Abstract
Background
Oxidative stress is associated with the pathogenesis of MS. Edaravone (EDV) has been proposed as a therapeutic resource for central nervous system diseases, and it was effective in reducing oxidative stress. However, the antioxidant mechanisms of EDV are poorly studied.
Objective
This study aimed to evaluate the effects of EDV on resting, phagocytosis, and PKC-activated granulocytes derived from MS patients and a healthy control group.
Methods
The effects of EDV on ROS production in phagocytosis (ROS production in the presence of opsonized particles) and PKC-stimulated granulocytes were evaluated in a luminol-dependent chemiluminescence method. Calphostin C was used in some experiments to compare with those of EDV.
Results
EDV inhibited ROS production in phagocytosis of opsonized particles and PKC-stimulated granulocytes from MS patients and healthy control group. In the presence of calphostin C, the inhibition of ROS production was similar to that observed with EDV.
Conclusion
These findings suggest the involvement of EDV on the ROS-PKC-NOX signaling pathways modulating oxidative stress in MS. EDV represents a promising treatment option to control oxidative innate immune response for MS.
Keywords: edaravone, multiple sclerosis, innate immunity, reactive oxygen species, phagocytosis, protein kinase C
Introduction
Multiple sclerosis (MS) is a chronic immune-mediated inflammatory disease of the central nervous system (CNS). Neuroinflammation, a key characteristic of MS, is orchestrated by the influx of leukocytes in the CNS and the loss of blood-brain barrier (BBB) integrity causing oxidative injury and inflammation.1-5 The role of the innate immune system appears to be relevant in chronic degenerative diseases, such as MS. Oxidative stress, a state from an imbalance between oxidizing species and antioxidant response, is associated with the pathogenesis of MS. Excessive ROS production plays a crucial role in demyelination, axonal/neuronal injury, and BBB integrity modulation.4,6-11 In neurodegenerative diseases, the primary generator of ROS is NADPH-oxidase (NOX), a membrane enzyme composed of several subunits that is activated via p38 MAPK (mitogen-activated protein kinases), extracellular signal-regulated kinase (ERK) 1/2, MEK (MAP kinase) 1/2, PI3K/AKT pathway, and protein kinase C (PKC).12-22 Although the inflammatory process has been extensively researched, modulation, or suppression of oxidative stress is not the focus of immunotherapies currently available to MS patients, possibly due to the lack of translational success in clinical studies. Novel therapeutic targets proposals to MS must take into account the signaling pathways involved in ROS generation. In this context, studies have shown that Edaravone (EDV, 3-methyl-1-phenyl-2-pyrazolin-5-one) effectively reduced oxidative stress in CNS diseases.14,23-28 EDV is a free radical scavenger previously approved in Japan for treating patients who had an acute ischemic stroke, and due to its neuroprotective effect, EDV was also accepted for amyotrophic lateral sclerosis (ALS) treatment.26,29 The scavenging activity of EDV occurs via an electron-donating mechanism over a wide range of radical species.30-33 However, the antioxidant mechanisms of EDV are not fully understood. According to the above, we hypothesize that EDV could modulate oxidative stress by up-regulating the ROS-NOX signaling pathways. The objective of the present study was to evaluate the effects of EDV on resting, phagocytosis, and PKC-activated granulocytes derived from MS patients and healthy controls.
Material and Methods
Study Population
The Ethics Committee from Santa Casa Hospital of Belo Horizonte, Brazil approved this comparative cross-sectional parallel-group study (approval number 3-2017-0168). Written informed consent was obtained from patients or guardians of all patients., and all participants gave written informed consent (approval number 69385917.7.0000.5138). Forty-five adult subjects were included in the study, twenty-five MS patients (age 37 ± 9.5) and twenty healthy individuals (control group) with an approximate mean age (43 ± 13.5). All patients included in the MS group are non-smokers, and they were on treatment with immunotherapies. Exclusion criteria were the following: pregnancy, dementia, inflammation, malignant disease, infection, or tobacco/alcohol dependence. The detailed profile of both studied populations is shown in Table 1.
Table 1.
Characteristics of Multiple Sclerosis patients and Control group.
| Control Group | Multiple Sclerosis | |
|---|---|---|
| Female/Male, n | 15/5 | 20/5 |
| Age, years a | 43 ± 13.5 | 37 ± 9.5 |
| Disease duration, years b | na | 5 (1-20) |
| Scale of EDSS, n | — | |
| 0–1.5 | na | 8 |
| 2–8 | na | 17 |
| Disease course, n | — | |
| Relapsing-remitting | na | 23 |
| Progressive relapsing | na | 2 |
aValues expressed in mean ± standard deviation.
bValues expressed in median (minimum–maximum).
Expanded Disability Status Scale (EDSS)
The EDSS is a method of quantifying disability progression in MS patients based on an examination by a neurologist. The EDSS ranges from 0 to 10.0, with higher scores indicating worse disability. 34
Reagents
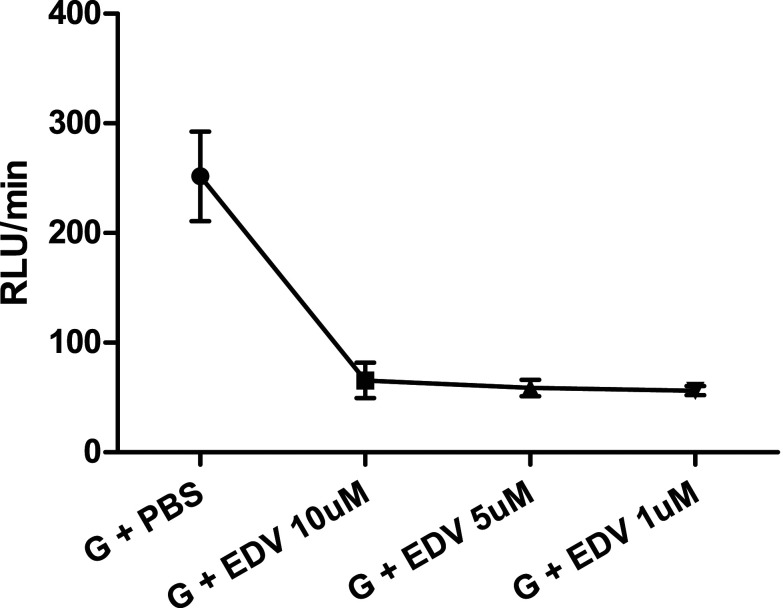
The following reagents were purchased from Merk KGaA (Darmstadt, German): Edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one, cat. #M70800), calphostin C from Cladosporium cladosporioides (cat. #C6303), Phorbol 12,13-dibutyrate (PDB, cat. #1269), and zymosan A from Saccharomyces cerevisiae (cat. #Z4250). Isopropyl alcohol was used to dilute 50 mg of Edaravone (37ºC/30min), and the work solution was diluted in saline. Figure 1 shows the dose response curve of Edaravone, no difference was observed between the 3 different concentrations on inhibition of ROS production. The dose of 1 µM of EDV was based on the study from Shi et al. 35
Figure 1.
Dose response curve of Edaravone. Values expressed in mean ± standard deviation. n = 3 for each concentration. EDV: Edaravone; G: granulocytes; PBS: phosphate buffered saline; RLU/min: Relative Light Units/minute.
Preparation of Granulocytes
Granulocytes were obtained from peripheral blood, according to Bicalho et al., 36 through a modified version of the Ficoll–Hypaque gradient method. Briefly, samples of heparinized venous blood (10 mL) were applied to double Ficoll–Hypaque gradients of different densities (1.08 and 1.12) to generate 3 interfaces after centrifugation (30–40 minutes). The first interface was rich in peripheral blood mononuclear cells, while the second interface contained granulocytes. The cells were identified and counted based on morphology, granulation, and size using a stereoscopic microscope with 400X magnification. The cellular viability of each sample was determined using the trypan blue exclusion test and was found to be > 90% in all cases.
Oxidative Responses
A luminol-based chemiluminescence method was employed to assess the oxidative responses of granulocytes. In each assay, 200 μL of luminol dissolved in .4 M dimethyl sulfoxide was mixed with a 100 μL aliquot of granulocyte suspension (1 x 106 cells/mL) in phosphate-buffered saline (PBS). Assays to establish the basal level of ROS production in granulocytes were carried out over a 20 min period, and reactions were monitored using a Turner Biosystems (Promega, Madison, WI, USA) model 20/20n luminometer. The effects of modulators on ROS production in granulocytes were assessed in sequential reactions whereby the basal granulocyte level was maintained for 20 min. Subsequently, the modulators were added, and the assay continued for a further 20 min. The modulators employed were EDV (1 μM, 100 uL), opsonized particles (100 μL of a 13.6 mg/mL zymosan-C3b suspension, ZyC3b), PKC-activator phorbol 12,13-dibutyrate (PDB; 10−4 M, 100 μL), and PKC-inhibitor calphostin C (1 μM, 100uL). In order to test the effects of EDV on ROS production in phagocytosis and PKC-activated granulocytes, the associations ZyC3b + EDV, PDB + EDV, and PDB + calphostin C were investigated. EDV and/or calphostin C were added to the corresponding assay mixture in these experiments, and the reaction was monitored for an additional 20 min.
Statistical Analysis
The D'Agostino and Pearson test was used to assess the normality of the continuous data. Normally distributed data were expressed as mean ± standard error (SE) and nonparametric data as median (minimum-maximum). The differences in the samples were compared using the unpaired Student t-test or the Mann–Whitney U-test and, in some cases, the χ2 test. P < .05 was considered statistically significant. All analyses were performed using GraphPad Prism 5 (GraphPad Software, Inc).
Results
Table 1 shows the detailed profile of the studied populations, which comprised patients diagnosed with multiple sclerosis and healthy individuals (control group). The median duration of the disease was 5 years (minimum 1 and maximum 20 years). Twenty-three MS patients were diagnosed in a relapsing-remitting course, and 2 in progressive relapsing. According to the EDSS scale, 8 MS patients with no-minimal disability were classified in EDSS 0–1.5, and seventeen patients were in the EDSS 2–8, moderate to severe disability.
EDV Inhibited ROS Production in Phagocytosis-Stimulated Granulocytes
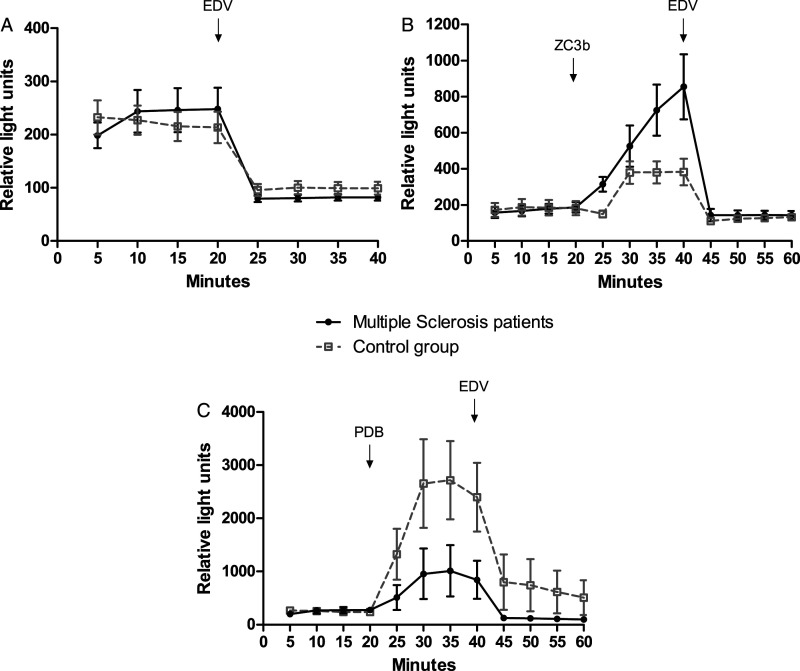
The results shown in Table 2 demonstrated similar levels of ROS formation in resting granulocytes from MS patients and the control group. However, the activation of ROS following opsonized particles stimulation (phagocytosis) was significantly (P<.05) higher in granulocytes from MS patients than in healthy individuals. Comparably, the addition of EDV inhibited ROS generation in resting cells and phagocytosis-stimulated granulocytes from both sources. Typical curves obtained in kinetic studies of the effects of EDV on ROS generation in granulocytes from the healthy control group and MS patients are presented in Figure 2. The basal level of ROS generation in resting (Figure 2A) and phagocytosis-stimulated granulocytes was rapidly down-regulated following the addition of EDV (Figure 2B). These results showed that EDV inhibited phagocytosis (ROS production in the presence of opsonized particles) in granulocytes.
Table 2.
EDV inhibited ROS production during phagocytosis of opsonized ZC3b particles in granulocytes from Multiple Sclerosis patients and control group.
| Assay Components | ROS Production (RLU/min) | ||
|---|---|---|---|
| Control group | Multiple Sclerosis patients | P | |
| 1. G + PBS | 219.2 ± 18.6 | 239.4 ± 28.3 | ns |
| 2. G + EDV | 94.5 ± 9.5 a | 75.6 ± 4.2 a | ns |
| 3. G + ZyC3b | 634.1 ± 87.8 a | 834.8 ± 108.7 a | <.05 |
| 4. G + ZyC3b + EDV | 128.9 ± 14.6 b | 143.9 ± 21.5 b | ns |
Values expressed in mean ± standard error; n = 12 for each group.EDV: Edaravone; G: granulocytes; PBS: phosphate buffered saline; RLU/min: Relative Light Units/minute; ROS: reactive oxygen species; ZC3b: Zymosan recovered using C3b fragments (opsonized particles).
aP<.05 vs G + PBS, Student t-test.
bP<.05 vs G + ZyC3b, Student t-test.
Figure 2.
EDV-induced down-regulation on ROS generation in resting (A), phagocytosis-stimulated cells (B), and PDB-stimulated granulocytes (C) from healthy control group and Multiple Sclerosis patients. Typical curves obtained in kinetic studies of 5 experimental protocols for each group. EDV: Edaravone; G: granulocytes; PDB: Phorbol Dibutyrate; ZC3b: Zymosan recovered using C3b fragments (opsonized particles).
Inhibition of ROS Production by EDV Involves PKC
In order to investigate the signaling pathway involved in the inhibition of ROS generation by EDV, the levels of ROS production by PDB (a selective activator of PKC)-stimulated granulocytes from MS patients and healthy controls assayed in the absence or presence of EDV and the calphostin C (a selective PKC inhibitor) are shown in Table 3. EDV or/and calphostin C significantly downregulated ROS production in resting granulocytes from MS patients and controls. The activation of ROS production by PDB was significantly more enhanced (P < .05) in cells from the control group than in those from MS patients. In the presence of EDV or/and calphostin C, ROS generation was significantly lower in cells from MS patients compared to controls (P < .05). Typical curves of the EDV-induced down-regulation on ROS generation in PDB-stimulated granulocytes from the healthy control group and MS patients are shown in Figure 2C. These findings suggest that the inhibitory effect of EDV involves PKC signaling pathway.
Table 3.
EDV inhibited ROS production in PKC-stimulated granulocytes from Multiple Sclerosis patients and control group.
| Assay Components | ROS Production (RLU/min) | ||
|---|---|---|---|
| Control group | Multiple Sclerosis patients | P | |
| 1. G + PBS | 224 (101–623) | 193 (100–476) | ns |
| 2. G + EDV | 108 (55–208) a | 78 (53–205) a | ns |
| 3. G + Calphostin C | 159 (89–355) a | 145 (85–201) a | ns |
| 4. G + EDV/Calphostin C | 77 (51–114) a | 66 (54–92) a | ns |
| 4. G + PDB | 1331 (335–7507) a | 328 (200–3525) a | <.05 |
| 5. G + PDB + EDV | 156 (83–689) b | 82 (52–309) b | <.05 |
| 6. G + PDB + Calphostin C | 708 (156–4247) b | 254 (76–2043) b | <.05 |
| 7. G + PDB + EDV/Calphostin C | 107 (68–940) b | 68 (58–112) b | <.05 |
Values expressed in median (minimum – maximum); n = 13 for each group.
aP<.05 vs G + PBS, Mann–Whitney U-test.
bP<.05 vs G + PDB, Mann–Whitney U-test.
EDV: Edaravone; G: granulocytes; PBS: phosphate buffered saline; PDB: Phorbol Dibutyrate; PKC: protein kinase C; RLU/min: Relative Light Units/minute; ROS: reactive oxygen species.
Discussion
Although EDV is not yet used in MS treatment, drug repositioning is increasing in therapeutic applications. Moriya et al. 23 and Zhao et al. 14 suggested that EDV may also apply to neurodegenerative disorders treatment in which oxidative stress has been primarily implicated. In the current study, EDV inhibits ROS generation in resting, phagocytosis, and PKC-stimulated granulocytes from MS patients and healthy controls (Figure 2, Tables 2 and 3).
EDV, an antioxidant that crosses BBB, has been used to treat acute ischemic stroke and ALS.26,29 In ALS patients, the use of EDV delayed the progression of functional motor disturbances by reducing oxidative stress. 37 Experimental studies have been shown that EDV generates neuroprotective effects,28,38,39 ameliorates the clinical severity of experimental autoimmune encephalomyelitis (EAE) by reducing the lymphocytes infiltration and the expression of inducible nitric oxide synthase (iNOS) 23 , attenuates oxidative stress induced by chronic cerebral hypoperfusion injury 40 , protects against retinal damage caused by oxidative stress in streptozotocin-induced diabetic mice, 41 and decreases the levels of different isoforms of PKC and mitogen-activated protein kinase (MAPK) signaling proteins in experimental autoimmune myocarditis. 42 The signaling pathways of EDV have been associated with the inhibition of AKT, AMP-activated protein kinase (AMPK), and MAPKs, such as ERK1/2.14,42,43 In contrast, studies also demonstrated that EDV increases the antioxidant system by activating ERK/Nrf2/HO-1, 40 alleviates neuronal injury, and has anti-apoptotic effects via a pathway involving activation of ERK1/2.44,45
The production of ROS is necessary for cell activity, proliferation, and the effectiveness of phagocyte cells. Nevertheless, increased ROS production can participate in demyelination, axonal/neuronal injury, BBB integrity modulation, secretion of pro-inflammatory cytokines, and reacts with lipids, proteins, and nucleic acids, leading to functional disabilities.4-11,44-49 The results presented here indicated that ROS produced through phagocytosis can be downregulated by EDV (Table 2). Phagocytosis of the myelin sheath is an important mechanism to eliminate myelin debris, preventing the accumulation of neurotoxic lipid peroxidation products, even though it causes damage in the CNS and the stimulation of ROS production are toxic to oligodendrocytes and axons.50-54 A considerable body of evidence suggests that phagocytosis and generation of ROS seem to be altered in granulocytes from MS patients, and oxidative stress, one of the most significant harmful conditions for the CNS, may be involved directly in several processes underlying disease pathogenesis.4,6,7,9,10,55-58
The presence of infiltrating T cells in CNS mediates the influx and activity of granulocytes that initiated axonal demyelination and represent a major source of ROS.5,59,60 PKC, a serine/threonine kinase family with at least 11 isoforms involved in different intracellular effects signal transduction in various cell types, stimulates ROS production through the phosphorylation of NOX subunits.61,62 PDB, a membrane-permeable activator of PKC, activated ROS generation in cells from both studied groups, although the ROS production was significantly lower in cells from MS patients than in the control group (Table 3). Similar results have been reported with PMA (also an activator of PKC) in MS patients with a severe course and during bouts of MS.63,64 Both calphostin C (an inhibitor of PKC) and EDV inhibited ROS production in PDB-stimulated granulocytes either from MS patients or healthy control (Table 3). Our findings suggest EDV could act on the PKC, but other signaling pathways are possibly involved.
Targeting the ROS-generating pathway may be a possible treatment of CNS disorders. Apocynin and DPI (diphenyliodonium chloride) are chemical compounds with NOX-inhibitory properties. The activities of those NOX inhibitors have been studied in EAE, showing reduction of BBB permeability,65,66 inhibition of ROS formation and blockage of myelin phagocytosis, 58 prevention of activated microglia from killing oligodendrocytes, 67 reduction of demyelination, infiltration of immune cells, and reduction of clinical symptoms. 66 The inhibition of NOX assembly in EAE by blockage or deletion of NOX subunits, such as p47phox, attenuated ROS production and neuroinflammation, 68 decreased EAE severity, 69 reduced toxicity to oligodendrocytes, prevented the weight loss, attenuated oligodendrocyte loss, and reduced microglia reactivity. 67
The trigger of MS remains unknown. Nonetheless, the activation of innate immune response is well characterized by inflammation and oxidative damage. Although inflammation can lead to oxidative stress and vice versa, oxidative stress precedes the inflammatory response in MS patients. 70 Hence, the generation of ROS can be considered as an inducer phase in MS pathology.
This study has some limitations, including the precise molecular mechanisms by which edaravone inhibits ROS production is unknown, and the evaluation of other signaling pathways such as NOX complex and intracellular oxidative production.
Collectively, these results indicate that EDV is effective as a ROS inhibitor in various in vitro models, including those involving resting, phagocytosis, and PKC-activated granulocytes. We suggest that EDV acts on the ROS-PKC-NOX signaling pathways modulating oxidative stress in MS. Thus, EDV might be considered as a possible complementary option to MS treatment.
Conclusion
Due to its use in other neurologic pathologies and the downregulating ROS generation, we suggest that EDV can be considered a promising medication for auxiliary treatment for MS. Therefore, further investigations are necessary to elucidate the precise activity of EDV in the modulation of oxidative stress.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Caroline Maria Oliveira Volpe https://orcid.org/0000-0003-0791-8538
References
- 1.Becher B, Spath S, Goverman J. Cytokine networks in neuroinflammation. Nat Rev Immunol. 2017;17(1):49-59. doi: 10.1038/nri.2016.123. [DOI] [PubMed] [Google Scholar]
- 2.Chiurchiù V, Orlacchio A, Maccarrone M. Is modulation of oxidative stress an answer? The state of the art of redox therapeutic actions in neurodegenerative diseases. Oxid Med Cell Longev. 2016;2016:1-11. doi: 10.1155/2016/7909380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friese MA, Schattling B, Fugger L. Mechanisms of neurodegeneration and axonal dysfunction in multiple sclerosis. Nat Rev Neurol. 2014;10(4):225-238. doi: 10.1038/nrneurol.2014.37. [DOI] [PubMed] [Google Scholar]
- 4.Lassmann H, Van Horssen J. The molecular basis of neurodegeneration in multiple sclerosis. FEBS (Fed Eur Biochem Soc) Lett. 2011;585(23):3715-3723. doi: 10.1016/j.febslet.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Trapp BD, Nave K-A. Multiple sclerosis: An immune or neurodegenerative disorder? Annu Rev Neurosci. 2008;31:247-269. doi: 10.1146/annurev.neuro.30.051606.094313. [DOI] [PubMed] [Google Scholar]
- 6.Haider L, Fischer MT, Frischer JM, et al. Oxidative damage in multiple sclerosis lesions. Brain. 2011;134(7):1914-1924. doi: 10.1093/brain/awr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilms H, Arnold P, Mojumder D, DeToledo J, Lucius R. Pathophysiological processes in multiple sclerosis: Focus on nuclear factor erythroid-2-related factor 2 and emerging pathways. J Clin Pharmacol. 2014;6(1):35-42. doi: 10.2147/CPAA.S35033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bae EH, Kim HY, Kang YU, Kim CS, Ma SK, Kim SW. Risk factors for in-hospital mortality in patients starting hemodialysis. Kidney Research and Clinical Practice. 2015;34(3):154-159. doi: 10.1016/j.krcp.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Horssen J, Witte ME, Schreibelt G, de Vries HE. Radical changes in multiple sclerosis pathogenesis. Biochim Biophys Acta (BBA) - Mol Basis Dis. 2011;1812(2):141-150. doi: 10.1016/j.bbadis.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Smith KJ, Kapoor R, Felts PA, Demyelination: The role of reactive oxygen and nitrogen species. In: Brain Pathol. 9. International Society of Neuropathology; 1999:69-92. 10.1111/j.1750-3639.1999.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller E, Walczak A, Saluk J, Ponczek MB, Majsterek I. Oxidative modification of patient’s plasma proteins and its role in pathogenesis of multiple sclerosis. Clin Biochem. 2012;45(1-2):26-30. doi: 10.1016/j.clinbiochem.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 12.Hingtgen SD, Tian X, Yang J, et al. Nox2-containing NADPH oxidase and Akt activation play a key role in angiotensin II-induced cardiomyocyte hypertrophy. Physiol Genom. 2006;26(3):180-191. doi: 10.1152/physiolgenomics.00029.2005. [DOI] [PubMed] [Google Scholar]
- 13.Bae YS, Sung J-Y, Kim O-S, et al. Platelet-derived growth factor-induced H2O2 production requires the activation of phosphatidylinositol 3-kinase. J Biol Chem. 2000;275(14):10527-10531. doi: 10.1074/jbc.275.14.10527. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Z-Y, Luan P, Huang S-X, et al. Edaravone protects HT22 neurons from H2O2-induced apoptosis by inhibiting the MAPK signaling pathway. CNS Neurosci Ther. 2013;19(3):163-169. doi: 10.1111/cns.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Groemping Y, Rittinger K. Activation and assembly of the NADPH oxidase: A structural perspective. Biochem J. 2005;386(3):401-416. doi: 10.1042/BJ20041835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang XN, Cairns B, Kim JY, Yenari MA. NADPH oxidase in stroke and cerebrovascular disease. Neurol Res. 2012;34(4):338-345. doi: 10.1179/1743132812Y.0000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan H, Zhang X, Huang X, et al. NADPH Oxidase 2-Derived Reactive Oxygen Species Mediate FFAs-Induced Dysfunction and Apoptosis of β-Cells via JNK, p38 MAPK and p53 Pathways. PLoS One. 2010;5(12):e15726. doi: 10.1371/journal.pone.0015726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao H-M, Zhou H, Hong J-S. NADPH oxidases: Novel therapeutic targets for neurodegenerative diseases. Trends Pharmacol Sci. 2012;33(6):295-303. doi: 10.1016/j.tips.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bedard K, Krause K-H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol Rev. 2007;87(1):245-313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 20.Furst R, Brueckl C, Kuebler WM, et al. Atrial natriuretic peptide induces mitogen-activated protein kinase phosphatase-1 in human endothelial cells via Rac1 and NAD(P)H oxidase/Nox2-activation. Circ Res. 2005;96(1):43-53. doi: 10.1161/01.RES.0000151983.01148.06. [DOI] [PubMed] [Google Scholar]
- 21.Borchi E, Parri M, Papucci L, et al. Role of NADPH oxidase in H9c2 cardiac muscle cells exposed to simulated ischaemia‐reperfusion. J Cell Mol Med. 2009;13(8 B):2724-2735. doi: 10.1111/j.1582-4934.2008.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barua S, Kim JY, Yenari MA, Lee JE. The role of NOX inhibitors in neurodegenerative diseases. IBRO Reports. 2019;7(July):59-69. doi: 10.1016/j.ibror.2019.07.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moriya M, Nakatsuji Y, Miyamoto K, et al. Edaravone, a free radical scavenger, ameliorates experimental autoimmune encephalomyelitis. Neurosci Lett. 2008;440(3):323-326. doi: 10.1016/j.neulet.2008.05.110. [DOI] [PubMed] [Google Scholar]
- 24.Zhou S, Yu G, Chi L, et al. Neuroprotective effects of edaravone on cognitive deficit, oxidative stress and tau hyperphosphorylation induced by intracerebroventricular streptozotocin in rats. Neurotoxicology. 2013;38:136-145. doi: 10.1016/j.neuro.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Jangra A, Kwatra M, Singh T, et al. Edaravone alleviates cisplatin-induced neurobehavioral deficits via modulation of oxidative stress and inflammatory mediators in the rat hippocampus. Eur J Pharmacol. 2016;791:51-61. doi: 10.1016/j.ejphar.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe K, Tanaka M, Yuki S, Hirai M, Yamamoto Y. How is edaravone effective against acute ischemic stroke and amyotrophic lateral sclerosis? J Clin Biochem Nutr. 2018;62(1):20-38. doi: 10.3164/jcbn.17-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ueno Y, Zhang N, Miyamoto N, Tanaka R, Hattori N, Urabe T. Edaravone attenuates white matter lesions through endothelial protection in a rat chronic hypoperfusion model. Neuroscience. 2009;162(2):317-327. doi: 10.1016/j.neuroscience.2009.04.065. [DOI] [PubMed] [Google Scholar]
- 28.Lee BJ, Egi Y, van Leyen K, Lo EH, Arai K. Edaravone, a free radical scavenger, protects components of the neurovascular unit against oxidative stress in vitro. Brain Res. 2010;1307:22-27. doi: 10.1016/j.brainres.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bailly C, Hecquet P-E, Kouach M, Thuru X, Goossens J-F. Chemical reactivity and uses of 1-phenyl-3-methyl-5-pyrazolone (PMP), also known as edaravone. Bioorg Med Chem. 2020;28(10):115463. doi: 10.1016/j.bmc.2020.115463. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto Y, Kuwahara T, Watanabe K, Watanabe K. Antioxidant activity of 3-methyl-1-phenyl-2-pyrazolin-5-one. Redox Rep. 1996;2(5):333-338. doi: 10.1080/13510002.1996.11747069. [DOI] [PubMed] [Google Scholar]
- 31.Ohara K, Fujii A, Ichimura Y, Sato K, Mukai K. Kinetic Study of Radical-Scavenging and Vitamin E-Regenerating Actions of Edaravone (3-Methyl-1-phenyl-2-pyrazolin-5-one). Bull Chem Soc Jpn. 2006;79(3):421-426. doi: 10.1246/bcsj.79.421. [DOI] [Google Scholar]
- 32.Hu CL, Nydes M, Shanley KL, Morales Pantoja IE, Howard TA, Bizzozero OA. Reduced expression of the ferroptosis inhibitor glutathione peroxidase‐4 in multiple sclerosis and experimental autoimmune encephalomyelitis. J Neurochem. 2019;148(3):426-439. doi: 10.1111/jnc.14604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferretti G, Bacchetti T. Peroxidation of lipoproteins in multiple sclerosis. J Neurol Sci. 2011;311(1-2):92-97. doi: 10.1016/j.jns.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology. 1983;33(11):1444-1444. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 35.Shi Y, Nan C, Yan Z, et al. Synaptic plasticity of human umbilical cord mesenchymal stem cell differentiating into neuron-like cells in vitro induced by edaravone. Stem Cell Int. 2018;2018:1-11. doi: 10.1155/2018/5304279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bicalho HMS, Gontijo CM, Nogueira-Machado JA. A simple technique for simultaneous human leukocytes separation. J Immunol Methods. 1981;40(1):115-116. doi: 10.1016/0022-1759(81)90087-9. [DOI] [PubMed] [Google Scholar]
- 37.Yoshino H, Kimura A. Investigation of the therapeutic effects of edaravone, a free radical scavenger, on amyotrophic lateral sclerosis (phase II study). Amyotroph Lateral Scler. 2006;7(4):247-251. doi: 10.1080/17482960600881870. [DOI] [PubMed] [Google Scholar]
- 38.Yoshida H, Yanai H, Namiki Y, Fukatsu-Sasaki K, Furutani N, Tada N. Neuroprotective effects of edaravone: A novel free radical scavenger in cerebrovascular injury. CNS Drug Rev. 2006;12(1):9-20. doi: 10.1111/j.1527-3458.2006.00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ji Yuan W, Yasuhara T, Shingo T, et al. Neuroprotective effects of edaravone-administration on 6-OHDA-treated dopaminergic neurons. BMC Neurosci. 2008;9. doi: 10.1186/1471-2202-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang D, Xiao Y, Lv P, et al. Edaravone attenuates oxidative stress induced by chronic cerebral hypoperfusion injury: role of ERK/Nrf2/HO-1 signaling pathway. Neurol Res. 2018;40(1):1-10. doi: 10.1080/01616412.2017.1376457. [DOI] [PubMed] [Google Scholar]
- 41.Yuan D, Xu Y, Hang H, et al. Edaravone protect against retinal damage in streptozotocin-induced diabetic mice. PLoS One. 2014;9(6):e99219. doi: 10.1371/journal.pone.0099219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arumugam S, Thandavarayan RA, Veeraveedu PT, et al. Involvement of AMPK and MAPK signaling during the progression of experimental autoimmune myocarditis in rats and its blockade using a novel antioxidant. Exp Mol Pathol. 2012;93(2):183-189. doi: 10.1016/j.yexmp.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 43.Kawasaki T, Kitao T, Nakagawa K, et al. Nitric oxide-induced apoptosis in cultured rat astrocytes: Protection by edaravone, a radical scavenger. Glia. 2007;55(13):1325-1333. doi: 10.1002/glia.20541. [DOI] [PubMed] [Google Scholar]
- 44.Wang G, Su J, Li L, et al. Edaravone alleviates hypoxia-acidosis/reoxygenation-induced neuronal injury by activating ERK1/2. Neurosci Lett. 2013;543:72-77. doi: 10.1016/j.neulet.2013.02.067. [DOI] [PubMed] [Google Scholar]
- 45.Liu X-Y, Yao L-L, Chen Y-J, et al. Survivin is involved in the anti-apoptotic effect of edaravone in PC12 cells. Mol Cell Biochem. 2009;327(1-2):21-28. doi: 10.1007/s11010-009-0037-1. [DOI] [PubMed] [Google Scholar]
- 46.Evans MD, Dizdaroglu M, Cooke MS. Oxidative DNA damage and disease: Induction, repair and significance. Mutat Res Rev Mutat Res. 2004;567(1):1-61. doi: 10.1016/j.mrrev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 47.Cooke MS, Evans MD, Dizdaroglu M, Lunec J. Oxidative DNA damage: mechanisms, mutation, and disease. Faseb J. 2003;17(10):1195-1214. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- 48.1a KU, 2d MM, 1d ZA. systems for oxidized biomolecules Oxidative stress-repair systems of oxidatively damaged biomolecules. An Elimin Prog Heal Sci. 2018;8(1):2018-2145. doi: 10.5604/01.3001.0012.1118. [DOI] [Google Scholar]
- 49.Lassmann H. Axonal injury in multiple sclerosis. J Neurol Neurosurg Psychiatr. 2003;74(6):695-697. doi: 10.1136/jnnp.74.6.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Epstein LG, Prineas JW, Raine CS. Attachment of myelin to coated pits on macrophages in experimental allergic encephalomyelitis. J Neurol Sci. 1983;61(3):341-348. doi: 10.1016/0022-510X(83)90167-3. [DOI] [PubMed] [Google Scholar]
- 51.Lin RF, Lin TS, Tilton RG, Cross AH. Nitric oxide localized to spinal cords of mice with experimental allergic encephalomyelitis: An electron paramagnetic resonance study. J Exp Med. 1993;178(2):643-648. doi: 10.1084/jem.178.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Toft-Hansen H, Nuttall RK, Edwards DR, Owens T. Key metalloproteinases are expressed by specific cell types in experimental autoimmune encephalomyelitis. J Immunol. 2004;173(8):5209-5218. doi: 10.4049/jimmunol.173.8.5209. [DOI] [PubMed] [Google Scholar]
- 53.Mantovani RM, Rocha NP, Magalhães DM, Barbosa IG, Teixeira AL, Simões e Silva AC. Early changes in adipokines from overweight to obesity in children and adolescents. J Pediatr. 2016;92(6):624-630. doi: 10.1016/j.jped.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 54.Sosa RA, Murphey C, Robinson RR, Forsthuber TG. IFN-γ ameliorates autoimmune encephalomyelitis by limiting myelin lipid peroxidation. Proc Natl Acad Sci Unit States Am. 2015;112(36):E5038-E5047. doi: 10.1073/pnas.1505955112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Naegele M, Tillack K, Reinhardt S, Schippling S, Martin R, Sospedra M. Neutrophils in multiple sclerosis are characterized by a primed phenotype. J Neuroimmunol. 2012;242(1-2):60-71. doi: 10.1016/j.jneuroim.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 56.Ferretti G, Bacchetti T, DiLudovico F, et al. Intracellular oxidative activity and respiratory burst of leukocytes isolated from multiple sclerosis patients. Neurochem Int. 2006;48(2):87-92. doi: 10.1016/j.neuint.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 57.Podikoglou DG, Lianou PE, Tsakanikas CD, Papavassiliou JT. Polymorphonuclear leukocyte functions and multiple sclerosis. Neurology. 1994;44(1):129-129. doi: 10.1212/wnl.44.1.129. [DOI] [PubMed] [Google Scholar]
- 58.Van Der Goes A, Brouwer J, Hoekstra K, Roos D, Van Den Berg TK, Dijkstra CD. Reactive oxygen species are required for the phagocytosis of myelin by macrophages. J Neuroimmunol. 1998;92(1-2):67-75. doi: 10.1016/S0165-5728(98)00175-1. [DOI] [PubMed] [Google Scholar]
- 59.Dhib-Jalbut S, Arnold DL, Cleveland DW, et al. Neurodegeneration and neuroprotection in multiple sclerosis and other neurodegenerative diseases. Journal of NeuroimmunologyJ Neuroimmunol. 2006;176:198-215. doi: 10.1016/j.jneuroim.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 60.Stadelmann C, Wegner C, Brück W. Inflammation, demyelination, and degeneration - Recent insights from MS pathology. Biochim Biophys Acta (BBA) - Mol Basis Dis. 2011;1812(2):275-282. doi: 10.1016/j.bbadis.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 61.Dempsey EC, Newton AC, Mochly-Rosen D, et al. Protein kinase C isozymes and the regulation of diverse cell responses. In: American Journal of Physiology - Lung Cellular and Molecular Physiology, 279. American Physiological Society; 2000:L429-L438. 10.1152/ajplung.2000.279.3.l429. [DOI] [PubMed] [Google Scholar]
- 62.Park J-W, Babior BM. Activation of the Leukocyte NADPH Oxidase Subunit p47phox by Protein Kinase C. A phosphorylation-dependent change in the conformation of the C-Terminal End of p47phox. Biochemistry. 1997;36(24):7474-7480. doi: 10.1021/bi9700936. [DOI] [PubMed] [Google Scholar]
- 63.Mossberg N, Movitz C, Hellstrand K, Bergström T, Nilsson S, Andersen O. Oxygen radical production in leukocytes and disease severity in multiple sclerosis. J Neuroimmunol. 2009;213(1-2):131-134. doi: 10.1016/j.jneuroim.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 64.Vladimirova O, Lu FM, Shawver L, Kalman B. The activation of protein kinase C induces higher production of reactive oxygen species by mononuclear cells in patients with multiple sclerosis than in controls. Inflamm Res. 1999;48(7):412-416. doi: 10.1007/s000110050480. [DOI] [PubMed] [Google Scholar]
- 65.Seo J-E, Hasan M, Rahaman KA, Kang M-J, Jung B-H, Kwon O-S. A leading role for NADPH oxidase in an in-vitro study of experimental autoimmune encephalomyelitis. Mol Immunol. 2016;72:19-27. doi: 10.1016/j.molimm.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 66.Choi BY, Kim JH, Kho AR, et al. Inhibition of NADPH oxidase activation reduces EAE-induced white matter damage in mice. J Neuroinflammation. 2015;12(1). doi: 10.1186/s12974-015-0325-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li J, Baud O, Vartanian T, Volpe JJ, Rosenberg PA. Peroxynitrite generated by inducible nitric oxide synthase and NADPH oxidase mediates microglial toxicity to oligodendrocytes. Proc Natl Acad Sci Unit States Am. 2005;102(28):9936-9941. doi: 10.1073/pnas.0502552102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu Y, Hao W, Letiembre M, et al. Suppression of microglial inflammatory activity by myelin phagocytosis: Role of p47-PHOX-mediated generation of reactive oxygen species. J Neurosci. 2006;26(50):12904-12913. doi: 10.1523/JNEUROSCI.2531-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van der Veen RC, Dietlin TA, Hofman FM, Pen L, Segal BH, Holland SM. Superoxide prevents nitric oxide-mediated suppression of helper T lymphocytes: Decreased autoimmune encephalomyelitis in nicotinamide adenine dinucleotide phosphate oxidase knockout mice. J Immunol. 2000;164(10):5177-5183. doi: 10.4049/jimmunol.164.10.5177. [DOI] [PubMed] [Google Scholar]
- 70.Wang P, Xie K, Wang C, Bi J. Oxidative stress induced by lipid peroxidation is related with inflammation of demyelination and neurodegeneration in multiple sclerosis. Eur Neurol. 2014;72:249-254. doi: 10.1159/000363515. [DOI] [PubMed] [Google Scholar]