Abstract
Introduction
Universal availability and affordability of essential medicines are determined by effective design and implementation of relevant policies, typically involving multiple stakeholders. This paper examined stakeholder engagements, powers and resultant influences over design and implementation of four medicines pricing policies in Ghana: Health Commodity Supply Chain Master Plan, framework contracting for high demand medicines, Value Added Tax (VAT) exemptions for selected essential medicines, and ring-fencing medicines for local manufacturing.
Methods
Data were collected using reviews of policy documentation (n=16), consultative meetings with key policy actors (n=5) and in-depth interviews (n=29) with purposefully identified national-level policymakers, public and private health professionals including members of the National Medicine Pricing Committee, pharmaceutical wholesalers and importers. Data were analysed using thematic framework.
Results
A total of 46 stakeholders were identified, including representatives from the Ministry of Health, other government agencies, development partners, pharmaceutical industry and professional bodies. The Ministry of Health coordinated policy processes, utilising its bureaucratic mandate and exerted high influences over each policy. Most stakeholders were highly engaged in policy processes. Whereas some led or coproduced the policies in the design stage and participated in policy implementation, others were consulted for their inputs, views and opinions. Stakeholder powers reflected their expertise, bureaucratic mandates and through participation in national level consultation meetings, influences policy contents and implementation. A wider range of stakeholders were involved in the VAT exemption policies, reflecting their multisectoral nature. A minority of stakeholders, such as service providers were not engaged despite their interest in medicines pricing, and consequently did not influence policies.
Conclusions
Stakeholder powers were central to their engagements in, and resultant influences over medicine pricing policy processes. Effective leadership is important for inclusive and participatory policymaking, and one should be cognisant of the nature of policy issues and approaches to policy design and implementation.
Keywords: Health policy, Health systems
WHAT IS ALREADY KNOWN ON THIS TOPIC.
Design and implementation of any health policies involves multiple stakeholders with varied powers, roles, interests and agendas, though little is known about stakeholder roles and engagement in medicines pricing policies in low-income and middle-income countries.
WHAT THIS STUDY ADDS
In Ghana, stakeholder engagements across four national medicine pricing policies were generally high-to-medium with strong leadership from the Ministry of Health but limited engagements from the private sector and local health workers.
Stakeholder power was central to their engagements and resultant influences over policy design and implementation. All these were also affected by the nature of policy issue and approaches to policymaking.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE AND/OR POLICY
Medicines pricing policies can be improved by: identifying key stakeholders and considering their experiences, perspectives and expectations; greater understanding of the nature of a policy issue and approaches to policy design and implementation and developing feasible and context-specific ways of enhancing stakeholder engagements within complex policy processes.
Introduction
Improving availability and affordability of essential medicines is critical to achieving better health outcomes,1 2 and requires effective design and implementation of medicines pricing policies.3–5 Policymaking typically involves multiple stakeholders with varying roles, agendas and interests.6 7 Stakeholders are ‘…actors (individuals or organizations) who have interest in the issue under consideration, who are affected by the issue, or who—because of their position—have or could have an active or passive influence on the decision-making and implementation processes’.8 Stakeholder engagements in policymaking reflect their interests, and together with their exercise of power, determine their influences over policy decisions.6 8–11 Understanding these can reveal how policies are framed, whether and how policy implementation occurs and how all these can be improved. For example, understanding who led, who contributed and who were excluded from the policy design, can reveal how policy agenda for medicines pricing was determined and what evidence may have informed policy decisions; and the rationale for regulating (or not) medicines prices. This knowledge can inform effective interventions in regulating and reducing pricing of medicines, contributing to improved access to medicines.
Guidance is available on conducting stakeholder analyses8 10 and studies examined stakeholder roles in health insurance systems,12 health research priority-setting13 and provider payment systems.14 However, we found no publications from low-income and middle-income countries (LMICs) which examined stakeholder roles in design and implementation of medicines pricing policies. This paper contributes to bridging this knowledge gap by reporting our stakeholder analysis from four medicines pricing policies from Ghana. We answer the following question: what were the roles, engagements and powers of different stakeholders, and how did these translate into stakeholder influences over the design and implementation of four medicine pricing policies in Ghana? In doing so, we also enhance the understanding of processes of designing and implementing medicines pricing policies, although our primary focus remains on the stakeholder analysis. We anticipate this paper to be of interest and relevance to policymakers, practitioners and academics, who are interested and engaged in, understanding stakeholder roles and influences for improving medicines pricing policies and access to essential medicines, and ensuring effective design and implementation of health policy processes more generally.
Four medicines pricing policies in Ghana
The Ghanaian health sector has a predominately, publicly administered and delivered services model, although with growing private sector participation in service provision. In addition to policy design and formulation, the Ministry of Health (MoH) has the mandate to coordinate activities within the health sector and authority over health policy processes. In doing so, the MoH works with its implementing agencies such as Ghana Health Service and National Health Insurance Authority (NHIA), as well as other government and non-government actors.15
Ghana’s pharmaceutical industry is the second largest in West Africa and total market value was estimated at $586 million in 2019.16 The pharmaceutical industry is the key component of the health sector, and its performance is driven by the health sector demand for medicines. The main pharmaceutical policy priorities are to ensure access to affordable, efficacious and safe medicines for everyone, strengthen local pharmaceutical industry and improve supply chain.17 Yet, medicine prices in Ghana are considered to be high, and several interventions were introduced to reduce price build-ups along the supply chain and improve access to medicines.17
Reducing medicines prices is high on government’s political agenda.17 Between 2012 and 2017, four medicine pricing policies were introduced, targeting the supply chain and incentives to medicines importers and local manufactures to improve access and contribute to improved health outcomes of Ghanaians. These policies include: (i) Health Commodity Supply Chain Master Plan (HCSCMP);18 (ii) framework contracting for medicines in high demand;19 20 (iii) Value Added Tax (VAT) exemptions for selected essential medicines21 22 and (iv) ring-fencing medicines for local manufacturing.21 Table 1 summarises key features of each policy.
Table 1.
Summary of medicines pricing policies introduced between 2012 and 2017
| Policy/year | Aim | Type of medicines | Pharmaceutical sector affected/beneficiary |
| HCSCMP (2012) | To address numerous challenges in the supply chain, for example, overlapping tasks, high costs and payment delays in procurement. | Essential medicines | Supply chain/public sector |
| Framework Contracting (2012) | To outline a centralised procurement process for bulk purchase and negotiation of medicine prices. | High demand essential medicines | Supply chain/public sector |
| VAT exemption for medicines importers (2017) | To remove build up costs due to taxes | 392 selected essential medicines (imported finished products) | Importation/cost build-up due to taxes. Importers agreed to reduce prices of essential medicines by a minimum of 30%. Public and private sector |
| VAT exemption for active pharmaceutical inputs (API), manufacturing inputs and packaging materials (reviewed in 2012 and 2017) | To remove the build-up of costs due to taxes, and ring-fenced some selected essential medicines for local manufacturing | 552 (active ingredients, and selected inputs) for essential medicines | Local manufacturing/cost build-up due to taxes Private |
HCSCMP, Health Commodity Supply Chain Master Plan; VAT, Value Added Tax.
The HCSCMP sought to streamline procurement systems of essential medicines within the public sector, and was subsequently operationalised into the framework contracting, a strategy of HCSCMP which sought to address fragmented medicines procurement contracts.18 19 The two VAT exemption policies provided incentives to medicines importers and local manufacturers, respectively.
Methods
We report results from a wider study which examined implementation of four medicine pricing policies in Ghana. Examining stakeholder roles, engagement and influences in the design and implementation of these policies was a critical component of the analysis and is the focus of this article.
Conceptual framework
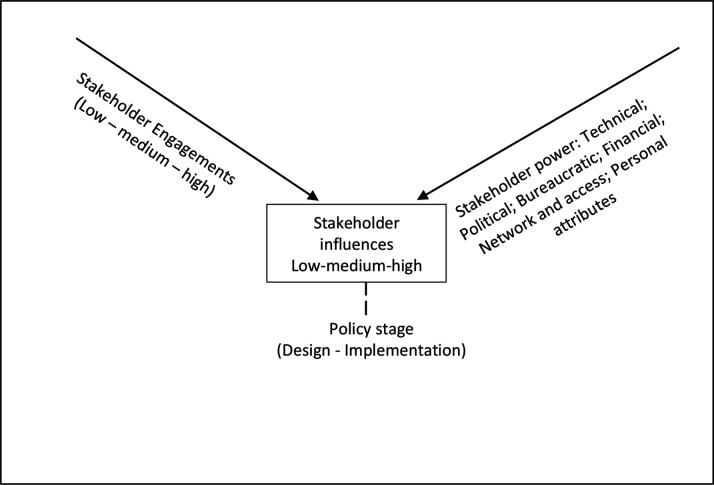
Our understanding of stakeholder engagements, powers and resultant influences over policy processes (figure 1) drew on the available conceptualisations of stakeholder powers, engagements and influences over health policy processes.23–27 Each stakeholder that is, actor with interest in a policy issue, has own sources of power which they can exercise to influence policy decisions as they engage in policy design and/or implementation.
Figure 1.
Theoretical framework (drawing upon).23–26
The literature highlights six sources of stakeholder power: (a) technical that is, derived from knowledge, skills and information, (b) political that is, derived from political authority, (c) bureaucratic that is, derived from knowledge and authority of bureaucracies where policies are designed and implemented, (d) financial that is, derived from accessibility to financial resources, (e) network that is, derived from actors’ collective knowledge and action and (f) personal attributes that is, charismatic authority.24 Fundamentally, all stakeholders possess one or more sources of power, which they must exercise to be able to influence policy processes.25
Stakeholder engagements can be across the low–medium–high spectrum. Low degrees of engagement are when policy actors are mere recipients of information and are not actively involved in policy decisions. Medium degrees refer to cases in which stakeholders are consulted to express their knowledge, views and opinions. High degrees is when stakeholders had actively participated, led or coproduced the policy design or implementation.23 Stakeholder engagements arguably reflect their levels of interest, and willingness to participate, in the policy processes. Degrees of stakeholder engagement can also reflect spaces that stakeholders occupy at the policy table, such as provided (eg, policymakers), invited (eg, knowledge experts) and claimed (eg, advocacy coalitions).7 26 Thus, stakeholder powers, combined with engagements, inform stakeholder influences over policy processes. As with degrees of engagement, stakeholder influences can be along the low–medium–high spectrum,10 adapted to contexts of specific countries and policies.
Three points are worth noting on stakeholder influences. First, although more powerful stakeholders are more likely to exert greater influences over policies, these are subject to their engagements, which reflect their initial interests and willingness to participate in policymaking. For example, powerful stakeholders with expertise in the subject matter may be disinterested in a policy issue due to competing interest, resulting in their low engagement and consequently low influences. Second, stakeholder engagements require some power to exert influences over policy decisions. For example, knowledge experts with high interests and degrees of engagement can provide critical inputs when consulted in technical working grouping (TWG) forum to express their professional perspectives and thus are able to exert high influences over policy design and implementation decisions, perhaps similar to those who actively lead and implement those policies. Additionally, stakeholders with expertise and good understanding of the policy issue and are able to navigate the decision-making processes during TWGs and review meetings create opportunities to express their views and convince others to support their views to influence decisions.28 Conversely, some stakeholders views can be overlooked and ignored, despite their high interests and proactive engagements in policy processes. Furthermore, during policy design and implementation processes stakeholders who are not invited or do not claim space to participate in review meetings can miss the opportunity to express their views and consequently influence decisions. Third, sources of power, degrees of engagement and the resultant policy influences, can differ across the policy design and implementation. For example, frontline health workers may not be engaged in the policy design, but their actions essentially determine whether and how policies are implemented.29
Data collection
We collected data using document reviews, in-depth interviews and consultative meetings with key policy actors.
We started by mapping policy actors and their roles from policy documentation, broadly defined as actual policies, regulatory documents and reports related to the design and implementation of the four policies (n=16). Our main inclusion criterion was relevance to the four policies, so documents covering other medicine pricing policies were excluded. Examples of specific documents included the Ghana supply chain assessment (2020), National Medicines Policy (2017), meeting minutes of the VAT exemption TWG (January 2018) and reports of VAT exemption implementation committee (February 2018). The documents were sourced from the MoH website (https://www.moh.gov.gh), Pharmacy Directorate and google scholar.
A total of 29 in-depth interviews (IDIs) were then conducted during August 2020–February 2021 (see table 2) by AK and LB. The respondents were purposefully identified from the documents and using snowballing from the IDIs and consultative meetings. Stakeholders who participated in policy decisions during policy design and implementation were considered. The IDIs sought to understand views and experiences of key policy actors in the development and implementation of four policies. The IDIs were conducted with national level policymakers, public and private health professionals, pharmaceutical wholesalers and importers and members of the National Medicines Pricing Committee (NMPC). The NMPC was inaugurated in 2019 to manage the medicine pricing system and comprises 22 members from the public and private sectors with direct or indirect interests in medicine pricing policies.
Table 2.
List of respondents
| Sector | Agency/institution | Number of respondents |
| Government agencies | Ghana Revenue Authority | 1 |
| Ministry of Health | 5 | |
| Ministry of Finance | 1 | |
| National Health Insurance Authority (NHIA) | 1 | |
| Ghana Health Service Regional Health Directorate (GHS-RHD) | 1 | |
| Ghana Health Service Regional Medical Store (GHS-RMS) | 1 | |
| Ghana Health Service Headquarters (GHS-HQ) | 2 | |
| Service providers | Teaching Hospital | 1 |
| Regional Health Facility | 1 | |
| Public Hospital | 3 | |
| Public Polyclinic | 1 | |
| Private Hospital | 1 | |
| Christian Health Association of Ghana72 | 1 | |
| Development partner | WHO33 | 1 |
| Professional association | Pharmaceutical Society of Ghana (PSGH) | 1 |
| Society of Private Medical and Dental Practitioners | 2 | |
| Pharmaceutical industry | Pharmaceutical Manufacturers Association of Ghana | 2 |
| Community Pharmacy Practice Association (CPPA) | 1 | |
| Pharmaceutical Wholesaler/Importer/Retailer | 1 | |
| NGO | Coalition of Non-Governmental Organisations in Health | 1 |
NGO, Non-Governmental Organisations.
The IDIs utilised a question guide (online supplemental material 1), semi-structured by: framing of medicine pricing, roles of policy actors (expected and actual); implementation processes, approaches and timelines (anticipated and actual). The IDIs were conducted via telephone, zoom or in person as feasible and each was preceded by obtaining verbal or written informed consent. All IDIs were in English, lasted on average 45 min, were digitally recorded, transcribed verbatim and anonymised for analysis.
bmjgh-2021-008225supp001.pdf (31KB, pdf)
Five consultative meetings were held between October 2020 and February 2021, primarily to validate emerging findings but also to collect further data. These meetings involved purposefully identified NMCP members (October 2020, April 2021), pharmaceutical sector stakeholders (December 2020), medicine price mark-up working group (December 2020) and Society of Private Medical and Dental Practitioners (February 2021). The consultative meetings were an integral part of the study and were organised in collaboration with the MoH Pharmacy Directorate. The MoH invited attendees and the meeting averagely lasted 4 hours. During meetings, the MoH representatives and study members led discussions on the four policies and the meetings proceedings documented. To address potential recall bias, data sourced from interviews, document reviews and consultative meetings were triangulated.
Data analysis
Data were analysed using thematic approach. The themes were organised according to the three dimensions from our conceptual framework (powers, engagements and influences) disaggregated by policy design and implementation. All authors participated in data analysis and interpretation after initial analysis by AK and TM. Emerging results were collated in tabular format for discussion and further analysis. The interview transcripts were the primary data sources for analysis, and insights from the documents and researcher notes from consultative meetings were used mainly to triangulate results from analysis of IDIs.
Our approach to determining low–medium–high degrees of stakeholder engagements and levels of influences has been informed by our analysis of data from the documents, IDIs and consultative meetings. In reporting our results and analysis, we primarily focused on stakeholders as organisations rather than individuals. This approach was driven by the emerging findings from the data, perhaps also reflecting the fluid and dynamic nature of policy processes (eg, with methodological questions of counting different individuals who were replacing their organisational colleagues in policy meetings, and those who were involved on one-off basis).
The study received approvals from ethics committees of the Ghana Health Service (GHS-ERC006/02/20) and the University of Leeds School of Medicine (MREC 19-060).
Results
Individuals from 46 organisations, were identified from the documents, IDIs and consultative meetings, as key stakeholders with respect to the four policies (table 3).
Table 3.
Stakeholder organisation
| Sector | Stakeholder organisation | Abbreviation |
| Government agencies | Ministry of Health | MOH |
| Minister for Health | Minister-MOH | |
| MOH-Procurement and Supplies Directorate | MOH-PS | |
| MOH-Pharmacy Department | MOH-PD | |
| MOH-Office of Chief Pharmacist | MOH-OCP | |
| MOH Ghana National Drugs P/rogramme | MOH-GNDP | |
| MOH-National Drug Information Resources Centre | MOH-NDIRC | |
| MOH-Central Medical Stores | MOH-CMS | |
| Ghana Health Service | GHS | |
| GHS-Regional Health Administrations | GHS-RHA | |
| GHS-Stores, Supplies and Drug Management | GHS-SSDM | |
| GHS-Regional Chief Pharmacist | GHS-RCP | |
| GHS-Regional Medical Stores | GHS-RMS | |
| GHS-Institutional Care Division | GHS-ICD | |
| GHS-Expanded Programme on Immunisation | GHS-EPI | |
| GHS-National Malaria Control Programme | GHS-NMCP | |
| GHS-National TB Control Programme | GHS-NTCP | |
| GHS-National AIDS Control Programme | GHS-NACP | |
| National Health Insurance Authority | NHIA | |
| NHIA-Provider Payment Directorate | NHIA-PPD | |
| Nurses and Midwives Council | NMC | |
| Pharmacy Council | PC | |
| Food and Drugs Authority | FDA | |
| Ministry of Finance | MOF | |
| Ghana Revenue Authority | GRA | |
| Ministry of Trade and Industry | MOTI | |
| Attorney General Department | AGD | |
| Public Procurement Authority | PPA | |
| Service providers | Public Service Providers | |
| GHS Health Facilities | GHS-HF | |
| Teaching Hospital Pharmacy Department | TH-PD | |
| Private Service Providers | ||
| Society of Private Medical and Dental Practitioners | SPMDP | |
| Christian Health Association of Ghana | CHAG | |
| Community Pharmacy Practice Association | CPPA | |
| Development Partners | WHO33 | WHO |
| USAID|DELIVER project | DELIVER | |
| USAID|Global Health Supply Chain-Procurement and Supply Management | GHSC-PSM | |
| United Nations Industrial Development Organisation | UNIDO | |
| Professional Associations | Pharmaceutical Society of Ghana | PSGH |
| Ghana Medical Association | GMA | |
| Pharmaceutical industry | Pharmaceutical Importers and Wholesalers Association | PIWA |
| Association of Representatives of Ethical Pharmaceutical Institutions | AREPI | |
| Ghana National Chamber of Pharmacy | GNCOP | |
| Pharmaceutical Manufacturers Association of Ghana | PMAG | |
| Pharmaceutical Suppliers | PS | |
| NGOs | Private Health Sector Alliance | PHSA |
| Politicians | Parliament Select Committee on Health | PSCoH |
| Parliamentarians | Parliament | |
| Patient groups | Cancer Connect Ghana | CCG |
| National Diabetes Association | NDA |
NGO, Non-Governmental Organisations.
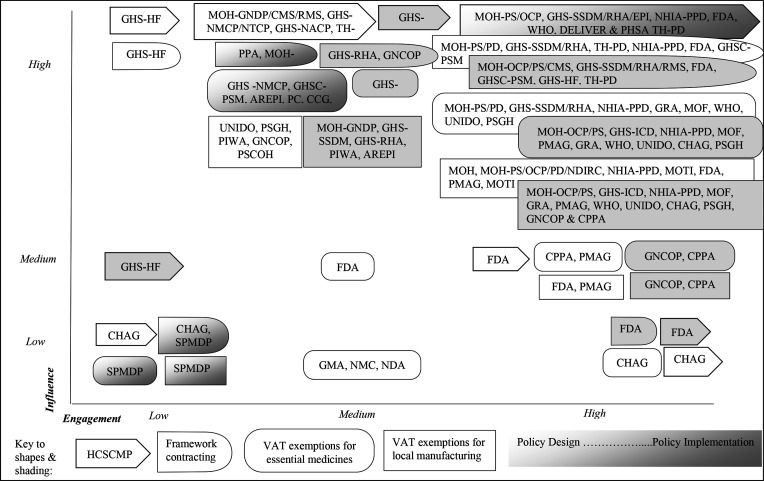
Stakeholder roles, degrees of engagement, sources of power and resultant levels of influence in the design and implementation of the four policies are summarised in figure 2, tables 4 and 5 and are discussed next.
Figure 2.
Engagements and influence by key stakeholders across the four medicines pricing policies.
Table 4.
Stakeholder roles and degrees of engagement in the design and implementation of medicine pricing policies in Ghana
| Policy stage | Policy design | ||
| Stakeholder role/degrees engagement | Low | Medium | High |
| Health commodity supply chain master plan (HCSCMP) | GHS-HF, CHAG—informed, supportive but not actively involved in the design process | MOH-GNDP, MOH-CMS, GHS-RMS, GHS-NMCP, GHS-NTCP, GHS-NACP, TH-PD—reviewed plans, participated in consultative meetings | MOH-PS—led and chaired Technical Working Group (TWG); drafted and reviewed plans and milestone MOH-PS, MOH-OCP, GHS-SSDM, GHS-RHA, GHS-EPI, NHIA-PPD, FDA, WHO, DELIVER and PHSA—drafted and reviewed plans and milestones; members of TWG DELIVER-technical advice, financial and secretarial support |
| Framework contracting for high demand medicines | GHS-HF, CHAG, SPMDP—informed but not involved in the design process | PPA—aware and supportive MOH-GNDP—reviewed medicine list |
MOH-PS—led in identifying and developing the list of pharmaceuticals to be procured through a competitive tendering process MOH-PD, GHS-SSDM, GHS-RHA, TH-PD, NHIA-PPD, FDA and GHSC-PSM—participated in identifying and developing the list of pharmaceuticals to be procured through a competitive tendering process |
| Value Added Tax (VAT) exemptions for essential medicines | SPMDP—informed but not involved in the design process | GHS -NMCP, FDA, GHSC-PSM, AREPI, GMA, PC, NMC, CCG, NDA, PSCOH—reviewed the list of medicines for exemption and participated in stakeholder forum. AGD—drafted the policy into legislative content Parliamentarians—approved the list in parliament |
MINISTER-MOH and MOH-PS—led the process and chaired the TWG MOH-PD, GHS-SSDM, GHS-RHA, NHIA-PPD, GRA, MOF, WHO, UNIDO, CHAG, PSGH, GNCOP, CPPA and PMAG—considered, agreed and recommended a list of selected imported pharmaceutical products for VAT exemption; members of the TWG MOH-NDIRC—secretarial support MOF—laid the Legislative Instrument before Parliament |
| VAT exemption for active pharmaceutical inputs (API), manufacturing inputs and packaging materials | SPMDP—informed but not involved in the design process | UNIDO, PSGH, PIWA, GNCOP, PSCOH—participated stakeholder meetings to build consensus Parliamentarians—approved the list in parliament AGD—drafted the policy into legislative content Parliamentarians—approved the list in parliament |
MINISTER-MOH, MOH-PS, MOH-OCP and MOH-PD—led the process and chaired the TWG to review and update the list of restricted medicines and APIs for local manufacturing. MOH-NDIRC—secretarial support NHIA-PPD, MOTI, FDA, PMAG—reviewed and updated the restricted list of restricted medicines and APIs for local manufacturing. MOTI—approved the recommended list and participated in stakeholder meetings |
| Policy stage | Policy implementation | ||
| Stakeholder role/degrees engagement | Low | Medium | High |
| Health commodity supply chain master plan (HCSCMP) | GHS-HF—implementing some aspect of the plans | GHS-RHA (non-ISC members), implementing aspects of the plan | MINISTER-MOH and MOH-PS—chaired the implementation steering committee (ISC) to develop and coordinate implementation modalities GHS-SSDM, GHS-RHA, MOH-CMS, GHS-RMS TH-PD, NHIA-PPD, FDA, WHO, DELIVER and PHSA-ISC members |
| Framework contracting for high demand medicines | SPMDP—informed but not implementing partner | PPA—evaluated tendering offers based on PPA guidelines, technical advice GHS-HF—submitted requests/demands to evaluation team GNCOP—participated in pre-bid conference |
MOH-PS, GHS-RHA, TH-PD, NHIA-PPD—chaired tendering evaluation team, participated in the pre-bid conference and tendering process, reviewed and finalised the draft tender document MOH-OCP, GHS-SSDM, GHS-RHA, GHS-RMS, FDA, GHSC-PSM—members of tendering evaluation team. MINISTER-MOH—reviewed, approved and signed tender documents MINISTER-MOH-PS, GHS-RHA, MOH-CMS, GHS-RMS, GHS-HF, TH-PD—entered into a framework contract with the identified/successful suppliers |
| Value Added Tax (VAT) exemptions for essential medicines | SPMDP—informed but not implementing partner | MOH-GNDP, GHS-SSDM, GHS-RHA, AREPI—participated in stakeholder meetings to agree on modalities and methods of implementation | MINISTER-MOH-Deputy Minister of Health—approved implementation modalities and methods MOH-OCP—led the implementation technical working group (TWG) and coordinated implementation MOH-PS, GHS-ICD, NHIA-PPD, MOF, PMAG, GRA, WHO, UNIDO, CHAG, PSGH, GNCOP and CPPA—members of TWG |
| VAT exemption for active pharmaceutical inputs (API), manufacturing inputs and packaging materials | SPMDP—informed but not implementing partner | MOH-GNDP, GHS-SSDM, GHS-RHA, PIWA, AREPI—participated in stakeholder meetings to agree on modalities and methods of implementation | MINISTER-MOH-Deputy Minister of Health—approved implementation modalities and methods MOH-OCP—led the implementation technical working group (TWG) and coordinated implementation PMAG—implementing the policy MOH-PS, GHS-ICD, NHIA-PPD, MOF, GRA, PMAG, WHO, UNIDO, CHAG, PSGH, GNCOP and CPPA—members of TWG |
Table 5.
Stakeholders’ power sources and influence over the design and implementation of medicine pricing policies in Ghana
| Policy/stage | HCSCMP | Framework contracting | VAT exemption for essential medicines | VAT exemption for local manufacturing | ||||||||
| Policy design | ||||||||||||
| Power/level of influence | Bureaucratic (approval) | Political | Technical | Bureaucratic (approval) | Political | Technical | Bureaucratic (approval) | Political | Technical | Bureaucratic (approval) | Political | Technical |
| Minister for Health | +++ | +++ | +++ | +++ | ||||||||
| MOH-Procurement and Supplies Directorate | +++ | +++ | +++ | +++ | +++ | +++ | ||||||
| MOH-Office of Chief Pharmacist | ++ | +++ | +++ | |||||||||
| MOH-Pharmacy Department | +++ | +++ | +++ | +++ | ||||||||
| GHS-Stores, supplies and drug management | +++ | +++ | ++ | ++ | ||||||||
| GHS-Expanded Programme on Immunisation | ++ | |||||||||||
| GHS-Institutional Care Division | ++ | ++ | ||||||||||
| NHIA-Provider Payment Directorate | +++ | +++ | +++ | +++ | ||||||||
| Food and Drugs Authority | ++ | +++ | ++ | ++ | ||||||||
| Ministry of Finance | +++ | +++ | +++ | |||||||||
| Ghana Revenue Authority | +++ | +++ | +++ | |||||||||
| Ministry of Trade and Industry | +++ | |||||||||||
| Pharmaceutical Manufacturers Association of Ghana (PMAG) | ++ | +++ | ||||||||||
| WHO | +++ | +++ | ++ | |||||||||
| United Nations Industrial Development Organisation (UNIDO) | +++ | +++ | ||||||||||
| USAID|DELIVER | +++ | |||||||||||
| USAID|Global Health Supply Chain-Procurement and Supply Management | +++ | ++ | ||||||||||
| Private Health Sector Alliance | ++ | |||||||||||
| Christian Health Association of Ghana72 | + | ++ | ++ | |||||||||
| Pharmaceutical Society of Ghana | +++ | ++ | ||||||||||
| Ghana National Chamber of Pharmacy | +++ | ++ | ||||||||||
| Community Pharmacy Practice Association (CPPA) | ++ | ++ | ||||||||||
| MOH Ghana National Drugs Programme | ++ | ++ | ++ | ++ | ||||||||
| MOH Central Medical Stores | ++ | |||||||||||
| MOH-National Drug Information Resources Centre | +++ | +++ | ||||||||||
| GHS-Regional Health Administrations | ++ | +++ | ++ | + | ||||||||
| GHS Regional Medical Stores | ++ | ++ | ||||||||||
| GHS National TB Control Programme | + | |||||||||||
| GHS National AIDS Control Programme | + | |||||||||||
| Teaching hospital Pharmacy Department | ++ | +++ | ||||||||||
| GHS National Malaria Control Programme | + | |||||||||||
| GHS Health facilities | + | ++ | ||||||||||
| Pharmaceutical Importer and Wholesalers Association (PIWA) | ++ | |||||||||||
| Association of Representatives of Ethical Pharmaceutical Institutions (AREPI) | ++ | + | ||||||||||
| Ghana Medical Association (GMA) | + | + | ||||||||||
| Nurses and Midwives Council (NMC) | + | + | ||||||||||
| Pharmacy Council | ++ | ++ | ||||||||||
| Parliament Select Committee on Health/Parliamentarians | +++ | +++ | ||||||||||
| Attorney General Department | +++ | +++ | ||||||||||
| Society of Private Medical and Dental Practitioners | ||||||||||||
| Public Procurement Authority | +++ | |||||||||||
| Cancer Connect Ghana | + | |||||||||||
| National Diabetes Association | + | |||||||||||
| Policy implementation | ||||||||||||
| Power/level of influence | Financial | Authority to implement | Authority to enforce, monitor and evaluate | Financial | Authority to implement | Authority to enforce, monitor and evaluate | Financial | Authority to implement | Authority to enforce, monitor and evaluate | Financial | Authority to implement | Authority to enforce, monitor and evaluate |
| Minister for Health | +++ | + | +++ | +++ | + | +++ | +++ | + | +++ | +++ | + | +++ |
| MOH-Procurement and Supplies Directorate | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | ++ | ||
| MOH-Office of Chief Pharmacist | ++ | ++ | ||||||||||
| MOH-Pharmacy Department | ++ | +++ | ++ | +++ | +++ | +++ | +++ | +++ | ||||
| GHS- Stores, supplies and drug management | ++ | ++ | ++ | ++ | ||||||||
| GHS-Expanded Programme on Immunisation | ++ | |||||||||||
| GHS-Institutional Care Division | ||||||||||||
| NHIA-Provider Payment Directorate | ++ | +++ | +++ | +++ | +++ | +++ | +++ | |||||
| Food and Drugs Authority | + | + | + | + | ||||||||
| Ministry of Finance | +++ | +++ | +++ | +++ | +++ | +++ | ||||||
| Ghana Revenue Authority | +++ | +++ | +++ | +++ | ||||||||
| Ministry of Trade and Industry | +++ | +++ | ||||||||||
| Pharmaceutical Manufacturers Association of Ghana (PMAG) | +++ | +++ | +++ | |||||||||
| WHO | + | ++ | +++ | |||||||||
| United Nations Industrial Development Organisation (UNIDO) | ||||||||||||
| USAID |DELIVER | ++ | ++ | +++ | |||||||||
| USAID|Global Health Supply Chain-Procurement and Supply Management | +++ | ++ | +++ | |||||||||
| Private Health Sector Alliance | + | |||||||||||
| Christian Health Association of Ghana72 | ||||||||||||
| Pharmaceutical Society of Ghana | ++ | |||||||||||
| Ghana National Chamber of Pharmacy | ++ | ++ | +++ | +++ | +++ | ++ | + | ++ | ||||
| Community Pharmacy Practice Association (CPPA) | ++ | |||||||||||
| MOH Ghana National Drugs Programme | + | + | + | |||||||||
| MOH Central Medical Stores | ++ | + | +++ | + | ||||||||
| MOH- National Drug Information Resources Centre | + | + | ||||||||||
| GHS-Regional Health Administrations | +++ | +++ | +++ | ++ | ||||||||
| GHS Regional Medical Stores | ++ | + | +++ | + | ||||||||
| GHS National TB Control Programme | ||||||||||||
| GHS National AIDS Control Programme | ||||||||||||
| Teaching hospital Pharmacy Department | +++ | + | +++ | ++ | ||||||||
| GHS National Malaria Control Programme | ||||||||||||
| GHS Health facilities | +++ | + | +++ | + | ||||||||
| Pharmaceutical Importer and Wholesalers Association (PIWA) | +++ | +++ | ||||||||||
| Association of Representatives of Ethical Pharmaceutical Institutions (AREPI) | +++ | +++ | ||||||||||
| Ghana Medical Association (GMA) | ||||||||||||
| Nurses and Midwives Council (NMC) | ||||||||||||
| Pharmacy Council | ||||||||||||
| Parliament Select Committee on Health/ Parliamentarians | ||||||||||||
| Pharmaceutical Suppliers | +++ | + | +++ | ++ | ||||||||
| Attorney General Department | ||||||||||||
| Society of Private Medical and Dental Practitioners | ||||||||||||
| Public Procurement Authority | +++ | + | ||||||||||
| Cancer Connect Ghana | ||||||||||||
| National Diabetes Association | ||||||||||||
Notes:+++, high influence; ++, medium influence; +, low influence; empty cells—no influence identified.
HCSCMP, Health Commodity Supply Chain Master Plan; VAT, Value Added Tax.
Most stakeholders had high–medium engagements (26/46, 56.5% and 27/46, 58.7%, respectively) and very few (3/46, 6.5%) had low engagements. The most highly engaged stakeholders were individuals from the MoH, other government agencies, development partners, pharmaceutical industry and professional bodies. The least engaged stakeholders were some service providers.
Two mechanisms were used by the MoH to facilitate stakeholder engagement, which contributed to inclusive and participatory policy development and implementation. First, eight TWGs were established by the MoH Procurement and Supplies, and Pharmacy directorates, for design and implementation of each policy. The TWGs met on average once a week for 2 months and collectively drafted, reviewed and coproduced plans and milestones, and implementation strategies. Some stakeholders were part of both the design and the implementation TWGs, while others were involved in one only. Stakeholder involvement in the TWGs reflected their technical expertise, roles, political, bureaucratic and financial mandates and interests. Second, the MoH conducted extensive stakeholder consultations. These involved meetings with a range of stakeholders to share knowledge, views, opinions and … to finalise the policies and how to ensure that the policies are implemented as intended (Governmental Officer). These meetings lasted typically 0.5–1 day, with the TWG members presenting plans for review and discussion.
Next, we report powers, engagements and resultant influences by five main stakeholder groups which emerged from our analysis.
Health sector agencies
Two MoH directorates featured prominently across all four policies: the Procurement and Supplies, and the Pharmacy. They formed, and actively engaged in, all TWGs, reflecting their technical expertise, high interest, mandates and high influence over policy design and implementation, which ultimately contributed to inclusive and participatory policy development and implementation:
National medicine pricing policies are initiated by government through the MoH, and we have all come to accept that. (Professional association)
The Procurement and Supply directorate led the design and implementation of the pooled procurement, tendering and negotiations of medicine pricing in HCSCMP and framework contracting, whereas the Pharmacy directorate led the design and implementation of the VAT exemption for selected essential medicines and local manufacturing. However, the Pharmacy directorate exerted medium influence over the implementation of the HCSCMP and the Framework contracting, since implementation of pooled procurement policies was outside their remit.
The GHS had representatives in all TWGs except in the design of VAT exemptions for local manufacturing. This reflected their mandate, for example that supporting manufactures and providing technical expertise was not a GHS’s core mandate. The following GHS representatives were highly engaged and contributed expertise in supply chain management in the TWGs for the HCSCMP and framework contracting policy processes: Supplies, Stores and Drug Management (SSDM) directorate (GHS headquarters) and the Regional Health Administrations (RHAs) for Eastern, Central and Western regions. The RHAs controlled implementation structures at peripheral level and consequently had high influence over implementation of these policies. This allowed for local ownership of policy implementation and therefore contributed to implementation feasibility within local context. However, the SSDM at the GHS headquarters with oversight role on implementation had medium influence over actual policy implementation.
Meanwhile, the Institutional Care division (GHS headquarters) was highly engaged only in the implementation TWGs for VAT exemptions for essential medicines and local manufacturing, but with no reported influence on policy implementation. On the other hand, selected GHS health facilities who were implementing framework contracting, engaged with the TWG on their region-specific requests during the pre-bidding conference, and as implementers wielded high influences over policy implementation by ensuring that implementation is grounded within realities of their local organisational and cultural contexts.
Document review also highlighted that the representatives from the GHS programmes for TB, Malaria and AIDS and the Regional Medical Stores were engaged in a consultative meeting to review the HCSCMP implementation but exerted low influences due to their disease-specific focus and medical stores activities.
As far as I know, all major players were involved. The GHS a government agency was involved, and also other provider groups including CHAG, pharmaceutical supplies, and community pharmacies. (Industry association)
The NHIA with interest in access to affordable essential medicines was highly engaged in TWGs across all four policies. Its provider payment directorate drew on their experiences in negotiating medicine prices and exerted high influences over the implementation of framework contracting and VAT exemptions policies, for example through chairing tenders for framework contracting creating the opportunity to share experiences and expertise and through this making the process more rigorous. Similarly, a representative from the Korle-Bu Teaching Hospital’s Pharmacy Department with high interest in affordable medicines and expertise in tendering, chaired one of tendering evaluation processes for framework contracting creating the opportunity to collate and synthesise other views and perspectives and also contributing to policy implementation inclusiveness. Two other teaching hospitals (Cape Coast and Tamale) were also highly engaged in the TWGs for framework contracting and contributed experiences in bulk purchasing and price negotiations.
A representative from the Central Medical Store (CMS) with technical expertise and authority to implement had medium influence over implementation of the HCSCMP across the peripherals. On the other hand, an individual from the National Drug Information Resource Centre used technical skills to provide secretariat support for the VAT exemptions TWGs and had control over the document wording exerting high influence in the design and implementation of the VAT exemptions.
A broader range of stakeholders were interested and engaged in the consultative meetings on the VAT exemptions policies, than the HCSCMP and framework contracting, reflecting multisectoral nature of the VAT exemptions. While that meant more inclusive policy processes with more views and perspectives been considered that also meant greater number of consultations to harness the diverse views and perspectives. These stakeholders included two MoH regulatory agencies (Pharmacy Council and Nurses and Midwife Council); Ghana Medical Association, which represents physicians, surgeons and dentists; and two patient groups (Cancer Connect Ghana and National Diabetes Association). Although consulted based on their technical expertise, interviews showed that these stakeholders had low influences on the implementation of the VAT exemption policies, perhaps reflecting their limited political and bureaucratic powers.
Government agencies
The MoH closely engaged other interested government ministries in all policies, through inviting them to the TWGs and stakeholder consultation meetings. This reflected the MoH’s desire for participatory policymaking, and enabled leveraging bureaucratic, political and technical powers of government agencies to ensure inclusion of multiple stakeholder views and perspectives during policy development and implementation.
For consensus building and common understanding, the MoH organised meetings bringing all stakeholders on board including representatives from other ministries. (Health policymaker)
The high-level government stakeholders with political powers highly influenced policy design, but not the implementation. The Parliamentary Select Committee on Health, using its legislative mandate, reviewed and approved the VAT exempted medicines list and the Parliament of Ghana with legislative power passed the Law on VAT exemptions.30 While both exerted high influences over policy design, neither influenced policy implementation reflecting their disinterest in policy implementation. Similarly, the Attorney General Department (AGD), a legislative drafting session of the Office of the Attorney General and Ministry of Justice, provided legal advice in drafting the VAT exemptions policies into a legislative Bill for Parliament to approve. The resulting regulation30 is the law that guides the implementation of VAT exemptions for medicines. While the AGD highly influenced the design of VAT policies, they did not influence policy implementation. Similarly, the Food and Drugs Authority (FDA) was highly engaged as TWGs member for the HCSCMP and framework contracting but had reportedly low influence over their implementations. This is because their TWG members provided technical advice on medicines quality, registration status and local manufacturing capacity, but were not involved in policy implementation.
We found examples of government agencies influencing both policy design and implementation. The Ministry of Finance (MoF) and the Ghana Revenue Agency (GRA) were highly engaged as TWG members and advised on the design of 30% reduction in medicines prices, cost build-up for wholesalers and manufacturers and establishing average period for VAT exemption approval.22 During implementation, the MoF and GRA who were involved in the design of implementation modalities were processing applications for VAT exemptions for imported essential medicines and restricted medicines for local manufacturing, and thus were enforcing, financing, monitoring and evaluating VAT exemptions policies. This allowed for consistent key stakeholder ownership across the policy design and implementation and consequently contributing to feasibility of policy activities within national context.
Our results also reveal two examples of government agencies influencing policy implementation only. First, the Ministry of Trade and Industry (MOTI), which also promotes private sector development, was in TWG for the VAT exemptions for local manufacturing and highly influenced policy implementation because of their political and bureaucratic powers. The MOTI advocated for a list of restricted medicine that would protect the interest of the patients, wholesalers, importers and local manufacturers, thus contributing to more equitable policies that is, consideration of benefits from these policies to wide range of stakeholders. Second, the Public Procurement Authority (PPA), a state organisation with a mandate to ensure efficient and transparent public procurement, was highly engaged in framework contracting and reviewed the tender offers against their guidelines.31 Consequently, it wielded high influence over the implementation of the framework contracting policy contributing to a transparent and accountable implementation modalities.
Development partners
The interviews and TWG meeting minutes showed that roles of three developmental partners were spread across the policies, reflected their technical expertise and interests.
You know the donors have certain areas that they focus on. For example, the USAID projects were interested in procurement related policies. (Industry Association)
The USAID-funded projects supported procurement, logistics and supply chain management.32 For example, representatives from the DELIVER project and Global Health Supply Chain-Procurement and Supply Management (GHSC-PSM) were highly engaged as TWG members for HCSCMP and framework contracting, respectively. They provided technical advice, financial and secretarial support and as such highly influenced feasibility and technical rigour of policy design and implementation coproducing policy content. On the other hand, the GHSC-PSM also reviewed implementation modalities for VAT exemptions for essential medicines but had no reported influence over their implementation.
The UNIDO engaged in the design and implementation of the VAT exemptions for essential medicines and was a TWG member for the design of VAT exemptions for local manufacturing, providing economic and industrial advice. Through their expertise and knowledge, the UNIDO representative participated in the coproduction of the policy content wielding high influence over the technical rigour of the policy design but had no influence over policy implementation.
The representative from WHO33 provided technical advice for HCSCMP and both VAT exemptions policies (implementation only) wielding high influence over evidence-informed nature of policy design by sharing guides on access to medicines and other countries’ examples, but with low-to-medium influence over policy implementations. The WHO also provided general support to health policymaking, for example the design of the National Medicine Policy, the National Standard Treatment Guidelines and Essential Medicines Lists.34–36
Industry and professional associations
These were highly engaged by the MoH in the design and implementation of all policies.
The MoH involved the pharmaceutical sector players for their buy in so that as they participate in the drafting of the policies, they will accept its implementation in good faith and ensure reduction in medicine prices. (Governmental Officer)
However, professional associations had varying influences over policies, with most notable influences exerted over two VAT exemption policies. Their involvement reflected level of interest and differing roles in policy design and implementation, as we explain next.
The representatives from Pharmaceutical Society of Ghana (PSGH), Ghana National Chamber of Pharmacy, Community Pharmacy Practice Association (CPPA) and Pharmaceutical Manufacturers Association of Ghana (PMAG) with high interest contributed professional expertise as TWG members for the design and implementation of the VAT exemptions. The PSGH exerted medium influence over implementation of the VAT exemptions for local manufacturing, as a body with monitoring role over the pharmacy practices including manufacturing of pharmaceuticals. The CPPA, representing the community pharmacies, exerted high influence over the technical rigour and feasibility of design and implementation of the VAT exemptions for essential medicines, drawing on their experience in designing and implementing mark-up regimes. However, they had no identified influence over implementation of the VAT exemptions for local manufacturing.
The Association of Representatives of Ethical Pharmaceutical Institutions (AREPI) and Pharmaceutical Importers and Wholesalers Association (PIWA), as policy implementers representing wholesalers and importers, wielded medium influences over policy contents as they reviewed the VAT exemptions for selected essential medicines and local manufacturing. The Ghana Chamber of Pharmacy, however, reviewed only plans for VAT exemption for local manufacturing and had low influence over policy implementation reflecting their different focus. Although framework contracting was implemented through the public health facilities,18 the pharmaceutical suppliers and Ghana National Chamber of Pharmacy were engaged in, and influenced, policy implementation.
Service providers
These stakeholders, even with technical skills, interest and technical expertise, were not specifically invited, engaged and did not claim space to influence the four policies. Although we do not have data that directly explain this, it is likely to be due to their limited bureaucratic and political powers in relation to national-level medicines pricing. The Society of Private Medical and Dental Practitioners (SPMDP) was not actively involved in any policies, neither as a group nor as individuals. Although the policies were designed to reduce medicines prices and improve access within public and private sectors, the SPMDP did not contribute their expertise and experiences.
The private health facilities were not involved and has not benefited from implementation of these medicine pricing policies. (Professional Association)
Representatives of some GHS health facilities who were interviewed were unaware of the implementation modalities of the four policies. As noted by one respondent:
I work in the public sector and in a district hospital, but I was not involved in any other discussions or stakeholder engagements. (Service Provider).
CHAG, which represents 344 faith-based facilities,37 were not involved in the design of the HCSCMP and framework contracting. A CHAG member implemented a pooled procurement programme in 2012,38 but their expertise was not considered. The CHAG representatives were highly engaged in the VAT exemptions policies, but with no visible influences over policy implementation.
Discussion
We reported analysis of stakeholder roles, engagements, powers and resultant influences over design and implementation of four medicines pricing policies in Ghana. In doing so, we also highlighted the critical role of stakeholder analysis in understanding, informing and improving health policy processes.8–11
Five out of six sources of power24 featured in our results: technical (expertise of meeting contributors and chairs), political (legislative authority to approve policy), bureaucratic (authority to formulate, implement, enforce, monitor and evaluate policies), financial (access to/control over budgets) and network (collective power within TWGs). Personal characteristics did not feature explicitly, suggesting that stakeholders were primarily understood as organisations and not as individuals, contrasting the literature on individual charismatic policy champions.39–43 Our results also reveal closed and invited policy spaces and visible powers, with no claimed spaces or hidden or invisible powers.7 26 This may reflect a strong MoH coordination role but may also reinforce methodological challenges of identifying these phenomena. Our results also reinforce the importance of identifying specific manifestations of power,24–26 such as authority to formulate or implement within bureaucratic power.
All four policies had high-to-medium stakeholder engagements in their design and implementation. The high engagements by non-health stakeholders in the VAT exemption policies highlight the importance of multisectoral approaches to medicines pricing18 19 22 and broader health policymaking.10 12 By the virtue of their mandate, influence and interests, policymakers, pharmaceutical industry and developmental partners played prominent roles. This is consistent with earlier-reported similar roles of policymakers,44 pharmaceutical manufactures,45 wholesalers and retailers,46 47 private sector and other state actors.48–52
Stakeholder engagements, combined with the exercise of power, determine their influences over policy processes,10 24 such as inclusive, participatory and evidence-informed policy development or feasible implementation grounded within local contexts. However, a mere existence of power alone is not sufficient and requires adequate engagements to influence policy decisions, illustrated by powerful government officials and development partners only engaging in some policies reflecting their mandates and interests. Similarly, high degree of engagements by less powerful stakeholders may not result in policy influence, as exemplified by the high engagements of the GHS-SSDM in both HCSCMP and the VAT exemptions policies but with different influences over each policy.
Two MoH directorates led all four policies by establishing TWGs and facilitated stakeholder consultations. Such an approach can facilitate opening up a ‘decision space’53 and ensure inclusive, transparent and evidence-informed policymaking. Our findings echo the Thailand’s experience where Ministry of Public Health as ‘progressive bureaucrats’ also engaged others in the UHC reforms.54 However, policymakers require adequate capacity to ensure availability of policy spaces and avoid omissions of stakeholders with relevant expertise,26 55 56 as was the case with the exclusion of CHAG from HCSCMP and framework contracting policies in our study. Prominent roles of development partners within the Ghanaian health sector57 mirror other LMICs,58 and also reinforce the importance of strong leadership by the national policymakers.55 57 59 60
Limited awareness of the four policies by the grassroots-level health service providers in Ghana is worth noting. It may reflect insufficient communication by the policymakers but may also be due to limited information sharing by the TWG members within their organisations and networks. If unaddressed, limited local awareness can constrain engagements, and contribute to lack of ownership, detachment, and fuel resentment towards policy implementation.29 61
The nature of the policy issue and the approach to policy design and implementation42 62–65 appear to have influenced stakeholder engagements and interest in the four policies. For example, minimal engagements from the private sector in the implementations of the HCSCMP and the framework contracting policies (such as SPMDP despite them being beneficiaries of both policies) was because the implementations of these policies were done primarily through the public sector and perhaps through more enclosed TWGs as policy networks.66 67 In contrast, the multisectoral VAT exemptions policies had engagements and interest from a wider range of stakeholders.
Our results helped to understand stakeholder roles within the four policies, and also provided deeper insights into the determinants of health policy processes more generally. Robust health policy processes are typically understood as being participatory, inclusive, clear, transparent and evidence-informed.55 64 All these characteristics are affected by degrees of stakeholder engagement, power dynamics and the resultant influences on policy processes.6 24 43 68 69 Adequate capacity of national policymakers for strong leadership and coordination of health policy processes is particularly important.42 55 57 70 71
Our results suggest four policy implications and lessons for improving the medicine pricing policy processes in Ghana and similar contexts. First, identifying who has a stake in issues of pricing, availability and affordability of medicines and the process of generating an inclusive list of stakeholders is a critical step in ensuring participatory policymaking. Second, considering stakeholder experiences, perspectives, needs, interest and expectations are critical steps in reducing gaps between the policy intent and actual implementation. Third, the nature of a policy issue and the approach to policy design and implementation can enable or constrain stakeholder involvements in policy processes, and consequently their inputs into decision-making. Finally, given a complex and time-consuming nature of policymaking, there is a need to identify the most feasible and context-specific ways of ensuring adequate stakeholder engagements within policy processes.
We acknowledge four study limitations. First, we mainly analysed stakeholder roles within the four policies. While we also reported key effects on policy processes, a more detailed analysis of policy processes was outside the primary scope of our paper and represents an agenda for future research. Second, the views of representatives in TWGs may not be generalisable to their respective organisations, and a larger study may be appropriate where resources permit. Third, implementations of four policies are ongoing, so stakeholder powers, roles, interests and engagements and resultant influences may change. While this highlights a more general limitation of stakeholder analysis, it also provides an opportunity for follow-on analyses to compare trends over time. Finally, documented evidence on stakeholder powers were understandably limited, and our strategy was to draw on data from the interviews and consultive meetings. Despite these limitations, our analysis should provide useful information for improving stakeholder involvements in the design and implementation of medicine pricing policies in Ghana and beyond.
Conclusion
Effective leadership is important for ensuring inclusive and participatory policymaking. Policymakers and analysts should also be cognisant of the nature of policy issues and approaches to policy design and implementation. Current and future medicines pricing policies can be improved by identifying key stakeholders and considering their experiences, perspectives, needs and expectations; greater understanding of the nature of a policy issue and approaches to policy design and implementation, and their implications on stakeholder engagements and developing feasible and context-specific ways of enhancing stakeholder engagements within complex policy processes.
Acknowledgments
We acknowledge the National Medicine Pricing Committee members, Ministry of Health and our respondents.
Footnotes
Handling editor: Seye Abimbola
Twitter: @tmirzoev
Contributors: AK and TM jointly conceived the study; AK, LB, IAK, IAA, ACdC, AD-A, TE and TM contributed to data collection and analysis; AK and TM wrote the first draft. All contributed to manuscript revisions, read and approved final version of the manuscript. AK and TM are the overall content guarantors.
Funding: This research was commissioned by the National Institute for Health Research (NIHR) NIHR Global Health Policy and Systems Research Development Award using UK aid from the UK Government (grant number 130219).
Disclaimer: The views expressed in this publication are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study involves human participants. Ethical approval received from the ethics committees of the Ghana Health Service (GHS-ERC006/02/20) and the University of Leeds School of Medicine (MREC 19-060). Participants gave informed consent to participate in the study before taking part.
References
- 1.Bigdeli M, Laing R, Tomson G, et al. Medicines and universal health coverage: challenges and opportunities. J Pharm Policy Pract 2015;8:8. 10.1186/s40545-015-0028-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acosta A, Ciapponi A, Aaserud M, et al. Pharmaceutical policies: effects of reference pricing, other pricing, and purchasing policies. Cochrane Database Syst Rev 2014:CD005979. 10.1002/14651858.CD005979.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hinsch M, Kaddar M, Schmitt S. Enhancing medicine price transparency through price information mechanisms. Global Health 2014;10:34–11. 10.1186/1744-8603-10-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen TA, Knight R, Roughead EE, et al. Policy options for pharmaceutical pricing and purchasing: issues for low- and middle-income countries. Health Policy Plan 2015;30:267–80. 10.1093/heapol/czt105 [DOI] [PubMed] [Google Scholar]
- 5.Vogler S, Paris V, Ferrario A. How can pricing and reimbursement policies improve affordable access to medicines? In: Lessons learned from European countries, 2017. [DOI] [PubMed] [Google Scholar]
- 6.Walt G, Gilson L. Reforming the health sector in developing countries: the central role of policy analysis. Health Policy Plan 1994;9:353–70. 10.1093/heapol/9.4.353 [DOI] [PubMed] [Google Scholar]
- 7.Gaventa J. Reflections on the Uses of the 'Power Cube' Approach for Analyzing the Spaces, Places and Dynamics Of Civil Society Participation and Engagement. Sussex: Institute of Development Studies, University of Sussex, 2005. [Google Scholar]
- 8.Varvasovszky Z, Brugha R. A stakeholder analysis. Health Policy Plan 2000;15:338–45. 10.1093/heapol/15.3.338 [DOI] [PubMed] [Google Scholar]
- 9.Walt G. Health policy: an introduction to process and power. Johannesburg, London: Zed Brooks, Witwatersrand University Press, 1994. [Google Scholar]
- 10.Balane MA, Palafox B, Palileo-Villanueva LM, et al. Enhancing the use of stakeholder analysis for policy implementation research: towards a novel framing and operationalised measures. BMJ Glob Health 2020;5. 10.1136/bmjgh-2020-002661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilson L, Erasmus E, Borghi J, et al. Using stakeholder analysis to support moves towards universal coverage: lessons from the shield project. Health Policy Plan 2012;27 Suppl 1:i64–76. 10.1093/heapol/czs007 [DOI] [PubMed] [Google Scholar]
- 12.Heydari M, Seyedin H, Jafari M. Stakeholder analysis of Iran’s health insurance system. J Edu Health Promot 2018;7:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kapiriri L. Stakeholder involvement in health research priority setting in low income countries: the case of Zambia. research involvement and engagement. 4, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abiiro GA, McIntyre D. Universal financial protection through national health insurance: a stakeholder analysis of the proposed one-time premium payment policy in Ghana. Health Policy Plan 2013;28:263–78. 10.1093/heapol/czs059 [DOI] [PubMed] [Google Scholar]
- 15.Ministry of Health . National health policy: ensuring healthy lives for all. Accra, Ghana, 2020. [Google Scholar]
- 16.Pharmexcil. Ghana Pharma Market & Regulatory Report, 2020. Available: https://pharmexcil.com/uploads/countryreports/Ghana_Market_Regulatory_report2020.pdf [Accessed 6 Mar 2022].
- 17.Ministry of Health . National medicines policy. 3 ed. Accra, Ghana, 2017. [Google Scholar]
- 18.Ministry of Health . Health commodity supply chain master plan. Accra, Ghana: Ministry of Health, Ghana, 2012. [Google Scholar]
- 19.Ministry of Health . Justification for number of medicines to be selected for procurement under Costed framework contracting agreement project for Ghana. Accra, Ghana: Ministry of Health, Ghana, 2017. [Google Scholar]
- 20.Boyajian R, Gordon WJ, Kowtoniuk AM, et al. Development & Impact of a Virtual PSA Monitoring Clinic for Follow-up of Prostate Cancer Patients: An Efficient Model With Unique Benefits Relevant to COVID-19. Int J Radiat Oncol Biol Phys 2021;111:S65. 10.1016/j.ijrobp.2021.07.163 [DOI] [Google Scholar]
- 21.Government of Ghana . Legislative instrument (L.I) 2255 value added Tax (exemption of active ingredients, selected inputs and selected drugs for pharmaceuticals) (amendments) regulations, 2017. Accra, Ghana: Ghana Publishing Company Limited, 2017. [Google Scholar]
- 22.Ministry of Health . Report of the Vat exemption implementation Committee to the Minister for health Accra, Ghana. Ministry of Health, Ghana, 2018. [Google Scholar]
- 23.Schneider F, Buser T. Promising degrees of stakeholder interaction in research for sustainable development. Sustain Sci 2018;13:129–42. 10.1007/s11625-017-0507-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sriram V, Topp SM, Schaaf M, et al. 10 best resources on power in health policy and systems in low- and middle-income countries. Health Policy Plan 2018;33:611–21. 10.1093/heapol/czy008 [DOI] [PubMed] [Google Scholar]
- 25.Mintzberg H. Power in and around organizations. Englewood Cliffs, N.J.: Prentice-Hall, 1983. [Google Scholar]
- 26.Gaventa J. Introduction: exploring citizenship, participation and accountability. IDS Bulletin 33.2. Brighton, England: Institute of Development Studies, 2002. [Google Scholar]
- 27.Topp SM, Schaaf M, Sriram V, et al. Power analysis in health policy and systems research: a guide to research conceptualisation. BMJ Glob Health 2021;6:e007268. 10.1136/bmjgh-2021-007268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrow NC. Pharmaceutical policy Part 2 pharmaceutical engagement and policy development: a framework for influence. J Pharm Policy Pract 2015;8:5. 10.1186/s40545-015-0026-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lipsky M. Street-level bureaucracy: dilemmas of the individual in public services. New York: Russel Sage Foundation, 1980. [Google Scholar]
- 30.Government of Ghana . Value added Tax (exemption of active ingredients, selected inputs and selected drugs or pharmaceutical) (Amendment) regulation, 2017. Accra, Ghana: Ghana Publishing Company (Assembly Press), 2017. [Google Scholar]
- 31.Ministry of Health . Framework contracting evaluation report. Accra, Ghana: Ministry of Health, 2019. [Google Scholar]
- 32.USAID | DELIVER PROJECT . USAID | DELIVER PROJECT Final Country Report : Ghana.: Arlington, Va.: USAID | DELIVER PROJECT, Task Orders 4 and 7, 2016. [Google Scholar]
- 33.WHO . The selection and use of essential medicines. World Health Organization, 2008. [Google Scholar]
- 34.Ministry of Health . Standard treatment guidelines. 7th ed. Accra: Ghana National Drugs Programme, 2017. [Google Scholar]
- 35.Ministry of Health . Ghana national medicines policy. 3rd ed. Accra: Ghana National Drugs Programme, 2017. [Google Scholar]
- 36.Sinclair D, Gyansa-Lutterodt M, Asare B, et al. Integrating global and national knowledge to select medicines for children: the Ghana national drugs programme. PLoS Med 2013;10:e1001449. 10.1371/journal.pmed.1001449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Christian Health Association of Ghana. Number of Facilites. Available: https://chag.org.gh [Accessed 14 Jun 2021].
- 38.Domfeh KA. Pooled procurement program in the quality improvement of medicines of the National Catholic health service in Ghana: using the Donabedian model. J Pharm Health Serv Res 2021;12:133–41. 10.1093/jphsr/rmaa030 [DOI] [Google Scholar]
- 39.Martineau T, Mirzoev T, Pearson S, et al. Coherence between health policy and human resource strategy: lessons from maternal health in Vietnam, India and China. Health Policy Plan 2015;30:111–20. 10.1093/heapol/czt102 [DOI] [PubMed] [Google Scholar]
- 40.Ha BTT, Mirzoev T, Mukhopadhyay M. Shaping the health policy agenda: the case of safe motherhood policy in Vietnam. Int J Health Policy Manag 2015;4:741-6. 10.15171/ijhpm.2015.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mirzoev T, Green A, Gerein N, et al. Role of evidence in maternal health policy processes in Vietnam, India and China: findings from the HEPVIC project. evid policy 2013;9:493–511. 10.1332/174426413X669845 [DOI] [Google Scholar]
- 42.Green A, Gerein N, Mirzoev T, et al. Health policy processes in maternal health: a comparison of Vietnam, India and China. Health Policy 2011;100:167–73. 10.1016/j.healthpol.2010.11.016 [DOI] [PubMed] [Google Scholar]
- 43.Mirzoev T, Das M, Ebenso B, et al. Contextual influences on the role of evidence in health policy development: what can we learn from six policies in India and Nigeria? evid policy 2017;13:59–79. 10.1332/174426415X14454407579925 [DOI] [Google Scholar]
- 44.Abdel Rida N, Mohamed Ibrahim MI, Babar Z-U-D, et al. A systematic review of pharmaceutical pricing policies in developing countries. J Pharm Health Serv Res 2017;8:213–26. 10.1111/jphs.12191 [DOI] [Google Scholar]
- 45.Danzon PM, Chao Li‐Wei. Does regulation drive out competition in pharmaceutical markets? J Law Econ 2000;43:311–58. 10.1086/467458 [DOI] [Google Scholar]
- 46.de Jager H, Suleman F. The impact of generics and generic reference pricing on candesartan and rosuvastatin utilisation, price and expenditure in South Africa. Int J Clin Pharm 2019;41:81–7. 10.1007/s11096-018-0758-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moodley R, Suleman F. The impact of the single exit price policy on a basket of generic medicines in South Africa, using a time series analysis from 1999 to 2014. PLoS One 2019;14:e0219690. 10.1371/journal.pone.0219690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fink G, Dickens WT, Jordan M, et al. Access to subsidized ACT and malaria treatment--evidence from the first year of the AMFm program in six districts in Uganda. Health Policy Plan 2014;29:517–27. 10.1093/heapol/czt041 [DOI] [PubMed] [Google Scholar]
- 49.Tougher S, Mann AG, et al. , ACTwatch Group . Improving access to malaria medicine through private-sector subsidies in seven African countries. Health Aff 2014;33:1576–85. 10.1377/hlthaff.2014.0104 [DOI] [PubMed] [Google Scholar]
- 50.Ye Y, Arnold F, Noor A, et al. The Affordable medicines Facility-malaria (AMFm): are remote areas benefiting from the intervention? Malar J 2015;14:398. 10.1186/s12936-015-0904-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ali GKM, Yahia Y. Controlling medicine prices in Sudan: the challenge of the recently established medicines regulatory authority. East Mediterr Health J 2012;18:811–20. 10.26719/2012.18.8.811 [DOI] [PubMed] [Google Scholar]
- 52.Assefa Y, Hill PS, Ulikpan A, et al. Access to medicines and hepatitis C in Africa: can tiered pricing and voluntary licencing assure universal access, health equity and Fairness? Global Health 2017;13:73. 10.1186/s12992-017-0297-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roman TE, Cleary S, McIntyre D. Exploring the functioning of decision space: a review of the available health systems literature. Int J Health Policy Manag 2017;6:365–76. 10.15171/ijhpm.2017.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tangcharoensathien V, Patcharanarumol W, Kulthanmanusorn A, et al. The political economy of UHC reform in Thailand: lessons for low- and middle-income countries. Health Syst Reform 2019;5:195–208. 10.1080/23288604.2019.1630595 [DOI] [PubMed] [Google Scholar]
- 55.Mirzoev TN, Green A, Van Kalliecharan R. Framework for assessing the capacity of a health ministry to conduct health policy processes--a case study from Tajikistan. Int J Health Plann Manage 2015;30:173–85. 10.1002/hpm.2222 [DOI] [PubMed] [Google Scholar]
- 56.Gil A, Polikina O, Koroleva N, et al. Alcohol policy in a Russian region: a stakeholder analysis. Eur J Public Health 2010;20:588–94. 10.1093/eurpub/ckq030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koduah A, Agyepong IA, van Dijk H. ‘The one with the purse makes policy’: Power, problem definition, framing and maternal health policies and programmes evolution in national level institutionalised policy making processes in Ghana. Soc Sci Med 2016;167:79–87. 10.1016/j.socscimed.2016.08.051 [DOI] [PubMed] [Google Scholar]
- 58.Durham J, Warner M, Phengsavanh A, et al. Stakeholder analysis of community distribution of misoprostol in Lao PDR: a qualitative study. PLoS One 2016;11:e0162154. 10.1371/journal.pone.0162154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.COHRED . Alignment and harmonization in health research. AHA study. Geneva, Switzerland: Council for Health Research and Development, SIDA, 2008. [Google Scholar]
- 60.High Level Forum . Paris Declaration on aid effectiveness. ownership, harmonisation, alignment, results and mutual accountability. Paris, France: High Level Forum, 2005. [Google Scholar]
- 61.Erasmus E. The use of street-level bureaucracy theory in health policy analysis in low- and middle-income countries: a meta-ethnographic synthesis. Health Policy Plan 2014;29 Suppl 3:iii70–8. 10.1093/heapol/czu112 [DOI] [PubMed] [Google Scholar]
- 62.Bijlsma RM, Bots PWG, Wolters HA, et al. An Empirical Analysis of Stakeholders’ Influence on Policy Development: the Role of Uncertainty Handling. Ecology and Society 2011;16. 10.5751/ES-03865-160151 [DOI] [Google Scholar]
- 63.Barker C. The health care policy process. London: Sage Publications, 1996. [Google Scholar]
- 64.Gilson L, Raphaely N. The terrain of health policy analysis in low and middle income countries: a review of published literature 1994-2007. Health Policy Plan 2008;23:294–307. 10.1093/heapol/czn019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gilson L, ed. Health Policy and Systems Research. A Methodology Reader. Geneva, Switzerland: Alliance for Health Policy and Systems Research, World Health Organization, 2012. [Google Scholar]
- 66.Atkinson MM, Coleman WD, Networks P. Policy networks, policy communities and the problems of governance. Governance 1992;5:154–80. 10.1111/j.1468-0491.1992.tb00034.x [DOI] [Google Scholar]
- 67.Adam S, Kriesi H. The Network Approach. In: Sabatier PA, ed. Theories of the policy process. 132. Boulder, CO: Westview Press, 2009. [Google Scholar]
- 68.Erasmus E, Orgill M, Schneider H, et al. Mapping the existing body of health policy implementation research in lower income settings: what is covered and what are the gaps? Health Policy Plan 2014;29 Suppl 3:iii35–50. 10.1093/heapol/czu063 [DOI] [PubMed] [Google Scholar]
- 69.Erasmus E, Gilson L. How to start thinking about investigating power in the organizational settings of policy implementation. Health Policy Plan 2008;23:361–8. 10.1093/heapol/czn021 [DOI] [PubMed] [Google Scholar]
- 70.Omaswa F, Boufford JI. Strong Ministries for strong health systems. An overview of the study report: supporting Ministerial health leadership: a strategy for health systems strengthening. New York: The African Center for Global Health and Social Transformation (ACHEST) and The New York Academy of Medicine (NYAM), 2010. [Google Scholar]
- 71.WHO . Building leadership and management capacity in health Geneva: World Health Organization; 2007, 2010. Available: www.who.int/management/FrameworkBrochure.pdf [Accessed 15 Mar 2017].
- 72.Lazaridis II, Kraljević M, Schneider R, et al. The impact of the COVID-19 pandemic on bariatric surgery: results from a worldwide survey. Obes Surg 2020;30:4428–36. 10.1007/s11695-020-04830-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2021-008225supp001.pdf (31KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.