Abstract
l-Carnosine is an endogenous dipeptide that has high potential for therapeutic purposes, being an antioxidant with metal chelating, anti-aggregating, anti-inflammatory, and neuroprotective properties. Despite its potential therapeutic values, the biomolecular mechanisms involved in neuroprotection are not fully understood. Here, we demonstrate, at chemical and biochemical levels, that insulin-degrading enzyme plays a pivotal role in carnosine neuroprotection.
Keywords: diabetes, Alzheimer’s disease, insulin, IDE, carnosine, neuropeptides
1. Introduction
Amyloid ß-protein (Aβ) is a complex mixture of peptides of 37–43 amino acids in length that is present in the brain and the cerebrospinal fluid of human beings.1 Aβ represents the key peptide in the pathogenesis of Alzheimer’s disease (AD), a neurodegenerative disorder with a growing prevalence on a global scale. Although AD pathogenesis is not fully characterized yet, systemic accumulation of biomarkers of redox unbalance2 and inflammation,3 along with the deposition of insoluble proteinaceous aggregates in the brain, are hallmarks of disease progression.4−6
In the AD brain, Aβ, which is released upon enzymatic digestion of APP precursor protein in the monomeric state, typically enters a neurodegenerative pathway, which causes it to undergo aggregation characterized by the formation of larger and heavier species,7 among which the soluble oligomers, which anticipate the formation of insoluble aggregates, are supposed to represent the most toxic ones.8
Both peripheral macrophages and microglia, the brain-resident immune cells, represent two different specialized cell types activated during the immune response.9,10 There is a bidirectional cross-talk between microglia and neurons; in fact, neurons inform microglia regarding their status and control activation and the motility of microglia, while microglial cells are able to modulate neuronal homeostasis.11 Reactive microglia co-localize with Aβ within the neuritic plaques observed in the brain of AD subjects and could be implicated either in the removal or, paradoxically, in the formation of amyloid plaques.12−15 Microglia can promote Aβ clearance through different mechanisms including the internalization and degradation of the peptide through the endosome/lysosome pathway16 and the secretion of enzymes able to degrade Aβ such as insulin-degrading enzyme (IDE),17,18 a major enzyme responsible for the degradation of insulin (Ins) and Aβ in vitro and in vivo.19−22 In fact, despite Ins being the preferred substrate for IDE, the enzyme also cleaves different amyloidogenic peptides such as amylin23 and Aβ.24,25 The latter,26,27 as well as IDE itself,28 represents a well-recognized neurobiological link and a common pharmacological target between AD and type 2 diabetes (T2DM). Mice with the homozygous deletion of the IDE gene (IDE–/−) and an IDE deficiency show increased cerebral accumulation of endogenous Aβ, as well as hyperinsulinemia and glucose intolerance, hallmarks of T2DM.29 Positive allosteric modulators of the activity of IDE are currently studied as potential drugs for both pathologies, as shown by the use of a novel IDE inhibitor in a mouse model of T2DM, while activators of IDE have been considered for AD treatment.29 In addition, IDE has been recognized to be involved in many other biochemical pathways wherein it does not play a proteolytic action but rather a regulatory one,19,30−33 envisaging a multifaceted role of this enzyme within the dynamics of metabolism of living organisms.
l-Carnosine (Car) is a naturally occurring dipeptide synthesized by Car synthase composed of β-alanine and l-histidine,34,35 and it is highly concentrated in muscle and brain tissues. The concentration of this dipeptide is very high in cardiac and skeletal muscles, up to 20 mM,36,37 and remains in the millimolar range in the brain.38 Different studies have shown the therapeutic potential of Car in diseases characterized by abnormal oxidative stress,39 inflammation,40 and abnormal protein aggregation, such as diabetes,41,42 AD, but also retinal diseases.43 Nevertheless, the well-documented antioxidant, anti-inflammatory, and anti-aggregative activities of Car make this molecule very attractive for drug discovery approaches in neurodegenerative diseases.44 In addition, Car has shown the ability to interact with macrophage receptors,45 stimulating the phagocytic activities of these cells.46,47 These modulatory activities along with its ability to decrease oxidative stress and inflammation in two in vitro models (macrophages and microglia) of Aβ-induced stress47,48 make Car a very attractive pharmacological tool in the context of AD pathology. Indeed, in the context of AD, Car has been able to revert oxidative stress and microglial activation in a transgenic mouse model of AD,43 while its supplementation was shown to counteract cognitive decline in AD subjects.49 It is worth mentioning that the plasma concentration of Car in subjects with a presumptive diagnosis of AD is significantly lower than that detectable in age- and sex-matched healthy subjects.50
In the present study, we wondered whether Car can exert neuroprotective effects against Aβ oligomers through the modulation of IDE activity. We first investigated the toxic potential of Aβ1–42 oligomers in the absence or presence of Car and/or a highly selective IDE inhibitor (6bK). We conducted these studies in primary mixed neuronal cultures as a well-known and established in vitro model to study Aβ toxicity as well as the therapeutic potential of molecules of interest.48,51 Once the neuroprotective activity of Car was established to be IDE-mediated, we then investigated Car/IDE interaction and the molecular mechanisms underlying the protective effects of Car. For this purpose, we have applied high-performance liquid chromatography–mass spectrometry (HPLC-MS), surface plasmon resonance (SPR), dynamic light scattering (DLS), and fluorescent methods to determine the effect of Car on IDE activity, oligomerization, and cooperativity. Results indicate that the neuroprotective effect of Car is due to a modulation of IDE activity and oligomerization.
2. Results
2.1. Car Prevents the Toxicity of Aβ1–42 Induced in Mixed Neuronal Cultures via IDE
We investigated the neuroprotective activity of Car in mixed cultures of cortical cells consisting of neurons (35–40%) and glial (astrocytes and microglia; 60–65%) cells treated with Aβ1–42 oligomers (1 μM) for 48 h. Because Aβ1–42 is able to potentiate glutamate toxicity,52 the experiments were carried out in the presence of a cocktail of ionotropic glutamate receptor antagonists [MK-801 (10 μM) and DNQX (30 μM)] to exclude the contribution of endogenous excitotoxicity to the overall process of neuronal death. Using this model, neurotoxicity of Aβ oligomers showed faster kinetics, with a substantial increase (about 250%) in the number of trypan blue positive cells (dead neurons) being detected after 48 h of exposure to Aβ1–42 oligomers compared to untreated cells (p < 0.001) (Figure 1). Car significantly decreased the toxicity due to Aβ1–42 oligomers treatment in mixed neuronal cultures, giving a number of dead cells comparable to that observed for untreated cells. The highly selective IDE inhibitor, 6bK, prevented the neuroprotective activity of Car directly applied to mixed neuronal cultures treated with Aβ1–42 oligomers (p < 0.001 compared to Aβ1–42 oligomers + Car). Thus, these experiments show for the first time that IDE is implicated, at least in part, in mediating the neuroprotective effects of Car. The treatment with 6bK had no effect per se on mixed neuronal culture viability in the absence of Aβ1–42 oligomers.
Figure 1.
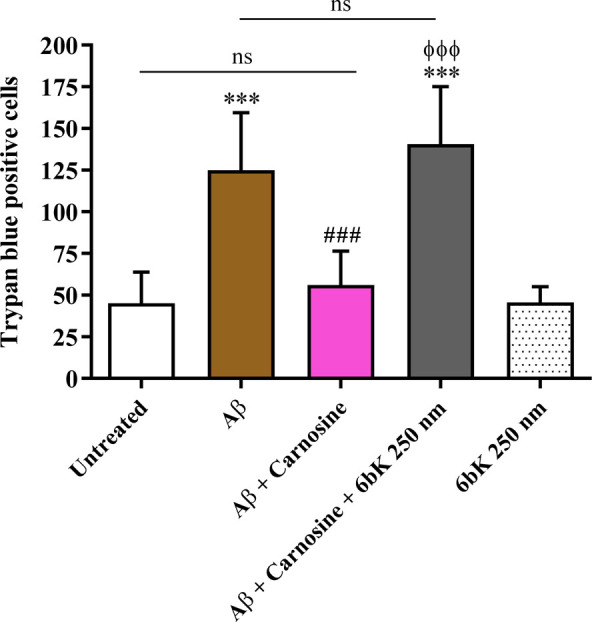
Neuroprotective effects of Car against the toxicity induced by Aβ1–42 oligomers are mediated by IDE. Primary mixed neuronal cultures were treated with Aβ1–42 oligomers (1 μM) for 48 h in the absence or presence of Car (10 mM). The effect of 6bK (highly selective IDE inhibitor) pretreatment (1 h; 250 nM) on the neuroprotective activity of Car against Aβ1–42 oligomer-induced toxicity is also shown. The toxicity of Aβ1–42 oligomers in mixed neuronal cultures was assessed by cell counting after trypan blue staining. Cell counts were performed in three to four random microscopic fields/well. Data are the mean of 7 to 8 determinations. Standard deviations are represented by vertical bars. ***Significantly different from untreated cells, p < 0.001, ###significantly different from Aβ1–42 oligomers, p < 0.001, ϕϕϕsignificantly different from Aβ1–42 oligomers + Car, p < 0.001; ns = not significant.
An additional set of experiments in which mixed neuronal cultures were treated with Aβ1–42 oligomers for 48 h in the absence or presence of increasing concentrations of 6bK (100 and 250 nM) was carried out. The results reported in Figure 1S show how the selective IDE inhibitor did not significantly modify the Aβ-induced cell death.
In order to understand the role played by glial cells in the neuroprotective effects of Car, we treated primary pure neuronal cultures with Aβ1–42 oligomers for 48 h in the absence or presence of Car. As shown in Figure 1S, the treatment of pure neurons with Aβ1–42 oligomers significantly decreased cell viability compared to untreated cells (p < 0.001). Differently from mixed neuronal cultures, Car was not able to revert the toxic effects mediated by Aβ1–42 oligomers, underlining the key role played by glial cells in the neuroprotection elicited by Car.
2.2. Car Induces IDE Oligomerization
DLS was performed to determine the average hydrodynamic diameter (dh) of IDE in the presence of Car. A dose-dependent effect of Car on the stability, conformation, and aggregation of IDE was revealed through thermal denaturation experiments (see Supporting Information). IDE alone showed a hydrodynamic diameter of 12.7 ± 0.9 nm, a finding consistent with previous studies.53 At a concentration of 1 mM, Car caused an increase of IDE diameter (22 ± 2 nm), suggesting that an oligomerization process of IDE actually occurred (Figure 2). DLS measurements were also performed using IDE R767A. This mutation hinders the oligomerization properties of IDE and, therefore, IDE R767A is mainly monomeric. The hydrodynamic diameter of the IDE mutant was found to be 10 ± 1 nm, supporting a minor presence of oligomeric species in solution.32,54 DLS data showed that Car did not significantly affect the hydrodynamic diameter of the IDE R767A. This result demonstrates that Car could induce the oligomerization of IDE.
Figure 2.
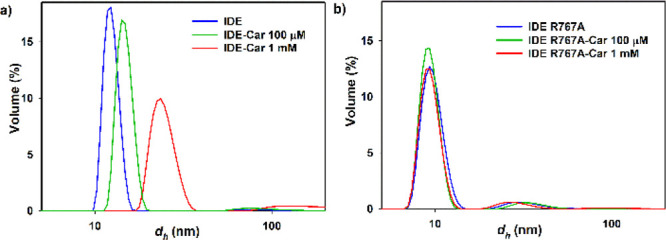
DLS measurements of (a) IDE wild type and (b) IDE R767A in the presence of increasing concentrations of Car.
In addition, the thermal denaturation of IDE was also followed to study the effects of Car on the stability, conformation, and aggregation of IDE at pH 7.4. Figure 3S shows the derived count rate (DCR) variation when the temperature of an IDE solution was increased in the presence/absence of Car. The DCR represents the scattering intensity measured at the detector in the absence of the laser light attenuation filter and therefore it is related to both size and concentrations of the protein.55 As for IDE alone, DCR increased over 50 °C in DLS experiments upon the protein denaturation. Car at 0.1 mM appeared to slightly anticipate the denaturation process, whereas at 1 mM, it promoted a change of IDE size at low temperature. These data further support a dose-dependent effect of Car on IDE stability and oligomerization.
2.3. Car Differently Modulates IDE Activity In Vitro toward Long and Short Substrates
The activity of IDE has been tested for different substrates and by different methods. Indeed, it is well known that IDE activity and allosteric mechanisms are different depending on the length of the substrates, as only long substrates are able to bind the catalytic site as well as the exosite.56 For this reason, Car has been tested as a possible IDE activator toward the degradation of Ins, Aβ1–40, and the short fluorogenic peptide, substrate V.53 In Figure 4S, the cleavage sites of IDE on Ins and Aβ1–40 as obtained by HPLC-MS detection of the peptide fragments generated by the incubation with IDE are reported, whereas in Figure 5S, the normalized areas of the peaks assigned to intact Ins and Aβ1–40 in absence and presence of Car 100 μM and 1 mM as a function of incubation time are plotted.23 In both cases, Car enhances peptide degradation by IDE as the decrease of the Ins and Aβ1–40 molecular peaks is more rapid in the presence of the dipeptide. For this reason, MS experiments confirmed that Car is an activator of IDE activity toward both long substrates tested, Ins and Aβ1–40.
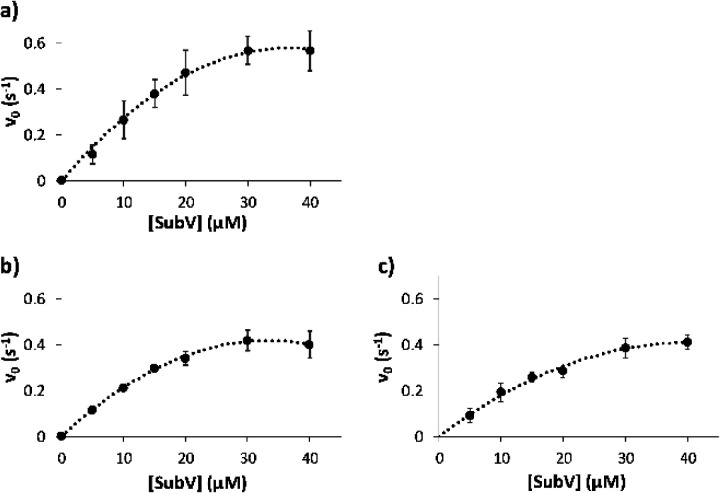
As for the IDE activity toward a short substrate, Figure 3 shows the IDE kinetic graphs, obtained by plotting the initial velocity as a function of the substrate V concentration (a), also in the presence of different amounts of Car (b, c). Based on these results, Car seems to slightly lower the maximum reaction rate, suggesting that Car affects the IDE-dependent degradation of substrate V in a noncompetitive manner. This effect of Car on the IDE activity toward substrate V, different from that reported for that on the IDE-mediated hydrolysis of both Ins and Aβ1–40, could reasonably be ascribed to a different substrate dimension. Bearing that in mind, the fluorimetric assay results showed that Car did not act as an IDE activator toward short peptides like it does for longer ones.
Figure 3.
Kinetic graphs related to the IDE-mediated hydrolysis of substrate V (a) also in the presence of Car 0.1 mM (b) or 1 mM (c).
Finally, it is important to highlight that, besides Car addition, the pH and all the other experimental values have been kept constant during all activity measurements. In order to check if the Car effect was molecule-specific, we have performed IDE activity modulation experiments in the presence of d-carnosine and carnitine, using Ins as a substrate. In neither cases, IDE activity modulation was observed (data not shown), demonstrating a specific modulatory activity for Car and the selective role of stereochemistry.57
2.4. Car Increases IDE Cooperativity
It has been reported that IDE has a Hill coefficient for the degradation of small fluorogenic substrates equal to about 2.0.58 In this work, we have measured IDE-Ins interaction kinetic parameters in the presence and in the absence of Car at different concentrations, by applying a novel SPR approach described in the Supporting Information. In Table 1, the obtained results are reported for both wild-type and IDE R767A. It is possible to see that the effect of Car on the Hill coefficient value n (second column of Table 1) is remarkable. Interestingly, such effect is dependent on the concentration of Car and is not detected in the case of IDE R767A, confirming the DLS results. The variation of the KD value observed in the presence of Car in the case of IDE R767A indicates that a contribution to the activation of the enzyme is possibly given also to a Car induced higher affinity of IDE toward Ins.
Table 1. Hill Coefficient (n) and the Dissociation Constant (KD) Extrapolated from the SPR Analysis of IDE-Ins and IDE R767A-Ins in the Absence and Presence of Cara.
| solution | n | KD | R2 |
|---|---|---|---|
| IDE-Ins | 1.89 ± 0.10 | 1.52 × 10–5 ± 1.19 × 10–6 | 0.9999 |
| IDE-Ins + Car 100 μM | 2.26 ± 0.21 | 1.70 × 10–5 ± 2.16 × 10–6 | 0.9998 |
| IDE-Ins + Car 1 mM | 3.36 ± 0.65 | 8.09 × 10–6 ± 5.79 × 10–7 | 0.9977 |
| IDE R767A-Ins | 1.24 ± 0.03 | 2.66 × 10–1 ± 7.91 × 10–2 | 0.9987 |
| IDE R767A-Ins + Car 100 μM | 1.25 ± 0.02 | 1.25 × 10–4 ± 3.07 × 10–5 | 0.9993 |
| IDE R767A-Ins + Car 1 mM | 1.31 ± 0.53 | 3.50 × 10–5 ± 6.07 × 10–5 | 0.9980 |
The R-square is also reported in the last column.
3. Conclusions
As experts in the field continue to advertise, “many of the most exciting new possibilities hinge on the development of powerful pharmacological modulators of IDE.”59 Car is an endogenous peptide that can be also given orally as a beta-alanine supplement, widely used by many people, especially athletes to improve their performances.60 Although the presence of Car in the serum is not detectable because of its rapid degradation by serum carnosinase,61 intact Car is excreted in urine up to 5 h after intake, indicating that the dipeptide resists somehow to degradation.62 As it is widely reported that Car is neuroprotective,63 here we have explored the possibility that Car exerts its beneficial effect through the modulation of IDE. Our results obtained in rat mixed neuronal cultures clearly show that Car is protective against Aβ1–42-induced toxicity and also that the neuroprotective activity of Car is lost in the presence of 6bK, a highly selective IDE inhibitor, supporting the recent findings described by Fu et al.18 demonstrating that microglia partially degrade Aβ via the secretion of IDE. In order to understand the molecular basis of such an intriguing result, we have applied various experimental approaches to assess the Car mechanism of action on IDE. Indeed, DLS measurements show that Car alters the average hydrodynamic radius of the enzyme, hinting to higher oligomeric forms induced by the presence of Car in a concentration-dependent manner. We exclude that the change in the hydrodynamic radius could be due to a change in enzyme conformation, as IDE R767A used to test such hypothesis did not show the same trend. In accordance to this result, SPR measurements applied to calculate the Hill coefficient gave a clear indication that Car directly affects the enzyme cooperativity, increasing the value of the Hill coefficient in a concentration-dependent manner. Last but not least, HPLC-MS experiments clearly show an increase in IDE activity toward both Ins and Aβ peptides in the presence of Car. On the contrary, the IDE degradation of a smaller fluorogenic substrate does not seem to be affected by the presence of Car. The latter findings give a clear indication on the possible mechanism involved in Car neuroprotection. Indeed, all results point at an IDE activating role of Car due to an increase in the oligomerization and in the cooperativity of the enzyme, which increase the enzyme capability to degrade long substrates such as Ins and Aβ peptides, but not shorter one such as substrate V. This specific regulatory mechanism indicates that Car does not bind to the IDE catalytic site, being a heterotropic modulator, as it is able to regulate the enzyme activity by binding to the exosite or to other not identified sites, causing a different interaction between the enzyme and long substrates, changing their reciprocal affinity and, in turn, IDE catalytic activity. Such a result is in accordance with previous findings already reported for IDE activity53,56 and opens a new path to explore the therapeutic potential of Car in AD.
Acknowledgments
We thank the University of Catania Programma Ricerca di Ateneo Unict 2020-2022-Linea 2 (Project: 3N-ORACLE) for financial support. We thank Prof. Graziella Vecchio for providing the d-carnosine. We also sincerely thank Prof. Andrea Bellelli and Prof. Massimo Coletta for fruitful discussion and comments.
Glossary
Abbreviations
- IDE
insulin-degrading enzyme
- Ins
insulin
- Car
l-carnosine
- AD
Alzheimer’s disease
- PD
Parkinson disease
- HPLC-MS
high performance liquid chromatography-mass spectrometry
- SPR
surface plasmon resonance
- DLS
dynamic light scattering
- T2DM
type 2 diabetes.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acschemneuro.2c00201.
Materials and reagents; preparation of Ab1–42 oligomers; primary pure and mixed neuronal cultures; measurement of cell viability and cell death by the MTT and trypan blue exclusion assays; statistical analysis; study approval; dynamic light scattering (DLS) measurements; HPLC-MS; enzymatic assays; and surface plasmon resonance (PDF).
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. A.D. and G.C. contributed equally.
This research was supported by University of Catania Programma Ricerca di Ateneo Unict 2020–2022-Linea 2 (Project: 3N-ORACLE). A.D. and G.A.Z. were supported by the PhD program in Chemical Sciences, University of Catania. G.C. and F.C. were supported by the Italian Ministry of Health Research Program 2018 (12 December 2018), grant number RC: 2635256.
The authors declare no competing financial interest.
Supplementary Material
References
- Haass C.; Schlossmacher M. G.; Hung A. Y.; Vigo-Pelfrey C.; Mellon A.; Ostaszewski B. L.; Lieberburg I.; Koo E. H.; Schenk D.; Teplow D. B. Amyloid Beta-Peptide Is Produced by Cultured Cells during Normal Metabolism. Nature 1992, 359, 322–325. 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- Huang W.-J.; Zhang X.; Chen W.-W. Role of Oxidative Stress in Alzheimer’s Disease. Biomed. Rep. 2016, 4, 519–522. 10.3892/br.2016.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastre M.; Klockgether T.; Heneka M. T. Contribution of Inflammatory Processes to Alzheimer’s Disease: Molecular Mechanisms. Int. J. Dev. Neurosci. 2006, 24, 167–176. 10.1016/j.ijdevneu.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Younkin S. G. Evidence That A Beta 42 Is the Real Culprit in Alzheimer’s Disease. Ann. Neurol. 1995, 37, 287–288. 10.1002/ana.410370303. [DOI] [PubMed] [Google Scholar]
- Haass C.; Hung A. Y.; Schlossmacher M. G.; Oltersdorf T.; Teplow D. B.; Selkoe D. J. Normal Cellular Processing of the Beta-Amyloid Precursor Protein Results in the Secretion of the Amyloid Beta Peptide and Related Molecules. Ann. N. Y. Acad. Sci. 1993, 695, 109–116. 10.1111/j.1749-6632.1993.tb23037.x. [DOI] [PubMed] [Google Scholar]
- Brion J. P. Neurofibrillary Tangles and Alzheimer’s Disease. Eur. Neurol. 1998, 40, 130–140. 10.1159/000007969. [DOI] [PubMed] [Google Scholar]
- Brorsson A.-C.; Kumita J. R.; MacLeod I.; Bolognesi B.; Speretta E.; Luheshi L. M.; Knowles T. P. J.; Dobson C. M.; Crowther D. C. Methods and Models in Neurodegenerative and Systemic Protein Aggregation Diseases. Front. Biosci. 2010, 15, 373–396. 10.2741/3626. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. Soluble Oligomers of the Amyloid Beta-Protein Impair Synaptic Plasticity and Behavior. Behav. Brain Res. 2008, 192, 106–113. 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez F. O.; Helming L.; Gordon S. Alternative Activation of Macrophages: An Immunologic Functional Perspective. Annu. Rev. Immunol. 2009, 27, 451–483. 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- Malagoli D.; Mandrioli M.; Tascedda F.; Ottaviani E. Circulating Phagocytes: The Ancient and Conserved Interface between Immune and Neuroendocrine Function. Biol. Rev. Cambridge Philos. Soc. 2017, 92, 369–377. 10.1111/brv.12234. [DOI] [PubMed] [Google Scholar]
- Szepesi Z.; Manouchehrian O.; Bachiller S.; Deierborg T. Bidirectional Microglia-Neuron Communication in Health and Disease. Front. Cell Neurosci. 2018, 12, 323. 10.3389/fncel.2018.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D.; Haverkamp L. J.; Yu J. H.; Karshin W.; Tom D.; Li J.; Kirkpatrick J.; Kuo L. M.; Roher A. E. Specific Domains of Beta-Amyloid from Alzheimer Plaque Elicit Neuron Killing in Human Microglia. J. Neurosci. 1996, 16, 6021–6037. 10.1523/JNEUROSCI.16-19-06021.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Cella M.; Mallinson K.; Ulrich J. D.; Young K. L.; Robinette M. L.; Gilfillan S.; Krishnan G. M.; Sudhakar S.; Zinselmeyer B. H.; Holtzman D. M.; Cirrito J. R.; Colonna M. TREM2 Lipid Sensing Sustains the Microglial Response in an Alzheimer’s Disease Model. Cell 2015, 160, 1061–1071. 10.1016/j.cell.2015.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren-Shaul H.; Spinrad A.; Weiner A.; Matcovitch-Natan O.; Dvir-Szternfeld R.; Ulland T. K.; David E.; Baruch K.; Lara-Astaiso D.; Toth B.; Itzkovitz S.; Colonna M.; Schwartz M.; Amit I. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 2017, 169, 1276–1290.e17. 10.1016/j.cell.2017.05.018. [DOI] [PubMed] [Google Scholar]
- Henstridge C. M.; Hyman B. T.; Spires-Jones T. L. Beyond the Neuron-Cellular Interactions Early in Alzheimer Disease Pathogenesis. Nat. Rev. Neurosci. 2019, 20, 94–108. 10.1038/s41583-018-0113-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco-Quinto J.; Eckman E. A. Endothelin-Converting Enzymes Degrade Intracellular β-Amyloid Produced within the Endosomal/Lysosomal Pathway and Autophagosomes. J. Biol. Chem. 2013, 288, 5606–5615. 10.1074/jbc.M112.422964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu W. Q.; Walsh D. M.; Ye Z.; Vekrellis K.; Zhang J.; Podlisny M. B.; Rosner M. R.; Safavi A.; Hersh L. B.; Selkoe D. J. Insulin-Degrading Enzyme Regulates Extracellular Levels of Amyloid Beta-Protein by Degradation. J. Biol. Chem. 1998, 273, 32730–32738. 10.1074/jbc.273.49.32730. [DOI] [PubMed] [Google Scholar]
- Fu H.; Liu B.; Li L.; Lemere C. A. Microglia Do Not Take Up Soluble Amyloid-Beta Peptides, But Partially Degrade Them by Secreting Insulin-Degrading Enzyme. Neuroscience 2020, 443, 30–43. 10.1016/j.neuroscience.2020.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacławczyk D.; Silberring J.; Grasso G. The Insulin-Degrading Enzyme as a Link between Insulin and Neuropeptides Metabolism. J. Enzyme Inhib. Med. Chem. 2021, 36, 183–187. 10.1080/14756366.2020.1850712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso G.; Rizzarelli E.; Spoto G. AP/MALDI-MS Complete Characterization of the Proteolytic Fragments Produced by the Interaction of Insulin Degrading Enzyme with Bovine Insulin. J. Mass Spectrom. 2007, 42, 1590–1598. 10.1002/jms.1348. [DOI] [PubMed] [Google Scholar]
- Grasso G.; Rizzarelli E.; Spoto G. The Proteolytic Activity of Insulin-Degrading Enzyme: A Mass Spectrometry Study. J. Mass Spectrom. 2009, 44, 735–741. 10.1002/jms.1550. [DOI] [PubMed] [Google Scholar]
- Bellia F.; Pietropaolo A.; Grasso G. Formation of Insulin Fragments by Insulin-Degrading Enzyme: The Role of Zinc(II) and Cystine Bridges. J. Mass Spectrom. 2013, 48, 135–140. 10.1002/jms.3060. [DOI] [PubMed] [Google Scholar]
- Bellia F.; Grasso G. The Role of Copper(II) and Zinc(II) in the Degradation of Human and Murine IAPP by Insulin-Degrading Enzyme. J. Mass Spectrom. 2014, 49, 274–279. 10.1002/jms.3338. [DOI] [PubMed] [Google Scholar]
- Duckworth W. C.; Bennett R. G.; Hamel F. G. Insulin Degradation: Progress and Potential. Endocr. Rev. 1998, 19, 608–624. 10.1210/edrv.19.5.0349. [DOI] [PubMed] [Google Scholar]
- Grasso G.; Mineo P.; Rizzarelli E.; Spoto G. MALDI, AP/MALDI and ESI Techniques for the MS Detection of Amyloid β-Peptides. Int. J. Mass Spectrom. 2009, 282, 50–55. 10.1016/j.ijms.2009.02.008. [DOI] [Google Scholar]
- Caruso G.; Fresta C. G.; Lazzarino G.; Distefano D. A.; Parlascino P.; Lunte S. M.; Lazzarino G.; Caraci F. Sub-Toxic Human Amylin Fragment Concentrations Promote the Survival and Proliferation of SH-SY5Y Cells via the Release of VEGF and HspB5 from Endothelial RBE4 Cells. Int. J. Mol. Sci. 2018, 19, E3659 10.3390/ijms19113659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso G.; Distefano D. A.; Parlascino P.; Fresta C. G.; Lazzarino G.; Lunte S. M.; Nicoletti V. G. Receptor-Mediated Toxicity of Human Amylin Fragment Aggregated by Short- and Long-Term Incubations with Copper Ions. Mol. Cell. Biochem. 2017, 425, 85–93. 10.1007/s11010-016-2864-1. [DOI] [PubMed] [Google Scholar]
- Pivovarova O.; Höhn A.; Grune T.; Pfeiffer A. F. H.; Rudovich N. Insulin-Degrading Enzyme: New Therapeutic Target for Diabetes and Alzheimer’s Disease?. Ann. Med. 2016, 48, 614–624. 10.1080/07853890.2016.1197416. [DOI] [PubMed] [Google Scholar]
- Farris W.; Mansourian S.; Chang Y.; Lindsley L.; Eckman E. A.; Frosch M. P.; Eckman C. B.; Tanzi R. E.; Selkoe D. J.; Guenette S. Insulin-Degrading Enzyme Regulates the Levels of Insulin, Amyloid Beta-Protein, and the Beta-Amyloid Precursor Protein Intracellular Domain in Vivo. Proc. Natl. Acad. Sci. U. S. A. 2003, 100, 4162–4167. 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso G.; Lanza V.; Malgieri G.; Fattorusso R.; Pietropaolo A.; Rizzarelli E.; Milardi D. The Insulin Degrading Enzyme Activates Ubiquitin and Promotes the Formation of K48 and K63 Diubiquitin. Chem. Commun. 2015, 51, 15724–15727. 10.1039/c5cc06786c. [DOI] [PubMed] [Google Scholar]
- Tundo G. R.; Sbardella D.; Ciaccio C.; Grasso G.; Gioia M.; Coletta A.; Polticelli F.; Di Pierro D.; Milardi D.; Van Endert P.; Marini S.; Coletta M. Multiple Functions of Insulin-Degrading Enzyme: A Metabolic Crosslight?. Crit. Rev. Biochem. Mol. Biol. 2017, 52, 554–582. 10.1080/10409238.2017.1337707. [DOI] [PubMed] [Google Scholar]
- Bellia F.; Lanza V.; Ahmed I. M. M.; Garcia-Vinuales S.; Veiss E.; Arizzi M.; Calcagno D.; Milardi D.; Grasso G. Site Directed Mutagenesis of Insulin-Degrading Enzyme Allows Singling out the Molecular Basis of Peptidase versus E1-like Activity: The Role of Metal Ions†. Metallomics 2019, 11, 278–281. 10.1039/c8mt00288f. [DOI] [PubMed] [Google Scholar]
- Zingale G. A.; Bellia F.; Ahmed I. M. M.; Mielczarek P.; Silberring J.; Grasso G. IDE Degrades Nociceptin/Orphanin FQ through an Insulin Regulated Mechanism. Int. J. Mol. Sci. 2019, 20, E4447 10.3390/ijms20184447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyankar G. D.; Meister A. Enzymatic Synthesis of Carnosine and Related Beta-Alanyl and Gamma-Aminobutyryl Peptides. J. Biol. Chem. 1959, 234, 3210–3218. 10.1016/S0021-9258(18)69651-6. [DOI] [PubMed] [Google Scholar]
- Winnick R. E.; Winnick T. Carnosine-Anserine Synthetase of Muscle I. Preparation and Properties of a Soluble Enyzme from Chick Muscle. Biochim. Biophys. Acta 1959, 31, 47–55. 10.1016/0006-3002(59)90437-8. [DOI] [PubMed] [Google Scholar]
- Boldyrev A. A.; Aldini G.; Derave W. Physiology and Pathophysiology of Carnosine. Physiol. Rev. 2013, 93, 1803–1845. 10.1152/physrev.00039.2012. [DOI] [PubMed] [Google Scholar]
- Gariballa S. E.; Sinclair A. J. Carnosine: Physiological Properties and Therapeutic Potential. Age Ageing 2000, 29, 207–210. 10.1093/ageing/29.3.207. [DOI] [PubMed] [Google Scholar]
- Hipkiss A. R.; Preston J. E.; Himsworth D. T.; Worthington V. C.; Keown M.; Michaelis J.; Lawrence J.; Mateen A.; Allende L.; Eagles P. A.; Abbott N. J. Pluripotent Protective Effects of Carnosine, a Naturally Occurring Dipeptide. Ann. N. Y. Acad. Sci. 1998, 854, 37–53. 10.1111/j.1749-6632.1998.tb09890.x. [DOI] [PubMed] [Google Scholar]
- Bellia F.; Vecchio G.; Cuzzocrea S.; Calabrese V.; Rizzarelli E. Neuroprotective Features of Carnosine in Oxidative Driven Diseases. Mol. Aspects Med. 2011, 32, 258–266. 10.1016/j.mam.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Di Paola R.; Impellizzeri D.; Salinaro A. T.; Mazzon E.; Bellia F.; Cavallaro M.; Cornelius C.; Vecchio G.; Calabrese V.; Rizzarelli E.; Cuzzocrea S. Administration of Carnosine in the Treatment of Acute Spinal Cord Injury. Biochem. Pharmacol. 2011, 82, 1478–1489. 10.1016/j.bcp.2011.07.074. [DOI] [PubMed] [Google Scholar]
- Albrecht T.; Schilperoort M.; Zhang S.; Braun J. D.; Qiu J.; Rodriguez A.; Pastene D. O.; Krämer B. K.; Köppel H.; Baelde H.; de Heer E.; Anna Altomare A.; Regazzoni L.; Denisi A.; Aldini G.; van den Born J.; Yard B. A.; Hauske S. J. Carnosine Attenuates the Development of Both Type 2 Diabetes and Diabetic Nephropathy in BTBR Ob/Ob Mice. Sci. Rep. 2017, 7, 44492. 10.1038/srep44492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miceli V.; Pampalone M.; Frazziano G.; Grasso G.; Rizzarelli E.; Ricordi C.; Casu A.; Iannolo G.; Conaldi P. G. Carnosine Protects Pancreatic Beta Cells and Islets against Oxidative Stress Damage. Mol. Cell. Endocrinol. 2018, 474, 105–118. 10.1016/j.mce.2018.02.016. [DOI] [PubMed] [Google Scholar]
- Herculano B.; Tamura M.; Ohba A.; Shimatani M.; Kutsuna N.; Hisatsune T. β-Alanyl-L-Histidine Rescues Cognitive Deficits Caused by Feeding a High Fat Diet in a Transgenic Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2013, 33, 983–997. 10.3233/JAD-2012-121324. [DOI] [PubMed] [Google Scholar]
- Caruso G.; Caraci F.; Jolivet R. B. Pivotal Role of Carnosine in the Modulation of Brain Cells Activity: Multimodal Mechanism of Action and Therapeutic Potential in Neurodegenerative Disorders. Prog. Neurobiol. 2019, 175, 35–53. 10.1016/j.pneurobio.2018.12.004. [DOI] [PubMed] [Google Scholar]
- Guiotto A.; Calderan A.; Ruzza P.; Borin G. Carnosine and Carnosine-Related Antioxidants: A Review. Curr. Med. Chem. 2005, 12, 2293–2315. 10.2174/0929867054864796. [DOI] [PubMed] [Google Scholar]
- Onufriev M. V.; Potanova G. I.; Silaeva S. A.; Nikolaev A. I. Carnosine as a stimulator of cytotoxic and phagocytic function of peritoneal macrophages. Biokhimiia 1992, 57, 1352–1359. [PubMed] [Google Scholar]
- Caruso G.; Benatti C.; Musso N.; Fresta C. G.; Fidilio A.; Spampinato G.; Brunello N.; Bucolo C.; Drago F.; Lunte S. M.; Peterson B. R.; Tascedda F.; Caraci F. Carnosine Protects Macrophages against the Toxicity of Aβ1-42 Oligomers by Decreasing Oxidative Stress. Biomedicines 2021, 9, 477. 10.3390/biomedicines9050477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso G.; Fresta C. G.; Musso N.; Giambirtone M.; Grasso M.; Spampinato S. F.; Merlo S.; Drago F.; Lazzarino G.; Sortino M. A.; Lunte S. M.; Caraci F. Carnosine Prevents Aβ-Induced Oxidative Stress and Inflammation in Microglial Cells: A Key Role of TGF-Β1. Cell 2019, 8, 64. 10.3390/cells8010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso G.; Godos J.; Castellano S.; Micek A.; Murabito P.; Galvano F.; Ferri R.; Grosso G.; Caraci F. The Therapeutic Potential of Carnosine/Anserine Supplementation against Cognitive Decline: A Systematic Review with Meta-Analysis. Biomedicines 2021, 9, 253. 10.3390/biomedicines9030253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonteh A. N.; Harrington R. J.; Tsai A.; Liao P.; Harrington M. G. Free Amino Acid and Dipeptide Changes in the Body Fluids from Alzheimer’s Disease Subjects. Amino Acids 2007, 32, 213–224. 10.1007/s00726-006-0409-8. [DOI] [PubMed] [Google Scholar]
- Caraci F.; Tascedda F.; Merlo S.; Benatti C.; Spampinato S. F.; Munafò A.; Leggio G. M.; Nicoletti F.; Brunello N.; Drago F.; Sortino M. A.; Copani A. Fluoxetine Prevents Aβ1-42-Induced Toxicity via a Paracrine Signaling Mediated by Transforming-Growth-Factor-Β1. Front. Pharmacol. 2016, 7, 389. 10.3389/fphar.2016.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraci F.; Molinaro G.; Battaglia G.; Giuffrida M. L.; Riozzi B.; Traficante A.; Bruno V.; Cannella M.; Merlo S.; Wang X.; Heinz B. A.; Nisenbaum E. S.; Britton T. C.; Drago F.; Sortino M. A.; Copani A.; Nicoletti F. Targeting Group II Metabotropic Glutamate (MGlu) Receptors for the Treatment of Psychosis Associated with Alzheimer’s Disease: Selective Activation of MGlu2 Receptors Amplifies Beta-Amyloid Toxicity in Cultured Neurons, Whereas Dual Activation of MGlu2 and MGlu3 Receptors Is Neuroprotective. Mol. Pharmacol. 2011, 79, 618–626. 10.1124/mol.110.067488. [DOI] [PubMed] [Google Scholar]
- Im H.; Manolopoulou M.; Malito E.; Shen Y.; Zhao J.; Neant-Fery M.; Sun C.-Y.; Meredith S. C.; Sisodia S. S.; Leissring M. A.; Tang W.-J. Structure of Substrate-Free Human Insulin-Degrading Enzyme (IDE) and Biophysical Analysis of ATP-Induced Conformational Switch of IDE. J. Biol. Chem. 2007, 282, 25453–25463. 10.1074/jbc.M701590200. [DOI] [PubMed] [Google Scholar]
- Ralat L. A.; Guo Q.; Ren M.; Funke T.; Dickey D. M.; Potter L. R.; Tang W.-J. Insulin-Degrading Enzyme Modulates the Natriuretic Peptide-Mediated Signaling Response. J. Biol. Chem. 2011, 286, 4670–4679. 10.1074/jbc.M110.173252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourlinos A. B.; Trivizas G.; Karakassides M. A.; Baikousi M.; Kouloumpis A.; Gournis D.; Bakandritsos A.; Hola K.; Kozak O.; Zboril R.; Papagiannouli I.; Aloukos P.; Couris S. Green and Simple Route toward Boron Doped Carbon Dots with Significantly Enhanced Non-Linear Optical Properties. Carbon 2015, 83, 173–179. 10.1016/j.carbon.2014.11.032. [DOI] [Google Scholar]
- Hulse R. E.; Ralat L. A.; Tang W.-J. Structure, Function, and Regulation of Insulin-Degrading Enzyme. Vitam. Horm. 2009, 80, 635–648. 10.1016/S0083-6729(08)00622-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciacca M. F. M.; Romanucci V.; Zarrelli A.; Monaco I.; Lolicato F.; Spinella N.; Galati C.; Grasso G.; D’Urso L.; Romeo M.; Diomede L.; Salmona M.; Bongiorno C.; Fabio G. D.; Rosa C. L.; Milardi D. Inhibition of A$\upbeta$ Amyloid Growth and Toxicity by Silybins: The Crucial Role of Stereochemistry. ACS Chem. Neurosci. 2017, 8, 1767–1778. 10.1021/acschemneuro.7b00110. [DOI] [PubMed] [Google Scholar]
- Song E. S.; Rodgers D. W.; Hersh L. B. A Monomeric Variant of Insulin Degrading Enzyme (IDE) Loses Its Regulatory Properties. PLoS One 2010, 5, e9719 10.1371/journal.pone.0009719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leissring M. A. Insulin-Degrading Enzyme: Paradoxes and Possibilities. Cell 2021, 10, 2445. 10.3390/cells10092445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale C.; Artioli G. G.; Gualano B.; Saunders B.; Hobson R. M.; Harris R. C. Carnosine: From Exercise Performance to Health. Amino Acids 2013, 44, 1477–1491. 10.1007/s00726-013-1476-2. [DOI] [PubMed] [Google Scholar]
- Sadikali F.; Darwish R.; Watson W. C. Carnosinase Activity of Human Gastrointestinal Mucosa. Gut 1975, 16, 585–589. 10.1136/gut.16.8.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppermann H.; Elsel S.; Birkemeyer C.; Meixensberger J.; Gaunitz F. Erythrocytes Prevent Degradation of Carnosine by Human Serum Carnosinase. Int. J. Mol. Sci. 2021, 22, 12802. 10.3390/ijms222312802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara M.; Tanaka K.; Kato-Negishi M. Zinc, Carnosine, and Neurodegenerative Diseases. Nutrients 2018, 10, 147. 10.3390/nu10020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.