Abstract
Introduction
Although obesity is one of the established risk factors of diabetes mellitus, the relationship between obesity and diabetic retinopathy (DR) remains unclear in different studies. This study aimed to investigate the association of DR with four obesity-related indexes, including body mass index (BMI), waist to hip ratio (WHR), waist to height ratio (WHtR) and body adiposity index (BAI) in patients with diabetes.
Research design and methods
We prospectively enrolled 2305 patients with diabetes (2305 eyes) in the Guangzhou Diabetic Eye Study between November 2017 and December 2019 to investigate the prevalence and the association of different types of obesity with DR using BMI, WHR, WHtR and BAI. DR, diabetic macular oedema (DME) and vision-threatening DR (VTDR) were selected as primary outcomes. BMI was categorised as normal (18.5–22.9 kg/m2), overweight (23.0–25.0 kg/m2) and obese (>25.0 kg/m2); WHR, WHtR and BAI were categorised into quarters.
Results
A total of 336 (14.58%), 93 (4.03%) and 98 (4.25%) developed DR, DME and VTDR, respectively. The prevalence of DR, DME and VTDR was higher in patients with higher BMI/WHR or lower WHtR/BAI. In the univariate regression model, WHR correlated positively with DR, while WHtR and BAI correlated negatively with DR, DME and VTDR. The association remained independent of age, sex and lipid metabolism parameters. In the multivariate model, obese presented as a protective factor for DME and VTDR, while the second quarter of WHtR(Q2-WHtR) presented as a risk factor.
Conclusions
As high as 67.8% of patients with diabetes were overweight or obese. Obese presented as a significant protective factor of VTDR, while Q2-WHtR presented as a significant risk factor. Therefore, more attention should be paid to centripetal obesity as well as general obesity. Further research is also needed to focus on the improvement of sex-specific weight management in patients with diabetes.
Keywords: Diabetic retinopathy, DIABETES & ENDOCRINOLOGY, NUTRITION & DIETETICS
Strengths and limitations of this study.
This study is a combined study that analysed the association of four obesity-related indexes (body mass index, waist to hip ratio, waist to height ratio, and body adiposity index) with the presence and the severity of diabetic retinopathy (DR).
Any DR, diabetic macular oedema (DME) and vision-threatening DR were selected as primary outcomes.
DR and DME were diagnosed and graded according to the International Clinical Severity Scale of Diabetic Retinopathy and DME, using 7-position fundus photos of participants.
To reduce the examination time and improve the compliance of participants, the measurement of waist circumference and hip circumference was not performed on every participant, while eventually 483 patients have undergone all the measurements.
The diabetic participants with severe conditions (eg, very poor eyesight, past DR treatment history, occurred with other combined eye diseases that could affect the retinal thickness) were excluded from our study.
Introduction
Diabetic retinopathy (DR) is one of the most common complications of diabetes mellitus and is a leading cause of vision loss and blindness throughout the world.1 It severely affects the life quality of patients with diabetes and increases the economic burden of treatment without timely management.1 Although obesity is one of the established risk factors that correlated positively with diabetes mellitus,2 3 the relationship between obesity and DR varies in different studies. For instance, in a cross-sectional study that enrolled 50 464 Saudi patients with diabetes, overweight and obesity presented as a protective factor for DR.4 However, in a meta-analysis of prospective cohort studies, obesity correlated with a significant increase in DR incidence.5 The methods to improve the weight management of patients with diabetes to decrease the presence and severity of DR have become a major public health problem.
Body mass index (BMI) has been commonly used to assess weight level in the previous study,4 6 7 but it could not distinguish whether a patient is general obese or abdominal obese. Moreover, combined or separate studies about the association of waist to hip ratio (WHR), waist to height ratio (WHtR) and body adiposity index (BAI) with DR are still limited. Studies to explore the relationship between obesity and DR among Chinese people are also limited.
Therefore, this study assessed the association of obesity-related indexes with DR, diabetic macular oedema (DME) and vision-threatening DR (VTDR) among T2DM patients using the data of the Guangzhou Diabetic Eye Study (GDES) in China.
Methods
Study design and participants
The GDES is an ongoing prospective study that enrolled patients with diabetes from communities in Guangzhou. Before enrolment, the participants were diagnosed with diabetes in the general hospitals, and were registered and followed up in the community health centres. They were referred to Zhongshan Ophthalmic Centre and underwent ophthalmic examinations and physical examinations at the baseline visit, 1-year visit and 2-year visit. Demographic information and medical history were also collected at the same time. However, patients with any evidence of the following conditions were excluded: (1) best corrected visual acuity worse than 20/200, axial length >30 mm or unmeasurable, spherical equivalent ≤−12.0°, astigmatism >4° or intraocular pressure (IOP) >21 mm Hg in the right eye; (2) except DR, other combined eye diseases that could affect retinal thickness in the right eye, such as glaucoma, age-related macular degeneration, and retinal detachment; (3) surgery or invasive treatment or laser treatment history on the right eye; (4) severe systemic diseases, such as uncontrolled hypertension, severe cardiovascular and cerebrovascular disease, malignant tumours and nephritis; (5) general surgery history, such as heart bypass, thrombolysis and kidney transplantation; (6) cognitive disorders or mental illness that would hinder the patient’s cooperation with tests and (7) inability to obtain clear fundus or SS-OCT images because of refractive media opacity or non-cooperation.
A total of 2372 patients with diabetes participated and completed the examinations between November 2017 and December 2019. Sixty-seven participants with ungradable fundus images were excluded, and 2305 participants were finally included. The baseline data of demographic information, medical history, ophthalmic examinations and physical examinations were extracted in the analysis. There was no missing data in the study.
Demographic information, medical history, and biometric parameter assessment
Demographic information and medical history (eg, age, sex, education, smoking and drinking history, duration of diabetes and insulin use) were collected using a standardised questionnaire. The previous medical records would be checked and confirmed by the doctors. The physical examination, including a blood pressure test, blood test, biochemical test and urine test, was carried out by a certified nurse.
Assessment of BMI, WHR, WHtR and BAI
The participants’ weight (in kilograms), height (in metres), waist circumference (in centimetres), and hip circumference were measured by certified nurses. Participants were required to remove their shoes and the heavy object (eg, mobile phones, keys, and wallets) on them. Weight was measured using a weight scale. Height was measured using a measuring stick on the weight scale. Waist and hip circumferences were assessed using a nonstretchable medical tape. Waist circumference was taken at the smallest horizontal girth between the costal margins and the iliac crests at the end of tidal expiration. Hip circumference was taken at the maximal protuberance of the buttocks. Every participant underwent the weight and height measurement, while 483 consecutive participants underwent hip circumference measurement, and 1484 consecutive participants underwent waist circumference measurement.
BMI was calculated as weight divided by height squared and was categorised into normal weight (18.5–22.9 kg/m2), overweight (23.0–25.0 kg/m2) and obese (>25.0 kg/m2), according to Asia-Pacific BMI cut-off points.8–10 Sixty underweight participants (BMI <18.5 kg/m2) were not included because of the small sample size. WHR was calculated as waist circumference divided by hip circumference, while WHtR was calculated by dividing waist circumference by height. BAI was calculated as hip circumference divided by ((height)1.5 minus 18). Because of the lack of standardised classifications, WHR, WHtR and BAI were categorised in quarters.
Assessment of DR, DME and VTDR
All the participants underwent ophthalmic examinations including vision test, IOP test, anterior segment examination, intraocular lens (IOL) master test, mydriatic fundus photography and optical coherence tomography examination, by trained ophthalmologists.
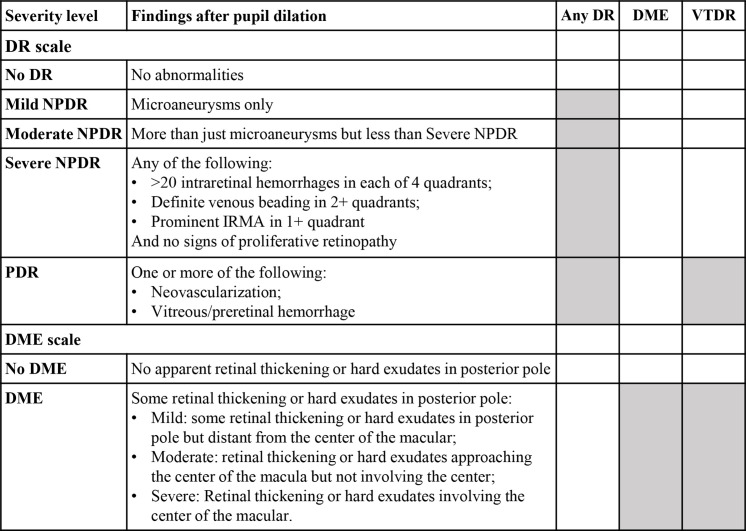
DR and DME were diagnosed and graded according to the International Clinical Severity Scale of Diabetic Retinopathy and DME (figure 1), using 7-position fundus photos of participants. Any DR, DME, and VTDR were selected as primary outcomes. Any DR was defined as the presence of mild non-proliferative DR (NPDR), moderate NPDR, severe NPDR or PDR. VTDR was defined as the presence of DME or PDR. For each participant, only the data of the worse eye would be used. If the DR grades of both eyes were consistent, then the right eye would be selected for analysis.
Figure 1.
International Clinical Severity Scale of Diabetic Retinopathy and Diabetic Macular Oedema. DME, diabetic macular oedema; DR, diabetic retinopathy; IRMA, intraretinal microvascular abnormalities; NPDR, non-proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; VTDR, vision-threatening DR.
Statistical analysis
All analyses were performed using STATA statistical software (Stata V.14.0, Stata). BMI, WHR, WHtR and BAI classifications were used as both continuous variables and categorical variables. To compare the differences in characteristics of participants with or without DR, DME and VTDR, the Student’s t-test was used for continuous variables that were normally distributed, the Mann-Whitney U test was used for other continuous variables (creatinine and microalbuminuria), and the χ2 test was used for categorical variables.
The binary and ordinal logistic regression model was used to assess the association of BMI, WHR, WHtR and BAI with the presence of any DR and VTDR. In special, the outcome of the ordinal logistic regression model of DR was set as no DR, mild NPDR, moderate NPDR and VTDR (including PDR and DME). In the multivariate logistic model, the association was adjusted for potential confounding factors established in previous research. These factors included continuous variables (eg, age, systolic blood pressure, Glycosylated Hemoglobin Type A1C (HbA1c), C reaction protein, total cholesterol, triglycerides, low-density cholesterol, high-density cholesterol, creatinine, microalbuminuria, uric acid and axial length) and categorical variables (eg, sex, smoking history, drinking history, education, duration of diabetes and insulin use). P values less than 0.05 were considered statistically significant.
Patient and public involvement statement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Results
In general, 336 (14.58%) participants developed DR, including 76 (3.30%) patients with mild NPDR, 197 (8.55%) patients with moderate NPDR, 45 (1.95%) patients with severe NPDR, 17 (0.74%) patients with PDR, and 93 (4.03%) patients with DME. 98 (4.25%) patients developed VTDR.
Compared with participants who did not have DR, participants with DR had a younger age, a lower level of education, a longer duration of diabetes and a higher proportion of males, smoking history, drinking history and insulin use (table 1). They also had a higher level of HbA1c, creatinine, microalbuminuria and systolic blood pressure, but shorter axis length (all p<0.05). Moreover, their BMI, WHR, WHtR and BAI were higher. According to Asia-Pacific BMI cut-off points, as high as 947 participants (41.1%) were obese, and 615 (26.7%) were overweight, while only 683 participants (29.6%) were normal weight.
Table 1.
The characteristics of participants with or without diabetic retinopathy (DR), diabetic macular oedema (DME) and vision threatening diabetic retinopathy (VTDR)
| DR | No DR | DME | No DME | VTDR | No VTDR | |
| n=336 | n=1970 | n=93 | n=2212 | n=98 | n=2207 | |
| Medical history | ||||||
| Age, median (IQR), year | 64.0 (10.0) | 65.0 (10.0) | 62.0 (9.0) | 65.0 (10.0) | 61.5 (9.0) | 65.0 (10.0) |
| Sex, % | ||||||
| Female | 49.4 | 58.35 | 43.01 | 57.64 | 42.86 | 57.68 |
| Male | 50.6 | 41.65 | 56.99 | 42.36 | 57.14 | 42.32 |
| Smoking history, % | ||||||
| No | 81.88 | 86.29 | 83.12 | 85.76 | 82.5 | 85.79 |
| Yes | 18.12 | 13.71 | 16.88 | 14.24 | 17.5 | 14.21 |
| Drinking history, % | ||||||
| No | 88.04 | 91.18 | 89.61 | 90.78 | 87.5 | 90.87 |
| Yes | 11.96 | 8.82 | 10.39 | 9.22 | 12.5 | 9.13 |
| Education, % | ||||||
| Educated | 16.42 | 11.26 | 16 | 11.83 | 15.09 | 11.85 |
| Not educated | 83.58 | 88.74 | 84 | 88.17 | 84.91 | 88.15 |
| Diabetes duration, %, year | ||||||
| <5 | 18.15 | 39.21 | 21.51 | 36.75 | 20.41 | 36.84 |
| 5–9 | 20.24 | 26.16 | 17.2 | 25.63 | 17.35 | 25.65 |
| 10–19 | 40.48 | 27.37 | 45.16 | 28.62 | 44.9 | 28.59 |
| ≥20 | 21.13 | 7.26 | 16.13 | 9 | 17.35 | 8.93 |
| Taking insulin, % | ||||||
| No | 52.38 | 82.73 | 51.61 | 79.43 | 51.02 | 79.52 |
| Yes | 47.62 | 17.27 | 48.39 | 20.57 | 48.98 | 20.48 |
| Examination and laboratory tests, median (IQR) | ||||||
| Systolic blood pressure, mm Hg | 136.00 (26.00) | 133.00 (24.00) | 133.00 (28.00) | 134.00 (24.00) | 132.50 (27.50) | 134.00 (24.00) |
| BMI | 23.97 (3.52) | 24.40 (4.06) | 23.72 (3.09) | 24.38 (3.99) | 23.59 (2.67) | 24.39 (4.02) |
| WHR | 0.91 (0.09) | 0.90 (0.07) | 0.93 (0.07) | 0.90 (0.08) | 0.91 (0.08) | 0.90 (0.08) |
| WHtR | 0.53 (0.07) | 0.54 (0.07) | 0.52 (0.06) | 0.54 (0.07) | 0.52 (0.06) | 0.54 (0.07) |
| BAI | 27.48 (5.13) | 28.86 (5.12) | 26.89 (3.70) | 28.74 (5.28) | 26.89 (3.99) | 28.74 (5.27) |
| HbA1c, % | 7.80 (2.20) | 6.60 (1.30) | 8.00 (2.40) | 6.60 (1.40) | 8.00 (2.50) | 6.60 (1.40) |
| C reaction protein | 1.35 (2.03) | 1.47 (2.00) | 1.19 (1.68) | 1.45 (2.02) | 1.17 (1.69) | 1.46 (2.02) |
| Total cholesterol | 4.73 (1.37) | 4.78 (1.40) | 4.91 (1.55) | 4.77 (1.38) | 4.90 (1.56) | 4.77 (1.38) |
| Triglycerides | 1.90 (1.60) | 1.91 (1.58) | 1.96 (1.50) | 1.90 (1.58) | 1.99 (1.49) | 1.90 (1.58) |
| Low-density cholesterol | 2.97 (1.16) | 3.00 (1.28) | 3.16 (1.24) | 2.98 (1.25) | 3.15 (1.23) | 2.98 (1.25) |
| High-density cholesterol | 1.22 (0.47) | 1.22 (0.51) | 1.21 (0.50) | 1.22 (0.50) | 1.21 (0.50) | 1.22 (0.50) |
| Creatinine | 76.00 (28.00) | 69.00 (25.00) | 79.00 (26.00) | 70.00 (25.00) | 80.00 (27.00) | 70.00 (25.00) |
| Microalbuminuria | 1.96 (8.22) | 0.85 (2.29) | 2.54 (9.62) | 0.91 (2.48) | 2.54 (9.89) | 0.91 (2.48) |
| Uric acid | 374.00 (123.00) | 368.00 (128.00) | 357.00 (144.00) | 369.00 (128.00) | 355.50 (132.00) | 369.00 (128.00) |
| Axial length, mm | 23.25 (1.20) | 23.44 (1.19) | 23.19 (1.15) | 23.43 (1.20) | 23.16 (1.17) | 23.43 (1.20) |
BAI, Body Adiposity Index; BMI, body mass index; HbaA1c, Glycosylated Hemoglobin Type A1C; WHR, waist to hip ratio; WHtR, waist to height ratio.
Association of BMI with any DR, DME and VTDR
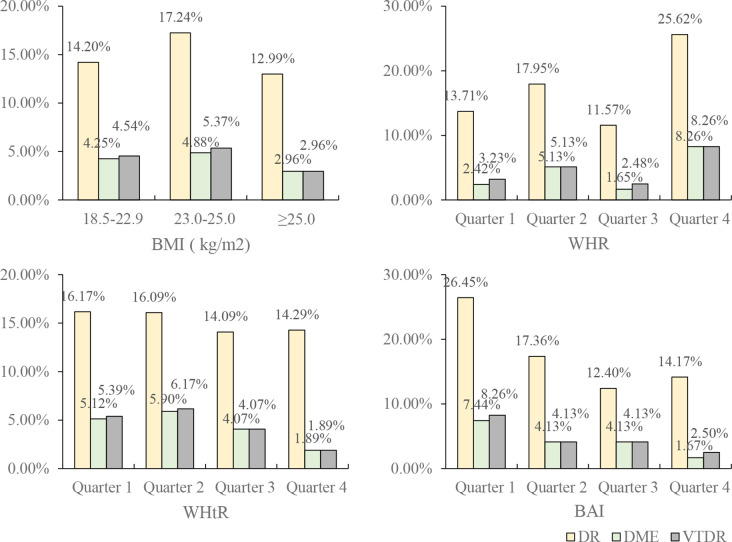
The prevalence of any DR, DME and VTDR in overweight patients with diabetes was higher than that in patients who were normal weight or obese (figure 2, table 2). However, there was no significance in the association of BMI with any DR in the univariate binary or ordinal logistic model.
Figure 2.
The prevalence of diabetic retinopathy (DR) and severe DR (DME and vision threatening diabetic retinopathy, VTDR) in different groups of the obesity-related indexes. BAI, body Adiposity Index; BMI, body mass index; DME, diabetic macular oedema; WHR, waist to hip ratio; WHtR, waist to height ratio.
Table 2.
The prevalence of DR, DME and VTDR in different groups of the obesity-related indexes
| No of patients | DR prevalence, % | DME prevalence, % | VTDR prevalence, % | |
| BMI, kg/m2 (n=2245) | ||||
| 18.5–22.9 (normal weight) | 683 | 14.20 | 4.25 | 4.54 |
| 23.0–25.0 (over weight) | 615 | 17.24 | 4.88 | 5.37 |
| ≥25.0 (obese) | 947 | 12.99 | 2.96 | 2.96 |
| WHR, (n=483) | ||||
| Quarter 1 | 124 | 13.71 | 2.42 | 3.23 |
| Quarter 2 | 117 | 17.95 | 5.13 | 5.13 |
| Quarter 3 | 121 | 11.57 | 1.65 | 2.48 |
| Quarter 4 | 121 | 25.62 | 8.26 | 8.26 |
| WHtR, (n=1484) | ||||
| Quarter 1 | 371 | 16.17 | 5.12 | 5.39 |
| Quarter 2 | 373 | 16.09 | 5.90 | 6.17 |
| Quarter 3 | 369 | 14.09 | 4.07 | 4.07 |
| Quarter 4 | 371 | 14.29 | 1.89 | 1.89 |
| BAI, (n=483) | ||||
| Quarter 1 | 121 | 26.45 | 7.44 | 8.26 |
| Quarter 2 | 121 | 17.36 | 4.13 | 4.13 |
| Quarter 3 | 121 | 12.40 | 4.13 | 4.13 |
| Quarter 4 | 120 | 14.17 | 1.67 | 2.50 |
BAI, Body Adiposity Index; BMI, body mass index; DME, diabetic macular oedema; DR, diabetic retinopathy; VTDR, vision-threatening diabetic retinopathy; WHR, waist to hip ratio; WHtR, waist to height ratio.
After adjusted for sex and age, obesity presented as a protective factor for VTDR (OR=0.57 (95% CI 0.33 to 0.96), p for trend=0.028, (online supplemental table S1). The association remained after the regression model was additionally adjusted for lipid metabolism parameter (online supplemental table S2).
bmjopen-2021-056332supp001.pdf (49.9KB, pdf)
In the full model that further adjusted for continuous variables (age, systolic blood pressure, HbA1c, C reaction protein, total cholesterol, triglycerides, low-density cholesterol, high-density cholesterol, creatinine, microalbuminuria, uric acid and axial length) and categorical variables (sex, smoking history, drinking history, education, duration of diabetes and insulin use), the association of BMI became significant with both DME and VTDR (for DME, p for trend = 0.031; for VTDR, p for trend = 0.016, Table 3). Obesity was inversely associated with DME and VTDR with a decreased OR (for DME, OR=0.40 (95% CI 0.16 to 0.96); for VTDR, OR=0.37 (95% CI 0.16 to 0.87), table 3). However, the association was only significant in female patients (for DME, p for trend =0.021, OR of obesity=0.10 (95% CI 0.01 to 0.77); for VTDR, p for trend =0.015, OR of obesity =0.09 (95% CI 0.01 to 0.76), online supplemental table S3-1), but not in male patients (online supplemental table S3-2).
Table 3.
The OR of BMI, WHR, WHtR and BAI in the binary logistic regression model additionally adjusted for other variables in all patients
| DR | DME | VTDR | ||||
| OR (95% CI) | P valve | OR (95% CI) | P valve | OR (95% CI) | P valve | |
| BMI, kg/m2 | ||||||
| 18.5–22.9 (normal weight) | Ref. | Ref. | Ref. | |||
| 23.0–25.0 (overweight) | 0.82 (0.52, 1.29) | 0.393 | 1.01 (0.43, 2.37) | 0.989 | 1.03 (0.46, 2.32) | 0.946 |
| ≥25.0 (obese) | 0.72 (0.47, 1.10) | 0.131 | 0.40 (0.16, 0.96) | 0.041 | 0.37 (0.16, 0.87) | 0.023 |
| P for trend | 0.134 | 0.031 | 0.016 | |||
| WHR | ||||||
| Quarter 1 | Ref. | Ref. | Ref. | |||
| Quarter 2 | 0.94 (0.39, 2.29) | 0.900 | 2.77 (0.30, 25.73) | 0.370 | 1.56 (0.22, 10.86) | 0.655 |
| Quarter 3 | 0.49 (0.19, 1.25) | 0.136 | 0.79 (0.09, 6.96) | 0.830 | 0.62 (0.11, 3.35) | 0.579 |
| Quarter 4 | 1.06 (0.46, 2.45) | 0.893 | 3.21 (0.42, 24.78) | 0.263 | 1.98 (0.34, 11.61) | 0.450 |
| P for trend | 0.834 | 0.459 | 0.645 | |||
| WHtR | ||||||
| Quarter 1 | Ref. | Ref. | Ref. | |||
| Quarter 2 | 1.07 (0.61, 1.87) | 0.820 | 3.04 (1.04, 8.85) | 0.041 | 2.74 (1.01, 7.43) | 0.048 |
| Quarter 3 | 0.64 (0.35, 1.16) | 0.142 | 1.13 (0.31, 4.03) | 0.856 | 0.93 (0.28, 3.07) | 0.906 |
| Quarter 4 | 0.81 (0.44, 1.48) | 0.494 | 0.57 (0.13, 2.59) | 0.468 | 0.48 (0.11, 2.09) | 0.330 |
| P for trend | 0.234 | 0.252 | 0.133 | |||
| BAI | ||||||
| Quarter 1 | Ref. | Ref. | Ref. | |||
| Quarter 2 | 0.77 (0.35, 1.69) | 0.512 | 1.15 (0.29, 4.52) | 0.845 | 1.05 (0.29, 3.74) | 0.943 |
| Quarter 3 | 0.60 (0.26, 1.37) | 0.226 | 0.89 (0.19, 4.13) | 0.879 | 0.77 (0.19, 3.08) | 0.706 |
| Quarter 4 | 0.57 (0.23, 1.39) | 0.216 | 0.63 (0.11, 3.55) | 0.605 | 0.92 (0.22, 3.77) | 0.909 |
| P for trend | 0.191 | 0.610 | 0.769 | |||
These variables included continuous variables (eg, age, systolic blood pressure, HbA1c, C reaction protein, total cholesterol, triglycerides, low-density cholesterol, high-density cholesterol, creatinine, microalbuminuria, uric acid and axial length) and categorical variables (eg, sex, smoking history, drinking history, education, duration of diabetes and insulin use). DR, DME and VTDR were set as outcomes of the regression model, respectively.
BAI, Body Adiposity Index; BMI, body mass index; DME, diabetic macular oedema; DR, diabetic retinopathy; HbaA1c, Glycosylated Hemoglobin Type A1C; VTDR, vision-threatening diabetic retinopathy; WHR, waist to hip ratio; WHtR, waist to height ratio.
Association of WHR with any DR and severe DR
The prevalence of DR, DME and VTDR was the highest in the fourth quarter of WHR (Q4-WHR) (figure 2, table 2). In the univariable logistic regression model, Q4-WHR presented as a risk factor for DR (in the binary model, OR=2.17 (95% CI 1.13 to 4.17); in the ordinal model, OR=2.25 (95% CI 1.18 to 4.32), table 3). When DME and VTDR were set as the outcome of the model, WHR presented a similar trend, although it was not significant.
After the logistic regression model was adjusted for sex and age, Q4-WHR remained a risk factor for DR (OR=2.02 (95% CI 1.03 to 3.98), (online supplemental table S1). The association remained independent of the lipid metabolism parameter (online supplemental table S2). However, in the full model, the association of WHR with DR and severe DR presented a similar trend, but was not significant (table 3).
Association of WHtR with any DR and severe DR
The prevalence of DR decreased slightly with the growth of WHtR, while the prevalence of DME and VTDR was the highest in the Q2-WHtR, and then decreased (table 2). In the univariate regression model, Q4-WHtR presented as a significant protective factor for DME (OR=0.36 (95% CI 0.15 to 0.86), table 4) and VTDR, (OR=0.34, (95% CI 0.14 to 0.81)).
Table 4.
The OR of BMI, WHR, WHtR, and BAI in the univariate logistic regression model
| Binary regression model of DR | Ordinal regression model of DR | Binary regression model of DME | Binary regression model of VTDR | |||||
| OR (95% CI) | P valve | OR (95% CI) | P valve | OR (95% CI) | P valve | OR (95% CI) | P valve | |
| BMI, kg/m2 | ||||||||
| 18.5–22.9 (normal weight) | Ref. | Ref. | Ref. | Ref. | ||||
| 23.0–25.0 (overweight) | 1.26 (0.93 to 1.70) | 0.133 | 1.28 (0.95 to 1.72) | 0.109 | 1.16 (0.69 to 1.95) | 0.585 | 1.19 (0.72 to 1.97) | 0.492 |
| ≥25.0 (obese) | 0.90 (0.68 to 1.20) | 0.479 | 0.89 (0.67 to 1.18) | 0.425 | 0.69 (0.40 to 1.17) | 0.164 | 0.64 (0.38 to 1.08) | 0.094 |
| P for trend | 0.381 | 0.329 | 0.147 | 0.083 | ||||
| WHR | ||||||||
| Quarter 1 | Ref. | Ref. | Ref. | Ref. | ||||
| Quarter 2 | 1.38 (0.69 to 2.76) | 0.368 | 1.39 (0.69 to 2.77) | 0.355 | 2.18 (0.53 to 8.93) | 0.279 | 1.62 (0.45 to 5.90) | 0.463 |
| Quarter 3 | 0.82 (0.39 to 1.75) | 0.615 | 0.85 (0.40 to 1.80) | 0.669 | 0.68 (0.11 to 4.13) | 0.673 | 0.76 (0.17 to 3.48) | 0.727 |
| Quarter 4 | 2.17 (1.13 to 4.17) | 0.021 | 2.25 (1.18 to 4.32) | 0.014 | 3.63 (0.97 to 13.54) | 0.055 | 2.70 (0.82 to 8.87) | 0.101 |
| P for trend | 0.056 | 0.040 | 0.093 | 0.152 | ||||
| WHtR | ||||||||
| Quarter 1 | Ref. | Ref. | Ref. | Ref. | ||||
| Quarter 2 | 0.99 (0.67 to 1.47) | 0.974 | 0.99 (0.67 to 1.46) | 0.962 | 1.16 (0.62 to 2.18) | 0.643 | 1.15 (0.62 to 2.14) | 0.651 |
| Quarter 3 | 0.85 (0.57 to 1.27) | 0.430 | 0.86 (0.57 to 1.28) | 0.457 | 0.79 (0.39 to 1.57) | 0.494 | 0.74 (0.37 to 1.48) | 0.397 |
| Quarter 4 | 0.86 (0.58 to 1.29) | 0.475 | 0.86 (0.57 to 1.28) | 0.444 | 0.36 (0.15 to 0.86) | 0.021 | 0.34 (0.14 to 0.81) | 0.015 |
| P for trend | 0.358 | 0.344 | 0.015 | 0.009 | ||||
| BAI | ||||||||
| Quarter 1 | Ref. | Ref. | Ref. | Ref. | ||||
| Quarter 2 | 0.64 (0.34 to 1.19) | 0.158 | 0.61 (0.33 to 1.13) | 0.117 | 0.54 (0.17 to, 1.65) | 0.277 | 0.48 (0.16 to 1.44) | 0.191 |
| Quarter 3 | 0.43 (0.22 to 0.85) | 0.015 | 0.43 (0.22 to 0.84) | 0.014 | 0.54 (0.17 to 1.65) | 0.277 | 0.48 (0.16 to 1.44) | 0.191 |
| Quarter 4 | 0.50 (0.26 to 0.97) | 0.039 | 0.49 (0.25 to 0.94) | 0.031 | 0.21 (0.04 to 1.00) | 0.05 | 0.28 (0.08 to 1.06) | 0.061 |
| P for trend | 0.017 | 0.015 | 0.042 | 0.051 | ||||
DR, DME and VTDR were set as outcomes of the regression model, respectively.
BAI, Body Adiposity Index; BMI, body mass index; DME, diabetic macular oedema; DR, diabetic retinopathy; VTDR, vision-threatening diabetic retinopathy; WHR, waist to hip ratio; WHtR, waist to height ratio.
In the logistic regression model adjusted for sex and age, Q4-WHtR remained as a protective factor of VTDR (OR=0.40 (95% CI 0.16 to 0.96), (online supplemental table S1), independent of lipid metabolism parameter (online supplemental table S2). In the full model, Q2-WHtR presented as a significant risk factor of DME (OR=3.04 (95% CI 1.04 to 8.85), table 3) and VTDR (OR=2.74 (95% CI 1.01 to 7.43)). The association was also more significant in female patients (for DME, p for trend=0.065, or of Q2-WHtR=6.79 (95% CI 1.19 to 38.57); for VTDR, p for trend=0.049, OR of Q2-WHtR=7.38 (95% CI 1.48 to 36.77) (online supplemental table S3-1), but not in male patients either (online supplemental table S3-2).
Association of BAI with any DR and severe DR
The prevalence of DR and severe VTDR showed a downward trend with the increase of BAI (figure 2, table 2). However, in the univariate logistic regression model, increased BAI was associated with a decreased risk of DR (table 4). After adjusted for sex and age, the association became less significant, while in the full model, the association with either any DR, DME or VTDR was not significant.
Discussion
In this study, we enrolled 2305 participants and analysed the association of obesity with any DR, DME and VTDR. There are three main findings in our study. First, only 29.6% of patients with diabetes had normal weight, while as high as 67.8% of patients with diabetes were overweight or obese. Second, obesity (BMI >25.0 kg/m2) presented as a significant protective factor of VTDR, while Q2-WHtR presented as a significant risk factor. Third, we found a significant negative association between BAI and DR in the univariate logistic regression model, while the association became less significant in the multivariable model.
Previous studies recognised obesity as a critical component of metabolic syndrome, which induces insulin resistance and advances the development of type 2 diabetes.2 3 Therefore, weight control is usually recommended in the management of diabetes and several systemic diseases to reduce the prevalence of complications.3 11 12 However, in this study, three obesity-related indexes, BMI, WHtR and BAI, were all negatively associated with DR. The result presented as an ‘obesity paradox’, which was also presented in several previous studies.13–15 Moreover, it was more significant in the association with VTDR. The first possible reason would be that VTDR, presented and DME or PDR, was more likely to appear in the patients with advanced diabetes. Advanced diabetes would manifest as weight loss as one of the metabolic complications, contributing to the inverse association of obesity with DR. Second, BMI could hardly differentiate general obesity and centripetal obesity, which may play a different role in the progress of diabetes. Third, all the participants were from the community. They were diagnosed with diabetes in the hospital before the enrollment. The patients who had severe complications, low willingness to seek doctor’s help or mobility problems, would be limited, contributing to the selection bias in the study.
Although obesity was recognised as one of the important biomarkers inducing insulin resistance,16 the obesity paradox has prevented scientists from making recommendations on weight management for patients with diabetes. The positive correlation between centripetal obesity (presented as higher WHR) and diabetic progression has shed light on this problem.17 WHR was regarded to assess centripetal obesity, and BAI is established and has a significant linear relationship with body fat rate.18 They demonstrated that abdominal obesity may be a more critical factor of DR than the generalised obesity. However, in our study, as the indicator of centripetal obesity, Q2-WHtR associated positively with DR, and WHtR generally shows an opposite trend, indicating a nonlinear relationship between centripetal obesity. Therefore, we are collecting follow-up data to further prospectively analyse the relationship between obesity and DR.
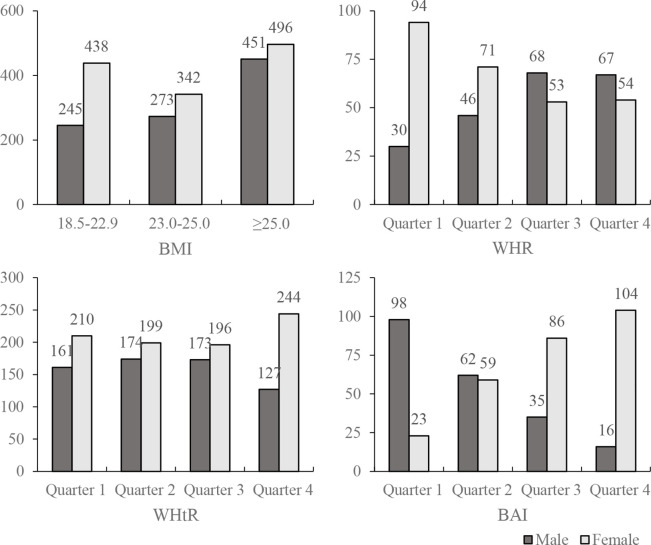
Our study also found that the associations between obesity-related index (both BMI and WHR) and VTDR were only significant in female patients, indicating that female patients would have a higher risk with the increase of centripetal obesity. The sex-specific obesity–diabetes association has been reported in several studies, but the association between obesity-related indexes (including BMI, WHR, WHtR, BAI) and DR was seldom reported. We furthered analysed the sex-specific distribution regarding different obesity-related indexes (figure 3). Male patients have a significantly higher WHR, lower WHtR and lower BAI. However, the results may be influenced by the small number of patients in some of the categories after they were grouped by sex (there were ‘no observation’ categories in online supplemental table S3-1 and online supplemental table S3-2). Therefore, more studies should be designed to investigate the weight control management and standard weight range regarding different sex in patients with diabetes.
Figure 3.
The number of male and female patients in different groups of the obesity-related indexes. BAI, body Adiposity Index; BMI, body mass index; WHR, waist to hip ratio; WHtR, waist to height ratio.
There are some other limitations in this study. First, in order to reduce the examination time and improve the compliance of participants, the measurement of waist circumference and hip circumference was not performed on every participant, while eventually 483 patients have undergone all the measurement including height, weight, waist circumference and hip circumference. Therefore, we are unable to put BMI, WHR, WHtR and BAI in the full model with 2305 patients at the same time. Second, although we used robust regression to make the OR more robust, we did not exclude the influence of collinearity in the full model, which may contribute to the variation of the association of factors such as BAI with DR. Third, the diabetic participants with severe condition (eg, very poor eye sight, past DR treatment history, occurred with other combined eye diseases that could affect retinal thickness) were excluded. On contrary, the participants usually had a less severe condition, which may affect the generalisability of the results.
In summary, this study provides medical data of 2305 participants, and analysed the relationship between obesity and DR. The results presented general obesity and centripetal obesity as a protective factor in the development of DR, which was more significant in female patients. Because the interactions between obesity and DR is not completely clear, further researches are needed to focus on the improvement of sex-specific weight management in patients with diabetes regarding different sex.
Supplementary Material
Footnotes
Contributors: WW and WH had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. WW and WH conceived and designed the study. WL, WW, XG, LW, JM, YL and KX collected and interpreted the data. WL, WW and LJ carried out the statistical analysis. WL wrote the manuscript. WH, WW, XG and XL reviewed and edited the manuscript. All authors have seen the final version of the manuscript and approved it for publication.
Funding: This study was supported by National Natural Science Foundation of China (82171084, 8200090, 72061137002), and Guangzhou Science & Technology Plan of Guangdong Pearl River Talents Program (202102010162).
Disclaimer: The funding organisations had no role in the design or conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
Ethics approval
This study followed the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board of Zhongshan Ophthalmic Center (2017KYPJ094), Guangzhou, China. Written informed consent was obtained from all participants. Patient records and information were anonymized and de-identified before analysis.
References
- 1.Ting DSW, Cheung GCM, Wong TY. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Exp Ophthalmol 2016;44:260–77. 10.1111/ceo.12696 [DOI] [PubMed] [Google Scholar]
- 2.Malone JI, Hansen BC. Does obesity cause type 2 diabetes mellitus (T2DM)? or is it the opposite? Pediatr Diabetes 2019;20:5–9. 10.1111/pedi.12787 [DOI] [PubMed] [Google Scholar]
- 3.Hitman GA, Finer S. Obesity and type 2 diabetes; achieving weight loss. Diabet Med 2011;28:627. 10.1111/j.1464-5491.2011.03319.x [DOI] [PubMed] [Google Scholar]
- 4.Hao Z, Huang X, Qin Y, et al. Analysis of factors related to diabetic retinopathy in patients with newly diagnosed type 2 diabetes: a cross-sectional study. BMJ Open 2020;10:e32095. 10.1136/bmjopen-2019-032095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu W, Wu Y, Meng Y-F, et al. Association of obesity and risk of diabetic retinopathy in diabetes patients. Medicine 2018;97:e11807. 10.1097/MD.0000000000011807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madsen LR, Bek T, Richelsen B. Diabetic retinopathy in people with type 2 diabetes and obesity treated by Roux‐en‐Y gastric bypass compared with non‐operated controls: with focus on the role of diabetes remission in a cross‐sectional and a 6‐year follow‐up study. Diabet. Med. 2018. 10.1111/dme.13876 [DOI] [PubMed] [Google Scholar]
- 7.Raum P, et al. Prevalence and cardiovascular associations of diabetic retinopathy and maculopathy: results from the Gutenberg health study. Plos One 2015;10:e127188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hills AP, Arena R, Khunti K, et al. Epidemiology and determinants of type 2 diabetes in South Asia. Lancet Diabetes Endocrinol 2018;6:966–78. 10.1016/S2213-8587(18)30204-3 [DOI] [PubMed] [Google Scholar]
- 9.Forthun I, Wilcox AJ, Strandberg-Larsen K, et al. Maternal prepregnancy BMI and risk of cerebral palsy in offspring. Pediatrics 2016;138. 10.1542/peds.2016-0874. [Epub ahead of print: 08 09 2016]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morisaki N, Nagata C, Jwa SC, et al. Pre-pregnancy BMI-specific optimal gestational weight gain for women in Japan. J Epidemiol 2017;27:492–8. 10.1016/j.je.2016.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.IOTF . Asia-Pacific perspective: redefining obesity and its treatment 2000.
- 12.Esc guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2013;34:3035–87. 10.1093/eurheartj/eht108 [DOI] [PubMed] [Google Scholar]
- 13.Smith JJ, Wright DM, Scanlon P, et al. Risk factors associated with progression to referable retinopathy: a type 2 diabetes mellitus cohort study in the Republic of Ireland. Diabet Med 2020;37:1000–7. 10.1111/dme.14278 [DOI] [PubMed] [Google Scholar]
- 14.Li Y-H, Sheu WH-H, Lee I-T. Influence of diabetic retinopathy on the relationship between body mass index and mortality in patients with poorly controlled type 2 diabetes. Diabetes Metab Syndr Obes 2020;13:907–14. 10.2147/DMSO.S246032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Rubeaan K, Abu El-Asrar AM, Youssef AM, et al. Diabetic retinopathy and its risk factors in a Society with a type 2 diabetes epidemic: a Saudi national diabetes registry-based study. Acta Ophthalmol 2015;93:e140–7. 10.1111/aos.12532 [DOI] [PubMed] [Google Scholar]
- 16.Arkan MC, Hevener AL, Greten FR, et al. Ikk-Beta links inflammation to obesity-induced insulin resistance. Nat Med 2005;11:191–8. 10.1038/nm1185 [DOI] [PubMed] [Google Scholar]
- 17.Man REK, Sabanayagam C, Chiang PP-C, et al. Differential association of generalized and abdominal obesity with diabetic retinopathy in Asian patients with type 2 diabetes. JAMA Ophthalmol 2016;134:251–7. 10.1001/jamaophthalmol.2015.5103 [DOI] [PubMed] [Google Scholar]
- 18.Bergman RN, Stefanovski D, Buchanan TA, et al. A better index of body adiposity. Obesity 2011;19:1083–9. 10.1038/oby.2011.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-056332supp001.pdf (49.9KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.