Abstract
Guillain-Barré syndrome (GBS) is a rare condition caused by autoimmune damage of peripheral nerves. We describe a case where a man in his 80s presented with subacute, progressive fatigue and weakness. He had received an outpatient work-up for possible haematological malignancy, but eventually presented to the emergency department for worsening weakness. A physical exam and cerebrospinal fluid analysis suggested a diagnosis of GBS. Subsequently, a pathological diagnosis of angioimmunoblastic T-cell lymphoma was made. The patient underwent intravenous immunoglobulin treatment for GBS and was started on cyclophosphamide, doxorubicin, vincristine and prednisone therapy. Prior research has suggested that incident malignancy may be associated with GBS, which may be caused by a paraneoplastic-type phenomenon, malignancy-associated immune dysregulation or an autoimmune reaction triggered by a common exposure. Clinicians should be aware of the possible association between these two conditions and should remain open minded to the possibility of non-infectious triggers for GBS.
Keywords: haematology (incl blood transfusion), neuromuscular disease, oncology
Background
Guillain-Barré syndrome (GBS) is a rare condition that presents with symptoms including progressive weakness, hyporeflexia or areflexia, pain and paresthesias, caused by underlying autoimmune damage of peripheral nerves. While it often is preceded by an infection,1 2 other precipitants have been suggested including malignancy.3 Timely recognition and appropriate treatment of GBS is important as some patients will experience serious sequelae, including respiratory depression.
This report describes the diagnosis of GBS in a patient being evaluated for an underlying haematological malignancy. It highlights the importance of maintaining an index of suspicion for non-infectious triggers for GBS. Additionally, it raises important questions about the underlying mechanisms of GBS and what might connect it to neoplastic processes.
Case presentation
A man in his 80s presented to the emergency department with progressive weakness, difficulty ambulating and a 2–3-week history of falls. In the preceding weeks, he had noted increasing fatigue and shortness of breath, initially while playing tennis, but eventually with his basic activities of daily living. Initial bloodwork performed by his outpatient cardiologist identified a pancytopenia and he was recently referred for a haematology consultation. Further investigations were pursued, including an abdominal ultrasound which demonstrated splenomegaly and bilateral iliac lymphadenopathy. In this context, a diagnosis of lymphoma was considered. A bone marrow biopsy was performed, the results of which were pending at the time of the patient’s presentation to the emergency department. His past medical history was otherwise remarkable for hypertension, dyslipidaemia, gout, prostate malignancy treated with radical prostatectomy, surgical hypothyroidism and hypoparathyroidism, and unilateral optic neuropathy causing monocular blindness. His medications included allopurinol, calcitriol, losartan, hydrochlorothiazide, rosuvastatin, levothyroxine, zopiclone, calcium and magnesium.
On examination, the patient was alert, haemodynamically stable and in no apparent distress. An examination of his head and neck revealed palpable bilateral cervical lymph nodes measuring less than 1 cm each. The patient’s cardiac and respiratory exams were within normal limits. His neurological evaluation revealed a pre-existing right sided relative afferent pupillary defect with otherwise unremarkable cranial nerve examination and normal muscle tone. Fasciculations were noted at the lateral thighs, as was reduced power on hip flexion (left: 4−, right: 3), knee flexion (left: 4+, right: 4), knee extension (left: 4+, right: 4) and great toe extension (left: 5, right: 4). The patient’s biceps and brachioradialis tendon reflexes were 2+ bilaterally; however, his triceps, patellar and Achilles tendon reflexes were absent. No clonus or positive Babinski sign was noted. His sensation of vibration was reduced at the level of his right ankle and left knee. His proprioception was impaired across his toes.
Investigations
Investigations performed immediately prior to the patient’s emergency department visitand subsequent admission to hospital showed a pancytopenia with a haemoglobin of 108 g/L (mean corpuscular volume 101.2 fL), white blood cells 3.5×109 /L (neutrophils 2.1×109 /L) and platelets 90×109/ L along with an elevated reticulocyte count (16%), lactate dehydrogenase (328 U/L), ferritin (682 ug/L), total bilirubin (46 umol/L), direct bilirubin (13 umol/L) and creatinine (142 umol/L). The patient’s haptoglobin, measured in the days before admission, was also reduced (0.06 g/L), however, direct antiglobulin tests were negative. His remaining bloodwork including electrolytes, calcium profile, thyroid stimulating hormone, liver enzymes, creatine kinase and serum protein electrophoresis was unremarkable. Analysis of cerebrospinal fluid (CSF) obtained by lumbar puncture demonstrated an albumin-cytological dissociation (white blood cells 1×106/ L (range 0–5), protein 1.33 g/L (range 0.15–0.45)) with further testing negative for cytomegalovirus, enterovirus, herpes simplex virus, varicella-zoster and cryptococcal antigen. Initial imaging, including a CT head and neck, thorax, abdomen and pelvis demonstrated extensive lymphadenopathy across the head and neck, thorax, abdomen and pelvis, in addition to hepatosplenomegaly. The patient’s CT brain and MRI spine were unremarkable.
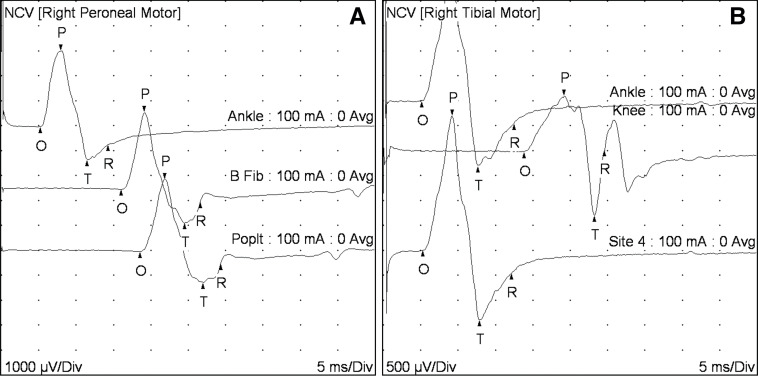
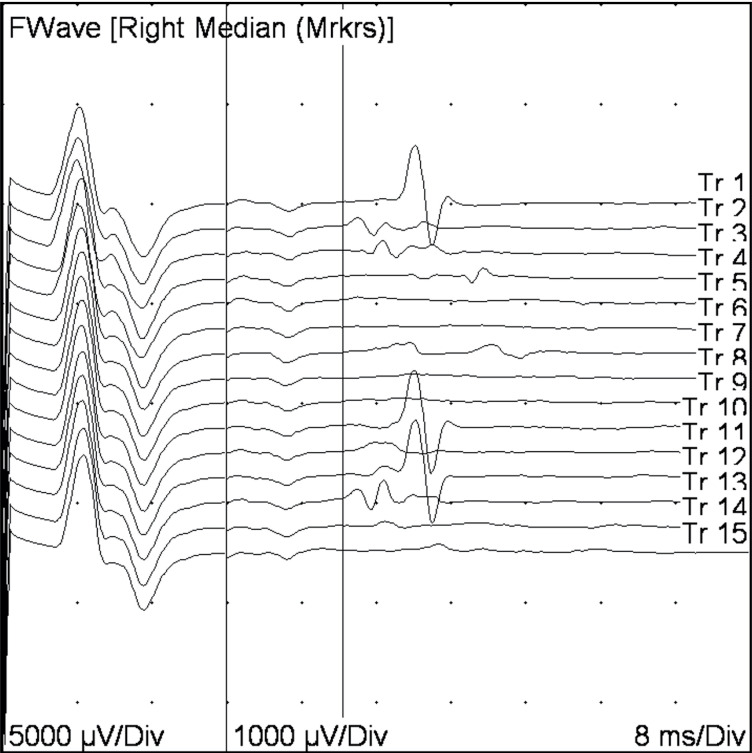
Given the constellation of physical exam and CSF findings, a presumptive clinical diagnosis of GBS was made. Electromyography testing, performed after treatment with intravenous immunoglobulin, showed reduced recruitment of all muscles tested (right first dorsal interosseal, deltoid, anterior tibialis, gastric, vastus lateralis). Nerve conduction studies showed severely slowed conduction velocity with probable conduction block (figure 1) along with markedly prolonged F wave responses (figure 2) that were consistent with a diagnosis of GBS.
Figure 1.
Nerve conduction studies. Nerve conduction studies for (A) the right peroneal and (B) right tibial nerves. The nerve conduction studies shown here represent severely slowed velocities, characteristic of demyelinating neuropathies. Normal conduction velocity (NCV) for peroneal (fibular) and tibial nerves is 42 m/s; here the velocities were 31 m/s for peroneal (measured between just below the fibular head and the ankle) and for the right tibial (measured between the knee and the ankle). In addition, there is a marked drop in the amplitude of tibial motor response (difference between points O and P on the graph) on stimulation at popliteal fossa (1.1 mV) compared with the amplitude of the response on stimulation at the ankle (2.7 mV). This finding is compatible with a conduction block. Conduction block is seen in the acquired demyelinating polyneuropathies.
Figure 2.
F-wave response from the right median nerve. The first waveform represents the direct motor response generated by stimulating the tested nerve, propagating to the motor end plate at the muscle. The smaller wave forms following F-waves, represent evoked activity generated by the stimulating motor neurons in the spinal cord (antidromic conduction), which in turn lead to another set of motor potentials. The F-wave responses here are markedly prolonged (initiation of the F-wave indicated by the first vertical bar). Normal values for the median nerve are 31 ms; the patient’s average measured F-wave latencies were 36.4 ms. Values for the right ulnar nerve (37.5 ms, normal <31 ms) and right tibial nerve (74.1 ms, normal <56 ms) were similarly elevated. In this patient, marked prolongation of F-wave responses is compatible with a primary demyelinating neuropathy.
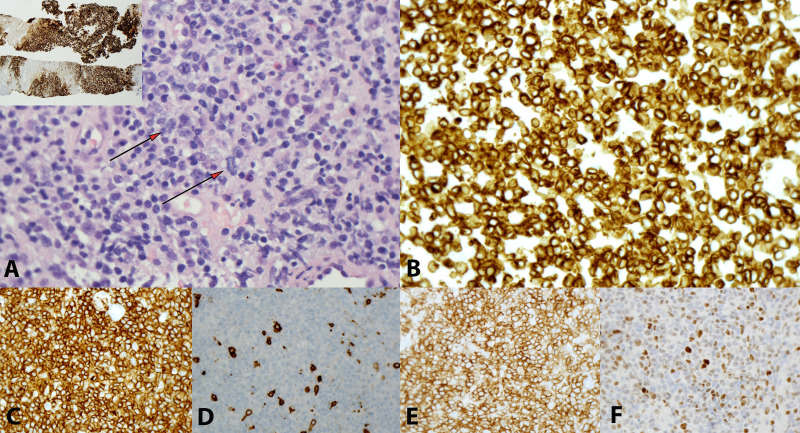
To further evaluate for an underlying malignancy, biopsies of the patient’s bone marrow and lymph nodes were obtained. The histological sections showed fragments of lymph node diffusely and heavily involved by neoplastic process (figure 3). The cellular population was composed predominantly of small to medium sized cells with slightly irregular nuclear contour, vesicular chromatin, small but distinct nucleoli and variable amounts of pale cytoplasm. A mild degree of vascular proliferation was also seen. The neoplastic cells were mixed with a polymorphous population of cells in the background, composed mostly of small lymphocytes, reactive histiocytes, plasma cells and rare eosinophils and neutrophils. The neoplastic cells were positive for CD3, CD4, CD5, CD57, BCL6 and PD-1 and were negative for CD8 and CD10. In situ hybridisation for Epstein-Barr virus (EBV)-encoded small RNA was negative in the neoplastic cells, with rare bystander cells having a positive response. Overall, these findings were consistent with a diagnosis of angioimmunoblastic T-cell lymphoma (AITL).
Figure 3.
Angioimmunoblastic T-cell lymphoma. (A) Tumour cells are small to intermediate with slightly vesicular chromatin, small distinct nucleoli and variable amounts of pale cytoplasm (arrows). Vessels are prominent. CD21 stain highlights expanded and distorted meshwork of follicular dendritic cells (inset); (B) CD3; (C) CD4; (D) CD8; (E) PD-1 and (F) BCL6 (magnification for all images ×400; inset; ×20).
Treatment
Given the presumptive clinical diagnosis of GBS and the patient’s worsening respiratory function, intravenous immunoglobulin was initiated and given over 2 days. The patient also underwent rehabilitation to improve motor function during his recovery. After the diagnosis of AITL was made, the patient was started on cyclophosphamide, doxorubicin, vincristine and prednisone therapy.
Outcome and follow-up
The patient initially responded well to first line chemotherapy from both a neurological and haematological perspective and completed six cycles of treatment with return of good physical function and quality of life. He was in remission for 3 months after completion of treatment. After this period of remission, however, unfortunately his lymphoma relapsed with recurrence of his lymphadenopathy, constitutional symptoms and functional decline. He was offered palliative chemotherapy or referral for entrance into a clinical trial, and on reflection, as quality of life was very important to him, he chose a home palliation approach and passed away peacefully at home a few weeks later.
Discussion
GBS, or acute inflammatory demyelinating polyneuropathy, encompasses a spectrum of disorders resulting from autoimmune damage of the peripheral nervous system. The aetiology of GBS is incompletely understood, although the disease is most often associated with a viral or bacterial infection (eg, Campylobacter jejuni, EBV, cytomegalovirus).1 2 Clinically, where an infectious prodrome or other putative trigger is not present in the context of a suspected case of GBS, it remains possible that a subclinical infection occurred prior to presentation. However, an index of suspicion should be maintained for other causes, including malignancy in the right clinical context, as this case illustrates. An association between GBS and an underlying malignancy has been proposed; although, previous research has been either limited to case reports or relatively small cohorts of GBS patients without controls. Vigliani and colleagues reported that the rate of incident malignancies (first diagnosis or recurrence) occurring within 6 months of a diagnosis of GBS was higher than that expected in the general population (OR=2.43, 95% CI: 1.11, 4.62).3 Hiew and Rajabally also found a higher rate of malignancy occurring among GBS patients than the general population of the United Kingdom. However, their results were sensitive to their outcome definition—after excluding cancers that developed two or more years after an episode of GBS, no significant increase in malignancy rate was observed.4 It thus remains unclear whether a robust statistical association exists and whether it would be causal or reflective of confounding or detection bias.
To date, GBS has been considered a ‘non-classical’ paraneoplastic syndrome in consensus diagnostic criteria for paraneoplastic neurological syndromes, recognising that there are no known onconeural antibodies identified and that the presence of GBS does not often associate with cancer, in contrast to conditions such as encephalomyelitis.5 The pathogenesis of paraneoplastic neurological syndromes remains incompletely understood, although they are generally considered to result from autoimmune responses triggered by ectopic cancer cell antigens. Anti-neuronal antibodies can be subdivided into onconeuronal antibodies, which target antigens expressed by both the cancer and neurons through a cytotoxic T-cell effect, and neuronal cell surface antibodies, which are direct-acting antibodies that may or may not be paraneoplastic.6 One possibility is that GBS in cancer patients may result from antigen expression by cancer cells causing autoimmune destruction of native peripheral nervous system tissue, as has been proposed with other paraneoplastic syndromes.7 However, in the case of AITL, the same outcome could also result from cancer-related immune dysregulation.
AITL is a rare form of non-Hodgkin's lymphoma, composing 1%–2% of cases.8 It derives from follicular T helper cells and has been associated with immune dysregulation. Individuals with AITL can suffer from immunodeficiencies,9 and can have positive autoimmune serologies including rheumatoid factor, anti-smooth muscle and cold agglutinins.8 Autoimmune associations with AITL have also been observed, including cases of autoimmune haemolytic anaemia10 and autoimmune thyroid disease.11 12 Whether a GBS could also arise from AITL-related derangements in immune function is unknown. Interestingly, EBV is a shared risk factor for AITL and GBS,1 8 although the cell population predominantly infected in AITL is B-cells.8 13 Some authors have proposed that EBV may promote the development of AITL,14 while others have suggested that the presence of EBV in AITL is a byproduct of a larger immunodeficiency associated with the condition.9 Whether there is a common aetiological role of EBV infection in both these entities or whether AITL-associated immunodeficiency predisposes patients to active EBV infection with consequent GBS is unclear.15
To assess evidence for these possibilities in other cases, we performed a literature review and identified a total of five additional cases (table 1; for details on search strategy see online supplemental appendix), which were not reported in a prior review of GBS and non-Hodgkin’s lymphomas.15 In all described cases, a diagnosis of GBS occurred within a year of the diagnosis of AITL (minimum: simultaneously, maximum: 7 months), which would categorise all cases as possible paraneoplastic syndromes under existing guidelines.5 With respect to possible infectious aetiologies, none of the described cases presented with positive testing for C. jejuni, cytomegalovirus or EBV, although one case identified EBV positive cells in the biopsied lymph node tissue, but not in the malignant cell population. In several reports no autoimmune testing was performed or described; however, one case presented with an autoimmune haemolytic anaemia,16 another with findings suggestive of haemolytic anaemia with negative direct antigen testing (the case described here) and in two other case reports, specific autoimmune conditions (haemolytic anaemia, collagen vascular disease) were reported as ruled out.15 17 From this review, there does not appear to be an obvious infectious trigger for GBS in the AITL population, although if the precipitating infection occurred in the weeks prior and was subsequently cleared, testing at time of presentation might be expected to be negative. There is mixed evidence of autoimmune phenomena in the cases identified, with only one case with positive result for autoantibodies. Given the small number of reported cases in the literature, it remains unclear if the occurrence of GBS in association with AITL is causally related or just mere coincidence.
Table 1.
Review of prior cases of Guillain-Barré syndrome associated with angioimmunoblastic T-cell lymphoma
| Reference | Onset of GBS relative to AITL | Infectious testing | Immunological testing |
| Howell et al, 2022 | Concomitant diagnosis |
|
|
| Pathak et al, 201915 | Concomitant diagnosis. Clinical signs of AITL (eg, anaemia) preceding neuromotor findings |
|
|
| Hiew & Rajabally, 20174 | Diagnosis of AITL within 2 years of GBS |
|
|
| Naidech et al, 200218 | Diagnosis of AITL 7 months prior to GBS |
|
|
| Lindahl et al, 200116 | Diagnosis of AITL7 days prior to GBS |
|
|
| Monteiro et al, 198417 | Diagnosis of AITL approximately 5 weeks before Miller Fisher diagnosis |
|
|
*Howell et al 2022 refers to the present case.
AITL, angioimmunoblastic T-cell lymphoma; CMV, cytomegalovirus; CSF, cerebrospinal fluid; EBV, Epstein-Barr virus; GBS, Guillain-Barré syndrome; HSV, herpes simplex virus.
bcr-2021-246176supp001.pdf (88.3KB, pdf)
In our patient, the concurrence of neurological and haematological disorders may argue in favour of a mechanism involving immune dysregulation in its pathophysiology. Further, his GBS symptoms improved with chemotherapy for his underlying AITL, a phenomenon observed with other autoimmune manifestations of lymphoma. The specific nature of this immune dysregulation is unclear. It may derive from an antigen being expressed by the neoplastic cells that is also expressed on peripheral nerves leading to unintentional destruction of peripheral nerves as a bystander (paraneoplastic hypothesis). Alternatively, it is possible that the underlying AITL predisposed the patient to form autoantibodies resulting in peripheral nerve damage, analogously to AITL-associated autoimmune haemolytic anaemia or thyroid disease, but with no associated ectopic protein produced by the neoplastic cells themselves (autoimmune hypothesis). Conversely, there is no evidence that his AITL or his GBS was associated with EBV or another infectious precursor.
In summary, several possible mechanisms may underly a relationship between AITL and GBS. However, the possibility that the diagnosis of GBS and AITL is coincidental cannot be excluded. Further population-based studies along with basic research exploring associations between GBS and cancer are warranted to explore the robustness of this clinical observation, possible heterogeneity across types of malignancy, including lymphomas and putative mechanisms explaining this relationship.
Learning points.
Guillain-Barré syndrome (GBS) has been reported to be associated with an underlying malignancy in several cases.
In cases where a typical infectious prodrome does not precede signs consistent with GBS, the treating clinician should maintain an index of suspicion for other underlying aetiologies, including malignancy in the right clinical context.
Hypothesised associations between GBS and lymphomas may be caused by a paraneoplastic response or a lymphoma-related autoimmune response.
Acknowledgments
We thank the patient and his family for their time and help with this publication.
Footnotes
Contributors: NAH contributed towards the conception and design, acquisition of data, analysis and interpretation of data; he also was responsible for drafting the initial version of the article. SA, HS, AS and AP contributed towards the acquisition of data and the analysis and interpretation of the data. PT and RW contributed towards the analysis and interpretation of data. All authors critically revised the manuscript for important intellectual content, provided final approval of the manuscript and agreed to be accountable for the article.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: PT reports non-financial support from Aurora, outside the submitted work. NAH, SA, HS, RW and AP have nothing to declare.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Consent obtained from next of kin.
References
- 1.Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barré syndrome. Lancet 2016;388:717–27. 10.1016/S0140-6736(16)00339-1 [DOI] [PubMed] [Google Scholar]
- 2.Jacobs BC, Rothbarth PH, van der Meché FG, et al. The spectrum of antecedent infections in Guillain-Barré syndrome: a case-control study. Neurology 1998;51:1110–5. 10.1212/wnl.51.4.1110 [DOI] [PubMed] [Google Scholar]
- 3.Vigliani M-C, Magistrello M, Polo P, et al. Risk of cancer in patients with Guillain-Barré syndrome (GBS). A population-based study. J Neurol 2004;251:321–6. 10.1007/s00415-004-0317-3 [DOI] [PubMed] [Google Scholar]
- 4.Hiew FL, Rajabally YA. Malignancy in Guillain-Barré syndrome: a twelve-year single-center study. J Neurol Sci 2017;375:275–8. 10.1016/j.jns.2017.02.024 [DOI] [PubMed] [Google Scholar]
- 5.Graus F, Delattre JY, Antoine JC, et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry 2004;75:1135–40. 10.1136/jnnp.2003.034447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berzero G, Psimaras D. Neurological paraneoplastic syndromes: an update. Curr Opin Oncol 2018;30:359–67. 10.1097/CCO.0000000000000479 [DOI] [PubMed] [Google Scholar]
- 7.Graus F, Dalmau J. Paraneoplastic neurological syndromes in the era of immune-checkpoint inhibitors. Nat Rev Clin Oncol 2019;16:535–48. 10.1038/s41571-019-0194-4 [DOI] [PubMed] [Google Scholar]
- 8.Lunning MA, Vose JM. Angioimmunoblastic T-cell lymphoma: the many-faced lymphoma. Blood 2017;129:1095–102. 10.1182/blood-2016-09-692541 [DOI] [PubMed] [Google Scholar]
- 9.Yabe M, Dogan A, Horwitz SM. Angioimmunoblastic T-cell lymphoma. In: Querfeld C, Zain J, Rosen ST, eds. T-Cell and NK-cell lymphomas: from biology to novel therapies. Cham, Switzerland: Springer, 2019: 99–126. [Google Scholar]
- 10.Brearley RL, Chapman J, Cullen MH, et al. Haematological features of angioimmunoblastic lymphadenopathy with dysproteinaemia. J Clin Pathol 1979;32:356–60. 10.1136/jcp.32.4.356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ambepitiya GB. Angioimmunoblastic lymphadenopathy associated with thyroid disease. J Clin Pathol 1989;42:668–9. 10.1136/jcp.42.6.668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pautier P, Devidas A, Delmer A, et al. Angioimmunoblastic-like T-cell non Hodgkin's lymphoma: outcome after chemotherapy in 33 patients and review of the literature. Leuk Lymphoma 1999;32:545–52. 10.3109/10428199909058412 [DOI] [PubMed] [Google Scholar]
- 13.Dogan A, Attygalle AD, Kyriakou C. Angioimmunoblastic T-cell lymphoma. Br J Haematol 2003;121:681–91. 10.1046/j.1365-2141.2003.04335.x [DOI] [PubMed] [Google Scholar]
- 14.Zhou Y, Attygalle AD, Chuang S-S, et al. Angioimmunoblastic T-cell lymphoma: histological progression associates with EBV and HHV6B viral load. Br J Haematol 2007;138:44–53. 10.1111/j.1365-2141.2007.06620.x [DOI] [PubMed] [Google Scholar]
- 15.Pathak P, Perimbeti S, Ames A, et al. Guillain Barré syndrome heralding the diagnosis of angioimmunoblastic T-cell lymphoma. Leuk Lymphoma 2019;60:1835–8. 10.1080/10428194.2018.1553299 [DOI] [PubMed] [Google Scholar]
- 16.Lindahl J, Kimby E, Björkstrand B, et al. High-dose chemotherapy and APSCT as a potential cure for relapsing hemolysing AILD. Leuk Res 2001;25:267–70. 10.1016/S0145-2126(00)00134-X [DOI] [PubMed] [Google Scholar]
- 17.Monteiro ML, Coppeto JR, Greco P, et al. Angioimmunoblastic lymphadenopathy with Fisher syndrome. Arch Neurol 1984;41:456–7. 10.1001/archneur.1984.04050160122028 [DOI] [PubMed] [Google Scholar]
- 18.Naidech A, Weisberg L, Palliyath S, et al. Sudden weakness in a patient with lymphoma. Cleve Clin J Med 2002;69:337–41. 10.3949/ccjm.69.4.337 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bcr-2021-246176supp001.pdf (88.3KB, pdf)