Abstract
Background
There has been insufficient attention to a fundamental force shaping healthcare policies—conflicts of interest (COI). We investigated COI, which results in the professional judgement of a policymaker or healthcare provider being compromised by a secondary interest, in relation to antimicrobial use, thereby illuminating challenges to the regulation of medicines use more broadly. Our objectives were to characterise connections between three groups—policymakers, healthcare providers and pharmaceutical companies—that can create COI, and elucidate the impacts of COI on stages of the policy process.
Methods
Using an interpretive approach, we systematically analysed qualitative data from 136 in-depth interviews and five focus group discussions in three Asian countries with dominant private healthcare sectors: Cambodia, Indonesia and Pakistan.
Findings
We characterised four types of connections that were pervasive between the three groups: financial, political, social and familial. These connections created strong COI that could impact all stages of the policy process by: preventing issues related to medicines sales from featuring prominently on the agenda; influencing policy formulation towards softer regulatory measures; determining resource availability for, and opposition to, policy implementation; and shaping how accurately the success of contested policies is reported.
Interpretation
Our multicountry study fills a gap in empirical evidence on how COI can impede effective policies to improve the quality of healthcare. It shows that COI can be pervasive, rather than sporadic, in influencing regulation of medicine use, and highlights that, in addition to financial connections, other types of connections should be examined as important drivers of COI.
Keywords: health policy, qualitative study
WHAT IS ALREADY KNOWN ON THIS TOPIC
There is evidence from a range of high-income to low-income country settings that conflicts of interest (COI) can compromise the professional judgement of policymakers or healthcare providers.
This is particularly salient in relation to policies on antimicrobial use, owing to the diversity of stakeholders that stand to lose or gain from policies that impact medicine sales.
WHAT THIS STUDY ADDS
The hidden nature of COI makes it challenging to research and address, and our multicountry study fills an important gap in empirical evidence on how COI impacts all stages of the policy process to regulate medicine use in pluralistic health systems.
It documents that COI is pervasive and characterises the connections between policymakers, healthcare providers and pharmaceutical companies that can create COI.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE AND/OR POLICY
Impediments to political will and the effectiveness of policies to improve quality of care and reduction of unnecessary antimicrobial may be addressed by recognising COI as a pervasive force, and applying a wide lens to assess its intrinsic role when planning policies, so that COI can be addressed explicitly.
Introduction
There is a trend in health policy recommendations toward emphasising the need for political commitment, stronger regulatory systems and greater investment in health systems strengthening.1–3 But in understanding challenges to progress on these recommendations, there has been insufficient attention to a fundamental force shaping health policy and systems: conflicts of interest (COI).4 We define COI as a situation in which the objectivity of a policymaker’s or healthcare provider’s professional judgement is compromised, or could be compromised, by a secondary interest.5 This definition of COI encompasses the set of circumstances and relationships that increase the risk that the primary professional judgement is compromised, even if the individual or institution is not consciously or tangibly influenced. Indeed, no action needs to be taken for COI to exist, which makes COI distinct from many other practices that fall under corruption, such as bribery or overbilling for services, in which entrusted power is consciously abused for private gain. It also makes COI challenging to investigate and tackle in any setting, but particularly in pluralistic health systems characterised by substantial healthcare provision by for-profit providers and out-of-pocket (OOP) payments by patients.
In many health systems worldwide, the failure of the public sector to adequately provide, regulate and monitor health service delivery has resulted in a dominant for-profit private health sector.6 There is evidence from a range of high-income to low-income country settings that the profit-generating model of private healthcare can result in a misalignment between providers’ financial incentives and optimisation of public health benefit.7 8 The influence of the pharmaceutical industry in shaping decisions of healthcare providers and policymakers should not be underestimated here.7 Described as ‘a robust sector that has been one of the pillars of industrialised economies’, the biopharmaceutical industry contributes almost US$2 billion to the world’s gross domestic product and employs approximately 5.5 million people.9 Politicians in many countries thus seek to strengthen their domestic pharmaceutical sectors, which can be at odds with efforts to reduce unnecessary use of medicines.10
In our study, we use COI in relation to policies on antimicrobial use to explore the challenges to regulate unnecessary use of medicines more broadly, owing to the diversity of stakeholders that stand to lose or gain from policies on the use of antimicrobials.11 12
Study objectives and setting
Our study objectives were two-fold. First, to identify and characterise connections between three groups—policymakers, healthcare providers and pharmaceutical companies—that can create COI. Second, to elucidate the impacts of these COI on different stages of the policy process (to better regulate the use of antimicrobials). Our study is based on data from linked studies conducted by the authors in Cambodia, Indonesia and Pakistan; these studies focused on antimicrobial use by for-profit healthcare providers, who play a major role in the delivery and financing of healthcare in all three settings. Our study countries have faced some similar health systems challenges, including insufficient trained human and material resources for health; competing external donor interests; systems of patronage that impede recruitment of technically competent practitioners; and a limited regulatory capacity to govern a growing private healthcare sector.13 14 These countries’ pharmaceutical industries have experienced rapid and extensive growth over the past decade, which is testing for regulatory institutions. However, a marked difference is that Cambodia, owing to its smaller population, has a substantially smaller pharmaceutical market size and fewer nationally-owned companies have emerged relative to Pakistan and Indonesia, where there is more money to be made from pharmaceuticals.9 15 Likewise, the number of private healthcare providers, and resources needed to regulate them, is much greater in Indonesia and Pakistan (box 1).
Box 1. Summary of study country contexts.
Located in Southeast Asia, Cambodia has a population of approximately 16.5 million people. Although the government has committed to health sector reforms in line with universal health coverage (UHC), OOP spending accounts for approximately 60% of total health expenditure.36 The health system is still recovering from decades of political instability, conflict and civil unrest, which virtually eliminated the country’s professional and intellectual classes, including Ministry of Health staff and qualified healthcare providers. By 1979, only 32 of 530 practicing doctors and 26 of 120 pharmacists had survived the Khmer Rouge regime.37 In recent years, the pharmaceutical industry has been growing at pace; between 2010 and 2015, there was a roughly 77% increase in the number of pharmaceutical companies operating (a total of 308), and pharmaceutical sales per annum are between US$0.2–0.3 billion.38 But most companies are not Cambodian-owned and, as of 2015, 90% of pharmaceuticals were imported. Cambodia was one of the first countries in the region to develop a national policy on antimicrobial resistance (AMR) in 2014 and establish a national AMR working group, which has since developed a Multi-Sectoral Action Plan 2019–23 and national guidelines for antimicrobial stewardship in 2019.39 40 Though challenges to address inappropriate use of antibiotics persist due to the widespread availability of antibiotics from formal and informal providers without a prescription, sometimes of substandard quality or fake.41
Also located in Southeast Asia, Indonesia is the fourth most populous country in the world, with a population of approximately 270.6 million. In 2014, the government launched a new compulsory national health insurance scheme, known as Jaminan Kesehatan Nasional, with the aim of providing affordable healthcare to all citizens.42 By 2020, the scheme covered around 220 million members or 82% of the national population, making it one of the world’s biggest single-payer health insurance schemes.43 But progress toward UHC has not been without its challenges. Government expenditure on health is approximately half that of other countries at a comparable income level.44 OOP spending accounts for approximately 35% of total health expenditure and even insured patients have reported high charges at the point-of-care, mainly for medicines.42 In addition to private doctors, private midwives, community pharmacies and drug shops are commonly used. Indonesia’s pharmaceutical industry is dominated by domestic manufacturers accounting for 88% of the 206 registered companies, yet around 95% of active ingredients are still imported. Pharmaceutical sales per annum are approximately US$6 billion.45 Progress on combatting AMR includes the development of a national action plan in 2017, awareness-raising and development of surveillance systems and stewardship programmes in hospital settings, as well as multiple governance mechanisms, such as the High Level Inter-Ministerial Steering Committee and the National AMR Coordination Committee.46 47 However, major challenges have been recognised, such as the enforcement of rules on appropriate use of antibiotics, especially challenging in the private healthcare sector, which is a major source of antibiotic dispensing in the community.48 49
Pakistan is the sixth most populous country in the world located in South Asia, with a population of approximately 216.6 million. OOP spending in Pakistan has been declining since the mid-2000s from around 78% to 56% in 2018, although studies suggest that between 57% and 80% of health service utilisation still occurs in the private sector.50 Since the early 1990s, funding for public services has been severely cut, meanwhile the availability of financing for private providers has increased and health policies have prioritised curative over preventive care, paving the way for exponential and unregulated growth of a for-profit healthcare market.51 The value of the pharmaceutical industry in Pakistan has doubled over the past decade, with more than 700 pharmaceutical companies operating, of which the vast majority are domestic.52 Pharmaceutical sales per annum are over US$2 billion.52 Although a national drug policy exists to regulate this sector, unethical drug promotion has become an acceptable norm.52 In 2017, the Ministry of National Health Services developed a national AMR action plan and established a national coordination group and multisectoral secretariat.53 The same year, as part of a WHO evaluation process, Pakistan scored the lowest in policy and capacity to combat AMR, signalling a weak regulatory environment for the monitoring and control of antibiotic use in both human and animal sectors.54
Methods
Study design
We analysed qualitative data collected using in-depth interviews and focus group discussions from three linked studies, as summarised in table 1. In these studies, the research teams worked with independent study advisors to purposively identify participants falling into predetermined categories (policymakers or advisors, healthcare providers and pharmaceutical industry stakeholders) for a first round of data collection, and then conducted a second round of data collection based on snowball sampling using suggestions from the first set of participants. Data was collected using a topic guide by experienced qualitative researchers familiar with the local context, in a language that was preferred by the participant. Further details on sampling and data collection methods can be found in Khan et al and Ferdiana et al.16 17 All questions in the topic guide were framed around regulation of antimicrobial use, covering the following main themes: motivations of different stakeholders that could impede effective regulations; types of connections or relationships held by policy actors, and ways these could impede effective regulations; and how influential various groups are in policy-setting and implementation. Where possible and appropriate, we introduced an interactive exercise using cards representing different types of healthcare providers, policy actors and pharmaceutical industry stakeholders, to encourage participants to talk about connections and COI that might influence policies and healthcare provider decisions. This exercise is described in more detail by Legido-Quigley et al along with the underlying conceptual framework.11
Table 1.
Characteristics of the three linked data sets used in this study
| Characteristics | Data sets | ||
| Country | Cambodia | Indonesia | Pakistan |
| Qualitative data collection method | IDI | FGD and IDI | IDI |
| Sample size | 55 IDIs | 5 FGDs and 31 IDIs | 50 IDIs |
| Study participants |
|
||
| Data collection process |
|
||
FGD, focus group discussion; IDI, in-depth interview.
Approach to data analysis
Our approach to synthesising qualitative data from these linked studies was based on Noblit and Hare’s methods for meta-ethnography18; our analysis examined COI in the individual studies and translated key constructs between studies to evolve a broader understanding of COI. We started with an initial inductive analysis of the raw data to identify different types of connections between policy actors, healthcare providers and pharmaceutical companies. We defined ‘connections’ in the broadest sense, to capture the myriad of direct, indirect or covert ways which might link policy actors with healthcare providers and pharmaceutical companies; pharmaceutical companies with healthcare providers; and within healthcare providers, licensed providers and other licensed providers, and licensed providers and unlicensed providers. By licensed/unlicensed healthcare provider, we are referring to those providers who have/have not received formally recognised training and are/are not typically registered with any government regulatory body. Common examples of unlicensed healthcare providers include drug shops, drug sellers and village doctors.
We mapped the types of connections identified to these five stakeholder pairings to form the basis of the coding framework. Three of the authors (AR-S, MK and LPLW) used this framework to organise data in the transcripts, following the Framework Method outlined by Gale et al.19 We then conducted a second round of inductive analysis (on the same set of transcripts) to identify the policy impacts of the connections previously identified. In this round, we first coded the data in terms of direct or indirect policy impacts using an interpretive approach in which findings are supported by excerpts from the raw data to ensure that data interpretation is linked to the words of the participants.20 We coded the data again, but this time, in terms of which stage(s) of the policy process the COI impacted using Fadlallah et al’s five-stages model21: agenda-setting, policy formulation, policy adoption, policy implementation and policy evaluation.
Role of the funding source
The funding source did not play a role in this research.
Patient and public involvement
Neither patients nor the public were involved in any way in this research.
Results
To address the first objective of our study, we identified four broad types of connections from the data that resulted in COI: financial, political, social and familial. Financial connections were associations owing to exchanges of monetary or non-monetary benefits from which an individual or institution can derive financial gain. We identified both overt and covert financial connections. Political connections were relationships based on the gain of power or influence in local or national political processes. Social connections were based on shared experiences, either within a professional or non-professional setting, and mutual assistance. Finally, there were familial connections, which were based on blood relations and through marriage. In table 2, we present these connections in relation to the actors or groups involved along with examples from one or more countries where this connection was identified. Although our analysis distinguished between financial, political, social and familial connections, we found that one type of connection would often create or reinforce another type of connection. For example, in Pakistan, it was reported that pharmaceutical company owners paid for politicians’ recreational travel, which helped forge friendships, which in turn strengthened their social connections, even leading to familial connections through marriages between children of politicians and wealthy pharmaceutical company owners.
Table 2.
Summary of the types of connections identified between different actor groups and illustrative examples of the resultant COI based on the data
| Groups involved | Type of connection | Examples |
| Policy actors and pharmaceutical industry | Financial (overt) |
|
| Financial (covert) |
|
|
| Political |
|
|
| Social and familial |
|
|
| Policy actors and healthcare providers | Financial (overt) |
|
| Financial (covert) |
|
|
| Political |
|
|
| Social and familial |
|
|
| Healthcare providers and pharmaceutical companies | Financial |
|
| Social |
|
|
| Licensed healthcare providers and other licensed healthcare providers | Financial |
|
| Licensed healthcare providers and unlicensed healthcare providers | Financial |
|
| Familial |
|
Connections creating COI for policy actors
We found that government officials and senior members of professional medical associations, both influential in terms of policy design and implementation, experienced COI owing to financial, political, social and familial connections with the pharmaceutical industry and with healthcare providers. Interviewees cited numerous examples of government officials holding multiple roles that created financial connections with the organisations or individuals that they are responsible for regulating. For example, in Cambodia and Pakistan, it was reported that some decision-makers (or their close family members) may own or work for pharmaceutical companies or healthcare facilities that they were responsible for regulating. In Cambodia, one interviewee explained that pharmaceutical companies wanting to expand sales in a new part of the country often start by making the village leaders their ‘business partners’. We also found that professional associations, which are responsible for providing independent technical guidelines for doctors and pharmacists, in some cases received support from the pharmaceutical industry.
In addition to financial connections described above, our data indicated a range of political, social and familial connections between policymakers and the pharmaceutical industry or healthcare providers. An interviewee in Pakistan described how domestic pharmaceutical companies pay for politicians to perform Hajj (Islamic pilgrimage) annually in order to build social connections and ‘buy favours’. COI created through political connections are typified by the quote below, which emphasises that health officials avoid upsetting unlicensed providers during their relatively short terms in office to maintain political support from this powerful group for their careers going forward.
[unlicensed healthcare provider’s] voice is very powerful when they speak out. If they criticise the government, it goes viral… Ministry of Health understands the situation very clearly. But this issue [of unlicensed medical practice] is untouchable at the moment. (Pakistan)
Ultimately, where policy actors were involved in COI, it resulted in them being incentivised to support the interests of specific pharmaceutical companies and/or healthcare providers. We found this impacted the agenda-setting stage of the policy process, largely by keeping things off the agenda. For example, interviewees in Pakistan indicated that discussions about stronger regulation of medicine sales or illegal health practices are less likely to reach the policy agenda—and in an unbiased way—when policy actors are connected either to the pharmaceutical industry or to private healthcare providers. If issues that touch on COI of government officials do end up high on the policy agenda, our analysis indicated that loopholes or ambiguity can be introduced during the policy formulation and adoption stages. For example, in Indonesia, policies have been formulated to allow a pharmacist to work in up to three pharmacies, without clarifying whether the pharmacist must be present in the pharmacy(ies) at all times during opening hours to oversee patient encounters. Similar ambiguity regarding pharmacy practices exists in Cambodia and Pakistan. In all three countries, it can result in pharmacists using their professional license to open a pharmacy where they do not work; such ‘license-renting’ maximises the income of pharmacists. When it comes to policy formulation, we also found that regulatory agency officials preferred softer measures to regulate the pharmaceutical industry and healthcare providers, such as mandating training and information sharing, rather than stricter measures, such as inspections and fines.
We try to suggest many interventions, but policymakers do not agree. So, it cannot work. (Cambodia)
[I know many people] who are often influenced by these pharma people. Hugely influenced. When I say hugely influenced, they are offered trips abroad, they had been given so many other incentives. So, I think again it means that [regulatory agency—name redacted] is not exercising its true powers and its mandate… they may issue some sort of warning to doctors, maybe in the press, but I have not seen any punitive action ever taken…’ (Pakistan)
There was also strong evidence that the connections between government officials and healthcare providers impact policy implementation. For instance, in Cambodia, some interviewees suggested that healthcare providers with social, familial or financial connections to government officials may experience an easier license approval process to set up pharmacies and clinics. One interviewee reported that, some years ago, raids on medicine importers suspected of bringing in substandard medicines could be blocked because of the importer’s connections. Interviewees in all three countries highlighted the tacit reluctance of regulators to enforce rules on those that they had social or familial connections with. For example, in Indonesia, several interviewees emphasised that social connections, such as those based on a shared alma mater, can be enough to soften the implementation of rules by regulators:
A huge conflict of interest [occurs when there is], for example, a senior doctor and the staff of the district health office [responsible for checking practice of the senior doctor] happens to be his former student. (Indonesia)
Another commonly cited impact of COI, across all three countries, relates to the deliberate under-resourcing of the agencies or government departments responsible for implementation of policies to regulate medicine sales, especially of those policies that influential stakeholders do not support. This under-resourcing was associated with agencies being hindered from effectively implementing policies and making regulators more susceptible to different forms of bribery and intimidation.
Presently the [regulatory agency—name redacted]… is the most neglected wing of the health department in the government of every province. Basically, the resources, meaning the infrastructure, manpower… We are not being provided any funds except our salaries… (Pakistan)
There should be strict monitoring… Drug stores are not supposed to sell antibiotics, but they often have ampicillin and amoxicillin. (Indonesia)
Additionally, the process by which regulators could take legal action against healthcare providers or pharmaceutical companies that break rules was sometimes described as an unnecessarily complicated or thwarted process. This was seen, by interviewees, as a deterrent for taking further action. Some interviewees also indicated concerns about the consequences of taking action against powerful individuals.
We have a saying in Cambodia: “before you beat a dog, you should know who owns the dog”. People worry about consequences when taking action. It’s really important. The [regulatory agency—name redacted] is very hesitant to do something serious. (Cambodia)
[In] many instances, where we tried to catch hold of the proprietors or persons running a medical store, they are not just giving a small threat but also threatening to kill us. (Pakistan)
Finally, at the policy evaluation stage, we found that accurate monitoring and reporting of the failure of specific polices was related to COI. In one of our study countries, not named owing to political sensitivities, some interviewees highlighted that official government evaluations show zero unlicensed healthcare providers, as a testament to the success of policies to curtail unlicensed practitioners, although this misrepresents the ground reality. Biased reporting from such evaluations has a knock-on effect on the agenda-setting stage, by diminishing the visibility of selected issues, both internationally and domestically.
Connections creating COI in healthcare providers
In contrast to the diverse nature of connections between government regulatory officials and healthcare providers or the pharmaceutical industry described above, we found that COI affecting healthcare providers’ decisions occurred mainly owing to financial connections with the pharmaceutical industry and with other healthcare providers. With regards to the latter, similarly to how pharmaceutical companies sought to build connections with policymakers, in all three countries they offered benefits to both licensed and unlicensed providers, as well as access to social and educational events, in order to influence prescribing practices.
When I worked at a branch of a pharmaceutical company, I was often visited by our antibiotic marketing division… we then also “pushed” to the field, to hospitals, to pharmacies to send their purchase order to us. I realised that this would mean a push towards a large sales target, and one day it will have its implications on the irrational use of antibiotics. (Indonesia)
There were also clear examples of COI owing to connections between providers. We found that licensed healthcare providers established financial connections with other licensed or unlicensed providers to maximise their income generation. For example, in all three countries, some doctors were known to direct patients to buy medicines from specific pharmacies, drug shops or unlicensed medicine sellers that they either own or receive kickbacks from. This made policy implementation particularly challenging because of dual practice in which some healthcare providers work in both the public and private sectors.
So, they [government agencies] cannot stop [unlicensed medicine sellers]. Because the ones [public health centre staff] who run the illegal [outlets] are also the same ones from the public sector! (Cambodia)
In Indonesia, we found that some larger pharmacies sold antimicrobials to smaller pharmacies, midwives and unlicensed drug shops as a way of side-stepping policies on prescription-only sales. A common type of financial connection in Cambodia and Pakistan involved license-renting whereby licensed doctors and pharmacists would rent their professional licenses to unqualified people to allow them to open clinics or drug shops.
Following our investigation, we noted that pharmacies which should have distributed and sold medicines to clients, had changed their business and acted as a wholesaler [which distributes medicines to other pharmacies, illegally]. (Indonesia)
Interviewees in Cambodia and Pakistan also frequently described familial connections in which licensed providers would rely on family members to run their clinic or pharmacy in their absence, or would train family members to run a separate unlicensed practice. Owing to these mainly financial connections between licensed and unlicensed providers, we found that licensed providers were motivated to use their power to (covertly) influence agenda setting and policy implementation to impede policies that might impact informal practices.
Discussion
Rather than sporadic occurrences, we found that COI was a fundamental component of the functioning of the political and health systems that we studied. Our analysis showed that connections between policy actors, healthcare providers (licensed and unlicensed) and pharmaceutical companies can create strong COI that impact on all stages of the policy process (figure 1). COI can prevent issues related to sales of medicines from featuring prominently on the policy agenda; influence policy formulation, such that softer or ambiguous measures are introduced, pushing the policy into the symbolic quadrant of Matland’s ambiguity-conflict matrix that characterises implementation processes22; impact the extent to which formulated policies are adopted, determine resource availability for, and opposition to, policy implementation; and shape how accurately the success of contested policies is evaluated and reported.
Figure 1.
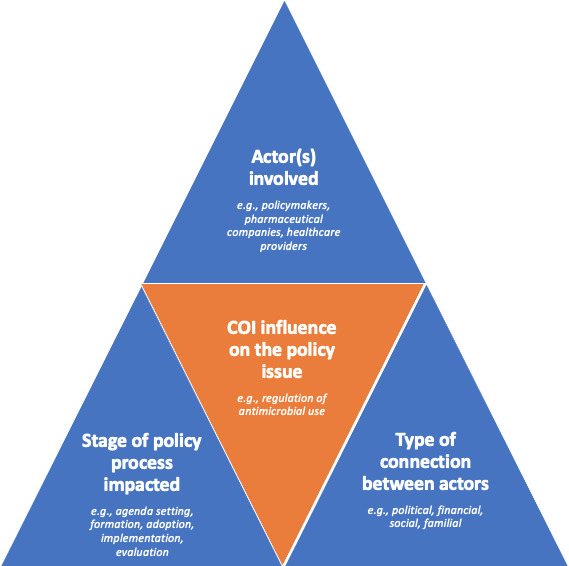
Conceptualisation of COI influencing a health policy issue (illustrated with reference to our analysis on policies to regulate antimicrobial use).
Overall, we found that when COI involved healthcare providers, it tended to impact most directly on policy implementation whereas in situations when COI involved policymakers, such as senior government officials, it could have a more direct influence on agenda-setting and policy formation, as well as on policy implementation and evaluation. This finding is important when considering strategies and solutions to address COI influencing physician-induced demand, unnecessary use of medicines and the broader consequences of these issues, such as AMR.
While others have highlighted the role of financial connections in creating COI that impede policies that prioritise public health,7 23 our study illuminates the role of familial, social and political connections in driving COI. Reflecting on the types of connections leading to COI identified through our detailed analysis across three countries, we note that some connections are more pervasive globally than others.24 25 COI arising from the reliance of healthcare providers on pharmaceutical companies to provide educational and professional development opportunities is a challenge in many higher-income and lower-income countries,26 27 as is funding from pharmaceutical companies to politicians. Resonating with our findings, an analysis in the USA found that the pharmaceutical and health product industry spent US$4.7 billion between 1999 and 2018 on lobbying the federal government, with contributions targeting senior legislators in Congress involved in drafting healthcare laws as well as committees involved in drug pricing and regulation.28 Financial ties between pharmaceutical companies and ‘independent’ policy advisors or government officials is also widespread. For example, in the UK, where the health system is not dominated by private healthcare provision, members of the Vaccine Taskforce were found to have financial interests in pharmaceutical companies from which the government purchased COVID-19 diagnostic tests and treatment.29 In contrast to these more widespread connections leading to COI, connections between licensed and unlicensed healthcare providers, and the power of unlicensed providers to hinder stronger regulation of informal healthcare practice, is more widely documented in low-income and middle-income countries (LMIC).30 31 Finally, others have shown that the role of independent professional medical associations is commonly compromised by COI. For example, prior to their decision to reject funding from formula milk companies in 2019, the Royal College of Paediatrics and Child Health in the UK accepted around £40 000 annually from formula milk companies toward event sponsorship and advertising.32 While this type of COI is an issue around the world, severe under-resourcing and dependency on donations in LMIC makes it perhaps more pronounced in such settings.33
Our multicountry study fills an important gap in empirical evidence on COI in pluralistic health systems, as the hidden and nuanced nature of COI makes it challenging to research and address. In light of the evidence on the urgent need for improvements in quality of care and reduction of unnecessary antimicrobial use,34 35 our findings and those from a 2021 study in India,31 suggest that action on COI is critical to address impediments to progress on this wicked problem. Actions that may help to address this issue could include strengthening medical curricula and teaching on COI, as well as wide dissemination of practical and accessible guidelines that are endorsed by respected national and international bodies. We acknowledge the limitations of our methodology, including possible differences in the extent to which subthemes, such as unlicensed healthcare provision, were covered by researchers in each country. Although we applied a methodological innovation to solicit open responses, we realise that interviewees may have held back in their responses given the sensitivity of the topic. Despite this reservedness, the data captured is highly revealing of COI in the respective settings, and the urgent need for further investigation.
Conclusion
Policymakers and healthcare providers typically hold a position of power and trust owing to the informational asymmetry that characterise healthcare decisions. Populations are thus left, to differing extents, reliant on policymakers and healthcare providers to prioritise the interests of the people they are responsible for looking after. It is therefore critical to address the misalignment between personal gain and public health benefit which occurs when policymakers and healthcare providers are affected by COI. Our study shows that COI can be pervasive, and illuminates routes through which it can have a pronounced impact on the policy process for regulating unnecessary use of antimicrobials and medicines more broadly. Going forward, we need to better understand systems of interactions and interconnectedness between stakeholder groups. This will require the development of new methods to investigate the impact of COI on health policies, generated by researchers who have a deep understanding of the cultural and political context. In the context of regulating antimicrobial use to combat AMR, given the pervasiveness of COI shown by our study, we expect impediments and hindered progress until COI is addressed. The first step is to move away from avoiding this contentious issue, and to instead apply a wide lens to assess the intrinsic role COI plays in the policy process for regulating medicine use—as we have done in this analysis—so that it can be addressed explicitly.
bmjgh-2022-008596supp001.pdf (58.7KB, pdf)
Footnotes
Handling editor: Seye Abimbola
Twitter: @DrMishalK, @MsLilaWulandari
Contributors: MK and JH conceptualised the analysis. MK, AD-B, RH, JH, AP, VW, LPLW, SB, SS, VS, SC, SP, CP and SH were involved in designing and implementing the methodology for data collection in relation to contributing data sets to this research, and in executing said methodologies in the respective country settings (including data collection and analysis). AR-S, LPLW and MK curated and analysed the data for this research. MK, AR-S and JH developed the original draft. All authors contributed equally to editing and reviewing, and approved the final manuscript. MK is responsible for the overall content as the study guarantor. Also, see Author Reflexivity Statement.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Author note: The reflexivity statement for this paper is linked as an online supplemental file1.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. The data sets generated and/or analysed from the research conducted in Cambodia and Pakistan are not publicly available but are available from the corresponding author on reasonable request. The anonymised data set from the research conducted in Indonesia is available upon reasonable request. The request can be made immediately following publication (no end date) with anyone who wishes to access the data sets for any purpose.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
For the Cambodia and Pakistan data sets, the study received ethical approval from the London School of Hygiene & Tropical Medicine, and the Cambodia National Ethics Committee for Health Research (NECHR). For the Indonesia data set, research was conducted in compliance with a protocol approved by the ethical committee of Universitas Gadjah Mada (KE/FK/0161/EC/2019), the University of New South Wales (HC190043). Participants gave informed consent to participate in the study before taking part.
References
- 1.Balabanova D, Mills A, Conteh L, et al. Good health at low cost 25 years on: lessons for the future of health systems strengthening. Lancet 2013;381:2133 10.1016/S0140-6736(12)62000-5 [DOI] [PubMed] [Google Scholar]
- 2.Victora CG, Hanson K, Bryce J, et al. Achieving universal coverage with health interventions. Lancet 2004;364:1541–8. 10.1016/S0140-6736(04)17279-6 [DOI] [PubMed] [Google Scholar]
- 3.Durski KN, Osterholm M, Majumdar SS, et al. Shifting the paradigm: using disease outbreaks to build resilient health systems. BMJ Glob Health 2020;5. 10.1136/bmjgh-2020-002499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rahman-Shepherd A, Balasubramaniam P, Gautham M, et al. Conflicts of interest: an invisible force shaping health systems and policies. Lancet Glob Health 2021;9:e1055–6. 10.1016/S2214-109X(21)00202-3 [DOI] [PubMed] [Google Scholar]
- 5.Ralston R, Hil SE, da Silva Gomes F, et al. Towards preventing and managing conflict of interest in nutrition policy? an analysis of submissions to a consultation on a draft who tool. Int J Health Policy Manag 2021;10:255-265–65. 10.34172/ijhpm.2020.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McPake B, Hanson K. Managing the public-private mix to achieve universal health coverage. Lancet 2016;388:622–30. 10.1016/S0140-6736(16)00344-5 [DOI] [PubMed] [Google Scholar]
- 7.Gagnon M-A. Corruption of pharmaceutical markets: addressing the misalignment of financial incentives and public health. J Law Med Ethics 2013;41:12066:571–80. 10.1111/jlme.12066 [DOI] [PubMed] [Google Scholar]
- 8.Currie J, Lin W, Meng J. Using audit studies to test for physician induced demand: the case of antibiotic abuse in China. NBER Working Paper Series - National Bureau of Economic Research 2012;18153. 10.3386/w18153 [DOI] [Google Scholar]
- 9.Facts and figures 2021: the pharmaceutical industry and global health - IFPMA. Available: https://www.ifpma.org/resource-centre/facts-and-figures-2021-the-pharmaceutical-industry-and-global-health/ [Accessed 7 Oct 2021].
- 10.Kaplan W, Boskovic N, Flanagan D. Pharmaceutical policy in countries with developing healthcare systems: synthesis of country case studies. Pharmaceutical Policy in Countries with Developing Healthcare Systems 2017:30. 10.1007/978-3-319-51673-8_20 [DOI] [Google Scholar]
- 11.Legido-Quigley H, Khan MS, Durrance-Bagale A, et al. Something borrowed, something new: a governance and social construction framework to investigate power relations and responses of diverse stakeholders to policies addressing antimicrobial resistance. Antibiotics 2018;8:8010003 10.3390/antibiotics8010003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Littmann J, Viens AM, Silva DS. The super-wicked problem of antimicrobial resistance 2020:421–43.
- 13.Khan MS, Meghani A, Liverani M, et al. How do external donors influence National health policy processes? experiences of domestic policy actors in Cambodia and Pakistan. Health Policy Plan 2018;33:czx145 10.1093/heapol/czx145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gryseels C, Kuijpers LMF, Jacobs J, et al. When ‘substandard’ is the standard, who decides what is appropriate? Exploring healthcare provision in Cambodia. Crit Public Health 2019;29:460–72. 10.1080/09581596.2019.1591614 [DOI] [Google Scholar]
- 15.Pharmaceutical sector in Indonesia. Cekindo.
- 16.Ferdiana A, Liverani M, Khan M, et al. Community pharmacies, drug stores, and antibiotic dispensing in Indonesia: a qualitative study. BMC Public Health 2021;21:10. 10.1186/s12889-021-11885-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan MS, Bory S, Rego S, et al. Is enhancing the professionalism of healthcare providers critical to tackling antimicrobial resistance in low- and middle-income countries? Hum Resour Health 2020;18:9. 10.1186/s12960-020-0452-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnett-Page E, Thomas J. Methods for the synthesis of qualitative research: a critical review. BMC Med Res Methodol 2009;9:11. 10.1186/1471-2288-9-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gale NK, Heath G, Cameron E, et al. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol 2013;13:117. 10.1186/1471-2288-13-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liamputtong Rice P, Ezzy D. Qualitative research methods: a health focus 1999;295. [Google Scholar]
- 21.Fadlallah R, El-Jardali F, Nomier M, et al. Using narratives to impact health policy-making: a systematic review. Health Res Policy Syst 2019;17. 10.1186/s12961-019-0423-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matland RE. Synthesizing the implementation literature: the ambiguity-conflict model of policy implementation, 1995. Available: https://www.jstor.org/stable/1181674 [Accessed 18 Oct 2021].
- 23.Dünnbier M. Exposed: the strategies big alcohol deploys to interfere in WHO alcohol policy consultation - Movendi International 2021. https://movendi.ngo/blog/2021/03/17/exposed-the-strategies-big-alcohol-deploys-to-interfere-in-who-alcohol-policy-consultation/
- 24.Marten R, Hawkins B. Stop the toasts: the Global Fund’s disturbing new partnership. The Lancet 2018;391:735–6. [DOI] [PubMed] [Google Scholar]
- 25.Sah S. Conflicts of interest and COVID, 2020. Available: https://www.scientificamerican.com/article/conflicts-of-interest-and-covid/ [Accessed 7 Oct 2021].
- 26.de AM, Jafarey A, Shekhani SS. The ethics of pharma–physician relations in Pakistan: “When in Rome”. Ethics and Behavior 2018;29:89 https://www.research.ed.ac.uk/en/publications/the-ethics-of-pharmaphysician-relations-in-pakistan-when-in-rome [Google Scholar]
- 27.Lemmens C. Pharma goes to the laundry: public relations and the business of medical education. Hastings Center Report 2004;34:23. [PubMed] [Google Scholar]
- 28.Wouters OJ. Lobbying expenditures and campaign contributions by the pharmaceutical and health product industry in the United States, 1999-2018. JAMA Intern Med 2020;180:97. 10.1001/jamainternmed.2020.0146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thacker PD. Conflicts of interest among the UK government’s covid-19 advisers. BMJ 2020;371. [DOI] [PubMed] [Google Scholar]
- 30.Goodman C, Kachur SP, Abdulla S, et al. Drug shop regulation and malaria treatment in Tanzania--why do shops break the rules, and does it matter? Health Policy Plan 2007;22:403 10.1093/heapol/czm033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gautham M, Spicer N, Chatterjee S, et al. What are the challenges for antibiotic stewardship at the community level? an analysis of the drivers of antibiotic provision by informal healthcare providers in rural India. Soc Sci Med 2021;275:113813. 10.1016/j.socscimed.2021.113813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lake L, Kroon M, Sanders D, et al. Child health, infant formula funding and South African health professionals: eliminating conflict of interest. S Afr Med J 2019;109:6. 10.7196/SAMJ.2019.v109i12.14336 [DOI] [PubMed] [Google Scholar]
- 33.Azimova A, Abdraimova A, Orozalieva G, et al. Professional medical associations in low-income and middle-income countries. Lancet Glob Health 2016;4:7:e606–7. 10.1016/S2214-109X(16)30139-5 [DOI] [PubMed] [Google Scholar]
- 34.Sheikh K, Josyula LK, Zhang X, et al. Governing the mixed health workforce: learning from Asian experiences. BMJ Glob Health 2017;2:e000267. 10.1136/bmjgh-2016-000267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kruk ME, Pate M. The Lancet global health commission on high quality health systems 1 year on: progress on a global imperative. Lancet Glob Health 2020;8:2:e30–2. 10.1016/S2214-109X(19)30485-1 [DOI] [PubMed] [Google Scholar]
- 36.Asante AD, Ir P, Jacobs B, et al. Who benefits from healthcare spending in Cambodia? Evidence for a universal health coverage policy. Health Policy Plan 2019;34:czz011 10.1093/heapol/czz011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santini H. Rebirth of the health-care system in Cambodia. Lancet 2002;360:s57–8. 10.1016/S0140-6736(02)11824-1 [DOI] [PubMed] [Google Scholar]
- 38.Bureau-Point E, Baxerres C, Chheang S. Self-Medication and the pharmaceutical system in Cambodia. Med Anthropol 2020;39:781 10.1080/01459740.2020.1753726 [DOI] [PubMed] [Google Scholar]
- 39.Cambodia strategy to combat AMR 2015.
- 40.Multi-Sectoral action plan on antimicrobial resistance in Cambodia 2019-2023, 2019. Available: https://rr-asia.oie.int/wp-content/uploads/2020/03/cambodia_final-msap-english-version-with-signed.pdf [Accessed 4 Nov 2021].
- 41.Suy S, Rego S, Bory S, et al. Invisible medicine sellers and their use of antibiotics: a qualitative study in Cambodia. BMJ Glob Health 2019;4:e001787. 10.1136/bmjgh-2019-001787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nugraheni WP, Mubasyiroh R, Hartono RK. The influence of Jaminan Kesehatan Nasional (JKN) on the cost of delivery services in Indonesia. PLoS One 2020;15:0235176. 10.1371/journal.pone.0235176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Social Security Agency for Health . JKN program participants, 2020. Available: https://bpjs-kesehatan.go.id/bpjs/ [Accessed 4 Nov 2021].
- 44.Wiseman V, Thabrany H, Asante A, et al. An evaluation of health systems equity in Indonesia: study protocol. Int J Equity Health 2018;17:138. 10.1186/s12939-018-0822-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hasnida A, Kok MO, Pisani E. Challenges in maintaining medicine quality while aiming for universal health coverage: a qualitative analysis from Indonesia. BMJ Glob Health 2021;6:e003663. 10.1136/bmjgh-2020-003663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parathon H, Kuntaman K, Widiastoety TH, et al. Progress towards antimicrobial resistance containment and control in Indonesia. BMJ 2017;358:j3808. 10.1136/bmj.j3808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.National action plan on antimicrobial resistance Indonesia 2017-2019. Available: https://www.flemingfund.org/wp-content/uploads/59f148b0482cda160087e29b9a5a21a0.pdf [Accessed 4 Nov 2021].
- 48.Wulandari LPL, Wiseman V. Engaging the private sector to improve antimicrobial use in the community. Public Health and Preventive Medicine Archive 2018;6:79. 10.15562/phpma.v6i2.187 [DOI] [Google Scholar]
- 49.Wulandari LPL, Khan M, Liverani M, et al. Prevalence and determinants of inappropriate antibiotic dispensing at private drug retail outlets in urban and rural areas of Indonesia: a mixed methods study. BMJ Glob Health 2021;6:004993 10.1136/bmjgh-2021-004993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khalid F, Raza W, Hotchkiss DR, et al. Health services utilization and out-of-pocket (OOP) expenditures in public and private facilities in Pakistan: an empirical analysis of the 2013-14 OOP health expenditure survey. BMC Health Serv Res 2021;21:178. 10.1186/s12913-021-06170-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jooma R, Sabatinelli G. Political determinants of health: lessons for Pakistan. Pak J Med Sci 2014;30:457. 10.12669/pjms.303.5487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gul R, Saeed H, Saleem Z, et al. Perceptions of and barriers to ethical promotion of pharmaceuticals in Pakistan: perspectives of medical representatives and doctors. BMC Med Ethics 2021;22:16. 10.1186/s12910-020-00569-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.National AMR Action Plan for Pakistan Antimicrobial Resistance National Action Plan Pakistan Ministry of National Health Services Regulations & Coordination Government of Pakistan 2017.
- 54.Khan MS, Durrance-Bagale A, Mateus A, et al. What are the barriers to implementing national antimicrobial resistance action plans? a novel mixed-methods policy analysis in Pakistan. Health Policy Plan 2020;35:973–82. 10.1093/heapol/czaa065 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2022-008596supp001.pdf (58.7KB, pdf)
Data Availability Statement
Data are available upon reasonable request. The data sets generated and/or analysed from the research conducted in Cambodia and Pakistan are not publicly available but are available from the corresponding author on reasonable request. The anonymised data set from the research conducted in Indonesia is available upon reasonable request. The request can be made immediately following publication (no end date) with anyone who wishes to access the data sets for any purpose.


