Abstract
Here, we show how signal amplification by reversible exchange hyperpolarization of a range of 15N-containing synthons can be used to enable studies of their reactivity by 15N nuclear magnetic resonance (NO2– (28% polarization), ND3 (3%), PhCH2NH2 (5%), NaN3 (3%), and NO3– (0.1%)). A range of iridium-based spin-polarization transfer catalysts are used, which for NO2– work optimally as an amino-derived carbene-containing complex with a DMAP-d2 coligand. We harness long 15N spin-order lifetimes to probe in situ reactivity out to 3 × T1. In the case of NO2– (T1 17.7 s at 9.4 T), we monitor PhNH2 diazotization in acidic solution. The resulting diazonium salt (15N-T1 38 s) forms within 30 s, and its subsequent reaction with NaN3 leads to the detection of hyperpolarized PhN3 (T1 192 s) in a second step via the formation of an identified cyclic pentazole intermediate. The role of PhN3 and NaN3 in copper-free click chemistry is exemplified for hyperpolarized triazole (T1 < 10 s) formation when they react with a strained alkyne. We also demonstrate simple routes to hyperpolarized N2 in addition to showing how utilization of 15N-polarized PhCH2NH2 enables the probing of amidation, sulfonamidation, and imine formation. Hyperpolarized ND3 is used to probe imine and ND4+ (T1 33.6 s) formation. Furthermore, for NO2–, we also demonstrate how the 15N-magnetic resonance imaging monitoring of biphasic catalysis confirms the successful preparation of an aqueous bolus of hyperpolarized 15NO2– in seconds with 8% polarization. Hence, we create a versatile tool to probe organic transformations that has significant relevance for the synthesis of future hyperpolarized pharmaceuticals.
Introduction
Positron emission tomography (PET) is a very sensitive technique that uses gamma cameras to image changes in metabolic processes, blood flow, and agent absorption in the body. It takes long-lived radionuclides generated using a cyclotron that are then embedded into a suitable receptor to create the radiopharmaceuticals that convey the diagnostic response. Unfortunately, this process can be complex and costly. Magnetic resonance imaging (MRI) is another powerful diagnostic method, but inherent low sensitivity means that routine clinical measurements probe highly abundant water.
Consequently, there has been a great deal of excitement in the clinical community with a method called hyperpolarization that gives MRI the sensitivity needed to visualize changes in metabolic flux by detecting biomolecules that encode disease. The most clinically developed method currently involves the use of dissolution dynamic nuclear polarization (d-DNP).1−3 One alternative method to create hyperpolarization is parahydrogen (p-H2)-induced polarization (PHIP), which despite being discovered in the 1980s is only now receiving worldwide attention. Three recent significant PHIP advances that utilize p-H2 are signal amplification by reversible exchange (SABRE),4p-H2-induced polarization with side-arm hydrolysis,5 and the rapid hyperpolarization and purification of the metabolite fumarate.6,7 As p-H2 can be prepared to a level of 50% purity by simply cooling H2 gas by liquid nitrogen,8 one could imagine the widespread future use of this MR sensitization approach.
Here, we demonstrate how it is possible to turn PHIP into a versatile tool for the in situ synthesis of a family of long-lived and highly MR visible precursors containing 15N, akin to the radionuclides of PET. These reactive intermediates are rapidly embedded into important molecular reporters to illustrate the creation of the hyperpharmaceutical. We achieve this by harnessing reactive species like nitrite (NO2–), nitrosonium (NO+), and ammonia (NH3), representing simple building blocks which can be transformed into a wide range of hyperpolarized materials.
Our method takes their 15N isotopologues and uses PHIP to first hyperpolarize them. Thus we create an imbalance in one of the 15N’s two possible nuclear spin orientations (+1/2 or −1/2) which can potentially be maintained for 10’s of minutes if stored in an appropriate magnetic field.9−11 We focus on establishing this concept by reference to nuclear magnetic resonance (NMR), a technique that is used by many scientific disciplines. NMR mainly detects 1H responses, because the most commonly probed alternative nucleus, 13C, is 6400 times harder to detect than 1H. This is due to 13C’s 1% abundance and small gyromagnetic ratio (γ). Consequently, the Zeeman splitting yielding the resonance frequency is four times smaller than 1H, and a minute macroscopic nuclear magnetization occurs, which is detected by NMR.
Less utilized 15N has a highly informative 1350 ppm chemical shift range and long T1,9 but as 15N is only 0.36% abundant and has a γ 10 times smaller than 1H, it is 260,000 times harder to detect. Hence, high-concentration samples and extensive signal averaging are needed for NMR studies at natural abundance. Despite this limitation, nitrite ions have been probed by 15N NMR in solution and the solid state12 and used to study chemodenitrification in humic substances13 and nitric oxide release from copper sites, so its utility is established.14 Importantly, the 15N isotope can be sourced cheaply in materials like 15NH4Cl and Na15NO2, and this offers routes to synthesize other isotopically labeled compounds such as pharmaceuticals. Hyperpolarized Na15NO2, created by d-DNP, has been studied.15
The sensitivity gains provided by PHIP have already been used widely to aid the study of organic and inorganic chemicals, and it has made the detection of previously hidden intermediates possible.16−19 Our aim here is to illustrate the hyperpharmaceutical concept while establishing that we can track chemical reactivity, complete the diagnostic fingerprinting of materials, and dramatically expand chemical diversity in the field of hyperpolarization.
We start with nitrite (NO2–), a reagent that is formed during the nitrification of ammonia by nitrosomas within the nitrogen cycle.20 While mammals do not absorb nitrites directly, plants use it to form essential nitrogen-containing molecules such as amino acids and further aerobic oxidation of nitrite leads to nitrate. Nitrite is used as a food additive for cured meats21 and approximately 7% of our ingested nitrite comes this source, while the remainder comes from the enterosalivary pathway.3,22 While nitrites are noncarcinogenic, their ability to form nitrosamines can lead to toxicity23 as examined by the research community and mainstream media.24,25 The action of methmyogolbin production by nitrite is, however, beneficial in the treatment of cyanide poisoning and sodium nitrite remains as one of the primary antidotes for acute intoxication.26
The nitrite ion, usually as sodium nitrite, finds widespread use in the chemical industry, because of its oxidizing properties and role in organic transformations; common examples are the Sandmeyer reaction, which transforms aryl amines into aryl halides, and the diazotization reaction that is used en route to the formation of dyes and pigments.27
Nitrite is also an ambidentate ligand that can bind to metals via the N- or O-atoms to form nitro or nitrito complexes, respectively,28,29 with Ni30−33 and Pt34−36 examples being the most prevalent. As the PHIP hyperpolarization method SABRE works through reversible binding of the agent that is set to become hyperpolarized to a metal complex, we hypothesized that polarization of NO2– via such a route is possible.4,11,37,38
In fact, there are a few examples of ionic species such as sodium pyruvate,39,40 sodium acetate,41 and naicin42 that undergo SABRE. This method requires the creation of a scalar coupling network between the target agent and p-H2-derived protons in a catalyst.43−46 Hence, an η1-NO2 (N-nitro) complex with a potentially large hydride-15N coupling would be preferred over η1-ONO (O-nitrito) or η2-O–N–O (O,O-bidentate) linkage isomers. Theoretical descriptions of SABRE are provided by Barskiy and others43,44,47 and account for the magnetization transfer conditions needed to sensitize a range of agents.45 Transfer is optimized at low magnetic fields, typically 6 mT for 1H, or through r.f. excitation at high field.48 When combined with suitable catalyst lifetimes, this has driven the efficient sensitization of 1H, 13C, 15N, 19F, 31P, and 29Si (etc.)39−41,49−59 nuclei.
Here, we also evaluate the azide anion, an excellent nucleophile that readily forms organic azides such as the antiretroviral AZT, Avapro, Diova, and Tamiflu. This functionality can be readily reduced to create amines, and through the Curtius rearrangement carbamates. Copper-catalyzed azide-alkyne cycloadditions or click reactions are also important. Consequently, azide represents an important precursor to agrochemicals, pharmaceuticals, and natural products so its successful hyperpolarization is also desirable.
Typically, when an iridium N-heterocyclic carbene (NHC) catalyst is used, products can be created whose NMR signal strengths are many orders of magnitude higher than those which would be obtained at thermal equilibrium.60,61 Warren et al. in particular stand-out for their work on 15N68 in a refinement called SABRE-SHEATH,51,56 and up to 79% 15N polarization has recently been reported for a range of neutral Lewis bases.62 Several alternative radio-frequency transfer strategies have also been exemplified,48,63,64 and given one of the goals of SABRE is in vivo detection, water-soluble SABRE catalysts have been described,65,66 with the in vitro MRI detection of an 15N response already illustrated.66 Tessari et al. have developed a number of analytical science applications for SABRE67 and other catalyst types have been reported.68 Furthermore, hyperpolarized long-lived singlet states, as pioneered by Levitt,69 have been created and detected after their formation.50,70−74 Consequently, we might expect the benefits of such a simple approach to sensitize a range of 15N-containing reagents to be substantial.
Results and Discussion
Demonstration That an Active SABRE Catalyst Forms with Na15NO2
As indicated, for successful SABRE transfer to occur, the formation of a complex exhibiting spin–spin couplings between the bound substrate and p-H2-derived hydride nuclei is required. Classically, this involves the reaction of a precatalyst (most commonly [IrCl(COD)(IMes)] (1) (IMes = 1,3-bis(2,4,6-trimethylphenyl)imidazolylidene)), with an excess of the selected substrate under a H2 atmosphere. Complexes of type [Ir(H)2(IMes)(sub)3]Cl, when the substrate is a neutral N-heterocycle such as pyridine, meet this requirement.4 Consequently, our initial efforts targeted the synthesis of an active SABRE catalyst with bound NO2– rather than pyridine.
When Na15NO2 (1 equiv) was added to a solution of [IrCl(COD)(IMes)] (1, 5 mM) in methanol-d4, the complete conversion to [Ir(15NO2)(COD)(IMes)] (2) at 298 K (Figure 1a) is observed. This change was readily evident as the 15N signal for free Na15NO2 at δN 611.8 moved to δN 490.7 for the bound NO2– at 255 K (see the Supporting Information). When 2 was then exposed to a 3 bar pressure of H2 at 254 K, the oxidative addition of hydrogen took place to form [Ir(H)2(15NO2)(COD)(IMes)] (3). This complex exhibits 1H NMR for its hydride ligands at δH −18.77 (hydride trans to 15NO2–, 2JHN = 23.1 Hz and 2JHH = 3.3 Hz) and δH −14.17 (hydride trans to COD, 2JHH = 3.3 Hz). Additionally, a signal for bound 15NO2– appears at δN 476.1. Hence, there is a strong hydride-15N coupling in this material that would be commensurate with SABRE. Subsequently, this sample was warmed to 298 K for 20 min. This led to the formation of multiple hydride-containing products, some of which display PHIP on exposure to p-H2 (see the Supporting Information). Pleasingly, a hyperpolarized signal for free Na15NO2 is observed at 298 K in the 15N NMR spectrum after SABRE transfer at −5 mG; a field in the mG range will be needed for efficient SABRE transfer.51,55,56 The resulting 15N signal enhancement was 134-fold and confirms reversible binding of NO2–. Unfortunately, when this sample was left at room temperature for >2 h, SABRE activity was lost because of catalyst decomposition. Hence, we sought to create alternative catalysts that would not only improve the 15N signal enhancement level but also be suitable for repeated measurement over long periods. Coligands have been used to achieve stability in conjunction with weakly binding substrate,40,41,73 to reduce spin dilution42,75,76 and to create hydride ligand chemical, rather than magnetic, inequivalence.77 Hence, we chose to follow this path as it offers a multitude of benefits to SABRE.
Figure 1.
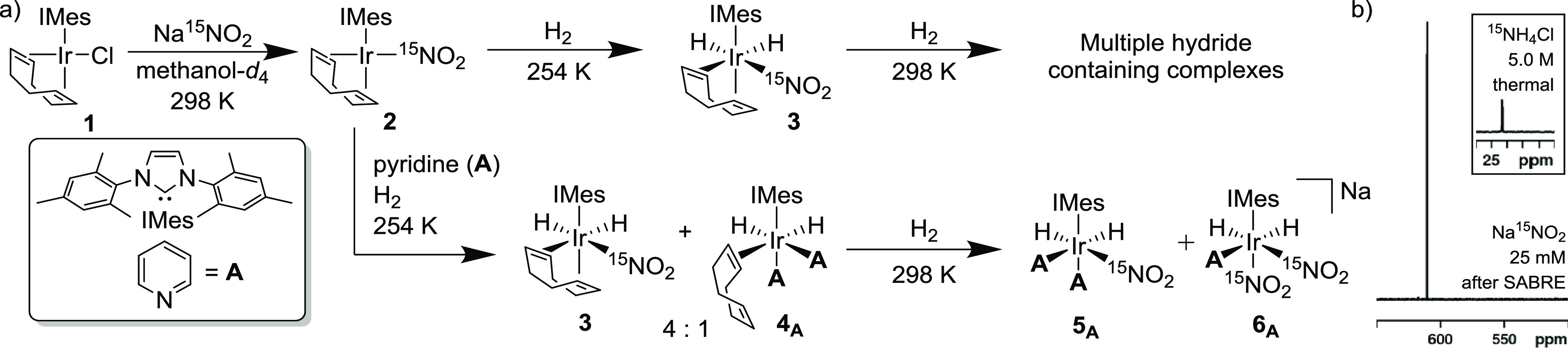
(a) Reaction of [IrCl(COD)(IMes)] with Na15NO2 in the presence of hydrogen and pyridine (inset: structure of the IMes and pyridine ligand). (b) 15N NMR spectrum of Na15NO2 after SABRE hyperpolarization under 3 bar p-H2 (inset: thermally polarized 15N NMR spectrum of a 5.0 M solution of 15NH4Cl in D2O for comparison).
Thus, a sample containing [IrCl(COD)(IMes)] (1, 5 mM), Na15NO2 (5 equiv), and pyridine (3 equiv) was investigated by NMR spectroscopy. The initial formation of [Ir(15NO2)(COD)(IMes)] (2) was indicated. Clearly, nitrite outcompetes pyridine for the [Ir(COD)(IMes)]+ center. Subsequently, exposing this sample to 3 bar H2 at 254 K led again to the formation of neutral 3. Characterization data are provided in the Supporting Information. We note that known [Ir(H)2(IMes)(η1-COD)(pyridine)2]Cl (4A) forms alongside 3 in a 1:4 ratio. After warming the sample for 1 h at room temperature, further reaction to form two additional hydride-containing products takes place. Of these, [Ir(H)2(15NO2)(IMes)(pyridine)2] (5A), with characteristic hydride peaks at δH −21.24 and −22.45, dominates. The former resonance exhibits a 2JNH splitting of 29 Hz, and both show 2JHH couplings of −8 Hz.
A further minor product formed in this reaction proved to be Na[Ir(H)2(15NO2)2(IMes)(pyridine)] (6A). It yields hydride resonances at δH −22.02 (2JNH = 29 Hz) and −23.01 with mutual 2JHH splittings of −7 Hz. Interestingly, no evidence for the formation of tris pyridine-containing [Ir(H)2(IMes)(pyridine)3]Cl was observed78 and unlike the complexes formed in the absence of pyridine, pyridine-derived 5A and 6A proved stable when left at room temperature for >24 h. Given this stability, these species were suitable probes for rigorous assessment of their SABRE performance. When the ratio of Na15NO2 to pyridine was set to 5:3, the ratio of 5A to 6A in solution proved to be 85:15. Further addition of Na15NO2 (25 equiv), while maintaining the pyridine concentration, only moderately shifted the equilibrium between 5A and 6A to 80:20 thereby confirming that neutral [Ir(H)2(15NO2)(IMes)(pyridine)2] (5A) is the most thermodynamically stable of these products.
SABRE Assessment of [Ir(H)2(15NO2)(IMes)(A)2] (5A) and Na[Ir(H)2(15NO2)2(IMes)(A)] (6A) Activity
In order for effective SABRE, the lifetime of the active catalyst must match with the propagating couplings and a level anticrossing condition should be met.43,44,46 To assess the SABRE performance of 5A and 6A, a series of shake and drop measurements were undertaken using a mu-metal shield to attenuate the Earth’s field by a factor of 300 to bring it into the range needed for efficient transfer. These measurements involved first exposing an NMR tube equipped with a J. Youngs Tap containing a solution of [IrCl(COD)(IMes)] (1, 5 mM), Na15NO2 (5 equiv), and pyridine (4 equiv) in methanol-d4 (0.6 mL) to H2 (3 bar) for 1 h to form an 85:15 ratio of 5A to 6A in solution. Subsequently, the H2 atmosphere was replaced with p-H2 (3 bar), and the sample was shaken for 10 s inside the mu-metal shield. After shaking, the sample was transferred into the 9.4 T detection field and an 15N NMR spectrum was recorded immediately.
Spectral analysis revealed that the free 15N signal of Na15NO2 was now ∼880-fold larger than that of the corresponding thermally polarized NMR spectrum, corresponding to a 0.29% 15N polarization level (Figure 1b). SABRE transfer to the 15N of unlabeled pyridine was also observed, and a 172-fold signal gain was quantified for its resonance at δN 301. 15N NMR signals for coordinated NO2– ligands were also readily visible at δN 511.28 (JHN = 29 Hz) for 5A and at δN 509.7 (JHN = 29 Hz) for the NO2– in the equatorial position and at δN 483.7 for the ligand in the axial position of 6A. Repeating the experiment after polarization transfer at 70 G and subsequently recording a 1H NMR spectrum revealed that PHIP-enhanced hydride resonances for 5A and 6A. SABRE hyperpolarization was also quantified for the 1H resonances of free pyridine as ∼230, 60, and 150-fold for its ortho, meta, and para positions, respectively, after 60 G transfer. No evidence for a PHIP-enhanced hydride resonance for [Ir(H)2(IMes)(py)3]Cl at δH −22.7 was observed. We conclude therefore that 15NO2– sensitization is possible through the action of this coligand-supported catalyst.
Effect of Polarization Transfer Field on the Level of 15N NMR Signal Gain in Na15NO2
To improve the levels of signal gain, a more precise polarization transfer field needs to be used. To investigate this effect, a sample containing [IrCl(COD)(IMes)] (1, 5 mM), Na15NO2 (5 equiv), and pyridine (4 equiv) in methanol-d4 (0.6 mL) was exposed to p-H2 (3 bar), and polarization transfer fields from +10 to −10 mG were deployed; these were created by a solenoid located within an mu-metal shield. A profile of the resulting SABRE enhanced resonance for Na15NO2 is presented in the Supporting Information. The highest signal enhancements were observed when the polarization transfer field was nominally +5 or −3 mG with gains of 1948 and 2054-fold, respectively. More precise probing of the polarization transfer field around these maxima revealed that improvement could be achieved using a −3.5 mG value. At this polarization transfer field, a 2329-fold signal gain was quantified through subsequent measurement at 9.4 T which corresponds to an 15N polarization level of 0.77%.
Effect of the Coligand on SABRE Catalysis
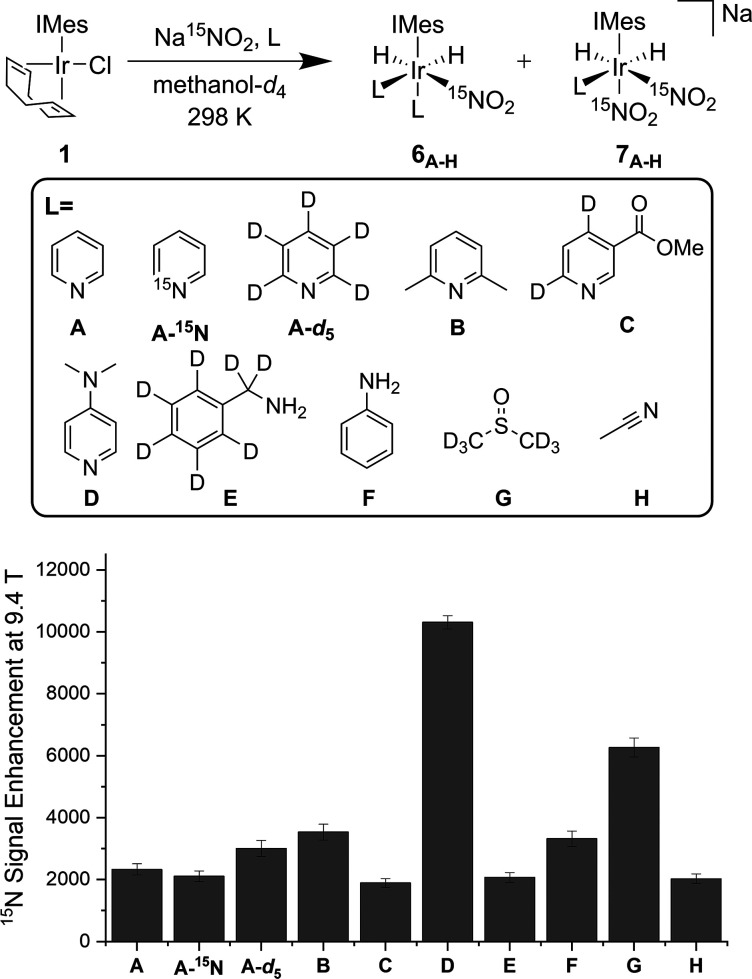
To form 5A and 6A, 15NO2– must out-bind the stabilizing coligand pyridine. Consequently, we hypothesized that the SABRE processes will be sensitive to the identity of this coligand; such a behavior has been observed previously during the SABRE polarization of sodium pyruvate by sulfoxides,39 and there are other examples.41,72,79 Additionally, isotopic labeling of these coligands, which reduces the number of polarization-acceptor spins at the metal center, has proven beneficial while additionally attenuating the effect of relaxation.42,80,81 A suitable range of coligands were therefore examined to test if it were possible to improve the measured polarization levels in free Na15NO2, as detailed in Figure 2.
Figure 2.
Graphical representation of the effect the coligand, A-H, has on the resulting SABRE polarization efficiency for Na15NO2 based on the precatalyst [IrCl(COD)(IMes)], Na15NO2 (25 equiv), and coligand (4 equiv) in methanol-d4 at 298 K. The 15N NMR signal enhancements occur after polarization transfer from 3 bar p-H2 in a −3.5 mG field.
In each case, samples containing [IrCl(COD)(IMes)], Na15NO2 (25 equiv), and the coligand (A-H, 4 equiv) were prepared and then exposed to 3 bar H2 at 298 K for 1 h to form the corresponding complexes 6 and 7. Subsequently, a sample was exposed to 3 bar p-H2, in a −3.5 mG field, prior to its rapid insertion into the 9.4 T detection field. The second coligand tested was 15N labeled pyridine (A-15N), and this reduced the signal enhancement level for Na15NO2 from 2329-fold to 2107-fold. This is likely to reflect the increase in spin dilution associated by increasing the proportion of spin-1/2 nuclei that can accept polarization. The resonance for free 15N-pyridine at δN 301 now exhibits a signal gain of 1558-fold. In contrast, the use of pyridine-d5 (A-d5) improves the SABRE hyperpolarization for Na15NO2 as the new enhancement level increases to 3007-fold. As expected, all the pyridine isotopologues yield analogous complexes, 5 to 6, in a common 85:15 ratio. Hence, catalyst speciation is constant and the ca. 30% improvement, compared to undeuterated A, likely reflects both slower relaxation in the active catalyst and reduced polarization.
To further modulate the coligand, other pyridyl derivatives having different steric and electronic properties were examined. Recently reported 2,6-lutidine (B)82,83 was chosen as its ortho methyl groups hinder binding to the metal center, a change which might promote ligand loss. When B is employed with Na15NO2, an increase in the SABRE polarization level is indeed observed when compared to pyridine. Interestingly, the ratio of 5B to 6B has changed to 95:5. However, slow activation means that 3 (c.f. Figure 1a) is still visible after 1 h at room temperature; at this stage, it exists in a 1:1 ratio with 5B. Unfortunately, when this sample is left under a 3 bar atmosphere of H2 for a longer time to allow full activation, sample degradation and the formation of multiple hydride-containing complexes are noted. Hence, B fails to provide the stability needed.
The use of electron-deficient methyl 4,6-d2-nicotinate (C), which has been shown to exhibit 1H polarization levels of ca. 60% itself under SABRE,42,60 as a coligand was found to decrease the signal enhancement of Na15NO2 to 1894. The formation of mono-C-substituted 6C is favored by this change as the ratio of 5C to 6C became 1:2. In contrast, electron-rich dimethylamino pyridine (D, DMAP) forms 5D in a 17:1 ratio with 6D. Consequently, electron-donating coligands favor the formation of a bis-co-ligand substituted complex. Additionally, a significantly improved 10,313-fold 15N signal enhancement is now observed for Na15NO2 which corresponds to the creation of a 3.4% 15N polarization level.
Nonheterocyclic ligands can also be utilized for SABRE. As such, amine ligands have been shown to be able to form stable SABRE catalysts and are effective agents for SABRE-relay polarization transfer.80,81,84−86 When utilized as a coligand for the hyperpolarization of Na15NO2, benzylamine-d7 (E-d7) led to a 15N signal gain of 2070-fold. The two hydride-containing complexes 5E and 6E were formed under these conditions in a ca. 1:1 ratio with hydride resonances at δH −22.10 and −23.40 and δH −22.36 and −22.72, respectively. When aniline (F) was used as the coligand, a 3322-fold signal gain for Na15NO2 was quantified. In this sample, 5F now dominates.
Similarly, sulfoxides have proven to be efficacious for the hyperpolarization of sodium pyruvate and weakly coordinating substrates.39,40,79,87 The coligand DMSO-d6 (G) gave a 6270-fold signal enhancement for Na15NO2. Interestingly, while Na[Ir(H)2(15NO2)2(IMes)(DMSO-d6)] (6G) is now dominant, a second isomer 7G, where the two 15NO2 ligands lie cis to one another and trans to hydride is observed. This complex gives rise to a single hydride resonance at δH −22.32 where JNHcis + JNHtrans is 27.6 Hz. The 15NO2 resonance of 7G appears at δH 502. Isomer 5G is also detected, but now as a minor species, with the ratio of 5G:6G:7G in solution being ∼1:9:5. Characterization data for these complexes are provided in the Supporting Information. Finally, acetonitrile88 gave a 2029-fold 15N signal gain for NO2–. For acetonitrile, the neutral complex [Ir(H)2(15NO2)(IMes)(acetonitrile)2] (5H), with hydride resonances at δH −22.66 (2JNH = 26.7 Hz, 2JHH = −7 Hz) and −21.77 (2JHH = −7 Hz), was the only complex observed. Clearly substantial coligand effects occur, with D proving optimal.
Identifying the Optimum DMAP (D):Na15NO2 Ratio
Interestingly, this ligand yielded the highest concentration of isomer 5. We postulated that the concentration of 5D in solution could be further manipulated by changing the number of equivalents of D in relation to [IrCl(COD)(IMes)] (1) and Na15NO2. Therefore, a series of samples were prepared with between 3 and 20 equiv of D relative to 1. After activation, they were exposed to 3 bar p-H2 while located in a −3.5 mG polarization transfer field. The resulting signal enhancements at 9.4 T are shown in the Supporting Information.
When three equivalents of D (with respect to iridium) are utilized, a 9086-fold signal enhancement is observed with the corresponding 5D:6D ratio being 8:1. Increasing the concentration of DMAP to four equivalents improved the signal gain seen at 9.4 T to 11,019-fold. The ratio of complex 5D:6D also increased to 17:1. Further incremental increases in DMAP concentration, to 6, 8, and 10 equiv, gave signal enhancements of 12,036, 12,079, and 11,888-fold, respectively. The ratio 5D:6D was now 24:1 in all three samples. At higher loadings of D, the formation of [Ir(H)2(IMes)(D)3]Cl is observed, as a single hydride resonance at δH −23.00. Clearly, this catalyst does not transfer hyperpolarization to Na15NO2 and hinders the overall 15N signal gain because of consumption of p-H2. Therefore, we conclude that a sensible DMAP level lies between 6 and 10 equivalents with respect to iridium. This creates SABRE beneficial 5D as the dominant species in solution.
Synthesis and Utilization of DMAP-d2 for SABRE
As stated, deuteration of ligands within the active catalyst can be beneficial.42,70,76,77,88 We postulated that deuteration of the ortho protons in D, to give DMAP-d2, may lead to further improvements in 15N polarization. Thus, DMAP-d2 was synthesized via H/D exchange from DMAP in D2O under microwave irradiation as reported in the literature.89 Examination of a sample containing [IrCl(COD)(IMes)] (5 mM), DMAP-d2 (6 equiv), and Na15NO2 (25 equiv) in methanol-d4 and exposure to 3 bar p-H2 at a polarization transfer at −3.5 mG led to a signal gain of 13,811 after investigation at 9.4 T (4.56% 15N polarization level). The corresponding value with 1H-DMAP was 12,036, and hence introducing the 2H is beneficial to SABRE.
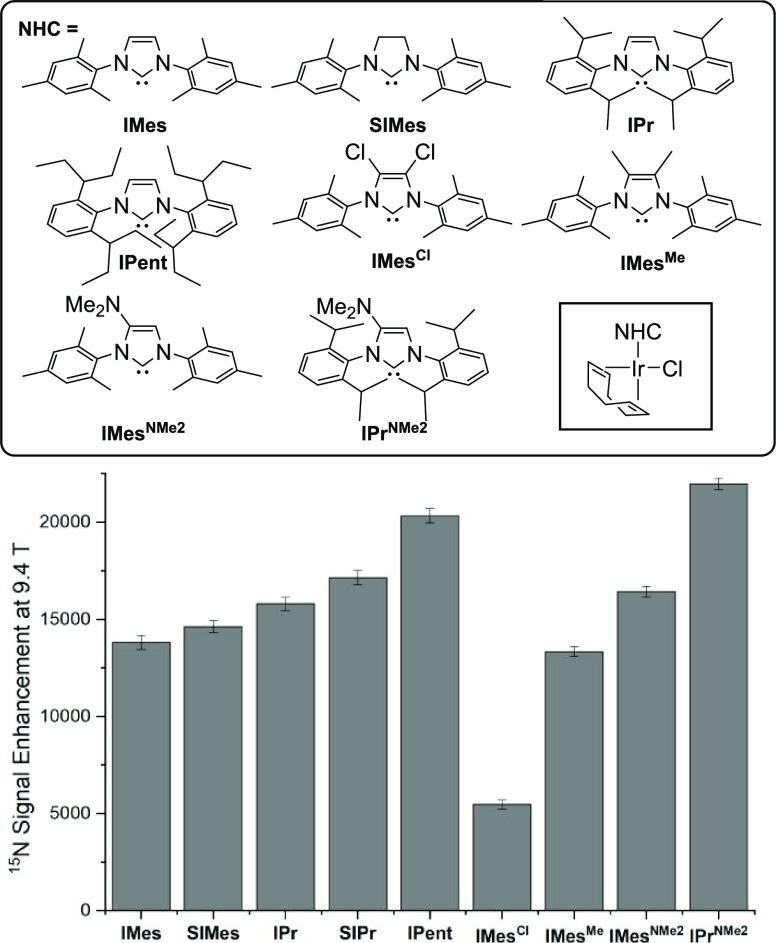
Effect of NHC Identity on the Efficiency of Na15NO2 Polarization
Aiming to improve the polarization outcome still further, a study of the effect of the NHC ligand was completed in conjunction with DMAP-d2. Previously, we have shown how manipulation of the steric and electronic properties of this ancillary ligand can result in improved 1H, 13C, and 15N signal enhancements because of changes in the rate of ligand exchange.60 We sequentially increased the steric bulk of the NHC (quantified by the magnitude of %BurV90,91) to drive ligand exchange (Figure 3). On moving from the IMes ligand (%BurV = 31.2) to SIMes (32.7), we saw a 14,628-fold signal gain which is a slight improvement from the 13,811-fold signal gain previously observed for IMes. IPr (33.6) and SIPr (35.7) both also led to increased signal enhancements of 15,799 and 17,149-fold. However, the best result was obtained for IPent as a 20,337-fold signal gain which has the highest %BurV of 43.4.
Figure 3.
Effect of NHC on the 15N NMR signal gain for Na15NO2 with precatalyst [IrCl(COD)(NHC)] (5 mM), DMAP-d2 (6 equiv), and Na15NO2 (25 equiv) in methanol-d4 after polarization transfer at −3.5 mG under 3 bar p-H2.
Next our focus turned to the electronic properties of the NHC ligand (Figure 3). As expected, electron-deficient IMesCl, which has chloro substituents on the imidazole ring, reduced the signal enhancement to just 5471. Introducing methyl groups on the imidazole ring showed a minimal effect when compared to IMes (13,336-fold vs 13,811-fold, respectively). However, introduction of a single −NMe2 group increased the signal enhancement level to 16,427-fold at 9.4 T.
To combine the steric and electronic effects, we utilized the ligand IPrNMe2, which has previously proven to be effective for Buchwald–Hartwig amination catalysis,92,93 to form the precatalyst [IrCl(COD)(IPrNMe2)]. This catalyst system gave the highest 15N signal enhancement for free Na15NO2 seen, 21,967-fold at 9.4 T which is equivalent to 7.2% polarization.
Effect of Na15NO2 Concentration on Signal Enhancement
A series of samples were prepared which contained varying excesses of Na15NO2 relative to 5 mM of [IrCl(COD)(IPrNMe2)] and a constant 30 mM of DMAP-d2. The corresponding hyperpolarization results are presented in the Supporting Information. Reducing the substrate excess to 10 equiv with respect to iridium increased the 15N signal enhancement to 36,629-fold, but for just 4 equiv a value of 62,470-fold was found which is equivalent to 20.6% 15N polarization. Conversely, increasing the substrate loading to 50 equiv reduced the signal gain to 10,382 (3.17%), albeit with an improved signal to noise ratio. When the concentration of [IrCl(COD)(IPrNMe2)] is reduced to 0.25 mM, while maintaining the 1:4 molar ratio with Na15NO2, the 15N polarization level increases to 28.42%. This phenomenon is likely the result of increasing the excess of p-H2, the limiting reagent, relative to this substrate.53,60
Detection of Unlabeled NaNO2
Given the high signal gains obtained for Na15NO2, we tested a sample where the 15N label was present at natural abundance. This sample contained 20 mM of NaNO2 and was examined with [IrCl(COD)(IPrNMe2)] (5 mM) and DMAP-d2 (6 equiv,). An 15N signal was easily seen whose signal enhancement was 115,592-fold at 9.4 T; this corresponds to a 38.1% polarization level.
Effect of 15-Crown-5 and Alternative Solvents
Unfortunately, the ionic nature of NaNO2 acts to limit its solubility in the range of organic solvents that are typically employed for SABRE catalysis; it has a moderate solubility in methanol; however, it is sparingly soluble in other primary alcohols and insoluble in most apolar solvents. In an attempt to increase methanol-d4 solubility, the macrocycle 15-crown-5 was added, which has a high chelating affinity for Na+.94,95 SABRE transfer was therefore undertaken on a sample containing [IrCl(COD)(IMes)] (5 mM), Na15NO2 (25 equiv), DMAP (6 equiv), and 15-crown-5 (25 equiv) in methanol-d4. This led to an 15N signal enhancement of 12,044-fold at 9.4 T for 15NO2– which corresponds to 3.97% and reflects a 10% improvement over the analogous measurement with no 15-crown-5. Interestingly, the ratio of 5D to 6D in solution was 99:1, as opposed to 91:9 seen in the absence of 15-crown-5. For the optimized catalyst and coligand ([IrCl(COD)(IPrNMe2)] (5 mM), Na15NO2 (25 equiv), and DMAP-d2 (6 equiv)), the effect of 15-crown-5 proved to be less pronounced, with the 15N signal gain improving from 21,967-fold to just 23,114-fold. Hence, solvent effects on this catalysis are substantial and likely to change ligand exchange rates in addition to catalyst speciation.
When an NMR sample containing [IrCl(COD)(IMes)] (5 mM), Na15NO2 (25 equiv), and D (6 equiv) was prepared in dichloromethane-d2 (0.6 mL), the impact of insolubility of Na15NO2 was immediately evident. After sonication for 30 min, the sample was exposed to H2 (3 bar). Investigation by NMR spectroscopy revealed just [Ir(H)2Cl(IMes)(DMAP)2]. However, when an analogous sample was prepared containing 15-crown-5 in a 1:1 ratio with Na15NO2, a different hydride-containing complex formed. Its hydride resonances appear at δH −22.66 (2JHN = 27.5 Hz and 2JHH = −7 Hz) and δ −23.00 (2JHH = −7 Hz) and match those of 5D. After SABRE transfer in a −3.5 mG field, a 3586-fold signal enhancement was observed at 9.4 T for the free 15NO2– resonance at δN 618. Warming this sample to 308 K prior to polarization transfer significantly improved the signal gain to 7248-fold and indicates that slow ligand exchange limits the polarization level attained. However, warming further to 323 K yielded no further increase. Using the electron-rich and sterically encumbered precatalyst [IrCl(COD)(IPrNMe2)] also yielded improved polarization transfer as an 8149-fold signal gain is seen at 9.4 T. Warming this sample, however, had no benefit. Hence, we have demonstrated how significant polarization levels for 15NO2– result in dichloromethane-d2 if 15-crown-5 is present.
Assessment of Na15NO2 Relaxation Rates
DNP hyperpolarized Na15NO2 is reported to have a T1 of 14.8 s in D2O at 5.8 T.15 We used a low-tip angle approach to assess the T1 of this SABRE-polarized product at 9.4 T. It was found to be comparable at 16.45 s in the presence of a SABRE catalyst. This value was also determined using an automated hyperpolarization device under reversible flow,96 after first conducting the SABRE process at −3.5 mG, prior to turning off the p-H2 supply and holding the sample in a defined magnetic field for a period of time, prior to transfer to 9.4 T to acquire a spectrum. Repeating this process for a number of time points enables the effective low field T1 value to be calculated. This analysis was undertaken on samples that were stored in the mu-metal shield (ca. 300-fold shielding) or at −3.5 mT. The new T1 values were 14.9 and 11.2 s, respectively (see the Supporting Information). These values suggest that there will be sufficient time to use the hyperpolarized 15NO2– resulting from SABRE synthetically to create other hyperpolarized products as 3 × T1 is available before a signal vanishes. Interestingly, as the T1 values for 15N nuclei can dramatically be extended when they are located in an appropriate magnetic field, accessing reaction times of many minutes may be possible.55,97,98 We are currently exploring this, but here we show how rapid reactions can be evaluated through 15N NMR at high field is detailed in the following sections.
Conversion of Hyperpolarized Na15NO2 to a Diazonium via NO+
The first reaction we consider is the important Sandmeyer reaction that rapidly converts arylamines into arylhalides via a diazonium salt intermediate.99 Since the first reported example in 1884,100 it has become a mainstay of organic chemistry and many related reactions have been discovered.101 Classically, it utilizes either stoichiometric or catalytic amounts of a copper halide, although a number of metal free variants are known.102−104 The formation of the diazonium salt intermediate proceeds via nitrous acid addition, which is formed in situ from the reaction of NaNO2 and a strong acid. We sought to follow a diazotization reaction by 15N hyperpolarized NMR spectroscopy. To do this, we first created a solution of hyperpolarized Na15NO2 using the previously optimized conditions ([IrCl(COD)(IPrNMe2)] (5 mM), DMAP (30 mM), Na15NO2 (125 mM) in methanol-d4 (0.6 mL)). A solution of aniline (150 mM) and conc. HCl (100 μL) in methanol-d4 (100 μL) was then added, and the resulting NMR tube was immediately transferred into the spectrometer and investigated using a T1-corrected variable flip angle pulse sequence. It took between 3 and 5 s to start this series of measurements, and the resulting hyperpolarized signals were indicative of nitrous acid (H15NO2, δN 563) forming phenyl diazonium chloride (δN 314) and ortho-15N2 (δN 308). Their identity was confirmed by their independent synthesis and comparison to literature data.105 Over the course of 30 s, the response for H15NO2 vanished.
When this process was repeated with aniline-15N, the reaction monitoring step revealed the detection of hyperpolarized responses for both of the 15N centers in the diazo product at δN 315.0 and 232.6 in agreement with the literature (Figure 4a).106 The hyperpolarization of both of the 15N sites happens even though aniline itself was not hyperpolarized. Consequently, efficient polarization transfer between them takes place during their time as a coupled spin pair at low field. As a control, we exposed a sample of phenyl diazonium chloride and the catalyst to p-H2 at −3.5 mG and noted no hyperpolarized 15N resonance result. Consequently, all the hyperpolarized signals seen during this reaction originate from the initially hyperpolarized Na15NO2 synthon.
Figure 4.
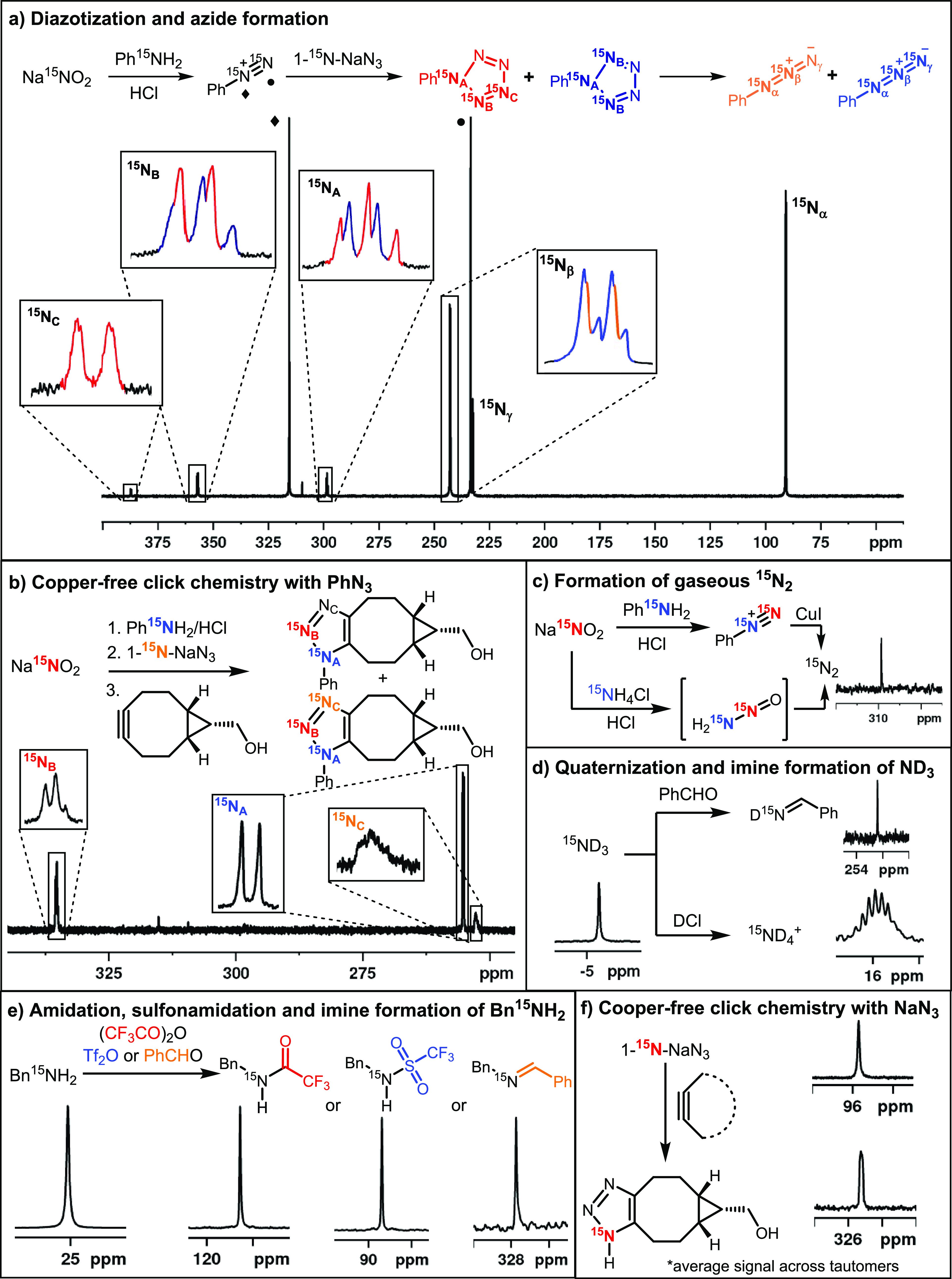
Establishing SABRE hyperpolarization allows the reactivity of 15N-containing synthons to be assessed. (a) Multistep reaction from Na15NO2 that tracks diazotization with aniline-15N and the subsequent formation of two isotopomers of phenyl azide after reaction with 1-15N-NaN3 in a process that proceeds through a cyclic intermediate; (b) copper-free click reaction of PhN3 formed as in part (a); (c) formation of N2(g); (d) ND3 quaternization with DCl(aq) and imine formation with benzaldehyde; (e) amidation, sulfonamidation, and imine formation of benzylamine-15N; (f) copper-free click reaction of 1-15N-NaN3.
Figure S12 of the Supporting Information details how the hyperpolarization level of unlabeled NaNO2 is sufficient to allow the detection of phenyl diazonium chloride without the need for isotopic labeling.
Reactions of Hyperpolarized 15N2-Phenyl Diazonium Chloride
Phenyl diazonium chloride proved to have hyperpolarized T1 values for 15N1 and 15N2 of 29.4 and 39.2 s, respectively, at 9.4 T. Additionally, it proved to be relatively stable under these conditions as only limited decomposition to hyperpolarized 15N2(g) (δN 308) was seen. This meant that we could explore the reactivity of this diazonium salt in situ. It is known that such salts liberate N2 under photochemical or transition-metal-catalyzed processes.107,108 Under our hyperpolarized regime, addition of CuI saw its rapid conversion into N2 and consequently a strong signal was seen at δN 308.
A similar hyperpolarized diazonium salt solution was prepared and then treated with NaN3 to examine the formation of phenyl azide. Rapid monitoring enabled the collection of a hyperpolarized 15N NMR spectrum with strong resonances at δN 242.2 and 90.1 that share a common 2JNN of 13.8 Hz because of this species. According to the literature, this reaction could proceed via a cyclic and/or acyclic intermediate, species which would deliver five and three distinct 15N signals, respectively.109−111 Interestingly, we detect transient signals at δN 356.8 and 298.2 (both with 2JNN = 16.7 Hz) for the site connected to the C6H5 ring which we assign to this product. Upon repeating this study with 1-15N NaN3, these two signals gain further complexity and appear alongside one other at δN 387.3 (d, 17 Hz). These additional features are reflective of the two possible isotopologues that can result from 15N1–N3– addition to form a cyclic intermediate, which place a Ph–15N next to two chemically equivalent 15N groups (a triplet at δN 298.6 of 17 Hz is seen for it alongside a doublet of 17 Hz at δN 356.9) or one (a doublet at δN 298.6 of 17 Hz is now seen) alongside a further triplet at 356.9 of 17 Hz and a doublet at δN 387.3 (d 17 Hz) for the next and more remote center (Figure 4a) 15N of the N5 ring. Hence, all three unique signals for this cyclic intermediate have been detected. We note that its conversion into phenyl azide (Ph–15N=15N+=15N– and Ph–15N=15N+=N–) proceeds rapidly at 298 K, and the signals for this product also appear, δN 90.3, 242.5, and 232, with apparent T1 values of 56, 192, and 101 s at 9.4 T, all respectively.
These long T1 values enable the creation of strongly hyperpolarized phenyl azide. When the reactive alkyne, (1R,8S,9s)-bicyclo[6.1.0]non-4-yn-9-ylmethanol,112 is added to this hyperpolarized sample in a third synthetic step, further reaction to form the corresponding triazole occurs. Despite the corresponding 15N signal’s T1 in this product proving to be <10 s, its formation is readily indicated in the associated hyperpolarized 15N NMR measurements through three signals at δN 335.4, 255.3, and 252.7 that can be linked through mutual 2JNN couplings of 12.8 Hz (Figure 4b). As copper-free click chemistry is used widely for bioconjugation with nuclei acids, we expect such measurements to help in the optimization of pharmaceutical preparations and/or in vivo detection.113,114
These data have clearly illustrated the successful examination of a multistep reaction as it proved possible to simultaneously see 15N signals for the phenyl diazonium salt, the pentazole intermediate and phenyl azide (Figure 4a) or the pentazole intermediate, phenyl azide, and the triazole (Figure 4b). We plan to develop methods to extract precise kinetic data for these changes in the future.
Conversion of Hyperpolarized Na15NO2 to 15N2 through Reaction with 15NH4Cl/HCl
N2 gas spontaneously forms from the diazonium salts of primary amines. Consequently, as 15NH4Cl is readily available we monitored its reaction with Na15NO2 and saw strong signals for 15N2 in solution (see Figure 4c). We have therefore detailed two facile approaches to hyperpolarized 15N2, we are currently exploring as routes to potentially important p-N2.54,74
Utilization of 15N Hyperpolarized Azide, Amines, and Ammonia as Probes of Reactivity
The SABRE hyperpolarization of NH3 and amines, such as benzylamine, and their use in SABRE-relay have been extensively reported.80,81,84−86 Additionally, ammonia and amines have been used as a coligand that leads to improved SABRE catalysis.62,115 The 15N polarization of benzylamine-15N (E-15N) is reported to be ca. 800-fold at 9.4 T.84 This involves the action of [Ir(H)2(IMes)(E-15N)3]Cl in dichloromethane-d2 solution. We restudied this process to improve the SABRE outcome and thereby provide access to a further functional group to demonstrate hyperpolarized reactivity screening. Using the same conditions as previously reported ([IrCl(COD)(IMes)] (5 mM) and benzylamine-15N (E-15N, 7 equiv)), we determined that optimal SABRE transfer occurs at −4 mG. At this transfer field, a 7751-fold signal enhancement was achieved at 9.4 T. As the rate of benzylamine dissociation from [Ir(H)2(IMes)(E-15N)3]Cl in dichloromethane-d2 is slow,45,85 we found that warming the sample to 308 K further improved the enhancement level to 11,211-fold which corresponds to 3.7% 15N polarization; it has 14 s T1 at 9.4 T in the absence of the catalyst and 12.8 s when it is present. We predict that further optimizations could improve this value; however, the resulting signal strengths are sufficient to explore its reactivity. We exemplify now the utilization of hyperpolarized E-15N as a synthon for amidation, sulfonamidation, and imine formation. This resulted in the 15N detection of the products shown in Figure 4e. Their identity was verified by independent synthesis as described in the Supporting Information or by comparison to literature data. In particular, the addition of trifluoroacetic anhydride to hyperpolarized E-15N led to the formation and detection of N-benzyl-trifluoroacetamide-15N in the resulting 15N NMR spectrum through a signal at δN 116.4. Similarly, triflic anhydride reacted to yield the analogous sulfonamide with a resonance at δN 88.6. Finally, addition of benzaldehyde to E-15N produced the imine condensation product as evident from a peak at δN 327.7.
Ammonia is also widely used in synthetic chemistry and we sought to test whether its reactivity could be probed while hyperpolarized. As gaseous 15NH3 was expensive and difficult to handle, we used an alternative ammonia source.
This involved taking a 1:1 mixture of 15NH4Cl/KOtBu and adding it to 1 in the presence of H2 with the result that [Ir(H)2(IMes)(15ND3)3]Cl forms in methanol-d4. After polarization transfer at −4 mG, a 3268-fold 15N signal gain was quantified for the free 15ND3 signal. However, over the course of ca. 1 h, the signal enhancement diminishes when the SABRE process is repeated. In contrast, the use of 15NH4OH (available as a 14 molar solution in H2O) yielded the same active catalyst, but the sample was now stable for >24 h. The 15N signal enhancement is also slightly improved to 3765-fold. Changing the NHC ligand proved to have a modest effect on SABRE efficacy (see the Supporting Information), and warming the sample derived from 1 to 308 K improved the signal gain to 4521-fold. However, dramatic improvements are observed with a coligand. While the coligands DMSO-d6, CD3CN, NO2–, and DMAP (see the Supporting Information) were explored, pyridine-d5 proved to give the highest signal gain of 15,145-fold (5.0% polarization). The 15N T1 value for 15ND3 at 9.4 T proved to be 37 s so there is again a wide time window over which a reaction can be examined. Protonation of 15ND3 with DCl in D2O led to the detection of hyperpolarized 15ND4+ as a signal δN 15.93 with resolved 15N-D scalar couplings, JND, of 10.8 Hz and a hyperpolarized T1 of 33.6 s (Figure 4d).
The SABRE hyperpolarization of 1-15N NaN3 itself using the coligand strategy with DMAP and 1 also proved successful. The reaction was found to proceed to form [Ir(H)2(DMAP)2(IMes)(15N=N=N)] which exhibits hydride signals at δH −23.1 (2JHH = 8 Hz) and δH −25.0 (2JHH = 8 Hz and 2JNH = 8 Hz) alongside [Ir(H)2(DMAP)3(IMes)]Cl (δH –22.8). SABRE transfer at −3.5 mG yielded 3.2% hyperpolarization of the N3– signal at δN 95.7. A hyperpolarized solution of NaN3 was then reacted directly with (1R,8S,9s)-bicyclo[6.1.0]non-4-yn-9-ylmethanol to form the triazole. Under these conditions, a single hyperpolarized 15N response for the product was visible at δN 321.3 as expected and is shown in Figure 4f.
Producing Hyperpolarized NO2– in Water
For biological applications, it is desirable to produce hyperpolarized NO2– in water. Unfortunately, 5A did not form when the analogous reaction was conducted in this solvent. Preforming 5D in methanol-d4 prior to removing the solvent and replacing it with D2O was also unsuccessful. One further way to achieve an aqueous bolus is to use a biphasic116 approach with dichloromethane-d2, which could benefit from the fact that the catalyst is not present in the aqueous layer.65,117 A sample containing [IrCl(COD)(IMes)] (5 mM), Na15NO2 (25 equiv) and DMAP (6 equiv) and 15-crown-5 (25 equiv) in dichloromethane-d2 (0.3 mL) was prepared and exposed to H2 (3 bar) to form the active catalyst. D2O (0.3 mL) was then added under a nitrogen atmosphere. After SABRE transfer at −3.5 mG and phase separation, two hyperpolarized signals were seen in the corresponding 15N NMR spectrum for Na15NO2 at δN 618 and δN 609. These peaks had relative intensities of 1:70 and were assigned to Na15NO2 dissolved in the dichloromethane-d2 and D2O phases respectively by comparison with data from independent solutions. Assuming that all of the Na15NO2 was present, the D2O layer results in a 15N signal gain of 4667-fold. This will be an underestimate of the actual signal gain. As 15-crown-5 can also play a role as a phase-transfer catalyst,118 a further 25 equiv was added to the sample and this proved to increase the signal enhancement level to 13,794-fold. Further additions of 15-crown-5 did not improve on this; however, warming the sample to 308 K resulted in a 26,327-fold signal gain at 9.4 T. This is equivalent to an 8.69% polarization level in aqueous Na15NO2. Hence, we have created a simple route to hyperpolarized Na15NO2 in biocompatible water. The level of signal gain compares favorably with the <1% reported with DNP.15 To confirm the phase distribution of Na15NO2, a series of 15N MRI images were recorded on a 10 mm-diameter sample tube as detailed in the Supporting Information. The data in Figure 5 details these results which confirm both separation and the fact that the associated signal strengths are sufficient to allow for high-sensitivity 15N imaging of NO2–.
Figure 5.
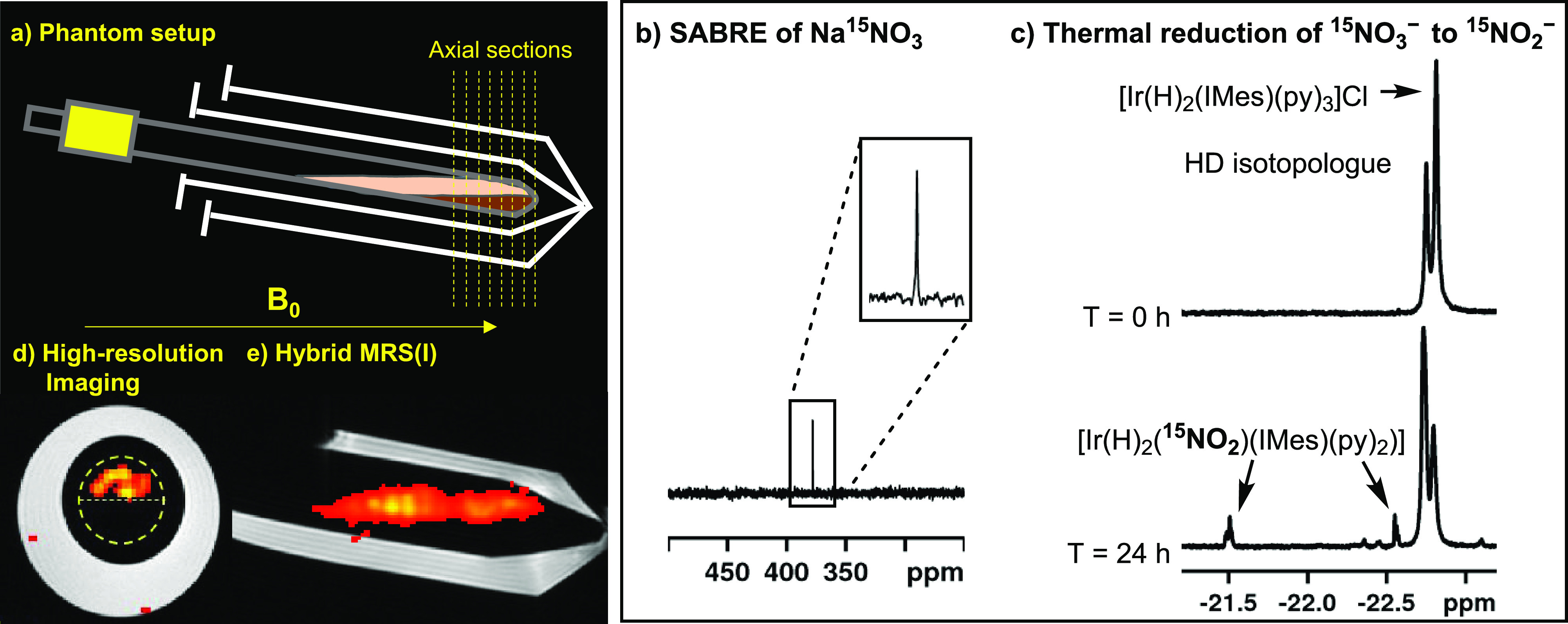
Establishing the imaging capability of SABRE hyperpolarized 15N O2– in biocompatible solvents. (a) Setup for hyperpolarized imaging at 7 T of a reaction cell used for the biphasic preparation of 15NO2–; (b) SABRE hyperpolarized 15N NMR spectrum of Na15NO3 created with [Ir(COD)(IMes)(pyridine)]BF4, DMSO-d6 (2 equiv), Na15NO3 (25 equiv), and 3 bar p-H2 at 9.4 T; (c) 1H NMR spectra of the hydride region establish the conversion of NO3– to NO2– via the detection of Ir(H)(15NO2)(IMes)(py)2]; (d) high-resolution three-dimensional axial acquisition using spiral (out) encoding of 6 cm3 over a 64 × 64 × 8 matrix with a 6 s acquisition time; (e) hybrid MRS(I) data collected using an EPSI pulse sequence and a 64 × 64 matrix (512 spectral points).
SABRE Hyperpolarization of Na15NO3
In contrast to nitrite, nitrate is usually noncoordinating; however, there are examples of it functioning as a weak monodentate or bidentate ligand.29,119−123 To further explore SABRE’s use as a tool to polarize materials featuring in the nitrogen cycle, we explored the SABRE hyperpolarization of Na15NO3. As expected, in the absence of a coligand no active SABRE catalyst formed in the reaction between [IrCl(COD)(IMes)] and Na15NO3 under a H2 atmosphere (3 bar) in methanol-d4. We screened a number of coligands (DMAP, 2-picoline, DMSO-d6, DPSO, or CD3CN) and saw no evidence for the 15N polarization of Na15NO3. In each case, the dominant hydride-containing species in solution was [Ir(H)2(IMes)(coligand)3]Cl or [IrCl(H)2(IMes)(coligand)2]. However, when the ionic precatalyst [Ir(COD)(IMes)(pyridine)]BF4 was used with DMSO-d6 (2 equiv), a 547-fold signal enhancement for the 15NO3– signal at 9.4 T (0.18% 15N polarization) is observed (Figure 5b). No direct evidence for an NO3– containing complex could be found, and therefore, polarization transfer must occur through a very low concentration species.
Unexpected Reduction of Sodium Nitrate
During the course of these investigations, a hyperpolarized 15N NMR signal at δN 511.28 (JHN = 28.5 Hz) appears over the course of 0.5 h when pyridine alone is used as a coligand. This matches the equatorial NO2– resonance previously observed for [Ir(H)2(15NO2)(IMes)(pyridine)2] (5A). Relatively strong polarized signals for free pyridine and the pyridine ligand trans to hydride in [Ir(H)2(IMes)(py)3]Cl (δN 299.6 and 255.7, respectively) were also observed in these NMR spectra. As expected, the corresponding 1H NMR spectrum is dominated by the hydride signal of [Ir(H)2(IMes)(py)3]Cl which appears at δH −22.7, although a weakly PHIP-enhanced signal for 5A is visible in this spectrum at δH −21.49 (the peak at δH −22.71 cannot be observed because of overlap). No evidence for 6A was observed in either the 1H or 15N NMR spectra which indicates that 5A is likely to be the kinetic product of this reaction. After waiting for a further 1 h, refreshing the sample with p-H2, and repeating the SABRE process, a polarized signal for free Na15NO2 (δN 611.9) could also be detected and the observed signal for 5A increased in size. We therefore suspect that the reducing environment of this medium converts nitrate to nitrite in a metal-catalyzed reduction. To further probe this reduction, a sample containing [IrCl(COD)(IMes)] (20 mM), pyridine (3 equiv) and Na15NO3 (25 equiv) was exposed to 3 bar of H2 at 298 K for 24 h and the growth of the hydride ligand resonance for 5A at δH −21.49 was monitored by thermally polarized 1H NMR spectroscopy over the course of 24 h. The resulting integral data for this peak could be fitted to an exponential growth curve (see the Supporting Information). After 24 h and refreshing the H2 atmosphere, further conversion to 5A could again be seen which indicates that H2 is needed to drive this reaction. While the electrochemical reduction of nitrate is widely known124,125 and limited examples of heterogeneous hydrogenative reduction of nitrate are also reported,126−128 to the best of our knowledge the molecular reduction of nitrate using transition-metal catalysis has not received significant attention. Optimization of the phenomenon reported here may therefore provide a useful alternative.
Conclusions
In this work, we have demonstrated how the 15N hyperpolarization of a range of important 15N-synthons, including some which feature in the important nitrogen cycle (NO2– (28% polarization), NH3 (3%), PhCH2NH2 (5%), NaN3 (3%), and NO3– (0.1%)), is possible. When monitored by 15N NMR, all these species yield strong signals that can be detected readily. The in-field, T1 values of NO2– (17 s), ND3 (36 s), PhCH2NH2 (12 s), and NaN3 (50 s) mean that sufficient time exists to monitor their reactivity through hyperpolarized product responses. This has been demonstrated for the formation of phenyl diazonium, phenyl azide, a triazole, an amide, a sulfonamide, and two imines (Figure 4). In the case of phenyl azide formation, a pentazole intermediate was detected whose cyclic, rather than acyclic, formulation has been confirmed. Studies of the unlabeled formation of phenyl diazonium are also detailed.
Hence, these results demonstrate how SABRE reflects a versatile tool capable of tracking the preparation of a range of nitrogen rich products. We expect the future application of this approach to aid in achieving the optimized the synthesis of many materials, including important pharmaceuticals.
Furthermore, we demonstrate a biphasic method using a 15-crown-5 as a phase-transfer agent that yields >8% aqueous NO2– polarization. We expect this route to help SABRE deliver biocompatible products in the future as we expect it to produce large amounts of such hyperpolarized reagents in seconds. The recent report of 79% 15N-derived SABRE hyperpolarization62 suggests that with further optimization a currently unrivaled low-cost approach to rapidly deliver 15N NMR sensitivity will therefore be obtained. Hence, the pioneering work of Bowers and Weitekamp again continues to expand beyond its original horizons.129
Acknowledgments
We are grateful for the help from Dr. Victoria Annis.
Glossary
Abbreviations
- SABRE
signal amplification by reversible exchange
- p-H2
para-hydrogen
- PHIP
parahydrogen induced polarization
- d-DNP
dissolution dynamic nuclear polarization
- PET
positron emission tomography
- DMAP
dimethylamino pyridine
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.2c02619.
Full experimental methods, hyperpolarized NMR spectra, and characterization data (PDF)
S.B.D. and A.J.K. thank the following for supporting this work: The EPSRC (EP/R51181X/1, G0065601) and the University of York and Norman Turner (N0013902).
The authors declare no competing financial interest.
Supplementary Material
References
- Keshari K. R.; Wilson D. M. Chemistry and biochemistry of C-13 hyperpolarized magnetic resonance using dynamic nuclear polarization. Chem. Soc. Rev. 2014, 43, 1627–1659. 10.1039/C3CS60124B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugler J. P.; Altes T. A. Hyperpolarized 129Xe MRI of the human lung. J. Magn. Reson. Imaging 2013, 37, 313–331. 10.1002/jmri.23844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzberg E.; Hezel M.; Lundberg J. O. Nitrate-Nitrite-Nitric Oxide Pathway: Implications for Anesthesiology and Intensive Care. Anesthesiology 2010, 113, 1460–1475. 10.1097/ALN.0b013e3181fcf3cc. [DOI] [PubMed] [Google Scholar]
- Adams R. W.; Aguilar J. A.; Atkinson K. D.; Cowley M. J.; Elliott P. I.; Duckett S. B.; Green G. G.; Khazal I. G.; López-Serrano J.; Williamson D. C. Reversible interactions with para-hydrogen enhance NMR sensitivity by polarization transfer. Science 2009, 323, 1708–1711. 10.1126/science.1168877. [DOI] [PubMed] [Google Scholar]
- Reineri F.; Boi T.; Aime S. ParaHydrogen Induced Polarization of C-13 carboxylate resonance in acetate and pyruvate. Nat. Commun. 2015, 6, 5858. 10.1038/ncomms6858. [DOI] [PubMed] [Google Scholar]
- Knecht S.; Blanchard J. W.; Barskiy D.; Cavallari E.; Dagys L.; Dyke E. V.; Tsukanov M.; Bliemel B.; Münnemann K.; Aime S.; Reineri F.; Levitt M. H.; Buntkowsky G.; Pines A.; Blümler P.; Budker D.; Eills J. Rapid hyperpolarization and purification of the metabolite fumarate in aqueous solution. Proc. Natl. Acad. Sci. U. S. A. 2021, 118, e2025383118 10.1073/pnas.2025383118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eills J.; Cavallari E.; Kircher R.; Di Matteo G.; Carrera C.; Dagys L.; Levitt M. H.; Ivanov K. L.; Aime S.; Reineri F.; Münnemann K.; Budker D.; Buntkowsky G.; Knecht S. Singlet-Contrast Magnetic Resonance Imaging: Unlocking Hyperpolarization with Metabolism**. Angew. Chem., Int. Ed. 2021, 60, 6791–6798. 10.1002/anie.202014933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazina D.; Duckett S. B.; Dunne J. P.; Godard C. Applications of the parahydrogen phenomenon in inorganic chemistry. Dalton Trans. 2004, 2601–2609. 10.1039/B409606A. [DOI] [PubMed] [Google Scholar]
- Shchepin R. V.; Birchall J. R.; Chukanov N. V.; Kovtunov K. V.; Koptyug I. V.; Theis T.; Warren W. S.; Gelovani J. G.; Goodson B. M.; Shokouhi S.; Rosen M. S.; Yen Y. F.; Pham W.; Chekmenev E. Y. Hyperpolarizing Concentrated Metronidazole (NO2)-N-15 Group over Six Chemical Bonds with More than 15% Polarization and a 20 Minute Lifetime. Chem. – Eur. J. 2019, 25, 8829–8836. 10.1002/chem.201901192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shchepin R. V.; Barskiy D. A.; Coffey A. M.; Feldman M. A.; Kovtunova L. M.; Bukhtiyarov V. I.; Kovtunov K. V.; Goodson B. M.; Koptyug I. V.; Chekmenev E. Y. Robust Imidazole-N-15(2) Synthesis for High-Resolution Low-Field (0.05 T) (15)NHyperpolarized NMR Spectroscopy. ChemistrySelect 2017, 2, 4478–4483. 10.1002/slct.201700718. [DOI] [Google Scholar]
- Hövener J.-B.; Pravdivtsev A. N.; Kidd B.; Bowers C. R.; Glöggler S.; Kovtunov K. V.; Plaumann M.; Katz-Brull R.; Buckenmaier K.; Jerschow A.; Reineri F.; Theis T.; Shchepin R. V.; Wagner S.; Bhattacharya P.; Zacharias N. M.; Chekmenev E. Y. Parahydrogen-Based Hyperpolarization for Biomedicine. Angew. Chem., Int. Ed. 2018, 57, 11140–11162. 10.1002/anie.201711842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko Y.; Bonner F. T.; Crull G. B.; Harbison G. S. Protonation nitrogen shielding and NOE in aqueous nitrite and solid-state nitrogen-15 NMR of nitrosyl and nitryl tetrafluoroborate. Inorg. Chem. 1993, 32, 3316–3319. 10.1021/ic00067a021. [DOI] [Google Scholar]
- Thorn K. A.; Mikita M. A. Nitrite Fixation by Humic Substances Nitrogen-15 Nuclear Magnetic Resonance Evidence for Potential Intermediates in Chemodenitrification. Soil Sci. Soc. Am. J. 2000, 64, 568–582. 10.2136/sssaj2000.642568x. [DOI] [Google Scholar]
- Sakhaei Z.; Kundu S.; Donnelly J. M.; Bertke J. A.; Kim W. Y.; Warren T. H. Nitric oxide release via oxygen atom transfer from nitrite at copper(ii). Chem. Commun. 2017, 53, 549–552. 10.1039/C6CC08745K. [DOI] [PubMed] [Google Scholar]
- Gamliel A.; Uppala S.; Sapir G.; Harris T.; Nardi-Schreiber A.; Shaul D.; Sosna J.; Gomori J. M.; Katz-Brull R. Hyperpolarized [15N]nitrate as a potential long lived hyperpolarized contrast agent for MRI. J. Magn. Reson. 2019, 299, 188–195. 10.1016/j.jmr.2019.01.001. [DOI] [PubMed] [Google Scholar]
- Godard C.; Lopez-Serrano J.; Galvez-Lopez M. D.; Rosello-Merino M.; Duckett S. B.; Khazal I.; Lledos A.; Whitwood A. C. Detection of platinum dihydride bisphosphine complexes and studies of their reactivity through para-hydrogen-enhanced NMR methods. Magn. Reson. Chem. 2008, 46, S107–S114. 10.1002/mrc.2342. [DOI] [PubMed] [Google Scholar]
- Vazquez-Serrano L. D.; Owens B. T.; Buriak J. M. Catalytic olefin hydrogenation using N-heterocyclic carbene-phosphine complexes of iridium. Chem. Commun. 2002, 21, 2518–2519. 10.1039/B208403A. [DOI] [Google Scholar]
- Giernoth R.; Heinrich H.; Adams N. J.; Deeth R. J.; Bargon J.; Brown J. M. PHIP detection of a transient rhodium dihydride intermediate in the homogeneous hydrogenation of dehydroamino acids. J. Am. Chem. Soc. 2000, 122, 12381–12382. 10.1021/ja002516o. [DOI] [Google Scholar]
- Torres O.; Procacci B.; Halse M. E.; Adams R. W.; Blazina D.; Duckett S. B.; Eguillor B.; Green R. A.; Perutz R. N.; Williamson D. C. Photochemical Pump and NMR Probe: Chemically Created NMR Coherence on a Microsecond Time Scale. J. Am. Chem. Soc. 2014, 136, 10124–10131. 10.1021/ja504732u. [DOI] [PubMed] [Google Scholar]
- Delwiche C. C. The Nitrogen Cycle. Sci. Am. 1970, 223, 136–146. 10.1038/scientificamerican0970-136. [DOI] [PubMed] [Google Scholar]
- Cammack R.; Joannou C. L.; Cui X.-Y.; Torres Martinez C.; Maraj S. R.; Hughes M. N. Nitrite and nitrosyl compounds in food preservation. Biochim. Biophys. Acta, Bioenerg. 1999, 1411, 475–488. 10.1016/S0005-2728(99)00033-X. [DOI] [PubMed] [Google Scholar]
- Shiva S. Nitrite: A physiological store of nitric oxide and modulator of mitochondrial function. Redox Biol. 2013, 1, 40–44. 10.1016/j.redox.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chui J. S. W.; Poon W. T.; Chan K. C.; Chan A. Y. W.; Buckley T. A. Nitrite-induced methaemoglobinaemia – aetiology, diagnosis and treatment. Anaesthesia 2005, 60, 496–500. 10.1111/j.1365-2044.2004.04076.x. [DOI] [PubMed] [Google Scholar]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS);; Mortensen A.; Aguilar F.; Crebelli R.; Di Domenico A.; Dusemund B.; Frutos M. J.; Galtier P.; Gott D.; Gundert-Remy U.; Lambré C.; Leblanc J.-C.; Lindtner O.; Moldeus P.; Mosesso P.; Oskarsson A.; Parent-Massin D.; Stankovic I.; Waalkens-Berendsen I.; Woutersen R. A.; Wright M.; van den Brandt P.; Fortes C.; Merino L.; Toldrà F.; Arcella D.; Christodoulidou A.; Cortinas Abrahantes J.; Barrucci F.; Garcia A.; Pizzo F.; Battacchi D.; Younes M. Re-evaluation of potassium nitrite (E 249) and sodium nitrite (E 250) as food additives. EFSA J. 2017, 15, e04786 10.2903/j.efsa.2017.4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R. Nitrates, nitrites and N-nitrosocompounds: A review of the occurrence in food and diet and the toxicological implications. Food Addit. Contam. 1990, 7, 717–768. 10.1080/02652039009373938. [DOI] [PubMed] [Google Scholar]
- Bebarta V. S.; Brittain M.; Chan A.; Garrett N.; Yoon D.; Burney T.; Mukai D.; Babin M.; Pilz R. B.; Mahon S. B.; Brenner M.; Boss G. R. Sodium Nitrite and Sodium Thiosulfate Are Effective Against Acute Cyanide Poisoning When Administered by Intramuscular Injection. Ann. Emerg. Med. 2017, 69, 718–725.e4. 10.1016/j.annemergmed.2016.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S.; Batra S. Applications of Sodium Nitrite in Organic Synthesis. Eur. J. Org. Chem. 2019, 2019, 6424–6451. 10.1002/ejoc.201900951. [DOI] [Google Scholar]
- Halfen J. A.; Mahapatra S.; Wilkinson E. C.; Gengenbach A. J.; Young V. G.; Que L.; Tolman W. B. Synthetic Modeling of Nitrite Binding and Activation by Reduced Copper Proteins. Characterization of Copper(I)–Nitrite Complexes That Evolve Nitric Oxide. J. Am. Chem. Soc. 1996, 118, 763–776. 10.1021/ja952691i. [DOI] [Google Scholar]
- Timmons A. J.; Symes M. D. Converting between the oxides of nitrogen using metal–ligand coordination complexes. Chem. Soc. Rev. 2015, 44, 6708–6722. 10.1039/C5CS00269A. [DOI] [PubMed] [Google Scholar]
- Takeuchi A.; Sato K.; Sone K.; Yamada S.; Yamasaki K. Preparation of some nitro-amine complexes of nickel and their properties. Inorg. Chim. Acta 1967, 1, 399–402. 10.1016/S0020-1693(00)93210-9. [DOI] [Google Scholar]
- Goodgame D. M. L.; Hitchman M. A. Studies of Nitro and Nitrito Complexes. I. Some Nitrito Complexes of Nickel(II). Inorg. Chem. 1964, 3, 1389–1394. 10.1021/ic50020a010. [DOI] [Google Scholar]
- Goodgame D. M. L.; Hitchman M. A. Studies of Nitro and Nitrito Complexes. III. Some Nitro Complexes of Nickel(II) and a Nitro-Nitrito Equilibrium. Inorg. Chem. 1966, 5, 1303–1307. 10.1021/ic50042a001. [DOI] [Google Scholar]
- Gwak J.; Ahn S.; Baik M.-H.; Lee Y. One metal is enough: a nickel complex reduces nitrate anions to nitrogen gas. Chem. Sci. 2019, 10, 4767–4774. 10.1039/C9SC00717B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura I.; Funasako Y.; Mochida T. Nitro–Nitrito Photoisomerization of Platinum(II) Complexes with Pt(NO2)42– and (FSO2)2N– Anions: Correlation between Isomerization Ratio and Reaction Cavity. Cryst. Growth Des. 2020, 20, 8047–8052. 10.1021/acs.cgd.0c01294. [DOI] [Google Scholar]
- Badar Ud D.; Bailar J. C. Observations on the oxidation and reduction of platinum(II) nitro complexes. J. Inorg. Nucl. 1961, 22, 241–245. 10.1016/0022-1902(61)80439-9. [DOI] [Google Scholar]
- Gel’fman M. I.; Starkina N. A.; Salishcheva O. V.; Moldagulova N. E. Trans-influence of a nitro group in platinum complexes. Russ. J. Inorg. Chem. 2007, 52, 1551–1556. 10.1134/S0036023607100130. [DOI] [Google Scholar]
- Rayner P. J.; Duckett S. Signal Amplification by Reversible Exchange (SABRE): From Discovery to Diagnosis. Angew. Chem., Int. Ed. 2018, 57, 6742–6753. 10.1002/anie.201710406. [DOI] [PubMed] [Google Scholar]
- Kovtunov K. V.; Pokochueva E. V.; Salnikov O. G.; Cousin S. F.; Kurzbach D.; Vuichoud B.; Jannin S.; Chekmenev E. Y.; Goodson B. M.; Barskiy D. A.; Koptyug I. V. Hyperpolarized NMR Spectroscopy: d-DNP, PHIP, and SABRE Techniques. Chem. – Asian J. 2018, 13, 1857–1871. 10.1002/asia.201800551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tickner B. J.; Semenova O.; Iali W.; Rayner P. J.; Whitwood A. C.; Duckett S. B. Optimisation of pyruvate hyperpolarisation using SABRE by tuning the active magnetisation transfer catalyst. Catal. Sci. Technol. 2020, 10, 1343–1355. 10.1039/C9CY02498K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iali W.; Roy S. S.; Tickner B. J.; Ahwal F.; Kennerley A. J.; Duckett S. B. Hyperpolarising Pyruvate through Signal Amplification by Reversible Exchange (SABRE). Angew. Chem., Int. Ed. 2019, 58, 10271–10275. 10.1002/anie.201905483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemeinhardt M.; Limbach M.; Gebhardt T.; Eriksson C.; Eriksson S.; Lindale J.; Goodson E.; Warren W.; Chekmenev E.; Goodson B. “Direct” 13C Hyperpolarization of 13C-Acetate by MicroTesla NMR Signal Amplification by Reversible Exchange (SABRE). Angew. Chem., Int. Ed. 2020, 59, 418–423. 10.1002/anie.201910506. [DOI] [PubMed] [Google Scholar]
- Rayner P. J.; Burns M. J.; Olaru A. M.; Norcott P.; Fekete M.; Green G. G. R.; Highton L. A. R.; Mewis R. E.; Duckett S. B. Delivering strong 1H nuclear hyperpolarization levels and long magnetic lifetimes through signal amplification by reversible exchange. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, E3188–E3194. 10.1073/pnas.1620457114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams R. W.; Duckett S. B.; Green R. A.; Williamson D. C.; Green G. G. R. A theoretical basis for spontaneous polarization transfer in non-hydrogenative parahydrogen-induced polarization. Chem. Phys. 2009, 131, 194505. 10.1063/1.3254386. [DOI] [PubMed] [Google Scholar]
- Barskiy D. A.; Pravdivtsev A. N.; Ivanov K. L.; Kovtunov K. V.; Koptyug I. V. A simple analytical model for signal amplification by reversible exchange (SABRE) process. Phys. Chem. Chem. Phys. 2016, 18, 89–93. 10.1039/C5CP05134G. [DOI] [PubMed] [Google Scholar]
- Knecht S.; Pravdivtsev A. N.; Hovener J.-B.; Yurkovskaya A. V.; Ivanov K. L. Quantitative description of the SABRE process: rigorous consideration of spin dynamics and chemical exchange. RSC Adv. 2016, 6, 24470–24477. 10.1039/C5RA28059A. [DOI] [Google Scholar]
- Pravdivtsev A. N.; Yurkovskaya A. V.; Vieth H.-M.; Ivanov K. L.; Kaptein R. Level Anti-Crossings are a Key Factor for Understanding para-Hydrogen-Induced Hyperpolarization in SABRE Experiments. ChemPhysChem 2013, 14, 3327–3331. 10.1002/cphc.201300595. [DOI] [PubMed] [Google Scholar]
- Pravdivtsev A. N.; Ivanov K. L.; Yurkovskaya A. V.; Petrov P. A.; Limbach H. H.; Kaptein R.; Vieth H. M. Spin polarization transfer mechanisms of SABRE: A magnetic field dependent study. J. Magn. Reson. 2015, 261, 73–82. 10.1016/j.jmr.2015.10.006. [DOI] [PubMed] [Google Scholar]
- Theis T.; Truong M.; Coffey A. M.; Chekmenev E. Y.; Warren W. S. LIGHT-SABRE enables efficient in-magnet catalytic hyperpolarization. J. Magn. Reson. 2014, 248, 23–26. 10.1016/j.jmr.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barskiy D. A.; Shchepin R. V.; Tanner C. P. N.; Colell J. F. P.; Goodson B. M.; Theis T.; Warren W. S.; Chekmenev E. Y. The Absence of Quadrupolar Nuclei Facilitates Efficient 13C Hyperpolarization via Reversible Exchange with Parahydrogen. ChemPhysChem 2017, 18, 1493–1498. 10.1002/cphc.201700416. [DOI] [PubMed] [Google Scholar]
- Zhou Z.; Yu J.; Colell J. F. P.; Laasner R.; Logan A.; Barskiy D. A.; Shchepin R. V.; Chekmenev E. Y.; Blum V.; Warren W. S.; Theis T. Long-Lived 13C2 Nuclear Spin States Hyperpolarized by Parahydrogen in Reversible Exchange at Microtesla Fields. J. Phys. Chem. Lett. 2017, 8, 3008–3014. 10.1021/acs.jpclett.7b00987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis T.; Truong M. L.; Coffey A. M.; Shchepin R. V.; Waddell K. W.; Shi F.; Goodson B. M.; Warren W. S.; Chekmenev E. Y. Microtesla SABRE Enables 10% Nitrogen-15 Nuclear Spin Polarization. J. Am. Chem. Soc. 2015, 137, 1404–1407. 10.1021/ja512242d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S. S.; Rayner P. J.; Burns M. J.; Duckett S. B. A simple and cost-efficient technique to generate hyperpolarized long-lived 15N-15N nuclear spin order in a diazine by signal amplification by reversible exchange. J. Chem. Phys. 2020, 152, 014201 10.1063/1.5132308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shchepin R. V.; Barskiy D. A.; Mikhaylov D. M.; Chekmenev E. Y. Efficient Synthesis of Nicotinamide-1-15N for Ultrafast NMR Hyperpolarization Using Parahydrogen. Bioconjugate Chem. 2016, 27, 878–882. 10.1021/acs.bioconjchem.6b00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis T.; Ortiz G. X.; Logan A. W. J.; Claytor K. E.; Feng Y.; Huhn W. P.; Blum V.; Malcolmson S. J.; Chekmenev E. Y.; Wang Q.; Warren W. S. Direct and cost-efficient hyperpolarization of long-lived nuclear spin states on universal 15N2−diazirine molecular tags. Sci. Adv. 2016, 2, e1501438 10.1126/sciadv.1501438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barskiy D. A.; Shchepin R. V.; Coffey A. M.; Theis T.; Warren W. S.; Goodson B. M.; Chekmenev E. Y. Over 20% 15N Hyperpolarization in Under One Minute for Metronidazole, an Antibiotic and Hypoxia Probe. J. Am. Chem. Soc. 2016, 138, 8080–8083. 10.1021/jacs.6b04784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong M. L.; Theis T.; Coffey A. M.; Shchepin R. V.; Waddell K. W.; Shi F.; Goodson B. M.; Warren W. S.; Chekmenev E. Y. 15N Hyperpolarization by Reversible Exchange Using SABRE-SHEATH. J. Phys. Chem. C 2015, 119, 8786–8797. 10.1021/acs.jpcc.5b01799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhivonitko V. V.; Skovpin I. V.; Koptyug I. V. Strong 31P nuclear spin hyperpolarization produced via reversible chemical interaction with parahydrogen. Chem. Commun. 2015, 51, 2506–2509. 10.1039/C4CC08115C. [DOI] [PubMed] [Google Scholar]
- Burns M. J.; Rayner P. J.; Green G. G. R.; Highton L. A. R.; Mewis R. E.; Duckett S. B. Improving the Hyperpolarization of 31P Nuclei by Synthetic Design. J. Phys. Chem. B 2015, 119, 5020–5027. 10.1021/acs.jpcb.5b00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaru A. M.; Burt A.; Rayner P. J.; Hart S. J.; Whitwood A. C.; Green G. G. R.; Duckett S. B. Using signal amplification by reversible exchange (SABRE) to hyperpolarise 119Sn and 29Si NMR nuclei. Chem. Commun. 2016, 52, 14482–14485. 10.1039/C6CC07109K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner P. J.; Norcott P.; Appleby K. M.; Iali W.; John R. O.; Hart S. J.; Whitwood A. C.; Duckett S. B. Fine-tuning the efficiency of para-hydrogen-induced hyperpolarization by rational N-heterocyclic carbene design. Nat. Commun. 2018, 9, 4251. 10.1038/s41467-018-06766-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley M. J.; Adams R. W.; Atkinson K. D.; Cockett M. C. R.; Duckett S. B.; Green G. G. R.; Lohman J. A. B.; Kerssebaum R.; Kilgour D.; Mewis R. E. Iridium N-Heterocyclic Carbene Complexes as Efficient Catalysts for Magnetization Transfer from para-Hydrogen. J. Am. Chem. Soc. 2011, 133, 6134–6137. 10.1021/ja200299u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete M.; Ahwal F.; Duckett S. B. Remarkable Levels of N-15 Polarization Delivered through SABRE into Unlabeled Pyridine, Pyrazine, or Metronidazole Enable Single Scan NMR Quantification at the mM Level. J. Phys. Chem. B 2020, 124, 4573–4580. 10.1021/acs.jpcb.0c02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svyatova A.; Skovpin I. V.; Chukanov N. V.; Kovtunov K. V.; Chekmenev E. Y.; Pravdivtsev A. N.; Hovener J. B.; Koptyug I. V. N-15 MRI of SLIC-SABRE Hyperpolarized N-15-Labelled Pyridine and Nicotinamide. Chem. – Eur. J. 2019, 25, 8465–8470. 10.1002/chem.201900430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravdivtsev A. N.; Yurkovskaya A. V.; Zimmermann H.; Vieth H. M.; Ivanov K. L. Enhancing NMR of insensitive nuclei by transfer of SABRE spin hyperpolarization. Chem. Phys. Lett. 2016, 661, 77–82. 10.1016/j.cplett.2016.08.037. [DOI] [Google Scholar]
- Kidd B. E.; Gesiorski J. L.; Gemeinhardt M. E.; Shchepin R. V.; Kovtunov K. V.; Koptyug I. V.; Chekmenev E. Y.; Goodson B. M. Facile Removal of Homogeneous SABRE Catalysts for Purifying Hyperpolarized Metronidazole, a Potential Hypoxia Sensor. J. Phys. Chem. C 2018, 122, 16848–16852. 10.1021/acs.jpcc.8b05758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skovpin I. V.; Svyatova A.; Chukanov N.; Chekmenev E. Y.; Kovtunov K. V.; Koptyug I. V. N-15 Hyperpolarization of Dalfampridine at Natural Abundance for Magnetic Resonance Imaging. Chem. – Eur. J. 2019, 25, 12694–12697. 10.1002/chem.201902724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshuis N.; van Weerdenburg B. J. A.; Feiters M. C.; Rutjes F. P. J. T.; Wijmenga S. S.; Tessari M. Quantitative Trace Analysis of Complex Mixtures Using SABRE Hyperpolarization. Angew. Chem., Int. Ed. 2015, 54, 1481–1484. 10.1002/anie.201409795. [DOI] [PubMed] [Google Scholar]
- van Weerdenburg B. J. A.; Gloeggler S.; Eshuis N.; Engwerda A. H. J.; Smits J. M. M.; de Gelder R.; Appelt S.; Wymenga S. S.; Tessari M.; Feiters M. C.; Blumich B.; Rutjes F. P. J. T. Ligand effects of NHC-iridium catalysts for signal amplification by reversible exchange (SABRE). Chem. Commun. 2013, 49, 7388–7390. 10.1039/c3cc43423k. [DOI] [PubMed] [Google Scholar]
- Levitt M. H.; Singlet N. M. R. Annu. Rev. Phys. Chem. 2012, 89–105. 10.1146/annurev-physchem-032511-143724. [DOI] [PubMed] [Google Scholar]
- Roy S. S.; Norcott P.; Rayner P. J.; Green G. G. R.; Duckett S. B. A Hyperpolarizable 1H Magnetic Resonance Probe for Signal Detection 15 Minutes after Spin Polarization Storage. Angew. Chem., Int. Ed. 2016, 55, 15642–15645. 10.1002/anie.201609186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S. S.; Norcott P.; Rayner P. J.; Green G. G. R.; Duckett S. B. A Simple Route to Strong Carbon-13 NMR Signals Detectable for Several Minutes. Chem. – Eur. J. 2017, 23, 10496–10500. 10.1002/chem.201702767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G. N.; Colell J. F. P.; Glachet T.; Lindale J. R.; Reboul V.; Theis T.; Warren W. S. Terminal Diazirines Enable Reverse Polarization Transfer from N-15(2) Singlets. Angew. Chem., Int. Ed. 2019, 58, 11118–11124. 10.1002/anie.201904026. [DOI] [PubMed] [Google Scholar]
- Shen K.; Logan A. W. J.; Colell J. F. P.; Bae J.; Ortiz G. X. Jr.; Theis T.; Warren W. S.; Malcolmson S. J.; Wang Q. Diazirines as Potential Molecular Imaging Tags: Probing the Requirements for Efficient and Long-Lived SABRE-Induced Hyperpolarization. Angew. Chem., Int. Ed. 2017, 56, 12112–12116. 10.1002/anie.201704970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procacci B.; Roy S. S.; Norcott P.; Turner N.; Duckett S. B. Unlocking a Diazirine Long-Lived Nuclear Singlet State via Photochemistry: NMR Detection and Lifetime of an Unstabilized Diazo-Compound. J. Am. Chem. Soc. 2018, 140, 16855–16864. 10.1021/jacs.8b10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norcott P.; Burns M. J.; Rayner P. J.; Mewis R. E.; Duckett S. B. Using 2H Labelling to Improve the NMR Detectability of Pyridine and its Derivatives by SABRE. Magn. Reson. Chem. 2018, 663. 10.1002/mrc.4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norcott P.; Rayner P. J.; Green G. G. R.; Duckett S. Achieving High 1H Nuclear Hyperpolarization Levels with Long Lifetimes in a Range of Tuberculosis Drug Scaffolds. Chem. – Eur. J. 2017, 23, 16990–16997. 10.1002/chem.201703278. [DOI] [PubMed] [Google Scholar]
- Fekete M.; Bayfield O.; Duckett S. B.; Hart S.; Mewis R. E.; Pridmore N.; Rayner P. J.; Whitwood A. Iridium(III) Hydrido N-Heterocyclic Carbene–Phosphine Complexes as Catalysts in Magnetization Transfer Reactions. Inorg. Chem. 2013, 52, 13453–13461. 10.1021/ic401783c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd L. S.; Asghar A.; Burns M. J.; Charlton A.; Coombes S.; Cowley M. J.; Dear G. J.; Duckett S. B.; Genov G. R.; Green G. G. R.; Highton L. A. R.; Hooper A. J. J.; Khan M.; Khazal I. G.; Lewis R. J.; Mewis R. E.; Roberts A. D.; Ruddlesden A. J. Hyperpolarisation through reversible interactions with parahydrogen. Catal. Sci. Technol. 2014, 4, 3544–3554. 10.1039/C4CY00464G. [DOI] [Google Scholar]
- Rayner P. J.; Gillions J. P.; Hannibal V. D.; John R. O.; Duckett S. B. Hyperpolarisation of weakly binding N-heterocycles using signal amplification by reversible exchange. Chem. Sci. 2021, 12, 5910–5917. 10.1039/D0SC06907H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner P. J.; Tickner B. J.; Iali W.; Fekete M.; Robinson A. D.; Duckett S. B. Relayed hyperpolarization from para-hydrogen improves the NMR detectability of alcohols. Chem. Sci. 2019, 10, 7709–7717. 10.1039/C9SC02765C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iali W.; Rayner P. J.; Duckett S. B. Using parahydrogen to hyperpolarize amines, amides, carboxylic acids, alcohols, phosphates, and carbonates. Sci. Adv. 2018, 4, eaao6250 10.1126/sciadv.aao6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shchepin R. V.; Truong M. L.; Theis T.; Coffey A. M.; Shi F.; Waddell K. W.; Warren W. S.; Goodson B. M.; Chekmenev E. Y. Hyperpolarization of “Neat” Liquids by NMR Signal Amplification by Reversible Exchange. J. Phys. Chem. Lett. 2015, 6, 1961–1967. 10.1021/acs.jpclett.5b00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colell J.; Logan A. W. J.; Zhou Z.; Lindale J. R.; Laasner R.; Shchepin R.; Chekmenev E.; Blum V.; Warren W. S.; Malcolmson S. J.; Theis T. Rational ligand choice extends the SABRE substrate scope. Chem. Commun. 2020, 56, 9336–9339. 10.1039/D0CC01330G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iali W.; Rayner P. J.; Alshehri A.; Holmes A. J.; Ruddlesden A. J.; Duckett S. B. Direct and indirect hyperpolarisation of amines using parahydrogen. Chem. Sci. 2018, 9, 3677–3684. 10.1039/C8SC00526E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner P. J.; Richardson P. M.; Duckett S. B. The Detection and Reactivity of Silanols and Silanes Using Hyperpolarized 29Si Nuclear Magnetic Resonance. Angew. Chem., Int. Ed. 2020, 59, 2710–2714. 10.1002/anie.201915098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson P. M.; Iali W.; Roy S. S.; Rayner P. J.; Halse M. E.; Duckett S. B. Rapid 13C NMR hyperpolarization delivered from para-hydrogen enables the low concentration detection and quantification of sugars. Chem. Sci. 2019, 10, 10607–10619. 10.1039/C9SC03450A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tickner B. J.; Lewis J. S.; John R. O.; Whitwood A. C.; Duckett S. B. Mechanistic insight into novel sulfoxide containing SABRE polarisation transfer catalysts. Dalton Trans. 2019, 48, 15198–15206. 10.1039/C9DT02951F. [DOI] [PubMed] [Google Scholar]
- Mewis R. E.; Green R. A.; Cockett M. C. R.; Cowley M. J.; Duckett S. B.; Green G. G. R.; John R. O.; Rayner P. J.; Williamson D. C. Strategies for the Hyperpolarization of Acetonitrile and Related Ligands by SABRE. J. Phys. Chem. B 2015, 119, 1416–1424. 10.1021/jp511492q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagley M. C.; Alnomsy A.; Sharhan H. I. Rapid Protium–Deuterium Exchange of 4-Aminopyridines in Neutral D2O under Microwave Irradiation. Synlett 2016, 27, 2467–2472. 10.1055/s-0035-1562479. [DOI] [Google Scholar]
- van Weerdenburg B. J. A.; Eshuis N.; Tessari M.; Rutjes F. P. J. T.; Feiters M. C. Application of the [small pi]-accepting ability parameter of N-heterocyclic carbene ligands in iridium complexes for signal amplification by reversible exchange (SABRE). Dalton Trans. 2015, 44, 15387–15390. 10.1039/C5DT02340H. [DOI] [PubMed] [Google Scholar]
- Poater A.; Cosenza B.; Correa A.; Giudice S.; Ragone F.; Scarano V.; Cavallo L. SambVca: A Web Application for the Calculation of the Buried Volume of N-Heterocyclic Carbene Ligands. Eur. J. Inorg. Chem. 2009, 2009, 1759–1766. 10.1002/ejic.200801160. [DOI] [Google Scholar]
- Zhang Y.; Lavigne G.; Lugan N.; César V. Buttressing Effect as a Key Design Principle towards Highly Efficient Palladium/N-Heterocyclic Carbene Buchwald–Hartwig Amination Catalysts. Chem. – Eur. J. 2017, 23, 13792–13801. 10.1002/chem.201702859. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; César V.; Storch G.; Lugan N.; Lavigne G. Skeleton Decoration of NHCs by Amino Groups and its Sequential Booster Effect on the Palladium-Catalyzed Buchwald–Hartwig Amination. Angew. Chem., Int. Ed. 2014, 53, 6482–6486. 10.1002/anie.201402301. [DOI] [PubMed] [Google Scholar]
- Izatt R. M.; Bradshaw J. S.; Nielsen S. A.; Lamb J. D.; Christensen J. J.; Sen D. Thermodynamic and kinetic data for cation-macrocycle interaction. Chem. Rev. 1985, 85, 271–339. 10.1021/cr00068a003. [DOI] [Google Scholar]
- More M. B.; Ray D.; Armentrout P. B. Intrinsic Affinities of Alkali Cations for 15-Crown-5 and 18-Crown-6: Bond Dissociation Energies of Gas-Phase M+–Crown Ether Complexes. J. Am. Chem. Soc. 1999, 121, 417–423. 10.1021/ja9823159. [DOI] [Google Scholar]
- Mewis R. E.; Atkinson K. D.; Cowley M. J.; Duckett S. B.; Green G. G. R.; Green R. A.; Highton L. A. R.; Kilgour D.; Lloyd L. S.; Lohman J. A. B.; Williamson D. C. Probing signal amplification by reversible exchange using an NMR flow system. Magn. Reson. Chem. 2014, 52, 358–369. 10.1002/mrc.4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colell J. F. P.; Logan A. W. J.; Zhou Z.; Shchepin R. V.; Barskiy D. A.; Ortiz G. X.; Wang Q.; Malcolmson S. J.; Chekmenev E. Y.; Warren W. S.; Theis T. Generalizing, Extending, and Maximizing Nitrogen-15 Hyperpolarization Induced by Parahydrogen in Reversible Exchange. J. Phys. Chem. C 2017, 121, 6626–6634. 10.1021/acs.jpcc.6b12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G.; Colell J. F. P.; Glachet T.; Lindale J. R.; Reboul V.; Theis T.; Warren W. S. Terminal Diazirines Enable Reverse Polarization Transfer from 15N2 Singlets. Angew. Chem., Int. Ed. 2019, 58, 11118–11124. 10.1002/anie.201904026. [DOI] [PubMed] [Google Scholar]
- Hodgson H. H. The Sandmeyer Reaction. Chem. Rev. 1947, 40, 251–277. 10.1021/cr60126a003. [DOI] [PubMed] [Google Scholar]
- Sandmeyer T. Ueber die Ersetzung der Amidgruppe durch Chlor in den aromatischen Substanzen. Ber. Dtsch. Chem. Ges. 1884, 17, 1633–1635. 10.1002/cber.18840170219. [DOI] [Google Scholar]
- Mo F.; Qiu D.; Zhang Y.; Wang J. Renaissance of Sandmeyer-Type Reactions: Conversion of Aromatic C–N Bonds into C–X Bonds (X = B, Sn, P, or CF3). Acc. Chem. Res. 2018, 51, 496–506. 10.1021/acs.accounts.7b00566. [DOI] [PubMed] [Google Scholar]
- Leas D. A.; Dong Y.; Vennerstrom J. L.; Stack D. E. One-Pot, Metal-Free Conversion of Anilines to Aryl Bromides and Iodides. Org. Lett. 2017, 19, 2518–2521. 10.1021/acs.orglett.7b00771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q.; Sun B.; Liu Z.; Kao Y.; Dong B.-W.; Jiang S.-D.; Li F.; Liu G.; Yang Y.; Mo F. A general electrochemical strategy for the Sandmeyer reaction. Chem. Sci. 2018, 9, 8731–8737. 10.1039/C8SC03346C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong T.; Pang M.-K.; Chen Z.-D.; Zhang B.; Weng J.; Lu G. Copper-free Sandmeyer-type Reaction for the Synthesis of Sulfonyl Fluorides. Org. Lett. 2020, 22, 3072–3078. 10.1021/acs.orglett.0c00823. [DOI] [PubMed] [Google Scholar]
- Olah G. A.; Herges R.; Laali K.; Segal G. A. Onium ions. 34. The methoxydiazonium ion: preparation, proton, carbon-13, and nitrogen-15 NMR and IR structural studies, theoretical calculations, and reaction with aromatics. Attempted preparation and the intermediacy of the hydroxydiazonium ion. J. Am. Chem. Soc. 1986, 108, 2054–2057. 10.1021/ja00268a054. [DOI] [Google Scholar]
- Elofson R. M.; Cyr N.; Laidler J. K.; Schulz K. F.; Gadallah F. F. Correlation of 13C and 15N nuclear magnetic resonance chemical shifts with polarographic reduction potentials of para-substituted benzenediazonium salts and their electronic structures. Can. J. Chem. 1984, 62, 92–95. 10.1139/v84-018. [DOI] [Google Scholar]
- Barraclough R.; Jones F.; Patterson D.; Tetlow A. The Photochemical Decomposition of Aryldiazonium Salts I—Stability and Quantum Yields. J. Soc. Dyers Colour. 1972, 88, 22–25. 10.1111/j.1478-4408.1972.tb03035.x. [DOI] [Google Scholar]
- Mo F.; Dong G.; Zhang Y.; Wang J. Recent applications of arene diazonium salts in organic synthesis. Org. Biomol. 2013, 11, 1582–1593. 10.1039/c3ob27366k. [DOI] [PubMed] [Google Scholar]
- Ritchie C. D.; Wright D. J. Anion-cation combination reactions. III. Reaction of diazonium ions with azide ion in aqueous solution. J. Am. Chem. Soc. 1971, 93, 2429–2432. 10.1021/ja00739a012. [DOI] [Google Scholar]
- Butler R. N.; Fox A.; Collier S.; Burke L. A. Pentazole chemistry: the mechanism of the reaction of aryldiazonium chlorides with azide ion at −80 °C: concerted versus stepwise formation of arylpentazoles, detection of a pentazene intermediate, a combined 1H and 15N NMR experimental and ab initio theoretical study. J. Chem. Soc., Perkin Trans. 2 1998, 2243–2248. 10.1039/a804040k. [DOI] [Google Scholar]
- Joshi S. M.; de Cózar A.; Gómez-Vallejo V.; Koziorowski J.; Llop J.; Cossío F. P. Synthesis of radiolabelled aryl azides from diazonium salts: experimental and computational results permit the identification of the preferred mechanism. Chem. Commun. 2015, 51, 8954–8957. 10.1039/C5CC01913C. [DOI] [PubMed] [Google Scholar]
- Dommerholt J.; Schmidt S.; Temming R.; Hendriks L. J. A.; Rutjes F. P. J. T.; van Hest J. C. M.; Lefeber D. J.; Friedl P.; van Delft F. L. Readily Accessible Bicyclononynes for Bioorthogonal Labeling and Three-Dimensional Imaging of Living Cells. Angew. Chem., Int. Ed. 2010, 49, 9422–9425. 10.1002/anie.201003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E.; Koo H. Biomedical applications of copper-free click chemistry: in vitro, in vivo, and ex vivo. Chem. Sci. 2019, 10, 7835–7851. 10.1039/C9SC03368H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewett J. C.; Bertozzi C. R. Cu-free click cycloaddition reactions in chemical biology. Chem. Soc. Rev. 2010, 39, 1272–1279. 10.1039/b901970g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaneeckhaute E.; De Ridder S.; Tyburn J.-M.; Kempf J. G.; Taulelle F.; Martens J. A.; Breynaert E. Long-Term Generation of Longitudinal Spin Order Controlled by Ammonia Ligation Enables Rapid SABRE Hyperpolarized 2D NMR. ChemPhysChem 2021, 22, 1170–1177. 10.1002/cphc.202100079. [DOI] [PubMed] [Google Scholar]
- Iali W.; Olaru A. M.; Green G. G. R.; Duckett S. B. Achieving High Levels of NMR-Hyperpolarization in Aqueous Media With Minimal Catalyst Contamination Using SABRE. Chem. – Eur. J. 2017, 23, 10491–10495. 10.1002/chem.201702716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoharan A.; Rayner P. J.; Iali W.; Burns M. J.; Perry V. H.; Duckett S. B. Achieving Biocompatible SABRE: An in vitro Cytotoxicity Study. ChemMedChem 2018, 13, 352–359. 10.1002/cmdc.201700725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landini D.; Maia A.; Montanari F.; Pirisi F. M. Crown ethers as phase-transfer catalysts. A comparison of anionic activation in aqueous–organic two-phase systems and in low polarity anhydrous solutions by perhydrodibenzo-18-crown-6, lipophilic quaternary salts, and cryptands. J. Chem. Soc., Perkin Trans. 2 1980, 1, 46–51. 10.1039/P29800000046. [DOI] [Google Scholar]
- Krushna C.; Mohapatra C.; Dash K. C. 4-, 5- and 6-coordinate complexes of copper(II) with substituted imidazoles. J. Inorg. Nucl. 1977, 39, 1253–1258. 10.1016/0022-1902(77)80363-1. [DOI] [Google Scholar]
- Mu J.; Perlmutter D. D. Thermal decomposition of metal nitrates and their hydrates. Thermochim. Acta 1982, 56, 253–260. 10.1016/0040-6031(82)87033-0. [DOI] [Google Scholar]
- Dollimore D.; Gamlen G. A.; Taylor T. J. Degradation studies on nickel nitrate hexahydrate: Part 2. evolved gas analysis. Thermochim. Acta 1985, 91, 287–297. 10.1016/0040-6031(85)85221-7. [DOI] [Google Scholar]
- Dollimore D.; Gamlen G. A.; Taylor T. J. Degradation studies on nickel nitrate hexahydrate. Part 1. Effect of experimental conditions. Thermochim. Acta 1985, 86, 119–132. 10.1016/0040-6031(85)87040-4. [DOI] [Google Scholar]
- Wheeler M. T.; Walmsley F. Transition metal nitrate complexes of 1,4,5-triazanaphthalene. Thermochim. Acta 1986, 108, 325–336. 10.1016/0040-6031(86)85101-2. [DOI] [Google Scholar]
- Lu X.; Song H.; Cai J.; Lu S. Recent development of electrochemical nitrate reduction to ammonia: A mini review. Electrochem. Commun. 2021, 129, 107094 10.1016/j.elecom.2021.107094. [DOI] [Google Scholar]
- Wang Y.; Wang C.; Li M.; Yu Y.; Zhang B. Nitrate electroreduction: mechanism insight, in situ characterization, performance evaluation, and challenges. Chem. Soc. Rev. 2021, 50, 6720–6733. 10.1039/D1CS00116G. [DOI] [PubMed] [Google Scholar]
- Wei L.; Liu D.-J.; Rosales B. A.; Evans J. W.; Vela J. Mild and Selective Hydrogenation of Nitrate to Ammonia in the Absence of Noble Metals. ACS Catal. 2020, 10, 3618–3628. 10.1021/acscatal.9b05338. [DOI] [Google Scholar]
- Xu P.; Agarwal S.; Lefferts L. Mechanism of nitrite hydrogenation over Pd/γ-Al2O3 according a rigorous kinetic study. J. Catal. 2020, 383, 124–134. 10.1016/j.jcat.2020.01.003. [DOI] [Google Scholar]
- Zhu I.; Getting T. A review of nitrate reduction using inorganic materials. Environ. Technol. Rev. 2012, 1, 46–58. 10.1080/09593330.2012.706646. [DOI] [Google Scholar]
- Bowers C. R.; Weitekamp D. P. Para-Hydrogen And Synthesis Allow Dramatically Enhanced Nuclear Alignment. J. Am. Chem. Soc. 1987, 109 (18), 5541–5542. 10.1021/ja00252a049. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.