Abstract
Background and Objectives:
Pancreatic neuroendocrine tumors (PNETs) represent a rare form of pancreatic cancer. Racial/ethnic disparities have been documented in pancreatic ductal adenocarcinoma, but health disparities have not been well described in patients with PNETs.
Methods:
A retrospective review of patients with PNETs in the National Cancer Database was performed for 2004–2014. 16,605 patients with PNETs and available vital status were identified. Survival was compared by race/ethnicity and socioeconomic status using Kaplan-Meier methods and Cox regression.
Results:
There were no significant differences in survival between Non-Hispanic, White; Hispanic, White; or Non-Hispanic, Black patients on univariate analysis. Kaplan-Meier analysis showed that patients from communities with lower median household income and education level had worse survival (p<0.001). Patients age <65 without insurance, similarly, had worse survival (p<0.001). Multivariable modeling found no association between race/ethnicity and risk of mortality (p=0.37). Lower median household income and lower education level were associated with increased mortality (p<0.001).
Conclusions:
Unlike most other malignancies, race/ethnicity is not associated with survival differences in patients with PNETs. Patients with lower socioeconomic status had worse survival. The presence of identifiable health disparities in patients with PNETs represents a target for intervention and opportunity to improve survival in patients with this malignancy.
Keywords: healthcare disparities, neuroendocrine carcinoma, pancreas cancer
Introduction
Primary pancreatic neuroendocrine tumors (PNETs) represent a rare form of pancreatic cancer, comprising only 1–3% of pancreatic neoplasms.1,2 The incidence of PNETs is approximately 0.82 per 100,000 persons, as of 2012. This represents a greater than fourfold increase from 20 years prior3, which is thought to be partially due to the increase in incidental, non-functional PNETs diagnosed on Computed Tomography (CT) imaging. PNETs exhibit varied malignant potential2 and treatment options include hormonal or chemotherapeutic agents, radiation/nuclear medicine therapies, and surgical intervention. Treatment patterns and outcomes vary by provider and institution and the optimal treatment of PNETs remains controversial.1,4,5 Tumor size and functionality are important clinical factors that affect treatment decisions. Nevertheless, PNETs prove deadly, with a five-year overall survival of 52%.6
Health disparities are known to underlie outcomes in nearly every malignancy.7,8,9 Racial/ethnic and other socioeconomic disparities exist for patients with pancreatic ductal adenocarcinoma (PDAC), the most common type of pancreatic cancer.10,11,12,13 Incidence and survival with PDAC vary by race/ethnicity, with Non-Hispanic, Black (NHB) patients having higher incidence and worse overall survival compared to Non-Hispanic, White (NHW) patients. Hispanic patients have the best overall survival, which appears to be independent of socioeconomic status. Other socioeconomic factors such as lower income, urban/rural residence, geographic location, and insurance status are also associated with survival in PDAC across racial/ethnic groups, but these factors do not fully explain the observed disparities, suggesting biologic differences across races/ethnicities that drive tumor pathogenesis.14 However, the presence of these disparities remains underexplored in PNETs. A single study of disparities in PNETs in the Surveillance, Epidemiology, and End Results (SEER) database exists, but only compares Black patients to all others, rather than comparing data across races/ethnicities.15
Further exploration into health disparities in PNETs is needed and may help elucidate optimal management, as well as improve outcomes. This work seeks to evaluate the presence of health disparities in patients with PNETs captured in the National Cancer Database (NCDB), as well as to characterize these disparities. The objective of this study is to examine survival by race/ethnicity and other socioeconomic factors in patients diagnosed with PNETs. We hypothesize that health disparities exist in patients with PNETs.
Materials and Methods
Data source and study population
An application was submitted to the National Cancer Database (NCDB) for a Participant User File (PUF) for all cases of pancreatic malignancies diagnosed between 2004–2014. The NCDB is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. It includes data from more than 1,500 Commission-accredited cancer programs in the United States. The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator. The database includes about 70% of all newly diagnosed cancers in the United States 16. The data are de-identified and IRB exempt. The PUF was queried for all cases of PNETs. The following International Classification of Diseases for Oncology (ICD-O) codes were used to identify these cases: 8150, 8151, 8152, 8153, 8155, 8156, 8240, 8243, 8246, and 8249. There were 20,601 identified cases. Vital status was missing for 2,991 patients. An additional 1,022 patients were excluded because their case counts by race/ethnicity were too low for inclusion; their race/ethnicity was listed as American Indian/Native Alaskan (N=49), Asian (N=448), Hispanic, Black (N=318), Native Hawaiian/Pacific Islander (N=36) or other/unknown (N=171), leaving 16,605 cases for analysis.
Variables and outcomes
Patient characteristics and socioeconomic variables included age, sex, race, ethnicity, Charlson/Deyo score (a comorbidity index), insurer (private, Medicare/Medicaid, or uninsured), distance lived from the hospital that reported the case, urban vs rural geographic location, median household income and percentage of population without a high school degree in the patient’s ZIP code, whether surgery was performed, facility type, histology, and presence of metastasis. The median household income for patients’ area of residence at the time of diagnosis was determined by ZIP code and 2012 American Community Survey data. Race (black, white, etc.) was combined with ethnicity (Hispanic vs Non-Hispanic). Patients were classified as Non-Hispanic, White; Non-Hispanic, Black; or Hispanic, White. Tumors were classified as secretory (ICD-O codes 8150 or 8246), non-secretory (8151, 8152, 8153, 8155, 8156) or carcinoid (8240, 8243, 8249) as recommended by the Neuroendocrine Tumor Task Force of the National Cancer Institute GI Steering Committee.17 Pathologic T, N, and M stage are reported. The primary outcome of interest was survival across race/ethnicity. Secondary outcomes included survival by other socioeconomic factors.
Statistical analysis
Statistical analysis was conducted using the R statistical software package (V.3.6.3, The R Foundation for Statistical Computing).18 Descriptive statistics were calculated from variables of interest noted above. P-values are the results of Fisher’s exact or chi-square tests (categorical variables) or Mann-Whitney tests (continuous variables). Kaplan-Meier methods were used for univariate analysis of survival by patient variables. Pairwise comparisons were calculated for variables with >2 groups and reported when significant.
Mixed-effects Cox proportional hazards models were used for multivariable analysis of long-term survival. A random effect for hospital was included to account for the clustering of observations by medical center. Facility type was excluded as a covariate because it was a marker for severity of case (patients needing surgery tended to be reported by academic centers and patients with more advanced disease more often were reported by community centers). Similarly, N stage was excluded as it was highly correlated with T stage. After exclusions, covariates for all models included gender, age, geographic region (East, Midwest, New England or Pacific), population (metro, metro adjacent, non-metro adjacent or rural), race, distance from center reporting the case, Charlson/Deyo score, year of diagnosis, tumor type (secretory, non-secretory or carcinoid), tumor site (C250, C251, C252, C258, C259 or other), tumor grade (well-differentiated, moderately differentiated, poor or undifferentiated, or undetermined), tumor size, T stage (T0/T1, T2, T3, or T4/TX), and metastasis. The relationship between age and mortality was non-linear, with risk increasing rapidly for older patients, so a quadratic term was included in the models. The increasing risk of tumor size plateaued at 39mm. Therefore, the model assumed a linear effect for tumors <39mm and static effect for tumors >39mm.
Education and income levels were highly correlated (78% of those in the highest income bracket were also in the highest education bracket, while only 3% of those in the lowest two income brackets were in the highest education bracket). To avoid possible masking of effects introduced by collinearity, we ran two models, one with all covariates above plus income, and a second replacing income with education. Also, because Medicare causes insurance status to be highly correlated with age, the effect of insurance status was estimated in two subanalyses that included all covariates above plus income, but considered patients <65 and 65 and older separately. The non-linear age terms were dropped from these models because it did not improve model fit.
Most covariates were 100% complete (gender, age, race, Charlson/Deyo score, year of diagnosis, metastasis, tumor type, site and grade) or had missingness rates <3% (income, education, insurance, population area and distance from reporting center). Only region (8%), tumor size (13%) and T stage (13%) had higher missingness rates. All missing data was multiply imputed (10 iterations) via the R package “mice”, which draws values for missing data from a likely distribution given the patient’s values on other variables and the distribution of the data across all patients 19. Results of the 10 model runs were pooled according to the methods of Barnard and Rubin to yield final effect estimates20.
Results
Patient characteristics
There were 16,605 patients with PNETs meeting inclusion criteria in the NCDB from 2004–2015 (Table 1). Of those, 13,095 were NHW; 2,061 were NHB; 1,449 were Hispanic, White (HW). The patient characteristics for these populations are displayed in Table 1. The average age at diagnosis (mean ± standard deviation) for NHW patients was higher (61 ± 13.6 years) than for all other groups. Black patients were younger (57 ± 13.3 years) and more often female (58.9%, compared to < 47.9% for all other groups). NHB and HW patients had higher uninsured rates (5.6% and 7.4%, respectively) compared to NHW (2.3%). NHW patients more often lived in ZIP codes with median income > $63,000 (38%), compared to 17.9% of NHB and 28.8 % of HW. Additionally, NHB and HW patients more often lived in ZIP codes with greater than 21% of the population lacking a high school degree (32.1% and 29.3%, respectively) compared to NHW (10.5%). NHB and HW more often lived in metro locations (91.1% and 89.8%, respectively) compared to NHW (82.7%). NHW patients lived a closer distance to the hospital compared to NHB and HW (61.2 ± 170 miles vs 35.0 ± 121 miles and 38.1 ±115 miles, respectively). There were significant differences by race/ethnicity for a number of oncologic factors including disease site (p<0.0001), tumor size (p<0.0001), grade (p=0.006), type (p=.005), T stage (p=0.0003), and N stage (p=0.002). There were not significant differences by race/ethnicity on the presence of metastasis at diagnosis (p=0.10).
Table 1.
Patient Characteristics
| White, Non-Hispanic (N = 13,095) | Black, Non-Hispanic (N = 2,061) | White, Hispanic (N = 1,449) | p-value | |
|---|---|---|---|---|
| Age | 61.0 (13.6) | 56.9 (13.3) | 58.2 (14.7) | <0.0001 |
| Male | 7311 (55.8) | 848 (41.1) | 755 (52.1) | <0.0001 |
| Charlson/Deyo Score | <0.0001 | |||
| 0 | 9648 (73.7) | 1367 (66.3) | 1060 (73.2) | |
| 1 | 2650 (20.2) | 533 (25.9) | 312 (21.5) | |
| 2 | 556 (4.2) | 105 (5.1) | 52 (3.6) | |
| 3+ | 241 (1.8) | 56 (2.7) | 25 (1.7) | |
| Surgery | 7163 (55.9) | 1101 (54.9) | 782 (55.1) | 0.667 |
| Distance from Hospital | 61.1 (170) | 35.0 (120) | 38.1 (115) | <0.0001 |
| Insurer | <0.0001 | |||
| Medicare/Medicaid | 5879 (46.2) | 964 (47.9) | 603 (43.5) | |
| Private | 6557 (51.5) | 937 (46.5) | 681 (49.1) | |
| None | 288 (2.3) | 112 (5.6) | 103 (7.4) | |
| Median Income | <0.0001 | |||
| < $38,000 | 1490 (11.5) | 824 (40.3) | 293 (20.4) | |
| $38,000 – $47,999 | 2869 (22.2) | 441 (21.6) | 335 (23.4) | |
| $48,000 – $62,999 | 3648 (28.2) | 413 (20.2) | 393 (27.4) | |
| > $63,000 | 4942 (38.2) | 365 (17.9) | 413 (28.8) | |
| % of Population with Less than High School Education | <0.0001 | |||
| >21% | 1366 (10.5) | 656 (32.1) | 420 (29.3) | |
| 13–21% | 3039 (23.5) | 689 (33.7) | 355 (24.8) | |
| 7–13% | 4567 (35.3) | 486 (23.8) | 377 (26.3) | |
| <7% | 3984 (30.8) | 213 (10.4) | 282 (19.7) | |
| Facility Location | <0.0001 | |||
| Midwest | 2148 (17.6) | 279 (15.1) | 304 (23.7) | |
| East | 2925 (24.0) | 471 (25.6) | 248 (19.4) | |
| New England | 5178 (42.4) | 1001 (54.3) | 478 (37.3) | |
| Pacific | 1960 (16.1) | 91 (4.9) | 251 (19.6) | |
| Facility Type | <0.0001 | |||
| ARP | 6797 (55.7) | 1086 (59.0) | 604 (47.2) | |
| CCCP | 554 (4.5) | 66 (3.6) | 45 (3.5) | |
| CCP | 3650 (29.9) | 429 (23.3) | 459 (35.8) | |
| INCP | 1210 (9.9) | 261 (14.2) | 173 (13.5) | |
| Urban/Rural | <0.0001 | |||
| Metro | 10475 (82.7) | 1834 (91.1) | 1269 (89.8) | |
| Metro adjacent | 1362 (10.8) | 114 (5.7) | 81 (5.7) | |
| Non-metro adjacent | 572 (4.5) | 40 (2.0) | 48 (3.4) | |
| Rural | 260 (2.1) | 26 (1.3) | 15 (1.1) | |
| Tumor Type | 0.005 | |||
| Secretory | 9964 (76.1) | 1562 (75.8) | 1161 (80.1) | |
| Non-secretory | 284 (2.2) | 37 (1.8) | 31 (2.1) | |
| Carcinoid | 2847 (21.7) | 462 (22.4) | 257 (17.7) | |
| Disease Site | <0.0001 | |||
| Head of pancreas | 3842 (29.3) | 668 (32.4) | 468 (32.3) | |
| Body of pancreas | 1759 (13.4) | 325 (15.8) | 189 (13.0) | |
| Tail of pancreas | 4196 (32.0) | 497 (24.1) | 426 (29.4) | |
| Pancreatic duct | 8 (0.1) | 4 (0.2) | 1 (0.1) | |
| Islets of Langerhans | 415 (3.2) | 54 (2.6) | 38 (2.6) | |
| Other | 271 (2.1) | 56 (2.7) | 29 (2.0) | |
| Overlapping | 854 (6.5) | 131 (6.4) | 85 (5.9) | |
| Pancreas, NOS | 1750 (13.4) | 326 (15.8) | 213 (14.7) | |
| Tumor Size (mm) | 43.6 (59.9) | 41.3 (49.8) | 46.2 (53.3) | <0.0001 |
| Grade | 0.006 | |||
| Poor | 841 (6.4) | 113 (5.5) | 104 (7.2) | |
| Moderate | 1375 (10.5) | 177 (8.6) | 155 (10.7) | |
| Well | 5711 (43.6) | 947 (45.9) | 578 (39.9) | |
| Undifferentiated | 165 (1.3) | 21 (1.0) | 21 (1.4) | |
| Undetermined | 5003 (38.2) | 803 (39.0) | 591 (40.8) | |
| T Stage | 0.0003 | |||
| T0 | 17 (0.1) | 3 (0.2) | 2 (0.2) | |
| T1 | 1892 (16.6) | 278 (15.8) | 166 (13.0) | |
| T2 | 1845 (16.2) | 326 (18.5) | 215 (16.9) | |
| T3 | 1745 (15.3) | 257 (14.6) | 181 (14.2) | |
| T4 | 136 (1.2) | 23 (1.3) | 32 (2.5) | |
| TX | 5737 (50.4) | 875 (49.7) | 677 (53.2) | |
| N Stage | 0.002 | |||
| N0 | 3479 (30.8) | 533 (30.5) | 336 (26.6) | |
| N1 | 1698 (15.0) | 305 (17.4) | 205 (16.2) | |
| NX | 6106 (54.1) | 912 (52.1) | 724 (57.2) | |
| Distant Metastasis | 0.096 | |||
| No | 7820 (59.7) | 1264 (61.3) | 836 (57.7) | |
| Yes or unavailable | 5275 (40.3) | 797 (38.7) | 613 (42.3) |
Categorical variables displayed as N (%); Continuous variables are displayed as mean (SD)
P-values are the results of chi-square tests (categorical variables) or Kruskal-Wallis tests (continuous variables).
ARP: Academic/Research Program; CCCP: Comprehensive Community Cancer Program; CCP: Community Cancer Program; INCP: Integrate Network Cancer Program; NOS: Not otherwise specified
Patient characteristics and survival
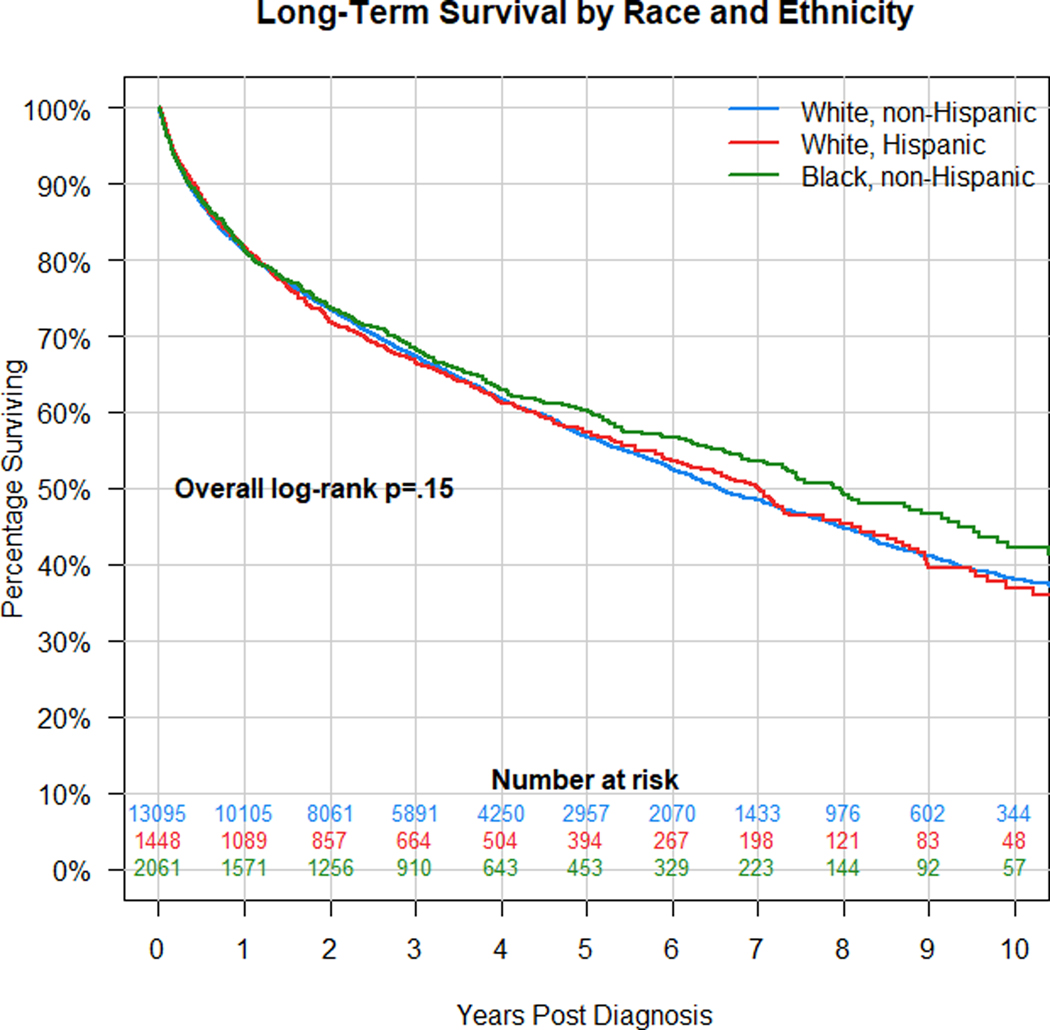
As a preliminary step prior to risk-adjustment using Cox regression models, Kaplan-Meier analysis was performed to assess the effect of individual socioeconomic parameters on overall survival. Fig. 1 demonstrates that there is no significant difference in overall survival by race/ethnicity (log-rank p=0.151). Median survival times in months (95% CI) were 78.8 (75.6–82.0), 84.0 (74.0–93.1), and 95.6 (87.5–110.8) in the NHW, HW, and NHB groups, respectively.
Figure 1.
Kaplan-Meier analysis of survival in patients with PNETs by race/ethnicity. There is no significant difference in survival by race/ethnicity, overall log-rank p=0.151.
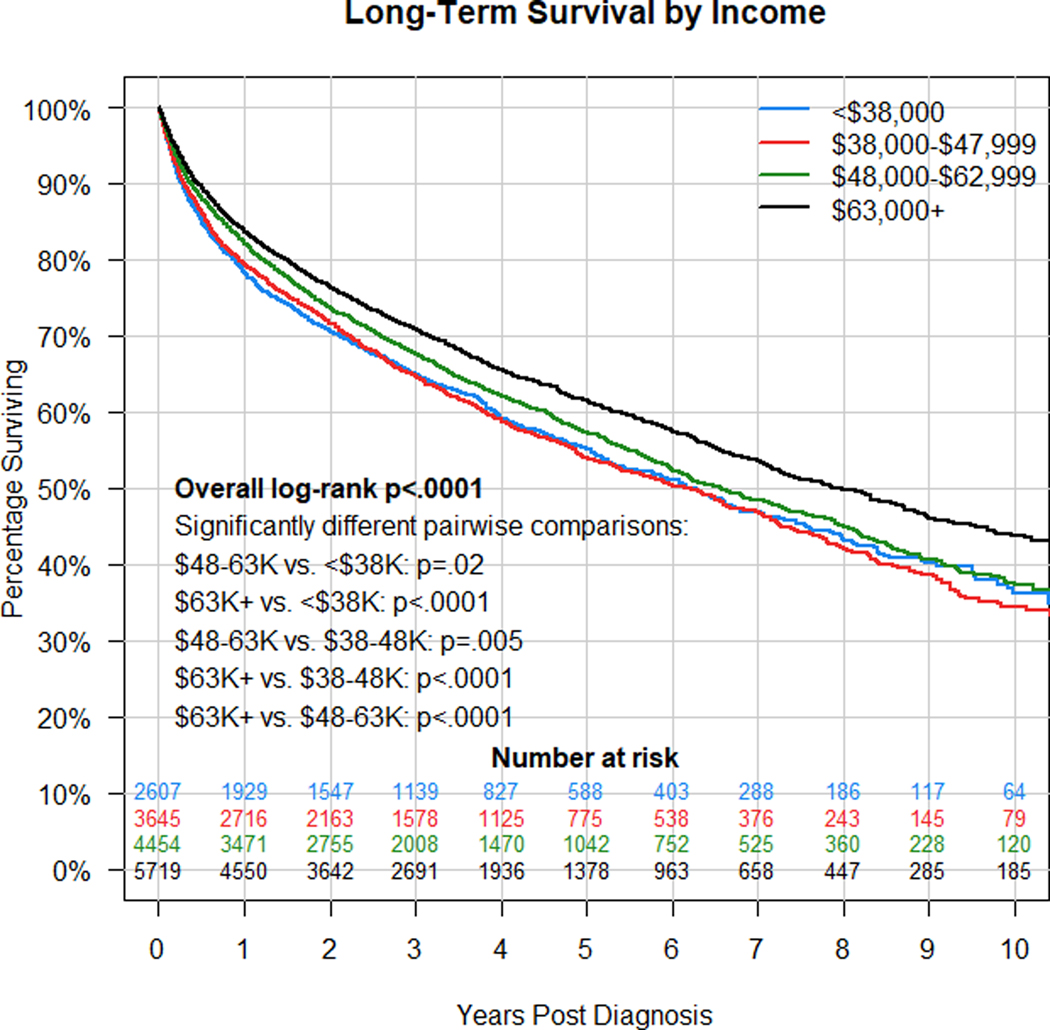
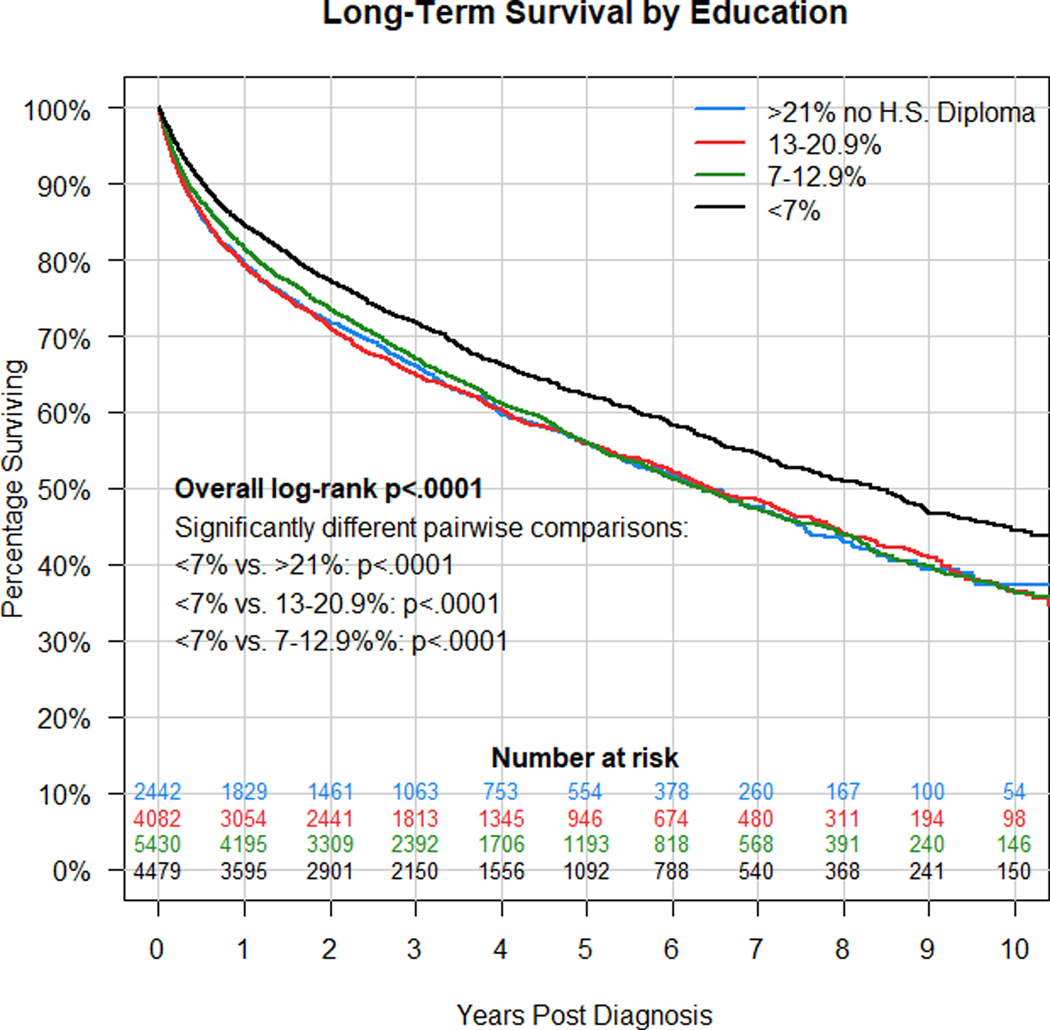
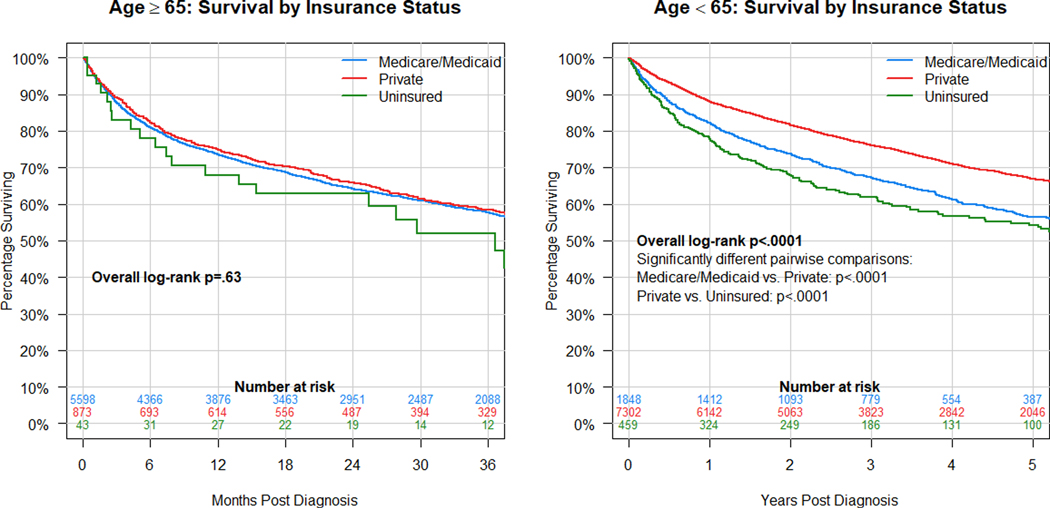
The effect of socioeconomic factors on survival were similarly evaluated using Kaplan-Meier analysis. Fig. 2 shows that there is a significant difference in survival by median household income in patient area of residence (log-rank p<0.001). Pairwise comparisons show that patients living in ZIP codes with median household income of > $63,000 live significantly longer than all other groups. Median survival times in months (95% CI) were 74.8 (66.9–81.0), 74.2 (67.3–79.4), 78.8 (73.2–86.1) and 95.0 (87.7–104.2) in the <$38,000, $38,000-$47,999, $48,000-$62,999 and ≥$63,000 groups, respectively. Fig. 3 demonstrates that there is a significant difference in survival by percentage of the population without a high school diploma in the patient’s area of residence (log rank p<0.001). Pairwise comparisons show that patients living in ZIP codes with <7% of the population without a high school diploma live significantly longer than all other groups. Median survival times in months (95% CI) were 76.4 (69.8–85.0), 77.4 (72.4–85.8), 76.0 (70.8–81.8), and 100.1 (91.3–107.1) in the >21%, 13–20.9%, 7–12.9%, and <7% groups, respectively. Finally, the effect of insurer on overall survival was evaluated (Fig. 4). Because of the age restriction for Medicare, we considered the effect of insurance for patients 65 or older (N=6,674) and those <65 (N=9,931) separately. Among patients 65 or older, there were no significant survival differences among those on public, private or no insurance. Median survival times in months (95% CI) were 36.7 (CI non-estimable because of small group size) for uninsured, 51.3 (48.1–55.7) for Medicare/Medicaid, and 53.5 (45.8–61.3) for private insurance. In contrast, for patients <65, those on private insurance had better survival than those on public insurance or those uninsured (p<.0001 for both pairwise comparisons). Median survival times in months (95% CI) were 65.4 (56.7–100.7) for uninsured, 80.3 (73.0–95.7) for Medicare/Medicaid, and 116.7 (109.7–130.4) for private insurance.
Figure 2.
Kaplan-Meier analysis of survival in patients with PNETs by median household income in patients’ area of residence. There is a significant difference in survival by median household income (log-rank p<0.001). Pairwise comparisons demonstrated that patients residing in ZIP codes with median household income > $63,000 had significantly longer survival than all other groups (p<0.001).
Figure 3.
Kaplan-Meier analysis of survival in patients with PNETs by percentage of population without a high school diploma in patients’ area of residence. There is a significant difference in survival by percentage without high school diploma (log-rank p<0.001). Pairwise comparisons demonstrated that patients residing in ZIP codes with <7% of the population without a high school diploma had significantly longer survival than all other groups (p<0.001).
Figure 4.
Kaplan-Meier analysis of survival in patients with PNETs by insurance type. Among patients 65 or older at diagnosis, there is no significant difference in survival by insurance type (p=.625), but among patients <65, those with private insurance have better survival than those on public insurance or without insurance (p<.0001).
Multivariable analysis of patient characteristics and survival
The effect of covariates on survival was evaluated using mixed-effects Cox proportional hazards models. Because of their high correlation, the effects of income and education level were estimated in two otherwise identical models. Table 2 shows the results of these models, each pooled over 10 multiply imputed datasets. Similarly, because of the Medicare-induced confounding of age and insurance status, the effect of insurance was estimated in two models, one including only patients 65 and older and an otherwise identical model including only younger patients (Table 3). Notably, despite the large sample size, race/ethnicity was not significantly associated with survival in any of the four models (overall p>0.50 for all), although there was marginal evidence that HW have a slight survival advantage over NHW. As expected, as age increased, the risk of mortality also increased (p <0.0001). Male patients had significantly higher risk of mortality than female patients (p<.002 in all models). There was a significant difference in mortality by median household income (p<.0002 in all three models that included income as a covariate) and by education level (p<.0001). Patients who live in ZIP codes with median household income >$63,000 and/or in the highest education bracket (<7% without high school diploma) had significantly lower risk of mortality compared to those with lowest income and/or education (p<.0001). Insurance status had a significant effect for patients <65, with those on private insurance having better survival than those on public insurance, presumably largely Medicaid (p<.0001); however, this effect disappeared for patients 65 and older, where there was no difference in outcome between those with private insurance and those with public insurance, presumably mostly Medicare (p=.792). Other notable clinical and pathologic variables that were associated with increased mortality include lesion in the head or body of the pancreas, worse tumor grade, increased tumor size, increased T stage, and the presence of metastasis. Race, year of diagnosis and population area were not significantly associated with survival in any of the four models.
Table 2.
Multivariable results for Income and Education level: Pooled effects from two mixed-effects Cox models for long-term survivala,b
| Factor | Model 1: HR (95% CI), p-value | Model 2: HR (95% CI), p-value |
|---|---|---|
| Education (vs. ≥21% no high school diploma) | Overall p<.0001 | |
| 13–20.9% | NA | 0.98 (.903,1.06), .572 |
| 7–12.9% | NA | 0.97 (.894,1.05), .399 |
| <7% | NA | 0.81 (.742,.877), <.0001 |
| Race (vs. White, non-Hispanic) | Overall p=.565 | Overall p=.666 |
| White, Hispanic | 0.92 (.843,1.00), .055 | 0.92 (.840,1.00), .051 |
| Black, Non-Hispanic | 1.0 (.938,1.11), .656 | 1.0 (.956,1.12), .381 |
| Male gender (vs. female) | 1.1 (1.09,1.20), <.0001 | 1.1 (1.09,1.20), <.0001 |
| Age overall p-value (for combined linear and non-linear terms) | <.0001 | <.0001 |
| Age (linear component) | 0.97 (.955,.979), <.0001 | 0.97 (.955,.979), <.0001 |
| Age (quadratic component) | 1.1 (1.04,1.06), <.0001 | 1.1 (1.04,1.06), <.0001 |
| Year of diagnosis (HR multiplies per year) | 1.0 (.990,1.01), .797 | 1.0 (.990,1.01), .827 |
| Income (vs. <$38K/year) | Overall p<.0001 | |
| $38,000-$47,999 | 0.94 (.870,1.02), .145 | NA |
| $48,000-$62,999 | 0.90 (.829,.973), .009 | NA |
| $63,000+ | 0.80 (.738,.867), <.0001 | NA |
| Charlson/Deyo score (HR multiplies by per unit increase) | 1.2 (1.13,1.21), <.0001 | 1.2 (1.12,1.20), <.0001 |
| Miles from hospital (HR multiplies by per 10-mile increment) | 0.99 (.993,.998), .0001 | 0.99 (.994,.998), .0001 |
| Region (vs. West) | Overall p<.0001 | Overall p<.0001 |
| East | 1.2 (1.06,1.24), .001 | 1.1 (1.06,1.24), .001 |
| New England | 1.0 (.952,1.10), .515 | 1.0 (.935,1.08), .866 |
| Pacific/mountain | 1.1 (1.01,1.20), .031 | 1.1 (.993,1.19), .070 |
| Population area (vs. Rural) | Overall p=.119 | Overall p=.086 |
| Metro | 1.1 (.904,1.31), .376 | 1.1 (.884,1.27), .527 |
| Metro adjacent | 1.2 (.969,1.43), .102 | 1.2 (.965,1.42), .109 |
| Not metro adjacent | 1.0 (.813,1.26), .921 | 1.0 (.815,1.26), .907 |
| Disease site (vs. Other) | Overall p<.0001 | Overall p<.0001 |
| C250 | 1.5 (1.29,1.64), <.0001 | 1.5 (1.29,1.64), <.0001 |
| C251 | 1.2 (1.05,1.37), .009 | 1.2 (1.05,1.37), .008 |
| C252 | 1.1 (.969,1.24), .141 | 1.1 (.969,1.24), .143 |
| C258 | 1.2 (.990,1.33), .067 | 1.2 (.988,1.33), .071 |
| C259 | 1.4 (1.23,1.59), <.0001 | 1.4 (1.22,1.58), <.0001 |
| Tumor type (vs. carcinoid) | Overall p<.0001 | Overall p<.0001 |
| Non-secretory | 1.2 (1.01,1.45), .037 | 1.2 (1.01,1.44), .041 |
| Secretory | 1.4 (1.30,1.54), <.0001 | 1.4 (1.30,1.53), <.0001 |
| Tumor grade (vs. well-differentiated) | Overall p<.0001 | Overall p<.0001 |
| Poor/Undifferentiated | 3.8 (3.46,4.10), <.0001 | 3.8 (3.46,4.10), <.0001 |
| Moderately differentiated | 1.4 (1.25,1.53), <.0001 | 1.4 (1.25,1.53), <.0001 |
| Undetermined | 1.7 (1.63,1.85), <.0001 | 1.7 (1.63,1.85), <.0001 |
| Tumor size ≤39mm (HR multiplies per mm increase) | 1.01 (1.005,1.015), .0003 | 1.01 (1.005,1.016), .0002 |
| Tumor >39mm | 1.5 (1.26,1.70), <.0001 | 1.5 (1.27,1.71), <.0001 |
| T stage (vs. 0/1) | Overall p<.0001 | Overall p<.0001 |
| T2 | 1.0 (.844,1.21), .903 | 1.0 (.840,1.21), .946 |
| T3 | 1.2 (1.00,1.43), .048 | 1.2 (1.00,1.43), .051 |
| T4/X | 1.9 (1.57,2.18), <.0001 | 1.8 (1.56,2.17), <.0001 |
| Metastasis | 2.9 (2.72,3.06), <.0001 | 2.9 (2.72,3.07), <.0001 |
Model 1=All covariates+income (pooled adjusted R2=0.34, pooled AUC=0.79)
Model 2=All covariates+education (pooled adjusted R2=0.34, pooled AUC=0.79)
Table 3.
Multivariable results for Insurance Status: Pooled effects from two mixed-effects Cox models for long-term survival.
| Factor | Model 1: HR (95% CI), p-value | Model 2: HR (95% CI), p-value |
|---|---|---|
| Insurance (vs. Medicare/Medicaid) | Overall p=.335 | Overall p<.0001 |
| Private | 1.0 (.914,1.13), .792 | 0.68 (.620,.741), <.0001 |
| Uninsured | 1.2 (.751,1.81), .497 | 1.0 (.869,1.20), .813 |
| Race (vs. White, non-Hispanic) | Overall p=.549 | Overall p=.953 |
| White, Hispanic | 0.92 (.816,1.05), .207 | 0.89 (.786,1.00), .058 |
| Black, Non-Hispanic | 0.89 (.776,1.01), .077 | 1.1 (.971,1.20), .156 |
| Male gender (vs. female) | 1.2 (1.08,1.24), <.0001 | 1.1 (1.05,1.21), .001 |
| Age (HR multiplies for each year increase) | 1.1 (1.041,1.053), <.0001 | 1.02 (1.013,1.021), <.0001 |
| Year of diagnosis (HR multiplies per year) | 1.0 (.985,1.01), .739 | 1.0 (.984,1.01), .648 |
| Income (vs. <$38K/year) | Overall p<.0001 | Overall p=.0001 |
| $38,000-$47,999 | 0.93 (.835,1.05), .241 | 0.98 (.875,1.10), .730 |
| $48,000-$62,999 | 0.89 (.797,.999), .049 | 0.95 (.849,1.07), .384 |
| $63,000+ | 0.80 (.718,.902), .0002 | 0.86 (.767,.968), .012 |
| Miles from hospital (HR multiplies by per 10-mile increment) | 0.99 (.991,.998), .006 | 0.99 (.994,.999), .015 |
| Region (vs. West) | Overall p=.018 | Overall p=.001 |
| East | 1.1 (1.01,1.26), .033 | 1.2 (1.05,1.32), .006 |
| New England | 1.0 (.918,1.12), .764 | 1.1 (.950,1.17), .321 |
| Pacific/mountain | 1.1 (.968,1.24), .150 | 1.1 (.967,1.24), .150 |
| Population area (vs. Rural) | Overall p=.240 | Overall p=.749 |
| Metro | 1.1 (.845,1.40), .515 | 1.1 (.803,1.38), .705 |
| Metro adjacent | 1.2 (.899,1.53), .241 | 1.1 (.844,1.49), .425 |
| Not metro adjacent | 0.95 (.701,1.28), .730 | 1.1 (.766,1.44), .754 |
| Charlson/Deyo score (HR multiplies by per unit increase) | 1.2 (1.15,1.26), <.0001 | 1.1 (1.03,1.15), .002 |
| Disease site (vs. Other) | Overall p<.0001 | Overall p<.0001 |
| C250 | 1.6 (1.32,1.89), <.0001 | 1.3 (1.09,1.53), .003 |
| C251 | 1.3 (1.07,1.59), .008 | 1.1 (.896,1.31), .412 |
| C252 | 1.2 (.969,1.40), .105 | 1.0 (.853,1.20), .887 |
| C258 | 1.2 (.985,1.53), .067 | 1.1 (.859,1.29), .627 |
| C259 | 1.6 (1.32,1.92), <.0001 | 1.2 (1.00,1.43), .046 |
| Tumor type (vs. carcinoid) | Overall p<.0001 | Overall p<.0001 |
| Non-secretory | 1.2 (.884,1.48), .304 | 1.3 (1.02,1.68), .037 |
| Secretory | 1.3 (1.17,1.45), <.0001 | 1.6 (1.38,1.79), <.0001 |
| Tumor grade (vs. well-differentiated) | Overall p<.0001 | Overall p<.0001 |
| Poor/Undifferentiated | 3.3 (2.88,3.68), <.0001 | 4.5 (3.97,5.03), <.0001 |
| Moderately differentiated | 1.4 (1.21,1.60), <.0001 | 1.4 (1.18,1.58), <.0001 |
| Undetermined | 1.6 (1.49,1.79), <.0001 | 1.9 (1.69,2.03), <.0001 |
| Tumor size ≤39mm (HR multiplies per mm increase) | 1.01 (1.002,1.015), .016 | 1.01 (1.006,1.021), .0007 |
| Tumor >39mm | 1.4 (1.15,1.73), .001 | 1.6 (1.28,1.96), <.0001 |
| T stage (vs. 0/1) | Overall p<.0001 | Overall p<.0001 |
| T2 | 1.0 (.787,1.25), .956 | 1.1 (.806,1.37), .719 |
| T3 | 1.1 (.893,1.41), .322 | 1.3 (1.03,1.71), .031 |
| T4/X | 1.7 (1.34,2.03), <.0001 | 2.1 (1.66,2.64), <.0001 |
| Metastasis | 2.6 (2.41,2.85), <.0001 | 3.2 (2.93,3.48), <.0001 |
Model 1= patients 65 and older (pooled adjusted R2=0.33, pooled AUC=0.76)
Model 2= patients <65 (pooled adjusted R2=0.30, pooled AUC=0.80)
Discussion
The extent of health disparities underlying outcomes in pancreatic neuroendocrine tumors has not been described. This work represents an in-depth evaluation of the impact of a number of socioeconomic factors on survival at the national level. There were no differences in univariable or multivariable analysis of survival by race/ethnicity. Patients who were uninsured or who resided in communities with lower median household income and lower education level had worse overall survival. The evidence in this study demonstrates that while race/ethnicity is not associated with survival, patients from disadvantaged socioeconomic backgrounds are at risk of worse survival. Clinical factors that increased the risk of death from PNETs included larger tumor size, increased T stage, and the presence of metastasis.
A single national study using the SEER database evaluated for health disparities in patients with PNETs.15 This study found that Black patients had worsened overall survival and disease specific survival compared to “White/Other” patients. Unfortunately, making significant conclusions about race-specific survival when comparing Black patients to all other patients is difficult. Further, it is important to understand the differences between the NCDB and SEER database. The advantage of the SEER database is the ability to derive population level data and disease specific survival. The advantage of the NCDB is that it captures about 70% of all new cancer cases compared to 28% of cancer cases captured by SEER. We chose to perform this study using the NCDB due to the rarity of PNETs and desire for increased samples size. No conclusions can be made about how the differences in the databases may account for observed differences between studies. Importantly, provided a large database with robust race/ethnicity data, studies to evaluate for racial/ethnic disparities should avoid combining large, diverse groups together to make conclusions about a single race.
Our results differ significantly from many other malignancies. For instance, Black patients with invasive breast cancer are more likely to die from stage 1 disease than non-Hispanic, White, and Asian women, even after adjustment for income.21 Similar trends have been observed in Black men with prostate cancer.22 In lung cancer, 5-year survival is lower among Black patients than White patients.23 In evaluating other GI malignancies, Black patients have been found to have worsened survival in gastric, colorectal, liver cancer, and the most common pancreatic cancer, pancreatic ductal adenocarcinoma14,24,25,26,27,28 In our study, there were not significant differences in unadjusted or adjusted survival in NHW, NWB, and HW patients. A study using the NCDB to evaluate for racial/ethnic disparities in pancreatic ductal adenocarcinoma found that Hispanic patients, particularly those of Dominican descent, had improved survival.28 Due to sample size limitations among Hispanic patients with PNETs, further subpopulation analysis was not feasible. Similar to our study, other studies in the NCDB have found socioeconomic factors such as median household income, education, insurance status, and urban/rural residence to be associated with survival in pancreatic, colorectal, lung, and oral cancer14,29,30,31
There are a number of important limitations to note about our study. Although it utilizes a national database with a large sample set, it is retrospective in nature. Misclassification bias may occur. There is no ability to classify multi-racial persons in the NCDB, although they are certainly present, thus the extent of misclassification of race/ethnicity, particularly for patients of diverse ancestry, is unknown. Additionally, analysis of all reported racial/ethnic groups was not feasible due to low case counts for certain groups. Since ZIP codes are used to determine median household income, these are not representative of an individual’s socioeconomic status, but reflect the community in which the patient resides. Finally, some variables that may affect disease stage at presentation and overall survival had incomplete or unavailable data. While these limitations are important to understand, given the large patient population in the NCDB, the results reported remain valuable to understanding how socioeconomic status affects survival in patients with PNETs.
Conclusion
In conclusion, unlike many other malignancies, there were not significant survival differences by race/ethnicity in patients with PNETs. More importantly, socioeconomic factors are associated with survival. Patients who come from disadvantaged backgrounds have worse survival. Further work to identify factors contributing to these health disparities, such as barriers to follow-up surveillance, or ability to adhere to recommended treatment plans, may further characterize possible access-related issues associated with these socioeconomic factors. Identifying patients at risk of poorer outcomes and implementing mechanisms to overcome these disparities represent important next steps in improving survival in PNETs.
Synopsis:
Race/ethnicity is not associated with survival differences in patients with PNETs. Patients with lower socioeconomic status had worse survival. The presence of identifiable health disparities in patients with PNETs represents a target for intervention and opportunity to improve survival.
Acknowledgments
Financial Support: This work is supported by the Joseph and Ann Matella Fund for Pancreatic Cancer Research (to JGT) and the National Cancer Institute (NCI) of the National Institutes of Health (NIH) (Grants: U54CA233444 and R01CA242003 to JGT). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCI. Dr. Riner is supported by T32 HG008958 through the National Human Genome Research Institute of the NIH. The final peer-reviewed manuscript is subject to the National Institutes of Health Public Access Policy.
Footnotes
Conflicts of Interest: The authors declare no potential conflicts of interest.
Data availability statement
The primary dataset (National Cancer Database) is publicly available by request through the American College of Surgeons (https://www.facs.org/quality-programs/cancer/ncdb). The dataset analyzed for this study may be made available by the corresponding author upon request.
References
- 1.Cloyd JM, Poultsides GA. Non-functional neuroendocrine tumors of the pancreas: Advances in diagnosis and management. World J Gastroenterol. Aug 28 2015;21(32):9512–9525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang J, Francois R, Iyer R, Seshadri M, Zajac-Kaye M, Hochwald SN. Current understanding of the molecular biology of pancreatic neuroendocrine tumors. J Natl Cancer Inst. Jul 17 2013;105(14):1005–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dasari A, Shen C, Halperin D, et al. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. Oct 1 2017;3(10):1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Assi HA, Mukherjee S, Kunz PL, et al. Surgery Versus Surveillance for Well-Differentiated, Nonfunctional Pancreatic Neuroendocrine Tumors: An 11-Year Analysis of the National Cancer Database. Oncologist. Feb 2020;25(2):e276–e283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bar-Moshe Y, Mazeh H, Grozinsky-Glasberg S. Non-functioning pancreatic neuroendocrine tumors: Surgery or observation? World J Gastrointest Endosc. Apr 16 2017;9(4):153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu J, Sun C, Li E, et al. Non-functional pancreatic neuroendocrine tumours: emerging trends in incidence and mortality. BMC Cancer. Apr 8 2019;19(1):334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newman LA. Breast Cancer Disparities: Socioeconomic Factors versus Biology. Ann Surg Oncol. Oct 2017;24(10):2869–2875. [DOI] [PubMed] [Google Scholar]
- 8.Ryan BM. Lung cancer health disparities. Carcinogenesis. May 28 2018;39(6):741–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlottmann F, Gaber C, Strassle PD, Charles AG, Patti MG. Health care disparities in colorectal and esophageal cancer. Am J Surg. Dec 23 2019. [DOI] [PubMed] [Google Scholar]
- 10.Lutfi W, Zenati MS, Zureikat AH, Zeh HJ, Hogg ME. Health Disparities Impact Expected Treatment of Pancreatic Ductal Adenocarcinoma Nationally. Ann Surg Oncol. Jul 2018;25(7):1860–1867. [DOI] [PubMed] [Google Scholar]
- 11.Riner AN, Underwood PW, Yang K, et al. Disparities in Pancreatic Ductal Adenocarcinoma-The Significance of Hispanic Ethnicity, Subgroup Analysis, and Treatment Facility on Clinical Outcomes. Cancer Med. 06 2020;9(12):4069–4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swords DS, Mulvihill SJ, Brooke BS, Skarda DE, Firpo MA, Scaife CL. Disparities in utilization of treatment for clinical stage I-II pancreatic adenocarcinoma by area socioeconomic status and race/ethnicity. Surgery. Apr 2019;165(4):751–759. [DOI] [PubMed] [Google Scholar]
- 13.Tavakkoli A, Singal AG, Waljee AK, et al. Racial Disparities and Trends in Pancreatic Cancer Incidence and Mortality in the United States. Clin Gastroenterol Hepatol. Jan 2020;18(1):171–178 e110. [DOI] [PubMed] [Google Scholar]
- 14.Moaven O, Richman JS, Reddy S, Wang T, Heslin MJ, Contreras CM. Healthcare disparities in outcomes of patients with resectable pancreatic cancer. Am J Surg. Apr 2019;217(4):725–731. [DOI] [PubMed] [Google Scholar]
- 15.Zhou H, Zhang Y, Wei X, et al. Racial disparities in pancreatic neuroendocrine tumors survival: a SEER study. Cancer Med. Nov 2017;6(11):2745–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohanty S, Bilimoria KY. Comparing national cancer registries: The National Cancer Data Base (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) program. J Surg Oncol. Jun 2014;109(7):629–630. [DOI] [PubMed] [Google Scholar]
- 17.Kulke MH, Siu LL, Tepper JE, et al. Future directions in the treatment of neuroendocrine tumors: consensus report of the National Cancer Institute Neuroendocrine Tumor clinical trials planning meeting. J Clin Oncol. Mar 2011;29(7):934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.R: A Language and Environment for Statistical Computing [computer program]. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 19.S vB, K G-O Multivariate Imputation by Chained Equations in R. Journal of Statistical Software. 2011;45(3):1–67. [Google Scholar]
- 20.Barnard J, Rubin DB. Small sample degrees of freedom with multiple imputation. Biometrika. 1999;86:948–955. [Google Scholar]
- 21.Iqbal J, Ginsburg O, Rochon PA, Sun P, Narod SA. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA. Jan 13 2015;313(2):165–173. [DOI] [PubMed] [Google Scholar]
- 22.Dess RT, Hartman HE, Mahal BA, et al. Association of Black Race With Prostate Cancer-Specific and Other-Cause Mortality. JAMA Oncol. Jul 1 2019;5(7):975–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richards TB, Henley SJ, Puckett MC, et al. Lung cancer survival in the United States by race and stage (2001–2009): Findings from the CONCORD-2 study. Cancer. Dec 15 2017;123 Suppl 24:5079–5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howard JH, Hiles JM, Leung AM, Stern SL, Bilchik AJ. Race influences stage-specific survival in gastric cancer. Am Surg. Mar 2015;81(3):259–267. [PubMed] [Google Scholar]
- 25.Momin BR, Pinheiro PS, Carreira H, Li C, Weir HK. Liver cancer survival in the United States by race and stage (2001–2009): Findings from the CONCORD-2 study. Cancer. Dec 15 2017;123 Suppl 24:5059–5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White A, Joseph D, Rim SH, Johnson CJ, Coleman MP, Allemani C. Colon cancer survival in the United States by race and stage (2001–2009): Findings from the CONCORD-2 study. Cancer. Dec 15 2017;123 Suppl 24:5014–5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nipp R, Tramontano AC, Kong CY, et al. Disparities in cancer outcomes across age, sex, and race/ethnicity among patients with pancreatic cancer. Cancer Med. Feb 2018;7(2):525–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riner AN, Underwood PW, Yang K, et al. Disparities in Pancreatic Ductal Adenocarcinoma-The Significance of Hispanic Ethnicity, Subgroup Analysis, and Treatment Facility on Clinical Outcomes. Cancer Med. Apr 13 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melvan JN, Sancheti MS, Gillespie T, et al. Nonclinical Factors Associated with 30-Day Mortality after Lung Cancer Resection: An Analysis of 215,000 Patients Using the National Cancer Data Base. J Am Coll Surg. Aug 2015;221(2):550–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robbins AS, Pavluck AL, Fedewa SA, Chen AY, Ward EM. Insurance status, comorbidity level, and survival among colorectal cancer patients age 18 to 64 years in the National Cancer Data Base from 2003 to 2005. J Clin Oncol. Aug 1 2009;27(22):3627–3633. [DOI] [PubMed] [Google Scholar]
- 31.Shin JY, Yoon JK, Shin AK, Blumenfeld P, Mai M, Diaz AZ. Association of Insurance and Community-Level Socioeconomic Status With Treatment and Outcome of Squamous Cell Carcinoma of the Pharynx. JAMA Otolaryngol Head Neck Surg. Sep 1 2017;143(9):899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The primary dataset (National Cancer Database) is publicly available by request through the American College of Surgeons (https://www.facs.org/quality-programs/cancer/ncdb). The dataset analyzed for this study may be made available by the corresponding author upon request.