Abstract
Background:
Reproduction of the perfusion used in therapy (hyperthermic intraperitoneal chemotherapy) procedures preclinically represents a valuable asset for investigating new therapeutic agents that may improve patient outcomes. This article provides technical descriptions of our execution of closed and open “coliseum” abdominal perfusion techniques in a mouse model of peritoneal carcinomatosis of colorectal cancer.
Materials and Methods:
BALB/c mice presenting with disseminated colorectal cancer (CT26-luciferin cells) underwent 30-min perfusions mimicking either the closed perfusion or the coliseum perfusion technique. Disease burden was monitored by bioluminescence signaling using an in vivo imaging system. Perfusion circuits consisted of single inflow lines with either a single or dual outflow line.
Results:
Twelve mice presenting with disseminated disease underwent the closed perfusion technique. Surgical complications included perfusate leakage and organ constriction/suction into the outflow line(s). Nine mice underwent the coliseum perfusion technique with surgical debulking, using bipolar cauterization to remove tumors attached to the peritoneum. All mice survived the coliseum perfusion with limited intraoperative complications.
Conclusions:
Fewer intraoperative complications were experienced with our coliseum perfusion technique than the closed perfusion. The methods described here can be used as a guideline for developing future perfusion murine models for investigating perfusion models useful for delivery of chemotherapy or other tumor-sensitization agents, including selective targeted agents, nanoparticles, and heat.
Keywords: Peritoneal carcinomatosis, Colorectal cancer, Intraabdominal perfusion, Murine model
Introduction
Peritoneal carcinomatosis (PC) is an advanced (stage IV) manifestation of cancers of pelvic and abdominal organs presenting as a widespread dissemination of tumor nodules over the surface of the peritoneum. The most common primary cancers associated with development of PC are gastric, colorectal, ovarian, pancreatic, and appendiceal.1,2 The presence of peritoneal metastases portends a poor prognosis and is associated with significant morbidity and mortality. For example, in colorectal cancer (CRC), the 5-y survival rate for localized disease is nearly 90%, whereas metastatic disease survival plummets to below 14%; without treatment, CRC patients with PC have a mean survival time of only 6 mo.3–5
Historically, PC has been poorly amenable to conventional cancer treatments like surgery and chemotherapy. This poor response is in part due to the architecture and physiology of the peritoneal cavity. Structured like a fluid-filled “sac”, it allows cancer cells to bathe and seed the large surface of the peritoneum and abdominal organs, leading to widespread disease dissemination and concealment of microscopic disease. The large surface area combined with CRC and appendiceal cancer subtypes that produce excessive mucus secretion upsurges the surgical challenges.6 Cytoreductive surgery (CRS) and/or peritonectomy are most commonly performed to remove all macroscopic lesions in patients with PC.7 Tumor nodules and micrometastases that escape CRS have the potential to reseed the peritoneal surface, leading to regrowth of tumors.8 Traditionally, the issue of residual disease has been solved by administration of systemic chemotherapy; however, chemotherapeutic agents cannot reach significant concentrations inside the peritoneal cavity due to the presence of the peritoneum-plasma barrier.9–11
To bypass the peritoneum-plasma barrier, the incorporation of intraperitoneal (IP) chemotherapy for malignancies was investigated in the 1980s.12,13 The justification for using IP chemotherapy include: 1) higher doses of chemotherapy (5–30 fold higher) can be used while reducing systemic toxicity due to peritoneal containment by the peritoneal plasma barrier, 2) the chemotherapy comes into direct contact with surface malignancies (PC tumors) that would otherwise receive minimal drug from an intravenous route due to abnormal vasculature.14,15 IP delivery was then combined with CRS, a surgical approach to remove substantial tumor burden, to prolong survival.16 The treatment of disseminated abdominal cancers was further transformed by the addition of hyperthermic intraperitoneal chemotherapy (HIPEC), following cytoreduction surgery in the 1990s.16–18
Following CRS, HIPEC is a technique where a warm chemotherapy solution (40°C–43°C) is perfused throughout the peritoneal cavity for 30–120 min, depending on the drug and drug dose.17 Hyperthermia augments the activity of chemotherapy by affecting both drug pharmacokinetics and pharmacodynamics.19 Most cytotoxic drugs used for HIPEC synergistically affect chemotherapy at target temperatures between 40°C and 45°C, and studies have shown that the penetration depth of the warm chemotherapy through tumors and tissues is 1.0–3.0 mm.17,20–22
HIPEC can be delivered by a few different perfusion methods but this article will focus on the two most common methods: the open abdomen (“coliseum”) method or the closed abdomen method.22 In the open abdominal perfusion method, the edges of the longitudinal abdominal incision are suspended with the use of a Thompson retractor, creating an open “bowl” to contain the chemotherapy solution.22,23 Inflow lines are placed in the upper quadrant and outflow lines are placed in the lower quadrant and perfused at a rate of ~ 1 L/min16 A plastic sheet is placed over the open cavity to prevent exposure of the surgical staff to chemotherapy by containing the liquid and aerosolized chemotherapy. A slit through the sheet allows the surgeon’s hand to enter the peritoneum and manipulate its contents to distribute the chemotherapy. The advantages of the open technique are the ability to achieve homogenous temperature and even distribution of chemotherapy within the peritoneal cavity.22 The disadvantages include the potential for exposing the operating room staff to chemotherapy as well as heat loss.22,24
In the closed abdominal perfusion method, the layers of the abdominal cavity are closed with a continuous running suture following CRS. Inflow and outflow lines are placed and heated chemotherapy is subsequently perfused varying from 400 mL/min to 1 L/min24 The abdomen is vigorously compressed for the duration of perfusion to agitate the contents. The temperature of the perfusate is monitored on inflow and outflow to maintain the perfusate temperature.25 The closed technique reduces the risk of operating room staff exposure to chemotherapy, increases chemotherapy perfusion through the peritoneal surfaces, and quickly reaches and maintains hyperthermic conditions.22,26 However, the main caveats of using the closed technique encompass nonhomogenous distribution of chemotherapy and temperature within the cavity, thus leading to regions of undertreated and overtreated tissue.26,27
To quickly and more efficiently investigate efficacy of new adjuvants and methods for an HIPEC regimen, clearly defined animal models for reproducible studies are essential. Various animal models including mouse, rat, porcine, and rabbit have been utilized to evaluate the optimal HIPEC technique.28 Specifically, several rodent models for developing disseminated abdominal cancer for PC treatments have been found to be translatable.28–33 However, there is quite a bit of variation amongst the models, with most procedures using closed perfusion, which does not allow for direct manipulation of the abdominal organs during perfusion. In addition to perfusion of classical chemotherapy agents through the abdomen, researchers have been investigating the potential to deploy targeted therapies, such as nanoparticles- or radiation-inducing materials, directly to the tumor and spare the adjacent tissues from unnecessary therapy.31,34,35 There has also been interest in using perfusates that can disrupt cells by changing the osmotic pressure or by utilizing agents that initiate the production of reactive oxygen species.36–38 Only Graziosi et al.,30 describes an open abdominal perfusion model in mice, and the other literature describes the use of closed technique. Although their model is excellent, it focused on dissemination of human gastric cancer in an immune-compromised mouse. A syngeneic mouse model is more preferred for evaluation with hyperthermia treatments though, so that the impact of heat on immune function can be included. For example, the use of MC38 CRC cells in C5Bl/6 mice or CT26 CRC cells in BALB/c mice.38 Part of the challenges with utilizing a model of abdominal perfusion is the setup/instrumentation required for the perfusion circuit. This work outlines all the equipment needed and the approach taken to establish perfusion in both open and closed abdominal models of PC from CRC. The perfusion methods described here are meant to focus on the experimental setup of the perfusion model and can be adjusted accordingly to the users’ needs. One of the goals of the work was to minimize the perfusion circuit volume, which is especially important when costly new agents (antibodies, drugs, H2O2, antiseptic solutions, nanoparticles, or other selective tumor-targeting agents) are being evaluated.
Methods
Cell line
CT26.WT-Fluc-Neo cells (Imanis Life Sciences, CL043), a polyclonal population of the CT26.WT (ATCC CRL-2638) mouse colorectal carcinoma line transduced with lentivirus encoded with firefly luciferase flanked by a neomycin resistance gene, were purchased from Imanis Life Sciences. The CT26 cell line is an N-nitroso-N-methylurethane induced, undifferentiated colon carcinoma cell line derived from BALB/C mice. Cells were grown in DMEM media (Gibco) supplemented with 10% FBS, 1X Penicillin/Streptomycin, and 0.4 mg/mL G418 in T225 flasks until reaching 80% confluency. Cells were trypsinized with 0.25% (w/v) Trypsin-0.03% (w/v) EDTA until cells lifted and neutralized with culture media. For in vivo injections, cell suspensions were spun down gently, washed once with 1X PBS, and finally resuspended in 1X PBS for injection.
Animals
Care of the mice used in the described experiments was done in accordance with the National Institutes of Health guide for the care and use of laboratory animals. All procedures were reviewed and approved by the Wake Forest University Health Sciences Animal Care and Use Committee. Six-week-old female BALB/c mice weighing 15–20 g were purchased from Charles River Laboratories, housed with five animals per cage, maintained in a vivarium on a 12-h light/dark schedule, and had food and water available ad libitum. A total of 21 animals were used in this study, 12 to evaluate the closed perfusion technique and nine to evaluate the open technique. For any procedures that could lead to pain, the animals were properly anesthetized during the procedure and they were provided appropriate analgesia following the procedure. Pain was monitored according to Mouse Grimace Scale and also by observation of mobility.39 In addition, although IP injection of the of the CRC tumors cells and in vivo imaging system (IVIS) imaging are minimally or temporarily painful, mice were anesthetized for these procedures. Surgical procedures were done under aseptically and no preoperative or postoperative antibiotics were given.
Surgical equipment
Adson Forceps, 12 cm, Straight, Serrated, TC Jaws (World Precise Instruments Sarasota, FL Item#: 500222).
Noyes Scissors, 12 cm, Straight, Sharp/Sharp, 15 mm blades, SS (World Precise Instruments Sarasota, FL Item # 500228).
Fine Scissors, 9 cm, Straight, Sharp Tip, Swiss (World Precise Instruments Sarasota, FL Item#: 504613).
Webster Needle Holder, 12.5 cm, Straight, Serrated, Extra Delicate (World Precise Instruments Sarasota, FL Item #: 14,109).
Gauze pads.
6-0 Prolene suture
4-0 and 6-0 Vicryl suture
Green surgical towel, sterile
Blue surgical drape, sterile
Ring stand and 10 cm ring support
Perfusion circuit
Dual outflow circuit components
Masterflex L/S Digital Pump
Masterflex XX8000004 Head
Masterflex 96,400-15 Tubing (0.189″ I.D.)
SmartSite Extension Set (Alaris 30262E) (2.7 mm I.D.)
Tygon AAQ04127 Tubing (0.040″ I.D., 0.070″ O.D.)
BD Vacutainer Safety-Lok Blood Collection Set (367282)
AIRCARE Cuffed Endotracheal Tube (100/100/050) (5.0 mm I.D., 6.9 mm O.D.)
Y-drain (Nalgene 15-320-10A, 1/8″ ID)
Single ouflow circuit components
Masterflex L/S Digital Pump
Masterflex XX8000004 Head
Tygon AAQ04127 Tubing (0.040″ I.D., 0.070″ O.D.)
BD Vacutainer Push Button Blood Collection Set (367344)
White Polypropylene Straight Barbed Connector (1/8″ ID)
Tygon R-3603 Tubing (3/32″ I.D., 5/32″ O.D.)
Fisherbrand PolyEthylene Quick Disconnect (15-315-27C) (0.125″ I.D.)
Masterflex 96,400-15 Tubing (0.189″ I.D.)
Fisherbrand Tygon S3 E-3603 Flexible Tubings (14-171-104) (3/16″ I.D., 3/8″ O.D.)
Additional equipment
Baker’s Biopsy Punch BakerCummins 0889 2 mm
Acuderm Acu-Punch Biopsy Punch, sterile, 3 mm (P325)
Sixteen gauge stainless steel blunt needle with leur PPE (component supply NE-161PL-25)
18½ gauge needle
4-0 and 6-0 Vicryl absorbable suture
5-0 Ethilon monofilament suture
#10 or #15 surgical blades
SurgiStatTM II-20 electrosurgical generator
Bipolar handles
Animal preparation equipment
Hair dilapatory cream
7.5% Betadine solution
0.9 % NaCl solution
1 and 5 mL sterile syringes
Lactated Ringers solution
Buprenorphine Hydrochloride (0.01 mg/mL)
Development of the dissemination model
- Six-week-old BALB/c mice were briefly anesthetized under 2% isofluorane. The abdomens were swabbed with alcohol and 3.0 × 106 CT26.WT-Fluc-Neo (Imanis Life Sciences) cells suspended in 1X PBS were injected intraperitoneally. The abdomens of the mice were thoroughly massaged to distribute the cell suspension throughout the abdominal cavity.
- The Living Image Software (PerkinElmer) was used to quantify the total bioluminescence signal (photons/second) of the disease in the animals. D-Luciferin (200 μL of 15 mg/mL stock; PerkinElmer) was injected intraperitoneally into the mice 24 h after implantation of the CT26 cells and every 72 h subsequently. The bioluminescence signal was detected using an in vivo imaging system (IVIS: Caliper-PerkinElmer) with 1 and 10 s exposures on the ventral and dorsal sides of the animals, respectively, using subject heights of 1.5 cm and medium binning.
Surgery for this model must be conducted within 5 d of inoculation, otherwise tumor burden and vascular supply to tumors will be too excessive for a survival surgery. For the number of tumor cells injected, we found that if we waited longer than 5 d to begin the surgical procedures, the animals had too much disease, there were many tumor nodules binding various organs together, or causing intestinal blockages that led to animal death before surgical intervention could proceed. We tried an alternate approach of using fewer cells, and although this extended the time at which carcinomatosis presented, as determined by BLI, this led to the formation of less diffuse disease and the growth of larger tumor nodules having significant vasculature. Removal of these larger tumors led to irrecoverable bleeding and animal death. Hence, we found that the best methods for developing a disseminated CRC within the peritoneal cavity to be 5 d for this cell line and mouse strain.
Manufacture of circuit components
We tested our perfusion models using a single inflow line and either a single or double outflow (drainage) line(s). Dual drainage lines are commonly used in human perfusions, but run the risk of developing inconsistencies in line pressure in mice thus leading to fluid backflow of loss of circulation of the perfusate. In the later versions of both our closed and open models, we deviated from the double outflow lines to a single outflow line to avoid pressure loss and backflow in the circuit. Using a single circuit line also decreased the necessary volumes needed for the perfusion and decreases the amount of heat loss that can occur with longer circuit lines. The tubing diameter and lengths were selected to reduce the circuit volume and lessen heat loss.
Below we describe the methodology for forming both the double and single circuits. Circuit perforations were made to mimic the fenestrated tubing at the outlets used clinically. The fenestration avoids suction of any organs, which occurs from outflow blockage and subsequent circuit pressure increases that occurs in nonfenestrated outflows.
Double outflow circuit
Cut Masterflex 96,400-15 tubing to the length of 16 cm.
Cut the first SmartSite Extension tubing to the length of 25.5 cm from the top of female luer.
Cut the second SmartSite Extension Set to the length of 33.0 cm from the top female luer. At the distal 0.5 cm of the tubing, create 30 perforations circumferentially using 18½ gauge needle. Cut Tygon tubing to the length of 5.5 cm.
Cut BD Vacutainer Safety-Lok Blood Collection Set to the length of 5 cm from the top of the male luer lock.
Cut AIRCARE endotracheal tube to the length of 1 cm. Use 2 mm biopsy punch to make 12 perforations circumferentially in a staggered pattern.
Cut a 1 × 1 mm strip from AIRCARE endotracheal tube. Insert into the perforated tip of SmartSite tubing so that it is partially occluded.
Single outflow circuit
Cut the Tygon AAQ04127 tubing to the length of 6 cm.
Cut BD Vacutainer Safety-Lok blood collection set to the length of 4 cm from the top of the male luer lock.
Cut the Tygon R-3603 tubing to the lengths of both 20 and 30 cm.
Cut Masterflex 96,400-15 tubing to the length of 17 cm.
Using the Acuderm Acu-Punch Biopsy Punch, Sterile, 3 mm (P325), create a circular punch cut from the Fisherbrand Tygon S3 E-3603 Flexible Tubings to serve as a circuit cap.
Figure 1: Demonstrates the single outflow circuit assembly. Of specific note is the outflow tubing image provided in part g, where holes have been placed at the end of the tubing to minimize the suction of organs at the end of the drain line.
Fig. 1 –
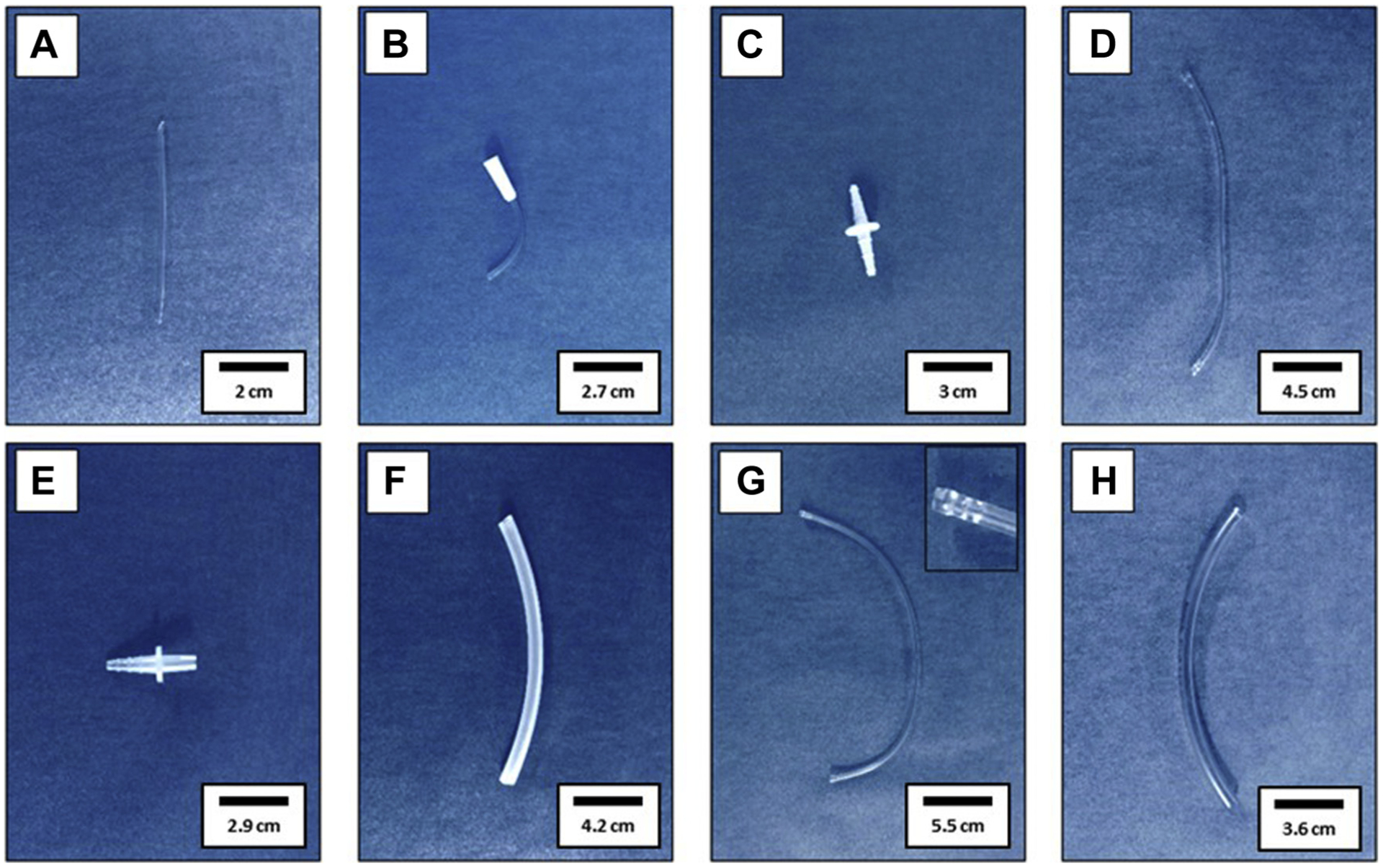
Single outflow circuit assembly. (A) Inflow Tygon AAQ04127 tubing; (B) Inflow BD Vacutainer Push Button Collection Set Male Luer Lock; (C) Inflow White Polypropylene Straight Barbed Connector; (D) Inflow Tygon R-3603 tubing; (E) Fisherbrand Polyethylene Quick Disconnect; (F) Masterflex 96,400-15 tubing; (G) Outflow Tygon R-3603 with inset displaying perforations formed with 16 Gauge Stainless Steel Blunt Needle; (H) Fisherbrand Tygon S3 E-3603 Flexible Tubings used to form 3 mm diameter plug to insert at inset of picture (G)
Figure 2: Illustrates the final circuit design (single outflow) that was the most reliable and beneficial for this study.
Fig. 2 –
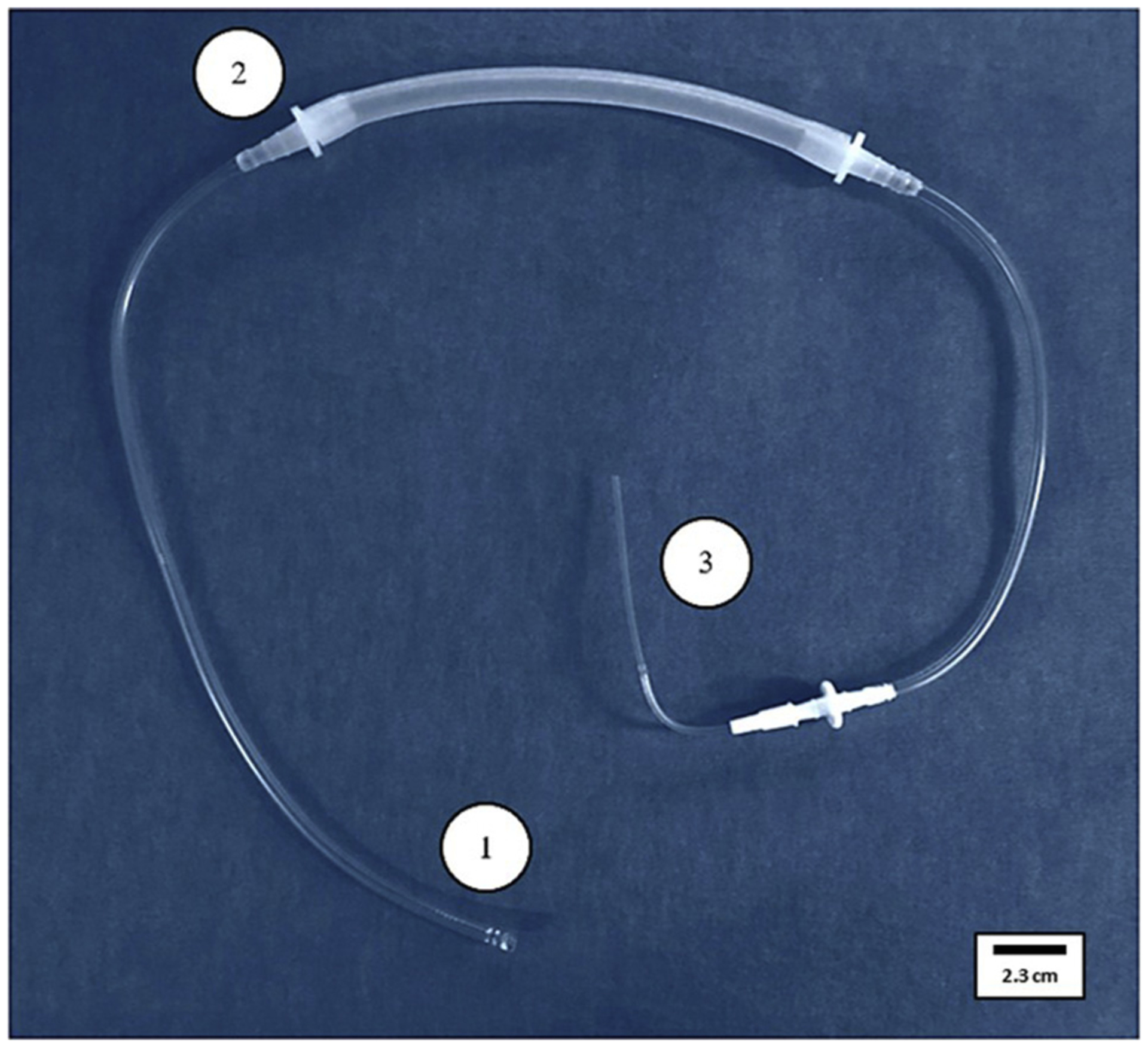
Final circuit design: (1) Outflow end with perforations, (2) Masterflex tubing, and (3) Inflow end.
Complete circuits were sterilized by ethylene oxide gas.
Circuit assembly
Double outflow circuit
- Inflow tube assembly:
- Attach the BD Vacutainer luer lock to cut end of 25.5 cm smartsite extension tube.
- Insert Tygon tubing into cut end of BD vacutainer tubing.
Load Masterflex tubing into pump head.
Insert female luer of inflow tube into left end of Masterflex tubing.
Insert female luer of outflow tube into right end of Masterflex tubing.
Single outflow circuit
Insert the Tygon AAQ04127 Tubing approximately 1 mm into the tubing of the prepared BD vacutainer push button blood collection set component to serve as the perfusion inflow.
Connect the assembled inflow to the 20 cm Tygon R-3603 Tubing via the white polypropylene straight barbed connector.
Insert the female end of the smallest inner diameter connection of the Fisherbrand PolyEthylene Quick Disconnect into one side of the Masterflex 96,400-15 tubing and connect assembled 20 cm Tygon R-3603 tubing to the male end.
At one end of the 30 cm Tygon R-3603 tubing, form three rows of four perforations to a maximum length of 0.5 cm from the end using the 16 gauge stainless steel blunt needle.
Plug the perforated end of the circuit with the prepared Fisherbrand Tygon S3 E-3603 Flexible Tubings circular punch cut.
Insert the female end of the smallest inner diameter connection of the Fisherbrand PolyEthylene Quick Disconnect into the other side of the Masterflex 96,400-15 tubing and connect prepared 30 cm Tygon R-3603 tubing to the male end.
Perfusate preparation
Warm 0.9% saline solution to desired hyperthermic temperature before the start of surgical operations. For our needs, we warmed our solutions using a water bath before loading the circuit.
Customization option 1
Heat exchangers for rodent HIPEC perfusion models have been used before, such as a water bath, to maintain the temperature of the perfusates.29,37 The techniques we describe here can be modified for the addition of a heat exchanger if desired.
Customization option 2
IP perfusion using cytotoxic agents in rodents has been described and demonstrated IP tolerability of commonly used human chemotherapy agents in peritoneum of mice.32,40
Circuit priming
Flush the circuit with 0.9% saline solution.
Preload circuit lines with perfusate to remove air.
Operative procedure (closed)
Closed abdominal perfusion in mice was explored first in 12 mice. The animals were survived for 24 h following surgery. Below is the technical description for executing the closed perfusion model.
Anesthesia and surgical preparation of the animal
Induce general anesthesia with 2% isoflurane in oxygen (2 L/min).
- Following induction of anesthesia, transfer the animal onto operative platform and maintain anesthesia by isoflurane inhalation through a nose cone at 1.5% (1.5 L/min).
- We used an acrylic water pad platform to maintain the outside body temperature of the mouse above 30°C.
Inject 1.0 cc of Lactated Ringers Solution subcutaneously near the dorsal base of the neck and add one drop Rugby Artificial Tears Ointment to each eye to prevent the eyes from drying out. The Lactated Ringers solution will keep the animal hydrated during the surgery, and in our experience, this has led to the mice regaining consciousness quicker once they were removed from anesthesia.
Apply hair removal cream to abdominal skin for 5 min and remove with 0.9% saline solution.
Disinfect the exposed skin with Betadine solution three times then move mouse onto a dry surgical cloth.
Secure mouse to operating surface with medical tape.
Incisions and tube insertion
Make a horizontal incision of 2 mm in length in the right upper subcostal area halfway through right hemifield. Insert inflow tube to the depth of 5 mm and secure with a single simple interrupted 5-0 Ethilon suture on each side.
Make a second incision of 5 mm in length in oblique orientation in the left lower quadrant in the midline of left hemifield.
At the second incision, lift the skin and the peritoneal membrane with forceps and insert the outflow device up to the proximal edge of the cap and place gently on top of the organs.
Secure the opening around the tube with purse-string 5-0 Ethilon suture to create a tight seal.
Performing closed perfusion
Establishing perfusion parameters.
The perfusion flow rate for the closed perfusion model was derived from the rates used in humans and scaled down for use in a mouse. Reported human perfusion rates vary from 400 mL/min to 1 L/min and rodent perfusions can safely be upward of 6 mL/min28,41,42 Mouse body surface area constitutes 0.43% of human body surface area.43 We based our calculations for murine perfusion on the lowest reported human flow rate we found in literature and began our perfusion at rates at half of the value obtained to avoid exceeding the tolerated perfusion rate and hemodynamic effects of IP perfusion in mice41 Figure 3.
Fig. 3 –
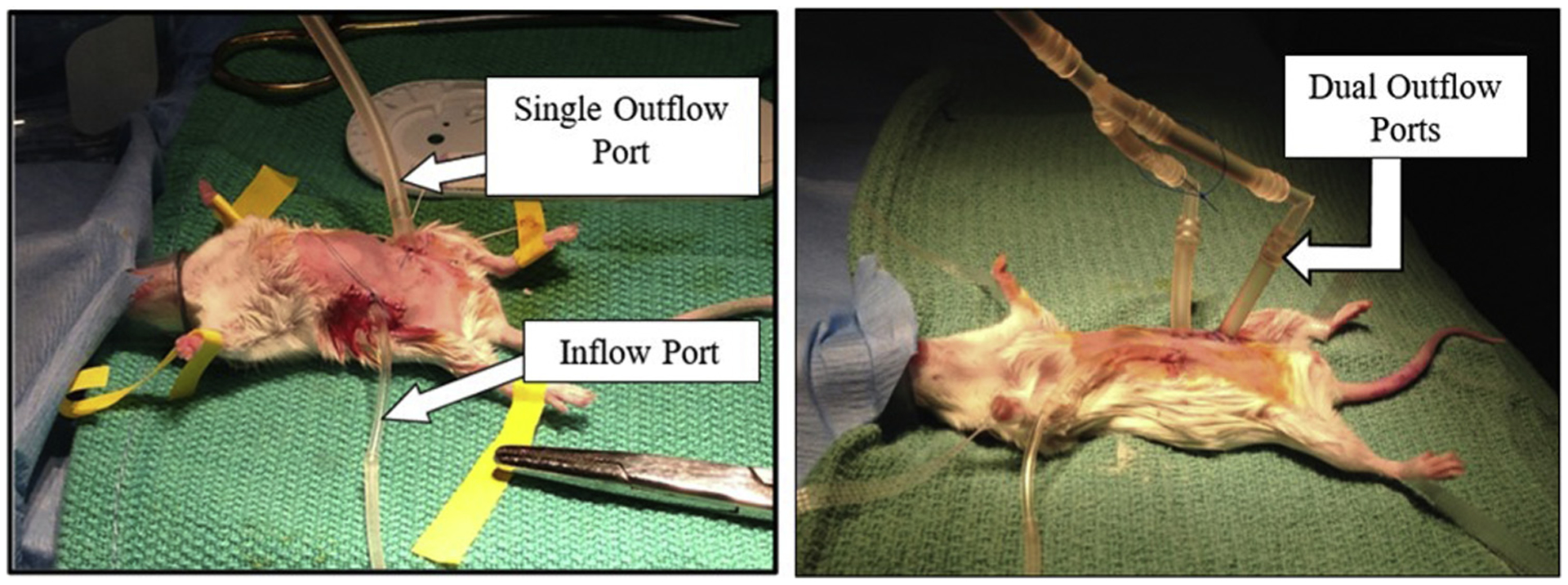
Closed Perfusion Circuit—(Left): Perfusion circuit in a mouse with a single drain, (Right): Closed circuit in mouse with dual drain port.
Human perfusion times for standard HIPEC procedure range between 30 and 120 min. We used a flow rate of 50 mL/h for the closed perfusion and perfusion time of 30 min. The 30-min time frame was chosen to match the shortest perfusion time reported in humans, to increase the likelihood of animal survival.44 Perfusion time was carefully selected to balance approximating human parameters and avoiding previously reported complications with excess peritoneal pressures in small rodents.45 In our experience, we found that higher flow rates using in the closed perfusion model impeded mouse respiration.
Set the Masterflex L/S Digital Pump to continuous flow at 50 mL/h with flow direction oriented such that the perfusion inlet is dispensing fluid.
Perfuse desired solution for 30 min with firm circular massage of the abdominal cavity every 2–5 min.
Drain perfusate and flush abdomen by perfusing warm 0.9% saline three times.
Removal and recovery
After final drainage, carefully remove inflow tubing, and close peritoneal sac with 1–2 interrupted absorbable 6-0 vicryl suture. Close skin with 2–3 interrupted monofilament 5-0 ethion suture; repeat for outflow tube site.
Wean mouse off isoflurane and once the animal is conscious administer 0.05 mg/kg subcutaneous buprenorphine in 800 μL Lactated Ringers solution.
Postoperative care of the animals
Animals were monitored in the immediate postop period to ensure full return of mobility, acceptable pain level as monitored by rodent grimace scales, and acceptance by cage mates. Staff veterinarians conducted routine animal checks.
Animals survived up to 24 h postoperative endpoint and were euthanized in accordance with institutional policy for humane euthanasia.
Postmortem examinations
Following euthanasia, animals were dissected and organs inspected for signs of injury related to the procedure.
Operative procedure (open/coliseum)
After evaluating the closed perfusion model and 24-h postsurgery survival, we switched the perfusion model to the open abdomen “coliseum” technique and short-term survival. By switching to the coliseum technique, a surgical debulking step was incorporated to remove larger tumor nodules on the peritoneum using bipolar cauterizers (Fig. 4). We did not resect any other organs, besides the peritoneum, on which tumors may have seeded to maintain high survivability from the surgery and minimize morbidity. The rationale for doing surgical debulking of larger tumors in this model was three-fold: i) the larger tumors tended to have significant vascularity and their rapid progression limited survival time, ii) the larger tumors impede the flow of the perfusate, and iii) development of a model that more closely mimics PC observed in humans. To help the mice thermoregulate, we covered their bodies with a plastic surgical draping. The open abdomen required more perfusate by volume, so the flow rate was increased up to 200 mL/h to ensure at least five total exchanges of the perfusate during a 30-min perfusion. For delivery of targeted agents (future studies), the number of volume exchanges is more important than the overall time of perfusion. This was an additional reason why the length of time for perfusion could be shortened. Both dual and single drain circuits were assessed. A total of nine mice were used in the coliseum technique perfusion group.
Fig. 4 –
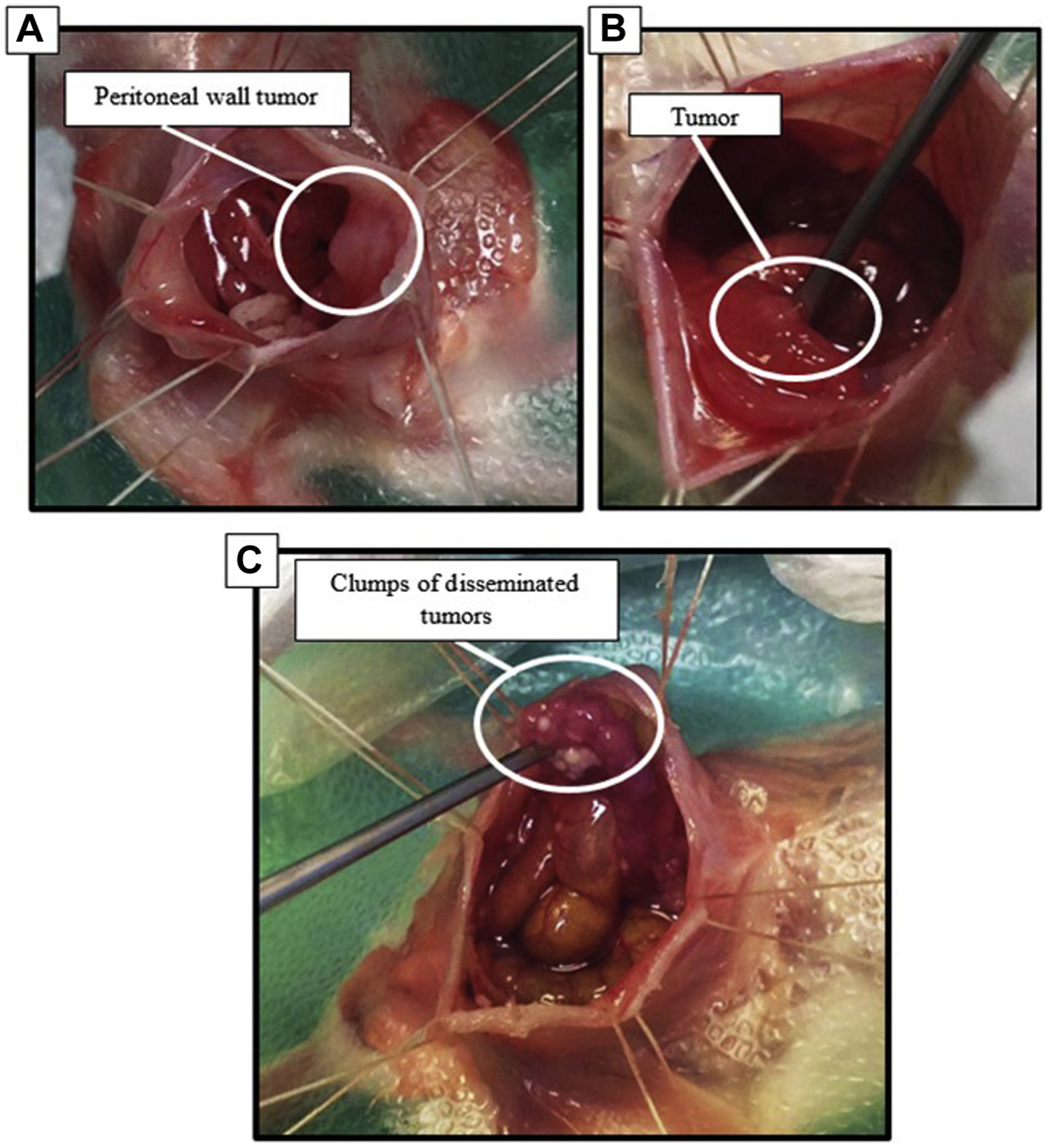
Tumors that developed in IP injection dissemination model. (A) Tumors adhered to internal peritoneal wall (B) tumors integrated onto surface of the large intestine. (C) Clumps of tumors with poor vasculature; these tumors were easily disturbed and produced mucus.
Repeat (VI-A [a-f]) anesthesia and surgical preparation
Apply a surgical draping (Glad Press’n Seal) over the entire body, exposing only the tail and 10 mm clearance from the nosecone to the base of the neck.
Incision
Make a 20 mm midline incision starting from the base of the sternum, through the drape, skin, and peritoneal sac.
Use 4-0 vicryl suture to secure 4–6 points of the skin and peritoneum to the ring support, approximately 10 cm above the open abdomen to form the “coliseum”.
Apply the Rugby Artificial Tears Ointment to the edges of the skin using a sterile swab to prevent drying of the skin.
Remove ascitic abdominal fluid and any cancer-secreted mucus with sterile gauze pads. Gently move organs with blunted probes, as needed, to access organ surfaces.
If small, nonadherent tumors are present, remove with saline rinses, pickups, or cotton tips–(Fig. 4).
If small tumor nodules are present on the peritoneal wall, excise them with bipolar cauterizer handles with cut and coagulation set to 10W. Nodule removal using bipolar cauterization reduces the risk of bleeding (many PC tumors can develop significant vascularization).
Coliseum technique perfusion
Saline Flush—Using a 5 mL syringe, slowly add 5 mL of desired temperature 0.9% saline to the open cavity; manipulate the organs with the blunted probe to aid in removing the ascitic abdominal fluid and any cancer-secreted mucus Figures 5 and 6.
Insert the suction outflow line(s) to remove saline flush (200 mL/h). Position the outflow line(s) against internal peritoneal sac on the left lower quadrant to prevent organ suction.
Insert inflow line at the upper right quadrant against the peritoneal wall.
Set the Masterflex L/S Digital Pump to continuous flow counter-clockwise at 200 mL/h (Fig. 6).
Perfuse for 30 min using blunted probes to manipulate organs to ensure an even distribution of perfusate throughout the abdominal cavity every 2–5 min.
Drain perfusate and flush abdomen with warm 0.9% saline three times.
Fig. 5 – –
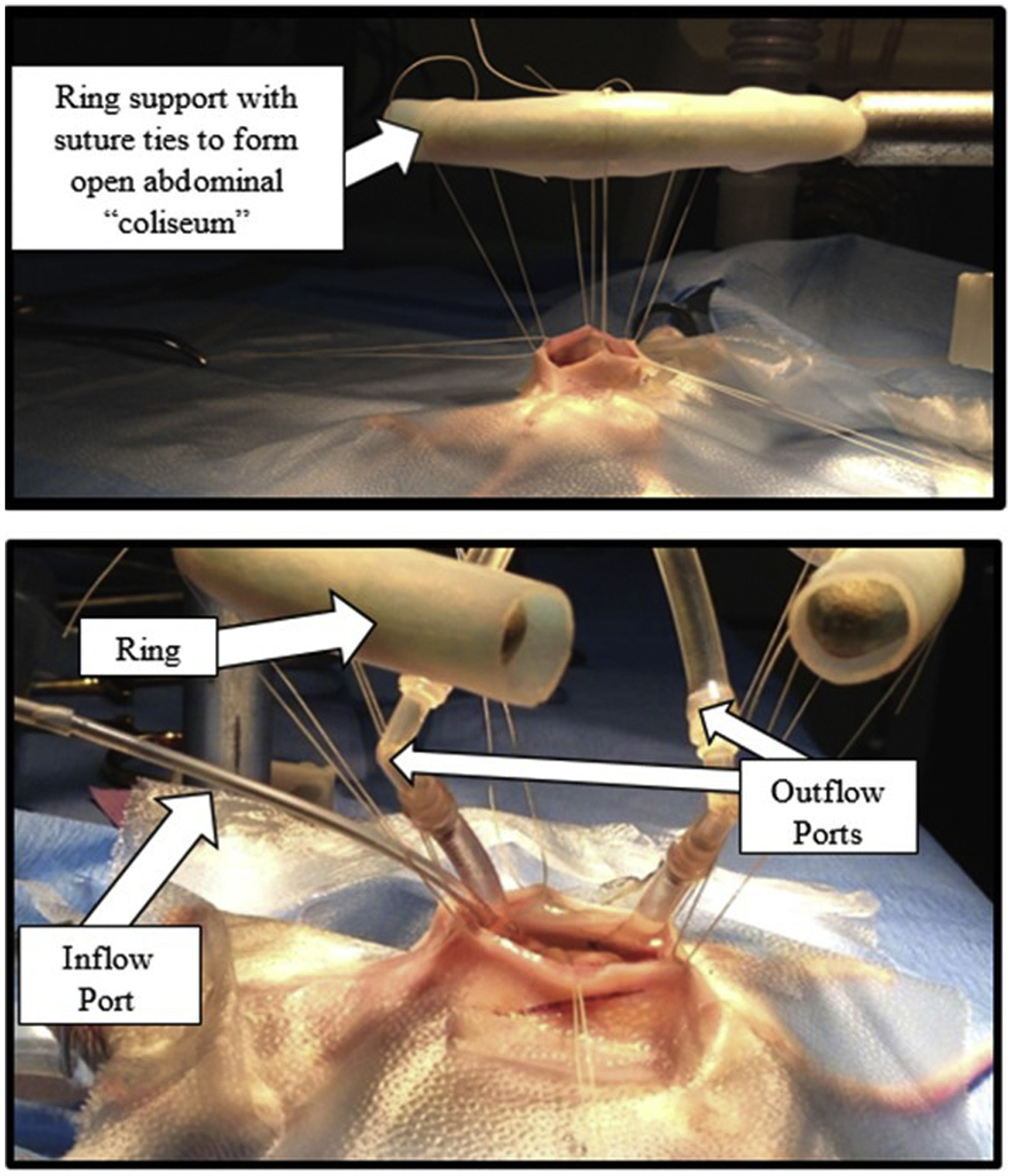
Open abdominal “Coliseum” technique perfusion: (Top): Mouse with open abdomen sutured to a ring stand to form the “coliseum”. (Bottom): Mouse with perfusion circuit inserted into the coliseum. This circuit has a single inflow tube and two outflow tubes.
Fig. 6 –
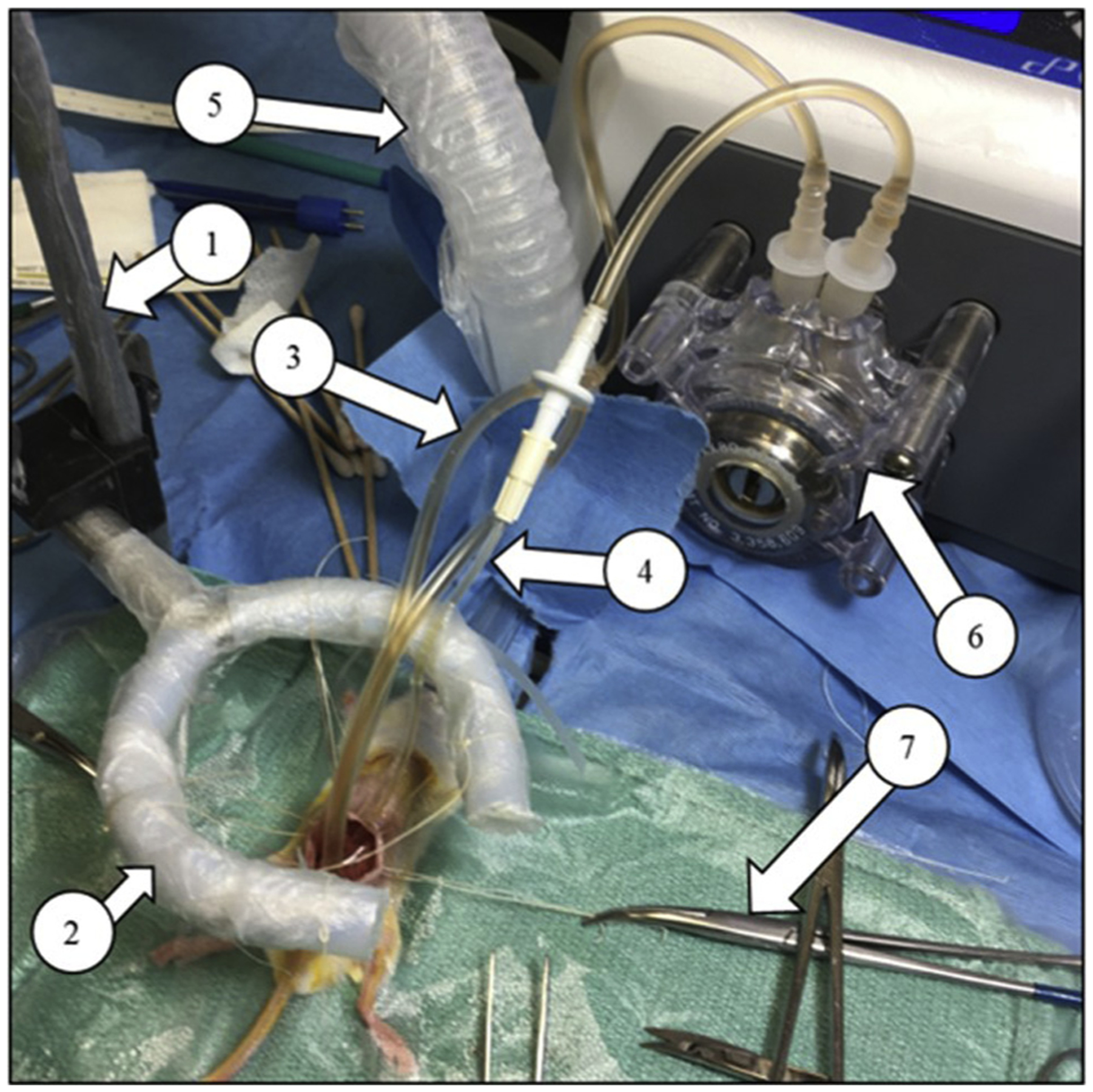
Complete Open “Coliseum” Technique Perfusion Circuit—The perfusate can be customized to drug or therapeutic of choice. (1) Ring stand, (2) Ring support for sutures to be tied onto to secure the open abdomen, (3) Dual outflow circuit with perfusate flowing, (4) Inflow line of the circuit, (5) Anesthesia airline, (6) Masterflex pump with 200 mL/h continuous flow rate, (7) Hemostat securing sutures used to maintain shape of the coliseum.
Removal and recovery
Remove sutures connected to the ring support, remove surgical draping, and suture peritoneal sac using absorbable 6-0 vicryl with a continuous stitch.
Close skin with 5-0 ethilon monofilament suture using 8–9 interrupted stitches.
Wean mouse from isoflurane and clean mouse abdomen with warm saline.
Administer 0.05 mg/kg subcutaneous buprenorphine in 800 μL Lactated Ringers solution once mouse has attained consciousness.
Disease progression monitoring/endpoint
Disease progression in the mice was monitored by measuring tumor burden via the detected bioluminescence signal of the luciferase transfected CT26 cells using a PerkinElmer IVIS. Bioluminescence monitoring is an effective method for estimating tumor localization and tumor size in live rodents.46–48
- The mice undergoing closed perfusion constituted the initial study group and were survived for 24 h. After switching to the coliseum technique, the mice were survived until a humane endpoint was reached to determine the survivability of the mice to both the debulking surgery and the coliseum technique.
- Humane endpoints were defined by:
- Bioluminescence signal saturation at 1 s of imaging.
- Postoperative weight loss that is greater than 20% of preoperative weight.
- Mouse exhibiting distress or extreme lethargic characteristics.
- Presence of blood pooling within the abdomen and confirmed by an IP draw via a 25-gauge needle and 1 mL syringe.
Results
Closed perfusion results
Surgical morbidity and mortality (closed)
All mice in the closed perfusion study were survived for 24 h to evaluate the effective utilization of the closed technique and short-term complications.
Of 12 mice studied, 11 survived to the 24-h postoperative endpoint. There was one intraoperative death.
- Intraoperative complications included suction of organs into outflow tube (3) and incomplete circuit closure at outflow with leakage of perfusate (4). The intraoperative death was due to the intestine being sucked into the capped outflow line.
- Circuit complications included bubble formation during every surgery and loss of suction in one of the outflow drain lines when using a dual outflow circuit.
Coliseum technique results
Surgical morbidity and mortality
All mice that underwent the coliseum technique were subject to a surgical debulking that included removal of easily disturbed disseminated tumors that were not seeded onto the surfaces of tissues (Fig. 4C). After the debulking step, mice were perfused for 30 min, then had three abdominal flushes of saline.
- Of the nine mice studied, all mice survived surgery and survived until disease burden reached a humane endpoint, which was between 4 and 8 d postsurgery.
- Organ suction was not observed in the open model, nor was there any leakage of perfusate.
Intraoperative complications included bleeding following midline incision and some bleeding of the tumor vasculature.
Model notes—coliseum technique
Circuit continuity was often disrupted in the double outflow circuit; the perfusate would backwash into the abdomen of the animals as if the circuit failed. This phenomenon was most likely due to a pressure difference between the two prongs that is created by the establishment of any difference in height of the two prongs.26
Many tumors excised from the mice in the coliseum model exhibited mucus production and were very easy to detach from organ surfaces.
Nearly all mice exhibited ascites by the time of endpoint. Pathology reports indicated that the ascitic fluid originated from the tumors and not from infection or inflammation.
As shown in Figure 7, some mice that underwent the coliseum perfusion technique had increased bioluminescence following the procedure. Since the perfusate was saline and not intended for therapeutic purpose, the increase in intensity could be due to tumor growth, or that the perfusion disrupted small microtumors, leading to greater diffusion of the disease, as seen most clearly in the seventh mouse.
Fig. 7 –
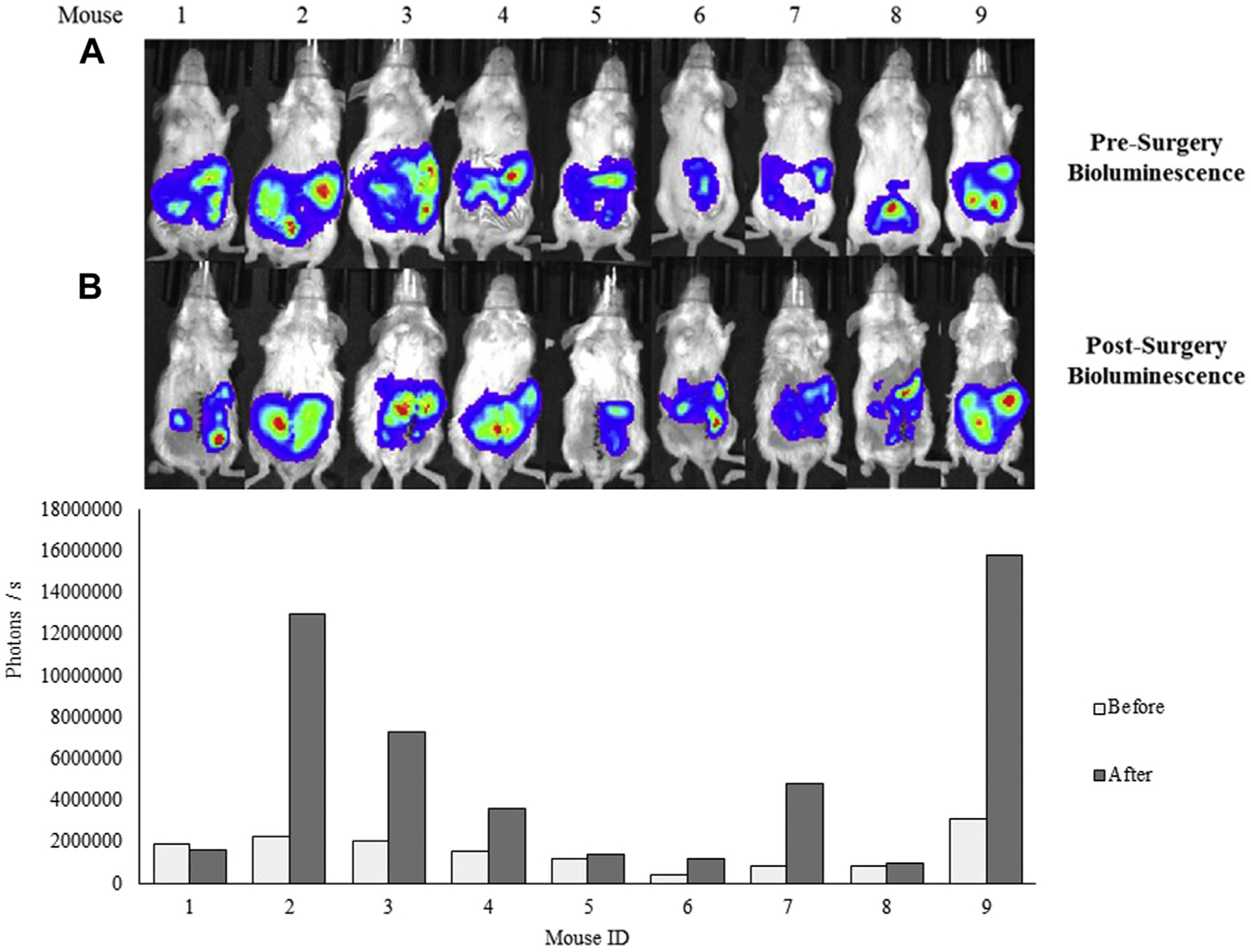
Bioluminescence signal presurgery and postsurgery of the nine mice that underwent the debulking surgery with the coliseum technique. (A) Bioluminescence signal from CT26 cell in live mice 2 h before and (B) 12–24 h following surgery imaged with IVIS. (Bottom) Quantified bioluminescence signal (photons/s) using the IVIS software. In some instances, mice presented with a stronger bioluminescence signal following surgery. Abbreviation: IVIS = in vivo imaging system.
Discussion and conclusions
Animal models are beneficial for the advancement of research regarding the treatment of PC. Many murine models for CRC studies have been developed and offer unique advantages for studying disease progression and treatments.29,30,38,40,49–51 Some of the challenges for developing a good model of PC from CRC is the selection of cell line and mouse strain. Although in theory it seems promising to use human CRC cells, these models require immune-deficient mice; hence, the involvement of the immune system in response to therapy is negated. The model present in this work uses immune competent BALB/c mice and the CT26 CRC cell line, which is derived from this strain of mouse and made to exhibit bioluminescence so that disease progression can be monitored. Francescutti et al.50 also used the animal model and cell line presented here to evaluate a model of peritoneal fluid instillation with whole animal orbital shaking. An alternative mouse strain is the C57Bl/6 mouse and MC38 CRC line, as described by Lehmann et al.38 Of significant note is that both immune-competent mouse models develop PC within a few days of tumor inoculation. Immune-suppressed models typically take a few weeks to develop tumors, depending on the cell line.29,30,37,40 An important detail to consider with this model design is that there is no primary tumor site. The goal of this model was to develop a murine perfusion model that could be used for future experiments aimed at treating late stage dissemination CRC with PC presentation, and many of the tumors produced ascites, a common hurdle in human CRC.52
We chose not to continue using the closed technique due to the inability to visualize the tumor burden directly, perfusate distribution, bubble formation in the circuit, and organ suction. The coliseum technique became our preferred model due to ease of access to the entire cavity, possibility of debulking of large tumors before perfusion, easier circuit blockage corrections, and the ability to better control the distribution of the perfusate. The closest mimic of the human HIPEC procedure would ideally involve surgical debulking, involving an open abdomen in the mouse, followed by closed abdominal perfusion for therapeutic solutions. However, this complete method has not yet been described in the literature, most likely due to the challenges of surgical debulking in the small mouse. The best option to mimic PC is the development of small, but well-dispersed tumors throughout the abdomen, which has been done in this work. Although closed perfusion is preferable in human HIPEC, for evaluation of new agents, especially agents that are designed to home in specifically to the microtumors, where optimal organ manipulation can be accomplished in a murine model, the coliseum perfusion model is superior. The setup could be improved from what has been presented in this work, as the use of a ring stand is cumbersome in the surgical field. An alternative technique for making a “bowl-shaped” abdomen was described by Ito et al.,36 where Lego building blocks were used as support structures for securing the sutures.
The aim of the present work was a technical description of a base procedure of IP perfusion in mice to allow additional modifications as required for a given project. As this was a first step toward determining perfusion parameters, and all equipment needed, no chemotherapeutic agents were tested. In humans, morbidity of HIPEC is attributed to both the hemodynamic stress of the perfusion and the toxicity of chemotherapeutic agents. In theory, addition of cytotoxic agents could compromise animal survival; however, previous reports of HIPEC in mice have demonstrated the use of commonly used human agents mitomycin, oxaliplatin, and cisplatin with excellent survival. Table 1 provides a summary of previous studies using rodent models of hyperthermic chemoperfusion.
Table 1 –
A list of previous published works, where hyperthermic chemotherapy has been perfused. The specific cancer type that was used is also included.
| Reference | PC origin | Agent | Heating technique | Target temperature(s) |
|---|---|---|---|---|
| Muenyi et al.29 | Ovarian | Cisplatin 3 mg/kg | Saline bag submerged in water | 42°C |
| Graziosi et al.30 | Gastric |
Mitomycin 8.25 μg/L Cisplatin 62.5 μg/L |
Tube coil Water tank |
40°C 43°C |
| Miailhe et al.40 | Ovarian | Oxaliplatin 920 mg/m2 | Saline bag submerged in water | 43°C |
| Trépanier et al.51 | No tumor | Oxaliplatin 460 mg/m2 Mitomycin C 35 mg/m2 |
Water tank | 43°C |
There are other parameters of this model, which can be modified according to the needs of the study. These include lengthening the circuit tubing, but for hyperthermic procedures, longer tubing can lead to heat loss, although this can be accounted for by modifying the heat source to account for the cooling that occurs. For evaluation of new agents, where the supply may be limited, having a shorter perfusion circuit that requires less volume, can be beneficial for keeping the agent concentrated and using the least amount of material as possible. In reviewing the current literature, there is a wide variation in the flow rates that are used, and this is a parameter which can easily be adjusted, if necessary, for increasing the number of circuit volume exchanges, or introducing greater shear flow to dissociate microtumors from host tissues. In our experience, however, adjustment of the flow rate must be carefully managed because higher rates create too much suction and the delicate organs of the mouse can be damaged. Excessive suction forces on the organs can be mediated somewhat by using a drain line with perforations along the tube length. This increases the total open area over which the perfusate flows and reduces the suction forces that occur if only the end of the tubing is used to remove the fluids.
A survival mouse model of abdominal perfusion is achievable and can be customized to either open or closed abdominal perfusion techniques. The methods described in this article can provide guidelines for developing future research that will involve abdominal perfusion in mice. Although the use of bioluminescence imaging is a well-accepted standard for determining the extent of disease, the results are still challenging to interpret. For example, the extent of disease determined by BLI may look quite extensive, but on evaluating the abdominal contents visually at the time of open procedure, there may be the presence of small nodules that are not easily identifiable individually via IVIS imaging.
In summary, this work has provided a comparison of using the open and closed abdominal perfusion techniques in an immune-competent mouse model of PC. The details for setting up a perfusion circuit that was safe (no significant suction on the animal organs) and utilized the minimal amount of tubing were described. We hope that the methods presented here are beneficial to researchers looking to evaluate new therapies that can be translated to HIPEC procedures in humans in the future.
Acknowledgment
This work was supported by NIH (R21-EB019748) and the Department of Plastic and Reconstructive Surgery at Wake Forest School of Medicine. We would also like to thank the Wake Forest’s Cell Viral Vector Core Laboratory for supplying tissue culture media and reagents and for the use of the in vivo imaging system. Pathology support was provided by the Tumor Tissue and Pathology Shared Resource of Wake Forest Baptist Comprehensive Cancer Center’s NCI Cancer Center Support Grant P30CA012197.
Footnotes
Disclosure
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
REFERENCES
- 1.Chua TC, Yan TD, Saxena A, Morris DL. Should the treatment of peritoneal carcinomatosis by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy still be regarded as a highly morbid procedure?: a systematic review of morbidity and mortality. Ann Surg. 2009;249:900–907. [DOI] [PubMed] [Google Scholar]
- 2.Sadeghi B, Arvieux C, Glehen O, et al. Peritoneal carcinomatosis from non-gynecologic malignancies. Cancer. 2000;88:358–363. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177–193. [DOI] [PubMed] [Google Scholar]
- 4.Colorectal cancer - cancer stat facts. Available at: https://seer.cancer.gov/statfacts/html/colorect.html. Accessed January 22, 2018.
- 5.Razenberg LGEM, Lemmens VEPP, Verwaal VJ, et al. Challenging the dogma of colorectal peritoneal metastases as an untreatable condition: results of a population-based study. Eur J Cancer Oxf Engl. 2016;65:113–120. [DOI] [PubMed] [Google Scholar]
- 6.Carmignani CP, Sugarbaker TA, Bromley CM, Sugarbaker PH. Intraperitoneal cancer dissemination: mechanisms of the patterns of spread. Cancer Metastasis Rev. 2003;22:465–472. [DOI] [PubMed] [Google Scholar]
- 7.Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995;221:29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montori G, Coccolini F, Ceresoli M, et al. The treatment of peritoneal carcinomatosis in advanced gastric cancer: state of the art. Int J Surg Oncol. 2014;2014:912418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flessner MF. Pharmacokinetic problems in peritoneal drug administration: an update after 20 years. Pleura Peritoneum. 2016;1:183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flessner MF. The transport barrier in intraperitoneal therapy. Am J Physiol Renal Physiol. 2005;288:F433–F442. [DOI] [PubMed] [Google Scholar]
- 11.Flessner MF. Peritoneal transport physiology: insights from basic research. J Am Soc Nephrol. 1991;2:122–135. [DOI] [PubMed] [Google Scholar]
- 12.Casper ES, Kelsen DP, Alcock NW, Lewis JJ. Ip cisplatin in patients with malignant ascites: pharmacokinetic evaluation and comparison with the iv route. Cancer Treat Rep. 1983;67:235–238. [PubMed] [Google Scholar]
- 13.Speyer JL, Collins JM, Dedrick RL, et al. Phase I and pharmacological studies of 5-fluorouracil administered intraperitoneally. Cancer Res. 1980;40:567–572. [PubMed] [Google Scholar]
- 14.Sugarbaker PH, Graves T, DeBruijn EA, et al. Early postoperative intraperitoneal chemotherapy as an adjuvant therapy to surgery for peritoneal carcinomatosis from gastrointestinal cancer: pharmacological studies. Cancer Res. 1990;50:5790–5794. [PubMed] [Google Scholar]
- 15.Sugarbaker PH, Cunliffe WJ, Belliveau J, et al. Rationale for integrating early postoperative intraperitoneal chemotherapy into the surgical treatment of gastrointestinal cancer. In: Proceedings of the 3rd International Congress on Neo-Adjuvant Chemotherapy. Paris: Springer; 1991:272–275. [Google Scholar]
- 16.Stephens AD, Alderman R, Chang D, et al. Morbidity and mortality analysis of 200 treatments with cytoreductive surgery and hyperthermic intraoperative intraperitoneal chemotherapy using the coliseum technique. Ann Surg Oncol. 1999;6:790–796. [DOI] [PubMed] [Google Scholar]
- 17.Turaga K, Levine E, Barone R, et al. Consensus guidelines from the American society of peritoneal surface malignancies on standardizing the delivery of hyperthermic intraperitoneal chemotherapy (HIPEC) in colorectal cancer patients in the United States. Ann Surg Oncol. 2014;21:1501–1505. [DOI] [PubMed] [Google Scholar]
- 18.Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737–3743. [DOI] [PubMed] [Google Scholar]
- 19.Sugarbaker PH. Laboratory and clinical basis for hyperthermia as a component of intracavitary chemotherapy. Int J Hyperthermia. 2007;23:431–442. [DOI] [PubMed] [Google Scholar]
- 20.Kusamura S, Dominique E, Baratti D, Younan R, Deraco M. Drugs, carrier solutions and temperature in hyperthermic intraperitoneal chemotherapy. J Surg Oncol. 2008;98:247–252. [DOI] [PubMed] [Google Scholar]
- 21.Ihemelandu CU, Shen P, Stewart JH, Votanopoulos K, Levine EA. Management of peritoneal carcinomatosis from colorectal cancer. Semin Oncol. 2011;38:568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glehen O, Cotte E, Kusamura S, et al. Hyperthermic intraperitoneal chemotherapy: nomenclature and modalities of perfusion. J Surg Oncol. 2008;98:242–246. [DOI] [PubMed] [Google Scholar]
- 23.Neuwirth MG, Alexander HR, Karakousis GC. Then and now: cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC), a historical perspective. J Gastrointest Oncol. 2016;7:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esquivel J Technology of hyperthermic intraperitoneal chemotherapy in the United States, Europe, China, Japan, and Korea. Cancer J. 2009;15:249–254. [DOI] [PubMed] [Google Scholar]
- 25.González-Moreno S, González-Bayón LA, Ortega-Pérez G. Hyperthermic intraperitoneal chemotherapy: rationale and technique. World J Gastrointest Oncol. 2010;2:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elias D, Antoun S, Goharin A, Otmany AE, Puizillout JM, Lasser P. Research on the best chemohyperthermia technique of treatment of peritoneal carcinomatosis after complete resection. Int J Surg Investig. 2000;1:431–439. [PubMed] [Google Scholar]
- 27.Thomas F, Ferron G, Gesson-Paute A, Hristova M, Lochon I, Chatelut E. Increased tissue diffusion of oxaliplatin during laparoscopically assisted versus open heated intraoperative intraperitoneal chemotherapy (HIPEC). Ann Surg Oncol. 2008;15:3623–3624. [DOI] [PubMed] [Google Scholar]
- 28.Gremonprez F, Willaert W, Ceelen W. Intraperitoneal chemotherapy (IPC) for peritoneal carcinomatosis: review of animal models. J Surg Oncol. 2014;109:110–116. [DOI] [PubMed] [Google Scholar]
- 29.Muenyi CS, States VA, Masters JH, Fan TW, Helm CW, States JC. Sodium arsenite and hyperthermia modulate cisplatin-DNA damage responses and enhance platinum accumulation in murine metastatic ovarian cancer xenograft after hyperthermic intraperitoneal chemotherapy (HIPEC). J Ovarian Res. 2011;4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graziosi L, Mencarelli A, Renga B, et al. Gene expression changes induced by HIPEC in a murine model of gastric cancer. In Vivo. 2012;26:39–45. [PubMed] [Google Scholar]
- 31.Aarts F, Hendriks T, Boerman OC, Koppe MJ, Oyen WJG, Bleichrodt RP. A comparison between radioimmunotherapy and hyperthermic intraperitoneal chemotherapy for the treatment of peritoneal carcinomatosis of colonic origin in rats. Ann Surg Oncol. 2007;14:3274–3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pelz JO, Doerfer J, Hohenberger W, Meyer T. A new survival model for hyperthermic intraperitoneal chemotherapy (HIPEC) in tumor-bearing rats in the treatment of peritoneal carcinomatosis. BMC Cancer. 2005;5:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terracina KP, Aoyagi T, Huang W-C, et al. Development of a metastatic murine colon cancer model. J Surg Res. 2015;199:106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levi-Polyachenko NH, Merkel EJ, Jones BT, Carroll DL, Stewart JH. Rapid photothermal intracellular drug delivery using multiwalled carbon nanotubes. Mol Pharm. 2009;6:1092–1099. [DOI] [PubMed] [Google Scholar]
- 35.Levi-Polyachenko NH, Stewart IVJH. Clinical relevance of nanoparticle induced hyperthermia for drug delivery and treatment of abdominal cancers. Survival. 2011;1:1E–3E. [Google Scholar]
- 36.Ito F, Camoriano M, Seshadri M, Evans SS, Kane JM, Skitzki JJ. Water: a simple solution for tumor spillage. Ann Surg Oncol. 2011;18:2357–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Razavi R, Harrison LE. Thermal sensitization using induced oxidative stress decreases tumor growth in an in vivo model of hyperthermic intraperitoneal perfusion. Ann Surg Oncol. 2010;17:304–311. [DOI] [PubMed] [Google Scholar]
- 38.Lehmann K, Rickenbacher A, Jang J-H, et al. New insight into hyperthermic intraperitoneal chemotherapy: induction of oxidative stress dramatically enhanced tumor killing in in vitro and in vivo models. Ann Surg. 2012;256:730–738. [DOI] [PubMed] [Google Scholar]
- 39.Leach MC, Klaus K, Miller AL, Di Perrotolo MS, Sotocinal SG, Flecknell PA. The assessment of post-vasectomy pain in mice using behaviour and the Mouse Grimace Scale. PLoS One. 2012;7:e35656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miailhe G, Arfi A, Mirshahi M, Eveno C, Pocard M, Touboul C. A new animal model for hyperthermic intraperitoneal chemotherapy (HIPEC) in tumor-bearing mice in the treatment of peritoneal carcinomatosis of ovarian origin. J Visc Surg. 2018;155:183–189. [DOI] [PubMed] [Google Scholar]
- 41.Wu H-T, Peng K-W, Ji Z-H, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy with lobaplatin and docetaxel to treat synchronous peritoneal carcinomatosis from gastric cancer: results from a Chinese center. Eur J Surg Oncol. 2016;42:1024–1034. [DOI] [PubMed] [Google Scholar]
- 42.Ceelen W, Somer FD, Nieuwenhove YV, Putte DV, Pattyn P. Effect of perfusion temperature on glucose and electrolyte transport during hyperthermic intraperitoneal chemoperfusion (HIPEC) with oxaliplatin. Eur J Surg Oncol. 2013;39:754–759. [DOI] [PubMed] [Google Scholar]
- 43.Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7:27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klaver CEL, Musters GD, Bemelman WA, et al. Adjuvant hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with colon cancer at high risk of peritoneal carcinomatosis; the COLOPEC randomized multicentre trial. BMC Cancer. 2015;15:428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Avital S, Itah R, Szomstein S, et al. Correlation of CO2 pneumoperitoneal pressures between rodents and humans. Surg Endosc. 2009;23:50–54. [DOI] [PubMed] [Google Scholar]
- 46.Gould SJ, Subramani S. Firefly luciferase as a tool in molecular and cell biology. Anal Biochem. 1988;175:5–13. [DOI] [PubMed] [Google Scholar]
- 47.Dothager RS, Flentie K, Moss B, Pan M-H, Kesarwala A, Piwnica-Worms D. Advances in bioluminescence imaging of live animal models. Curr Opin Biotechnol. 2009;20:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smakman N, Martens A, Kranenburg O, Rinkes IHB. Validation of bioluminescence imaging of colorectal liver metastases in the mouse. J Surg Res. 2004;122:225–230. [DOI] [PubMed] [Google Scholar]
- 49.Karim BO, Huso DL. Mouse models for colorectal cancer. Am J Cancer Res. 2013;3:240–250. [PMC free article] [PubMed] [Google Scholar]
- 50.Francescutti V, Rivera L, Seshadri M, et al. The benefit of intraperitoneal chemotherapy for the treatment of colorectal carcinomatosis. Oncol Rep. 2013;30:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trépanier J-S, Sidéris L, Lee L, Tremblay J-F, Drolet P, Dubé P. Impact of electrocautery and hyperthermic intraperitoneal chemotherapy on intestinal microvasculature in a murine model. Int J Hyperthermia. 2016;32:483–487. [DOI] [PubMed] [Google Scholar]
- 52.Adam RA, Adam YG. Malignant ascites: past, present, and future. J Am Coll Surg. 2004;198:999–1011. [DOI] [PubMed] [Google Scholar]


