: controls mean 62.1 years, cases mean 69.0 years M%/F%: cases 70.6/29.4, controls 78.8/21.2 Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Unclear time interval. Symptoms recorded on admission. Timing of reference test not specified | ||
| Comparative | |||
| Notes | KT and MHD patients: very specific population at higher risk of infection Funding: none declared |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Arslan 2021.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to identify the clinical findings and outcomes of children with COVID‐19 and factors predicting RT‐PCR positivity Design: cross‐sectional cohort study, retrospective data collection Recruitment: all suspected COVID‐19 patients (children) admitted to and treated in the ED, inpatient clinic, or paediatric ICU of a tertiary hospital (suspected if a child had contact with a confirmed COVID‐19 case, lived in an epidemic area where COVID‐19 case(s) were reported, or had any family member hospitalised due to a respiratory infection or experiencing symptoms such as cough, fever, or shortness of breath in the last 2 weeks and if the children had respiratory or GI symptoms) Sample size: n = 404 (176 cases) Inclusion criteria: children between 1 month and 18 years of age presenting to the ED, inpatient clinic, or paediatric ICU, suspected of COVID‐19 Exclusion criteria: none specified |
||
| Patient characteristics and setting |
Facility cases: positive SARS‐CoV‐2 test Facility controls: negative SARS‐CoV‐2 test Country: Turkey Dates: 20 March 2020‐31 May 2020 Symptoms and severity: asymptomatic: 33.5% of cases; mild: 53.4% of cases; moderate: 12.5% of cases; severe: 0.0% of cases; critical: 0.6% of cases Demographics: median age cases 79 months, controls 30.5 months M%/F%: cases 55.7/44.3, controls 59.2/40.8 Exposure history: previous exposure to SARS‐CoV‐2‐infected person: cases 93%, controls 23% |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Timing not specified | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Barbhaya 2021.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to investigate and characterise mild to moderate COVID‐19 and risk factors Design: cross‐sectional cohort study, retrospective data collection Recruitment: patients presenting to a dedicated ambulatory COVID‐19 clinic in Washington DC Sample size: n = 2471 (846) Inclusion criteria: patients from the community and hospital associates; PCR tested, prescreened for symptoms or exposure; decisions for testing made according to CDC guidelines in place at the time of each encounter, most often only if the patient was symptomatic Exclusion criteria: none specified |
||
| Patient characteristics and setting |
Facility cases: positive SARS‐CoV‐2 test Facility controls: negative SARS‐CoV‐2 test Country: USA Dates: 11 March 2020‐14 June 2020 Symptoms and severity: mild to moderate disease 88.9%, severe disease (= hospitalisation) 11.1% Demographics: mean age cases 43.2 years, controls 43.5 years M%/F%: cases 47.8/52.0, controls 35.5/64.4, 0.1% not specified Exposure history: exposure to patient with COVID‐19 58.7% |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Index tests and RS both taken at presentation | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Bhattacharya 2021.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to evaluate the clinical symptoms among patients with suspected COVID‐19 presenting to screening outpatient clinics to develop and validate a clinical symptom‐based scoring system Design: cross‐sectional cohort study, prospective data collection Recruitment: patients presenting to an outpatient clinic at a tertiary care hospital Sample size: n = 378 (125) Inclusion criteria: patients who were suspected of having COVID‐19 and tested, provided informed consent and were contacted successfully by phone, from 1066 suspected patients who were tested during this period, 384 patients were enrolled in the study based on the availability of informed consent and successful telephonic communication). Suspicion was based on the testing advisory developed by the Indian Council of Medical Research (ICMR), Version 5, dated 18 May 2020. "ILI symptoms", defined as acute respiratory infection with fever ≥ 38 °C AND cough. Exclusion criteria: no PCR test result |
||
| Patient characteristics and setting |
Facility cases: positive SARS‐CoV‐2 test Facility controls: negative SARS‐CoV‐2 test Country: India Dates: 17 June 2020‐1 July 2020 Symptoms and severity: not specified, mostly mild to moderate severity Demographics: mean age overall 35.6 years M%/F% overall: 65.1/34.9 Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Index tests and RS both at presentation, phone interview after test was taken but before result was generated | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Bouzid 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to identify clinical or biological characteristics to help distinguish SARS‐CoV‐2 from other respiratory viruses Design: cross‐sectional cohort study, prospective data collection Recruitment: all consecutive patients admitted through the ED and presenting with respiratory symptoms Sample size: n = 596 (268 cases) (from 03 March 2020) Inclusion criteria: all consecutive patients presenting with an influenza‐like illness (ILI: fever with a temperature > 38.5 °C, malaise, headache, and myalgia; and 1 respiratory symptom (cough, sore throat, and dyspnoea)) and admitted to the hospital through the ED Exclusion criteria: none specified |
||
| Patient characteristics and setting |
Facility cases: positive SARS‐CoV‐2 test Facility controls: negative SARS‐CoV‐2 test Country: France Dates: 03 March 2020‐30 March 2020 Symptoms and severity: not specified, all patients presented with ILI, 13% needed ICU admission, 13% died Demographics: median age cases 59 years, controls 62 years M%/F%: cases 71.0/29.0, controls 50.0/50.0 Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Index tests and RS both taken at ED admission | ||
| Comparative | |||
| Notes | Conflicts of interest: DB and BV declare having received past personal fees and grant from Qiagen (Hilden, Germany) Funded by the AP‐HP (Assistance Publique – Hôpitaux de Paris). This study was supported by Qiagen in the form of a grant funding the data management of the RespiFast2 study targeting to assess the impact of respiratory viruses and of discounted equipment and consumables in the context of the COVID‐19 outbreak. |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Unclear | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Brendish 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to examine and compare the clinical characteristics, symptoms, and outcomes of adult patients presenting to the ED or Acute Medicine Unit (AMU), testing positive and negative for COVID‐19 Design: cross‐sectional cohort study, prospective data collection from a large, controlled, non‐randomised trial of molecular POC testing vs laboratory RT‐PCR for SARS‐CoV‐2 Recruitment: all consecutive patients presenting to the ED or AMU or other admissions area of Southampton, with an acute respiratory illness or otherwise clinically suspected of having COVID‐19 (= all patients included in the CoV‐19 POC trial) Sample size: n = 1054 (352 cases) Inclusion criteria: all consecutive adults (≥ 18 years old), presenting to the ED or AMU or other admissions area of Southampton, with an acute respiratory illness or otherwise clinically suspected of having COVID‐19 Exclusion criteria: not fulfilling all the inclusion criteria; declines nasal/pharyngeal swabbing; consent declined or consultee consent declined; already recruited to the study in the last 14 days |
||
| Patient characteristics and setting |
Facility cases: PCR‐positive patients by either molecular POC testing or laboratory RT‐PCR Facility controls: PCR‐negative patients by either molecular POC testing or laboratory RT‐PCR Country: UK Dates: 20 March 2020‐29 April 2020 Symptoms and severity: mild to moderate to severe
Demographics: median age cases 68 years, controls 69 years M%/F%: cases 57.4/42.6, controls 51.7/48.3 Exposure history: not specified, 21% of cases a HCW, 5% of controls |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Index tests and RS both taken at admission | ||
| Comparative | |||
| Notes | Unclear how decision was made which patients received which RS. Funding: the CoV‐19 POC trial was funded by University of Southampton and University Hospital Southampton NHS Foundation Trust. NJB is supported by a National Institute of Health Research (NIHR) Clinical Lecturer post. TWC is supported by a NIHR Fellowship (PDF 2016‐09‐061). |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Buonafine 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to investigate the prevalence and clinical characteristics of HCWs with COVID‐19 symptoms Design: cross‐sectional cohort study, prospective data collection Recruitment: HCW from the Santa Casa de São Paulo Hospital were defined as symptomatic and invited to participate in the study if presented with self‐reported fever or symptoms suspicious of COVID‐19 Sample size: n = 295 (125 cases) Inclusion criteria: HCW with self‐reported fever or any of the following: acute respiratory symptoms (cough, nasal congestion, sore throat, shortness of breath), loss or changed sense of smell or taste, ocular symptoms, headache, arthralgia, myalgia, fatigue, diarrhoea, nausea, and vomiting Exclusion criteria: none specified |
||
| Patient characteristics and setting |
Facility cases: RT‐PCR‐positive for SARS‐CoV‐2 Facility controls: RT‐PCR‐negative for SARS‐CoV‐2 Country: Brazil Dates: 21 March 2020‐22 May 2020 Symptoms and severity: mild to moderate to severe: 7% hospitalised 2% died, 91% of included individuals had headache, 88% nasal congestion, 85% cough, 85% fatigue, 81% myalgia Demographics: mean age cases 35 years, controls 34 years M%/F%: cases 40.0/60.0, controls 23.6/76.4 Exposure history: all HCW. Close contact with confirmed COVID‐19: cases 73%; controls 74% |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Timing not specified | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Chan 2021.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease) Design: cross‐sectional cohort study, retrospective data collection Recruitment: all patients aged ≥ 18 years in custody in a New York City jail facility or hospital units housing patients from the jail system who were tested for COVID‐19 Sample size: n = 721 (510 cases) Inclusion criteria: all patients aged ≥ 18 (as of 11 March 2020) in custody in a New York City jail facility or hospital units housing patients from the jail system and tested for COVID‐19 Exclusion criteria: none specified |
||
| Patient characteristics and setting |
Facility cases: RT‐PCR positive for SARS‐CoV‐2 Facility controls: RT‐PCR negative for SARS‐CoV‐2 Country: New York, USA Dates: 11 March 2020‐28 April 2020 Symptoms and severity: mild to moderate to severe: 8% hospitalised, 1% ICU admission, 1% died Demographics: median age cases 37 years, controls 33 years M%/F%: cases 91.0/9.0, controls 92.0/8.0 Exposure history: not specified, all participants in custody in the New York City jail system during a COVID‐19 outbreak |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Timing not specified | ||
| Comparative | |||
| Notes | Very specific population Funding: none declared |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Cheng 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: to identify the clinical features and CT manifestations of COVID‐19 and compare them with those of pneumonia occurring in patients who do not have COVID‐19 Design: cross‐sectional, single‐centre, retrospective study Recruitment: pneumonia patients who presented at a fever observation department in Shanghai Sample size: n = 33 (11 cases) Inclusion criteria: patients with clinical and radiological features of pneumonia, and a normal or reduced total leukocyte count or total lymphocyte count, plus an epidemiologic history that included travel or a history of residence in Hubei Province or other areas where continuous transmission of local cases occurred within 14 days before onset of symptoms, a history of contact with patients who had fever or respiratory symptoms and were from Hubei Province or other areas with continuous transmission of local cases within 14 days before onset of the disease, or clustering or epidemiologic association with the new coronavirus infection Exclusion criteria: not defined |
||
| Patient characteristics and setting |
Facility cases: confirmed case: positive RT‐PCR test result obtained by a throat swab. Test was repeated when the first test was negative Facility controls: pneumonia patients confirmed not to be infected by SARS‐CoV‐2 (2 PCR tests) Country: China Dates: 19 January 2020‐6 February 2020 Symptoms and severity: pneumonia was defined as patients with at least 1 clinical symptom (i.e. cough, sputum, fever, dyspnoea, or pleuritic chest pain), a finding of either coarse crackles on auscultation or elevated inflammatory biomarkers, and observation of a new pulmonary opacification on chest CT Demographics: median age ± SD cases 50.36 ± 15.5, controls 43.59 ± 16.02, gender distribution cases (M/F: 8/3), controls (M/F: 7/15) Exposure history: cases 8/11, controls 7/22 (in the last 14 days with patients with fever or respiratory symptoms or with known cases) |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
Tests were repeated if the first test was negative |
||
| Flow and timing | Time interval not specified, reference test at day 0 (or later when the first test was negative), index tests were questionnaired at day 0 for the presence of symptoms in the past period of time | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Chew 2021.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to develop a prediction model to identify patients who are at low risk of having COVID‐19 Design: cross‐sectional cohort study, retrospective data collection Recruitment: all patients admitted to the Pneumonia and Acute Respiratory Infection (PARI) wards of Changi General Hospital Sample size: n = 1228 (52 cases) Inclusion criteria: all patients admitted to PARI wards Exclusion criteria: none specified, for patients with multiple admissions during the study period, analysis was limited to the first PARI admission |
||
| Patient characteristics and setting |
Facility cases: RT‐PCR‐positive for SARS‐CoV‐2 Facility controls: RT‐PCR‐negative for SARS‐CoV‐2 Country: Singapore Dates: 10 February 2020‐30 April 2020 Symptoms and severity: not specified, mild to moderate severity Demographics: median age cases 48 years, controls 64 years M%/F%: total cohort 59.9/40.1 Exposure history: contact with people with acute respiratory infection, cases 36%, controls 4%; travel history: cases 3%, controls 2% |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Timing not specified | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Chua 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to evaluate the utility of acute olfactory loss as a risk‐ stratifying tool for COVID‐19 Design: retrospective cohort study Recruitment: chart review was performed for all patients who presented with acute respiratory symptoms, and in those who fulfilled the prevailing Ministry of Health suspect or surveillance case definition, at ED of tertiary hospital Sample size: n = 688 (24 cases) Inclusion criteria: all patients with suspected SARS‐CoV‐2 infection (suspicion based on presence of acute respiratory symptoms, and fulfilling the prevailing Ministry of Health suspect or surveillance case definition) Exclusion criteria: patients with pre‐existing olfactory loss, and those who were unable to give a history of olfactory loss reliably (e.g. those with cognitive impairment) |
||
| Patient characteristics and setting |
Facility cases: suspected patients with a positive PCR test Facility controls: suspected patients with a negative PCR test Country: Singapore Dates: 23 March 2020‐04 April 2020 Symptoms and severity: not specified Demographics: age: not specified gender: not specified Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | RS and index tests both taken at presentation | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Chung 2021.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease) ‐ compare symptoms and characteristics of people with and without laboratory‐confirmed SARS‐CoV‐2 infection Design: cross‐sectional cohort study, retrospective data collection Recruitment: research staff screened for study eligibility among people of all ages who had sought medical care (e.g. tele health, primary care, urgent care, and EDs) and/or COVID‐19 testing for an acute respiratory illness Sample size: n = 4961 (916 cases) Inclusion criteria: reported acute illness with fever/feverishness, cough, or shortness of breath/difficulty breathing and had a respiratory specimen collected for SARS‐CoV‐2 testing within 10 days of illness onset Exclusion criteria: people tested by antigen detection assays alone; swabbed, tested, or interviewed > 10 days after symptom onset; inconclusive RT‐PCR results |
||
| Patient characteristics and setting |
Facility cases: positive SARS‐CoV‐2 test Facility controls: negative SARS‐CoV‐2 test Country: USA Dates: 26 March 2020‐15 August 2020 Symptoms and severity: not specified, mostly mild to moderate Demographics: age groups ≤ 4 years: cases 1% controls 6%; 5‐17 years: cases 4% controls 8%; 18‐49 years: cases 65% controls 56%; 50‐64 years: cases 21% controls 22 years; ≥ 65 years: cases 9% controls 9% M%/F%: cases 61.0/39.0, controls 67.0/33.0 Exposure history: previous exposure to person with known COVID‐19: cases 59%, controls 18% |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Timing not specified | ||
| Comparative | |||
| Notes | Funding: supported through cooperative agreements funded by the US Centers for Disease Control and Prevention and, at the University of Pittsburgh, by infrastructure funding from the National Institutes of Health (UL1 TR001857). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Clemency 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to develop symptom‐based criteria for screening of HCW for SARS‐CoV‐2 Design: prospective observational cohort Recruitment: HCW with symptoms concerning for COVID‐19 infection were evaluated for potential testing through a centralised nurse call centre and referred to outpatient drive‐through testing sites if any suspicion of infection Sample size: n = 961 (225 cases) Inclusion criteria: all HCW tested for SARS‐CoV‐2, based on symptom‐based triage ("symptoms concerning for COVID‐19 infection" Exclusion criteria: none specified (141 excluded because symptoms were not documented, 12 excluded because test results not available) |
||
| Patient characteristics and setting |
Facility cases: all consecutive HCW with a single positive RT‐PCR test for SARS‐CoV‐2 Facility controls: all consecutive HCW with a single negative RT‐PCR test for SARS‐CoV‐2 Country: New York, USA Dates: 26 March 2020‐16 April 2020 Symptoms and severity: mild to moderate severity, inclusion based on presenting symptoms Demographics: mean age not presented; gender not presented Exposure history: not presented (likely a high rate of exposure, because HCW) |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | HCW referred for reference test after index test, but exact time interval not specified | ||
| Comparative | |||
| Notes | Funding: supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award UL1TR001412 to the University at Buffalo | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Clifford 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to investigate the association between different triage chief complaints and COVID‐19 status Design: cross‐sectional cohort study, retrospective data collection Recruitment: all adult ED visits (structured data) of patients who underwent nasopharyngeal swab RT‐PCR testing Sample size: n = 11992 (6524 cases) Inclusion criteria: all adult patients undergoing RT‐PCR at the ED Exclusion criteria: none specified |
||
| Patient characteristics and setting |
Facility cases: RT‐PCR‐positive for SARS‐CoV‐2 Facility controls: RT‐PCR‐negative for SARS‐CoV‐2 Country: New York, USA Dates: 01 March 2020‐13 May 2020 Symptoms and severity: mild to moderate to severe; hospital admission cases 65% controls 47%; mortality cases 20% controls 4%; intubation and ventilation cases 16% controls 4% Demographics: median age cases 62 years, controls 54 years M%/F%: cases 55.5/44.5, controls 48.5/51.5 Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Timing of RS not specified | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Cunarro‐Lopez 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to examine maternal‐perinatal outcomes in pregnant women with suspected COVID‐19 according to the result of a RT‐PCR test and to investigate possible variables that could be useful for predicting a negative RT‐PCR result Design: cross‐sectional cohort study, retrospective data collection Recruitment: obstetrics patient (pregnant, in labour or puerperium) with suspected COVID‐19 who attended the hospital Sample size: n = 111 (68 cases) Inclusion criteria: all obstetrics patients (pregnant, in labour or puerperium) with suspected COVID‐19 who attended the hospital Exclusion criteria: non‐conclusive RT‐PCR result, those patients who did not undergo obstetric follow‐up in the hospital and asymptomatic patients with SARS‐CoV‐2 infections |
||
| Patient characteristics and setting |
Facility cases: RT‐PCR positive for SARS‐CoV‐2 Facility controls: RT‐PCR negative for SARS‐CoV‐2 Country: Spain Dates: 10 March 2020‐12 May 2020 Symptoms and severity
Demographics: mean age cases 34 years, controls 32 years M%/F%: cases 0.0/100.0, controls 40.0/100.0 Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Timing of RS not specified | ||
| Comparative | |||
| Notes | Funding: by University of Alcalá (COVID‐19 UAH 2019/00003/016/001/023) and by (FIS‐PI18/00912) the Instituto de Salud Carlos III (Plan Estatal de I + D+I 2013‐2016) and co financed by the European Development Regional Fund | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Drager 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to analyse clinical characteristics of patients with SARS‐CoV‐2 suspicion Design: cross‐sectional cohort study, prospective data collection Recruitment: all symptomatic patients presenting at the Corona outpatient clinic at the Carl Gustav Carus University hospital of Dresden Sample size: n = 2257 (163 cases) Inclusion criteria: all patients presenting themselves at the outpatient clinic: patients with symptoms (not further specified) + high‐risk contacts or returning from a high‐risk area where tested for SARS‐CoV‐2 Exclusion criteria: non specified |
||
| Patient characteristics and setting |
Facility cases: RT‐PCR‐positive for SARS‐CoV‐2 Facility controls: RT‐PCR‐negative for SARS‐CoV‐2 Country: Germany Dates: 09 March 2020‐31 March 2020 Symptoms and severity: mostly cough and upper airway symptoms, mild to moderate, no hospitalisations specified Demographics: median age overall 39 years overall 45% male 55% female 32% had pre‐existing Illness Exposure history: 5% tested based on epidemiology (returning from high‐risk area), 27% had exposure to COVID‐19‐positive patient(s) |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Timing not specified | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Feng 2021.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of COVID‐19 pneumonia Design: cross‐sectional, retrospective, single‐centre study Recruitment: patients admitted to ED with history of exposure to COVID‐19 Sample size: n = 132 (cases = 7) inclusion criteria: all patients admitted to the fever clinic of the ED of the First Medical Center, Chinese People's Liberation Army General Hospital (PLAGH) in Beijing with the epidemiological history of exposure to COVID‐19 according to WHO interim guidance Exclusion criteria: < 14 years old, no other criteria specified |
||
| Patient characteristics and setting |
Facility cases: among clinically suspected patients: those with a positive RT‐PCR Facility controls: clinically non‐suspected patients + suspected patients with negative RT‐PCR Country: China Dates: 14 January 2020‐9 February 2020 Symptoms and severity: all patients admitted, with exposure history to COVID‐19, so all levels of severity; days from illness onset until admission (median, IQR): 2.0 (1.0‐5.0); patient population with general mild disease and limited presence of comorbidities (range 0%‐2.3% (COPD)) Demographics: age: controls median 40.0 years (IQR 32.5‐54.5), cases median 39.0 years (IQR 37.0‐41.5) M%/F%: cases 71.4/28.6, controls 63.2/36.8 Exposure history: epidemiological history of exposure to COVID‐19 (as per WHO guidance) |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Index test and RS both taken on admission | ||
| Comparative | |||
| Notes | Funding: supported by grants from the PLA Science and Technology Project (14CXZ005, AWS15J004, 16BJZ19), National Key R&D Program of China (2019 yearsFF0302300), Construction Project of Key Disciplines in the 13th Five‐Year Plan of the PLA (Traumatic Surgery in the Battlefield, 2019‐126, 2019‐513), Beijing Science and Technology New Star Project (XX2018019/Z181100006218028), the PLA General Hospital Science and technology Project (2019XXJSYX20, 2018XXFC‐20, ZH19016) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Fiel‐Ozores 2021.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease) ‐ to identify the main differential clinical features of infection by SARS‐CoV‐2 Design: cross‐sectional cohort study, retrospective data collection Recruitment: every paediatric patient (ages 0‐15 years) that had undergone a RT‐PCR test for detection of SARS‐CoV‐2 in a nasopharyngeal sample due to suspected infection was invited for a serology test and interview, setting unclear Sample size: n = 126 (33 cases) Inclusion criteria: patients aged 0‐15 years who underwent RT‐PCR test due to clinical suspicion of SARS‐CoV‐2 during the period under study whose parents/legal guardians provided consent and did not meet any of the exclusion criteria Exclusion criteria: patients with serum immunoglobulin (Ig) deficiency. Patients who underwent the RT‐PCR test when asymptomatic in the context of contact tracing. Study period: March‐May 2020, the months that followed the declaration of the state of alert and the population‐wide lock down |
||
| Patient characteristics and setting |
Facility cases: RT‐PCR‐positive for SARS‐CoV‐2 Facility controls: RT‐PCR‐negative for SARS‐CoV‐2 Country: Spain Dates: March 2020‐May 2020 Symptoms and severity: not specified, mild to moderate severity Demographics: mean age cases 8.4 years, controls 6.5 years M%/F%: cases 66.7/33.3, controls 59.1/40.9 Exposure history
|
||
| Index tests | All registered at onset of symptoms and during the course of disease
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Timing not specified | ||
| Comparative | |||
| Notes | Median time elapsed to the PCR was 8 days and to the first antibody test was 51 days, but interviews were done at time of serology testing (asking about onset of symptoms and evolution); unclear which test (PCR or serology) was eventually used as RS Funding: none declared |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Could the patient flow have introduced bias? | High risk | ||
Fink 2021.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease) ‐ to define clinical and radiological characteristics of COVID‐19 patients within a cohort with respiratory infections in the emergency department Design: cross‐sectional cohort study, prospective data collection Recruitment: patients who presented at the ED of the University Hospital, LMU Munich with signs of a respiratory infection suspicious for COVID‐19 Sample size: n = 219 (72 cases) Inclusion criteria: patients who presented at ED with signs of a respiratory infection suspicious for COVID‐19 and received radiological imaging (chest radiographs/chest X‐ray and/or CT) as well as RT‐PCR for SARS‐CoV‐2 Exclusion criteria: none specified |
||
| Patient characteristics and setting |
Facility cases: positive RT‐PCR for SARS‐CoV‐2 Facility controls: negative RT‐PCR for SARS‐CoV‐2 Country: Germany Dates: 16 March 2020‐12 April 2020 Symptoms and severity: mild to moderate to severe. ICU admission: cases 27%, controls 14%. Deaths: cases 5%, controls 3% Demographics: age: controls mean 59.5 years, cases mean 60.0 years M%/F%: cases 68.1/31.9, controls 56.5/43.5 Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | RS and index test both taken at presentation | ||
| Comparative | |||
| Notes | Funding: open access funding enabled and organised by Projekt DEAL | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Gilbert 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease) Design: prospective cohort, including consecutive patients with suspected SARS‐CoV‐2 infection Recruitment: all patients presenting to the ED triage centre with symptoms suggestive of COVID‐19 Sample size: n = 598 (175 cases) Inclusion criteria: all consecutive patients suspected of SARS‐CoV‐2 infection and directed to the triage centres located close to the EDs and subjected to SARS‐CoV‐2 testing; suspicion = respiratory symptoms and/or fever in a healthcare provider, an immunosuppressed patient or a nursing home resident, and all patients who required admission to the hospital Exclusion criteria: none |
||
| Patient characteristics and setting |
Facility cases: RT‐PCR‐positive patients Facility controls: RT‐PCR‐negative patients Country: Belgium Dates: 02 March 2020‐23 March 2020 Symptoms and severity: consecutive patients (selection based on PCR testing), mild to moderate severity (83% sent home for self‐isolation, 1.9% ICU, 15% hospital admission) Demographics: mean age (all): 41.1 years gender: % female (all): 59.0% Exposure history: travel to endemic country: cases 5.1%, controls 12.5% contact with positive patients: cases: 10.9%, controls 9.0% |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Index tests followed by reference standard | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Haehner 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to investigate the frequency of olfactory loss in an outpatient population who presented to a coronavirus testing centre. To evaluate the diagnostic value of the symptom "sudden smell loss" for screening procedures Design: cross‐sectional cohort study (prospective data collection) Recruitment: patients who presented with symptoms of a common cold to a coronavirus testing centre and fulfilled coronavirus testing criteria Sample size: n = 500 (cases 34) Inclusion criteria: patients with common cold complaints who met the criteria for SARS‐CoV‐2 testing to WHO recommendations Exclusion criteria: none |
||
| Patient characteristics and setting |
Facility cases: RT‐PCR for SARS‐CoV‐2‐positive Facility controls: RT‐PCR for SARS‐CoV‐2‐negative Country: Germany Dates: not specified Symptoms and severity: olfactory loss Demographics: mean age: 41.3 years gender % female: 54.6% Exposure history: not specified |
||
| Index tests | Olfactory loss | ||
| Target condition and reference standard(s) |
|
||
| Flow and timing | RS and index test taken on the same day | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Haliga 2021.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease) ‐ to analyse whether the evaluation of clinical symptoms and signs upon admission to hospital can be useful for the differentiation of patients with acute medical pathology and suspicion of SARS‐CoV‐2 infection Design: cross‐sectional cohort study, retrospective data collection Recruitment: unclear, adult patients presenting to an ED, cohort of patients admitted to our internal medicine clinic at the emergency clinical county hospital; admission through ED, only RT‐PCR for patients who were hospitalised Sample size: n = 253 (20 cases) Inclusion criteria: > 18 years of age, irrespective of gender, admitted to ED due to an acute medical illness as a consequence of exacerbation of their chronic illness as well as symptoms or clinical signs included in the case definition of suspected cases of SARS‐CoV‐2 infection Exclusion criteria: no informed consent, acute pathology requiring specific emergency treatment or PCR already positive at presentation |
||
| Patient characteristics and setting |
Facility cases: RT‐PCR‐positive for SARS‐CoV‐2 Facility controls: RT‐PCR‐negative for SARS‐CoV‐2 Country: Romania Dates: 01 April 2020‐ 31 May 2020 Symptoms and severity: not specified, mild to moderate to severe: 236 admitted to the clinic and 17 admitted to ICU Demographics: mean age overall 64 years M%/F% overall 57.7/42.3 Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Index tests and RS both taken on admission | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Huang 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to explore a novel risk score to predict diagnosis with COVID‐19 among all suspected patients at admission Design: retrospective, multicentric observational study Recruitment: retrospective chart review of patients admitted into 26 COVID‐19 designated hospitals in Sichuan Province, China Sample size: n = 475 (336 cases) Inclusion criteria: patients with suspected COVID‐19 (suspected case is defined as having exposure history and 2 clinical manifestations. Patients without epidemiological exposure histories could also be seen as "suspected COVID‐19" only if 3 clinical manifestations were present. Exclusion criteria: none |
||
| Patient characteristics and setting |
Facility cases: suspected patients with a positive RT‐PCR test Facility controls: suspected patients with a negative RT‐PCR test. If the first test was negative, at least a second test was done, 24 h apart. Country: China Dates: 21 January 2020‐07 February 2020 Symptoms and severity: mild to moderate severity, all suspected patients included Demographics: mean age, cases: 43 years, controls: 34 years. Gender: % female cases 45.8%, controls: 41.0% Exposure history: epidemiological exposure history: cases: 69.6%, controls 12.9% |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | RS and index tests both taken on admission | ||
| Comparative | |||
| Notes | Funding: Emergency Response Project for New Coronavirus of Science and Technology Department of Sichuan Provincial, Grant/Award Numbers: 2020YFS0005, 2020YFS0009; Special Funds for COVID‐19 Prevention and Control of West China Hospital of Sichuan University, Grant/Award Number: HX‐ 2019‐nCoV‐068; Science and Technology Benefit People Project of Chengdu Municipality, Grant/Award Number: 2016‐HM02‐00099‐SF | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Hüfner 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease) ‐ to identify patients in the ED early using a score so that they can be isolated pre‐emptively Design: cross‐sectional cohort study, retrospective data collection Recruitment: all patients who presented at 1 of 3 ED's Sample size: n = 697 (64 cases) Inclusion criteria: all patients presenting at one of the participating EDs and scoring at least 1 item of the COVID score Exclusion criteria: none specified |
||
| Patient characteristics and setting |
Facility cases: positive RT‐PCR for SARS‐CoV‐2 Facility controls: negative RT‐PCR for SARS‐CoV‐2 Country: Germany Dates: 9 March 2020‐30 April 2020 Symptoms and severity: all symptomatic patients, 79.7% of cases hospitalised, 53.1% of controls Demographics: age: controls mean 54.7 years, cases mean 60.3 years M%/F%: cases 68.8/31.2, controls 46.9/53.1 Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Timing not specified | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Unclear | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Ide 2021.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease) ‐ to evaluate the SARS‐CoV‐2‐positive ratio among mildly ill patients in Tokyo, Japan, their characteristics, the Ministry of Health, Labour and Welfare criteria, and to identify better criteria Design: cross‐sectional cohort study, retrospective data collection Recruitment: patients who underwent SARS‐CoV‐2 PCR testing at the National Center for Global Health and Medicine in Tokyo (= outpatient screening centre) Sample size: n = 277 (25 cases) Inclusion criteria: those who met ≥ 1 of the following criteria were eligible: patients who have fever or symptoms (e.g. respiratory, fatigue, headache, myalgia); patients who had exposure to COVID‐19 patient; patients who did not meet 1) or 2) but referred by another physician due to possible exposure to COVID‐19 patient or travel history. Exclusion criteria: patients who were non‐ambulatory upon presentation (referred to the infectious disease clinic for further evaluation) |
||
| Patient characteristics and setting |
Facility cases: RT‐PCR‐positive for SARS‐COV‐2 Facility controls: RT‐PCR‐negative for SARS‐COV‐2 Country: Japan Dates: 09 March 2020‐29 March 2020 Symptoms and severity: mild to moderate severity Demographics: age: controls median 38.9 years, cases median 44.1 years M%/F%: cases 88.0/12.0, controls 52.0/48.0 Exposure history: exposure to case: cases 52%, controls 7%; travel: cases 72%, controls 20% |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Timing not specified | ||
| Comparative | |||
| Notes | Funding: grant for International Health Research from the Ministry of Health Labor and Welfare of Japan (grant no. 20A2003D) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Ishii 2021.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to identify the predictors of SARS‐CoV‐2 test positivity for more efficient and evidenced selection of suspected individuals Design: cross‐sectional cohort study, prospective data collection Recruitment: all consecutive individuals, tested in a drive‐through outpatient test centre during the first 7 months of the testing programme Sample size: n = 3540 (164 cases) Inclusion criteria: all consecutive participants who underwent drive‐through nasopharyngeal swab testing at an outpatient clinic. Reason for testing: upon request of the participant or participants who had been confirmed to have contacted COVID‐19 patients based on contact tracing. No clinical suspicion needed per se, but 54% of individuals were symptomatic, suggestive of COVID‐19 Exclusion criteria: none specified |
||
| Patient characteristics and setting |
Facility cases: positive RT‐PCR test for SARS‐CoV‐2 Facility controls: negative RT‐PCR test for SARS‐CoV‐2 Country: Japan Dates: 16 July 2020‐31 January 2021 Symptoms and severity: not specified, 54% of individuals presented with symptoms suggestive of COVID‐19, mostly mild severity Demographics: age: controls median 27 years, cases median 25 years M%/F%: cases 63.4/36.6, controls 49.4/50.6 Exposure history: history of close contact with COVID‐19 patient: cases 44%, controls 26% |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Timing not specified | ||
| Comparative | |||
| Notes | No clinical suspicion needed for testing Funding: none declared |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Jeyashree 2021.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to describe the symptom profile of people who underwent testing for COVID‐19 Design: cross‐sectional cohort study, prospective data collection Recruitment: all consecutive adults who visited COVID‐19 testing centres in Chennai city in Southern India Sample size: n = 277 (58 cases) Inclusion criteria: all consenting adults aged 18‐80 years belonging to any gender, who visited COVID‐19 testing centres in Chennai city in Southern India Exclusion criteria: none specified |
||
| Patient characteristics and setting |
Facility cases: positive RT‐PCR test for SARS‐CoV‐2 Facility controls: negative RT‐PCR test for SARS‐CoV‐2 Country: India Dates: not specified (2020) Symptoms and severity: not specified, mild to moderate severity Demographics: overall age: mean 40.7 years M%/F%: cases 51.7/48.3, controls 63.5/36.5 Exposure history: history of close contact with COVID‐19 patient: cases 9%, controls 5% |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Index tests were collected on same day of the specimen collection, and prior to the declaration of COVID‐19 test results | ||
| Comparative | |||
| Notes | Funding: supported by the Indian Council of Medical Research‐ National Institute of Epidemiology (ICMR‐NIE) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Just 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to identify predictive risk factors for a positive SARS‐CoV‐2 RT‐PCR result in a primary care setting Design: multicentre, cross‐sectional cohort study Recruitment: 26 office‐based specialists for internal and/or general medicine with a full primary care mandate from 14 different locations participated in the study. Suspected COVID‐19 patients for whom a PCR was taken were included. Sample size: n = 374 (40 cases) Inclusion criteria: convenience sample of patients who received PCR in the participating GPs' practices within the study period Exclusion criteria: patients whose tests had been carried out for procedural reasons and did not correspond to a specific clinical indication were excluded (e.g. testing of recovered patients after end of quarantine). There were no other exclusion criteria. |
||
| Patient characteristics and setting |
Facility cases: suspected patients with a positive PCR test Facility controls: suspected patients with a negative PCR test Country: Germany Dates: 24 March 2020‐17 April 2020 Symptoms and severity: mild to moderate severity Demographics: median age: cases: 52.0 years, controls: 43.5 years gender: % female cases: 65.0%, controls: 57.2% Exposure history: first grade contact (with symptoms): cases: 35.0%, controls 17.4% |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | RS and index tests both taken on admission | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Kalayjian 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to assess the rate of COVID‐19 positivity in a community‐based health centre, evaluate the clinical symptoms, and follow patient outcomes Design: cross‐sectional cohort study, prospective data collection Recruitment: clients entering the health centre (walk‐in clinic) were screened for symptoms and triaged to the COVID‐19 clinic. Testing was performed for patients with a documented or subjective fever within the past 72 h. Sample size: n = 345 (117 cases) Inclusion criteria: people ≥ 17 years old, screened for COVID‐19 symptoms and triaged, a documented or subjective fever within the past 72 h Exclusion criteria: none specified |
||
| Patient characteristics and setting |
Facility cases: patients with a positive Labcorp’s nucleic‐acid amplification nasopharyngeal swab for SARS‐CoV‐2 Facility controls: patients with a negative Labcorp’s nucleic‐acid amplification nasopharyngeal swab for SARS‐CoV‐2 Country: Louisiana, USA Dates: 16 March 2020‐10 April 2020 Symptoms and severity: walk‐in clinic for people with COVID‐19 symptoms; 9 required ED assessment of whom 6 were admitted to hospital; all patients had to have fever in the past 72 h Demographics: age: controls mean 44.4 years, cases mean 42.3 years M%/F%: cases 49.6/50.4, controls 46.5/50.4 (3.1% "other") Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Index tests and reference standard taken at the same clinical encounter | ||
| Comparative | |||
| Notes | Only feverish patients included Funding: none declared |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Low risk | ||
Kelen 2021.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to understand the impact of COVID‐19 on EDs Design: cross‐sectional cohort study, retrospective data collection Recruitment: consecutive: all patients of at least 15 years of age presenting at ED with symptoms suggestive of COVID‐19 or with high acuity Sample size: n = 11402 (2484 cases) Inclusion criteria: at least 15 years old and symptoms suggestive of COVID‐19 symptoms or high acuity Exclusion criteria: none specified |
||
| Patient characteristics and setting |
Facility cases: positive RT‐PCR test for SARS‐CoV‐2 Facility controls: negative RT‐PCR test for SARS‐CoV‐2 Country: USA Dates: 16 March 2020‐15 May 2020 Symptoms and severity: mild to moderate severity; acuity triage score 1 (highest) 4.7%; 2 22.9%; 3 55.3%; 4 14.1%; 5 1.6%; not listed 1.3% Demographics: age overall: 15‐24 years: 31.8%, 25‐34 years: 33.7%, 35‐44 years: 39.3%, 45‐54 years: 42.1%, 55‐64 years: 45.2%, 65‐74 years: 48.1%, 75+ years: 52.6% M%/F%:overall (all those tested) 40.7/59.3 Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Index test and RS both taken upon arrival at ED | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Kempker 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to describe the most common and distinguishing clinical symptoms among HCWs who underwent screening for COVID‐19 Design: cross‐sectional cohort study, prospective data collection Recruitment: HCW with a viral‐like illness, triaged to the employee health services staff for a virtual clinical assessment and then scheduled for SARS‐CoV‐2 testing Sample size: n = 283 (51 cases) Inclusion criteria: HCW with symptoms consistent with a viral‐like illness Exclusion criteria: none specified |
||
| Patient characteristics and setting |
Facility cases: HCW with positive RT‐PCR test for SARS‐CoV‐2 Facility controls: HCW with negative RT‐PCR test for SARS‐CoV‐2 Country: Georgia, USA Dates: 18 March 2020‐14 April 2020 Symptoms and severity: not specified, mild to moderate severity (from table) Demographics: age: not specified M%/F%: overall 19/81 Exposure history: patient contact in general: cases: 96%, controls 88%. Contact with COVID‐19 patients: cases 33%, control 36% |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Not specified | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Kim 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to develop a prediction rule for COVID‐19 Design: cross‐sectional cohort study, retrospective data collection Recruitment: consecutive: all patients > 18 years visiting ED who had undergone COVID‐19 testing Sample size: n = 242 (54 cases) Inclusion criteria: > 18 years and visit to one of the EDs Exclusion criteria: ‐ if initial signs and symptoms were not recorded ‐ in the case of a revisit |
||
| Patient characteristics and setting |
Facility cases: positive RT‐PCR test for SARS‐CoV‐2 Facility controls: negative RT‐PCR test for SARS‐CoV‐2 Country: South Korea Dates: 1 March 2020‐21 March 2020 Symptoms and severity: mild to moderate to severe: admission to ward: cases 48.1% controls 0% Admission to ICU cases 11.5% controls 0%, in‐hospital death cases 20.4% controls 0% Demographics: age: controls median 43.5 years, cases median 70.0 years M%/F%: cases 55.6/44.4, controls 45.2/54.8 Exposure history: any exposure risk: 37.2% overall |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Index test and RS both taken upon arrival at ED | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Low risk | ||
King 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to assess symptom patterns among children tested for SARS‐CoV‐2 Design: cross‐sectional cohort study, retrospective data collection Recruitment: database study (Alberta Health Services Communicable Disease Outbreak Management database), including data on children with a positive test result or children tested for contact with a case or in an outbreak Sample size: n = 3249 (2264 cases), after exclusions: n = 2463 (1978 cases) Inclusion criteria: patients < 18 years with a positive PCR test or were tested for being in a high‐risk group (close contact with a case or in an outbreak) Exclusion criteria: second tests or patients tested prior to 13 April 2020. Children who were tested for symptoms and were negative, were not contacted for the symptom questionnaire. |
||
| Patient characteristics and setting |
Facility cases: positive RT‐PCR test for SARS‐CoV‐2 Facility controls: negative RT‐PCR test for SARS‐CoV‐2 Country: Canada Dates: 13 April 2020‐30 September 2020 Symptoms and severity: mild to moderate severity Demographics: age: controls mean 8.4 years, cases mean 9.8 years; M%/F%: cases 49.4/50.6, controls 53.6/46.4 Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Maximum 5 days between index tests and RS (RS came first in some cases) | ||
| Comparative | |||
| Notes | Funding: FM was funded by an Alberta Health Services Chair in Cardiovascular Outcomes Research; project was funded by the Alberta Strategy for Patient Oriented Research Support Unit | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Did the study avoid inappropriate inclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Krastinova 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to describe infection rates, clinical characteristics, occupational exposure, living conditions and household transmission of symptomatic HCWs Design: cross‐sectional cohort study, prospective data collection Recruitment: symptomatic HCWs in 1 hospital were tested on a voluntary basis (ED/outpatient setting) Sample size: n = 314 (110 cases) Inclusion criteria: symptomatic HCWs, defined as the presence of fever and/or respiratory symptoms Exclusion criteria: none specified |
||
| Patient characteristics and setting |
Definition cases: positive RT‐PCR test for SARS‐CoV‐2 Definition controls: negative RT‐PCR test for SARS‐CoV‐2 Country: France Dates: 17 March 2020‐20 April 2020 Symptoms and severity: mild to moderate severity, 0% of controls hospitalised, 8% of cases hospitalised Demographics: age: controls mean 40.2 years, cases mean 40.3 years M%/F%: cases 20.0/80.0, controls 18.0/82.0 Exposure history: not specified |
||
| Index tests | At illness onset:
At screening:
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Timing not specified | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Langer 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to develop a model to predict the results of RT‐PCR Design: cross‐sectional cohort study, retrospective data collection Recruitment: all patients admitted to ED with symptoms compatible with COVID‐19 Sample size: n = 199 (124 cases) Inclusion criteria: all patients presenting with symptoms compatible with COVID‐19 Exclusion criteria: < 12 years, no leukocyte formula |
||
| Patient characteristics and setting |
Definition cases: positive RT‐PCR test for SARS‐CoV‐2 Definition controls: negative RT‐PCR test for SARS‐CoV‐2 Country: Italy Dates: 22 February 2020‐16 March 2020 Symptoms and severity: inclusion based on potential COVID‐19 symptoms, mostly mild to moderate symptoms, oxygen supplementation needed in 22% of cases and 32% of controls Demographics: age: controls median 66 years, cases median 65 years M%/F%: cases 62.9/37.1, controls 65.3/34.7 Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Index tests and RS both on admission, but in case of a negative PCR the test was repeated after 48 h | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Lazzerini 2021.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to describe characteristics and risk factors for COVID‐19 in children Design: cross‐sectional cohort study, retrospective data collection Recruitment: all children aged 0‐18 years tested for SARS‐CoV‐2 at 1 of 20 paediatric centres across Italy because of symptoms/signs suggestive of COVID‐19 Sample size: n = 2148 (159 cases) Inclusion criteria: children (0‐18 years) tested because of symptoms suggestive of COVID‐19 Exclusion criteria: none |
||
| Patient characteristics and setting |
Definition cases: positive RT‐PCR test for SARS‐CoV‐2 Definition controls: negative RT‐PCR test for SARS‐CoV‐2 Country: Italy Dates: 23 February 2020‐24 May 2020 Symptoms and severity: inclusion based on potential COVID‐symptoms, mostly mild to moderate symptoms, among the cases, only 2.1% required respiratory support and 1.1% were admitted to intensive care; all recovered Demographics: age: < 6 months: cases 12%, controls 8%; 6‐24 months: cases 10.7%, controls 23.7%; 2‐9 years: cases 23.3%, controls 42%; 10‐18 years: cases 54.1%, controls 26% M%/F%: cases 48.4/51.6, controls 55.8/44.2 Exposure history: contact with person having COVID‐19: cases 79.2%, controls 6.1%; relatives with respiratory symptoms: cases 72.3%, controls 11.5% |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Timing not specified | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Leal 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to describe the clinical features predictive for SARS‐CoV‐2 infection in primary care Design: prospective population‐based cohort Recruitment: residents of the municipality aged ≥ 12 years with suspected COVID‐19 symptoms were encouraged to contact the dedicated platform via the website or phone. They were invited to complete an initial screening questionnaire. Participents were then called by a medical student to complete a risk assessment. Sample size: n = 1583 (444 cases (only the PCR‐positive patients) Inclusion criteria: patients meeting the suspected COVID‐19 case definition (having at least 2 of the following symptoms: fever, cough, sore throat, coryza or change in/loss of smell (anosmia); or 1 of these symptoms plus at least 2 other symptoms consistent with COVID‐19 Exclusion criteria: all pregnant women, and patients meeting pre‐defined triage criteria for severe disease |
||
| Patient characteristics and setting |
Facility cases: patients with suspected COVID‐19 who tested positive (RT‐PCR, testing at home) Facility controls: patients with suspected COVID‐19 who tested negative (RT‐PCR, testing at home) Country: Brazil Dates: 13 April 2020‐13 May 2020 Symptoms and severity: mild to moderate severity, severe cases were excluded Demographics: all age groups represented from ≥ 10 years. Gender: % female cases: 55.0%, controls: 66.5% Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Swabs were taken within 5 days of symptom onset | ||
| Comparative | |||
| Notes | Funding: the municipal health department of São Caetano do Sul funded the establishment and implementation of the platform. Plus award from FAPESP (2018/14389‐0) and the UK Medical Research Council (MR/S0195/1) to the Brazil‐UK Centre for Arbovirus Discovery | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Leung 2021.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to review the characteristics and outcomes of individuals who attended a testing centre Design: cross‐sectional cohort study, retrospective data collection Recruitment: all patients attending the testing centre (temporary outpatient testing centre at the AsiaWorldExpo) Sample size: n = 1258 (86 cases) Inclusion criteria: all patients presenting to the testing centre Exclusion criteria: missing data and immediate referral to regional ED |
||
| Patient characteristics and setting |
Definition cases: positive RT‐PCR test for SARS‐CoV‐2 Definition controls: negative RT‐PCR test for SARS‐CoV‐2 Country: Hong Kong (China) Dates: 20 March 2020‐19 April 2020 Symptoms and severity: 96% of all participants symptomatic, mostly mild severity, acutely ill patients were immediately referred to ED Demographics: age: controls mean 27.1 years, cases mean 30.6 years M%/F%: cases 53.5/46.5, controls 51.7/48.3 Non‐Chinese ethnicity overall 10% Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Timing not specified, but both index tests and RS taken at outpatient test centre, so all tests and assessments should have been done at the time of presentation | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Low risk | ||
Maechler 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to describe epidemiological and clinical characteristics; to identify risk factors for SARS‐CoV‐2 infection Design: cross‐sectional cohort study, prospective data collection Recruitment: symptomatic patients presenting at the test site. Subgroups not by testing criteria at that time: (1) high‐risk contacts at a nightclub (26/94 positive). (2) Charité employees 125 asymptomatic. Sample size: n = 4333 (333 cases) Inclusion criteria: until 24 March 2020: symptomatic patients with high‐risk contacts or return from high‐risk area. From 24 March: also symptomatic people with risk factors and if the test capacity allowed also only symptomatic patients. Plus 2 subgroups of high‐risk patients in a nightclub and Charité employees Exclusion criteria: none specified |
||
| Patient characteristics and setting |
Facility cases: patients with a positive RT‐PCR for SARS‐CoV‐2 infection Facility controls: patients with a negative RT‐PCR for SARS‐CoV‐2 infection Country: Germany Dates: 03 March 2020‐13 April 2020 Symptoms and severity: mild to relatively more severe. Asymptomatic: 12 cases, 431 controls Demographics: age: controls median 34.0 years, cases mean 34.0 years M%/F%: cases 56.8/43.2, controls 48.5/51.5 Exposure history: high‐risk contact: cases 56.8%, controls 36.4% |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Index tests and reference standard both at presentation | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Mansella 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to evaluate safety and feasibility of low‐threshold testing process; to identify clinical predictors for severe acute COVID‐19 Design: cross‐sectional cohort study, prospective data collection Recruitment: all patients presenting at the test centre with respiratory symptoms (such as shortness of breath), other flu‐like symptoms (fever, sore throat, cough) and self‐reported exposure to COVID‐19 Sample size: n = 4815 (572 cases) Inclusion criteria: all patients presenting at the test centre with respiratory symptoms, flu‐like symptoms and self‐reported exposure to COVID‐19 Exclusion criteria: no informed consent, no nasopharyngeal swab and missing data |
||
| Patient characteristics and setting |
Facility cases: positive RT‐PCR test for SARS‐CoV‐2 Facility controls: negative RT‐PCR test for SARS‐CoV‐2 Country: Switzerland Dates: 19 March 2020‐19 April 2021 Symptoms and severity: mostly mild to moderate severity, 41/4815 (0.9%) participants were hospitalised Demographics: age: controls median 41.2 years, cases median 45.7 years M%/F%: cases 50.5/49.5, controls 44.8/55.2 Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Index tests and RS probably both at presentation, but not specified | ||
| Comparative | |||
| Notes | Funding: supported by Scientific funds from the University Hospital Basel Switzerland | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Low risk | ||
Mao 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to ascertain the effectiveness of the screening strategy and provide insight for early diagnosis of COVID‐19 Design: multicenter, retrospective, observational cohort study Recruitment: all patients visiting the fever clinics within the study period Sample size: n = 1004 (cases = 188) Inclusion criteria: all patients visiting the fever clinics within the study period. Patients with fever (body temperature > 37.5 °C), or patients with pulmonary symptoms and epidemiological exposure history were requested to visit the fever clinics. All patients visiting the fever clinics during the study period were included. Exclusion criteria: patients with missing data |
||
| Patient characteristics and setting |
Facility cases: RT‐PCR‐positive patients Facility controls: RT‐PCR‐negative patients Country: China Dates: 17 January 2020‐16 February 2020 Symptoms and severity: not specified Demographics: median age: cases 46 years, controls 39 years. Female; gender %: cases 50%, controls 47% Exposure history: recent visit to epidemic region: cases 51%, controls 28%; contact with infected person: cases 34%, controls 13% |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | RS and index tests taken on the same day | ||
| Comparative | |||
| Notes | Funding: National Natural Science Foundation of China | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Did the study avoid inappropriate inclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Martín‐Sánchez 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild to severe COVID‐19 disease); to describe the clinical characteristics and 30‐day mortality rates in ED patients with COVID‐19 in different diagnostic groupings Design: cross‐sectional cohort study, retrospective data collection Recruitment: COVID‐19 suspects treated in the emergency room Sample size: n = 1417 (1190 cases) (after exclusion of all participants without an RT‐PCR) Inclusion criteria: all suspicious cases of COVID‐19 served in the ED of the San Carlos Clinical Hospital Exclusion criteria: patients with a positive PCR test prior to assessment in the ED |
||
| Patient characteristics and setting |
Facility cases: positive RT‐PCR for SARS‐CoV‐2 Facility controls: negative RT‐PCR for SARS‐CoV‐2 Country: Spain Dates: 28 February 2020‐31 March 2020 Symptoms and severity: 30‐day mortality was 11.5% (56.5% in hospitalised cases and 19.6% in cases classified as severe) Demographics: age: controls median 50.7 years, cases median 61.5 years M%/F%: cases 53.5/46.5, controls 37.4/62.6 Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Index tests and reference standard both taken at the ED | ||
| Comparative | |||
| Notes | Strong preselection of participants, unclear why some were not tested, very high disease prevalence Funding: none declared |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Martin‐Sanz 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to evaluate the incidence of certain symptoms in a population of HCWs (exposed to COVID‐19‐positive patients) Design: cross‐sectional cohort study, prospective data collection Recruitment: HCW of the University Hospital of Getafe (Madrid, Spain) with suspicion of COVID‐19 infection Sample size: n = 355 (215 cases) Inclusion criteria: HCW with suspicion of COVID‐19 infection. Suspicion of COVID‐19 was determined by the presence of either cough, fever (> 37.5 °C), headache, or breathlessness, regardless of contact with a COVID‐19 patient Exclusion criteria: inconclusive PCR results |
||
| Patient characteristics and setting |
Facility cases: positive RT‐PCR for SARS‐CoV‐2 Facility controls: negative RT‐PCR for SARS‐CoV‐2 Country: Spain Dates: 15 March 2020‐07 April 2020 Symptoms and severity: mild to moderate severity (only 14 patients (3.94%) developed pneumonia or severe symptoms that required hospitalisation) Demographics: age: overall mean 42.9 years (SD = 0.67) M%/F%: cases 20.5/79.5, controls 17.1/82.9 Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Not specified | ||
| Comparative | |||
| Notes | Article states that it is a case‐control study, while it is not (all consecutive suspected HCW's enrolled and tested) Funding: none declared |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Nazerian 2021.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to formally evaluate the diagnostic testing characteristics of physicians' gestalt for COVID‐19 Design: cross‐sectional cohort study, prospective data collection Recruitment: patients with suspected COVID‐19 were prospectively enrolled in 2 EDs Sample size: n = 838 (193 cases) Inclusion criteria: age ≥ 18 years, presence of any sign or symptom for COVID‐19 and first evaluation at ED during the study period Exclusion criteria: known diagnosis of COVID‐19, loss to follow‐up and refusal to participate |
||
| Patient characteristics and setting |
Definition cases: positive RT‐PCR test for SARS‐CoV‐2 or suggestive symptoms plus chest imaging of acute interstitial lung disease in the absence of an alternative diagnosis taking into account all 30‐day follow‐up data including medical data and a structured telephone interview Definition controls: negative RT‐PCR test for SARS‐CoV‐2 Country: Italy Dates: 01 April 2020‐30 April 2020 Symptoms and severity: mostly mild to moderate severity, need of oxygen supplementation or ventilation: cases 37%, controls 25% Demographics: age: controls median 70 years, cases mean 69 years M%/F%: cases 52.3/47.7, controls 49.5/50.5 Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Max 5 days for PCR test, up to 30 days for clinical information | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Low risk | ||
Nitecki 2021.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to assess the utility of self‐reported symptoms in identifying positive COVID‐19 cases among predominantly healthy young adults in a military setting Design: cross‐sectional cohort study, retrospective data collection Recruitment: all individuals who were deemed eligible for COVID‐19 testing by the Israel Defence Forces COVID Centre, including those voluntarily calling to report symptoms, those actively addressed following epidemiological investigation Sample size: n = 24362 (1338 cases) Inclusion criteria: all individuals who were deemed eligible for COVID‐19 testing by the Israel Defence Forces COVID Centre (suspicious symptoms, those quarantined), including those voluntarily calling to report symptoms, those actively addressed following epidemiological investigation Exclusion criteria: no informed consent, no nasopharyngeal swab and missing data |
||
| Patient characteristics and setting |
Facility cases: positive RT‐PCR test for SARS‐CoV‐2 Facility controls: negative RT‐PCR test for SARS‐CoV‐2 Country: Israel Dates: 26 March 2020‐02 August 2020 Symptoms and severity: mostly mild to moderate severity, mostly cough (56.1%) and fever (28.3%) Demographics: age: controls median 21 years, cases median 21 years M%/F%: cases 61.1/38.9, controls 59.0/41.0 Exposure history: suspected exposure 50.1% (defined as close contact with a confirmed COVID‐19 patient or recent (< 14 days) international travel) |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Timing not specified | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
O'Reilly 2020a.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to determine the clinical and epidemiological predictors of a positive SARS‐CoV‐2 test result and the requirement for intensive respiratory support Design: cross‐sectional cohort study, prospective data collection Recruitment: adult patients who meet testing criteria for COVID‐19 and have a SARS‐CoV‐2 PCR test requested in the ED Sample size: n = 240 (cases = 11) Inclusion criteria: all adults who met the testing criteria for COVID‐19 and who presented at the ED Exclusion criteria: patients who attended the screening clinic and did not present for medical assessment in the ED (no clinical data available) |
||
| Patient characteristics and setting |
Facility cases: positive RT‐PCR for SARS‐CoV‐2 Facility controls: negative RT‐PCR for SARS‐CoV‐2 Country: Australia Dates: 01 April 2020‐14 April 2020 Symptoms and severity: moderate to severe Demographics: mean age: cases 51 years, controls 61 years M%/F%: cases 72.0/28.0, controls 55.0/45.0 Exposure history: contact with infected person: cases 56%, controls 7% |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | RS and index tests taken on the same day | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
O'Reilly 2020b.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to describe the epidemiology and clinical features of patients presenting to the ED with suspected and confirmed COVID‐19 Design: cross‐sectional cohort study, prospective data collection Recruitment: all adult patients who met criteria for "suspected COVID‐19" and underwent testing for SARS‐CoV‐2 were eligible for inclusion. "Testing criteria are guided by the various health jurisdictions and have evolved throughout the project." Sample size: n = 1334 (50 cases) Inclusion criteria: adult patients who had a SARS‐CoV‐2 PCR test requested in the ED and were managed as "suspected COVID‐19" Exclusion criteria: patients who underwent testing for surveillance purposes |
||
| Patient characteristics and setting |
Facility cases: positive RT‐PCR for SARS‐CoV‐2 Facility controls: negative RT‐PCR for SARS‐CoV‐2 Country: Australia Dates: 01 July 2020‐31 July 2020 Symptoms and severity: all levels of severity, mostly mild to moderate Demographics: mean age: cases 53 years, controls 56 years M%/F%: cases 48.0/52.0, controls 50.0/50.0 Exposure history: 50% reported close contact with a confirmed case of COVID‐19 or a positive SARS‐CoV‐2 PCR swab result in the 14 days prior to their ED presentation |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Index tests and reference standard both taken at presentation in the ED | ||
| Comparative | |||
| Notes | Thresholds only specified for vital parameters, not for most symptoms Funding: none declared |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Olivar Lopez 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to describe the profile of patients < 18 years of age treated at a paediatric COVID centre and its association with test confirmation, endotracheal intubation and death Design: cross‐sectional cohort study, prospective data collection Recruitment: all patients < 18 years who presented with a clinical picture compatible with COVID‐19 (= fever, respiratory symptoms or general malaise) at the ED of a COVID paediatric reference hospital Sample size: n = 510 (79 cases) Inclusion criteria: all patients < 18 years with symptoms compatible with COVID‐19 (= fever, respiratory symptoms or general malaise), and who underwent PCR testing Exclusion criteria: unreported test results, insufficient or poorly taken samples |
||
| Patient characteristics and setting |
Facility cases: positive RT‐PCR test for SARS‐CoV‐2 Facility controls: negative RT‐PCR test for SARS‐CoV‐2 Country: Mexico Dates: March 2020‐June 2020 Symptoms and severity: mild to moderate to severe, 12.3% of controls and 19.3% of cases intubated Demographics: age: controls mean 5 years, cases mean 4.9 years M%/F%: cases 59.5/40.5, controls 51.7/48.3 Exposure history: contact with case: 57.9% of cases, 27.6% of controls |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Timing not specified | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Peng 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: analyse the clinical features and imaging manifestations of COVID‐19 Design: cross‐sectional, single‐centre, retrospective study Recruitment: clinically suspected cases who were sent to hospital for screening Sample size: n = 86 (n = 11) Inclusion criteria: clinically suspected patients Exclusion criteria: not specified |
||
| Patient characteristics and setting |
Facility cases: positive RT‐PCR via nasopharyngeal swab Facility controls: negative RT‐PCR via nasopharyngeal swab (once) Country: China Dates: 23 January 2020‐16 February 2020 Symptoms and severity: fever, cough, dyspnoea, sore throat, fatigue, systemic soreness, runny nose Demographics: M/F: total 39/47, cases: 5/6, controls 34/40 Case group: mean age 40.73 ± 11.32 years, 5 men. Control group: mean age 39.67 ± 13.90 years, 34 men Exposure history: 7/11 COVID‐19 patients (63.6%) had a history of travel to Hubei (5 Wuhan, 1 Huanggang, 1 Xiaogan), 2 patients had close contact with the COVID‐19 patients, and 2 taxi drivers |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Time interval not specified | ||
| Comparative | |||
| Notes | Funding: supported by the Key Discipline of Pudong Area, Shangai | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Peyrony 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to assess utility of clinical parameters, physician clinical judgment, and lung ultrasonography to accurately identify SARS‐CoV‐2‐infected patients at ED presentation Design: prospective cohort study Recruitment: cohort of all adult (≥ 18 years) patients with suspected COVID‐19 who were tested for SARS‐CoV‐2 prospectively enrolled at university ED (not every patient was tested for SARS‐CoV‐2: testing was left to the clinician’s discretion) Sample size: n = 391 (225 cases) Inclusion criteria: no predefined inclusion criteria. Testing was mostly performed in patients who had severe symptoms such as dyspnoea, reported shortness of breath, presented with comorbidities, or were > 70 years. Some patients without COVID‐19 symptoms were also tested when they needed admission to hospital. Exclusion criteria: patients who attended the ED more than once (only the last visit was included). There were no other exclusion criteria. |
||
| Patient characteristics and setting |
Facility cases: all patients who tested positive for SARS‐CoV‐2 by RT‐PCR Facility controls: all patients who tested negative for SARS‐CoV‐2 by RT‐PCR Country: France Dates: 09 March 2020‐04 April 2020 Symptoms and severity: moderate to mild severity, inclusion based on signs and symptoms suggestive of SARS‐CoV‐2 infection, 82% of included patients with comorbidities; not all included patients had COVID‐19 symptoms Demographics: all included patients (pos + neg): median age: 62 years % female: 38.4% Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | RS and index tests both taken at presentation | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Pisapia 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to compare the characteristics at hospital admission of confirmed and not‐confirmed COVID‐19 patients, in the early phase of the epidemic Design: retrospective cohort study Recruitment: all patients consecutively admitted in selected medical wards (ED + lab) of the mono‐specialist infectious diseases referral centre because of clinical suspicion of COVID‐19 Sample size: n = 37 (17 cases) Inclusion criteria: all patients consecutively admitted in the selected medical wards because of clinical suspicion of COVID‐19. No specification of 'suspicion' Exclusion criteria: none |
||
| Patient characteristics and setting |
Facility cases: suspected cases with a positive RT‐PCR (second test after 24 h if first negative) Facility controls: suspected cases with a negative RT‐PCR (2 negative tests) Country: Italy Dates: 10 February 2020‐10 March 2020 Symptoms and severity: mild to moderate severity Demographics: median age cases: 49 years controls: 29 years. Gender: % female cases: 35%, controls: 35% Exposure history: travel to affected area: cases 35%, controls 95%. Contact with a confirmed case: cases 47%, controls: 0%. Contact with people from affected area: cases: 12% controls: 0% |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | RS and index tests both taken on admission | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Pivetta 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of COVID‐19 pneumonia; to explore whether the integration of lung ultrasound and clinical evaluation increases the sensitivity of the diagnosis of COVID‐19 pneumonia Design: cross‐sectional cohort study, prospective data collection Recruitment: all adult patients visiting an ED and screened positive for SARS‐CoV‐2‐associated symptoms Sample size: n = 228 (107 cases) Inclusion criteria: all adults (≥ 18 years) who screened positive for acute symptoms associated with SARS‐CoV‐2 infection at triage (= fever, dyspnoea, new or worsening cough, sore throat, diarrhoea, ageusia, anosmia and asthenia) Exclusion criteria: patients known to be infected by SARS‐CoV‐2, requiring an urgent psychiatric assessment, or already intubated at arrival, no attending physician with expertise in lung ultrasonography available |
||
| Patient characteristics and setting |
Facility cases: positive RT‐PCR test for SARS‐CoV‐2, 1 test in patients with an initial positive PCR result, 2 tests in patients with an initial negative PCR result when clinical, sonographic, lab or imaging results were suggestive of COVID‐19 Facility controls: negative RT‐PCR test for SARS‐CoV‐2 and all other test results are concordant Country: Italy Dates: 01 April 2020‐20 April 2020 Symptoms and severity: ED outcome: home discharge 25.2% cases, 78.5% controls, ward admission 60.8% cases, 20.7% controls, ICU admission 7.5% cases, 0.8% controls, ED death 6.5% cases, 0% controls Demographics: age: controls median 50.3 years, cases median 62.8 years M%/F%: cases 54.1/45.9, controls 43.7/56.3 Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Maximum 72 h between index tests and RS | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Pokorska‐Śpiewak 2021.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to compare clinical severity and epidemiological spectrum between COVID‐19 and influenza in children Design: cross‐sectional cohort study, prospective data collection Recruitment: all consecutive paediatric patients referred to a tertiary healthcare department Sample size: n = 319 (15 cases) Inclusion criteria: clinical symptoms (WHO definition) of the disease or positive epidemiological history (international travel or contact with infected person) Exclusion criteria: not specified |
||
| Patient characteristics and setting |
Facility cases: positive RT‐PCR test for SARS‐CoV‐2 Facility controls: negative RT‐PCR test for SARS‐CoV‐2 Country: Poland Dates: 01 February 2020‐15 April 2020 Symptoms and severity: mild to moderate severity, hospitalisation needed in 73% of cases and 37% of controls, none needed mechanical ventilation Demographics: age: controls median 84 months, cases median 128 months M%/F%: cases 53.3/46.7, controls 50.7/49.3 Exposure history: household contacts with confirmed positive patient: 100% of cases, 9.2% of controls; history of travel: 6.7% of cases, 25.3% of controls |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Timing not specified | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Porto 2021.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to identify the symptoms associated with early stage SARS‐CoV‐2 (COVID‐19) infections in HCWs using both clinical and laboratory data Design: cross‐sectional cohort study, prospective data collection Recruitment: all patients presenting themselves at a polyclinic (a designated diagnostic site for personnel working in the Brazilian public health system, for screening of COVID‐19) Sample size: n = 1297 (410 cases) Inclusion criteria: all patients presenting at the Piquet Carneiro Polyclinic, test indication not specified, but high proportion of symptomatic individuals in recruited population Exclusion criteria: none specified |
||
| Patient characteristics and setting |
Facility cases: positive RT‐PCR test for SARS‐CoV‐2 Facility controls: negative or inconclusive RT‐PCR test for SARS‐CoV‐2 Country: Brazil Dates: 19 March 2020‐08 April 2020 Symptoms and severity: not specified, mostly symptomatic presentation (mild to moderate severity) Demographics: age: overall 42 years M%/F%: overall 28.2/71.8 Exposure history: confirmed contact with SARS‐CoV‐2 infected person: 43.8% of cases 43.3% in controls |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Timing not specified | ||
| Comparative | |||
| Notes | Funding: the Laboratory of Histocompatibility and Cryopreservation received a thermo cycler and a −80 °C freezer from COVID‐19 donation open account for Pedro Ernesto University Hospital. Rio de Janeiro Health Secretary provided nucleic acid extraction and RT‐PCR | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Raberahona 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to identify clinical signs and symptoms and epidemiological features that could help discriminate confirmed cases of COVID‐19 from SARS‐CoV‐2‐negative patients Design: cross‐sectional cohort study, retrospective data collection Recruitment: patients visiting the screening centre Sample size: n = 3154 (1288 cases) Inclusion criteria: patients visiting the screening centre Exclusion criteria: PCR unknown or inconclusive result |
||
| Patient characteristics and setting |
Facility cases: positive RT‐PCR test for SARS‐CoV‐2 Facility controls: negative RT‐PCR test for SARS‐CoV‐2 Country: Madagascar Dates: 06 May 2020‐01 July 2020 Symptoms and severity: mostly mild to moderate severity, 27.8% asymptomatic Demographics: age: controls median 31 years, cases median 39 years M%/F%: cases 48.6/51.4, controls 51.1/48.9 Exposure history: self‐reported contact cases 19.2%, controls 26.2% |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Not specified | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Romero‐Gameros 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to determine the diagnostic yield of a self‐assessment questionnaire on smell alterations and the psychophysical olfactory test as screening instruments for COVID‐19 Design: cross‐sectional cohort study, prospective data collection Recruitment: patients who sought a respiratory triage assessment at ED of tertiary care hospital due to COVID‐19 suspicion Sample size: n = 139 (72 cases) Inclusion criteria: > 18 years, nasopharyngeal swab taken, present with mild to moderate form of the disease Exclusion criteria: > 65 years, Parkinson or Alzheimer's history, chronic rhino sinusitis, allergic rhinitis, no SARS‐CoV‐2 PCR result, requiring hospitalisation |
||
| Patient characteristics and setting |
Facility cases: positive RT‐PCR test for SARS‐CoV‐2 Facility controls: negative RT‐PCR test for SARS‐CoV‐2 Country: Mexico Dates: 25 May 2020‐30 June 2020 Symptoms and severity: mild to moderate severity, patients in need of hospitalisation were excluded Demographics: age: controls mean 39.2 years, cases mean 38.9 years M%/F%: cases 37.5/62.5, controls 35.8/64.2 Medium‐high academic level: 99% Exposure history: not specified |
||
| Index tests |
(Self‐assessment questionnaire of smell disorders, pocket smell test) |
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | RS taken at presentation, after ENT team assessment of symptoms | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Romero‐Gameros 2021.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to evaluate and establish the diagnostic performance of symptoms and signs in patients with suspected COVID‐19 Design: cross‐sectional cohort study, prospective data collection Recruitment: patients who came to the ED for suspected COVID‐19. Patients were selected through a non probabilistic sampling of consecutive cases according to the order of arrival at the ED Sample size: n = 2137 (1148 cases) Inclusion criteria: > 17 years, high clinical probability of SARS‐CoV‐2, confirmatory RT‐PCR available Exclusion criteria: no RT‐PCR test done |
||
| Patient characteristics and setting |
Facility cases: positive RT‐PCR test for SARS‐CoV‐2 Facility controls: negative RT‐PCR test for SARS‐CoV‐2 Country: Mexico Dates: 14 April 2020‐21 July 2020 Symptoms and severity: mild to moderate severity, high prevalence (> 50%) of symptoms such as cough, asthenia, myalgia and headache; oxygen saturation < 93% in 11% of cases and 4% of controls Demographics: age: controls mean 42.8 years, cases mean 48.6 years M%/F%: cases 55.7/44.2, controls 46.8/53.1 Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Timing not specified | ||
| Comparative | |||
| Notes | Funding: supported by the Mexican Institute of Social Security | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Rutten 2020a.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); describing (a)typical symptoms and disease course in confirmed COVID‐19 patients Design: cross‐sectional cohort study, prospective data collection Recruitment: all patients in a nursing home that were suspected of COVID‐19 underwent a diagnostic test Sample size: n = 1969 (857 cases) Inclusion criteria: patients with at least 2 of the following symptoms: fever/feverish feeling, cough and shortness of breath ‐ later on (from 10 April 2020) patients with atypical symptoms were added. Exclusion criteria: patients who didn't undergo a diagnostic test. Patients with unknown results |
||
| Patient characteristics and setting |
Facility cases: positive RT‐PCR for SARS‐CoV‐2 Facility controls: negative RT‐PCR for SARS‐CoV‐2 Country: the Netherlands Dates: 18 March 2020‐15 April 2020 Symptoms and severity: mild, moderate and severe cases, 30‐day mortality almost 50% in the cases Demographics: mean age: cases 84 years, controls 83 years M%/F%: cases 35.0/65.0, controls 39.0/61.0 Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Timing not specified | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Rutten 2020b.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); describing (a)typical symptoms and disease course in confirmed COVID‐19 patients Design: cross‐sectional cohort study, prospective data collection Recruitment: all patients in a nursing home that were suspected of COVID‐19 (based on the physician's assessment) Sample size: n = 4007 (1538 cases) Inclusion criteria: all nursing home residents with a clinical suspicion of COVID‐19 based on the physician's assessment and for whom they had the result of the RT‐PCR Exclusion criteria: residents of whom results of follow‐up diagnostics were not (yet) available |
||
| Patient characteristics and setting |
Facility cases: positive RT‐PCR for SARS‐CoV‐2 Facility controls: negative RT‐PCR for SARS‐CoV‐2 Country: the Netherlands Dates: 18 March 2020‐13 May 2020 Symptoms and severity: decreased oxygen saturation in 44% of cases and 47% of controls, 30‐ day mortality 3 times higher in cases (42%) than in controls Demographics: mean age: cases 84 years, controls 83 years M%/F%: cases 36.0/64.0, controls 39.0/61.0 Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Index tests and RS taken at same visit | ||
| Comparative | |||
| Notes | Data overlap with Rutten 2020a Funding: supported by the Dutch Ministry of Health, Welfare, and sport |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Sacks 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to determine whether and which specific symptoms are associated with a positive COVID‐19 test result Design: cross‐sectional cohort study, retrospective data collection Recruitment: mandatory, large‐scale programme with onsite capacity to test all HCWs with acute symptoms that were potentially consistent with COVID‐19 on an outpatient basis Sample size: n = 1747 (157 cases) Inclusion criteria: all HCWs with symptoms including fever, myalgia, GI symptoms, runny nose, cough, shortness of breath, sore throat, and anosmia Exclusion criteria: none specified |
||
| Patient characteristics and setting |
Facility cases: positive RT‐PCR for SARS‐CoV‐2 Facility controls: negative RT‐PCR for SARS‐CoV‐2 Country: Massachusetts, USA Dates: 13 March 2020‐02 April 2020 Symptoms and severity: mild to moderate severity Demographics: mean age overall cohort: 39 years M%/F%: cases 32.0/68.0, controls 26.0/74.0 Exposure history: 69% reported direct patient contact as part of their routine work, 15% reported known contact inside or outside the hospital with someone who had been diagnosed with COVID‐19 |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Timing not specified | ||
| Comparative | |||
| Notes | Funded by Mass CPR Grant and the Massachusetts General Hospital Executive Committee on Research (Carney Family Foundation Award to CAS and Steve and Deborah Gorlin Research Scholars Award to RPW). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Saegerman 2021.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to develop a clinical decision support tool for diagnosis of COVID‐19 in hospitals Design: cross‐sectional cohort study, prospective data collection Recruitment: all patients directed to the triage centres of 2 university hospital EDs Sample size: n = 2152 (573 cases) Inclusion criteria: all suspected patients directed to the triage centres (no definition of 'suspected') Exclusion criteria: patient with missing data in clinical records or missing RT‐PCR result |
||
| Patient characteristics and setting |
Facility cases: positive RT‐PCR for SARS‐CoV‐2 Facility controls: negative RT‐PCR for SARS‐CoV‐2 Country: Belgium Dates: 02 March 2020‐15 June 2020 Symptoms and severity: not specified, clinical suspicion at presentation Demographics: mean age: cases 58 years, controls 52 years M%/F%: cases 48.7/51.3, controls 42.2/57.8 Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Index tests and RS both taken at presentation | ||
| Comparative | |||
| Notes | Funded by the Liège University Hospital | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Salmon Ceron 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); second part of the study: to assess the diagnostic accuracy of olfactory/gustatory dysfunction for SARS‐CoV‐2 infection in the overall population tested for SARS‐CoV‐2 Design: prospective cohort study Recruitment: all consecutive patients who were tested for SARS‐CoV‐2 in the Paris‐based screening centre for COVID‐19 Sample size: n = 1824 (849 cases) Inclusion criteria: (second part of the study): all consecutive patients with a suspicion of SARS‐CoV‐2 infection, independent of loss of smell no specification of 'suspicion' Exclusion criteria: (second part of the study): none |
||
| Patient characteristics and setting |
Facility cases: all suspected patients with a positive RT‐PCR Facility controls: all suspected patients with a negative RT‐PCR Country: France Dates: 17 March 2020‐25 March 2020 Symptoms and severity: mild to moderate severity Demographics: not specified for second part of this study Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | RS and index tests both taken at presentation | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Shah 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to describe characteristics, diagnostics and outcomes of patients with respiratory illness, comparing patients with and without COVID‐19 disease Design: retrospective cohort Recruitment: all patients presenting to an ED with an acute respiratory illness and tested for SARS‐CoV‐2 Sample size: n = 316 (33 cases) Inclusion criteria: all patients ≥ 18 years who underwent testing for COVID‐19 within 24 h of presentation to the ED. Patients with acute respiratory symptoms, influenza‐like illness Exclusion criteria: not specified |
||
| Patient characteristics and setting |
Facility cases: positive RT‐PCR for SARS‐CoV‐2 Facility controls: negative RT‐PCR for SARS‐CoV‐2 Country: California, USA Dates: 03 February 2020‐31 March 2020 Symptoms and severity: not specified Demographics: median age: cases 63, controls 62. %. Female: cases 36%, controls 50% Exposure history: travel in last 21 days or known COVID exposure: cases 46%, controls 11% |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | RS performed maximum 24 h later than index tests | ||
| Comparative | |||
| Notes | Funding: supported by the National Center for Advancing Translational Sciences, the National Heart Lung Blood Institute, National Institute of Allergy and Infectious Diseases, the Chan Zuckerberg Biohub, the Chan Zuckerberg Initiative | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Simpson 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to describe the demographics, clinical features, and test results of children referred from their primary provider for SARS‐CoV‐2 testing in the community setting Design: cross‐sectional cohort study, retrospective data collection Recruitment: all children (0‐22 years) who were referred by paediatricians to an outpatient paediatric testing site Sample size: n = 1210 (378 cases) Inclusion criteria: children presenting with mild symptoms, children who were at high risk for serious infection, children who lived with high‐risk household members, or children who lived with household members whose work status would be impacted by the presence of infection Exclusion criteria: patients were excluded from the geospatial analysis if they had invalid addresses (n = 12) |
||
| Patient characteristics and setting |
Facility cases: positive RT‐PCR for SARS‐CoV‐2 Facility controls: negative RT‐PCR for SARS‐CoV‐2 Country: USA Dates: 21 March 2020‐16 May 2020 Symptoms and severity: mostly mild symptoms Demographics: median age overall: 8 years M%/F%: cases 47.6/49.3 controls: 52.0/47.8 Exposure history: not specified, self‐reported high‐risk contacts vs no high‐risk contacts: OR = 1.6 |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Timing not specified | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Sonoda 2021.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to investigate the clinical symptoms to discriminate between COVID‐19 and non‐COVID‐19 cases among outpatients in GP clinics Design: cross‐sectional cohort study, retrospective data collection Recruitment: outpatients visiting the screening centre (GP clinic) Sample size: n = 360 (17 cases) Inclusion criteria: patients visiting the centre with suspicion of COVID‐19 (no definition of 'suspicion') Exclusion criteria: patients who didn't receive a RT‐PCR test |
||
| Patient characteristics and setting |
Facility cases: positive RT‐PCR for SARS‐CoV‐2 Facility controls: negative RT‐PCR for SARS‐CoV‐2 Country: Japan Dates: 01 August 2020‐14 August 2020 Symptoms and severity: clinical spectrum of cases (n = 17) was 14 moderate, 2 moderate and 1 severe illness Demographics: mean age: cases 39.6 years, controls 41.2 years M%/F%: cases 58.8/41.2, controls 50.7/49.2 Exposure history: close contact to patients with COVID‐19: cases 13.3%, controls 8.1% |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Timing not specified | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Sun 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: algorithm development for estimating risk of COVID‐19 Design: cross‐sectional cohort study, retrospective data collection Recruitment: patients presenting at the designated national outbreak screening centre and tertiary care hospital in Singapore for SARS‐CoV‐2 testing. Patients were either self‐referred, referred from primary care facilities, or were at‐risk cases identified by national contact tracing efforts (recruited n = 991) Sample size: n = 788 (n = 54) Inclusion criteria: patients presenting to the centre:
Exclusion criteria: PCR results not available at time of data collection ‐ no electronic medical records ‐ unavailable vital sign records |
||
| Patient characteristics and setting |
Facility cases: positive SARS‐CoV‐2 RT‐PCR test Facility controls: all SARS‐CoV‐2 RT‐PCR results were negative (minimum 2 test negatives in high‐risk patients, minimum 1 test low‐risk patients) Country: Singapore Dates: 26 January 2020‐16 February 2020 Symptoms and severity: 252 (33.2%) symptoms > 5 days at presentation, 75 (9.5%) any comorbidity Demographics: median age 34 years, range 7 years‐98 years, IQR 27‐45; cases median 42 years, range 16‐79; controls 34 years, range 7‐98 M/F: 48.3%/51.7% F (cases M: 88 (88.9%)) Exposure history: contact with a known COVID‐19 case (20.1% (32/54 cases (59.3%)); 126/734 controls (17.2%), contact with travellers from China (22.1%, 15/54 cases (27.8%); 42/734 controls (5.7%)), recent travel history, and visit to hospital in China within 14 days prior to symptom onset (0.8%) |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Time interval not specified | ||
| Comparative | |||
| Notes | Funding: supported by the following grants from Singapore Ministry of Health’s National Medical Research Council: collaborative Solutions Targeting Antimicrobial Resistance Threats in Health Systems (CoSTAR‐HS) [NMRC CGAug16C005], NMRC Clinician Scientist Award [MOH‐000276] and NMRC Clinician Scientist Individual Research Grant [MOH‐CIRG18nov‐0006]. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Tan 2021.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to describe the differences in clinical presentation between COVID‐19 and other respiratory viruses and to determine independently associated clinical features with COVID‐19 Design: cross‐sectional multicenter cohort study, retrospective data collection Recruitment: all patients who fulfilled the hospital's COVID‐19 suspect criteria were admitted and tested for both SARS‐CoV‐2 and non‐SARS‐CoV‐2 respiratory viruses. The hospital's suspect criteria were based on a combination of clinical symptoms, and a constant changing criteria for a history of (international) travel based on the current epidemiological risks Sample size: n = 469 (287 cases) (18 asymptomatic patients excluded from the analysis) Inclusion criteria: patients who were positive for either SARS‐CoV‐2 or a non‐SARS‐CoV‐2 respiratory virus Exclusion criteria: testing negative for both SARS‐CoV‐2 and a non‐SARS‐CoV‐2 respiratory virus; co‐infection of SARS‐CoV‐2 and another non‐SARS‐CoV‐2 respiratory virus; asymptomatic patients (screening) |
||
| Patient characteristics and setting |
Facility cases: positive RT‐PCR for SARS‐CoV‐2 (2, if first was negative) Facility controls: negative RT‐PCR for SARS‐CoV‐2 Country: Singapore Dates: 17 January 2020‐15 April 2020 Symptoms and severity: mild to moderate severity, some severe to death Demographics: mean age: ≤ 30 years: cases 32.4%, controls 37.9%; 31‐60 years: cases 56.1%, controls 48.9%; > 60 years: cases 11.5%, controls 13.2% M%/F%: cases 81.2/18.8, controls 56.6/43.4 Exposure history: exposure to possible COVID‐19‐positive case: cases 54%, controls 46.2% Exposure to confirmed COVID‐19‐positive case: cases 24.7%, controls 11%. Resides in foreign worker dormitory: cases 51.6%, controls 17%. Positive contact with a person with acute respiratory infection symptoms: cases 17.4%, controls 27.5% |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Timing not specified | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Tolia 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of acute SARS‐CoV‐2 infection Design: cross‐sectional cohort, retrospective data collection Recruitment: all patients presenting to 1 of 2 EDs, located at an urban teaching hospital, and academic quaternary medical centre, within the same healthcare system who had targeted testing based on clinician's decision during the initial 10 days of test availability Sample size: n = 283 (29 cases) Inclusion criteria:
Exclusion criteria: not specified |
||
| Patient characteristics and setting |
Facility cases: positive SARS‐CoV‐2 test Facility controls: negative SARS‐CoV‐2 test, visiting the same EDs and being tested Country: USA (San Diego, CA) Dates: 10 March 2020‐19 March 2020 Symptoms and severity: not specified, all patients presenting with symptoms related to COVID‐19 infection (fever and cough or shortness of breath) Demographics: age (< 18 years: 0.7%, 18‐64 years: 83.4%, > 65 years: 15.9%); gender: cases M/F%: 55.2/44.8; controls M/F%: 52.8/47.2; all M/F%: 53.0/47.0 Exposure history: recent travel (5.5%), 90.6% symptom‐based criteria for testing, no known exposure history based |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Probably no time interval between index test and RS, but not specified | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Tordjman 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of COVID‐19 pneumonia; to determine the effectiveness of a pre‐test probability score for SARS‐CoV‐2 infection Design: cross‐sectional cohort, retrospective data collection Recruitment: a retrospective cohort of 200 patients with both RT‐PCR and CT scan results available with a 1:1 patient:control inclusion ratio from ED at Cochin Hospital (Paris, France) with a suspicion of SARS‐CoV‐2 infection: 100 consecutive infected patients and 100 consecutive controls + a validation cohort: consecutive recruitment of outpatients suspected with COVID‐19 (different from derivation cohort) at Cochin Hospital, Ambroise Paré and Raymond Poincaré hospitals Sample size: n = 605 (361) (no clinical data available from validation cohort) Inclusion criteria: clinical suspicion of SARS‐CoV‐2 infection, and both RT‐PCR and CT scan available, 'suspicion' not defined Exclusion criteria: absence of confirmed diagnosis (diagnosis still under investigation; lack of blood test including complete white blood cell count and serum electrolytes; absence of reported clinical characteristics) |
||
| Patient characteristics and setting |
Facility cases: suspected patients with a positive RT‐PCR or positive CT scan (positive signs of COVID‐19 pneumonia: usually bilateral and peripheral ground‐glass and consolidated pulmonary opacities) Facility controls: suspected patients with a negative RT‐PCR and negative findings on CT scan Country: France Dates: 10 March 2020‐30 April 2020 Symptoms and severity: not specified, mild to moderate severity Demographics: median age: cases 60.8 years, controls 54.1 years. Female %: cases 40%, controls 50% Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | RS and index tests both taken at first presentation | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Trubiano 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease) Design: prospective cohort study Recruitment: data on all patients presenting at a COVID‐19 rapid assessment screening clinic were prospectively collected in an electronic database. Only those patients who met the DHHS (Victorian Department of Health and Human Services) criteria for SARS‐CoV‐2 testing had nasopharyngeal swab collected for SARS‐CoV‐2 nucleic acid detection by PCR Sample size: n = 2935 (108 cases) Inclusion criteria: all people meeting DHHS criteria for testing: fever or chills in the absence of an alternative diagnosis that explains the clinical presentation or acute respiratory infection symptoms (e.g. cough, sore throat, shortness of breath, runny nose, loss of smell or loss of taste) Exclusion criteria: pending or intermediate results |
||
| Patient characteristics and setting |
Facility cases: patients with suspected COVID‐19 with a positive RT‐PCR for SARS‐CoV‐2 Facility controls: suspected patients with a negative RT‐PCR for SARS‐CoV‐2 Country: Australia Dates: 11 March 2020‐22 April 2020 Symptoms and severity: mild to moderate severity Demographics: median age: cases 51 years, controls 38 years. Female %: cases 49.1%, controls 64.1% Exposure history: overseas health facility exposure: cases 1.9%, controls 4.0%. Australian health facility exposure: cases 11.1%, controls 31.5%. Contact with known COVID‐19‐positive patient: cases 57.4%, controls 15.8% |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | RS and index tests both taken at presentation | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Tudrej 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to diagnose SARS‐CoV‐2 infection in primary care settings based on signs and symptoms Design: cross‐sectional cohort study, prospective data collection Recruitment: recruitment in 2 clinical laboratories in Lyon (France) to which GPs refer patients with suspected COVID–19 for a nasopharyngeal smear (RT‐PCR) Sample size: n = 816 (198 cases) Inclusion criteria: all consecutive patients referred by GPs for PCR testing Exclusion criteria: none specified |
||
| Patient characteristics and setting |
Facility cases: all suspected patients with a positive RT‐PCR Facility controls: all suspected patients with a negative RT‐PCR Country: France Dates: 24 March 2020‐14 April 2020 Symptoms and severity: not specified Demographics: all included patients: median age: 45 years, % female: 65% Exposure history: not specified, 37% of participants were healthcare professionals |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | RS specimen taken right after index tests, at presentation | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Van Loon 2021.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to identify early symptoms of SARS‐CoV‐2 infection among HCWs Design: single‐centre, cross‐sectional cohort study, prospective data collection Recruitment: all hospital HCWs self‐reporting mild symptoms of an acute upper or lower respiratory tract infection were tested in a large non‐academic hospital Sample size: n = 373 (185 cases) Inclusion criteria: HCWs with respiratory complaints and fever, according to the national guidelines for PCR‐testing for SARS‐CoV‐2 by Sciensano, based on those of the WHO and the European Center for Disease Control (ECDC). Exclusion criteria: inconclusive PCR results |
||
| Patient characteristics and setting |
Facility cases: positive RT‐PCR for SARS‐CoV‐2 (2, if first was negative) Facility controls: negative RT‐PCR for SARS‐CoV‐2 Country: Belgium Dates: 09 March January 2020‐17 April 2020 Symptoms and severity: mild symptoms of an acute upper or lower respiratory tract infection Demographics: mean age: < 30 years: total 20.4%, controls 60.5%; 30‐39 years: total 30.0 %, controls 41.4%; 40‐49 years: total 27.3%, controls 49.5%; 50‐59 years: total 17.4%, controls 56.9%; ≥ 60 years: cases 4.8%, controls 33.3% M%/F%: cases 53.1/46.9, overall 22.2/77.8 Exposure history: HCWs in a hospital with a high number of admitted COVID‐19 patients |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Timing not specified | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Van Walraven 2021.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to derive and assess a model to predict the risk of SARS‐CoV‐2 in community‐based people Design: cross‐sectional study, prospective data collection Recruitment: all people who were tested between 13 March and 21 April 2020 at a community‐based COVID‐19 testing centre Sample size: n = 9172 (571 cases) Inclusion criteria: presence of symptoms including rhinorrhoea; fever symptoms including rigor, chills, perceived fever, or documented fever at home or at the screening clinic; cough; and shortness of breath. Any infection risk factor including close contact with a person with known or presumed COVID‐19 disease or recent travel outside of Canada. In the absence of these indications, HCWs (or people cohabiting with a HCW) were included if they had symptoms of sore throat, sputum production, or rhinorrhoea. In the event of extenuating circumstances or if the person was referred to the screening clinic by public health officials for testing Exclusion criteria: result not known, people with previous testing |
||
| Patient characteristics and setting |
Facility cases: positive RT‐PCR for SARS‐CoV‐2 Facility controls: negative RT‐PCR for SARS‐CoV‐2 Country: Canada Dates: 13 March 2020‐21 April 2020 Symptoms and severity: severity not specified. Mostly symptomatic presentation: cough or shortness of breath was present in > 80% of cases and controls Demographics: mean age: cases 45.4 years, controls 42.5 years M%/F%: cases 44.5/55.5, controls 43.3/56.7 Exposure history: contact %: 72.5 cases, 47 controls travel %: 25.7 cases, 24.2 controls |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Timing not specified, likely both at presentation | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Vieceli 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to develop a tool to identify patients with higher probability of COVID‐19 diagnosis at admission Design: cross‐sectional cohort study, retrospective data collection Recruitment: the first 118 consecutive patients aged ≥ 18 admitted to the hospital due to suspected COVID‐19 were assessed Sample size: n = 100 (29 cases) Inclusion criteria: aged ≥ 18 years and suspected of COVID‐19 Exclusion criteria: patients discharged within 24 h of admission |
||
| Patient characteristics and setting |
Facility cases: positive RT‐PCR for SARS‐CoV‐2 (2, if first was negative) Facility controls: negative RT‐PCR for SARS‐CoV‐2 Country: Brazil Dates: 17 March January 2020‐10 April 2020 Symptoms and severity: mild to severe Demographics: mean age: cases 62 years, controls 54 years M%/F%: cases 51.7/48.3, controls 39.4/60.6 Exposure history: no patients in the negative group (controls) had travelled in the previous 3 weeks, compared to 8 (28.6%) patients in the confirmed (cases) group |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Index tests and reference standard both on admission | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Vilke 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to assess the frequency of fever and other symptoms associated with COVID‐19 among patients presenting to ED Design: cross‐sectional study, retrospective data collection Recruitment: all patients presenting to 1 of 2 EDs, who were tested for acute COVID‐19 while in the ED or within 2 h of their triage temperature Sample size: n = 6894 (330 cases) Inclusion criteria: all patients presenting to the ED who were tested for acute COVID‐19 infection while in the ED or within 2 h of their triage temperature (if they were admitted and tested after they left the ED) Exclusion criteria: none specified |
||
| Patient characteristics and setting |
Facility cases: positive test on rapid antigen test for SARS‐CoV‐2 or positive RT‐PCR for SARS‐CoV‐2 Facility controls: negative test on rapid antigen test for SARS‐CoV‐2 or negative RT‐PCR for SARS‐CoV‐2 Country: USA (California) Dates: 10 March 2020‐30 June 2020 Symptoms and severity: severity not specified. Mostly symptomatic presentation: cough was present in 64.5% of cases and 26.6% of controls, fever at presentation in 19.4% of cases and 5.6% of controls Demographics: not specified Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | RS was taken within 2 h after the triage temperature | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Villerabel 2021.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to evaluate the diagnostic value of a semi‐objective olfactory test in patients with self‐reported chemosensory dysfunction before COVID‐19 testing; to describe the diagnostic value of suggestive patient‐reported COVID‐19 symptoms Design: cross‐sectional study, prospective data collection Recruitment: all HCWs and adult patients presenting themselves at the COVID‐19 screening facility of the university hospital of Montpellier Sample size: n = 809 (58 cases) Inclusion criteria: all HCW or outpatients with symptoms or with close contact with an index case presenting at the screening facility Exclusion criteria: prior chemosensory dysfunction, testing inability, or contraindications |
||
| Patient characteristics and setting |
Facility cases: positive test on rapid antigen test for SARS‐CoV‐2 or positive RT‐PCR for SARS‐CoV‐2 Facility controls: negative test on rapid antigen test for SARS‐CoV‐2 or negative RT‐PCR for SARS‐CoV‐2 Country: France Dates: 23 March 2020‐22 April 2020 Symptoms and severity: severity not specified. Mild to moderate symptoms: 42.0% of patients presenting were asymptomatic, most common symptoms were cough, fever, headache, signs of upper respiratory tract infection and fatigue Demographics: mean age: cases 43.6 years, controls 41.7 years M%/F%: cases 32.8/67.2, controls 25.8/74.2 Exposure history: 71.1% had contact with COVID‐19 cases |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Index tests and RS within 10 min | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Low risk | ||
Wee 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to analyse OTDs as a diagnostic criterion for COVID‐19 Design: cross‐sectional, prospective single‐centre study Recruitment: all suspected cases presenting to the ED Sample size: n = 870 (cases = 154) Inclusion criteria:
Exclusion criteria: not specified |
||
| Patient characteristics and setting |
Facility cases: positive RT‐PCR for COVID‐19 Facility controls: negative RT‐PCR for COVID‐19 Country: Singapore Dates: 26 March 2020‐10 April 2020 Symptoms and severity: loss of sense of smell/taste Demographics: not specified Exposure history: close contact of a confirmed COVID‐19 case: cases 42/112, controls 37/679 |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Time interval: same day | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Wei 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); diagnosis of SARS‐CoV‐2 in outpatients visiting a fever clinic Design: retrospective cohort study Recruitment: all febrile patients visiting the fever clinic of Tongji Hospital Sample size: n = 936 (628 cases) Inclusion criteria: all febrile patients visiting the fever clinic Exclusion criteria: none specified |
||
| Patient characteristics and setting |
Facility cases: all febrile patients with a positive RT‐PCR for SARS‐CoV‐2 (tested twice in 24 h) Facility controls: all febrile patients with a negative RT‐PCR for SARS‐CoV‐2 (tested twice in 24 h) Country: China Dates: 30 January 2020‐04 February 2020 Symptoms and severity: cases: 88.1% mild, 11.5% severe, 0.5% critical; controls: 90.3% mild, 9.1% severe, 0.7% critical Demographics: median age: cases: 53 years, controls: 49 years. Gender: % female cases: 52.9%, controls: 53.9% Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | RS and index tests both taken at presentation | ||
| Comparative | |||
| Notes | Funding: supported by the National Natural Science Foundation of China [Grants 81874149, 81974456, and 81530024]; the Clinical Research Physician Program of Tongji Medical College, Huazhong University of Science and Technology [Grant 5001540075]; SARS‐CoV‐2 Pneumonia Emergency Technology Public Relations Project [2020FCA009] | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Wernhart 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to compare methods of outpatient management and testing strategies Design: cross‐sectional study, prospective data collection Recruitment: all patients with respiratory symptoms reporting to 3 rural GP offices in North Rhine‐Westphalia, Germany Sample size: n = 489; only 80 people RT‐PCR tested (5 cases) Inclusion criteria: all patients from 3 GP offices reporting symptoms of respiratory tract infection Exclusion criteria: none specified, only 80 suspected patients tested of the 489, following the strict test criteria of the Robert Koch Instritute (RKI) |
||
| Patient characteristics and setting |
Facility cases: patients receiving a smear test according to the RKI criteria and testing RT‐PCR‐positive Facility controls: patients receiving a smear test according to the RKI criteria and testing RT‐PCR‐negative Country: Germany Dates: 27 January 2020‐20 April 2020 Symptoms and severity: severity not specified. Mild to moderate symptoms: rhinopharyngitis in 40% and acute bronchitis in 60% of cases Demographics: mean age all patients 52.69 years; mean age of tested patients 47.03 years. Gender not specified Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Index tests and RS both on the same day (patients referred directly to the smear centre) | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Low risk | ||
Xie 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of COVID‐19 pneumonia; to compare the epidemiological, clinical, laboratory and radiological characteristics, treatment and outcomes between patients with confirmed COVID‐19 pneumonia and those with suspected COVID‐19 infection (71% of SARS‐CoV‐2‐positive patients had CT‐confirmed pneumonia) Design: retrospective 2‐centre cohort Recruitment: patients in whom a RT‐PCR test was performed at 2 Shangai hospitals Sample size: n = 105 (21 cases) Inclusion criteria: not specified Exclusion criteria: not specified |
||
| Patient characteristics and setting |
Facility cases: patients with a positive RT‐PCR test for SARS‐CoV‐2 Facility controls: patients with a negative RT‐PCR test for SARS‐CoV‐2 Country: China Dates: 01 January 2020‐15 February 2020 Symptoms and severity: 72% of all participants were hospitalised, 71% of the cases had pneumonia, 88% of controls had pneumonia ("clinical symptoms usually mild") Demographics: mean age: cases: 54.0 years, controls: 41.6 years. Gender: % female cases: 38.1%, controls: 51.2% Exposure history: recently been to Wuhan: cases: 42.9%, controls: 17.9%. Contact with people from Wuhan: cases: 14.3%, controls: 0%. Recently been to supermarkets and groceries: cases: 28.6%, controls: 34.5%. Recently travelled: cases: 14.3%, controls: 47.6% |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | RS and index tests both taken at admission | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Yombi 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); diagnosis of SARS‐CoV‐2 infection, using clinical signs in HCWs Design: cross‐sectional cohort study, retrospective data collection Recruitment: period 1: (before 30 March 2020) HCWs were tested only if they had fever and respiratory symptoms (some physicians were tested without fever); period 2 (after 30 March 2020), HCWs were tested if they had respiratory symptoms with or without fever Sample size: n = 536 (175 cases) Inclusion criteria: not specified (all suspected HCWs) Exclusion criteria: not specified |
||
| Patient characteristics and setting |
Facility cases: all suspected HCWs with a positive RT‐PCR Facility controls: all suspected HCWs with a negative RT‐PCR Country: Belgium Dates: 16 March 2020‐24 April 2020 Symptoms and severity: not specified (from tables: mild to moderate severity) Demographics: % age < 45 years: cases: 56.6%, controls: 62.3% gender: % female cases: 67.4%, controls: 73.1% Exposure history: not specified (all HCWs) |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Not specified | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Yonker 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease) in children; to describe the paediatric impact of COVID‐19, specifically focusing on viral burden, susceptibility to disease and immune responses Design: cross‐sectional cohort study, retrospective data collection Recruitment: paediatric patients ≤ 22 years of age presenting to Infection Control clinics for medical evaluation of symptoms concerning for COVID‐19 or admitted for acute symptoms related to COVID‐19 or multisystem inflammatory syndrome in children (MIS‐C) were offered enrolment in the paediatric COVID‐19 bio repository Sample size: n = 174 (49 cases), excluding 18 MIS‐C patients Inclusion criteria: children ages 0‐22 years; symptoms concerning for COVID‐19 or admitted for acute symptoms related to COVID‐19 or MIS‐C; informed consent, and if appropriate, assent,were verbally obtained by the patients or parent/guardian Exclusion criteria: none specified |
||
| Patient characteristics and setting |
Facility cases: positive RT‐PCR for SARS‐CoV‐2 Facility controls: negative RT‐PCR for SARS‐CoV‐2 Country: Massachusetts, USA Dates: not stated (before 29 July 2020 (date of article submission)) Symptoms and severity: asymptomatic to mild symptoms to MIS‐C Demographics: mean age: cases 12.7 years, controls 9.6 years M%/F%: cases 46.9/53.1, overall 53.6/46.4 Exposure history: household exposures cases: mother: 40.8%, father: 26.5%, sibling: 18.4%, other: 18.4%, no household exposure: 18.4% Household exposures controls: mother: 16.8%, father: 8.8%, sibling: 6.4%, other: 15.2%, no household exposure: 56.0% |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Index tests and reference standard both taken at presentation | ||
| Comparative | |||
| Notes | MIS‐C patients were excluded for our analyses Funding: Supported by the National Heart, Lung, and Blood Institute (5K08HL143183 to L.Y.), the Cystic Fibrosis Foundation (YONKER18Q0 to L.Y.), the National Institute of Child Health and Human Development (K08 HD094638 [to A.N.] and R01HD100022 [to A.E.]), Mark and Lisa Schwartz (to J.L.), the National Institute of Diabetes and Digestive and Kidney Diseases (DK039773, DK072381 [to J.B.] and DK104344 [to A.F.]), the National Institute of Allergy and Infectious Disease (K24AI141036 to I.B.), the Centers for Disease Control and Prevention (U01CK000490 to E.R.), and the Department of Pediatrics and the Department of Obstetrics/Gynecology at Massachusetts General Hospital (to L.Y. and A.E.) |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Zayet 2020a.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to compare the symptoms of patients with positive and negative SARS‐CoV‐2 RT‐PCR results and to determine the sensitivity, specificity, positive predictive value and negative predictive value for each of these symptoms in regard to SARS‐CoV‐2 RT‐PCR Design: retrospective cohort study Recruitment: all adult patients (≥ 18 years) who presented for possible COVID‐19 at the outpatient department Sample size: n = 217 (95 cases) Inclusion criteria: all adult patients (≥ 18 years) who presented for possible COVID‐19 at the outpatient department Exclusion criteria: pregnant women, children (< 18 years) and patients with dementia (unable to report functional symptoms) |
||
| Patient characteristics and setting |
Facility cases: patients with suspected COVID‐19 with a positive RT‐PCR Facility controls: patients with suspected COVID‐19 with a negative RT‐PCR Country: France Dates: 30 March 2020‐03 April 2020 Symptoms and severity: mild to moderate severity Demographics: mean age: cases: 39.8 years, controls: 39.6 years. Gender: % female cases: 83.2%, controls: 86.9% Exposure history: not specified (mostly HCWs) |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Not specified | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Zhu 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: description of initial clinical features in patients with suspected and confirmed SARS‐CoV‐2 infection Design: cross‐sectional cohort, retrospective data collection Recruitment: all patients with suspected COVID‐19 who presented to the ED of the First Affiliated Hospital of USTC and the Infectious Hospital of the First Affiliated Hospital of USTC for the first time Sample size: n = 116 (32 cases) Inclusion criteria:
Exclusion criteria: transfer from another hospital or previous visit to First Affiliated Hospital and previous diagnosis of COVID‐19 |
||
| Patient characteristics and setting |
Facility cases: positive nucleic acid amplification test on admission or 24 h later Facility controls: SARS‐CoV‐2 PCR test negative Country: China, Anhui Dates: 24 January 2020‐20 February 2020 Symptoms and severity: all suspected COVID‐19 patients included; days since onset of symptoms median 5 (IQR 2‐7) Demographics: median age: all: 40 years (IQR 27‐53), cases: 46 years (IQR 35‐52), controls: 35 years (IQR 27‐53); gender distribution M%/F%: all 46/54, cases 47/53, controls 46/54 Exposure history: no specific exposure history common to all patients with suspected disease: 8 (25%) diagnosed patients had visited Wuhan in the previous 2 weeks and 12 (38%) had been exposed to patients with infection in the previous 2 weeks |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Index tests and RS both taken on admission or after 24 h | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Zimmerman 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to develop a data‐driven set of clinical indicators for COVID‐19 that would help to identify outpatient symptoms and those who most benefit from limited testing availability Design: cross‐sectional cohort study, prospective data collection Recruitment: symptomatic individuals who had either exposure to a case of COVID‐19 or a typical respiratory illness symptom were scheduled at a centralised outpatient COVID‐19 testing facility Sample size: n = 736 (55 cases) Inclusion criteria: either exposure to a case of COVID‐19 or a typical respiratory illness symptom Exclusion criteria: asymptomatic individuals |
||
| Patient characteristics and setting |
Facility cases: adult patients testing positive for SARS‐CoV‐2 infection Facility controls: adult patients testing negative for SARS‐CoV‐2 infection Country: Pennsylvania, USA Dates: 29 March 2020‐26 April 2020 Symptoms and severity: mild to moderate severity Demographics: not specified Exposure history: contact with COVID‐19 case: cases: 70%, controls: 21% |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Not specified | ||
| Comparative | |||
| Notes | Funding: supported through a co‐operative agreement with the Centers for Disease Control and Prevention (CDC) through grant number U01 IP000467 and the National Institutes of Health grant number 1UL1 TR001857. The US Flu VE Network is supported through co‐operative agreements funded by CDC. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Zurl 2021.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to analyse the infection rate in symptomatic children Design: cross‐sectional cohort, retrospective data collection Recruitment: children presenting at university hospital's children's ED Sample size: n = 1105 (10 cases) Inclusion criteria: children with symptoms and anamnestic details according to national criteria for suspicion of SARS‐CoV‐2, and no alternative diagnosis Exclusion criteria: not specified |
||
| Patient characteristics and setting |
Facility cases: SARS‐CoV‐2 PCR test‐positive Facility controls: SARS‐CoV‐2 PCR test‐negative Country: Austria Dates: 19 March 2020‐15 August 2020 Symptoms and severity: not specified, mostly mild to moderate severity, 50% of cases requiring hospital admission, 32.4% of controls Demographics: median age: cases: 8.4 years, controls: 3.2 years gender M%/F%: cases 50/50, controls 52.1/47.9 Exposure history: known contact with a case: cases 0%; controls 1.4% |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Timing not specified | ||
| Comparative | |||
| Notes | Funding: none declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
BP: blood pressure; CDC: Centre for Disease Control; COPD: constructive obstructive pulmonary disease; COVID‐19: coronavirus disease 2019; CT: computed tomography; ED: emergency department; ENT: ear, nose and throat; F: female; FiO2: fraction of inspired oxygen; GI: gastrointestinal; GP: general practitioner; HCW: healthcare workers; ICU: intensive care unit; IgM: immunoglobulin M; IQR: interquartile range; M: male; NCP: novel coronavirus pneumonia; OTD: olfactory and taste disorder; PaO2: partial pressure of oxygen; POC: point‐of‐care; RS: reference standard; RT‐PCR: reverse transcription polymerase chain reaction; SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2; SD: standard deviation; SpO2: oxygen saturation; TC: target condition; WBC: blood white blood cell; WHO: World Health Organization; 2019‐nCoV: 2019 novel coronavirus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Accorsi 2020 | Ineligible population |
| Afshar 2020 | Ineligible design |
| Agarwal 2021 | Ineligible design |
| Ai 2020 | Preprint |
| Akinbami 2021 | Ineligible population |
| Akyala 2020 | Ineligible population |
| Aleebrahim‐Dehkordi 2020 | Conference abstract |
| Al‐Rifai 2021 | Only SARS‐CoV‐2‐positive patients |
| Altınbilek 2020 | Ineligible outcomes |
| Andina‐Martinez 2021 | Only SARS‐CoV‐2‐positive patients |
| Antonelli 2021 | Index test not assessed on admission |
| Aubert 2021 | Ineligible design |
| Auvinen 2021 | Ineligible population |
| Baghaei 2020 | Ineligible outcomes |
| Bailey 2020 | No data |
| Bailey 2021 | Ineligible outcomes |
| Bartlett 2020 | Conference abstract |
| Bastiani 2021 | Ineligible design |
| Bhatta 2021 | Only SARS‐CoV‐2‐positive patients |
| Bidkar 2021 | Ineligible population |
| Bonadio 2020 | Conference abstract |
| Brotons 2020 | Preprint |
| Burrel 2021 | Ineligible outcomes |
| Cadegiani 2021 | Ineligible design |
| Cai 2020 | Timing of index test not specified |
| Calagnan 2020 | Ineligible design |
| Carignan 2020 | Ineligible design |
| Challener 2020 | Ineligible design |
| Chen 2020 | Ineligible design |
| Chen 2021 | Ineligible population |
| Concheiro‐Guisan 2020 | Index test not assessed on admission |
| D'Souza 2020 | No clinical suspicion at inclusion |
| Dai 2021 | Timing of index test not specified |
| Dantas 2021 | Ineligible population |
| De Angelis 2020 | Ineligible population |
| Deng 2020 | Ineligible population |
| Dixon 2021 | Index test not assessed on admission |
| Dreyer 2020 | Ineligible population |
| Duan 2020 | No data |
| Duque 2021 | Ineligible design |
| Duramaz 2021 | Only SARS‐CoV‐2‐positive patients |
| Elimian 2020 | Timing of index test not specified |
| Escosteguy 2020 | Ineligible population |
| Feehan 2021 | Ineligible population |
| Fisher 2021 | Index test not assessed on admission |
| Foster 2021 | Ineligible design |
| Gale 2020 | Ineligible population |
| Gerkin 2021 | Ineligible design |
| Giavedoni 2020 | Only SARS‐CoV‐2‐positive patients |
| Gibbons 2021 | Index test not assessed on admission |
| Gnanasambantham 2020 | Ineligible design |
| Goel 2020 | Ineligible index test |
| Gombos 2021 | No data |
| Goodacre 2020 | Ineligible population |
| Guillén 2020 | Only SARS‐CoV‐2‐positive patients |
| Gurrola 2021 | Only SARS‐CoV‐2‐positive patients |
| Haddadin 2021 | Ineligible population |
| Hamed 2021 | Ineligible design |
| Hernández‐Cruz 2021 | Ineligible population |
| Hosseinzadeh 2021 | Ineligible design |
| Hosseninasab 2020 | Timing of index test not specified |
| Hubiche 2021 | Conference abstract |
| Hurst 2020 | No data |
| Indini 2021 | Ineligible population |
| Islam 2020 | Conference abstract |
| Jain 2021 | Case report |
| Karni 2020 | Ineligible design |
| Kasiukiewicz 2020 | Conference abstract |
| Kline 2021 | Ineligible design |
| Lechner 2021 | Ineligible design |
| Lee 2020 | Ineligible design |
| Lee 2021 | Only SARS‐CoV‐2‐positive patients |
| Li 2020a | Timing of index test not specified |
| Li 2020b | Timing of index test not specified |
| Li 2021 | Ineligible outcomes |
| Liang 2020 | Preprint |
| Liu 2021 | Ineligible design |
| Loftus 2020 | Index test not assessed on admission |
| Lu 2020 | Ineligible population |
| Madan 2020 | Ineligible design |
| Makaronidis 2020 | Ineligible population |
| Manley 2020 | Conference abstract |
| McDonald 2020 | Ineligible design |
| McGovern 2020 | Conference abstract |
| Medetalibeyoglu 2020 | Conference abstract |
| Membrilla 2020 | Only SARS‐CoV‐2‐positive patients |
| Mizrahi 2020 | Ineligible design |
| Möckel 2021 | Ineligible design |
| Moolla 2021 | Index test not assessed on admission |
| Muhammad 2021 | Ineligible design |
| Munblit 2020 | Only SARS‐CoV‐2‐positive patients |
| Murillo‐Zamora 2020 | Ineligible index test |
| Nakajima 2021 | Ineligible design |
| Nakanishi 2020 | Ineligible design |
| Nayan 2021 | No data |
| Nobel 2020 | Ineligible design |
| Ortiz 2020 | Only SARS‐CoV‐2‐positive patients |
| Oshman 2020 | Ineligible outcomes |
| Ozcan 2021 | No data |
| Paar 2021 | Ineligible population |
| Pigott 2020 | Conference abstract |
| Platten 2021 | No clinical suspicion at inclusion |
| Popovych 2021 | Ineligible population |
| Pullen 2020 | Ineligible design |
| Quer 2020 | Ineligible design |
| Ravani 2020 | No clinical suspicion at inclusion |
| Rentsch 2020 | Preprint |
| Ronan 2021 | No data |
| Rubel 2020 | Index test not assessed on admission |
| Sabetian 2021 | Only SARS‐CoV‐2‐positive patients |
| Sabetta 2020 | Ineligible outcomes |
| Sehanobish 2021 | Index test not assessed on admission |
| Senok 2020 | Ineligible design |
| Shanbehzadeh 2021 | Ineligible design |
| Shayganmehr 2021 | Ineligible population |
| Sheen 2020 | No data |
| Shoer 2021 | No clinical suspicion at inclusion |
| Sieber 2021 | Ineligible population |
| Song 2020 | Preprint |
| Sorlini 2020 | Ineligible design |
| Spangler 2021 | Ineligible outcomes |
| Stacevičienė 2021 | Ineligible design |
| Tabacof 2020 | Only SARS‐CoV‐2‐positive patients |
| Taziki 2020 | Index test not assessed on admission |
| Ticinesi 2020 | Conference abstract |
| Trevisan 2021 | Ineligible design |
| Verma 2020 | Index test not assessed on admission |
| Viana dos Santos 2021 | Ineligible design |
| Visconti 2021 | Ineligible design |
| Vos 2020 | Ineligible design |
| Weiss 2021a | Ineligible design |
| Weiss 2021b | Ineligible population |
| Wells 2020 | No clinical suspicion at inclusion |
| Wu 2020 | Only SARS‐CoV‐2‐positive patients |
| Xia 2021 | Ineligible population |
| Yan 2020 | Ineligible design |
| Yang 2020 | Preprint |
| Yousef 2021 | Ineligible design |
| Žaja 2021 | Only SARS‐CoV‐2‐positive patients |
| Zavascki 2020 | Preprint |
| Zayet 2020b | Ineligible design |
| Zhao 2020 | Ineligible design |
| Zhao 2021 | Ineligible design |
Differences between protocol and review
As the evidence base evolves over the course of the pandemic, we have made some adjustments to our original approach with the following changes between earlier versions of the review and this second update:
Clarification regarding inclusion criteria: suspicion of infection was interpreted as: clinical suspicion of SARS‐CoV‐2 infection based on a symptomatic presentation. At least 50% of the study population had to present with COVID‐19 compatible symptoms.
Both studies using a retrospective and a prospective data collection were included, but the main findings of this review were based on the prospective studies only, as retrospective studies tend to overestimate the diagnostic accuracy of the index tests.
Preprints that were included in previous versions of the review were now excluded (unless they were published in the meantime), and they were no longer included from this update onwards. Consequently, this second review update does not contain any preprints. Records from preprint archives were no longer evaluated for eligible studies because of an increase in COVID‐19 study references from recommended diagnostic test accuracy sources (MEDLINE and Embase). For the baseline review, preprint archives were essential to identify emerging evidence; but for the second update, it was no longer necessary to search these sources.
Search sources included in the protocol and the previous version of this review, the Cochrane COVID‐19 Study Register and the CDC Database of COVID‐19 Research Articles, were not included in this version as the single source from the University of Bern living search database proved more efficient to process as it did not involve manual effort to deduplicate.
We did not set out to identify any ongoing studies for this 2022 review version since the review will no longer be updated in its current form (see Objectives).
Contributions of authors
JD, JDi, YT, CD, ML, RS, LH, AVdB, and DE, contributed clinical, methodological and/or technical expertise to drafting the protocol. JD co‐ordinated contributions from all co‐authors and drafted the protocol. ML drafted the QUADAS‐2 criteria. AVdB oversaw the overall progress of this review, participated in the selection process, data extraction, quality appraisal and drafting of the manuscript. TS co‐ordinated the review process, analysed the data, drafted the manuscript and participated in the selection, data extraction and quality appraisal. JDo, DW, AT, VL, SJ and SH participated in the data extraction, quality appraisal, interpretation of the findings and commented on the manuscript.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK
University of Birmingham, UK
External sources
-
Foreign, Commonwealth and Development Office, UK
Project number 300342‐104
National Institute for Health Research (NIHR), UK
NIHR Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham, UK
Declarations of interest
Thomas Struyf: none known
Jonathan J Deeks: no relevant interests; published eight podcasts, including Talk Evidence (BMJ), More‐or‐Less (Radio 4), Inside Science (Radio 4), The Newscast (Radio 4). Five opinion pieces in Guardian, unHerd and the BMJ. Numerous television, radio and mainstream media interviews giving substantial coverage of scientific issues related to test evaluation for COVID‐19. Presented evidence to the House of Lords Select Committee, and the All Parliamentary Party Investigation on COVID‐19. Two invited editorials on COVID‐19 for the BMJ; Editor, Cochrane Diagnostic Test Accuracy Review editorial team
Jacqueline Dinnes: no relevant interests; Editor, Cochrane Diagnostic Test Accuracy Review editorial team
Yemisi Takwoingi: no relevant interests; Editor, Cochrane Infectious Diseases; Statistical Editor, Cochrane Bone, Joint and Muscle Trauma; Editor, Cochrane Diagnostic Test Accuracy Review editorial team
Clare Davenport: no relevant interests; Contact Editor for Cochrane Diagnostic Test Accuracy Review editorial team and was not involved in the editorial process for this review
Mariska MG Leeflang: no relevant interests; team member, Cochrane Diagnostic Test Accuracy Review editorial team
René Spijker: none known
Lotty Hooft: no relevant interests; editorial roles with the Cochrane Diagnostic Test Accuracy Review editorial team and Prognosis Methods Group implementation team
Devy Emperador: no relevant interests; employed by FIND with funding from DFID and KFW. FIND is a global non‐for profit product development partnership and World Health Organization Diagnostic Collaboration Centre. It is FIND’s role to accelerate access to high‐quality diagnostic tools for low‐resource settings and this is achieved by supporting both research and development, and access activities for a wide range of diseases, including COVID‐19. FIND has several clinical research projects to evaluate multiple new diagnostic tests against published Target Product Profiles that have been defined through consensus processes. These studies are for diagnostic products developed by private sector companies who provide access to know‐how, equipment/reagents, and contribute through unrestricted donations as per FIND policy and external SAC review
Julie Domen: no relevant interests; works as a general practitioner
Anouk Tans: none known
Stéphanie Janssens: no relevant interests; works as a general practitioner in training in 'De Wijkpraktijk' in Antwerp
Dakshitha Wickramasinghe: none known
Viktor Lannoy: none known
Sebastiaan Horn: no relevant interests; works as a resident general practitioner: Praktijkhuis Baarle, University of Antwerp, Antwerp, Belgium
Ann Van den Bruel: none known
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Ahmed 2021 {published data only}
- Ahmed SM, Shah RU, Fernandez V, Grineski S, Brintz B, Samore MH, et al.Robust testing in outpatient settings to explore COVID-19 epidemiology: disparities in race/ethnicity and age, Salt Lake County, Utah, 2020. Public Health Reports 2021;136(3):345-53. [DOI: 10.1177/0033354920988612] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aldobyany 2020 {published data only}
- Aldobyany A, Touman A, Ghaleb N, Alsaggaf R, Murtaza N, Hamada A, et al.Correlation between the COVID-19 Respiratory Triage Score and SARS-COV-2 PCR test. Frontiers in Medicine 2020;7(903):605689. [DOI: 10.3389/fmed.2020.605689] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alizadehsani 2021 {published data only}
- Alizadehsani R, Alizadeh SZ, Behjati M, Roshanzamir Z, Hussain S, Abedini N, et al.Risk factors prediction, clinical outcomes, and mortality in COVID-19 patients. Journal of Medical Virology 2021;93(4):2307-20. [DOI: 10.1002/jmv.26699] [DOI] [PMC free article] [PubMed] [Google Scholar]
Allegorico 2020 {published data only}
- Allegorico E, Buonerba C, Bosso G, Pagano A, Porta G, Serra C, et al.The use of chest ultrasonography in suspected cases of COVID-19 in the emergency department. Future Science OA 2020;7(1):Fso635. [DOI: 10.2144/fsoa-2020-0127] [DOI] [PMC free article] [PubMed] [Google Scholar]
Arenas 2020 {published data only}
- Arenas MD, Crespo M, Perez-Saez MJ, Collado S, Redondo-Pachón D, Llinàs-Mallol L, et al.Clinical profiles in renal patients with COVID-19. Journal of Clinical Medicine 2020;9(8):2665. [DOI: 10.3390/jcm9082665] [DOI] [PMC free article] [PubMed]
Arslan 2021 {published data only}
- Arslan G, Aktürk H, Duman M.Clinical characteristics of pediatric COVID-19 and predictors of PCR positivity. Pediatrics International 2021;63(9):1055-1061. [DOI: 10.1111/ped.14602] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barbhaya 2021 {published data only}
- Barbhaya D, Franco S, Gandhi K, Arya R, Neupane R, Foroughi N, et al.Characteristics and outcomes of COVID-19 infection from an urban ambulatory COVID-19 clinic-guidance for outpatient clinicians in triaging patients. Journal of Primary Care & Community Health 2021;12:21501327211017016. [DOI: 10.1177/21501327211017016] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhattacharya 2021 {published data only}
- Bhattacharya A, Ranjan P, Kumar A, Brijwal M, Pandey R M, Mahishi N, et al.Development and validation of a clinical symptom-based scoring system for diagnostic evaluation of COVID-19 patients presenting to outpatient department in a pandemic situation. Cureus 2021;13(3):e13681. [DOI: 10.7759/cureus.13681] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bouzid 2020 {published data only}
- Bouzid D, Mullaert J, Le Hingrat Q, Laurent O, Duval X, Lescure X, et al.Characteristics associated with COVID-19 or other respiratory viruses' infections at a single-center emergency department. PLoS One 2020;15(12):e0243261. [DOI: 10.1371/journal.pone.0243261] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brendish 2020 {published data only}
- Brendish NJ, Poole S, Naidu VV, Mansbridge CT, Norton N, Borca F, et al.Clinical characteristics, symptoms and outcomes of 1054 adults presenting to hospital with suspected COVID-19: a comparison of patients with and without SARS-CoV-2 infection. Journal of Infection 2020;81(6):937-43. [DOI: 10.1016/j.jinf.2020.09.033] [DOI] [PMC free article] [PubMed] [Google Scholar]
Buonafine 2020 {published data only}
- Buonafine CP, Paiatto BN, Leal FB, Matos SF, Morais CO, Guerra GG, et al.High prevalence of SARS-CoV-2 infection among symptomatic healthcare workers in a large university tertiary hospital in São Paulo, Brazil. BMC Infectious Diseases 2020;20(1):917. [DOI: 10.1186/s12879-020-05662-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2021 {published data only}
- Chan J, Burke K, Bedard R, Grigg J, Winters J, Vessell C, et al.COVID-19 in the New York City jail system: epidemiology and health care response, March-April 2020. Public Health Reports 2021;136(3):375-83. [DOI: 10.1177/0033354921999385] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheng 2020 {published data only}
- Cheng Z, Lu Y, Cao Q, Qin L, Pan Z, Yan F, et al.Clinical features and chest CT manifestations of coronavirus disease 2019 (COVID-19) in a single-center study in Shanghai, China. American Journal of Roentgenology 2020;215(1):121-6. [DOI: 10.2214/AJR.20.22959] [DOI] [PubMed] [Google Scholar]
Chew 2021 {published data only}
- Chew WM, Loh CH, Jalali A, Fong GS, Senthil Kumar L, Sim RH, et al.A risk prediction score to identify patients at low risk for COVID-19 infection. Singapore Medical Journal 2021 Mar 12 [Epub ahead of print]. [DOI: 10.11622/smedj.2021019] [DOI] [PMC free article] [PubMed]
Chua 2020 {published data only}
- Chua AJ, Chan EC, Loh J, Choong Charn T.Acute olfactory loss is specific for COVID-19 at the emergency department. Annals of Emergency Medicine 2020;76(4):550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chung 2021 {published data only}
- Chung JR, Kim SS, Jackson ML, Jackson LA, Belongia EA, King JP, et al.Clinical symptoms among ambulatory patients tested for SARS-CoV-2. Open Forum Infectious Diseases 2021;8(1):ofaa576. [DOI: 10.1093/ofid/ofaa576] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clemency 2020 {published data only}
- Clemency BM, Varughese R, Scheafer DK, Ludwig B, Welch JV, McCormack RF, et al.Symptom criteria for COVID-19 testing of health care workers. Academic Emergency Medicine 2020;27(6):469-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clifford 2020 {published data only}
- Clifford CT, Pour TR, Freeman R, Reich DL, Glicksberg BS, Levin MA, et al.Association between COVID-19 diagnosis and presenting chief complaint from New York City triage data. American Journal of Emergency Medicine 2020;46:520-4. [DOI: 10.1016/j.ajem.2020.11.006] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cunarro‐Lopez 2020 {published data only}
- Cuñarro-López Y, Cano-Valderrama Ó, Pintado-Recarte P, Cueto-Hernández I, González-Garzón B, García-Tizón S, et al.Maternal and perinatal outcomes in patients with suspected COVID-19 and their relationship with a negative RT-PCR result. Journal of Clinical Medicine 2020;9(11):3552. [DOI: 10.3390/jcm9113552] [DOI] [PMC free article] [PubMed] [Google Scholar]
Drager 2020 {published data only}
- Drager S, Kather A, Fricke M, With K.Corona outpatient clinic at Dresden university hospital: clinical characteristics of SARS-CoV-2 tested patients. Zeitschrift fur Allgemeinmedizin 2020;96(9):343-7. [DOI: ] [DOI] [Google Scholar]
Feng 2021 {published data only}
- Feng C, Wang L, Chen X, Zhai Y, Zhu F, Chen H, et al.A novel artificial intelligence-assisted triage tool to aid in the diagnosis of suspected COVID-19 pneumonia cases in fever clinics. Annals of Translational Medicine 2021;9(3):201. [DOI: 10.21037/atm-20-3073] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fiel‐Ozores 2021 {published data only}
- Fiel-Ozores A, González-Durán ML, Novoa-Carballal R, Portugués-de la Red MD, Fernández-Pinilla I, Cabrera-Alvargonzález JJ, et al.Differential clinic in children infected by SARS-CoV-2, traceability of contacts and cost-effectiveness of diagnostic tests: cross-sectional observational study [Clínica diferencial en niños infectados por SARS-CoV-2, trazabilidad de contactos y rentabilidad de pruebas diagnósticas: estudio observacional transversal]. Anales de Pediatría (English Edition) 2021;94(5):318-26. [DOI: 10.1016/j.anpedi.2020.12.001] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fink 2021 {published data only}
- Fink N, Rueckel J, Kaestle S, Schwarze V, Gresser E, Hoppe B, et al.Evaluation of patients with respiratory infections during the first pandemic wave in Germany: characteristics of COVID-19 versus non-COVID-19 patients. BMC Infectious Diseases 2021;21(1):167. [DOI: 10.1186/s12879-021-05829-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gilbert 2020 {published data only}
- Gilbert A, Brasseur E, Petit M, Donneau AF, Diep AN, Hetzel Campbell S, et al.Immersion in an emergency department triage center during the COVID-19 outbreak: first report of the Liège University hospital experience. Acta Clinica Belgica 2020 Jun 12 [Epub ahead of print]. [DOI: 10.1080/17843286.2020.1778348] [DOI] [PubMed] [Google Scholar]
Haehner 2020 {published data only}
- Haehner A, Draf J, Draeger S, With K, Hummel T.Predictive value of sudden olfactory loss in the diagnosis of COVID-19. Karger 2020;82(4):175-80. [DOI: 10.1159/000509143] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haliga 2021 {published data only}
- Haliga RE, Sorodoc V, Lionte C, Petris OR, Bologa C, Coman AE, et al.Acute clinical syndromes and suspicion of SARS-CoV-2 infection: the experience of a single Romanian center in the early pandemic period. Medicina (Kaunas) 2021;57(2):121. [DOI: 10.3390/medicina57020121] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2020 {published data only}
- Huang D, Wang T, Chen Z, Yang H, Yao R, Liang Z.A novel risk score to predict diagnosis with coronavirus disease 2019 (COVID-19) in suspected patients: a retrospective, multicenter, and observational study. Journal of Medical Virology 2020;92(11):2709-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hüfner 2020 {published data only}
- Hüfner A, Kiefl D, Baacke M, Zöllner R, Loza Mencía, Schellein O, et al.Risk stratification through implementation and evaluation of a COVID-19 score: a retrospective diagnostic study [Risikostratifizierung durch Implementierung und Evaluation eines COVID-19-Scores]. Medizinische Klinik - Intensivmedizin und Notfallmedizin 2020;115 Suppl 3:132-8. [DOI: 10.1007/s00063-020-00754-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ide 2021 {published data only}
- Ide S, Hayakawa K, Yamamoto K, Tsuzuki S, Tanuma J, Ohara K, et al.Positive ratio of polymerase chain reaction (PCR) and validity of pre-screening criteria at an outpatient screening center during the early phase of the COVID-19 epidemic in Japan. Japanese Journal of Infectious Diseases 2021;74(5):481-6. [DOI: 10.7883/yoken.JJID.2020.813] [DOI] [PubMed] [Google Scholar]
Ishii 2021 {published data only}
- Ishii T, Kushimoto S, Katori Y, Kure S, Igarashi K, Fujita M, et al.Predictors of SARS-CoV-2 positivity based on RT-PCR swab tests at a drive-through outpatient clinic for COVID-19 screening in Japan. Tohoku Journal of Experimental Medicine 2021;253(2):101-8. [DOI: 10.1620/tjem.253.101] [DOI] [PubMed] [Google Scholar]
Jeyashree 2021 {published data only}
- Jeyashree K, Raju M, Ponnaiah M, Muthappan S, Rozario AG, Raichel R, et al.Self-reported and clinically identified loss of smell and taste among persons tested for COVID-19 in Chennai, southern India, July-August 2020: a cross sectional study. Clinical Epidemiology and Global Health 2021;11:100718. [DOI: 10.1016/j.cegh.2021.100718] [DOI] [PMC free article] [PubMed] [Google Scholar]
Just 2020 {published data only}
- Just J, Puth M-T, Regenold F, Weckbecker K, Bleckwenn M.Risk factors for a positive SARS-CoV-2 PCR in patients with common cold symptoms in a primary care setting - a retrospective analysis based on a joint documentation standard. BMC Family Practice 2020;21(1):251. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kalayjian 2020 {published data only}
- Kalayjian BC, Conner K, Butler I, Myers L, Telleria C, Panchang D, et al.Race, heart rate, and temperature are strongly associated with COVID-19 at a community-based clinic in New Orleans. Mayo Clinic Proceedings: Innovations, Quality & Outcomes 2020;4(6):683-6. [DOI: ] [DOI] [PMC free article] [PubMed]
Kelen 2021 {published data only}
- Kelen GD, Swedien D, Hansen J, Klein E, Peterson S, Saheed M, et al.Characterization and impact of COVID-19-tested and infected patients: experience of The Johns Hopkins Health System regional emergency departments. Journal of the American College of Emergency Physicians Open 2021;2(1):e12321. [DOI: 10.1002/emp2.12321] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kempker 2020 {published data only}
- Kempker RR, Kempker JA, Peters M, Rebolledo PA, Carroll K, Toomer L, et al.Loss of smell and taste among healthcare personnel screened for coronavirus 2019. Clinical Infectious Diseases 2021;72(7):1244-6. [DOI: DOI: 10.1093/cid/ciaa877] [DOI] [PMC free article] [PubMed]
Kim 2020 {published data only}
- Kim C, Yeo IH, Kim JK, Cho Y, Lee MJ, Jung H, et al.Confirmation of COVID-19 in out-of-hospital cardiac arrest patients and postmortem management in the emergency department during the COVID-19 outbreak. Infection & Chemotherapy 2020;52(4):562-72. [DOI: 10.3947/ic.2020.52.4.562] [DOI] [PMC free article] [PubMed] [Google Scholar]
King 2020 {published data only}
- King JA, Whitten TA, Bakal JA, McAlister FA.Symptoms associated with a positive result for a swab for SARS-CoV-2 infection among children in Alberta. CMAJ 2020;193(1):E1-9. [DOI: 10.1503/cmaj.202065] [DOI] [PMC free article] [PubMed] [Google Scholar]
Krastinova 2020 {published data only}
- Krastinova E, Garrait V, Lecam MT, Coste A, Varon E, Delacroix I, et al.Household transmission and incidence of positive SARS-CoV-2 RT-PCR in symptomatic healthcare workers, clinical course and outcome: a French hospital experience. Occupational and Environmental Medicine 2020;78(7):479-85. [DOI: 10.1136/oemed-2020-106866] [DOI] [PMC free article] [PubMed] [Google Scholar]
Langer 2020 {published data only}
- Langer T, Favarato M, Giudici R, Bassi G, Garberi R, Villa F, et al.Development of machine learning models to predict RT-PCR results for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in patients with influenza-like symptoms using only basic clinical data. Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine 2020;28(1):113. [DOI: 10.1186/s13049-020-00808-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lazzerini 2021 {published data only}
- Lazzerini M, Sforzi I, Trapani S, Biban P, Silvagni D, Villa G, et al.Characteristics and risk factors for SARS-CoV-2 in children tested in the early phase of the pandemic: a cross-sectional study, Italy, 23 February to 24 May 2020. Eurosurveillance 2021;26(14):2001248. [DOI: 10.2807/1560-7917.ES.2021.26.14.2001248] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leal 2020 {published data only}
- Leal FE, Mendes-Correa MC, Buss LF, Costa SF, Bizario JC, Souza SR, et al.Clinical features and natural history of the first 2073 suspected COVID-19 cases in the Corona São Caetano primary care programme: a prospective cohort study. BMJ Open 2021;11(1):e042745. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leung 2021 {published data only}
- Leung WL, Yu EL, Wong SC, Leung M, Lee LL, Chung KL, et al.Findings from the first public COVID-19 temporary test centre in Hong Kong. Hong Kong Medical Journal 2021;27(2):99-105. [DOI: 10.12809/hkmj208909] [DOI] [PubMed] [Google Scholar]
Maechler 2020 {published data only}
- Maechler F, Gertler M, Hermes J, Van Loon W, Schwab F, Piening B, et al.Epidemiological and clinical characteristics of SARS-CoV-2 infections at a testing site in Berlin, Germany, March and April 2020-a cross-sectional study. Clinical Microbiology and Infection 2020;26(12):1685.e7-12. [DOI: 10.1016/j.cmi.2020.08.017] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mansella 2020 {published data only}
- Mansella G, Rueegg M, Widmer AF, Tschudin-Sutter S, Battegay M, Hoff J, et al.COVID-19 triage and test center: safety, feasibility, and outcomes of low-threshold testing. Journal of Clinical Medicine 2020;9(10):3217. [DOI: 10.3390/jcm9103217] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mao 2020 {published data only}
- Mao B, Liu Y, Chai YH, Jin XY, Lu HW, Yang JW, et al.Assessing risk factors for SARS-CoV-2 infection in patients presenting with symptoms in Shanghai, China: a multicentre, observational cohort study. Lancet Digital Health 2020;2(6):e323-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martín‐Sánchez 2020 {published data only}
- Martín-Sánchez FJ, González Del Castillo J, Valls Carbó A, López Picado A, Martínez-Valero C, Miranda JD, et al.Diagnostic groups and short-term outcomes in suspected COVID-19 cases treated in an emergency department [Categorías diagnósticas y resultados a corto plazo en los pacientes con sospecha de COVID-19 atendidos en un servicio de urgencias]. Emergencias 2020;32(4):242-52. [1137-6821] [PubMed] [Google Scholar]
Martin‐Sanz 2020 {published data only}
- Martin-Sanz E, Riestra J, Yebra L, Larran A, Mancino F, Yanes-Diaz J, et al.Prospective study in 355 patients with suspected COVID-19 infection: value of cough, subjective hyposmia, and hypogeusia. Laryngoscope 2020;130(11):2674-9. [DOI: 10.1002/lary.28999] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nazerian 2021 {published data only}
- Nazerian P, Morello F, Prota A, Betti L, Lupia E, Apruzzese L, et al.Diagnostic accuracy of physician's gestalt in suspected COVID-19. Prospective bicentric study. Academic Emergency Medicine 2021;28(4):404-11. [DOI: 10.1111/acem.14232] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nitecki 2021 {published data only}
- Nitecki M, Taran B, Ketko I, Geva G, Yosef R, Toledo I, et al.Self-reported symptoms in healthy young adults to predict potential COVID-19 disease. Clinical Microbiology and Infection 2021;27(4):618-23. [DOI: 10.1016/j.cmi.2020.12.028] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Reilly 2020a {published data only}
- O'Reilly GM, Mitchell RD, Rajiv P, Wu J, Brennecke H, Brichko L, et al.Epidemiology and clinical features of emergency department patients with suspected COVID-19: initial results from the COVID-19 Emergency Department Quality Improvement Project (COVED-1). Emergency Medicine Australasia 2020;32(4):638-45. [DOI] [PubMed] [Google Scholar]
O'Reilly 2020b {published data only}
- O'Reilly GM, Mitchell RD, Mitra B, Akhlaghi H, Tran V, Furyk JS, et al.Epidemiology and clinical features of emergency department patients with suspected and confirmed COVID-19: a multisite report from the COVID-19 Emergency Department Quality Improvement Project for July 2020 (COVED-3). Emergency Medicine Australasia 2020;33(1):114-24. [DOI: 10.1111/1742-6723.13651] [DOI] [PMC free article] [PubMed] [Google Scholar]
Olivar Lopez 2020 {published data only}
- Olivar-Lopez V, Leyva-Barrera A, Lopez-Martinez B, Parra-Ortega I, Marquez-Gonzalez H.Clinical risk profile associated with SARS-CoV-2 infection and complications in the emergency area of a pediatric COVID-19 center. Boletín Médico del Hospital Infantil de México 2020;77(5):221-7. [DOI: 10.24875/BMHIM.20000198] [DOI] [PubMed] [Google Scholar]
Peng 2020 {published data only}
- Peng L, Liu KY, Xue F, Miao YF, Tu PA, Zhou C.Improved early recognition of coronavirus disease-2019 (COVID-19): single-center data from a Shanghai screening hospital. Archives of Iranian Medicine 2020;23(4):272-6. [DOI] [PubMed] [Google Scholar]
Peyrony 2020 {published data only}
- Peyrony O, Marbeuf-Gueye C, Truong V, Giroud M, Rivière C, Khenissi K, et al.Accuracy of emergency department clinical findings for diagnosis of coronavirus disease 2019. Annals of Emergency Medicine 2020;76(4):405-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pisapia 2020 {published data only}
- Pisapia R, Pisaturo M, Fusco FM, Parrella G, Iodice V, Tambaro O, et al.Differences among confirmed and not-confirmed COVID-19 patients at "D.Cotugno" hospital, Naples (Italy): what we learned from first suspected cases? Infezioni in Medicina 2020;28 Suppl 1:84-8. [PubMed] [Google Scholar]
Pivetta 2020 {published data only}
- Pivetta E, Goffi A, Tizzani M, Locatelli SM, Porrino G, Losano I, et al.Lung ultrasonography for the diagnosis of SARS-CoV-2 pneumonia in the emergency department. Annals of Emergency Medicine 2020;77(4):385-94. [DOI: 10.1016/j.annemergmed.2020.10.008] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pokorska‐Śpiewak 2021 {published data only}
- Pokorska-Śpiewak M, Talarek E, Popielska J, Nowicka K, Ołdakowska A, Zawadka K, et al.Comparison of clinical severity and epidemiological spectrum between coronavirus disease 2019 and influenza in children. Scientific Reports 2021;11(1):5760. [DOI: 10.1038/s41598-021-85340-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Porto 2021 {published data only}
- Porto LC, Costa CH, Nunes AS, Bouzas I, Ferreira TF, Porto VM, et al.Clinical and laboratory characteristics in outpatient diagnosis of COVID-19 in healthcare professionals in Rio de Janeiro, Brazil. Journal of Clinical Pathology 2021 Feb 10 [Epub ahead of print]. [DOI: 10.1136/jclinpath-2020-206797] [DOI] [PubMed] [Google Scholar]
Raberahona 2020 {published data only}
- Raberahona M, Rakotomalala R, Rakotomijoro E, Rahaingoalidera T, Andry CE, Mamilaza N, et al.Clinical and epidemiological features discriminating confirmed COVID-19 patients from SARS-CoV-2 negative patients at screening centres in Madagascar. International Journal of Infectious Diseases 2020;103:6-8. [DOI: 10.1016/j.ijid.2020.11.151] [DOI] [PMC free article] [PubMed] [Google Scholar]
Romero‐Gameros 2020 {published data only}
- Romero-Gameros CA, Waizel-Haiat S, Mendoza-Zubieta V, Anaya-Dyck A, López-Moreno MA, Colin-Martinez T, et al.Evaluation of predictive value of olfactory dysfunction, as a screening tool for COVID-19. Laryngoscope Investigative Otolaryngology 2020;5(6):983-91. [DOI: 10.1002/lio2.482] [DOI] [PMC free article] [PubMed] [Google Scholar]
Romero‐Gameros 2021 {published data only}
- Romero-Gameros CA, Colin-Martínez T, Waizel-Haiat S, Vargas-Ortega G, Ferat-Osorio E, Guerrero-Paz JA, et al.Diagnostic accuracy of symptoms as a diagnostic tool for SARS-CoV 2 infection: a cross-sectional study in a cohort of 2,173 patients. BMC Infectious Diseases 2021;21(1):255. [DOI: 10.1186/s12879-021-05930-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rutten 2020a {published data only}
- Rutten JS, Van Loon AM, Joling KJ, Smalbrugge M, Van Buul LW, Hertogh CM.COVID-19 in verpleeghuizen. Een studie naar diagnostiek, ziektepresentatie en ziektebeloop. Nederlands Tijdschrift voor Geneeskunde 2020;164:D5173. [ntvg.nl/D5173] [PubMed] [Google Scholar]
Rutten 2020b {published data only}
- Rutten JJS, Van Loon AM, Van Kooten J, Van Buul LW, Joling KJ, Smalbrugge M, et al.Clinical suspicion of COVID-19 in nursing home residents: symptoms and mortality risk factors. Journal of the American Medical Directors Association 2020;21(12):1791-7.e1. [DOI: 10.1016/j.jamda.2020.10.034] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sacks 2020 {published data only}
- Sacks CA, Dougan M, McCoy TH Jr, Zheng A, Buonomo G, North CM, et al.The association between symptoms and COVID-19 test results among healthcare workers. Annals of Surgery 2020;272(6):e329-32. [DOI: 10.1097/SLA.0000000000004483] [DOI] [PMC free article] [PubMed]
Saegerman 2021 {published data only}
- Saegerman C, Gilbert A, Donneau AF, Gangolf M, Diep AN, Meex C, et al.Clinical decision support tool for diagnosis of COVID-19 in hospitals. PLoS One 2021;16(3):e0247773. [DOI: 10.1371/journal.pone.0247773] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salmon Ceron 2020 {published data only}
- Salmon Ceron D, Bartier S, Hautefort C, Nguyen Y, Nevoux J, Hamel AL, et al.Self-reported loss of smell without nasal obstruction to identify COVID-19. The multicenter Coranosmia cohort study. Journal of Infection 2020;81(4):614-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shah 2020 {published data only}
- Shah SJ, Barish PN, Prasad PA, Kistler AL, Neff N, Kamm J, et al.Clinical features, diagnostics, and outcomes of patients presenting with acute respiratory illness: a retrospective cohort study of patients with and without COVID-19. eClinicalMedicine 2020;27:100518. [DOI: 10.1016/j.eclinm.2020.100518] [DOI] [PMC free article] [PubMed] [Google Scholar]
Simpson 2020 {published data only}
- Simpson JN, Goyal MK, Cohen JS, Badolato GM, McGuire M, Ralph A, et al.Results of testing children for SARS-CoV-2 through a community-based testing site. Journal of Pediatrics 2020;231:157-61.e1. [DOI: 10.1016/j.jpeds.2020.12.030] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sonoda 2021 {published data only}
- Sonoda S, Kuramochi J, Matsuyama Y, Miyazaki Y, Fujiwara T.Validity of clinical symptoms score to discriminate patients with COVID-19 from common cold out-patients in general practitioner clinics in Japan. Journal of Clinical Medicine 2021;10(4):854. [DOI: 10.3390/jcm10040854] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sun 2020 {published data only}
- Sun Y, Koh V, Marimuthu K, Ng OT, Young B, Vasoo S, et al.Epidemiological and clinical predictors of COVID-19. Clinical Infectious Diseases 2020;71(15):786-92. [DOI: 10.1093/cid/ciaa322] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tan 2021 {published data only}
- Tan JY, Sim XY, Wee LE, Chua YY, Cherng BP, Ng IM, et al.A comparative study on the clinical features of COVID-19 with non-SARS-CoV-2 respiratory viral infections. Journal of Medical Virology 2021;93(3):1548–55. [DOI: ] [DOI] [PubMed] [Google Scholar]
Tolia 2020 {published data only}
- Tolia VM, Chan TC, Castillo EM.Preliminary results of initial testing for coronavirus (COVID-19) in the emergency department. Western Journal of Emergency Medicine 2020;21(3):503-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tordjman 2020 {published data only}
- Tordjman M, Mekki A, Mali RD, Saab I, Chassagnon G, Guillo E, et al.Pre-test probability for SARS-CoV-2-related infection score: the PARIS score. PLoS ONE 2020;15(12):e0243342. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Trubiano 2020 {published data only}
- Trubiano JA, Vogrin S, Smibert OC, Marhoon N, Alexander AA, Chua KY, et al.COVID-MATCH65 - a prospectively derived clinical decision rule for severe acute respiratory syndrome coronavirus 2. PLoS One 2020;15(12):e0243414. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tudrej 2020 {published data only}
- Tudrej B, Sebo P, Lourdaux J, Cuzin C, Floquet M, Haller DM, et al.Self-reported loss of smell and taste in SARS-CoV-2 patients: primary care data to guide future early detection strategies. Journal of General Internal Medicine 2020;35(8):2502-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Loon 2021 {published data only}
- Van Loon N, Verbrugghe M, Cartuyvels R, Ramaekers D.Diagnosis of COVID-19 based on symptomatic analysis of hospital healthcare workers in Belgium: observational study in a large Belgian tertiary care center during early COVID-19 outbreak. Journal of Occupational and Environmental Medicine 2021;63(1):27-31. [DOI: 10.1097/JOM.0000000000002015] [DOI] [PMC free article] [PubMed]
Van Walraven 2021 {published data only}
- Van Walraven C, Manuel DG, Desjardins M, Forster AJ.Derivation and internal validation of a model to predict the probability of severe acute respiratory syndrome coronavirus-2 infection in community people. Journal of General Internal Medicine 202;36(1):162-9. [DOI: 10.1007/s11606-020-06307-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vieceli 2020 {published data only}
- Vieceli T, Oliveira Filho CM, Berger M, Saadi MP, Salvador PA, Anizelli LB, et al.A predictive score for COVID-19 diagnosis using clinical, laboratory and chest image data. Brazilian Journal of Infectious Diseases 2020;24(4):343-8. [DOI: 10.1016/j.bjid.2020.06.009] [DOI] [PMC free article] [PubMed]
Vilke 2020 {published data only}
- Vilke GM, Brennan JJ, Cronin AO, Castillo EM.Clinical features of patients with COVID-19: is temperature screening useful? Journal of Emergency Medicine 2020;59(6):952-6. [DOI: 10.1016/j.jemermed.2020.09.048] [DOI] [PMC free article] [PubMed] [Google Scholar]
Villerabel 2021 {published data only}
- Villerabel C, Makinson A, Jaussent A, Picot MC, Nègre-Pagès L, Rouvière JA, et al.Diagnostic value of patient-reported and clinically tested olfactory dysfunction in a population screened for COVID-19. JAMA Otolaryngology–Head & Neck Surgery 2021;147(3):271-9. [DOI: 10.1001/jamaoto.2020.5074] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wee 2020 {published data only}
- Wee LE, Chan YZ, Teo NY, Cherng BZ, Thien SY, Wong M, et al.The role of self-reported olfactory and gustatory dysfunction as a screening criterion for suspected COVID-19. European Archives of Oto-Rhino-Laryngology 2020;277(8):2389-90. [DOI: 10.1007/s00405-020-05999-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wei 2020 {published data only}
- Wei Y, Lu Y, Xia L, Yuan X, Li G, Li X, et al.Analysis of 2019 novel coronavirus infection and clinical characteristics of outpatients: an epidemiological study from a fever clinic in Wuhan, China. Journal of Medical Virology 2020;92:2758-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wernhart 2020 {published data only}
- Wernhart S, Förster TH, Weihe E.Outpatient management of oligosymptomatic patients with respiratory infection in the era of SARS-CoV-2: experience from rural German general practitioners. BMC Infectious Diseases 2020;20(1):811. [DOI: 10.1186/s12879-020-05538-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Xie 2020 {published data only}
- Xie S, Zhang G, Yu H, Wang J, Wang S, Tang G, et al.The epidemiologic and clinical features of suspected and confirmed cases of imported 2019 novel coronavirus pneumonia in north Shanghai, China. Annals of Translational Medicine 2020;8(10):637. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yombi 2020 {published data only}
- Yombi JC, De Greef J, Marsin A-S, Simon A, Rodriguez-Villalobos H, Penaloza A, et al.Symptom-based screening for COVID-19 in health care workers: the importance of fever. Journal of Hospital Infection 2020;105(3):428-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yonker 2020 {published data only}
- Yonker LM, Neilan AM, Bartsch Y, Patel AB, Regan J, Arya P, et al.Pediatric severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): clinical presentation, infectivity, and immune responses. Journal of Pediatrics 2020;227:45–52.e5. [DOI: 10.1016/j.jpeds.2020.08.037] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zayet 2020a {published data only}
- Zayet S, Klopfenstein T, Mercier J, Kadiane-Oussou NJ, Lan Cheong Wah L, Royer PY, et al.Contribution of anosmia and dysgeusia for diagnostic of COVID-19 in outpatients. Infection 2020;14:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhu 2020 {published data only}
- Zhu W, Xie K, Lu H, Xu L, Zhou S, Fang S.Initial clinical features of suspected coronavirus disease 2019 in two emergency departments outside of Hubei, China. Journal of Medical Virology 2020;92(9):1525-32. [DOI: 10.1002/jmv.25763 ] [DOI] [PMC free article] [PubMed]
Zimmerman 2020 {published data only}
- Zimmerman RK, Nowalk MP, Bear T, Taber R, Clarke KS, Sax TM, et al.Proposed clinical indicators for efficient screening and testing for COVID-19 infection using Classification and Regression Trees (CART) analysis. Human Vaccines & Immunotherapeutics 2020;17(4):1109-12. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zurl 2021 {published data only}
- Zurl C, Eber E, Siegl A, Loeffler S, Stelzl E, Kessler HH, et al.Low rate of SARS-CoV-2 infections in symptomatic patients attending a pediatric emergency department. Frontiers in Pediatrics 2021;9:637167. [DOI: 10.3389/fped.2021.637167] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Accorsi 2020 {published data only}
- Accorsi TA, Amicis K, Brígido AR, Belfort DS, Habrum FC, Scarpanti FG, et al.Assessment of patients with acute respiratory symptoms during the COVID-19 pandemic by Telemedicine: clinical features and impact on referral. Einstein (Sao Paulo) 2020;18:eAO6106. [DOI: 10.31744/einstein_journal/2020AO6106] [DOI] [PMC free article] [PubMed] [Google Scholar]
Afshar 2020 {published data only}
- Afshar Y, Gaw SL, Flaherman VJ, Chambers BD, Krakow D, Berghella V, et al.Clinical presentation of coronavirus disease 2019 (COVID-19) in pregnant and recently pregnant people. Obstetrics and Gynecology 2020;136(6):1117-25. [DOI: 10.1097/AOG.0000000000004178] [DOI] [PMC free article] [PubMed] [Google Scholar]
Agarwal 2021 {published data only}
- Agarwal R, Gupta E, Dubey S, Padhi A, Khodare A, Kumar G, et al.Pooled nasopharyngeal swab collection in a single vial for the diagnosis of SARS CoV-2 infection: an effective cost saving method. Indian Journal of Medical Microbiology 2021;39(2):231-4. [DOI: 10.1016/j.ijmmb.2020.11.002] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ai 2020 {published data only}
- Ai JW, Zhang HC, Xu T, Wu J, Zhu M, Yu YQ, et al.Optimizing diagnostic strategy for novel coronavirus pneumonia, a multi-center study in Eastern China. medRxiv [Preprint] 2020. [DOI: ] [Google Scholar]
Akinbami 2021 {published data only}
- Akinbami LJ, Petersen LR, Sami S, Vuong N, Lukacs SL, Mackey L, et al.COVID-19 symptoms and SARS-CoV-2 antibody positivity in a large survey of first responders and healthcare personnel, May-July 2020. Clinical Infectious Diseases 2021;73(3):e822-5. [DOI: 10.1093/cid/ciab080] [DOI] [PMC free article] [PubMed] [Google Scholar]
Akyala 2020 {published data only}
- Akyala AI, Awayimbo JR, Elayo MI, Olugbade OT, Akabe EA, Akinyoade A.Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection among health care workers in Nasarawa State, Nigeria: implications for infection prevention and control measures. Pan African Medical Journal 2020;37 Suppl 1:21. [DOI: 10.11604/pamj.supp.2020.37.21.25767] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aleebrahim‐Dehkordi 2020 {published data only}
- Aleebrahim-Dehkordi E, Reyhanian A, Hasanpour-Dehkordi A.Clinical manifestation and the risk of exposure to SARS-CoV-2 (COVID-19). International Journal of Preventive Medicine 2020;11:86. [DOI: 10.4103/ijpvm.IJPVM_145_20] [DOI] [PMC free article] [PubMed] [Google Scholar]
Al‐Rifai 2021 {published data only}
- Al-Rifai RH, Acuna J, Al Hossany FI, Aden B, Al Memari SA, Al Mazrouei SK, et al.Epidemiological characterization of symptomatic and asymptomatic COVID-19 cases and positivity in subsequent RT-PCR tests in the United Arab Emirates. PLoS One 2021;16(2):e0246903. [DOI: 10.1371/journal.pone.0246903] [DOI] [PMC free article] [PubMed] [Google Scholar]
Altınbilek 2020 {published data only}
- Altınbilek E, Öztürk D, Atasoy C, Özlem M, Yılmaz F, Kavalci C.Analysis of the patients who admitted to a Turkish emergency department during COVID-19 pandemic. Acta Biomedica 2020;91(4):e2020201. [DOI: 10.23750/abm.v91i4.10227] [DOI] [PMC free article] [PubMed] [Google Scholar]
Andina‐Martinez 2021 {published data only}
- Andina-Martinez D, Nieto-Moro M, Alonso-Cadenas JA, Añon-Hidalgo J, Hernandez-Martin A, Perez-Suarez E, et al.Mucocutaneous manifestations in children hospitalized with COVID-19. Journal of the American Academy of Dermatology 2021;85(1):88-94. [DOI: 10.1016/j.jaad.2021.03.083] [DOI] [PMC free article] [PubMed] [Google Scholar]
Antonelli 2021 {published data only}
- Antonelli M, Capdevila J, Chaudhari A, Granerod J, Canas LS, Graham MS, et al.Optimal symptom combinations to aid COVID-19 case identification: analysis from a community-based, prospective, observational cohort. Journal of Infection 2021;82(3):384-90. [DOI: 10.1016/j.jinf.2021.02.015] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aubert 2021 {published data only}
- Aubert J, Durán D, Monsalves MJ, Rodríguez MF, Rotarou ES, Gajardo J, et al.Diagnostic properties of case definitions of suspected COVID-19 in Chile, 2020 [Propiedades diagnósticas de las definiciones de caso sospechoso de COVID-19 en Chile, 2020]. Revista Panamericana de Salud Publica [Pan American Journal of Public Health] 2021;45:e14. [DOI: 10.26633/RPSP.2021.14] [DOI] [PMC free article] [PubMed] [Google Scholar]
Auvinen 2021 {published data only}
- Auvinen R, Nohynek H, Syrjänen R, Ollgren J, Kerttula T, Mäntylä J, et al.Comparison of the clinical characteristics and outcomes of hospitalized adult COVID-19 and influenza patients - a prospective observational study. Infectious Diseases 2021;53(2):111-21. [DOI: 10.1080/23744235.2020.1840623] [DOI] [PubMed] [Google Scholar]
Baghaei 2020 {published data only}
- Baghaei P, Nadji SA, Marjani M, Moniri A, Hashemian SM, Sheikhzade H, et al.Clinical manifestations of patients with coronavirus disease 2019 (COVID-19) in a referral center in Iran. Tanaffos 2020;19(2):122-8. [PMC free article] [PubMed] [Google Scholar]
Bailey 2020 {published data only}
- Bailey L, O'Donoghue A, Martin A.Acute presentations of nursing home residents to hospital during COVID-19 pandemic. European Geriatric Medicine 2020;11 Suppl 1:S52. [Google Scholar]
Bailey 2021 {published data only}
- Bailey LC, Razzaghi H, Burrows EK, Bunnell HT, Camacho PE, Christakis DA, et al.Assessment of 135 794 pediatric patients tested for severe acute respiratory syndrome coronavirus 2 across the United States. JAMA Pediatrics 2021;175(2):176-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bartlett 2020 {published data only}
- Bartlett B, Ploog N, Heaton H, Mullan A, Knutson B.287 likelihood of COVID-19 positive test results in patients who present to the emergency department with key COVID chief symptoms. Annals of Emergency Medicine 2020;76(4):S111. [DOI: 10.1016/j.annemergmed.2020.09.301] [DOI] [Google Scholar]
Bastiani 2021 {published data only}
- Bastiani L, Fortunato L, Pieroni S, Bianchi F, Adorni F, Prinelli F, et al.Rapid COVID-19 screening based on self-reported symptoms: psychometric assessment and validation of the EPICOVID19 Short Diagnostic Scale. Journal of Medical Internet Research 2021;23(1):e23897. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhatta 2021 {published data only}
- Bhatta S, Gandhi S, Saindani SJ, Ganesuni D, Ghanpur AD.Otorhinolaryngological manifestations of coronavirus disease 2019: a prospective review of 600 patients. Journal of Laryngology and Otology 2021;135(3):206-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bidkar 2021 {published data only}
- Bidkar V, Mishra M, Selvaraj K, Joshi P, Shrikrishna BH, Dabhekar S, et al.Testing olfactory and gustatory dysfunctions among quarantine COVID-19 suspects. Indian Journal of Otolaryngology and Head & Neck Surgery 2021;73(3):304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bonadio 2020 {published data only}
- Bonadio W, Bonadio W, Jackson K, Gottlieb L.379 accuracy of an emergency department clinical protocol for early identification of coronavirus infection. Annals of Emergency Medicine 2020;76(4):S146. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brotons 2020 {published data only}
- Brotons C, Serrano J, Fernández D, Garcia-Ramos C, Ichazo B, Lemaire J, et al.Seroprevalence against COVID-19 and follow-up of suspected cases in primary health care in Spain. medRxiv [Preprint]. [DOI: ] [Google Scholar]
Burrel 2021 {published data only}
- Burrel S, Hausfater P, Dres M, Pourcher V, Luyt CE, Teyssou E, et al.Co-infection of SARS-CoV-2 with other respiratory viruses and performance of lower respiratory tract samples for the diagnosis of COVID-19. International Journal of Infectious Diseases 2021;102:10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cadegiani 2021 {published data only}
- Cadegiani FA, Zimerman RA, Campello de Souza B, McCoy J, Pereira E, Costa RA, et al.The AndroCoV Clinical Scoring for COVID-19 Diagnosis: a prompt, feasible, costless, and highly sensitive diagnostic tool for COVID-19 based on a 1757-patient cohort. Cureus 2021;13(1):e12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cai 2020 {published data only}
- Cai X, Jiang H, Zhang S, Xia S, Du W, Ma Y, et al.Clinical manifestations and pathogen characteristics in children admitted for suspected COVID-19. Frontiers of Medicine 2020;14(6):776-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Calagnan 2020 {published data only}
- Calagnan E, Gobbato M, Burba I, Del Zotto S, Toffolutti F, Serraino D, et al.COVID-19 infections in the Friuli Venezia Giulia Region (northern Italy): a population-based retrospective analysis. Epidemiologia & Prevenzione 2020;44(5-6 Suppl 2):323-9. [DOI] [PubMed] [Google Scholar]
Carignan 2020 {published data only}
- Carignan A, Valiquette L, Grenier C, Musonera JB, Nkengurutse D, Marcil-Héguy A, et al.Anosmia and dysgeusia associated with SARS-CoV-2 infection: an age-matched case-control study. CMAJ 2020;192(26):E702-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Challener 2020 {published data only}
- Challener DW, Challener GJ, Gow-Lee VJ, Fida M, Shah AS, O'Horo JC.Screening for COVID-19: patient factors predicting positive PCR test. Infection Control and Hospital Epidemiology 2020;41(8):968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2020 {published data only}
- Chen X, Tang Y, Mo Y, Li S, Lin D, Yang Z, et al.A diagnostic model for coronavirus disease 2019 (COVID-19) based on radiological semantic and clinical features: a multi-center study. European Radiology 2020;30(9):4893-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2021 {published data only}
- Chen R, Xu G, Yang L, Deng Z, Hu Q, Hu H, et al.A new rapid screening program based on risk scores for COVID-19 patients. Internal and Emergency Medicine 2021;16(4):925-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Concheiro‐Guisan 2020 {published data only}
- Concheiro-Guisan A, Fiel-Ozores A, Novoa-Carballal R, González-Duran ML, Portugués de la Red M, Martínez-Reglero C, et al.Subtle olfactory dysfunction after SARS-CoV-2 virus infection in children. International Journal of Pediatric Otorhinolaryngology 2020;140:110539. [DOI] [PMC free article] [PubMed] [Google Scholar]
D'Souza 2020 {published data only}
- D'Souza G, Springer G, Gustafson D, Kassaye S, Alcaide M L, Ramirez C, et al.COVID-19 symptoms and SARS-CoV-2 infection among people living with HIV in the US: the MACS/WIHS combined cohort study. HIV Research & Clinical Practice 2020;21(5):130-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dai 2021 {published data only}
- Dai YN, Zheng W, Wu QQ, Hui TC, Sun NN, Chen GB, et al.A rapid screening model for early predicting novel coronavirus pneumonia in Zhejiang Province of China: a multicenter study. Scientific Reports 2021;11(1):3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dantas 2021 {published data only}
- Dantas LF, Peres IT, Bastos LS, Marchesi JF, Souza GF, Gelli JG, et al.App-based symptom tracking to optimize SARS-CoV-2 testing strategy using machine learning. PLoS One 2021;16(3):e0248920. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Angelis 2020 {published data only}
- De Angelis G, Posteraro B, Biscetti F, Ianiro G, Zileri Dal Verme L, Cattani P, et al.Confirmed or unconfirmed cases of 2019 novel coronavirus pneumonia in Italian patients: a retrospective analysis of clinical features. BMC Infectious Diseases 2020;20(1):775. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deng 2020 {published data only}
- Deng LS, Yuan J, Ding L, Chen YL, Zhao CH, Chen GQ, et al.Comparison of patients hospitalized with COVID-19, H7N9 and H1N1. Infectious Diseases of Poverty 2020;9(1):163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dixon 2021 {published data only}
- Dixon BE, Wools-Kaloustian KK, Fadel WF, Duszynski TJ, Yiannoutsos C, Halverson PK, et al.Symptoms and symptom clusters associated with SARS-CoV-2 infection in community-based populations: results from a statewide epidemiological study. PLoS One 2021;16(3):e0241875. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dreyer 2020 {published data only}
- Dreyer NA, Reynolds M, DeFilippo Mack C, Brinkley E, Petruski-Ivleva N, Hawaldar K, et al.Self-reported symptoms from exposure to COVID-19 provide support to clinical diagnosis, triage and prognosis: an exploratory analysis. Travel Medicine and Infectious Disease 2020;38:101909. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duan 2020 {published data only}
- Duan J, Liang M, Li Y, Wu D, Chen Y, Gao S, et al.Definition and retrospective application of a clinical scoring system for COVID-19 triage at presentation. Therapeutic Advances in Respiratory Disease 2020;14:1753466620963019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duque 2021 {published data only}
- Duque MP, Lucaccioni H, Costa C, Marques R, Antunes D, Hansen L, et al.COVID-19 symptoms: a case-control study, Portugal, March-April 2020. Epidemiology and Infection 2021;149:e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duramaz 2021 {published data only}
- Duramaz BB, Turel O, Korkmaz C, Karadogan MT, Yozgat CY, Iscan A, et al.A snapshot of pediatric patients with COVID-19 in a pandemic hospital. Klinische Pädiatrie 2021;233(1):24-30. [DOI] [PubMed] [Google Scholar]
Elimian 2020 {published data only}
- Elimian KO, Ochu CL, Ebhodaghe B, Myles P, Crawford EE, Igumbor E, et al.Patient characteristics associated with COVID-19 positivity and fatality in Nigeria: retrospective cohort study. BMJ Open 2020;10(12):e044079. [DOI] [PMC free article] [PubMed] [Google Scholar]
Escosteguy 2020 {published data only}
- Escosteguy CC, Eleuterio TA, Pereira AG, Marques MR, Brandão AD, Batista JP.COVID-19: a cross-sectional study of suspected cases admitted to a federal hospital in Rio de Janeiro, Brazil, and factors associated with hospital death. Epidemiologia e Serviços de Saúde 2020;30(1):e2020750. [DOI] [PubMed] [Google Scholar]
Feehan 2021 {published data only}
- Feehan AK, Fort D, Velasco C, Burton JH, Garcia-Diaz J, Price-Haywood EG, et al.The importance of anosmia, ageusia and age in community presentation of symptomatic and asymptomatic SARS-CoV-2 infection in Louisiana, USA; a cross-sectional prevalence study. Clinical Microbiology and Infection 2021;27(4):633.e9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fisher 2021 {published data only}
- Fisher KA, Olson SM, Tenforde MW, Self WH, Wu M, Lindsell CJ, et al.Symptoms and recovery among adult outpatients with and without COVID-19 at 11 healthcare facilities-July 2020, United States. Influenza and Other Respiratory Viruses 2021;15(3):345-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Foster 2021 {published data only}
- Foster CE, Marquez L, Davis AL, Tocco E, Koy TH, Dunn J, et al.A surge in pediatric coronavirus disease 2019 cases: the experience of Texas Children's Hospital from March to June 2020. Journal of the Pediatric Infectious Diseases Society 2021;10(5):593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gale 2020 {published data only}
- Gale J, Clark DA, Bohm C, Canney M, Davis I, LeBlanc JJ, et al.COVID-19 status, symptom burden, and characteristics of dialysis patients residing in areas of community transmission: research letter. Canadian Journal of Kidney Health and Disease 2020;7:2054358120964178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gerkin 2021 {published data only}
- Gerkin RC, Ohla K, Veldhuizen MG, Joseph PV, Kelly CE, Bakke AJ, et al.Recent smell loss is the best predictor of COVID-19 among individuals with recent respiratory symptoms. Chemical Sense 2021;46:bjaa081. [DOI] [PMC free article] [PubMed] [Google Scholar]
Giavedoni 2020 {published data only}
- Giavedoni P, Podlipnik S, Pericàs JM, Fuertes de Vega I, García-Herrera A, Alós L, et al.Skin manifestations in COVID-19: prevalence and relationship with disease severity. Journal of Clinical Medicine 2020;9(10):3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gibbons 2021 {published data only}
- Gibbons C, Hussain M, O'Keeffe DT, Simpkin AJ.An analysis of patient self-reported COVID-19 symptoms during the first wave of the pandemic in Ireland. Irish Journal of Medical Science 2021 Mar 25 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gnanasambantham 2020 {published data only}
- Gnanasambantham K, Aitken G, Morris B, Simionato J, Chua EH, Ibrahim JE.Developing a clinical screening tool for identifying COVID-19 infection in older people dwelling in residential aged care services. Australasian Journal on Ageing 2020;40(1):48-57. [DOI] [PubMed] [Google Scholar]
Goel 2020 {published data only}
- Goel A, Raizada A, Bansal K, Gaur N, Abraham J, Yadav A.Profile of patients suspected to be COVID-19: a retrospective analysis of early pandemic data. Cureus 2020;12(8):e10125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gombos 2021 {published data only}
- Gombos K, Földi M, Kiss S, Herczeg R, Gyenesei A, Geiger L, et al.Analysis of COVID-19-related RT-qPCR test results in Hungary: epidemiology, diagnostics, and clinical outcome. Frontiers in Medicine 2021;7:625673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goodacre 2020 {published data only}
- Goodacre S, Thomas B, Lee E, Sutton L, Loban A, Waterhouse S, et al.Characterisation of 22445 patients attending UK emergency departments with suspected COVID-19 infection: observational cohort study. PLoS One 2020;15(11):e0240206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guillén 2020 {published data only}
- Martínez AG, Gálvez MA, Rodriguez Sanz S, Hernandez Ruiz P, García Morillas A, Sánchez ET.Incidence of smell and taste disorders and associated factors in patients with mild to moderate COVID-19. Polish Journal of Otolaryngology 2020;75(2):1-5. [DOI] [PubMed] [Google Scholar]
Gurrola 2021 {published data only}
- Gurrola JG 2nd, Chang JL, Roland LT, Loftus PA, Cheung SW.Short-term chemosensory distortions and phantoms in COVID-19. Laryngoscope Investigative Otolaryngology 2021;6(2):172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haddadin 2021 {published data only}
- Haddadin Z, Chappell J, McHenry R, Pulido CG, Rahman H, Gu W, et al.Coronavirus surveillance in a pediatric population in Jordan from 2010 to 2013: a prospective viral surveillance study. Pediatric Infectious Disease Journal 2021;40(1):e12-e7. [DOI] [PubMed] [Google Scholar]
Hamed 2021 {published data only}
- Hamed E, Alnuaimi AS, Syed MA, Elhamid MA, Sedeeq ST, Alemrayat B, et al.Clinical characteristics of 51815 patients presenting with positive and negative SARS-CoV-2 swab results in primary health care settings. Journal of Infection 2021;82(4):84-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hernández‐Cruz 2021 {published data only}
- Hernández-Cruz RG, Sánchez-Cobo D, Acevedo-Gallegos S, Helguera-Repetto AC, Rodriguez-Bosch MR, Ramirez-Santes VH, et al.Clinical characteristics and risk factors for SARS-CoV-2 infection in pregnant women attending a third level reference center in Mexico City. Journal of Maternal-fetal & Neonatal Medicine 2021:1-5. [DOI] [PubMed] [Google Scholar]
Hosseinzadeh 2021 {published data only}
- Hosseinzadeh A, Rezapour M, Rohani-Rasaf M, Emamian MH, Talebi S, Goli S, et al.Epidemiological patterns of syndromic symptoms in suspected patients with covid-19 in Iran: a latent class analysis. Journal of Research in Health Sciences 2021;21(1):e00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hosseninasab 2020 {published data only}
- Hosseninasab A, Bafti MS, Ebrahimi S, Anjomshoaa A, Zerandi FS, Jafari M, et al.Coronavirus disease 2019 in children with acute respiratory infection: a study from southeastern Iran. Shiraz E Medical Journal 2020;21(12):1-7. [Google Scholar]
Hubiche 2021 {published data only}
- Hubiche T, Phan A, Leducq S, Rapp J, Fertitta L, Aubert H, et al.Acute acral eruptions in children during the COVID-19 pandemic: characteristics of 103 children and their family clusters. Annales de Dermatologie et de Vénéréologie 2021;148(2):94-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hurst 2020 {published data only}
- Hurst JH, Heston SM, Chambers HN, Cunningham HM, Price MJ, Suarez L, et al.SARS-CoV-2 infections among children in the Biospecimens from Respiratory Virus-Exposed Kids (BRAVE Kids) Study. Clinical Infectious Diseases 2020 Nov 03 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Indini 2021 {published data only}
- Indini A, Cattaneo M, Ghidini M, Rijavec E, Bareggi C, Galassi B, et al.Triage process for the assessment of coronavirus disease 2019-positive patients with cancer: the ONCOVID prospective study. Cancer 2021;127(7):1091-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Islam 2020 {published data only}
- Islam N, Ara Rahman M, Uz Zaman A, Ferdous N, Bin Nazrul F, Tuz Zahura F, et al.Frequency and characteristics of COVID-19 infection in rheumatic patients-an online survey from Bangladesh. International Journal of Rheumatic Diseases 2020;23 Suppl 1:171. [Google Scholar]
Jain 2021 {published data only}
- Jain A, Kaur J, Rai AK, Pandey AK.Anosmia: a clinical indicator of COVID-19 reinfection. Ear, Nose, & Throat Journal 2021;100(2 Suppl):180S-181S. [DOI] [PubMed] [Google Scholar]
Karni 2020 {published data only}
- Karni N, Klein H, Asseo K, Benjamini Y, Israel S, Nammary M, et al.Self-rated smell ability enables highly specific predictors of COVID-19 status: a case-control study in Israel. Open Forum Infectious Diseases 2020;8(2):ofaa589. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kasiukiewicz 2020 {published data only}
- Kasiukiewicz A, Wojszel ZB, Kazberuk M, Sitkiewicz J, Olszewska J.Symptoms and final diagnoses of patients referred to one of the COVID-19 dedicated hospitals in Poland - a cross-sectional study. European Geriatric Medicine 2020;11 Suppl 1:S83. [Google Scholar]
Kline 2021 {published data only}
- Kline JA, Camargo CA, Courtney DM, Kabrhel C, Nordenholz KE, Aufderheide T, et al.Clinical prediction rule for SARS-CoV-2 infection from 116 U.S. emergency departments 2-22-2021. PLoS One 2021;16(3):e0248438. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lechner 2021 {published data only}
- Lechner M, Liu J, Counsell N, Ta NH, Rocke J, Anmolsingh R, et al.Course of symptoms for loss of sense of smell and taste over time in one thousand forty-one healthcare workers during the COVID-19 pandemic: our experience. Clinical Otolaryngology 2021;46(2):451-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2020 {published data only}
- Lee DJ, Lockwood J, Das P, Wang R, Grinspun E, Lee JM.Self-reported anosmia and dysgeusia as key symptoms of coronavirus disease 2019. CJEM 2020;22(5):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2021 {published data only}
- Lee DJ, Daliyot D, Wang R, Lockwood J, Das P, Zimlichman E, et al.Comparative study of chemosensory dysfunction in COVID-19 in 2 geographically distinct regions. Ear, Nose & Throat Journal 2021:1455613211000170. [DOI] [PubMed] [Google Scholar]
Li 2020a {published data only}
- Li Y, Li H, Han J, Yang L.The preliminary comparative results between COVID-19 and non-COVID-19 patients in western China. BMC Infectious Diseases 2020;20(1):935. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2020b {published data only}
- Li Y, Shang Y, Yang Y, Wang M, Yu D, Su D, et al.Factors associated with a positive severe acute respiratory syndrome coronavirus 2 testing in suspected cases presenting with pneumonia: a retrospective cohort study in a single medical center. Respiration 2020;99(9):739-47. [DOI] [PubMed] [Google Scholar]
Li 2021 {published data only}
- Li S, Liu SY, Zhao YQ, Li QY, Liu DY, Liu ZC, et al.Spatial and temporal distribution and predictive value of chest CT scoring in patients with COVID-19. Zhonghua Jie He He Hu Xi Za Zhi 2021;44(3):230-6. [DOI] [PubMed] [Google Scholar]
Liang 2020 {published data only}
- Liang Y, Liang J, Zhou Q, Li X, Lin F, Deng Z, et al.Prevalence and clinical features of 2019 novel coronavirus disease (COVID-19) in the fever clinic of a teaching hospital in Beijing: a single-center, retrospective study. medRxiv [Preprint]. [DOI: ] [Google Scholar]
Liu 2021 {published data only}
- Liu Y, Chen H, Tan W, Kuang Y, Tang K, Luo Y, et al.Clinical characteristics and outcome of SARS-CoV-2 infection during pregnancy. Journal of Infection 2021;82(6):e9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Loftus 2020 {published data only}
- Loftus PA, Roland LT, Gurrola JG 2nd, Cheung SW, Chang JL.Temporal profile of olfactory dysfunction in COVID-19. OTO Open 2020;4(4):2473974X20978133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lu 2020 {published data only}
- Lu X, Zhang H, Adu IK, Xiong Z, Zheng Y, Wang J.A retrospective study of the related common factors of COVID-19. Electronic Journal of General Medicine 2020;18(1):em262. [Google Scholar]
Madan 2020 {published data only}
- Madan S, Beri S.Conjunctivitis in novel coronavirus disease (COVID-19). Indian Journal of Occupational and Environmental Medicine 2020;24(2):129-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Makaronidis 2020 {published data only}
- Makaronidis J, Mok J, Balogun N, Magee CG, Omar RZ, Carnemolla A, et al.Seroprevalence of SARS-CoV-2 antibodies in people with an acute loss in their sense of smell and/or taste in a community-based population in London, UK: an observational cohort study. PLoS Medicine 2020;17(10):e1003358. [DOI] [PMC free article] [PubMed] [Google Scholar]
Manley 2020 {published data only}
- Manley HJ, Majchrzak KM, Sanders R, Cumber S, Aweh GN, Ladik V, et al.Screening for SARS-CoV-2 (COVID) infection in chronic dialysis patients: a nonprofit provider's experience. Journal of the American Society of Nephrology 2020;31:32. [Google Scholar]
McDonald 2020 {published data only}
- McDonald SA, Medford RJ, Basit MA, Diercks DB, Courtney DM.Derivation with internal validation of a multivariable predictive model to predict COVID-19 test results in emergency department patients. Academic Emergency Medicine 2020;28(2):206-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
McGovern 2020 {published data only}
- McGovern A, Brooks F, Downs T, Drennan M, Frizzell E, Knox L, et al.Delirium may be the only symptom in older adults with COVID19 disease. European Geriatric Medicine 2020;11 Suppl 1:S6. [Google Scholar]
Medetalibeyoglu 2020 {published data only}
- Medetalibeyoglu A, Bahat G, Senkal N, Kose M, Catma Y, Yesil Y, et al.Comparison of clinical characteristics and outcome measures of PCR-positive and PCR-negative patients diagnosed as COVID19: analyses focusing on the older adults. European Geriatric Medicine 2020;11 Suppl 1:S87. [Google Scholar]
Membrilla 2020 {published data only}
- Membrilla JA, de Lorenzo Í, Sastre M, Díaz de Terán J.Headache as a cardinal symptom of coronavirus disease 2019: a cross-sectional study. Headache 2020;60(10):2176-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mizrahi 2020 {published data only}
- Mizrahi B, Shilo S, Rossman H, Kalkstein N, Marcus K, Barer Y, et al.Longitudinal symptom dynamics of COVID-19 infection. Nature Communications 2020;11(1):6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Möckel 2021 {published data only}
- Möckel M, Stegemann MS, Burst V, Kümpers P, Risse J, Koehler FC, et al.Which parameters support disposition decision in suspected COVID-19 cases in the emergency department (ED): a German clinical cohort study. BMJ Open 2021;11(3):e044853. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moolla 2021 {published data only}
- Moolla MS, Parker A, Parker MA, Sithole S, Amien L, Chiecktey R, et al.Staff testing for COVID-19 via an online pre-registration form. Southern African Journal of Infectious Diseases 2021;36(1):232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Muhammad 2021 {published data only}
- Muhammad LJ, Algehyne EA, Usman SS, Ahmad A, Chakraborty C, Mohammed IA.Supervised machine learning models for prediction of COVID-19 infection using epidemiology dataset. SN Computer Science 2021;2(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Munblit 2020 {published data only}
- Munblit D, Nekliudov NA, Bugaeva P, Blyuss O, Kislova M, Listovskaya E, et al.StopCOVID cohort: an observational study of 3,480 patients admitted to the Sechenov University hospital network in Moscow city for suspected COVID-19 infection. Clinical Infectious Diseases 2020;73(1):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murillo‐Zamora 2020 {published data only}
- Murillo-Zamora E, Aguilar-Sollano F, Delgado-Enciso I, Hernandez-Suarez CM.Predictors of laboratory-positive COVID-19 in children and teenagers. Public Health 2020;189:153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nakajima 2021 {published data only}
- Nakajima M, Yamamoto Y, Kaszynski RH, Yamauchi Y, Yamamoto K, Nakajima Y, et al.A comparison on the percentage of polymerase chain reaction positivity for SARS-CoV-2 between Public Health Center referrals and direct walk-in patients: a single center retrospective analysis in Tokyo. Journal of Infection and Chemotherapy 2021;27(6):852-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nakanishi 2020 {published data only}
- Nakanishi H, Suzuki M, Maeda H, Nakamura Y, Ikegami Y, Takenaka Y, et al.Differential diagnosis of COVID-19: importance of measuring blood lymphocytes, serum electrolytes, and olfactory and taste functions. Tohoku Journal of Experimental Medicine 2020;252(2):109-19. [DOI] [PubMed] [Google Scholar]
Nayan 2021 {published data only}
- Nayan N, Kumar MK, Nair RK, Manral I, Ghosh S, Bhalla S, et al.Clinical triaging in cough clinic alleviates COVID-19 overload in emergency department in India. SN Comprehensive Clinical Medicine 2021;3:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nobel 2020 {published data only}
- Nobel YR, Phipps M, Zucker J, Lebwohl B, Wang TC, Sobieszczyk ME, et al.Gastrointestinal symptoms and coronavirus disease 2019: a case-control study from the United States. Gastroenterology 2020;159(1):373-5.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ortiz 2020 {published data only}
- Ortiz Molina E, Hernandez Pailos R, Pola Guillen M, Pascual Pedreno A, Rodriguez Rodriguez E, Hernandez Martinez A.COVID-19 infection in symptomatic pregnant women at the midpoint of the pandemic in Spain: a retrospective analysis. Ginekologia Polska 2020;91(12):755-63. [DOI] [PubMed] [Google Scholar]
Oshman 2020 {published data only}
- Oshman L, Caplan A, Ali R, Singh L, Khalid R, Jameel M, et al.Whom should we test for COVID-19? Performance of a symptom and risk factor questionnaire on COVID-19 test results and patient outcomes in an immediate care setting. Journal of Primary Care & Community Health 2020;11:2150132720981297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ozcan 2021 {published data only}
- Ozcan E, Yavuzer S, Borku Uysal B, Islamoglu MS, Ikitimur H, Unal OF, et al.The relationship between positivity for COVID-19 RT-PCR and symptoms, clinical findings, and mortality in Turkey. Expert Review of Molecular Diagnostics 2021;21(2):245-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Paar 2021 {published data only}
- Paar M, Strumann C, Giesen H.Rate and predictive parameters of novel coronavirus 2019 (SARS-CoV-2) infections in a German general practice. Irish Journal of Medical Science 2021;191(1):31-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pigott 2020 {published data only}
- Pigott JS, Jackman C, Lindenberg N, Beaumont L, Patel S, Sun K, et al.Clinical presentation of COVID-19 in older people. European Geriatric Medicine 2020;11 Suppl 1:S5-6. [Google Scholar]
Platten 2021 {published data only}
- Platten M, Cranen R, Peters C, Wisplinghoff H, Nienhaus A, Bach AD, et al.Prevalence of SARS-CoV-2 in employees of a general hospital in Northrhine-Westphalia, Germany [Prävalenz von SARS-CoV-2 bei Mitarbeitern eines Krankenhauses der Regel-/Schwerpunktversorgung in Nordrhein-Westfalen]. Deutsche Medizinische Wochenschrift 2021;146(5):e30-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Popovych 2021 {published data only}
- Popovych VI, Koshel I, Haman Y, Leschak V, Duplikhin R.Diagnostic accuracy and predictive value of clinical symptoms for the diagnosis of mild COVID 19. medRxiv [Preprint]. [DOI: ] [Google Scholar]
Pullen 2020 {published data only}
- Pullen MF, Skipper CP, Hullsiek KH, Bangdiwala AS, Pastick KA, Okafor EC, et al.Symptoms of COVID-19 outpatients in the United States. Open Forum Infectious Diseases 2020;7(7):ofaa271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Quer 2020 {published data only}
- Quer G, Radin JM, Gadaleta M, Baca-Motes K, Ariniello L, Ramos E, et al.Wearable sensor data and self-reported symptoms for COVID-19 detection. Nature Medicine 2020;27(1):73-7. [DOI] [PubMed] [Google Scholar]
Ravani 2020 {published data only}
- Ravani P, Saxinger L, Chandran U, Fonseca K, Murphy S, Lang E, et al.COVID-19 screening of asymptomatic patients admitted through emergency departments in Alberta: a prospective quality-improvement study. CMAJ Open 2020;8(4):E887-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rentsch 2020 {published data only}
- Rentsch CT, Kidwai-Khan F, Tate JP, Park LS, King JT, Skanderson M, et al.COVID-19 testing, hospital admission, and intensive care among 2,026,227 United States veterans aged 54-75 years. medRxiv [Preprint]. [DOI: ] [Google Scholar]
Ronan 2021 {published data only}
- Ronan G, Kumar L, Davey M, O'Leary C, McAleer S, Lynch J, et al.Factors associated with SARS-CoV-2 infection in patients attending an acute hospital ambulatory assessment unit. Journal of Medical Virology 2021;93(7):4488-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rubel 2020 {published data only}
- Rubel K, Sharma D, Campiti V, Yedlicka G, Burgin SJ, Illing EA, et al.COVID-19 status differentially affects olfaction: a prospective case-control study. OTO Open 2020;4(4):2473974X20970176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sabetian 2021 {published data only}
- Sabetian G, Moghadami M, Hashemizadeh Fard Haghighi L, Shahriarirad R, Fallahi MJ, Asmarian N, et al.COVID-19 infection among healthcare workers: a cross-sectional study in southwest Iran. Virology Journal 2021;18(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sabetta 2020 {published data only}
- Sabetta E, Campagner A, Ferrari D, Ceriotti D, Di Resta C, Colombini A, et al.Development, evaluation, and validation of machine learning models for COVID-19 detection based on complete blood count test from 1,624 patients. Biochimica Clinica 2020;44 Suppl 2:S6-7. [DOI] [PubMed] [Google Scholar]
Sehanobish 2021 {published data only}
- Sehanobish E, Barbi M, Fong V, Kravitz M, Matsumura C, Tejera D, et al.Risk factors associated with COVID-19 related anosmia and ageusia. Journal of Allergy and Clinical Immunology 2021;147(2 Supplement):AB134. [Google Scholar]
Senok 2020 {published data only}
- Senok A, Alsuwaidi H, Atrah Y, Ayedi OA, Zahid JA, Han A, et al.Saliva as an alternative specimen for molecular COVID-19 testing in community settings and population-based screening. Infection and Drug Resistance 2020;13:3393-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shanbehzadeh 2021 {published data only}
- Shanbehzadeh M, Nopour R, Kazemi-Arpanahi H.Determination of the most important diagnostic criteria for COVID-19: a step forward to design an intelligent clinical decision support system. Journal of Advances in Medical and Biomedical Research 2021;29(134):176-82. [Google Scholar]
Shayganmehr 2021 {published data only}
- Shayganmehr A, Dorosti AA, Saboktakin L, Abbasi M, Khaiatzadeh S, Khoshmaram N, et al.Clinical pediatric screening for COVID-19. Iranian Journal of Pediatrics 2021;31(1):e107780. [Google Scholar]
Sheen 2020 {published data only}
- Sheen F, Tan V, Haldar S, Sengupta S, Allen D, Somani J, et al.Evaluating the onset, severity and recovery from smell and taste changes associated with COVID-19 Infection in a Singaporean population: a prospective case controlled study (the COV-OSMIA-19 Trial). JMIR Research Protococol 2020;9(12):e24797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shoer 2021 {published data only}
- Shoer S, Karady T, Keshet A, Shilo S, Rossman H, Gavrieli A, et al.A prediction model to prioritize individuals for SARS-CoV-2 test built from national symptom surveys. Med (New York, N.Y.) 2021;2(2):196-208.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sieber 2021 {published data only}
- Sieber P, Flury D, Güsewell S, Albrich WC, Boggian K, Gardiol C, et al.Characteristics of patients with coronavirus disease 2019 (COVID-19) and seasonal influenza at time of hospital admission: a single center comparative study. BMC Infectious Diseases 2021;21(1):271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Song 2020 {published data only}
- Song CY, Xu J, He JQ, Lu YQ.COVID-19 early warning score: a multi-parameter screening tool to identify highly suspected patients. medRxiv [Preprint]. [DOI: ] [Google Scholar]
Sorlini 2020 {published data only}
- Sorlini C, Femia M, Nattino G, Bellone P, Gesu E, Francione P, et al.The role of lung ultrasound as a frontline diagnostic tool in the era of COVID-19 outbreak. Internal and Emergency Medicine 2021;16(3):749-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spangler 2021 {published data only}
- Spangler D, Blomberg H, Smekal D.Prehospital identification of COVID-19: an observational study. Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine 2021;29(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stacevičienė 2021 {published data only}
- Stacevičienė I, Burokienė S, Steponavičienė A, Vaičiūnienė D, Jankauskienė A.A cross-sectional study of screening for coronavirus disease 2019 (COVID-19) at the pediatric emergency department in Vilnius during the first wave of the pandemic. European Journal of Pediatrics 2021;180(7):2137-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tabacof 2020 {published data only}
- Tabacof L, Kellner C, Breyman E, Dewil S, Braren S, Nasr L, et al.Remote patient monitoring for home management of coronavirus disease 2019 in New York: a cross-sectional observational study. Telemedicine and e-Health 2020;27(6):641-8. [DOI] [PubMed] [Google Scholar]
Taziki 2020 {published data only}
- Taziki Balajelini MH, Vakili MA, Saeidi M, Tabarraei A, Hosseini SM.Using anti-SARS-CoV-2 IgG and IgM antibodies to detect outpatient cases with olfactory and taste disorders suspected as mild form of COVID-19: a retrospective survey. SN Comprehensive Clinical Medicine 2020;2(12):2554-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ticinesi 2020 {published data only}
- Ticinesi A, Nouvenne A, Cerundolo N, Guida L, Chiussi G, Morelli I, et al.Age-related differences in clinical presentation of COVID-19 and factors associated with mortality: a retrospective analysis of patients hospitalized during the pandemic peak. European Geriatric Medicine 2020;11 Suppl 1:S21-2. [Google Scholar]
Trevisan 2021 {published data only}
- Trevisan C, Noale M, Prinelli F, Maggi S, Sojic A, Di Bari M, et al.Age-related changes in clinical presentation of COVID-19: the EPICOVID19 web-based survey. European Journal of Internal Medicine 2021;86:41-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Verma 2020 {published data only}
- Verma RK, Kannaujia S, Khurana N, Singh A, Singh DP, Kumar A.Clinical correlation of severe acute respiratory syndrome-coronavirus-2 cases in selected districts of Uttar Pradesh: a cross-sectional hospital-based study. Journal of Education and Health Promotion 2020;9:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
Viana dos Santos 2021 {published data only}
- Viana dos Santos Santana Í, Cm da Silveira A, Sobrinho Á, Chaves E Silva L, Dias da Silva L, Santos DF, et al.Machine learning classification models for COVID-19 test prioritization in Brazil. Journal of Medical Internet Research 2021;23(4):e27293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Visconti 2021 {published data only}
- Visconti A, Bataille V, Rossi N, Kluk J, Murphy R, Puig S, et al.Diagnostic value of cutaneous manifestation of SARS-CoV-2 infection. British Journal of Dermatology 2021;184(5):880-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vos 2020 {published data only}
- Vos ER, den Hartog G, Schepp RM, Kaaijk P, Van Vliet J, Helm K, et al.Nationwide seroprevalence of SARS-CoV-2 and identification of risk factors in the general population of the Netherlands during the first epidemic wave. Journal of Epidemiology and Community Health 2020;75(6):489-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weiss 2021a {published data only}
- Weiss JJ, Attuquayefio TN, White EB, Li F, Herz RS, White TL, et al.Tracking smell loss to identify healthcare workers with SARS-CoV-2 infection. PLoS One 2021;16(3):e0248025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weiss 2021b {published data only}
- Weiss R, Guchlerner L, Loth AG, Leinung M, Wicker S, Kempf VA, et al.Typical symptoms of common otorhinolaryngological diseases may mask a SARS-CoV-2 infection. European Archives of Oto-Rhino-Laryngology 2021;278(9):3551-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wells 2020 {published data only}
- Wells PM, Doores KJ, Couvreur S, Nunez RM, Seow J, Graham C, et al.Estimates of the rate of infection and asymptomatic COVID-19 disease in a population sample from SE England. Journal of Infection 2020;81(6):931-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2020 {published data only}
- Wu M, Xie C, Wu R, Shu Y, Wang L, Li M, et al.Epidemiological and clinical characteristics of SARS-CoV-2 infection among healthcare workers in Hubei Province of China. Infection Control & Hospital Epidemiology 2020 Nov 18 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xia 2021 {published data only}
- Xia Y, Chen W, Ren H, Zhao J, Wang L, Jin R, et al.A rapid screening classifier for diagnosing COVID-19. International Journal of Biological Sciences 2021;17(2):539-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yan 2020 {published data only}
- Yan CH, Faraji F, Prajapati DP, Boone CE, DeConde AS.Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. International Forum of Allergy & Rhinology 2020;10(7):806-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2020 {published data only}
- Yang Z, Lin D, Chen X, Qiu J, Li S, Huang R, et al.Distinguishing COVID-19 from influenza pneumonia in the early stage through CT imaging and clinical features. medRxiv [Preprint]. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yousef 2021 {published data only}
- Yousef M, Showe LC, Ben Shlomo I.Clinical presentation of COVID-19 - a model derived by a machine learning algorithm. Journal of Integrative Bioinformatics 2021;18(1):3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Žaja 2021 {published data only}
- Žaja R, Kerner I, Macan J, Milošević M.Characteristics of work-related COVID-19 in Croatian healthcare workers: a preliminary report. Archives of Industrial Hygiene and Toxicology 2021;72(1):36-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zavascki 2020 {published data only}
- Zavascki AP, Gazzana MB, Bidart JP, Fernandes PS, Galiotto A, Kawski CT, et al.Development of a predictive score for COVID-19 diagnosis based on demographics and symptoms in patients attended at a dedicated screening unit. medRxiv [Preprint]. [DOI: ] [Google Scholar]
Zayet 2020b {published data only}
- Zayet S, Kadiane-Oussou NJ, Lepiller Q, Zahra H, Royer PY, Toko L, et al.Clinical features of COVID-19 and influenza: a comparative study on Nord Franche-Comte cluster. Microbes and Infection 2020;22(9):481-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhao 2020 {published data only}
- Zhao D, Yao F, Wang L, Zheng L, Gao Y, Ye J, et al.A comparative study on the clinical features of COVID-19 pneumonia to other pneumonias. Clinical Infectious Diseases 2020;71(15):756-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhao 2021 {published data only}
- Zhao J, Grabowska ME, Kerchberger VE, Smith JC, Eken HN, Feng Q, et al.ConceptWAS: a high-throughput method for early identification of COVID-19 presenting symptoms and characteristics from clinical notes. Journal of Biomedical Informatics 2021;117:103748. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Bossuyt 2015
- Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, et al.STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015;351:h5527. [DOI: 10.1136/bmj.h5527] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2020a
- Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Spijker R, Taylor-Phillips S, et al, Cochrane COVID-19 Diagnostic Test Accuracy Group.Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database of Systematic Reviews 2020, Issue 6. Art. No: CD013652. [DOI: 10.1002/14651858.CD013652] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dinnes 2021
- Dinnes J, Deeks JJ, Berhane S, Taylor M, Adriano A, Davenport C, et al, Cochrane COVID-19 Diagnostic Test Accuracy Group.Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database of Systematic Reviews 2021, Issue 3. Art. No: CD013705. [DOI: 10.1002/14651858.CD013705.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Griffith 2020
- Griffith G, Morris TT, Tudball M, Herbert A, Mancano G, Pike L, et al.Collider bias undermines our understanding of COVID-19 disease risk and severity. Nature Communications 2020;11(1):5749. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Islam 2021
- Islam N, Ebrahimzadeh S, Salameh J-P, Kazi S, Fabiano N, Treanor L, et al, Cochrane COVID-19 Diagnostic Test Accuracy Group.Thoracic imaging tests for the diagnosis of COVID‐19. Cochrane Database of Systematic Reviews 2021, Issue 3. Art. No: CD013639. [DOI: 10.1002/14651858.CD013639.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jaeschke 1994
- Jaeschke R, Guyatt GH, Sackett DL.Users' guides to the medical literature. III. How to use an article about a diagnostic test. B. What are the results and will they help me in caring for my patients? The Evidence-Based Medicine Working Group. JAMA 1994;271(9):703-7. [DOI] [PubMed] [Google Scholar]
Macaskill 2013
- Macaskill P, Gatsonis C, Deeks JJ, Harbord RM, Takwoingi Y.Chapter 10: Analysing and presenting results. In: Deeks JJ, Bossuyt PM, Gatsonis C editor(s). Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0. The Cochrane Collaboration, 2013. Available from srdta.cochrane.org.
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group.Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
NIH 2021
- National Institutes of Health.Clinical Spectrum of SARS-CoV-2 Infection. www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/ (accessed 15 February 2022). [https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/]
R 2020 [Computer program]
- R core team R Foundation for Statistical Computing.Version 3.5.1. Vienna, Austria: R core team, 2020.
Review Manager 2020 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5).Version 5.4.1. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Rutjes 2006
- Rutjes AW, Reitsma JB, Di Nisio M, Smidt N, Van Rijn JC, Bossuyt PM.Evidence of bias and variation in diagnostic accuracy studies. CMAJ 2006;174(4):469476. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stegeman 2020
- Stegeman I, Ochodo EA, Guleid F, Holtman GA, Yang B, Davenport C, et al, Cochrane COVID-19 Diagnostic Test Accuracy Group.Routine laboratory testing to determine if a patient has COVID-19. Cochrane Database of Systematic Reviews 2020, Issue 11. Art. No: CD013787. [DOI: 10.1002/14651858.CD013787] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tande 2022
- Tande AJ, Pollock BD, Shah ND, Farrugia G, Virk A, Swift M, et al.Impact of the coronavirus disease 2019 (COVID-19) vaccine on asymptomatic infection among patients undergoing preprocedural COVID-19 molecular screening. Clinical Infectious Diseases 2021;74(1):59-65. [DOI: 10.1093/cid/ciab229] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van den Bruel 2010
- Van den Bruel A, Haj-Hassan T, Thompson M, Buntinx F, Mant D.Diagnostic value of clinical features at presentation to identify serious infection in children in developed countries: a systematic review. Lancet 2010;375(9717):834-45. [DOI] [PubMed] [Google Scholar]
Whiting 2011
- Whiting PF, Rutjes AW, Westwood ME, Mallett S Deeks JJ, Reitsma JB, et al.QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529-36. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Deeks 2020b
- Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Leeflang MM, Spijker R, et al.Diagnosis of SARS-CoV-2 infection and COVID-19: accuracy of signs and symptoms; molecular, antigen, and antibody tests; and routine laboratory markers. Cochrane Database of Systematic Reviews 2020, Issue 4. Art. No: CD013596. [DOI: 10.1002/14651858.CD013596] [DOI] [Google Scholar]
Struyf 2020
- Struyf T, Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Leeflang M, et al, Cochrane COVID-19 Diagnostic Test Accuracy Group.Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19 disease. Cochrane Database of Systematic Reviews 2020, Issue 7. Art. No: CD013665. [DOI: 10.1002/14651858.CD013665] [DOI] [PMC free article] [PubMed] [Google Scholar]
Struyf 2021
- Struyf T, Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Leeflang MMG, et al, Cochrane COVID-19 Diagnostic Test Accuracy Group.Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19. Cochrane Database of Systematic Reviews 2021, Issue 2. Art. No: CD013665. [DOI: 10.1002/14651858.CD013665.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]