Abstract
Enterocin A is a small, heat-stable, antilisterial bacteriocin produced by Enterococcus faecium DPC1146. The sequence of a 10,879-bp chromosomal region containing at least 12 open reading frames (ORFs), 7 of which are predicted to play a role in enterocin biosynthesis, is presented. The genes entA, entI, and entF encode the enterocin A prepeptide, the putative immunity protein, and the induction factor prepeptide, respectively. The deduced proteins EntK and EntR resemble the histidine kinase and response regulator proteins of two-component signal transducing systems of the AgrC-AgrA type. The predicted proteins EntT and EntD are homologous to ABC (ATP-binding cassette) transporters and accessory factors, respectively, of several other bacteriocin systems and to proteins implicated in the signal-sequence-independent export of Escherichia coli hemolysin A. Immediately downstream of the entT and entD genes are two ORFs, the product of one of which, ORF4, is very similar to the product of the yteI gene of Bacillus subtilis and to E. coli protease IV, a signal peptide peptidase known to be involved in outer membrane lipoprotein export. Another potential bacteriocin is encoded in the opposite direction to the other genes in the enterocin cluster. This putative bacteriocin-like peptide is similar to LafX, one of the components of the lactacin F complex. A deletion which included one of two direct repeats upstream of the entA gene abolished enterocin A activity, immunity, and ability to induce bacteriocin production. Transposon insertion upstream of the entF gene also had the same effect, but this mutant could be complemented by exogenously supplied induction factor. The putative EntI peptide was shown to be involved in the immunity to enterocin A. Cloning of a 10.5-kb amplicon comprising all predicted ORFs and regulatory regions resulted in heterologous production of enterocin A and induction factor in Enterococcus faecalis, while a four-gene construct (entAITD) under the control of a constitutive promoter resulted in heterologous enterocin A production in both E. faecalis and Lactococcus lactis.
In recent years there has been considerable interest in bacteriocins produced by lactic acid bacteria (LAB), many of which are active against organisms involved in foodborne disease and food spoilage. The potential for use of these naturally produced inhibitory substances lies in their ability to control undesirable microorganisms in food. This has largely been prompted by the successful exploitation of nisin, one of the most widely studied of the LAB bacteriocins and the subject of many reviews (13, 14, 15, 21, 27, 36, 62). On the basis of genetic and biochemical studies, three defined classes of bacteriocins in LAB have been established (50). Class II are small heat-stable peptides usually preceded by a leader peptide with a double-glycine processing site. The gene clusters of many class II bacteriocin systems have been described to date and, while the individual gene products of each system are not fully characterized as yet, much information can be obtained by comparative analyses (for reviews, see references 40, 42, and 50).
Enterocin 1146 was originally described as a small heat-stable antilisterial bacteriocin produced by Enterococcus faecium DPC1146 (55, 56). In this study, we report that this bacteriocin is identical to enterocin A, produced by E. faecium CTC492 (8). Enterocin A is a member of the class IIa subgroup of class II bacteriocins, otherwise known as pediocin-like bacteriocins. This group comprises several Listeria-active peptides with a -Y-G-N-G-V-X-C- consensus in the N terminus of the mature peptide which is cleaved from an inactive prepeptide during export from the cell, generally by a transporter of the ATP-binding-cassette (ABC) type (22, 28, 50). No other modifications are thought to take place, apart from the formation of one to two disulfide bridges thought to play a role in activity (2, 19). Production of enterocin A is an inducible phenomenon, and the induction factor has been described (51). It is a small peptide molecule with a similar type of leader sequence to the bacteriocin it induces. Several other bacteriocins are known to be induced by such induction factors (9, 11, 16, 66). Induction is proposed to occur through a two-component signal transduction pathway (43).
This study presents evidence that enterocin A production is also inducible in E. faecium DPC1146. In addition, we demonstrate that a 10.5-kb region of the chromosome is sufficient for bacteriocin production, immunity, and induction in a heterologous strain. The putative immunity gene identified by Aymerich et al. (8) is confirmed to confer immunity on previously sensitive strains. Four additional bacteriocin-related genes are identified, two of which are observed to be necessary, along with the structural and immunity genes, to allow production of active bacteriocin in Lactococcus lactis. Another five open reading frames (ORFs) are also described, some of which may have a role in the EntA+ phenotype. Furthermore, two regulatory mutants of DPC1146 are characterized, thus enabling the identification of possible sites at which regulation of the bacteriocin system occurs.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
Bacterial strains and plasmids used in this study are listed in Table 1. E. faecium DPC3675 was generated after Tn916 mutagenesis of DPC1146 (data not shown). The plasmid pGEM-T was supplied by Promega (Madison, Wis.) as part of their pGEM-T Vector System I.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristicsa | Reference or source |
|---|---|---|
| Enterococcus faecium | ||
| DPC1146 | Wild-type strain, EntA+ EntI+ EntF+ | 55 |
| DPC3342 | Acridine orange mutant of DPC1146, EntA− EntI− EntF− | 55 |
| DPC3675 | Tn916 mutant derivative of DPC1146, Tcr EntA− EntI− | This study |
| Enterococcus faecalis OG1X | Plasmid-free host strain, enterocin sensitive | 39 |
| Listeria innocua DPC1770 | Indicator organism | 55 |
| Listeria monocytogenes | ||
| LO28 | Sensitive to enterocin A (much less sensitive than DPC1770) | P. Cossart |
| EGD(Sv 1/2a) | Sensitive to enterocin A | W. Goebel |
| Lactococcus lactis subsp. lactis | ||
| IL1403 | Plasmid-free host strain, contains lcnC and lcnD analogues | 77 |
| MG1363 | Plasmid-free host strain, slightly sensitive to enterocin A | 25 |
| Pediococcus acidilactici LMG2351 | Ped+ | I. F. Nes |
| Lactobacillus sake LMG2334 | Sak+ | I. F. Nes |
| Plasmids | ||
| pGEM-T | PCR product cloning vector, AprlacZ, linearized | Promega |
| pMG36e | Expression vector, can replicate in E. coli and L. lactis | 76 |
| pCI372 | Shuttle vector, replicates in E. coli and L. lactis, Cmr | 30 |
| pENT01 | pMG36e containing entA and entI downstream of promoter | This study |
| pENT02 | pENT01 containing entT and entD downstream from entA and entI | This study |
| pENT03 | pCI372 containing >10 kb of DPC1146 DNA (all genes needed for EntA production) | This study |
EntA, enterocin A production; EntI, immunity to enterocin A; EntF, enterocin induction factor production; Ped, pediocin PA-1 production; Sak, sakacin A production; lcnC and lcnD, the lactococcin transport and accessory factor genes, respectively; Tc, tetracycline. Ap, ampicillin; lacZ, β-galactosidase gene; Em, erythromycin; Cm, chloramphenicol.
Unless otherwise stated, enterococci, listeriae, and lactococci were grown in M17 medium (70), which was supplied by Difco Laboratories (Detroit, Mich.), supplemented with 0.5% glucose (GM17) at 37, 37, and 30°C, respectively. Escherichia coli strains XL1-Blue and DH5α (Stratagene, Inc., La Jolla, Calif.) were grown in Luria-Bertani (LB) medium (64) with vigorous agitation at 37°C. Lactobacillus sake LMG2334 and Pediococcus acidilactici LMG2351 were cultured in MRS broth (Difco Laboratories) at 30°C. Antibiotics used in the selective media were added at the following concentrations: tetracycline, 10 μg/ml; ampicillin, 100 μg/ml; erythromycin, 100 μg/ml (E. coli) and 5 μg/ml (L. lactis and Enterococcus spp.); and chloramphenicol, 20 μg/ml (E. coli) and 5 μg/ml (L. lactis and Enterococcus spp.). Color screening for transformants containing pGEM-T with inserts was carried out on LB plates containing IPTG (isopropyl-β-d-thiogalactopyranoside) and X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside). IPTG was dissolved in water, filter sterilized, and added to selective plates at a final concentration of 0.5 mM. An X-Gal stock solution in N,N-dimethylformamide stored at −20°C was added to plates at a final concentration of 80 μg/ml. Chemical reagents were obtained from Sigma Chemical Co., Dorset, England. Chemically synthesized enterocin A induction factor was a kind gift of Ingolf Nes, Laboratory of Microbial Gene Technology, Agricultural University of Norway, Ås, Norway.
Bacteriocin activity and immunity.
The ability to produce bacteriocin was detected by deferred antagonism (48, 63). The strain under examination was spotted onto the appropriate agar and allowed to form colonies overnight at the appropriate temperature. These plates were then overlaid with 3 ml of soft agar seeded with 100 μl of the indicator strain (ca. 107 stationary-phase cells) and incubated overnight at 37°C. Bacteriocin production was detected by the formation of clear zones of inhibition around the test colonies in the indicator lawn. Bacteriocin production in broth was quantified by using a critical dilution assay (55). Heat-treated (80°C for 5 min) cell-free supernatant was serially diluted (twofold), and 10-μl aliquots were spotted onto GM17 plates and allowed to dry. The plates were overlaid as described above and incubated at 37°C overnight. One arbitrary unit (AU) was defined as the reciprocal of the highest dilution giving a zone of growth inhibition on the indicator lawn. When a clear inhibition zone was followed by a turbid one, the critical dilution was taken to be the average of the final two dilutions. Listeria innocua DPC1770 was the standard indicator organism used to assay for bacteriocin activity.
The immunity of transformants was tested against concentrated enterocin A, which was obtained by ammonium sulfate precipitation (55% saturation) from 1 liter of cell-free supernatant from an 8-h MRS culture. The precipitate, which was resuspended in sterile distilled water, was then dialyzed (Cellu-Sep-T1; Membrane Filtration Products, Inc., San Antonio, Tex.) overnight at 4°C against sterile distilled water. Transformants which displayed insensitivity to enterocin, relative to the strains from which they were derived, were deemed immune.
DNA manipulations and transformations.
Chromosomal DNA was isolated either by using a rapid DNA extraction method (32) or by a protocol with CsCl gradients to obtain high-quality concentrated DNA (46). Plasmid DNA from E. coli (3 ml of fresh overnight culture) was isolated by using a QIAprep Spin Miniprep Kit (Qiagen Gmbh, Hilden, Germany) and resuspended in sterile distilled water. Plasmid DNA was extracted from lactococci and enterococci (3 ml of fresh overnight culture) by the rapid lysis method of Anderson and McKay (4) and dialyzed on filters with a 0.025-μm pore size (Millipore Corp., Bedford, Mass.) before subsequent manipulations. Recovery of DNA fragments from low-melting-point agarose gels was achieved by using a GeneClean kit (Bio 101, La Jolla, Calif.) according to the manufacturer’s instructions. Restriction enzymes were supplied by Boehringer Mannheim (Boehringer Corp., Ltd., East Sussex, United Kingdom) and New England Biolabs, Ltd. (Hertfordshire, United Kingdom), and reactions were carried out according to the manufacturers’ instructions. RNase-free DNase and DNase-free RNase were also supplied by Boehringer Mannheim. Ligations were performed with T4 DNA ligase (Boehringer Mannheim) at 15 to 18°C overnight. Electrocompetent cells of lactococci and enterococci were prepared and transformed by the method of Holo and Nes (33) with 2.5% glycine for growth (or 2.0% in the case of E. faecium DPC3342). Cells were transformed with a Gene Pulser apparatus (Bio-Rad Laboratories, Richmond, Calif.) set at 2.5 kV and 25 μF, with the pulse controller at 200 Ω. E. coli transformations were performed under the conditions outlined in the Bio-Rad manual. DNA was dialyzed as described previously for 20 min prior to electrotransformation to reduce the ionic strength of the solution.
PCR amplification.
DNA was amplified by PCR (49) by using Biotaq DNA Polymerase (Bioline UK, Ltd., London, England) in a GeneAmp PCR Systems 2400 DNA thermal cycler (Perkin-Elmer Corp., Norwalk, Conn.). DNA fragments for cloning were amplified by using the Expand High-Fidelity PCR System (Boehringer Mannheim). Large fragments (5 kb or greater) were amplified by using the Expand Long Template PCR System. Reactions were carried out as detailed in the Boehringer Mannheim PCR applications manual. To perform PCR amplification directly from colonies, Nonidet P-40 (Sigma) at a final concentration of 0.5% was included in the reaction to lyse the cells and expose the DNA.
Inverse PCR (10, 72) was performed as described above with the following modifications to the template DNA. Gradient-quality DNA was digested with an appropriate restriction enzyme for at least 3 h. The restricted DNA was then cleaned by phenol-chloroform extraction, precipitated in ethanol at −20°C for a minimum of 2 h, dissolved in sterile distilled water, and religated at a concentration of 2 to 5 ng/μl. These ligations were similarly cleaned by phenol-chloroform extraction and precipitation and then resuspended in 10 μl of sterile distilled water. PCR was carried out as described above with the entire 10-μl volume. The primers used in cloning and inverse PCR experiments, together with their relative locations (presented in Fig. 1B), include P1, ATCGAGCAGATTATGGAG; P2, ACCTAAAAAACCACCTAT; P3, GGTGCTGGAACAAAACCTCAAG; P4, CAATTCCTTCTTGAAACGTAGC; P5, GTATAGCATGAAGGCCCCAAC; P6, AGCCCGTTTGCATTTTCACTTG; P7, GCACGTTTCAAGAAGGAATTGC; P8, GAGGATCCGTAGCTCATCTTCG; P9, AACTCAAAGTCGACTGTAGCCC; P10, CTTCTAAGCTTTCTTCTGTGATTTC; and P11, ATAGCATGCATTCAGGAATGAAAAAGTTAGTG.
FIG. 1.
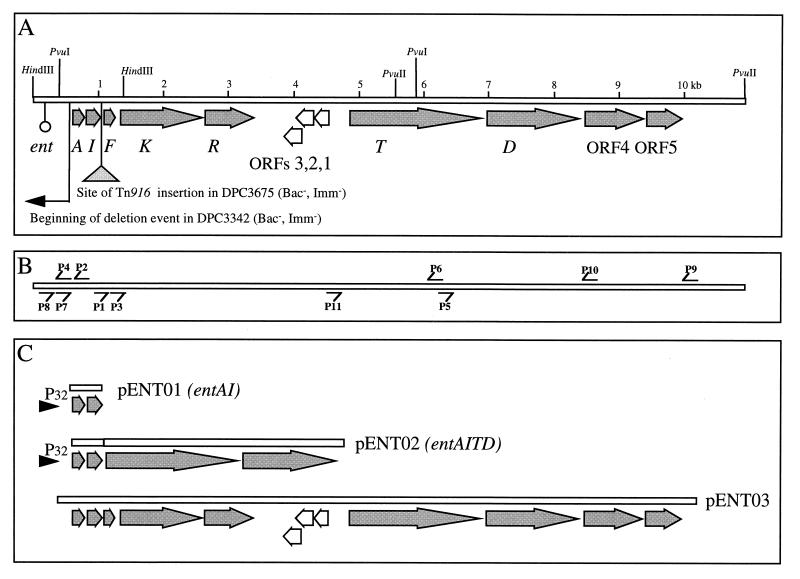
(A) Graphic illustration of ORFs. The site of insertion of Tn916 in DPC3675 and the start of the deletion in DPC3342 are indicated, as are the restriction sites used in the inverse PCR strategy. (B) Relative locations of primers used in this study. (C) Regions cloned in pENT01, pENT02, and pENT03. The presence of a constitutive promoter (P32) in pENT01 and pENT02 is indicated.
The primer GATCTATAGAATAAGGCTTTACGAGC was used to amplify from the left end of Tn916.
DNA sequencing and analysis.
DNA for sequencing was purified by using a High Pure PCR Product Purification Kit (Boehringer Mannheim) according to the manufacturer’s instructions. Sequencing was performed with a 373 DNA Sequencer STRETCH automated sequencer (Applied Biosystems, Foster City, Calif.) and was initiated with DNA primers based downstream of the amplification primers. It was then continued with specific synthetic 15- or 16-mer primers prepared on a DNA synthesizer (391 PCR-MATE; Applied Biosystems). Antisense primers were used to confirm the sequence on the complementary strand of DNA. Both strands were sequenced completely. Sequencing data were compiled and analyzed by using Genejockey (Biosoft, Cambridge, United Kingdom), DNAstar (Apple Computers, Inc., Cupertino, Calif.) and the BLAST program (3).
Nucleotide sequence accession number.
The sequence presented here has been submitted to GenBank, where it has been assigned the accession number AF099088.
RESULTS
Sequence analysis of the enterocin A gene cluster.
A number of PCR products were amplified from the chromosome of DPC1146 with primers based on the known sequence of enterocin A (8). These products were sequenced, and E. faecium DPC1146 was confirmed to possess a gene with the same sequence as that of enterocin A (8). That this is also the structural gene for the bacteriocin previously termed enterocin 1146 was confirmed by N-terminal sequencing of the isolated enterocin 1146 bacteriocin (data not shown). Therefore, enterocin 1146 will be henceforth designated enterocin A. The resulting sequence spanned from 88 nucleotides (nt) upstream of the start of the gene, entA, to 42 nt beyond the end of the gene designated ORF2 by Aymerich et al. (8) and was identical to the sequence elucidated by that group with the exception of 2 nt in the gene corresponding to ORF2. One of these did not alter the encoded amino acid (Asn79), the other changed Ser48 to a glycine. This second gene was designated entI since biological evidence confirms that it plays a role in immunity to enterocin A (see below).
An inverse PCR strategy for chromosome walking, based on the entAI region, was employed to amplify and sequence the flanking DNA as described in Materials and Methods. The restriction enzymes and primers used are shown in Fig. 1. With primers facing outwards on the known entAI sequence and DPC1146 DNA (HindIII digested and religated) as a template, ca. 1.1 kb of additional DNA was amplified. Sequence analysis of this DNA revealed that the sequence of DPC1146 diverged from that of CTC492 (8) at a point 41 nucleotides downstream from the stop codon of the immunity gene. Since the upstream DNA showed no homology to the bacteriocin-related sequences in the database, sequencing efforts concentrated on the downstream DNA. PvuI-digested and religated DNA was used to generate a large fragment of downstream DNA by using the inverse PCR method incorporating the Expand Long Template PCR System. The resulting 5.4-kb PCR product was sequenced on both strands by primer walking. To amplify the remainder of the enterocin-coding region also using inverse PCR, PvuII was used to generate a further 4.9 kb of DNA, which was sequenced as described above.
The completed DNA sequence is graphically represented in Fig. 1A. Ten ORFs, each preceded by putative ribosome binding sites, were identified downstream of entAI. The homologues for each ORF are listed in Table 2. The first of these ORFs (designated entF) starts 93 nt downstream from the end of entI and is identical to the gene encoding the precursor of the induction peptide for enterocin A synthesis (51). Originally, a single nucleotide difference was observed (GG instead of GGG at nt 628 to 630 in the sequence previously published by Nilsen et al. [51]) which altered the predicted leader region of the prepeptide from that previously reported. However, the sequence reported here is considered the correct version (49a).
TABLE 2.
Homologues of the enterocin gene cluster ORFs
| Enterocin (amino acids) | Homologue
|
|||
|---|---|---|---|---|
| Name | % Identity | % Similarity | Comment (reference) | |
| EntA (65) | EntA | 100 | NA | Enterocin A prepeptide (8) |
| EntI (103) | ORF2 | 96 | NA | Putative immunity gene (8) |
| EntF (48) | EntF | 100 | NA | Enterocin induction factor (51) |
| EntK (427) | SapK | 45 | 60 | Histidine kinase, sakacin A system (7) |
| CbnK | 38 | 63 | Histidine kinase, carnobacteriocin B2 (59) | |
| EntR (250) | SapR | 62 | 78 | Response regulator, sakacin A (6) |
| CbnR | 63 | 70 | Response regulator, carnobacteriocin B2 (59) | |
| EntT (717) | CbnT | 74 | 86 | ABC transporter, carnobacteriocin B2 system (59) |
| MesI | 48 | 68 | ABC transporter, mesenterocin Y105 system (24) | |
| SapT | 47 | 67 | ABC transporter, sakacin A system (6) | |
| ComA | 47 | 66 | ATP-binding transport protein, competence factor secretion in S. pneumoniae (34) | |
| HlyB | 31 | 50 | ATP-binding transport protein, hemolysin A secretion in E. coli (67) | |
| EntD (455) | CbnD | 48 | 67 | Accessory factor for CbnT (59) |
| ComB | 27 | 48 | Accessory factor for ComA (35) | |
| SapE | 24 | 49 | Accessory factor for SapT (6) | |
| ORF1 (59) | ||||
| ORF2 (71) | LafX | 43 | 61 | One component of lactacin F (1, 23) |
| LcnM | 34 | 40 | One component of lactococcin M (73) | |
| LcnN | 28 | 38 | Second component of lactococcin M (73) | |
| ORF3 (71) | ||||
| ORF4 (166) | YteI | 43 | 65 | Putative protease from B. subtilis (45) |
| SppA | 29 | 47 | Protease IV, signal peptide peptidase from E. coli (38, 54) | |
| ORF5 (115) | YteJ | 39 | 63 | Unknown function, encoded directly after the gene for YteI (45) |
Located 326 nt upstream of the entA start codon is an inverted repeat possibly representing a rho-independent transcription termination signal with a calculated ΔG of −33.4 kcal/mol (71). The 3′ end of a potential ORF is observed 20 nt upstream of this inverted repeat. Since the product of this ORF has homology (55% identity) with the last 51 amino acids of ProX, a putative glycine betaine-binding protein precursor from Streptococcus mutans (26), this putative terminator seems to denote the upstream limit of the genes involved in bacteriocin production. No obvious transcription termination signal was detected in the region downstream of entR (or upstream of ORF3). Interestingly, a pair of short direct repeats with a single mismatch are located 72 nt upstream of entA (TTCAGGAA[14 nt]TTCAAGAA) and an identical arrangement (same repeat, same mismatch, and same spacing) is also located 107 nt upstream of entT.
Characterization of the entKRTD, ORF4, and ORF5 gene products.
The putative proteins encoded by entK and entR exhibited sequence similarity to a large number of histidine protein kinases (HPKs) and response regulator proteins, respectively, of two-component signal transduction systems (43, 57, 69), in particular, those of sakacin A and carnobacteriocin B2 (Table 2). In general, HPKs autophosphorylate at a conserved histidine residue. The phosphate group is then transferred to a conserved aspartate residue in the response regulator, thus activating it. In addition to the conserved histidine, HPKs of the AgrC-ComD type are characterized by five to eight transmembrane sequences in their membrane domains as well as several regions of conserved sequence near the C terminus (29, 69). Response regulators, on the other hand, are more highly conserved at the N terminus, containing within this region a conserved lysine residue and a second aspartate as well as the one already mentioned (69). EntK and EntR display all of these general features of HPKs and response regulator proteins.
The deduced EntT protein is homologous to several putative ATP-dependent translocators or ABC transporters, proteins which are involved in the signal-sequence-independent transport of peptides across the bacterial membrane (Table 2; references 22 and 31). The highest similarity was observed to the ABC transporters of other bacteriocins and to ComA, the proposed translocator of a competence factor which coordinates the induction of genetic competence in Streptococcus pneumoniae (34). EntT also exhibited sequence similarity to HlyB, a protein which is essential for hemolysin A secretion in E. coli (67) and which is referred to as the prototype bacterial ABC exporter (22). This HlyB family of transporters usually contains a C-terminal ATP-binding domain of about 200 conserved amino acids located on the cytoplasmic face of the membrane as well as several (normally six) membrane-spanning domains towards the N-terminal region, which are thought to recognize and translocate the substrate across the cytoplasmic membrane (22, 31). The similarity between these proteins is highly significant around two conserved regions in the C-terminal A and B sites, which together constitute the ATP-binding motif (22). In addition, a proteolytic domain is observed in many of the bacteriocin ABC exporters that is responsible for cleavage of the bacteriocin leader peptide during export (28). Conserved cysteine and histidine residues, which are observed in EntT, are thought to be important in this active site.
The entD gene product is homologous to several accessory proteins for ABC transporters (Table 2). These are required for successful externalization of the bacteriocin, although their exact role has not yet been elucidated (22, 50). A common feature of EntD and other HlyD homologues is that they are largely hydrophilic except for a small region of hydrophobic amino acids close to the N terminus.
ORF4 and ORF5 are homologous to yteI and yteJ, respectively (Table 2), two genes which appear to be transcribed as a unit in Bacillus subtilis (45). ORF4 is also similar to the gene encoding protease IV (Table 2), a signal peptide peptidase from E. coli (38, 54). This is a cytoplasmic membrane protease which digests the signal peptide of the outer membrane lipoprotein after its release from the precursor protein (37). No function has been assigned to yteJ, the only homologue of the largely hydrophobic ORF5.
ORF1, -2, and -3.
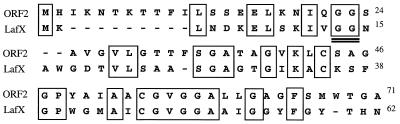
Only the ORF2 putative product displays homology with bacteriocin-related proteins and possesses most of the molecular features of class II bacteriocins, including a hydrophobic profile and a possible leader sequence with a consensus GG processing site (Fig. 2). In addition, the C terminus is similar to LafX and to LcnM and LcnN (Table 2), peptides implicated in lacticin F and lactococcin MN biosynthesis, respectively. The role, if any, of these three ORFs in enterocin biosynthesis has not been elucidated.
FIG. 2.
Alignment of the product of ORF2 with LafX, one of the peptides required for lactacin F activity. Identical amino acids are boxed, and the GG processing site of LafX is double underlined.
Bacteriocin production is inducible in DPC1146.
That enterocin A production in E. faecium DPC1146 is an inducible phenomenon was confirmed by serially diluting a producing culture into fresh broth. When cultures were diluted 10−6-fold or more, bacteriocin synthesis ceased to occur. Addition of chemically synthesized induction factor (51) at levels above 10−12 M restored bacteriocin production. In all cases, whether bacteriocin production was switched on or off in liquid culture, the plating of individual cells onto solid agar surfaces resulted in bacteriocin-producing colonies.
Isolation and phenotypic characterization of two mutants of DPC1146 unable to produce enterocin A.
In an attempt to learn more about the genes involved in the biosynthesis of enterocin A, two Bac− mutants were created. In one instance, bacteriocin production was disrupted in E. faecium DPC3675 by Tn916 insertion. The other mutant, Bac− DPC3342, was isolated after acridine orange mutagenesis (55). The relevant phenotypic properties of both mutants are listed in Table 1. Both mutants failed to inhibit L. innocua DPC1770, which is very sensitive to enterocin A. Loss of production also coincided with loss of immunity in both mutants. This loss of immunity to enterocin did not affect the degree of sensitivity of the mutants to other pediocin-like bacteriocins, including pediocin PA-1 and sakacin A.
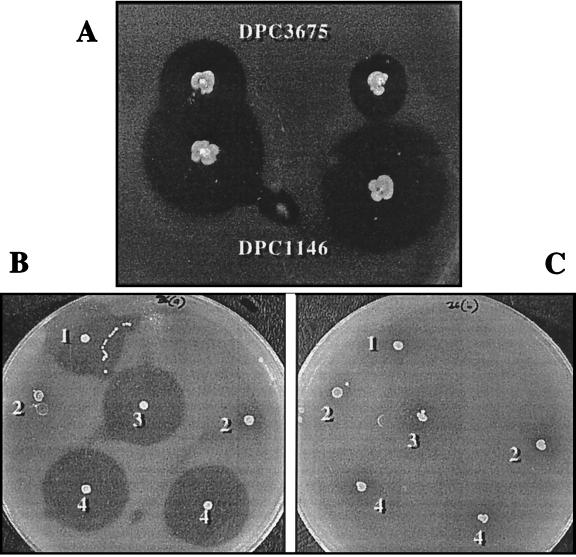
It proved possible to complement the Bac− phenotype of DPC3675 in a number of ways. Single colonies of DPC3675 could produce enterocin A when spotted within 15 mm of a colony of the enterocin-producing parent (Fig. 3A). The extent of the complementation depended inversely on the distance between parent and mutant. Ammonium sulfate-concentrated culture supernatant of DPC1146 was also capable of inducing bacteriocin synthesis in DPC3675 in a similar manner. That this induction was due to the product of the entF gene was confirmed when synthetic enterocin induction peptide (51) was also observed to switch on bacteriocin production in DPC3675 when incorporated in GM17 plates at 10−12 M or above. The minimal amount required to switch on bacteriocin production in liquid GM17 was also 10−12 M. However, only 50 AU/ml was produced by DPC3675 at concentrations up to 10−10 M inducer (levels of 300 AU/ml are normally observed from the wild type, DPC1146). The induction is medium dependent, since no bacteriocin synthesis by DPC3675 could be detected in MRS broth even in the presence of 10−7 M inducer. Other bacteriocin producers were also examined for their ability to induce enterocin A production in DPC3675. Colonies of the pediocin PA-1 producer LMG2351 and the sakacin A producer LMG2334 failed to complement DPC3675.
FIG. 3.
(A) Complementation of colonies of E. faecium DPC3675 (EntA−) by DPC1146 is dependent on the distance between colonies. (B) The Bac+/− phenotype of various enterococcal strains against Listeria monocytogenes EGD, including the parent E. faecium DPC1146 (colony 1), plasmid-free E. faecalis OG1X (colony 2), E. faecalis OG1X(pENT02) (colony 3), and E. faecalis OG1X(pENT03) (colony 4). (C) The same colonies as in panel B, overlaid with EntI+ L. monocytogenes EGD(pENT01).
The acridine orange Bac− Imm− mutant, DPC3342, could neither complement DPC3675 nor be complemented by the induction peptide, thus implying a mutation of a different nature.
Genotypic characterization of the Bac− mutants, DPC3675 and DPC3342.
The insertion point of Tn916 was mapped precisely by PCR analysis and sequencing by using primers based on the enterocin operon and the termini of Tn916. The transposon was shown to have inserted 66 nt upstream of the start codon of entF (Fig. 1A). Apart from the introduction of a 5-bp coupling sequence at the Tn916 insertion site, the DNA at either side of the transposon was undisturbed. It is perhaps noteworthy that the point of insertion of Tn916 coincides with the point at which the CTC492 sequence diverges from that of DPC1146. In fact, the sequence information already presented for the entA and entF genes and flanking regions in CTC492 (8, 51) strongly suggests that an insertion sequence (IS6770-like) has inserted at this same point in that strain, suggesting that this sequence may represent a transposon hot spot.
Locating the site of the DPC3342 lesion was achieved following multiple inverse PCRs based on the known sequence of the enterocin operon. A significant deletion was detected which did not affect any of the ent genes but rather occurred some 84 nt upstream of the entA start codon. The deletion event extended at least beyond the HindIII site some 450 bp upstream of the deletion start point, but the full extent remains unknown. It is important to note that the deletion includes one of the two direct repeats found upstream of entA (Fig. 1).
entI is the enterocin immunity gene.
The entAI genes were amplified on a 710-bp PCR fragment (which did not include any upstream signals other than the ribosomal binding sites of both genes) and cloned into pGEM-T. The fragment was subsequently recovered as a SacI/SphI fragment and directionally cloned into the expression vector pMG36e under the control of the constitutive P32 promoter to generate pENT01 (Fig. 1C). The expected nucleotide sequence of the insert and promoter region of this construct was confirmed.
pENT01 was electroporated into L. lactis MG1363 and Listeria monocytogenes LO28, both of which are slightly sensitive to enterocin. Transformants of both strains became totally insensitive to the bacteriocin even at the highest concentration available (51,200 AU/ml). L. monocytogenes EGD, whose sensitivity is comparable to that of the standard indicator, L. innocua DPC1770, was also examined. The sensitivity of the EGD transformant was reduced at least 12-fold (Fig. 3C), though it was still slightly sensitive to highly concentrated enterocin. Thus, the insert on pENT01 is capable of conferring enterocin immunity on previously sensitive strains. As expected, no bacteriocin production was detected in any of these transformants given that genes for production-export are not present on pENT01. The presence of pENT01 did not alter the sensitivity of an MG1363 transformant to a number of other bacteriocins, including lacticin 3147, nisin, lactococcin, lacticin 481, pediocin PA-1, and sakacin A.
Several attempts were made to express enterocin A in L. lactis IL1403 with the plasmid pENT01. L. lactis IL1403 encodes a transporter and accessory factor capable of undertaking the secretion and maturation of the lactococcins (77). However, IL1403(pENT01) transformants failed to produce enterocin A either in broth or on solid media even though reverse transcriptase PCR examination confirmed that the enterocin genes were being transcribed (data not shown).
Heterologous expression of EntA from a four-gene cassette, entAITD.
A 4-kb fragment containing entTD was amplified by high-fidelity PCR and cloned into pENT01 to create an entAITD gene cassette under the control of P32 (SphI and HindIII sites were incorporated into the primers to permit directional cloning). This plasmid was designated pENT02 (Fig. 1C). When pENT02 was introduced into L. lactis IL1403, the resulting transformants were inhibitory to L. monocytogenes on a solid medium, with zones of inhibition ranging from 5 to 12 mm in diameter. However, little (50 AU/ml) or no bacteriocin activity was produced in broth from the IL1403 host. Plasmid isolation and digestion subsequently revealed several fragments smaller than those expected for pENT02, suggesting that deletions had occurred. Production of bacteriocin by transformants was also observed to be quite unstable after a number of generations, as manifested by a decrease in zone size or the complete absence of zones of inhibition on plate assays. The introduction of pENT02 into L. lactis MG1363 did not result in any detectable bacteriocin production.
Enterococcus faecalis OG1X was also transformed with pENT02, and the resulting transformants were examined for enterocin production. Transformants produced zones of inhibition varying in diameter from 4 to 12 mm on solid agar. An Imm+ derivative of a sensitive strain of L. monocytogenes, EGD, (containing pENT01 [see above]) was not inhibited, confirming that E. faecalis OG1X (pENT02) was producing enterocin A (Fig. 3), but no production was observed in broth. Plasmid and phenotypic instability similar to that demonstrated in L. lactis was also observed.
Cloning and expression of the entire enterocin operon.
A fragment of ca. 10.5 kb was amplified from the chromosome of DPC1146 by using long-template PCR. This fragment contained all of the enterocin-related genes. BamHI and SalI (which had been incorporated into the primers) were used to clone the fragment into cognate sites of the E. coli-Lactococcus shuttle vector, pCI372, to generate pENT03 (Fig. 1C). E. faecalis OG1X was electroporated with the plasmid pENT03. The resulting transformants produced inhibition zones against L. monocytogenes EGD which varied from 4 to 13 mm in diameter. These zones were absent when tested against Imm+ L. monocytogenes EGD(pENT01), thus confirming that the zones were due to enterocin A (Fig. 3). pENT03 was isolated from a representative transformant and digested to confirm the presence of the correct 10.5-kb insert. No bacteriocin production was initially observed in GM17 broth. However, when induction factor was added at a concentration of 10−11 M, 300 AU/ml were detected in overnight cultures, which is comparable to the wild-type producer. That E. faecalis OG1X(pENT03) is capable of producing induction factor on solid medium was demonstrated by its ability to induce enterocin production in colonies of E. faecium DPC3675.
pENT03 did not result in any detectable bacteriocin activity when introduced into either L. lactis IL1403 or MG1363, even when screened on plates containing 10−11 M induction factor. No deletions appeared to have occurred, since examination of plasmids from several MG1363 transformants revealed an intact insert in all cases.
Complementation of DPC3342 by supplying enterocin genes in trans.
The Bac− Imm− mutant DPC3342 could be complemented by the enterocin structural and immunity genes encoded by pENT01. Exogenously supplied induction factor was not necessary for production. The amount of bacteriocin produced in broth was comparable to that produced by the wild type, DPC1146. The transformants, however, remained EntF− as demonstrated by an inability to complement DPC3675.
In addition, DPC3342 could be complemented by introducing the entire enterocin-coding region on the plasmid, pENT03. These transformants produced enterocin A, were immune, and could also induce production in DPC3675 colonies. To determine whether complementation was due to the plasmid-borne genes or due to a homologous recombination event, the plasmid was cured at 48°C. Loss of chloramphenicol resistance coincided with loss of bacteriocin production, thus confirming that the mutation in DPC3342 had been complemented in trans.
DISCUSSION
An inverse PCR strategy based on a core sequence consisting of the enterocin A structural and immunity genes was successfully used to obtain and sequence approximately 11 kb of the genome of E. faecium DPC1146. This locus contains the genetic information required for production of enterocin A in a heterologous strain. In addition to three previously sequenced genes, several new ORFs were identified, some of which had homologies to genes known to function in either regulation or processing and export of bacteriocins.
The first three genes in the enterocin cluster, entA, entI, and entF, were previously identified as the structural gene for enterocin A, the putative immunity gene, and the gene encoding the enterocin induction factor, respectively (8, 51). To confirm the role of entI in immunity, the entAI region was introduced into previously sensitive strains on the plasmid pENT01. As this fragment resulted in either complete or partial immunity in transformants and as entA had been confirmed as the structural gene, immunity can be attributed to entI. That only partial immunity was observed in L. monocytogenes EGD may be due to poor levels of transcription of entI under the control of the pMG36e promoter or perhaps due to the fact that another gene product, in addition to EntI, is needed for full immunity in strains that would otherwise be highly sensitive. A similar “immunity breakdown” at higher bacteriocin levels was observed upon introduction of the carnobacteriocin B2 and lactococcin A immunity proteins into sensitive strains (61, 75). Several mechanisms have been proposed for immunity to class I bacteriocins, including inhibition of pore formation, proteolytic degradation of the active compound, or active extrusion from the cell (20, 65, 68). Information concerning the immunity mechanism(s) of class II bacteriocins is quite scarce, but it is thought that the immunity protein binds the same membrane receptor as the bacteriocin (52, 61, 78). It is conceivable that higher receptor numbers in more-sensitive strains, such as EGD, may require a higher level of the immunity protein than is provided by pENT01. Elsewhere it has been reported that cross-resistance to class IIa bacteriocins can be correlated with the expression of immunity genes (19). This does not appear to be the case for enterocin immunity, since transformants harboring the immunity gene were still equally sensitive to other bacteriocins, including members of the pediocin-like group. Equally, the Bac− Imm− derivatives of DPC1146 did not become more sensitive to other bacteriocins.
Many LAB are known to produce more than one antimicrobial peptide (5, 19, 48, 60, 74). Combined biological and PCR results, with primers based on the known sequence of enterocin B, suggest that DPC1146 is also capable of producing at least both enterocins A and B (data not shown), which are known to have synergistic activity and are both induced by the entF gene product (12, 51). Another candidate for a bacteriocin structural gene is ORF2, which is located in the intergenic region between entR and entT. The putative peptide product bears comparison with class II bacteriocins and is especially similar to inhibitory peptides of the two-component lactacin F and lactococcin M bacteriocin systems (2, 23, 73). The possible roles of ORF1 and -3 are not evident, since neither product contains any of the unusual motifs of a bacteriocin prepeptide and/or immunity peptides. The fact that ORF1, -2, and -3 are encoded on the opposite strand to all the other genes in the enterocin gene cluster suggests that this intergenic region may be as a result of a recombination event and, as such, may play no role in enterocin A synthesis.
Two genes, entT and entD, with homology to several bacteriocin ABC transporters and accessory factors resulted in production of active enterocin A in L. lactis IL1403 and E. faecalis OG1X when introduced on a cassette with the bacteriocin structural and immunity genes under the control of the constitutive lactococcal P32 promoter. The structural and immunity genes alone on pENT01 resulted in no bacteriocin production. This indicates that entT and entD are necessary for enterocin A secretion. It is also likely that the products of ORF4 and ORF5 play some role in enterocin synthesis, since it seems likely from sequence context that they are cotranscribed with entT and entD. ORF4 possibly encodes a serine protease similar to the E. coli sppA gene product which degrades signal peptides in the cell membrane to maintain proper secretion of mature peptides from the cell (37, 38). A role for ORF4 in removing membrane-bound leader peptides from secreted enterocin and induction factor might be envisaged in DPC1146. The fact that production of enterocin occurred from the entAITD cassette on pENT02 in the IL1403 and OG1X backgrounds suggests that these ORFs are nonessential or, alternatively, that these strains may encode functional homologues. However, the instability of the pENT02 plasmid relative to pENT03 might indicate that enterocin production cannot be properly maintained in an ORF4− ORF5− background.
Bacteriocin production has been observed to be a regulated phenomenon in many strains of LAB (11, 16, 18, 44, 50, 59). An induction factor for enterocin A was previously identified in E. faecium CTC492. The same gene, entF, is also present in DPC1146. The DPC1146 gene encodes a prepeptide with a 23-amino-acid leader sequence of the double-glycine type (51). This represents a changed interpretation of the originally published entF gene product (51), which had a shorter leader due to a sequencing error. The combination of induction peptide and the two-component signal transduction system encoded by entK and entR is likely to provide the cell with a means to monitor and respond to cell density. Similar quorum-sensing mechanisms have been observed in other gram-positive bacteria where, in addition to control of bacteriocin synthesis, they are responsible for the onset of a state of competence in B. subtilis and S. pneumoniae, as well as regulation of the virulence response in Staphylococcus aureus (29, 41, 47, 53, 58). The point at which the EntIKR three-component system exerts control over the production of enterocin A is almost certainly at the level of transcription. Identical direct repeats are present upstream of entAIF and entD ORF4 and ORF5. Similar repeats have been observed in other bacteriocin systems and were suggested to function as binding sites for activated transcriptional regulators (11, 17, 43, 50). Evidence to support the involvement of the direct repeats upstream of entAIF was provided in the acridine orange mutant, DPC3342, which is missing one of these repeats and no longer produces enterocin A; nor can it be complemented by exogenously supplied induction factor. Constitutively expressing entAI in DPC3342 complements both of these negative phenotypes in trans, as does providing the whole enterocin cluster with its intact repeats upstream of entA.
A model for enterocin A regulation in DPC1146 can be proposed based on our results. This assumes that entFKR and entTD ORF4 and ORF5 are expressed at a basal level. In this regard, weak consensus promoters can be predicted upstream of entA and entT. This leads to a slow accumulation of induction factor in the environment. Once a certain threshold level is reached, sufficient EntK becomes phosphorylated to activate EntR. These may then bind to the direct repeats upstream of entAIFKR and entTD ORF4 and ORF5. This switches on high-level production of enterocin, the immunity factor, and the induction factor, as well as the transporter and accessory factor. If a culture with a fully activated system is inoculated at a level above 0.01% into fresh liquid medium, then sufficient induction factor is introduced to keep the system “primed” and essentially a constitutive “on” state is achieved. However, if cells are extensively diluted, insufficient induction factor is provided and enterocin production switches off. This is in agreement with our biological data. Cell-density-dependent regulation of bacteriocin production could be important from an ecological point of view, as it may give a producing cell an advantage over related bacteria when the competition for resources increases. It is also possible that the induction system may function as a quorum-sensing mechanism to regulate the expression of other genes once a certain cell density has been reached.
It is possible that there are other, as-yet-unidentified factors involved in enterocin A regulation. For example, although expression of enterocin A was observed in L. lactis, this was only possible by constitutively expressing entAITD under the control of a lactococcal promoter. When the entire region was introduced under its own control signals, as in pENT03, no production was observed, even though the same region encoded sufficient information to allow bacteriocin production in another enterococcal strain. However, even in this instance enterocin production in broth was only observed upon the addition of exogenous induction factor. It is possible that this is due to the lack of expression of the entKR system, but this remains to be proven. The plasmid pENT03 was constructed after amplification of a large fragment, and the possibility exists that the sequence was corrupted during this process. However, several independent clones were examined, and all produced the same phenotype. Therefore, even though it has been established that heterologous expression of enterocin A can take place, certain problems must be overcome. Enterocin A may have potential applications in fermented food due to its previously observed high specificity for Listeria spp. relative to many lactic acid bacteria (55). In this regard, heterologous production in a lactococcal strain likely to be used as a starter is desirable. Other applications of the enterocin system may lie in the development of gene expression systems. Further study is now needed, particularly regarding the regulation of the whole system, to facilitate the development of these heterologous secretion systems with a view to potential applications.
ACKNOWLEDGMENTS
We thank Ingolf Nes for the kind gift of chemically synthesized enterocin A induction factor.
This research has been part funded by a Teagasc Research Award and by grant aid under the Food Sub-Programme of the Operational Programme for Industrial Development which is administered by the Department of Agriculture, Food and Forestry and is supported by national and EU funds.
REFERENCES
- 1.Allison G E, Fremaux C, Klaenhammer T R. Expansion of bacteriocin activity and host range upon complementation of two peptides encoded within the lactacin F operon. J Bacteriol. 1994;176:2235–2241. doi: 10.1128/jb.176.8.2235-2241.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allison G E, Klaenhammer T R. Functional analysis of the gene encoding immunity to lactacin F, lafF, and its use as a Lactobacillus-specific, food-grade genetic marker. Appl Environ Microbiol. 1996;62:4450–4460. doi: 10.1128/aem.62.12.4450-4460.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 4.Anderson D G, McKay L L. Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl Environ Microbiol. 1983;46:549–552. doi: 10.1128/aem.46.3.549-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderssen E L, Diep D B, Nes I F, Eijsink V G H, Nissen-Meyer J. Antagonistic activity of Lactobacillus plantarum C11: two new peptide bacteriocins, plantaricins EF and JK, and the induction factor plantaricin A. Appl Environ Microbiol. 1998;64:2269–2272. doi: 10.1128/aem.64.6.2269-2272.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Axelsson L, Holck A. The genes involved in production of an immunity to sakacin A, a bacteriocin from Lactobacillus sake Lb706. J Bacteriol. 1995;177:2125–2137. doi: 10.1128/jb.177.8.2125-2137.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Axelsson L, Holck A, Birkeland S, Aukrust T, Blom H. Cloning and nucleotide sequence of a gene from Lactobacillus sake Lb706 necessary for sakacin A production and immunity. Appl Environ Microbiol. 1993;59:2868–2875. doi: 10.1128/aem.59.9.2868-2875.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aymerich T, Holo H, Håverstein L S, Hugas M, Garriga M, Nes I F. Biochemical and genetic characterization of enterocin A from Enterococcus faecium, a new antilisterial bacteriocin in the pediocin family of bacteriocins. Appl Environ Microbiol. 1996;62:1676–1682. doi: 10.1128/aem.62.5.1676-1682.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barefoot S F, Chen Y, Hughes T A, Bodine A B, Shearer M Y, Hughes M D. Identification and purification of a protein that induces production of the Lactobacillus acidophilus bacteriocin lactacin B. Appl Environ Microbiol. 1994;60:3522–3528. doi: 10.1128/aem.60.10.3522-3528.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benkel B F, Fong Y. Long range-inverse PCR (LR-IPCR): extending the useful range of inverse PCR. Genet Anal. 1996;13:123–127. doi: 10.1016/s1050-3862(96)00161-1. [DOI] [PubMed] [Google Scholar]
- 11.Brurberg M B, Nes I F, Eijsink V G H. Pheromone-induced production of antimicrobial peptides in Lactobacillus. Mol Microbiol. 1997;26:347–360. doi: 10.1046/j.1365-2958.1997.5821951.x. [DOI] [PubMed] [Google Scholar]
- 12.Casaus P, Nilsen T, Cintas L M, Nes I F, Hernandez P E, Holo H. Enterocin B, a new bacteriocin from Enterococcus faecium T136 which can act synergistically with enterocin A. Microbiology. 1997;143:2287–2294. doi: 10.1099/00221287-143-7-2287. [DOI] [PubMed] [Google Scholar]
- 13.Delves-Broughton J. Nisin and its uses as a food preservative. Food Technol. 1990;44:100–117. [Google Scholar]
- 14.Delves-Broughton J, Blackburn P, Evans R J, Hugenholtz J. Applications of the bacteriocin, nisin. Antonie Leeuwenhoek. 1996;69:193–202. doi: 10.1007/BF00399424. [DOI] [PubMed] [Google Scholar]
- 15.de Vos W M, Kuipers O P, van der Meer J R, Siezen R J. Maturation pathway of nisin and other lantibiotics: post-translationally modified antimicrobial peptides exported by gram-positive bacteria. Mol Microbiol. 1995;17:427–437. doi: 10.1111/j.1365-2958.1995.mmi_17030427.x. [DOI] [PubMed] [Google Scholar]
- 16.Diep D B, Håvarstein L S, Nes I F. A bacteriocin-like peptide induces bacteriocin synthesis in Lactobacillus plantarum C11. Mol Microbiol. 1995;18:631–639. doi: 10.1111/j.1365-2958.1995.mmi_18040631.x. [DOI] [PubMed] [Google Scholar]
- 17.Diep D B, Håverstein L S, Nes I F. Characterization of the locus responsible for the bacteriocin production in Lactobacillus plantarum C11. J Bacteriol. 1996;178:4472–4483. doi: 10.1128/jb.178.15.4472-4483.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eijsink V G H, Brurbeg M B, Middelhoven P H, Nes I F. Induction of bacteriocin production in Lactobacillus sake by a secreted peptide. J Bacteriol. 1996;178:2232–2237. doi: 10.1128/jb.178.8.2232-2237.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eijsink V G H, Skeie M, Middelhoven P H, Brurberg M B, Nes I F. Comparative studies of class IIa bacteriocins of lactic acid bacteria. Appl Environ Microbiol. 1998;64:3275–3281. doi: 10.1128/aem.64.9.3275-3281.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engelke G, Gutowski-Eckel Z, Kiesau P, Siegers K, Hammelmann M, Entian K D. Regulation of nisin biosynthesis and immunity in Lactococcus lactis 6F3. Appl Environ Microbiol. 1994;60:814–825. doi: 10.1128/aem.60.3.814-825.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Entian K D, de Vos W. Genetics of subtilin and nisin biosyntheses: biosynthesis of lantibiotics. Antonie Leeuwenhoek. 1996;69:109–117. doi: 10.1007/BF00399416. [DOI] [PubMed] [Google Scholar]
- 22.Fath M J, Kolter R. ABC transporters: bacterial exporters. Microbiol Rev. 1993;57:995–1017. doi: 10.1128/mr.57.4.995-1017.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fremaux C, Ahn C, Klaenhammer T R. Molecular analysis of the lactacin F operon. Appl Environ Microbiol. 1993;59:3906–3915. doi: 10.1128/aem.59.11.3906-3915.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fremaux C, Hechard Y, Cenatiempo Y. Mesenterocin Y105 gene clusters in Leuconostoc mesenteroides Y105. Microbiology. 1995;141:1637–1645. doi: 10.1099/13500872-141-7-1637. [DOI] [PubMed] [Google Scholar]
- 25.Gasson M J. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J Bacteriol. 1983;154:1–9. doi: 10.1128/jb.154.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gutierrez J A. Insertional mutagenesis and recovery of interrupted genes of Streptococcus mutans by using transposon Tn917: preliminary characterization of mutants displaying acid sensitivity and mutational requirements. J Bacteriol. 1996;178:14166–14175. doi: 10.1128/jb.178.14.4166-4175.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansen J N. Nisin as a model food preservative. Crit Rev Food Sci Nutr. 1994;34:69–93. doi: 10.1080/10408399409527650. [DOI] [PubMed] [Google Scholar]
- 28.Håverstein L S, Diep D B, Nes I F. A family of bacteriocin ABC transporters carry out proteolytic processing of their substrates concomitant with export. Mol Microbiol. 1995;16:229–240. doi: 10.1111/j.1365-2958.1995.tb02295.x. [DOI] [PubMed] [Google Scholar]
- 29.Håverstein L S, Gaustad P, Nes I F, Morrison D A. Identification of the streptococcal competence-pheromone receptor. Mol Microbiol. 1996;21:863–869. doi: 10.1046/j.1365-2958.1996.521416.x. [DOI] [PubMed] [Google Scholar]
- 30.Hayes F, Daly C, Fitzgerald G F. Identification of the minimal replicon of Lactococcus lactis subsp. lactis UC317 plasmid pCI305. Appl Environ Microbiol. 1990;56:202–209. doi: 10.1128/aem.56.1.202-209.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgins C F. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 32.Hoffman C S, Winston F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene. 1987;57:267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- 33.Holo H, Nes I F. High-frequency transformation by electroporation of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media. Appl Environ Microbiol. 1989;55:3119–3123. doi: 10.1128/aem.55.12.3119-3123.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hui F M, Morrison D A. Genetic transformation in Streptococcus pneumoniae: nucleotide sequence analysis shows comA, a gene required for competence induction, to be a member of the bacterial ATP-dependent transport protein family. J Bacteriol. 1991;173:372–381. doi: 10.1128/jb.173.1.372-381.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hui F M, Zhou L, Morrison A. Competence for genetic transformation in Streptococcus pneumoniae: organization of a regulatory locus with homology to two lactococcin A secretory genes. Gene. 1995;153:25–31. doi: 10.1016/0378-1119(94)00841-f. [DOI] [PubMed] [Google Scholar]
- 36.Hurst A. Nisin. Adv Appl Microbiol. 1981;27:263–265. [Google Scholar]
- 37.Ichihara S, Beppu N, Mizushima S. Protease IV, a cytoplasmic protein of Escherichia coli, has signal peptide peptidase activity. J Biol Chem. 1984;259:9853–9857. [PubMed] [Google Scholar]
- 38.Ichihara S, Suzuki T, Suzuki M, Mizushima S. Molecular cloning and sequencing of the sppA gene and characterization of the encoded protease IV, a signal peptide peptidase, of Escherichia coli. J Biol Chem. 1986;261:9405–9411. [PubMed] [Google Scholar]
- 39.Ike Y, Craig R A, White B A, Yagi Y, Clewell D B. Modification of Streptococcus faecalis sex pheromones after acquisition of plasmid DNA. Proc Natl Acad Sci USA. 1983;80:5369–5373. doi: 10.1073/pnas.80.17.5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jack R W, Tagg J R, Ray B. Bacteriocins of gram-positive bacteria. Microbiol Rev. 1995;59:171–200. doi: 10.1128/mr.59.2.171-200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ji G, Beavis R C, Novick R P. Cell density control of staphylococcal virulence mediated by an octapeptide pheromone. Proc Natl Acad Sci USA. 1995;92:12055–12059. doi: 10.1073/pnas.92.26.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klaenhammer T R. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol Rev. 1993;12:39–86. doi: 10.1111/j.1574-6976.1993.tb00012.x. [DOI] [PubMed] [Google Scholar]
- 43.Kleerebezem M, Quadri L E, Kuipers O P, de Vos W M. Quorum sensing by peptide pheromones and two-component signal-transduction systems in gram-positive bacteria. Mol Microbiol. 1997;24:895–904. doi: 10.1046/j.1365-2958.1997.4251782.x. [DOI] [PubMed] [Google Scholar]
- 44.Kuipers O P, Beerthuyzen M M, de Ruyter P G, Luesink E J, de Vos W M. Autoregulation of nisin biosynthesis in Lactococcus lactis by signal transduction. J Biol Chem. 1995;270:27299–27304. doi: 10.1074/jbc.270.45.27299. [DOI] [PubMed] [Google Scholar]
- 45.Lapidus A, Galleron N, Sorokin A, Ehrlich S D. Sequencing and functional annotation of the Bacillus subtilis genes in the 200 kb rrnB-dnaB region. Microbiology. 1997;143:3431–3441. doi: 10.1099/00221287-143-11-3431. [DOI] [PubMed] [Google Scholar]
- 46.Leenhouts K J, Gietema J, Kok J, Venema G. Chromosomal stabilization of the proteinase genes in Lactococcus lactis. Appl Environ Microbiol. 1991;57:2568–2575. doi: 10.1128/aem.57.9.2568-2575.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Magnusson R, Solomon J, Grossman A D. Biochemical and genetic characterization of a competence pheromone from Bacillus subtilis. Cell. 1994;77:207–216. doi: 10.1016/0092-8674(94)90313-1. [DOI] [PubMed] [Google Scholar]
- 48.Morgan S, Ross R P, Hill C. Bacteriolytic activity caused by the presence of a novel lactococcal plasmid encoding lactococcins A, B, and M. Appl Environ Microbiol. 1995;61:2995–3001. doi: 10.1128/aem.61.8.2995-3001.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mullis K, Faloona F, Schark S, Saiki R, Erhlich H. Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harbor Symp Quant Biol. 1986;51:263–273. doi: 10.1101/sqb.1986.051.01.032. [DOI] [PubMed] [Google Scholar]
- 49a.Nes, I. F. Personal communication.
- 50.Nes I F, Diep D B, Håverstein L S, Brurberg M B, Eijsink V, Holo H. Biosynthesis of bacteriocins in lactic acid bacteria. Antonie Leeuwenhoek. 1996;70:113–128. doi: 10.1007/BF00395929. [DOI] [PubMed] [Google Scholar]
- 51.Nilsen T, Nes I F, Holo H. An exported inducer peptide regulates bacteriocin production in Enterococcus faecium CTC492. J Bacteriol. 1998;180:1848–1854. doi: 10.1128/jb.180.7.1848-1854.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nissen-Meyer J, Håverstein L S, Holo H, Sletten K, Nes I F. Association of the lactococcin A immunity factor with the cell membrane: purification and characterization of the immunity factor. J Gen Microbiol. 1993;139:1503–1509. doi: 10.1099/00221287-139-7-1503. [DOI] [PubMed] [Google Scholar]
- 53.Novick R P, Projan S J, Kornblum J, Ross H F, Ji G, Kreisworth B, Vandenesch F, Moghazeh S. The agr P2 operon: an autocatalytic sensory transduction system in Staphylococcus aureus. Mol Gen Genet. 1995;248:446–458. doi: 10.1007/BF02191645. [DOI] [PubMed] [Google Scholar]
- 54.Pacaud M. Purification and characterization of two novel proteolytic enzymes in membranes of Escherichia coli. J Biol Chem. 1981;257:4333–4339. [PubMed] [Google Scholar]
- 55.Parente E, Hill C. Characterization of enterocin 1146, a bacteriocin from Enterococcus faecium inhibitory to Listeria monocytogenes. J Food Prot. 1992;55:497–502. doi: 10.4315/0362-028X-55.7.497. [DOI] [PubMed] [Google Scholar]
- 56.Parente E, Hill C. Inhibition of Listeria in buffer, broth and milk by enterocin 1146, a bacteriocin produced by Enterococcus faecium. J Food Prot. 1992;55:503–508. doi: 10.4315/0362-028X-55.7.503. [DOI] [PubMed] [Google Scholar]
- 57.Parkinson J S. Signal transduction schemes of bacteria. Cell. 1993;73:857–871. doi: 10.1016/0092-8674(93)90267-t. [DOI] [PubMed] [Google Scholar]
- 58.Pestova E V, Håverstein L S, Morrison D A. Regulation of competence for genetic transformation in Streptococcus pneumoniae by an auto-induced peptide pheromone and a two-component regulatory system. Mol Microbiol. 1996;21:853–862. doi: 10.1046/j.1365-2958.1996.501417.x. [DOI] [PubMed] [Google Scholar]
- 59.Quadri L, Kleerebezem M, Kuipers O P, de Vos W, Roy K L, Vederas J C, Stiles M. Characterization of a locus from Carnobacterium piscicola LV17B involved in bacteriocin production and immunity: evidence for global inducer-mediated transcriptional regulation. J Bacteriol. 1997;179:6163–6171. doi: 10.1128/jb.179.19.6163-6171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quadri L E, Sailer M, Roy K L, Vederas J C, Stiles M E. Chemical and genetic characterization of bacteriocins produced by Carnobacterium piscicola LV17B. J Biol Chem. 1994;269:12204–12211. [PubMed] [Google Scholar]
- 61.Quadri L E, Sailer M, Terebiznik M R, Roy K L, Vederas J C, Stiles M E. Characterization of the protein conferring immunity to the antimicrobial peptide carnobacteriocin B2 and expression of carnobacteriocins B2 and BM1. J Bacteriol. 1995;177:1144–1151. doi: 10.1128/jb.177.5.1144-1151.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rodriguez J M, Dodd H M. Genetic determinants for the biosynthesis of nisin, a bacteriocin produced by Lactococcus lactis. Microbiologia. 1996;12:61–74. [PubMed] [Google Scholar]
- 63.Ryan M P, Rea M C, Hill C, Ross R P. An application in cheddar cheese manufacture for a strain of Lactococcus lactis producing a novel broad-spectrum bacteriocin, lacticin 3147. Appl Environ Microbiol. 1996;62:612–619. doi: 10.1128/aem.62.2.612-619.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 65.Saris P E J, Immonen T, Reis M, Sahl H. Immunity to lantibiotics. Antonie Leeuwenhoek. 1996;69:151–159. doi: 10.1007/BF00399420. [DOI] [PubMed] [Google Scholar]
- 66.Saucier L, Poon A, Stiles M E. Induction of bacteriocin in Carnobacterium piscicola LV17. J Appl Bacteriol. 1995;78:684–690. [Google Scholar]
- 67.Schmidt H, Kernbach C, Karch H. Analysis of the EHEC hly operon and its location in the physical map of the large plasmid of enterohaemorrhagic Escherichia coli O157:h7. Microbiology. 1996;142:907–914. doi: 10.1099/00221287-142-4-907. [DOI] [PubMed] [Google Scholar]
- 68.Siegers K, Entian K D. Genes involved in immunity to the lantibiotic nisin produced by Lactococcus lactis 6F3. Appl Environ Microbiol. 1995;61:1082–1089. doi: 10.1128/aem.61.3.1082-1089.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stock J B, Ninfa A J, Stock A M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989;53:450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Terzaghi B E, Sandine W E. Improved medium for lactic streptococci and their bacteriophage. Appl Microbiol. 1975;29:807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tinoco I, Jr, Borer P N, Dengler B, Levine M D, Uhlenbeck O C, Crothers D M, Gralla J. Improved estimation of secondary structure in ribonucleic acids. Nature (London) New Biol. 1973;246:40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- 72.Triglia T, Peterson M G, Kemp J. A procedure for in vitro amplification of DNA segments that lie outside the boundaries of known sequences. Nucleic Acids Res. 1988;16:8186. doi: 10.1093/nar/16.16.8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Van Belkum M J, Hayema B J, Jeeninga R E, Kok J, Venema G. Organization and nucleotide sequences of two lactococcal bacteriocin operons. Appl Environ Microbiol. 1991;57:492–498. doi: 10.1128/aem.57.2.492-498.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Van Belkum M J, Kok J, Venema G. Cloning, sequencing, and expression in Escherichia coli of lcnB, a third bacteriocin determinant from the lactococcal bacteriocin plasmid p9B4-6. Appl Environ Microbiol. 1992;58:572–577. doi: 10.1128/aem.58.2.572-577.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Van Belkum M J, Kok J, Venema G, Holo H, Nes I F, Konings W N, Abee T. The bacteriocin lactococcin A specifically increases permeability of lactococcal cytoplasmic membranes in a voltage-independent, protein-mediated manner. J Bacteriol. 1991;173:7934–7941. doi: 10.1128/jb.173.24.7934-7941.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Van de Guchte M, Van der Vossen J M B M, Kok J, Venema G. Construction of a lactococcal expression vector: expression of hen egg white lysozyme in Lactococcus lactis subsp. lactis. Appl Environ Microbiol. 1989;55:224–228. doi: 10.1128/aem.55.1.224-228.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Venema K, Dost M H, Beun P A, Haandrikman A J, Venema G, Kok J. The genes for secretion and maturation of lactococcins are located on the chromosome of Lactococcus lactis IL1403. Appl Environ Microbiol. 1996;62:1689–1692. doi: 10.1128/aem.62.5.1689-1692.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Venema K, Haverkort R E, Abee T, Haandrikman A J, Leenhouts K J, deLeij L, Venema G, Kok J. Mode of action of LciA, the lactococcin A immunity protein. Mol Microbiol. 1994;14:521–533. doi: 10.1111/j.1365-2958.1994.tb02186.x. [DOI] [PubMed] [Google Scholar]