Abstract
Introduction
Patients have contributed <1% of spontaneous adverse drug reaction (ADR) reports in Uganda’s pharmacovigilance database. Peer support combined with mobile technologies could empower people living with HIV (PLHIV) to report ADRs and improve ADR management through linkage to care. We seek to test the feasibility and effect of a peer support intervention on ADR reporting by PLHIV receiving combination antiretroviral therapy (cART) in Uganda; identify barriers and facilitators to the intervention; and characterise ADR reporting and management.
Methods and analysis
This is a quasi-experimental study to be implemented over 4 months at 12 intervention and 12 comparison cART sites from four geographical regions of Uganda. Per region, two blocks each with a tertiary, secondary and primary care cART site will be selected by simple random sampling. Blocks per region will be randomly assigned to intervention and comparison arms.
Study units will include cART sites and PLHIV receiving cART. PLHIV at intervention sites will be assigned to peer supporters to empower them to report ADRs directly to the National Pharmacovigilance Centre (NPC). Peer supporters will be expert clients from among PLHIV and/or recognised community health workers.
Direct patient reporting of ADRs to NPC will leverage the Med Safety App and toll-free unstructured supplementary service data interface to augment traditional pharmacovigilance methods.
The primary outcomes are attrition rate measured by number of study participants who remain in the study until the end of follow-up at 4 months; and number of ADR reports submitted to NPC by PLHIV as measured by questionnaire and data abstraction from the national pharmacovigilance database at baseline and 4 months.
Ethics and dissemination
The study received ethical approval from: School of Health Sciences Research and Ethics Committee at Makerere University (MAKSHSREC-2020-64) and Uganda National Council for Science and Technology (HS1206ES). Results will be shared with PLHIV, policy-makers, the public and academia.
Trial registration number
ISRCTN75989485.
Keywords: Adverse events, SOCIAL MEDICINE, Epidemiology, Pharmacology
Strengths and limitations of this study.
The study will blend a novel peer support intervention with mobile data transmission technologies to promote the detection and reporting of suspected adverse drug reactions (ADRs) by people living with HIV.
People living with HIV who experience serious ADRs will be linked directly to health facilities for ADR management.
An implementation research approach will be employed to identify the factors that could influence the uptake of peer support in patient reporting of ADRs while documenting predefined outputs and outcomes relevant to the research objectives.
The study will generate pilot data on effect sizes to aid the planning of future randomised controlled trials using peer support to promote patient reporting of ADRs.
Introduction
Adverse drug reactions (ADRs) are a leading cause of morbidity, mortality and increased healthcare costs.1–3 The timely detection and reporting of ADRs promotes their appropriate management, more accurate prediction and prevention.4 Pharmacovigilance systems worldwide have identified and led to withdrawal from the market of at least 462 harmful medicines, primarily through passive spontaneous ADR reporting by healthcare professionals (HCPs),5 thereby contributing to patient safety. The major drawback of the spontaneous pharmacovigilance system is its reliance on individual HCP motivation. It is estimated that only about 10% of ADRs are reported through the spontaneous pharmacovigilance system, which is a very low rate of ADR reporting.6–8 Several factors hinder ADR reporting by HCPs including medical specialty, lower-level healthcare facility, older HCP age, heavy workloads, shortage of reporting tools, ignorance and fear of litigation.8 9
Patient reporting of suspected ADRs is given little attention in low-income and middle-income countries (LMICs). Yet, patients are a known complementary source of pharmacovigilance data.10–12 Patients can make detailed ADR reports and with similar quality as ADR reports from HCPs. Patients can also report previously unknown ADRs.13 Thus, patients are well placed to participate in ADR reporting because they have first-hand experience of their own state of health and treatment. Patient involvement in ADR-reporting aligns with the increasing global momentum towards patient-centred healthcare.14 Yet, patient participation in pharmacovigilance is underexplored with little empirical data, especially in LMICs. In Uganda, patients’ contribution to ADR reporting is very low indeed and is estimated at less than 1% of the reports in the national pharmacovigilance database (Victoria Nambasa, Pharmacovigilance Manager at National Drug Authority (NDA); personal communication; 6 April 2020).
The quest for expanded avenues to increase the reporting of suspected ADRs has never been more apparent than in Uganda where dolutegravir (DTG) and isoniazid preventive therapy (IPT) have been massively rolled out since 2018 and 2019, respectively. Anecdotal evidence in Uganda suggests that increased use of DTG and IPT has increased the burden of associated serious ADRs, for example, hyperglycaemia, hepatotoxicity and neuropsychiatric effects15 16; necessitating a more robust pharmacovigilance system that leverages patient reporting of suspected ADRs to DTG regimens and/or IPT. This study proposes to test the feasibility and effect of a peer support intervention combined with mobile phone-based tools to promote the reporting of ADRs by people living with HIV (PLHIV) on DTG-based antiretroviral therapy (ART) and/or IPT in Uganda. If successful, this study will contribute to the development of a more robust pharmacovigilance system to better document serious ADRs in Uganda.
Patient-centred peer support has shown promise in the management of chronic illnesses such as diabetes and mental health17 18; and in improving retention in HIV care and adherence to ART.19 20 Thus, peer support could substantially promote the detection, reporting and management of ADRs among PLHIV. In this study, peer support is based on the premise that PLHIV who have previously experienced ADRs linked to ART can—as peer supporters—encourage, mentor and support other similarly affected but less experienced PLHIV to detect and report ADRs.21 Peer supporters could serve as positive role models to improve the self-efficacy of other PLHIV whom they could guide to identify and report ADRs using the available tools. Direct patient-reporting of ADRs could use the Med Safety mobile application, a toll-free unstructured supplementary service data (USSD) interface and the traditional pharmacovigilance methods of paper-form, online forms and voice call. The aim remains to have all suspected ADR reports submitted to the National Pharmacovigilance Centre (NPC) database for analysis and subsequent processing. However, those that require clinical management should be brought to the attention of the HCP for appropriate management and prevention.21–23 From guiding less experienced PLHIV, expert clients serving as peer supporters could equally be empowered to build their own self-esteem.24 25
Our peer support intervention for strengthening the Ugandan pharmacovigilance system through patient-reporting of ADRs is intended to leverage the available mobile technologies, for example, the USSD platform available for both low-tech non-smartphones and high-tech smartphones26; and the Med Safety mobile application for high-tech smartphones.27 USSD is a real-time text-driven technology which allows users to interact directly from their mobile phones by making a selection from a menu. It allows for faster two-way communication of information and enables rapid exchange of data - up to seven times faster than SMS.28 The USSD interface has been a key success factor in the extensive penetration of mobile money banking in rural unbanked sub-Saharan Africa.29 No internet connection is needed. This project’s toll-free USSD code has been developed by a private Ugandan software company.30 Med Safety is a smartphone mobile application for ADR reporting that was recently adapted for LMICs from the prototype app funded by the European Union’s Innovative Medicines Initiative—the WEB-RADR project. Adaptation of the mobile app is led by UK’s Medicines and Healthcare products Regulatory Agency in collaboration with WHO and the WHO Collaborating Centre for International Drug Monitoring, the Uppsala Monitoring Centre.31 Med Safety was launched in Uganda in February 2020. Using both USSD and Med Safety alongside existing pharmacovigilance methods could strengthen peer support-enhanced patient-driven pharmacovigilance in Uganda.
Lastly, the study will use an implementation science approach to evaluate the peer support intervention among PLHIV. Implementation research is critical in identifying factors that could influence uptake of the intervention while documenting predefined outputs and outcomes relevant to the research objectives.32 Our ultimate goal is to increase patient reporting of ADRs in LMICs such as Uganda with weak pharmacovigilance systems. Hence, this study aims to develop and assess the feasibility of a peer support intervention combined with mobile phone-based tools to promote the detection and reporting of ADRs in PLHIV on DTG-based ART and/or IPT in Uganda. It will identify the barriers and facilitators to implementing the intervention, characterise ADR reporting and management and estimate the effect of the intervention on ADR reporting among PLHIV.
Research hypotheses and objectives
Research hypotheses
We hypothesise that the patient-centred peer support intervention combined with existing mobile data transmission technologies for promoting the detection, reporting and management of ADRs in PLHIV is feasible and acceptable. We also hypothesise that this peer support intervention combined with mobile data transmission technologies will significantly increase the number of ADR reports submitted to NPC by PLHIV who receive the intervention during 4 months of follow-up when compared with PLHIV who do not receive the intervention.
Specific objectives
To develop a peer support intervention combined with mobile data transmission technologies to promote the detection, reporting and management of ADRs in PLHIV receiving DTG and/or IPT in Uganda.
To explore the barriers and facilitators to implementation of the peer support intervention combined with mobile data transmission technologies to promote ADR detection, reporting and management among PLHIV on DTG and/or IPT in Uganda.
To describe the patterns of ADR reporting (number, rate, quality, time to reporting, seriousness, etc) by PLHIV receiving DTG and/or IPT in whom the peer support intervention combined with mobile data transmission technologies is implemented in Uganda.
To estimate the effect of the peer support intervention combined with mobile data transmission technologies on the rate of ADR reporting by PLHIV receiving DTG and/or IPT in Uganda.
Methods
Study setting
Uganda has a tiered healthcare system with different levels of healthcare from the National Referral Hospitals which provide tertiary and super-specialised healthcare, through Regional Referral Hospitals (RRHs), General Hospitals, level IV Health Centres (HC IV), level III Health Centres (HC III), to level II HC (HC II) that progressively offer less scope and breadth of health services to out-patient services.33 HIV treatment and care is provided from HC III and upwards giving a total of 1832 accredited centres that provide ART services in Uganda. Uganda adopted the Differentiated Service Delivery Models (DSDM), where stable clients have less frequent clinical assessment visits. In 2019, about 80% (1466/1832) of the ART accredited sites and 78% (975 675/1 241 478) of PLHIV on ART had access to the DSDM model. An additional, 12% (114 363/975 675) of clients enrolled on DSDM received ART services from the community through Community Drug Distribution Points (CDDPs) and Community Client-Led ART Distribution (CCLAD).34
Uganda has an estimated 1.46 million PLHIV, of whom prevalence among people aged 15–49 years is 5.8% with women having a higher prevalence (7.1%) than men (4.3%). Among the PLHIV, 93% are aged >15 years and 60% of the HIV-infected adults are women. In 2019, there were 53 413 new HIV infections of which 40 000 were among adults and 21 000 Ugandans died of AIDS-related illnesses.35 Following the ‘Test and Treat’ policy for HIV adopted in 2016 and scaled up in 2017, the ART coverage was at 89% in 2019. Approximately 96% of PLHIV on ART are taking first-line regimens and >443 000 PLHIV are on TLD. About 17% of PLHIV are ART-naïve at treatment initiation. In 2019, about 41% of TB patients were HIV-positive and 97% of HIV-positive TB patients were receiving ART.35 36 By the end of 2019, 477 190 of PLHIV were enrolled on IPT. Strategies to strengthen pharmacovigilance were instituted as part of DTG/IPT roll-out in the 2020 revised Consolidated Guidelines for Prevention and Treatment of HIV and AIDS in Uganda.16 The guidelines support ADR identification, monitoring and reporting, particularly for DTG and IPT. Pharmacovigilance sentinel sites were established at 18 sentinel sites (RRHs and Centres of Excellence). These ART sites received training and ADR-reporting tools. ADRs are reported to the NPC at NDA through a paper-based system, online system, toll-free phone line or through NDA’s Med Safety App.
For this study, the authors have divided the country into four geographical regions to establish a sampling framework that leads to selection of national level representation of health facilities and factors that influence provision of care to PLHIV and their pharmacovigilance-related needs. In each region, two blocks of health facilities with ART-sites will be selected of which one block will implement the intervention and the other will serve as the comparison block of health facilities. Each block will consist of an ART site at an RRH (tertiary care), an HC IV (secondary care) and HC III (primary care), respectively. Therefore, 12 intervention ART sites will be matched by level of care and region with 12 comparison ART sites from the four regions of Uganda.
Study design
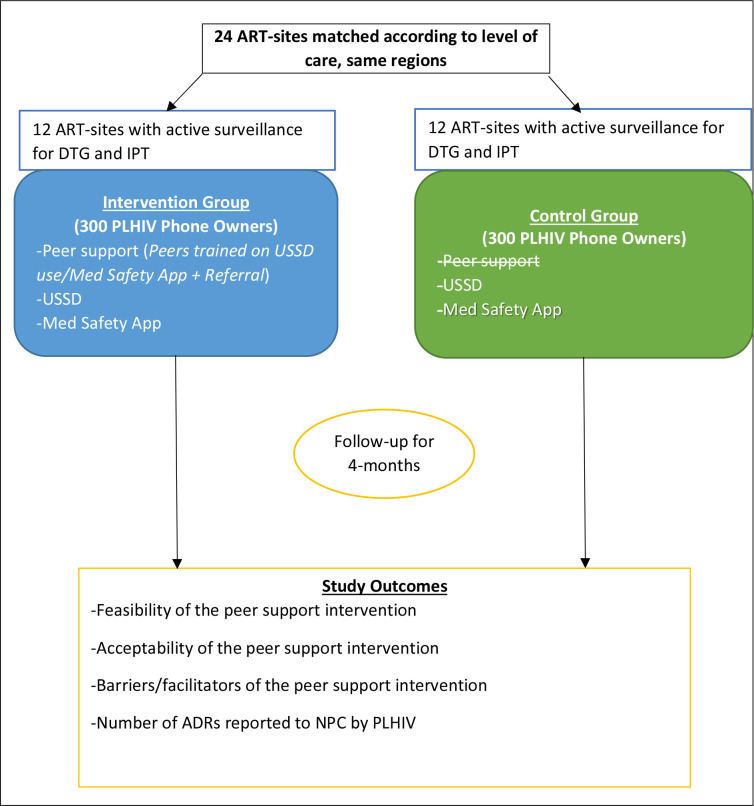
The study will employ a quasi-experimental design with pre–post and there-there comparisons to measure the preliminary impact of the peer support intervention on ADR reporting by PLHIV (figure 1). The study will use both quantitative and qualitative methods to triangulate the research findings. The qualitative research methods aim to understand the barriers and facilitators to implementing the peer support intervention for promoting ADR detection, reporting and management from the perspective of PLHIV and in the context of their interface with Uganda’s healthcare system37 and thus they will be predominantly implemented in the intervention arm. We will explore the experiences of PLHIV in the utilisation of the peer support intervention and elicit their preferences to further refine the intervention and implementation strategy.
Figure 1.
Before-and-after and there-there quasi-experimental study design for a peer support intervention to improve adverse drug reaction reporting by people living with HIV in Uganda. ADR, adverse drug reaction; ART, antiretroviral therapy; DTG, dolutegravir; IPT, isoniazid preventive therapy; NPC, National Pharmacovigilance Centre; PLHIV, people living with HIV; USSD, unstructured supplementary service data.
The intervention
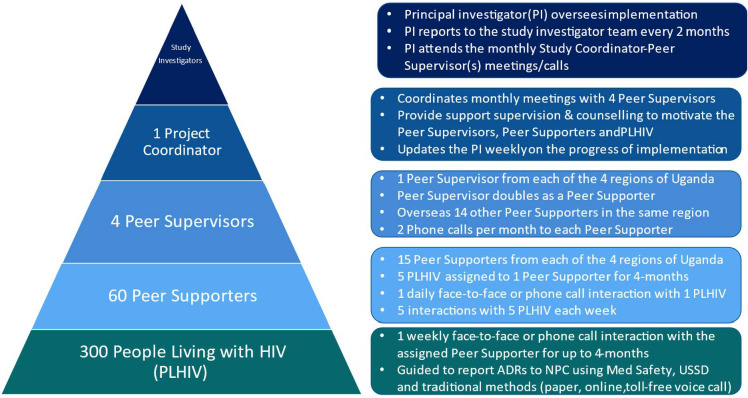
The peer support intervention leverages mobile data transmission technologies (Med Safety, USSD) in addition to traditional pharmacovigilance methods (paper, online, voice call). The peer support mechanism has several layers of supervision from the mentored PLHIV, through peer supporters, peer supervisors, study coordinator to study investigators at the top of the hierarchy (figure 2).
Figure 2.
Layers of supervision in the peer support mechanism. ADRs, adverse drug reactions; NPC, National Pharmacovigilance Centre; USSD, unstructured supplementary service data.
The PLHIV to be mentored in the intervention arm will be assigned to peer supporters to guide their ART care for 4 months. The peer supporters will constitute a mixed group of lay people, namely: (1) expert clients who are PLHIV with more experience in the use of ART and (2) recognised community health workers (CHWs). Most CHWs in Uganda’s HIV programmes are expert clients. Thus, it is possible to recruit CHWs all of whom are expert clients. Peer supporters in the intervention arm will guide the mentored PLHIV to report ADRs to NPC and improve the latter’s healthcare-seeking behaviour. The PLHIV should own mobile phones.
The PLHIV to be mentored will be identified by verbal communication/written announcements on noticeboards at the study sites and matched with the respective peer supporters of similar age, gender and proximity of residence. The non-random matching of PLHIV to peer supporters is intended to promote easier and faster bonding of the peer-relationships. Five PLHIV will be assigned to one peer supporter from the same community. A weekly (minimum fortnightly) face-to-face/phone call interaction will be held between a peer supporter and each assigned PLHIV. Thus, a peer supporter will be expected to interact with one PLHIV per day and five PLHIV in 5 days each week. Each PLHIV to be supported will be introduced to an assigned PLHIV by the research team and focal health facility staff. The procedure for the weekly interaction will be illustrated to the PLHIV-peer supporter pair. The mentored PLHIV and peer supporter will be provided with the telephone contacts of the study coordinator/focal health facility staff whom they could notify at any time when they want to terminate engagement.
Peer supporters will use one-on-one in-person support blended with mobile phone-based interaction to guide each assigned PLHIV to recognise and report suspected ADRs to NPC. The peer supporter will also administer a short weekly questionnaire to each assigned PLHIV regarding ADR experience in the past 1 week.
This peer support intervention adapts the ‘humanising healthcare model’ developed by peers for progress, a group that demonstrates the value and best practices of peer support. The model is based on four functions, namely; assistance in daily management, providing social and emotional support, linking to clinical and community resources and ongoing support38 (figure 3).
Figure 3.
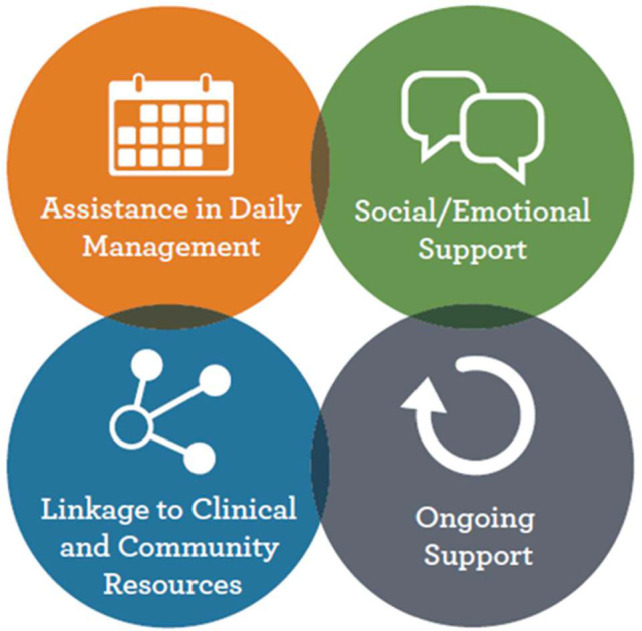
Four key functions of the humanising healthcare model for peer support as adapted from the framework by peers for progress. Source: Peers for Progress, Global Evidence for Peer Support; Humanizing Healthcare (September 2014)
Both the supported PLHIV and peer supporters will be trained on the following aspects: ART; how to live positively with HIV; recognition of suspected ADRs and how to report them via Med Safety, USSD or traditional methods (paper, online, voice call) to NPC; and about linkage or referral to health facility care for example, when a serious ADR occurs. The supported PLHIV and peer-supporters will be trained to interact in a manner that ensures confidentiality. The data generated from Med Safety and USSD will be safeguarded according to applicable laws on data protection. The linkage to appropriate care of PLHIV by the peer supporter will aim to: (1) promote healthcare-seeking behaviour of the PLHIV, (2) improve the monitoring of HIV treatment (management of serious ADRs, ART adherence, retention in care), (3) enhance timely refill of ART prescriptions and/or (4) provide for any other special care that PLHIV might require.
Peer supporters will be separately trained and skilled in interpersonal interaction to be responsive to PLHIV and encourage them to identify and report any suspected ADRs. The training components for peer supporters will include care in chronic illness, ART, adherence to ART, ADRs, ADR-reporting, care-seeking, counselling and facilitative supervision. Training for the peer supporters will take up to 3 days. It will include a 1-day didactic session followed by 2 days of on-the-job, one-on-one training. In addition to being trained, peer supporters will receive supplementary educational materials. Four follow-up supervisory visits/phone calls at 2-week intervals will be conducted by the trainers to reinforce the knowledge, skills and attitudes gained by the peer supporters. Each supported PLHIV will receive 1 day’s training during his/her clinic visit which will include a didactic session and one-on-one discussion in a non-classroom environment. The trainers will be qualified individuals carefully identified by the project team with the requisite knowledge to offer the training and expertise in adult learning and counselling.
The peer support mechanism will have two additional layers of supervision (figure 2). The first level of additional oversight will be provided by four peer supervisors identified from among the peer supporters at each of the four selected RRHs. Peer supervisors will be seconded by the study sites and collaborating patient safety groups involved in the recruitment of peer supporters. Each peer supervisor will oversee 15 peer supporters in his/her region (10 from RRH, 3 from HC IV, 2 from HC III). The peer supervisor will call each peer supporter twice a month. During these ‘booster’ sessions, the peer supervisor will review, emphasise and re-educate peer supporters on expectations of the intervention for example, setting and reviewing goals with PLHIV. The second level of oversight will be provided by the project coordinator who will oversee the four peer supervisors whom he/she will meet/call every month. At least one study investigator, mostly the principal investigator, will participate in these meetings/calls. The project coordinator will have the requisite knowledge, skills and competence to train PLHIV and peer supporters. The project coordinator will provide support supervision and counselling to motivate the peer supervisors, peer supporters and PLHIV.
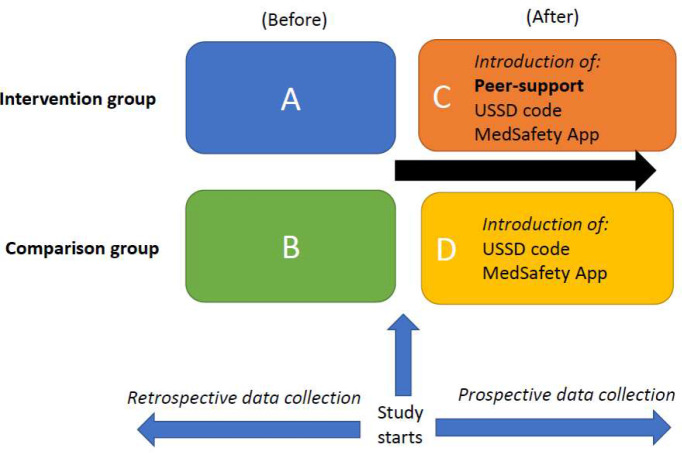
The comparison group
PLHIV in the comparison group will be mobile phone owners who will be trained to recognise suspected ADRs and report them to NPC via Med Safety, USSD or the traditional pharmacovigilance methods (paper, online, toll-free voice call) to a peer-supporter, HCP or NPC (figure 4). Smartphone owners will be guided to instal Med Safety for ADR reporting. PLHIV with non-smartphones or smartphone owners who will not instal Med Safety will report ADRs by USSD or the traditional reporting methods. PLHIV in this group will not receive dedicated peer support.
Figure 4.
Intervention and comparison groups with two-way comparisons (before-after for each group (A&C, B&D), and between groups after intervention (C&D). USSD, unstructured supplementary service data.
Study units, participants and selection
Study units and participants
This study has multiple study units and layers to assess the feasibility of implementation and effect of the intervention on promoting the detection and reporting of suspected ADRs in PLHIV on DTG-based ART and/or IPT in Uganda. From microlevel to macrolevel, the study units include PLHIV receiving DTG regimens and/or IPT; the pair of PLHIV and peer supporter; the peer supporter; the combination of the PLHIV with peer supporter and ART site; peer supervisor; the pair of peer supervisor and peer supporter; and the ART site. At the study ART site, HCPs and health facility managers will be included. Lastly, the reporting of suspected ADRs by PLHIV, peer supporters, HCPs and ART sites to the NPC will also be examined.
About 17% of PLHIV are ART naive, 95% are aged 15 years and older, and 89% receive first-line ART either as treatment-naive or treatment-experienced PLHIV.36
Eligibility criteria
Inclusion criteria: Selection of study units will occur at three levels: First, eligible PLHIV should (1) be aged >15 years, (2) receive ART at the selected study sites, (3) own a mobile phone (smartphone, basic feature phone) and (4) provide written/thumb-printed informed consent. Child consent can be given by emancipated minors aged 15–17 years in Uganda39 Second, eligible peer supporters (expert clients, CHWs) will be those that are recognised and seconded by the study sites or collaborating patient safety groups. These peer supporters will be those that are attached to the study sites and have already received institutional training in their role as expert clients/CHWs; they should own mobile phones. A focal clinical staff assigned to the study by the health facility administration will approach and recruit the peer supporters. The recruited peer supporters will be screened by the research team to gauge their ability to be peer supporters, for example, the ability to use the Med Safety App/USSD, ability to read and write in English and good interpersonal skills. Satisfactory peer supporters will give written informed consented. Peer supporters will participate only in the intervention arm of the study. Third, study health facilities will be selected and enrolled as follows; in each of the four geographical regions, blocks of three health facilities each with an ART site, including at least an RRH, HC IV and HC III will be created based on the catchment of each RRH. From the created blocks of three health facilities in each region, two blocks will be selected by simple random sampling to participate in the study as the intervention and comparison health facilities, respectively. This will give 24 ART sites consisting of 12 intervention sites (4 RRHs, 4 HC IVs, 4 HC IIIs) matched by level of care and region with 12 comparison sites (4 RRHs, 4 HC IVs, 4 HC IIIs) selected from the four geographical regions of Uganda.
Exclusion criteria: Exclusion will apply only at the level of PLHIV and CHWs. We shall exclude PLHIV on ART for <6 months and expert clients/ CHWs who will be unable to commit, from the outset, at least 5 hours per week to the study for up to 4 months.
Many ADRs happen when starting ART although such PLHIV tend to be unstable on treatment. The priority of this pilot is to understand the dynamics (feasibility and acceptability) of the peer support intervention in a stable group of PLHIV on ART (for ≥6 months). If found to be feasible, the peer support intervention will be introduced, in future initiatives, to the unstable group of PLHIV on ART (for <6 months).
Sample size and sampling considerations
Sample size computation is based on the possible effect of the peer support intervention on the rate of ADR reporting by PLHIV, with adjustment for clustering. We assume a conservative intracluster correlation coefficient of 0.045 and a priori increase of 50% in the rate of ADR reporting to NDA, from 6 ADR reports per 100 person-years at baseline40 to 9 ADR reports per 100 person-years at end-line evaluation. We assume an SD of 12 ADR reports per 100 person-years computed from the monthly ADR reports submitted to NPC for 1 year (October 2018 to September 2019). The study is designed to have at least 80% power to estimate an effect size of 1.5. Thus, 126 PLHIV will be required in the intervention arm and 126 PLHIV in the control arm.
Since the caseload for each peer supporter will be 5 PLHIV in the intervention arm, 60 peer supporters (15 from each of the 4 regions) will be responsible for 300 PLHIV on DTG and/or IPT. Thus, the peer support arm will include 300 PLHIV and the control arm 300 PLHIV all of whom should own functional mobile phones (smartphone or non-smartphone or both). Thus, a total of 600 PLHIV will be enrolled; 400 from RRHs, 120 from HC IVs and 80 from HC IIIs.
The PLHIV will be enrolled consecutively until the required sample size is attained. Smartphone owners will be guided to instal Med Safety for ADR reporting. We assume that 7 in 10 PLHIV at the ART sites will have mobile phones and only one in 10 will possess smartphones.41 Thus, only up to 10% (or 60) of PLHIV with smartphones will be helped (with maximal support from peer supporters) to download Med Safety. The rest of the 540 PLHIV without smartphones or smartphone owners who will not instal Med Safety will report ADRs by USSD or the traditional methods.
Study variables
Primary outcomes: Feasibility of the peer support intervention—attrition rate recorded as the number of study participants who remain in the study until the end of follow-up at 4 months; Number of suspected ADR reports submitted to NPC by PLHIV as measured by questionnaire and data abstracted from the national pharmacovigilance database at baseline and 4 months
Other process/output/outcome variables
Acceptability of the peer support intervention measured using a questionnaire and qualitative interviews at 4 months postintervention.
Barriers/facilitators of the peer support intervention measured using a questionnaire during the intervention and qualitative interviews at 4 months postintervention.
Fidelity to the peer support intervention measured using a questionnaire and qualitative interviews at 4 months postintervention.
Rate of ADR reporting to NPC by PLHIV as measured by questionnaire and data abstraction from the national pharmacovigilance database at baseline and 4 months.
Quality of ADR reports by PLHIV measured by questionnaire and data abstraction from the national pharmacovigilance database at baseline and 4 months.
Time to ADR reporting to NPC by PLHIV since enrolment measured by questionnaire and data abstraction from the national pharmacovigilance database during 4 months.
Time from ADR onset to registration in the national pharmacovigilance database measured by questionnaire and data abstraction from the database during 4 months.
Health-related quality of life measured by questionnaire at baseline and 4 months.
Management of ADRs recorded using a questionnaire during the 4 months.
Number of PLHIV linked to health facilities by peer supporters for ADR management as measured by questionnaire during the 4-month intervention period.
Health-seeking behaviour measured using a questionnaire at baseline and 4 months.
Self-efficacy to report ADRs measured by questionnaire at baseline and 4 months.
Self-reported ART adherence measured by questionnaire at baseline and 4 months.
Mood (positive/negative affect) measured by questionnaire at baseline and 4 months.
Patient and public involvement
Direct involvement of PLHIV in the detection and reporting of suspected ADRs, and patient safety groups in recruitment of PLHIV, will have value in improving the public’s awareness of ADRs and the available pharmacovigilance tools (Med Safety, USSD, toll-free voice call, etc). Together, these will be essential for ensuring that changes to clinical practice to promote patient safety based on our work are acceptable to the public.
The study team will work with PLHIV to assess whether the available pharmacovigilance tools meet their needs, to identify potential improvements and to understand facilitators and barriers to using these pharmacovigilance tools. Wider public input into the refinement of the tools and mechanisms to encourage uptake will add value to our work. This work will also be of value to the wider public as Med Safety can be used to report ADRs to any drug, and users can receive drug safety information directly from NPC.
Data management and statistical analysis
Quantitative data
Data collection and management
Baseline and end-line semistructured questionnaires will be administered to the PLHIV and peer supporters (expert clients) in the intervention arm and PLHIV only in the comparison arm.
The baseline questionnaire will record sociodemographics (age, sex, monthly income, education level, residence) of all study participants. Clinical details (ART adherence; ADRs; ART regimen; ART status that is, first line, second line, third line; duration on ART; comorbidities) and healthcare-seeking behaviour of study PLHIV will be measured. Data will be transmitted to a password-protected online database via the Open Data Kit (ODK) suite of tools. Participating PLHIV will be asked at enrolment if they experienced suspected ADRs in the 4 months preceding the study. The self-reported suspected ADRs will be corroborated with additional information on documented suspected ADRs from retrospective clinical chart review of the 4 month period prior to study enrolment. The clinical charts will be accessed by the health facility staff.
Additional data collection for the intervention group: On a weekly basis for up to 4 months, peer supporters will inquire from each assigned PLHIV (during a 1 hour face-to-face or phone call interaction) if he/she experienced one or more suspected ADR(s) and if the ADR had any impact on quality of life and/or ART adherence. Peer supporters will document if the ADR(s) was/were reported; and, if reported, by which means (Med Safety, USSD, voice call, other methods). Peer supporters will document all ADRs experienced by the PLHIV during the previous 1 week (using a tool designed to capture the medicines and ADRs); and will guide the PLHIV to report ADRs directly to NPC using the available pharmacovigilance methods. Active surveillance of ADRs linked to DTG and/or IPT will be prioritised but ADRs linked to other medicines will also be documented. PLHIV who experience serious ADRs will be linked directly to the health facilities where they receive ART for ADR management. Peer supporters will refer serious ADR cases to peer supervisors who will, in turn, refer these cases to focal clinical staff assigned to the study by the health facility administration; usually stationed at triage to connect the cases to clinicians. We will document the management of serious and non-serious ADRs (number of serious and non-serious ADR cases referred for health facility management; actions taken by health facilities in the management of serious and non-serious ADRs, for example, stopping treatment, changing treatment, continuing treatment with adherence counselling, doing nothing, etc).
The end-line questionnaire for PLHIV will measure their healthcare-seeking behaviour, linkage to care for ADR management and adherence to ART. The PLHIV will also be asked to report their experiences while receiving peer support to assess the intervention’s feasibility and acceptability (eg, user satisfaction). The study will also assess the participants’ experiences when using the various pharmacovigilance methods (Med Safety, USSD, toll-free voice call, etc). We shall assess the ease of use, language and costs of the available pharmacovigilance methods (Med Safety, USSD, toll-free voice call, etc) alongside peer support.
Med safety APP and USSD data collection
PLHIV will submit ADR reports via Med Safety and/or USSD with initial assistance from peer supporters. Each app-based ADR report will be automatically converted into the standard E2B (R2) format prior to its receipt in the Vigilance Hub.42 The app is hosted by Uganda’s NDA which manages the reported ADR data. For USSD reporting, PLHIV will dial the USSD code and answer a set of questions. The data will be stored in real-time on a dashboard accessible to the project staff.
Statistical analysis
All ADR data in both the national and project databases and received from the study sites during the study period will be exported into Stata V.15.0 MP for descriptive analysis—frequencies, proportions and their 95% CIs (StataCorp). Duplicate ADR reports will be identified and analysed accordingly. Summary estimates will be reported by pharmacovigilance method (Med Safety, USSD, toll-free voice call, etc).
To assess the feasibility to retain peer supporters and PLHIV, we shall compute the attrition rate which is the proportion of study participants who remain in the study until the end of follow-up at 4 months.
The number of suspected ADRs reported to NPC by the PLHIV overall and in each study arm will be described by subgroup: serious ADR (yes/no); peer supporter guided (yes/no); DTG-linked (yes/no); IPT-linked (yes/no); DTG/IPT-linked (yes/no); linked to other medicines (yes/no); level of reporting (PLHIV, peer supporter, HCP, health facility), etc.
The rate of ADR reporting (ma) by PLHIV (per site, overall) per completed-month (m1) of follow-up will be computed as follows: ma = [na reports/(Na completed-months of follow-up)], where na is the number of reported ADRs and Na the number of completed-months of follow-up. Reporting rates of same-day ADR onsets will be documented; and time from ADR onset to registration in the national database recorded for all other events.33 Time to ADR reporting to NPC for a PLHIV will be the time from the day a PLHIV is enrolled into the peer support intervention to the time he/she reports the first suspected ADR to NPC. Time-to-event data will be analysed by survival analysis techniques.
We will explore the influence of level of care on the uptake of the peer support intervention in Uganda’s healthcare system—such as whether rolling it out at primary care facilities or tertiary hospitals influences uptake.
The change in outcome measures (eg,) between preintervention and postintervention in PLHIV will be assessed using a linear mixed model with random effect for peer supporter. Random effect will be included to account for clustering of PLHIV with peer supporters. The intra-cluster correlation coefficient (ICC) will be estimated from this model. Since supporters are a mixed population of expert clients and CHWs, a stratified analysis will be conducted. To aid the planning of future randomised controlled trials from this pilot’s data, we shall report effect sizes.
Qualitative data
Data collection
Postintervention, a combination of focus group discussions (FGDs), in-depth interviews (IDIs) and key informant interviews (KIIs) will be conducted with purposively selected study participants. A lead qualitative researcher will be assisted by two well-trained research assistants. Semistructured interviews informed by the Consolidated Framework for Implementation Research (CFIR)43 will be used to elicit participants’ perspectives on the facilitators and barriers to implementing the peer support intervention at four purposively selected health facilities.
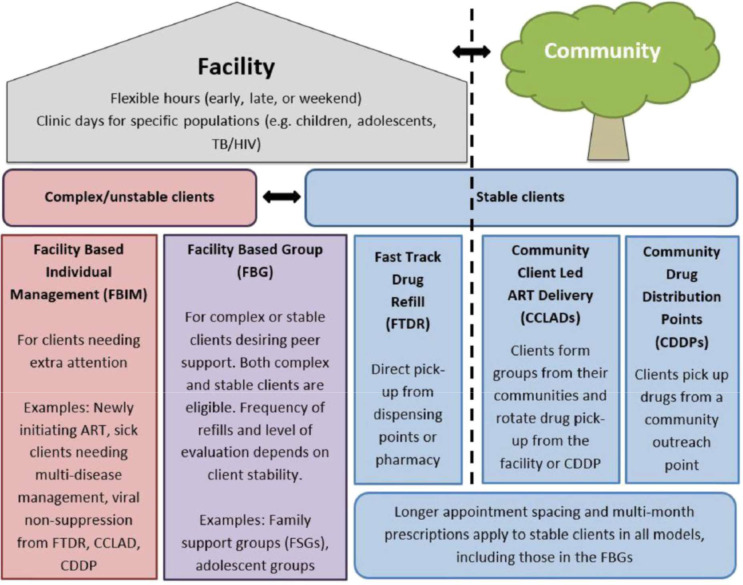
We will conduct six FGDs with three categories of PLHIV in the intervention arm; two with those enrolled in CDDPs, two in CCLAD and two in facility-based ART delivery models.
A total of 12 IDIs will be conducted with peer supporters (expert clients, CHWs) attached to each of the three ART delivery models: (1) CDDP, (2) CCLAD, (3) facility based (figure 5).
Figure 5.
The five differentiated service delivery models of HIV and TB care in Uganda.2 ART, antiretroviral therapy; TB, tuberculosis. Source: Ministry of Health, Implementation Guide for Differentiated TB Services in Uganda (June 2017).
Four KIIs will be conducted with HCPs/facility managers with insights in the implementation experience of the peer support intervention at their respective host facilities from an organisational context.
As a first step, participants will complete a written informed consent form. We will then capture baseline characteristics: age, gender and educational level. A CFIR-informed semistructured guide will be used for the interviews. The semistructured guide will explore participants’ experiences with the peer support intervention, their preferences and suggestions for improvement of the intervention and the challenges encountered in using USSD and/or Med Safety. On average, the duration of the FGDs and IDIs will be approximately 45–60 min. The FGDs, IDIs and KIIs will be conducted until theoretical saturation is reached. Theoretical saturation means that no new knowledge is generated and all aspects of a theory are covered. All the data generated from the focus groups and interviews will be explored for themes and subthemes.
Guiding qualitative analytical framework
The CFIR will be adopted as the overall guiding analytical framework for this study. The CFIR is a comprehensive ‘meta-theoretical’ implementation research framework compiled from more than 20 sources and is cross-cutting in more than 13 scientific disciplines; it guides systematic assessment of multilevel implementation settings to identify factors that influence intervention implementation and effectiveness.44 The CFIR informs the conceptualisation of this study, will guide the development of data collection tools and will serve as an overarching deductive thematic framework in analysis of study findings and the overall synthesis and interpretation of results for this study. The CFIR is widely applied because of its multilevel, ‘ecological’ dimensions on multifaceted influences on healthcare intervention implementation outcomes.45 The CFIR has been applied across diverse interventions and varied content fields.44
More specifically, the CFIR-derived domains that will guide the study are the following:
Intervention characteristics: Implementation of the peer support intervention could potentially be impacted by factors including its perceived effectiveness in ADR reporting, relative advantages over alternative reporting approaches, adaptability in varied resource-constrained settings, trialability, complexity, design quality and presentation and cost-effectiveness.
Outer setting: external influences on implementation of peer support may include external policies and incentives, socio-cultural belief systems, peer pressure dynamics and socioeconomic context.
Inner setting: characteristics of the implementing organisation (or host health facility) such as organisational culture, the relative priority assigned to the peer support intervention (including funding support), presence of intervention ‘champions’, availability of supportive administrative or physical infrastructure, congruence with host organisation’s mission and vision, quality of leadership support and implementation climate(s).
Characteristics of individuals: Patients’ beliefs, knowledge, level of income, self‐efficacy and personal attributes that may affect the implementation and uptake of the peer support intervention.
Process of implementation: Influences on implementation outcomes may derive from different implementation phases involved in roll-out of the peer support strategy such as degree and quality of involvement of primary beneficiaries in designing the intervention, planning, execution, degree of effectiveness of monitoring and evaluation strategies and presence of key intervention stakeholders and influencers including opinion leaders, stakeholder engagement and intervention champions.
The CFIR will be used to identify barriers and facilitators of the peer support intervention for promoting ADR reporting by PLHIV.
Data analysis
Our qualitative data analysis will follow the procedures recommended by Miles & Huberman.46 Interviews and FGDs will be audio-recorded and transcribed verbatim into text transcripts by three research assistants (and translated into English where necessary). Data will be analysed, in an iterative process, involving four major steps:
Data familiarisation: An experienced qualitative researcher and one other investigator will read the interview transcripts multiple times for data familiarisation.
Developing a coding framework: We shall adopt the five CFIR-derived domains (Intervention characteristics, outer setting, inner setting, characteristics of individuals, and process of implementation) as an overarching deductive thematic framework, combined with an inductive approach based on the data.47
Data abstraction: The coded data will be categorised into thematic categories.
Overall interpretation and synthesis: Our overall synthesis of study findings will adopt a team-based process of peer-debriefing involving all investigators to resolve disagreements in interpretation of study findings.
Quality assurance
To ensure uniform study procedures and high-quality data, all research assistants recruited for the study will receive face-to-face training on the following: the informed consent process, participant interviewing techniques, confidentiality issues, pharmacovigilance, use of the Med Safety App, use of the USSD, ADRs, use of the ODK software for data entry into an online password-protected database; and qualitative and quantitative study designs, among others.
The FGDs and KIs will be led by an expert in qualitative research. Research assistants with prior training in qualitative research methods will also be hired for the qualitative study component. All research assistants will receive face-to-face training in both qualitative and quantitative research methods.
Questionnaire data will be transmitted through ODK to an online database by the research assistants while still in the field. The study statistician will check the online data for integrity and contact field staff as soon as possible while still in the field to correct any data entry errors. Prior to entry into ODK, all research assistants shall be required to cross-check the data on study questionnaires to eliminate errors and ensure data completeness.
Results uptake and use
Outcomes/Impact/Outreach: The peer support intervention is expected to increase patient-reporting of ADRs to NPC. It is anticipated that the patients will subsequently: (1) find it easier and faster to report ADRs (including DTG-related and IPT-related reactions) anywhere and at any time using their mobile phones and (2) receive medication-safety alerts directly from NPC to their phones. We expect this project to promote pharmacovigilance in Uganda by improving: (1) the exchange of medication-safety information between patients, peer supporters, HCPs and NPC, (2) the awareness of pharmacovigilance by patients and the public through the mobile phone and other awareness campaigns and (3) the rate of ADR reporting by patients.
Potential impact on policy or programmes: This project could foster the increased involvement of patients in pharmacovigilance activities and improve the efficiency of pharmacovigilance systems in Uganda with real-time monitoring of DTG and INH safety in PLHIV in the first instance, thus, increasing the volume of analysable data for quick decision-making by both clinicians and policy-makers.
We expect to promote collaboration between consumers/public and the NPC, national AIDS Control Programme—Ministry of Health and the National TB and Leprosy Control Programme (NTLP). The accumulation of relevant medication-safety data from spontaneous and active ADR reports permits robust detection of safety signals at the national and international levels.
Scalability: After this pilot project, we expect the peer support intervention to be tested in a nationwide randomised controlled trial; and the USSD and Med Safety App to be modified accordingly and implemented at all 1832 ART sites in Uganda to complement the existing active and passive pharmacovigilance methods for ART and TB treatment. We hope to embed peer support in routine pharmacovigilance practice to promote the detection and reporting of ADRs by PLHIV in Uganda. The Med Safety App is available in English and will be subsequently translated into other local languages according to need.
The USSD and Med Safety are potentially invaluable tools for the pharmacovigilance of drugs used for other diseases for example, non-communicable diseases like cancers, diabetes mellitus, hypertension, etc.
Peer support, USSD and Med Safety will be scaled up to support spontaneous ADR reporting in both public and private health facilities at all levels of healthcare ranging from hospitals, medical centres and clinics to pharmacies and drug shops, not least, the general public.
The pharmacovigilance data at NPC could be linked with the patients’ clinical data at ART sites, stock consumption data from the Supply Management Chain system; and the electronic Health Management Information System. Machine learning/artificial intelligence analytical techniques could then be used on big data in the near future to foster improved systems.
Sustainability: Peer support to promote the detection and reporting of ADRs by PLHIV can be embedded in the HIV/AIDS programme of Uganda just as community engagement programmes have been successful in Maternal and Child Health programmes; and are being rolled out in the COVID-19 Community Engagement Strategy and the Young people and Adolescent Peer Support Model for improving HIV care and treatment outcomes for48 in Uganda.48 49 The USSD and Med Safety will be integrated into NPC’s routine pharmacovigilance functions to complement existing pharmacovigilance methods. Regional pharmacovigilance centres have pharmacovigilance focal persons who will continue to support the NPC. All ADR reports received by NPC are reviewed and submitted into an existing national medication-safety database. The equipped peer supporters are a valuable resource for scaling up peer support in the ART sites after the study is concluded.
The USSD interface and Med Safety will be freely available. Med Safety can be downloaded and installed from both Google Play and Apple iOS stores. NPC pays the salaries of its full-time pharmacovigilance staff who receive and process the reported medication-safety data.
The research collaboration between Makerere University’s Department of Pharmacology, Department of Pharmacy, NPC, ACP, MHRA and other stakeholders will continue to source for additional research grants to support the future scale-up of evidence-based digital pharmacovigilance in Uganda. The findings could be helpful to other countries to inform their own pharmacovigilance activities.
Dissemination
Med Safety users will immediately benefit from the app’s two-way communication functionality as they will receive medication-safety alerts from NPC in addition to their submission to NPC of ADR reports.
We plan to present the project’s research findings at local stakeholders’ workshops organised to ensure the balanced representation of HCPs, administrators, policy-makers, patient safety groups, the public and other local and international partners. At least one policy brief will be prepared from this work. We shall also disseminate the results at three or more local and international conferences, engage the public through local and international television channels, and through social media (Facebook, Twitter, WhatsApp, blogging, etc). We shall publish at least two manuscripts in internationally-recognised peer-reviewed journals.
Ethical and environmental considerations
The study received ethical approval from the School of Health Sciences Research and Ethics Committee at Makerere University College of Health Sciences (MAKSHSREC-2020-64); and was registered with the Uganda National Council for Science and Technology (HS1206ES). Administrative clearance will be obtained from participating ART sites and written/thumb-printed informed consent from participating PLHIV and expert clients/CHWs. We consider the introduction of USSD and Med Safety for ADR reporting to be a minimal risk intervention. However, we shall remind participants to mind their own confidentiality which could be lost due to phone sharing. On the contrary, participants in the intervention group could potentially benefit from peer-support. We received a waiver of consent from the ethics committee to access anonymised clinical and medication data of PLHIV at the health facilities. The data will be extracted by staff of the respective health facilities. Applicable international laws on data protection will be observed as well as the Data Protection and Privacy Act, 2019 of the Republic of Uganda.50
Risk management
Small number of patients (<10%) expected to own functional smartphones: Our main goal is to demonstrate that Med Safety can be downloaded and used by PLHIV, which we can achieve without the requirement for strict sample size and power calculations. Also, we shall use the USSD which can work on both basic low-tech mobile phones and high-tech smartphones.
Duplicate ADR reports: Duplicates will be identified by the NPC staff and study statistician.
Lost to follow-up of peer supporters and PLHIV: A major goal is to demonstrate the feasibility of peer support for PLHIV to get involved in ADR reporting. The study will provide preliminary data on the magnitude of loss to follow-up to be expected in future studies.
Compromise in data quality by the research assistants: The research assistants will be trained by the study team. Questionnaire data will be transmitted online immediately using ODK—thus giving a chance to the centrally located statistician to verify data integrity.
COVID-19: We shall observe the Standard Operating Procedures (SOPs) of social distancing, washing hands and wearing masks by study participants and investigators to minimise the risk of spreading. COVID-19. The pandemic could limit face-to-face contact but is also an opportunity to show how more remote engagement can support pharmacovigilance in a developing country setting. Remote engagement could be more cost-effective to support participants through phone calls and other forms of online interaction.
Supplementary Material
Acknowledgments
Uganda’s National Drug Authority provided technical and logistical support during the planning phase of this project.
Footnotes
Twitter: @fredkitutu
Collaborators: MHRA adapted Med Safety for Uganda with NDA’s approval. The NPC staff at NDA, where NPC is located, will participate in this project. Involvement of the ACP in this pharmacovigilance project will promote the integration of peer support-driven pharmacovigilance in the HIV care and treatment programme of Uganda. The Department of Pharmacology & Therapeutics and Department of Pharmacy, Makerere University conceived this project and will coordinate the study. The WHO contracted MHRA to adapt the app for Uganda and will, together with UMC, provide technical support.
Contributors: RK, HZ, FEK, SN and JNS designed the study. RK drafted the manuscript. RK, HBN, VN, CK, HZ, SN, JNS, PT, KH, CSM, M-ER and FEK critically reviewed and revised the final version of the manuscript. All authors read and approved the final manuscript.
Funding: This project was supported by the Special Programme for Research and Training in Tropical Diseases (TDR), a cosponsored programme of the United Nations Children’s Fund, the United Nations Development programme, the World Bank and the WHO (no grant number). TDR funding was provided by governmental and cosponsor core contributions to TDR.
Disclaimer: CS Merle and ME Raguenaud are staff members of the WHO; the authors alone are responsible for the views expressed in this publication and they do not necessarily represent the decisions, policies or views of WHO.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Johnson JA, Bootman JL. Drug-related morbidity and mortality and the economic impact of pharmaceutical care. Am J Health Syst Pharm 1997;54:554–8. 10.1093/ajhp/54.5.554 [DOI] [PubMed] [Google Scholar]
- 2.Kiguba R, Karamagi C, Bird SM. Incidence, risk factors and risk prediction of hospital-acquired suspected adverse drug reactions: a prospective cohort of Ugandan inpatients. BMJ Open 2017;7:e010568. 10.1136/bmjopen-2015-010568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tumwikirize WA, Ogwal-Okeng JW, Vernby A. Adverse drug reactions in patients admitted on internal medicine wards in a district and regional hospital in Uganda. Afr Health Sci 2011;11:72–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Coleman JJ, Pontefract SK. Adverse drug reactions. Clin Med 2016;16:481–5. 10.7861/clinmedicine.16-5-481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onakpoya IJ, Heneghan CJ, Aronson JK. Post-marketing withdrawal of 462 medicinal products because of adverse drug reactions: a systematic review of the world literature. BMC Med 2016;14:10. 10.1186/s12916-016-0553-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bates DW, et al. Incidence of adverse drug events and potential adverse drug events. JAMA 1995;274:29–34. 10.1001/jama.1995.03530010043033 [DOI] [PubMed] [Google Scholar]
- 7.Hazell L, Shakir SAW. Under-reporting of adverse drug reactions : a systematic review. Drug Saf 2006;29:385–96. 10.2165/00002018-200629050-00003 [DOI] [PubMed] [Google Scholar]
- 8.Kiguba R, Karamagi C, Waako P, et al. Recognition and reporting of suspected adverse drug reactions by surveyed healthcare professionals in Uganda: key determinants. BMJ Open 2014;4:e005869. 10.1136/bmjopen-2014-005869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez-Gonzalez E, Herdeiro MT, Figueiras A. Determinants of under-reporting of adverse drug reactions: a systematic review. Drug Saf 2009;32:19–31. 10.2165/00002018-200932010-00002 [DOI] [PubMed] [Google Scholar]
- 10.Defer G, Fedrizzi S, Chevanne D, et al. Adverse drug reaction reporting using a mobile device application by persons with multiple sclerosis: a cluster randomized controlled trial. Drug Saf 2021;44:223–33. 10.1007/s40264-020-01009-z [DOI] [PubMed] [Google Scholar]
- 11.Margraff F, Bertram D. Adverse drug reaction reporting by patients: an overview of fifty countries. Drug Saf 2014;37:409–19. 10.1007/s40264-014-0162-y [DOI] [PubMed] [Google Scholar]
- 12.Inácio P, Cavaco A, Airaksinen M. The value of patient reporting to the pharmacovigilance system: a systematic review. Br J Clin Pharmacol 2017;83:227–46. 10.1111/bcp.13098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avery AJ, Anderson C, Bond CM, et al. Evaluation of patient reporting of adverse drug reactions to the UK 'Yellow Card Scheme': literature review, descriptive and qualitative analyses, and questionnaire surveys. Health Technol Assess 2011;15:1–234. 10.3310/hta15200 [DOI] [PubMed] [Google Scholar]
- 14.Ramlakhan JU, Foster AM, Grace SL, et al. What constitutes patient-centred care for women: a theoretical rapid review. Int J Equity Health 2019;18:182. 10.1186/s12939-019-1048-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ministry of Health Uganda . Consolidated guidelines for prevention and treatment of HIV in Uganda, 2018. Available: http://library.health.go.ug/publications/hivaids/consolidated-guidelines-prevention-and-treatment-hiv-uganda [Accessed 19 Sep 2019].
- 16.Ministry of Health Uganda, . Consolidated guidelines for the prevention and treatment of HIV and AIDS in Uganda. Kampala, Uganda: Ministry of Health Uganda, 2020. [Google Scholar]
- 17.Johnson S, Lamb D, Marston L, et al. Peer-supported self-management for people discharged from a mental health crisis team: a randomised controlled trial. Lancet 2018;392:409–18. 10.1016/S0140-6736(18)31470-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kong L-N, Hu P, Yang L, et al. The effectiveness of peer support on self-efficacy and quality of life in adults with type 2 diabetes: a systematic review and meta-analysis. J Adv Nurs 2019;75:711–22. 10.1111/jan.13870 [DOI] [PubMed] [Google Scholar]
- 19.Ammon N, Mason S, Corkery JM. Factors impacting antiretroviral therapy adherence among human immunodeficiency virus-positive adolescents in sub-Saharan Africa: a systematic review. Public Health 2018;157:20–31. 10.1016/j.puhe.2017.12.010 [DOI] [PubMed] [Google Scholar]
- 20.Snyder K, Wallace M, Duby Z, et al. Preliminary results from Hlanganani (coming together): a structured support group for HIV-infected adolescents piloted in Cape town, South Africa. Child Youth Serv Rev 2014;45:114–21. 10.1016/j.childyouth.2014.03.027 [DOI] [Google Scholar]
- 21.Genberg BL, Shangani S, Sabatino K, et al. Improving engagement in the HIV care cascade: a systematic review of interventions involving people living with HIV/AIDS as Peers. AIDS Behav 2016;20:2452–63. 10.1007/s10461-016-1307-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davidson L, Chinman M, Sells D, et al. Peer support among adults with serious mental illness: a report from the field. Schizophr Bull 2006;32:443–50. 10.1093/schbul/sbj043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawn S, Smith A, Hunter K. Mental health peer support for hospital avoidance and early discharge: an Australian example of consumer driven and operated service. J Ment Health 2008;17:498–508. 10.1080/09638230701530242 [DOI] [Google Scholar]
- 24.Ratzlaff S, McDiarmid D, Marty D, et al. The Kansas consumer as provider program: measuring the effects of a supported education initiative. Psychiatr Rehabil J 2006;29:174–82. 10.2975/29.2006.174.182 [DOI] [PubMed] [Google Scholar]
- 25.Repper J. Peer support workers: theory and practice, 2013. Available: https://www.centreformentalhealth.org.uk/publications/peer-support-workers-theory-and-practice [Accessed 28 May 2021].
- 26.GSMA . Low tech, high impact: USSD in the time of COVID-19, 2020. Available: https://www.gsma.com/mobilefordevelopment/uncategorized/low-tech-high-impact-ussd-in-the-time-of-covid-19/
- 27.WEB-RADR . Med safety APP. Available: https://web-radr.eu/mobile-apps/med-safety/
- 28.smsone. Available: https://smsone.co.ug/ [Accessed 26 Jul 2021].
- 29.Ayo CK, Ukpere WI, Oni A, et al. A prototype mobile money implementation in Nigeria. Afr J Bus Manag 2012;6:2195–201. [Google Scholar]
- 30.Kolastudios. Available: https://kolastudios.com/#contact [Accessed 26 Jul 2021].
- 31.Ghosh R, Lewis D. Aims and approaches of Web-RADR: a Consortium ensuring reliable ADR reporting via mobile devices and new insights from social media. Expert Opin Drug Saf 2015;14:1845–53. 10.1517/14740338.2015.1096342 [DOI] [PubMed] [Google Scholar]
- 32.Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health 2011;38:65–76. 10.1007/s10488-010-0319-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiguba R, Ndagije HB, Nambasa V, et al. Adverse Drug Reaction Onsets in Uganda's VigiBase®: Delayed International Visibility, Data Quality and Illustrative Signal Detection Analyses. Pharmaceut Med 2018;32:413–27. 10.1007/s40290-018-0253-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.CQUIN . Differentiated service delivery in Uganda, 2020. Available: https://cquin.icap.columbia.edu/the-work/uganda/
- 35.Ministry of Health Uganda . The 2019 HIV epidemiological surveillance report for Uganda, 2020. Available: https://www.health.go.ug/cause/the-2019-hiv-epidemiological-surveillance-report-for-uganda/
- 36.PEPFAR . PEPFAR Uganda country operational plan (COP) 2019 strategic direction summary, 2019. Available: https://www.state.gov/wp-content/uploads/2019/09/Uganda_COP19-Strategic-Directional-Summary_public.pdf
- 37.Levesque J-F, Harris MF, Russell G. Patient-Centred access to health care: conceptualising access at the interface of health systems and populations. Int J Equity Health 2013;12:18. 10.1186/1475-9276-12-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peers for Progress and National Council of La Raza . Global evidence for peer support; humanizing healthcare. Report from an international Conference. Available: http://peersforprogress.org/
- 39.Uganda National Council for Science and Technology . National guidelines for research involving humans as research participants, 2014. Available: https://engage.avac.org/wp-content/uploads/grassblade/12230-testing-tincan/story_content/external_files/Uganda%202014%20National%20guidelines%20for%20research%20involving%20humans%20as%20research%20participants.pdf [Accessed 27 Jul 2021].
- 40.Batista CJB, Correa RG, Evangelista LR, et al. The Brazilian experience of implementing the active pharmacovigilance of dolutegravir. Medicine 2019;98:e14828. 10.1097/MD.0000000000014828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.The Collaboration on International ICT Policy for East and Southern Africa . National information technology survey, 2017. Available: https://www.nita.go.ug/sites/default/files/publications/National%20IT%20Survey%20April%2010th.pdf [Accessed 28 May 2021].
- 42.Pierce CE, de Vries ST, Bodin-Parssinen S, et al. Recommendations on the use of mobile applications for the collection and communication of pharmaceutical product safety information: lessons from ImI WEB-RADR. Drug Saf 2019;42:477–89. 10.1007/s40264-019-00813-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Damschroder LJ, Aron DC, Keith RE, et al. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci 2009;4:50. 10.1186/1748-5908-4-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Safaeinili N, Brown-Johnson C, Shaw JG, et al. CFIR simplified: pragmatic application of and adaptations to the consolidated framework for implementation research (CFIR) for evaluation of a patient-centered care transformation within a learning health system. Learn Health Syst 2020;4:e10201. 10.1002/lrh2.10201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Means AR, Kemp CG, Gwayi-Chore M-C, et al. Evaluating and optimizing the consolidated framework for implementation research (CFIR) for use in low- and middle-income countries: a systematic review. Implement Sci 2020;15:17. 10.1186/s13012-020-0977-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miles MB, Huberman AM. Qualitative data analysis: an expanded sourcebook. 2nd ed. Thousand Oaks, CA, US: Sage Publications, Inc, 1994. [Google Scholar]
- 47.Fereday J, Muir-Cochrane E. Demonstrating rigor using thematic analysis: a hybrid approach of inductive and deductive coding and theme development. Int J Qual Methods 2006;5:80–92. 10.1177/160940690600500107 [DOI] [Google Scholar]
- 48.Ministry of Health Uganda . Young people and adolescent peer support model for improving HIV care and treatment outcomes for adolescents and young PLHIV of 2019 in Uganda, 2019. [Google Scholar]
- 49.Ministry of Health Uganda . Technical Inter-sectoral Committee on COVID-19; national community engagement strategy for COVID-19 response for Uganda, 2020. Available: https://www.redcrossug.org/about/more-info/covid-19-community-engagement-strategy [Accessed 28 May 2021].
- 50.Government of Uganda . Data protection and privacy act, 2019 of the Republic of Uganda, 2019. Available: https://ict.go.ug [Accessed 28 May 2021].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.