Abstract
Background:
Head and neck cancer (HNC) patients undergoing radiation therapy (RT) experience significant side effects, presenting challenging care tasks for their informal (unpaid) caregivers. HNC caregivers report low caregiving self-efficacy, high distress, and interest in supportive care interventions.
Objective:
This randomized pilot trial assessed the feasibility and acceptability of a 6 to 7 week supported self-management intervention (Prepare to Care) offering psychoeducation and stress management skills building for caregivers of patients receiving RT for HNC.
Methods:
Caregivers were randomized to Prepare to Care or standard of care. Primary feasibility measures included participation and retention percentages. Assessments were completed before the intervention, at intervention completion, and 6-weeks later after intervention completion.
Results:
Caregivers (N = 38) were predominantly female (88.6%), an average age of 56 years old, and a spouse/partner to the patient (71.4%). Participation percent was 42.2%; retention at intervention conclusion was 80% and 77% at the 6-week follow-up. Quantitative and qualitative results support acceptability, with 64% to 88% reporting each intervention module was helpful (quite a bit or very). Intervention caregivers reported a significantly greater improvement in self-efficacy for progressive muscle relaxation (PMR).
Conclusions:
Prepare to Care and the randomized pilot trial methods are feasible and acceptable for HNC caregivers of patients receiving RT. A significant treatment effect was observed for self-efficacy for PMR, and findings were in the expected direction regarding improved caregiving self-efficacy. Further research is necessary to determine the efficacy of this intervention with a focus on increased engagement strategies and longer-term outcomes.
Trial Registration:
Keywords: head and neck cancer, oncology, caregiving, intervention, self-efficacy, progressive muscle relaxation
Introduction
Treatment for the 66 000 people diagnosed with HNC each year in the United States is complex and often includes daily radiotherapy (RT) for 6 to 7 weeks, sometimes in combination with chemotherapy and/or surgery.1,2 Patients experience significant side effects that impede core aspects of daily life and report higher care needs relative to other cancer patients, presenting challenging care tasks for informal caregivers.3-5 Caring for a patient with HNC may include special food preparation (eg, pureeing food) and feeding tube management, as well as more traditional informal caregiving tasks such as providing emotional support, administering medications, managing medical appointments and insurance claims, communicating with health care providers, and providing hands-on medical care with little to no training.3,6-8 Cancer caregivers, including HNC caregivers, commonly report low self-efficacy for specific caregiving tasks. 6 Low self-efficacy (ie, one’s confidence in capacity to engage in a particular behavior) among cancer caregivers has been shown to be associated with worse caregiver distress, strain, coping, quality of life, adaptation, caregiver functioning, and physical health, as well as patient physical and emotional symptom burden.9-11
HNC caregivers also report high rates of and clinically significant emotional distress, caregiver burden, fear of patient cancer recurrence, and poorer physical and mental health compared to patients and the general population.12-16 One study demonstrated that during and shortly following the patient’s course of RT, HNC caregivers reported increased schedule burden, decreased self-esteem, poorer self-reported health, and dysregulation of the psychoneuroendocrine system. 17 Similarly, another study showed that HNC caregivers experienced a steady increase in distress, peaking 5 weeks into the patient’s radiotherapy treatment. 18 Collectively, these findings suggest a critical need to support HNC caregivers during patient radiotherapy, a time of substantial patient care needs.19-21
HNC caregivers have a need for, and are interested in, supportive care interventions.4,22-26 The few existing interventions for HNC caregivers have demonstrated promise, but have focused solely on optimizing delivery of patient care or have intervened on both members of the patient-caregiver dyad.27-30 The majority were time and resource-intensive, requiring several in-person or lengthy telephone sessions. Resource-intensive interventions requiring significant provider or support staff engagement can be costly to deliver and result in limited translation to clinical practice. 31 In supported self-management interventions, the recipient uses self-directed educational materials to learn information and strategies to manage emotions and promote healthy behaviors with the support of a health care provider.32,33 Such interventions are often used for individuals affected by chronic diseases, such as cancer. 34 Supported self-management interventions can be low cost, reduce health care utilization, and have greater dissemination potential.32,35 Further, supported self-management interventions can be used according to the participant’s preferences for place and time and lessen logistical barriers to in-person delivered interventions. As such, they may be especially attractive to HNC caregivers navigating the hectic radiotherapy treatment period. Supported self-management interventions have shown improvements in fatigue, depression, anxiety, and overall quality of life; however, to date, these interventions have largely been applied in cancer patients, particularly among cancer populations not including HNC (eg, breast, lung, prostate), and have been under-utilized in the cancer caregiving field.32-34
In this study, we conducted a randomized pilot trial to evaluate Prepare to Care, a supported self-management intervention that teaches psycho-education and stress-management skills for caregivers of patients receiving radiation therapy. Our primary aim was to assess the feasibility and acceptability of the intervention and the randomized pilot trial methods. We also assessed potential improvements in self-efficacy for caregiving and other caregiver outcomes including distress (anxiety and depression), quality of life, and self-efficacy for abbreviated progressive muscle relaxation.
Methods
Study Design and Overview
We assessed the feasibility and acceptability of a 6 to 7-week self-management intervention and the randomized pilot trial methods for caregivers of HNC patients undergoing radiation therapy using a parallel group design. Caregivers were randomized 1:1 to the intervention or standard of care control group using a computer-generated randomization scheme with mixed block sizes developed by the biostatistician. Caregivers completed assessments before the intervention (T1), at intervention completion (T2), and 6-week after intervention completion (T3). Intervention caregivers completed a semi-structured qualitative interview at T2. Sociodemographics and caregiving characteristics were collected at recruitment. We also collected clinical characteristics for the patient at study recruitment from the electronic health record. This study was approved by the local Institutional Review Board (IRB00038084) and registered with clinicaltrials.gov (NCT03032250) and adheres to CONSORT recommendations for reporting a pilot feasibility trial. 36
Participants
We approached patient-caregiver dyads at 2 outpatient radiation clinics at the Comprehensive Cancer Center of Wake Forest Baptist Medical Center. If a primary, informal (unpaid) caregiver was not present, with permission of the patient we contacted the caregiver by telephone to gage interest in the study. Though Prepare to Care is a caregiver-directed intervention, at study initiation, both members of the dyad provided written informed consent. In order to increase accrual, we later revised the eligibility criteria so that patient consent was not required for caregiver participation. Patient eligibility included: (1) new or recurrent AJCC stage I to IV squamous cell carcinoma of the upper aerodigestive tract (including lip/oral cavity, nasopharynx, salivary gland, oropharynx, hypopharynx, paranasal sinus, and larynx cancers), (2) planned external beam radiotherapy (± chemotherapy) for 6 to 7 weeks (with curative intent), (3) ≥18 years of age, and (4) having an informal (unpaid) caregiver during RT who is also willing to participate in the study. Patients were excluded if they could not read/communicate in English. Caregivers were eligible if they were: (1) providing the majority of the informal (unpaid) care for an adult meeting the above criteria, and (2) ≥18 years of age. Caregivers were excluded if they (1) had a current cancer diagnosis or (2) could not read/communicate in English. Caregivers received $20 gift cards at the completion of T1, T2, and T3 assessments ($60 in gift cards total).
Intervention Development and Theoretical Underpinnings
Intervention content, timing, and delivery format were developed prior to the current study using a 3-step process including: (1) assessing preferences for wellness programs, including delivery and timing preferences, among a different sample of HNC caregivers 12 ; (2) obtaining input regarding intervention content and delivery from an advisory panel including HNC caregivers and clinicians; and (3) reviewing empirical literature to identify HNC challenges, especially during the radiotherapy period. The Prepare to Care intervention is guided by the self-efficacy construct from Social Cognitive Theory. 11 Self-efficacy refers to one’s confidence in his or her capacity to engage in a particular behavior 11 and is a central component for self-management interventions. 33 Prepare to Care’s self-directed educational modules aim to improve caregiving self-efficacy by promoting the development of skills to manage caregiver emotions and coping, improve communication with the patient and health care providers, promote healthy behaviors, and support patient care.
Intervention Content and Procedures
The multi-component intervention, Prepare to Care, includes: (1) a brief introduction video on a DVD and study website; (2) 8 modules (Table 1) available as a hard copy workbook and on the study website; and (3) CD with audio instructions to facilitate Progressive Muscle Relaxation (PMR) Training (to accompany a module on PMR). PMR training teaches caregivers to identify muscle tension signals and to use the signals as cues to trigger a relaxation response. 37 Prepare to Care is a 6 to 7 week intervention aligned with a patient’s RT (ie, intervention began at the start of radiotherapy and was completed at the end of radiotherapy), in recognition that the severity of multiple RT-related acute toxicities and associated care burdens increase during the treatment regimen. 21 To maximize intervention engagement, modules were designed to be succinct, and study iPads linking directly to the website were available for use at the radiotherapy clinic. Each module includes an introduction and rationale for the topic, strategies to overcome relevant challenges, and an activity to facilitate skills building. Caregiver vignettes are interspersed throughout the modules.
Table 1.
Description of Prepare to Care Intervention Modules.
| Module | Description |
|---|---|
| Cancer education | Information about HNC, radiotherapy (± chemotherapy), side effects, and managing patient side effects |
| Using your resources | Information linking caregivers to supportive resources available within (a) the Comprehensive Cancer Center of Wake Forest University, (b) the local community, and (c) the internet |
| Managing time | Managing a busy schedule, including recognizing competing outside demands and prioritizing |
| Seeking/accepting support | Importance of social support, learning to identify supporters, and ask for help when needed |
| Communicating with others | Strategies to optimize communication with provider, patient, and other friends/family members |
| Healthy behaviors | Importance of healthy behaviors (diet, physical activity, sleep) as a cancer caregiver |
| Positive coping | Learning to identify and re-frame negative thoughts 46 |
| Muscle relaxation | Importance of engaging in relaxation; also includes a CD with audio instructions for Progressive Muscle Relaxation (PMR) |
After recruitment and randomization, caregivers in the intervention group met with the study interventionist at the radiation clinic or by telephone to review the intervention. All participants were provided access to the online website and hard-copy materials (provided in person or by mail). Interventionist involvement was minimal; sessions were brief (approximately 10 minutes) and supported caregivers’ independent engagement with the materials by checking in on caregivers’ module completion and answering potential questions. Each week, caregivers completed a brief assessment to help guide them to relevant modules, though caregivers were encouraged to complete the modules they felt would be most helpful. The assessment included a 24-item checklist with items that corresponded to topics covered in the 8 modules. Modules with the most items selected were recommended. Participants had the option to complete this with the interventionist or on their own. This preference-based approach empowers caregivers to build skills to address their evolving challenges. Caregivers were asked to complete at least 1 module per week and re-visit modules as needed and did not need to complete all modules as part of their participation. Caregivers were encouraged to utilize intervention materials at a time that best fit their schedule. Modules included time logs to track use. The interventionist also sent weekly text or email reminders to complete modules, based on participants’ communication preferences.
Control Group
Caregivers participating in the control group received standard care, which may have included referral to cancer center support services, though this was not a systematic process. At the conclusion of the study, caregivers participating in the control group were provided with Prepare to Care written materials.
Feasibility and Acceptability
Our primary feasibility measures were participation and retention proportions. Prepare to Care acceptability was assessed with a quantitative survey developed for the study and a semi-structured qualitative interview at T2. The quantitative survey asked caregivers to rate how helpful each component of the intervention was from 0 (“Not at all helpful”) to 4 (“Very much helpful”). Qualitative interviews (lasting approximately 30 minutes to 1 hour) explored factors influencing overall acceptability. Specifically, caregivers were asked about their overall experience with the intervention and what they liked or wished was different about each of the intervention components or features. Interviews were recorded and transcribed verbatim.
Measures
Measures were completed online using REDCap, by telephone, or with paper versions completed in person at the clinic or at home and returned by mail. The Caregiver Inventory (CI) 38 is a 21-item instrument that assesses caregiving self-efficacy including self-efficacy for managing medical information, caring for the patient, caring for oneself, and managing difficult interactions/emotions. The CI uses a 9-point Likert scale (“not at all confident” to “totally confident”). Total scores range from 21 to 189, with higher scores indicating greater caregiving self-efficacy. The CI has demonstrated adequate validity and reliability and has been previously used with HNC caregivers.38,39 Depressive symptoms were assessed with the Center for Epidemiological Studies Depression Scale (CES-D), 40 a 20-item instrument assessing frequency of symptoms associated with depression (ie, restless sleep, poor appetite, and feeling lonely). Scores range from 0 to 60, with higher scores indicating more depressive symptoms. The CES-D has been widely used in caregiver populations.12,41,42 Anxiety was assessed with the PROMIS Emotional Distress Anxiety Short Form- 8a, 43 a brief 8-item assessment of anxiety. T-scores range from 37.1 to 83.1, with higher scores reflecting greater anxiety. We assessed quality of life using the total score of the Caregiver Quality of Life Index-Cancer (CqoL-Canc). The CqoL-Canc is a 35-item instrument that assesses burden, disruptiveness, positive adaptation, and financial concerns and provides a total quality of life score. The CqoL-Canc uses a 5-point Likert scale (“not at all” to “very much”), with scores ranging from 0 to 100 and higher scores indicating better quality of life. The instrument has demonstrated reliability and validity and is strongly recommended as quality of life assessment for cancer caregivers.44,45 Self-efficacy for engaging in progressive muscle relaxation (PMR) was assessed with 3-items developed for the purpose of this study (Cronbach’s alpha .89). The instrument uses a 9-point Likert scale (“not at all confident” to “totally confident”), with scores ranging from 3 to 27. Higher scores reflect greater self-efficacy for PMR. These items assess self-efficacy for: (1) distinguishing between tense and relaxed muscles, (2) relaxing each muscle one by one, and (3) relaxing quickly when in stressful situations.
Data Analysis
The sample size was based upon the primary outcome of feasibility. For the targeted sample size of 40, two-sided 95% confidence interval around the expected recruitment (70%) and retention (80%) percentages were within ±12%, yielding 95% confidence that the true recruitment percentage would exceed 59% and the true retention percentage would exceed 67%, both of which would indicate a feasible study. Descriptive statistics (means and standard deviations for continuous variable and frequencies/percentages for categorical variables) were used to summarize sociodemographic characteristics and caregiving characteristics at T1. Participation and retention proportions and their associated 95% confidence intervals (CI) were estimated. Participation proportion was estimated as proportion of all eligible screened caregivers who agreed to participate; retention proportions were estimated as proportion of all enrolled participants completing T1 surveys who completed all subsequent (T2 and T3) surveys.
Acceptability of the intervention was summarized quantitatively and qualitatively by caregivers in the intervention arm following the conclusion of the intervention. Percentages of caregivers reporting that an intervention component was quite a bit or very much helpful were calculated to demonstrate acceptability of each intervention component. Qualitative interviews were coded by 2 raters using a thematic analysis procedure.46,47 Specifically, coders read and re-read interview transcripts and identified themes related to acceptability and suggestions for improvement. Discrepancies were resolved with team discussion to achieve consensus.
Finally, though our study was not powered to detect intervention effects, as a secondary goal we examined differences in key outcomes. In an intent-to-treat analysis, we used mixed models with a random subject effect and fixed effects of time (T1, T2, T3, treated as 3 categories), intervention (yes/no), and included the first-order interaction between intervention assignment and time. We used linear contrasts to estimate average within-person changes by time by intervention group. A 2-sided alpha level of .05 was used to indicate statistical significance. Outcomes examined included self-efficacy for caregiving (CI total score as well as the 4 subscales), self-efficacy for progressive muscle relaxation, depression, anxiety, and overall CqoL-Canc total index score). All analyses were conducted in SAS (version 9.4, Cary, NC).
Results
Feasibility
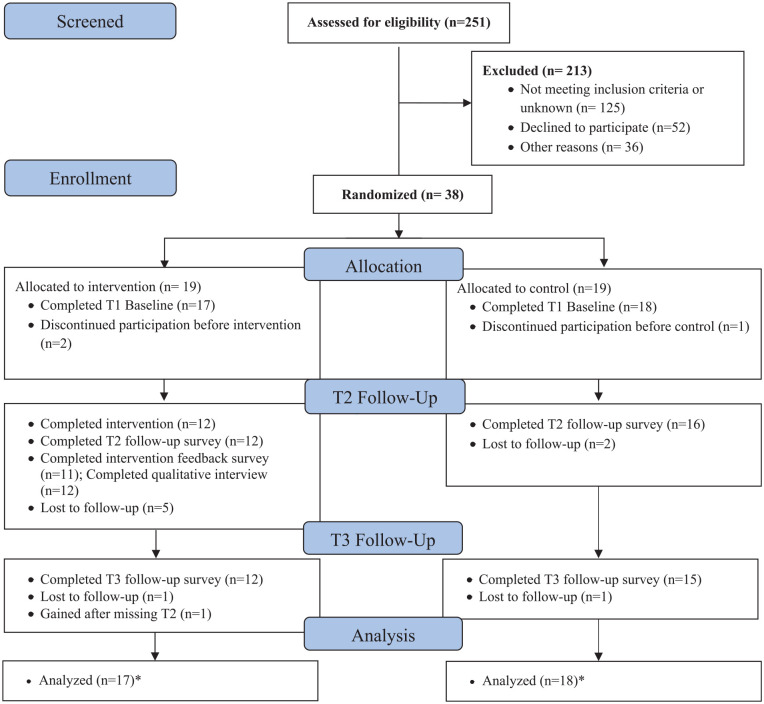
Caregivers were approached for recruitment between May 2017 and December 2019, with brief disruptions due to staffing issues. Of the 90 eligible caregivers, 38 (42.2%, 95% CI 32.0%-52.4%) agreed to participate and were randomized to the Prepare to Care intervention (n = 19) or control group (n = 19). See Table 2 for sociodemographic and caregiving characteristics. Of the 38 caregivers randomized, 35 completed T1 assessments (92%; 95% CI 79%-98%; n = 17 intervention; n = 18 control). The 3 caregivers who did not return T1 surveys or initiate intervention activity are not included in our primary analyses. Of the 35 caregivers who completed T1 (38.9% of the 90 eligible; 95% CI 28.8%-49.0%, n = 17 intervention; n = 18 control), 28 (80%; 95% CI 63.1%-91.6%) were retained at T2 (12 [70.6%; 95% CI 44%-89.7%] intervention; 16 [88.9%; 95% CI 65.3%-98.6%] control). Twenty-seven caregivers (77.1%; 95% CI 63.2%-91.1%) were retained at T3 (12 [70.6%; 95% CI 44%-89.7%] intervention; 15 [83.3%; 95% CI 58.6%-96.4%] control) (See Figure 1). Data collection was completed February 2020, at which point the trial was considered complete.
Table 2.
Baseline Sociodemographic and Caregiving Characteristics.
| Intervention (n = 17) | Control (n = 18) | Total (n = 35) | |
|---|---|---|---|
| Sociodemographic characteristics | |||
| Sex, n (%) | |||
| Male | 0 (0) | 4 (22.22) | 4 (11.43) |
| Female | 17 (100) | 14 (77.78) | 31 (88.57) |
| Age (years), mean (SD) | 55.3 (15.5) | 57.4 (11.8) | 56.4 (13.6) |
| Race/Ethnicity, n (%) | |||
| White | 16 (94.1) | 13 (72.2) | 29 (82.9) |
| Black | 1 (5.9) | 4 (22.2) | 5 (14.3) |
| Other | 0 (0) | 1 (5.6) | 1 (2.9) |
| Non-Hispanic | 16 (94.1) | 18 (100) | 34 (97.1) |
| Missing | 1 (5.9) | 0 (0) | 1 (2.9) |
| Education, n (%) | |||
| <High School/High School Graduate or GED | 5 (29.4) | 6 (33.3) | 11 (31.4) |
| Some College/College Graduate | 10 (58.9) | 10 (55.6) | 20 (57.1) |
| Graduate degree | 1 (5.9) | 2 (11.1) | 3 (8.6) |
| Other | 1 (5.9) | 0 (0) | 1 (2.7) |
| Employment status, n (%)* | |||
| Employed | 9 (52.9) | 11 (61.1) | 20 (57.1) |
| Retired | 5 (29.4) | 3 (16.7) | 8 (22.9) |
| Other | 3 (17.6) | 4 (22.2) | 7 (20.0) |
| Marital status, n (%) | 13 (76.5) | 12 (66.6) | 25 (71.4) |
| Married or has partner single/widowed | 4 (23.5) | 6 (33.3) | 10 (28.6) |
| Caregiving characteristics | |||
| Caregiver relationship to patient, n (%) | |||
| Spouse or partner | 12 (70.6) | 13 (72.2) | 25 (71.4) |
| Other family member | 5 (29.4) | 4 (22.3) | 9 (25.7) |
| Friend | 0 (0) | 1 (5.6) | 1 (2.9) |
| Lives with patient, n (%) | |||
| Yes | 14 (82.4) | 14 (77.8) | 28 (80.0) |
| Daily hours of caregiving, n (%) | |||
| 1-4 | 5 (29.5) | 7 (38.9) | 12 (34.2) |
| 5-8 | 5 (29.5) | 4 (22.3) | 9 (25.7) |
| 9+ | 7 (41.2) | 6 (33.3) | 13 (37.1) |
| Missing | 0 (0) | 1 (5.6) | 1 (2.9) |
| Has help from additional caregiver in household**, n (%) | |||
| Yes | 3 (21.4) | 1 (7.1) | 4 (14.3) |
| Provides household childcare, n (%) | |||
| Yes | 6 (35.3) | 6 (33.3) | 12 (34.4) |
Other includes unemployed, disabled, or keeping house.
Calculated among caregivers (n = 28 total) who report living with patient.
Figure 1.
Study flow diagram.
*Participants with data at any time point were included in the intent to treat analysis.
Acceptability
Eleven of the intervention caregivers (65%) provided quantitative ratings of helpfulness of each intervention component. Given the preference-based nature of the intervention, caregivers only rated helpfulness for modules used. The majority of caregivers rated each of the used modules as quite a bit/ very much helpful, ranging from 64.0% of caregivers (eg, Managing Time Module) to 88.0% of caregivers (Utilizing Resources Module). All modules were completed by all participants who completed the survey, with the exception of the Utilizing Resources Module (completed by 80% of participants) and the Seeking and Accepting Support Module (completed by 91% of participants). See Table 3 for acceptability ratings for modules and other intervention components and features.
Table 3.
Intervention Use and Acceptability Survey Results among Intervention Caregivers who completed the Survey at T2 (n = 11).
| Intervention components and features | Participants who used component, n | Participant ratings* | ||
|---|---|---|---|---|
| Not at all helpful, n (%) | A little bit/ somewhat helpful, n (%) | Quite a bit/very much helpful, n (%) | ||
| Modules | ||||
| Cancer education | 11 | 0 | 2 (18.2) | 9 (82.0) |
| Utilizing resources | 8 | 0 | 1 (13.0) | 7 (88.0) |
| Managing time | 11 | 1 (9.1) | 3 (27.3) | 7 (64.0) |
| Seeking/accepting support | 10 | 1 (10.0) | 2 (20.0) | 7 (70.0) |
| Communicating with others | 11 | 0 | 4 (36.4) | 7 (64.0) |
| Healthy behaviors | 11 | 0 | 1 (9.1) | 7 (64.0) |
| Positive coping | 11 | 0 | 1 (9.1) | 7 (64.0) |
| Muscle relaxation | 11 | 0 | 4 (36.4) | 7 (64.0) |
| Supplemental components | ||||
| Introductory video | 8 | 0 | 4 (50.0) | 4 (50.0) |
| Relaxation CD | 9 | 0 | 2 (22.2) | 7 (77.8) |
| Reminders | ||||
| Weekly text reminders | 11 | 0 | 5 (45.5) | 6 (55.0) |
| Weekly email reminders | 9 | 4 (44.4) | 2 (22.2) | 4 (44.4) |
The intervention allowed caregivers to select intervention components; therefore, caregivers only responded to acceptability survey items for the components they used.
Denominator equals number of participants who used the component, among those who responded to the survey.
Qualitative findings (n = 12 intervention caregivers, 71%) provided additional support for caregivers’ acceptance of the Prepare to Care Intervention. All caregivers reported a positive experience using the Prepare to Care Intervention, citing several ways that the intervention was helpful. Caregivers noted that the intervention normalized what they were feeling, prompted them to ask for help, and linked them to additional helpful resources (See Table 4). Caregivers reported the modules increased their understanding of module content and what to expect, which better equipped the caregiver to care for the patient. Similarly, it was noted that the intervention allowed caregivers to focus their energy on their role as a caregiver. Importantly, caregivers also indicated that they developed new skills as a result of Prepare to Care such as relaxing and coping, learning to ask for help, and communicating with their loved one and others. One caregiver expressed that the intervention was a catalyst for a newfound desire to help others in similar situations.
Table 4.
Exemplar Quotes from Caregivers in the Prepare to Care Intervention.
| Theme | Exemplar quote |
|---|---|
| Advantages of Prepare to Care intervention participation | |
| Developed self-care skills | “I liked that it told you, taught you, showed you that it’s okay to worry about yourself too. And to help keep yourself organized and try not to completely freak out over every little thing and the way it taught you to kind of calm down, and focus, and meditate, and just chill out for a while.” (Participant 2, 56) |
| “I think communicating with others was good because it kinda helped me, I wouldn’t say venting, but it helped me to get some of my emotions out by talking about it with other people.”(Participant 9, Age 61) | |
| “It helped me tremendously. It helped me learn how to relax and cope with it.” (Participant 3, 44) | |
| Increased awareness of their own needs | “Knowledge is power, so if I can have something to hold on to and, you know, know that it’s gonna help me, even the breathing techniques and things like that, it covered so many different areas. . . that sometimes caregivers might, you know, put themselves aside or their needs aside, but this sort of really brought it all to the forefront, so, no I was really fortunate to have had this experience.” (Participant 11, 58) |
| Normalized their own feelings | “Just knowing that what you’re feeling is natural, I guess you could say” (Participant 6, 41) |
| “My naked feelings were okay” (Participant 1, 58) | |
| Better equipped to help patient | “I was able to read those modules, and, just educate myself, not just for my benefit, but for his. I would know what to expect or, I felt like I had great resources that helped me help him.” (Participant 11, 58) |
| “. . .from the first minute I heard, my brother say that he had cancer, you sort of don’t know where to start. it’s sort of like an atom spewing out all these electrons I guess. but having that information eventually, just sort of brought it all together, and maybe. . . it gave me as we as my siblings the opportunity to sort of focus our energy better, rather than just going randomly to help.”(Participant 11, 58) | |
| Easy to participate in intervention | “I found it easier to take care . . . to do the intervention . . . while taking care of him. I didn’t feel any guilt while doing that. Taking the time. But other things, I mean, to the point of feeling guilty for being able to eat food.”(Participant 7, 44) |
| Challenges associated with Prepare to Care intervention participation | |
| Unable to use information at the time of participation | “Definitely, there was so much useful information in there. None that I necessarily could use at the time as I was going through it but that I will definitely access now that we’re almost through this part of the journey, that I can calm down and settle down and look at it and use it.”(Participant 8, 58) |
| Desire for more in depth information | “Some of them, I was like, ‘Okay, you know, maybe deeper in depth on some of the topics’.” (Participant 7, 44) |
| Challenging to remember to engage in intervention | “Between work and then with his schedule and everything else. . .but with everything else, you know, picking up the household slack and everything, sometimes it was just hard to remember.” (Participant 7, 44) |
Caregivers also made suggestions for improving the intervention. Though 2 caregivers noted that they would have liked more information, 1 caregiver reported that it was too much information and another caregiver thought the activities were too time consuming. While several caregivers commented on ease of intervention participation, 1 caregiver indicated that they were unable to utilize the information learned at the time, though they were still planning to do so now that their situation was calmer. Some caregivers also commented on how challenging it was to remember to participate and suggested sending additional text reminders or sending the text reminders at a different time of the day. The website was not an appealing option to caregivers, in part because it was duplicative of the hard-copy workbooks; suggestions included developing a website accessible on a cell phone, such as a web-app, and including an option to complete activities on a more interactive website.
Preliminary Examination of Intervention Effects
Caregivers participating in the intervention demonstrated a larger improvement in self-efficacy for caregiving (total score and subscales) compared to caregivers in the control group, though this difference was not significant (See Table 5). Among the instrument subscales, the intervention group showed the largest improvements in self-efficacy for caring for oneself and managing difficult interactions/emotions over time. We observed a significant improvement in self-efficacy for progressive muscle relaxation over time in intervention caregivers compared to control group caregivers. Significant intervention effects were not observed for distress or quality of life.
Table 5.
Analyzes of Intervention Effects from Repeated Measures Models (Intent-to-Treat Analysis, N = 35).
| Outcome measure | Intervention vs control | Estimated least squares mean (SE) | Estimated T2-T1 difference (95% CI)* | Difference of estimated differences (95% CI) | P | Estimated T3-T1 difference (95% CI)* | Difference of estimated differences (95% CI) | P | ||
|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | ||||||||
| Self-efficacy for caregiving total | Intervention | 135.5 (6.3) | 142.2 (2.0) | 147.8 (7.0) | 6.7 (−5.9, 19.2) | 4.0 (−12.8, 20.9) | .63 | 12.2 (−0.3, 24.8) | 4.9 (−12.1, 21.9) | .56 |
| Control | 144.3 (6.1) | 146.9 (6.3) | 151.6 (6.4) | 2.6 (−9.5, 13.8) | 7.3 (−4.1, 18.8) | |||||
| Self-efficacy-managing medical information | Intervention | 22.4 (0.9) | 23.0 (1.0) | 23.9 (1.0) | 0.7 (−1.5, 2.9) | 0.3 (−2.7, 3.3) | .83 | 1.5 (−0.7, 3.7) | 0.6 (−2.4, 3.6) | .69 |
| Control | 22.0 (0.8) | 22.3 (.9) | 22.9 (0.9) | 0.3 (−1.6, 2.3) | .9 (−1.1, 2.9) | |||||
| Self-efficacy-caring for care-recipient | Intervention | 52.9 (1.9) | 53.3 (2.2) | 55.2 (2.2) | 0.4 (−3.8, 4.6) | 0.9 (−4.7, 6.5) | .76 | 2.3 (−1.9, 6.5) | 2.8 (−2.9, 8.4) | .33 |
| Control | 55.1 (1.9) | 54.6 (2.0) | 54.6 (2.0) | −0.5 (−4.2, 3.2) | −0.5 (−4.3, 3.3) | |||||
| Self-efficacy- caring for oneself | Intervention | 25.7 (2.2) | 28.9 (2.4) | 29.0 (2.4) | 3.2 (−1.3,7.6) | 0.4 (−5.6,6.5) | .89 | 3.3 (−1.2, 7.8) | −1.7 (−7.8, 4.3) | .57 |
| Control | 28.1 (2.1) | 30.8 (2.2) | 33.1 (2.3) | 2.7 (−1.3,6.7) | 5.0 (1.0, 9.1) | |||||
| Self-efficacy-managing difficult interactions/emotions | Intervention | 34.5 (2.3) | 37.1 (2.5) | 39.6 (2.5) | 2.6 (−1.7,6.9) | 2.4 (−3.4,8.2) | .40 | 5.1 (0.8, 9.4) | 3.1 (−2.8,8.9) | .29 |
| Control | 39.1 (2.2) | 39.3 (2.3) | 41.1 (2.3) | 0.2 (−3.7,4.0) | 2.0 (−1.9, 5.9) | |||||
| Self-efficacy for progressive muscle relaxation total | Intervention | 11.9 (1.3) | 15.7 (1.5) | 17.3 (1.5) | 3.8 (1.2, 6.4) | 5.4 (1.9, 8.9) | .0034 | 5.4 (2.8, 8.0) | 5.9 (2.3, 9.5) | .0016 |
| Control | 13.3 (1.3) | 11.8 (1.4) | 12.8 (1.4) | −1.6 (−3.9, 0.8) | −0.5 (−2.9,1.9) | |||||
| Center for Epidemiological Studies-Depression (CES-D) | Intervention | 19.7 (3.0) | 19.9 (3.3) | 17.2 (3.3) | 0.2 (−.5.3, 5.7) | 4.9 (−2.5,12.2) | .19 | −2.6 (−8.1,3.0) | 3.8 (−3.6, 11.3) | .31 |
| Control | 19.3 (2.9) | 14.6 (3.0) | 12.9 (3.1) | −4.7 (−9.5, 0.2) | −6.4 (−11.4, −1.4) | |||||
| PROMIS Emotional Distress Anxiety Short Form-8a | Intervention | 59.2 (2.3) | 56.3 (2.6) | 53.8 (2.6) | −2.9 (−8.1, 2.2) | 0.8 (−6.1,7.7) | .83 | −5.4 (−10.6, −0.3) | 0.6 (−6.4, 7.5) | .87 |
| Control | 60.0 (2.2) | 56.3 (2.3) | 54.0 (2.4) | −3.7 (−8.3,0.9) | −6.0 (−10.7, −1.3) | |||||
| Caregiver Quality of Life Index-Cancer (CqoL-Canc) total | Intervention | 87.6 (5.5) | 88.6 (5.9) | 92.8 (5.9) | 0.9 (−7.8, 9.6) | −1.7 (−13.4, 9.9) | .49 | 5.1 (−3.6, 13.8) | −4.4 (−16.2, 7.3) | .45 |
| Control | 87.3 (5.8) | 89.9 (5.4) | 96.8 (5.5) | 2.7 (−5.1, 10.4) | 9.6 (1.6, 17.5) | |||||
Estimated as difference of least squares means from mixed model containing time (T1,T2, T3, corresponding to pre-intervention, intervention conclusion, and 6-week post intervention conclusion weeks) and intervention condition and first-order time × intervention condition interaction terms.
Bolded numbers reflect significant findings.
Discussion
Results from this randomized pilot trial study support the feasibility and acceptability of Prepare to Care for caregivers of patients receiving RT for HNC. The retention percentage (80% at intervention completion and 77% at follow-up) was consistent with the expected percentage and supported the feasibility of the intervention. The participation percentage of 42% was lower than anticipated but consistent with previous studies evaluating psycho-educational interventions including cancer caregivers, although there is wide variability across studies, many of which recruited breast and prostate cancer caregivers.27,48,49 The hypothesized percentage of 70% was based on a prior study that was observational, which may have been an unrealistic estimate for a randomized pilot trial. 50 In this study, many caregivers who declined participation reported feeling overwhelmed. HNC caregivers are an especially vulnerable population tasked with providing complex care and are underrepresented with behavioral intervention approaches. Consequently, when intervening with this population, expectations regarding feasibility metrics such as participation should be considered carefully and in the context of this highly burdensome disease. Further, our recruitment challenges underscore the need for creative strategies to market programs for busy HNC caregivers during patient RT. For example, some cancer centers have caregiver advocates or peer support programs that include volunteers or paid staff that have experience as an individual affected by cancer. These individuals may be particularly well suited to share details about the study to boost recruitment efforts. Another option is partnering with supportive care programs or patient advocacy non-profit groups to advertise psychosocial caregiver interventions.
Our findings demonstrated acceptability of the intervention. Caregivers reported that the intervention modules were helpful and these findings were also supported by qualitative feedback. The highest levels of acceptability were reported for the “Utilizing Resources” and “Cancer Education” modules relative to the other modules. This aligns with reports of high unmet educational/information needs as they pertain to cancer and available resources from prior studies of HNC caregivers.4,12,22,24-26 Importantly, qualitative data highlighted the variability in caregivers’ preferences regarding the amount of information provided, the timing of the intervention and their engagement in intervention activities, highlighting that the preference-based approach of the intervention was appropriate and could be enhanced to potentially improve the intervention. Caregivers also recommended a more interactive website for completing the intervention activities to improve accessibility.
Our findings showed an improvement in caregiving self-efficacy for intervention caregivers relative to control group caregivers, though these findings were not statistically significant. Similarly, other psychosocial interventions for cancer patients and caregivers have successfully improved self-efficacy constructs.38,51,52 Our focus on caregiving self-efficacy for HNC caregivers is especially important, given the challenges associated with caring for this high-needs patient population while simultaneously taking care of oneself.3-6 Our results also suggest that HNC caregivers can engage in PMR skills training during patient RT and improve their PMR-specific self-efficacy. PMR and other integrative medicine modalities have been primarily tested in cancer patient populations rather than HNC caregivers or cancer caregivers more broadly.53,54 Of note, our qualitative data revealed that the Prepare to Care intervention may have influenced some unmeasured outcomes in this study. For example, intervention participants highlighted that the intervention helped caregivers know what to expect, improved their communication with patients, and improved their ability to ask for help. A larger, fully-powered study with a more distal assessment and consideration of additional outcome measures is needed to determine both proximal and distal intervention effects for caregivers.
This study builds upon the sparse literature for behavioral interventions targeted at HNC caregivers.28-30,49 While most HNC caregiver behavioral interventions have focused solely on optimizing patient care or have been dyadic in nature, Prepare to Care focuses on providing psycho-education and stress-management skills training for caregivers. Another promising self-management pilot intervention targeted both HNC patients and spouse caregivers and demonstrated intervention effects for patient and caregiver depressive symptoms and patient cancer-specific distress. 27 An important question for future caregiver-directed interventions, such as Prepare to Care, is whether or not patient outcomes can be improved by caregiver participation alone, thereby preventing additional burden for HNC patients who are already managing multi-modality treatment and side effects.2,19-21
There are several strengths to this study. Prepare to Care was developed with input from stakeholders including HNC caregivers, providers, and psychosocial oncology professionals, an important consideration for supporting translation into practice. 55 In addition, this study is an important contribution that builds upon the limited psychosocial interventions that have been developed for and tested in HNC caregivers.27-30 Our focus on a vulnerable population of caregivers (ie, HNC caregivers) who care for patients receiving complex treatment, has been highlighted by the National Cancer Institute and National Institute for Nursing Research as an important gap to move the cancer caregiving field forward. 56 Further, the self-management format of Prepare to Care allows the intervention to be easily delivered to those providing care for HNC patients treated at diverse oncology clinics, with potential for adaption to other cancer caregiving populations as well. Caregiving self-efficacy has also not traditionally been a focus of behavioral interventions for HNC caregivers, despite caregivers reporting poor self-efficacy and feeling underprepared for their caregiving roles.6,7 Finally, we supplemented quantitative outcome assessments using validated instruments with qualitative interviews to allow a more in-depth exploration of caregiver experiences.
This feasibility study was limited by a small sample; however, our primary interest was ability to recruit and retain HNC caregivers during patient RT. We were not powered to determine efficacy of our preliminary outcomes and our findings should be interpreted with caution. Since participants could access materials in hard-copy, online, or a mixture of both, we cannot confirm level of intervention engagement among caregivers who were lost to follow-up. For example, it is possible that a caregiver continued to utilize intervention materials despite not completing the T2 assessment. Though our participation percentage is similar or better than other caregiver intervention studies, we did not meet our participation goal of 70%.48,49 In addition, participants were randomized prior to baseline and due to the purpose of the RCT (to compare Prepare to Care with a standard care control group), we were unable to blind participants to their assignment group, introducing the potential for performance bias. 57 Given the scope of this feasibility study, we did not include a long-term follow-up assessment to assess potential sustained or delayed intervention effects, which may be especially important for this population since HNC patients experience morbidity beyond the treatment period. 58 The majority of participants were white females who were a spouse/partner to the patient, limiting the generalizability of these findings to additional caregiver types. However, spouses/partners commonly serve as a caregiver for HNC patients and HNC most commonly afflicts white males.1,14,25 Finally, we did not stratify randomization based on gender in this study and all 4 male caregivers were randomized to the control group. Female cancer caregivers experience greater stress and mental and physical health outcomes compared to male caregivers and we cannot rule out potential gender effects in this study. 59
Conclusions
Prepare to Care, our novel self-management intervention for HNC caregivers of patients receiving RT, and the randomized pilot trial methodology, were feasible and acceptable in this pilot study. This study is an important first step that should be expanded upon in future research. A larger, fully-powered efficacy trial is necessary to determine treatment effects on caregiving self-efficacy, as well as secondary outcomes, including potential impacts on the patients as well. Additionally, a longer follow-up assessment is warranted to determine distal intervention effects and mechanisms of change. In accordance with qualitative feedback, future work should include a more interactive, mobile-accessible website which may also ease participation burden by allowing caregivers to access materials and complete activities on a mobile phone. Though we previously included a DVD introduction that provided a brief overview of the intervention, we recommend including an improved introduction that addresses perceived barriers to engagement to help caregivers prepare with information about what to expect with the intervention and how to best use the intervention within the busy RT schedule. Similarly, to improve engagement, we also suggest making the PMR audio file available on the study website. Finally, testing in diverse community oncology clinics as a multi-site trial would provide valuable information, given the careful consideration to scalability in the intervention development phase of Prepare to Care.
Acknowledgments
We thank the participants for their participation during this challenging time. We also thank Michelle Bishop, PhD, for lending her collaboration in developing the intervention materials and to Sara Boyd, MPH, for her contribution to the qualitative analyses.
Footnotes
Data Available: Data are available by request.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Cancer Institute of the NIH under Award Number R03CA208560, through the NCI Community Oncology Research Program (NCORP) under Award Number UG1CA189824, and the Comprehensive Cancer Center of Wake Forest Baptist Medical Center under Award Number P30CA012197. Dr. Nightingale’s effort on this work was in part supported by the National Center for Advancing Translational Sciences of the NIH under Award Number KL2TR001421. This study also received study coordinator support through the Wake Forest Clinical Translational Science Institute (UL1TR001420). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ORCID iD: Chandylen Nightingale  https://orcid.org/0000-0002-2275-9849
https://orcid.org/0000-0002-2275-9849
References
- 1. American Cancer Society. Cancer Facts & Figures; 2021. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-and-figures-2021.pdf
- 2. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Head and Neck Cancers; 2019. https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf
- 3. Bond SM, Schumacher K, Sherrod A, et al. Development of the head and neck cancer caregiving task inventory. Eur J Oncol Nurs. 2016;24:29-38. [DOI] [PubMed] [Google Scholar]
- 4. Baghi M, Wagenblast J, Hambek M, et al. Demands on caring relatives of head and neck cancer patients. Laryngoscope. 2007;117:712-716. [DOI] [PubMed] [Google Scholar]
- 5. Chen SC, Tsai MC, Liu CL, Yu WP, Liao CT, Chang JT. Support needs of patients with oral cancer and burden to their family caregivers. Cancer Nurs. 2009;32:473-481. [DOI] [PubMed] [Google Scholar]
- 6. Penner JL, McClement S, Lobchuk M, Daeninck P. Family members’ experiences caring for patients with advanced head and neck cancer receiving tube feeding: a descriptive phenomenological study. J Pain Symptom Manag. 2012;44:563-571. [DOI] [PubMed] [Google Scholar]
- 7. Blum K, Sherman DW. Understanding the experience of caregivers: a focus on transitions. Semin Oncol Nurs. 2010;26:243-258. [DOI] [PubMed] [Google Scholar]
- 8. Locher JL, Robinson CO, Bailey FA, et al. Disruptions in the organization of meal preparation and consumption among older cancer patients and their family caregivers. Psychooncology. 2010;19:967-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Porter LS, Keefe FJ, Garst J, McBride CM, Baucom D. Self-efficacy for managing pain, symptoms, and function in patients with lung cancer and their informal caregivers: associations with symptoms and distress. Pain. 2008;137:306-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thomas Hebdon MC, Coombs LA, Reed P, Crane TE, Badger TA. Self-efficacy in caregivers of adults diagnosed with cancer: an integrative review. Eur J Oncol Nurs. 2021;52:101933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Prentice-Hall; 1986. [Google Scholar]
- 12. Nightingale CL, Sterba KR, Tooze JA, et al. Vulnerable characteristics and interest in wellness programs among head and neck cancer caregivers. Support Care Cancer. 2016;24:3437-3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Drabe N, Zwahlen D, Büchi S, Moergeli H, Zwahlen RA, Jenewein J. Psychiatric morbidity and quality of life in wives of men with long-term head and neck cancer. Psychooncology. 2008;17:199-204. [DOI] [PubMed] [Google Scholar]
- 14. Longacre ML, Ridge JA, Burtness BA, Galloway TJ, Fang CY. Psychological functioning of caregivers for head and neck cancer patients. Oral Oncol. 2012;48:18-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee Y, Lin PY, Chien CY, Fang FM. Prevalence and risk factors of depressive disorder in caregivers of patients with head and neck cancer. Psychooncology. 2015;24:155-161. [DOI] [PubMed] [Google Scholar]
- 16. Hodges LJ, Humphris GM. Fear of recurrence and psychological distress in head and neck cancer patients and their carers. Psychooncology. 2009;18:841-848. [DOI] [PubMed] [Google Scholar]
- 17. Nightingale CL, Pereira DB, Curbow BA, Wingard JR, Carnaby GD. A prospective biopsychosocial investigation into head and neck cancer caregiving. Biol Res Nurs. 2017;19:87-96. doi: 10.1177/1099800416660760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Badr H, Carmack CL, Diefenbach MA. Psychosocial interventions for patients and caregivers in the age of new communication technologies: opportunities and challenges in cancer care. J Health Commun. 2015;20:328-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Trotti A, Bellm LA, Epstein JB, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol. 2003;66:253-262. [DOI] [PubMed] [Google Scholar]
- 20. Rischin D, King M, Kenny L, et al. Randomized trial of radiation therapy with weekly cisplatin or cetuximab in low-risk HPV-associated oropharyngeal cancer (TROG 12.01) - a trans-Tasman Radiation oncology group study. Int J Radiat Oncol. 2021;111:876-886. doi: 10.1016/j.ijrobp.2021.04.015 [DOI] [PubMed] [Google Scholar]
- 21. Van den Bosch L, van der Laan HP, van der Schaaf A, et al. Patient-reported toxicity and quality-of-life profiles in patients with head and neck cancer treated with definitive radiation therapy or chemoradiation. Int J Radiat Oncol. 2021;111:456-467. doi: 10.1016/j.ijrobp.2021.05.114 [DOI] [PubMed] [Google Scholar]
- 22. Richardson AE, Morton R, Broadbent E. Psychological support needs of patients with head and neck cancer and their caregivers: a qualitative study. Psychol Health. 2015;30:1288-1305. [DOI] [PubMed] [Google Scholar]
- 23. Meyer A, Keszte J, Wollbrück D, et al. Psychological distress and need for psycho-oncological support in spouses of total laryngectomised cancer patients-results for the first 3 years after surgery. Support Care Cancer. 2015;23:1331-1339. [DOI] [PubMed] [Google Scholar]
- 24. Girgis A, Lambert SD, McElduff P, et al. Some things change, some things stay the same: a longitudinal analysis of cancer caregivers’ unmet supportive care needs. Psychooncology. 2013;22:1557-1564. [DOI] [PubMed] [Google Scholar]
- 25. Aung SHH, White K, Bloomfield J. The experiences and the needs of caregivers of patients with head and neck cancer: an integrative review. Cancer Nurs. 2021;44:E361-E373. doi: 10.1097/NCC.0000000000000833 [DOI] [PubMed] [Google Scholar]
- 26. Wang T, Mazanec SR, Voss JG. Needs of informal caregivers of patients with head and neck cancer: a systematic review. Oncol Nurs Forum. 2021;48:11-29. [DOI] [PubMed] [Google Scholar]
- 27. Badr H, Herbert K, Chhabria K, Sandulache VC, Chiao EY, Wagner T. Self-management intervention for head and neck cancer couples: results of a randomized pilot trial. Cancer. 2019;125:1176-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sterba KR, Armeson K, Zapka J, et al. Evaluation of a survivorship needs assessment planning tool for head and neck cancer survivor-caregiver dyads. J Cancer Surviv. 2019;13:117-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Badr H, Herbert K, Bonnen MD, Asper JA, Wagner T. Dyadic coping in patients undergoing radiotherapy for head and neck cancer and their spouses. Front Psychol. 2018;9:1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wall LR, Cartmill B, Ward EC, Hill AJ, Isenring E, Porceddu SV. Evaluation of a weekly speech pathology/dietetic service model for providing supportive care intervention to head and neck cancer patients and their carers during (chemo)radiotherapy. Support Care Cancer. 2016;24:1227-1234. [DOI] [PubMed] [Google Scholar]
- 31. Northouse L, Williams AL, Given B, McCorkle R. Psychosocial care for family caregivers of patients with cancer. J Clin Oncol. 2012;30:1227-1234. [DOI] [PubMed] [Google Scholar]
- 32. Bodenheimer T. Patient self-management of chronic disease in primary care. JAMA. 2002;288:2469-2475. [DOI] [PubMed] [Google Scholar]
- 33. Lorig KR, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med. 2003;26:1-7. [DOI] [PubMed] [Google Scholar]
- 34. Kim AR, Park HA. Web-based self-management support interventions for cancer survivors: a systematic review and meta-analyses. Stud Health Technol Inform. 2015;216:142-147. [PubMed] [Google Scholar]
- 35. Jacobsen PB, Meade CD, Stein KD, Chirikos TN, Small BJ, Ruckdeschel JC. Efficacy and costs of two forms of stress management training for cancer patients undergoing chemotherapy. J Clin Oncol. 2002;20:2851-2862. [DOI] [PubMed] [Google Scholar]
- 36. Eldridge SM, Chan CL, Campbell MJ, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. Pilot Feasibility Stud. 2016;2:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bernstein DA, Borkovec TD. Progressive Relaxation Training: A Manual for the Helping Professions. Research Press; 1973. [Google Scholar]
- 38. Merluzzi TV, Philip EJ, Vachon DO, Heitzmann CA. Assessment of self-efficacy for caregiving: the critical role of self-care in caregiver stress and burden. Palliat Support Care. 2011;9:15-24. [DOI] [PubMed] [Google Scholar]
- 39. Sandstrom M, SR, Coletta K D, Building family caregiver skills using a simulation-based intervention: a randomized pilot trial. Oncol Nurs Forum. 2019;46:419-427. [DOI] [PubMed] [Google Scholar]
- 40. Vilagut G, Forero CG, Barbaglia G, Alonso J. Screening for depression in the general population with the Center for Epidemiologic Studies Depression (CES-D): a systematic review with meta-analysis. PLoS One. 2016;11:e0155431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cochrane A, Reid O, Woods S, Gallagher P, Dunne S. Variables associated with distress amongst informal caregivers of people with lung cancer: a systematic review of the literature. Psychooncology. 2021;30:1246-1261. [DOI] [PubMed] [Google Scholar]
- 42. Given B, Wyatt G, Given C, et al. Burden and depression among caregivers of patients with cancer at the end of life. Oncol Nurs Forum. 2004;31:1105-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pilkonis PA, Choi SW, Reise SP, Stover AM, Riley WT, Cella D. Item banks for measuring emotional distress from the patient-reported outcomes measurement information system (PROMIS®): depression, anxiety, and anger. Assess. 2011;18:263-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Deeken JF, Taylor KL, Mangan P, Yabroff KR, Ingham JM. Care for the caregivers: a review of self-report instruments developed to measure the burden, needs, and quality of life of informal caregivers. J Pain Symptom Manag. 2003;26:922-953. [DOI] [PubMed] [Google Scholar]
- 45. Weitzner MA, Jacobsen PB, Wagner H, Jr, Friedland J, Cox C. The caregiver quality of life index–cancer (CQOLC) scale: development and validation of an instrument to measure quality of life of the family caregiver of patients with cancer. Qual Life Res. 1999;8:55-63. [DOI] [PubMed] [Google Scholar]
- 46. Sandelowski M, Leeman J. Writing usable qualitative health research findings. Qual Health Res. 2012;22:1404-1413. [DOI] [PubMed] [Google Scholar]
- 47. Green J, Thorogood N. Qualitative Methods for Health Research. 4th ed. Sage Publications, Inc; 2018. [Google Scholar]
- 48. Laudenslager ML, Simoneau TL, Mikulich-Gilbertson SK, et al. A randomized control trial of stress management for caregivers of stem cell transplant patients: effect on patient quality of life and caregiver distress. Psychooncology. 2019;28:1614-1623. [DOI] [PubMed] [Google Scholar]
- 49. Badr H, Krebs P. A systematic review and meta-analysis of psychosocial interventions for couples coping with cancer. Psychooncology. 2013;22:1688-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nightingale C, Curbow B, Winga J, Per D, Carn G. Burden, quality of life, and social support in caregivers of patients undergoing radiotherapy for head and neck cancer: a pilot study. Chronic Illn. 2016;12:236-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. El-Jawahri A, Jacobs JM, Nelson AM, et al. Multimodal psychosocial intervention for family caregivers of patients undergoing hematopoietic stem cell transplantation: a randomized clinical trial. Cancer. 2020;126:1758-1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gong J, Hu C, Chen M, Cao Q, Li Q. Interventions to improve self-efficacy in colorectal cancer patients and/or caregivers: a systematic review and meta-analysis. J Oncol. 2021;2021:4553613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pelekasis P, Matsouka I, Koumarianou A. Progressive muscle relaxation as a supportive intervention for cancer patients undergoing chemotherapy: a systematic review. Palliat Support Care. 2017;15:465-473. [DOI] [PubMed] [Google Scholar]
- 54. Tian X, Tang RY, Xu LL, et al. Progressive muscle relaxation is effective in preventing and alleviating of chemotherapy-induced nausea and vomiting among cancer patients: a systematic review of six randomized controlled trials. Support Care Cancer. 2020;28:4051-4058. [DOI] [PubMed] [Google Scholar]
- 55. Ratcliff CG, Vinson CA, Milbury K, Badr H. Moving family interventions into the real world: what matters to oncology stakeholders? J Psychosoc Oncol. 2019;37:264-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kent EE, Rowland JH, Northouse L. Caring for caregivers and patients: research and clinical priorities for informal cancer caregiving. Cancer. 2016;122:1987-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Friedman LM, Furberg CD, DeMets DL, Reboussin DM, Granger CB. Blinding. Fundamentals of Clinical Trials. Springer International Publishing; 2015. [Google Scholar]
- 58. Miller MC, Shuman AG; American Head and Neck Society’s Committee on Survivorship. Survivorship in head and neck cancer: a primer. JAMA Otolaryngol Head Neck Surg. 2016;142:1002-1008. [DOI] [PubMed] [Google Scholar]
- 59. Kim Y, Mitchell HR, Ting A. Application of psychological theories on the role of gender in caregiving to psycho-oncology research. Psychooncology. 2019;28:228-254. [DOI] [PMC free article] [PubMed] [Google Scholar]