Abstract
OBJECTIVES:
Kaposi sarcoma (KS) is one of the most common childhood cancers in eastern and central Africa. It has become a treatable disease with increasing availability of antiretroviral therapy (ART) and chemotherapy. We aim to fill the data gap in establishing whether long-term survival is achievable for children in low-income countries.
METHODS:
We retrospectively analyzed children and adolescents ≤18.9 years diagnosed with HIV-related or endemic KS from 2006-2015 who received standardized institutional treatment regimens utilizing chemotherapy plus ART (if HIV-positive) at a tertiary care public hospital in Lilongwe, Malawi. Long-term survival was analyzed and mortality was associated with KS for those with refractory/progressive disease at time of death.
RESULTS:
There were 207 children/adolescents with KS (90.8% HIV-related); 36.7% were alive, 54.6% died, and 8.7% were lost to follow-up. Median follow-up time for survivors was 6.9 years (range 4.2-13.9). Death occurred at median 5.3 months after KS diagnosis (range 0.1-123). KS progression was associated with mortality for most (61%) early deaths (less than 6 months survival); conversely, KS was associated with a minority (31%) of late-onset deaths (after 24 months). The 7-year overall survival was 37% (95% CI: 30-44%) and was higher for those diagnosed between 2011-2015 compared to 2006-2010: 42% (95% CI: 33-51%) versus 29% (95% CI: 20-39%), p = 0.01. Among the 66 HIV-positive survivors, 58% were still on first-line ART.
CONCLUSIONS:
Long-term survival is possible for pediatric KS in low-resource settings. Despite better survival in more recent years, there remains room for improvement.
Keywords: Kaposi sarcoma, pediatric oncology, global health, HIV, KSHV, Africa, KS, HHV-8
INTRODUCTION
Kaposi sarcoma (KS) is among the most common childhood cancers in eastern and central Africa, a region where infection with its causative agent, Kaposi sarcoma herpesvirus/human herpesvirus-8 (KSHV/HHV-8), is endemic, and HIV-prevalence is high.(1, 2) With increasing availability of both antiretroviral therapy (ART) and multi-agent chemotherapy regimens, KS has become a treatable disease.
The Lilongwe Pediatric KS Program has treated children with HIV-related and endemic KS since 2006.(3) Previous publications on sub-sets of this cohort have helped establish some distinctions of pediatric KS. Studies have described the high frequency of the unique, primarily lymphadenopathic clinical phenotype in children, identified prognostic factors, and highlighted significant overlap between HIV-related and endemic pediatric KS.(4-6) Our work has established favorable responses to chemotherapy for children with lymphadenopathic disease, the high KS-related mortality for patients with visceral and/or disseminated disease, and the relatively frequent association between pediatric KS and HHV-8 lytic activation.(5, 7-11) However, survival data for childhood KS in sub-Saharan Africa has been limited to short-term 1-2 year duration.(4, 5, 8, 12-14) This study aims to fill existing gaps in the literature by describing long-term outcomes into and beyond the decade after KS diagnosis for children and adolescents in Lilongwe, Malawi.
METHODS
We performed a retrospective review of pediatric patients with a clinical or histological diagnosis of KS from January 2006 through December 2015 at the Baylor College of Medicine Children’s Foundation Clinical Center of Excellence in Lilongwe, Malawi with an end point for censoring data in March 2020. Specific criteria for clinical diagnosis in low-income settings without reliable access to diagnostic pathology resources have been previously defined and guidelines have been described in extensive detail.(5) Clinical features and associated short-term outcomes (1-2 years) have been reported for subsets of this cohort: for HIV-positive patients diagnosed between January 2006-October 2009 and August 2010-June 2013 and HIV-negative patients with endemic KS diagnosed between January 2011-December 2015.(4-7) Therefore, this study sought not to validate previous findings, but rather to define long-term patterns of survival and mortality in this cohort where survivors had at least four years of follow-up.
Patients were included if ≤18.9 years of age at time of KS diagnosis. They were confirmed as (1) alive, (2) having died, or (3) lost to follow-up (LTFU). Patients were excluded if their records were incomplete (e.g. insufficient details of clinical presentation). Data granularity for detailed clinical factors at time of original diagnosis were limited for patients diagnosed prior to 2010 due to lack of systematic and comprehensive clinical documentation. Therefore, these analyses do not investigate specific clinical features associated with survival, but rather focus on general survival trends. De-identified data were extracted from an electronic medical record and standardized patient treatment forms. Due to the retrospective nature of this study, informed consent was not obtained. This study was approved by the Baylor College of Medicine Institutional Review Board and the Malawi National Health Sciences Research Committee.
Our team has previously defined a pediatric-specific staging system to address distinct clinical features of childhood KS observed in HHV-8 endemic regions of Africa.(7) This staging system stratifies patients by clinical phenotype with prognostic implications, defining four groups with progressively worse survival outcomes—mild/moderate mucocutaneous KS, lymphadenopathic KS, woody edema, and visceral and/or severe, disseminated mucocutaneous disease.(7, 8, 11) Chemotherapy was uniform for both HIV-positive and -negative patients as we have previously described.(5, 6) First line chemotherapy consisted of eight cycles of bleomycin and vincristine (BV); second and third line options for relapsed/refractory and/or advanced-stage disease included non-liposomal doxorubicin plus BV or paclitaxel.(5) Chemotherapy was typically initiated prior to initiation of ART in ART-naïve HIV-positive patients, or at the time of KS diagnosis for HIV-negative patients. Definitions for treatment response have also been previously described.(5) Treatment responses included complete remission (CR, defined as no clinical evidence of KS lesions and a response sustained off chemotherapy) and partial response (PR, defined as a subjectively determined reduction of greater than 50% in burden of KS lesions but without achieving CR). Refractory disease included patients who progressed on chemotherapy, while relapsed disease was defined as the recurrence of KS or new lesions that developed after having achieved CR or PR.
HIV testing and treatment with first-line ART were provided per national guidelines, which evolved considerably over the time period of this study but generally included two nucleoside analog reverse-transcriptase inhibitors and one non-nucleoside reverse transcriptase inhibitor or integrase inhibitor. Considering the extensive clinical and prognostic overlap between HIV-negative endemic and HIV-related pediatric KS, coupled with the paucity of literature describing endemic disease in the childhood population, our team feels that it is important to include the HIV-negative subset to avoid further marginalizing this orphan disease.(6) Deaths occurring <6 months from KS diagnosis were defined as early deaths, between 6-24 months as intermediate, and >24 months as late. Cause of death was associated with KS if patients died in the clinical context of overt KS progression, in the absence of any other plausible etiology, including those patients who died while on chemotherapy. Other deaths that occurred on chemotherapy were categorized as treatment-related mortality and non-KS deaths.(5)
We evaluated demographic and clinical information using standard descriptive statistics. Survivorship variables included duration of overall survival (OS). Survival time was measured from date of KS diagnosis established at our referral center—defined as either date of clinical diagnosis or date of biopsy confirmation. Probability of OS was reported using standard Kaplan-Meier curves. Patients who were LTFU were considered to have died for estimations of OS; dates of last follow-up were used as date of death for the curves. A log-rank test estimated survival differences across strata of interest, and p-values were considered significant at α = 0.05. All statistical analyses were performed using STATA v13.1.
RESULTS
A total of 234 patients were identified with KS between the included dates, however 27 patients were excluded due to insufficient or incomplete clinical details. In total, 207 patients were included in our analyses with 36.7% (n=76) being alive, 54.5% (n=113) having died and 8.7% (n=18) LTFU (Table 1). The median age at diagnosis was 8.3 years (range 1.7-18.7), 41.1% (n=85) were female, 33.8% (n=70) were pathology-confirmed diagnoses, and 90.8% (n=188) were HIV-positive. Baseline CD4 counts at time of KS diagnosis were available for 146 patients (77.7%); median CD4 was 324 (interquartile range [IQR] 116-592).
Table 1.
Characteristics of children and adolescents diagnosed with Kaposi sarcoma between 2006-2015 categorized by long-term outcome. Legend: LTFU = Lost to follow-up
| Alive | Died | LTFU | Total | |
|---|---|---|---|---|
| Number of Patients | 76 | 113 | 18 | 207 |
| Female gender, n (%) | 38 (50%) | 41 (36%) | 6 (33%) | 85 (41.1%) |
| Median Age in Years (range) * | 9.3 (1.7-18.7) | 8.0 (1.8-17.9) | 7.0 (2.4-15.6) | 8.3 (1.7-18.7) |
| HIV-status, n (%) | ||||
| Positive | 66 (87%) | 105 (93%) | 17 (94%) | 188 (90.8%) |
| Negative | 10 (13%) | 8 (7%) | 1 (6%) | 19 (9.2%) |
| Median CD4 (interquartile range) * | 367 (151-610) | 292 (66-532) | 438 (210-606) | 324 (113-597) |
Indicates at time of Kaposi sarcoma diagnosis
Among the 76 survivors, the median follow-up duration was 6.9 years (range 4.2-13.9); the median time off chemotherapy was 5.8 years (range 1.5-11.9). Forty-seven survivors remained in first CR, while an additional six achieved stable PR that was sustained without additional chemotherapy. Twenty-three survivors were alive after salvage treatment for relapsed/refractory disease. All but four survivors received chemotherapy as part of their KS treatment; the four exceptions received ART alone. Amongst 39 survivors, median pulse oximetry at last follow up was 99% (IQR 97-99%), measured at a median of 6.5 years of survival (range 4.4-12.7). Of the 76 survivors, 28% (n=21) were diagnosed between 2006-2010 (median follow-up 11.7 years) and 72% (n=55) between 2011-2015 (median follow-up 6.2 years). Among 66 HIV-positive survivors, 38 (58%) were on first-line ART at time of last follow-up, the remainder on second-line ART.
Deaths occurred at a median time of 5.3 months after KS diagnosis (range 0.1-123, Supplemental Table 1). Death was identified as either occurring in the context of KS progression or non-KS-related for 83.2% (n=94). For non-KS mortality where specific cause of death was ascertained, the most common causes were HIV-related complications (e.g. severe malnutrition, opportunistic infections). Notably, KS was associated with death for the majority of early deaths, while the frequency of non-KS deaths increased after 6 months. The distribution of early, intermediate, and late deaths was similar for patients diagnosed between 2006-2010 compared to 2011-2015, with approximately half of deaths occurring early and one-quarter occurring late. Of the 29 patients who died >24 months from diagnosis, 21 experienced relapsed/refractory disease at some time point in their treatment course. For 12 of those 21, cause of death was ascertained as non-KS with their underlying malignancy under control; the remaining nine died in the context of overt KS progression.
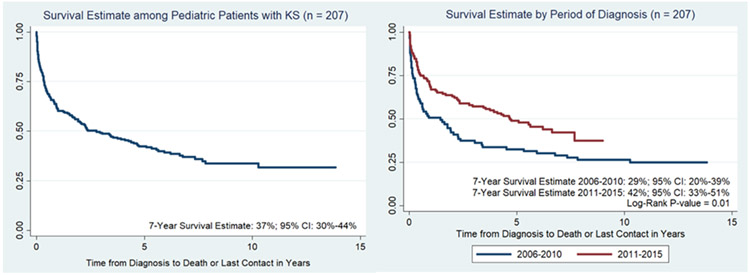
Kaplan-Meier curves demonstrating the probability of OS (with LTFU subset presumed to have died) revealed a 7-year estimate of 37% (95% CI: 30-44%), Figure 1. For the 18 patients that were LTFU, the median time of occurrence of LTFU was 23.6 months from time of KS diagnosis (range 0.4-92.4 months). Seventeen of 188 (9%) children with HIV-related KS and 1/19 (5%) with endemic KS were LTFU. When the LTFU cohort was considered unknown and excluded altogether, 40.2% (76/189) survived. When stratified by period of diagnosis—2006-2010 versus 2011-2015—the 7-year probability of survival was significantly higher for those diagnosed between 2011-2015: 42% (95% CI: 33-51%) versus 29% (95% CI: 20-39%), p = 0.01.
Figure 1.
Kaplan-Meier curves demonstrating probability of overall survival (OS) among pediatric patients with KS and stratified by period of diagnosis.
DISCUSSION
Through evaluation of long-term outcomes for childhood KS in Lilongwe, Malawi, we have gained insight into survival and mortality patterns. With the longest documented survival reaching nearly 14 years, we have shown that long-term survival into and beyond a decade is possible for children and adolescents with KS despite extreme limitations in healthcare infrastructure. Amongst the 76 long-term survivors, over half are in their first CR and nearly 60% remain on first-line ART. While CR is uncommonly reported in adults with KS, children with KS—particularly those with the lymphadenopathic variant—have long been described to achieve CR.(5, 7, 8, 15, 16)
While this study has highlighted that long-term survival is possible, we acknowledge room for continued improvement to combat both KS-related and non-KS causes of death. When survival was stratified based on time period of KS diagnosis, results revealed a greater probability of 7-year OS in more recent times. Recent optimization of outcomes is likely multi-factorial including: greater clinical awareness of the unique features of childhood KS, improved diagnostic pathology resources, consistent availability of chemotherapy, and better control of HIV.
It is notable that 23 of 76 long-term survivors and 21 of 29 patients with late deaths experienced relapsed/refractory KS during their disease course. Among the 21 who died, 12 deaths were secondary to other causes with their KS under control. This ability to successfully salvage patients with relapsed disease makes a case for diligent long-term follow-up of patients beyond their initial treatment. In situations where close follow-up at the tertiary-level KS treatment center is not possible, we educate and empower families prior to transfer to self-monitor and identify relapse early. We also provide support to return to care for any concerns and advocate for increased outreach education to regional health centers to help recognize pediatric KS. Long-term oncology management has helped keep patients alive longer, and some ultimately died of other HIV-related complications. This underlies the complexity of managing HIV-associated malignancies in children and the challenges of overcoming not only the cancer diagnosis, but also the myriad of other illnesses.
To achieve these outcomes, chemotherapy was administered for the vast majority of patients in combination with ART for HIV-positive patients, with only a small fraction of HIV-positive patients (n=4 of 188, 2.1%) achieving long-term survival with ART alone. This contrasts with experiences treating adult KS in high-income countries where HHV-8 is not endemic. Those cohorts are characterized by higher proportions of patients with T0 limited-stage disease, the subset most likely to respond favorably to treatment with ART alone.(17) Our experience is consistent with reports of adult KS from sub-Saharan Africa, where presentation with limited-stage disease is uncommon, and ART alone is usually insufficient treatment.(16, 18, 19) Further, while ART coverage has increased in sub-Saharan Africa over time, up to 75% of children diagnosed with KS have already initiated ART, indirectly demonstrating that ART alone is inadequate to completely prevent or treat pediatric KS.(8, 12) Anecdotally, we express caution that during the early years of our pediatric KS experience in Lilongwe, delays in chemotherapy initiation often led to KS-related deaths despite starting ART, particularly for lymphadenopathic KS.(11)
An important limitation in therapeutic options includes reliance on bleomycin in the chemotherapy regimen. While paclitaxel has recently emerged as a safe and effective option for adults with KS in sub-Saharan Africa, access to paclitaxel or liposomal doxorubicin (standard first-line therapy for adult KS in high-income countries) remains limited due to affordability and availability.(20) Because BV has proven to be well-tolerated, modestly effective, and widely available, clinical programs are forced to accept the long-term risk of pulmonary toxicity associated with bleomycin in order to avoid the acute, life-threatening consequences of untreated KS. Standard of care to monitor for lung toxicity typically involves pulmonary function testing—spirometry and diffusing lung capacity for carbon monoxide—neither of which are available in our setting. However, while not a validated proxy of pulmonary function, measured pulse oximetry levels were all within normal ranges and no patient demonstrated clinical evidence of chronic pulmonary disease.
Limitations include the retrospective nature of the study. Challenges arose in data collection due to lack of systematic and comprehensive documentation of baseline clinical features at time of diagnosis prior to 2010, when standardized clinical data collection forms were introduced. As we stratify the cohort based on outcomes, comparisons within groups is limited by sample size. Inclusion of the 8.7% LTFU as having died leads to underestimation of survival estimates and results must be interpreted accordingly.(21) Information on treatment abandonment was not available for this cohort, but in our subset of patient diagnosed between 2010-2013, overall treatment abandonment occurred in 7%.(5) Further, stratification of cause of death as related to KS or a non-KS etiology involves some aspect of speculation given limited resources for testing in the final days of life or post-mortem. We therefore chose to assign KS as the driver of mortality only in those patients whose death occurred in the context of overt KS progression (typically manifest as progressive visceral involvement or multi-organ failure from an HHV-8 related systemic inflammatory syndrome). Ideally, future outcome studies will also include objective measures of quality of life to comprehensively report the nature of long-term survivorship, especially for those patients with woody edema. Nevertheless, this is the largest study of long-term survival of pediatric KS ever reported, establishing evidence of long-term survival for children and adolescents treated amidst severe limitations in medical infrastructure in a low-income country. These data advocate for continued investment in strengthening systems to treat childhood cancer for both HIV-infected and HIV-negative children alike.
Supplementary Material
Acknowledgments:
We wish to honor our long-time mentor and friend, Dr. Peter Kazembe, who passed away in 2020. He was a world-recognized leader in the field of pediatrics for over 30 years and a pioneer in the treatment of childhood HIV and cancer in Malawi. We express admiration to our patients and their families fighting a brave battle against severe illnesses in the setting of limitations in societal infrastructure and medical resources. We thank our many colleagues at the Baylor College of Medicine Children’s Foundation Malawi, the Tingathe Outreach Program, and the Baylor College of Medicine International Pediatric AIDS Initiative at Texas Children’s Hospital. We are grateful for our colleagues at the Texas Children’s Cancer and Hematology Centers and the Global HOPE (Hematology-Oncology Pediatric Excellence) Program, as well as Kamuzu Central Hospital with particular acknowledgment of the Pathology Laboratory and the Department of Paediatrics, the University of North Carolina Project Malawi and the University of North Carolina Vironomics Core Laboratory in Chapel Hill.
Source of Funding:
This work of the pediatric Kaposi sarcoma program at the Baylor College of Medicine Children’s Foundation Malawi in Lilongwe was supported by the National Cancer Institute at the National Institutes of Health [U54CA254569 & R21CA217137], Celgene Cancer Care Links Grant Program, and the Baylor-UT Houston Center for AIDS Research through support from the National Institute of Allergy and Infectious Diseases [AI36211]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the other funders. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflicts of Interest: All authors report no conflicts of interest.
DATA AVAILABILITY STATEMENT:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES:
- 1.Stefan DC. Patterns of distribution of childhood cancer in Africa. J Trop Pediatr. 2015;61(3):165–73. [DOI] [PubMed] [Google Scholar]
- 2.Dollard SC, Butler LM, Jones AM, Mermin JH, Chidzonga M, Chipato T, et al. Substantial regional differences in human herpesvirus 8 seroprevalence in sub-Saharan Africa: insights on the origin of the "Kaposi's sarcoma belt". Int J Cancer. 2010;127(10):2395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Mallawany NK, Villiera J, Kamiyango W, Mhango J, Slone JS, Mehta PS, et al. Increasing Numbers of New Kaposi Sarcoma Diagnoses in HIV-Infected Children and Adolescents Despite the Wide Availability of Antiretroviral Therapy in Malawi. Clin Infect Dis. 2017;64(6):818–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cox CM, El-Mallawany NK, Kabue M, Kovarik C, Schutze GE, Kazembe PN, et al. Clinical characteristics and outcomes of HIV-infected children diagnosed with Kaposi sarcoma in Malawi and Botswana. Pediatr Blood Cancer. 2013;60(8):1274–80. [DOI] [PubMed] [Google Scholar]
- 5.El-Mallawany NK, Kamiyango W, Slone JS, Villiera J, Kovarik CL, Cox CM, et al. Clinical Factors Associated with Long-Term Complete Remission versus Poor Response to Chemotherapy in HIV-Infected Children and Adolescents with Kaposi Sarcoma Receiving Bleomycin and Vincristine: A Retrospective Observational Study. PloS one. 2016;11(4):e0153335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Mallawany NK, Villiera J, Kamiyango W, Peckham-Gregory EC, Scheurer ME, Allen CE, et al. Endemic Kaposi sarcoma in HIV-negative children and adolescents: an evaluation of overlapping and distinct clinical features in comparison with HIV-related disease. Infect Agent Cancer. 2018;13:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Mallawany NK, Kamiyango W, Villiera J, Slone JS, Kovarik CL, Campbell LR, et al. Proposal of a Risk-Stratification Platform to Address Distinct Clinical Features of Pediatric Kaposi Sarcoma in Lilongwe, Malawi. J Glob Oncol. 2018;4(4):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Mallawany NK, McAtee CL, Campbell LR, Kazembe PN. Pediatric Kaposi sarcoma in context of the HIV epidemic in sub-Saharan Africa: current perspectives. Pediatric Health Med Ther. 2018;9:35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Mallawany NK, Mehta PS, Kamiyango W, Villiera J, Peckham-Gregory EC, Kampani C, et al. KSHV viral load and Interleukin-6 in HIV-associated pediatric Kaposi sarcoma-Exploring the role of lytic activation in driving the unique clinical features seen in endemic regions. Int J Cancer. 2019;144(1):110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Mallawany NK, Kamiyango W, Villiera J, Peckham-Gregory EC, Scheurer ME, McAtee CL, et al. Kaposi Sarcoma Herpesvirus Inflammatory Cytokine Syndrome-like Clinical Presentation in Human Immunodeficiency Virus-infected Children in Malawi. Clin Infect Dis. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamiyango W, Villiera J, Silverstein A, Peckham-Gregory E, Campbell LR, El-Mallawany NK. Navigating the heterogeneous landscape of pediatric Kaposi sarcoma. Cancer Metastasis Rev. 2019;38(4):749–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macken M, Dale H, Moyo D, Chakmata E, Depani S, Israels T, et al. Triple therapy of vincristine, bleomycin and etoposide for children with Kaposi sarcoma: Results of a study in Malawian children. Pediatr Blood Cancer. 2018;65(2). [DOI] [PubMed] [Google Scholar]
- 13.Gantt S, Kakuru A, Wald A, Walusansa V, Corey L, Casper C, et al. Clinical presentation and outcome of epidemic Kaposi sarcoma in Ugandan children. Pediatr Blood Cancer. 2010;54(5):670–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stefan DC, Stones DK, Wainwright L, Newton R. Kaposi sarcoma in South African children. Pediatr Blood Cancer. 2011;56(3):392–6. [DOI] [PubMed] [Google Scholar]
- 15.Ziegler JL, Katongole-Mbidde E. Kaposi's sarcoma in childhood: an analysis of 100 cases from Uganda and relationship to HIV infection. Int J Cancer. 1996;65(2):200–3. [DOI] [PubMed] [Google Scholar]
- 16.Hosseinipour MC, Kang M, Krown SE, Bukuru A, Umbleja T, Martin JN, et al. As-Needed Vs Immediate Etoposide Chemotherapy in Combination With Antiretroviral Therapy for Mild-to-Moderate AIDS-Associated Kaposi Sarcoma in Resource-Limited Settings: A5264/AMC-067 Randomized Clinical Trial. Clin Infect Dis. 2018;67(2):251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bower M, Dalla Pria A, Coyle C, Andrews E, Tittle V, Dhoot S, et al. Prospective stage-stratified approach to AIDS-related Kaposi's sarcoma. J Clin Oncol. 2014;32(5):409–14. [DOI] [PubMed] [Google Scholar]
- 18.Herce ME, Kalanga N, Wroe EB, Keck JW, Chingoli F, Tengatenga L, et al. Excellent clinical outcomes and retention in care for adults with HIV-associated Kaposi sarcoma treated with systemic chemotherapy and integrated antiretroviral therapy in rural Malawi. J Int AIDS Soc. 2015;18:19929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mosam A, Shaik F, Uldrick TS, Esterhuizen T, Friedland GH, Scadden DT, et al. A randomized controlled trial of highly active antiretroviral therapy versus highly active antiretroviral therapy and chemotherapy in therapy-naive patients with HIV-associated Kaposi sarcoma in South Africa. J Acquir Immune Defic Syndr. 2012;60(2):150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krown SE, Moser CB, MacPhail P, Matining RM, Godfrey C, Caruso SR, et al. Treatment of advanced AIDS-associated Kaposi sarcoma in resource-limited settings: a three-arm, open-label, randomised, non-inferiority trial. Lancet. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stanley CC, Westmoreland KD, Itimu S, Salima A, van der Gronde T, Wasswa P, et al. Quantifying bias in survival estimates resulting from loss to follow-up among children with lymphoma in Malawi. Pediatr Blood Cancer. 2017;64(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.