Abstract
Lead may be passed on from a mother to their unborn fetus. If she has been exposed to lead for an extended period, the lead deposited in their bones can be stimulated to be released into the bloodstream during gestation. This study was planned to examine blood lead level at the prenatal stage and its response to markers of iron deficiency during gestation. We collected 396 samples during the second trimester of gestation from women age 19 to 45 years. Hematological markers including hemoglobin, hepcidin, total iron-binding capacity (TIBC), ferritin, and blood iron were analyzed. For the detection of blood lead, we used Atomic absorption spectroscopy. The mean blood lead level of the control group was 3.25 ± .407 μg/dL, and in the iron deficiency group, it was 7.96 ± .502 μg/dL. At the same time, the women with iron deficiency anemia showed 22.12 ± 1.02 μg/dL of mean blood lead. Pearson’s approach showed a non-significant negative correlation between blood lead and hepcidin, while hemoglobin, total iron-binding capacity, ferritin, and serum iron showed a significant (.01) negative correlation with blood lead. Blood lead has no direct effect on iron deficiency markers. In contrast, iron deficiency contributes to an increase in lead accumulation during pregnancy. Iron and lead both have an impact on the heme-biosynthetic pathways. The study revealed that pre-existing iron deficiency is connected with increased lead intake and can negatively impact health in gestational females.
Keywords: lead, iron deficiency, anemia, prenatal exposures, Pakistan
Introduction
Lead (Pb), a non-biodegradable heavy metal that remains in the environment, is widely utilized in various sectors, including autos, paint, batteries, plastics, cosmetics, and jewelry.1,2 Pb pollution is a severe concern to human health, particularly for pregnant women and fragile babies, who are more sensitive to Pb exposure due to Pb’s ability to cross the placenta effortlessly. 3 High levels of Pb exposure have been related to preeclampsia, pregnancy-induced hypertension, miscarriage, preterm labor, congenital abnormalities, premature membrane rupture, and even cognitive difficulties.4-9 Lead is dispersed throughout the body, including the brain, liver, kidneys, and bones. It is stored in the teeth and bones, where it builds up over time. Lead in bone is released into the bloodstream during pregnancy, exposing the growing baby. There is no amount of lead exposure that is known to be without adverse consequences. Center for disease control recommended there are no known safe blood lead levels for children and pregnant women. 10 Lead exposure is vertically transferred (i.e., mother-to-fetus) before birth and continues throughout early childhood. A research finding implies that testing women for the lead during pregnancy may be necessary to determine the danger to their future children. 11 Pregnancy causes heightened susceptibility to hazardous chemicals. Blood lead levels may rise during pregnancy, either from an endogenous source (bone saved) or ambient pollution, affecting pregnancy health and potentially endangering the mother and growing fetus. 12 Few studies published a significant hematological effect identified in females exposed to lead even at very low lead levels. It is discovered that females are more prone to develop lead-induced anemia than males. 13
Lead can cause abnormalities in iron homeostasis and interference with erythropoiesis. The effect of BLL on iron hematological and biochemical markers were also investigated in few studies. Few studies have been published on the interaction of lead levels with different hematological markers of iron deficiency. 14 The definition of iron deficiency has been updated to include additional criteria with demonstrated diagnostic usefulness. Systemic iron homeostasis has been rethought in less than 2 decades.15,16
Iron levels must rise significantly throughout pregnancy to promote fetoplacental development and maternal pregnancy adaption. To satisfy these iron needs, both dietary iron absorption and iron mobilization from reserves increase, a system that is heavily reliant on the iron-regulatory hormone hepcidin. 17 According to few findings, circulating hepcidin is only a minor predictor of dietary iron bioavailability in people. 18
Blood lead levels rose throughout pregnancy, from 24 weeks through delivery, due to increased gastrointestinal absorption and increased bone turnover during this time. During pregnancy, bone resorption accelerates to meet the fetus’s mineral demands, which may result in temporary elevations in endogenous blood lead levels. 12
Low-level lead exposure is an occupational and public health issue that should be addressed more aggressively, especially in pregnant females. On this background, we planned the current study to check BLL in the second trimester of pregnancy. Mainly, we work on the question, do iron deficiency and elevated blood lead have a similar hematological profile during pregnancy and what is the correlation between BLL and markers of iron deficiency?
Methods
Study Design and Setting
We conducted a multicenter analysis of pregnant females. The two hospitals, Said Mitha Teaching Hospital Lahore and Govt, Kot Khawaja Saeed Teaching Hospital, were chosen using a lottery system.
Inclusion criteria: All the females were during the second trimester of pregnancy, from 13 to 26 weeks. Ten cc blood was taken from pregnant females. Every participant in the study provided informed permission, and their privacy rights have strictly adhered to it. The WHO guidelines for the age range of 18–45 years were followed. For the sample, both primigravida and multigravida women were selected.
Sample collection: Following previous permission, data on location, education, parity, and socioeconomic level were gathered. A skilled phlebotomist took a 10 cc blood sample from each participant once. Two cc blood were put in CBC vials, and the remaining 8 cc were transferred to gel tubes for blood separation. All aliquots were transferred in a cold chain from OPD to the lab.
Biochemical Procedures
After calibrating standard curves for all tests mentioned above, samples are conducted by measuring absorbance [19, 20]. Each sample had a complete blood count (CBC) done using an automated hematology analyzer named Sysmex-KX21. Sysmex-XP-100 three-part differential techniques were used to quantify blood iron. In contrast, ELISA was used to check levels of ferritin, TIBC, and hepcidin-25 using antibody-coated 96-well plates (Science glory).
Anemia and Iron Deficiency
The WHO classified anemia in pregnant women based on hemoglobin levels. Hemoglobin 11 g/dL anemia is classified as mild (10 g/dL), moderate (9 g/dL), and severe (7 g/dL).19-21
Iron deficiency was identified by lower blood iron and ferritin concentrations 22 Hepcidin, a newly discovered marker for iron homeostasis, is also considered to identify anemia during pregnancy.23,24
Detection of Lead
Shimadzu AA-7000G (Graphite Furnace Atomizer) and platform-type Graphite Tube were used to analyze Pb in blood. Because the sample was combined with matrix modifier solution before GF-AAS analysis, the method provided a fast way to detect Pb in blood.
To prepare the matrix modifier, 5 mL of 10% Triton X-100, 2 mL of NH4PO4, and 4 drops of 70% HNO3 acid were combined and diluted to volume with deionized water in a 100 mL volumetric flask. To create a multi-point calibration curve, working standard solutions of 0, 50, 100, 300, and 600 ppb Pb in 1% HNO3 were produced. In the autosampler vessels, 100 μl of the working standard solution and 900 μl of matrix modifier were mixed to generate 0, 5, 10, 30, and 60 ppb standard solution. The prepared solutions were left alone until bubbles disappeared.
The samples were made by combining 100 μl of whole blood and anticoagulant and 900 μl of matrix modifier. On the other hand, the spiked recovery samples were created by combining 100 μl blood sample, 100 μl working standard solution, and 800 μl matrix modifier.
The graphite tube design enables more uniform sample atomization, while the matrix modifier releases and avoids loss of the target element while reducing matrix interference. The calibration curve demonstrated high linearity (r= .998) for standard solutions that were less than the CDC blood lead levels of 100 ppb. 25
Statistical Analysis
Raosoft® was used to calculate sample size, with a margin of error of 3% and a confidence level of 95%. All demographic and biochemical characteristics were subjected to descriptive analysis. We were identifying significant connections between discrete variables and outcomes (lead level). Absolute and relative frequencies were used to describe qualitative factors, whereas means, standard deviations, and amplitude ranges were used to represent quantitative variables (minimum and maximum). In the event of significant association, the risk of a 95% confidence interval was calculated.
Pearson’s correlation coefficients were calculated in the study of 396 women to explore the connection between Blood lead levels and iron storage indices-ferritin, TIBC, hepcidin, Blood iron, and hemoglobin. All statistical analyses were performed using SPSS 20.0 program.26,27
Results
Increased Blood lead levels are observed in all three groups of pregnant females. The mean blood lead level of the non-iron deficiency group is 3.25 ± .407 μg/dL, and in the iron deficiency group, it is 7.96 ± .502 μg/dL. At the same time, the women with iron deficiency anemia showed 22.12 ± 1.02 μg/dL of mean blood lead. Although the mean values among the three groups are different, no statistically significant differences were detected between the groups.
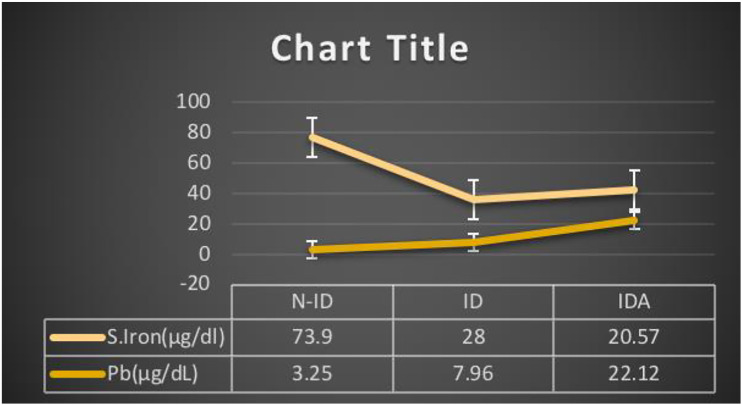
As per Pearson’s Correlation among blood lead and hematological markers of iron deficiency, blood lead showed a non-significant negative correlation with hepcidin, but it shows a significant negative correlation with hemoglobin (P = .01). (Figures 1 and 2). Ferritin, blood iron, hematocrit, mean corpuscular volume, and RBCs are significantly negatively correlated with blood lead levels (Figures 3 and 4). In contrast, TIBC and MCHC are positively correlated with significance. But when the N-ID, ID, and IDA groups are compared, these show a non-significant correlation.
Figure 1.
Blood lead (Pb) level and hemoglobin. Comparison of blood lead levels with hemoglobin in non-iron deficiency, iron deficiency, and iron deficiency anemia groups. Results expressed as mean ± S.E.M. (n = 396).
Figure 2.
Blood lead (Pb) level and hepcidin. Comparison of blood lead levels with hepcidin in non-iron deficiency, iron deficiency, and iron deficiency anemia groups. Results expressed as mean ± S.E.M. (n = 396).
Figure 3.
Blood lead (Pb) level and ferritin. Comparison of blood lead levels with Ferritin in non-iron deficiency, iron deficiency, and iron deficiency anemia groups. Results expressed as mean ± S.E.M. (n = 396).
Figure 4.
Blood lead (Pb) level and serum iron. Comparison of blood lead levels with Blood iron in non-iron deficiency, iron deficiency, and iron deficiency anemia groups. Results expressed as mean ± S.E.M. (n = 396).
Discussion
Lead may be passed on from a woman to her unborn child. Increased lead levels in the blood during pregnancy can: increase the chance of miscarriage. Cause the baby to be born prematurely or at a tiny size. The baby’s brain, kidney, and neurological system will be harmed. 28 So, blood lead level screening during pregnancy is highly recommended. Lead risk assessment is required since the previously acknowledged “safe” lead level has also been linked to poor health effects. 29
Although it is well acknowledged that some toxic substances may have positive benefits at low concentrations, integrating these effects into risk assessments often overlooks well-established exposure and human susceptibility considerations. 30 Hormesis refers to the phenomena of adaptive health effects at low dosages of the toxic chemicals and micronutrients. Like nitric oxide in the central nervous system at neuroprotection and neurotoxicity31,32.
Pregnant women who were in the non-iron deficiency group were also detected with 3.25± .407 μg/dL blood lead. (Table 1) According to the latest release of the World Health Organization, there are no safe levels of lead during pregnancy even in very minute concentrations it is going to interrupt the cellular mechanisms for RNA synthesis. 33 While in early ninety’s there have been several studies that show lead-induced hormetic-like biphasic dose-response correlations. Low-level lead (Pb) exposure may cause compensatory reactions in extremely sensitive central nervous system cells such as astroglial cells. 34
Table 1.
Result of lead (Pb) detection in three groups of pregnant females, that is, non-iron deficiency, iron deficiency, and iron deficiency anemia. Values are presented as mean ± S.E.M. (n = 396).
| Group | Hemoglobin (g/dl) | Ferritin (ng/dl) | TIBC (μg/dl) | Hepcidin (μg/L) | S. Iron (μg/dl) | Pb (μg/dL) |
|---|---|---|---|---|---|---|
| N-ID | 13.04 ± .10 | 79.12 ± 3.24 | 326.88 ± 5.30 | 32.52 ±1.06 | 74.00 ± 2.005 | 3.25 ± .407 |
| ID | 12.57 ± .05 | 21.87 ± 1.82 | 519.24 ± 4.95 | 21.17 ± 2.33 | 27.00 ± .88 | 7.96 ± .502 |
| IDA | 8.11 ± .12 | 19.17 ± 1.33 | 482.71 ± .39 | 17.13 ± 1.05 | 20.37 ± .62 | 22.12 ± 1.02 |
Developing fetuses are also at risk for dire health consequences as few researchers discovered a link between lead and microRNA. It might be linked to processes that govern DNA methylation, a physiological process necessary to govern gene expression and guarantee that the gene operates appropriately. 35 Group of pregnant females diagnosed with iron deficiency based on blood iron levels. Blood-based hematological markers are used to measure iron status biochemically. These primarily focus on detecting iron-deficient conditions. 36 Estimation of hepcidin as well as regular iron profile parameters not only aid in the diagnosis of iron deficiency anemia but also the understanding of the severity/stage of iron deficiency in females (Table 2).
Table 2.
Pearson’s correlation coefficient between various Pb and hematological and biochemical parameters of groups with iron deficiency.
| Parameters | N-ID | ID | IDA | Overall |
|---|---|---|---|---|
| Pb × Hepcidin | .025NS | −.076NS | −.019NS | −.096NS |
| Pb × Hb | −.051NS | −.105NS | .022NS | −.481 a |
| Pb × Hct | −.051NS | −.105NS | .022NS | −.481 a |
| Pb × RBCs | −.101NS | −.098NS | .024NS | −.480 a |
| Pb × MCV | −.157NS | .023NS | −.011NS | −.486 a |
| Pb × MCHC | −.050NS | −.102NS | .025NS | .524 a |
| Pb × TIBC | .262* | .028NS | −.009NS | .169 a |
| Pb × Ferritin | .031NS | −.034NS | .113NS | −.241 a |
| Pb × S. Iron | −.233NS | .033NS | −.052NS | −.325 a |
aCorrelation is significant at the .01 level (2-tailed), *Correlation is significant at the .05 level (2-tailed), and N.S. for non-significant.
The blood lead levels average of the iron deficiency group is 12.66 ± .05, which can be responsible for many hematological effects, as Agency has explained it for Toxic Substances and Disease Registry (ATSDR). Lead (Pb) interferes with numerous enzymatic stages in the heme production pathway, impairing the body’s capacity to produce hemoglobin. Lead (Pb) inhibits heme production by decreasing the activities of d-aminolevulinic acid dehydratase (ALAD) and ferrochelatase (FECH). Ferrochelatase, the enzyme that catalyzes the incorporation of iron into protoporphyrin IX, is extremely sensitive to lead. A reduction in the activity of this enzyme causes an increase in the substrate erythrocyte protoporphyrin (EP) in red blood cells, and ZPP-bound to zinc rather than iron was also discovered37,38. Lead influences the heme synthesis pathway, which is implicated in many other systems in the body, including renal, neural, hepatic, and endocrine systems. 37 We categorically focused on the relation of blood lead and markers of iron deficiency concerning the Pakistani population. Women diagnosed with anemia are at high-risk levels of blood lead. Because iron deficiency anemia is related to higher blood lead levels and may enhance lead absorption, it also has a detrimental influence on fetal development. 39 Nan-Haung et al found that lead (Pb) exposure was substantially related to anemia risk. Low-level lead exposure is an occupational and public health issue that should be addressed more aggressively. 13 The danger of lead exposure (low level, acute, and chronic) and the resulting health consequences necessitates the development of suitable prevention and treatment methods.
The current study has several strengths, including biochemical examination of pregnant women. The primary strength of our study findings is the ability to detect blood lead levels during pregnancy and compare it to hematological markers of iron deficiency. It is not a single illness based on its origin, pathophysiology, and clinical characteristics. 24 Synergistic effects of Blood lead and iron deficiency anemia are harmful during pregnancy. The study’s findings point to the need for health agencies to focus on the equitable allocation of resources to eradicate Blood lead health effects and iron deficiency.
However, the study has some limitations. Women are hesitant to provide samples due to a lack of understanding and research culture. We believe that looking at other biomarkers, such as soluble transferrin receptors and C reactive proteins, would have been beneficial. Because of a lack of resources, we could not improve the analysis of lead concentration in all three trimesters of pregnancy. 40
Blood lead bioavailability must be reduced in any way possible, and actions are made to minimize the occurrence of iron deficiency and anemia during pregnancy. Regulatory and health organizations should consider this a priority and make more serious efforts to resolve this public health concern as eradicating iron deficiency and anemia in women with associated problems highlights Sustainable Development Goals (SDGs) 2025 for Pakistan. 41
Conclusion
Markers of body iron status were evaluated as a possible modifying factor in the preceding investigation, and there was a strong negative association between these variables. The high prevalence of anemia and iron deficiency in our research sample may potentially explain some of the higher BLL we found. Further study and public health studies in Pakistan should be carried out by monitoring BLL in various contexts, particularly in pregnant women. The combined health impacts of these can have a negative impact on pregnancy outcomes and developing fetus. Blood lead bioavailability must be reduced in every method that is practical.
Acknowledgments
We acknowledge the support of Dr Sajida Hassan, and Dr Rafiq Ahmed, Director of Health Department, for providing accessibility and facilities during sampling at hospitals.
Footnotes
Author’s Contributions: Conceptualization, S.A., A.A and J.I.W; methodology, S.A. and A.A; formal analysis and investigation, S.A and A.A.; writing—original draft preparation, S.A.; writing—review and editing, A.A., and J.I.W; resources, A.A.; supervision, A.A. All authors have read and agreed to the published version of the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the University of Central Punjab, Pakistan.
Ethical Approval: The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Human Research Ethics Committee of the University of Central Punjab, Lahore via letter-number UCP/FLS/2020/136.
Informed Consent: Informed consent was obtained from all the participants of the study.
Data Availability: Data sharing does not apply to this publication since it is an original research study that includes biochemical and hematological parameters. For manuscript preparations, no previously accessible data is used. This work makes no use of gene sequences, crystallographic models, or computer models.
ORCID iD
Shafia Arshad https://orcid.org/0000-0002-9838-2937
References
- 1.Flora G, Gupta D, Tiwari A. Toxicity of lead: A review with recent updates. Interdiscipl Toxicol. 2012;5(2):47-58. doi: 10.2478/v10102-012-0009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng H, Hu Y. Lead (Pb) isotopic fingerprinting and its applications in lead pollution studies in China: A review. Environ Pollut. 2010;158(5):1134-1146. doi: 10.1016/j.envpol.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 3.Shannon M. Severe lead poisoning in pregnancy. Ambul Pediatr. 2003;3(1):37-39. doi:. [DOI] [PubMed] [Google Scholar]
- 4.Omeljaniuk W, Socha K, Soroczynska J, et al. Cadmium and lead in women who miscarried. Clin Lab. 2018;64(1-2):59-67. doi: 10.7754/Clin.Lab.2017.170611. [DOI] [PubMed] [Google Scholar]
- 5.Huang S, Xia W, Sheng X, et al. Maternal lead exposure and premature rupture of membranes: A birth cohort study in China. BMJ Open. 2018;8(7):e021565. doi: 10.1136/bmjopen-2018-021565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dórea JG. Environmental exposure to low-level lead (Pb) co-occurring with other neurotoxicants in early life and neurodevelopment of children. Environ Res. 2019;177:108641. doi: 10.1016/j.envres.2019.108641. [DOI] [PubMed] [Google Scholar]
- 7.Rimbaud D, Restrepo M, Louison A, et al. Blood lead levels and risk factors for lead exposure among pregnant women in western French Guiana: the role of manioc consumption. J Toxicol Environ Health, Part A. 2017;80(6):382-393. doi: 10.1080/15287394.2017.1331490. [DOI] [PubMed] [Google Scholar]
- 8.Musa Obadia P, Kayembe-Kitenge T, Haufroid V, Banza Lubaba Nkulu CC, Nemery B. Preeclampsia and blood lead (and other metals) in Lubumbashi, DR Congo. Environ Res. 2018;167(July):468-471. doi: 10.1016/j.envres.2018.07.032. [DOI] [PubMed] [Google Scholar]
- 9.Rameshbhai JA, Kumar JK, Gopalkrishanan S. Occupational and environmental exposure to lead and reproductive health impairment. Indian Journal of Public Health Research & Development. 2019;10(11):1524-1530. doi: 10.5958/0976-5506.2019.03752.5. [DOI] [Google Scholar]
- 10.WHO . Lead poisoning and health. https://www.who.int/news-room/fact-sheets/detail/lead-poisoning-and-health#:∼:text=Thereisnoknown’safe,symptomsandeffectsalsoincreases.
- 11.Cassidy-Bushrow AE, Sitarik AR, Havstad S, et al. Burden of higher lead exposure in African-Americans starts in utero and persists into childhood. Environ Int. 2017;108:221-227. doi: 10.1016/j.envint.2017.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jameil NA. Maternal serum lead levels and risk of preeclampsia in pregnant women: A cohort study in a maternity hospital, Riyadh, Saudi Arabia. Int J Clin Exp Pathol. 2014;7(6):3182-3189. [PMC free article] [PubMed] [Google Scholar]
- 13.Hsieh N-H, Chung S-H, Chen S-C, et al. Anemia risk in relation to lead exposure in lead-related manufacturing. BMC Publ Health. 2017;17(1):1-12. doi: 10.1186/s12889-017-4315-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peters JL, Perry MJ, McNeely E, Wright RO, Heiger-Bernays W, Weuve J. The association of cadmium and lead exposures with red cell distribution width. PLoS One. 2021;16:e0245173. doi: 10.1371/journal.pone.0245173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coffey R, Ganz T. Iron homeostasis: An anthropocentric perspective. J Biol Chem. 2017;292(31):12727-12734. doi: 10.1074/jbc.R117.781823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Means RT. Iron deficiency and iron deficiency anemia: Implications and impact in pregnancy, fetal development, and early childhood parameters. Nutrients. 2020;12(2):447. doi: 10.3390/nu12020447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher AL, Nemeth E. Iron homeostasis during pregnancy. Am J Clin Nutr. 2017;106:1567S-1574S. doi: 10.3945/ajcn.117.155812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zimmermann MB, Troesch B, Biebinger R, Egli I, Zeder C, Hurrell RF. Plasma hepcidin is a modest predictor of dietary iron bioavailability in humans, whereas oral iron loading, measured by stable-isotope appearance curves, increases plasma hepcidin. Am J Clin Nutr. 2009;90(5):1280-1287. doi: 10.3945/ajcn.2009.28129. [DOI] [PubMed] [Google Scholar]
- 19.Suchdev PS, Namaste SM, Aaron GJ, et al. Overview of the biomarkers reflecting inflammation and nutritional determinants of anemia (BRINDA) project. Adv Nutr. 2016;7(7):349-356. doi: 10.3945/an.115.010215.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Api O, Breyman C, Çetiner M, Demir C, Ecder T. Gebelikte ve postpartum dönemde demir eksikliği anemisi tanı ve tedavisi: Demir eksikliği anemisi çalışma grubu ortak görüş raporu. Turk Jinekoloji ve Obstetrik Dernegi Dergisi. 2015;12(3):173-181. doi: 10.4274/tjod.01700. [DOI] [Google Scholar]
- 21.Who, Chan M. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Geneva, Switzerland: World Health Organization; 2011:1-6. Published online. [Google Scholar]
- 22.Johnson-Wimbley TD, Graham DY. Diagnosis and management of iron deficiency anemia in the 21st century. Therapeutic Advances in Gastroenterology. 2011;4(3):177-184. doi: 10.1177/1756283X11398736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amstad Bencaiova G, Vogt DR, Hoesli I. Serum hepcidin and iron status parameters in pregnant women and the association with adverse maternal and fetal outcomes: A study protocol for a prospective cohort study. BMJ Open. 2019;9(11):e032280. doi: 10.1136/bmjopen-2019-032280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zaman B, Rasool S, Jasim S, Abdulah D. Hepcidin as a diagnostic biomarker of iron deficiency anemia during pregnancy. J Matern Fetal Neonatal Med. 2021;34(8):1288-1296. doi: 10.1080/14767058.2019.1635112. [DOI] [PubMed] [Google Scholar]
- 25.Chua M, Parreñas T, Kierulf A. Application news direct determination of pb in whole blood by graphite furnace atomic absorption spectro. Excellence in Science. 1991;11(1221):112-224. [Google Scholar]
- 26.Marković M, Majkić‐Singh N, Subota V. Usefulness of soluble transferrin receptor and ferritin in iron deficiency and chronic disease. Scand J Clin Lab Investig. 2005;65(7):571-576. doi: 10.1080/00365510500206542. [DOI] [PubMed] [Google Scholar]
- 27.Laflamme EM. Maternal hemoglobin concentration and pregnancy outcome: A study of the effects of elevation in EL alto, Bolivia. McGill J Med. 2010;13(1):47-55. doi: 10.26443/mjm.v13i1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention . Lead Poisoning: Are You Pregnant? Published online; 2018. [Google Scholar]
- 29.Mitra P, Sharma S, Purohit P, Sharma P. Clinical and molecular aspects of lead toxicity: An update. Crit Rev Clin Lab Sci. 2017;54(7-8):506-528. doi: 10.1080/10408363.2017.1408562. [DOI] [PubMed] [Google Scholar]
- 30.Trovato Salinaro A, Pennisi M, di Paola R, et al. Neuroinflammation and neurohormesis in the pathogenesis of Alzheimer’s disease and Alzheimer-linked pathologies: modulation by nutritional mushrooms. Immun Ageing. 2018;15(1):8. doi: 10.1186/s12979-017-0108-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cornelius C, Perrotta R, Graziano A, Calabrese EJ, Calabrese V. Stress Responses, Vitagenes and Hormesis as Critical Determinants in Aging and Longevity: Mitochondria as a “Chi”; 2013. http://www.immunityageing.com/content/10/1/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calabrese V, Mancuso C, Calvani M, Rizzarelli E, Butterfield DA, Giuffrida Stella AM. Nitric oxide in the central nervous system: Neuroprotection versus neurotoxicity. Nat Rev Neurosci. 2007;8(10):766-775. doi: 10.1038/nrn2214. [DOI] [PubMed] [Google Scholar]
- 33.Lead levels. https://www.who.int/news-room/fact-sheets/detail/lead-poisoning-and-health.
- 34.Calabrese EJ, Baldwin LA. Inorganics and Hormesis. Crit Rev Toxicol. 2003;33:215-304. [DOI] [PubMed] [Google Scholar]
- 35.Lead Levels and Epigenetics. https://www.eurekalert.org/pub_releases/2021-05/fda-etl051721.php. [Google Scholar]
- 36.Pfeiffer CM, Looker AC, Milman N, Pfeiffer CM, Looker AC. Laboratory methodologies for indicators of iron status: Strengths, limitations, and analytical challenges. Am J Clin Nutr. 2017;106:1606S-1614S. doi: 10.3945/ajcn.117.155887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.ATSDR . https://www.atsdr.cdc.gov/csem/leadtoxicity/physiological_effects.html.
- 38.Patharkar SA, Shaikh UMJ, Patil NJ, Nerukar AV, Shinde U. Estimation of urinary delta aminolevulinic acid levels in females of reproductive age as an index of lead exposure. Biomed Pharmacol J. 2021;14:823-827. https://biomedpharmajournal.org/vol14no2/estimation-of-urinary-delta-aminolevulinic-acid-levels-in-females-of-reproductive-age-as-an-index-of-lead-exposureestimation-of-urinary-delta-aminolevulinic-acid-levels-in-females-of-reproductive-age/. [Google Scholar]
- 39.Tiwari AKM, Mahdi AA, Mishra S, Parveen H, Fatima G. Effect of iron and folate supplementation on Pb levels in pregnant anemic women: a prospective study. Free Radic Res. 2020;54(8-9):662-669. doi: 10.1080/10715762.2020.1825704. [DOI] [PubMed] [Google Scholar]
- 40.Sullivan KM, Mei Z, Grummer-Strawn L, Parvanta I. Haemoglobin adjustments to define anaemia. Trop Med Int Health. 2008;13(10):1267-1271. doi: 10.1111/j.1365-3156.2008.02143.x. [DOI] [PubMed] [Google Scholar]
- 41.Sharma D. Achieving sustainable development nutrition targets: The challenge for South Asia. Journal of Global Health. 2020;10(1):1-4. doi: 10.7189/jogh.10.010303. [DOI] [PMC free article] [PubMed] [Google Scholar]