Abstract
Among solid organ transplant recipients, donor cytomegalovirus (CMV) seropositive (D+) and recipient seronegative (R−) status are associated with an increased risk of graft loss and mortality after kidney or lung transplantation. Whether a similar relationship exists among liver transplant recipients (LTR) is unknown. We assessed graft loss and mortality among adult LTRs from January 1, 2010, to March 14, 2020, in the Organ Procurement and Transplantation Network database. We used multivariable mixed Cox proportional hazards regression to analyze the association of donor and recipient CMV serostatus group with graft loss and mortality, with donor seronegative (D−) and recipient seronegative (R−) as the reference group. Among 54,078 LTRs, the proportion of D−R−, D− and recipient seropositive (R+), D+R−, and D+R+ was 13.4%, 22.5%, 22%, and 42%, respectively. By unadjusted Kaplan-Meier survival curve estimates, survival by the end of follow-up was 73.3%, 73.5%, 70.1%, and 69.7%, among the D−R−, D−R+, D+R−, and D+R+ groups, respectively. By multivariable Cox regression, the CMV D+R− serogroup, but not other serogroups, was independently associated with increased risks of graft loss (adjusted hazard ratio [aHR], 1.13; 95% confidence interval [CI], 1.05–1.22) and mortality (aHR, 1.13; 95% CI, 1.05–1.22). The magnitude of the association of the CMV D+R− serostatus group with mortality was similar when the Cox regression analysis was restricted to the first year after transplant and beyond the first year after transplant: aHR, 1.13 (95% CI, 1.01–1.27) and aHR, 1.13 (95% CI, 1.02–1.25), respectively. Even in an era of CMV preventive strategies, CMV D+R− serogroup status remains independently associated with increased graft loss and mortality in adult LTRs. Factors in addition to direct CMV-associated short-term mortality are likely, and studies to define the underlying mechanism(s) are warranted.
Cytomegalovirus (CMV) disease has been associated with significant morbidity and mortality in solid organ transplant recipients.(1–3) CMV seronegative recipients (R−) of an organ transplant from CMV seropositive donors (D+) are at highest risk for developing CMV infection and disease. The underlying mechanism is the impaired ability of CMV R− recipients, who are immunologically naïve to CMV, to develop an effective immune response to primary CMV infection transmitted from the D+ in the context of immunosuppression. This is in contrast to CMV seropositive recipients (R+), who already have some degree of preexisting immunity from prior CMV infection before transplantation and are therefore at lower risk of developing CMV infection and disease compared with the D+R− subgroup.
Despite widespread implementation of CMV preventive strategies, CMV disease continues to occur, most commonly within the first year following transplant.(2,3) CMV disease directly causes morbidity and mortality.(1,4,5) In addition to these direct short-term effects of CMV disease, CMV has also been implicated in a broad range of indirect (ie, not directly related to overt CMV disease) biological effects that could contribute to worse clinical outcomes. These indirect effects include increased systemic inflammation that could increase the risk for cardiovascular and thrombotic complications,(6,7) allograft inflammation that could lead to allograft injury,(8) and systemic immunosuppression that could predispose to secondary infection.(6,9,10) These biological effects of CMV can be mediated even during CMV latency or subclinical CMV reactivation (ie, independent of clinically recognized CMV disease) and occur more frequently in D+R− patients.(6,10) Thus, it has been hypothesized that both the direct and indirect effects of CMV would disproportionately impact D+R− patients and lead to worse overall outcomes in this specific group of transplant recipients, as previously demonstrated among kidney and lung transplant recipients.(11,12)
There are important differences among organ transplant populations, and whether a similar relationship between donor and recipient CMV serostatus and outcomes exists in liver transplant recipients (LTR) is unknown. Thus, the aim of this study was to analyze the association of donor and recipient CMV serostatus with graft and patient survival using the Organ Procurement and Transplantation Network (OPTN) database in adult LTRs.
Patients and Methods
STUDY POPULATION AND DATA SOURCE
We conducted a retrospective analysis of all US recipients undergoing liver transplantation (LT) from January 1, 2010, to March 14, 2020, who had complete graft and patient survival data in the OPTN database. This time period was selected to encompass the current era of transplant practices including immunosuppression and CMV preventive strategies. Recipients were excluded if they were younger than 18 years old at the time of transplant, were missing recipient and/or donor CMV serostatus, had a prior history of LT, received simultaneous organ transplants, or received a living donor transplant. Patients were categorized into the following 4 CMV serogroups based on the recipient and donor CMV serostatus: donor seronegative (D−) and R−, D−R+, D+R−, and D+R+. The D−R− group was the reference group for all analyses.
The United Network for Organ Sharing (UNOS), as the contractor for OPTN, supplied these data. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the OPTN or the US government. The University of Washington Human Subjects Division deemed the OPTN database as de-identified and publicly available and thus not human subject data. Therefore, this study was exempt from human subject review. The OPTN released these data on April 1, 2020.
BASELINE RECIPIENT, DONOR, AND TRANSPLANT CHARACTERISTICS
Baseline donor, recipient, and transplant characteristics were defined as those collected at the time of transplant. In addition to these variables, we also used donor and recipient height and weight to calculate the body surface area. The donor’s body surface area was divided by the recipient’s body surface area to calculate the donor-to-recipient body surface area ratio used in the final analysis.
OUTCOME MEASURES
We evaluated the association between CMV donor and recipient serostatus group with graft loss and all-cause mortality. Graft loss is defined in the OPTN database as either death or retransplant before the last OPTN follow-up date. Date of death or retransplant date was used as the graft loss date. Any subsequent death or retransplant after this initial graft loss date is not included in the analyses.
STATISTICAL ANALYSIS
Continuous variables are given as mean ± standard deviation (SD) or median and interquartile range (IQR), and categorical variables are presented as count and percentages. Kaplan-Meier survival curve analysis was used to determine the graft and patient survival of the donor and recipient CMV groups. The log-rank test was used to compare survival curves. We used univariable and multivariable mixed Cox proportional hazards models to determine the association between donor and recipient CMV serostatus group with graft loss or death. As the goal of the multivariable analysis was to assess whether CMV serostatus group was independently associated with graft loss and/or recipient survival, we included in our multivariable model potential confounders including recipient, donor, and transplant variables previously described to be associated with graft loss or mortality. These variables include those used to calculate the donor risk index and also donor-to-recipient body surface area ratio, which has been previously associated with long-term graft survival.(13–19) In the mixed Cox model, we also used the de-identified center code and the year of LT as random effect variables to address any differences in management by donor and recipient CMV serostatus by center or transplant year.
The donor, recipient, and transplant factors used as covariates are described in Supporting Table 1. To minimize the risk of bias by inclusion of a large number of covariates, we assessed for collinearity between CMV donor and recipient serostatus group and other covariates and also stratified analyses to assess for potential effect modifications. Follow-up time started at the time of LT and extended until time of death or last follow-up date as reported to UNOS, whichever date was earlier. In our primary analyses, follow-up occurred until death or last follow-up as reported to UNOS, whichever date was earlier. In the primary analyses we compared survival by CMV donor and recipient serostatus groups during the entire duration of follow-up.
We also performed 2 separate sensitivity analyses. In the first analysis, we analyzed associations between CMV serostatus group and graft loss or mortality and up to the first year after transplant. The entire study population was included in this sensitivity analysis. In the second sensitivity analysis, we performed a subgroup analysis: we analyzed these associations only for patients who had a minimum 1 year of follow-up time after transplant (ie, patients who died or were lost to follow-up in the first year were excluded from this sensitivity analysis). The baseline characteristics, stratified by follow-up time (≤1 compared with >1 year) are shown in Supporting Table 2. The rationale for these sensitivity analyses was to explore whether the association of CMV D+R− status differed between the first year after transplant, during which CMV disease is most likely to occur, and the subsequent posttransplant time period beyond the first year, when CMV disease is uncommon. Although recipient cause of death (COD) is included in the OPTN database, we excluded the analysis of this outcome for the following reasons: a high proportion of missing values, the lack of independent verification of this outcome, and known challenges in accurately establishing COD.
Results were considered statistically significant if the P value was less than 0.05. All analyses were done with R version 4.0.0 (R Foundation for Statistical Computing, Vienna, Austria). The mixed Cox proportional hazards model was performed using the coxme 2.2–16 package in R.
Results
BASELINE CHARACTERISTICS OF THE RECIPIENT AND DONOR POPULATION
Among 54,078 adult LTRs, 7251 (13.4%) were D−R−; 12,194 (22.5%) were D−R+; 11,903 (22%) were D+R−; and 22,730 (42%) were D+R+ (Table 1). There were important differences in recipient and donor characteristics according to CMV donor/recipient serostatus groups: in the D−R− and D+R− CMV groups, compared with the D−R+ and D+R+ groups, recipients were younger, more likely to be male and Caucasian, and less likely to have viral hepatitis as the etiology of their liver disease (P < 0.001; Table 1). Among the donors, in the CMV D−R− and D−R+ groups, compared with the D+R− and D+R+ groups, donors were younger and had higher rates of donation after circulatory death (DCD; 9% in D−R− and 8.9% in D−R+ versus 5.7% in D+R− and 5.6% in D+R+; P < 0.001; Table 2).
TABLE 1.
LTR Characteristics by CMV Donor and Recipient Serostatus
| Recipient Baseline Characteristic | CMV Donor and Recipient Serostatus at Time of Transplant |
||||
|---|---|---|---|---|---|
| D−R− |
D−R+ |
D+R− |
D+R+ |
P Value | |
| n = 7251 | n = 12,194 | n = 11,903 | n = 22,730 | ||
| Age, years | 57 (50–64) | 58 (51–63) | 57 (50–62) | 58 (51–64) | <0.001 |
| Male | 76.1 | 63 | 73.9 | 61.6 | <0.001 |
| Race/ethnicity | <0.001 | ||||
| Caucasian | 86.3 | 64.6 | 84.5 | 63.1 | |
| Asian | 1 | 6 | 1 | 6.2 | |
| Black, non-Hispanic | 4.8 | 10.6 | 5.3 | 10.5 | |
| Hispanic | 6.9 | 17.1 | 8.1 | 18.3 | |
| Other | 1 | 1.9 | 1.1 | 1.9 | |
| Reason for LT | <0.001 | ||||
| Acute liver failure | 2.7 | 3.7 | 3.3 | 3.9 | |
| Autoimmune hepatitis | 2.2 | 2.8 | 2 | 2.7 | |
| Malignancy | 26.1 | 29.4 | 26.8 | 29.3 | |
| Cholestatic liver disease | 9.1 | 7.2 | 8 | 6.5 | |
| Cryptogenic | 3.9 | 4.2 | 4 | 4.1 | |
| Alcohol | 21.6 | 16 | 21.7 | 16 | |
| Metabolic | 3.1 | 2.5 | 3.2 | 2.4 | |
| NASH | 13.2 | 11.1 | 12.9 | 11.8 | |
| Viral | 16.9 | 22.1 | 16.5 | 22.4 | |
| Other | 1.1 | 1 | 1.5 | 0.9 | |
| Concurrent HCC | 9.4 | 9.3 | 8.2 | 9.3 | 0.004 |
| Recipient diabetes mellitus | 25.9 | 27.3 | 26.5 | 27.8 | 0.004 |
| Location at time of graft offer | <0.001 | ||||
| Home | 70.6 | 66.1 | 69.8 | 66.9 | |
| Hospital | 18.8 | 19.4 | 18.8 | 18.3 | |
| ICU | 10.6 | 14.6 | 11.5 | 14.8 | |
| Status 1 | 2.3 | 2.8 | 2.6 | 3 | 0.01 |
| On life support | 6 | 8.2 | 6.2 | 8.8 | <0.001 |
| Allocation MELD score | 22.1 ± 10.7 | 22.7 ± 11.3 | 22.2 ± 10.6 | 22.6 ± 11.2 | 0.002 |
| Albumin, g/dL | 3.2 ± 0.7 | 3.2 ± 0.7 | 3.1 ± 0.7 | 3.1 ± 0.7 | 0.10 |
| Encephalopathy | 0.03 | ||||
| None | 39 | 37 | 38 | 37 | |
| Grade 1–2 | 50.3 | 50.9 | 50.3 | 51 | |
| Grade 3–4 | 10.8 | 12 | 11.9 | 11.9 | |
| Ascites | 0.4 | ||||
| Absent | 26.7 | 26.6 | 26.6 | 26.5 | |
| Slight | 43 | 43 | 42.7 | 43.7 | |
| Moderate | 30.4 | 30.4 | 30.8 | 29.8 | |
| Dialysis in the week preceding LT | 8.7 | 11.2 | 9.2 | 11.3 | <0.001 |
| PVT | 14 | 15.3 | 14.7 | 14.7 | 0.13 |
| History of abdominal surgery | 49.4 | 52.3 | 49.2 | 52.3 | <0.001 |
| History of TIPS | 10.3 | 10.5 | 11.7 | 10.8 | 0.004 |
| HBV sAg positive | 2.1 | 5.3 | 2.1 | 5.5 | <0.001 |
| HCV-antibody positive | 29.6 | 36.4 | 28.8 | 36.3 | <0.001 |
| EBV donor and recipient status | <0.001 | ||||
| D−R− | 0.9 | 0.6 | 0.4 | 0.3 | |
| D−R+ | 5.6 | 5.7 | 2.3 | 2.3 | |
| D+R− | 10.8 | 6.7 | 10.8 | 6.7 | |
| D+R+ | 65.8 | 66.6 | 65.9 | 65.2 | |
| Unknown | 16.9 | 20.4 | 20.6 | 25.5 | |
NOTE: Data are expressed as proportion, median (IQR), or mean ± SD.
TABLE 2.
Donor and Transplant Characteristics by CMV Donor and Recipient Serostatus Group
| Donor Baseline Characteristic | CMV Donor and Recipient Serostatus at Time of Transplant | ||||
|---|---|---|---|---|---|
| D−R− |
D−R+ |
D+R− |
D+R+ |
||
| n = 7251 | n = 12,194 | n = 11,903 | n = 22,730 | P Value | |
| Age, years | 40 (27–53) | 38 (25–52) | 45 (30–56) | 44 (29–56) | <0.001 |
| Male | 68.1 | 66.7 | 56.8 | 54.9 | <0.001 |
| Race/ethnicity | <0.001 | ||||
| Caucasian | 79.9 | 79.1 | 58.6 | 55.4 | |
| Asian | 0.8 | 1.1 | 2.8 | 3.5 | |
| Black, non-Hispanic | 11.5 | 10.9 | 22.2 | 21.8 | |
| Hispanic | 6.5 | 7.4 | 14.3 | 17.1 | |
| Other | 1.4 | 1.6 | 2.2 | 2.2 | |
| Whole donor graft | 98.4 | 98.3 | 99.1 | 98.8 | <0.001 |
| DCD liver donation | 9 | 8.9 | 5.7 | 5.6 | <0.001 |
| Donor laboratory results | |||||
| AST, units/L | 43 (25–83) | 43 (25–82) | 43 (25–83) | 43 (25–85) | 0.6 |
| ALT, units/L | 38 (22–79) | 38 (22–79) | 36 (21–72) | 35 (21–70) | <0.001 |
| Total bilirubin, mg/dL | 0.6 (0.4–1) | 0.6 (0.4–1) | 0.7 (0.4–1.1) | 0.7 (0.4–1.1) | <0.001 |
| COD | <0.001 | ||||
| Anoxia | 42.7 | 41.3 | 32.8 | 30 | |
| CVA | 29.4 | 29.2 | 35 | 35.8 | |
| Head trauma | 25.4 | 26.7 | 30 | 31.9 | |
| Other | 2.6 | 2.9 | 2.2 | 2.3 | |
| Donor diabetes mellitus | 10.9 | 9.7 | 14.5 | 14.1 | <0.001 |
| UNOS sharing area | <0.001 | ||||
| Local | 70.3 | 68 | 69.6 | 68.7 | |
| Regional | 25.3 | 28.2 | 25.1 | 27 | |
| National | 4.3 | 3.8 | 5.3 | 4.3 | |
| Donor hypertension | 32.9 | 30.8 | 40.4 | 39.6 | <0.001 |
| Donor blood infection | 5.9 | 11% | 11% | 11% | 0.9 |
| Donor HCV-antibody positive | 5.9 | 6.4 | 6.8 | 6.3 | 0.08 |
| CIT, hours | 6.1 ± 2.3 | 6.2 ± 2.5 | 6.1 ± 2.4 | 6.2 ± 2.5 | 0.047 |
| ABO blood group match | <0.001 | ||||
| Compatible | 5.1 | 5.6 | 5.3 | 5.8 | |
| Identical | 93.5 | 93.1 | 93.7 | 93.2 | |
| Incomplete | 1.4 | 1.3 | 1 | 1 | |
NOTE: Data are expressed as proportion, median (IQR), or mean ± SD.
ASSOCIATION BETWEEN CMV DONOR AND RECIPIENT SEROSTATUS GROUPS AND GRAFT LOSS
During a mean follow-up of 3.3 ± 2.7 years after transplant, 9703 (17.9%) recipients had graft loss (Table 3). Overall unadjusted graft survival by the end of the study period was 71.6% for D−R−, 71.4% for D−R+, 68.2% for D+R−, and 67.8% for D+R+ (Fig. 1). The observed cumulative incidence rate for graft loss, per 100 person-years, was highest in D+R− (5.98 per 100 person-years [P-Ys]) and D+R+ (5.91 per 100 P-Ys) and lower in D−R+ (5.49 per 100 P-Ys) and D−R− (5.19 per 100 P-Ys). On univariable analysis, both D+R− serogroup status (hazard ratio [HR], 1.15; 95% confidence interval [CI], 1.07–1.24) and D+R+ serostatus (HR, 1.15; 95% CI, 1.08–1.23) compared with the reference group D−R− were significantly associated with graft loss. However, on multivariable analyses, only CMV D+R− (adjusted hazard ratio [aHR], 1.13; 95% CI, 1.05–1.22), but not D+R+ serostatus (aHR, 1.07; 95% CI, 0.99–1.15), remained significantly associated with graft loss.
TABLE 3.
Association of Donor and Recipient CMV Serostatus Group With Overall Graft Loss and Mortality
| Donor/Recipient CMV Status | Number of Patients | P-Y | Number with Outcome, % | Incidence per 100 P-Ys | HR | aHR* |
|---|---|---|---|---|---|---|
| Overall graft loss | ||||||
| D−R− | 7251 | 23,109 | 1200 | 5.19 | 1 | 1 |
| D−R+ | 12,194 | 40,966 | 2251 | 5.49 | 1.07 (0.99–1.15) | 1.00 (0.93–1.08) |
| D+R− | 11,903 | 37,731 | 2257 | 5.98 | 1.15 (1.07–1.24) | 1.13 (1.05–1.22) |
| D+R+ | 24,100 | 76,817 | 4540 | 5.91 | 1.15 (1.08–1.23) | 1.07 (0.99–1.15) |
| Overall recipient mortality | ||||||
| D−R− | 7251 | 23,141 | 1058 | 4.57 | 1 | 1 |
| D−R+ | 12,194 | 40,997 | 1970 | 4.80 | 1.06 (0.99–1.15) | 1.00 (0.93–1.08) |
| D+R− | 11,903 | 37,776 | 1981 | 5.24 | 1.15 (1.07–1.24) | 1.13 (1.05–1.22 ) |
| D+R+ | 24,100 | 76,904 | 4,030 | 5.24 | 1.16 (1.08–1.24) | 1.07 (0.99–1.15) |
Adjusted for recipient sociodemographic characteristics (age, sex, race/ethnicity), etiology of liver disease, HCC, recipient diabetes mellitus, recipient location at time of LT, recipient life support status, ascites, hepatic encephalopathy, status 1, allocation MELD/MELD-Na score, recipient albumin, recipient receipt of dialysis in the week preceding transplant, recipient HBV sAg status, recipient HCV antibody status, PVT, TIPS, prior abdominal surgery, donor sociodemographic characteristics (age, sex, race/ethnicity), donor-to-recipient body surface area ratio, donor AST, donor ALT, donor blood infection, CIT, geographic allocation status, donor HBV sAg status, donor HCV antibody status, donor COD, type of graft (whole versus partial), donor hypertension, donor diabetes mellitus, EBV donor and recipient serostatus, and ABO donor/recipient match.
FIG. 1.
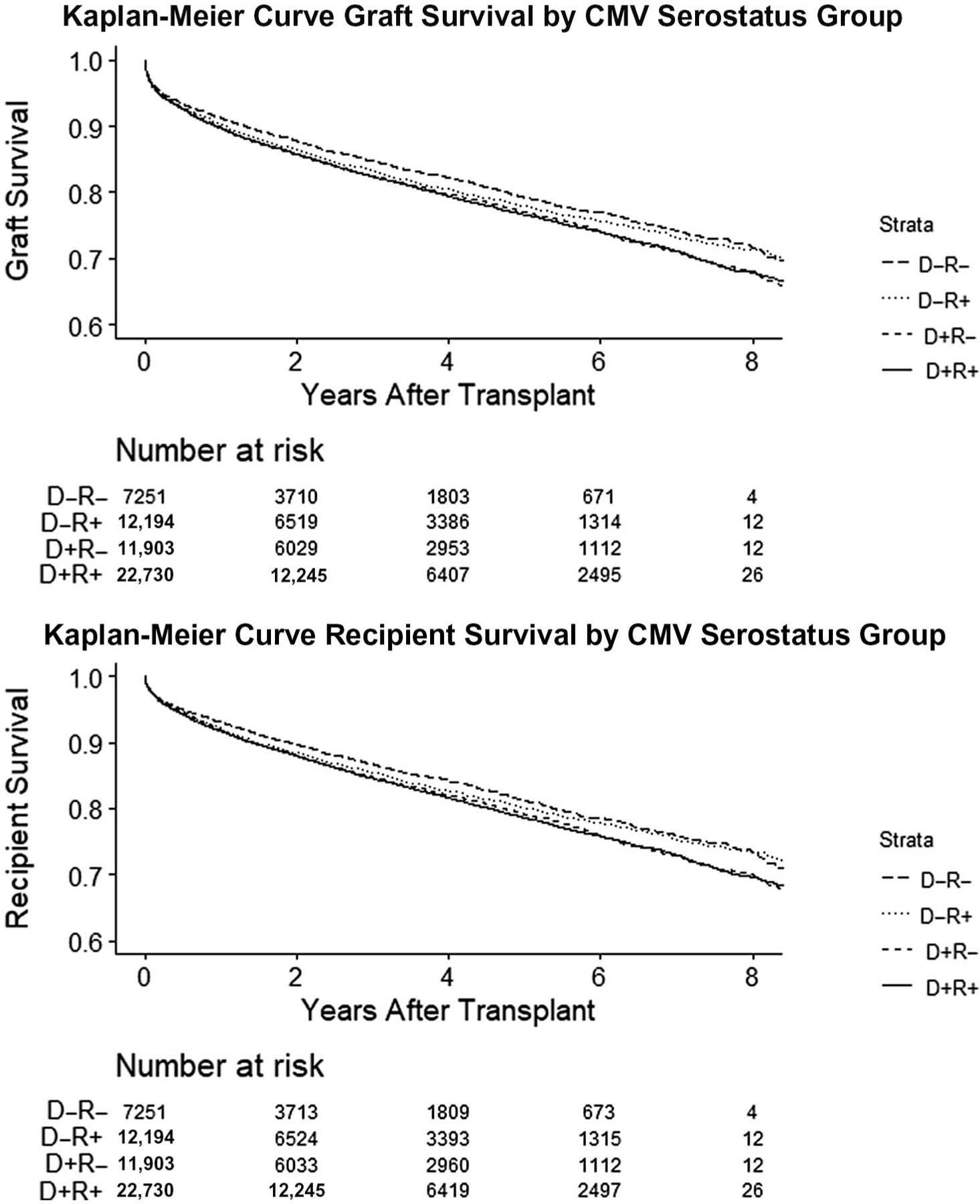
Unadjusted Kaplan-Meier curve graft or recipient survival stratified by CMV donor and recipient serostatus group. Graft survival was highest in the D−R− (71.6%) group, followed by the D−R+ (71.4%), D+R− (68.2%), and D+R+ (67.8%, P < 0.001) groups. Patient survival was highest in the D−R+ (73.5%) group, followed by the D−R− (73.3%), D+R− (70.1%), and D+R+ (69.7%, P < 0.001) groups.
On sensitivity analyses, the magnitude of the association of CMV D+R− serogroup with graft loss was similar when multivariable regression analysis was restricted to those with follow-up beyond the first year after transplant: aHR, 1.13 (95% CI, 1.02–1.25) for the analysis within 1 year after transplant. The magnitude of the association of CMV D+R− serogroup with graft loss was also similar for the analysis restricted within 1 year post-transplant, however this association was just below the threshold for statistical significance (aHR, 1.09; 95% CI, 0.99–1.21).
ASSOCIATION BETWEEN DONOR AND RECIPIENT CMV SEROSTATUS GROUP AND MORTALITY
During a mean follow-up of 3.31 ± 2.7 years after LT, 9039 (16.7%) of patients had died (Table 3). In unadjusted Kaplan-Meier survival curve estimates, survival by the end of the study period was 73.3% for D−R−, 73.5% for D−R+, 70.1% for D+R−, and 69.7% for D+R+ (Fig. 1). The crude mortality rate was highest in the D+R+ cohort (5.24 per 100 P-Ys) and D+R− (5.24 per 100 P-Ys) versus D−R+ (4.8 per 100 P-Ys) versus D−R− (4.57 per 100 P-Ys). On unadjusted Cox regression analysis, with CMV D−R− as the reference, the D+R− (HR, 1.15; 95% CI, 1.07–1.24 [versus D−R−]) and D+R+ serostatus groups (HR, 1.16; 95% CI, 1.08–1.24) were both associated with an increased risk of mortality. However, on multivariable regression analysis, only CMV D+R− status (aHR, 1.13; 95% CI, 1.05–1.22) remained significantly associated with mortality. D−R+ CMV serostatus was not associated with increased mortality risk on either univariable or multivariable analysis. We also explored, before constructing these regression models, potential interactions and collinearity between CMV serostatus groups and other covariates in our final multivariable regression model including recipient age, etiology of liver disease, and recipient comorbidities; no effect modification of other covariates on the association between CMV serostatus group and risk of graft loss or mortality was found. We did not find evidence for collinearity between CMV donor and recipient serostatus and any other variables.
On sensitivity analyses, the magnitude of the association of CMV D+R− serogroup with mortality was similar when the Cox regression analysis was restricted to the first year after transplant and beyond the first year after transplant: aHR, 1.13 (95% CI, 1.01–1.27) and aHR, 1.13 (95% CI, 1.02–1.25), respectively.
Discussion
Using rigorous statistical approaches to adjust for potential confounders and data from a large national transplant database with well-validated outcomes, we demonstrated that CMV D+R− serogroup status was independently associated with an increased risk of graft loss and mortality among adult LTRs, even in an era of CMV preventive strategies. The magnitude of the association between CMV D+R− status and patient survival also did not differ between the first year (the time period when the majority of CMV disease occurs) and the subsequent time period after transplant, suggesting that factors beyond short-term CMV disease-associated mortality (eg, “indirect effects of CMV”) are likely to be important in mediating this association. These results confirm and extend the previous demonstration of an independent association of CMV D+R− serostatus with graft and patient survival in kidney and lung transplant recipients to LTRs and have implications for future CMV prevention strategies.
The strength and independence of the association of CMV D+R− serostatus with graft loss and mortality in this study, combined with similar findings in kidney and lung transplant recipient populations, and the presence of biologically plausible mechanisms (direct CMV-associated mortality and indirect effects of CMV), support a possible causal association. However, as for all observational studies, causality cannot be definitively concluded. Leeaphorn and colleagues(12) created a “paired kidney cohort” using OPTN data and assessed outcomes among CMV serostatus discordant recipients from a single donor (ie, donor seropositive, 1 recipient seronegative/1 recipient seropositive). In their analysis of 9134 kidney transplant recipients, CMV D+R−, compared with D+R+ serostatus, had a 21% higher risk of all-cause mortality and 47% higher risk of infection-associated mortality.(12) This was despite a more favorable comorbidity profile in CMV D+R− compared with D+R+ serostatus, including a lower rate of diabetes mellitus and a higher proportion of preemptive transplants. A similar association between CMV D+R− serostatus and mortality has also been described in adult LTRs: in the annual report from the International Thoracic Organ Transplant Registry, CMV D+R− was independently associated with a higher risk of 5-year mortality (aHR, 1.23; 95% CI, 1.15–1.31).(11) Collectively, these studies demonstrate a consistent body of evidence linking D+R− status with an increased risk for worse long-term graft and patient outcomes across several solid organ transplant populations.
The mechanism(s) underlying the observed association of CMV D+R− status is/are uncertain. In the current era of CMV preventive strategies, CMV disease occurs in ~10% to 30% of D+R− LTRs, and the majority of CMV disease occurs within the first transplant year.(2,4) The short-term mortality rate for those who develop CMV disease is <10%.(2,20,21) In addition, in this current study, the magnitude of the association between CMV D+R− serostatus and graft and patient survival was similar in the analysis restricted to the first year after transplant and for the analysis of those who had follow-up beyond 1 year after transplant. If the association had been driven primarily by direct CMV disease-associated short-term mortality, the magnitude of the mortality association for those with follow-up beyond 1 year would have been anticipated to be lower than that for the analysis restricted to the first posttransplant year (the period when CMV disease was most likely). Both of these lines of evidence suggest that direct CMV-associated mortality is unlikely to be the only mechanism underlying the observed association of CMV D+R− serostatus with worse graft and patient survival. Instead, these results are compatible with the hypothesis that the association is mediated by a combination of direct CMV disease-associated short-term mortality and CMV-associated indirect biological effects, including increased risk of thrombotic events, more severe hepatitis C virus (HCV) recurrence, and graft rejection.(2,6,7,10,12,22,23) Unfortunately, the accuracy and level of detail in the OPTN database preclude such assessments. Future studies to define the mechanism(s) through which CMV D+R− serostatus increases the risk for graft loss and death are warranted. Given the known significantly increased risk for CMV-associated direct and indirect effects conferred by D+R− serostatus, these findings raise the testable hypothesis that improved prevention or control of CMV infection and disease would lead to improved graft and patient survival.
The strengths of the current study include the use of a large database with well-validated and clinically relevant endpoints, a well-defined prestudy hypothesis based on data from other solid organ transplant populations, and rigorous analytic approaches to control for potential confounding. Specifically, we used multivariable modeling to assess whether CMV D+R− serostatus group was independently associated with graft and mortality loss, even after adjusting for differences and potential confounding of these baseline characteristics. We also acknowledge potential limitations. The results of this study do not demonstrate causality but raise a specific and potentially testable hypothesis: better prevention of CMV infection/disease in the D+R− group leads to improved long-term graft and patient survival in LTRs. This is relevant with the advent of new strategies such as CMV vaccines and other immune-based prevention and treatment strategies that hold significant promise for improved control of CMV infection and disease.(24,25) The OPTN registry data do not include details on CMV prevention methods or CMV disease events, so we specifically chose a study period, between 2010 and 2020, during which all major transplant guidelines recommended CMV prevention and treatment guidelines and were therefore in widespread clinical use. The OPTN data are also not granular with respect to cause of recipient death and had a high proportion of missing data. Thus, we were unable to perform a robust analysis of the association between CMV serostatus and specific cause(s) of death.
In summary, we demonstrated a robust and independent association of CMV D+R− serostatus with increased graft loss and mortality in LTRs that is unlikely to be explained solely by direct CMV disease-associated short-term mortality. We hypothesize that a combination of direct CMV disease–associated short-term mortality and longer term CMV-associated indirect biological effects may underlie the observed association, but future studies to precisely define the mechanism(s) are warranted. New strategies such as CMV vaccination or immune therapies that could improve control of CMV in D+R− patients have the potential to reduce the negative impact of D+R− serostatus on important clinical outcomes in LTRs.
Supplementary Material
Acknowledgments:
The authors acknowledge Teresa Jewell, MLIS, for providing expertise on the literature review for this article.
Philip Vutien reports funding from National Institutes of Health T32 DK007742. All other authors disclose no funding interests in relation to this manuscript.
Hannah Imlay is a site principal investigator for Gilead. Ajit P. Limaye consults for and has received grants from Merck and also consults for AlloVir and Novartis.
Philip Vutien and James Perkins participated in the study concept, study design, analysis and interpretation, and critical review of the manuscript. Scott W. Biggins, Jorge Reyes, and Hannah Imlay participated in the study design, data interpretation, and critical review of the manuscript. Ajit P. Limaye participated in the study concept, study design, data interpretation, and critical review of the manuscript.
Abbreviations:
- aHR
adjusted hazard ratio
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- CIT
cold ischemia time
- CMV
cytomegalovirus
- CVA
cerebrovascular accident
- COD
cause of death
- D−
donor seronegative
- D+
donor seropositive
- DCD
donation after circulatory death
- EBV
Epstein-Barr virus
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HR
hazard ratio
- ICU
intensive care unit
- IQR
interquartile range
- LT
liver transplantation
- LTR
liver transplant recipient
- MELD
Model for End-Stage Liver Disease
- MELD-Na
Model for End-Stage Liver Disease–sodium
- NASH
nonalcoholic steatohepatitis
- OPTN
Organ Procurement and Transplantation Network
- P-Y
person-year
- PVT
portal vein thrombosis
- R−
recipient seronegative
- R+
recipient seropositive
- sAg
surface antigen
- SD
standard deviation
- TIPS
transjugular intrahepatic portosystemic shunt
- UNOS
United Network for Organ Sharing
Footnotes
Additional supporting information may be found in the online version of this article.
REFERENCES
- 1).Bosch W, Heckman MG, Diehl NN, Shalev JA, Pungpapong S, Hellinger WC. Association of cytomegalovirus infection and disease with death and graft loss after liver transplant in high-risk recipients. Am J Transplant 2011;11:2181–2189. [DOI] [PubMed] [Google Scholar]
- 2).Limaye AP, Bakthavatsalam R, Kim HW, Randolph SE, Halldorson JB, Healey PJ, et al. Impact of cytomegalovirus in organ transplant recipients in the era of antiviral prophylaxis. Transplantation 2006;81:1645–1652. [DOI] [PubMed] [Google Scholar]
- 3).Razonable RR, Humar A. Cytomegalovirus in solid organ transplant recipients—guidelines of the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant 2019;33:e13512. [DOI] [PubMed] [Google Scholar]
- 4).Katsolis JG, Bosch W, Heckman MG, Diehl NN, Shalev JA, Pungpapong S, et al. Evaluation of risk factors for cytomegalovirus infection and disease occurring within 1 year of liver transplantation in high-risk patients. Transpl Infect Dis 2013;15:171–180. [DOI] [PubMed] [Google Scholar]
- 5).Limaye AP, Bakthavatsalam R, Kim HW, Kuhr CS, Halldorson JB, Healey PJ, et al. Late-onset cytomegalovirus disease in liver transplant recipients despite antiviral prophylaxis. Transplantation 2004;78:1390–1396. [DOI] [PubMed] [Google Scholar]
- 6).Belga S, MacDonald C, Chiang D, Kabbani D, Shojai S, Abraldes JG, et al. Donor graft CMV-serostatus and the risk of arterial and venous thrombotic events in seronegative recipients after non-thoracic solid organ transplantation. Clin Infect Dis 2020;72:845–852. [DOI] [PubMed] [Google Scholar]
- 7).Madalosso C, de Souza NF Jr., Ilstrup DM, Wiesner RH, Krom RAF. Cytomegalovirus and its association with hepatic artery thrombosis after liver transplantation. Transplantation 1998;66:294–297. [DOI] [PubMed] [Google Scholar]
- 8).Yadav SK, Saigal S, Choudhary NS, Saha S, Kumar N, Soin AS. Cytomegalovirus infection in liver transplant recipients: current approach to diagnosis and management. J Clin Exp Hepatol 2017;7:144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Simanek AM, Dowd JB, Pawelec G, Melzer D, Dutta A, Aiello AE. Seropositivity to cytomegalovirus, inflammation, all-cause and cardiovascular disease-related mortality in the United States. PLoS One 2011;6:e16103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Caston JJ, Castells L, Varo E, Gomez MA, de la Mata M, Campos-Varela I, et al. Impact of cytomegalovirus infection on severe hepatitis C recurrence in patients undergoing liver transplantation. Transplantation 2016;100:593–599. [DOI] [PubMed] [Google Scholar]
- 11).Chambers DC, Cherikh WS, Harhay MO, Hayes D Jr., Hsich E, Khush KK, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-sixth adult lung and heart-lung transplantation report-2019; focus theme: donor and recipient size match. J Heart Lung Transplant 2019;38:1042–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Leeaphorn N, Garg N, Thamcharoen N, Khankin EV, Cardarelli F, Pavlakis M. Cytomegalovirus mismatch still negatively affects patient and graft survival in the era of routine prophylactic and preemptive therapy: a paired kidney analysis. Am J Transplant 2019;19:573–584. [DOI] [PubMed] [Google Scholar]
- 13).Kwong A, Kim WR, Lake JR, Smith JM, Schladt DP, Skeans MA, et al. OPTN/SRTR 2018 annual data report: liver. Am J Transplant 2020;20(suppl 1):193–299. [DOI] [PubMed] [Google Scholar]
- 14).Reyes J, Perkins J, Kling C, Montenovo M. Size mismatch in deceased donor liver transplantation and its impact on graft survival. Clin Transplant 2019;33:e13662. [DOI] [PubMed] [Google Scholar]
- 15).Jay C, Ladner D, Wang E, Lyuksemburg V, Kang R, Chang Y, et al. A comprehensive risk assessment of mortality following donation after cardiac death liver transplant—an analysis of the national registry. J Hepatol 2011;55:808–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant 2006;6:783–790. [DOI] [PubMed] [Google Scholar]
- 17).Schaubel DE, Sima CS, Goodrich NP, Feng S, Merion RM. The survival benefit of deceased donor liver transplantation as a function of candidate disease severity and donor quality. Am J Transplant 2008;8:419–425. [DOI] [PubMed] [Google Scholar]
- 18).Humar A, Beissel J, Crotteau S, Kandaswamy R, Lake J, Payne W. Whole liver versus split liver versus living donor in the adult recipient: an analysis of outcomes by graft type. Transplantation 2008;85:1420–1424. [DOI] [PubMed] [Google Scholar]
- 19).Rana A, Hardy MA, Halazun KJ, Woodland DC, Ratner LE, Samstein B, et al. Survival outcomes following liver transplantation (SOFT) score: a novel method to predict patient survival following liver transplantation. Am J Transplant 2008;8:2537–2546. [DOI] [PubMed] [Google Scholar]
- 20).Gane E, Saliba F, Valdecasas GJ, O’Grady J, Pescovitz MD, Lyman S, Robinson CA. Randomised trial of efficacy and safety of oral ganciclovir in the prevention of cytomegalovirus disease in liver-transplant recipients. Lancet 1997;350: 1729–1733. [DOI] [PubMed] [Google Scholar]
- 21).McCloskey M, Huhn L, Horgan P, Shabir S, Ball S, Borrows R. Increased graft failure and mortality following cytomegalovirus disease in kidney transplant recipients undergoing contemporary immunosuppression and CMV prophylaxis. Transplantation 2014;98:621. [Google Scholar]
- 22).Humar A, Snydman D, Practice ASTIDCo. Cytomegalovirus in solid organ transplant recipients. Am J Transplant 2009;9(suppl 4):S78–S86. [DOI] [PubMed] [Google Scholar]
- 23).Bosch W, Heckman MG, Pungpapong S, Diehl NN, Shalev JA, Hellinger WC. Association of cytomegalovirus infection and disease with recurrent hepatitis C after liver transplantation. Transplantation 2012;93:723–728. [DOI] [PubMed] [Google Scholar]
- 24).Vincenti F, Budde K, Merville P, Shihab F, Ram Peddi V, Shah M, et al. A randomized, phase 2 study of ASP0113, a DNA-based vaccine, for the prevention of CMV in CMV-seronegative kidney transplant recipients receiving a kidney from a CMV-seropositive donor. Am J Transplant 2018;18:2945–2954. [DOI] [PubMed] [Google Scholar]
- 25).Griffiths PD, Stanton A, McCarrell E, Smith C, Osman M, Harber M, et al. Cytomegalovirus glycoprotein-B vaccine with MF59 adjuvant in transplant recipients: a phase 2 randomised placebo-controlled trial. Lancet 2011;377:1256–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


