Dear editor,
We read with interest the article by Tré-Hardy et al. on the kinetics of anti-spike IgG (S-IgG) levels after vaccination among healthcare workers who received the mRNA-1273 (Moderna) vaccine.1 The authors reported that the antibody levels markedly reduced between 3 and 6 months after the second dose. We also investigated the kinetics of S-IgG levels for 6 months after receiving the BNT162b2 (Pfizer-BioNTech) vaccine and the T-cell response among low responders in a cohort of workers at the National Center for Geriatrics and Gerontology, including a hospital and a research institute, in Japan.
We have previously reported that seroprevalence against the nucleocapsid of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) among our staff was equivalent to that observed among the local community and that 99.4% of the participants had S-IgG.2 , 3 However, the kinetics of the antibody titer against S-IgG after vaccination remain unclear. Additionally, T-cell-mediated cellular immunity may affect COVID-19 recovery, even with a low antibody response.4 The relationship between humoral and cellular immune responses to vaccination has been rarely investigated.
Of the 878 employees, 800 agreed to participate in the survey (participation rate: 91.1%). They received the first vaccination between February and June 2021 and the second dose 3 weeks later. Blood samples were obtained between June 14 and 18, 2021. This study was approved by the Institutional Review Board of the Ethics and Conflicts of Interest Committee (approval no 1481). All participants provided written informed consent.
We performed all laboratory tests in-house using two S-IgG chemiluminescence enzyme immunoassays: the SARS-CoV-2 S-IgG from Sysmex and ARCHITECT SARS-CoV-2 IgG assay from Abbott. The positive cutoff value was 20 BAU/mL for Sysmex's and 50 AU/mL for Abbot's assays, respectively.
The participants’ characteristics are summarized in Table S1 (N = 800). The mean age ± SD was 41.0 ± 11.6 years, and 66.4% were women. Clinical staff (doctors, nurses, and allied healthcare professionals) accounted for 63.8%, whereas the others were engaged in basic research and investigation, general office duties, and other nonclinical work. Most participants (n = 642, 80.3%) received two doses of BNT162b2 vaccination at least 1 week before their annual health checkups in June. Seven participants received the second dose in March, seven in April, 515 in May, and 113 in June.
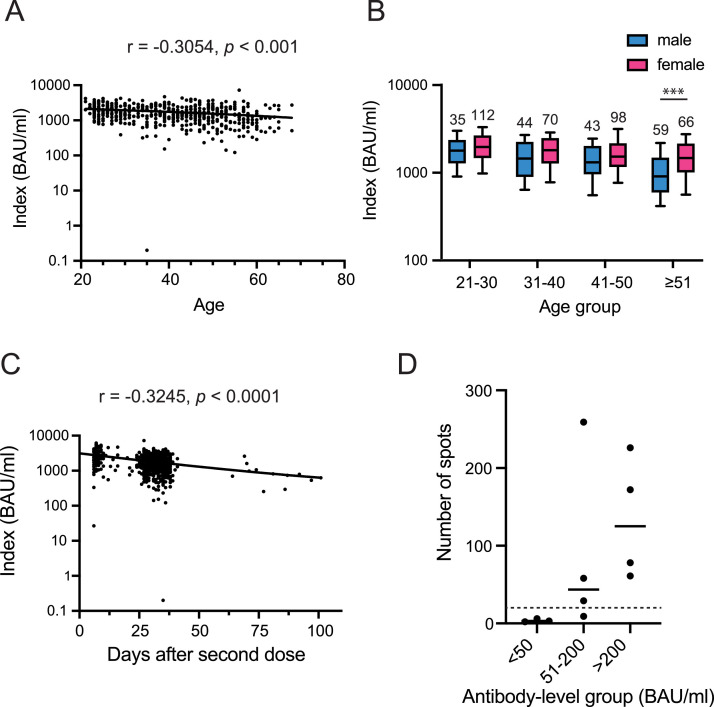
Of the 527 eligible participants who had received both vaccine doses by May 2021, all were positive for S-IgG except one participant. An age-dependent decline in antibody response was observed with Spearman correlation coefficient of –0.305 for the Sysmex tests (p < 0.001; Fig. 1 A). Women tended to develop a higher S-IgG titer in each age group. The difference was significant in the age group ≥51 years (Mann–Whitney U test with Bonferroni correction, p < 0.001), and the age difference was statistically significant regardless of sex (Kruskal–Wallis test, p < 0.001) (Fig. 1B).
Fig. 1.
Antibody titer and T-cell response following the second dose of the BNT162b2 vaccine. (A) Correlation between age and S-IgG levels 1–4 months after the second vaccination dose was measured using Sysmex test. Spearman r, as well as p-values, are presented in each graph. (B) Comparison of antibody levels among different age groups and between the sexes. Data are expressed as box plots: middle line, median; box edges, 25th–75th centile. ***p < 0.001. (C) Kinetics of the antibody titers following the second dose of the vaccine. Correlation between S-IgG levels and the number of days after the second dose. (D) Cellular immunity of participants who failed to develop antibodies against SARS-CoV-2 after the second dose of the vaccine. The number of spot-forming cells per 250,000 in the panel 1 of T-SPOT test was plotted based on the antibody-level groups. The horizontal solid lines indicate the mean of spot-forming cell numbers. The dashed line indicates the threshold of T-SPOT values.
The antibody titer tended to decline post-vaccination in a time-dependent manner, with a Spearman correlation coefficient of −0.325 (p < 0.001; Fig. 1C). The median titer of samples collected 6–20 days after the second vaccination (n = 118) was 2636 BAU/mL for the Sysmex test. Among the individuals who received the second dose 21–50 days before the survey (n = 512), the median antibody titers decreased to 1599 BAU/mL. The antibody titer of participants who received the second dose >50 days before sample collection (n = 12) was reduced to 28.5%. Similar results were also obtained in the Abbott test (Fig. S1).
Seven participants with low antibody titers were invited to the additional investigation of T-cell-dependent cellular immunity in October 2021. We collected peripheral blood mononuclear cells and detected T cells secreting interferon-γ (IFN-γ) in response to SARS-CoV-2 peptides using the T-SPOT Discovery SARS-CoV-2 kit (Oxford Immunotec). Two participants had a count that was comparable with those showing higher antibody responses. One participant was receiving an immune suppressant for kidney transplantation; he showed negative responses in the cellular immunity and seroprevalence tests. Two participants— one was immunocompromised and the other had a history of Klippel–Trénanay–Weber syndrome and protein-losing gastroenteropathy—with a titer of <50 also showed negative responses. A 20-week pregnant woman showed a negative result in the cellular immunity test, although her antibody titers were relatively high compared with that of the other participants.
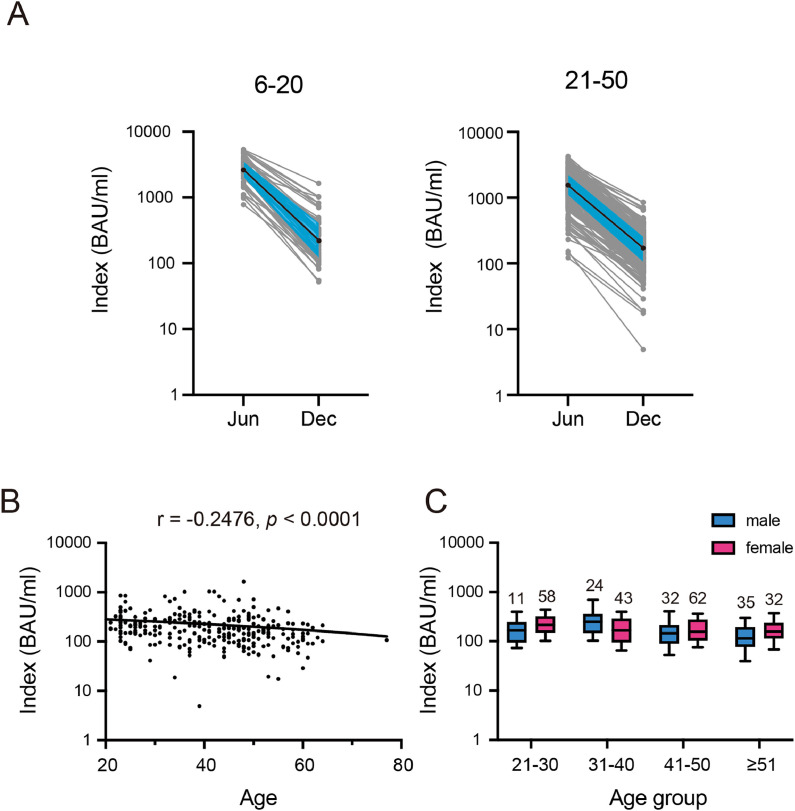
Further, 297 participants received additional medical checkups on December 16 or 17, 2021, who are required for employees engaged in specific work activities, such as night shift, donated a blood sample. The participant characteristics in the follow-up survey are presented in Table S2. A sharp decline in S-IgG titer was observed in the 6 months after receiving both vaccine doses (Fig. 2 A). Median titers were reduced by 88.4% (from 2821 to 327 BAU/mL) for those who participated in the June survey 6–20 days at post-vaccination and by 88.9% (1537 to 172 BAU/mL) for those who participated at 21–50 days. However, most participants (n = 293, 98.7%) maintained a positive humoral immune response. Sex-dependent differences were not observed, whereas the titer significantly declined with age, with a Spearman correlation coefficient of −0.248 (Fig. 2B, C).
Fig. 2.
Anti-spike IgG levels at 5–6 months following the second dose of the BNT162b2 vaccine. (A) Kinetics of anti-spike IgG levels of each participant as measured by the Sysmex test. The median values of each data point are displayed as solid black circles connected with solid black lines. Shaded areas in blue indicate the 25%–75% percentile. (B) Correlation between age and S-IgG levels in the December survey as measured with the Sysmex test. (C) Comparison of antibody levels among the different age groups and between the sexes. Data are expressed as box plots: middle line, median; box edges, 25th–75th centile.
Consistent with the previous reports,1 , 5 , 6 the anti-spike antibody titer resulting from mRNA vaccination was markedly reduced within a few months and continued to decline thereafter, suggesting the requirement of additional doses. Conversely, cellular responses, which remain broad to wild type and variants,7 were maintained even at 5–6 months after the second dose in those who developed a sufficient antibody titer. A survey of larger cohorts in the future is warranted to confirm our observation.
Funding
This work was supported by the Japan Health Research Promotion Bureau Research Fund [grant number 2020-B-09] and research funding for longevity sciences from the National Center for Geriatrics and Gerontology [grant number 21-48].
Data availability statement
Not applicable.
Declarations of Competing Interest
None.
Acknowledgements
The authors thank Shuji Nakamura, Junko Hirokawa, Megumi Banno, Yukari Kido, and Kanno Fujikawa for their technical assistance.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2022.05.016.
Appendix. Supplementary materials
References
- 1.Tré-Hardy M., Cupaiolo R., Wilmet A., Antoine-Moussiaux T., Della Vecchia A., Horeanga A., et al. Immunogenicity of mRNA-1273 COVID vaccine after 6 months surveillance in health care workers; a third dose is necessary. J Infect. 2021;83:559–564. doi: 10.1016/j.jinf.2021.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishikimi A., Kojima M., Watanabe K., Watanabe A., Yasuoka M., Oshima H., et al. Seroprevalence of antibodies against SARS-CoV-2 among workers in a national research institute and hospital in Central Japan. GHM Open. 2021;1:40–42. doi: 10.35772/ghmo.2021.01026. [DOI] [Google Scholar]
- 3.Nishikimi A., Watanabe K., Watanabe A., Yasuoka M., Watanabe R., Oshima H., et al. Prevalence of SARS-CoV-2 antibodies after one-year follow up among workers in a research institute in Japan. J Infect. 2022;84:e23–e25. doi: 10.1016/j.jinf.2021.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bange E.M., Han N.A., Wileyto P., Kim J.Y., Gouma S., Robinson J., et al. CD8+ T cells contribute to survival in patients with COVID-19 and hematologic cancer. Nat Med. 2021;27:1280–1289. doi: 10.1038/s41591-021-01386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levin E.G., Lustig Y., Cohen C., Fluss R., Indenbaum V., Amit S., et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. 2021;385:e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maeda K., Amano M., Uemura Y., Tsuchiya K., Matsushima T., Noda K., et al. Correlates of neutralizing/SARS-CoV-2-S1-binding antibody response with adverse effects and immune kinetics in BNT162b2-vaccinated individuals. Sci Rep. 2021;11:22848. doi: 10.1038/s41598-021-01930-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu X., Munro A.P.S., Feng S., Janani L., Aley P.K., Babbage G., et al. Persistence of immunogenicity after seven COVID-19 vaccines given as third dose boosters following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK: three month analyses of the COV-BOOST trial. J Infect. 2022 doi: 10.1016/j.jinf.2022.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.