Abstract
A woman in her 70s presented to the hospital being generally unwell 8 days following the first dose of the AstraZeneca COVID-19 vaccination. She was in stage III acute kidney injury (AKI) with hyperkalaemia and metabolic acidosis. Urinalysis showed haematoproteinuria. Renal immunology screen was negative. She subsequently underwent two renal biopsies. The second biopsy showed features consistent with acute tubulointerstitial nephritis. She was commenced on oral steroids, which led to marked improvement of her renal function.
There are reasons why AKI can occur post vaccination such as prerenal AKI from reduced oral intake postvaccination due to feeling unwell or developing vomiting or diarrhoea. Intravenous fluids were given to this patient but with no meaningful improvement in renal function. She developed a possible reaction to the AstraZeneca COVID-19 vaccine, which led to AKI as supported by the interstitial inflammation and presence of eosinophils on renal biopsy.
Keywords: COVID-19, Immunological products and vaccines, Unwanted effects / adverse reactions, Acute renal failure, Renal medicine
Background
With the COVID-19 pandemic, vaccination programmes are being rolled out worldwide. As the population receives vaccination, there may be cases identified of possible COVID-19 vaccination-induced acute interstitial nephritis (AIN). Report of minimal change disease following Pfizer-BioNTech COVID-19 vaccine improves with administration of prednisolone.1 The plausible mechanism includes cell-mediated response initiated by COVID-19 vaccination.1 This will need one to consider avoiding the second dose of the same vaccine and potential increasing vaccine mixing. Although rare, it may in the future cause autoimmune diseases as seen with some other vaccines such as the thrombocytopaenia with the Measles–Mumps–Rubella vaccine, swine influenza vaccine and Guillain-Barre syndrome in 1976. This case report simply describes AIN after COVID-19 vaccination where causality has not been established. More research is required to determine causality between AIN and COVID-19 vaccination.
The authors felt the importance to increase awareness of the potential for COVID-19 vaccination-induced AIN as more and more people will receive the vaccination worldwide to avoid potential end-stage kidney disease if the same vaccination type is repeated.
Case presentation
A woman in her 70s presented to the hospital with a 1-week history of being generally unwell 8 days following the AstraZeneca COVID-19 vaccination. She also described transient right-sided weakness and a generalised headache. The patient was passing good amount of urine and had no symptoms of lower urinary tract infection, vomiting or diarrhoea. She also had no symptoms of systemic vasculitis or myeloma such as a purpuric rash, gritty eyes, joint pains, mouth ulcers and back pain. On presentation, she had no history of fever. She did not self-medicate with herbal remedies or take any over-the-counter medications including non-steroidal anti-inflammatory drugs. This part of history was important to ascertain any intrinsic renal cause or drug-induced AKI.
Past medical history included type II diabetes mellitus on oral hypoglycaemic agents and hypertension. She was diagnosed with type II diabetes mellitus in 2004 and suffered from background diabetic retinopathy. The chronicity and control of type II diabetes could have an impact on her renal function, although it was unsure if she had proteinuria prior to hospital admission. She had no known drug allergies, and her drug history included alogliptin, glimepiride, metformin, olmesartan and simvastatin.
She received the first dose of AstraZeneca COVID-19 vaccination 8 days prior to presentation. She lived with her husband and was independent with all her activities of daily living. She was a non-smoker and did not drink any alcohol or use recreational medications.
There was no family history of renal disease of note.
Investigations
It was interesting to note that her blood sugar was 1.6 mmol/L with the ambulance crew. CT scan of the head for the headache and weakness showed no acute intracranial pathology.
Renal function showed AKI stage III with metabolic acidosis and hyperkalaemia of 6.1 mmol/L. Her serum creatinine on admission was 416 μmol/L, which rose to 618 μmol/L at its peak. Her baseline creatinine was around 80 μmol/L. Other than a normocytic anaemia with a haemoglobin of 97 g/L, all her other blood investigations including liver function test and bone profile were unremarkable.
Urinalysis showed haematoproteinuria. Urine protein–creatinine ratio was 135 mg/mmol. These two results could either indicate an intrinsic renal cause of AKI or the fact that she suffered from hypertension and type II diabetes mellitus. There was no evidence of eosinophiluria.
Renal screen including vasculitis and myeloma screens were unremarkable. Ultrasound scan of the kidneys demonstrated two normal size kidneys, with good cortical preservation, no evidence of renal calculi, mass or obstructive hydronephrosis.
The ultrasound scan was important to determine the chronicity or renal injury (cortical preservation with normal sized kidneys) and to rule out any reversible post renal cause of AKI.
Renal biopsy was then arranged to look for intrinsic causes of AKI. The first biopsy produced an insufficient sample for analysis. The second biopsy (figures 1–3) revealed mild to moderate infiltrates of lymphocytes, plasma cells, histiocytes, neutrophils and eosinophils with evidence of tubulitis. There were no pus cells, and immunofluorescence was negative for staining immune deposits. These are features consistent with AIN.
Figure 1.
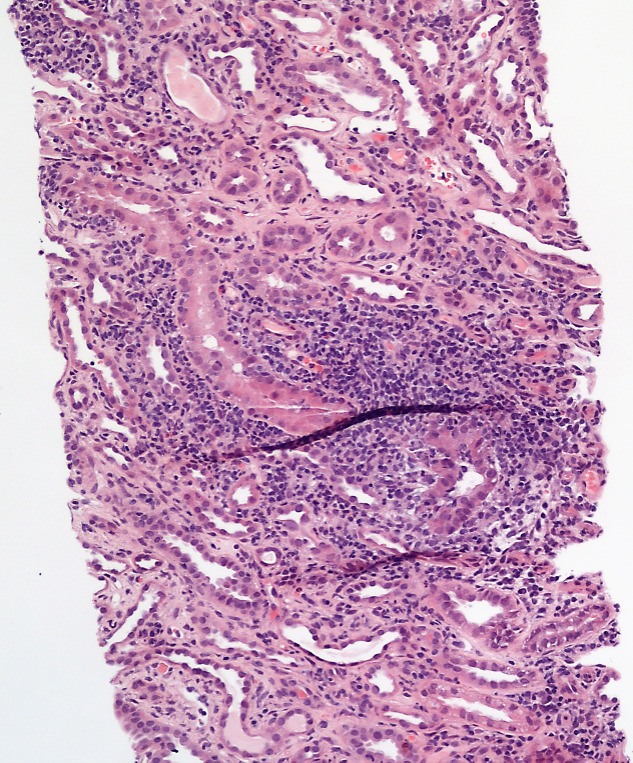
Renal biopsy demonstrated Interstitial inflammation in the tubules (100x).
Figure 2.
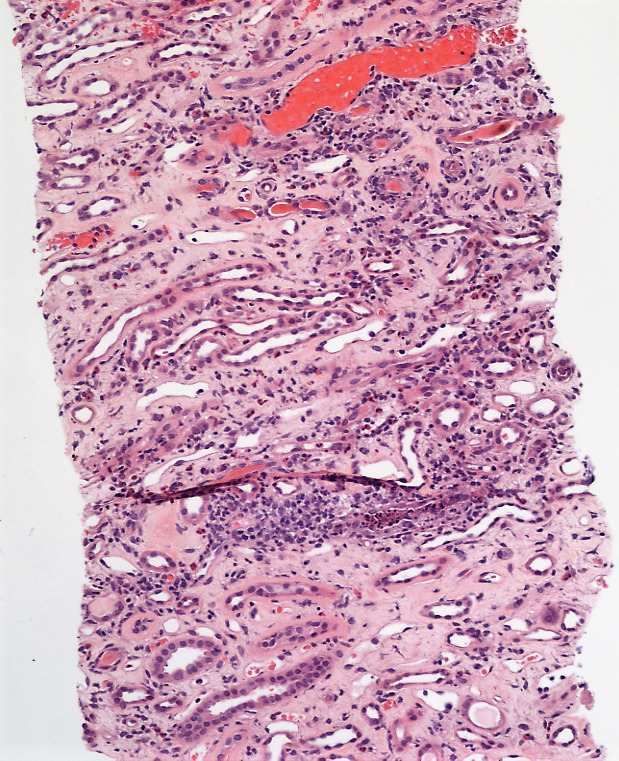
Interstitial inflammation infiltrates the tubules (100x).
Figure 3.
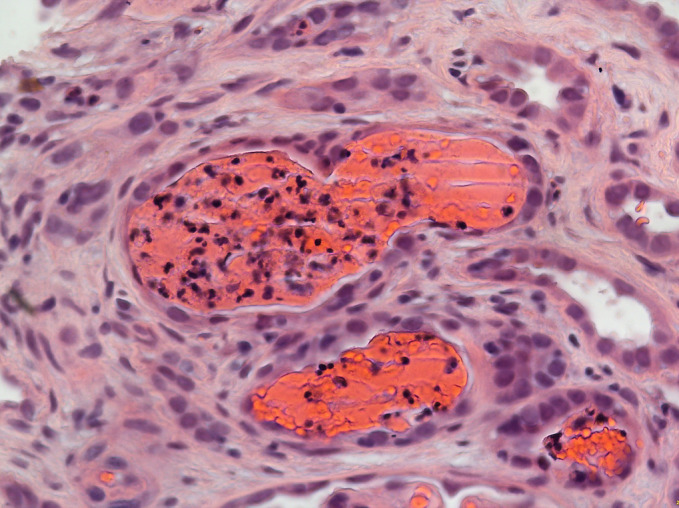
Interstitial inflammation infiltrates the tubules (400x).
Differential diagnosis
It was thought that hypoglycaemia was the cause of stroke mimic, explaining the transient right-sided weakness, which subsequently improved with glucose ingestion.
The unexpected AKI was thought to be either prerenal in combination with medications (Olmesartan), as she was generally unwell following vaccination and prerenal accounts for the majority of AKI cases. However, her renal function did not improve with volume repletion but continued to worsen.
Ultrasound scan of urinary tract ruled out post-renal causes of AKI.
The urinalysis and urine protein–creatinine ratio raised suspicions of an intrinsic renal cause despite the unremarkable renal vasculitic and myeloma screens. Renal biopsy confirmed AIN, which we believe was due to the COVID-19 vaccination.
Treatment
The patient received 60 mg of oral prednisolone following the results of the biopsy. After 28 days, prednisolone was weaned down and eventually stopped 4 months postdiagnosis. The patient was given patient education on her condition prior to discharge. She was advised to monitor her blood sugars closely due to the side effects of glucocorticoids.
The patient’s renal function had continued to improve despite weaning off steroids, coupled with the fact that she had also not been started on any steroid-sparing agents suggesting that seronegative autoimmune diseases were unlikely.
Outcome and follow-up
She was seen in clinic 2 weeks postdischarge and her creatinine had improved to 234 μmol/L. She was then seen again 2 weeks following that and her creatinine was 183 μmol/L whereupon her glucocorticoid regime was gradually reduced. The patient will continue to be seen in clinic until at least the normalisation of renal function.
Discussion
A COVID-19 vaccine known as ChAdOx1 nCoV-19 or AZD1222 was developed by the University of Oxford and AstraZeneca to treat SARS-CoV-2 infection (the cause of COVID-19). In this vaccine, a modified version of a chimpanzee adenovirus (ChAdOx1) is used, which can enter human cells but not replicate inside. A gene for the COVID-19 vaccine was added into the adenovirus DNA, allowing the vaccine to target the spike proteins that SARS-CoV-2 uses to enter human cells. The vaccine was given emergency authorisation by the UK in December 2020 of the pandemic.2 It is given intramuscularly and is being evaluated as two doses 4–12 weeks apart. The WHO recommends that the two doses be given 8–12 weeks apart.3 Evaluated in four trials across three continents, this vaccine had an efficacy of 70.4% after two doses and protection of 64.1% after at least one standard dose, against symptomatic disease to original viruses and some variants, with no safety concerns.4 In a subsequent analysis of this trial, vaccine efficacy for symptomatic COVID-19 was 76% from 21 days after receipt of the first dose until receipt of the second dose or day 90, whichever came first, suggesting protection with a single dose.5
Immunisation is one of the 10 great public health achievements of the 20th century and is considered ‘one of the greatest tools in the public health arsenal’. Most vaccines are safe to administer and cause only minor side effects. The common side effects of many vaccines and toxoids include fever, local reactions at the injection site or even serum sickness-like reaction.6 Although very rare, case reports published in the literature have associated vaccines to renal complications including different glomerular diseases and acute kidney injury (AKI) (table 1).
Table 1.
Vaccine-associated kidney diseases6
| Vaccine | Kidney disease/pathology reported in literature |
| Influenza |
|
| Hepatitis B |
|
| Pneumococcal |
|
| Pertussis |
|
| Diphtheria, pertussis, tetanus |
|
| Tetanus diphtheria poliomyelitis |
|
| Smallpox |
|
| Measles |
|
| Rabies |
|
| Meningococcal |
|
| BCG |
|
AIN, acute interstitial nephritis; ATN, acute tubular necrosis; GBM, glomerular basement membrane; GN, glomerulonephritis; HSP, Henoch–Schonlein purpura; MCD, minimal change disease; MN, membranous nephropathy.
There are reports of AIN following influenza vaccine in both the adult and the paediatric groups and report of minimal change disease (MCD) following Pfizer-BioNTech COVID-19 vaccine.1 7 8 This is the first report of AIN caused by any of the various available COVID-19 vaccines, as far as we know.
AIN is one of the leading causes of AKI. There are multiple factors for tubulointerstitial nephritis and the most important aetiology is medication, which consists of 50% to 85%, sometimes 92% of total cases.9 There is no typical range of time of onset for medication-induced AIN. The onset of drug-induced AIN following drug exposure may range from 3 to 10 days (as occurs with a second exposure to an offending drug), to as long as several weeks, to many months (as occurs following a first exposure to an offending drug).10 11 Our case presented with AKI 8 days after AstraZeneca COVID-19 vaccine. Simvastatin is also a possibility but less likely as the renal function improved with continued use. Renal biopsy showed evidence of AIN. In the absence of any other causative factor, we were inclined to think this was COVID-19 vaccine induced.
AIN can present in many ways as detailed below, from two large series in patients with AIN, that included 121 patients (table 2).
Table 2.
Clinical and laboratory features at presentation in patients with AIN14
| Features | Frequency in renal biopsy series % |
| AKI | 100 |
| AKI requiring dialysis | 40 |
| Arthralgia | 45 |
| Skin rash | 22 |
| Fever | 36 |
| Non-visible haematuria | 67 |
| Visible haematuria | 5 |
| Proteinuria | 93 |
| Nephrotic range proteinuria | 2.5 |
| Nephrotic syndrome | 0.8 |
| Eosinophilia | 35 |
| Eosinophiluria | 66 |
AIN, acute interstitial nephritis; AKI, acute kidney injury.
Drug-induced TIN is a result of allergic reactions and is thought to be immune-mediated. Only small proportions of the population taking the same medications have TIN mainly due to variable responses of individuals to the same drug. The main pathogenic mechanism of drug-induced TIN is type IV hypersensitive reactions in which T cells (71% CD8 + and CD4+ equally) have a central role with contributions from monocytes (15%), B cells (7%).9 We suspect a similar pathophysiology in our patient.
Apart from drugs, there are other causes of AIN mentioned in literature, as summarised below (table 3).
Table 3.
Causes of AIN (original)
| Frequency (%) | Causes | References |
| 50–75 | Allergic
|
14–17 |
| 10–30 | Toxic
Other
|
18–28 |
| 5–15 | Immune
|
14 29–31 |
| 1–10 | Infection
|
14 32 |
AIN, acute interstitial nephritis.
Patient showed excellent response to corticosteroids, similar to the report of MCD after Pfizer-BioNTech vaccine.8 We started steroids on the face of a rising serum creatinine, once the biopsy showed results compatible with AIN. The best supportive data for glucocorticoid treatment comes from a retrospective, multicentre study. This showed that the cohort treated with corticosteroids had a lower frequency of dialysis (4% vs 44%) and a lower serum creatinine level at 18 months (186 and 327 μmol/L).12 There is a retrospective study to refute this.13 However, one must note that the median creatinine was 670 μmol/L in this study and a large number of cases (44%) were NSAID induced, which do not respond to corticosteroids.
The pertinent question from this case report is whether the AIN and AKI are coincidental or causally related to vaccination. As described in this report, the association between development of AIN and COVID-19 vaccination can only be based on timing and exclusion of other precipitating factors.
Learning points.
Presentation with acute kidney injury (AKI) after a COVID-19 vaccine should be managed following standard guidelines.
A renal biopsy should be carried out in unresolved AKI following COVID-19 vaccine. If features are compatible with acute interstitial nephritis, we suggest consideration to using corticosteroids in patients with rising creatinine or imminent dialysis.
Further research is required to determine the causality between COVID-19 vaccination and acute interstitial nephritis.
Footnotes
Contributors: FT and MK contributed to conceptualisation and write up; SB to review and editing.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
References
- 1.Lebedev L, Sapojnikov M, Wechsler A, et al. Minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. American Journal of Kidney Diseases 2021;78:142–5. 10.1053/j.ajkd.2021.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dutta SS. What are adenovirus-based vaccines? 2021. News-Medical. Available: https://www.news-medical.net/health/What-are-Adenovirus-Based-Vaccines.aspx
- 3.Connors M, Graham BS, Lane HC, et al. SARS-CoV-2 vaccines: much accomplished, much to learn. Ann Intern Med 2021;174:687–90. 10.7326/M21-0111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021;397:99–111. 10.1016/S0140-6736(20)32661-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voysey M, Costa Clemens SA, Madhi SA, et al. Single-Dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet 2021;397:881–91. 10.1016/S0140-6736(21)00432-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel C, Shah HH. Vaccine associated kidney diseases: a narrative review of the literature. Saudi J Kidney Dis Transpl 2019;30:1002–9. 10.4103/1319-2442.270254 [DOI] [PubMed] [Google Scholar]
- 7.Novati R, Nebiolo PE, Galotto C, et al. Acute renal failure after influenza vaccination: a case report. J Prev Med Hyg 2014;55:31–2. [PMC free article] [PubMed] [Google Scholar]
- 8.Sokoda T, Sawai T, Iwai M. Japanese Journal of paediatric nephrology 2007;20:55–9. [Google Scholar]
- 9.Bhandari J, Thada PK, Arif H. Tubulointerstitial Nephritis. In: StatPearls. Treasure Island (FL: StatPearls Publishing, 2021. https://www.ncbi.nlm.nih.gov/books/NBK557537/ [Google Scholar]
- 10.Neilson EG. Pathogenesis and therapy of interstitial nephritis. Kidney Int 1989;35:1257–70. 10.1038/ki.1989.118 [DOI] [PubMed] [Google Scholar]
- 11.Ten RM, Torres VE, Milliner DS, et al. Acute interstitial nephritis: immunologic and clinical aspects. Mayo Clin Proc 1988;63:921–30. 10.1016/S0025-6196(12)62697-4 [DOI] [PubMed] [Google Scholar]
- 12.González E, Gutiérrez E, Galeano C, et al. Early steroid treatment improves the recovery of renal function in patients with drug-induced acute interstitial nephritis. Kidney Int 2008;73:940–6. 10.1038/sj.ki.5002776 [DOI] [PubMed] [Google Scholar]
- 13.Clarkson MR, Giblin L, O'Connell FP, et al. Acute interstitial nephritis: clinical features and response to corticosteroid therapy. Nephrol Dial Transplant 2004;19:2778–83. 10.1093/ndt/gfh485 [DOI] [PubMed] [Google Scholar]
- 14.Praga M, González E. Acute interstitial nephritis. Kidney Int 2010;77:956–61. 10.1038/ki.2010.89 [DOI] [PubMed] [Google Scholar]
- 15.Goicoechea M, Rivera F, López-Gómez JM, et al. Increased prevalence of acute tubulointerstitial nephritis. Nephrol Dial Transplant 2013;28:112–5. 10.1093/ndt/gfs143 [DOI] [PubMed] [Google Scholar]
- 16.Leonard CE, Freeman CP, Newcomb CW, et al. Proton pump inhibitors and traditional nonsteroidal anti-inflammatory drugs and the risk of acute interstitial nephritis and acute kidney injury. Pharmacoepidemiol Drug Saf 2012;21:1155–72. 10.1002/pds.3329 [DOI] [PubMed] [Google Scholar]
- 17.Härmark L, van der Wiel HE, de Groot MCH, et al. Proton pump inhibitor-induced acute interstitial nephritis. Br J Clin Pharmacol 2007;64:070717090808006–???. 10.1111/j.1365-2125.2007.02927.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jelaković B, Karanović S, Vuković-Lela I, et al. Aristolactam-DNA adducts are a biomarker of environmental exposure to aristolochic acid. Kidney Int 2012;81:559–67. 10.1038/ki.2011.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet 2012;379:721–8. 10.1016/S0140-6736(11)61516-X [DOI] [PubMed] [Google Scholar]
- 20.Bassilios N, Martel P, Godard V, et al. Monitoring of glomerular filtration rate in lithium-treated outpatients--an ambulatory laboratory database surveillance. Nephrol Dial Transplant. In Press 2008;23:562–5. 10.1093/ndt/gfm567 [DOI] [PubMed] [Google Scholar]
- 21.Bendz H, Schön S, Attman P-O, et al. Renal failure occurs in chronic lithium treatment but is uncommon. Kidney Int 2010;77:219–24. 10.1038/ki.2009.433 [DOI] [PubMed] [Google Scholar]
- 22.Grünfeld J-P, Rossier BC. Lithium nephrotoxicity revisited. Nat Rev Nephrol 2009;5:270–6. 10.1038/nrneph.2009.43 [DOI] [PubMed] [Google Scholar]
- 23.Ball ST, Lapsley M, Norden AGW, et al. Urinary retinol binding protein in Indo-Asian patients with idiopathic interstitial nephritis. QJM 2003;96:363–7. 10.1093/qjmed/hcg052 [DOI] [PubMed] [Google Scholar]
- 24.Klaus R, Niyazi M, Lange-Sperandio B. Radiation-Induced kidney toxicity: molecular and cellular pathogenesis. Radiat Oncol 2021;16:43. 10.1186/s13014-021-01764-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan JC. Environmental injury to the kidney: interstitial nephritis. Hong Kong Journal of Nephrology 2014;16:23–8. 10.1016/j.hkjn.2014.09.001 [DOI] [Google Scholar]
- 26.Kirchmair M, Carrilho P, Pfab R, et al. Amanita poisonings resulting in acute, reversible renal failure: new cases, new toxic Amanita mushrooms. Nephrol Dial Transplant 2012;27:1380–6. 10.1093/ndt/gfr511 [DOI] [PubMed] [Google Scholar]
- 27.Cohen LJ, Rennke HG, Laubach JP, et al. The spectrum of kidney involvement in lymphoma: a case report and review of the literature. Am J Kidney Dis 2010;56:1191–6. 10.1053/j.ajkd.2010.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Long Y, Aljamal AA, Bahmad HF, et al. Multiple myeloma presenting as acute tubulointerstitial nephritis. Autops Case Rep 2021;11:e2021328. 10.4322/acr.2021.328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maripuri S, Grande JP, Osborn TG, et al. Renal involvement in primary Sjögren's syndrome: a clinicopathologic study. Clin J Am Soc Nephrol 2009;4:1423–31. 10.2215/CJN.00980209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Göbel U, Kettritz R, Schneider W, et al. The protean face of renal sarcoidosis. J Am Soc Nephrol 2001;12:616–23. 10.1681/ASN.V123616 [DOI] [PubMed] [Google Scholar]
- 31.Raissian Y, Nasr SH, Larsen CP, et al. Diagnosis of IgG4-related tubulointerstitial nephritis. J Am Soc Nephrol 2011;22:1343–52. 10.1681/ASN.2011010062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joyce E, Glasner P, Ranganathan S, et al. Tubulointerstitial nephritis: diagnosis, treatment, and monitoring. Pediatr Nephrol 2017;32:577–87. 10.1007/s00467-016-3394-5 [DOI] [PMC free article] [PubMed] [Google Scholar]


