Abstract
Background:
MOBILE and ENHANCE were similarly designed randomized trials of walking-impaired adults with relapsing-remitting or progressive multiple sclerosis (MS) who received placebo or 10 mg prolonged-release (PR)-fampridine twice daily for 24 weeks. Both studies showed sustained and clinically meaningful improvement in broad measures of walking and balance over 24 weeks of PR-fampridine treatment.
Objective:
To evaluate the functional benefits and safety of PR-fampridine versus placebo using a post hoc integrated efficacy analysis of MOBILE and ENHANCE data.
Methods:
Data from the intention-to-treat (ITT) populations of MOBILE and ENHANCE studies were pooled in a post hoc analysis based on the following outcome measures: 12-item MS Walking Scale (MSWS-12), Timed Up and Go (TUG) speed, Berg Balance Scale (BBS), MS Impact Scale physical impact subscale (MSIS-29 PHYS), EQ-5D utility index score, visual analogue scale (VAS), and adverse events. The primary analysis was the proportion of people with MS (PwMS) with a mean improvement in MSWS-12 score (⩾8 points) from baseline over 24 weeks. A subgroup analysis based on baseline characteristics was performed.
Findings:
In the ITT population (N = 765; PR-fampridine, n = 383; placebo, n = 382), a greater proportion of PR-fampridine–treated PwMS than placebo-treated PwMS achieved a clinically meaningful improvement in the MSWS-12 scale over 24 weeks (44.3% versus 33.0%; p < 0.001). PR-fampridine MSWS-12 responders demonstrated greater improvements from baseline in TUG speed, BBS score, MSIS-29 PHYS score, and EQ-5D utility index and VAS scores versus PR-fampridine MSWS-12 nonresponders and placebo. Subgroup analyses based on baseline characteristics showed consistency in the effects of PR-fampridine.
Conclusion:
The pooled analysis of MOBILE and ENHANCE confirms previous evidence that treatment with PR-fampridine results in clinically meaningful improvements in walking, mobility and balance, self-reported physical impact of MS, and quality of life and is effective across a broad range of PwMS.
Keywords: balance, fampridine, multiple sclerosis, quality of life, walking
Introduction
Impaired mobility is the hallmark physical manifestation of multiple sclerosis (MS), reported by 45%, 67%, and 93% of people with MS (PwMS) within 1 month, 2 years, and 10 years of diagnosis, respectively. 1 Commonly reported deficits in mobility include reductions in walking speed, gait disturbances, and deterioration in balance.2,3 Walking impairments emerge early in the disease course even when overall neurologic disability levels are low.2,3 These early changes in walking have been attributed to possible motor pathway damage in the central nervous system. 4 Among functional domains, PwMS place a high value on walking. 5 Difficulty walking results in disruptions in daily life for PwMS, including impact on employment, with consequential impact on their families and caregivers.6,7
Disease-modifying therapies for MS reduce relapses and delay disability worsening, but there is little evidence that they reverse walking impairments in PwMS. 8 Rehabilitative programs (i.e. exercise and gait training, physical therapy) are recognized as effective nonpharmacologic treatments to maintain mobility and improve walking in PwMS.9–12 Prolonged-release (PR)-fampridine (known as dalfampridine extended-release tablets in the United States) is the only symptomatic pharmacotherapy approved for the improvement of walking in PwMS with walking disability [Expanded Disability Status Scale (EDSS) score between 4.0 and 7.0]. PR-fampridine is thought to block voltage-dependent potassium channels, resulting in improvements in action potential conduction in demyelinated nerve fibers. 13 PR-fampridine has been prescribed to approximately 410,036 PwMS globally in the postmarketing setting, corresponding to 602,802 person-years of exposure through 30 September 2021.
In two multicenter, randomized, double-blind, phase III trials, PR-fampridine demonstrated improvements in walking speed versus placebo, as measured by the objective Timed 25-foot Walk (T25FW).14,15 In addition, a strong efficacy signal was evident in an open-label extension study of the two phase III clinical trials, whereby improvements in walking speed were lost after PR-fampridine was discontinued in the parent trial only to return by the 2-week assessment after re-initiation of the drug. 16 In the phase III pivotal trials, participants treated with PR-fampridine who were considered responders on the T25FW test rated their improvement in walking speed as clinically meaningful on the self-reported 12-item Multiple Sclerosis Walking Scale (MSWS-12).14,15,17 These results demonstrated that treated participants were aware of their improvement in walking disability, but were regarded as tentative confirmation of the objective findings because of complexities and limitations inherent in individuals’ self-assessments of health.
MOBILE was a 6-month exploratory phase II study that evaluated the effects of PR-fampridine versus placebo on self-reported walking and balance in PwMS with walking disability. 18 Based on data from MOBILE, a reduction of ⩾8 points in mean score was identified as the threshold for a clinically meaningful improvement in MSWS-12 score in individuals with MS. 19
The phase III ENHANCE study was a randomized, double-blind, placebo-controlled study similar in design to MOBILE. 20 The ⩾8-point threshold for a clinically meaningful improvement in MSWS-12 score 19 was the prespecified primary endpoint in ENHANCE. 20 Walking-impaired PwMS treated with PR-fampridine 10 mg twice daily had a greater likelihood of experiencing clinically meaningful improvements in self-reported walking ability over 24 weeks compared with placebo [odds ratio (OR) 1.61; 95% confidence interval (CI) 1.15–2.26; p = 0.006). 20 In addition, PR-fampridine showed significant benefits when compared with placebo on Timed Up and Go (TUG) speed and the self-reported MS Impact Scale physical subscale (MSIS-29 PHYS) score. In the PR-fampridine group compared with the placebo group, there was a higher percentage of PwMS with clinically meaningful improvement (⩾15%) in TUG speed (43.4% versus 34.7%; OR 1.46, 95% CI 1.04−2.07; p = 0.03) and greater improvements from baseline in least-squares mean (LSM) MSIS-29 PHYS scores over 24 weeks (8.00 versus 4.68 points; LSM improvement: 3.31; 95% CI −5.13 to −1.50; p < 0.001). 20 Numerical improvements in the Berg Balance Scale (BBS) scores over 24 weeks were observed in the PR-fampridine group (LSM improvement: 1.75 points) and placebo group (LSM improvement: 1.34 points), but the treatment difference was not significant (LSM difference: 0.41; 95% CI −0.13 to 0.95; p = 0141). 20
The similar design of the MOBILE and ENHANCE studies provides the opportunity to combine the data and perform analyses that allow for more robust evaluations, including subgroup analyses. Here, we report the results of a post hoc integrated analysis of individual-level data from the MOBILE and ENHANCE studies that allows further exploration of the effects of PR-fampridine across efficacy and safety measures and based on demographic and clinical characteristics at baseline.
Methods
Study design and participants
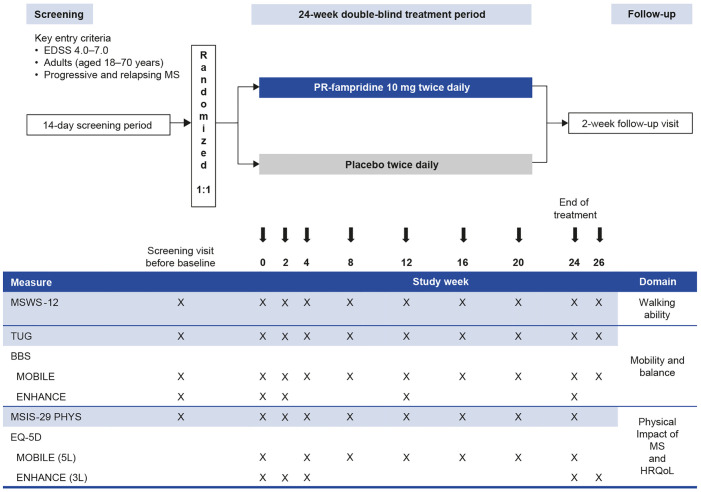
Independent ethics committees (IECs) or institutional review boards (IRBs) approved the MOBILE (NCT01597297) and ENHANCE (NCT02219932) study protocols and all their amendments. A full list of the IEC/IRB names and approval numbers is available upon request. Both studies were conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization guidelines on Good Clinical Practice. Written informed consent was obtained for all participants. Details of the MOBILE and ENHANCE study designs have been previously described.18,20 Combining participant-level data from MOBILE and ENHANCE was appropriate because of the many similarities, including similarity in the population of PwMS included (Table 1) and only limited differences between their study designs (Figure 1).18,20 Both studies featured multicenter, randomized, double-blind, parallel-group designs that evaluated the effects of 24 weeks of PR-fampridine 10 mg twice daily versus placebo in PwMS using the same efficacy endpoints.18,20
Table 1.
Baseline demographic and disease characteristics.
| MOBILE | ENHANCE | Pooled analysis | ||||
|---|---|---|---|---|---|---|
| Characteristic | Placebo n = 64 |
PR-fampridine n = 68 |
Placebo n = 318 |
PR-fampridine n = 315 |
Placebo n = 382 |
PR-fampridine n = 383 |
| Age in years, mean (95% CI) | 49.8 (47.5–52.1) |
49.8 (47.7–51.9) |
48.8 (47.6–50.0) |
49.0 (47.9–50.0) |
49.0 (47.9–50.0) |
49.1 (48.1–50.1) |
| Female, % (95% CI) | 52 (39.3–63.8) |
56 (44.1–67.7) |
57 (51.2–62.1) |
59 (53.6–64.5) |
56 (50.8–60.7) |
58 (53.6–63.4) |
| Body mass index in kg/m2, mean (95% CI) | 26.5 (24.9–28.0) |
26.8 (25.6–28.0) |
25.1 (24.6–25.6) |
25.6 (25.1–26.2) |
25.3 (24.9–25.8) |
25.8 (25.4–26.3) |
| Time since first MS diagnosis in years, mean (95% CI) | 12.4 (10.3–14.5) a |
10.9 (9.2–12.5) |
11.4 (10.5–12.2) |
11.5 (10.6–12.3) |
11.5 (10.7–12.3) a |
11.4 (10.6–12.1) |
| EDSS score, mean (95% CI) | 5.85 (5.63–6.07) |
5.58 (5.35–5.81) |
5.48 (5.38–5.58) |
5.49 (5.39–5.59) |
5.54 (5.45–5.63) |
5.51 (5.41–5.60) |
| Outcome measure, mean (SD) | ||||||
| MSWS-12 score | 75.90 (19.76) | 71.69 (19.29) | 65.39 (21.93) | 63.61 (21.67) | 67.15 (21.91) | 65.04 (21.46) |
| TUG speed, m/s | 0.34 (0.17) b | 0.38 (0.15) | 0.38 (0.20) | 0.38 (0.19) | 0.37 (0.20) | 0.38 (0.18) |
| BBS score | 39.27 (12.34) b | 40.92 (11.91) | 40.24 (11.84) | 40.55 (11.64) | 40.05 (11.91) | 40.62 (11.67) |
| MSIS-29 PHYS score | 53.0 (19.09) | 50.93 (19.40) | 55.29 (21.04) | 52.44 (21.12) | 54.90 (20.72) | 52.17 (20.81) |
| EQ-5D utility index score c | 0.51 (0.23) | 0.54 (0.20) | 0.61 (0.20) d | 0.61 (0.21) e | 0.59 (0.21) f | 0.60 (0.21) f |
| EQ-5D VAS | 59.10 (19.76) b | 61.63 (17.74) | 56.98 (18.31) d | 60.91 (18.03) e | 57.33 (18.55) g | 61.04 (17.96) f |
| MS subtype, % (95% CI) | ||||||
| Relapsing-remitting | 31 (19.9–42.6) |
35 (23.9–46.7) |
49 (43.2–54.2) |
54 (48.1–59.2) |
46 (40.8–50.8) |
50 (45.4–55.4) |
| Secondary progressive | 58 (45.7–69.9) |
46 (33.8–57.4) |
31 (26.0–36.2) |
30 (25.1–35.2) |
36 (30.8–40.4) |
33 (28.2–37.6) |
| Primary progressive | 9 (2.2–16.5) |
18 (8.6–26.7) |
14 (10.3–18.0) |
13 (9.3–16.7) |
13 (9.9–16.8) |
14 (10.4–17.3) |
| Progressive relapsing | 2 (0.0–4.6) |
1 (0.0–4.3) |
6 (3.4–8.6) |
3 (1.2–5.1) |
5 (3.0–7.5) |
3 (1.2–4.5) |
BBS, Berg Balance Scale; CI, confidence interval; EDSS, Expanded Disability Status Scale; ITT, intention-to-treat; MS, multiple sclerosis; ITT, intention-to-treat; MS, multiple sclerosis; MSIS-29 PHYS, Multiple Sclerosis Impact Scale physical subscale; MSWS-12, 12-item Multiple Sclerosis Walking Scale; PR, prolonged-release; SD, standard deviation; TUG, Timed Up and Go; VAS, visual analogue scale.
N = 63 in MOBILE and in the pooled analysis N = 381; time since diagnosis was not available for one participant in the placebo group in MOBILE.
n = 63; number in the ITT population with data available at baseline and any post-baseline visit.
MOBILE used the EQ-5D-5L; 18 ENHANCE used the EQ-5D-3L; 28 and the pooled analysis used the ‘crosswalk’ method, developed by the EuroQol Group, to map the EQ-5D-5L data to the EQ-5D-3L UK value set before calculating the utility index score. 29
n = 316; number in the ITT population with data available at baseline.
n = 312; number in the ITT population with data available at baseline.
n = 380; number in the ITT population with data available at baseline.
n = 379; number in the ITT population with data available at baseline.
Figure 1.
Study design and assessment schedule in MOBILE and ENHANCE.
BBS, Berg Balance Scale; EDSS, Expanded Disability Status Scale; HRQoL, health-related quality of life; MS, multiple sclerosis; MSIS-29 PHYS, Multiple Sclerosis Impact Scale physical subscale; MSWS-12, 12-item Multiple Sclerosis Walking Scale; PR, prolonged-release; TUG, Timed Up and Go.
The inclusion/exclusion criteria of the studies were highly comparable: enrollment of participants aged 18–70 years with a diagnosis of primary-progressive MS, secondary-progressive MS, progressive-relapsing MS, or relapsing-remitting MS per revised McDonald criteria21,22 of ⩾3 months’ duration.18,20 Also mandatory for inclusion was an EDSS score of 4–7.18,20 Presence of a walking impairment (as deemed by the investigator) was an inclusion criterion of ENHANCE only. 20
Assessments
Instruments used in both trials were selected based on their suitability for this integrated analysis. Self-reported walking ability was assessed using the MSWS-12 (used for the primary endpoint in ENHANCE). 20 Objectively assessed mobility and dynamic balance were assessed using the TUG test,23,24 and clinician-reported static and dynamic balance were measured using the BBS.23,25 Self-reported physical impact of MS and health-related quality of life were assessed using the MSIS-29 PHYS 26 and the generic EQ-5D utility index and visual analogue scale (VAS) scores, respectively. 27 The 5-level classification system of the EQ-5D (EQ-5D-5L) was used in MOBILE, 18 whereas the 3-level classification system of the EQ-5D (EQ-5D-3L) was used in ENHANCE. 28 To pool these data sets, the ‘crosswalk’ method, developed by the EuroQol Group, was used to map the EQ-5D-5L data to the EQ-5D-3L UK value set before calculating the combined utility index score. 29
Study visits were scheduled at screening, day 1, and weeks 2, 4, 8, 12, 16, 20, and 24 of the on-treatment period, and at the week 26 follow-up visit in MOBILE and ENHANCE (Figure 1).18,20 The MSWS-12, TUG, and MSIS-29 PHYS questionnaires were completed at these times in both studies, except the MSIS-29 PHY was not assessed at the week 26 follow-up visit. The BBS was also assessed at these times in MOBILE, but less frequently on treatment in ENHANCE (Figure 1). The EQ-5D was administered at all on-treatment visits except week 2 in MOBILE, and was administered at day 1, weeks 2, 4, 24, and the week 26 follow-up visit in ENHANCE. Safety evaluated via physical examination, electrocardiograms, vital signs, clinical laboratory tests, and adverse event (AE) reporting as previously described.18,20 All AEs were recorded using Medical Dictionary for Regulatory Activities (MedDRA®; version 18.1) terms. Treatment-emergent AEs were defined as AEs that started on or after the first dose of study drug, or pre-existing conditions that worsened in severity after the first dose of study drug; a participant was only counted once within each PT (Preferred Term). Severe AEs were defined as symptoms causing severe discomfort, incapacitation, or significant impact on daily life. Investigators assessed whether the AE was related to study drug. A serious AE was any untoward medical occurrence that resulted in death/risk of death, hospitalization/prolonged hospitalization, persistent or significant disability/incapacity, or resulted in a congenital anomaly/birth defect.
Statistical analyses
These post hoc pooled analyses were performed on the intention-to-treat (ITT) populations of the MOBILE and ENHANCE studies, defined as all randomized participants who received at least one dose of study drug and had at least one post-baseline assessment for any of the efficacy measures.18,20 For analysis of MSWS-12, BBS, and MSIS-29 PHYS, baseline was defined as the mean of the screening and day 1 visits. For analysis of EQ-5D utility index and VAS, baseline was defined as day 1 only, given that this parameter was not measured at screening.
The primary analysis was the proportion of PwMS with a mean improvement in MSWS-12 score exceeding the predetermined threshold (⩾8 points) from baseline over 24 weeks (i.e. PwMS who met the definition of PR-fampridine responders for this analysis), analyzed using a logistic regression model with treatment group as the classification variable and study, baseline MSWS-12 score, baseline TUG speed, age, and screening EDSS score as covariates.
For both the MOBILE and ENHANCE studies for each visit, if ⩾50% of the MSWS-12 component items were answered but ⩾1 was not answered, item scores from the unanswered items were imputed using the respondent-specific mean score using the scores from answered items. If < 50% of the MSWS-12 component items were answered, then those unanswered component items were considered missing (and therefore, the total score would be missing, as well) for that visit. For the MOBILE study, missing data for the MSWS-12 total score were imputed using the last observation carried forward (LOCF) approach. For the ENHANCE study, missing data for MSWS-12 total score were imputed using the Markov Chain Monte Carlo (MCMC) method.30,31 For the pooled analysis, missing data were imputed using the MCMC method. A similar analysis compared the proportion of PwMS with a ⩾15% mean improvement in TUG speed between treatment groups, and missing TUG speed individual post-baseline scores were handled as described above for MSWS-12.
A mixed model for repeated measures (MMRM) compared changes from baseline over 24 weeks between treatment groups for the MSWS-12, TUG speed, BBS, MSIS-29 PHYS, and the EQ-5D-3L utility index and VAS score. Treatment, visit, and treatment by visit interaction were included in the models as explanatory variables. Corresponding baseline values for each measure, study, and screening EDSS score were included as covariates in the model. The MSWS-12 model also adjusted for baseline TUG speed and age before fitting the model. For BBS and EQ-5D-3L endpoints, only the post-baseline assessments that were measured at the same study visits in both studies were included in the model. In MOBILE, the missing values of MSWS-12, BBS, MSIS-29 PHYS, and TUG were imputed with LOCF approach and EQ-5D was not imputed. In ENHANCE, missing MSWS-12, BBS, MSIS-29 PHYS, and TUG individual post-baseline scores were imputed using the multiple imputation (MI) method as described above; whereas for the EQ-5D endpoint, the data from both studies were first combined, and then the missing scores were imputed using the MMRM method.
Safety analyses were based on the safety sample (i.e. all participants randomized and exposed to study drug). Any AE with a missing onset date and a resolution date after the first dose of study treatment was considered treatment emergent.18,20
Previous studies have demonstrated that there is a subgroup of PwMS who respond to PR-fampridine;14,15,20 and because the European Medicines Agency prescribing information states that only PwMS who respond to PR-fampridine should remain on treatment after a trial of 2–4 weeks, 32 we conducted analyses using the pooled data to evaluate each study outcome in people who met the criteria of a PR-fampridine MSWS-12 responder compared with PR-fampridine MSWS-12 nonresponders and placebo-treated participants. A PR-fampridine MSWS-12 responder was defined as a participant who received treatment with PR-fampridine and had a mean improvement of ⩾8 points in MSWS-12 score from baseline over 24 weeks. 19
Ad hoc sensitivity analyses were conducted with no adjustments for baseline covariates and also separate analyses with observed data only to understand the impact of adjustments and imputation on the results. The percentages of PwMS with mean MSWS-12 score improvement of ⩾8 points over 24 weeks or with a ⩾15% mean improvement in TUG speed were evaluated using a logistic regression model with treatment group as a classification variable without adjusting for baseline covariates. Missing data were imputed using MI methods described above. Analyses of mean improvement in MSWS-12 score ⩾8 points from baseline over 24 weeks for PR-fampridine versus placebo were conducted using observed data only.
All summaries and statistical analyses were generated using SAS® version 9.4 (SAS® Institute Inc., Cary, NC, USA).
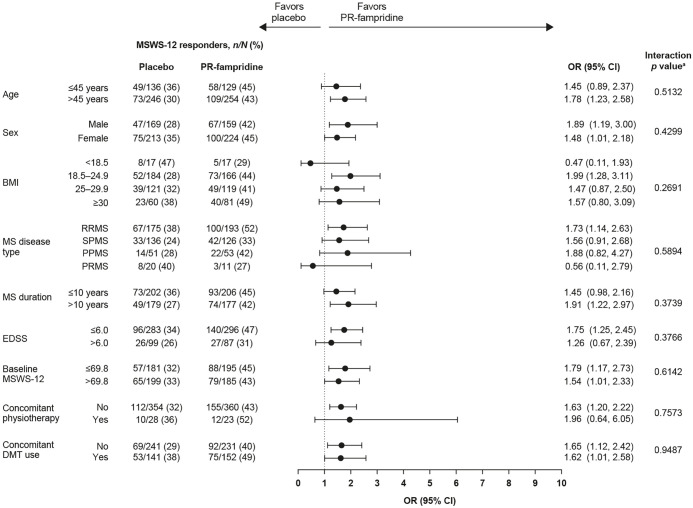
Subgroup analyses
Additional efficacy analyses were performed in specific subgroups of participants to determine whether the treatment effects on percentage of PwMS with mean MSWS-12 score improvement of ⩾ 8 points over 24 weeks were heterogeneous across subgroups. The prespecified subgroups were stratified by age (⩽45 or > 45 years), sex, body mass index, MS disease type, median MS duration (⩽10 or > 10 years), EDSS score (⩽6.0 or > 6.0), median baseline MSWS-12 score [⩽69.8 (equal to or below the median MSWS-12 score at baseline) or > 69.8 (greater than the median MSWS-12 score at baseline)], concomitant physiotherapy, and concomitant DMT use. In the pooled MOBILE and ENHANCE studies, concomitant DMTs included alemtuzumab, fingolimod, dimethyl fumarate, glatiramer acetate, interferon beta-1a, interferon beta-1b, natalizumab, and teriflunomide. For each baseline characteristic subgroup, the proportion of MSWS-12 responders was based on binomial proportions and the OR and 95% CI were calculated using logistic regression model with treatment as the only predictor. The p value was calculated using a logistic regression model fitted with the complete data for that characteristic that considered the interaction effect of treatment and subgroup. In the interaction test, a p value < 0.05 means that the treatment effect of PR-fampridine was significantly different among the subgroups analyzed.
Results
Participants
The ITT population for this integrated efficacy analysis comprised 765 participants who were randomized to receive PR-fampridine (n = 383) or placebo (n = 382). Baseline characteristics between MOBILE and ENHANCE were generally similar between the treatment groups with respect to demographics (age, sex, and body mass index) and clinical characteristics, except that MOBILE enrolled more participants with secondary-progressive MS and ENHANCE enrolled more participants with relapsing-remitting MS (Table 1).
Efficacy
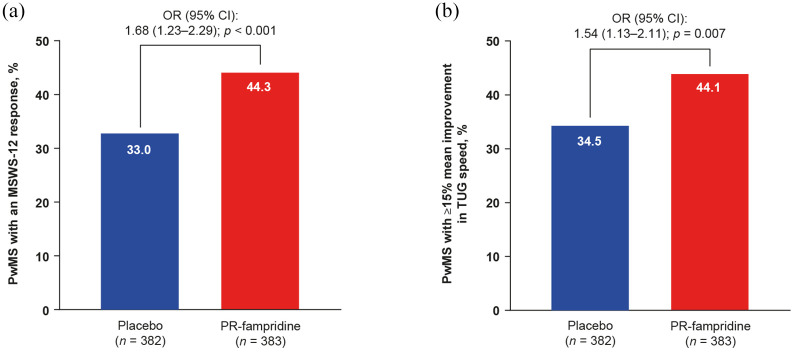
Figure 2(a) shows that a greater proportion of PR-fampridine–treated PwMS than placebo-treated PwMS achieved a clinically meaningful ⩾8-point mean improvement in the MSWS-12 over 24 weeks (44.3% versus 33.0%; p < 0.001). PwMS receiving PR-fampridine were more likely than PwMS receiving placebo to obtain a ⩾8-point mean improvement in the MSWS-12 over 24 weeks (OR 1.68, 95% CI 1.23–2.29; p < 0.001). Similar results were observed in sensitivity analyses that did not include adjustment for baseline covariates in the model (PR-fampridine versus placebo: OR 1.62, 95% CI 1.19–2.19; p < 0.0019).
Figure 2.
Percentage of PwMS in the pooled MOBILE/ENHANCE ITT population with (a) mean MSWS-12 score improvement of ⩾8 points over 24 weeksa and (b) ⩾15% mean improvement in TUG speed (m/s).b
CI, confidence interval; EDSS, Expanded Disability Status Scale; ITT, intention-to-treat; MSWS-12, 12-item Multiple Sclerosis Walking Scale; OR, odds ratio; PR, prolonged-release; PwMS, people with multiple sclerosis; TUG, Timed Up and Go.
aPercentage based on binomial proportions. OR, 95% CI, and p value calculated using logistic regression model adjusted for study, baseline MSWS-12 score, baseline TUG speed, age, and screening EDSS score (missing data imputed using multiple imputation).
bPercentage based on binomial proportions. OR, 95% CI, and p value calculated using logistic regression model adjusted for study, baseline TUG speed, and screening EDSS score (missing data imputed using multiple imputation).
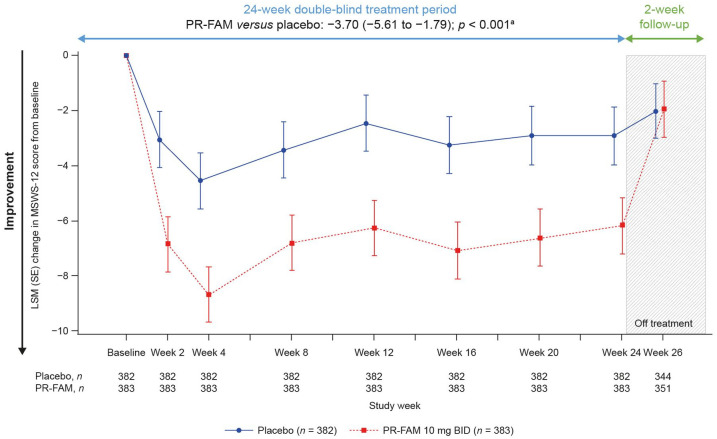
Figure 3 and Table S1 (Online Supplement) show the adjusted LSM change from baseline in MSWS-12 score at every visit up to week 24, and over the 24-week treatment period. The LSM improvements from baseline in the PR-fampridine group relative to the placebo group were detected by week 2 and sustained over 24 weeks. Overall, PR-fampridine was associated with an LSM improvement of −3.70 points in MSWS-12 score relative to placebo over 24 weeks of treatment (95% CI −5.61 to −1.79; p < 0.001). Similar results were seen when only observed data were included in the analysis (Figure S1).
Figure 3.
LSM change from baseline in MSWS-12 score over 24 weeks. Analyses were done in the pooled ITT population with a mixed model for repeated measures.
BID, twice daily; CI, confidence interval; ITT, intention-to-treat; LSM, least-squares mean; MSWS-12, 12-item Multiple Sclerosis Walking Scale; PR-FAM, prolonged-release fampridine.
aLSM (95% CI) treatment difference for PR-FAM versus placebo over 24 weeks.
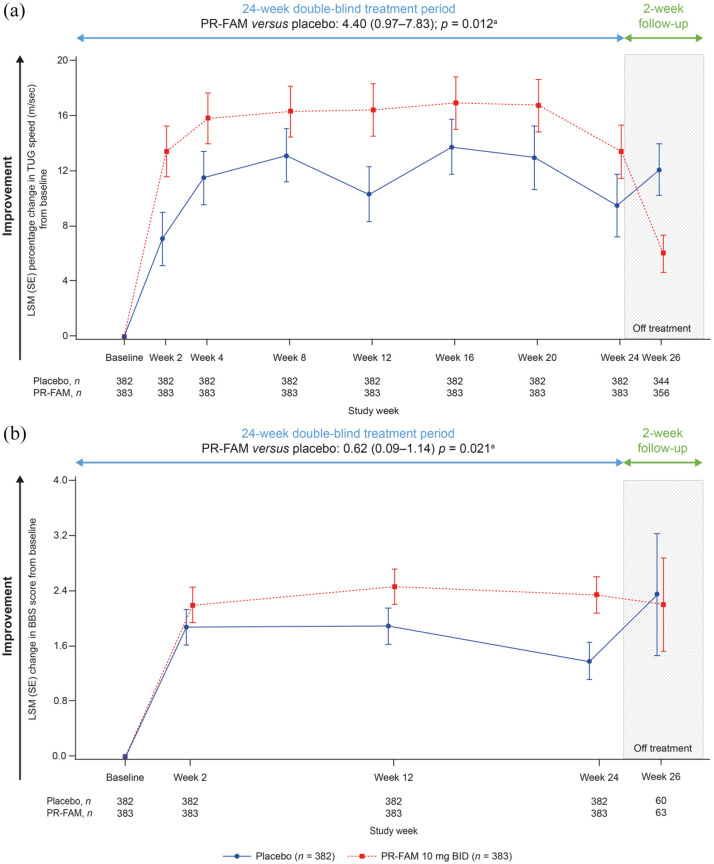
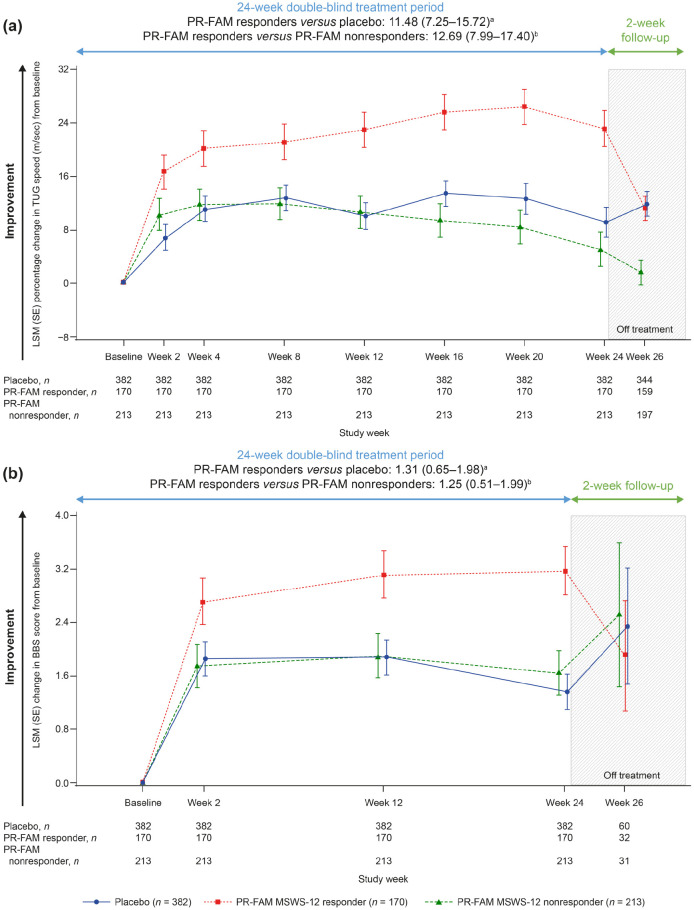
A greater percentage of PR-fampridine–treated participants had a ⩾15% mean improvement in TUG speed versus placebo-treated participants (44.1% versus 34.5%; OR 1.54; 95% CI 1.13–2.11; p = 0.007; Figure 2(b)). Similar results were observed in sensitivity analyses that did not include adjustment for baseline covariates in the model (PR-fampridine versus placebo: OR 1.50, 95% CI 1.10–2.03; p = 0.0094). Greater LSM improvements from baseline in percentage change in TUG speed (LSM difference: 4.40, 95% CI 0.97–7.83; p = 0.012) and BBS score (LSM difference: 0.62, 95% CI 0.09–1.14; p = 0.021) over 24 weeks were observed for PR-fampridine group compared with the placebo group (Figure 4(a) and (b)).
Figure 4.
LSM change from baseline in percentage change in TUG speed (a) and BBS scoreb (b) over 24 weeks. Analyses were done in the pooled ITT population with a mixed model for repeated measures.
BBS, Berg Balance Scale; BID, twice daily; CI, confidence interval; ITT, intention-to-treat; LSM, least-squares mean; PR-FAM, prolonged-release fampridine; TUG, Timed Up and Go.
aLSM (95% CI) treatment difference for PR-FAM versus placebo over 24 weeks.
bAnalysis includes only those visits that were common between the MOBILE ENHANCE studies.
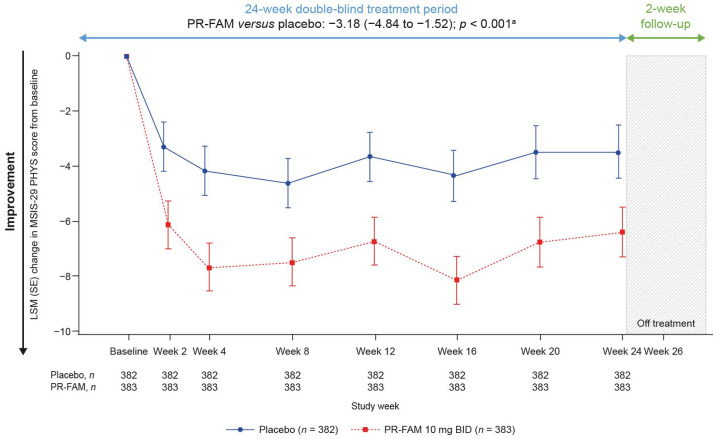
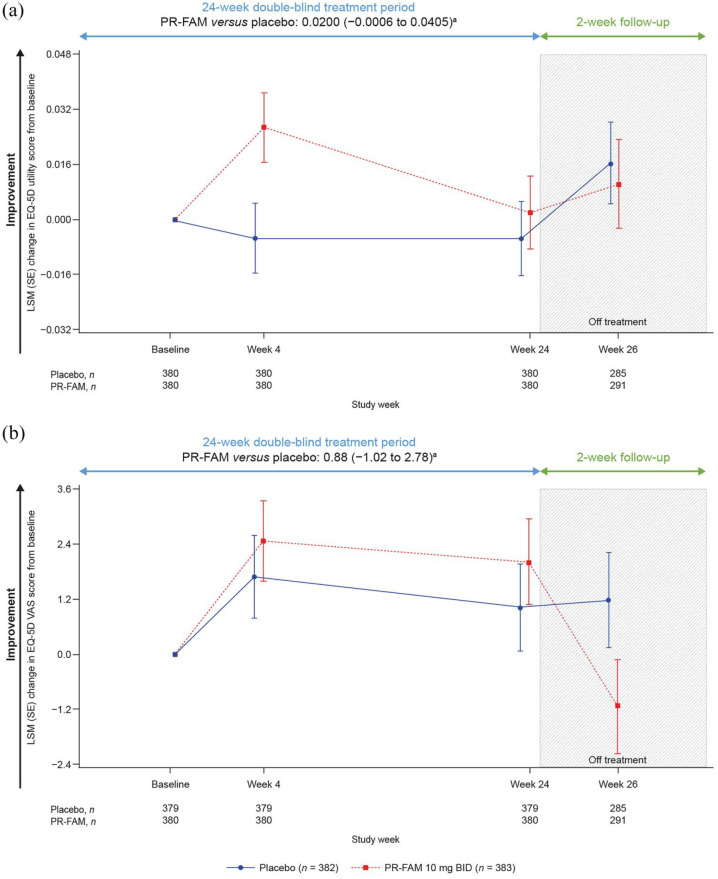
Over 24 weeks of treatment, the PR-fampridine–treated group had significantly greater LSM improvements in MSIS-29 PHYS score compared with the placebo group (LSM difference: −3.18, 95% CI −4.84 to −1.52; p < 0.001; Figure 5). Numerical improvements from baseline over 24 weeks were observed with PR-fampridine versus placebo on the EQ-5D-3L utility index and VAS scores; however, these results were not statistically significant (Figure 6(a) and (b)).
Figure 5.
LSM change from baseline in MSIS-29 PHYS score over 24 weeks. Analyses were done in the pooled ITT population with a mixed model for repeated measures.
BID, twice daily; CI, confidence interval; ITT, intention-to-treat; LSM, least-squares mean; MSIS-29 PHYS, Multiple Sclerosis Impact Scale physical subscale; PR-FAM, prolonged-release fampridine.
aLSM (95% CI) treatment difference for PR-FAM versus placebo over 24 weeks.
Figure 6.
LSM change from baseline in EQ-5D-3L utility index scoreb (a) and EQ-5D-3L VAS scoreb (b) and score over 24 weeks. Analyses were done in the pooled ITT population with a mixed model for repeated measures.
BID, twice daily; CI, confidence interval; ITT, intention-to-treat; LSM, least-squares mean; PR-FAM, prolonged-release fampridine; VAS, visual analogue scale.
aLSM (95% CI) treatment difference for PR-FAM versus placebo over 24 weeks.
bAnalysis includes only those visits that were common between the MOBILE ENHANCE studies.
PR-fampridine MSWS-12 responder analyses
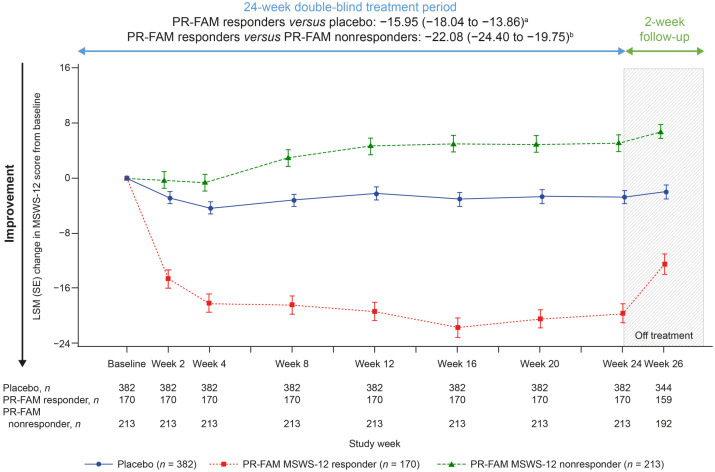
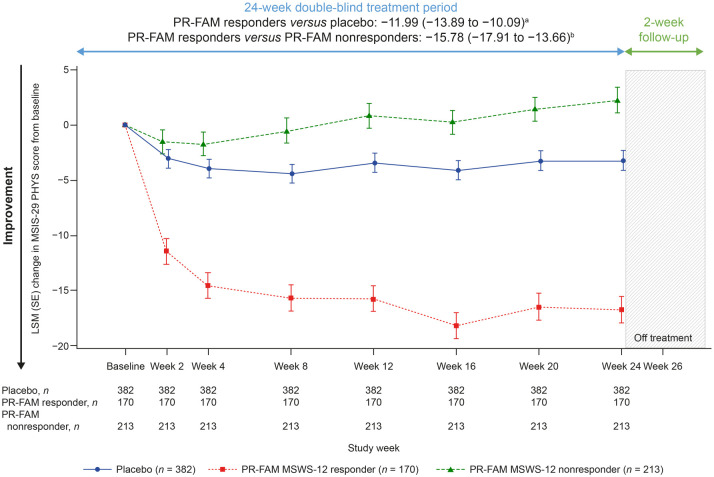
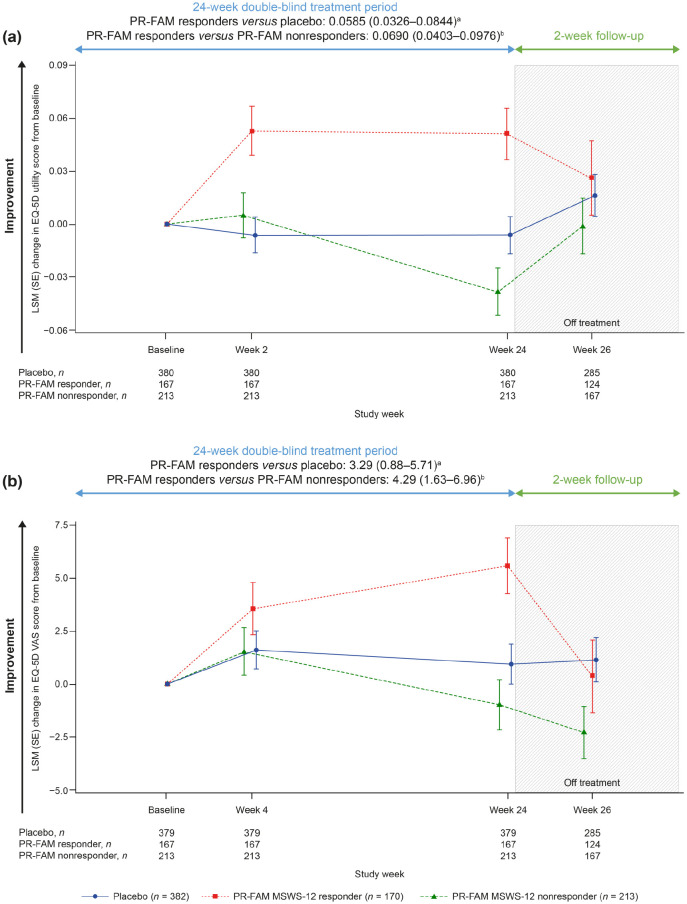
To better understand outcomes in PwMS who would remain on PR-fampridine over the longer term in real-world clinical practice, we evaluated each of the outcome measures in PR-fampridine MSWS-12 responders versus PR-fampridine MSWS-12 nonresponders and the placebo group. Compared with PR-fampridine MSWS-12 nonresponders and placebo-treated participants, PR-fampridine MSWS-12 responders demonstrated greater LSM improvements across all efficacy outcomes over 24 weeks. LSM improvement in MSWS-12 score from baseline over 24 weeks was −15.95 points versus placebo and −22.08 points versus PR-fampridine nonresponders (Figure 7). Similar results were also observed when only observed data were included in the analysis (Figure S2). The percentage of PR-fampridine MSWS-12 responders with a ⩾15% mean improvement in TUG speed was 53.9% compared with 36.3% among PR-fampridine nonresponders (OR 2.33; 95% CI 1.50–3.61; p < 0.001) and 34.5% among placebo-treated PwMS (OR 2.46; 95% CI 1.66–3.65; p < 0.001). PR-fampridine MSWS-12 responders demonstrated greater LSM improvements from baseline in TUG speed, BBS score, MSIS-29 PHYS score, and EQ-5D-3L utility index and VAS scores versus PR-fampridine MSWS-12 nonresponders and placebo based on LSM 95% CIs that did not include zero (Figures 8–10, Tables S2–S6). Differences between responders and nonresponders in MSWS-12, TUG, BBS, and MSIS-29 PHYS outcomes were evident by week 2 and in the EQ-5D-3L utility index by the first assessment at week 4 (Figures 8–10). PR-fampridine MSWS-12 nonresponders and placebo-treated participants had similar LSM changes from baseline in TUG speed and BBS score over 24 weeks (Tables S2 and S3). However, PR-fampridine MSWS-12 nonresponders had LSM worsening from baseline over 24 weeks in MSWS-12 and MSIS-29 PHYS scores, while the placebo group demonstrated some improvement (Tables S1 and S4). Both the PR-fampridine MSWS-12 nonresponders and placebo groups worsened from baseline in EQ-5D-3L utility index by week 24, with greater worsening occurring in nonresponders (Figure 10(a); Table S5). PR-fampridine MSWS-12 nonresponders had a small improvement from baseline in EQ-5D-3L VAS score over 24 weeks, although not as much as in placebo patients (Table S6).
Figure 7.
LSM change from baseline in MSWS-12 score over 24 weeks in PR-fampridine MSWS-12 responders, PR-fampridine MSWS-12 nonresponders, and placebo-treated people. Analyses were done in the pooled ITT population with a mixed model for repeated measures.
CI, confidence interval; ITT, intention-to-treat; LSM, least-squares mean; MSWS-12, 12-item Multiple Sclerosis Walking Scale; PR-FAM, prolonged-release fampridine.
aLSM (95% CI) treatment difference for PR-FAM responders versus placebo over 24 weeks.
bLSM (95% CI) treatment difference for PR-FAM responders versus PR-FAM nonresponders over 24 weeks.
Figure 8.
LSM change from baseline in percentage change in TUG speed (a), BBS score (b)c over 24 weeks in PR-fampridine MSWS-12 responders, PR-fampridine MSWS-12 nonresponders, and placebo-treated people. Analyses were done in the pooled ITT population with a mixed model for repeated measures.
BBS, Berg Balance Scale; CI, confidence interval; ITT, intention-to-treat; LSM, least-squares mean; MSWS-12, 12-item Multiple Sclerosis Walking Scale; PR-FAM, prolonged-release fampridine; TUG, Timed Up and Go.
aLSM (95% CI) treatment difference for PR-FAM responders versus placebo over 24 weeks.
bLSM (95% CI) treatment difference for PR-FAM responders versus PR-FAM nonresponders over 24 weeks.
cAnalysis includes only those visits that were common between the MOBILE ENHANCE studies.
Figure 9.
LSM change from baseline in MSIS-29 PHYS score over 24 weeks in PR-fampridine MSWS-12 responders, PR-fampridine MSWS-12 nonresponders, and placebo-treated people. Analyses were done in the pooled ITT population with a mixed model for repeated measures.
CI, confidence interval; ITT, intention-to-treat; LSM, least-squares mean; MSIS-29 PHYS, Multiple Sclerosis Impact Scale physical subscale; MSWS-12, 12-item Multiple Sclerosis Walking Scale; PR-FAM, prolonged-release fampridine.
aLSM (95% CI) treatment difference for PR-FAM responders versus placebo over 24 weeks.
bLSM (95% CI) treatment difference for PR-FAM responders versus PR-FAM nonresponders over 24 weeks.
Figure 10.
LSM change from baseline in EQ-5D-3L utility index scorec (a) and EQ-5D-3L VAS scorec (b) over 24 weeks in PR-fampridine MSWS-12 responders, PR-fampridine MSWS-12 nonresponders, and placebo-treated people. Analyses were done in the pooled ITT population with a mixed model for repeated measures.
CI, confidence interval; ITT, intention-to-treat; LSM, least-squares mean; MSWS-12, 12-item Multiple Sclerosis Walking Scale; PR-FAM, prolonged-release fampridine.
aLSM (95% CI) treatment difference for PR-FAM responders versus placebo over 24 weeks.
bLSM (95% CI) treatment difference for PR-FAM responders versus PR-FAM nonresponders over 24 weeks.
cAnalysis includes only those visits that were common between the MOBILE ENHANCE studies.
Safety
Overall, there was no marked difference in AEs, serious AEs, or AEs leading to treatment discontinuation or study withdrawal between the PR-fampridine MSWS-12 responders, PR-fampridine MSWS-12 nonresponders, and placebo-treated subgroups (Table 2). Treatment-emergent AEs were slightly more common in MSWS-12 responders (22%) than MSWS-12 nonresponders (15%) or placebo (14%). Urinary tract infections were the most common treatment-emergent AE by MedDRA PT in all three groups, MSWS-12 nonresponders (14%), MSWS-12 responders (9%), and placebo (11%). There were two deaths reported, one each in the MSWS-12 responder and placebo groups. Both deaths were considered unrelated to study treatment (coronary artery stenosis and acute myocardial infarction) and occurred after the participant had completed study treatment but before completing the 2-week post-treatment follow-up.
Table 2.
Adverse events.
| AE, n (%) | |||
|---|---|---|---|
| PR-fampridine 10 mg
BID Nonresponder n = 214 |
PR-fampridine 10 mg
BID Responder n = 170 |
Placebo n = 383 |
|
| Any AE | 147 (69) | 111 (65) | 239 (62) |
| Any severe AE a | 13 (6) | 3 (2) | 12 (3) |
| Any treatment-related AE b | 33 (15) | 38 (22) | 52 (14) |
| Serious AE c | 16 (7) | 11 (6) | 26 (7) |
| Serious AE in >1 participant by MedDRA PT c | |||
| MS relapse | 12 (6) | 4 (2) | 11 (3) |
| Fall | 0 | 2 (1) | 2 (<1) |
| Any treatment-related serious AEb,c | 0 | 0 | 2 (<1) |
| AE leading to dose interruption | 13 (6) | 11 (6) | 15 (4) |
| AE leading to treatment discontinuation | 12 (6) | 9 (5) | 23 (6) |
| AE leading to study withdrawal | 12 (6) | 10 (6) | 24 (6) |
| Death d | 0 | 1 (<1) | 1 (<1) |
| Most common treatment-emergent AE by MedDRA SOC (⩾5% in any treatment group) e | |||
| Infections and infestations | 74 (35) | 48 (28) | 113 (30) |
| Nervous system disorders | 62 (29) | 43 (25) | 81 (21) |
| Musculoskeletal and connective tissue disorders | 38 (18) | 31 (18) | 57 (15) |
| Gastrointestinal disorders | 26 (12) | 24 (14) | 34 (9) |
| General disorders and administration site conditions | 23 (11) | 22 (13) | 46 (12) |
| Injury, poisoning, and procedural complications | 22 (10) | 18 (11) | 39 (10) |
| Psychiatric disorders | 9 (4) | 18 (11) | 13 (3) |
| Renal and urinary disorders | 8 (4) | 16 (9) | 8 (2) |
| Investigations | 19 (9) | 14 (8) | 25 (7) |
| Skin and subcutaneous tissue disorders | 13 (6) | 14 (8) | 13 (3) |
| Respiratory, thoracic, and mediastinal disorders | 11 (5) | 8 (5) | 13 (3) |
| Most common treatment-emergent AE by MedDRA PT (⩾5% in any treatment group) e | |||
| Urinary tract infection | 31 (14) | 16 (9) | 42 (11) |
| Fall | 14 (7) | 14 (8) | 27 (7) |
| MS relapse | 23 (11) | 14 (8) | 36 (9) |
| Nasopharyngitis | 14 (7) | 12 (7) | 27 (7) |
| Back pain | 11 (5) | 11 (6) | 14 (4) |
| Insomnia | 4 (2) | 11 (6) | 3 (<1) |
| Upper respiratory tract infection | 7 (3) | 9 (5) | 10 (3) |
| Headache | 12 (6) | 8 (5) | 20 (5) |
| Treatment-emergent AEs of special interest by MedDRA PT (⩾1% in any treatment group) c | |||
| Urinary tract infections | 41 (19) | 28 (16) | 49 (13) |
| Urinary tract infection | 31 (14) | 16 (9) | 42 (11) |
| Micturition urgency | 1 (<1) | 4 (2) | 0 |
| Dysuria | 2 (<1) | 2 (1) | 1 (<1) |
| Pollakiuria | 0 | 2 (1) | 0 |
| Proteinuria | 1 (<1) | 2 (1) | 1 (<1) |
| Urinary retention | 0 | 2 (1) | 0 |
| Cystitis | 4 (2) | 1 (<1) | 2 (<1) |
| Cardiovascular disorders | 2 (<1) | 4 (2) | 2 (<1) |
| Palpitations | 2 (<1) | 2 (1) | 1 (<1) |
| Tachycardia | 0 | 2 (1) | 0 |
| Serious hypersensitivity | 13 (6) | 16 (9) | 14 (4) |
| Rash | 5 (2) | 5 (3) | 4 (1) |
| Pruritus | 1 (<1) | 3 (2) | 1 (<1) |
| Erythema | 0 | 2 (1) | 0 |
| Seasonal Allergy | 0 | 2 (1) | 1 (<1) |
AE, adverse event; BID, twice daily; MedDRA, Medical Dictionary of Regulatory Activities; MS, multiple sclerosis; PR, prolonged-release; PT, Preferred Term; SOC, system organ class.
Severe AEs were defined as symptoms causing severe discomfort, incapacitation, or significant impact on daily life.
Investigators assessed whether the AE was related to study drug.
A serious AE was any untoward medical occurrence that resulted in death/risk of death, hospitalization/prolonged hospitalization, persistent or significant disability/incapacity, or resulted in a congenital anomaly/birth defect.
Both deaths were considered unrelated to study treatment (coronary artery stenosis and acute myocardial infarction) and occurred after the participant had completed study treatment but before completing the 2-week post-treatment follow-up.
Treatment-emergent AEs were defined as AEs that started on or after the first dose of study drug, or pre-existing conditions that worsened in severity after the first dose of study drug; a participant was only counted once within each PT.
Subgroup analyses
Subgroup analyses were performed to evaluate whether there was a differential response to PR-fampridine on MSWS-12 response in subgroups of PwMS based on demographic variables and clinical characteristics, including concomitant physiotherapy or DMT use. The subgroups and p values for the interaction test for each subgroup are shown in Figure 11. There was no significant interaction between the treatment effect and any of the subgroup variables evaluated. This indicates there was no apparent heterogeneity of effect of PR-fampridine across demographic and baseline clinical characteristics variables tested. Furthermore, the effect of PR-fampridine on MSWS-12 response was not significantly different in patients who did and did not receive concomitant physiotherapy or concomitant DMTs.
Figure 11.
Subgroup analyses based on demographic and clinical characteristics at baseline.
BMI, body mass index; CI, confidence interval; DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale; MS, multiple sclerosis; MSWS-12,12-item Multiple Sclerosis Walking Scale; OR, odds ratio; PPMS, primary-progressive multiple sclerosis; PR, prolonged-release; PRMS, primary-relapsing multiple sclerosis; RRMS, relapsing-remitting multiple sclerosis; SPMS, secondary-progressive multiple sclerosis.
aIn the interaction test, a p value <0.05 means that the treatment effect of PR-fampridine was significantly different among the subgroups analyzed.
Discussion
This integrated analysis of MOBILE and ENHANCE provides a more robust estimate of the AEs and benefits of PR-fampridine on self-reported walking, objectively measured mobility and balance, self-reported physical impact of MS, and quality of life. The pooled analysis included more than 380 PwMS per treatment group with a range of MS subtypes. At 765 patients, this is the largest randomized controlled data set ever analyzed with PR-fampridine to our knowledge. The results confirm the findings from related work. 33
Consistent with subgroup analyses of the pivotal studies of PR-fampridine that used an objective definition of response to PR-fampridine based on the T25FW, 33 data from the current subgroup analysis using improvement in the self-reported MSWS-12 as the basis for the definition of response confirm that the PR-fampridine treatment effects were not significantly different in the subgroups evaluated. In addition, these analyses show that PR-fampridine is effective in the presence or absence of concomitant DMTs and expands the range of DMTs evaluated beyond that in pooled analysis of the pivotal studies when only a limited number of DMTs were available. 33 Based on these results, we would expect consistent benefits for PR-fampridine across a broad range of PwMS, including those receiving concomitant therapies, such as physiotherapy or DMTs. Subgroup analyses based on two different definitions of response, one objective (Timed-walk responder) 33 and the other subjective (MSWS-12 responder), indicate that PR-fampridine has a consistent effect across a broad range of PwMS.
As in ENHANCE and MOBILE,18,20 the pooled PR-fampridine group had significantly higher proportions of PwMS with clinically meaningful improvements in MSWS-12 and TUG speed compared with the placebo group over 24 weeks. In addition, similar to the individual studies,18,20 significant differences in favor of PR-fampridine over placebo regarding LSM improvements in MSWS-12 score, TUG speed, and MSIS-29 PHYS over 24 weeks of treatment were detected, with improvements evident by week 2 and sustained over 24 weeks. Numerical improvements that were not statistically significant were observed in EQ-5D-3L utility index and VAS scores.
LSM improvements from baseline in BBS score were significantly greater in the PR-fampridine-treated group versus the placebo group over 24 weeks. In MOBILE, 18 PR-fampridine resulted in greater improvements from baseline in BBS score compared with placebo (during the 24-week treatment period). However, due to the exploratory nature of MOBILE, formal statistical testing between treatment groups was not conducted for this endpoint. 18 In ENHANCE, the improvement in BBS observed with PR-fampridine versus placebo did not reach statistical significance. 20 One of the key advantages of this integrated pooled analysis is that sample size is increased, providing greater power in the evaluation of treatment differences.
The prespecified analyses in the original MOBILE and ENHANCE studies included adjustments for baseline covariates to control for potential imbalances in the treatment groups and for imputation of missing data. The primary analyses of the MOBILE-ENHANCE pooled data were conducted using analytical models with adjustments for covariates and imputation to remain faithful to the prespecified analyses in the protocol. Statistical models that adjust for covariates and imputation for missing data are the primary analyses in the protocol based on requests from regulatory authorities. However, to understand the impact of adjusting for covariates or imputing data, we conduced sensitivity analyses without adjustments and with observed data only. These sensitivity analyses yielded ORs and significant treatment differences that were similar to the prespecified analyses, indicating the data were not imbalanced and imputation of data did not influence the results.
It is important to consider these results within the context of the potential limitations of the outcomes measures to detect change in the populations of MOBILE and ENHANCE. An examination of BBS measurement performance using MOBILE data suggested that the BBS has limited ability to detect change, thus potentially underestimating the impact of PR-fampridine on balance in MOBILE and ENHANCE.20,34 Despite these limitations, the increased statistical power of the pooled analysis confirmed the benefits on PR-fampridine on clinician-reported static and dynamic balance.
The magnitude of improvement was consistently greater with PR-fampridine, although some improvements were observed in the placebo group on a range of efficacy measures. The high placebo response rate in the pooled analysis is likely a result of the inherently greater variability associated with self-reported measures. In these studies, the definition of a responder was based on a magnitude of change in a self-reported measure with response options that are subject to individual interpretation. In the pivotal clinical studies of PR-fampridine, the definition of consistent timed-walk responder status was independent of the magnitude of change on the T25FW.14,15 Individuals participating in clinical trials have considerable expectations that could influence how they respond on subjective measures and can lead to greater variability than would be observed with an objective measure. 35 PR-fampridine nonresponders behave similar to placebo-treated PwMS on the objective outcome measures, but show worse outcomes over time than placebo on the self-reported measures, which may be a result of selection bias in the responder analyses or because of inherently higher variability in subjective measures.
The use of a mean improvement of ⩾8 points in MSWS-12 score to define a responder was supported by marked benefits in TUG speed, BBS, and MSIS-29 PHYS, and EQ-5D-3L utility index and VAS among PR-fampridine MSWS-12 responders compared with PR-fampridine MSWS-12 nonresponders and placebo-treated participants. Differences between PR-fampridine MSWS-12 responders and nonresponders were evident by week 2 and were sustained over 24 weeks. As would be expected, greater benefits on MSWS-12 scores were observed in PR-fampridine MSWS-12 responders than PR-fampridine MSWS-12 nonresponders or the placebo group.
PR-fampridine MSWS-12 nonresponders and placebo showed similar results from baseline over 24 weeks in the objective assessments – TUG and BBS tests (Tables S2 and S3). Conversely, these two groups showed different results in the subjective assessments – MSWS-12 and MSIS-29 PHYS scores – where nonresponders showed a worsening result but the placebo group demonstrated some improvement (Tables S1 and S4). We further examined if these findings could be due to a difference in AEs; we found no marked difference between the PR-fampridine MSWS-12 responders, nonresponders, or placebo-treated subgroups for AEs, serious AEs, or AEs leading to treatment discontinuation or study withdrawal.
A key strength of this analysis was the robustness of the efficacy data, which were collected from two nearly identical, double-blind, randomized, placebo-controlled clinical trials; this allowed for the analyses to explore the effect of PR-fampridine across a range of patient subgroups. The MSWS-12, TUG, and MSIS-12 were assessed at the same time points in each study, and temporal BBS and EQ-5D-3L data were reported only when there was overlap between MOBILE and ENHANCE.
This was a post hoc pooled analysis that was confirmatory because the previous studies showed similar results; however, by pooling the data of the individual studies the sample size increased, making the data set more robust and therefore allowed us to explore the effect of PR-fampridine in subgroups of PwMS which was not feasible in the individual studies due to lower numbers. The larger sample size is important given the diversity of the MS disease in this patient population, as suggested by EDSS scores ranging from 4.0 to 7.0. Since EDSS ⩾6 indicates patients are likely in a progressive phase with different pathophysiology, it may be of benefit for the treating physicians to know if PR-fampridine is expected to have the same magnitude of effect in a patient with an EDSS score of 4 compared with a patient with an EDSS score of 6.5. This larger sample size allowed a subgroup analysis of this question, demonstrating that PR-fampridine seems to work equally well in most of these subgroups.
Specifically, there were no significant differences in treatment effect based on EDSS score ⩽6.0 and > 6.0 and more participants had EDSS scores ⩽6.0, so the results may be generalizable to the indicated population. The treatment effects of PR-fampridine beyond 24 weeks on the outcomes assessed in this pooled analysis could not be determined. One of the biases of responder analyses is that PwMS who respond on one measure are more likely to show a positive response on other outcome measures.
The EQ-5D self-reported questionnaire includes the EQ-5D descriptive system, which comprises five dimensions of health: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. However, in EQ-5D-3L, each dimension of the EQ-5D descriptive system will have three levels of potential functioning, while in EQ-5D-5L, it will have five levels. Through the crosswalk method, the EQ-5D-5L data were mapped into the EQ-5D-3L data, and then the mapped data were used to calculate the EQ-5D utility index score. 29 This mapping method ensures that each unique health state measured by EQ-5D-5L will have a corresponding health state measured by EQ-5D-3L. Both the EQ-5D-3L and EQ-5D-5L are appropriate for use in a wide range of populations; however, the EQ-5D-5L yields more precise and sensitive measurement of health status.36,37 Limitations of the mapping approach are that it results in a more narrow range of index values, which can result in an artificial floor effect on the resulting values, and that different translations of the EQ-5D-5L descriptive system might be subject to cultural interpretation that can influence the crosswalk when mapped to EQ-5D-3L value sets. 29 Because the EQ-5D is not a disease-specific measure, it may not appropriately capture all changes in quality of life in PwMS treated with PR-fampridine. In addition, the EQ-5D-3L may not be sensitive enough to discern the impact of walking changes in PwMS with mobility impairments treated with PR-fampridine.
Results from the MOBILE-ENHANCE pooled analyses augment the available evidence that demonstrate positive effects of PR-fampridine on objectively measured walking speed, self-assessed walking ability, functional mobility, and quality of life. 38 Notably, in the pooled analyses there was a significant treatment effect of PR-fampridine on improvement in balance as measured by the BBS, which supports the numerical improvements observed in the ENHANCE cohort alone. 20 The effects of PR-fampridine have been studied on other functional domains with mixed results. 38 Assessments in MOBILE and ENHANCE did not evaluate long-term walking capacity or the contributions of fatigue from MS on walking capacity. Some studies have reported improvements in fatigue with PR-fampridine treatment.39–41 It would be interesting to evaluate whether reductions in fatigue from PR-fampridine impact long-term walking capacity. There is a need for randomized controlled studies with cognitive, emotional, and speech measures powered as primary endpoints to more confidently evaluate the impact of PR-fampridine on these domains. Definitions of response based on other functional outcomes would allow us to understand whether the definitions of response based on objective or self-perceived walking criteria are too narrow to detect patients who could be benefiting in other ways from PR-fampridine treatment. 38
Conclusion
Results of this integrated analysis provide a more robust estimate of the effects of PR-fampridine on self-perceived walking ability, dynamic and static balance, self-reported physical impact of MS, quality of life, and AEs over 24 weeks across a broad range of PwMS, including those with progressive MS. Use of a prospectively defined MSWS-12 responder analysis is supported by the substantial benefit observed across all ambulatory outcome measures, which was accompanied by improvements in disease-specific and generic measures of health states. The effects of PR-fampridine versus placebo were not different in the subgroups analyzed based on demographic and clinical characteristics, which indicates that PR-fampridine is effective across a broad range of PwMS, including in those who are receiving concomitant treatment with DMTs and/or physiotherapy.
Supplemental Material
Supplemental material, sj-pdf-1-tan-10.1177_17562864221090398 for Efficacy of prolonged-release fampridine versus placebo on walking ability, dynamic and static balance, physical impact of multiple sclerosis, and quality of life: an integrated analysis of MOBILE and ENHANCE by Raymond Hupperts, Claudio Gasperini, Jan Lycke, Tjalf Ziemssen, Peter Feys, Shan Xiao, Carlos Acosta, Thijs Koster and Jeremy Hobart in Therapeutic Advances in Neurological Disorders
Acknowledgments
Manjit McNeill, of Biogen Maidenhead, UK, provided initial statistical support and guidance for the analyses in this manuscript. Biogen provided funding for medical writing support in the development of this paper. Malcolm Darkes, PhD, MPS, and Bess Reinoso, PhD, from Excel Scientific Solutions wrote the first draft of the manuscript based on input from authors, and Jackie Parker from Excel Scientific Solutions copyedited and styled the manuscript per journal requirements. Biogen reviewed and provided feedback on the paper to the authors. The authors had full editorial control of the paper and provided their final approval of all content.
Footnotes
Author contributions: Raymond Hupperts: Conceptualization; Data curation; Investigation; Project administration; Supervision; Writing – review & editing.
Claudio Gasperini: Data curation; Investigation; Project administration; Supervision; Writing – review & editing.
Jan Lycke: Data curation; Investigation; Project administration; Supervision; Writing – review & editing.
Tjalf Ziemssen: Conceptualization; Investigation; Methodology; Project administration; Supervision; Writing – review & editing.
Peter Feys: Data curation; Investigation; Resources; Supervision; Writing – review & editing.
Shan Xiao: Data curation; Formal analysis; Methodology; Validation; Writing – review & editing.
Carlos Acosta: Conceptualization; Methodology; Writing – review & editing.
Thijs Koster: Conceptualization; Project administration; Writing – review & editing.
Jeremy Hobart: Conceptualization; Data curation; Investigation; Methodology; Supervision; Writing – review & editing.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Raymond Hupperts has received compensation for consulting, advisory boards and research grants and as a speaker for lectures from Biogen, and Merck.
Claudio Gasperini has received compensation for consulting from Bayer HealthCare and Biogen and as a speaker for lectures from Biogen, Bayer HealthCare, Genzyme, Merck Serono, Novartis and Teva.
Jan Lycke has received travel support and/or lecture honoraria from Biogen, Novartis, Merck, Alexion, Roche and Sanofi Genzyme; has served on scientific advisory boards for Biogen, Novartis, Merck, Roche, BSM and Sanofi Genzyme; serves on the editorial board of the Acta Neurologica Scandinavica; and has received unconditional research grants from Biogen and Novartis.
Tjalf Ziemssen has received compensation for advisory boards, trial steering committees and data and safety monitoring committees, and speaker fees and project support, from Bayer, Biogen, Celgene, Merck, Novartis, Roche, Sanofi, and Teva.
Peter Feys has received advisory board fees for Biogen and Novartis, speaker fees from Excemed, and is an editorial board member for Multiple Sclerosis Journal and Neurorehabilitation and Neural Repair.
Jeremy Hobart has received consulting/advisor fees, honoraria, and research support from Acorda, Biogen, Genzyme, Global Blood Therapeutics, Merck Serono, Novartis, Tigercat, and Vanita.
Shan Xiao was an employee of and held stock/stock options in Biogen at the time the research was conducted. Carlos Acosta and Thijs Koster are employees of and hold stock/stock options in Biogen.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: the MOBILE and ENHANCE studies and this integrated analysis were funded by Biogen.
ORCID iDs: Raymond Hupperts  https://orcid.org/0000-0003-2106-2158
https://orcid.org/0000-0003-2106-2158
Jan Lycke  https://orcid.org/0000-0002-7891-8466
https://orcid.org/0000-0002-7891-8466
Tjalf Ziemssen  https://orcid.org/0000-0001-8799-8202
https://orcid.org/0000-0001-8799-8202
Data availability: Requests for data supporting this manuscript should be submitted to the Biogen Clinical Data Request Portal (www.biogenclinicaldatarequest.com).
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Raymond Hupperts, Department of Neurology, Zuyderland Medical Center, 6130 MB Sittard, The Netherlands.
Claudio Gasperini, Department of Neurosciences, S. Camillo Forlanini Hospital, Rome, Italy.
Jan Lycke, Department of Clinical Neuroscience, Institute of Neuroscience and Physiology, Sahlgrenska Academy and Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden.
Tjalf Ziemssen, Center of Clinical Neuroscience, Carl Gustav Carus University Clinic, Technical University of Dresden, Dresden, Germany.
Peter Feys, REVAL, Faculty of Rehabilitation Sciences, Hasselt University, Diepenbeek, Belgium; UMSC Hasselt, Pelt, Belgium.
Shan Xiao, Biogen, Cambridge, MA, USA.
Carlos Acosta, Biogen, Baar, Switzerland.
Thijs Koster, Biogen, Cambridge, MA, USA.
Jeremy Hobart, Plymouth University Peninsula Schools of Medicine and Dentistry, Plymouth Hospitals NHS Trust, Plymouth, UK.
References
- 1. van Asch P. Impact of mobility impairment in multiple sclerosis 2 – patients’ perspectives. Eur Neurol Rev 2011; 6: 115–120. [Google Scholar]
- 2. Martin CL, Phillips BA, Kilpatrick TJ, et al. Gait and balance impairment in early multiple sclerosis in the absence of clinical disability. Mult Scler 2006; 12: 620–628. [DOI] [PubMed] [Google Scholar]
- 3. Langeskov-Christensen D, Feys P, Baert I, et al. Performed and perceived walking ability in relation to the Expanded Disability Status Scale in persons with multiple sclerosis. J Neurol Sci 2017; 382: 131–136. [DOI] [PubMed] [Google Scholar]
- 4. Hubbard EA, Wetter NC, Sutton BP, et al. Diffusion tensor imaging of the corticospinal tract and walking performance in multiple sclerosis. J Neurol Sci 2016; 363: 225–231. [DOI] [PubMed] [Google Scholar]
- 5. Heesen C, Böhm J, Reich C, et al. Patient perception of bodily functions in multiple sclerosis: gait and visual function are the most valuable. Mult Scler 2008; 14: 988–991. [DOI] [PubMed] [Google Scholar]
- 6. Sutliff MH. Contribution of impaired mobility to patient burden in multiple sclerosis. Curr Med Res Opin 2010; 26: 109–119. [DOI] [PubMed] [Google Scholar]
- 7. Larocca NG. Impact of walking impairment in multiple sclerosis: perspectives of patients and care partners. Patient 2011; 4: 189–201. [DOI] [PubMed] [Google Scholar]
- 8. Baird JF, Sandroff BM, Motl RW. Therapies for mobility disability in persons with multiple sclerosis. Expert Rev Neurother 2018; 18: 493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pearson M, Dieberg G, Smart N. Exercise as a therapy for improvement of walking ability in adults with multiple sclerosis: a meta-analysis. Arch Phys Med Rehabil 2015; 96: 1339–1348.e1337. [DOI] [PubMed] [Google Scholar]
- 10. Motl RW, Sandroff BM, DeLuca J. Exercise training and cognitive rehabilitation: a symbiotic approach for rehabilitating walking and cognitive functions in multiple sclerosis? Neurorehabil Neural Repair 2016; 30: 499–511. [DOI] [PubMed] [Google Scholar]
- 11. Ramari C, Hvid LG, David AC, et al. The importance of lower-extremity muscle strength for lower-limb functional capacity in multiple sclerosis: systematic review. Ann Phys Rehabil Med 2020; 63: 123–137. [DOI] [PubMed] [Google Scholar]
- 12. Leone C, Kalron A, Smedal T, et al. Effects of rehabilitation on gait pattern at usual and fast speeds depend on walking impairment level in multiple sclerosis. Int J MS Care 2018; 20: 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Judge SI, Bever CT., Jr. Potassium channel blockers in multiple sclerosis: neuronal Kv channels and effects of symptomatic treatment. Pharmacol Ther 2006; 111: 224–259. [DOI] [PubMed] [Google Scholar]
- 14. Goodman AD, Brown TR, Krupp LB, et al. Sustained-release oral fampridine in multiple sclerosis: a randomised, double-blind, controlled trial. Lancet 2009; 373: 732–738. [DOI] [PubMed] [Google Scholar]
- 15. Goodman AD, Brown TR, Edwards KR, et al. A phase 3 trial of extended release oral dalfampridine in multiple sclerosis. Ann Neurol 2010; 68: 494–502. [DOI] [PubMed] [Google Scholar]
- 16. Goodman AD, Bethoux F, Brown TR, et al. Long-term safety and efficacy of dalfampridine for walking impairment in patients with multiple sclerosis: results of open-label extensions of two Phase 3 clinical trials. Mult Scler 2015; 21: 1322–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hobart JC, Riazi A, Lamping DL, et al. Measuring the impact of MS on walking ability: the 12-item MS Walking Scale (MSWS-12). Neurology 2003; 60: 31–36. [DOI] [PubMed] [Google Scholar]
- 18. Hupperts R, Lycke J, Short C, et al. Prolonged-release fampridine and walking and balance in MS: randomised controlled MOBILE trial. Mult Scler 2016; 22: 212–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mehta L, McNeill M, Hobart J, et al. Identifying an important change estimate for the Multiple Sclerosis Walking Scale-12 (MSWS-12v1) for interpreting clinical trial results. Mult Scler J Exp Transl Clin 2015; 1: 2055217315596993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hobart J, Ziemssen T, Feys P, et al. Assessment of clinically meaningful improvements in self-reported walking ability in participants with multiple sclerosis: results from the randomized, double-blind, phase III ENHANCE trial of prolonged-release fampridine. CNS Drugs 2019; 33: 61–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol 2001; 50: 121–127. [DOI] [PubMed] [Google Scholar]
- 22. Polman CH, Wolinsky JS, Reingold SC. Multiple sclerosis diagnostic criteria: three years later. Mult Scler 2005; 11: 5–12. [DOI] [PubMed] [Google Scholar]
- 23. Cattaneo D, Regola A, Meotti M. Validity of six balance disorders scales in persons with multiple sclerosis. Disabil Rehabil 2006; 28: 789–795. [DOI] [PubMed] [Google Scholar]
- 24. Podsiadlo D, Richardson S. The timed ‘Up & Go’: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991; 39: 142–148. [DOI] [PubMed] [Google Scholar]
- 25. Cattaneo D, Jonsdottir J, Repetti S. Reliability of four scales on balance disorders in persons with multiple sclerosis. Disabil Rehabil 2007; 29: 1920–1925. [DOI] [PubMed] [Google Scholar]
- 26. Hobart J, Lamping D, Fitzpatrick R, et al. The Multiple Sclerosis Impact Scale (MSIS-29): a new patient-based outcome measure. Brain 2001; 124: 962–973. [DOI] [PubMed] [Google Scholar]
- 27. EuroQoL Group. EQ-5D: a standardized instrument for use as a measurement of health outcome, http://www.euroqol.org (accessed 30 August 2018).
- 28. Hobart J, Hupperts R, Linnebank M, et al. Health-related quality of life improved in people with multiple sclerosis who had clinically meaningful changes in walking ability with PR-fampridine: post hoc analysis of ENHANCE. J Neurol Sci 2017; 381: 452. [Google Scholar]
- 29. van Hout B, Janssen MF, Feng YS, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012; 15: 708–715. [DOI] [PubMed] [Google Scholar]
- 30. Schafer JL. Analysis of incomplete multivariate data. 1st ed. London: Chapman & Hall/CRC Press, 1997, p. 430. [Google Scholar]
- 31. Schafer JL. Multiple imputation: a primer. Stat Methods Med Res 1999; 8: 3–15. [DOI] [PubMed] [Google Scholar]
- 32. Biogen. Fampyra 10 mg prolonged-release tablets [summary of product characteristics], https://www.ema.europa.eu/en/documents/product-information/fampyra-epar-product-information_en.pdf (accessed 3 June 2021).
- 33. Goodman AD, Brown TR, Schapiro RT, et al. A pooled analysis of two phase 3 clinical trials of dalfampridine in patients with multiple sclerosis. Int J MS Care 2014; 16: 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hobart J. Clinical outcomes assessment in MS trials: on balance, the Berg Balance Scale is a precarious measure. Mult Scler 2018; 24: 365. [Google Scholar]
- 35. Institute of Medicine (US) Forum on Drug Discovery, Development, and Translation. Transforming clinical research in the United States: challenges and opportunities: workshop summary, https://www.ncbi.nlm.nih.gov/books/NBK50889/ (2010, accessed 5 April 2021). [PubMed]
- 36. Buchholz I, Janssen MF, Kohlmann T, et al. A systematic review of studies comparing the measurement properties of the three-level and five-level versions of the EQ-5D. Pharmacoeconomics 2018; 36: 645–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Janssen MF, Bonsel GJ, Luo N. Is EQ-5D-5L better than EQ-5D-3L? A head-to-head comparison of descriptive systems and value sets from seven countries. Pharmacoeconomics 2018; 36: 675–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Valet M, Quoilin M, Lejeune T, et al. Effects of fampridine in people with multiple sclerosis: a systematic review and meta-analysis. CNS Drugs 2019; 33: 1087–1099. [DOI] [PubMed] [Google Scholar]
- 39. Allart E, Benoit A, Blanchard-Dauphin A, et al. Sustained-released fampridine in multiple sclerosis: effects on gait parameters, arm function, fatigue, and quality of life. J Neurol 2015; 262: 1936–1945. [DOI] [PubMed] [Google Scholar]
- 40. Broicher SD, Filli L, Geisseler O, et al. Positive effects of fampridine on cognition, fatigue and depression in patients with multiple sclerosis over 2 years. J Neurol 2018; 265: 1016–1025. [DOI] [PubMed] [Google Scholar]
- 41. Rodriguez-Leal FA, Haase R, Akgun K, et al. Nonwalking response to fampridine in patients with multiple sclerosis in a real-world setting. Ther Adv Chronic Dis 2019; 10: 2040622319835136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tan-10.1177_17562864221090398 for Efficacy of prolonged-release fampridine versus placebo on walking ability, dynamic and static balance, physical impact of multiple sclerosis, and quality of life: an integrated analysis of MOBILE and ENHANCE by Raymond Hupperts, Claudio Gasperini, Jan Lycke, Tjalf Ziemssen, Peter Feys, Shan Xiao, Carlos Acosta, Thijs Koster and Jeremy Hobart in Therapeutic Advances in Neurological Disorders