Abstract
A recent randomised controlled trial failed to demonstrate a beneficial effect of recombinant human thrombomodulin (rhTM) on sepsis. However, there is still controversy in the effects of rhTM for sepsis due to the heterogeneity of the study population. We previously identified patients with a distinct phenotype that could be a potential target of rhTM therapy (rhTM target phenotype). However, for application in the clinical setting, a simple tool for determining this target is necessary. Thus, using three multicentre sepsis registries, we aimed to develop and validate a machine learning model for predicting presence of the target phenotype that we previously identified for targeted rhTM therapy. The predictors were platelet count, PT-INR, fibrinogen, fibrinogen/fibrin degradation products, and D-dimer. We also implemented the model as a web-based application. Two of the three registries were used for model development (n = 3694), and the remaining registry was used for validation (n = 1184). Approximately 8–9% of patients had the rhTM target phenotype in each cohort. In the validation, the C statistic of the developed model for predicting the rhTM target phenotype was 0.996 (95% CI 0.993–0.998), with a sensitivity of 0.991 and a specificity of 0.967. Among patients who were predicted to have the potential target phenotype (predicted target patients) in the validation cohort (n = 142), rhTM use was associated with a lower in-hospital mortality (adjusted risk difference, − 31.3% [− 53.5 to − 9.1%]). The developed model was able to accurately predict the rhTM target phenotype. The model, which is available as a web-based application, could profoundly benefit clinicians and researchers investigating the heterogeneity in the treatment effects of rhTM and its mechanisms.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-022-04020-1.
Keywords: Recombinant human thrombomodulin, Sepsis, Phenotype, Prediction model, Coagulopathy
Background
Recombinant human thrombomodulin (rhTM) has been suggested as an adjunct therapy for patients with sepsis [1]. A recent randomised controlled trial (RCT) failed to demonstrate its beneficial effect on 28-day mortality [2], but there remains controversy in the results of this study due to the heterogeneity of its study population. Indeed, 22% of patients in the RCT did not meet protocol-specified coagulopathy. In addition, an updated meta-analysis including the RCT reported an association between rhTM use and a lower risk of mortality [3]. These findings collectively suggest the importance of appropriately targeting the study population prior to conducting studies to gain maximum benefit [4–6]. We identified a distinct phenotype that could be a potential target of rhTM therapy [7], a finding consistent with previously suggested targets, including coagulation disorder and high disease severity [8, 9]. However, for application in the clinical setting, a simple tool for determining this target is necessary [10]. Thus, we aimed to develop and validate a model for predicting the potential target phenotype for rhTM therapy and to implement the model as a web-based application to facilitate further research.
Methods
Study design and settings
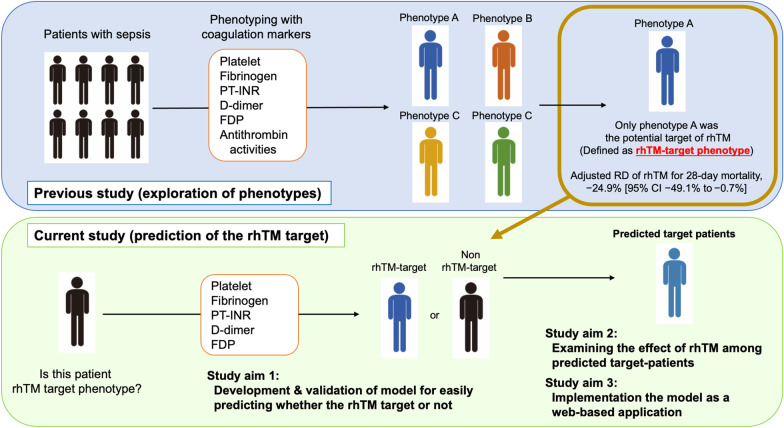
The concept of this study is shown in Fig. 1. Details of this study are provided in Additional file 1. This was a secondary analysis of the following multicentre registries: the Japan Septic Disseminated Intravascular Coagulation (JSEPTIC-DIC) study (42 ICUs at 40 institutions, 2011–2013) [11], Tohoku Sepsis Registry (10 institutions, 2015) [12], and Focused Outcomes Research in Emergency Care for Acute Respiratory Distress Syndrome, Sepsis, and Trauma (FORECAST) sepsis study (59 ICUs, 2016–2017) [13]. These studies were approved by the institutional review boards at the participating hospitals, and the need for informed consent was waived.
Fig. 1.
Current study (development and implementation of a prediction model of rhTM target phenotype)
Study samples
We included all patients (aged ≥ 16 years), who were admitted to the ICU with severe sepsis or septic shock as defined in the three registries, according to the International Sepsis Definitions Conference Criteria [14, 15]. We excluded patients with missing information on 28-day mortality, which is required for determining the phenotype of each patient [7].
Predictors
We used the following coagulation markers for predicting the presence of the target phenotype, in accordance with our previous study [7]: platelet counts, PT-INR, fibrinogen, fibrinogen/fibrin degradation products (FDP), and D-dimer.
Outcomes
The primary outcome was the presence of the clinical phenotype identified in our previous study [7], characterised as severe physiological status and organ dysfunction (high Acute Physiology and Chronic Health Evaluation [APACHE II] and Sequential Organ Failure Assessment [SOFA] scores), coagulopathy (low platelet count, prolonged PT-INR, low fibrinogen, and extremely high FDP and D-dimer levels), high lactate level, and high mortality. We termed this phenotype as “rhTM target phenotype”.
Statistical analysis
We derived our prediction model using the JSEPTIC-DIC study and Tohoku Sepsis Registry (derivation cohort) and validated the model using the FORECAST sepsis study (validation cohort). We imputed missing predictors using the random forest method with the missForest package (Additional file 2: Table S1) [16]. We did not calculate the sample size in advance because we used all available data. The sample size for model development (n = 3694, of which 9% had the target phenotype) was enough to ensure precise predictions and minimise overfitting [17].
For model development, we divided the derivation cohort into the training set (70% of the full sample randomly chosen for model development and hyperparameter tuning) and test set (30% of the full sample randomly chosen for internal validation). Using the training set, using log-transformed predictors, we constructed a prediction model with XGBoost. We used the grid search strategy to identify the best combination of hyperparameters using the ranger and caret packages with tenfold cross validation.
We measured the prediction performance of the developed model by computing the (1) C statistic (i.e., the area under the receiver operating characteristic [ROC] curve) and (2) prospective prediction results.
In addition, among patients those who were predicted to have the potential target phenotype (termed as “predicted target patients”), we assessed the effect of rhTM on in-hospital and 28-day mortality using a generalised estimating equation to account for patient clustering within hospitals. The adjusted variables were selected according to the previous study [7] (see Additional file 1). We also reported the number of patients who met the inclusion criteria for the SCARLET trial (cardiovascular and/or respiratory dysfunction, and PT-INR > 1.4 and a platelet count in the range from 30 to 150 × 109/L) to illustrate the difference in the target study population between studies [2].
Lastly, we uploaded the model online (URL: http://research-kudo-prediction.s3-website-ap-northeast-1.amazonaws.com/), so researchers interested in utilising the model could access it for free. All analyses were performed with R statistical software version 3.6.1 (R Foundation for Statistical Computing).
Results
Patient characteristics were similar between predicted target patients and patients with rhTM target phenotype (Table 1). However, predicted target patients in the current study were likely to have milder coagulopathy. Approximately 8–9% of patients had the rhTM target phenotype. Overall, patients who met the inclusion criteria for the SCARLET trial accounted for 20–30% of rhTM target phenotype.
Table 1.
Characteristics and clinical course of patients with sepsis in the derivation and validation cohorts
| Variables | Test set of the derivation cohort (n = 1108) |
Validation cohort (n = 1184) |
||
|---|---|---|---|---|
| Predicted target patients n = 118 |
Patients with rhTM target phenotype n = 85 |
Predicted target patients n = 142 |
Patients with rhTM target phenotype n = 108 |
|
| Age, median (IQR) | 71 (56, 79) | 70 (55, 79) | 73 (64, 82) | 73 (64, 82) |
| Sex, female | 60 (51%) | 42 (49%) | 61 (43%) | 45 (42%) |
| Body weight (kg), median (IQR) | 54 (49, 63) | 55 (49, 64) | 55.0 (47.0, 65.0) | 53.0 (46.5, 60.5) |
| Infection site | ||||
| Catheter-related | 2 (2%) | 0 (0%) | 7 (5%) | 6 (6%) |
| Bone/soft tissue | 10 (8%) | 7 (8%) | 11 (8%) | 7 (6%) |
| Cardiovascular | 5 (4%) | 5 (6%) | 5 (4%) | 5 (5%) |
| Central nervous system | 3 (3%) | 3 (4%) | 2 (1%) | 2 (2%) |
| Urinary tract | 28 (24%) | 21 (25%) | 39 (27%) | 31 (29%) |
| Lung/thoracic | 16 (14%) | 13 (15%) | 24 (17%) | 19 (18%) |
| Abdomen | 33 (28%) | 19 (22%) | 38 (27%) | 25 (23%) |
| Other/unknown | 21 (18%) | 17 (20%) | 16 (11%) | 13 (20%) |
| APACHE II, median (IQR) | 28 (21, 34) | 27 (21, 34) | 27 (22, 33) | 27 (22, 32) |
| SIRS score, median (IQR) | 3 (3, 4) | 3 (3, 4) | 3 (3, 4) | 3 (3, 4) |
| SOFA scores | 13 (10, 16) | 13 (10, 16) | 13 (11, 13) | 11 (9, 14) |
| Lab data | ||||
| White blood cell (103/μL), median (IQR) | 11.5 (2.7, 20.3) | 12.4 (2.7, 20.7) | 11.0 (6.1, 20.1) | 10.8 (6.3, 20.0) |
| Platelet (103/μL), median (IQR) | 54 (29, 99) | 47 (26, 76) | 73 (42, 122) | 68 (41, 121) |
| PT-INR, median (IQR) | 1.7 (1.4, 2.1) | 1.7 (1.4, 2.1) | 1.5 (1.3, 1.7) | 1.5 (1.3, 1.7) |
| Fibrinogen (mg/mL), median (IQR) | 237 (141, 328) | 220 (130, 311) | 269 (153, 378) | 277 (154, 381) |
| FDP (μg/mL), median (IQR) | 98 (68, 224) | 127 (80, 299) | 107 (73, 188) | 121 (93, 245) |
| D-dimer (μg/mL), median (IQR) | 42 (31, 94) | 51 (34, 119) | 48 (32, 83) | 60 (40, 106) |
| Antithrombin (%), median (IQR) | 51 (42, 60) | 50 (42, 58) | 54 (48, 65) | 55 (49, 66) |
| Lactate (mmol/L), median (IQR) | 6 (3, 10) | 6 (4, 10) | 5 (3, 7) | 5 (3, 7) |
| Patients who met the inclusion criteria for the SCARLET trial* | ||||
| Coagulopathy | 29 (25%) | 23 (27%) | 32 (23%) | 33 (31%) |
| Coagulopathy and respiratory/cardiovascular dysfunction | 25 (21%) | 21 (25%) | 27 (19%) | 20 (16%) |
| Management | ||||
| rhTM | 52 (44%) | 41 (48%) | 55 (39%) | 44 (44%) |
| Vasopressor use | 105 (89%) | 77 (91%) | 103 (73%) | 77 (71%) |
| Renal replacement therapy | 51 (43%) | 40 (47%) | 23 (16%) | 16 (15%) |
| Steroids | 35 (30%) | 26 (31%) | 65 (48%) | 48 (44%) |
| Intravenous immunoglobulin | 58 (49%) | 40 (47%) | 50 (35%) | 18 (17%) |
| Antithrombin | 59 (50%) | 41 (48%) | 28 (20%) | 22 (20%) |
| Prognosis | ||||
| 28-day death | 48 (41%) | 36 (42%) | 40 (28%) | 24 (25%) |
| In-hospital death | 62 (53%) | 47 (55%) | 47 (33%) | 28 (28%) |
APACHE, Acute Physiology and Chronic Health Evaluation; FDP, fibrinogen/fibrin degradation product; IQR, interquartile range; PT-INR, prothrombin time-international normalised ratio; SIRS, systemic inflammatory response syndrome; SOFA, Sequential Organ Failure Assessment; and WBC, white blood cells
Five coagulation markers (in bold) were used for prediction
*Defined as patients with (1) coagulopathy (PT-INR > 1.4 and platelet count 30 to 150 × 109/L) and (2) vasopressor use or mechanical ventilation use
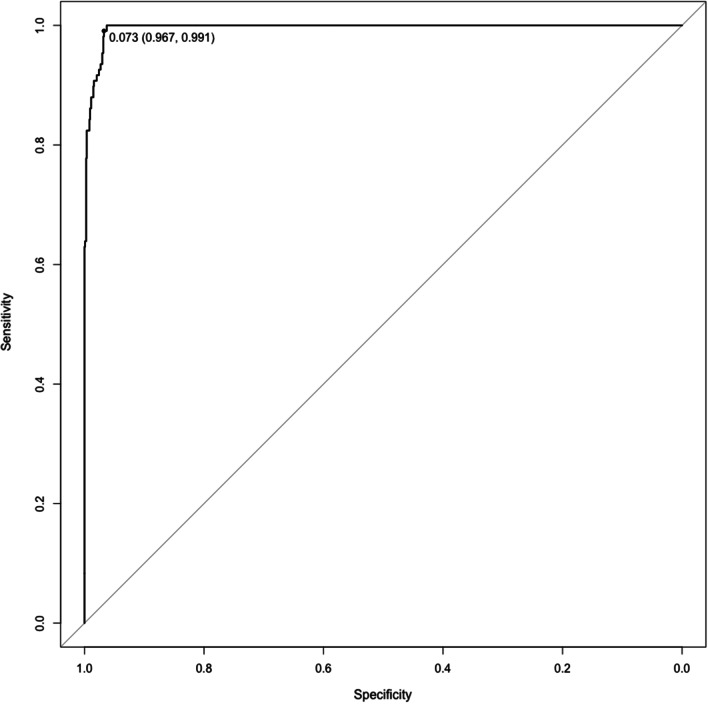
Using the test set of the derivation cohort, we found that the C statistic of the developed model was 0.993 (95% CI 0.989–0.997). Prospective prediction results were as follows: sensitivity 0.968, specificity 0.955, positive predictive value 0.669, and negative predictive value 0.997. Figure 2 shows the prediction ability of the developed model in the validation cohort. Using the validation cohort, we found that the model had high discrimination (C statistic, 0.996; 95% CI 0.993–0.998). Prospective prediction results were as follows: sensitivity 0.991, specificity 0.967, positive predictive value 0.754, and negative predictive value 0.999.
Fig. 2.
The receiver operating characteristic curve of the developed model for predicting the presence of the target phenotype in the external validation cohort
Among predicted target patients in the validation cohort, rhTM use was associated with a lower in-hospital mortality (adjusted risk difference, − 31.3% [− 53.5 to − 9.1%]; Table 2).
Table 2.
Unadjusted and adjusted risk difference between recombinant thrombomodulin use and outcomes among predicted target patients
| Predicted target patients | In-hospital mortality | 28-day mortality | ||
|---|---|---|---|---|
| Unadjusted risk Difference (95% CI) | Adjusted risk difference (95% CI) | Unadjusted risk Difference (95% CI) | Adjusted risk difference (95% CI) | |
| Test set of the derivation cohort (n = 118) |
− 22.0% (− 40.6 to − 3.4%) |
− 27.4% (− 41.8 to − 12.9%) |
− 20.0% (− 38.2 to − 1.8%) |
− 23.6% (− 39.8 to − 7.4%) |
| Validation cohort (n = 142) |
− 15.1% (− 31.1 to 1.0%) |
− 31.3% (− 53.5 to − 9.1%) |
− 8.4% (− 24.7 to 8.0%) |
− 21.1% (− 43.4 to 1.1%) |
In the test set of derivation cohort, the adjusted variables were age, sex, comorbidities, and Sequential Organ Failure Assessment (SOFA) scores
In the validation cohort, the adjusted variables were age, sex, comorbidities, SOFA scores, and in-hospital management, including renal replacement therapy, and treatment with steroids, intravenous immunoglobulin, antithrombin, and vasopressors
Discussion
We derived and validated a machine learning model that accurately predicts the rhTM target phenotype in patients with sepsis and released it online for clinical and research use. The C statistic was 0.994 in the validation cohort, with a sensitivity of 0.981 and a specificity of 0.944. The predicted target patients were likely to have milder coagulopathy compared to those with rhTM target phenotype.
The importance of considering the heterogeneity in the study population and the treatment effects has been emphasised in recent years [6]. As shown in the analysis of multiple sepsis registries and RCTs [5], clinical phenotypes were correlated with host-response patterns and clinical outcomes, and simulations suggested the presence of heterogeneity in treatment effects across phenotypes. Thus, such heterogeneity may at least partially explain the underlying mechanisms of RCTs that failed to reveal significant benefit of therapies in critical care [18, 19]. Indeed, patients who met the inclusion criteria for the SCARLET trial accounted for 20–30% of the patients with rhTM target phenotype, suggesting that further studies are needed to investigate the effects of rhTM for sepsis. Additionally, the process of identifying the target population to be treated is important and should be discussed in future cost–benefit analyses of treatment strategies, even if a small proportion of patients can be treated effectively (as was the case in our study sample).
Subgroup analyses have been widely used to address treatment effect heterogeneity despite its limitations [20]. In particular, conventional subgroup analyses assess one characteristic at a time, which may not reflect the biology or clinical practice where multiple factors often act synergistically [6]. To address this concern, several approaches have been proposed: clustering algorithms to identify distinct clinical phenotypes, Bayesian hierarchical models, and adaptive enrichment [6]. Building on these works, we used a clustering approach in a previous study to address the heterogeneity in our study population. While it is still challenging to find the true phenotypes that are responsible for the heterogeneity, we believe that our research process: (1) discovering the target phenotype, (2) implementing a model for predicting the phenotype, and (3) conducting studies for identifying the optimal target population or exploring underlying mechanisms—is an efficient way of conducting future studies and advancing personalised medicine. For example, our findings support the findings from a post hoc analysis of the SCARLET trial that reported an association between higher baseline thrombin generation biomarker levels and the effect of rhTM [9], by demonstrating that a subtype consisting of a high-dimensional coagulation profile could be a potential target of rhTM.
This study has several limitations. First, although we developed a model to predict the rhTM target phenotype, it remains unclear whether the rhTM target phenotype is the true target of rhTM therapy. Second, there may be diagnostic suspicion bias and unmeasured confounding. Additionally, the number of missing variables for prediction may have limited our findings. Thus, our findings should be validated in randomised controlled trials. Third, because machine learning models are generally difficult to interpret, our model itself does not provide information on the underlying mechanisms. Finally, our data were obtained from Japanese patients, and the generalisability of the results to other populations may be limited.
Conclusions
We developed a model that accurately predicted the rhTM target phenotype. Our model is available online, which could profoundly benefit clinicians and researchers investigating the heterogeneity in the treatment effects of rhTM and its mechanisms.
Supplementary Information
Additional file 2: Table S1. Missingness in predictors and outcome variables.
Acknowledgements
We thank Mr. Fujimori for implementing our prediction model as a web-based application and the core investigators of the FORECAST sepsis study (Appendix) for providing the dataset. We are grateful to all investigators involved in the JSEPTIC-DIC study, Tohoku Sepsis Registry, and the FORECAST sepsis study for contributing to the data collection and assessment.
Abbreviations
- APACHE
Acute Physiology and Chronic Health Evaluation
- FDP
Fibrinogen/fibrin degradation product
- FORECAST
Focused Outcomes Research in Emergency Care for Acute Respiratory Distress Syndrome, Sepsis, and Trauma
- ICU
Intensive care unit
- JSEPTIC-DIC
Japan Septic Disseminated Intravascular Coagulation
- PT-INR
Prothrombin time/international normalised ratio
- RCT
Randomised controlled trial
- rhTM
Recombinant human thrombomodulin
- ROC
Receiver operating characteristic
- SOFA
Sequential Organ Failure Assessment
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by DK, TG, RU, MH, TA, and AS. Statistical analyses were reviewed by RU, TA, and AS. The first draft of the manuscript was written by TG. The manuscript was reviewed and edited by DK, KY, TA, AS, and SK, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
JSEPTIC-DIC data are publicly available (Sci Data. 2018;5:180243). Tohoku Sepsis Registry and FORECAST sepsis study are not publicly available because participants of this study did not agree that their data can be shared publicly.
Declarations
Ethics approval and consent to participate
The three original studies were approved, and the need for informed consent was waived by the institutional review boards at the participating hospitals.
Consent for publication
Not applicable.
Competing interests
DK, MH, and SK received personal fees from Asahi Kasei Pharma Corporation. TG is the Chief Scientific Officer at TXP Medical Co. Ltd.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yamakawa K, Aihara M, Ogura H, Yuhara H, Hamasaki T, Shimazu T. Recombinant human soluble thrombomodulin in severe sepsis: a systematic review and meta-analysis. J Thromb Haemost. 2015;13(4):508–519. doi: 10.1111/jth.12841. [DOI] [PubMed] [Google Scholar]
- 2.Vincent JL, Francois B, Zabolotskikh I, Daga MK, Lascarrou JB, Kirov MY, Pettila V, Wittebole X, Meziani F, Mercier E, et al. Effect of a recombinant human soluble thrombomodulin on mortality in patients with sepsis-associated coagulopathy: The SCARLET Randomized Clinical Trial. JAMA. 2019;321(20):1993–2002. doi: 10.1001/jama.2019.5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamakawa K, Levy JH, Iba T. Recombinant human soluble thrombomodulin in patients with sepsis-associated coagulopathy (SCARLET): an updated meta-analysis. Crit Care. 2019;23(1):302. doi: 10.1186/s13054-019-2587-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angus DC, Chang CH. Heterogeneity of treatment effect: estimating how the effects of interventions vary across individuals. JAMA. 2021;326(22):2312–2313. doi: 10.1001/jama.2021.20552. [DOI] [PubMed] [Google Scholar]
- 5.Seymour CW, Kennedy JN, Wang S, Chang CH, Elliott CF, Xu Z, Berry S, Clermont G, Cooper G, Gomez H, et al. Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. JAMA. 2019;321(20):2003–2017. doi: 10.1001/jama.2019.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Granholm A, Alhazzani W, Derde LPG, Angus DC, Zampieri FG, Hammond NE, Sweeney RM, Myatra SN, Azoulay E, Rowan K, et al. Randomised clinical trials in critical care: past, present and future. Intensive Care Med. 2022;48(2):164–178. doi: 10.1007/s00134-021-06587-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kudo D, Goto T, Uchimido R, Hayakawa M, Yamakawa K, Abe T, Shiraishi A, Kushimoto S. Coagulation phenotypes in sepsis and effects of recombinant human thrombomodulin: an analysis of three multicentre observational studies. Crit Care. 2021;25(1):114. doi: 10.1186/s13054-021-03541-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamakawa K, Umemura Y, Hayakawa M, Kudo D, Sanui M, Takahashi H, Yoshikawa Y, Hamasaki T, Fujimi S, Japan Septic Disseminated Intravascular Coagulation Study G Benefit profile of anticoagulant therapy in sepsis: a nationwide multicentre registry in Japan. Crit Care. 2016;20(1):229. doi: 10.1186/s13054-016-1415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levi M, Vincent JL, Tanaka K, Radford AH, Kayanoki T, Fineberg DA, Hoppensteadt D, Fareed J. Effect of a recombinant human soluble thrombomodulin on baseline coagulation biomarker levels and mortality outcome in patients with sepsis-associated coagulopathy. Crit Care Med. 2020;48(8):1140–1147. doi: 10.1097/CCM.0000000000004426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujiogi M, Dumas O, Hasegawa K, Jartti T, Camargo CA. Identifying and predicting severe bronchiolitis profiles at high risk for developing asthma: analysis of three prospective cohorts. EClinicalMedicine. 2022;43:101257. doi: 10.1016/j.eclinm.2021.101257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayakawa M, Yamakawa K, Saito S, Uchino S, Kudo D, Iizuka Y, Sanui M, Takimoto K, Mayumi T. Nationwide registry of sepsis patients in Japan focused on disseminated intravascular coagulation 2011–2013. Sci Data. 2018;5:180243. doi: 10.1038/sdata.2018.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kudo D, Kushimoto S, Miyagawa N, Sato T, Hasegawa M, Ito F, Yamanouchi S, Honda H, Andoh K, Furukawa H, et al. The impact of organ dysfunctions on mortality in patients with severe sepsis: a multicenter prospective observational study. J Crit Care. 2018;45:178–183. doi: 10.1016/j.jcrc.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Abe T, Ogura H, Shiraishi A, Kushimoto S, Saitoh D, Fujishima S, Mayumi T, Shiino Y, Nakada TA, Tarui T, et al. Characteristics, management, and in-hospital mortality among patients with severe sepsis in intensive care units in Japan: the FORECAST study. Crit Care. 2018;22(1):322. doi: 10.1186/s13054-018-2186-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 15.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med. 2003;31(4):1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 16.Stekhoven DJ, Buhlmann P. MissForest–non-parametric missing value imputation for mixed-type data. Bioinformatics. 2012;28(1):112–118. doi: 10.1093/bioinformatics/btr597. [DOI] [PubMed] [Google Scholar]
- 17.Riley RD, Ensor J, Snell KIE, Harrell FE, Jr, Martin GP, Reitsma JB, Moons KGM, Collins G, van Smeden M. Calculating the sample size required for developing a clinical prediction model. BMJ. 2020;368:m441. doi: 10.1136/bmj.m441. [DOI] [PubMed] [Google Scholar]
- 18.Warren BL, Eid A, Singer P, Pillay SS, Carl P, Novak I, Chalupa P, Atherstone A, Penzes I, Kubler A, et al. Caring for the critically ill patient. High-dose antithrombin III in severe sepsis: a randomized controlled trial. JAMA. 2001;286(15):1869–1878. doi: 10.1001/jama.286.15.1869. [DOI] [PubMed] [Google Scholar]
- 19.Ranieri VM, Thompson BT, Barie PS, Dhainaut JF, Douglas IS, Finfer S, Gardlund B, Marshall JC, Rhodes A, Artigas A, et al. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366(22):2055–2064. doi: 10.1056/NEJMoa1202290. [DOI] [PubMed] [Google Scholar]
- 20.Burke JF, Sussman JB, Kent DM, Hayward RA. Three simple rules to ensure reasonably credible subgroup analyses. BMJ. 2015;351:h5651. doi: 10.1136/bmj.h5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 2: Table S1. Missingness in predictors and outcome variables.
Data Availability Statement
JSEPTIC-DIC data are publicly available (Sci Data. 2018;5:180243). Tohoku Sepsis Registry and FORECAST sepsis study are not publicly available because participants of this study did not agree that their data can be shared publicly.