Abstract
Hypertension is the most important modifiable cause of cardiovascular (CV) disease and all-cause mortality worldwide. Despite the positive correlations between blood pressure (BP) levels and later CV events since BP levels as low as 100/60 mmHg have been reported in numerous epidemiological studies, the diagnostic criteria of hypertension and BP thresholds and targets of antihypertensive therapy have largely remained at the level of 140/90 mmHg in the past 30 years. The publication of both the SPRINT and STEP trials (comprising > 8,500 Caucasian/African and Chinese participants, respectively) provided evidence to shake this 140/90 mmHg dogma. Another dogma regarding hypertension management is the dependence on office (or clinic) BP measurements. Although standardized office BP measurements have been widely recommended and adopted in large-scale CV outcome trials, the practice of office BP measurements has never been ideal in real-world practice. Home BP monitoring (HBPM) is easy to perform, more likely to be free of environmental and/or emotional stress, feasible to document long-term BP variations, of good reproducibility and reliability, and more correlated with hypertension-mediated organ damage (HMOD) and CV events, compared to routine office BP measurements. In the 2022 Taiwan Hypertension Guidelines of the Taiwan Society of Cardiology (TSOC) and the Taiwan Hypertension Society (THS), we break these two dogmas by recommending the definition of hypertension as ≥ 130/80 mmHg and a universal BP target of < 130/80 mmHg, based on standardized HBPM obtained according to the 722 protocol. The 722 protocol refers to duplicate BP readings taken per occasion ("2"), twice daily ("2"), over seven consecutive days ("7"). To facilitate implementation of the guidelines, a series of flowcharts encompassing assessment, adjustment, and HBPM-guided hypertension management are provided. Other key messages include that: 1) lifestyle modification, summarized as the mnemonic S-ABCDE, should be applied to people with elevated BP and hypertensive patients to reduce life-time BP burden; 2) all 5 major antihypertensive drugs (angiotensin-converting enzyme inhibitors [A], angiotensin receptor blockers [A], β-blockers [B], calcium-channel blockers [C], and thiazide diuretics [D]) are recommended as first-line antihypertensive drugs; 3) initial combination therapy, preferably in a single-pill combination, is recommended for patients with BP ≥ 20/10 mmHg above targets; 4) a target hierarchy (HBPM-HMOD- ambulatory BP monitoring [ABPM]) should be considered to optimize hypertension management, which indicates reaching the HBPM target first and then keeping HMOD stable or regressed, otherwise ABPM can be arranged to guide treatment adjustment; and 5) renal denervation can be considered as an alternative BP-lowering strategy after careful clinical and imaging evaluation.
Keywords: Blood pressure, Diagnosis, Drug, Guidelines, Hypertension, Treatment
TABLE OF CONTENTS
1. Introduction
1.1 Main themes
1.2 Development of the guidelines
2. Definition and grading of hypertension
2.1 Comparisons of different blood pressure measurement methods
2.2 Definitions and grading of hypertension
3. Blood pressure measurement, central blood pressure, and blood pressure variability
3.1 Devices for blood pressure measurement
3.2 Standardized blood pressure measurement
3.3 Blood pressure measurement in the clinic setting
3.4 Blood pressure measurement outside the clinic setting
3.5 White-coat hypertension and masked hypertension
3.6 Home blood pressure measurement
3.6.1 Measurement frequency, timing, and number per occasion of home blood pressure monitoring
3.7 Use of oscillometric blood pressure device in patients with atrial fibrillation
3.8 Ambulatory blood pressure monitoring
3.8.1 Emerging alternative approaches to blood pressure assessment in an ambulatory setting
3.9 Central blood pressure
3.10 Blood pressure variability
4. Evaluation
4.1 Medical history
4.2 Physical examination
4.3 Laboratory tests
4.4 Hypertension-mediated organ damage
5. Secondary hypertension
5.1 Overview
5.2 Primary aldosteronism
5.2.1 Screening
5.2.2 Confirmation
5.2.3 Lateralization
5.2.4 Treatment
5.3 Renal parenchymal disease
5.4 Renovascular disease and renal artery stenosis
5.5 Obstructive sleep apnea
5.6 Drug or alcohol-induced secondary hypertension
5.7 Other endocrine disorders
6. Principles of hypertension management
6.1 Objectives and thresholds of hypertension management
6.2 Risk chart-based universal blood pressure targets and management strategy
6.3 J-curve revisited
7. Lifestyle modifications
7.1 Sodium restriction
7.2 Alcohol limitation
7.3 Body weight reduction
7.4 Cigarette smoking cessation
7.5 Diet adaptation
7.6 Exercise adoption
8. Pharmacological therapy
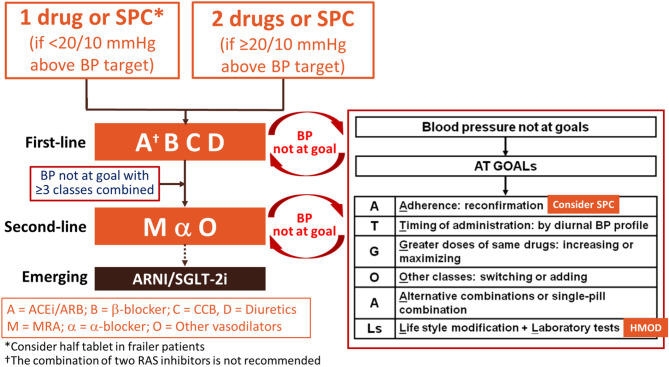
8.1 Initiation of pharmacological therapy: assessment flowchart
8.2 First-line antihypertensive drugs
8.3 Combination therapy
8.4 Single-pill combination
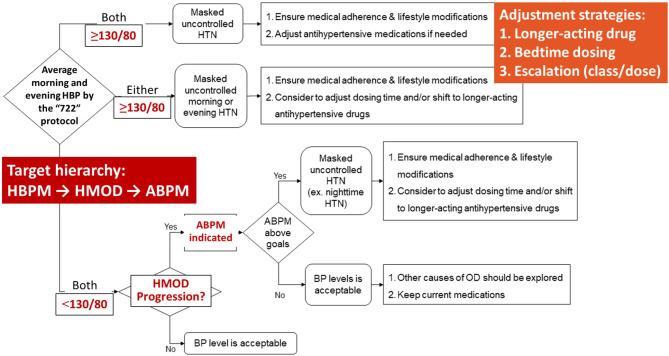
8.5 Adjustment flowchart and home blood pressure monitoring-guided management flowchart
8.6 Dose reduction and withdrawal of antihypertensive drugs
8.7 Classes of antihypertensive drugs
8.7.1 Angiotensin-converting enzyme inhibitors
8.7.2 Angiotensin receptor blockers
8.7.3 Direct renin inhibitors
8.7.4 Beta-blockers
8.7.5 Calcium channel blockers
8.7.5.1 Dihydropyridine calcium channel blockers
8.7.5.2 Non-dihydropyridine calcium channel blockers
8.7.6 Diuretics
8.7.6.1 Thiazides and thiazide-like diuretics
8.7.6.2 Loop diuretics
8.7.6.3 Mineralocorticoid receptor antagonists
8.7.6.4 Other potassium-sparing diuretics
8.7.7 Alpha-blockers
8.7.8 Centrally acting sympatholytic drugs
8.7.9 Direct vasodilators
8.7.10 Angiotensin receptor-neprilysin inhibitor
8.7.11 Sodium glucose cotransporter-2 inhibitors
9. Device therapy for hypertension
9.1 Evidence of renal denervation
9.2 Clinical application of renal denervation
10. Primary prevention patients with grade 1 hypertension
10.1 Post-hoc analysis
10.2 Meta-analysis
11. Patients with diabetes mellitus
12. Patients with coronary heart disease
13. Patients with cerebrovascular disease
13.1 Blood pressure control in the prehospital setting of suspected stroke
13.2 Blood pressure targets for patients with acute ischemic stroke
13.2.1 Patients not treated with intravascular thrombolysis or endovascular thrombectomy
13.2.2 Patients treated with intravascular thrombolysis
13.2.3 Patients treated with endovascular thrombectomy
13.2.4 Drugs of choice
13.3 Blood pressure targets for patients with acute hemorrhagic stroke
13.3.1 Acute intracranial hemorrhage
13.3.2 Acute aneurysmal subarachnoid hemorrhage
13.3.3 Drugs of choice
13.4 Blood pressure control for acute stroke in the convalescent and chronic stages
13.4.1 Blood pressure targets
13.4.2 When to target blood pressure for the secondary prevention of stroke
13.4.3 Drugs of choice for the secondary prevention of stroke
13.4.4 Blood pressure targets for ischemic stroke patients with symptomatic large vessel or cerebral small vessel disease
14. Patients with chronic kidney disease
14.1 Blood pressure targets for patients with non-dialysis chronic kidney disease
14.2 Blood pressure targets for patients with dialytic chronic kidney disease
14.3 Pharmacological treatment
15. Patients with heart failure
16. Patients receiving antithrombotic therapy
17. Elderly patients
18. Hypertension in women
18.1 Epidemiology and mechanisms
18.2 Hypertension in pregnancy
18.2.1 Diagnosis
18.2.2 Classification
18.2.3 Investigations
18.2.4 Risk classification
18.2.5 Prevention
18.2.6 Management
18.2.6.1 Mild hypertension in pregnancy (140-159/90-109 mmHg)
18.2.6.2 Severe hypertension in pregnancy (≥ 160/110 mmHg)
18.2.7 Post-partum hypertension and breastfeeding
18.2.8 Follow-up
18.3 Oral contraceptive pills and hormone replacement therapy
19. Patients with resistant hypertension
19.1 Definition
19.2 Phenotypes
19.3 Epidemiology
19.4 Causes
19.4.1 Non-adherence
19.4.2 Vasoactive substances
19.5 Treatment optimization
19.6 Lifestyle modifications
19.7 Device therapy
1. INTRODUCTION
1.1 Main themes
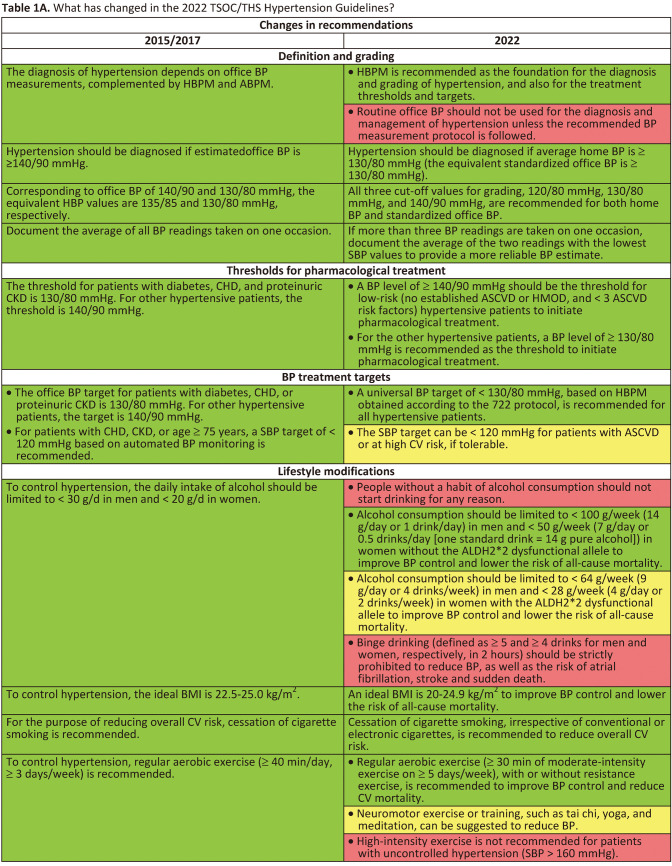
Hypertension is the most important modifiable cause of cardiovascular (CV) disease and all-cause mortality worldwide.1,2 Numerous epidemiological studies and pharmacological intervention trials have demonstrated that lower and lowering blood pressures (BP) are associated with fewer CV events and lower mortality.3,4 Despite the positive correlations between BP levels and later CV events since BP levels as low as 100/60 mmHg in almost all large-scale epidemiological studies,4-6 the diagnostic criteria of hypertension and BP thresholds and targets of antihypertensive treatment have largely remained at the level of 140/90 mmHg in the past 30 years (since the release of the Fifth Report of the Joint National Committee [JNC 5] on high BP in 1993).7 The publication of both the SPRINT and the STEP trials (comprising > 8,500 Caucasian/African and Chinese participants, respectively) provides enough evidence to shake this 140/90 mmHg dogma.8,9 In both trials, lowering systolic BP (SBP) to < 130 mmHg, compared to the traditional SBP target of < 140 (130-139) mmHg, was consistently associated with a 25-30% relative risk reduction in CV events. Another dogma regarding hypertension management is the dependence on office (or clinic) BP measurements.10,11 Although standardized office BP measurement has been widely recommended,12 the practice of office BP measurement has never been ideal in real-world practice. Further, the debate regarding the numerical equivalence between automated office BP (AOBP) measurement adopted in the SPRINT trial and office BP measurement has never been settled. The variations of office BP readings and the differences between office BP and home BP readings bewilder not only patients, but also healthcare professionals. On the other hand, out-of-office BP monitoring receives growing attention in contemporary hypertension guidelines.11,13 Home BP monitoring (HBPM) and ambulatory BP monitoring (ABPM) are two recognized approaches to obtaining out-of-office BP. HBPM is easy-to-use, more likely to be free of environmental and/or emotional stress (such as white-coat effect), feasible to document long-term BP variations, of good reproducibility and reliability, and more correlated with hypertension-mediated organ damage (HMOD) and CV events.1 The Taiwan Hypertension Society (THS) and the Taiwan Society of Cardiology (TSOC) jointly issued the Consensus Statement on HBPM in 2020.1 The "722" protocol to standardize HBPM has been advocated by both Societies and widely accepted by healthcare professionals. In the 2022 Taiwan Hypertension Guidelines, we break the dogma of "office BP-based management strategy" and further expand the role of HBPM to the whole hypertension management process, from diagnosis to long-term follow-up. The Task Force considers that, to improve the quality of long-term management of hypertension, patients themselves should take an active role and HBPM is the right tool to achieve this goal, regardless of many other advantages of HBPM.14 This approach is of particularly importance in the post-COVID era and can bridge the management with artificial intelligence technologies. To facilitate implementation of the guidelines, a series of flowcharts encompassing assessment, adjustment, and HBPM-guided hypertension management are provided. A total of 112 recommendations/keypoints are itemized. Changes between the 2022 and 2015/2017 Taiwan Hypertension Guidelines, new recommendations, and the "not to do" list are summarized in Tables 1A, Tables 1A Continued, Tables 1B, Tables 1B Continued, Tables 1C.
Tables 1A.
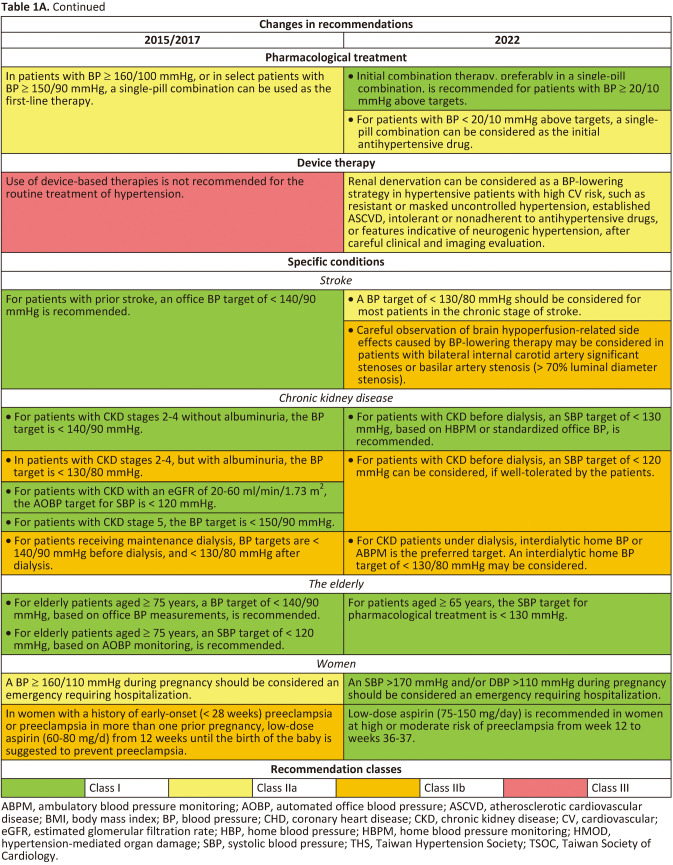
Tables 1A Continued.
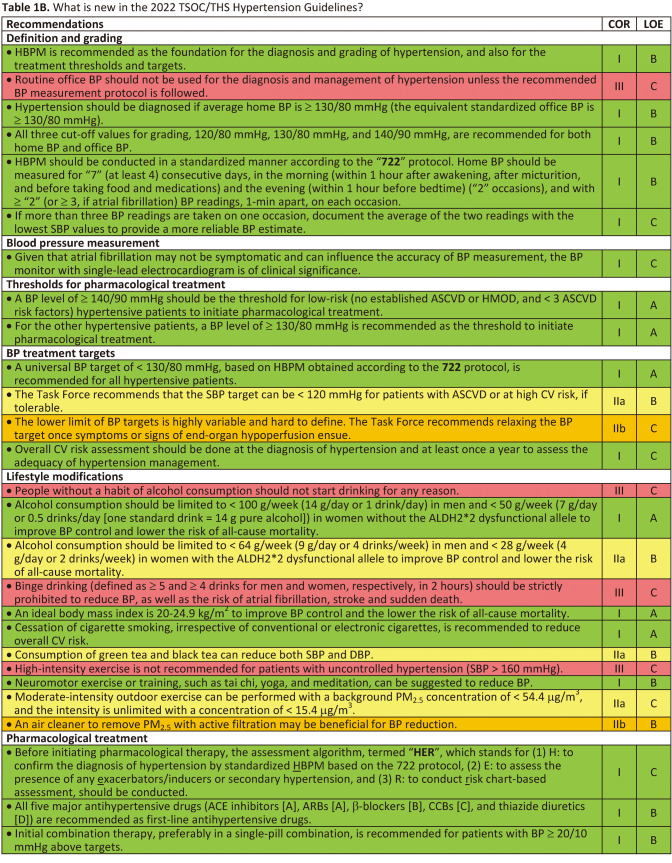
Tables 1B.
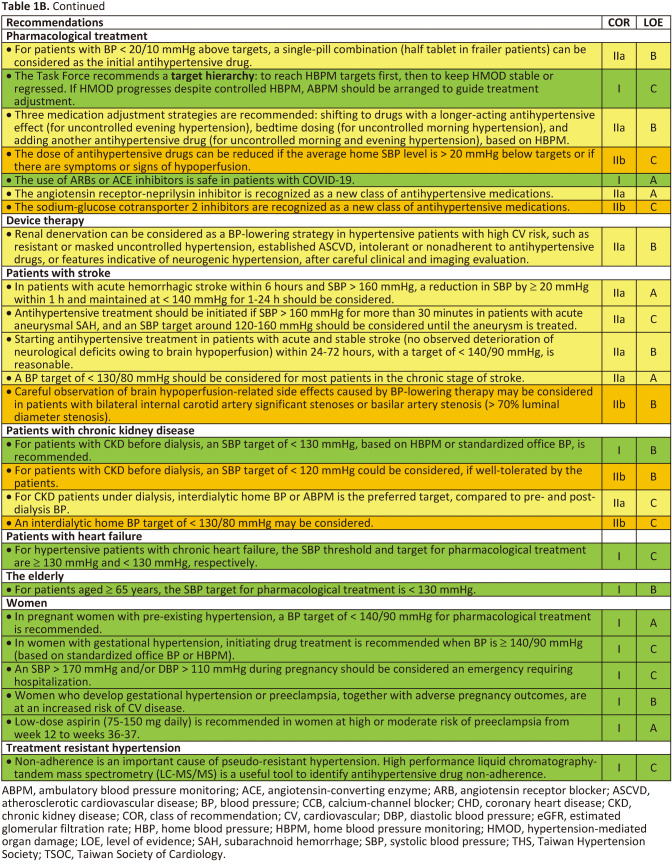
Tables 1B Continued.
Table 1C. “Not to do” messages from the 2022 TSOC/THS Hypertension Guidelines.
| • Routine office BP should not be used for the diagnosis and management of hypertension unless the recommended BP measurement protocol is followed. |
| • People without a habit of alcohol consumption should not start drinking for any reason. |
| • Binge drinking (defined as ≥ 5 and ≥ 4 drinks for men and women, respectively, in 2 hours) should be strictly prohibited to reduce BP, as well as the risk of atrial fibrillation, stroke and sudden death. |
| • High-intensity exercise is not recommended for patients with uncontrolled hypertension (SBP > 160 mmHg). |
| • Any combination of direct renin inhibitor, ACE inhibitors and ARBs is contraindicated. |
| • It is not recommended to lower BP in the prehospital setting without knowing the phenotypes of stroke. |
| • Routine aggressive BP lowering is not recommended unless BP ≥ 220/120 or in the presence of other situations needing immediate BP lowering (such as acute aortic dissection, congestive heart failure with lung edema, hypertensive encephalopathy) within 24 hours of acute ischemic stroke without undergoing thrombolytic or endovascular therapy. |
| • Salt reduction (less than 6 g/day) is not recommended as a non-drug therapy for gestational hypertension. |
| • ACE inhibitors, ARBs, DRI, ARNI, mineralocorticoid receptor antagonists, and chlorothiazide are teratogenic. Women with hypertension who become pregnant, are planning to become pregnant, or with child-bearing potential without reliable contraception, should avoid, or immediately withdraw these drugs in case of pregnancy. |
| • Oral contraceptives should not be used in women with uncontrolled hypertension. |
| • Hormone replacement therapy, as well as selective estrogen receptor modulators, should not be used for the primary or secondary prevention of CV diseases in postmenopausal women. |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; BP, blood pressure; CV, cardiovascular; DRI, direct renin inhibitor; SBP, systolic blood pressure; THS, Taiwan Hypertension Society; TSOC, Taiwan Society of Cardiology.
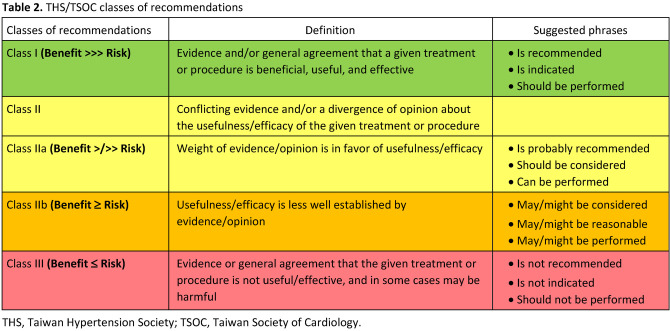
1.2 Development of the guidelines
Taiwan Hypertension Guidelines and related works (Focused Update/Consensus) evaluate and integrate available evidence with the purpose of assisting healthcare professionals in constructing the best management strategies for each individual patient. Members of this Task Force were jointly selected by the THS and the Hypertension Committee of TSOC to represent professionals from a broad array of backgrounds. The class of recommendation (COR) and level of evidence (LOE) were graded according to predefined scales as modified from the latest American and European guidelines for the management of arterial hypertension (Tables 2 and Table 3). Each member of the writing committee was assigned specific writing tasks, which were then reviewed and revised by three section coordinators. The text was developed over approximately 12 months, during which the Task Force members met collectively and communicate comprehensively between meetings. The TSOC/THS Guidelines undergo extensive review by the Task Force and external experts and are approved by all Task Force members. The guidelines and related works were developed independently without any involvement from the industry. The Task Force members’ comprehensive disclosure information is shown at the end of this Guideline. The TSOC/THS Hypertension Guidelines represent the official position of the TSOC and THS.
Tables 2.
Table 3. THS/TSOC levels of evidence (updated Mar 2019).
| Level A | Data derived from multiple (≥ 2) RCTs, or meta-analyses of high-quality RCTs |
| Level B | Data derived from a single RCT, large non-randomized studies, meta-analyses of moderate-quality RCTs or non-randomized studies |
| Level C | Subgroup analyses, post-hoc analyses, retrospective studies, cohort studies, registries, small studies, or consensus of expert opinion |
RCT, randomized controlled trial; THS, Taiwan Hypertension Society; TSOC, Taiwan Society of Cardiology.
Adherence to guidelines and related works can be improved by shared decision-making between healthcare professionals and patients, with patient engagement in choosing strategies based on individual preferences, values, and associated conditions. Guidelines and related works should not override clinical judgement, which is the right and responsibility of healthcare professionals. It is also the responsibility of healthcare professionals to verify the rules and regulations applicable to drugs and devices at the time of prescription.
2. DEFINITION AND GRADING OF HYPERTENSION
Recommendations/Keypoints
• There are 4 established methods of BP measurement: routine office BP (ROBP) measurement, automated office BP (AOBP) measurement, home BP monitoring (HBPM) measurement, and ambulatory BP monitoring (ABPM) measurement.
• BP readings obtained by AOBP, HBPM, and awake (daytime) ABPM are similar.
• The vast majority of cardiovascular outcome trials were based on "standardized" office BP measurement, rather than ROBP, to adjust medications or treatment strategies.
• HBPM is recommended as the foundation for the diagnosis and grading of hypertension, and also for the treatment thresholds and targets (COR I, LOE B).
• A lower threshold (≥ 130/80 mmHg) for defining hypertension is recommended (COR I, LOE B).
• All three cut-off values for grading, 120/80 mmHg, 130/80 mmHg, and 140/90 mmHg, are recommended for both home BP and office BP (if home BP not available) (COR I, LOE B).
• 7-day HBPM should be considered as the best approach for diagnosing hypertension (COR IIa, LOE B).
2.1 Comparisons of different blood pressure measurement methods
There are 4 established methods of BP measurement: routine office BP (ROBP) measurement, AOBP measurement, HBPM measurement, and ABPM measurement. The first 2 methods are performed in the clinic setting, while the latter 2 outside of clinics. ROBP was the most commonly performed and was less precise as only 1 or 2 BP measurements were usually obtained. There are many factors which could affect the accuracy of ROBP.15 One of the major concerns is the alerting response which causes the white-coat phenomena seen as white-coat hypertension in non-hypertensives and white-coat effect in known hypertensives.16 The accuracy of ROBP is a great concern in the crowded clinics in most regions in Taiwan. It should be emphasized that a vast majority of CV outcome trials were based on "standardized" office BP measurement, rather than ROBP, to adjust medications or treatment strategies. However, standardized office BP measurements are generally not applicable in busy clinics. Instructions regarding how to obtain standardized office BP are detailed in Section 3.1.
AOBP improves some drawbacks of ROBP. Though AOBP is also performed in clinics, it requires automated oscillometric devices with multiple readings, an averaged reading that can be stored, and an attended or un-attended quiet environment.16 The recent SPRINT trial used AOBP to enroll and follow-up hypertensive patients, and used the readings of AOBP as BP targets.8 AOBP is difficult to apply to the clinic settings in Taiwan as most hospitals and clinics cannot afford extra isolated spaces.
Out-of-office BP measurements include HBPM and ABPM. HBPM is referred to measurements of BP at home usually by oneself, or on occasion, by caregivers or research assistants.17 Compared to ROBP, HBPM is more likely to be free of environmental and/or emotional stress (such as white-coat effect).1 In the 2017 ACC/AHA Hypertension Guideline, the diagnosis of hypertension by ROBP should be confirmed by HBPM or ABPM18 HBPM is better than ROBP for the prediction of HMOD and CV outcomes.1 In a systematic review of 9 publications, HBPM was non-inferior to ABPM in predicting CV events and mortality.19 Four Asian studies have demonstrated that morning home BP is a better prognostic predictor of CV events than ROBP.20-23 The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014) recommended that a HBPM-guided approach was the most effective and practical approach in clinical practice.24 More importantly, HBPM is feasible and affordable in Taiwan. In the 2020 Consensus Statement of the Taiwan Hypertension Society and the Taiwan Society of Cardiology on Home Blood Pressure Monitoring for the Management of Arterial Hypertension, HBPM was recommended as an integral part in the diagnosis and management of hypertension in Taiwan.1
ABPM is the gold standard for diagnosing hypertension and assessing 24-hour BP and provides data on several important parameters that cannot be obtained using any other form of BP measurements.25 In addition, ABPM parameters provide better information on cardio- and cerebrovascular risk than ROBP. On the other hand, clinical studies and meta-analyses suggested that HBPM was as good as ABPM in their association with CV events or HMOD.26-28 Measurements with systolic and diastolic HBP for 1 week, compared with ROBP (3 visits) or ABPM, were more reliable and more strongly associated with left ventricular mass index, suggesting that 1 week of HBPM (7-day HBPM) may be the best approach for diagnosing hypertension.1,29 Compared with HBPM, ABPM is not tolerated by some patients, and the equipment is not widely available in Taiwan.
The SPRINT trial was a BP target-driven trial.8 AOBP was used in the SPRINT trial, in which attended- or unattended-AOBP showed similar BP values.30 In a cross-sectional study, BP measured with attended and unattended AOBP were similar to daytime BP from ABPM.31 Based on data from 14 studies involving 3,410 participants in different settings, an AOBP of 135/85 mmHg corresponded to 135/85 mmHg on awake ABPM.32 In a recent systematic review and meta-analysis of 31 articles comprising 9,279 participants, systolic BP readings from ROBP and systolic BP readings from research office BP measurement (standardized office BP) were substantially higher than systolic BP readings from AOBP, with pooled mean differences of 14.5 mmHg (p < 0.001) and 7.0 mmHg (p < 0.001), respectively.33 But systolic BP readings from AOBP were similar to systolic BP readings from awake (daytime) ABPM, with a pooled mean difference of only 0.3 mmHg.33 When HBPM was compared with awake ABPM by a dual-mode device, there was no significant difference between them (mean systolic BP difference 0.5 mmHg; mean diastolic BP difference 0.6 mmHg, both p value non-significant).34 Likewise, in a systematic review of 7,116 patients from 26 studies, no significant differences were found between AOBP, awake (daytime) ABPM, and HBPM.35 In a more recent analysis from 139 patients with hypertension, systolic BP measured with AOBP, HBPM, and awake ABPM were very similar (141.2 mmHg, 142.5 mmHg, and 142.1 mmHg, respectively) and much lower than ROBP (152.2 mmHg).36 We conclude that the BP readings obtained by AOBP, HBPM, and awake (daytime) ABPM are very similar. Table 4 shows the corresponding values of systolic BP/diastolic BP for HBPM, ROBP, AOBP, awake (daytime) ABPM, asleep (nighttime) ABPM, and 24-hour ABPM.
Table 4. Corresponding values of systolic BP/diastolic BP (mmHg) for HBPM, ROBP, AOBP, awake (daytime) ABPM, asleep (nighttime) ABPM, and 24-hour ABPM measurements.
| HBPM | ROBP | AOBP | Awake ABPM | Asleep ABPM | 24-hour ABPM |
| 120/80 | 120/80 | 120/80 | 120/80 | 100/65 | 115/75 |
| 130/80 | 130/80 | 130/80 | 130/80 | 110/65 | 125/75 |
| 135/85 | 140/90 | 135/85 | 135/85 | 120/79 | 130/80 |
| 145/90 | 160/90 | 145/90 | 145/90 | 140/85 | 145/90 |
ABPM, ambulatory blood pressure monitoring; AOBP, automated office blood pressure; BP, blood pressure; HBPM, home blood pressure monitoring; ROBP, routine office blood pressure.
2.2 Definitions and grading of hypertension
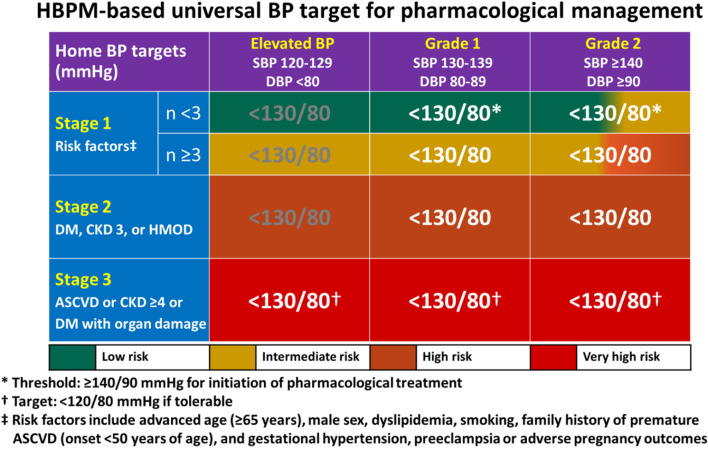
In an Asian consensus document, morning BP from HBPM was recommended as the initial focus for the management of out-of-office BP in Asians.37 There are several reasons to support this recommendation. Both morning BP surges detected by ABPM and HBPM were predictors of CV endpoints independent of ROBP level in Asian hypertensive patients.38,39 Morning BP measured at home, compared with evening BP, provided better discrimination for stroke.21 The multicenter HOMED-BP study demonstrated the feasibility of adjusting antihypertensive drug treatment based on morning BP measured by HBPM in Japanese hypertensive patients.22 Based on the evidence from Asia and special consideration of appropriateness of different BP measurement methods in Taiwan, the Task Force recommends to use HBPM for the diagnosis and grading of hypertension, and also for the treatment thresholds and targets (COR I, LOE B).
According to the Asia Pacific Cohort Studies Collaboration, the risks of coronary heart disease and stroke were higher in Asians compared with Caucasians, with the same BP readings.6 The hazard ratio of cardiovascular disease (CVD) for people from Australia and New Zealand in the prehypertension range (SBP 120-139 mmHg), previously defined by JNC 7,40 was 1.11 (95% confidence interval [CI] 0.97-1.27) when compared with normal BP (SBP < 120 mmHg). The hazard ratio, however, increased to 1.55 (95% CI: 1.41-1.70) for Asian people with prehypertension,41 suggesting an increased CV risk in the BP range of 120-139 mmHg for Asian people. Similar finding was reported from Taiwan.42 Furthermore, people with a SBP of 130-139 mmHg had an increased risk of CV diseases, based on independent reports from China,43 Hong Kong,44 and South Korea.45 All these lines of evidence are corroborated by the recent Strategy of Blood Pressure Intervention in the Elderly Hypertensive Patients (STEP) trial.9 In this multicenter, randomized controlled trial, 8,511 Chinese patients 60 to 80 years of age with hypertension from both mainland China and Taiwan were assigned to a SBP target of 110 to < 130 mmHg (intensive-treatment) or a target of 130 to < 150 mmHg (standard-treatment). During a median follow-up period of 3.34 years, the primary outcome events occurred in 147 patients (3.5%) in the intensive-treatment group, as compared with 196 patients (4.6%) in the standard-treatment group (hazard ratio, 0.74; 95% CI: 0.60 to 0.92; p = 0.007). The relative risk reduction divided by the between-group SBP difference is 2.8%/mmHg (26%/9.2 mmHg), which is consistent with the more pronounced impact of hypertension in Asian populations. Therefore, to define SBP ≥ 140 mmHg as hypertension that was previously defined by most Hypertension Guidelines seems not that appropriate to address the risk of hypertension in Asians.10,11,13 A lower threshold (≥ 130/80 mmHg), such as that defined by 2017 ACC/AHA Hypertension Guideline, would be more appropriate for Asian patients.18
In a prospective nationwide study of 2,081 randomized subjects aged 45 to 74 years from Finland (Finn-Home Study), CV events increased with SBP above 130 mmHg and diastolic BP (DBP) above 80 mmHg with HBPM.46 In a population-based cohort study from the Korean National Health Insurance Service of 2,488,101 adults aged 20 through 39 years with a median follow-up of 10 years, men with baseline BP of 130-139/80-89 mmHg compared with those with BP < 120/80 mmHg had higher risk of CV diseases (adjusted hazard ratio [aHR]: 1.25, 95% CI: 1.21-1.28), coronary heart disease (aHR: 1.23, 95% CI: 1.19-1.27), and stroke (aHR: 1.30, 95% CI: 1.25-1.36).45 The corresponding aHRs for baseline BP of ≥ 140/90 mmHg, compared with those BP < 120/80, were 1.76, 1.68, and 1.99, respectively.45 Data for women showed similar trends.45 In the Finn-Home study, an increment of 10 mmHg in SBP and 5 mmHg in DBP with HBPM significantly increased CVD risk by 22% and 15%, respectively.46 In a systematic review and meta-analysis that included Asian data, an increment of 10 mmHg in SBP with HBPM significantly increased CV disease risk by 20%, CV death by 29%, and total death by 14%.47 The incremental impact of HBPM on CV events, as shown above, is almost equivalent to that of office BP on CV events observed in the meta-analysis of 344,716 individual participant-level office BP data from 48 randomized trials of antihypertensive treatment by the Blood Pressure Lowering Treatment Trialists Collaboration.3 Taken together, the Task Force redefined hypertension by HBPM as shown in Table 5.
Table 5. Definition and grading of hypertension (based on home BP measurements following the 722 protocol or standardized office BP [if home BP is not available]).
| BP category | SBP (mmHg) | DBP (mmHg) | |
| Normal | < 120 | and | < 80 |
| Elevated | 120-129 | and | < 80 |
| Hypertension | |||
| Grade 1 | 130-139 | or | 80-89 |
| Grade 2 | ≥ 140 | or | ≥ 90 |
BP, blood pressure; DBP, diastolic blood pressure; SBP, systolic blood pressure.
The recommended BP cut-off values for grading of hypertension are traditionally based on office BP in all hypertension guidelines worldwide.10,11,13 In the 2022 Taiwan Hypertension Guideline, the Task Force recommends BP cut-off values based on HBPM for grading of hypertension. In Table 4, home BP values are equivalent to office BP values of ≤ 130/80 mmHg. Home BP value is 5 mmHg lower (135/85 mmHg) than office BP value of 140/90 mmHg. However, according to the 11-year follow-up data of 5,768 participants from the Dallas Heart Study, office SBP of 140 mmHg was equivalent to home SBP of 140 mmHg by outcome-derived approach and 135 mmHg by regression-based approach.48 Given that outcome-derived approach is of greater clinical significance, the Task Force recommends all three cut-off values for grading, 120/80 mmHg, 130/80 mmHg, and 140/90 mmHg, are identical for HBPM and office BP. The universal cut-off values should improve the implementation of guidelines in clinical practice.
3. BLOOD PRESSURE MEASUREMENT, CENTRAL BLOOD PRESSURE, AND BLOOD PRESSURE VARIABILITY
Recommendations/Keypoints
• Periodic calibration of automated electronic sphygmomanometer should be performed at an interval not greater than 12 months (COR I, LOE C).
• Key steps for proper BP measurements including preparation, the use of validated devices with appropriate-sized cuff, correct measurement process, and data recordings (COR I, LOE C).
• Routine office BP should not be used for the diagnosis and management of hypertension, unless the recommended BP measurement protocol is followed (COR III, LOE C).
• Home BP is one form of out-of-office BP; if measured correctly, can be used for diagnostic confirmation, identification of hypertension phenotypes (sustained hypertension, white-coat hypertension [effect], and masked [uncontrolled] hypertension), guidance of antihypertensive treatment, and improvement of hypertension control (COR I, LOE B).
• Hypertension should be diagnosed if average home BP is ≥ 130/80 mmHg (the equivalent standardized office BP is ≥ 130/80 mmHg)(COR I, LOE B).
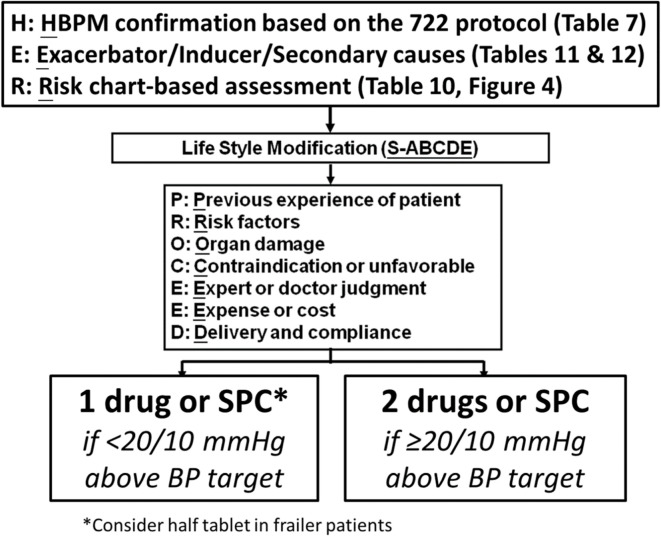
• To implement HBPM in the diagnosis and management of hypertension, the Task Force recommends that HBPM should be conducted according to the "722" protocol. Home BP should be measured for "7" (at least 4) consecutive days, in the morning (within 1 hour after awakening, but before taking food and medications) and the evening (within 1 hour before bedtime) ("2" occasions), and with ≥ "2" (≥ 3, if atrial fibrillation) BP readings, 1-min apart, on each occasion (COR I, LOE B).
• The measurement frequency, timing, and number per occasion of HBPM can be individualized to improve adherence and to establish the habit (COR IIa, LOE C).
• Multiple (≥ 3) measurements on one occasion and use of a specially validated device are recommended to obtain reliable HBPM readings in patients with AF (COR I, LOE C).
• If more than three BP readings are taken on one occasion, document the average of the two readings with the lowest SBP values to provide a more reliable BP estimate (COR I, LOE C).
• ABPM parameters provide better information on cardio- and cerebrovascular risk than office BP (COR I, LOE B).
• ABPM should be considered in all patients with elevated BP, particularly those with unstable office or home BP, or whom are suspected to have white-coat or masked hypertension, or progressive hypertension-mediated organ damage (COR IIa, LOE B).
• Measurement of central BP with a cut-off value of 130/80 mmHg for diagnosing hypertension is recommended (COR IIb, LOE B).
• BP variability (BPV) can be classified into short-term BPV (over 24 hours), mid-term (day-to-day), and long-term BPV (visit-to-visit) according to the length of BP recordings, which can be obtained by ABPM, HBPM, and office BP monitoring, respectively.
• Increased BPV was associated with organ damage, stroke, cardiovascular events, and mortality independent of average BP.
• Antihypertensive medications with longer duration of BP-lowering action could be considered to lower BPV (COR IIa, LOE B).
• Given that atrial fibrillation may not be symptomatic and can influence the accuracy of BP measurement, the BP monitor with single-lead electrocardiogram is of clinical significance (COR I, LOE C).
3.1 Devices for blood pressure measurement
Since the early 20th century, BP could be measured by using the auscultatory approach with a stethoscope and a manual manometer through the recognition of Korotkoff sounds. Subsequently, oscillometric approach was developed in 1970 and has been widely utilized in automated BP monitoring. The mercury sphygmomanometer, once regarded as the gold standard technique, has been banned for production in Taiwan since 2021 due to the concern of environmental safety of mercury. However, the mercury sphygmomanometer is permitted to be used as a reference standard for the validation of new BP monitors and for research purposes. There are two common types of non-mercury sphygmomanometer, oscillometric and aneroid devices. The oscillometric devices are operated automatically with the inflation and deflation of the cuff being controlled electronically. Periodic calibration of automated electronic sphygmomanometer should be performed at an interval not greater than 12 months.49 Aneroid sphygmomanometer, operated manually with a pressure cuff and a stethoscope using auscultatory approach, is a liquid-free device alternative of mercury sphygmomanometer.32 Many devices have been developed based on the oscillometric technique to measure BP outside of physicians’ clinic, including ABPM or self-monitoring BP. The latter includes BP taken at home, HBPM, or in public settings, such as kiosks, pharmacy, grocery store, and in the community.
The appropriate management of hypertension depends on accurate BP measurements. Using conventional office BP in the management of hypertension is not reliable since its value could be heavily influenced in the busy and hurry clinical environment. In a previous systematic review, it has been demonstrated that the office BP in routine clinical practice is substantially higher than research office BP and awake (daytime) ambulatory BP.50 Therefore, it could be risky and imprudent to prescribe antihypertensive medications solely based on ROBP. Many alternative strategies have been proposed to replace conventional office BP to guide the management of hypertension.51-54 To obtain precise office BP or self-monitoring BP for making proper management of hypertension, accurate BP measurement is an indispensable first step.
3.2 Standardized blood pressure measurement
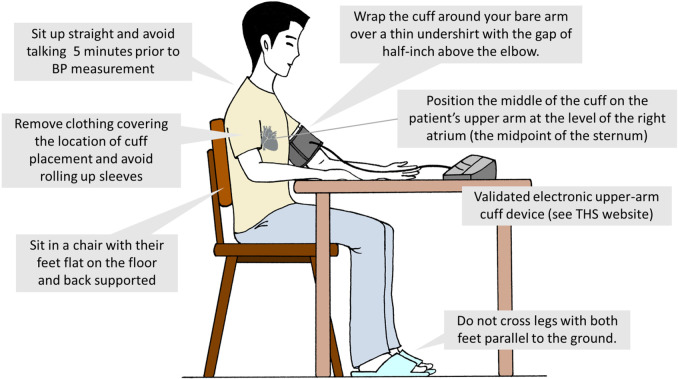
The accuracy of both office55 BP and self-monitoring BP measurements can be improved by adhering to the key steps of proper BP measurements.11,15 These key steps include proper preparation, use of proper techniques and validated devices, taking proper measurements needed for diagnosis and treatment, and proper BP readings recording. We summarize these important steps in Figure 1 and Tables 6 and 7. Unless the recommended BP measurement protocol is followed, routine office BP should not be used for the diagnosis and management of hypertension (COR III, LOE C).
Figure 1.
Standardized blood pressure measurement. BP, blood pressure; THS, Taiwan Hypertension Society.
Table 6. Recommended BP measurement protocol for office BP and home BP.
| Stage 1: Preparation | Empty bowel and stomach. |
| Before the measurement procedure, subjects should avoid caffeine, exercise, and smoking for at least 30 minutes. | |
| Sit calmly for at least 5 minutes and avoid talking during the rest period and the whole measurement process. | |
| Avoid conversation during the rest period and during the measurement. | |
| Remove clothing covering the location of cuff placement. Be sure to avoid rolling up sleeves; this may cause a (partial) tourniquet effect. | |
| Sit in a calm and comfortable place. | |
| Stage 2: Measurement equipment and position | Use validated BP devices and ensure that the device is calibrated at recommended intervals (at least every 12 months), and the device is better if equipped with capabilities of automatic data recording and/or auto-transmission. |
| Obtain and record subject’s mid-arm circumference. | |
| Support the patient’s arm with resting on a desk. | |
| Position the middle of the cuff on the patient’s upper arm at the level of the right atrium (the midpoint of the sternum). | |
| Use the correct cuff size, following the manufacturer’s instructions (cuff bladder width and length are at least 40% and 80% of the mid-arm circumference, respectively). | |
| Sit for 5 minutes without talking or moving around prior to recording the first BP reading in a chair with their feet flat on the floor and back supported. | |
| Stage 3: BP measurement process | If BP is measured for the first time, check the BP in right and left upper arms. If the between-arm BP difference is < 15 mmHg, use the higher BP for further management. |
| Position the center of the cuff over the upper arm brachial artery at least 2.5 cm (2 finger breadths) above the crease of the elbow. | |
| Separate repeated measurements by 1 minute. | |
| For an auscultatory determination of the BP level, inflate the cuff 20-30 mmHg above the estimated SBP assessed using the radial pulse obliteration method. | |
| Place the head of the stethoscope over the brachial artery for auscultatory determination. | |
| For auscultatory readings, deflate the cuff pressure 2 mmHg per second, and listen for Korotkoff sounds. | |
| To assess whether classic and delayed orthostatic hypotension are present, measure BP 1 and 3 minutes after assuming an upright posture, respectively. | |
| Stage 4: Documentation of accurate BP readings | Record SBP, DBP, and heart rate for each measurement using auto-transmission, an app on a digital device, or recording sheet. |
| Use an average of ≥ 2 readings for each measurement. | |
| If more than 3 readings are taken, document the average of the 2 readings with the lowest SBP values to provide a more reliable BP estimate. | |
| Use an average of ≥ 2 readings obtained on ≥ 2 occasions to estimate the BP. | |
| If using the auscultatory technique, record SBP as onset of the first of at least 2 consecutive beats and the last audible sound as DBP, Korotkoff phases 1 and 5, respectively. In cases where the sounds are audible at full deflation or until very low DBP levels (< 40 mmHg), then Korotkoff phase 4 (muffling of sounds) should be recorded and reported for DBP. | |
| If using the auscultatory approach, record SBP and DBP to the nearest even number. | |
| Provide information to help the patients interpret their BP values. |
BP, blood pressure; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Table 7. The “722” protocol for HBP monitoring modified from the TSOC/THS home BP consensus.
| The “722” protocol | Timing of HBP monitoring |
| “7” | 7 (at least 4) consecutive days |
| “2” | 2 occasions per day: in the morning (within 1 hour after awakening, after voiding, and before taking food and medications) and in the evening (within 1 hour before bedtime) |
| “2” | 2 or more BP readings, 1 minute apart, taken per occasion (≥ 3 BP readings if atrial fibrillation) |
| BP ranges | Frequency of HBP monitoring with the “722” protocol |
| Normal blood pressure (< 120/80 mmHg) | Every 1 year |
| Elevated blood pressure (120-129/< 80 mmHg) | Every 6 months |
| Hypertension (≥ 130/80 mmHg) | |
| Treatment-naïve | One “722” cycle, for confirmation of the diagnosis and phenotype identification |
| Initiation of drug therapy | 2 weeks later, then every 1 month if uncontrolled, or every 3 months if under control |
| Adjustment of drug therapy | 2 weeks later, then every 1 month if uncontrolled, or every 3 months if under control |
| Treated but uncontrolled | Every 1 month |
| Treated and controlled | Every 3 months |
BP, blood pressure; HBP, home blood pressure; THS, Taiwan Hypertension Society; TSOC, Taiwan Society of Cardiology.
3.3 Blood pressure measurement in the clinic setting
Previous hypertension management guidelines and quality improvement programs have mostly relied on BP measured in the clinic setting. Screening for abnormal BP and monitoring the response to treatments are the main purposes of measuring BP in routine clinical practice (routine office BP). However, the measurement of BP has been recognized to be the single clinical procedure of greatest importance but performed in the sloppiest manner.56,57 Most of the clinical practice settings are faced with time constraints which inevitably affect the accuracy of BP measurements. Besides, training in BP measurement, equipment used, and measurement methods vary widely across clinics, and can deviate from methods recommended by guidelines substantially. The research office BP in clinical trials is obtained with standardized protocols to minimize systematic errors and variability for BP measurements. However, a substantial white-coat effect, the difference between office and out-of-office BP, can be observed with both research and routine office BP measurements.1 Subsequently, unattended AOBP has been developed and regarded as a successful strategy to eliminate the white-coat effects.58 It has been shown in a previous systematic review that there are large discrepancies between routine office BP, research office BP, and AOBP with the difference of around 7 mmHg between routine and research office BP and between research office BP and AOBP.33
Unattended AOBP with its effect on eliminating white-coat effects was promoted by the Canadian hypertension guideline.59,60 There are 4 essential components for AOBP: electronic and automated device, multiple readings, averaged mean, unattended and undisturbed spaces (EMAU).50 Some studies have suggested that BP measured with staff present results in higher readings than those obtained with staff absent during measurements.61 However, whether the presence of staff would influence the accuracy of BP measurements has still been under debate.62,63 Recent publications claimed that the BP measurement technique used in the Systolic Blood Pressure Intervention Trial (SPRINT) was unattended.64 It was then clarified by the SPRINT researchers that the SPRINT protocol does not address the issue of attendance and similar BP levels and CV disease risk reduction were observed in the intensive group regardless of the measurement technique used being primarily attended or unattended.30 The average AOBP readings are shown to be comparable to the average awake ABPM reading and HBPM.33
3.4 Blood pressure measurement outside the clinic setting
BP measured in the clinic setting differs substantially from that obtained outside of the clinic setting.65-67 The prognostic value of out-of-office BP measurements has been shown to be superior to the traditional office BP.68 Therefore, it has been suggested out-of-office BP can be used to confirm the diagnosis of hypertension and for the management of high BP.69 The comparisons between routine office BP, AOBP, HBPM, and ABPM are provided in Table 8.
Table 8. Comparisons of different blood pressure measurement modalities.
| Home BP | Office BP | Automated office BP | Ambulatory BP | |
| Advantages | Stronger predictor of CV events than office BP. | BP measured in a clinical setting. | Eliminate white-coat effect. | Much stronger predictor of CV events than office BP. |
| Provides a larger number of BP readings. | Associated with CV outcomes. | Associated with CV outcomes. | Provides a larger number of BP readings during routine activities. | |
| Can be repeated more frequently than ABPM. | Method used in large outcome trials (standardized/research office BP). | Used in the SPRINT study. | Identifies white-coat and masked hypertension. | |
| Identifies white-coat and masked hypertension. | Identifies long-term visit-to-visit BP variability. | Obtains 3-5 BP readings with each measurement. | Discloses nocturnal hypertension and dipping patterns. | |
| Evaluates the efficacy of antihypertensive drugs at different times of the day and night, except sleep. | Provides average awake (daytime), asleep (nighttime) and 24-hour values. | |||
| Identifies mid-term day-to-day BP variability. | Identifies short-term and 24-hour BP variability. | |||
| High acceptance by patients. | Evaluates the 24-hour efficacy of antihypertensive drugs. | |||
| Relatively low cost. | ||||
| Disadvantages | Patient training required (simple for automated devices). | Lacks nighttime recordings. | Lacks nighttime recordings. | Cost (reimbursement issue). |
| Possible use of unvalidated devices. | No diurnal patterns of BP can be assessed. | No diurnal patterns of BP can be assessed. | Limited availability. | |
| Lacks nighttime recordings. | The accuracy of BP measurements hampered by time constraints in busy clinic conditions. | Higher cost of validated BP monitoring and more space and time required. | Patient discomfort. | |
| Patient may not correctly measure and report their BP. | Less precise with one or two measurements at each clinic visit. | Repeated measurements not likely in the short-term. | ||
| Less useful for evaluating the efficacy of antihypertensive drugs. | Requires two clinic visits to complete the test. |
BP, blood pressure; CV, cardiovascular.
As recommended by the United States Preventive Services Task Force and American College of Cardiology/ American Heart Association (ACC/AHA), one of the major utilities of out-of-office BP is to identify hypertension phenotypes of white-coat and masked hypertension.69
3.5 White-coat hypertension and masked hypertension
Because of the difference between office and out-of-office BP measurements, discrepancies in the diagnosis of hypertension arise when different criteria for hypertension based on different BP measurements are applied. Four BP phenotypes, normotension, white-coat hypertension, masked hypertension, and sustained hypertension, defined by the combination of hypertensive/non-hypertensive office and out-of-office BP are thus generated. In a previous study with 1,257 treatment-naïve subjects in Taiwan, the prevalence of white-coat hypertension among those with office SBP ≥ 140 mmHg or DBP ≥ 90 mmHg was 12.2%.70 In a sub-analysis of Taiwanese patients from the Asia BP at Home study, the prevalence of white-coat hypertension and masked hypertension were 21% and 11%, respectively, based on the diagnostic criteria of office and home BP of 130/80 mmHg.71 It has long been recognized that subjects with masked hypertension carry a comparable CV risk to those with sustained hypertension. Inconsistent evidence exists on whether white-coat hypertension is associated with a substantially increased risk for CVD compared with normotension.72-74 A previous community cohort study conducted in Kinmen suggested that the white-coat effect is mainly caused by arterial aging, and white-coat hypertension carries a higher risk for CV mortality compared to prehypertensive subjects.70 Besides, white-coat hypertension is also associated with a higher incidence of sustained hypertension versus normotension.75,76 A recent systematic review concluded that untreated white-coat hypertension, but not treated white-coat effect, is associated with an increased risk for CV events and all-cause mortality.77
It was shown that the prevalence of masked hypertension was higher in subjects with prehypertension vs. normal office BP,78 and office BP in the upper prehypertensive range can help predict masked hypertension.79 The prevalence of masked hypertension was also higher in patients with diabetes,80,81 and obstructive sleep apnea syndrome.82 As shown in an international cohort study, the proportion of masked uncontrolled hypertension in all hypertensives is not small (15.9% among treated subjects),74 suggesting that out-of-office BP should be considered in all hypertensive subjects.
3.6 Home blood pressure measurement
Accumulating evidence has demonstrated the relationship of HBPM with HMOD26,83,84 and CV outcomes.21,39,46,74,85-89 In previous systematic reviews, the prognostic value of HBPM was comparable to that of ABPM.19,27 Compared with office BP or 24-h ABPM, HBPM with one-week measurements was more reliable and more strongly associated with left ventricular mass index, suggesting that 7-day HBPM may be the best approach for diagnosing hypertension.29 Besides, with adequate feedback and intervention, HBPM can provide a better guiding strategy than conventional office BP.90 A better acceptability of 7-day HBPM over 24-hr ABPM was shown in a study surveying the preference.34 Morning and evening BP measured with HBPM were both able to predict future CV events.21,23,39 Asian populations have distinct presentations of hypertension and related CV disease from Westerners.37 For example, Asian patients have a higher rate of stroke and metabolic syndrome, which is often associated with higher morning and nighttime (asleep) BP reading.91 Recently, an innovative automated HBPM device has been developed for measuring nighttime BP.92 Its clinical applications await further verification.
HBPM can provide multiple measurements over longer periods and identify day-to-day BP variability. With the ability to detect morning and masked hypertension and a better tolerability than ABPM for long-term use, HBPM can therefore be considered as a strategy of choice to replace office BP monitoring for the diagnosis and treatment for hypertensive subjects.
To facilitate the application of HBPM in routine clinical practice, the Taiwan Hypertension Society and the Taiwan Society of Cardiology jointly put forward the consensus recommendations according to up-to-date scientific evidence and recommend the "722" protocol for HBPM measurement (Table 7), thus standardizing the ways to integrate HBPM in the diagnosis and management of hypertension.1
The proprietary algorithms for BP estimation vary considerably in oscillometric BP devices. Clinicians should recommend the use of BP monitoring devices which have been validated. Various societies and organizations have proposed different validation protocols for BP monitors.32 There are resources on the web that list validated BP monitors such as https://bihsoc.org/bp-monitors/provided by the British and Irish Hypertension Society and https://stridebp.org/ by the Stride BP.
3.6.1 Measurement frequency, timing, and number per occasion of home blood pressure monitoring
To determine the timing and number per occasion for BP measurement, the measurement protocols for HBPM in several clinical studies could be referenced.1,93,94 Basically, the more BP measurements taken, HBPM readings are more precise and reliable but at the expense of time consumed. Most clinical studies derived HBPM readings from the averages of morning and evening measurements together. Superior prognostic ability by averaging home BP of 14 measurements was demonstrated in the Ohasama study.86 Although the measurement protocols varied substantially between studies, a reliable diagnosis of hypertension can be made by means of at least 6 readings during 6 days, after excluding readings obtained on the first day.29,53,95-98
Taking the above evidence into considerations, the Task Force recommends that HBPM should be measured according to the "722" protocol for hypertension diagnosis and home BP-guided antihypertensive management (Table 7). The "722" protocol indicates that home BP should be measured for "7" (at least 4) consecutive days, in the morning (within 1 hour after awakening, but before taking food and medications) and the evening (within 1 hour before bedtime) ("2" occasions), and with ≥ "2" (≥ 3, if atrial fibrillation) BP readings, 1-min apart, on each occasion. Morning and evening home BP estimates are the averages of all morning and evening BP readings, respectively, except those obtained on the first day. The measurement frequency, timing, and number per occasion can be individualized to improve adherence and to establish the measurement habit.
3.7 Use of oscillometric blood pressure devices in patients with atrial fibrillation
The most prevalent cardiac arrhythmias in adults is atrial fibrillation (AF), in which hypertension is the most common comorbidity.99 According to the reimbursement database in Taiwan, the proportions of hypertensive subjects increased with CHA2DS2-VASc score (43.2%, 78.4%, 87%, 89.9% in score 1, 2, 3, and ≥ 4, respectively).100 Uncontrolled hypertension predisposes AF patients toward increased risk of stroke,101 which renders the detection and management of hypertension an utmost importance in AF patients.
The current automated BP monitors utilize oscillometric pressure wave amplitude during cuff deflation or inflation to determine SBP and DBP.102 The irregular R-R interval in AF results in less accurate BP values in these patients. How to measure BP in AF patients accurately remains challenging. It has been shown that increasing the number of consecutive measurements to ≥ 3 can achieve a better correlation of BP obtained from the noninvasive method and invasive BP measurements.103 Since the validation studies conducted in general population might not be applicable to AF patients, ANSI/AAMI/ISO currently considers patients with AF as a special population and requires additional validation studies. BP monitoring which has been validated specifically in AF patients should be recommend for HBPM in this special population.104
A progress in BP monitors is to combine with other diagnostic modalities, for example, single-lead electrocardiogram.105 The device can simultaneously monitor electrocardiogram and obtain BP readings. The sensitivity of atrial fibrillation detection was approximately 100% compared to 12-lead electrocardiogram. Given that atrial fibrillation might not be symptomatic and could influence the accuracy of BP measurement, this device is of clinical significance.
3.8 Ambulatory blood pressure monitoring
ABPM is generally considered the gold standard for diagnosing hypertension. ABPM can assess 24-hour BP profiles to derive several important parameters that cannot be obtained using any other form of BP measurement. In addition, ABPM parameters provide better information on cardio- and cerebrovascular risk than office BP.106,107 ABPM should be considered in all patients with elevated BP, particularly those with unstable office or home BP, or whom are suspected to have white-coat or masked hypertension, or progressive HMOD.37 ABPM needs to be performed using a validated device with good practice techniques, and has a role both in hypertension diagnosis and in monitoring the response to antihypertensive therapy to ensure strict BP control throughout the 24-hour period.25
The ABPM devices are typically programmed to take BP measurements every 15 to 30 minutes in the daytime and 30-60 minutes at night. ABPM could provide many important information, that includes details of all BP readings showing daytime and nighttime windows with an indication of normal BP, average SBP, average DBP, and heart rate, the percentage change in SBP and DBP at night, and summary statistics for time-weighted average SBP, DBP, and pulse rate for the 24-hour period, daytime, and nighttime, with standard deviations and number of valid BP readings.25 It has been shown that nocturnal (asleep) BP is the most reproducible and reliable ABPM parameter for risk stratification.108,109 Nocturnal hypertension could indicate the presence of comorbidities such as obstructive sleep apnea, and the riser pattern of nighttime BP is associated with a particularly poor prognosis with respect to the occurrence of stroke and cardiac events.110,111 In addition, morning hypertension defined as elevation of averaged BP over the 2 hours after awakening was associated with higher risk of stroke.112 Both HBPM and ABPM could be used to identify morning hypertension.113
3.8.1 Emerging alternative approaches to blood pressure assessment in an ambulatory setting
The BP measurement arena has been greatly expanded with the upsurge in numbers of iPhone and Android apps. Many apps use a combination of finger plethysmography and pulse transit time calculations to estimate BP.119 Non-invasive BP monitors should be approved by the regulatory agency (for example, the Taiwan FDA or FDA) because they are classified as moderate risk medical devices. Some wearable cuffless BP monitors may be accurate if used exactly as directed.120,121 Until more studies investigating the role of wearable BP monitors in clinical practice available, the Task Force recommends using a HBPM device that measures upper-arm BP instead of wrist or finger BP monitors.1,15
3.9 Central blood pressure
It has long been observed that BP levels increase from the central aorta to the peripheral arteries due to the well-recognized BP amplification phenomenon.122 The major determinants of central BP are increased arterial stiffness and wave reflections, which are also the dominant hemodynamic manifestations of vascular aging. However, all BP measuring modalities, including office BP measurement, HBPM, and ABPM, use recordings from the brachial arteries or wrist, which may be different from the central BP measured in the ascending aorta or carotid arteries. A previous cohort study in Taiwan and a meta-analysis suggested that central BP may be more relevant than peripheral BP in predicting HMOD and CV outcomes.123,124 Central and peripheral BP respond differently to antihypertensive medications as shown in previous s randomized controlled trials. The individual discrepancies between central BP and peripheral BP may be substantial and are highly variable, which may be magnified during hemodynamic changes or after pharmacological interventions.125,126 Changes of HMOD indices after antihypertensive medications are more closely related to changes in central BP than peripheral BP.127 Therefore, BP measurements in the peripheral arteries cannot serve as a direct substitute for their central counterpart.128,129 Currently, one can obtain non-invasive central BP with either tonometry-based or cuff-based techniques.128 A previous Taiwan study derived and validated the diagnostic threshold using an outcome-derived approach.130 Recent studies suggested that, as compared with the conventional strategy, it may be more cost-effective to central BP to confirm the diagnosis of hypertension,131 which may cause lesser use of medications to achieve BP control.132
With the available central BP devices burgeoning, a validation standard has been proposed which further classifies central BP devices into two types.133 According to whether BP amplification is preserved, some devices give an estimate of central BP relative to measured brachial BP (type I), while others estimate the intra-arterial central BP (type II). A previous study based on data from the 2013-2016 National Nutrition and Health Survey in Taiwan revealed similar central and brachial SBP and DBP levels.134 Therefore, the same BP threshold as that of HBPM and office BP is recommended for central BP.135 In 2019, to facilitate the clinical application of central BP in the management of hypertension, the THS and TSOC jointly put forward a consensus document on the Clinical Application of Central BP in the Management of Hypertension.128 More clinical trials are required to investigate the comparative effectiveness between these readily available BP monitoring strategies to inform clinical practice decisions.125,136
3.10 Blood pressure variability
BP fluctuations, also coined as BP variability (BPV), constitute a complex phenomenon. BPV has usually been considered a physiological indicator in response to internal and external stimulations.137 It can also be used as a risk predictor for cardiovascular and cerebrovascular events in patients with hypertension and CV diseases, and an index for evaluating the efficacy of antihypertensive medications.138
BPV comprises a range of estimation of the variations in SBP, DBP, or pulse pressure measured within different time frames (e.g., very short-term, short-term, mid-term and long-term) using different methods of measurement (e.g., beat-to-beat, ambulatory, day-to-day, and visit-to-visit BP measurements) and characterized by different patterns (e.g., nocturnal, postural, and postprandial).138 Different statistical indices (e.g., standard deviation, coefficient of variation, variation independent of the mean) were calculated to estimate the fluctuations of BP. In practice, BPV can be classified into short-term (over 24 hours), mid-term (day-to-day), and long-term BPV (visit-to-visit) according to the length of BP recordings, which can be obtained by ABPM, HBPM, and office BP monitoring, respectively.91,137
Increased BPV has been associated with HMOD, stroke, CV events, and mortality even after adjusting for average BP, indicating its independent role as a vascular risk factor.139-144 Recently, the association between BPV and the risk of dementia has also been suggested.145
As shown in a previous study in Taiwan, pressure wave reflection was the major hemodynamic determinant of short-term BPV.146 Different antihypertensive medications might exert variable effects on BPV.147 It has been shown that calcium-channel blocker-based regimen was associated with lower BPV and a lower incidence of stroke than a beta-blocker-based regimen. Among different classes of antihypertensive medications, the one with longer biological half-lives and potentially longer duration of BP-lowering action was considered to lower BPV.148,149 In a recent community study in Taiwan, subjects who had a stable and frequent BP measuring pattern were shown to have a significantly lower BPV.150 In addition, combined DASH diet and low sodium intake can not only lower BP but also reduce BPV.151
4. EVALUATION
Recommendations/Keypoints
• The purposes of physical examination include establishing the diagnosis and determining the severity of hypertension, searching for signs of secondary hypertension and HMOD, and assessing global cardiovascular risk.
• Serial assessment of HMOD to monitor regression determines the efficacy of antihypertensive treatment.
4.1 Medical history
A complete medical history should be taken during the first visit for patients with high BP. The information of interest to clinicians is related to treatment threshold, BP targets, and choice of management strategy. Medical history includes:
– Blood pressure pattern: previous BP levels, hypertension onset time, duration, anti-hypertensive medication use, including effectiveness and intolerance, and adherence to antihypertensive treatment.
– Previous atherosclerotic cardiovascular diseases (ASCVD) and associated risk factors: coronary heart disease (CHD), stroke or transient ischemic attack, diabetes, dyslipidemia, heart failure, renal disease, peripheral artery disease, and sleep disorder such as snoring and sleep apnea. Family history of hypertension and premature CVD should also be inquired.
– Personal history: dietary habit, salt intake, alcohol intake, smoking history, physical activity, exercise habit and personality/psychological state.
– Previous drug history: anti-hypertensive drugs, non-steroid anti-inflammatory drugs (NSAIDs), cyclooxygenase-2 inhibitors, steroids, oral contraceptives, antimigraine medications, antidepressants, cold remedies (containing liquorice or sympathomimetics like pseudoephedrine), herbal medication (such as ma-huang), cocaine, amphetamines, recombinant human erythropoietin, calcineurin inhibitor, systemic or intra-vitreal use of anti-vascular endothelial growth factor (anti-VEGF) antibody (bevacizumab), and certain tyrosine kinase inhibitors (sunitinib, sorafenib, and pazopanib).
– Others: symptoms and signs of hypertension, features favoring secondary hypertension, and possible symptoms of HMOD.
4.2 Physical examination
Physical examination plays an essential role in the assessment of hypertensive patients. The purposes of physical examination include establishing the diagnosis and determining the severity of hypertension, searching for signs of secondary hypertension and HMOD, and assessing global CV risk.152 Initially, BP should be measured correctly. Comprehensive physical examination should include the followings: 1) calculation of body mass index; 2) inspection of Cushingoid appearance including moon face, buffalo hump, truncal obesity, and wide purple striae and acromegaly appearance including abnormal enlargement of peripheral limbs and forehead protrusion; 3) evaluation of optic fundi for hypertensive retinopathy with fundoscopy or fundus camera; 4) palpation of the thyroid gland for goiter; 5) auscultation of carotid, abdominal and femoral bruits for renovascular disease and peripheral artery disease; 6) auscultation over the back for a loud murmur suggesting coarctation of aorta; 7) comprehensive examination of the heart and lungs for left ventricular hypertrophy, and ventricular gallop of congestive heart failure; 8) examination of the abdomen for enlarged kidneys, masses, and pulsation of abdominal aorta; 9) palpation of the lower extremities for edema and pulses; and 10) a complete neurological assessment.152 The aforementioned evaluation should be adapted according to the severity of hypertension and clinical situations.
4.3 Laboratory tests
Laboratory tests aim to search for additional risk factors, provide evidence of secondary hypertension, and look for HMOD (Table 9).153 A more detailed diagnostic work-up should be performed in younger patients, patients with very high BP, and patients with HMOD. Routine tests should be considered in every patient at the first visit. Recommended studies are optional (Table 9). Measurement of urinary albumin excretion or albumin/creatinine ratio is strongly recommended in Taiwan, a country with the highest prevalence of end-stage renal disease in the world.154 High-sensitivity C-reactive protein predicts the incidence of CV events and optimizes the use of statins in hypertensive patients who have a high CV risk.155
Table 9. Evaluation of hypertensive patients: laboratory tests.
| Laboratory tests |
| Routine tests |
| Hemoglobin and hematocrit |
| Serum creatinine with estimated creatinine clearance (Cockroft-Gault formula) or glomerular filtration rate (Modification of Diet in Renal Disease formula) |
| Serum sodium, potassium and calcium |
| Fasting glucose and glycated hemoglobin A1c (HbA1c) |
| Total cholesterol, LDL-cholesterol, HDL-cholesterol and triglycerides |
| Serum uric acid |
| Urinalysis |
| Electrocardiogram |
| Chest X-ray |
| Recommended tests |
| High-sensitivity C-reactive protein |
| Quantitative microalbuminuria/proteinuria |
| Fundoscopy or fundus camera |
| Echocardiography |
| Carotid ultrasound |
| Ambulatory blood pressure monitoring |
| Ankle-brachial index |
| Pulse wave velocity |
| Extended evaluations (domain of the specialist) |
| Further investigations for cerebral, cardiac, renal and vascular damage: mandatory for complicated hypertension |
| Search for secondary hypertension when suggested by history, physical examination or routine tests: measurement of renin, aldosterone, thyroid-stimulating hormone, corticosteroids, catecholamines in plasma and/or urine; angiographies; renal and adrenal ultrasound; computed tomography; magnetic resonance imaging |
HDL, high-density lipoprotein; LDL, low-density lipoprotein.
4.4 Hypertension-mediated organ damage
HMOD is defined by the presence of the structural or functional changes of end organ system caused by elevated BP.1 The end organs include the brain, the eyes, the heart, the kidneys and the blood vessels. The existence of HMOD hallmarks the poor control of hypertension and is associated with increased CV risk and mortality.156 The detection of HMOD can reclassify the Systemic Coronary Risk Estimation (SCORE) risk stratification for the hypertensive patients with low to moderate CV risks and help to select the appropriate drug class with benefit to specific HMOD.157,158 The prevention of HMOD should be a treatment target and a surrogate clinical marker of adequate BP control. Some types of HMOD can be reversed if BP has been treated early and/or aggressively. HMOD can become irreversible if it is caused by long-standing severe hypertension.159,160 Basic HMOD screening is recommended in all hypertensive patients during first visit and further detailed evaluation is required if necessary. Serial assessment of HMOD to monitor regression determines the efficacy of treatment. The various types of HMOD and related screening test are summarized in Table 10.
Table 10. Assessment of hypertension-mediated organ damage.
| Organ | HMOD | Screening test | Indication and interpretation |
| Brain | Stroke (ischemia/hemorrhage) | Brain imaging | To detect brain infarction, microbleeds and white matter lesions in hypertensive patients with neurological symptoms. |
| Transient ischemic attack | Early subclinical changes can be identified by MRI with the highest sensitivity, but routine MRI is not recommended due to costs, and should be evaluated by a specialist. | ||
| Cognitive impairment | |||
| Cognitive function testing | To assess cognition in hypertensive patients with symptoms suggestive of cognitive decline. | ||
| Eyes | Hypertensive retinopathy | Fundoscopy or fundus camera | To detect hypertensive retinopathy (retinal changes, hemorrhages, microaneurysms, hard exudates, cotton wool spots, papilledema, tortuosity and nipping), especially in hypertensive urgencies and emergencies. |
| Heart | LVH | ECG | To screen for LVH, atrial fibrillation, ischemic heart disease and other possible abnormalities, and to record baseline heart rate and rhythm. |
| Atrial fibrillation | The sensitivity of ECG is limited and requires further echocardiography to confirm the diagnosis. | ||
| Heart failure | |||
| Echocardiography | To evaluate cardiac structure and function (ventricular geometry, systolic and diastolic function, left atrial size, aortic root dimensions and subclinical systolic function impairment assessed by myocardial strain). | ||
| Kidney | Chronic kidney disease | eGFR | To evaluate kidney function and detect renal disease. |
| Proteinuria/albuminuria | |||
| Proteinuria | To assess albumin excretion in possible renal disease, the most commonly used tool is UACR in early morning spot urine. | ||
| Blood vessels | Carotid atherosclerosis | Carotid ultrasound | To determine the carotid plaque burden (atherosclerosis), stenosis and IMT, especially in hypertensive patients with cerebrovascular disease. |
| Aortic stiffness | |||
| Aortic aneurysm | |||
| Peripheral artery disease | |||
| Abdominal ultrasound | Evaluate abdominal aorta for the presence of aneurysmal dilatation and vascular disease. | ||
| To evaluate renal size and structure in patients with chronic kidney disease. In addition, renal artery Doppler echo may help to screen for the presence of renovascular disease. | |||
| ABI | To screen for peripheral arterial obstructive disease (lower extremities). | ||
| PWV | To evaluate the degree of arterial stiffness. |
ABI, ankle brachial index; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; HMOD, hypertension-mediated organ damage; IMT, intima media thickness; LVH, left ventricular hypertrophy; MRI, magnetic resonance imaging; PWV, pulse wave velocity; UACR, urinary albumin-creatinine ratio.
5. SECONDARY HYPERTENSION
Recommendations/Keypoints
• Newly diagnosed and/or uncontrolled hypertensive patients with high-risk features should be screened for secondary hypertension (Figure 2, Table 11).
Figure 2.
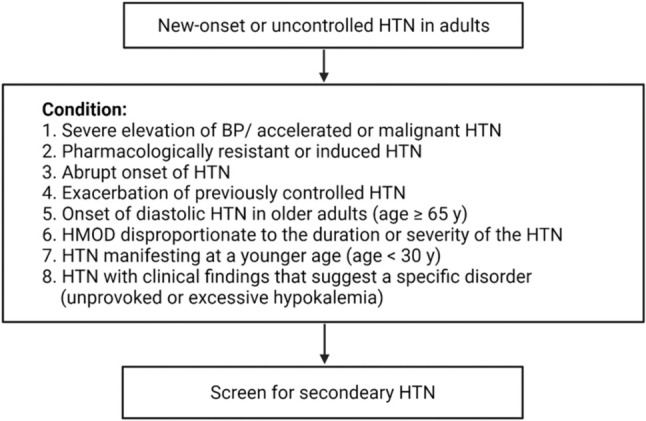
Algorithm of screening for secondary hypertension. BP, blood pressure; HMOD, hypertension-mediated organ damage; HTN, hypertension.
Table 11. Causes of secondary hypertension with clinical indications and diagnostic screening tests.
| Prevalence (HTN) | Prevalence (Resistant HTN) | Clinical Indications | Physical examination | Screening tests | Additional/confirmatory tests | |
| Common causes | ||||||
| Primary aldosteronism | 8-20% | 17-23% | Resistant hypertension; hypertension with hypokalemia (spontaneous or diuretic induced); hypertension and muscle cramps or weakness; hypertension and incidentally discovered adrenal mass; hypertension and obstructive sleep apnea; hypertension and family history of early-onset hypertension or stroke. | Arrhythmias (with hypokalemia); especially atrial fibrillation. | Plasma aldosterone concentration (PAC); plasma renin activity (PRA); plasma aldosterone/renin ratio (ARR). | Oral sodium loading test, IV saline infusion test, or captopril suppression test; adrenal CT or MRI scan, adrenal vein sampling; adrenal scintigraphy. |
| Renal parenchymal disease | 1-2% | 2-10% | Urinary tract infections; obstruction, hematuria; urinary frequency and nocturia; analgesic abuse; family history of polycystic kidney disease; elevated serum creatinine; abnormal urinalysis. | Abdominal mass (polycystic kidney disease); skin pallor. | Serum creatinine, renal ultrasound, urinalysis. | Specific tests to evaluate the cause of renal disease (toxins, biopsy). |
| Renal artery stenosis/renovascular disease | 5-34% | 2.5-20% | Resistant hypertension; hypertension of abrupt onset or worsening or increasingly difficult to control; flash pulmonary edema (atherosclerotic); early-onset hypertension, especially in women (fibromuscular hyperplasia). | Abdominal systolic or diastolic bruit; bruits over other arteries (carotid or femoral artery atherosclerotic stenosis, or fibromuscular dysplasia). | Renal duplex, or CT, or MRI/MRA. | Renal angiography. |
| Obstructive sleep apnea | 25-50% | > 30% | Resistant hypertension; snoring; unrefreshing sleep; breathing pauses during sleep; daytime sleepiness. | Obesity, Mallampati class III-IV; loss of normal nocturnal BP fall. | Berlin Questionnaire, Epworth Sleepiness Score, overnight oximetry. | Polysomnography. |
| Drug or alcohol induced | 2-4% | NA | Sodium-containing antacids; caffeine; nicotine (smoking); alcohol; NSAIDs; oral contraceptives; cyclosporine or tacrolimus; sympathomimetics (decongestants, anorectics); cocaine, amphetamines and other illicit drugs; neuropsychiatric agents; erythropoiesis-stimulating agents; clonidine withdrawal; herbal agents (Ma Huang, ephedra). | Fine tremor, tachycardia, sweating (cocaine, ephedrine, MAO inhibitors); acute abdominal pain (cocaine). | Urinary/hair drug screen (illicit drugs). | Response to withdrawal of suspected agent. |
| Uncommon causes | ||||||
| Pheochromocytoma | 0.1-0.6% | < 1% | Resistant hypertension; paroxysmal hypertension or crisis superimposed on sustained hypertension; “spells,” BP lability, headache, sweating, palpitations, pallor; positive family history of pheochromocytoma/paraganglioma; adrenal incidentaloma. | Skin stigmata of neurofibromatosis (café-au-lait spots; neurofibromas); orthostatic hypotension. | 24-h urinary fractionated metanephrines or plasma metanephrines. | CT or MRI scan of the abdomen/pelvis. |
| Cushing’s syndrome | < 0.1% | < 1% | Rapid weight gain, especially with central distribution; proximal muscle weakness; depression; hyperglycemia. | Central obesity, “moon” face, dorsal and supraclavicular fat pads, wide (1-cm) violaceous striae, hirsutism. | Overnight 1-mg dexamethasone suppression test/24-h urinary free cortisol excretion/midnight salivary cortisol. | Low dose dexamethasone suppression test. |
| Hypothyroidism | < 1% | 1-3% | Dry skin; cold intolerance; constipation; hoarseness; weight gain. | Delayed ankle reflex; periorbital puffiness; coarse skin; cold skin; slow movement; goiter. | Thyroid stimulating hormone; free thyroxine. | None. |
| Hyperthyroidism | < 1% | Warm, moist skin; heat intolerance; nervousness; tremulousness; insomnia; weight loss; diarrhea; proximal muscle weakness. | Lid lag; fine tremor of the outstretched hands; warm, moist skin. | Thyroid stimulating hormone; free thyroxine. | Radioactive iodine uptake and scan. | |
| Aortic coarctation | 0.001 | < 1% | Young patients with hypertension (< 30 years of age). | BP higher in the upper extremities than in the lower extremities; absent femoral pulses; continuous murmur over the back, chest, or abdominal bruit; left thoracotomy scar (postoperative). | Echocardiogram. | Thoracic and abdominal CT angiogram or MRA. |
| Primary hyperparathyroidism | Rare | Rare | Hypercalcemia. | Usually none. | Serum calcium. | Serum parathyroid hormone. |
• Hypertension with secondary causes can co-exist with primary hypertension, in which residual hypertension often remains after those pathogenetic causes are identified and removed.
• Primary aldosteronism is one of the most common causes of secondary hypertension with higher cardiovascular, renal, metabolic and other systemic damages.
• Screening of primary aldosteronism is beneficial because of the good clinical outcomes after appropriate treatment.
• Plasma aldosterone to renin ratio (ARR) is currently the most feasible screening method for primary aldosteronism. ARR is the ratio of plasma aldosterone concentration and plasma renin activity. The most commonly recommended cutoff value of ARR is 30 (or 35) ng/dl per ng/ml/h. The plasma aldosterone concentration of > 10 ng/dL is necessary for positive interpretation of ARR.
5.1 Overview
Secondary hypertension, defined as elevated systemic arterial BP due to an identifiable cause in hypertensive patients.161,162 Patients with secondary hypertension can be cured or experience a marked improvement in BP control, with reduction in CV risk, if a specific cause of hypertension can be correctly diagnosed and treated. All newly diagnosed hypertensive patients with high risk features should be screened for secondary hypertension especially before initiation of treatment.
The prevalence of secondary hypertension varies among selected populations and clinical studies according to age and other clinical conditions such as hypertensive severity, duration, or status of control. Hypertension with secondary causes can co-exist with primary hypertension, in which residual hypertension often remains after those pathogenetic causes are identified and removed.163 The overall prevalence of secondary hypertension is around 10% in hypertensive patients,164 while in patients with resistant hypertension, the prevalence of secondary hypertension is significantly higher (up to 20 to 35%).165,166 Prevalence also varies by the secondary causes. Simplified classification into common causes and uncommon causes is utilized by guidelines with cut-off value of 1% (Table 11).162
Secondary hypertension can manifest with 1) severe elevation of BP, i.e., accelerated or malignant hypertension, 2) pharmacologically resistant or induced hypertension, 3) abrupt onset of hypertension, 4) exacerbation of previously controlled hypertension, 5) onset of diastolic hypertension in older adults (age ≥ 65 years), 6) HMOD disproportionate to the duration or severity of hypertension, 7) hypertension manifesting at a younger age (age < 30 years, although it is not uncommon for primary hypertension), and 8) hypertension with clinical findings that suggest a specific disorder (unprovoked or excessive hypokalemia).
A carefully evaluation of secondary hypertension is crucial, especially in those with a treatable cause, such as primary aldosteronism, drug or alcohol-induced hypertension, renal artery stenosis, obstructive sleep apnea, or the other endocrine hypertension. The detailed list of clinical indications and diagnostic screening tests for secondary hypertension is shown in Table 11 and the list of drugs that can induce secondary hypertension is shown in Table 12. The algorithm of screening for secondary hypertension is shown in Figure 2.
Table 12. Drugs and other substances inducing or exacerbating hypertension.
| Alcohol |
| Amphetamines (eg, amphetamine, methylphenidate dexmethylphenidate, dextroamphetamine) |
| Angiogenesis inhibitor (eg, bevacizumab) and tyrosine kinase inhibitors (eg, sunitinib, sorafenib) |
| Antidepressants (eg, MAOIs, SNRIs, TCAs) |
| Atypical antipsychotics (eg, clozapine, olanzapine) |
| Caffeine |
| Decongestants (eg, phenylephrine, pseudoephedrine) |
| Erythropoietin |
| Herbal supplements (eg, Ma Huang [ephedra], St. John’s wort [with MAOIs, yohimbine]) |
| Immunosuppressants (eg, cyclosporine, tacrolimus) |
| Oral contraceptives |
| Nonsteroidal anti-inflammatory drugs |
| Recreational drugs (eg, “bath salts” [MDPV], cocaine, methamphetamine, etc.) |
| Systemic corticosteroids (eg, dexamethasone, fludrocortisone, methylprednisolone, prednisone, prednisolone) |
5.2 Primary aldosteronism
Primary aldosteronism (PA) is a state of autonomous aldosterone secretion which is unresponsive to renin regulation, resulting in hypertension and electrolyte imbalance. The prevalence of PA ranges from 4% to 20% in hypertensive patients.162,167-169 There are several subtypes of aldosteronism, including bilateral adrenal hyperplasia (BAH), aldosterone-producing adenoma (APA), and familial hyperaldosteronism type I (also named as glucocorticoid-remediable aldosteronism, GRA), type II or type III. The first two subtypes, APA and BAH, account for near 90% of the PA cases. Elevated aldosterone exerts effects on many organs and systems. Compared with patients with essential hypertension, PA patients have increased prevalence of left ventricular hypertrophy, cardiac fibrosis, arterial stiffness, and worse diastolic dysfunction.170-177 PA patients have higher prevalence of stroke, myocardial infarction, atrial fibrillation, and heart failure compared with essential hypertensive patients independent of BP levels.175,178-181 In addition to its detrimental effects on CV system, PA also contributes to metabolic syndrome, renal disease and bone metabolism.182-185 Adrenalectomy can potentially cure APA,174,186 reverse CV remodeling,170,176,187-190 and improved long-term all-cause outcomes.191,192 Timely detection of PA is crucial. Members from the Taiwan Society of Aldosteronism and Taiwan Primary Aldosteronism Investigator (TAIPAI) study group had published consensus documents on case detection/diagnosis193 and treatment of PA.194
5.2.1 Screening
Hypertension and hypokalemia are the typical characteristics of PA. In adults with resistant hypertension, hypokalemia, adrenal mass, young stroke or family history of early-onset hypertension,162 evaluation of PA is suggested. Nevertheless, hypokalemia is not as common as previously recognized,195 and is only identified in approximately 50% of cases.192,196 Normotension may occasionally be found in patients with documented PA. Hence, normokalemia and normotension may not exclude a diagnosis of PA.197 The presence of clinical characteristics listed in Table 13 is recommended to receive screening for PA.
Table 13. Patient characteristics that should be considered for primary aldosteronism screening.
| 1. Persistent systolic/diastolic blood pressure > 150/110 mmHg |
| 2. Resistant hypertension |
| 3. Hypertension with spontaneous or diuretic-induced hypokalemia |
| 4. Hypertension with adrenal mass |
| 5. Early-onset hypertension (< 30 years old) or a family history of early-onset hypertension |
| 6. Cerebral vascular accident at a younger age (< 40 years old) |
| 7. Hypertension with first-degree relatives with primary aldosteronism |
Modified from the consensus of Taiwan Society of Aldosteronism in the detection of Primary Aldosteronism.193
Plasma aldosterone to renin ratio (ARR) is currently the most feasible screening method for PA. ARR is the ratio of plasma aldosterone concentration (PAC) and plasma renin activity (PRA). ARR is most sensitive when samples from patients are collected in the morning. The recommended cutoff value of ARR varies among study groups and societies, ranging from 20-40 ng/dl per ng/ ml/h, with 30 ng/dl per ng/ml/h the most commonly used cutoff value.168 In Taiwan, the TAIPAI study group proposes a cutoff value of 35 ng/dl per ng/ml/h to meet higher specificity.193,198 The major drawback of ARR is that it can be influenced by the presence of very low renin levels with normal or even low plasma aldosterone concentration. Therefore, the plasma aldosterone concentration above 10 ng/dL is necessary for positive interpretation.195,199 Direct renin concentration (DRC) is also widely used instead of PRA. Because the heterogeneity of assay methods for measuring both PRA, DRC and PAC, various cut-off points were used in different centers.
Interpretation of ARR should be cautious for various factors interfering the ARR level. Anti-hypertensive medications have different effects on ARR:168 β-adrenergic blockers, direct renin inhibitor and central α-2 agonist would lower the renin level more than aldosterone, resulting a false positive ARR; whereas dihydropyridine calcium channel blockers,200 diuretics including mineralocorticoid receptor antagonists (MRAs), angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARB)201 cause a false negative ARR. Therefore, these antihypertensive medications with effects on ARR should be discontinued at least 2-4 weeks before ARR testing (4-6 weeks for MRAs).201,202 Besides, NSAIDs and contraceptives may result in a false positive ARR.203 Hypokalemia and sodium restriction status also cause falsely low ARR. Switching to non-dihydropyridine calcium channel blockers, hydralazine, and α1-adrenergic blocker is recommended to maintain adequate BP control in patients undergoing PA screening and confirmation tests.
5.2.2 Confirmation
For patients with screening positive ARR, at least one or more confirmatory tests are indicated to avoid a false positive result. However, in patients with spontaneous hypokalemia combined with PRA below assay detection limits and PAC > 20 ng/dL, confirmation test may not be needed (Figure 3).168 There are four tests currently recommended by the Endocrine Society:168 1) saline infusion test (SIT) in recumbent position; 2) captopril challenge test (CCT); 3) fludrocortisone suppression test, and 4) oral sodium loading test (SLT). The first two, SIT and CCT, are the most widely used168 and with similar accuracy.198,204 Currently, there is no conclusive evidence to recommend one test over the others. In clinical practice, the choice of confirmatory test depends on the considerations of laboratory routine, local expertise, patient compliance, and cost. Recently, Stowasser et al. showed higher sensitivity of seated SIT than traditional recumbent saline infusion test.205 However, there is wide variability in both confirmatory test choice and its cut-off values between centers.204
Figure 3.
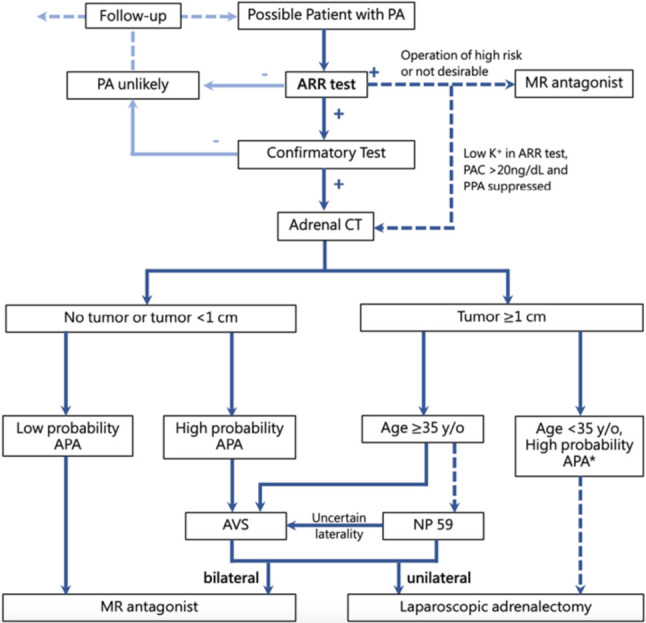
Diagnosis and treatment flowchart for primary aldosteronism. * Patients < 35 years of age with adrenal lesions < 1 cm can also undergo adrenal vein sampling if clinically indicated. APA, aldosterone-producing adenoma; ARR, aldosterone-to-renin ratio; AVS, adrenal venous sampling; CT, computed tomography; NP-59-SPECT, I-131-6-beta-iodomethyl-19-norcholesterol single-photon emission computed tomography.
5.2.3 Lateralization
Distinguishing between unilateral and bilateral disease is important because unilateral adrenalectomy is treatment of choice for unilateral PA. Adrenal venous sampling (AVS) is the recommended test for lateralization. 131I-6β-iodomethyl-19-norcholesterol (NP-59) scintigraphy is a reasonable substitution.193
Abdominal CT is the initial tool for evaluation and to exclude large tumors (> 3 cm in most cases), which may be suspected as adrenocortical carcinoma. However, the evaluation for lateralization of PA by CT is unreliable.206 For example, microadenoma is unlikely to be visualized in CT which may mis-diagnose bilateral PA as unilateral PA. In addition, nonfunctioning incidentaloma, which is not uncommon, is possibly to be interpreted as bilateral PA.207 However, for patients who are younger than 35-year-old with high probability of APA, including recent-onset typical PA presentations (marked PAC overproduction and spontaneous hypokalemia) and imaging evidence of unilateral adrenal nodule (≥ 1 cm), lateralization may not be necessary.208
AVS is the gold standard to distinguish unilateral from bilateral PA.168 AVS is indicated for patients who are going to undergo surgery to avoid unnecessary adrenalectomy. AVS can help physicians to distinguish which side to undergo adrenalectomy, especially in patients with bilateral adrenal adenoma, or with positive ARR but negative CT finding, or with inconclusive NP-59 scintigraphy results. Adrenal scintigraphy using 131I-labled cholesterol analog, such as NP-59, which is uptaken by adrenal cortex in proportion to the degree of hyper-function, is indicated when AVS is not available, contraindicated, or inconclusive compared with CT or MRI results. Although NP-59 scintigraphy is not used in the United States, it is till frequently used in Asia and Europe.209
5.2.4 Treatment
The treatment strategy is based on the lateralization result.193 Surgical treatment is recommended for lateralized PA and medical treatment is suggested for non-lateralized PA, PA patients of high surgical risk or no desire for operation.194 Both strategies could improve the outcomes of PA patients. An analysis of Taiwan National Health Insurance data suggested a reduced hazard ratio in all-cause mortality or mortality plus new-onset atrial fibrillation in PA patients receiving adrenalectomy but not MRA treatment.210 However, whether operation leads to a better long-term outcome than MRA treatment is still controversial.191,211,212
Laparoscopic adrenalectomy is the gold standard treatment for unilateral PA. It reduces long-term all-cause mortality independent of the effects on hypertension.191 By the Primary Aldosterone Surgical Outcome (PASO) study, the results of adrenalectomy to unilateral PA could be classified into 6 categories: complete, partial, and absent success of clinical and biochemical outcomes.213 In PASO, female and younger PA patients had higher likelihood of complete clinical success or clinical benefits (complete plus partial clinical success). Those with more pre-operative antihypertensive medications were less likely to have complete clinical success. In researches from the TAIPAI study group, patients with APA would have decreased long-term mortality,191 renal function progression,214 stroke risk,215 new onset-heart failure216 and atrial fibrillation,210 improved LV diastolic dysfunction,176 and reversed myocardial fibrosis,192 arterial wall thickness190 and stiffness190,217 after adrenalectomy.
MRA is the drug of choice to treat non-lateralized PA or for those with no desire for surgery. Spironolactone is the first-line medication effective in blocking the influences from excessive secretion of aldosterone. However, it lacks specificity while might also work as androgen receptor antagonist and result in gynecomastia and impotence in men.218 It would also act as progesterone receptor agonist and cause amenorrhea in pre-menopausal women.219 Eplerenone is a second generation MRA much more selective to mineralocorticoid receptor (MR) than spironolactone, with potent BP lowering and aldosterone blocking effects.220 Finerenone is a novel non-steroid MRA that could have both high potency and high MR selectivity. Compared with spironolactone, it has a lower risk for hyperkalemia.219 However, its clinical role is still under investigation in clinical trials.219,221
5.3 Renal parenchymal disease
Renal parenchymal disease is the one of the leading causes of secondary hypertension in adult hypertensive patients.161,163 Bilateral abdominal masses palpated during physical examination warrant survey of polycystic kidney disease. Serum creatinine concentration and urinalysis (protein, erythrocytes, and leukocytes) are the best screening tests for renal parenchymal disease.161-163 Renal ultrasound evaluation of kidney morphology and identification of abnormal masses or urinary tract pathology can further provide clues about etiology and pathogenesis.161-163 Other tests to evaluate causes of renal disease would be indicated if specific renal disease suspected.
5.4 Renovascular disease and renal artery stenosis
Renal artery stenosis (RAS) results from narrowing of renal artery causing restricted kidney blood perfusion. The most common cause of RAS in adult patients is atherosclerotic disease. Nonatherosclerotic disease such as fibromuscular dysplasia is the most common cause of RAS in young adults.162
Clinical conditions suggesting RAS include abdominal bruits, signs and symptoms of peripheral vascular disease, and multiple risk factors contributing to generalized atherosclerosis. Resistant hypertension, recent onset or progression of severe hypertension, recent renal function deterioration, acute renal function deterioration after ACE inhibitors or ARB usage, and flash pulmonary edema are other clinical clues pointing to RAS.163 RAS could be screened with renal duplex and doppler ultrasound, abdominal MRA or CT, and further confirmatory tests.161-163
Current guidelines recommend medical therapy optimization for hypertension and risk factor control for adults with atherosclerotic RAS because prior studies failed to show clinical advantages with endovascular intervention.162,222 Revascularization with angioplasty or stenting may be considered only if failed medically controlled atherosclerotic RAS (refractory hypertension, worsening renal function, and/or intractable heart failure). Revascularization with angioplasty (but not stenting) was effective in patients with nonatherosclerotic RAS due to fibromuscular dysplasia.
5.5 Obstructive sleep apnea
Obstructive sleep apnea (OSA) is caused by recurrent and intermittent upper airway collapse during sleep, inducing apnea or hypopnea, hypoxemia, and sleep disruption. This chronic medical condition correlates with other systemic diseases and presents as a strong risk factor for including hypertension, coronary and cerebrovascular diseases, heart failure, and atrial fibrillation.223-226 OSA is highly prevalent in hypertensive adults, especially in patients with resistant hypertension, with variant prevalence of 60-80% in different studies.227,228 Clinically, patients with OSA often present with obesity, large neck, and macroglossia and complain of daytime somnolence, impaired concentration, snoring during sleep and witnessed apneas. In addition, nocturnal non-dipper pattern, elevated daytime BP, tachycardia and/or bradycardia are frequently seen during ambulatory BP testing in OSA patients. OSA could be screened with questionnaire of Berlin Questionnaire or Epworth Sleepiness scale. Once positive, further gold standard diagnostic tool, polysomnography, can be used and the severity of OSA can be evaluated based on the apnea-hypopnea index.162,163 Although continuous positive airway pressure (CPAP) is effective in treating OSA, the effects on hypertension are small, about 2-3 mmHg reduction.162
5.6 Drug or alcohol-induced secondary hypertension
Medication history should be carefully reviewed since BP is affected by numerous substances, including prescription medications, over-the-counter medications, herbals, and food substances (Table 12).166,229 Substance affects BP through several mechanisms: substance itself is associated with hypertension development, drug-drug, or drug-food interactions, which are associated with the development of hypertension, worsening control of previously well-managed hypertension, or attenuation of the BP-lowering effects of pre-existing antihypertensive therapy. When feasible, drugs affecting BP should be reduced or discontinued, and alternative agents should be used; new or pre-existing antihypertensive therapy should be adjusted according to individual’s BP status.
5.7 Other endocrine disorders
Pheochromocytoma, Cushing’s syndrome and thyroid disorder are rare in hypertensive patients with unique clinical presentation. In pheochromocytoma, paroxysmal increase of plasma catecholamine causes intermittent hyperadrenergic spells, which induces clinical symptoms such as paroxysmal hypertension, palpitation, perspiration, pallor, and pounding headache.161-163 Twenty-four-hour urine catecholamines and metanephrine or plasma fractionated metanephrine are used as screening tools. Abdominal CT or MRI and scintigraphy localization are indicated as confirmatory tests.162,163
Hypertension is commonly found in 80% of patients with Cushing’s syndrome. Long-term excessive endogenous or exogenous glucocorticoids can cause a typical body habitus with central obesity, facial plethora, buffalo hump, hirsutism, and purple striae. Overnight 1 mg dexamethasone suppression test and 24-hour urinary free cortisol excretion are both used as screening test.162
Both hypothyroidism and hyperthyroidism could cause secondary hypertension. Body compensation to low cardiac output with increased systemic vessel resistance raises diastolic pressure in patients with hypothyroidism; high cardiac output causes raised systolic pressure in patients with hyperthyroidism. Free thyroxine and thyroid-stimulating hormone plasma concentrations are the screening method of choice.162,163
6. PRINCIPLES OF HYPERTENSION MANAGEMENT
Recommendations/Keypoints
• Lifestyle modification (LSM)-based non-pharmacological therapy should be applied to people with elevated BP and hypertensive patients to reduce life-time BP burden (COR I, LOE A).
• A BP level of ≥ 140/90 mmHg should be the threshold for low-risk (no established ASCVD or HMOD, and < 3 ASCVD risk factors) hypertensive patients to initiate pharmacological treatment (COR I, LOE A).
• For the other hypertensive patients, a BP level of ≥ 130/80 mmHg is recommended as the threshold to initiate pharmacological treatment (COR I, LOE A).
• The Task Force recommends a universal BP target of < 130/80 mmHg, based on HBPM obtained according to the 722 protocol, for all hypertensive patients (COR I, LOE A).
• The Task Force recommends that the SBP target can be < 120 mmHg for patients with ASCVD or at high CV risk, if tolerable (COR IIa, LOE B).
• The lower limit of BP targets is highly variable and hard to define. The Task Force recommends relaxing the BP target if symptoms or signs of end-organ hypoperfusion ensue (COR IIb, LOE C).
• Overall CV risk assessment should be done at the diagnosis of hypertension and at least once a year to assess the adequacy of hypertension management (COR I, LOE C).
6.1 Objectives and thresholds of hypertension management
The objectives of antihypertensive treatment are to prevent the development and progression of atherosclerotic cardio- and cerebrovascular diseases. Effective BP control can even reverse the existing atherosclerotic vascular changes.230,231 According to the most recently published meta-analysis of 344,716 individual participant-level data from 48 randomized trials of antihypertensive treatment, a 5 mmHg decrease in SBP reduced the risks of major CV events by 10%, stroke by 13%, ischemic heart disease by 8%, heart failure by 13%, CV mortality by 5%, and all-cause mortality by 2% after a median 4.2 years’ follow-up.3 The extent of reduction in the relative risk for CV diseases achieved by antihypertensive treatment did not differ significantly among subjects who have different ages, sex, presence or absence of associated diseases, or baseline SBP levels (ranging from < 120 to ≥ 170 mmHg).3,232,232 It should be emphasized that the average major CV event rate was > 3.0% annually in these meta-analyses, suggesting the majority of patients enrolled were at high CV risk. These lines of evidence indicate that a fixed degree of pharmacological BP lowering can confer CV benefits for high-risk patients with SBP ranging from < 120 to ≥ 170 mmHg. On the other hand, there is still no evidence to demonstrate a clear CV or survival benefit of a fixed degree of pharmacological BP lowering in patients with low CV risk (10-year ASCVD [nonfatal myocardial infarction, stroke, or CV death] risk < 5%) and baseline SBP < 140 mmHg after 3-5 years’ follow-up.9,233-236 According to ESC/ESH, ISH, and JSH guidelines, low-risk status is defined as patients with fewer than 3 CV risk factors and no evidence of HMOD or established ASCVD (stage 1, risk factors < 3, and BP < 140/90 mmHg in Figure 4).10,11,13 The Task Force thus recommends that a BP level of ≥ 140/90 mmHg should be the threshold for low-risk (no ASCVD or HMOD, and number of ASCVD risk factors < 3) hypertensive patients to initiate pharmacological treatment (COR I, LOE A). For the rest of hypertensive patients, a BP level of ≥ 130/80 mmHg is recommended as the threshold for initiation of pharmacological treatment (COR I, LOE A). However, mid-life elevated BP was associated with long-term (> 15 years) CV and dementia events.237,238 Lifestyle modification (LSM)-based non-pharmacological therapy should be applied to people with elevated BP and hypertensive patients to reduce life-time BP burden, which seems to be the root cause of all vascular events.239
Figure 4.
Risk chart-based blood pressure thresholds and targets for the initiation of pharmacological treatment of hypertension. ASCVD, atherosclerotic cardiovascular disease; BP, blood pressure; CKD, chronic kidney disease; DBP, diastolic blood pressure; DM, diabetes mellitus; HBPM, home blood pressure monitoring; HMOD, hypertension-mediated organ damage; SBP, systolic blood pressure.
6.2 Risk chart-based universal blood pressure targets and management strategy
There are two principles we have to emphasize before the introduction of risk chart-based BP targets for pharmacological antihypertensive treatment. First, all individuals with BP levels of ≥ 120/80 mmHg require lifestyle modifications to keep their BP below 120/80 mmHg, or higher if not tolerable, to alleviate life-time BP burden. Second, healthcare professionals should instruct patients to measure their BP at home, preferably following the "722" protocol and instructions (Figure 1 and Tables 6 and 7).1 The Task Force recommends that healthcare professionals should use HBPM, rather than non-standardized ROBP, to guide their decisions regarding hypertension management, especially if there is a large discrepancy between office BP and averaged home BP.
Given that the purpose of BP control is to prevent the occurrence of CV events, we should consider the two essential factors, the magnitude of BP reductions and inherent CV risk, which determine the absolute benefits obtained from BP management, in recommending BP targets and designing trials to fill the evidence gap. The combination of risk chart and BP targets embodies the comprehensive consideration in the determination of hypertension management strategy (Figure 4). This is also the first-ever risk chart-based BP thresholds and targets to facilitate its implementation. In the risk chart, different categories of BP are listed in the horizontal axis, whereas different categories of overall CV risks are listed in the vertical axis. We categorize BP itself into different "grades". While we categorize overall CV risks as "stages" to make this classification scheme consistent with that being used in the classification of heart failure.240,241 Further, stages, compared with grades, have a broader meaning concerning BP burden, damages incurred, and prognostic prediction.
Risk factors (stage 1), the extent of subclinical HMOD (stage 2), and the existence of ASCVD and hypertension-related CVD (stage 3) are incrementally associated with worse prognosis in hypertensive patients, as shown in cohort studies worldwide and several meta-analyses of antihypertensive drug trials. To determine the BP targets in each category of the risk chart, we should consider the balance of benefits and harms associated with BP reductions, as well as evidence from high-quality randomized controlled trials (RCTs). The following consensus was reached in the Task Force. First, pharmacological BP lowering to < 130/80 mmHg can generally confer CV benefits in patients with an annual CV event rate of > 1.0% (based on the broader definition of ASCVD), as demonstrated in the STEP, SPRINT, HOPE-3, and various meta-analyses.3,8,9,234 Second, among patients with an annual CV risk of > 3% (very high risk, Figure 4), further BP reductions down to < 120/80 mmHg seem beneficial, particularly for patients with their SBP in the range between 120-130 mmHg, based on evidence from the meta-analyses3,232 and the 2021 KDIGO guideline.12 Third, the Task Force recognizes that, together with aggressive BP targets, excessive BP reductions can cause harms.242 Lowering BP below a critical limit may compromise organ perfusion despite adequate physiological adaptation.243 End-organ damage can be further precipitated by blunted vasoregulatory responses with anti-hypertensive therapy.244 Tolerability to the BP target is the first priority in hypertension management. The Task Force recommends to relax the BP target once symptoms or signs of end-organ hypoperfusion ensue (COR IIb, LOE C). There is no RCT designed to explore the lower limit of target BP levels. All suggestive reports are from post-hoc analyses, which cannot exclude the possibility of reverse causality (see Section 6.3). The Task Force therefore considered the lower limit of BP targets is highly variable and hard to define. Age alone is not a prerequisite for poor tolerability to aggressive BP targets. Instead, both SPRINT and STEP trials demonstrated that, compared to younger adults, older adults (70-80 s) are associated with similar relative risk reductions and greater absolute risk reductions with the SBP target of < 130 mmHg compared to ≥ 130 mmHg. Finally, mid-life elevated BP was associated with long-term (> 15 years) CV and dementia events even in low-risk patients.237,238 Taken together, the Task Force recommends a universal BP target of < 130/80 mmHg, based on HBPM obtained according to the 722 protocol, for all hypertensive patients (COR I, LOE A).9,234,245,246 The Task Force also recommends that the SBP target can be < 120 mmHg for patients with established CV diseases or at high CV risk, if tolerable (COR IIa, LOE B) (Figure 4).
The evidence supporting the recommended BP targets is from standardized office BP obtained in RCTs. In the 2017 American hypertension guidelines, the corresponding values of home BP are the same as standardized office BP for 130/80 mmHg and 120/80 mmHg. The targets of home BP are set 5 mmHg lower (135/85 mmHg) than standardized office BP for 140/90 mmHg in most hypertension guidelines.10,11,13 However, according to the 11-year follow-up data of 5,768 participants from the Dallas Heart Study, office SBP threshold of 140 mmHg was equivalent to home BP threshold of 140 mmHg by outcome-derived approach and 135 mmHg by regression-based approach.48 Given that outcome-derived approach is of greater clinical significance, the Task Force recommends all three BP cut-off values, 140/90 mmHg, 130/80 mmHg, and 120/80 mmHg, are identical for home BP and office BP to facilitate implementation.
Risk factors used in the risk chart include advanced age (≥ 65 years), male sex, dyslipidemia, smoking, family history of premature ASCVD (onset < 50 years of age) and gestational hypertension or preeclampsia with adverse pregnancy outcomes (see Section 18.2) (Figure 4). We adopt the age criterion (65 years and over) from JSH guidelines since, compared to Western populations, the incidences of CV events are much closer between Japanese and Taiwanese populations. Obesity is not included because the results regarding its prognostic significance are conflicting.247,248 Stage 2 is defined as stage 3 chronic kidney disease (CKD) or with proteinuria, diabetes mellitus without organ damage, or HMOD, including left ventricular hypertrophy, increased arterial stiffness (increased pulse wave velocity), and obstructive atherosclerosis and carotid artery plaques/stenosis (See Section 4). Stage 3 is defined as stage 4 or 5 CKD, diabetes mellitus with organ damage, nonvalvular atrial fibrillation, and established cardio- and cerebrovascular diseases (brain hemorrhage, brain infarction, acute coronary syndrome/myocardial infarction, prior coronary or peripheral intervention, peripheral artery disease, heart failure, and aortic dissection). The Task Force recommends that overall risk assessment should be done at the diagnosis of hypertension and at least once a year (COR I, LOE C). Assessment of the severity of HMOD should be used as a guide to evaluate the appropriateness of BP-lowering therapy. Once there is progression in HMOD, more aggressive and sustained BP control should be considered.
In both American and European guidelines, the absolute CV risk in patients without established CV diseases is also assessed by calculating the risk score (Atherosclerotic Cardiovascular Diseases Risk Score and Systematic Coronary Risk Estimation [SCORE], respectively).249,250 There are at least the following limitations which make applying these scores directly in Taiwan not appropriate: first, the absolute incidences of various CV diseases in Taiwan and Western societies are different; second, these scores have not been adequately validated in Taiwanese population; and third, it is not convenient to calculate these scores without the help of App or software.
6.3 J-curve revisited
Coronary blood flow predominantly occurs in the diastole, and a myocardial perfusion pressure of < 40 mmHg may cease coronary blood flow.251 It is generally believed that "J-curve" phenomenon is true, and there must be a lowest value of DBP (nadir) and a level lower than that nadir may compromise coronary blood flow. The questions are where the nadir is, and does this J-curve phenomenon also apply to SBP.
Evidence from large-scaled epidemiological studies in healthy people did not support the concept of J-curve phenomenon. In a meta-analysis of 61 prospective cohort studies comprised of 1 million subjects with or without risk factors, but free from CV diseases, both CHD and stroke mortality appeared to increase at around 115/75 mmHg, without any J-curve phenomenon above these levels.252 In a cohort of 1.25 million subjects, initially free from CV disease, the lowest risk for CV disease was in people with SBP of 90-114 mmHg and DBP of 60-74 mmHg, without any evidence of J-curve phenomenon above these levels.4 Based on 1.3 million adults in a general outpatient population from Kaiser Permanente Northern California, the composite CV endpoints were lowest at SBP of 110 mmHg and at DBP of 62 mmHg, after adjustment of age and other covariates.253 Among 1,235,246 individuals who participated in routine medical examinations in Korea, the hazard ratios (HRs) were adjusted for potential confounders. During 22.7 million person-years of follow-up, an increase in SBP was directly related to an increase in vascular mortality at SBP above 100 mmHg. SBP < 90 mmHg may portend death from vascular causes, particularly from ischemic heart disease.254 These data suggested that SBP can be safely reduced to a level of 100-110 mmHg, while a DBP around 60 mmHg seemed to be safe.
Among RCTs, it is also uncommon to find any J-curve phenomenon if CV endpoints were evaluated prospectively, though the BP levels obtained in RCTs were generally higher than what we have mentioned in the epidemiological studies. In the three most important RCTs in isolated systolic hypertension (SHEP, Syst-Eur, Syst-China), the risk of stroke was significantly decreased while myocardial infarction was also reduced in the treatment group compared to the placebo group.255-257 No J-curve phenomenon was observed. In fact, the DBP in the treatment group in the SHEP trial was only 68 mmHg, and the risk of myocardial infarction was still significantly reduced by 33%.255 In the final report of the SPRINT trial, comprised of 9,361 patients at a mean age of 67.9 years and pre-treatment SBP of 139.7 mmHg, an intensive treatment target (SBP < 120 mmHg) versus a standard treatment target (SBP < 140 mmHg) reduced composite CV endpoints (HR: 0.73, 95% CI: 0.63-0.86, p value < 0.001) and all-cause death (HR: 0.75, 95% CI: 0.61-0.92, p = 0.006).8 The final achieved SBP was 120.0 mmHg versus 133.9 mmHg and DBP 68.7 mmHg versus 76.3 mmHg,8,258 with a significant reduction in myocardial infarction (HR: 0.72, 95% CI: 0.56-0.93, p = 0.01).8 These evidence suggested that SBP and DBP could be safely reduced to 120 mmHg and 70 mmHg, respectively.
Most of the data claiming a J-curve phenomenon came from post-hoc analyses of RCTs in patients with pre-existing CVD or high CV risk.259-264 For example, in the post-hoc analysis of the ONTARGET trial, the achieved SBP < 130 mmHg had higher CV event rates compared with those who achieved SBP > 150 mmHg. This may be due to higher ages and higher percentages of pre-existing CVD and other unmeasured confounders in the former group (reverse causality).261 Similar findings were observed in the CLARIFY registry and the APPEAR study.265,266 These "Pseudo-J curves" should be interpreted more cautiously.
7. LIFESTYLE MODIFICATIONS
Recommendations/Keypoints
• Lifestyle modification measures can be summarized as the mnemonic S-ABCDE: Sodium restriction, Alcohol limitation, Body weight reduction, Cigarette smoke cessation, Diet adaptation, and Exercise adoption.
• The major limitation of lifestyle modification is poor persistence over time. Cognitive behavioral strategies and multimodal interventions are recommended to facilitate LSM (COR I, LOE C).
• Sodium intake should be restricted within 2-4 g/day (5-10 g of salt per day) for a better BP control and a lower CV risk (COR I, LOE A).
• People without a habit of alcohol consumption should not start drinking for any reason (LOR III, LOE C).
• Alcohol consumption should be limited to < 100 g/ week (14 g/day or 1 drink/day) in men and < 50 g/ week (7 g/day or 0.5 drinks/day [one standard drink = 14 g pure alcohol]) in women without the ALDH2*2 dysfunctional allele to improve BP control and lower the risk of all-cause mortality (LOR I, LOE A).
• Alcohol consumption should be limited to < 64 g/week (9 g/day or 4 drinks/week) in men and < 28 g/week (4 g/day or 2 drinks/week) in women with the ALDH2*2 dysfunctional allele to improve BP control and lower the risk of all-cause mortality (LOR IIa, LOE B).
• Binge drinking (defined as ≥ 5 and ≥ 4 drinks for men and women, respectively, in 2 hours) should be strictly prohibited to reduce BP, as well as the risk of atrial fibrillation, stroke and sudden death (LOR III, LOE C).
• An ideal body mass index is 20-24.9 kg/m2 to improve BP control and lower the risk of all-cause mortality (COR I, LOE A).
• Cessation of cigarette smoking, irrespective of conventional or electronic cigarettes, should be an integral part of LSM to reduce overall CV risk (COR I, LOE A).
• DASH diet is recommended to improve BP control and reducethe overall CV risk (COR I, LOE A).
• Consumption of green tea and black tea can reduce both SBP and DBP (COR IIa, LOE B).
• Regular aerobic exercise (at least 30 min of moderate-intensity exercise on 5-7 days/week), with or without resistance exercise, is recommended to improve BP control and reduce CV mortality (COR I, LOE A).
• High-intensity exercise is not recommended for patients with uncontrolled hypertension (SBP > 160 mmHg) (COR III, LOE C).
• Neuromotor exercise or training, such as tai chi, yoga, and meditation, can be suggested to reduce BP (COR I, LOE B).
• Moderate-intensity outdoor exercise can be performed with a background PM2.5 concentration of < 54.4 μg/ m3, and the intensity is unlimited with a concentration of < 15.4 μg/m3 (COR IIa, LOE C).
• An air cleaner to remove PM2.5 with active filtration may be beneficial for BP reduction (COR IIb, LOE B).
Healthy lifestyle can effectively modify and prevent CV risk factors, including hypertension, and is highly recommended for general population.267,268 Sticking to lifestyle modification (LSM) is able to delay the initiation of pharmacological therapy in patients with elevated BP or grade 1 hypertension269 and augment the effect of BP-lowering therapy. LSM should never delay the initiation of drug therapy in patients with HMOD or a high CV risk.10 The major limitation of LSM is poor persistence over time.270 Although there were trends of improving the prevalence of healthy lifestyle in general population according to the surveys in the United States and Germany, the prevalence rates were actually only ~3-7%.271,272 Therefore, cognitive behavioral strategies and multimodal interventions have been highly suggested to facilitate LSM.267 LSM can be summarized as the mnemonic S-ABCDE: Sodium restriction, Alcohol limitation, Body weight reduction, Cigarette smoke cessation, Diet adaptation, and Exercise adoption (Table 14).
Table 14. Lifestyle modifications for the management of hypertension (S-ABCDE).
| Modification | Recommendation | Expected benefits in SBP reduction | COR | LOE |
| Sodium restriction | 2-4 g/day (5-10 g of salt per day) | 3.1 mmHg per 1 g/day of sodium reduction | I | A |
| Alcohol limitation | 1. People without a habit of alcohol consumption should not start drinking for any reason (LOR III, LOE C). | III | C | |
| 2. Alcohol consumption should be limited to < 100 g/week (14 g/day) in men and < 50 g/week (7 g/day) in women without the ALDH2*2 dysfunctional allele | 2-4 mmHg | I | A | |
| 3. Alcohol consumption should be limited to < 64 g/week (9 g/day or 4 drinks/week [one standard drink =14 g pure alcohol) in men and < 28 g/week (4 g/day or 2 drinks/week) in women with the ALDH2*2 dysfunctional allele | IIa | B | ||
| 4. Binge drinking (defined as ≥ 5 and ≥ 4 drinks for men and women, respectively, in 2 hours) should be strictly prohibited to reduce BP, as well as the risk of atrial fibrillation, stroke and sudden death (COR III, LOE C). | III | C | ||
| Body weight reduction | An ideal BMI is 20-24.9 kg/m2 | A weight reduction of 5.1 kg reduces SBP by 4.44 mmHg (approximately 1 mmHg reduction in SBP per 1 kg reduction) | I | A |
| Cigarette smoking cessation | Complete abstinence irrespective of conventional or electronic cigarettes | No independent effect | I | A |
| Diet adaptation | 1. DASH diet: high quantity of fruits and vegetables, low-fat dairy foods, whole grains, nuts, fish, and poultry, but reduced amounts of red meat, beverages, saturated fat, sweets and snacks | 10-12 mmHg | I | A |
| 2. Green tea or black tea | 1-2 mmHg | |||
| Exercise adoption | 1. Regular aerobic exercise (at least 30 min of moderate-intensity exercise on 5-7 days/week), with or without resistance exercise | 3-11 mmHg | I | A |
| 2. Neuromotor exercise or training, such as tai chi, yoga, and meditation | 6-14 mmHg | I | B | |
| 3. High-intensity exercise is not recommended for patients with uncontrolled hypertension (SBP > 160 mmHg) | ||||
| 4. Moderate-intensity outdoor exercise can be performed with a background PM2.5 concentration < 54.4 μg/m3, and the intensity is unlimited with a concentration < 15.4 μg/m3 | III | C |
BMI, body mass index; COR, class of recommendation; DASH, dietary approaches to stop hypertension; LOE, level of evidence; SBP, systolic blood pressure.161
7.1 Sodium restriction
It is a generally accepted concept that a reduction in sodium intake reduces BP. A modest reduction in sodium intake of 1 g/day has led to SBP reduction by 3.1 mmHg in hypertensive and by 1.6 mmHg in normotensive subjects from an early meta-analysis.273 In agreement with this finding, the PURE study measured 24-hour sodium excretion in urine from 102,216 participants from 18 countries and found a similar result, with greater BP reduction in response to sodium restriction observed in the older and hypertensive participants.274 It is also reported that salt restriction has a more prominent effect in patients with metabolic syndrome, diabetes, and CKD.275 The benefit of this BP reduction from sodium restriction also reflected on the subsequent CV outcomes, with the optimal range of sodium intake estimated to be 3-6 g/day for a lower risk of death and CV events.276 A more extensive study, NUTRICODE, collected sodium intake in persons from 66 countries (3,830 millions) and calculated its global impact on CV mortality by 107 randomized interventions. In this modeling study, 1.65 million CV deaths occurring in 2010 were attributed to sodium intake above 2 g/day.277 The durations of most sodium intake interventions are less than 3 months. TOHP trial is known for their long-term intervention, with follow-up duration up to 18-48 months and net sodium excretion reduction by 0.76-1.0 g/day. TOHP study observed a 30% reduction of CV events in the intervention group.278 On the other hand, an increase of sodium intake significantly raised stroke and coronary mortality in a meta-analysis.279
The J-curve phenomenon between sodium intake and CV outcomes had been noticed in the PURE study, with an increase of composite CV events (CV death, myocardial infarction, stroke, and heart failure) in subjects taking less than 3 g/day of sodium.276 A meta-analysis of pooled 4 prospective cohort studies, including 133,118 participants, also demonstrated a consistent trend of increased CV events among participants who had less than 3 g/day of sodium intake, despite a continuous BP reduction effect still observed along with sodium restriction to below 3 g/day. Interestingly, this phenomenon was robust irrespective of patients with or without hypertension.280 The J-curve phenomenon in terms of BP reduction was also demonstrated in a Taiwanese prospective cohort study which enrolled 1,520 participants to observe the relationship between the incidence of hypertension and urinary excretion of sodium during a median follow-up period of 7.93 years. The nadir of risk for incident hypertension occurred at 100 mmol/day (~2.3 g/day) of sodium intake.281 The mechanism of the increased risk at low sodium intake remains unclear and might be confounded by reverse causality.
Taken together, the Task Force recommends restricting sodium intake within 2-4 g/day (5-10 g of salt per day). A vigorous reduction of sodium intake to < 2 g/day is difficult in real-world practice and might be harmful in terms of a paradoxical increase of CV events. It is estimated that 80% of daily salt intake comes from processed food, therefore more basic food consumption is recommended for an optimal sodium restriction.267 Apart from sodium intake, a growing body of evidence also shows that potassium supplement is beneficial for better BP control and CV outcomes.276,282,283 The PURE study demonstrated that each increment of 1 g in estimated potassium excretion per day, there was a decrement of 0.75 mmHg in SBP, and this benefit was seemed to be dominant in Chinese participants.274 Mirroring this benefit, a lower risk of death and CV events was also observed in those with an estimated potassium excretion of > 1.5 g/day as compared to those of < 1.5 g/day.276 The recently published the Salt Substitute and Stroke Study (SSaSS) examined whether salt substitutes (75% sodium chloride and 25% potassium chloride by mass), compared to regular salt (100% sodium chloride), could provide beneficial effects on CV and safety outcomes in an open-label, cluster-randomized trial involving persons from 600 villages in rural China.283 A total of 20,995 persons who had a history of stroke or aged ≥ 60 years and had SBP ≥ 140 mmHg if receiving antihypertensive medications or ≥ 160 mmHg if not were enrolled. After a mean follow-up of 4.74 years, the mean difference in 24-hour urinary sodium excretion, 24-hour urinary potassium excretion, and SBP between the salt-substitute group and the regular-salt group was -350 mg, 803 mg, and -3.3 mmHg, respectively. The rate of stroke was 14% lower (29.1 events vs. 33.7 events per 1000 person-years; rate ratio: 0.86; 95% CI: 0.77 to 0.96; p = 0.006) with the salt substitute than with regular salt, as were the rates of major CV events (13% relative risk reduction) and death (12% relative risk reduction). There was no difference in adverse events attributed to hyperkalemia.
7.2 Alcohol limitation
It is not recommended that individuals without a habit of drinking alcohol start drinking for any reason.284 Contrary to previous results from epidemiologic studies and related meta-analyses which suggested a lower risk of CVD in subjects with moderate alcohol consumption (< 60 g/day) compared with non-drinkers,285 a more recent large-scale Mendelian randomization study shed a skeptical view on any potential benefit of moderate alcohol consumption.286 This study performed Mendelian randomization meta-analysis of 56 epidemiological studies and found that alcohol dehydrogenase 1B (ADH1B) variant allele carriers who had higher abstention, lower alcohol consumption, and lower prevalence of binge drinking had significantly lower SBP (-0.88 [-1.19–-0.56] mmHg) and, more importantly, lower risks of coronary artery disease (OR 0.90 [0.84-0.96]) and ischemic stroke (OR 0.83 [0.72-0.95]).286 Another more recent study analyzing 599,912 current drinkers from 83 prospective studies clearly demonstrated that all-cause mortality started to rise for drinkers consuming > 100 g/week of alcohol compared to those consuming 0-25 g/week, even though they indeed had a lower risk for myocardial infarction.287 Moreover, the report also revealed that the younger the drinkers were, the more years-of-life were lost. Consistent with this finding, another analysis aiming at elucidating the global disease burden due to alcohol use from 195 countries concluded that the risk of all-cause mortality rose along with increasing quantity of alcohol consumption. The consumption level that minimized health loss was actually "zero".149
Taken together, growing evidence suggests that the overall detrimental effect from moderate alcohol consumption outweighs its potential coronary benefit. This harmful effect of alcohol drinking could be even more pronounced in ~40-50% of the Taiwanese people carrying the aldehyde dehydrogenase-2 (ALDH2) dysfunctional allele (the ALDH2*2 variant). The ALDH2*2 dysfunctional allele delays acetaldehyde metabolism after alcohol consumption and causes the "Asian alcohol flushing syndrome" or "alcohol intolerance syndrome".288 The accumulation of toxic and carcinogenic acetaldehyde is known to cause cell damage and health loss.289 A recent large-scale survey comparing conventional with genetic epidemiological analyses (including both ADH1B and ALDH2 variants) from over 500,000 Chinese database revealed a clearer relationship between alcohol use and vascular disease burden. Surprisingly, the J-curve cardiovascular protective effect from moderate alcohol consumption shown in the conventional epidemiological analysis, completely disappeared in the genetic epidemiological analysis. Using the genetic epidemiological analysis, alcohol consumption was shown to be unprotective to coronary events but positively correlated to SBP levels and risk of total stroke.290 In consideration of all above-mentioned evidence, alcohol consumption in current European Society of Cardiology (ESC) guideline for CVD prevention has been reduced to 20 g/day (140 g/week) for men and 10 g/day (70 g/week) for women,267 a limit much stricter than that in the hypertension guidelines,10,135 highlighting the fact that alcohol consumption increases not only BP but also overall CV risk. This is also in line with the current (2018) daily alcohol consumption guideline published by the Health Promotion Administration in Taiwan.291 However, considering a relatively high prevalence of stroke incidences in Taiwan, the Task Force recommends limiting alcohol consumption further to < 100 g/week (14 g/day or 1 drink/day) for men and < 50 g/week (7 g/day or 0.5 drink/day) for women. One standard drink is defined as 14 g of pure alcohol.284 For people carrying the common ALDH2*2 dysfunctional allele (facial flushers or alcohol intolerant), alcohol abstention is recommended. If alcohol consumption is unavoidable, the Task Force recommends limiting alcohol consumption to < 64 g/week (9 g/day or 4 drinks/week) for men and < 28 g/week (4 g/day or 2 drinks/week) for women in people with alcohol intolerance, or alcohol facial flushing.292 Consistent with this guideline, there has been a recent alcohol guideline published for the alcohol flushers in South Korea where the ALDH2*2 dysfunctional allele is also prevalent.293 In addition, binge drinking (defined as ≥ 5 and ≥ 4 drinks for men and women, respectively, in 2 hours) should be strictly prohibited, because it has a strong pressor effect and is associated with a higher risk of atrial fibrillation, stroke and sudden death.292
7.3 Body weight reduction
Obesity increases CV death, especially stroke death.294,295 Compatible with this notion, weight reduction has been found to reduce BP. An early meta-analysis of 25 RCTs found that a net weight reduction of 5.1 kg reduced SBP by 4.44 mmHg and DBP by 3.57 mmHg, and that the extent of BP reduction perfectly paralleled the extent of weight reduction among the studies included, i.e. approximately 1 mmHg reduction in SBP per 1 kg reduction.296 Two large-scale studies have shown the relationship between body mass index (BMI) and all-cause mortality, with one including 19 prospective studies encompassing 1.46 million white adults294 and another enrolling 220,000 Chinese men for a 15-year follow-up.295 Both studies demonstrated a J-curve phenomenon, with the lowest mortality at the BMI of around 20-24.9 kg/m2. Therefore, the Task Force recommends an ideal weight of 20-24.9 kg/m2, but the healthy weight can be slightly higher for the elderly267,294 and those after coronary revascularization.297 Weight reduction can be better achieved by a multidisciplinary approach including regular exercise, dietary advice, and motivation counseling.267 For patients with morbid or severe obesity, anti-obesity medication and bariatric surgery can be adopted to reduce overall CV risk.298
7.4 Cigarette smoking cessation
Despite little impact of smoking on BP,299 smoking is deemed as a lethal addictive disorder.267 A lifetime smoker on average will lose 10 years of life,300 in comparison with only 3 years in men with severe hypertension.301 Smoking in general doubles the 10-year risk of myocardial infarction, with a much prominent trend among younger (< 55 years) female smokers whose risk is ~7-fold higher than that in non-smokers.302 Furthermore, several lines of evidence also identify smoking as a risk factor for stroke in Taiwan.303,304 Therefore, a wholistic LSM should include cessation of smoking which has a substantial impact on many hypertension-related CV outcomes.
Electronic cigarettes (EC) have been emerging as a popular way in aid of tobacco cessation in recent years. In England, the prevalence of EC has been positively associated with the success rates of quit attempts.305 However, large-scale meta-analysis of whether EC is superior to non-EC methods for tobacco cessation still yielded conflicting results.306,307 Growing evidence has raised the concerns regarding EC as an alternative to cigarette in many ways:308 first, those who successfully abstain from tobacco have a high rate of long-term EC use;309 second, there is potential EC or vaping product use-associated lung injury (EVALI) (Blount BC, et al. New England Journal of Medicine 2020;382:697-705.); third, there have been reports and systematic review demonstrating that short-term action of EC increased BP and arterial stiffness.310,311 Taken together, there’s still no solid evidence supporting that EC is a safer alternative for tobacco cessation, neither is there sufficient evidence to claim its long-term CV safety.
7.5 Diet adaptation
The most evidence-based diet pattern beneficial for BP lowering is the diet approach to stop hypertension (DASH) diet, characterized by high amounts of fruits-and-vegetables, low-fat dairy foods, whole grains, nuts, fish, and poultry, but reduced amounts of red meat, beverages, saturated fat, sweets and snacks. DASH diet was shown to reduced SBP and DBP by 11.4 and 5.5 mmHg, respectively, in patients with hypertension.312 Not surprisingly, sticking to DASH diet was also found to improve hypertension-related CV outcomes, such as stroke, coronary heart disease, peripheral artery disease, and heart failure.313,314 A more recent network meta-analysis assessing 22 non-pharmacological interventions has concluded that DASH diet is the most effective intervention in lowering BP (SBP/DBP by 6.97/3.54 mmHg) for adults with pre-hypertension or established hypertension.315
Apart from the DASH diet, the Mediterranean diet was recommended by the recent ESC hypertension guideline.10 The basic principle of two diet patterns is actually very similar except for olive oil and moderate red wine consumption which are exclusively recommended by the Mediterranean diet. Considering our specific genetic background (see Section 7.2) and a higher prevalence of stroke in Taiwan than that in Europe, the DASH diet is more appropriate for Taiwanese people.
In addition to diet pattern, tea and coffee consumptions also have evidence towards BP and CV benefits. Consumption of both green tea and black tea has been shown from meta-analyses to have a slight but significant effect on BP reduction (~1-2 mmHg for both SBP and DBP).316,317 A meta-analysis of 36 prospective studies enrolling 1,279,804 participants for a median of 10-year follow-up has demonstrated that moderate coffee drinking (1-4 cups/day) has led to a modest risk reduction (~10-15%) of composite CV outcomes (CV death, coronary heart disease, stroke, heart failure).318
7.6 Exercise adoption
Patients with hypertension who participate in any level of physical activity have been shown to reduce CV mortality by 16-67%.319 In line with this observation, runners, irrespective of the "doses" of exercise, has 30% and 45% lower risks of all-cause and CV mortality, respectively, compared with nonrunners in general population.320 In a meta-analysis of 93 RCTs, totaling 5,223 participants, demonstrated that endurance, dynamic resistance, isometric resistance exercises for at least 4 weeks significantly reduced SBP/DBP by 3.5/2.5, 1.8/3.2, and 10.9/6.2 mmHg, respectively. And there were graded increase of BP reductions from subjects with normal BP to those with prehypertension and hypertension.321 Patients with hypertension are advised to participate in at least 30 min of moderate-intensity aerobic exercise (walking briskly, slow cycling, jogging, or swimming)10,322 for the intensities of aerobic exercise) on 5-7 days/week.10,135 Resistance exercise which reduces bone loss and preserves muscle mass also has some evidence of BP benefit,323 particularly in combination with aerobic exercise. Performance of 2-3 sets of 8-12 repetitions at the intensity of 60-80% of personal 1 repetition maximum (1 RM, the maximal load that can be lifted one time) on 2-3 days per week can be advised.10,135,267 Of note, high-intensity exercise is not recommended for individuals with uncontrolled hypertension (SBP > 160 mmHg) until BP has been controlled.322
For older or debilitated adults unable to do aerobic exercise, neuromotor exercise or training, such as tai chi, yoga, and meditation, can be suggested.324 Three meta-analyses have demonstrated tai chi significantly reduced SBP/DBP by 6-14/0.6-7 mmHg.325-327 Another meta-analysis enrolling 13 studies, totaling 753 participants, showed that both yoga and meditation significantly reduced SBP and DBP, particularly in those whose age > 60 years.328
Recently, the interaction between air quality and physical activity has drawn growing attention. Using air cleaner to remove PM2.5 with active filtration, in comparison with sham filtration, for a median of 2 weeks was found to significantly reduce SBP by ~4 mmHg in a meta-analysis,329 suggesting that environmental PM2.5 per se contributes to hypertension development. Consistently, an increase of ambient PM2.5 concentration 5 days before cardiac rehabilitation visit also significantly increased BP on the day of visit.330 Apart from BP, an analysis of global burden of diseases attributable to ambient air pollution from 1990 to 2015 also found that the risks of ischemic heart disease and cerebrovascular disease were increased along with the increase of ambient PM2.5 concentrations above the reference level (0-2.4 μg/m3), and that this burden had substantially increased during the 25 years studied.331 People may imagine that exercise in the environment with air pollution may substantially offset its benefits or even cause harm in terms of BP control. However, a prospective analysis including 140,072 Taiwanese people without hypertension who joined a health screening program between 2001 and 2016 demonstrated that the risk of hypertension was indeed positively associated with PM2.5 concentrations (mean 26.1 μg/m3, ranged 5.7-50.3 μg/m3) but the benefit of exercise remained stable at various levels of PM2.5. It concludes that habitual exercise is an appropriate hypertension prevention strategy even for people residing in relatively polluted regions.332 In agreement with this finding, it is estimated that for global average of urban background PM2.5 concentration (22 μg/ m3), the benefit of exercise far outweighs the risk of air pollution, in terms of all-cause mortality. Cities with extremely high PM2.5 levels are very rare and only people there should avoid exercises of long duration. For example, the estimated harm would exceed the benefit after > 1.5 hours of cycling or > 10 hours of walking per day in areas with a PM2.5 concentration of 100 μg/m3.333 "The Recommendation for Exercise with Different Background Air Qualities" from the Health Promotion Administration in Taiwan suggests that moderate-intensity outdoor exercise can be performed with a background PM2.5 concentration of < 54.4 μg/m3, and the intensity is unlimited with a concentration of < 15.4 μg/m3.334
The timing of physical activity has recently been shown to be crucial as well. A cohort of 104,046 participants in the Copenhagen General Population Study with median 10-year follow-up has concluded that higher leisure time physical activity was associated with reduced risks of CV disease and all-cause mortality, whereas higher occupational physical activity was conversely associated with increased risks. The two kinds of physical activity are distinct and cannot be combined together in terms of CV benefit.335
8. PHARMACOLOGICAL THERAPY
Recommendations/Keypoints
• Before initiating pharmacological therapy, healthcare professionals should follow the assessment algorithm (Figure 5), dubbed "HER", which stands for 1) H: to confirm the diagnosis of hypertension by standardized HBPM based on the 722 protocol, 2) E: to assess the presence of any exacerbators/inducers or secondary hypertension (Tables 11 and 12), and 3) R: to conduct risk chart-based assessment, including risk factors, HMOD, and established ASCVD (COR I, LOE C).
Figure 5.
Assessment flowchart for the initiation of hypertension management. BP, blood pressure; HBPM, home blood pressure monitoring; SPC, single-pill combination.
• For all patients whose BP levels are above risk chart-based thresholds, both LSM and antihypertensive medications should be implemented once diagnosis is established (COR I, LOE A).
• Throughout all phases of hypertension management, HBPM based on the 722 protocol should be regularly obtained (Table 7). HBPM should be additionally performed while symptoms occur to elucidate whether symptoms are related to excessive BP reductions (COR I, LOE C).
• Task Force recommends that all 5 major antihypertensive drugs (ACE inhibitors [A], ARBs [A], β-blockers [B], CCBs [C], and thiazides diuretics [D]) are first-line antihypertensive drugs (COR I, LOE B).
• Spironolactone is recommended as one of the second-line antihypertensive drugs (COR I, LOE A).
• The Task Force recommends initial combination therapy, preferably in a single-pill combination, for patients with BP ≥ 20/10 mmHg above targets (COR I, LOE B).
• For patients with BP < 20/10 mmHg above targets, a single-pill combination (half tablet in frailer patients) could be considered as the initial antihypertensive drug (COR IIa, LOE B).
• Any combination between direct renin inhibitor, ACE inhibitor and ARB is contraindicated (COR III, LOE A).
• The concomitant use of drugs of the same class, such as DHP and non-DHP CCBs, and thiazides and loop diuretics, is allowed (COR IIa, LOE C).
• The Task Force recommends a target hierarchy (HBPM-HMOD-ABPM): to reach HBPM targets first, then to keep HMOD stable or regressed. If HMOD remains progression despite controlled HBPM, ABPM should be arranged to guide treatment adjustment (COR IIa, LOE C).
• Three medication adjustment strategies are recommended: shifting to drugs with a longer-acting antihypertensive effect (for uncontrolled evening hypertension), bedtime dosing (for uncontrolled morning hypertension), and adding another antihypertensive drug (for uncontrolled morning and evening hypertension) could be adopted according to results of HBPM (COR IIa, LOE B).
• The Task Force recommends that dose reduction could be performed if the average home SBP levels of > 20 mmHg below targets or symptoms or signs of hypoperfusion documented (COR IIb, LOE C).
• The use of ARBs or ACE inhibitors is safe in patients with COVID-19 (COR I, LOE A).
• The angiotensin receptor-neprilysin inhibitor is recognized as a new class of antihypertensive medications (COR IIa, LOE A).
• The sodium-glucose cotransporter 2 inhibitors are recognized as a new class of antihypertensive medications (COR IIb, LOE C).
8.1 Initiation of pharmacological therapy: assessment flowchart
Before initiation of pharmacological therapy, healthcare professionals should follow the assessment algorithm (Figure 5), dubbed " HER ", which comprises149 H: to confirm the diagnosis of hypertension by standardized HBPM based on the 722 protocol (Table 7), 2) E: to assess the presence of any exacerbators/inducers or secondary hypertension (Tables 11 and 12), and 3) R: to conduct risk chart-based assessment, including risk factors, HMOD, and established ASCVD (Table 10 and Figure 4). After completion of the 3 essential evaluations, we can determine the BP targets (based on risk chart category) (Figure 4), and doses, types, and timing of initial pharmacological therapy. Education about the importance of BP control, lifestyle modifications, regular HBPM, and shared decision-making regarding the choice of therapeutic strategies are of vital importance for the successful long-term control of hypertension.
Blood pressure measurement and management strategies based on hypertension grades and stages are shown in Table 7 and Figure 4. For individuals with normal BP levels (< 120/80 mmHg), we recommend to continue HBPM for at least one 722 cycle each year (Table 7). For individuals with elevated BP (120-129/< 80 mmHg), HBPM for at least one 722 cycle every 6 months, together with LSM, is recommended for stage 1 and stage 2 patients (Figure 4). Whereas for stage 3 patients with elevated BP, pharmacological therapy can be initiated once diagnosis is confirmed. For individuals with grade 1 hypertension (130-139/80-89 mmHg), HBPM for at least one 722 cycle every 3 months, together with LSM, is recommended for those who have fewer than 3 risk factors and no HMOD or established ASCVD. For the rest grade 1 hypertensive patients (stages 2, 3, and 1 with risk factors ≥ 3), pharmacological therapy should be initiated directly. For grade 2 hypertensive patients, pharmacological therapy should be initiated once diagnosis is confirmed. In summary, for all patients whose BP levels are above risk chart-based thresholds (Figure 4), both LSM and antihypertensive medications should be implemented once diagnosis is established.
Throughout all phases of hypertension management, HBPM based on the 722 protocol should be regularly obtained (Table 7). The 722 protocol denotes, first, to measure home BP for 7 consecutive days;20,336,337 second, on 2 occasions (in the morning and in the evening) per day; and third, 2 readings, 1 minute apart, on each occasion. The minimal consecutive days could be shortened to 4 days (first day data discarded) since at least 6 measurements are required to reach adequate diagnosis as shown in the IDHOCO study.98 Morning and evening HBP estimates are the averages of all morning and evening BP readings, respectively, except those obtained on the first day. The 722 protocol should be applied in the confirmation of hypertension diagnosis and 2 weeks after adjustment of antihypertensive medications. The effect of antihypertensive drugs reached 50% and80% of their full BP-lowering capacity 1 week and 2 weeks after use, respectively.338 Therefore, a period of 2 weeks is recommended to re-assess the efficacy of medication adjustment. In uncontrolled hypertensive patients, HBPM with one 722 cycle should be performed at least monthly, because single-digit number of SBP differences within 3 months could result in significant differences in the occurrence of CV diseases.339 In well-controlled hypertensive patients, HBP monitoring could be performed at least once weekly or following the 722 protocol at least every 3 months.340 During the acute stage of initiation or adjustment of antihypertensive therapy, close attention should be paid to symptoms and signs of adverse events. HBPM should be additionally performed while symptoms occur to elucidate whether symptoms are related to excessive BP reductions (COR I, LOE A).
The following general principles for initiation of pharmacological antihypertensive therapy are recommended (Figures 5 and 6). First, drugs which can provide sustained 24-hour BP control (once daily dosing) are preferred. The goal is to keep averaged morning and evening home BP within targets (Figure 7). Second, when the BP are ≥ 20/10 mmHg above targets, initial combination therapy or single-pill combination should be administered. This recommendation is based on the 10/5 rule regarding the magnitudes of BP reductions of a given antihypertensive drug with standard dose.341 Based on a meta-analysis of 354 randomized, double-blind, placebo-control trials comprising 40,000 drug-treated patients and 16,000 placebo-treated patients, an approximately 10 mmHg decrease in SBP and 5 mmHg decrease in DBP (10/5 rule) (after placebo-subtraction) can be anticipated with any one of the 5 major classes of antihypertensive drugs with standard dose, if the baseline BP is 154/97 mmHg. For a 10 mmHg increase in baseline SBP or DBP, further decrease of 1.0 mmHg in SBP and 1.1 mmHg in DBP can be observed. The 10/5 rule was first raised in the 2015 Taiwan Hypertension Guidelines.161 The BP-lowering effects of different categories of drugs are additive, whereas doubling of standard dose of a given antihypertensive drug would result in only 20% increase in BP reductions (additional 2/1 mmHg reductions). In contrast to BP reductions, side effects attributable to thiazides, β-blockers, and CCBs are dose-dependent. Initial combination therapy is universally recommended to all hypertensive patients in the 2018 European hypertension guidelines. Third, compelling indications (Table 15) should be considered first in choosing antihypertensive drugs for hypertensive patients with coexisting medical conditions. In the 2015 Taiwan Hypertension Guidelines, the acronym PROCEED was proposed to encompass all aspects to be considered for initiating pharmacological therapy (Figure 5). The Task Force still recommends the PROCEED principle, which includes "Previous unfavorable experience" of the individual patient to antihypertensive drugs, "Risk factors" which are essential for staging determination, "Organ damages" which are compelling indications for antihypertensive drugs, "Contraindications or unfavorable conditions" (Table 16), "Expert’s or doctor’s judgment" which is always of the utmost importance in making treatment decisions, "Expenses", and "Delivery and adherence" which should be regularly assessed since poor adherence is quite common in the management of any chronic diseases.342
Figure 6.
Adjustment flowchart for the pharmacological treatment of hypertension. ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor-neprilysin inhibitor; BP, blood pressure; CCB, calcium channel blocker; MRA, mineralocorticoid receptor antagonist; RAS, renin angiotensin system; SGLT-2i, sodium glucose cotransporter-2 inhibitor; SPC, single-pill combination.
Figure 7.
Home blood pressure monitoring-guided hypertension management flowchart. ABPM, ambulatory blood pressure monitoring; HBP, home blood pressure; HBPM, home blood pressure monitoring; HMOD, hypertension-mediated organ damage; HTN, hypertension; OD, organ damage.
Table 15. Recommended drugs: compelling indications.
| Clinical conditions | Drugs |
| Hypertension-mediated organ damage | |
| Left ventricular hypertrophy | ACEI, ARB, ARNI, CCB, thiazide diuretic |
| Microalbuminuria | ACEI, ARB |
| Clinical events | |
| History of myocardial infarction | ACEI, ARB, BB |
| Coronary heart disease | ACEI, ARB, BB, CCB (long-acting) |
| Heart failure | ACEI, ARB, ARNI, BB, MRA, thiazide diuretic, loop diuretic, SGLT2 inhibitor |
| Stroke | ACEI, ARB, CCB, thiazide diuretic |
| Chronic kidney disease | ACEI, ARB, loop diuretic, SGLT2 inhibitor, ARNI |
| Peripheral artery disease | ACEI, ARB, CCB, thiazide diuretic |
| Aortic dissection | BB |
| Diabetes mellitus | ACEI, ARB, SGLT-2 inhibitor |
| Associated conditions | |
| Isolated systolic hypertension | ACEI, ARB, CCB, thiazide diuretic |
| Metabolic syndrome | ACEI,ARB |
| Benign prostate hypertrophy | Alpha-blocker |
| Erectile dysfunction | ACEI, ARB, vasodilating BB, CCB |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor-neprilysin inhibitor; BB, beta blocker; CCB, calcium channel blocker; MRA, mineralocorticoid receptor antagonist; SGLT2, sodium-glucose cotransporter 2.
Table 16. Contraindications or unfavorable conditions.
| Contraindications | Unfavorable conditions | |
| ACEI | Bilateral renal artery stenosis, pregnancy, angioedema | Hyperkalemia |
| ARB | Bilateral renal artery stenosis, pregnancy, angioedema | Hyperkalemia |
| BB | Bronchial asthma, sick sinus syndrome, 2nd and 3rd degree AV block | Peripheral artery disease, metabolic syndrome |
| CCB (non-DHP) | Sick sinus syndrome, 2nd and 3rd degree AV block | Heart failure with reduced ejection fraction (class III or IV) |
| Thiazide diuretic | Gout, hypokalemia, hyponatremia, metabolic syndrome, pregnancy | |
| MRA | Hyperkalemia | |
| Alpha blocker | Heart failure with reduced ejection fraction (class III or IV) |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BB, beta blocker; CCB, calcium channel blocker; DHP, dihydropyridine; MRA, mineralocorticoid receptor antagonist.
8.2 First-line antihypertensive drugs
Several meta-analyses of large-scale RCTs of antihypertensive drugs have consistently shown that the clinical benefits of antihypertensive drugs are directly proportional to the magnitude of BP reductions, rather than the classes of antihypertensive drugs.232,233,343 These meta-analyses also demonstrated that five major classes of antihypertensive drugs including ACE inhibitors [A], ARBs [A], β-blockers [B], calcium-channel blockers (CCBs) [C], and thiazides diuretics [D] are all effective in preventing the occurrence of CVD (Figure 6). There is evidence that β-blockers were inferior to the other 4 major classes of drugs for the prevention of major CV diseases, stroke, and renal failure.232,344,345 Hypertension Guidelines issued by ESC/ESH, ACC/AHA, and International Society of Hypertension all recommend ACE inhibitors, ARBs, CCBs and thiazides diuretics, but not β-blockers, as first-line antihypertensive drugs. However, most trials involving β-blockers are based on the use of atenolol. No RCTs have evaluated the effects of newer-generation β-blockers, such as bisoprolol, carvedilol and nebivolol, on all-cause mortality. All these newer-generation b-blockers have been shown to provide morbidity and mortality benefits in patients with heart failure and reduced ejection fraction. In the most recently updated meta-analysis including 66,625 hypertensive patients from 45 RCTs to compare the 5 major antihypertensive drugs, all-cause death is similar for renin-angiotensin system (RAS) inhibitors, CCBs, thiazides and β-blockers.346 Chinese population is more sensitive to the effects of β-blocker propranolol on heart rate and BP than Caucasian populations.347 The evidence demonstrating the differential effects of 5 major antihypertensive drugs in Asian populations is lacking.348 Considering the above lines of evidence, the Task Force recommends that all 5 major antihypertensive drugs (ACE inhibitors [A], ARBs [A], β-blockers [B], CCBs [C], and thiazides diuretics [D]) are first-line antihypertensive drugs (COR I, LOE B).
8.3 Combination therapy
To achieve the target BP levels, combination antihypertensive therapy is usually required. In the SPRINT trial, the mean number of antihypertensive medications was 2.8, with the mean achieved SBP of 121.5 mmHg, in the intensive-treatment group throughout the 3.3 years of follow-up.8,258 In the STEP trial, patients began treatment with olmesartan medoxomil (20 mg, once daily) as the preferred ARB, or amlodipine besylate (5-10 mg, once daily) as the preferred CCB. Hydrochlorothiazide was not administered as an initial therapy in the STEP trial, in contrast to the SPRINT trial. The mean number of antihypertensive medications was 1.9, with the mean achieved SBP of 126.7 mmHg, in the intensive-treatment group throughout the 3.3 years of follow-up.9 A meta-analysis showed that the antihypertensive effect of a combination of two classes of antihypertensive drugs were 5 times more effective than those of doubling the dose of one antihypertensive drug.349
Hypertension is a multifactorial disease. Targeting a specific mechanism may trigger activation of counter-regulatory mechanisms. Initial combination therapy with antihypertensive drugs of different mechanisms, compared to sequential addition of antihypertensive drugs, can achieve earlier control of BP and fewer adverse events.350 In a population-based, nested case-control study of 209,650 patients, those who were treated with initial combination therapy and maintained throughout the course had 26% lower CV risk, compared with patients who maintained monotherapy.351 Initiating treatment with a combination of two drugs is also associated with a reduced risk of treatment discontinuation.352 Initial combination therapy is recommended to all hypertensive patients in the 2018 European hypertension guidelines and 2020 International Society of Hypertension guidelines. The Task Force recommends initial combination therapy, preferably in a single-pill combination, for patients with BP ≥ 20/10 mmHg above targets (COR I, LOE B). Adverse events are almost half at half of the standard dose, whereas BP-lowering effects reduce only 20%. Combination of half-dose of antihypertensive drugs increases efficacy and reduces adverse events. Given that multiple mechanisms are involved in the pathogenesis of hypertension and multiple compelling indications might coexist, the Task Force considers initial half-dose combination therapy a rational option for patients with BP < 20/10 mmHg above targets (COR IIa, LOE B).
Combinations among the 5 first-line, major antihypertensive drugs are reasonable,353-355 except the combination of ACE inhibitor and ARB. Several studies showed that the combination of ACE inhibitor and ARB, compared to monotherapy, was associated with a higher rate of progression to dialysis and mortality.356 Any combination between direct renin inhibitor, ACE inhibitor and ARB is contraindicated (COR III, LOE A). In the prematurely terminated ALTITUDE study, combination therapy with aliskiren and an ACE inhibitor or an ARB, compared to ACE inhibitor or ARB alone, was associated with significantly higher hyperkalemia and hypotension, and similar CV and renal events in high-risk type 2 diabetic patients.357 The other concomitant use of drugs of the same class is allowed, such as dihydropyridine (DHP) and non-DHP CCBs, and thiazides and loop diuretics (COR IIa, LOE C).
The persistence of uncontrolled hypertension with 2-drug combination therapy is often associated with volume overload resulting from excessive salt intake and/or salt sensitivity.358 In this circumstance, salt restriction and appropriate use of diuretics are important in keeping BP under control. In patients with an estimated glomerular filtration rate (eGFR) of ≥ 30 mL/min/1.73 m2, thiazides diuretics should be used. In patients with an eGFR of < 30 mL/min/1.73 m2, loop diuretics should be used. There have been no RCTs to compare the efficacy of any 3-drug combination in reducing CV events. Among the 13,551 patients who were concurrently receiving three antihypertensive drugs of different classes from the National Health Insurance Research Database of Taiwan during 2004-2006, there were no differences in the incidence of CV events between patients treated with a thiazides diuretic or a β-blocker on top of ACE inhibitor or ARB and CCB.359
In addition to the combinations of different classes of first-line antihypertensive drugs, it has been shown that sufficient BP reductions can be achieved by the addition of spironolactone at a low to moderate dose (25 to 50 mg per day), irrespective of the levels of plasma renin activity, plasm aldosterone concentration, and serum potassium.360,361 Eplerenone, a more selective MRA without the anti-androgen effects, was associated with a 10 mmHg reduction in 24-hour SBP, when used as a fourth-line agent at the dose of 50 mg twice daily.362 Other MRA, like finerenone and esaxerenone, have not been studied in patients with resistant hypertension. Spironolactone is recommended as one of the second-line antihypertensive drugs (COR I, LOE A). Spironolactone can cause adverse effects, such as gynecomastia, impotence and menorrhagia, whereas eplerenone and other MRA cause much fewer anti-androgen-related adverse effects. Other MRA should be considered in hypertensive patients responsive to spironolactone but intolerant to its adverse effects. The equivalent doses of other MRA to spironolactone in terms of BP reductions have not been determined yet.
Combination therapy with sympatholytic drugs, such as α-blockers [α], clonidine [O], and methyldopa [O], and direct vasodilators, such as hydralazine [O], could also be considered in patients with resistant hypertension or specific compelling indications. The Task Force considered all these drugs, including spironolactone and other MRAs, the second-line antihypertensive drugs (Figure 6).
8.4 Single-pill combination
The use of single-pill combination (SPC) (also called fixed-dose combination) drugs is advantageous for improving adherence by reducing the number of tablets to be taken and simplification of the prescription.363,364 A meta-analysis demonstrated that the use of SPC drugs, compared to free combinations, was associated with greater BP reductions, better adherence, and fewer adverse events.363 Another meta-analysis of RCTs regarding the antihypertensive effects of combination therapy with respective drugs and a SPC drug showed that there were no differences in BP reductions or adverse events between the two groups.365 The benefits of SPC compared to free combinations of drugs are more evident in the real world setting. In the Simplified Treatment Intervention to Control Hypertension (STITCH) trial done in Canada, initial use of SPC drugs was associated with a significant decrease of 5.4 mmHg in SBP and a 20% greater control rate compared to the free combination group.366 In the Kaiser Permanente Northern California (KPNC) hypertension program, the control rate of hypertension increased from 43.6% to 80.4% with the widespread use of SPC.367 In a real-world database analysis among 44,534 residents in Lombardy, Italy, treatment with initial combination therapy using SPC, compared to initial monotherapy, was associated with a 21% lower rate of hospitalization for any CV event within 1 year.368 By analyzing the National Health Insurance Research Database in Taiwan, switching from 2-drug free combinations to the corresponding SPCs resulted in a relative 75% increase in adherence within 1 year.342 All these lines of evidence indicate that initial therapy with SPCs provides earlier and better hypertension control than free combinations and monotherapy. The Task Force recommends that initial SPC use should be considered in patients with BP ≥ 20/10 mmHg above targets (COR I, LOE B). For patients with BP < 20/10 mmHg above targets, half-dose of SPC could be considered as the initial antihypertensive strategy (COR IIa, LOE B).
8.5 Adjustment flowchart and HBPM-guided management flowchart
The adjustment flowchart is shown in Figure 6. If BP arenot at goals after 4 weeks of treatment, the adjustment algorithm should be executed before swiftly adjusting the medications. An adjustment algorithm called "ATGOALs" is recommended: Adherence, Timing of administration, Greater doses, Other classes of drugs, Alternative combination or SPC, and LSM (and Laboratory tests). The first priority is to re-confirm drug adherence, because non-adherence is very common in daily practice. Early (or initial) adoption of single-pill combination drugs is a useful approach to improve adherence.342 Timing of drug administration can be adjusted according to the diurnal BP profile of individual patients, according to morning and evening home BP or ABPM. If early morning hypertension is observed, switching of medication from morning dosing to bedtime dosing may beuseful. Increasing or maximizing doses should be considered thereafter. The next step is to add or switch to other classes of drugs, or to use different combination of drugs, including SPC. Lifestyle modifications need to be optimized. Medications should be adjusted based on findings from laboratory tests, which reflect the extent of organ damages. The Task Force recommends that the goals of antihypertensive therapy should include the stability or regression of HMOD. Relevant laboratory tests, including electrocardiogram and urinalysis, should be regularly monitored no less than once yearly.
Figure 7 is the flowchart showing the HBPM-based hypertension management strategy. The Task Force recommends a target hierarchy of HBPM-HMOD-ABPM: to reach HBPM targets first, then to keep HMOD stable or regressed. If HMOD remains progression despite controlled HBPM, ABPM should be arranged to guide treatment adjustment (COR IIa, LOE C). An important aspect affecting the clinical efficacy of antihypertensive drugs is whether the BP reduction is sustained.144 Regular HBPM in patients treated with antihypertensive drugs could provide reliable assessment and guide the adjustment of antihypertensive drugs.369 Three medication adjustment strategies are recommended: shifting to drugs with longer-acting antihypertensive effect (for uncontrolled evening hypertension), bedtime dosing (for uncontrolled morning hypertension), and adding another antihypertensive drug (for uncontrolled morning and evening hypertension) could be adopted according to results of HBPM (COR IIa, LOE B).
8.6 Dose reduction and withdrawal of antihypertensive drugs
There are seasonal variations in BP,370 which makes adjustments of antihypertensive drugs on a seasonal basis a frequently encountered scenario. To avoid untoward fluctuations of BP above targets during dose reduction, the Task Force recommends that dose reduction could be performed if the average home BP levels of ≥ 20/10 mmHg below targets or symptoms or signs of hypoperfusion documented (COR IIb, LOE C). Dose reduction should be started with only one drug and half-dose each time. The next move should be initiated at least 2 weeks after the previous adjustment if the prior indications of dose reduction remain. The characteristics of patients in whom a normal BP could be maintained even after withdrawal include having grade I hypertension before treatment, younger age, normal body weight, low salt intake, nondrinker, using only one antihypertensive drug and having no organ damage.371
8.7 Classes of antihypertensive drugs
8.7.1 Angiotensin-converting enzyme (ACE) inhibitors
ACE inhibitors have been extensively studies in many RCTs for the treatment of hypertension.372,373 Even in high risk patients with elevated BP, several RCTs have confirmed the efficacy and safety compared to placebo or other antihypertensive drugs. ACE inhibitors are indicated in patients with left ventricular hypertrophy, proteinuria, heart failure, diabetes, and chronic kidney disease.374,375
The major adverse effects of ACE inhibitors include cough and angioedema. The incidence of ACE inhibitor-induced cough, due to enhanced bradykinin activity, is reported to be 5-35%. Cough due to ACE inhibitors is more common in Asians.376 The induction of cough prevents aspiration pneumonia.377 The incidence of potentially life-threatening angioedema caused by ACE inhibitors is < 1%, and especially rare in Chinese.378 A study reported that ACE inhibitors combined with DPP-4 inhibitors could increase the incidence of angioedema.379
8.7.2 Angiotensin receptor blockers (ARBs)
ARBs are effective in reducing CV and renal events. Because ARBs are well tolerated and have effects similar to ACE inhibitors, they are generally preferred over ACE inhibitors. ARBs specifically bind to angiotensin II type 1 (AT1) receptors and inhibit angiotensin II-mediated actions. The feedback increase in circulating angiotensin II level can stimulate angiotensin II type 2 (AT2) receptors, which further antagonize the actions of AT1 receptors. ARBs can also activate the ACE2-angiotensin (1-7)-Mas system.380 ACE2 is the receptor that mediates the entry of SARS-CoV-2 into the cells.381 RCTs have unequivocally demonstrated that the use of ARBs or ACE inhibitors is safe in patients contracting COVID-19.382,383
The tolerability of ARBs is excellent, and the discontinuation rate is the lowest among all 5 classes of first-line antihypertensive drugs. Cough and angioedema are rarely reported in patients receiving ARBs. ARBs, as well as ACE inhibitors, are contraindicated for pregnant or breast-feeding women. ARBs and ACE inhibitors should not be used in patients with bilateral renal artery stenoses or those with one kidney and unilateral renal artery stenosis because of the risk of rapid decline of renal function. The eGFR and serum potassium level should be measured within 2 weeks after the start of an ARB or an ACE inhibitor in patients with stage 3b CKD.12 ARBs should not be combined with ACE inhibitors or direct renin inhibitors because of increased risks of hyperkalemia, progression to dialysis, and mortality.356,357
8.7.3 Direct renin inhibitor (DRI)
The only available DRI, aliskiren, has been shown to be effective in lowering BP and exert favorable effects on organ damages, such as proteinuria or left ventricular hypertrophy, and on biomarkers for heart failure.384,385 In both large RCTs (the ALTITUDE and ASTRONAUT trials), aliskiren on top of pre-existing ACE inhibitor or ARB were associated with increased hyperkalemia, hypotension, and renal impairment in patients with high-risk diabetes (the ALTITUDE trial) or heart failure (the ASTRONAUT trial).357,386 DRI is not listed as first-line antihypertensive drugs as ACE inhibitors and ARBs. Aliskiren can be safely combined with hydrochlorothiazide or amlodipine in hypertensive patients aged ≥ 65 years.387 The contraindications for DRI are the same as for ACE inhibitors or ARBs.
8.7.4 Beta-blockers
Beta-blockers are classified into 3 subtypes: non-selective, β1-selective, and vasodilating beta-blockers. Beta-blockers are effective in preventing recurrent coronary events in people with a history of coronary heart disease, with a risk reduction of 29% (95% CI, 22% to 34%) compared with 15% (11% to 19%) in trials of other drugs, though the additional benefits were limited within the first few years after myocardial infarction.343 In the meta-analysis including 145,811 patients, it was shown that, compared with other anti-hypertensive drugs, atenolol was associated with an increased risk of stroke (relative risk 1.17, p < 0.05) in patients aged ≥ 60 years.388 The risk of stroke for non-atenolol beta-blockers compared with other drugs did not reach statistical significance. In patients aged < 60 years, atenolol was associated with reduced risk of stroke compared with other drugs (relative risk 0.78, p < 0.05), whereas non-atenolol beta-blockers were associated with a lower risk of composite cardiac events (relative risk 0.86, p < 0.05) compared with placebo, with no significant differences in events compared with other drugs. It seems that all the beta-blockers performed equally well in patients younger than 60 years, whereas for patients with age ≥ 60 years, atenolol was inferior to other antihypertensive drugs in reducing stroke. The possible reasons for the inferior effects of atenolol on reducing stroke include its less effectiveness in reducing central aortic pressure and shorter half-life (6-9 hours), which makes once daily dosing inadequate to provide 24-hour sustained BP reduction.126,389
There are no RCTs examining the effects of newer-generation beta-blockers, such as metoprolol, bisoprolol, carvedilol and nebivolol, on all-cause mortality in hypertensive patients. All these newer-generation beta-blockers have been shown to provide morbidity and mortality benefits in patients with heart failure and reduced ejection fraction.390-392 In the recent meta-analysis including 66,625 hypertensive patients from 45 RCTs to compare the 5 major antihypertensive drugs, all-cause death is similar for RAS inhibitors, CCBs, thiazides and beta-blockers.346 Chinese population is more sensitive to the effects of non-selective beta-blocker propranolol on heart rate and BP than Caucasian populations.347 The Task Force recommends that beta-blockers are one of the first-line classes of antihypertensive drugs, particularly in patients with coronary heart disease, history of myocardial infarction, higher heart rate (≥ 80 beats/min), hyperthyroidism, and aortic dissection. Given the inferior performance of atenolol in older populations, long-acting beta-blockers are preferred.
Active bronchial asthma is an absolute contraindication for the use of all beta-blockers, but chronic obstructive pulmonary disease (COPD) is not a contraindication for beta-blockers. In a retrospective cohort study, beta-1 selective, but not non-selective beta-blockers were suggested to be safe in patients hospitalized with acute exacerbation of COPD with underlying coronary heart disease, heart failure, or hypertension.393 While in the retrospective analysis of the OPTIMIZE-HF registry, both beta-1 selective and non-selective beta-blockers were associated with lower risk-adjusted mortality in patients with COPD.394 The major side effects with beta-blockers are reduced sexual function, fatigue, reduced exercise capacity, body weight increase, and new-onset diabetes, especially in combination with diuretics.395 Discontinuation of beta-blockers often induce withdrawal symptoms such as palpitations, headache, angina pectoris, and hypertensive attacks. The dose should be gradually reduced before withdrawal.396
8.7.5 Calcium channel blockers (CCBs)
Calcium channel blockers (CCBs) have potent BP-lowering effects, and have been the most widely used antihypertensive drugs, especially in Asia. Several recent large clinical trials have confirmed their efficacy not only in lowering BP but also in reducing CV morbidity and mortality in hypertensive patients with an average or high CV risk profile. CCBs can be broadly classified into 2 groups: dihydropyridine (DHP) and non-dihydropyridine (non-DHP) groups. Most of recent RCTs were testing DHP CCBs.
8.7.5.1 DHP CCB
Short-acting DHP CCBs cause reflex tachycardia and are not recommended as first-line anti-hypertensive drugs.397 Sublingual administration of the contents of nifedipine capsules are not recommended in patients with hypertensive urgency or emergency, since it may induce reflex tachycardia and trigger cerebral infarction or myocardial ischemia due to excessive BP reductions.398 The effect of nitrendipine versus placebo in reducing stroke in isolated systolic hypertension had been confirmed in the Syst-Eur and Syst-China trials.256,257 Other DHP CCBs have also been studied in RCTs, including the INSIGHT, HOT, and FEVER trials.245,399,400 An amlodipine-based therapy was either as effective as or better than other antihypertensive drugs in lowering BP and preventing organ damages and CV events in the ALLHAT, CAMELOT, VALUE, and ASCOT trials.339,354,401,402 In the ACCOMPLISH trial, the combination of ACE inhibitor and amlodipine was superior to the combination of ACE inhibitor and a thiazide diuretic in reducing composite CV endpoints.355 The efficacy of CCBs, particularly amlodipine, may be due to their potent and sustained BP-lowering effect, and thereby reduced BP variability.147 A meta-analysis of 12 trials reported that DHP CCB was more effective than other antihypertensive drugs in lowering daytime and nighttime SBP in East Asians.403 DHP CCBs might be less effective in preventing heart failure.
The main side effect of DHP CCBs is peripheral edema, which is more prevalent at high doses. Other side effects include palpitations, headache, facial flushes, gingival growth and constipation. There is no contraindication for the use of DHP CCBs.
8.7.5.2 Non-dihydropyridines CCBs
Non-DHP CCBs, including verapamil and diltiazem, are less potent than DHP CCBs in BP-lowering, but generally non-inferior to other antihypertensive drugs in several RCTs.404-406 Using real-world data from 4.9 million patients worldwide in the LEGEND-HTN study, initial treatment with the non-dihydropyridine CCBs were significantly inferior to ACE inhibitors, ARBs, DHP CCBs, and thiazides in preventing CV events.407 Non-DHP CCBs are more negatively chronotropic and inotropic than DHP CCBs, and have more contraindications. Both verapamil and diltiazem are metabolized by CYP3A4, and have more drug-drug interactions than DHP CCBs.
8.7.6 Diuretics
8.7.6.1 Thiazides and thiazide-like diuretics
Thiazide diuretics and thiazide-like diuretics (e.g., indapamide, chlorthalidone, etc) are the first-line antihypertensive drugs. The ALLHAT trial confirmed the equivalent effect of chlorthalidone in reducing CHD as compared to CCB and ACE inhibitor.401 Chlorthalidone outperformed CCB and ACE inhibitor in reducing heart failure events in the ALLHAT trial. The efficacy of thiazide diuretic in reducing heart failure has also been demonstrated in a large meta-analysis of 147 RCTs.343 There is no RCT for head-to-head comparison of different thiazides. In a study comparing hydrochlorothiazide 50 mg per day with chlorthalidone 25 mg per day, the latter provided a greater decrease in ambulatory SBP, with the greatest difference occurring at nighttime.408 In a retrospective observational cohort study from the Multiple Risk Factor Intervention Trial (MRFIT) dataset, chlorthalidone displayed significantly lower SBP, lower potassium, and higher uric acid over time compared with hydrochlorothiazide.409 Indapamide has outperformed placebo in several RCTs.410-412 Based on data from meta-analysis, chlorthalidone outperformed hydrochlorothiazide in reducing CV events, after correction for differences in BP.413 Until a head-to-head RCT is available, it is premature to draw conclusions regarding which thiazide diuretic is superior.
A major concern regarding the use of thiazide diuretics is the metabolic side effects. Thiazides reduce serum sodium and potassium, and increase uric acid, total cholesterol and triglycerides. According to data from Canada, the long-term persistence rate was lowest for users of diuretics, compared with users of other antihypertensive drugs.414
The annual incidence of thiazide-induced hyponatremia (≤ 130 mmol/L) is about 14%.415 Thiazide exposure was associated with a 5 times higher risk of hyponatremia than no exposure.416 The risk did not differ between men and women. A study indicated the involvement of SLCO2A1 (prostaglandin transport protein) gene mutations in thiazide-induced hyponatremia.417 Low-dose thiazide is preferred to avoid these electrolyte abnormalities.
The prevalence of hypokalemia (< 3.5 mmol/l) varied between approximately 7.2 and 8.5% at doses of 12.5-25 mg of chlorthalidone, and up to 56% with 50 mg hydrochlorothiazide.418 Thiazide-induced hypokalemia was more than twice as prevalent in men as in women, and was related to doses and age.
Thiazide diuretic can induce new-onset diabetes.415 The long-term impact of diuretic-induced diabetes on future CV events is controversial. In a post-hoc analysis of ALLHAT, patients with impaired fasting glucose had significantly fewer coronary events in chlorthalidone group compared with amlodipine group in the 4 to 8-year follow-up period, in spite of an increase in diabetes rate. In a 28-year follow-up of treated hypertensive patients, new-onset diabetes carried a significantly higher CV risk. The mean observation period from onset of diabetes to the first stroke was 9.1 years, and 9.3 years to the first myocardial infarction.419
8.7.6.2 Loop diuretics
To patients with severe CKD or end-stage renal disease (eGFR < 30 mL/min/1.73 m2), loop diuretics should be the drug of choice. Loop diuretics show more marked diuretic effects but less potent BP-lowering effects compared with thiazide diuretics. They can be combined with thiazide diuretics.420
8.7.6.3 Mineralocorticoid receptor antagonists (MRAs)
Aldosterone and its receptor play important roles in the pathogenesis of hypertension and hypertension-related CV outcomes.421 The prevalence of primary aldosteronism in hypertensive patients was increased along with grades of hypertension, approaching 15-20% in patients with resistant hypertension.422 Patients with higher aldosterone levels but matched levels of BP have higher rates of myocardial infarction, stroke and atrial fibrillation.423 Treatment with spironolactone in patients with heart failure and preserved ejection fraction (HFpEF), whom are characterized by a high prevalence rate (> 90%) of hypertension, resulted in significant improvement in left ventricular diastolic function, left ventricular remodeling, and lower NT-proBNP levels, but no difference in clinical symptoms and outcomes.424 In the TOPCAT trial, treatment with spironolactone did not reduce the incidence of death from CV causes in patients with HFpEF.425
Overwhelming evidence has confirmed the effect of MRA in the treatment of resistant hypertension, even at low doses.360,361,426-428 Eplerenone, a more selective MRA, achieved a 10 mmHg reduction in SBP in ABPM, when used as a fourth-line agent at the dose of 50 mg twice daily.362 The antihypertensive effects of spironolactone and eplerenone were observed even in the presence of normal serum aldosterone levels. Cautions should be taken when adding MRA to RAS inhibitors. The occurrence of hyperkalemia and rapid decline of eGFR should be monitored. Addition of MRA is relatively contraindicated if serum potassium levels > 5.0 mmol/l or eGFR < 30 ml/min/1.73 m2.
8.7.6.4 Other potassium-sparing diuretics
Other potassium-sparing diuretics, such as amiloride and triamterene, block the epithelial sodium channel. In the PATHWAY-2 study, amiloride (10 mg once daily) was as effective as spironolactone in BP-lowering in patients with resistant hypertension.429 They are usually prescribed with thiazide diuretics for hypertension control.
8.7.7 Alpha-blockers
Alpha-blockers are less widely prescribed as the first-line drug for hypertension, especially after the ALLHAT trial showing increased heart failure with the use of doxazosin compared with the use of chlorthalidone.430 There are still debates regarding whether the designs of the ALLHAT trial caused this finding. Doxazosin can be used for the treatment of resistant hypertension.354 Alpha-blockers are effective in the treatment of benign prostate hypertrophy, particularly beneficial for men with urination disorder. They are used for BP control in patients with pheochromocytoma. Orthostatic hypotension is occasionally encountered in patients treated with alpha-blockers. Increase in salt intake might be helpful in alleviating orthostatic hypotension.
8.7.8 Centrally acting sympatholytic drugs
Centrally acting drugs, such as clonidine and alpha-methyldopa, are considered as second-line agents. When the BP targets are not reached despite the use of an RAS inhibitor, a beta-blocker, a CCB, and a thiazide diuretic, the addition of a centrally acting sympatholytic drug could be considered following the administration of an MRA antagonist and an α-blocker.
A meta-analysis of RCTs involving patients with essential hypertension showed that alpha-methyldopa reduced BP significantly compared to a placebo.431 Alpha-methyldopa is indicated for patients with renal dysfunction. It can be safely used during pregnancy.432
Clonidine inhibits sympathetic activities by stimulating α2-receptors in the rostral ventrolateral area of the medulla oblongata. Adverse effects, such as sleepiness, thirst, malaise and impotence, are frequent. Sudden discontinuation may induce withdrawal symptoms. As sodium and water retention is observed, the concomitant use of a diuretic is sometimes necessary.
8.7.9 Direct vasodilators
Direct vasodilators, such as hydralazine and minoxidil, cause fluid retention and tachycardia. No RCTs for the treatment of hypertension have been done for hydralazine, nor for minoxidil.433 Adverse effects of hydralazine include reflex tachycardia, hemolytic anemia, vasculitis, glomerulonephritis, and a lupus-like syndrome. Hydralazine in combination with isosorbide dinitrate is effective in African American patients with heart failure.434 Because of the severity of adverse effects with minoxidil, its usage is limited to persons with severe hypertension unresponsive to other treatments.
8.7.10 Angiotensin receptor neprilysin inhibitor (ARNI)
Sacubitril/valsartan is a first-in-class angiotensin receptor neprilysin inhibitor (ARNI) that is effective in reducing morbidity and mortality, compared to enalapril, in patients with heart failure with reduced ejection fraction.435 Sacubitril inhibits neprilysin, a metallopeptidase that degrades natriuretic peptides. Natriuretic peptides exert sympatholytic, diuretic, natriuretic, vasodilatory, and insulin-sensitizing effects mostly via cyclic guanosine monophosphate (cGMP) mediated pathways.436 As an antihypertensive agent, sacubitril/valsartan has outperformed ARBs, with additional reductions of office SBP ranging between 5-7 mmHg, in multiple studies in Asia and around the globe.437 Sacubitril/valsartan has been shown to be effective in Asian patients with salt-sensitive hypertension, and can preferably lower nighttime BP with morning dosing.358 Sacubitril/valsartan is well tolerated in the elderly and those with CKD. Further investigations are needed to validate its safety for long-term use, and to explore other potentials such as in the management of insulin resistance and obesity, which often coexist with hypertension.
8.7.11 Sodium glucose cotransporter 2 (SGLT2) inhibitors
Empaglifolzin, canagliflozin, and dapagliflozin are SGLT2 inhibitors for treatment of type 2 diabetes mellitus that also reduce BP, heart failure hospitalization, and mortality, and slow the progressive loss of glomerular filtration rate. SGLT2 inhibitors inhibit the coupled reabsorption of sodium and glucose from the proximal tubules, thereby increasing renal glucose and sodium excretion. They increase the delivery of sodium to the loop of Henle and can thereby activate the tubuloglomerular feedback response to correct glomerular hyperfiltration. A meta-analysis including 27 RCTs comprising 12,960 patients with at least 28 weeks’ duration showed an average SBP/DBP reduction of 4.0/1.6 mmHg.438 The 4 mmHg reduction of SBP with SGLT2 inhibitors is consistent with the findings shown in EMPA-REG, CANVAS, and DAPA-CKD trials. Just like ARNI, SGLT2 inhibitors, which also enhance sodium excretion, can preferentially reduce nighttime BP.439 The greater magnitude of BP reductions achieved by SGLT2 inhibitors compared to the other glucose-lowering agents has been recognized by the Clinical Practice Guideline Update from the American College of Physicians.440
9. DEVICE THERAPY FOR HYPERTENSION
Recommendations/Keypoints
• Renal denervation can be considered as a BP-lowering strategy in hypertensive patients with high CV risk, such as resistant or masked uncontrolled hypertension, established ASCVD, intolerant or nonadherent to antihypertensive drugs, or features indicative of neurogenic hypertension after careful clinical and imaging evaluation (COR IIa, LOE B).
9.1 Evidence of renal denervation
Given the dominant role of renal sympathetic nerves in regulating CV systems, the application of device-based therapies aimed at renal neuromodulation is exploited.441 Catheter-based renal denervation (RDN) is currently the only feasible device therapy for hypertension.442 RDN was designed by means of radiofrequency, ultrasound, or alcohol injection for resistant hypertension initially. However, the emerging data has moved the indicated population towards uncontrolled hypertension, regardless of the number of concomitant antihypertensive medications.443-447
The SPYRAL HTN-OFF MED and SPYRAL HTN-ON MED trials randomized patients with combined hypertension (office SBP, 150-179 mmHg; office DBP ≥ 90 mmHg; and 24-hour ambulatory SBP, 140-169 mmHg) with and without antihypertensive medications to undergo radiofrequency RDN vs. sham procedure.448,449 The SPYRAL HTN-OFF MED Pivotal trial (n = 331) was designed to be powered for the coprimary efficacy endpoint of baseline-adjusted changes in 24-hour and office SBP at 3 months.450 The effect of RDN on 24-hour and office SBP reductions (-3.9 and -6.5 mmHg) was statistically significant in the drug-naïve cohort, compared to patients randomized to the sham-controlled group. Also, no major procedure-related safety events occurred in 3 months. The SPYRAL HTN-ON MED pilot study (n = 80) was conducted in mild-to-moderate hypertensive patients receiving 1-3 classes of antihypertensive medications.451 At 6 months, the decrease in 24-hour SBP was significantly larger in the RDN group (-9.0 mmHg vs. -1.6 mmHg, p = 0.0059).
The RADIANCE-HTN SOLO trial (n = 146) randomized drug-naïve patients with combined hypertension (24-hour BP 135-169/85-104 mmHg) to observe the effect of ultrasound-based RDN.452 The ultrasound-based RDN achieved a significant daytime SBP reduction (-6.3 mmHg, p < 0.001) at 2 months. The RADIANCE-HTN TRIO trial enrolled patients with uncontrolled hypertension on a triple combination pill. The median between-group difference was -4.5 mmHg of daytime ambulatory SBP at 2 months (p = 0.022).453
Regarding alcohol injection-based RDN, among 45 patients with uncontrolled hypertension on multiple medications, bilateral infusion of 0.6-mL alcohol in each renal artery caused significant reductions in ambulatory and office BP at 6 months.454
The 3-year follow-up of real-world patients, mostly with resistant hypertension and comorbidities, in the Global SYMPLICITY Registry demonstrated durable effectiveness and safety.455,456 However, identifying potential candidates with greater BP-lowering response following RDN remains an unmet clinical need. In addition to neurogenic hypertension or RAS overactivation, several clinical features and comorbidities have been proposed to predict RDN responses, but none of them appears to have a high discriminative power.457-460
9.2 Clinical application of renal denervation
BP targets are difficult to achieve and maintain, since adherence to medication was commonly suboptimal and dynamic. Only less than 50% of patients were fully adherent to antihypertensive medications in previous studies.444,451 Based on the National Reimbursement Claims Database in Taiwan from 2001 to 2007, only 18.6% patients had medications refilled for ≥ 80% of days in the year after initiation of antihypertensive treatment.444 Several consensus documents on RDN have been published worldwide based on the consistent positive results of a series of RDN sham-controlled clinical trials.445-447 The Taiwan Hypertension Society and Taiwan Society of Cardiology play a leading role in issuing the first Consensus Statement on RDN based on the second-generation RDN trial results in 2019. In the Consensus, an acronym "RDNi2" was created to assist proper patient selection for RDN.444 Given the featured "always-on effect" and "one-time procedure" of catheter-based RDN, it is generally regarded as an evidence-based complimentary or alternative tool to help hypertension under control, in addition to lifestyle modification and antihypertensive medications.446,447
The Task Force recommends that RDN could be considered as a BP-lowering strategy in hypertensive patients with higher CV risk, such as resistant or masked uncontrolled hypertension, established ASCVD, intolerant or nonadherent to antihypertensive drugs, or features indicative of neurogenic hypertension (COR IIa, LOE B).444,445 A structured shared decision-making process is recommended for patients and healthcare professionals considering RDN in daily practice (Figure 8).444,446,447,461 Patients’ preference as well as physicians’ perspective including BP control status, comorbidities and pathophysiology should lead to an individualized BP management strategy.445,461
Figure 8.
Components of the structured shared decision-making process for renal denervation. AF, atrial fibrillation; ASCVD, atherosclerotic cardiovascular disease; HCVD, hypertensive cardiovascular disease; HTN, hypertension.
10. PRIMARY PREVENTION PATIENTS WITH GRADE 1 HYPERTENSION
Recommendations/Keypoints
• For primary prevention patients with grade 1 hypertension and at intermediate-to-high risk for ASCVD (with ≥ 3 CV risk factors and/or with HMOD), a BP target of < 130/80 mmHg should be considered (COR IIa, LOE B).
• For primary prevention patients with grade 1 hypertension and at low-to-intermediate risk for ASCVD (no HMOD and < 3 ASCVD risk factors), a BP target of < 130/80 mmHg may be reasonable (COR IIb, LOE B).
The evidence for the BP target for this population was limited because we do not have any target-driven RCT to support this, and the following recommendation was mainly based on post-hoc analyses and meta-analysis of RCTs.
10.1 Post-hoc analysis
The best evidence comes from the primary prevention subgroup analysis of the SPRINT trial.462 Of the 9,361 participants with follow-up data, 6,875 participants with a median predicted 10-year ASCVD risk of 15.9%, based on the AHA/ACC Pooled Cohort Equation, met the criteria of primary prevention. Baseline BP was 140 ± 16/80 ± 11 mmHg. Of these, 3,435 were randomized to standard BP control (< 140 [130 to 139] mmHg, by AOBP) and 3,440 to intensive BP control (SBP < 120 mmHg, by AOBP). Median follow-up was 3.3 years. In this subgroup, intensive BP control significantly reduced the hazard of incident ASCVD by 25% (HR: 0.75, 95% CI: 0.58-0.97, p = 0.03) and was associated with a non-significant 8% (HR: 1.08, 95% CI: 1.00-1.17 p = 0.06) increased risk in serious adverse events. The net clinical benefit was similar across the spectrum of baseline predicted 10-year ASCVD risk quartiles for both absolute and relative risk reduction. Of note, nearly a quarter of participants had a baseline predicted 10-year ASCVD risk < 10% (low-to-intermediate risk) at entry, the findings from the subgroup analysis of SPRINT trial suggest that the benefits of intensive BP intervention targeting systolic AOBP to < 120 mmHg may extend to even lower risk patients with grade 1 hypertension in the setting of primary prevention. Blood pressure in the SPRINT was measured using unattended AOBP, which corresponds more closely with mean daytime ambulatory BP or HBPM, thus a target SBP of < 120 mmHg in the SRPINT trial maybe equal to a target SBP of < 130 mmHg in the real-world HBPM setting. The Task Force recommends that lower-risk primary prevention patients with grade 1 hypertension may have the same BP targets as that for primary prevention patients who are at higher risk (COR IIb, LOE B).
The other lines of evidence come from three large trials of low-to-intermediate risk patients that compared antihypertensive therapy with placebo. Two of these (the Medical Research Council [MRC] trial and the Hypertension Detection and Follow-up Program [HDFP] trial) enrolled patients whose baseline ROBP was ≥ 140/90 mmHg; in the Heart Outcomes Prevention Evaluation [HOPE]-3 trial, approximately two-thirds of the study population had a ROBP at entry that was < 140/90 mmHg. In general, these studies suggest benefits from ROBP lowering to < 140/90 mmHg, that might be equal to a target HBPM of < 130/80 mmHg. BP targets differ depending upon the technique of measurement because "ROBP" methods typically provide higher BP readings by ~10 mmHg compared with the preferred "HBPM" methods.
The MRC trial was single-blind and based almost entirely on general practices. A total of 17,354 patients with a baseline DBP of 90 to 109 mmHg were randomly assigned to bendrofluazide, propranolol, or placebo for up to five years.246 Overall, 85,572 patient-years of observation had accrued. The mean baseline BP was approximately 161/98 mmHg; the mean attained BP was approximately 137/86 mmHg in the two treated groups and 150/92 mmHg in the placebo group. The treated groups had significantly lower rates of all CV events (6.7 vs. 8.2 per 1000 patient-years; p < 0.05 on sequential analysis) and of stroke (1.4 vs. 2.6 per 1000 patient-years; p < 0.01) but not of coronary events or mortality.
In the HDFP trial, 7,825 (71.5%) of the 10,940 participants had DBP averaging between 90 and 104 mmHg on entry into the study and were designated stratum 1.463 In stratum 1 of the study, these patients were randomly assigned to intensive therapy by stepped care. Particularly noteworthy was the beneficial effect of intensive treatment on persons with DBP of 90 to 104 mmHg who had no evidence of end-organ damage and were not receiving antihypertensive medication when they entered the study. Five-year mortality from all causes was 17% lower for the intensive therapy group (6.4 vs. 7.7%, p < 0.01) and 20% lower for the intensive therapy subgroup with entry DBP of 90 to 104 mmHg compared to the corresponding subgroup (5.9 vs. 7.4%, p < 0 .01). The magnitude of benefit was similar but not quite significant for the almost 3,000 patients with an entry DBP of 90 to 94 mmHg (absolute benefit 1.6%, 95% CI: -0.2 to +3.4%). The average attained DBP by ROBP was 85 to 90 mmHg in the intensive therapy group.464
The findings from the recent HOPE-3 trial provided additional evidence to support this BP target.234 This trial randomly assigned 12,705 participants (only 38% with BP ≥ 140/90 mmHg at baseline) at intermediate risk (mean 10-year CV risk ~8% by Framingham Risk Score) who did not have CV disease to receive either candesartan at a dose of 16 mg per day plus hydrochlorothiazide at a dose of 12.5 mg per day or placebo. The mean BP of the participants at baseline was 138.1/81.9 mmHg; the decrease in BP was 6.0/3.0 mmHg greater in the active-treatment group than in the placebo group. At 5.6 years, fewer CV events occurred among those treated with the fixed-dose combination, although this was not statistically significant. Of note, we looked at the association between mean in-trial BP as recorded in many measurements and vascular outcomes. Among the 6,356 subjects on candesartan/hydrochlorothiazide, those with a mean on-treatment SBP of 160 mmHg or more had a 2.61% per year rate of the composite of CV death, MI, stroke, rescue from cardiac arrest, heart failure, or revascularization. This was more than three-fold higher than the 0.75% per year rate in patients with an on-treatment SBP of 120-140 mmHg. The composite event rate was also significantly higher in those with a mean on-treatment SBP of 140-160 mmHg, at 1.4% per year. The event rate in patients with an on-treatment SBP below 120 mmHg was identical to that of patients with a value of 120-140 mmHg. Only among patients with an on-treatment DBP of 90 mmHg or more was the composite event rate significantly greater than in those with a DBP of 70-80 mmHg, who had the lowest event rate by a margin of 1.89% versus 0.75% per year. In this landmark trial, optimal outcomes were seen with an achieved, on-treatment SBP of 130-140 mmHg and a DBP of 75-80 mmHg, by ROBP.
10.2 Meta-analysis
The most informative data come from a recently published meta-analysis. This meta-analysis was conducted by the Blood Pressure Lowering Treatment Trialists’ Collaboration in individual participant-level data from 48 randomized trials of BP lowering medications versus placebo or other classes of BP-lowering medications, or between more versus less intensive treatment regimens.3 Data were pooled to investigate the stratified effects of BP-lowering treatment in participants with and without prevalent CV disease overall and across seven SBP categories (ranging from < 120 to ≥ 170 mmHg). Mean pre-randomization SBP/DBP were 157/89 mmHg in participants without previous CVD (54%). There was substantial spread in BP at baseline, with 8.0% of individuals without CVD having a SBP of < 130 mmHg, and 19% without CVD having a DBP < 80 mmHg in primary setting. The relative effects of BP-lowering treatment were proportional to the intensity of SBP reduction. At 4.15 years of follow-up, those without previous CVD at baseline, the incidence rate for developing a MACE per 1,000 person-years was 31.9 (95% CI: 31.3-32.5) in the comparator group and 25.9 (95% CI: 25.4-26.4) in the intervention group. Hazard ratios associated with a reduction of SBP by 5 mmHg for a major CV event were 0.91 (95% CI: 0.89-0.94) for participants without previous CVD. That is comparable for participants with previous CVD. These findings do not substantiate concerns about a J-shaped association between BP and CV outcomes in post-hoc analyses of several RCTs (see Section 6.3). In this large-scale analysis of RCTs, a 5 mmHg reduction of SBP reduced the risk of MACE by about 10%, irrespective of primary or secondary prevention, and even at normal or elevated BP levels.
11. PATIENTS WITH DIABETES MELLITUS
Recommendations/Keypoints
• For patients with diabetes mellitus, a BP target of < 130/80 mmHg, based on HBPM or standardized office BP, are recommended (COR I, LOE B).
Guidelines vary with target BP of < 130/80 mmHg to < 140/90 mmHg for patients with diabetes mellitus. Evidence supports lower mortality when achieving SBP ≤ 135 mmHg and DBP ≤ 80 mmHg in patients with diabetes. Diabetic patients were excluded from the SPRINT trial, so we do not have information about the optimal BP targets by AOBP measurement. After the ACCORD trial, there are many debates regarding the traditional office BP targets for diabetes.465 There are several limitations in the design of the ACCORD trial: 1) patients aged > 80 years were excluded, 2) patients with dyslipidemia were excluded, and 3) patients with serum creatinine > 1.5 mg/dL were excluded.465 The number of enrollment in the ACCORD trial was too low to have enough power to show difference of intensive (SBP < 120 mmHg) and conventional (SBP < 140 mmHg) strategies in the composite CV endpoints. Despite this, the annual rates of stroke, a pre-specified secondary outcome, were decreased by 41% in the intensive treatment group (p = 0.01).466 More importantly, in the standard glycemic control group, the intensive BP treatment group had a lower 5-year CV events compared with the standard BP treatment group (6.9% vs. 9.2%, p < 0.05).467 In a recent analysis combining the ACCORD trial and the SPRINT trial,465 the primary CV endpoints, stroke, and heart failure all favored the intensive treatment group, without significant heterogeneity between the 2 trials.465
In a recent meta-analysis comprising 40 trials with a total of 100,354 participants with type 2 diabetes, the effects of BP lowering on all-cause mortality, 4 macrovascular outcomes (CVD, coronary heart disease, stroke, and heart failure), and 3 microvascular outcomes (retinopathy, renal failure, and albuminuria) were examined.468 Patients with an achieved SBP < 130 mmHg had a 28% reduction in stroke, though coronary heart disease and mortality were un-changed. Since stroke is an important CV disease in East Asia, the Task Force recommends an SBP target of < 130 mmHg for diabetic patients, based on HBPM or standardized office BP (COR I, LOE B.)
For the DBP target for diabetes, the HOT trial is the only RCT available.400 The details of the rationale for choosing a DBP target of < 80 mmHg had been extensively described in the 2017 guideline updates and 2018 consensus.469,470
12. PATIENTS WITH CORONARY HEART DISEASE
Recommendations/Keypoints
• For patients with coronary heart disease, a BP target of < 130/80 mmHg is recommended (COR I, LOE A).
Many observational studies and meta-analyses have shown that there is a proportional correlation between BP levels and incidence of CVD, including stroke and myocardial infarction (MI), and adequate BP control is associated with improved CV outcomes. For instance, a meta-analysis enrolling one million adults without CVD from 61 prospective observational studies demonstrated that BP is positively correlated with vascular mortality if the value is above 115/75 mmHg.252 Another two meta-analyses have shown that BP reduction is correlated with CHD, stroke, or MI event reductions.343,471
However, in recent years, many observational studies and subgroup analyses/post-hoc analyses of RCTs have demonstrated the "J curve phenomenon" between BP targets and clinical outcomes. The "J curve phenomenon" means if the BP is lower than a certain nadir point, the pressure would become too low to maintain adequate perfusions to vital organs including heart and brain, which may result in adverse cerebrovascular and CV outcomes. For example, in the post-hoc analysis of the INVEST study, BP lower than a nadir value of 129.5/73.8 mmHg was associated with an increased risk of primary outcomes in CHD patients with hypertension,472 and the J curve phenomenon was consistent among different age groups. In addition, patients receiving revascularization were shown to tolerate lower DBP than those without intervention.473 Similarly, analysis in the PROVE-IT study also showed an increased recurrent MI risk in acute coronary syndrome (ACS) patients with both lower SBP and DBP.474 Another analysis of the pooled data from the ONTARGET and the TRANSCEND trials, which enrolled high-risk patients with coronary artery diseases, cerebrovascular disease, peripheral artery disease, or diabetes with end-organ damage, also showed increased CV risks in subjects with achieved BP lower than 120/70 mmHg.244 In the CLARIFY registry, BP of < 120/70 mmHg was shown to be associated with adverse CV outcomes in patients with stable CHD.265
Nevertheless, the observation of J curve phenomenon was refuted by other large-scale epidemiological studies such as UKPDS, MRFIT, and Asia Pacific Cohort Studies.475-477 The J curve phenomenon should also be interpreted cautiously because clinical trials were not designed to compare different BP targets. The J curve phenomenon obtained from post-hoc analysis may be the result of reverse causality due to lack of randomization. Besides, the patient numbers of lower BP groups were mostly small, which hindered conclusive analysis of these data. According to another analysis from pooled data of ACCORD and SPRINT studies, the J curve was identical in shape for patients randomized to target SBP < 120 mmHg and target SBP < 140mmHg. This observation implied that lower attained BP than target BP values may be a marker of unfavorable baseline characteristics confounders, rather than the causative factor of worsened clinical outcomes.478 Therefore, the efficacy and safety associated with intensive BP lowering can only be established via RCTs which were designed to compare different treatment BP targets.
Evidence from other studies supports the benefits of intensive BP control for patients with CHD. According to a coronary IVUS sub-study of the CAMELOT trial, normal attained BP (< 120/80 mmHg) after 2 years of treatment was associated with reduced coronary atheroma volume in patient with CHD.230 A cohort study including 1.25 million patients demonstrated that the lowest CVD risk was in people with SBP of 90-114 mmHg and DBP of 60-74 mmHg.4 Most importantly, the SPRINT trial randomized 9,361 patients with CVD or at high risk for CVD to intensive BP treatment (SBP goal < 120 mmHg) or to standard BP treatment (SBP goal < 140 mmHg). The mean SBP in the intensive and standard treatment arms were 121.5 mmHg and 134.6 mmHg, respectively. Throughout the 3.26 years of follow-up, incidences of composite primary outcomes and all-cause mortality were reduced by 25% [1.65% per year vs. 2.19% per year; HR: 0.75; 95% CI: 0.64 to 0.89; p < 0.001) and 27% (HR: 0.73; 95% CI: 0.60-0.90), respectively. The benefits of intensive BP control are consistent across all subgroups, including patients with or without previous CVD.258 Furthermore, data from several meta-analyses also support intensive BP control in patients with CHD. A meta-analysis including 123 studies and 613,815 subjects showed that every 10 mmHg SBP reduction significantly reduced vascular risk irrespective of baseline BP levels and comorbidities. The benefit of BP reduction is consistent in patients with baseline SBP < 130 mmHg and in patients with CHD.232 Another network meta-analysis also demonstrated that more intensive SBP reduction to 120-124 mmHg was still beneficial in CVD and all-cause mortality reductions.479 Finally, according to the data from the latest meta-analysis, which included 344,716 participants from 48 RCTs, each 5 mmHg SBP reduction reduced major CV risks by around 10%, irrespective of underlying CVD status or SBP level. The benefit of BP reduction is even consistent in CVD patients with baseline SBP ≤ 120 mmHg.3 In summary, based on current available clinical evidence, the BP target in patients with CHD should be less than 130/80 mmHg (COR I, LOE A).
13. PATIENTS WITH CEREBROVASCULAR DISEASE
Recommendations/Keypoints
• It is not recommended to lower BP in the prehospital setting without knowing the phenotypes of stroke (COR III, LOE B).
• Routine aggressive BP lowering is not recommended unless BP ≥ 220/120 mmHg or in the presence of other situations needing immediate BP lowering (such as acute aortic dissection, congestive heart failure with lung edema, hypertensive encephalopathy) within 24 hours of acute ischemic stroke without undergoing IVT or EVT (COR III, LOE A).
• BP should be controlled to < 185/110 mmHg before starting IVT or EVT for acute ischemic stroke (COR I, LOE C).
• BP should be controlled to < 180/105 mmHg within 24 h after IVT or EVT for acute ischemic stroke (COR IIa, LOE B).
• Before successful recanalization, avoidance of a large BP reduction (> 40%) during EVT should be considered (COR IIa, LOE B), and strict SBP control around 140-180 mmHg may be considered (COR IIb, LOE C).
• Keeping lower BP to < 140/90 mmHg may be considered within 24 hours after successful EVT for acute ischemic stroke (COR IIb, LOE C).
• BP-lowering treatment is recommended if SBP exceeds 220 mmHg in patients with acute phase of intracranial hemorrhage (COR I, LOE C).
• In patients with acute hemorrhagic stroke within 6 hours and SBP > 160 mmHg, a reduction in SBP by ≥ 20 mmHg within 1 h and maintained at < 140 mmHg for 1-24 h should be considered (COR IIa, LOE A).
• Antihypertensive treatment should be initiated if SBP > 160 mmHg for more than 30 minutes in patients with acute aneurysmal SAH, and an SBP target around 120-160 mmHg should be considered until the aneurysm is treated (COR IIa, LOE C). Personalized BP targets may be considered based on cerebral blood flow measurement and continuous monitoring intracranial pressure (COR IIb, LOE C).
• Starting antihypertensive treatment in patients with acute and stable stroke (no observed deterioration of neurological deficits owing to brain hypoperfusion) within 24-72 hours is reasonable (COR IIa, LOE B).
• The initial BP target is < 140/90 mmHg in the convalescence stage regardless of extracranial/intracranial large vessel disease or cerebral small vessel disease (COR I, LOE B) and a BP target of < 130/80 mmHg should be considered for most patients in the chronic stage of stroke (COR IIa, LOE A).
• Careful observation of brain hypoperfusion-related side effects caused by BP-lowering therapy may be considered in patients with bilateral internal carotid artery significant stenoses or basilar artery stenosis (> 70% luminal diameter stenosis) (COR IIb, LOE B).
• An ACE inhibitor, ARB, diuretic, or calcium channel blocker should be the first-line drug for secondary prevention of stroke (COR IIa, LOE B).
Hypertension is the most important and modifiable risk factor for primary and secondary prevention of stroke.480 Nevertheless, it is complicated to recommend BP targets for patients with stroke, owing to its various phenotypes (ischemic or hemorrhagic) and subtypes, different stages and treatment modalities, and status of brain perfusion.
13.1 Blood pressure control in the prehospital setting of suspected stroke
High BP is common in the acute stage of stroke and is associated with poor clinical outcomes.481 The RIGHT-2 study, a prospective RCT, included 1,149 patients with presumed stroke within 4-hour onset and SBP of 120 mmHg or higher were randomly assigned to transdermal glyceryl trinitrate or placebo in the ambulance.482 The results showed that there was no significant difference in the risk of primary endpoint (modified Ranson score [mRS]) or other endpoints between both groups despite a significantly lower BP by 5.8/2.6 mmHg in patients treated with active compound than those with placebo.482 Therefore, it is not recommended to lower BP in the prehospital setting without knowing the phenotypes of stroke.
13.2 Blood pressure targets for patients with ischemic stroke (IS)
13.2.1 Patients not treated with intravascular thrombolysis (IVT) or endovascular thrombectomy (EVT)
High BP after acute ischemic stroke (AIS) is significantly associated with unfavorable clinical outcomes without J-curve phenomenon.481,483 However, efforts exercised in lots of RCTs484-489 to lower BP in patients with AIS showed no significant benefit. The COSSACS trial included 763 patients with acute stroke (AS) (59.5% AIS) within 48 hours of onset who had a history of hypertension and BP-lowering drugs before index stroke and these participants were randomized into 2 groups: continuing or discontinuing BP-lowering drugs. The results showed a similar risk of primary endpoint (death or dependence at 14 days) (relative risk [RR]: 0.86, 95% CI: 0.65-1.14), 6-month CV events or mortality between both groups despite a significant difference in SBP (13 mmHg) and DBP (8 mmHg).484 The SCAST trial including 2,029 patients with AS (85% AIS) within 30-hour onset who had baseline BP higher than 140/90 mmHg was designed to evaluate the outcome effect of active BP lowering by candesartan treatment for 7 days (achieved mean BP 147/ 82 mmHg) versus placebo (achieved mean BP 152/84 mmHg).485 The results of the SCAST trial revealed a similar risk of 3-point major adverse CV events (MACEs). (CV death, myocardial infarction or stroke) (RR: 1.09, 95% CI: 0.84-1.41) and a higher risk of poor mRS at 6 months (RR: 1.17, 95% CI: 1.00-1.38).485 A post-hoc analysis of the SCAST trial showed that patients with a large decrease in SBP has the highest risk of MACEs.490 A long-term follow-up data from the SCAST study did not show a different risk of the MACEs between both groups (RR: 0.87, 95% CI: 0.71-1.07).491 The CATIS trial included 4,071 Chinese patients with AIS within 48 hours of onset who were randomly assigned to immediate BP lowering by 10-25% initially and subsequently controlling BP to target SBP < 140 mmHg at 7 days or discontinuing all anti-hypertensive drugs.486 The achieved mean SBP was 144.7 mmHg versus 152.9 mmHg initially, and 137.3 mmHg versus 146.5 mmHg at 7 days, respectively. The results of the CATIS trial showed a similar risk of primary composite endpoint (death or major disability) at 14 days (RR: 1.00, 95% CI: 0.86-1.15)486 regardless of baseline BP492 or stroke severity.493 The ENOS trial included 4,011 patients with AS (83% AIS) within 48 hours of onset who had raised SBP around 140-220 mmHg and were randomly assigned to transdermal trinitrate treatment or placebo up to 7 days.487 The achieved mean SBP was 160 mmHg versus 163.5 mmHg at one day, and 157.5 mmHg versus 162 mmHg at 7 days, respectively.487 The results did not show superior effect with active BP lowering or continuing anti-hypertensive drugs on the risk of primary endpoint (mRS at 90 days).487 The CHASE study included 483 AS patients (50% AIS) within 72 hours of onset who had elevated SBP around 150-210 mmHg and were randomly assigned to achieve SBP reduction by 10-15% or to < 200 mmHg in patients with AIS.488 The achieved mean SBP was 144 mmHg versus 148 mmHg initially, and 138.1 mmHg versus 139.7 mmHg at 7 days, respectively.488 The primary endpoint (mRS at 90 days) was undoubtful no difference due to similar SBP achieved in both groups.488 The MAPAS trial included 218 AIS patients within 12 hours of onset who were randomized into 3 groups (targeting and maintaining SBP 140-160 mmHg, 161-180, or 181-200).489 The achieved mean SBP was 153 mmHg, 163 mmHg and 178 mmHg at 24 hours, respectively.489 The results show a similar risk of functional outcome between 3 groups; however, the higher SBP group was associated with a higher risk of symptomatic intracranial hemorrhage (ICH).489 Finally, two meta-analyses revealed no beneficial effect of early BP lowering in patients with AIS.494,495
Taken together, there is no evidence for routine aggressive BP lowering in the acute stage of ischemic stroke without receiving IVT or EVT.
13.2.2 Patients treated with IVT
Two time points should be specifically addressed: before and after IVT. There was no RCT aimed to investigate the BP target for AIS before administration of IVT. The recommendations from the current guidelines13,161,496 were based on the exclusion criteria of the GUSTO trial for acute myocardial infarction497 and the MIND tPA trial for AIS.498 Protocol violation with uncontrolled high BP before IVT was associated with an increased risk of ICH499,500 and poor functional recovery.500 Therefore, BP should be controlled to < 185/110 mmHg before starting IVT.
The current guidelines161,496 recommended that SBP should be controlled to < 180/105 mmHg within 24 hours after IVT. Two observational studies501,502 showed that protocol violation or high SBP after IVT was associated with an increased risk of ICH and poor clinical outcome. The optimal SBP after IVT appeared to be around 140-160 mmHg.501 The ENCHANTED trial included 2,196 AIS patients within 6 hours of onset who had an elevated SBP of > 150 mmHg before IVT and were randomly assigned to targeting SBP to 130-140 mmHg or < 180 mmHg within one hour and keeping it for 72 hours.503 The mean achieved SBP was 144.3 mmHg in the lower target group and 149.8 mmHg in the higher target group, respectively.503 The results of the ENCHANTED study showed that aggressive BP lowering after IVT was associated with a similar risk of primary endpoint (mRS at 90 days) or death but a lower risk of any ICH (RR: 0.75, 95% CI: 0.60-0.94).503 Therefore, SBP lowering to 140-150 (or 160) after IVT is feasible and might reduce ICH risk; however, the effect on functional outcome is controversial. Nevertheless, SBP should be controlled to < 180/105 mmHg within 24 h after IVT due to lack of strong evidence and being aligned with the recommendations from the 2020 Taiwan Stroke Society guideline.504
Two observational studies505,506 showed that higher SBP variability after IVT was associated with a higher risk of poor clinical outcome (RR: 1.68, 95% CI: 1.05-2.69)505 and severe hemorrhagic transformation (RR : 2.785, 95% CI: 1.294-5.994)506 but a similar risk of ICH.505 Therefore, close monitoring BP and keeping stable BP are needed for AIS after IVT.
13.2.3 Patients treated with EVT
Three time points should be addressed: before, during, and after EVT. There was no RCT aimed at the investigation of the BP target for AIS before starting EVT. The current guidelines recommended the same SBP target before EVT based on the exclusion criteria of all RCTs regarding EVT for AIS.504 A post-hoc analysis of the MERCI and Multi MERCI trials showed that pre-EVT SBP > 150 mmHg was associated with recanalization failure for EVT.507 A post-hoc analysis of the MR CLEAN trial revealed the U-curve relation of pre-EVT SBP and functional recovery with the optimal SBP being approximately 120 mmHg.508 Although some observations suggested a lower BP target, Although some observations suggested a lower BP target, BP should be controlled to < 185/110 mmHg before starting EVT due to lack of strong evidence and being aligned with the recommendations from the 2020 Taiwan Stroke Society guideline.504
There was no RCT with regard to BP control during EVT procedure. BP drop is a common phenomenon during EVT procedure due to sedation or general anesthesia and should be seriously concerned given that profound BP drop will affect brain perfusion with subsequent infarct progression and poor functional recovery before successful recanalization.509 Some observational studies identified BP reduction (mean BP reduction > 40%510 or ≥ 10%509,511) during EVT as one of the independent predictors of poor functional recovery. However, the difference became insignificant if peri-procedural SBP was strictly maintained around 140-180 mmHg.512 In addition to BP levels, the duration of BP changes was also a risk factor of poor neurological outcome (mean BP < 70 mmHg for > 10 minutes or mean BP > 90 mmHg for > 45 minutes).513 Taken together, close monitoring and managing BP are recommended for AIS during EVT. Before successful recanalization, avoidance of a large BP reduction (> 40%) may be needed and strict SBP control around 140-180 mmHg is reasonable.
Whether occluded vessel is successfully opened or not is an important issue in considering BP target after EVT. A retrospective analysis of 217 AIS patients with large vessel occlusion undergoing EVT (without mention of successful recanalization or not) showed that moderate BP control after EVT (BP < 160/90 mmHg) was associated with a lower risk of 3-month mortality but a similar risk of 3-month functional independence compared with permissive hypertension or intensive BP control (BP < 140/90 mmHg).514 The BEST trial, a prospective multi-center registry, including 485 AIS patients undergoing EVT (without mention of successful recanalization or not) showed that peak SBP > 158 within 24 hours after EVT had an increased likelihood of having a bad functional outcome in unadjusted, butnot in adjusted analysis.515 Another retrospective analysis of 690 AIS patients undergoing EVT revealed that lower mean SBP (132 mmHg vs. 137 or 138 mmHg) within 24 hours after EVT was associated with better functional outcome in patients with successful recanalization but the association became insignificant in those without successful recanalization.516 A larger observation study including 1,019 AIS patients undergoing EVT showed that intensive BP control (SBP < 140 mmHg) within 24 hours after successful EVT was associated with a lower risk of worse functional outcome and hemicraniectomy, whereas mo-derate BP control (SBP < 160 mmHg) was associated with a lower risk of 90-day mortality.517 A retrospective analysis of 166 AIS patients with successful recanalization in Taiwan showed that achieved SBP levels ranging from 90 to 150 mmHg at 6 hours after EVT were linearly correlated with the risk of poor functional outcome without U-curve phenomenon.518 Some observational studies showed that higher BP variability within 24 hours after EVT was significantly associated with a higher risk of poor clinical outcome.519-521 Taken together, because of lack of strong evidence for aggressive BP lowering after EVT, keeping BP < 180/105 mmHg is reasonable within 24 hours after EVT. However, keep lower BP to < 140/90 may be considered within 24 hours after successful EVT.
13.2.4 Drugs of choice
There is no evidence to recommend routine use of specific BP-lowering agents for the acute BP management of AIS.504 Drugs of choice should be individualized. In general, BP-lowering agents with rapid onset and short duration of action are reasonable (Table 17).486
Table 17. Anti-hypertensive drugs for acute blood pressure-lowering treatment.
| Drug | Route and dosage | Onset of action | Duration of action | Side effect | Contraindication |
| Nicardipine | 5 mg/h IVD, uptitrate 2.5 mg/h every 15-30 min, maximum 15 mg/h | 5-15 min | 30-40 min | Headache; reflex tachycardia | Liver failure |
| Labetalol | 0.25-0.5 mg/kg IVB, 2-4 mg/min IVD | 5-10 min | 3-6 h | Bradycardia; bronchoconstriction | Second or third degree AVB; asthma; bradycardia; HFrEF |
| Esmolol | 0.5-1 mg/kg IVB, 50-300 μg/kg/min IVD | 1-2 min | 10-30 min | Bradycardia | Second or third degree AVB; asthma; bradycardia; HFrEF |
| Glyceryl trinitrate | 5 mg/d transdermally | 30-60 min | 12-14 h | Allergy | Concomitant PDE-5 inhibitor |
| Nitroglycerine | 5-10 μg/min IVD, uptitrate 5 μg/min every 5 min, maximum 200 μg/min | 1-5 min | 3-5 min | Headache; reflex tachycardia | None |
| Hydralazine | 10-20 mg IVB, repeat every 4-6 h, maximum 40 mg | 10-20 min | 12 h | Reflex tachycardia; IICP | IICP |
| Nitroprusside | 0.3-0.5 μg/min IVD, uptitrate 0.5 μg/min every 5 min, maximum 10 μg/min | Immediate | 1-2 min | Headache; reflex tachycardia; IICP | IICP |
AVB, atrioventricular block; HFrEF, heart failure with reduced ejection fraction; IICP, increased intracranial pressure; IVB, intravenous bolus; IVD, intravenous drip; PDE-5, phosphodiesterase-5.
13.3 Blood pressure targets for patients with acute hemorrhagic stroke
13.3.1 Acute ICH
ICH accounts for approximately 15% (up to 45% in East Asians522) of total strokes and carries high morbidity and mortality.523 BP often becomes elevated and up to very high level in the acute stage of ICH.481,524 A substantial amount of evidence suggests that higher BP in the acute stage of hemorrhagic stroke is associated with higher case fatality and worse functional outcome.481,524 In the acute stage of ICH, it is arguable that BP lowering would result in cerebral blood flow reduction around peri-hematoma area. The ICH ADAPT trial revealed that there was a similar peri-hematoma and borderzone cerebral blood flow regardless of targeting SBP < 150 mmHg or < 180 mmHg and regardless of the magnitude of BP reduction in patients with acute ICH within 24 hours of onset.525,526 There were 3 RCTs aimed to investigate the BP lowering effects on clinical outcomes in patients with acute ICH.524,527,528 The INTERACT study including 404 patients with acute ICH within 6 hours of onset who had a baseline SBP around 150-220 mmHg and were randomly assigned to target SBP < 140 mmHg compared to < 180 mmHg within 1 hour after hospital presentation.527 The mean achieved SBP was 153 mmHg vs. 167 mmHg at 1 hour and 146 mmHg vs. 157 mmHg within 1-24 hours, respectively.527 The results of the INTERACT study revealed a 26% more reduction in hematoma volume at 24 hours in the intensive BP-lowering group as compared to the conventional group without any safety concern.527 Owing to the encouraging data from that pilot study, the INTERACT2 trial included more participants (2,839; 68% Asians) with a similar study design but a different primary composite endpoint.524 The mean achieved SBP was 150 mmHg in the intensive BP-lowering group and 164 mmHg in the conventional group at 1 hour, respectively.524 However, the results of the INTERACT2 trial showed a trend for a lower risk of primary composite endpoint (death or major disability) (RR: 0.87, 95% CI: 0.75-1.01) in the intensive BP-lowering group than the conventional group despite no statistical significance.524 Nevertheless, a significantly lower risk of poor functional outcome (RR: 0.87, 95% CI: 0.77-1.00) with better quality of life was noted in the intensive BP-lowering group without any safety concern.524 The ATACH-2 study including 1,000 patients with acute ICH within 4.5 hours of onset who had a baseline SBP > 180 mmHg and were randomly assigned to intensive BP control (targeting SBP 110-139 mmHg) or standard BP control (targeting SBP 140-179 mmHg) within 2 hours and through 24 hours. The mean achieved SBP was 128.9 mmHg in the intensive group and 141.1 mmHg in the standard group, respectively. However, the ATACH-2 study did not show a different risk of primary composite endpoint (death or disability) at 3 months or acute hematoma growth at 24 hours between both groups. Moreover, participants assigned to intensive BP target experienced more serious adverse events, mainly driven by renal events.528 Nevertheless, a sub-analysis of the ATACH-2 study showed that a 30% relative reduction in the hematoma growth in favor of intensive BP lowering treatment was observed in the subgroup with moderate-to-severe ICH.529 A post-hoc analysis of the INTERACT2 trial showed that more SBP reduction (≥ 20 mmHg vs. 10-20 mmHg or < 10 mmHg) was associated with a lower risk of death/major disability, deterioration of physical function, or death regardless of the time window of BP reduction (15-60 minutes, 1-24 hours, or 2-7 days).530 Another post-hoc analysis of the INTERACT2 trial revealed that a shorter time to achieve SBP < 140 mmHg was significantly associated with a more reduction in the absolute hematoma growth (≤ 1 hour vs. 1-6 hours or ≥ 6 hours).531 Early achieving SBP < 160 mmHg was associated with less hematoma growth, as described in the SAMURAI observational study.532 A meta-analysis of the INTERACT2 and the ATACH-2 studies revealed that achieving lower and stable SBP earlier was safe and associated with favorable outcomes in patients with acute ICH.533
Taken together, intensive SBP lowering is safe without subsequent perihematoma and borderzone hypoperfusion in the acute stage of ICH. SBP reduced by ≥ 20 mmHg within 1 h and maintained < 140 mmHg for 1-24 h are beneficial with an acceptable safety profile.
13.3.2 Acute aneurysmal subarachnoid hemorrhage (SAH)
Rebleeding of the ruptured aneurysm is associated with high morbidity and mortality.534 It usually occurs within 2-12 hours of onset.534,535 SBP > 160 mmHg is one of the risk factors of rebleeding.534,535 However, there is no high-grade and evidence-based guidelines so far to recommend BP target and BP management in the acute stage of aneurysmal SAH, resulting in variation in clinical practice among different physicians and institutes.536 BP control should be tried to balance the risk of stroke, the risk of BP-related rebleeding, and the maintenance of the cerebral blood flow.534 Recently, personalized BP targets were suggested based on measuring the surrogate of the cerebral blood flow and continuous monitoring intracranial pressure.537
13.3.3 Drugs of choice
There is also no evidence to recommend routine use of specific BP-lowering agents for the acute BP management of hemorrhagic stroke.504 The candidate drugs for hemorrhagic stroke are similar to AIS (Table 17).
13.4 Blood pressure control for acute stroke in the convalescent and chronic stages
13.4.1 Blood pressure targets
Recurrent stroke is common in patients with history of stroke.504,523 The recurrence rate is around 3-22% within 1 year after index IS504,538 and the cumulative risk of ICH recurrence is 1% to 5% per year.523,539,540 Hypertension is the most important and modifiable risk factor for recurrent IS480,504,522 or hemorrhagic stroke.523,540 Therefore, BP control is theoretically the most valuable strategy for prevention of recurrent stroke.
There are 7 RCTs aimed to evaluate the outcome effect of BP-lowering treatment for secondary prevention of stroke (Table 18).410,541-546 The PATS study including 5,665 Chinese patients with a history of stroke (64.4% IS, 10.5% transient ischemic accident [TIA], and 14.4% hemorrhagic stroke) within 1-120 months after the index event who had a mean baseline BP approximately 154/93 mmHg (83.9% with a history of hypertension) and were randomly assigned to indapamide treatment 2.5 mg per day or matching placebo.541 The mean achieved BP was 141.4/84.1 mmHg in the active treatment group and 147.3/87.2 mmHg in the placebo group.541 The results of the PATS study showed a lower risk of all stroke (RR: 0.69, 95% CI: 0.54-0.89) and CV events (RR: 0.75, 95% CI: 0.62-0.89) in the active treatment group as compared to placebo during 2-year follow-up.541 The PROGRESS study including 6,105 patients with a history of stroke (71% IS, 22% TIA, and 11% ICH) within 2-22 months after the index event who had a mean baseline BP approximately 147/86 mmHg (48% with a history of hypertension defined as BP ≥ 160/90 mmHg) and were randomly assigned to perindopril 4 mg ± indapamide 2.5 mg per day or matching placebo.410 The mean difference of achieved BP between active treatment group and placebo group was 9/4 mmHg (4.9/2.8 mmHg for single drug and 12.3/5 mmHg for combination therapy, respectively).410 The results of the PROGRESS study revealed that active treatment was associated with a lower risk of total stroke (RR reduction 28%, 95% CI: 17-38%), IS (RR reduction 24%, 95% CI: 10-35%), or major vascular events (RR reduction 26%, 95% CI: 16-34%)410 regardless of stroke subtype,547 baseline medications,547 or hypertension phenotype.548 The post-hoc analysis of the PROGRESS study showed that there was no J-curve relationship between BP levels and stroke risks.549 Moreover, Asians appeared to get more outcome benefits from active BP-lowering treatment than Western participants in the PROGRESS study.550 The MOSES study including 1,405 hypertensive patients with a history of stroke (61% IS, 27% TIA, and 5% ICH) and mean 11.6 months of onset before randomization who were randomly assigned to eprosartan 600 mg per day or nitrendipine 10 mg per day.542 The mean achieved BP was similar between both groups (138/81 mmHg vs. 136/ 80 mmHg).542 This study showed that eprosartan treatment was associated with a lower risk of primary composite endpoint (total death, all CV or all cerebrovascular events) (RR: 0.79, 95% CI: 0.66-0.96) or all cerebrovascular events (RR: 0.75, 95% CI: 0.58-0.97) than nitrendipine treatment.542 The ProFESS study including 20,332 patients with a history of noncardiac IS within 90 days who had a mean baseline BP 144/84 mmHg (74% with a history of hypertension) and were randomly assigned to telmisartan 80 mg per day or matching placebo.543 The difference of mean achieved BP levels between both groups was 3.8/2 mmHg.543 However, there was no significant difference of total stroke (RR: 0.95, 95% CI: 0.86-1.04) or MACEs (OR: 0.94, 95% CI: 0.87-1.01) between both groups during 2.5-year follow-up probably owing to a small difference of achieved BP levels between both groups. The SPS3 trial including 3,020 patients with a recent (≤ 180 days) symptomatic lacunar infarct documented by magnetic resonance imaging who had a mean baseline BP 143/78.5 mmHg and were randomized into lower BP group (targeting SBP < 130 mmHg) and higher BP group (targeting SBP around 130-149 mmHg).544 The mean achieved SBP was 127 mmHg in the lower BP group and 138 mmHg in the higher BP group at 1 year and the BP difference was approximately 11 mmHg at the last study visit.69 The results of the SPS3 study showed that targeting lower BP tended to reduce total stroke risk (RR: 0.81, 95% CI: 0.64-1.03, p = 0.08) or MACEs (RR: 0.84, 95% CI: 0.68-1.01, p = 0.10) despite no statistical significance.544 Nevertheless, a lower BP level was significantly associated with a lower risk of ICH (RR: 0.37, 95% CI: 0.14-0.89).544 However, intensive BP lowering was associated with a greater likelihood of rapid renal function deterioration in 2,610 participants of the SPS3 trial who had a normal baseline renal function.551 Nevertheless, renal function deterioration was not associated with MACEs in the intensive BP lowering arm.551 The PAST-BP trial included 529 patients with a history of stroke or TIA who had a baseline SBP ≥ 125 mmHg and were randomly assigned to intensive BP lowering group (SBP < 130 mmHg or 10 mmHg reduction if baseline SBP < 140 mmHg) or standard BP lowering group (SBP < 140 mmHg).545 The mean achieved SBP was 127.4 mmHg in the intensive arm and 129.4 mmHg in the standard arm, respectively.545 The results of the PAST-BP trial showed a similar risk of total stroke between both groups (RR: 0.14, 95% CI: 0.01-2.72).545 The RESPECT study included 1,280 hypertensive patients (eventually 1,263 were analyzed) with acute stroke (85% IS and 15% ICH) within 1 month to 3 years who had a baseline BP 145.4/83.6 mmHg and were randomized into intensive BP control group (BP target < 120/80) and standard control group (BP target < 140/90, or < 130/80 if diabetes, chronic kidney disease, or coronary artery disease).546 The mean achieved BP was 126.7/77.4 mmHg in the intensive BP control group and 133.2/77.7 mmHg in the standard control group, respectively.546 The results of the RESPECT study showed that intensive BP control was associated with a lower risk of ICH (RR: 0.09, 95% CI: 0.01-0.70) and tended to reduce total stroke risk despite no statistical significance (RR: 0.73, 95% CI: 0.49-1.11).546 A meta-analysis of 42,736 patients showed that SBP reduction was linearly related to the lower risk of recurrent stroke, myocardial infarction, total death, and CV death, while DBP reduction was linearly related to a lower risk of recurrent stroke and total death.552 This observational study indicated a BP target < 130/85 was reasonable.552 The investigators of the RESPECT study performed a meta-analysis including the SPS3, the PAST-BP, and the RESPECT trials and found that intensive BP treatment was associated with a lower risk of recurrent stroke (RR: 0.78, 95% CI: 0.64-0.96) without significant heterogeneity.546 Taken together, a BP target of < 130/80 is beneficial for secondary prevention of stroke, especially ICH risk.
Table 18. RCTs regarding BP control for the secondary prevention of stroke.
| Trial | Patients | Stroke subtype | History of HT | Intervention | Timing | Baseline BP (mmHg) | Achieved BP or difference (Δ) (mmHg) | Outcomes |
| PATS | 5665 Chinese | 64.4% IS, 10.5% TIA, and 14.4% HS | 83.9% | Indapamide 2.5 mg QD vs. placebo | ≥ 1-120 months | 154/93 | 141.4/84.1 (active treatment), 147.3/87.2 (placebo) | ↓all stroke (RR 0.69, 95% CI 0.54-0.89); ↓CV events (RR 0.75, 95% CI 0.62-0.89) |
| PROGRESS | 6105 | 71% IS, 22% TIA, and 11% ICH | 48% (≥ 160/90 mmHg) | Perindopril 4 mg ± indapamide 2.5 mg QD vs. placebo | 2-22 months | 147/86 | Δ: 9/4 (single: 4.9/2.8; dual: 12.3/5) | ↓total stroke (RR reduction 28%, 95% CI 17-38%); ↓IS (RR reduction 24%, 95% CI 10-35%); ↓ MACEs (RR reduction 26%, 95% CI 16-34%) |
| MOSES | 1405 | 61% IS, 27% TIA, and 5% ICH | 100% | Eprosartan 600 mg vs. nitrendipine 10 mg QD | Mean 11.6 months | 151/84 vs. 152/87 | 138/81 vs. 136/80 | ↓primary composite endpoint (RR 0.79, 95% CI 0.66-0.96); ↓all cerebrovascular events (RR 0.75, 95% CI 0.58-0.97) |
| ProFESS | 20332 | Non-cardiogenic IS | 0.74 | Telmisartan 80 mg QD vs. placebo | Mean 15 days | 144/84 | Δ: 3.8/2 | ↔total stroke (RR 0.95, 95% CI 0.86-1.04); ↔MACEs (RR 0.94, 95% CI 0.87-1.01) |
| SPS3 | 3020 | Lacunar stroke | 75% | BP target < 130 mmHg vs. 130-149 mmHg | ≤ 180 days | 143/78.5 | SBP 127 (lower target) vs. 138 (higher target) at 1 year; Δ: 11 at last visit | ↔total stroke risk (RR 0.81, 95% CI 0.64-1.03, p = 0.08); ↔MACEs (RR 0.84, 95% CI 0.68-1.01, p = 0.10); ↓ICH (RR 0.37, 95% CI 0.14-0.89) |
| PAST-BP | 529 | 47.6% stroke, and 52.2% TIA | NA | SBP target < 130 (or 10 mmHg reduction) vs. < 140 | NA | SBP ≥ 125 | SBP 127.4 vs. 129.4 | ↔total stroke (RR 0.14, 95% CI 0.01-2.72) |
| RESPECT | 1280 (1263 were analyzed) | 85% IS and 15% ICH | 100% | BP target < 120/80 vs. < 140/90 (or < 130/80 if DM, CKD, or CAD) | 1 month to 3 years | 145.4/83.6 | 126.7/77.4 (lower target) vs. 133.2/77.7 (higher target) | ↔total stroke (RR 0.73, 95% CI 0.49-1.11); ↓ICH (RR 0.09, 95% CI 0.01-0.70) |
↓ denotes significantly reduced and ↔ denotes a similar risk.
BP, blood pressure; CAD, coronary artery disease; CI, confidence interval; CKD, chronic kidney disease; DM, diabetes mellitus; HS, hemorrhagic stroke; HT, hypertension; ICH, intracranial hemorrhage; IS, ischemic stroke; MACEs, major adverse cardiovascular events; NA, not available; RCTs, randomized controlled trials; RR, relative risk; SBP, systolic blood pressure; TIA, transient ischemic accident.
13.4.2 When to target blood pressure for the secondary prevention of stroke
There were 3% participants randomized and treated within one week of acute stroke in the MOSES trial542 and 40% participants within 10 days of onset in the ProFESS trial.543 A sub-analysis of the ProFESS trial showed that 6.7% participants started treatment within 72 hours of onset without a safety signal.553 Therefore, starting anti-hypertensive treatment in patients with acute and stable stroke within 24-72 hours is acceptable. The initial BP target for stable stroke in the convalescent stage is < 140/90 mmHg based on the encouraging data from acute ICH trials and AIS trials with successful recanalization.
13.4.3 Drugs of choice for the secondary prevention of stroke
Owing to the pleiotropic effects of ARB, this compound has long been paid more attention about its potential benefits for stroke prevention.554 As mentioned previously, the MOSES study showed that eprosartan treatment was associated with a lower risk of primary composite endpoint or all cerebrovascular events than nitrendipine treatment in patients with a history of stroke.542 However, the biggest RCT with respect to the outcome effect of ARBs, the ProFESS study, failed to demonstrate a superior effect of an ARB treatment to placebo for secondary prevention of stroke. According to an observational study from Taiwan, ACE inhibitors plus diuretics or diuretics alone is superior to placebo for secondary prevention of stroke; however, head-to-head comparisons of anti-hypertensive drugs did not show each given drug class was superior to any other class.555 Another meta-analysis of 143,095 patients showed that compared with placebo, ACE inhibitor, ARB, and diuretics were significantly associated with a reduced risk of CV events.556 Moreover, ACE inhibitors were also associated with a lower risk of all secondary outcomes, whereas CCBs and diuretics were associated with a reduced risk of stroke significantly as compared to placebo.556 However, there was no significant difference in head-to-head comparisons of each given drug class with any other class.556 Taken together, currently, no solid evidence can support which anti-hypertensive drug class is superior to any other class. However, an ACE inhibitor, ARB, diuretic, or CCB should be the first-line anti-hypertensive drug for secondary prevention of stroke.
13.4.4 Blood pressure targets for ischemic stroke patients with symptomatic large vessel or cerebral small vessel disease
It remains a subject of debate that if BP lowering would result in brain hypoperfusion in IS patients due to large vessel disease, a combination of extracranial and intracranial stenosis/occlusion, thereby leading to a worse clinical outcome.504
However, there was no RCT aimed to investigate the outcome effect of BP control and identify BP target specifically in symptomatic patients with extracranial and intracranial large vessel stenosis/occlusion. As for medical treatment for intracranial large vessel disease,557-560 post-hoc analyses of the RCTs regarding surgical or interventional therapy for symptomatic extracranial large vessel disease showed conflicting results.561,562 Nevertheless, aggressive BP lowering treatment is still warranted for patients with symptomatic extracranial and intracranial large vessel disease. However, we should keep it in mind that a lower BP may result in brain hypoperfusion560 and potential hazards in patients with symptomatic extracranial or intracranial large vessel disease, or those with inadequate posterior circulation.559
The SPS3 study was the only one RCT aimed to identify optimal BP target for secondary prevention of IS, specifically in patients with symptomatic cerebral small vessel disease.544 As mentioned previously, targeting lower BP tended to reduce total stroke risk or MACEs despite no statistical significance.544 Nevertheless, a lower BP was significantly associated with a lower risk of ICH.544 Moreover, patients with higher cerebral white matter intensities, a surrogate marker of cerebral small vessel disease, appeared to get more benefits from aggressive BP lowering therapy for secondary prevention of stroke.563 There was no signal for safety concern except for renal function deterioration.551 The INFINITY study, an RCT, including 199 hypertensive elderly people (≥ 75 years old) with small vessel disease showed that targeting SBP ≤ 130 mmHg was associated with a lower risk of MACEs and a reduction in accrual of subcortical white matter disease than targeting SBP ≤ 145 mmHg.564 A small-scale but elegant study showed that targeting a lower SBP (< 125 mmHg) was not associated with a reduction in cerebral blood flow in patients with symptomatic cerebral small vessel disease (magnetic resonance imaging-documented lacunar stroke and confluent white matter hyperintensities) than targeting a standard SBP (130-140 mmHg).565 Taken together, aggressive BP lowering treatment may be beneficial for patients with symptomatic cerebral small vessel disease.
The BP targets for patients with history of stroke are summarized in Table 19 according to its phenotypes (ischemic or hemorrhagic) and subtypes, different stages and treatment modalities, and the status of brain perfusion.
Table 19. BP thresholds and targets for patients with stroke.
| Stage | Hyperacute | Acute | Convalescence | Chronic | |||
| Timing | Ambulance-based | < 1 h | 1-24 h | 24-72 h (or before discharge) | > 72 h (or after discharge) | ||
| Decision | Threshold/target | Threshold | BP target | BP target | Threshold | BP target | BP target (HBPM) |
| IS w/o IVT or EVT | NR | BP ≥ 220/120 mmHg or others* | SBP ↓15% | Individualized | Stable stroke# | < 140/90 mmHg | < 130/80 mmHg† |
| IS with IVT | NR | BP ≥ 185/110 mmHg | Before IVT: < 185/110 mmHg | After IVT: < 180/105 mmHg | Stable stroke# | < 140/90 mmHg | < 130/80 mmHg† |
| IS with EVT | NR | BP ≥ 185/110 mmHg | Before EVT: < 185/110 mmHg; During EVT: 140-180 mmHg | After EVT: < 180/105 mmHg; < 140/90 mmHg (successful recanalization) | Stable stroke# | < 140/90 mmHg | < 130/80 mmHg† |
| ICH | NR | SBP ≥ 220 mmHg | SBP ↓15% | Individualized (approximately SBP < 140 mmHg) | Stable stroke# | < 140/90 mmHg | < 130/80 mmHg |
| SBP ≥ 160 mmHg | SBP↓ by 20-60 mmHg | < 140 mmHg | |||||
| SAH | NR | SBP ≥ 160 mmHg | 120-160 mmHg before the aneurysm is treated | Stable stroke# | 120-160 mmHg before the aneurysm is treated | < 130/80 mmHg (after or intentionally waiving aneurysm treatment) |
* Other situations needing immediate BP lowering include acute aortic dissection, congestive heart failure with lung edema, hypertensive encephalopathy. # Stable stroke means no observed deterioration of neurological deficits owing to brain hypoperfusion. † Careful observation of brain hypoperfusion-related side effects caused by BP-lowering therapy may be considered in patients with bilateral internal carotid artery significant stenoses or basilar artery stenosis (> 70% luminal diameter stenosis).
BP, blood pressure; EVT, endovascular thrombectomy; HBPM, home blood pressure measurement; ICH, intracranial hemorrhage; IS, ischemic stroke; IVT, intravenous thrombolysis; NR, not recommended; SAH, subarachnoid hemorrhage; SBP, systolic blood pressure; w/o, without.
14. PATIENTS WITH CHRONIC KIDNEY DISEASE
Recommendations/Keypoints
• For patients with non-dialysis CKD, an SBP target of < 130 mmHg, based on HBPM or standardized office BP, is recommended (COR I, LOE B). If patients tolerate well, an SBP target of < 120 mmHg could be considered (COR IIb, LOE B).
• For dialysis CKD patients, interdialytic home BP or ABPM is the preferred target, compared to pre- and post-dialytic BP (COR IIa, LOE C).
• Interdialytic home BP target of < 130/80 mmHg may be considered (COR IIb, LOE C).
• Renin-angiotensin system inhibitor is the antihypertensive drug of choice for CKD patients with or without diabetes (COR I, LOE A).
According to previous epidemiologic data, 67-92% of hypertensive patients had CKD.566 Hypertension contributes to the development and progression of CKD and vice versa. To date, emerging evidence supports that lowering BP reduces mortality and CV morbidities, as well as slows further loss of kidney function in patients with CKD.12,235,567 Given the heterogeneity of study design and BP measurements in previous studies, the BP target remains under debate. In the 2017 THS/TSOC hypertension guideline, based on the SPRINT study for patients with CKD and an eGFR of 20-60 ml/min/1.73 m2, the AOBP target for SBP is < 120 mmHg.258,469 In 2021, the Kidney Disease: Improving Global Outcomes (KDIGO) released the clinical practice guideline and underscored two important differences.12 First, the adoption of standardized office BP measurement as the preferred technique. Second, the adoption of a lower SBP target (< 120 mmHg) independent of the presence of proteinuria, diabetes, or older age. Hereby, we summarized the updated information regarding BP control in patients with CKD based on CV and kidney endpoints.
14.1 Blood pressure targets for patients with non-dialysis chronic kidney disease
Given the variances of BP as measured by different methods, standardized office BP measurement is preferred to routine office BP measurement.12,33,568 Although an oscillometric device may be preferable to automated office BP, standardization emphasizes adequate preparations for BP measurement, not the type of equipment.12,33,568 During the coronavirus disease 2019 (COVID-19) pandemic, out-of-office BP measurements, i.e., HBPM or ABPM, are also strongly recommended.1,12
In term of CV outcomes, the SPRINT is the largest trial for patients with CKD.258 The number of included patient is more than the total combined number of the three major CKD trials, including the blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2), the African American Study of Kidney Disease and Hypertension (AASK), and the Modification of Diet in Renal Disease (MDRD) trials.258,569,571 The SBP target is < 120 mmHg for CKD patients with an eGFR of 20-60 ml/min/1.73 m2, but it cannot be extended to patients with eGFR < 20 ml/min/1.73 m2, heavy proteinuria (> 1 gm/day), diabetic nephropathy or polycystic kidneys.258 For patients with advanced CKD, comparing treatment with a combination of ACE inhibitor plus diuretic (perindopril plus indapamide) to usual care without diuretic, ADVANCE trial provides evidences that the relative risk of macrovascular or microvascular event was reduced by 9% (HR: 0.91; 95% CI: 0.83-1.00) and all-cause mortality by 14% (HR: 0.86; 95% CI: 0.75-0.98).411
However, regarding kidney outcomes, a target SBP of 125-130 mmHg showed no significant benefits on end-stage renal disease (ESRD) or all-cause mortality compared with a target SBP of < 140 mmHg in CKD patients.569,571 Although previous meta-analyses did not support a target of < 130/80 mmHg either,572 a recent meta-analysis from the Blood Pressure Lowering Treatment Trialists’ Collaboration, including trials of different BP targets, found that the proportional reduction in CV events with more intensive BP treatment was independent of the presence of CKD.573 In the subgroup analysis of the SPRINT trial, albuminuria during follow-up was lower in the intensive SBP arm than in the standard SBP treatment arm.258,574 In the ACCORD trial, patients with type 2 diabetes were randomly assigned to standard (SBP < 140 mmHg) or intensive (SBP < 120 mmHg) therapy, the annual rate of the primary outcome was 1.87% in the intensive-therapy group and 2.09% in the standard-therapy group during the follow-up of 4.7 years.466 Likewise, among 104 patients with advanced CKD (serum creatinine levels of 1.5 to 3.0 mg/dL), benazepril was associated with a 43 percent reduction in the risk of a doubling of the serum creatinine level, ESRD, or death.575 Taken together, the long-term effects of intensive SBP control on kidney outcomes cannot be fully understood from those short-term observations.12 Although the presence of proteinuria is associated with increased CV risks in patients with CKD,576 given a lack of evidence supporting the necessity to set a proteinuria-specific BP target,577 the Task Force recommends a universal BP target for CKD patients instead. Generally, if CKD patient cannot tolerate SBP < 120 mmHg, efforts should focus on maintaining SBP < 130 mmHg or an even higher tolerated SBP goal, based on HBPM or standardized office BP.12,258,578
14.2 Blood pressure targets for patients with dialytic chronic kidney disease
Hypertension is common among patients under dialysis (50-85% in hemodialysis patients and 30% in peritoneal dialysis patients).579,580 Compared to office or peri-dialysis BP, ABPM and HBPM are the first choice.69 Pre-dialysis (tend to overestimate) and post-dialysis (tend to underestimate) BP measurements are less recommended to diagnose hypertension or titrate antihypertensive therapy.69,581 Median intradialytic SBP is considered to make diagnostic decisions instead.582 In a lack of RCTs, the BP targets for dialysis patients remain uncertain. Several observational studies indicated a U-shaped relationship between pre-dialytic and post-dialytic BP and mortality among dialysis patients.583-585 Thus, the 2005 National Kidney Foundation K/DOQI guidelines suggested that pre-dialytic and post-dialytic BPs should be < 140/90 and < 130/80 mmHg, respectively.586 However, the major BP parameter associated with mortality is the interdialytic BP.583 A prospective study of Chronic Renal Insufficiency Cohort (CRIC) focused on patients who started hemodialysis and found a positive correlation between out-of-dialysis-unit SBP and mortality.583 The authors emphasized that more efforts should be made to obtain out-of-dialysis-unit SBP which may merit more consideration as a target for clinical management.583 Therefore, current BP target for dialysis patients is considered based on an interdialytic home BP of < 130/80 mmHg.583 Nevertheless, BP goals should be individualized, upon patients’ comorbidities and clinical conditions.
14.3 Pharmacological treatment
Given the substantial number of trials supporting that RAS inhibitors could slow the progression of CKD in patients with and without hypertension or diabetes, RAS inhibitor is the first-line antihypertensive drug of choice.12,374,587 During treatment, changes of BP, serum creatinine and potassium should be checked within 2-4 weeks of initiation or increase in the dose of RAS inhibitors. If symptomatic hypotension, uncontrolled hyperkalemia or creatinine rises by more than 30% within one month, RAS inhibitors should be considered for a reducing dose or discontinuation.12,587 Drug-induced changes in serum creatinine level must be interpreted carefully. An early decrease in glomerular filtration rate often occurs after the initiation of RAS inhibitors but is recovered thereafter, suggesting reversible hemodynamic changes rather than progression of CKD.588
15. PATIENTS WITH HEART FAILURE
Recommendations/Keypoints
• For hypertensive patients with chronic heart failure, the SBP threshold for pharmacological therapy is ≥ 130 mmHg (COR I, LOE C).
• For hypertensive patients with chronic heart failure, the SBP target for pharmacological therapy is < 130 mmHg (COR I, LOE C).
In the Framingham Heart Study, higher levels of BP were associated with a higher risk of heart failure (HF). Compared with patients having SBP < 125 mmHg, those with SBP 126-141 mmHg had borderline higher risk of HF (HR: 1.48, 95% CI: 0.99-2.21, p = 0.06) and those with SBP ≥ 142 mmHg had significantly higher risk of HF (HR: 3.07, 95% CI: 2.10-4.49, p < 0.001).589 In the Atherosclerosis Risk in Communities (ARIC) Study, elevated SBP group (≥ 140 mmHg) had a higher rate of HF compared with the low SBP group (< 120 mmHg).590 There is a continuous positive association between SBP and HF risk in the elderly for levels of SBP from as low as < 115 mmHg and over half of incident HF events occur in individuals with SBP < 140 mmHg in the Cardiovascular Health Study and the Health, Ageing and Body Composition Study.591 In Taiwan Chin-Shan community cardiovascular study, a 1.0 mmHg increase in SBP increased left ventricular mass by 0.18 g. Left ventricular hypertrophy (LVH), a predictor of HF, can regress if BP was controlled.592
There is no RCT-driven trial to test the adequate BP goal in hypertensive patients with HF. In the SPRINT trial, 9,361 participants were randomly assigned to a SBP target of < 120 mmHg or a target of < 140 mmHg. Trial participants assigned to the lower SBP target (mean achieved SBP 121.4 mmHg) had a 38% lower relative risk of HF (RR: 0.62, 95% CI: 0.45-0.84).258 However, patients with symptomatic HF within the past 6 months or left ventricular ejection fraction (by any method) < 35% were excluded. In the ACCORD trial, the diabetic hypertensive patients had a non-significant 6% risk reduction of HF in the intensive-therapy group with a mean SBP of 119.3 mmHg.466
In a meta-analysis of RCTs, the active lowering of BP over a 3- to 5-year period is effective in reducing the 36% risk of LVH and 53% risk of HF. Network meta-analysis has shown that treatment of hypertension reduces 40% risk of HF.593 In a recent meta-analysis including 5 RCTs involving 15,859 participants, lower BP targets may reduce HF (RR: 0.75, 95% CI: 0.60 to 0.92, absolute risk reduction 0.6%, number needed to treat to benefit 167 over 3.7 years) and reduction in HF was not reflected in total serious adverse events.594
In a meta-analysis including 19 trials with 44,989 participants and mean 3.8 years of follow-up (range 1.0-8.4 years) that randomly assigned participants to more intensive versus less intensive BP-lowering treatment, SBP/DBP differences of -7.2/-4.0 mmHg were associated with a non-significant 15% HF risk reduction.471 Similar findings were reported: SBP/DBP treatment differences of -7.9/-3.2 mmHg were associated with a non-significant 20% risk reduction of HF. However, meta-regression analysis showed relative risk reductions proportional to the magnitude of the BP reductions achieved. Every 10 mmHg reduction in SBP significantly reduced 28% risk of heart failure (RR: 0.72, 95% CI: 0.67-0.78).232 Furthermore, another meta-regression analysis found effects of more (-25 mmHg) vs. less (-17 mmHg) intense BP-lowering on HF calculated as SBP reductions from baseline were statistically significant (p < 0.001).595
From the ONTARGET and TRANSCEND trials, the lowest risk for the hospital admission for HF was found in patients with SBP between 120-140 mmHg and there was an increased risk for the hospital admission for HF at an SBP < 120 mmHg or a DBP < 70 mmHg during treatment in the high CV risk patients.244 A propensity score-matched observational study of the Medicare-linked Organized Program found an SBP level < 120 mmHg was significantly associated with poor outcomes among hospitalized patients with HF with preserved ejection fraction (HFpEF).596 For patients with heart failure with reduced ejection fraction (HFrEF) in the PARADIGM-HF trial, there was a U-shaped relationship between SBP and HF hospitalization. The lowest HF events were found in patients with baseline SBP between 120-140 mmHg.597 In the PARAGON-HF trial, a mean achieved SBP of 120 to 129 mmHg identified the lowest risk in patients with HFpEF.598 The Korean Acute Heart Failure registry prospectively enrolled a total of 5,625 consecutive patients hospitalized for acute HF. Patients were followed for a median of 2.2 years. The relationship between on-treatment BP and all-cause mortality followed a reversed J-curve relationship. A nonlinear, multivariable Cox proportional hazard model identified a nadir of SBP and DBP of 132.4/74.2 mmHg in HF patients, for whom the mortality rate was the lowest.599 In the Medicare-linked OPTIMIZE-HF registry, SBP < 130 mmHg was associated with poor outcomes among hospitalized older patients with HFrEF.600
16. PATIENTS RECEIVING ANTITHROMBOTIC THERAPY
Recommendations/Keypoints
• For patients receiving antithrombotic therapy for stroke prevention, a BP target of < 130/80 mmHg is recommended (COR I, LOE B).
Elevated BP is closely related to the risk of intracerebral hemorrhage.601 In a prospective, multicenter, observational cohort study (BAT Study) of 4,009 Japanese patients taking oral antithrombotic agents for CV or cerebrovascular diseases, the optimal cutoff BP level to predict impending risk of ICH was ≥ 130/81 mmHg.602 Lowering SBP reduced ICH in the PROGRESS trial, in which the lowest risk of intracranial bleeding was observed in participants with the lowest follow-up SBP (median, 113 mmHg).603
The management of BP is an important issue for atrial fibrillation (AF) patients since hypertenion is the most common comorbidity associated with AF, which was present in 62.9% of Taiwan AF patients and the prevalence continuously increased to near 80%.604,605 The close link between hypertension and increasing risk of major bleeding have been reported for AF patients in the subanalysis of RCTs.606-608 BP control is even more crucial for Asian AF patients who had a higher risk of ICH treated with oral anticoagulants.609,610 In the subanalysis of ENGAGE trial, patients with a SBP higher than 140 mmHg were associated with a significantly higher risk of major bleeding compared to those with a SBP between 130-140 mmHg.607 Of note, the risk of ischemic stroke/systemic embolism events was also significantly lower for patients with a SBP of "110 to < 120 mmHg" or "120 to < 130 mmHg". In the ROCKET-AF trial, each 10 mmHg increase of DBP was associated with a higher risk of ICH with a HR of 1.17 (95% CI: 1.01-1.36; p < 0.042).606 In the J-RHYTHM registry which included 7,406 Japanese AF patients, a higher incidence of systemic thromboembolism and major bleeding for patients treated with warfarin compared to those without was only observed among patients with a SBP ≥ 136 mmHg.611 The findings supported the concept that a well-managed BP could alleviate the risk of bleeding associated with oral anticoagulants.612
17. ELDERLY PATIENTS
Recommendations/Keypoints
• For patients aged ≥ 65 years, the SBP threshold for pharmacological therapy is ≥ 130 mmHg (COR I, LOE B).
• For patients aged ≥ 65 years, the SBP target for pharmacological therapy is < 130 mmHg. (COR I, LOE B).
In a meta-analysis of individual data from one million adults from 61 prospective studies (Prospective Study Collaboration), BP was associated strongly with the age-specific mortality rates from stroke and CHD.252 In general, a 20 mmHg difference in SBP is approximately equivalent in its hazards to a 10 mmHg difference in DBP. These relationships with vascular mortality continued steeply down as far as a SBP of 115 mmHg and a DBP of 75 mmHg, below which there was little evidence.252 All of these proportional differences in vascular mortality were about half as extreme at ages 80-89 years as at ages 40-49 years, but the annual absolute differences in risk were greater in old age.252 Similar findings were observed in the Asia Pacific Cohort Studies Collaboration,6 and a Chinese cohort study.43 In the sub-analysis of the Felodipine Event Reduction (FEVER) trial, the relative risk reduction of CV events was greater in patients aged > 65 years compared with those aged ≤ 65 years.613 Taken together, controlling BP in the elderly is very important.
Isolated systolic hypertension (ISH) is more common in the elderly.614 The major concern in the hypertension management in the elderly is fear of the "J-curve" phenomenon that an aggressive BP lowering might increase the risk of coronary event given that the DBP is already in the lower ranges in these elderly patients. In a cohort study of 1.25 million subjects, the lowest risk for CV disease in people aged 60-79 years was 90-114 mmHg in SBP and 60-74 mmHg in DBP, without any evidence of J-curve phenomenon above these levels.615 Among 1,235,246 individuals who participated in routine medical examinations in Korea, the lowest risk of all-cause death and ASCVD death in the elderly (age 60-95 years) was observed in the range of 100-110 mmHg in SBP, and there was no J-curve above this BP level.254 In the three most important RCTs in the elderly (age > 60 years) with ISH (SHEP, Syst-Eur, Syst-China), the risk of myocardial infarction was reduced in the treatment group compared to the placebo group.616-618 No J-curve phenomenon was observed. Therefore, it seems to be safe to decrease SBP to a level above 110 mmHg and DBP to above 60 mmHg.
The Hypertension in the Very Elderly Trial (HYVET) is a placebo-controlled RCT to test the effect of antihypertensive therapy on the risk of stroke and all-cause death in very elderly patients (age ≥ 80 years) with a baseline BP of 173.0/90.8 mmHg.619 Use of indapamide, plus perindopril if necessary, decreased fatal or nonfatal stroke by 30% (p = 0.06) and all-cause death by 21% (p = 0.02) with final achieved BP of 140/80 mmHg. However, the HYVET trial is not a BP target-driven trial, and it cannot answer the question that whether the effects could be even better if lower BP levels are achieved. There were two BP target-driven trials for the elderly hypertensive patients before the SPRINT trial, the JATOS and the VALISH trials.620,621 The JATOS trial tested a SBP < 140 mmHg vs. < 160 mmHg in the Japanese elderly patients,620 while VALISH trial tested a SBP < 140 mmHg vs. < 150 mmHg in the Japanese elderly patients.621 A lower BP target, compared with a higher BP target, did not translate into better CV outcomes in both trials.620,621 However, the number of enrollment was too low to have enough power for analysis.621 In addition, follow-up durations were very short and the event rates were very low (1.1 to 1.2%/ year in JATOS, 0.82 to 0.85%/year in VALISH),620,621 making the conclusions not convincing.621 A larger trial with longer follow-up period was needed.
The SPRINT trial is a recent target-driven trial and probably the most important one.622 One of the inclusion criteria was the elderly patients with age ≥ 75 years, and about 28% of the total study population of 9,361 patients were the elderly. In the pre-defined sub-analysis of the elderly patients, a BP target of < 120 mmHg (intensive treatment group), compared with a BP target of < 140 mmHg (standard treatment group), reduced the composite endpoints by 34% (95% CI: 0.51-0.85) and all-cause mortality by 33% (95% CI: 0.49-0.91).623 The overall rate of serious adverse events was not different between treatment groups. Interestingly, the incidence of orthostatic hypotension of the two treatment groups (5.0% vs. 5.7%) did not differ.624 The final achieved SBP was 123.4 mmHg vs. 134.8 mmHg, and the DBP was 62.0 mmHg vs. 67.2 mmHg.623 Similar findings were reported in a more recent sub-analysis of very elderly patients (age ≥ 80 years) in the same trial.625 The STEP trial,9 comprising exclusively of Chinese patients aged 60 to 80 years, replicates what had been observed in the SPRINT trial, and reassures the safety and efficacy of a SBP target of < 130 mmHg in elderly patients.
18. HYPERTENSION IN WOMEN
Recommendations/Keypoints
• In pregnant women with pre-existing hypertension, a BP target of < 140/90 mmHg for pharmacological treatment is recommended (COR I, LOE A).
• In women with gestational hypertension, initiating drug treatment is recommended when BP is ≥ 140/90 mmHg (based on standardized office BP or HBPM) (COR I, LOE C).
• An SBP > 170 mmHg and/or DBP > 110 mmHg during pregnancy should be considered an emergency requiring hospitalization (COR I, LOE C).
• Women who develop gestational hypertension or preeclampsia, together with adverse pregnancy outcomes, are at an increased risk of CVD.
• Women with hypertension who become pregnant, or are planning to become pregnant, should be transitioned to methyldopa, labetalol, and/or nifedipine (COR I, LOE C).
• The recommended treatment for hypertensive crisis in pregnancy is intravenous labetalol or nicardipine and magnesium; and nitroglycerin for pulmonary edema (COR I, LOE C).
• In women with gestational hypertension or mild preeclampsia, delivery is recommended at 37 weeks (COR I, LOE B).
• Salt reduction (less than 6 g/day) is not recommended as a non-drug therapy for gestational hypertension (COR III, LOE C).
• ACE inhibitors, ARBs, DRI, ARNIs, mineralocorticoid receptor antagonists (MRA), and chlorothiazide are teratogenic. Women with hypertension who become pregnant, are planning to become pregnant, or with child-bearing potential without reliable contraception, should avoid, or immediately withdraw these drugs in case of pregnancy (COR III, LOE C).
• Low-dose aspirin (75-150 mg daily) is recommended in women at high or moderate risk of preeclampsia from week 12 to weeks 36-37 (COR I, LOE A).
• Oral contraceptives should not be used in women with uncontrolled hypertension (COR III, LOE C).
• Hormone replacement therapy, as well as selective estrogen receptor modulators, should not be used for primary or secondary prevention of CV diseases in postmenopausal women (COR III, LOE C).
18.1 Epidemiology and mechanisms
Hypertension is the primary modifiable risk factor for the development of CVD among men and women. Women are also at risk for developing hypertension. CVD is the leading cause of death among men and women,626-628 including Taiwan.629,630 Hypertension and CVD pose a greater burden for women than men especially in the aging population.
New evidence suggests that sex hormones, sex-specific molecular mechanisms including the renin-angiotensin system, bradykinin, nitric oxide (NO) system, endothelin-1, sympathetic nervous activity, and T-cell activation all contribute to sex differences in BP control. Some lines of evidence suggests that there is a higher percentage of treatment-resistant hypertension in women, probably related to salt sensitivity, stimulation of sympathetic nerve activity, etc.628
Hypertension affects women in all phases of life, with specific characteristics relating to risk factors and management, including teenage and young adult women; hypertension in pregnancy; hypertension during use of oral contraceptives and assisted reproductive technologies, lactation, menopause, or hormone replacement; hypertension in elderly women; and issues of race and ethnicity.
Gender differences in epidemiology, clinical characteristic, risk factors and awareness, treatment, and control of hypertension have been well established in humans. Assessment of risk factors unique to premenopausal and postmenopausal women can facilitate the management of hypertension and improve long-term outcomes. Moreover, gender differences are linked to several specific types of hypertension, including white coat hypertension and masked hypertension.626-628,631,632 Further studies in women are needed to accurately stratify women’s risks based on these risk factors.
In children and teenage, in addition to genetic disorders (ex. Turner syndrome), structural (e.g., coarctation of aorta, fibromuscular dysplasia) or endocrine disorders (e.g., primary aldosteronism), obesity, family history of hypertension, parent-related factors including obesity, hypertension, smoker in close proximity, extreme postnatal weight gain, sedentary behavior, and obstructive sleep apnea should be taken in to consideration. Obstructive sleep apnea has also been associated with higher BP and lack of nocturnal dip in children. Among younger women, long-term vascular consequences of preeclampsia, the under-reported prevalence of fibromuscular dysplasia (abdominal bruit), and widespread use of oral contraceptive pills in women confer unique risks for hypertension-related CV risk. For older women, insights on vascular aging and hormonal changes with menopause are shown to be gender-specific causal factors for hypertension. The prevalence of hypertension in postmenopausal women is more than twice the prevalence in premenopausal women. Even moderate or borderline hypertension (< 140/90 mmHg) causes more endothelial dysfunction and CV complications in women than in men.
From the historical clinical trial data and international hypertension guidelines from the perspective of both genders, the effective treatment and control of hypertension improves CV outcomes both in man and women. Therefore, healthcare professionals should consider the differences in the factors between the two genders to improve the treatment and control of hypertension.
The current guidelines emphasize that lifestyle modifications should be part of antihypertensive education and initial treatment. The amount of alcohol intake recommended is lower in women.
Although gender differences have been implicated in the prevalence and determinants of hypertension and prehypertension, the control rates and benefits are similar between men and women taking antihypertensive medications. There is some evidence showing that BP may not be as well-controlled in women as in men, despite the awareness of hypertension, prescription rate and number of antihypertensive medications is higher in women, and women usually adhere better to their therapeutic regimens and medications than do men, and have their BP measured more frequently than do men.
There are some sex-related differences in pharmacokinetics and pharmacodynamics, which might affect efficacy, adverse effects, and tolerability. However, most investigations and international hypertension guidelines agree that there is no evidence that BP control rate and outcome issues differ in antihypertensive pharmacological therapy for women versus men, and it is difficult to conclude something about gender-specific antihypertensive therapy.10,13,162,236,626-628,631,633-635
The LIFE (the Losartan Intervention for Endpoint reduction in hypertension) trial noted that treatment effects were consistent in both men and women, but more women in losartan group required hospitalization and having angina.636 The Second Australian Blood Pressure Group Study noted that ACE inhibitors-based regimen might benefit more restricted to men.637
In the Systolic Blood Pressure Intervention Trial (SPRINT), for several individual outcomes (e.g., all stroke [women HR: 1.21; men HR: 0.75], all nonfatal stroke [women HR: 1.28; men HR: 0.71], composite renal outcome [women HR: 1.43; men HR: 0.61]), risks by sex suggested a difference, although treatment group by sex interactions did not reach significance. Among multiple agents and strategies, none has proven clearly more beneficial for older women, except perhaps thiazide diuretics, which reduce calcium excretion and prevent osteoporosis to prevent fractures.258,638
Incidence of adverse reactions of antihypertensive medications in women is twice that in men. Higher incidences of dry cough due to ACE inhibitors, peripheral edema during use of CCB, more hypokalemia and hyponatremia during use of diuretics. The recent large study showed that women reported adverse effect in 6 out of 10 groups of antihypertensives, and aldosterone antagonist was the only group with higher prevalence of adverse effects among men.639
18.2 Hypertension in pregnancy10,162,236,633,634,640-642
Hypertensive disorders in pregnancy affect 5-10% of pregnancies worldwide and remain a major cause of maternal, fetal, and neonatal morbidity and mortality. Maternal risks include death, stroke, pulmonary edema, renal insufficiency and renal failure, myocardial infarction, preeclampsia, placental abruption, cesarean delivery, post-partum hemorrhage, gestational diabetes, multiple organ failure, and disseminated intravascular coagulation. The fetus is at high risk of intrauterine growth retardation (25% of cases of preeclampsia), prematurity (27% of cases of preeclampsia), intrauterine or perinatal death (4% of cases of preeclampsia), and congenital abnormalities (e.g., heart defects, hypospadias, esophageal atresia).
18.2.1 Diagnosis
BP in pregnancy should be measured in the sitting position (or the left lateral recumbent during labor) with an appropriately sized arm cuff at heart level and using Korotkoff V for diastolic BP. BP measurement at every clinical prenatal check-up visit is important. ABPM is superior to office BP measurement for the prediction of pregnancy outcome. Home monitoring may reduce the frequency of office visits in cases with marginal BP control. Presumed advantages of out-of-office and self-monitoring include patient convenience, increased therapeutic adherence, confirmation of white coat hypertension, and assistance with adjusting medications when there is uncertainty. It may be useful to have a patient bring in her home monitor to compare against measurements done in the office. Procedures for the use of HBPM are available and emphasize patient training, use of appropriately validated devices, and clear instructions.
The definition of hypertension in pregnancy is traditionally based on office BP values of SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg (or ≥ 140/90 mmHg according to HBPM) and is classified as mild (140-159/90-109 mmHg) or severe (≥ 160/110 mmHg), in contrast to the conventional hypertension grading. If BP is severe (systolic BP ≥ 160 and/or diastolic BP ≥ 110 mmHg), then the BP should be confirmed within 15 minutes; for less severe cases, repeated readings should be taken for a few hours.
18.2.2 Classification
Hypertension in pregnancy is classified as follows:
• Pre-existing or chronic hypertension: precedes pregnancy or develops before 20 weeks of gestation, and persists for more than 6 weeks post-partum and may be associated with proteinuria.
• Gestational hypertension: develops after 20 weeks of gestation and usually resolves within 6 weeks post-partum.
• Antenatally unclassifiable hypertension: this term is used when BP is first recorded after 20 weeks of gestation and it is unclear if hypertension was pre-existing. Reassessment 6 weeks post-partum will help distinguish pre-existing from gestational hypertension.
• Pre-existing hypertension plus superimposed gestational hypertension with proteinuria.
• Preeclampsia: gestational hypertension with significant proteinuria (> 0.3 g/24 h or ≥ 30 mg/mmol albumin:creatinine ratio [ACR]). It occurs more frequently during the first pregnancy, in multiple pregnancy, in hydatidiform mole, in antiphospholipid syndrome, or with pre-existing hypertension, renal disease, or diabetes. It is often associated with fetal growth restriction due to placental insufficiency and is a common cause of prematurity. The only cure for preeclampsia is delivery. As proteinuria may be a late manifestation of preeclampsia, it should be suspected when de novo hypertension is accompanied by headache, visual disturbances, abdominal pain, or abnormal laboratory tests, specifically low platelets and/or abnormal liver function.
18.2.3 Investigations
Basic laboratory investigations recommended for monitoring pregnant hypertensive women include urinalysis, blood count, hematocrit, liver enzymes, serum creatinine, and serum uric acid (increased in clinically evident preeclampsia). Hyperuricemia in hypertensive pregnancies identifies women at increased risk of adverse maternal and fetal outcomes. All pregnant women should be assessed for proteinuria in early pregnancy to detect pre-existing renal disease and, in the second half of pregnancy, to screen for preeclampsia. A dipstick test of ≥ 1+ should prompt evaluation of ACR in a single spot urine sample and a value < 30 mg/mmol can reliably rule out proteinuria in pregnancy.
• Other potential circulating biomarkers, such as plasma pregnancy-associated plasma protein A, placental protein 13, homocysteine, asymmetrical dimethylarginine, uric acid and leptin, urinary albumin, or calcium.
In addition to basic laboratory tests, the following investigations may be considered:
• Ultrasound investigation of the kidneys and adrenals, and plasma or urinary fractionated metanephrine assays in pregnant women with a history suggestive of pheochromocytoma.
• Doppler ultrasound of uterine arteries (performed after 20 weeks of gestation) to detect those at higher risk of gestational hypertension, preeclampsia, and intrauterine growth retardation.
• Measurement of angiogenic factors (such as soluble endoglin, PlGF, sFlt-1, and sFLt-1/PlGF ratio). sFLt-1/PIGF ratio of ≤ 38 can be used to exclude the development of preeclampsia in the next week when suspected clinically. However, no test of angiogenic factors should be used routinely until further clinical studies are conducted.
18.2.4 Risk classification
ISSHP recommends clinical risk factors for preeclampsia including prior preeclampsia, chronic hypertension, pregestational diabetes mellitus, maternal BMI > 30 kg/m2, antiphospholipid syndrome, and receipt of assisted reproduction.635
The 2018 ESC hypertension guideline defines that high risk of preeclampsia includes any of the following: hypertensive disease during a previous pregnancy, CKD, autoimmune disease such as systemic lupus erythematosus or antiphospholipid syndrome, diabetes mellitus, and chronic hypertension. Moderate-risk of preeclampsia includes one or more of the following risk factors: first pregnancy, age ≥ 40 years, pregnancy interval of > 10 years, BMI of ≥ 35 kg/m2 at first visit, family history of preeclampsia, and multiple pregnancy.10,640
The American College of Obstetricians and Gynecologists defines high-risk as history of preeclampsia, multi-fetal gestation, chronic hypertension, type 1 or 2 diabetes, renal disease, autoimmune disease; and moderate-risk as obesity (BMI > 30 kg/m2), sociodemographic characteristics (e.g., low socioeconomic status), age ≥ 35 years, personal history factors (e.g., low birth weight for small gestational age, previous adverse pregnancy outcomes, more than 10-year pregnancy interval), and low risk as previous uncomplicated full-term delivery.641,642
18.2.5 Prevention
For women with gestational hypertension, a normal diet without salt restriction is recommended. Women considered at increased risk for preeclampsia should receive supplemental calcium (1.2-2.5 g/d) if their intake is likely to be low (< 600 mg/d) or it cannot be assessed or predicted. Women should exercise during pregnancy to maintain health, appropriate body weight, and reduce the likelihood of hypertension.
Women at high or moderate-risk of preeclampsia should be advised to take low dose of aspirin (defined as 75-162 mg/d, as studied in RCTs from weeks 12 to 36-37. Treatment initiates ideally before 16 weeks but definitely before 20 weeks. Uterine artery Doppler can select women who may benefit from 150 mg/d of aspirin to prevent preterm (before 37 weeks gestation) but not term preeclampsia. On the other hand, low molecular weight heparin is not indicated to prevent preeclampsia, even with a history of prior early onset preeclampsia.
18.2.6 Management
18.2.6.1 Mild hypertension in pregnancy (140-159/90-109 mmHg)
The goal of drug treatment for hypertension in pregnancy is to reduce maternal risk. However, the agents selected must be safe for the fetus. Most women with pre-existing hypertension and normal renal function will not have severe hypertension and are at low risk for developing complications during pregnancy. Some may be even able to withdraw their medication in the first half of pregnancy due to the physiological BP fall. It is still unclear whether mild hypertension in pregnancy should be pharmacological treated. Current guidelines are based on expert consensus because the benefits of drug treatment for mother and fetus in hypertension in pregnancy have not been extensively studied, with the best data from a single trial using alpha-methyldopa, performed 40 years ago. A further study suggested that tighter vs. less tight control of BP in pregnancy showed no difference in the risk of adverse perinatal outcomes and overall serious maternal complications. However, secondary analysis suggested that tighter control of BP may reduce the risk of developing more severe hypertension and preeclampsia. In the just released Chronic Hypertension and Pregnancy (CHAP) trial,643 a strategy of targeting a BP of < 140/90 mmHg was associated with better pregnancy outcomes than a strategy of reserving treatment only for severe hypertension (BP ≥ 160/105 mmHg), with no increase in the risk of small-for-gestational-age birth weight, in 2,408 pregnant women with mild chronic hypertension. The primary outcome was a composite of preeclampsia with severe features, medically indicated preterm birth at less than 35 weeks’ gestation, placental abruption, or fetal or neonatal death. The incidence of a primary outcome event was lower in the active-treatment group than in the control group (30.2% vs. 37.0%), for an adjusted risk ratio of 0.82 (95% CI: 0.74 to 0.92; p < 0.001). The percentage of small-for-gestational-age birth weights below the 10th percentile was 11.2% in the active-treatment group and 10.4% in the control group (adjusted risk ratio: 1.04; 0.82 to 1.31; p = 0.76). The incidence of any preeclampsia in the two groups was 24.4% and 31.1%, respectively (risk ratio: 0.79; 95% CI: 0.69 to 0.89), and the incidence of preterm birth was 27.5% and 31.4% (risk ratio: 0.87; 95% CI: 0.77 to 0.99).
The task force therefore recommends that, in pregnant women with pre-existing hypertension, a BP target of < 140/90 mmHg for pharmacological treatment (COR I, LOE A). In women with gestational hypertension, initiating drug treatment is recommended when BP is ≥ 140/90 mmHg in most international guidelines, despite the paucity of evidence from RCT (COR I, LOE C).
Women with pre-existing hypertension should continue monitor their BP at home and adjust their current antihypertensive medications accordingly. ACE inhibitors, ARBs, ARNI, and direct renin inhibitors are contraindicated due to adverse fetal and neonatal outcomes. Methyldopa, labetalol, and CCBs are the drugs of choice. Basically, a long-acting preparation should be used. The sublingual administration of capsule preparations should not be performed. Beta-blockers may induce fetal bradycardia and their type and dose should be carefully selected, with atenolol best avoided. Diuretic therapy should be used with caution because plasma volume is reduced in women who develop preeclampsia, and fluid status should be carefully monitored. Combination of two drugs with different antihypertensive action mechanisms could be considered, as methyldopa and labetalol are classified as sympatholytic drugs, and hydralazine and sustained-release nifedipine as vasodilators.
18.2.6.2 Severe hypertension in pregnancy (≥ 160/110 mmHg)
There is no agreed definition of severe hypertension, with values ranging between 160-180 mmHg/> 110 mmHg. The conventional consensus is to lower BP to < 160/105 mmHg to prevent acute hypertensive complications in the mother. After the publication of the CHAP trial, a more aggressive BP target (< 140/90 mmHg) is recommended for pregnant women with pre-existing hypertension. This more aggressive BP target (< 140/90 mmHg) can be applied to pregnant women without pre-existing hypertension. The 2018 ESC Task Force on CV disease during pregnancy considers an SBP ≥ 170 mmHg or DBP ≥ 110 mmHg an emergency in a pregnant woman, who should be immediately admitted to hospital for treatment. The 2019 Japanese Society of Hypertension Guidelines for the Management of Hypertension recommends anti-hypertensive treatment should be started soon after recording of SBP ≥ 180 mmHg or DBP ≥ 120 mmHg.
The selection of the antihypertensive drug and its route of administration depends on the expected time of delivery. Pharmacological treatment with oral methyldopa, CCB or intravenous labetalol and nicardipine have shown to be safe and effective. In hypertensive crises, i.e., in patients with eclampsia or severe preeclampsia (with or without hemolysis, elevated liver enzymes, and low platelets syndrome), hospitalization and BP-lowering therapy is essential, and delivery needs to be considered after the maternal condition has stabilized.
Monitoring of fetal heart rate is necessary to prevent fetal bradycardia. Intravenous sodium nitroprusside is contraindicated in pregnancy because of an increased risk of fetal cyanide poisoning. The drug of choice when preeclampsia associated with pulmonary edema is nitroglycerin with titration. Intravenous magnesium sulfate (MgSO4) is recommended for the prevention of eclampsia and treatment of seizures. Intravenous hydralazine is no longer the drug of choice as it is associated with more perinatal adverse effects than other drugs. However, hydralazine is still used when other treatment regimens fail to achieve adequate BP control.
Women with preeclampsia should be delivered if they have reached 37 weeks’ gestation or if they develop any of the following: repeated episodes of severe hypertension despite maintenance treatment with 3 classes of anti-hypertensive agents; progressive thrombocytopenia; progressively abnormal renal or liver enzyme tests; pulmonary edema; abnormal neurological features, such as severe intractable headache, repeated visual scotomata, or convulsions or nonreassuring fetal status.
18.2.7 Post-partum hypertension and breastfeeding
Post-partum hypertension is common in the first week. Methyldopa should be avoided because of the risk of post-partum depression, and considerations should be given to drug choice in breastfeeding women. All antihypertensive drugs taken by the nursing mother are excreted into breast milk. Most are present at very low concentrations except for propranolol and nifedipine, with which breast milk concentrations are similar to those in maternal plasma.
Women experiencing hypertension in their first pregnancy are at increased risk in a subsequent pregnancy. The earlier the onset of hypertension in the first pregnancy, the higher the risk of recurrence in a subsequent pregnancy.
Women who develop gestational hypertension or preeclampsia are at increased risk of hypertension, stroke, and ischemic heart disease in later life. In addition to hypertensive disorders of pregnancy, adverse pregnancy outcomes (APOs) such as preterm delivery, gestational diabetes, small-for-gestational-age delivery, placental abruption, and pregnancy loss also increase a woman’s risk of developing CVD risk factors (including hypertension, diabetes, and dyslipidemia) and of developing subsequent CVD. Hypertensive disorders of pregnancy is associated with worse outcomes of ASCVD (including coronary heart disease, ischemic stroke, peripheral vascular disease), hemorrhagic stroke and heart failure.
Although their value in reclassifying risk warrants to be established, it is still important to recognize APOs when CVD risk is evaluated in women and could serve as a prompt for more vigorous primordial prevention of CVD risk factors and primary prevention of CVD. This approach is adopted in risk stratification in this guideline, as shown in Figure 4. Adopting a heart-healthy diet and increasing physical activity among women with APOs, starting in the postpartum setting and continuing across the life span, are important lifestyle interventions to decrease CVD risk. Lactation and breastfeeding may lower a woman’s later cardiometabolic risk. Evidence shows that Black and Asian women experience more APOs, with more severe clinical presentations and worse outcomes, than Caucasian women. Healthcare systems need to improve transitions of care for women with APOs and implement targeted strategies to reduce their long-term CVD risk, and future studies for primary CVD prevention among women who have had an APO are warranted.
18.3 Oral contraceptive pills and hormone replacement therapy
Oral contraceptive pills, especially estrogen-containing, may cause hypertension in about 5% of women taking pills, which is usually mild but can be severe. BP usually decreases promptly after cessation of these pills. Therefore, BP should be monitored before and during oral contraceptive pill treatments. Recent studies of newer generation of oral contraceptive pills have reported less concerns about venous thrombosis, myocardial infarction, or stroke in comparison of older studies. Concomitant CV risk factors such as smoking and obesity should be assessed, and oral contraceptive pill is not recommended if BP is elevated.648
The prevalence and severity of hypertension in postmenopausal women are increased. Cross-sectional studies have long established that menopause doubles the risk of developing hypertension, even after adjusting for factors such as age and BMI. In addition, early menopause (age at menopause < 45 years) or premature ovarian insufficiency is associated with increased risk of arterial hypertension compared with those of normal age at menopause (> 45 years) (OR: 1.10, 95% CI: 1.01-1.19, p = 0.03; I2 79%). The direction or the magnitude of this association remained significant when the analysis was restricted to studies including groups matched for potential confounders, such as age, BMI, smoking or the use of menopausal hormone therapy or oral contraceptives.651-655
The effects of hormone replacement therapy (HRT) are controversial and there is no recommendation regarding prescribing this kind of therapy in postmenopausal women because of its uncertain value and possible association with adverse outcome – stroke. Although HRT contains estrogens, there is no convincing evidence that significant rises in BP will occur in otherwise normotensive menopausal women due to this therapy, or that BP will increase further due to HRT in menopausal hypertensive women. Thus, current guidelines suggest that the use of HRT is not associated with an increase in BP, and is not contraindicated in women with hypertension, and women with hypertension may be prescribed HRT if BP levels can be controlled by antihypertensive medication. The presence of CV risk factors is not a contraindication to HRT and that it is essential to optimally manage any underlying CV risk factors (e.g., hypertension, high cholesterol). Elevated BP should be addressed and managed in women as it should be for women who are not taking HRT. Importantly, HRT and selective estrogen receptor modulators should not be used for primary or secondary prevention of CVD. It seems that timing of introduction of this therapy and route of its administration have the critical role for development of ischemic stroke in peri- and postmenopausal women.656-658
19. PATIENTS WITH RESISTANT HYPERTENSION
Recommendations/Keypoints
• Treatment resistant hypertension (TRH) is defined as uncontrolled BP ≥ 130/80 mmHg in a patient despite the optimal doses of 3 antihypertensive drug classes, or in a patient requiring ≥ 4 drug classes for adequate BP control.
• Refractory hypertension, a more severe version of TRH, is defined as uncontrolled BP when taking ≥ 5 antihypertensive medications, including a diuretic.
• Non-adherence is an important cause of pseudo-resistant hypertension. High performance liquid chromatography-tandem mass spectrometry (LC-MS/MS) is a useful tool to identify antihypertensive drug non-adherence.
• Drug therapy for TRH should begin with optimization of diuretic doses. When optimal BP target cannot be obtained with the three-drug regimen, a mineralocorticoid receptor antagonist needs to be added (COR I, LOE B).
• Recent randomized sham-controlled trials of renal denervation have demonstrated significant BP reductions in patients with uncontrolled or resistant hypertension (COR IIa, LOE B).
19.1 Definition
Treatment resistant hypertension (TRH) is defined as uncontrolled BP ≥ 130/80 mmHg in a patient despite the optimal doses of 3 antihypertensive drug classes, or in a patient requiring 4 or more drug classes for adequate BP control. These drug classes commonly include a long-acting CCB, a blocker of the renin-angiotensin system, beta blockers, and a diuretic.10,162,659 TRH can be further defined in two ways. Uncontrolled TRH is when an individual’s BP is still high after treatment with three or more antihypertensive drug classes. Controlled TRH is when an individual’s BP is within the target after treatment with four or more antihypertensives. Regardless, optimal BP targets in patients with resistant hypertension and non-resistant hypertension should be the same. The target BP should be < 130/80 mmHg in most hypertensive patients.660
19.2 Phenotypes
TRH represents a heterogeneous group of patients including those with both controlled and uncontrolled BP. Another type of TRH is called refractory hypertension, which is defined as uncontrolled BP when taking five or more antihypertensive medications, including a diuretic. In population-based studies, the term apparent treatment-resistant hypertension (aTRH) is used. In real world sometimes pseudo-resistance cannot be completely excluded because of missing data. Nevertheless, it is important to exclude common causes of pseudo-resistance like the white-coat effect, inaccurate BP measurements, or elevated BP because of drugs nonadherence. The prevalence of white-coat effect may be as high as 30% among patients with elevated office BP despite treatment with at least 3 drugs.661 Steps for evaluation of resistant hypertension to exclude other causes of pseudo-resistance are shown in Table 20.
Table 20. Steps for evaluation of resistant hypertension to exclude pseudo-resistance.
| Step 1 | White coat hypertension: Home BP monitoring (722) or 24-hour ambulatory BP monitoring |
| Step 2 | Blood pressure measurement technique re-evaluation |
| Step 3 | Education and reinforcement of life-style issues that affect BP, such as sodium restriction, alcohol abuse, and overweight |
| Step 4 | Screening for inappropriate use of vasoactive substances |
| Step 5 | Check adherence to prescribed medications |
| Step 6 | Check suboptimal dosing of antihypertensive agents or inappropriate combinations |
BP, blood pressure.
19.3 Epidemiology
The prevalence of resistant hypertension differs between various sources of literature. The prevalence of true resistant hypertension evaluated by 24-h ABPM in a meta-analysis of data from 3.2 million patients was found to be more than 10% of patients in the general population treated for hypertension.662 It is important to distinguish the prevalence, cause, and prognosis of TRH as separate from refractory hypertension. Apparent TRH incidence using the updated definition under intensive treatment may be high as around 30%.663 Regardless, patients with aTRH have greater risk for CV events compared with individuals with hypertension and without aTRH.664 Patients with TRH have a higher prevalence of comorbid conditions.661 Treatment-resistant hypertension is associated with greater risk for ESRD, ischemic heart disease, HF, stroke, and mortality compared with non-treatment-resistant hypertension. The risk of ESRD and stroke were 25% and 23% greater, respectively, in uncontrolled TRH compared to controlled TRH.665,666 At present, clinicians cannot predict TRH in individuals with high BP at the time of dose titrations.
19.4 Causes
19.4.1 Non-adherence
Non-adherence is an important cause of pseudo-resistant hypertension. Adherence to lifestyle and medication is the most important factor to achieve adequate BP control. Confirmation of adherence is required for the correct diagnosis of TRH. Barriers to medication adherence are usually multidimensional and complex.667 High performance LC-MS/MS is a useful tool to provide a highly sensitive and specific detection of commonly prescribed BP-lowering drugs.668 Nonadherent hypertensive patients may respond to LC-MS/MS-based biochemical urine analysis by using urinary adherence ratio (the ratio of detected to prescribed antihypertensive medications). The observed increase in the urinary adherence ratio associated with improved adherence and significant BP drop. Biochemical analyses should be considered as a therapeutic approach in nonadherent hypertensive patients.669
19.4.2 Vasoactive substances
Resistant hypertension may be encountered in patients who are ingesting vasoactive substances despite taking antihypertensive drugs regularly. Salt and alcohol are common examples. Others include cocaine, amphetamines, anabolic steroids, oral contraceptives, cyclosporine, antidepressants, and nonsteroidal anti-inflammatory drugs.670 Vasoactive substances affecting antihypertensive drugs are shown in Table 12.
19.5 Treatment optimization
Drug therapy for TRH should begin with optimization of diuretic use, which is a common component in the single-pill combination.671 When optimal BP target cannot be obtained with the three-drug regimen, a MRA needs to be added (Figure 6). The PATHWAY-2 study (Prevention and Treatment of Hypertension with Algorithm Based Therapy) included patients with uncontrolled resistant hypertension who were randomized to a double-blinded, four-way cross-over comparison of 3 months each of placebo, spironolactone (25 or 50 mg), bisoprolol (5 or 10 mg) and doxazosin modified release (4-8 mg).360 Spironolactone was superior to the other two classes of agents and to placebo in patients with uncontrolled TRH. The use of MRA as part of a multiple drug regimen is extremely important for treating TRH. However, it is important to notice the association of high serum potassium with all-cause mortality in patients with HF, CKD, and/or diabetes.672 In a phase 2, randomized, double-blind, placebo-controlled trial (AMBER), patiromer, a sodium-free, non-absorbed, K+-binding polymer was used to enable more patients to continue treatment with spironolactone with less hyperkalemia.673 Increased spironolactone use for TRH patients should have clinical relevance for the treatment of resistant hypertension.
Beta-blockers have been routine treatment for patients with hypertension for several decades. The effectiveness of these pharmacological agents when used as first-line treatment for hypertension has been challenged. Patients are more likely to withdraw from a beta-blocker because of the side effects. Third generation beta-blockers with less side effects can be used as the additional drugs. The evidence from RCTs for TRH patients who were treated with a third-generation beta-blocker is lacking. Alpha-blockers can also be considered as the additional drug after the use of MRA. Sacubitril/valsartan, a novel combination drug containing an existing ARB (valsartan) and a neprilysin inhibitor (sacubitril), has been evaluated for the treatment of patients with hypertension in multiple clinical trials (see Section 8.7.10 and Figure 6).358,674,675 Both safety and efficacy of sacubitril/valsartan have been demonstrated for the treatment of uncontrolled hypertension.148 The additional beneficial effects of sacubitril/valsartan in hypertension may be related to systemic vasodilation, natriuresis, and diuresis through inhibition of the catabolism of natriuretic peptides by neprilysin and blockade of angiotensin II.
19.6 Lifestyle modifications
Lifestyle modification has not been well studied in patients with TRH. Several small studies suggested that changes in diet and physical activity have the potential to lower BP substantially in patient with TRH.676,677 Although there are not many studies investigate the BP-lowering effects of lifestyle modifications in patients with TRH, this strategy appears promising.678 There is a need for healthcare professionals to give more attention to therapeutic lifestyles in patients with TRH.
19.7 Device therapy
TRH has been associated with an increase in sympathetic nervous system dysregulation related to obstructive sleep apnea, renin-angiotensin activation, or renal dysfunction. It was thought the heightened sympathetic tone can be solved by a focused intervention, such as baroreceptor stimulation or renal denervation.679-681 Recent randomized sham-controlled trials of renal denervation have demonstrated significant BP reduction in patients with uncontrolled or resistant hypertension.682-684
Acknowledgments
We thank Prof. Che-Hong Chen, the president of the Taiwan Alcohol Intolerance Education Society (TAIES), for his expert review of the "Alcohol limitation" section of the guidelines. We thank Yu-Xun Su for her editorial assistance.
DECLARATION OF CONFLICTS OF INTEREST
Tzung-Dau Wang has been on the speakers bureau and served as an advisor or consultant for Medtronic, Novartis, and Omron and has received research grants from Omron. All other authors report no potential conflicts of interest in relation to these guidelines.
TASK FORCE FOR THE 2022 GUIDELINES OF THE TAIWAN SOCIETY OF CARDIOLOGY AND THE TAIWAN HYPERTENSION SOCIETY FOR THE MANAGEMENT OF HYPERTENSION
(In alphabetical order) Wei-Ting Chang, Ting-Hsing Chao, Tzu-Fan Chao, Michael Yu-Chih Chen, Hao-Min Cheng, Chern-En Chiang (TSOC Guideline Committee Chairperson), Pao-Hsien Chu, Charles Jia-Yin Hou (TSOC President), Hsien-Li Kao (THS President), Ying-Hsiang Lee, Tsung-Hsien Lin (TSOC Hypertension Committee Chairperson), Yen-Hung Lin, Kwo-Chang Ueng, Tzung-Dau Wang (THS Scientific Committee Chairperson), Yu-Chen Wang, Yen-Wen Wu, Yi-Jer Wu.
ADVISORY BOARD MEMBER FOR THE 2022 GUIDELINES OF THE TAIWAN SOCIETY OF CARDIOLOGY AND THE TAIWAN HYPERTENSION SOCIETY FOR THE MANAGEMENT OF HYPERTENSION
(In alphabetical order) Chao-Chien Chang, Shih-Sheng Chang, Shih-Tai Chang, Chen-Huan Chen, Ching-Pei Chen, Wen-Jone Chen, Zhih-Cherng Chen, Zyh-Hong Chen, Shu-Meng Cheng, Wen-Jin Cherng, Chuen-Wang Chiou, Morgan Fu, Ching-Hui Huang, Jin-Long Huang, Huei-Fong Hung, Jing-Ren Jeng, Hsing-Li Liang, Wen-Ter Lai, Chia-Pin Lin, Shing-Jong Lin, Chun-Ming Shih, Kou-Gi Shyu, Han-Lin Tsai, Wei-Chuan Tsai, Ji-Hung Wang, Wen-Shiann Wu.
REFERENCES
- 1.Lin HJ, Wang TD, Chen YC, et al. 2020 Consensus Statement of the Taiwan Hypertension Society and the Taiwan Society of Cardiology on home blood pressure monitoring for the management of arterial hypertension. Acta Cardiol Sin. 2020;36:537–561. doi: 10.6515/ACS.202011_36(6).20201106A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan HY, Lin HJ, Chen WJ, et al. Prevalence, treatment, control and monitoring of hypertension: a nationwide community-based survey in Taiwan, 2017. Acta Cardiol Sin. 2020;36:375–381. doi: 10.6515/ACS.202007_36(4).20191220A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rahimi and Collaboration BPLTT. Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: an individual participant-level data meta-analysis. Lancet. 2021;397:1625–1636. doi: 10.1016/S0140-6736(21)00590-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rapsomaniki E, Timmis A, George J, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet. 2014;383:1899–1911. doi: 10.1016/S0140-6736(14)60685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi YJ, Kim SH, Kang SH, et al. Reconsidering the cut-off diastolic blood pressure for predicting cardiovascular events: a nationwide population-based study from Korea. Eur Heart J. 2019;40:724–731. doi: 10.1093/eurheartj/ehy801. [DOI] [PubMed] [Google Scholar]
- 6.Perkovic V, Huxley R, Wu Y, et al. The burden of blood pressure-related disease: a neglected priority for global health. Hypertension. 2007;50:991–997. doi: 10.1161/HYPERTENSIONAHA.107.095497. [DOI] [PubMed] [Google Scholar]
- 7.The fifth report of the Joint National Committee on detection, evaluation, and treatment of high blood pressure (JNC V). Arch Intern Med. 1993;153:154–183. [PubMed] [Google Scholar]
- 8.Group SR, Lewis CE, Fine LJ, et al. Final report of a trial of intensive versus standard blood-pressure control. N Engl J Med. 2021;384:1921–1930. doi: 10.1056/NEJMoa1901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang W, Zhang S, Deng Y, et al. Trial of intensive blood-pressure control in older patients with hypertension. N Engl J Med. 2021;385:1268–1279. doi: 10.1056/NEJMoa2111437. [DOI] [PubMed] [Google Scholar]
- 10.Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 11.Unger T, Borghi C, Charchar F, et al. 2020 International Society of hypertension global hypertension practice guidelines. Hypertension. 2020;75:1334–1357. doi: 10.1161/HYPERTENSIONAHA.120.15026. [DOI] [PubMed] [Google Scholar]
- 12.Group KDIGOKBPW KDIGO. 2021 Clinical Practice Guideline for the management of blood pressure in chronic kidney disease. Kidney Int. 2021;99:s1–s87. doi: 10.1016/j.kint.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Umemura S, Arima H, Arima S, et al. The Japanese Society of Hypertension Guidelines for the management of hypertension (JSH 2019). Hypertens Res. 2019;42:1235–1481. doi: 10.1038/s41440-019-0284-9. [DOI] [PubMed] [Google Scholar]
- 14.Yu WL, Toh HS, Liao CT, et al. Cardiovascular complications of COVID-19 and associated concerns: a review. Acta Cardiol Sin. 2021;37:9–17. doi: 10.6515/ACS.202101_37(1).20200913A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muntner P, Einhorn PT, Cushman WC, et al. Blood pressure assessment in adults in clinical practice and clinic-based research: JACC Scientific Expert Panel. J Am Coll Cardiol. 2019;73:317–335. doi: 10.1016/j.jacc.2018.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siddique S, Hameed Khan A, Shahab H, et al. Office blood pressure measurement: a comprehensive review. J Clin Hypertens (Greenwich) 2021;23:440–449. doi: 10.1111/jch.14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D S, NT A, JN B, et al. Self-measured blood pressure monitoring at home: a Joint Policy Statement from the American Heart Association and American Medical Association. Circulation. 2020;142:e42–e63. doi: 10.1161/CIR.0000000000000803. [DOI] [PubMed] [Google Scholar]
- 18.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2018;71:e127–e248. doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Shimbo D, Abdalla M, Falzon L, et al. Studies comparing ambulatory blood pressure and home blood pressure on cardiovascular disease and mortality outcomes: a systematic review. J Am Soc Hypertens. 2016;10:224–234 e17. doi: 10.1016/j.jash.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohkubo T, Imai Y, Tsuji I, et al. Home blood pressure measurement has a stronger predictive power for mortality than does screening blood pressure measurement: a population-based observation in Ohasama, Japan. J Hypertens. 1998;16:971–975. doi: 10.1097/00004872-199816070-00010. [DOI] [PubMed] [Google Scholar]
- 21.Hoshide S, Yano Y, Haimoto H, et al. Morning and evening home blood pressure and risks of incident stroke and coronary artery disease in the Japanese general practice population: The Japan Morning Surge-Home Blood Pressure Study. Hypertension. 2016;68:54–61. doi: 10.1161/HYPERTENSIONAHA.116.07201. [DOI] [PubMed] [Google Scholar]
- 22.Asayama K, Ohkubo T, Metoki H, et al. Cardiovascular outcomes in the first trial of antihypertensive therapy guided by self-measured home blood pressure. Hypertens Res. 2012;35:1102–1110. doi: 10.1038/hr.2012.125. [DOI] [PubMed] [Google Scholar]
- 23.Kario K, Saito I, Kushiro T, et al. Morning home blood pressure is a strong predictor of coronary artery disease: The HONEST Study. J Am Coll Cardiol. 2016;67:1519–1527. doi: 10.1016/j.jacc.2016.01.037. [DOI] [PubMed] [Google Scholar]
- 24.Shimamoto K, Ando K, Fujita T, et al. The Japanese Society of Hypertension Guidelines for the management of hypertension (JSH 2014). Hypertens Res. 2014;37:253–390. doi: 10.1038/hr.2014.20. [DOI] [PubMed] [Google Scholar]
- 25.Kario K, Hoshide S, Chia YC, et al. Guidance on ambulatory blood pressure monitoring: a statement from the HOPE Asia Network. J Clin Hypertens (Greenwich) 2021;23:411–421. doi: 10.1111/jch.14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stergiou GS, Argyraki KK, Moyssakis I, et al. Home blood pressure is as reliable as ambulatory blood pressure in predicting target-organ damage in hypertension. Am J Hypertens. 2007;20:616–621. doi: 10.1016/j.amjhyper.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 27.Bliziotis IA, Destounis A, Stergiou GS. Home versus ambulatory and office blood pressure in predicting target organ damage in hypertension: a systematic review and meta-analysis. J Hypertens. 2012;30:1289–1299. doi: 10.1097/HJH.0b013e3283531eaf. [DOI] [PubMed] [Google Scholar]
- 28.Gaborieau V, Delarche N, Gosse P. Ambulatory blood pressure monitoring versus self-measurement of blood pressure at home: correlation with target organ damage. J Hypertens. 2008;26:1919–1927. doi: 10.1097/HJH.0b013e32830c4368. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz JE, Muntner P, Kronish IM, et al. Reliability of office, home, and ambulatory blood pressure measurements and correlation with left ventricular mass. J Am Coll Cardiol. 2020;76:2911–2922. doi: 10.1016/j.jacc.2020.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson KC, Whelton PK, Cushman WC, et al. Blood pressure measurement in SPRINT (Systolic Blood Pressure Intervention Trial). Hypertension. 2018;71:848–857. doi: 10.1161/HYPERTENSIONAHA.117.10479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andreadis EA, Geladari CV, Angelopoulos ET, et al. Attended and unattended automated office blood pressure measurements have better agreement with ambulatory monitoring than conventional office readings. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.118.008994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muntner P, Shimbo D, Carey RM, et al. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension. 2019;73:e35–e66. doi: 10.1161/HYP.0000000000000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roerecke M, Kaczorowski J, Myers MG. Comparing automated office blood pressure readings with other methods of blood pressure measurement for identifying patients with possible hypertension: a systematic review and meta-analysis. JAMA Intern Med. 2019;179:351–362. doi: 10.1001/jamainternmed.2018.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stergiou GS, Tzamouranis D, Nasothimiou EG, et al. Are there really differences between home and daytime ambulatory blood pressure? Comparison using a novel dual-mode ambulatory and home monitor. J Hum Hypertens. 2010;24:207–212. doi: 10.1038/jhh.2009.60. [DOI] [PubMed] [Google Scholar]
- 35.Pappaccogli M, Di Monaco S, Perlo E, et al. Comparison of automated office blood pressure with office and out-off-office measurement techniques. Hypertension. 2019;73:481–490. doi: 10.1161/HYPERTENSIONAHA.118.12079. [DOI] [PubMed] [Google Scholar]
- 36.Myers MG, Kaczorowski J. Are automated office blood pressure readings more variable than home readings? Hypertension. 2020;75:1179–1183. doi: 10.1161/HYPERTENSIONAHA.119.14171. [DOI] [PubMed] [Google Scholar]
- 37.Kario K, Chen CH, Park S, et al. Consensus document on improving hypertension management in Asian patients, taking into account Asian characteristics. Hypertension. 2018;71:375–382. doi: 10.1161/HYPERTENSIONAHA.117.10238. [DOI] [PubMed] [Google Scholar]
- 38.Kario K, Pickering TG, Umeda Y, et al. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation. 2003;107:1401–1406. doi: 10.1161/01.cir.0000056521.67546.aa. [DOI] [PubMed] [Google Scholar]
- 39.Kario K, Saito I, Kushiro T, et al. Home blood pressure and cardiovascular outcomes in patients during antihypertensive therapy: primary results of HONEST, a large-scale prospective, real-world observational study. Hypertension. 2014;64:989–996. doi: 10.1161/HYPERTENSIONAHA.114.04262. [DOI] [PubMed] [Google Scholar]
- 40.Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 41.Arima H, Murakami Y, Lam TH, et al. Effects of prehypertension and hypertension subtype on cardiovascular disease in the Asia-Pacific region. Hypertension. 2012;59:1118–1123. doi: 10.1161/HYPERTENSIONAHA.111.187252. [DOI] [PubMed] [Google Scholar]
- 42.Chien KL, Hsu HC, Sung FC, et al. Incidence of hypertension and risk of cardiovascular events among ethnic Chinese: report from a community-based cohort study in Taiwan. J Hypertens. 2007;25:1355–1361. doi: 10.1097/HJH.0b013e3280d94313. [DOI] [PubMed] [Google Scholar]
- 43.Gu D, Kelly TN, Wu X, et al. Blood pressure and risk of cardiovascular disease in Chinese men and women. Am J Hypertens. 2008;21:265–272. doi: 10.1038/ajh.2007.59. [DOI] [PubMed] [Google Scholar]
- 44.Wan EYF, Yu EYT, Chin WY, et al. Association of blood pressure and risk of cardiovascular and chronic kidney disease in Hong Kong hypertensive patients. Hypertension. 2019;74:331–340. doi: 10.1161/HYPERTENSIONAHA.119.13123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Son JS, Choi S, Kim K, et al. Association of blood pressure classification in Korean young adults according to the 2017 American College of Cardiology/American Heart Association Guidelines with subsequent cardiovascular disease events. JAMA. 2018;320:1783–1792. doi: 10.1001/jama.2018.16501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niiranen TJ, Hänninen MR, Johansson J, et al. Home-measured blood pressure is a stronger predictor of cardiovascular risk than office blood pressure: the Finn-Home study. Hypertension. 2010;55:1346–1351. doi: 10.1161/HYPERTENSIONAHA.109.149336. [DOI] [PubMed] [Google Scholar]
- 47.Ward AM, Takahashi O, Stevens R, et al. Home measurement of blood pressure and cardiovascular disease: systematic review and meta-analysis of prospective studies. J Hypertens. 2012;30:449–456. doi: 10.1097/HJH.0b013e32834e4aed. [DOI] [PubMed] [Google Scholar]
- 48.Vongpatanasin W, Ayers C, Lodhi H, et al. Diagnostic thresholds for blood pressure measured at home in the context of the 2017 hypertension guideline. Hypertension. 2018;72:1312–1319. doi: 10.1161/HYPERTENSIONAHA.118.11657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turner MJ, Speechly C, Bignell N. Sphygmomanometer calibration--why, how and how often? Aust Fam Physician. 2007;36:834–838. [PubMed] [Google Scholar]
- 50.Myers MG. A short history of automated office blood pressure - 15 years to SPRINT. J Clin Hypertens (Greenwich) 2016;18:721–724. doi: 10.1111/jch.12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cappuccio FP, Kerry SM, Forbes L, et al. Blood pressure control by home monitoring: meta-analysis of randomised trials. BMJ. 2004;329:145. doi: 10.1136/bmj.38121.684410.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Staessen JA, Byttebier G, Buntinx F, et al. Antihypertensive treatment based on conventional or ambulatory blood pressure measurement. A randomized controlled trial. Ambulatory Blood Pressure Monitoring and Treatment of Hypertension Investigators. JAMA. 1997;278:1065–1072. [PubMed] [Google Scholar]
- 53.Staessen JA, DenHond E, Celis H, et al. Antihypertensive treatment based on blood pressure measurement at home or in the physician’s office: a randomized controlled trial. JAMA. 2004;291:955–964. doi: 10.1001/jama.291.8.955. [DOI] [PubMed] [Google Scholar]
- 54.McManus RJ, Mant J, Haque MS, et al. Effect of self-monitoring and medication self-titration on systolic blood pressure in hypertensive patients at high risk of cardiovascular disease: the TASMIN-SR randomized clinical trial. JAMA. 2014;312:799–808. doi: 10.1001/jama.2014.10057. [DOI] [PubMed] [Google Scholar]
- 55.Clinic M. Alcohol intolerance. Mayo Clinic Press [Google Scholar]
- 56.Kaplan NM. Commentary on the sixth report of the Joint National Committee (JNC-6). Am J Hypertens. 1998;11:134–136. [PubMed] [Google Scholar]
- 57.Staessen JA, Li Y, Hara A, et al. Blood pressure measurement anno 2016. Am J Hypertens. 2017;30:453–463. doi: 10.1093/ajh/hpw148. [DOI] [PubMed] [Google Scholar]
- 58.Myers MG, Godwin M, Dawes M, et al. Measurement of blood pressure in the office: recognizing the problem and proposing the solution. Hypertension. 2010;55:195–200. doi: 10.1161/HYPERTENSIONAHA.109.141879. [DOI] [PubMed] [Google Scholar]
- 59.Leung AA, Nerenberg K, Daskalopoulou SS, et al. Hypertension Canada’s 2016 Canadian hypertension education program guidelines for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2016;32:569–588. doi: 10.1016/j.cjca.2016.02.066. [DOI] [PubMed] [Google Scholar]
- 60.Leung AA, Daskalopoulou SS, Dasgupta K, et al. Hypertension Canada’s 2017 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults. Can J Cardiol. 2017;33:557–576. doi: 10.1016/j.cjca.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 61.Myers MG, Valdivieso M, Kiss A. Use of automated office blood pressure measurement to reduce the white coat response. J Hypertens. 2009;27:280–286. doi: 10.1097/HJH.0b013e32831b9e6b. [DOI] [PubMed] [Google Scholar]
- 62.Bauer F, Seibert FS, Rohn B, et al. Attended versus unattended blood pressure measurement in a real life setting. Hypertension. 2018;71:243–249. doi: 10.1161/HYPERTENSIONAHA.117.10026. [DOI] [PubMed] [Google Scholar]
- 63.Paini A, Bertacchini F, Stassaldi D, et al. Unattended versus attended blood pressure measurement: mean values and determinants of the difference. Int J Cardiol. 2019;274:305–310. doi: 10.1016/j.ijcard.2018.06.056. [DOI] [PubMed] [Google Scholar]
- 64.Kjeldsen SE, Lund-Johansen P, Nilsson PM, et al. Unattended blood pressure measurements in the systolic blood pressure intervention trial: implications for entry and achieved blood pressure values compared with other trials. Hypertension. 2016;67:808–812. doi: 10.1161/HYPERTENSIONAHA.116.07257. [DOI] [PubMed] [Google Scholar]
- 65.Schwartz JE, Burg MM, Shimbo D, et al. Clinic blood pressure underestimates ambulatory blood pressure in an untreated employer-based us population: results from the masked hypertension study. Circulation. 2016;134:1794–1807. doi: 10.1161/CIRCULATIONAHA.116.023404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dolan E, Stanton A, Thijs L, et al. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension. 2005;46:156–161. doi: 10.1161/01.HYP.0000170138.56903.7a. [DOI] [PubMed] [Google Scholar]
- 67.Conen D, Aeschbacher S, Thijs L, et al. Age-specific differences between conventional and ambulatory daytime blood pressure values. Hypertension. 2014;64:1073–1079. doi: 10.1161/HYPERTENSIONAHA.114.03957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fagard RH, Cornelissen VA. Incidence of cardiovascular events in white-coat, masked and sustained hypertension versus true normotension: a meta-analysis. J Hypertens. 2007;25:2193–2198. doi: 10.1097/HJH.0b013e3282ef6185. [DOI] [PubMed] [Google Scholar]
- 69.Siu AL. Screening for high blood pressure in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163:778–786. doi: 10.7326/M15-2223. [DOI] [PubMed] [Google Scholar]
- 70.Sung SH, Cheng HM, Wang KL, et al. White coat hypertension is more risky than prehypertension: important role of arterial wave reflections. Hypertension. 2013;61:1346–1353. doi: 10.1161/HYPERTENSIONAHA.111.00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheng HM, Lin HJ, Wang TD, et al. Asian management of hypertension: current status, home blood pressure, and specific concerns in Taiwan. J Clin Hypertens (Greenwich) 2020;22:511–514. doi: 10.1111/jch.13747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang Y, Huang W, Mai W, et al. White-coat hypertension is a risk factor for cardiovascular diseases and total mortality. J Hypertens. 2017;35:677–688. doi: 10.1097/HJH.0000000000001226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Muntner P, Booth JN, 3rd, Shimbo D, et al. Is white-coat hypertension associated with increased cardiovascular and mortality risk? J Hypertens. 2016;34:1655–1658. doi: 10.1097/HJH.0000000000000983. [DOI] [PubMed] [Google Scholar]
- 74.Stergiou GS, Asayama K, Thijs L, et al. Prognosis of white-coat and masked hypertension: international database of home blood pressure in relation to cardiovascular outcome. Hypertension. 2014;63:675–682. doi: 10.1161/HYPERTENSIONAHA.113.02741. [DOI] [PubMed] [Google Scholar]
- 75.Mancia G, Bombelli M, Facchetti R, et al. Long-term risk of sustained hypertension in white-coat or masked hypertension. Hypertension. 2009;54:226–232. doi: 10.1161/HYPERTENSIONAHA.109.129882. [DOI] [PubMed] [Google Scholar]
- 76.Ugajin T, Hozawa A, Ohkubo T, et al. White-coat hypertension as a risk factor for the development of home hypertension: the Ohasama study. Arch Intern Med. 2005;165:1541–1546. doi: 10.1001/archinte.165.13.1541. [DOI] [PubMed] [Google Scholar]
- 77.Cohen JB, Lotito MJ, Trivedi UK, et al. Cardiovascular events and mortality in white coat hypertension: a systematic review and meta-analysis. Ann Intern Med. 2019;170:853–862. doi: 10.7326/M19-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shimbo D, Newman JD, Schwartz JE. Masked hypertension and prehypertension: diagnostic overlap and interrelationships with left ventricular mass: the masked hypertension study. Am J Hypertens. 2012;25:664–671. doi: 10.1038/ajh.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hänninen MR, Niiranen TJ, Puukka PJ, et al. Determinants of masked hypertension in the general population: the Finn-Home study. J Hypertens. 2011;29:1880–1888. doi: 10.1097/HJH.0b013e32834a98ba. [DOI] [PubMed] [Google Scholar]
- 80.Franklin SS, Thijs L, Li Y, et al. Masked hypertension in diabetes mellitus: treatment implications for clinical practice. Hypertension. 2013;61:964–971. doi: 10.1161/HYPERTENSIONAHA.111.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gorostidi M, Sarafidis PA, de la Sierra A, et al. Differences between office and 24-hour blood pressure control in hypertensive patients with CKD: a 5,693-patient cross-sectional analysis from Spain. Am J Kidney Dis. 2013;62:285–294. doi: 10.1053/j.ajkd.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 82.Drager LF, Diegues-Silva L, Diniz PM, et al. Obstructive sleep apnea, masked hypertension, and arterial stiffness in men. Am J Hypertens. 2010;23:249–254. doi: 10.1038/ajh.2009.246. [DOI] [PubMed] [Google Scholar]
- 83.Mulè G, Caimi G, Cottone S, et al. Value of home blood pressures as predictor of target organ damage in mild arterial hypertension. J Cardiovasc Risk. 2002;9:123–129. doi: 10.1177/174182670200900208. [DOI] [PubMed] [Google Scholar]
- 84.Niiranen T, Jula A, Kantola I, et al. Home-measured blood pressure is more strongly associated with atherosclerosis than clinic blood pressure: the Finn-home study. J Hypertens. 2007;25:1225–1231. doi: 10.1097/HJH.0b013e3280d94336. [DOI] [PubMed] [Google Scholar]
- 85.Sega R, Facchetti R, Bombelli M, et al. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation. 2005;111:1777–1783. doi: 10.1161/01.CIR.0000160923.04524.5B. [DOI] [PubMed] [Google Scholar]
- 86.Ohkubo T, Asayama K, Kikuya M, et al. How many times should blood pressure be measured at home for better prediction of stroke risk? Ten-year follow-up results from the Ohasama study. J Hypertens. 2004;22:1099–1104. doi: 10.1097/00004872-200406000-00009. [DOI] [PubMed] [Google Scholar]
- 87.Ntineri A, Kalogeropoulos PG, Kyriakoulis KG, et al. Prognostic value of average home blood pressure and variability: 19-year follow-up of the Didima study. J Hypertens. 2018;36:69–76. doi: 10.1097/HJH.0000000000001497. [DOI] [PubMed] [Google Scholar]
- 88.Asayama K, Ohkubo T, Kikuya M, et al. Prediction of stroke by self-measurement of blood pressure at home versus casual screening blood pressure measurement in relation to the Joint National Committee 7 classification: the Ohasama study. Stroke. 2004;35:2356–2361. doi: 10.1161/01.STR.0000141679.42349.9f. [DOI] [PubMed] [Google Scholar]
- 89.Tientcheu D, Ayers C, Das SR, et al. Target organ complications and cardiovascular events associated with masked hypertension and white-coat hypertension: analysis from the Dallas heart study. J Am Coll Cardiol. 2015;66:2159–2169. doi: 10.1016/j.jacc.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tucker KL, Sheppard JP, Stevens R, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med. 2017;14:e1002389. doi: 10.1371/journal.pmed.1002389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sogunuru GP, Kario K, Shin J, et al. Morning surge in blood pressure and blood pressure variability in Asia: evidence and statement from the HOPE Asia Network. J Clin Hypertens (Greenwich) 2019;21:324–334. doi: 10.1111/jch.13451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kario K, Kanegae H, Tomitani N, et al. Nighttime blood pressure measured by home blood pressure monitoring as an independent predictor of cardiovascular events in general practice. Hypertension. 2019;73:1240–1248. doi: 10.1161/HYPERTENSIONAHA.118.12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kario K, Park S, Buranakitjaroen P, et al. Guidance on home blood pressure monitoring: a statement of the HOPE Asia Network. J Clin Hypertens (Greenwich) 2018;20:456–461. doi: 10.1111/jch.13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Parati G, Stergiou GS, Asmar R, et al. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779–785. doi: 10.1038/jhh.2010.54. [DOI] [PubMed] [Google Scholar]
- 95.Juhanoja EP, Johansson JK, Puukka PJ, et al. Optimal schedule for assessing home BP variability: the Finn-home study. Am J Hypertens. 2018;31:715–725. doi: 10.1093/ajh/hpy030. [DOI] [PubMed] [Google Scholar]
- 96.Bello NA, Schwartz JE, Kronish IM, et al. Number of measurements needed to obtain a reliable estimate of home blood pressure: results from the improving the detection of hypertension study. J Am Heart Assoc. 2018;7:e008658. doi: 10.1161/JAHA.118.008658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Powers BJ, Olsen MK, Smith VA, et al. Measuring blood pressure for decision making and quality reporting: where and how many measures? Ann Intern Med. 2011;154:781–788, w-289-90. doi: 10.7326/0003-4819-154-12-201106210-00005. [DOI] [PubMed] [Google Scholar]
- 98.Niiranen TJ, Asayama K, Thijs L, et al. Optimal number of days for home blood pressure measurement. Am J Hypertens. 2015;28:595–603. doi: 10.1093/ajh/hpu216. [DOI] [PubMed] [Google Scholar]
- 99.Chiang CE, Wu TJ, Ueng KC, et al. 2016 guidelines of the Taiwan Heart Rhythm Society and the Taiwan Society of Cardiology for the management of atrial fibrillation. J Formos Med Assoc. 2016;115:893–952. doi: 10.1016/j.jfma.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 100.Chao TF, Lip GYH, Liu CJ, et al. Relationship of aging and incident comorbidities to stroke risk in patients with atrial fibrillation. J Am Coll Cardiol. 2018;71:122–132. doi: 10.1016/j.jacc.2017.10.085. [DOI] [PubMed] [Google Scholar]
- 101.Kim TH, Yang PS, Yu HT, et al. Age threshold for ischemic stroke risk in atrial fibrillation. Stroke. 2018;49:1872–1879. doi: 10.1161/STROKEAHA.118.021047. [DOI] [PubMed] [Google Scholar]
- 102.Alpert BS, Quinn D, Gallick D. Oscillometric blood pressure: a review for clinicians. J Am Soc Hypertens. 2014;8:930–938. doi: 10.1016/j.jash.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 103.Halfon M, Wuerzner G, Marques-Vidal P, et al. Use of oscillometric devices in atrial fibrillation: a comparison of three devices and invasive blood pressure measurement. Blood Press. 2018;27:48–55. doi: 10.1080/08037051.2017.1383852. [DOI] [PubMed] [Google Scholar]
- 104.Clark CE, McDonagh STJ, McManus RJ. Accuracy of automated blood pressure measurements in the presence of atrial fibrillation: systematic review and meta-analysis. J Hum Hypertens. 2019;33:352–364. doi: 10.1038/s41371-018-0153-z. [DOI] [PubMed] [Google Scholar]
- 105.Senoo K, Miki T, Okura T, et al. Diagnostic value of atrial fibrillation by built-in electrocardiogram technology in a blood pressure monitor. Circ Rep. 2020;2:345–350. doi: 10.1253/circrep.CR-20-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Clement DL, De Buyzere ML, De Bacquer DA, et al. Prognostic value of ambulatory blood-pressure recordings in patients with treated hypertension. N Engl J Med. 2003;348:2407–2415. doi: 10.1056/NEJMoa022273. [DOI] [PubMed] [Google Scholar]
- 107.Conen D, Bamberg F. Noninvasive 24-h ambulatory blood pressure and cardiovascular disease: a systematic review and meta-analysis. J Hypertens. 2008;26:1290–1299. doi: 10.1097/HJH.0b013e3282f97854. [DOI] [PubMed] [Google Scholar]
- 108.Yang WY, Melgarejo JD, Thijs L, et al. Association of office and ambulatory blood pressure with mortality and cardiovascular outcomes. JAMA. 2019;322:409–420. doi: 10.1001/jama.2019.9811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hoshide S, Cheng HM, Huang Q, et al. Role of ambulatory blood pressure monitoring for the management of hypertension in Asian populations. J Clin Hypertens (Greenwich) 2017;19:1240–1245. doi: 10.1111/jch.13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li Y, Wang JG. Isolated nocturnal hypertension: a disease masked in the dark. Hypertension. 2013;61:278–283. doi: 10.1161/HYPERTENSIONAHA.111.00217. [DOI] [PubMed] [Google Scholar]
- 111.Li Y, Staessen JA, Lu L, et al. Is isolated nocturnal hypertension a novel clinical entity? Findings from a Chinese population study. Hypertension. 2007;50:333–339. doi: 10.1161/HYPERTENSIONAHA.107.087767. [DOI] [PubMed] [Google Scholar]
- 112.Kario K, Ishikawa J, Pickering TG, et al. Morning hypertension:the strongest independent risk factor for stroke in elderly hypertensive patients. Hypertens Res. 2006;29:581–587. doi: 10.1291/hypres.29.581. [DOI] [PubMed] [Google Scholar]
- 113.Stergiou GS, Nasothimiou EG, Roussias LG. Morning hypertension assessed by home or ambulatory monitoring: different aspects of the same phenomenon? J Hypertens. 2010;28:1846–1853. doi: 10.1097/HJH.0b013e32833b497d. [DOI] [PubMed] [Google Scholar]
- 114.O'Brien E, Parati G, Stergiou G, et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31:1731–1768. doi: 10.1097/HJH.0b013e328363e964. [DOI] [PubMed] [Google Scholar]
- 115.Argentino C, Toni D, Rasura M, et al. Circadian variation in the frequency of ischemic stroke. Stroke. 1990;21:387–389. doi: 10.1161/01.str.21.3.387. [DOI] [PubMed] [Google Scholar]
- 116.Li Y, Thijs L, Hansen TW, et al. Prognostic value of the morning blood pressure surge in 5645 subjects from 8 populations. Hypertension. 2010;55:1040–1048. doi: 10.1161/HYPERTENSIONAHA.109.137273. [DOI] [PubMed] [Google Scholar]
- 117.Sheppard JP, Hodgkinson J, Riley R, et al. Prognostic significance of the morning blood pressure surge in clinical practice: a systematic review. Am J Hypertens. 2015;28:30–41. doi: 10.1093/ajh/hpu104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cheng HM, Wu CL, Sung SH, et al. Prognostic utility of morning blood pressure surge for 20-year all-cause and cardiovascular mortalities: results of a community-based study. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.007667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ding XR, Zhang YT, Liu J, et al. Continuous cuffless blood pressure estimation using pulse transit time and photoplethysmogram intensity ratio. IEEE Trans Biomed Eng. 2016;63:964–972. doi: 10.1109/TBME.2015.2480679. [DOI] [PubMed] [Google Scholar]
- 120.Kuwabara M, Harada K, Hishiki Y, et al. Validation of two watch-type wearable blood pressure monitors according to the ANSI/AAMI/ISO81060-2: 2013 guidelines: Omron HEM-6410T-ZM and HEM-6410T-ZL. J Clin Hypertens (Greenwich) 2019;21:853–858. doi: 10.1111/jch.13499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Islam SMS, Cartledge S, Karmakar C, et al. Validation and acceptability of a cuffless wrist-worn wearable blood pressure monitoring device among users and health care professionals: mixed methods study. JMIR Mhealth Uhealth. 2019;7:e14706. doi: 10.2196/14706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Karamanoglu M, O'Rourke MF, Avolio AP, et al. An analysis of the relationship between central aortic and peripheral upper limb pressure waves in man. Eur Heart J. 1993;14:160–167. doi: 10.1093/eurheartj/14.2.160. [DOI] [PubMed] [Google Scholar]
- 123.Wang KL, Cheng HM, Chuang SY, et al. Central or peripheral systolic or pulse pressure: which best relates to target organs and future mortality? J Hypertens. 2009;27:461–467. doi: 10.1097/hjh.0b013e3283220ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vlachopoulos C, Aznaouridis K, O’Rourke MF, et al. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J. 2010;31:1865–1871. doi: 10.1093/eurheartj/ehq024. [DOI] [PubMed] [Google Scholar]
- 125.Cheng HM, Park S, Huang Q, et al. Vascular aging and hypertension: implications for the clinical application of central blood pressure. Int J Cardiol. 2017;230:209–213. doi: 10.1016/j.ijcard.2016.12.170. [DOI] [PubMed] [Google Scholar]
- 126.Williams B, Lacy PS, Thom SM, et al. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113:1213–1225. doi: 10.1161/CIRCULATIONAHA.105.595496. [DOI] [PubMed] [Google Scholar]
- 127.Hashimoto J, Imai Y, O’Rourke MF. Monitoring of antihypertensive therapy for reduction in left ventricular mass. Am J Hypertens. 2007;20:1229–1233. doi: 10.1016/j.amjhyper.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 128.Cheng HM, Chuang SY, Sung SH, et al. 2019 consensus of the Taiwan Hypertension Society and Taiwan Society of Cardiology on the clinical application of central blood pressure in the management of hypertension. Acta Cardiol Sin. 2019;35:234–243. doi: 10.6515/ACS.201905_35(3).20190415B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Agabiti-Rosei E, Mancia G, O'Rourke MF, et al. Central blood pressure measurements and antihypertensive therapy: a consensus document. Hypertension. 2007;50:154–160. doi: 10.1161/HYPERTENSIONAHA.107.090068. [DOI] [PubMed] [Google Scholar]
- 130.Cheng HM, Chuang SY, Sung SH, et al. Derivation and validation of diagnostic thresholds for central blood pressure measurements based on long-term cardiovascular risks. J Am Coll Cardiol. 2013;62:1780–1787. doi: 10.1016/j.jacc.2013.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cheng HM, Sung SH, Chuang SY, et al. Diagnostic performance of a stand-alone central blood pressure monitor: application of central blood pressure in the diagnosis of high blood pressure. Am J Hypertens. 2014;27:382–391. doi: 10.1093/ajh/hpt282. [DOI] [PubMed] [Google Scholar]
- 132.Sharman JE, Marwick TH, Gilroy D, et al. Randomized trial of guiding hypertension management using central aortic blood pressure compared with best-practice care: principal findings of the BP GUIDE study. Hypertension. 2013;62:1138–1145. doi: 10.1161/HYPERTENSIONAHA.113.02001. [DOI] [PubMed] [Google Scholar]
- 133.Sharman JE, Avolio AP, Baulmann J, et al. Validation of non-invasive central blood pressure devices: ARTERY Society task force consensus statement on protocol standardization. Eur Heart J. 2017;38:2805–2812. doi: 10.1093/eurheartj/ehw632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chuang SY, Chang HY, Cheng HM, et al. Prevalence of hypertension defined by central blood pressure measured using a type II device in a nationally representative cohort. Am J Hypertens. 2018;31:346–354. doi: 10.1093/ajh/hpx178. [DOI] [PubMed] [Google Scholar]
- 135.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2018;71:2199–2269. [Google Scholar]
- 136.Cheng HM, Sung SH, Chen CH, et al. Guiding Hypertension Management Using Different Blood Pressure Monitoring Strategies (GYMNs study): comparison of three different blood pressure measurement methods: study protocol for a randomized controlled trial. Trials. 2019;20:265. doi: 10.1186/s13063-019-3366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Parati G, Ochoa JE, Lombardi C, et al. Assessment and management of blood-pressure variability. Nat Rev Cardiol. 2013;10:143–155. doi: 10.1038/nrcardio.2013.1. [DOI] [PubMed] [Google Scholar]
- 138.Parati G, Stergiou GS, Dolan E, et al. Blood pressure variability: clinical relevance and application. J Clin Hypertens (Greenwich) 2018;20:1133–1137. doi: 10.1111/jch.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Stevens SL, Wood S, Koshiaris C, et al. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. Bmj. 2016;354:i4098. doi: 10.1136/bmj.i4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang H, Li M, Xie SH, et al. Visit-to-visit systolic blood pressure variability and stroke risk: a systematic review and meta-analysis. Curr Med Sci. 2019;39:741–747. doi: 10.1007/s11596-019-2100-9. [DOI] [PubMed] [Google Scholar]
- 141.Kikuya M, Ohkubo T, Metoki H, et al. Day-by-day variability of blood pressure and heart rate at home as a novel predictor of prognosis: the Ohasama study. Hypertension. 2008;52:1045–1050. doi: 10.1161/HYPERTENSIONAHA.107.104620. [DOI] [PubMed] [Google Scholar]
- 142.Mena LJ, Felix VG, Melgarejo JD, et al. 24-hour blood pressure variability assessed by average real variability: a systematic review and meta-analysis. J Am Heart Assoc. 2017;6:e006895. doi: 10.1161/JAHA.117.006895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Huang JT, Cheng HM, Yu WC, et al. Increased nighttime pulse pressure variability but not ambulatory blood pressure levels predicts 14-year all-cause mortality in patients on hemodialysis. Hypertension. 2019;74:660–668. doi: 10.1161/HYPERTENSIONAHA.119.13204. [DOI] [PubMed] [Google Scholar]
- 144.Rothwell PM, Howard SC, Dolan E, et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895–905. doi: 10.1016/S0140-6736(10)60308-X. [DOI] [PubMed] [Google Scholar]
- 145.Chiu TJ, Yeh JT, Huang CJ, et al. Blood pressure variability and cognitive dysfunction: a systematic review and meta-analysis of longitudinal cohort studies. J Clin Hypertens (Greenwich) 2021;23:1463–1482. doi: 10.1111/jch.14310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hsu PF, Cheng HM, Sung SH, et al. Hemodynamic determinants of the short-term blood pressure variability: differential roles of arterial stiffness and wave reflection. Am J Hypertens. 2017;30:256–263. doi: 10.1093/ajh/hpw144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rothwell PM, Howard SC, Dolan E, et al. Effects of beta blockers and calcium-channel blockers on within-individual variability in blood pressure and risk of stroke. Lancet Neurol. 2010;9:469–480. doi: 10.1016/S1474-4422(10)70066-1. [DOI] [PubMed] [Google Scholar]
- 148.Cheung DG, Aizenberg D, Gorbunov V, et al. Efficacy and safety of sacubitril/valsartan in patients with essential hypertension uncontrolled by olmesartan: a randomized, double-blind, 8-week study. J Clin Hypertens (Greenwich) 2018;20:150–158. doi: 10.1111/jch.13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Collaborators GA. Alcohol use and burden for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392:1015–1035. doi: 10.1016/S0140-6736(18)31310-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lin JY, Kuo KL, Kuo YH, et al. Association between real-world home blood pressure measurement patterns and blood pressure variability among older individuals with hypertension: a community-based blood pressure variability study. J Clin Hypertens (Greenwich) 2021;23:628–637. doi: 10.1111/jch.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chang HC, Wu CL, Lee YH, et al. Impact of dietary intake of sodium and potassium on short-term blood pressure variability. J Hypertens. 2021;39:1835–1843. doi: 10.1097/HJH.0000000000002856. [DOI] [PubMed] [Google Scholar]
- 152.Chiang CE, Wang TD, Li YH, et al. 2010 guidelines of the Taiwan Society of Cardiology for the management of hypertension. J Formos Med Assoc. 2010;109:740–773. doi: 10.1016/S0929-6646(10)60120-9. [DOI] [PubMed] [Google Scholar]
- 153.Pusuroglu H, Cizgici AY, Demir AR, et al. Long-term prognostic value of mean platelet volume in patients with hypertension. Acta Cardiol Sin. 2021;37:504–511. doi: 10.6515/ACS.202109_37(5).20210324A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wen CP, Cheng TY, Tsai MK, et al. All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462 293 adults in Taiwan. Lancet. 2008;371:2173–2182. doi: 10.1016/S0140-6736(08)60952-6. [DOI] [PubMed] [Google Scholar]
- 155.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 156.Sehestedt T, Jeppesen J, Hansen TW, et al. Risk prediction is improved by adding markers of subclinical organ damage to SCORE. Eur Heart J. 2010;31:883–891. doi: 10.1093/eurheartj/ehp546. [DOI] [PubMed] [Google Scholar]
- 157.Volpe M, Battistoni A, Tocci G, et al. Cardiovascular risk assessment beyond Systemic Coronary Risk Estimation: a role for organ damage markers. J Hypertens. 2012;30:1056–1064. doi: 10.1097/HJH.0b013e3283525715. [DOI] [PubMed] [Google Scholar]
- 158.Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36:1953–2041. doi: 10.1097/HJH.0000000000001940. [DOI] [PubMed] [Google Scholar]
- 159.Lønnebakken MT, Izzo R, Mancusi C, et al. Left ventricular hypertrophy regression during antihypertensive treatment in an outpatient clinic (the Campania Salute Network). J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hung CL, Wu YW, Lin CC, et al. 2021 TSOC Expert Consensus on the clinical features, diagnosis, and clinical management of cardiac manifestations of fabry disease. Acta Cardiol Sin. 2021;37:337–354. doi: 10.6515/ACS.202107_37(4).20210601A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chiang CE, Wang TD, Ueng KC, et al. 2015 guidelines of the Taiwan Society of Cardiology and the Taiwan Hypertension Society for the management of hypertension. J Chin Med Assoc. 2015;78:1–47. doi: 10.1016/j.jcma.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 162.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Hypertension. 2018;71:e13–e115. doi: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 163.Rimoldi SF, Scherrer U, Messerli FH. Secondary arterial hypertension: when, who, and how to screen? Eur Heart J. 2014;35:1245–1254. doi: 10.1093/eurheartj/eht534. [DOI] [PubMed] [Google Scholar]
- 164.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 165.Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008;117:e510–e526. doi: 10.1161/CIRCULATIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 166.Faselis C, Doumas M, Papademetriou V. Common secondary causes of resistant hypertension and rational for treatment. Int J Hypertens. 2011;2011:236239. doi: 10.4061/2011/236239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yen RF, Wu VC, Liu KL, et al. 131I-6beta-iodomethyl-19-norcholesterol SPECT/CT for primary aldosteronism patients with inconclusive adrenal venous sampling and CT results. J Nucl Med. 2009;50:1631–1637. doi: 10.2967/jnumed.109.064873. [DOI] [PubMed] [Google Scholar]
- 168.Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:1889–1916. doi: 10.1210/jc.2015-4061. [DOI] [PubMed] [Google Scholar]
- 169.Xu Z, Yang J, Hu J, et al. Primary aldosteronism in patients in China with recently detected hypertension. J Am Coll Cardiol. 2020;75:1913–1922. doi: 10.1016/j.jacc.2020.02.052. [DOI] [PubMed] [Google Scholar]
- 170.Rossi GP, Sacchetto A, Visentin P, et al. Changes in left ventricular anatomy and function in hypertension and primary aldosteronism. Hypertension. 1996;27:1039–1045. doi: 10.1161/01.hyp.27.5.1039. [DOI] [PubMed] [Google Scholar]
- 171.Rossi GP, Sacchetto A, Pavan E, et al. Remodeling of the left ventricle in primary aldosteronism due to Conn’s adenoma. Circulation. 1997;95:1471–1478. doi: 10.1161/01.cir.95.6.1471. [DOI] [PubMed] [Google Scholar]
- 172.Matsumura K, Fujii K, Oniki H, et al. Role of aldosterone in left ventricular hypertrophy in hypertension. Am J Hypertens. 2006;19:13–18. doi: 10.1016/j.amjhyper.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 173.Tanabe A, Naruse M, Naruse K, et al. Left ventricular hypertrophy is more prominent in patients with primary aldosteronism than in patients with other types of secondary hypertension. Hypertens Res. 1997;20:85–90. doi: 10.1291/hypres.20.85. [DOI] [PubMed] [Google Scholar]
- 174.Rossi GP, Di Bello V, Ganzaroli C, et al. Excess aldosterone is associated with alterations of myocardial texture in primary aldosteronism. Hypertension. 2002;40:23–27. doi: 10.1161/01.hyp.0000023182.68420.eb. [DOI] [PubMed] [Google Scholar]
- 175.Kozàkovà M, Buralli S, Palombo C, et al. Myocardial ultrasonic backscatter in hypertension: relation to aldosterone and endothelin. Hypertension. 2003;41:230–236. doi: 10.1161/01.hyp.0000052542.68896.2b. [DOI] [PubMed] [Google Scholar]
- 176.Chang YY, Liao CW, Tsai CH, et al. Left ventricular dysfunction in patients with primary aldosteronism: a propensity score-matching follow-up study with tissue doppler imaging. J Am Heart Assoc. 2019;8:e013263. doi: 10.1161/JAHA.119.013263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Strauch B, Petrák O, Wichterle D, et al. Increased arterial wall stiffness in primary aldosteronism in comparison with essential hypertension. Am J Hypertens. 2006;19:909–914. doi: 10.1016/j.amjhyper.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 178.Savard S, Amar L, Plouin PF, et al. Cardiovascular complications associated with primary aldosteronism: a controlled cross-sectional study. Hypertension. 2013;62:331–336. doi: 10.1161/HYPERTENSIONAHA.113.01060. [DOI] [PubMed] [Google Scholar]
- 179.Takeda R, Matsubara T, Miyamori I, et al. Vascular complications in patients with aldosterone producing adenoma in Japan: comparative study with essential hypertension. The Research Committee of Disorders of Adrenal Hormones in Japan. J Endocrinol Invest. 1995;18:370–373. doi: 10.1007/BF03347840. [DOI] [PubMed] [Google Scholar]
- 180.Catena C, Colussi G, Nadalini E, et al. Cardiovascular outcomes in patients with primary aldosteronism after treatment. Arch Intern Med. 2008;168:80–85. doi: 10.1001/archinternmed.2007.33. [DOI] [PubMed] [Google Scholar]
- 181.Monticone S, D'Ascenzo F, Moretti C, et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2018;6:41–50. doi: 10.1016/S2213-8587(17)30319-4. [DOI] [PubMed] [Google Scholar]
- 182.Catena C, Lapenna R, Baroselli S, et al. Insulin sensitivity in patients with primary aldosteronism: a follow-up study. J Clin Endocrinol Metab. 2006;91:3457–3463. doi: 10.1210/jc.2006-0736. [DOI] [PubMed] [Google Scholar]
- 183.Wu CH, Yang YW, Hung SC, et al. Effect of treatment on body fluid in patients with unilateral aldosterone producing adenoma: adrenalectomy versus spironolactone. Sci Rep. 2015;5:15297. doi: 10.1038/srep15297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Martín-Fernández B, Rubio-Navarro A, Cortegano I, et al. Aldosterone induces renal fibrosis and inflammatory M1-macrophage subtype via mineralocorticoid receptor in rats. PLoS One. 2016;11:e0145946. doi: 10.1371/journal.pone.0145946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ceccoli L, Ronconi V, Giovannini L, et al. Bone health and aldosterone excess. Osteoporos Int. 2013;24:2801–2807. doi: 10.1007/s00198-013-2399-1. [DOI] [PubMed] [Google Scholar]
- 186.Amar L, Plouin PF, Steichen O. Aldosterone-producing adenoma and other surgically correctable forms of primary aldosteronism. Orphanet J Rare Dis. 2010;5:9. doi: 10.1186/1750-1172-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Catena C, Colussi G, Lapenna R, et al. Long-term cardiac effects of adrenalectomy or mineralocorticoid antagonists in patients with primary aldosteronism. Hypertension. 2007;50:911–918. doi: 10.1161/HYPERTENSIONAHA.107.095448. [DOI] [PubMed] [Google Scholar]
- 188.Lin YH, Lee HH, Liu KL, et al. Reversal of myocardial fibrosis in patients with unilateral hyperaldosteronism receiving adrenalectomy. Surgery. 2011;150:526–533. doi: 10.1016/j.surg.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 189.Strauch B, Petrák O, Zelinka T, et al. Adrenalectomy improves arterial stiffness in primary aldosteronism. Am J Hypertens. 2008;21:1086–1092. doi: 10.1038/ajh.2008.243. [DOI] [PubMed] [Google Scholar]
- 190.Lin YH, Lin LY, Chen A, et al. Adrenalectomy improves increased carotid intima-media thickness and arterial stiffness in patients with aldosterone producing adenoma. Atherosclerosis. 2012;221:154–159. doi: 10.1016/j.atherosclerosis.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 191.Wu VC, Wang SM, Chang CH, et al. Long term outcome of aldosteronism after target treatments. Sci Rep. 2016;6:32103. doi: 10.1038/srep32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wu VC, Hu YH, Er LK, et al. Case detection and diagnosis of primary aldosteronism - the consensus of Taiwan Society of Aldosteronism. J Formos Med Assoc. 2017;116:993–1005. doi: 10.1016/j.jfma.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 194.Huang KH, Yu CC, Hu YH, et al. Targeted treatment of primary aldosteronism - the consensus of Taiwan Society of Aldosteronism. J Formos Med Assoc. 2019;118:72–82. doi: 10.1016/j.jfma.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 195.Selassie A, Wagner CS, Laken ML, et al. Progression is accelerated from prehypertension to hypertension in blacks. Hypertension. 2011;58:579–587. doi: 10.1161/HYPERTENSIONAHA.111.177410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wu VC, Huang KH, Peng KY, et al. Prevalence and clinical correlates of somatic mutation in aldosterone producing adenoma-Taiwanese population. Sci Rep. 2015;5:11396. doi: 10.1038/srep11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ito Y, Takeda R, Takeda Y. Subclinical primary aldosteronism. Best Pract Res Clin Endocrinol Metab. 2012;26:485–495. doi: 10.1016/j.beem.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 198.Chao CT, Wu VC, Kuo CC, et al. Diagnosis and management of primary aldosteronism: an updated review. Ann Med. 2013;45:375–383. doi: 10.3109/07853890.2013.785234. [DOI] [PubMed] [Google Scholar]
- 199.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 200.Grasko JM, Nguyen HH, Glendenning P. Delayed diagnosis of primary hyperaldosteronism. BMJ. 2010;340:c2461. doi: 10.1136/bmj.c2461. [DOI] [PubMed] [Google Scholar]
- 201.Mulatero P, Rabbia F, Milan A, et al. Drug effects on aldosterone/plasma renin activity ratio in primary aldosteronism. Hypertension. 2002;40:897–902. doi: 10.1161/01.hyp.0000038478.59760.41. [DOI] [PubMed] [Google Scholar]
- 202.Mulatero P, Monticone S, Deinum J, et al. Genetics,prevalence,screening and confirmation of primary aldosteronism: a position statement and consensus of the Working Group on Endocrine Hypertension of The European Society of Hypertension. J Hypertens. 2020;38:1919–1928. doi: 10.1097/HJH.0000000000002510. [DOI] [PubMed] [Google Scholar]
- 203.Funder JW, Carey RM, Fardella C, et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:3266–3281. doi: 10.1210/jc.2008-0104. [DOI] [PubMed] [Google Scholar]
- 204.Salvà M, Cicala MV, Mantero F. Primary aldosteronism: the role of confirmatory tests. Horm Metab Res. 2012;44:177–180. doi: 10.1055/s-0032-1304661. [DOI] [PubMed] [Google Scholar]
- 205.Stowasser M, Ahmed AH, Cowley D, et al. Comparison of seated with recumbent saline suppression testing for the diagnosis of primary aldosteronism. J Clin Endocrinol Metab. 2018;103:4113–4124. doi: 10.1210/jc.2018-01394. [DOI] [PubMed] [Google Scholar]
- 206.Kempers MJ, Lenders JW, van Outheusden L, et al. Systematic review: diagnostic procedures to differentiate unilateral from bilateral adrenal abnormality in primary aldosteronism. Ann Intern Med. 2009;151:329–337. doi: 10.7326/0003-4819-151-5-200909010-00007. [DOI] [PubMed] [Google Scholar]
- 207.Young WF, Stanson AW, Thompson GB, et al. Role for adrenal venous sampling in primary aldosteronism. Surgery. 2004;136:1227–1235. doi: 10.1016/j.surg.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 208.Küpers EM, Amar L, Raynaud A, et al. A clinical prediction score to diagnose unilateral primary aldosteronism. J Clin Endocrinol Metab. 2012;97:3530–3537. doi: 10.1210/jc.2012-1917. [DOI] [PubMed] [Google Scholar]
- 209.Citton M, Viel G, Rossi GP, et al. Outcome of surgical treatment of primary aldosteronism. Langenbecks Arch Surg. 2015;400:325–331. doi: 10.1007/s00423-014-1269-4. [DOI] [PubMed] [Google Scholar]
- 210.Pan CT, Liao CW, Tsai CH, et al. Influence of different treatment strategies on new-onset atrial fibrillation among patients with primary aldosteronism: a nationwide longitudinal cohort-based study. J Am Heart Assoc. 2020;9:e013699. doi: 10.1161/JAHA.119.013699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Reincke M, Fischer E, Gerum S, et al. Observational study mortality in treated primary aldosteronism: the German Conn’s registry. Hypertension. 2012;60:618–624. doi: 10.1161/HYPERTENSIONAHA.112.197111. [DOI] [PubMed] [Google Scholar]
- 212.Rossi GP, Cesari M, Cuspidi C, et al. Long-term control of arterial hypertension and regression of left ventricular hypertrophy with treatment of primary aldosteronism. Hypertension. 2013;62:62–69. doi: 10.1161/HYPERTENSIONAHA.113.01316. [DOI] [PubMed] [Google Scholar]
- 213.Williams TA, Lenders JWM, Mulatero P, et al. Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol. 2017;5:689–699. doi: 10.1016/S2213-8587(17)30135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Chen YY, Lin YH, Huang WC, et al. Adrenalectomy improves the long-term risk of end-stage renal disease and mortality of primary aldosteronism. J Endocr Soc. 2019;3:1110–1126. doi: 10.1210/js.2019-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Chang YH, Chung SD, Wu CH, et al. Surgery decreases the long-term incident stroke risk in patients with primary aldosteronism. Surgery. 2020;167:367–377. doi: 10.1016/j.surg.2019.08.017. [DOI] [PubMed] [Google Scholar]
- 216.Huang WC, Chen YY, Lin YH, et al. Incidental congestive heart failure in patients with aldosterone-producing adenomas. J Am Heart Assoc. 2019;8:e012410. doi: 10.1161/JAHA.119.012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Liao CW, Lin LY, Hung CS, et al. Time course and factors predicting arterial stiffness reversal in patients with aldosterone-producing adenoma after adrenalectomy: prospective study of 102 patients. Sci Rep. 2016;6:20862. doi: 10.1038/srep20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Greenblatt DJ, Koch-Weser J. Gynecomastia and impotence: complications of spironolactone therapy. JAMA. 1973;223:82. [PubMed] [Google Scholar]
- 219.Bramlage P, Swift SL, Thoenes M, et al. Non-steroidal mineralo-corticoid receptor antagonism for the treatment of cardiovascular and renal disease. Eur J Heart Fail. 2016;18:28–37. doi: 10.1002/ejhf.444. [DOI] [PubMed] [Google Scholar]
- 220.Weinberger MH, Roniker B, Krause SL, et al. Eplerenone, a selective aldosterone blocker, in mild-to-moderate hypertension. Am J Hypertens. 2002;15:709–716. doi: 10.1016/s0895-7061(02)02957-6. [DOI] [PubMed] [Google Scholar]
- 221.Bakris GL, Agarwal R, Anker SD, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383:2219–2229. doi: 10.1056/NEJMoa2025845. [DOI] [PubMed] [Google Scholar]
- 222.Cooper CJ, Murphy TP, Cutlip DE, et al. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med. 2014;370:13–22. doi: 10.1056/NEJMoa1310753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 224.Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 225.Marshall NS, Wong KK, Liu PY, et al. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008;31:1079–1085. [PMC free article] [PubMed] [Google Scholar]
- 226.Parati G, Lombardi C, Hedner J, et al. Position paper on the management of patients with obstructive sleep apnea and hypertension: joint recommendations by the European Society of Hypertension, by the European Respiratory Society and by the members of European COST (COoperation in Scientific and Technological research) ACTION B26 on obstructive sleep apnea. J Hypertens. 2012;30:633–646. doi: 10.1097/HJH.0b013e328350e53b. [DOI] [PubMed] [Google Scholar]
- 227.Pedrosa RP, Drager LF, Gonzaga CC, et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension. 2011;58:811–817. doi: 10.1161/HYPERTENSIONAHA.111.179788. [DOI] [PubMed] [Google Scholar]
- 228.Muxfeldt ES, Margallo VS, Guimarães GM, et al. Prevalence and associated factors of obstructive sleep apnea in patients with resistant hypertension. Am J Hypertens. 2014;27:1069–1078. doi: 10.1093/ajh/hpu023. [DOI] [PubMed] [Google Scholar]
- 229.Cheng KH, Wu YW, Hou CJ, et al. An overview of cardio-oncology, a new frontier to be explored. Acta Cardiol Sin. 2021;37:457–463. doi: 10.6515/ACS.202109_37(5).20210706A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Sipahi I, Tuzcu EM, Schoenhagen P, et al. Effects of normal, pre-hypertensive, and hypertensive blood pressure levels on progression of coronary atherosclerosis. J Am Coll Cardiol. 2006;48:833–838. doi: 10.1016/j.jacc.2006.05.045. [DOI] [PubMed] [Google Scholar]
- 231.Ishii H, Kobayashi M, Kurebayashi N, et al. Impact of angiotensin II receptor blocker therapy (olmesartan or valsartan) on coronary atherosclerotic plaque volume measured by intravascular ultrasound in patients with stable angina pectoris. Am J Cardiol. 2013;112:363–368. doi: 10.1016/j.amjcard.2013.03.038. [DOI] [PubMed] [Google Scholar]
- 232.Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957–967. doi: 10.1016/S0140-6736(15)01225-8. [DOI] [PubMed] [Google Scholar]
- 233.Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension. 1. Overview, meta-analyses, and meta-regression analyses of randomized trials. J Hypertens. 2014;32:2285–2295. doi: 10.1097/HJH.0000000000000378. [DOI] [PubMed] [Google Scholar]
- 234.Lonn EM, Bosch J, López-Jaramillo P, et al. Blood-pressure lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med. 2016;374:2009–2020. doi: 10.1056/NEJMoa1600175. [DOI] [PubMed] [Google Scholar]
- 235.Brunström M, Carlberg B. Association of blood pressure lowering with mortality and cardiovascular disease across blood pressure levels: a systematic review and meta-analysis. JAMA Intern Med. 2018;178:28–36. doi: 10.1001/jamainternmed.2017.6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2019;140:e596–e646. doi: 10.1161/CIR.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Qi Y, Han X, Zhao D, et al. Long-term cardiovascular risk associated with stage 1 hypertension defined by the 2017 ACC/AHA hypertension guideline. J Am Coll Cardiol. 2018;72:1201–1210. doi: 10.1016/j.jacc.2018.06.056. [DOI] [PubMed] [Google Scholar]
- 238.Walker KA, Sharrett AR, Wu A, et al. Association of midlife to late-life blood pressure patterns with incident dementia. JAMA. 2019;322:535–545. doi: 10.1001/jama.2019.10575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. 2020;75:285–292. doi: 10.1161/HYPERTENSIONAHA.119.14240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Wang CC, Wu CK, Tsai ML, et al. 2019 focused update of the guidelines of the Taiwan Society of Cardiology for the diagnosis and treatment of heart failure. Acta Cardiol Sin. 2019;35:244–283. doi: 10.6515/ACS.201905_35(3).20190422A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 242.Pan HY, Lin HJ, Chen WJ, et al. Increased mortality with intensive control in patients with higher baseline SBP and lower Framingham risk. J Hypertens. 2022 doi: 10.1097/HJH.0000000000003100. [DOI] [PubMed] [Google Scholar]
- 243.Polese A, De Cesare N, Montorsi P, et al. Upward shift of the lower range of coronary flow autoregulation in hypertensive patients with hypertrophy of the left ventricle. Circulation. 1991;83:845–853. doi: 10.1161/01.cir.83.3.845. [DOI] [PubMed] [Google Scholar]
- 244.Böhm M, Schumacher H, Teo KK, et al. Achieved blood pressure and cardiovascular outcomes in high-risk patients: results from ONTARGET and TRANSCEND trials. Lancet. 2017;389:2226–2237. doi: 10.1016/S0140-6736(17)30754-7. [DOI] [PubMed] [Google Scholar]
- 245.Liu L, Zhang Y, Liu G, et al. The Felodipine Event Reduction (FEVER) Study: a randomized long-term placebo-controlled trial in Chinese hypertensive patients. J Hypertens. 2005;23:2157–2172. doi: 10.1097/01.hjh.0000194120.42722.ac. [DOI] [PubMed] [Google Scholar]
- 246.Medical Research Council Working Party. MRC trial of treatment of mild hypertension: principal results. Br Med J (Clin Res Ed) 1985;291:97–104. doi: 10.1136/bmj.291.6488.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Harada A, Ueshima H, Kinoshita Y, et al. Absolute risk score for stroke, myocardial infarction, and all cardiovascular disease: Japan Arteriosclerosis Longitudinal Study. Hypertens Res. 2019;42:567–579. doi: 10.1038/s41440-019-0220-z. [DOI] [PubMed] [Google Scholar]
- 248.Arima H, Yonemoto K, Doi Y, et al. Development and validation of a cardiovascular risk prediction model for Japanese: the Hisayama study. Hypertens Res. 2009;32:1119–1122. doi: 10.1038/hr.2009.161. [DOI] [PubMed] [Google Scholar]
- 249.Goff DC, Jr., Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation. 2014;129:S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 250.Conroy RM, Pyörälä K, Fitzgerald AP, et al. Estimation of ten--year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003. doi: 10.1016/s0195-668x(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 251.Rabkin SW, Waheed A, Poulter RS, et al. Myocardial perfusion pressure in patients with hypertension and coronary artery disease: implications for DBP targets in hypertension management. J Hypertens. 2013;31:975–982. doi: 10.1097/HJH.0b013e32835e831c. [DOI] [PubMed] [Google Scholar]
- 252.Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 253.Flint AC, Conell C, Ren X, et al. Effect of systolic and diastolic blood pressure on cardiovascular outcomes. N Engl J Med. 2019;381:243–251. doi: 10.1056/NEJMoa1803180. [DOI] [PubMed] [Google Scholar]
- 254.Yi SW, Mok Y, Ohrr H, et al. Low systolic blood pressure and vascular mortality among more than 1 million Korean adults. Circulation. 2016;133:2381–2390. doi: 10.1161/CIRCULATIONAHA.115.020752. [DOI] [PubMed] [Google Scholar]
- 255.SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP) JAMA. 1991;265:3255–3264. [PubMed] [Google Scholar]
- 256.Staessen JA, Fagard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet. 1997;350:757–764. doi: 10.1016/s0140-6736(97)05381-6. [DOI] [PubMed] [Google Scholar]
- 257.Liu L, Wang JG, Gong L, et al. Comparison of active treatment and placebo in older Chinese patients with isolated systolic hypertension. Systolic Hypertension in China (Syst-China) Collaborative Group. J Hypertens. 1998;16:1823–1829. doi: 10.1097/00004872-199816120-00016. [DOI] [PubMed] [Google Scholar]
- 258.Wright JT, Jr., Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Cooper-DeHoff RM, Gong Y, Handberg EM, et al. Tight blood pressure control and cardiovascular outcomes among hypertensive patients with diabetes and coronary artery disease. JAMA. 2010;304:61–68. doi: 10.1001/jama.2010.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Messerli FH, Mancia G, Conti CR, et al. Dogma disputed: can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous. Ann Intern Med. 2006;144:884–893. doi: 10.7326/0003-4819-144-12-200606200-00005. [DOI] [PubMed] [Google Scholar]
- 261.Sleight P, Redon J, Verdecchia P, et al. Prognostic value of blood pressure in patients with high vascular risk in the Ongoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial study. J Hypertens. 2009;27:1360–1369. doi: 10.1097/HJH.0b013e32832d7370. [DOI] [PubMed] [Google Scholar]
- 262.Bangalore S, Messerli FH, Wun CC, et al. J-curve revisited: an analysis of blood pressure and cardiovascular events in the Treating to New Targets (TNT) Trial. Eur Heart J. 2010;31:2897–2908. doi: 10.1093/eurheartj/ehq328. [DOI] [PubMed] [Google Scholar]
- 263.Redon J, Mancia G, Sleight P, et al. Safety and efficacy of low blood pressures among patients with diabetes: subgroup analyses from the ONTARGET (ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial). J Am Coll Cardiol. 2012;59:74–83. doi: 10.1016/j.jacc.2011.09.040. [DOI] [PubMed] [Google Scholar]
- 264.Verdecchia P, Angeli F, Mazzotta G, et al. Aggressive blood pressure lowering is dangerous: the J-curve:con side of the arguement. Hypertension. 2014;63:37–40. doi: 10.1161/01.hyp.0000439102.43479.43. [DOI] [PubMed] [Google Scholar]
- 265.Vidal-Petiot E, Ford I, Greenlaw N, et al. Cardiovascular event rates and mortality according to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: an international cohort study. Lancet. 2016;388:2142–2152. doi: 10.1016/S0140-6736(16)31326-5. [DOI] [PubMed] [Google Scholar]
- 266.Peri-Okonny PA, Patel KK, Jones PG, et al. Low diastolic blood pressure is associated with angina in patients with chronic coronary artery disease. J Am Coll Cardiol. 2018;72:1227–1232. doi: 10.1016/j.jacc.2018.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37:2315–2381. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Kokubo Y. Prevention of hypertension and cardiovascular diseases: a comparison of lifestyle factors in Westerners and East Asians. Hypertension. 2014;63:655–660. doi: 10.1161/HYPERTENSIONAHA.113.00543. [DOI] [PubMed] [Google Scholar]
- 269.Elmer PJ, Obarzanek E, Vollmer WM, et al. Effects of comprehensive lifestyle modification on diet, weight, physical fitness, and blood pressure control: 18-month results of a randomized trial. Ann Intern Med. 2006;144:485–495. doi: 10.7326/0003-4819-144-7-200604040-00007. [DOI] [PubMed] [Google Scholar]
- 270.Whelton PK, Appel LJ, Espeland MA, et al. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of nonpharmacologic interventions in the elderly (TONE). TONE Collaborative Research Group. JAMA. 1998;279:839–846. doi: 10.1001/jama.279.11.839. [DOI] [PubMed] [Google Scholar]
- 271.Troost JP, Rafferty AP, Luo Z, et al. Temporal and regional trends in the prevalence of healthy lifestyle characteristics: United States, 1994-2007. Am J Public Health. 2012;102:1392–1398. doi: 10.2105/AJPH.2011.300326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Finger JD, Busch MA, Heidemann C, et al. Time trends in healthy lifestyle among adults in Germany: results from three national health interview and examination surveys between 1990 and 2011. PLoS One. 2019;14:e0222218. doi: 10.1371/journal.pone.0222218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.He FJ, MacGregor GA. Effect of modest salt reduction on blood pressure: a meta-analysis of randomized trials. Implications for public health. J Hum Hypertens. 2002;16:761–770. doi: 10.1038/sj.jhh.1001459. [DOI] [PubMed] [Google Scholar]
- 274.Mente A, O'Donnell MJ, Rangarajan S, et al. Association of urinary sodium and potassium excretion with blood pressure. N Engl J Med. 2014;371:601–611. doi: 10.1056/NEJMoa1311989. [DOI] [PubMed] [Google Scholar]
- 275.Suckling RJ, He FJ, Markandu ND, et al. Modest salt reduction lowers blood pressure and albumin excretion in impaired glucose tolerance and type 2 diabetes mellitus: a randomized double-blind trial. Hypertension. 2016;67:1189–1195. doi: 10.1161/HYPERTENSIONAHA.115.06637. [DOI] [PubMed] [Google Scholar]
- 276.O'Donnell M, Mente A, Rangarajan S, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med. 2014;371:612–623. doi: 10.1056/NEJMoa1311889. [DOI] [PubMed] [Google Scholar]
- 277.Mozaffarian D, Fahimi S, Singh GM, et al. Global sodium consumption and death from cardiovascular causes. N Engl J Med. 2014;371:624–634. doi: 10.1056/NEJMoa1304127. [DOI] [PubMed] [Google Scholar]
- 278.Cook NR, Cutler JA, Obarzanek E, et al. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP). BMJ. 2007;334:885–888. doi: 10.1136/bmj.39147.604896.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Aburto NJ, Ziolkovska A, Hooper L, et al. Effect of lower sodium intake on health: systematic review and meta-analyses. BMJ. 2013;346:f1326. doi: 10.1136/bmj.f1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Mente A, O'Donnell M, Rangarajan S, et al. Associations of urinary sodium excretion with cardiovascular events in individuals with and without hypertension: a pooled analysis of data from four studies. Lancet. 2016;388:465–475. doi: 10.1016/S0140-6736(16)30467-6. [DOI] [PubMed] [Google Scholar]
- 281.Chien KL, Hsu HC, Chen PC, et al. Urinary sodium and potassium excretion and risk of hypertension in Chinese: report from a community-based cohort study in Taiwan. J Hypertens. 2008;26:1750–1756. doi: 10.1097/HJH.0b013e328306a0a7. [DOI] [PubMed] [Google Scholar]
- 282.Binia A, Jaeger J, Hu Y, et al. Daily potassium intake and sodium-to-potassium ratio in the reduction of blood pressure: a meta-analysis of randomized controlled trials. J Hypertens. 2015;33:1509–1520. doi: 10.1097/HJH.0000000000000611. [DOI] [PubMed] [Google Scholar]
- 283.Neal B, Wu Y, Feng X, et al. Effect of salt substitution on cardiovascular events and death. N Engl J Med. 2021;385:1067–1077. doi: 10.1056/NEJMoa2105675. [DOI] [PubMed] [Google Scholar]
- 284.Services USDoAaUSDoHaH Nutrition and Health Across the Lifespan. The Guidelines and Key Recommendations. 9th ed:49. In: Dietary Guideline for Americans, 2020-2025; 2020. [Google Scholar]
- 285.Ronksley PE, Brien SE, Turner BJ, et al. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. 2011;342:d671. doi: 10.1136/bmj.d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Holmes MV, Dale CE, Zuccolo L, et al. Association between alcohol and cardiovascular disease: Mendelian randomisation analysis based on individual participant data. BMJ. 2014;349:g4164. doi: 10.1136/bmj.g4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Wood AM, Kaptoge S, Butterworth AS, et al. Risk thresholds for alcohol consumption: combined analysis of individual-participant data for 599,912 current drinkers in 83 prospective studies. Lancet. 2018;391:1513–1523. doi: 10.1016/S0140-6736(18)30134-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Li H, Borinskaya S, Yoshimura K, et al. Refined geographic distribution of the oriental ALDH2*504Lys (nee 487Lys) variant. Ann Hum Genet. 2009;73:335–345. doi: 10.1111/j.1469-1809.2009.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Brooks PJ, Enoch MA, Goldman D, et al. The alcohol flushing response: an unrecognized risk factor for esophageal cancer from alcohol consumption. PLoS Med. 2009;6:e50. doi: 10.1371/journal.pmed.1000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Millwood IY, Walters RG, Mei XW, et al. Conventional and genetic evidence on alcohol and vascular disease aetiology: a prospective study of 500,000 men and women in China. Lancet. 2019;393:1831–1842. doi: 10.1016/S0140-6736(18)31772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Health Promotion Administration. Welfare MoHa Dietary guidelines. 2018. [Google Scholar]
- 292.Britton A, McKee M. The relation between alcohol and cardiovascular disease in Eastern Europe: explaining the paradox. J Epidemiol Community Health. 2000;54:328–332. doi: 10.1136/jech.54.5.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Lee S, Kim JS, Jung JG, et al. Korean alcohol guidelines for moderate drinking based on facial flushing. Korean J Fam Med. 2019;40:204–211. doi: 10.4082/kjfm.19.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Berrington de Gonzalez A, Hartge P, Cerhan JR, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363:2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Chen Z, Yang G, Offer A, et al. Body mass index and mortality in China: a 15-year prospective study of 220 000 men. Int J Epidemiol. 2012;41:472–481. doi: 10.1093/ije/dyr208. [DOI] [PubMed] [Google Scholar]
- 296.Neter JE, Stam BE, Kok FJ, et al. Influence of weight reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension. 2003;42:878–884. doi: 10.1161/01.HYP.0000094221.86888.AE. [DOI] [PubMed] [Google Scholar]
- 297.Oreopoulos A, Padwal R, Norris CM, et al. Effect of obesity on short- and long-term mortality postcoronary revascularization: a meta-analysis. Obesity (Silver Spring) 2008;16:442–450. doi: 10.1038/oby.2007.36. [DOI] [PubMed] [Google Scholar]
- 298.Jordan J, Yumuk V, Schlaich M, et al. Joint statement of the European Association for the Study of Obesity and the European Society of Hypertension: obesity and difficult to treat arterial hypertension. J Hypertens. 2012;30:1047–1055. doi: 10.1097/HJH.0b013e3283537347. [DOI] [PubMed] [Google Scholar]
- 299.Primatesta P, Falaschetti E, Gupta S, et al. Association between smoking and blood pressure: evidence from the health survey for England. Hypertension. 2001;37:187–193. doi: 10.1161/01.hyp.37.2.187. [DOI] [PubMed] [Google Scholar]
- 300.Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Kiiskinen U, Vartiainen E, Puska P, et al. Long-term cost and life-expectancy consequences of hypertension. J Hypertens. 1998;16:1103–1112. doi: 10.1097/00004872-199816080-00004. [DOI] [PubMed] [Google Scholar]
- 302.Prescott E, Hippe M, Schnohr P, et al. Smoking and risk of myocardial infarction in women and men: longitudinal population study. Bmj. 1998;316:1043–1047. doi: 10.1136/bmj.316.7137.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Lee TK, Huang ZS, Ng SK, et al. Impact of alcohol consumption and cigarette smoking on stroke among the elderly in Taiwan. Stroke. 1995;26:790–794. doi: 10.1161/01.str.26.5.790. [DOI] [PubMed] [Google Scholar]
- 304.Hsieh FI, Chiou HY. Stroke: morbidity, risk factors, and care in Taiwan. J Stroke. 2014;16:59–64. doi: 10.5853/jos.2014.16.2.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Beard E, West R, Michie S, et al. Association between electronic cigarette use and changes in quit attempts, success of quit attempts, use of smoking cessation pharmacotherapy, and use of stop smoking services in England: time series analysis of population trends. BMJ. 2016;354:i4645. doi: 10.1136/bmj.i4645. [DOI] [PubMed] [Google Scholar]
- 306.Kalkhoran S, Glantz SA. E-cigarettes and smoking cessation in real-world and clinical settings: a systematic review and meta-analysis. Lancet Respir Med. 2016;4:116–128. doi: 10.1016/S2213-2600(15)00521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Hartmann-Boyce J, McRobbie H, Lindson N, et al. Electronic cigarettes for smoking cessation. Cochrane Database Syst Rev. 2021;4:Cd010216. doi: 10.1002/14651858.CD010216.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Teriba A, Mbama U, Sharma S, et al. Evidence against e-cigarettes for smoking cessation. J Am Pharm Assoc (2003) 2021;61:e55–e58. doi: 10.1016/j.japh.2021.05.001. [DOI] [PubMed] [Google Scholar]
- 309.Pierce JP, Benmarhnia T, Chen R, et al. Role of e-cigarettes and pharmacotherapy during attempts to quit cigarette smoking: The PATH Study 2013-16. PLoS One. 2020;15:e0237938. doi: 10.1371/journal.pone.0237938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Vlachopoulos C, Ioakeimidis N, Abdelrasoul M, et al. Electronic cigarette smoking increases aortic stiffness and blood pressure in young smokers. J Am Coll Cardiol. 2016;67:2802–2803. doi: 10.1016/j.jacc.2016.03.569. [DOI] [PubMed] [Google Scholar]
- 311.Martinez-Morata I, Sanchez TR, Shimbo D, et al. Electronic cigarette use and blood pressure endpoints: a systematic review. Curr Hypertens Rep. 2020;23:2. doi: 10.1007/s11906-020-01119-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 313.Fung TT, Chiuve SE, McCullough ML, et al. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168:713–720. doi: 10.1001/archinte.168.7.713. [DOI] [PubMed] [Google Scholar]
- 314.Struijk EA, May AM, Wezenbeek NL, et al. Adherence to dietary guidelines and cardiovascular disease risk in the EPIC-NL cohort. Int J Cardiol. 2014;176:354–359. doi: 10.1016/j.ijcard.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 315.Fu J, Liu Y, Zhang L, et al. Nonpharmacologic interventions for reducing blood pressure in adults with prehypertension to established hypertension. J Am Heart Assoc. 2020;9:e016804. doi: 10.1161/JAHA.120.016804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Greyling A, Ras RT, Zock PL, et al. The effect of black tea on blood pressure: a systematic review with meta-analysis of randomized controlled trials. PLoS One. 2014;9:e103247. doi: 10.1371/journal.pone.0103247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Li G, Zhang Y, Thabane L, et al. Effect of green tea supplementation on blood pressure among overweight and obese adults: a systematic review and meta-analysis. J Hypertens. 2015;33:243–254. doi: 10.1097/HJH.0000000000000426. [DOI] [PubMed] [Google Scholar]
- 318.Ding M, Bhupathiraju SN, Satija A, et al. Long-term coffee consumption and risk of cardiovascular disease: a systematic review and a dose-response meta-analysis of prospective cohort studies. Circulation. 2014;129:643–659. doi: 10.1161/CIRCULATIONAHA.113.005925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Rossi A, Dikareva A, Bacon SL, et al. The impact of physical activity on mortality in patients with high blood pressure: a systematic review. J Hypertens. 2012;30:1277–1288. doi: 10.1097/HJH.0b013e3283544669. [DOI] [PubMed] [Google Scholar]
- 320.Lee DC, Pate RR, Lavie CJ, et al. Leisure-time running reduces all-cause and cardiovascular mortality risk. J Am Coll Cardiol. 2014;64:472–481. doi: 10.1016/j.jacc.2014.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Cornelissen VA, Smart NA. Exercise training for blood pressure: a systematic review and meta-analysis. J Am Heart Assoc. 2013;2:e004473. doi: 10.1161/JAHA.112.004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Pelliccia A, Sharma S, Gati S, et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J. 2021;42:17–96. doi: 10.1093/eurheartj/ehaa735. [DOI] [PubMed] [Google Scholar]
- 323.Polito MD, Dias JR Jr, Papst RR. Resistance training to reduce resting blood pressure and increase muscle strength in users and non-users of anti-hypertensive medication: a meta-analysis. Clin Exp Hypertens. 2021;43:474–485. doi: 10.1080/10641963.2021.1901111. [DOI] [PubMed] [Google Scholar]
- 324.Wang CH, Yang HW, Huang HL, et al. Long-term effect of device-guided slow breathing on blood pressure regulation and chronic inflammation in patients with essential hypertension using a wearable ECG device. Acta Cardiol Sin. 2021;37:195–203. doi: 10.6515/ACS.202103_37(2).20200907A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Liang H, Luo S, Chen X, et al. Effects of Tai Chi exercise on cardiovascular disease risk factors and quality of life in adults with essential hypertension: a meta-analysis. Heart Lung. 2020;49:353–363. doi: 10.1016/j.hrtlng.2020.02.041. [DOI] [PubMed] [Google Scholar]
- 326.Zhong D, Li J, Yang H, et al. Tai Chi for essential hypertension: a systematic review of randomized controlled trials. Curr Hypertens Rep. 2020;22:25. doi: 10.1007/s11906-020-1031-y. [DOI] [PubMed] [Google Scholar]
- 327.Guan Y, Hao Y, Guan Y, et al. Effects of Tai Chi on essential hypertension and related risk factors: a meta-analysis of randomized controlled trials. J Rehabil Med. 2020;52:jrm00057. doi: 10.2340/16501977-2683. [DOI] [PubMed] [Google Scholar]
- 328.Park SH, Han KS. Blood pressure response to meditation and yoga: a systematic review and meta-analysis. J Altern Complement Med. 2017;23:685–695. doi: 10.1089/acm.2016.0234. [DOI] [PubMed] [Google Scholar]
- 329.Walzer D, Gordon T, Thorpe L, et al. Effects of home particulate air filtration on blood pressure: a systematic review. Hypertension. 2020;76:44–50. doi: 10.1161/HYPERTENSIONAHA.119.14456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Zanobetti A, Canner MJ, Stone PH, et al. Ambient pollution and blood pressure in cardiac rehabilitation patients. Circulation. 2004;110:2184–2189. doi: 10.1161/01.CIR.0000143831.33243.D8. [DOI] [PubMed] [Google Scholar]
- 331.Cohen AJ, Brauer M, Burnett R, et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet. 2017;389:1907–1918. doi: 10.1016/S0140-6736(17)30505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Guo C, Zeng Y, Chang LY, et al. Independent and opposing associations of habitual exercise and chronic PM(2.5) exposures on hypertension incidence. Circulation. 2020;142:645–656. doi: 10.1161/CIRCULATIONAHA.120.045915. [DOI] [PubMed] [Google Scholar]
- 333.Tainio M, de Nazelle AJ, Götschi T, et al. Can air pollution negate the health benefits of cycling and walking? Prev Med. 2016;87:233–236. doi: 10.1016/j.ypmed.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Welfare MoHa. The Recommendation for Exercise with Different Background Air Qualities. Health Promotion Administration: 2020. [Google Scholar]
- 335.Holtermann A, Schnohr P, Nordestgaard BG, et al. The physical activity paradox in cardiovascular disease and all-cause mortality: the contemporary Copenhagen General Population Study with 104,046 adults. Eur Heart J. 2021;42:1499–1511. doi: 10.1093/eurheartj/ehab087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Bobrie G, Chatellier G, Genes N, et al. Cardiovascular prognosis of "masked hypertension" detected by blood pressure self-measurement in elderly treated hypertensive patients. JAMA. 2004;291:1342–1349. doi: 10.1001/jama.291.11.1342. [DOI] [PubMed] [Google Scholar]
- 337.Okumiya K, Matsubayashi K, Wada T, et al. A U-shaped association between home systolic blood pressure and four-year mortality in community-dwelling older men. J Am Geriatr Soc. 1999;47:1415–1421. doi: 10.1111/j.1532-5415.1999.tb01559.x. [DOI] [PubMed] [Google Scholar]
- 338.Lasserson DS, Buclin T, Glasziou P. How quickly should we titrate antihypertensive medication? Systematic review modelling blood pressure response from trial data. Heart. 2011;97:1771–1775. doi: 10.1136/hrt.2010.221473. [DOI] [PubMed] [Google Scholar]
- 339.Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022–2031. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- 340.McManus RJ, Mant J, Franssen M, et al. Efficacy of self-monitored blood pressure, with or without telemonitoring, for titration of antihypertensive medication (TASMINH4): an unmasked randomised controlled trial. Lancet. 2018;391:949–959. doi: 10.1016/S0140-6736(18)30309-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Law MR, Wald NJ, Morris JK, et al. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003;326:1427. doi: 10.1136/bmj.326.7404.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Wang TD, Chen YH, Huang CH, et al. Bidirectional adherence changes and associated factors in patients switched from free combinations to equivalent single-pill combinations of antihypertensive drugs. Hypertension. 2014;63:958–967. doi: 10.1161/HYPERTENSIONAHA.113.02455. [DOI] [PubMed] [Google Scholar]
- 343.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Lindholm LH, Carlberg B, Samuelsson O. Should beta blockers remain first choice in the treatment of primary hypertension? A meta-analysis. Lancet. 2005;366:1545–1553. doi: 10.1016/S0140-6736(05)67573-3. [DOI] [PubMed] [Google Scholar]
- 345.Wiysonge CS, Bradley HA, Volmink J, et al. Beta-blockers for hypertension. Cochrane Database Syst Rev. 2017;1:Cd002003. doi: 10.1002/14651858.CD002003.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Chen YJ, Li LJ, Tang WL, et al. First-line drugs inhibiting the renin angiotensin system versus other first-line antihypertensive drug classes for hypertension. Cochrane Database Syst Rev. 2018;11:Cd008170. doi: 10.1002/14651858.CD008170.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Zhou HH, Koshakji RP, Silberstein DJ, et al. Racial differences in drug response. Altered sensitivity to and clearance of propranolol in men of Chinese descent as compared with American whites. N Engl J Med. 1989;320:565–570. doi: 10.1056/NEJM198903023200905. [DOI] [PubMed] [Google Scholar]
- 348.Wang KL, Fang CY, Lai WT, et al. Extended-release carvedilol in the treatment of hypertension: a double-blind, randomized, placebo-controlled trial. Acta Cardiol Sin. 2021;37:186–194. doi: 10.6515/ACS.202103_37(2).20200914B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Wald DS, Law M, Morris JK, et al. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am J Med. 2009;122:290–300. doi: 10.1016/j.amjmed.2008.09.038. [DOI] [PubMed] [Google Scholar]
- 350.Brown MJ, McInnes GT, Papst CC, et al. Aliskiren and the calcium channel blocker amlodipine combination as an initial treatment strategy for hypertension control (ACCELERATE): a randomised,parallel-group trial. Lancet. 2011;377:312–320. doi: 10.1016/S0140-6736(10)62003-X. [DOI] [PubMed] [Google Scholar]
- 351.Corrao G, Nicotra F, Parodi A, et al. Cardiovascular protection by initial and subsequent combination of antihypertensive drugs in daily life practice. Hypertension. 2011;58:566–572. doi: 10.1161/HYPERTENSIONAHA.111.177592. [DOI] [PubMed] [Google Scholar]
- 352.Corrao G, Parodi A, Zambon A, et al. Reduced discontinuation of antihypertensive treatment by two-drug combination as first step. Evidence from daily life practice. J Hypertens. 2010;28:1584–1590. doi: 10.1097/HJH.0b013e328339f9fa. [DOI] [PubMed] [Google Scholar]
- 353.Dahlöf B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 354.Dahlöf B, Sever PS, Poulter NR, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required,in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366:895–906. doi: 10.1016/S0140-6736(05)67185-1. [DOI] [PubMed] [Google Scholar]
- 355.Jamerson K, Weber MA, Bakris GL, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359:2417–2428. doi: 10.1056/NEJMoa0806182. [DOI] [PubMed] [Google Scholar]
- 356.Mann JF, Schmieder RE, McQueen M, et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372:547–553. doi: 10.1016/S0140-6736(08)61236-2. [DOI] [PubMed] [Google Scholar]
- 357.Parving HH, Brenner BM, McMurray JJ, et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med. 2012;367:2204–2213. doi: 10.1056/NEJMoa1208799. [DOI] [PubMed] [Google Scholar]
- 358.Wang TD, Tan RS, Lee HY, et al. Effects of sacubitril/valsartan (LCZ696) on natriuresis, diuresis, blood pressures, and NT-proBNP in salt-sensitive hypertension. Hypertension. 2017;69:32–41. doi: 10.1161/HYPERTENSIONAHA.116.08484. [DOI] [PubMed] [Google Scholar]
- 359.Tsai MS, Tang CH, Lin CY, et al. Diuretic or beta-blocker for hypertensive patients already receiving ACEI/ARB and calcium channel blocker. Cardiovasc Drugs Ther. 2017;31:535–543. doi: 10.1007/s10557-017-6765-7. [DOI] [PubMed] [Google Scholar]
- 360.Williams B, MacDonald TM, Morant S, et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. 2015;386:2059–2068. doi: 10.1016/S0140-6736(15)00257-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Zhao D, Liu H, Dong P, et al. A meta-analysis of add-on use of spironolactone in patients with resistant hypertension. Int J Cardiol. 2017;233:113–117. doi: 10.1016/j.ijcard.2016.12.158. [DOI] [PubMed] [Google Scholar]
- 362.Karns AD, Bral JM, Hartman D, et al. Study of aldosterone syn-thase inhibition as an add-on therapy in resistant hypertension. J Clin Hypertens (Greenwich) 2013;15:186–192. doi: 10.1111/jch.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Gupta AK, Arshad S, Poulter NR. Compliance, safety, and effectiveness of fixed-dose combinations of antihypertensive agents: a meta-analysis. Hypertension. 2010;55:399–407. doi: 10.1161/HYPERTENSIONAHA.109.139816. [DOI] [PubMed] [Google Scholar]
- 364.Bangalore S, Kamalakkannan G, Parkar S, et al. Fixed-dose combinations improve medication compliance: a meta-analysis. Am J Med. 2007;120:713–719. doi: 10.1016/j.amjmed.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 365.Mallat SG, Tanios BY, Itani HS, et al. Free versus fixed combination antihypertensive therapy for essential arterial hypertension: a systematic review and meta-analysis. PLoS One. 2016; 11:e0161285. doi: 10.1371/journal.pone.0161285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Feldman RD, Zou GY, Vandervoort MK, et al. A simplified approach to the treatment of uncomplicated hypertension: a cluster randomized, controlled trial. Hypertension. 2009;53:646–653. doi: 10.1161/HYPERTENSIONAHA.108.123455. [DOI] [PubMed] [Google Scholar]
- 367.Jaffe MG, Lee GA, Young JD, et al. Improved blood pressure control associated with a large-scale hypertension program. JAMA. 2013;310:699–705. doi: 10.1001/jama.2013.108769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Rea F, Corrao G, Merlino L, et al. Early cardiovascular protection by initial two-drug fixed-dose combination treatment vs. monotherapy in hypertension. Eur Heart J. 2018;39:3654–3661. doi: 10.1093/eurheartj/ehy420. [DOI] [PubMed] [Google Scholar]
- 369.Shao YY, Lin HJ. Correspondence to:management of venous thromboembolisms: part II. the consensus for pulmonary embolism and updates. Acta Cardiol Sin. 2021;37:213–214. doi: 10.6515/ACS.202103_37(2).20210118B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Sega R, Cesana G, Bombelli M, et al. Seasonal variations in home and ambulatory blood pressure in the PAMELA population. Pressione Arteriose Monitorate E Loro Associazioni. J Hypertens. 1998;16:1585–1592. doi: 10.1097/00004872-199816110-00004. [DOI] [PubMed] [Google Scholar]
- 371.Sugiyama T, Kiraku J, Ashida T, et al. Remission of hypertension: retrospective observations over a period of 20 years. Hypertens Res. 1998;21:103–108. doi: 10.1291/hypres.21.103. [DOI] [PubMed] [Google Scholar]
- 372.Li EC, Heran BS, Wright JM. Angiotensin converting enzyme (ACE) inhibitors versus angiotensin receptor blockers for primary hypertension. Cochrane Database Syst Rev. 2014;2014:Cd009096. doi: 10.1002/14651858.CD009096.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Cheng J, Zhang W, Zhang X, et al. Effect of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on all-cause mortality, cardiovascular deaths, and cardiovascular events in patients with diabetes mellitus: a meta-analysis. JAMA Intern Med. 2014;174:773–785. doi: 10.1001/jamainternmed.2014.348. [DOI] [PubMed] [Google Scholar]
- 374.Xie X, Liu Y, Perkovic V, et al. Renin-angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD: a Bayesian network meta-analysis of randomized clinical trials. Am J Kidney Dis. 2016;67:728–741. doi: 10.1053/j.ajkd.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 375.Palmer SC, Mavridis D, Navarese E, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet. 2015;385:2047–2056. doi: 10.1016/S0140-6736(14)62459-4. [DOI] [PubMed] [Google Scholar]
- 376.McDowell SE, Coleman JJ, Ferner RE. Systematic review and meta-analysis of ethnic differences in risks of adverse reactions to drugs used in cardiovascular medicine. BMJ. 2006;332:1177–1181. doi: 10.1136/bmj.38803.528113.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Caldeira D, Alarcão J, Vaz-Carneiro A, et al. Risk of pneumonia associated with use of angiotensin converting enzyme inhibitors and angiotensin receptor blockers: systematic review and meta-analysis. BMJ. 2012;345:e4260. doi: 10.1136/bmj.e4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Yu LT, Zhu J, Tan HQ, et al. Telmisartan, ramipril, or both in high-risk Chinese patients: analysis of ONTARGET China data. Chin Med J (Engl) 2011;124:1763–1768. [PubMed] [Google Scholar]
- 379.Brown NJ, Byiers S, Carr D, et al. Dipeptidyl peptidase-IV inhibitor use associated with increased risk of ACE inhibitor-associated angioedema. Hypertension. 2009;54:516–523. doi: 10.1161/HYPERTENSIONAHA.109.134197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Te Riet L, van Esch JH, Roks AJ, et al. Hypertension: renin-angiotensin-aldosterone system alterations. Circ Res. 2015;116:960–975. doi: 10.1161/CIRCRESAHA.116.303587. [DOI] [PubMed] [Google Scholar]
- 381.Sungnak W, Huang N, Bécavin C, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Cohen JB, Hanff TC, William P, et al. Continuation versus discontinuation of renin-angiotensin system inhibitors in patients admitted to hospital with COVID-19: a prospective, randomised, open-label trial. Lancet Respir Med. 2021;9:275–284. doi: 10.1016/S2213-2600(20)30558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Lopes RD, Macedo AVS, de Barros ESPGM, et al. Effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID-19: a randomized clinical trial. JAMA. 2021;325:254–264. doi: 10.1001/jama.2020.25864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Parving HH, Persson F, Lewis JB, et al. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med. 2008;358:2433–2446. doi: 10.1056/NEJMoa0708379. [DOI] [PubMed] [Google Scholar]
- 385.Solomon SD, Appelbaum E, Manning WJ, et al. Effect of the direct renin inhibitor aliskiren, the angiotensin receptor blocker losartan, or both on left ventricular mass in patients with hypertension and left ventricular hypertrophy. Circulation. 2009;119:530–537. doi: 10.1161/CIRCULATIONAHA.108.826214. [DOI] [PubMed] [Google Scholar]
- 386.Gheorghiade M, Böhm M, Greene SJ, et al. Effect of aliskiren on postdischarge mortality and heart failure readmissions among patients hospitalized for heart failure: the ASTRONAUT randomized trial. JAMA. 2013;309:1125–1135. doi: 10.1001/jama.2013.1954. [DOI] [PubMed] [Google Scholar]
- 387.Teo KK, Pfeffer M, Mancia G, et al. Aliskiren alone or with other antihypertensives in the elderly with borderline and stage 1 hypertension: the APOLLO trial. Eur Heart J. 2014;35:1743–1751. doi: 10.1093/eurheartj/ehu079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Kuyper LM, Khan NA. Atenolol vs nonatenolol β-blockers for the treatment of hypertension: a meta-analysis. Can J Cardiol. 2014;30:S47–S53. doi: 10.1016/j.cjca.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 389.Boutouyrie P, Achouba A, Trunet P, et al. Amlodipine-valsartan combination decreases central systolic blood pressure more effectively than the amlodipine-atenolol combination: the EXPLOR study. Hypertension. 2010;55:1314–1322. doi: 10.1161/HYPERTENSIONAHA.109.148999. [DOI] [PubMed] [Google Scholar]
- 390.Flather MD, Shibata MC, Coats AJ, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J. 2005;26:215–225. doi: 10.1093/eurheartj/ehi115. [DOI] [PubMed] [Google Scholar]
- 391.Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334:1349–1355. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- 392.Poole-Wilson PA, Swedberg K, Cleland JG, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362:7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- 393.Stefan MS, Rothberg MB, Priya A, et al. Association between β-blocker therapy and outcomes in patients hospitalised with acute exacerbations of chronic obstructive lung disease with underlying ischaemic heart disease, heart failure or hypertension. Thorax. 2012;67:977–984. doi: 10.1136/thoraxjnl-2012-201945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Mentz RJ, Wojdyla D, Fiuzat M, et al. Association of beta-blocker use and selectivity with outcomes in patients with heart failure and chronic obstructive pulmonary disease (from OPTIMIZE-HF). Am J Cardiol. 2013;111:582–587. doi: 10.1016/j.amjcard.2012.10.041. [DOI] [PubMed] [Google Scholar]
- 395.Bangalore S, Parkar S, Grossman E, et al. A meta-analysis of 94,492 patients with hypertension treated with beta blockers to determine the risk of new-onset diabetes mellitus. Am J Cardiol. 2007;100:1254–1262. doi: 10.1016/j.amjcard.2007.05.057. [DOI] [PubMed] [Google Scholar]
- 396.Karachalios GN, Charalabopoulos A, Papalimneou V, et al. Withdrawal syndrome following cessation of antihypertensive drug therapy. Int J Clin Pract. 2005;59:562–570. doi: 10.1111/j.1368-5031.2005.00520.x. [DOI] [PubMed] [Google Scholar]
- 397.Furberg CD, Psaty BM, Meyer JV. Nifedipine. Dose-related increase in mortality in patients with coronary heart disease. Circulation. 1995;92:1326–1331. doi: 10.1161/01.cir.92.5.1326. [DOI] [PubMed] [Google Scholar]
- 398.Conlin PR, Williams GH. Use of calcium channel blockers in hypertension. Adv Intern Med. 1998;43:533–562. [PubMed] [Google Scholar]
- 399.Brown MJ, Palmer CR, Castaigne A, et al. Morbidity and mortality in patients randomised to double-blind treatment with a long-acting calcium-channel blocker or diuretic in the International Nifedipine GITS study: Intervention as a Goal in Hypertension Treatment (INSIGHT). Lancet. 2000;356:366–372. doi: 10.1016/S0140-6736(00)02527-7. [DOI] [PubMed] [Google Scholar]
- 400.Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755–1762. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- 401.Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 402.Nissen SE, Tuzcu EM, Libby P, et al. Effect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure: the CAMELOT study: a randomized controlled trial. JAMA. 2004;292:2217–2225. doi: 10.1001/jama.292.18.2217. [DOI] [PubMed] [Google Scholar]
- 403.Wang JG, Kario K, Lau T, et al. Use of dihydropyridine calcium channel blockers in the management of hypertension in Eastern Asians: a scientific statement from the Asian Pacific Heart Association. Hypertens Res. 2011;34:423–430. doi: 10.1038/hr.2010.259. [DOI] [PubMed] [Google Scholar]
- 404.Pepine CJ, Handberg EM, Cooper-DeHoff RM, et al. A calcium antagonist vs a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil-Trandolapril Study (INVEST): a randomized controlled trial. JAMA. 2003;290:2805–2816. doi: 10.1001/jama.290.21.2805. [DOI] [PubMed] [Google Scholar]
- 405.Black HR, Elliott WJ, Grandits G, et al. Principal results of the Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE) trial. JAMA. 2003;289:2073–2082. doi: 10.1001/jama.289.16.2073. [DOI] [PubMed] [Google Scholar]
- 406.Hansson L, Hedner T, Lund-Johansen P, et al. Randomised trial of effects of calcium antagonists compared with diuretics and beta-blockers on cardiovascular morbidity and mortality in hypertension: the Nordic Diltiazem (NORDIL) study. Lancet. 2000;356:359–365. doi: 10.1016/s0140-6736(00)02526-5. [DOI] [PubMed] [Google Scholar]
- 407.Suchard MA, Schuemie MJ, Krumholz HM, et al. Comprehensive comparative effectiveness and safety of first-line antihypertensive drug classes: a systematic, multinational, large-scale analysis. Lancet. 2019;394:1816–1826. doi: 10.1016/S0140-6736(19)32317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Ernst ME, Carter BL, Goerdt CJ, et al. Comparative antihypertensive effects of hydrochlorothiazide and chlorthalidone on ambulatory and office blood pressure. Hypertension. 2006;47:352–358. doi: 10.1161/01.HYP.0000203309.07140.d3. [DOI] [PubMed] [Google Scholar]
- 409.Dorsch MP, Gillespie BW, Erickson SR, et al. Chlorthalidone reduces cardiovascular events compared with hydrochlorothiazide: a retrospective cohort analysis. Hypertension. 2011;57:689–694. doi: 10.1161/HYPERTENSIONAHA.110.161505. [DOI] [PubMed] [Google Scholar]
- 410.Group PC. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358:1033–1041. doi: 10.1016/S0140-6736(01)06178-5. [DOI] [PubMed] [Google Scholar]
- 411.Patel A, MacMahon S, Chalmers J, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370:829–840. doi: 10.1016/S0140-6736(07)61303-8. [DOI] [PubMed] [Google Scholar]
- 412.Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–1898. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 413.Roush GC, Holford TR, Guddati AK. Chlorthalidone compared with hydrochlorothiazide in reducing cardiovascular events: systematic review and network meta-analyses. Hypertension. 2012;59:1110–1117. doi: 10.1161/HYPERTENSIONAHA.112.191106. [DOI] [PubMed] [Google Scholar]
- 414.Tu K, Anderson LN, Butt DA, et al. Antihypertensive drug prescribing and persistence among new elderly users: implications for persistence improvement interventions. Can J Cardiol. 2014;30:647–652. doi: 10.1016/j.cjca.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 415.Elliott WJ, Meyer PM. Incident diabetes in clinical trials of antihypertensive drugs: a network meta-analysis. Lancet. 2007;369:201–207. doi: 10.1016/S0140-6736(07)60108-1. [DOI] [PubMed] [Google Scholar]
- 416.Rodenburg EM, Hoorn EJ, Ruiter R, et al. Thiazide-associated hyponatremia: a population-based study. Am J Kidney Dis. 2013;62:67–72. doi: 10.1053/j.ajkd.2013.02.365. [DOI] [PubMed] [Google Scholar]
- 417.Ware JS, Wain LV, Channavajjhala SK, et al. Phenotypic and pharmacogenetic evaluation of patients with thiazide-induced hyponatremia. J Clin Invest. 2017;127:3367–3374. doi: 10.1172/JCI89812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Musini VM, Nazer M, Bassett K, et al. Blood pressure-lowering efficacy of monotherapy with thiazide diuretics for primary hypertension. Cochrane Database Syst Rev. 2014:Cd003824. doi: 10.1002/14651858.CD003824.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Almgren T, Wilhelmsen L, Samuelsson O, et al. Diabetes in treated hypertension is common and carries a high cardiovascular risk: results from a 28-year follow-up. J Hypertens. 2007;25:1311–1317. doi: 10.1097/HJH.0b013e328122dd58. [DOI] [PubMed] [Google Scholar]
- 420.Sica DA, Carter B, Cushman W, et al. Thiazide and loop diuretics. J Clin Hypertens (Greenwich) 2011;13:639–643. doi: 10.1111/j.1751-7176.2011.00512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Maron BA, Leopold JA. Aldosterone receptor antagonists: effective but often forgotten. Circulation. 2010;121:934–939. doi: 10.1161/CIRCULATIONAHA.109.895235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Rossi GP, Bernini G, Caliumi C, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48:2293–2300. doi: 10.1016/j.jacc.2006.07.059. [DOI] [PubMed] [Google Scholar]
- 423.Milliez P, Girerd X, Plouin PF, et al. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45:1243–1248. doi: 10.1016/j.jacc.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 424.Edelmann F, Wachter R, Schmidt AG, et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA. 2013;309:781–791. doi: 10.1001/jama.2013.905. [DOI] [PubMed] [Google Scholar]
- 425.Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 426.Chapman N, Dobson J, Wilson S, et al. Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension. 2007;49:839–845. doi: 10.1161/01.HYP.0000259805.18468.8c. [DOI] [PubMed] [Google Scholar]
- 427.de Souza F, Muxfeldt E, Fiszman R, et al. Efficacy of spironolactone therapy in patients with true resistant hypertension. Hypertension. 2010;55:147–152. doi: 10.1161/HYPERTENSIONAHA.109.140988. [DOI] [PubMed] [Google Scholar]
- 428.Václavík J, Sedlák R, Plachy M, et al. Addition of spironolactone in patients with resistant arterial hypertension (ASPIRANT): a randomized, double-blind, placebo-controlled trial. Hypertension. 2011;57:1069–1075. doi: 10.1161/HYPERTENSIONAHA.111.169961. [DOI] [PubMed] [Google Scholar]
- 429.Williams B, MacDonald TM, Morant SV, et al. Endocrine and haemodynamic changes in resistant hypertension, and blood pressure responses to spironolactone or amiloride: the PATHWAY-2 mechanisms substudies. Lancet Diabetes Endocrinol. 2018;6:464–475. doi: 10.1016/S2213-8587(18)30071-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.ALLHAT. Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). ALLHAT Collaborative Research Group. JAMA. 2000;283:1967–1975. [PubMed] [Google Scholar]
- 431.Mah GT, Tejani AM, Musini VM. Methyldopa for primary hypertension. Cochrane Database Syst Rev. 2009;2009:Cd003893. doi: 10.1002/14651858.CD003893.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Hoeltzenbein M, Beck E, Fietz AK, et al. Pregnancy outcome after first trimester use of methyldopa: a prospective cohort study. Hypertension. 2017;70:201–208. doi: 10.1161/HYPERTENSIONAHA.117.09110. [DOI] [PubMed] [Google Scholar]
- 433.Cohn JN, McInnes GT, Shepherd AM. Direct-acting vasodilators. J Clin Hypertens (Greenwich) 2011;13:690–692. doi: 10.1111/j.1751-7176.2011.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Taylor AL, Ziesche S, Yancy C, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351:2049–2057. doi: 10.1056/NEJMoa042934. [DOI] [PubMed] [Google Scholar]
- 435.McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 436.Lin DS, Wang TD, Buranakitjaroen P, et al. Angiotensin receptor neprilysin inhibitor as a novel antihypertensive drug: evidence from Asia and around the globe. J Clin Hypertens (Greenwich) 2021;23:556–567. doi: 10.1111/jch.14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Kario K, Sun N, Chiang FT, et al. Efficacy and safety of LCZ696, a first-in-class angiotensin receptor neprilysin inhibitor, in Asian patients with hypertension: a randomized, double-blind, placebo-controlled study. Hypertension. 2014;63:698–705. doi: 10.1161/HYPERTENSIONAHA.113.02002. [DOI] [PubMed] [Google Scholar]
- 438.Baker WL, Smyth LR, Riche DM, et al. Effects of sodium-glucose co-transporter 2 inhibitors on blood pressure: a systematic review and meta-analysis. J Am Soc Hypertens. 2014;8:262–275. doi: 10.1016/j.jash.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 439.Kario K, Williams B. Nocturnal hypertension and heart failure: mechanisms, evidence, and new treatments. Hypertension. 2021;78:564–577. doi: 10.1161/HYPERTENSIONAHA.121.17440. [DOI] [PubMed] [Google Scholar]
- 440.Qaseem A, Barry MJ, Humphrey LL, et al. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166:279–290. doi: 10.7326/M16-1860. [DOI] [PubMed] [Google Scholar]
- 441.Schlaich MP, Krum H, Sobotka PA. Renal sympathetic nerve ablation: the new frontier in the treatment of hypertension. Curr Hypertens Rep. 2010;12:39–46. doi: 10.1007/s11906-009-0078-6. [DOI] [PubMed] [Google Scholar]
- 442.Lauder L, Azizi M, Kirtane AJ, et al. Device-based therapies for arterial hypertension. Nat Rev Cardiol. 2020;17:614–628. doi: 10.1038/s41569-020-0364-1. [DOI] [PubMed] [Google Scholar]
- 443.Liu LY, Lin PL, Liao FC, et al. Effect of radiofrequency-based renal denervation: the impact of unplanned medication change from a systematic review and meta-analysis. Acta Cardiol Sin. 2019;35:144–152. doi: 10.6515/ACS.201903_35(2).20181231A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Wang TD, Lee YH, Chang SS, et al. 2019 consensus statement of the Taiwan Hypertension Society and the Taiwan Society of Cardiology on renal denervation for the management of arterial hypertension. Acta Cardiol Sin. 2019;35:199–230. doi: 10.6515/ACS.201905_35(3).20190415A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Kario K, Kim BK, Aoki J, et al. Renal denervation in Asia: consensus statement of the Asia renal denervation consortium. Hypertension. 2020;75:590–602. doi: 10.1161/HYPERTENSIONAHA.119.13671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Kandzari DE, Townsend RR, Bakris G, et al. Renal denervation in hypertension patients: proceedings from an expert consensus roundtable cosponsored by SCAI and NKF. Catheter Cardiovasc Interv. 2021 doi: 10.1002/ccd.29884. [DOI] [PubMed] [Google Scholar]
- 447.Schmieder RE, Mahfoud F, Mancia G, et al. European Society of hypertension position paper on renal denervation 2021. J Hypertens. 2021;39:1733–1741. doi: 10.1097/HJH.0000000000002933. [DOI] [PubMed] [Google Scholar]
- 448.Kandzari DE, Kario K, Mahfoud F, et al. The SPYRAL HTN Global Clinical Trial Program: rationale and design for studies of renal denervation in the absence (SPYRAL HTN OFF-MED) and presence (SPYRAL HTN ON-MED) of antihypertensive medications. Am Heart J. 2016;171:82–91. doi: 10.1016/j.ahj.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 449.Townsend RR, Mahfoud F, Kandzari DE, et al. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet. 2017;390:2160–2170. doi: 10.1016/S0140-6736(17)32281-X. [DOI] [PubMed] [Google Scholar]
- 450.Bohm M, Kario K, Kandzari DE, et al. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomised, sham-controlled trial. Lancet. 2020;395:1444–1451. doi: 10.1016/S0140-6736(20)30554-7. [DOI] [PubMed] [Google Scholar]
- 451.Kandzari DE, Bohm M, Mahfoud F, et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. 2018;391:2346–2355. doi: 10.1016/S0140-6736(18)30951-6. [DOI] [PubMed] [Google Scholar]
- 452.Azizi M, Schmieder RE, Mahfoud F, et al. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet. 2018;391:2335–2345. doi: 10.1016/S0140-6736(18)31082-1. [DOI] [PubMed] [Google Scholar]
- 453.Azizi M, Sanghvi K, Saxena M, et al. Ultrasound renal denervation for hypertension resistant to a triple medication pill (RADIANCE-HTN TRIO): a randomised, multicentre, single-blind, sham-controlled trial. Lancet. 2021;397:2476–2486. doi: 10.1016/S0140-6736(21)00788-1. [DOI] [PubMed] [Google Scholar]
- 454.Fischell TA, Ebner A, Gallo S, et al. Transcatheter alcohol-mediated perivascular renal denervation with the peregrine system: first-in-human experience. JACC Cardiovasc Interv. 2016;9:589–598. doi: 10.1016/j.jcin.2015.11.041. [DOI] [PubMed] [Google Scholar]
- 455.Lee CK, Wang TD, Lee YH, et al. Efficacy and safety of renal denervation for patients with uncontrolled hypertension in Taiwan: 3-year results from the global SYMPLICITY Registry-Taiwan (GSR-Taiwan). Acta Cardiol Sin. 2019;35:618–626. doi: 10.6515/ACS.201911_35(6).20190826A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Mahfoud F, Mancia G, Schmieder R, et al. Renal denervation in high-risk patients with hypertension. J Am Coll Cardiol. 2020;75:2879–2888. doi: 10.1016/j.jacc.2020.04.036. [DOI] [PubMed] [Google Scholar]
- 457.Böhm M, Mahfoud F, Townsend RR, et al. Ambulatory heart rate reduction after catheter-based renal denervation in hypertensive patients not receiving anti-hypertensive medications: data from SPYRAL HTN-OFF MED, a randomized, sham-controlled, proof-of-concept trial. Eur Heart J. 2019;40:743–751. doi: 10.1093/eurheartj/ehy871. [DOI] [PubMed] [Google Scholar]
- 458.Lin SI, Huang CC, Sung SH, et al. Preprocedural features of patients under antihypertensive drugs may help identify responders to renal denervation: a hypothesis-generating study. Rev Cardiovasc Med. 2022;23:65. doi: 10.31083/j.rcm2302065. [DOI] [PubMed] [Google Scholar]
- 459.Mahfoud F, Townsend RR, Kandzari DE, et al. Changes in plasma renin activity after renal artery sympathetic denervation. J Am Coll Cardiol. 2021;77:2909–2919. doi: 10.1016/j.jacc.2021.04.044. [DOI] [PubMed] [Google Scholar]
- 460.Chu PH, Lin SI, Lan WR, et al. The effects of renal denervation beyond anti-hypertension. J Taiwan Cardiovasc Interv. 2021;11:82–89. [Google Scholar]
- 461.Schmieder RE, Kandzari DE, Wang TD, et al. Differences in patient and physician perspectives on pharmaceutical therapy and renal denervation for the management of hypertension. J Hypertens. 2021;39:162–168. doi: 10.1097/HJH.0000000000002592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Plante TB, Juraschek SP, Miller ER, 3rd, et al. Comparison of frequency of atherosclerotic cardiovascular and safety events with systolic blood pressure < 120 mmHg versus 135-139 mmHg in a systolic blood pressure intervention trial primary prevention subgroup. Am J Cardiol. 2018;122:1185–1190. doi: 10.1016/j.amjcard.2018.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Five-year findings of the hypertension detection and follow-up program. I. Reduction in mortality of persons with high blood pressure, including mild hypertension. Hypertension Detection and Follow-up Program Cooperative Group. JAMA. 1979;242:2562–2571. [PubMed] [Google Scholar]
- 464.The effect of treatment on mortality in "mild" hypertension: results of the hypertension detection and follow-up program. N Engl J Med. 1982;307:976–980. doi: 10.1056/NEJM198210143071603. [DOI] [PubMed] [Google Scholar]
- 465.Perkovic V, Rodgers A. Redefining blood-pressure targets--sprint starts the Marathon. N Engl J Med. 2015;373:2175–2178. doi: 10.1056/NEJMe1513301. [DOI] [PubMed] [Google Scholar]
- 466.Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Margolis KL, O'Connor PJ, Morgan TM, et al. Outcomes of combined cardiovascular risk factor management strategies in type 2 diabetes: the ACCORD randomized trial. Diabetes Care. 2014;37:1721–1728. doi: 10.2337/dc13-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313:603–615. doi: 10.1001/jama.2014.18574. [DOI] [PubMed] [Google Scholar]
- 469.Chiang CE, Wang TD, Lin TH, et al. The 2017 focused update of the guidelines of the Taiwan Society of Cardiology (TSOC) and the Taiwan Hypertension Society (THS) for the management of hypertension. Acta Cardiol Sin. 2017;33:213–225. doi: 10.6515/ACS20170421A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Chiang CE, Lin SY, Lin TH, et al. 2018 consensus of the Taiwan Society of Cardiology and the Diabetes Association of Republic of China (Taiwan) on the pharmacological management of patients with type 2 diabetes and cardiovascular diseases. J Chin Med Assoc. 2018;81:189–222. doi: 10.1016/j.jcma.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 471.Xie X, Atkins E, Lv J, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet. 2016;387:435–443. doi: 10.1016/S0140-6736(15)00805-3. [DOI] [PubMed] [Google Scholar]
- 472.Cooper-DeHoff RM, Handberg EM, Mancia G, et al. INVEST revisited: review of findings from the International Verapamil SR-Trandolapril Study. Expert Rev Cardiovasc Ther. 2009;7:1329–1340. doi: 10.1586/erc.09.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Denardo SJ, Gong Y, Nichols WW, et al. Blood pressure and outcomes in very old hypertensive coronary artery disease patients: an INVEST substudy. Am J Med. 2010;123:719–726. doi: 10.1016/j.amjmed.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Bangalore S, Qin J, Sloan S, et al. What is the optimal blood pressure in patients after acute coronary syndromes?: Relationship of blood pressure and cardiovascular events in the PRavastatin OR atorVastatin Evaluation and Infection Therapy-Thrombolysis In Myocardial Infarction (PROVE IT-TIMI) 22 trial. Circulation. 2010;122:2142–2151. doi: 10.1161/CIRCULATIONAHA.109.905687. [DOI] [PubMed] [Google Scholar]
- 475.Adler AI, Stratton IM, Neil HA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321:412–419. doi: 10.1136/bmj.321.7258.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Klag MJ, Whelton PK, Randall BL, et al. Blood pressure and end-stage renal disease in men. N Engl J Med. 1996;334:13–18. doi: 10.1056/NEJM199601043340103. [DOI] [PubMed] [Google Scholar]
- 477.Lawes CM, Rodgers A, Bennett DA, et al. Blood pressure and cardiovascular disease in the Asia Pacific region. J Hypertens. 2003;21:707–716. doi: 10.1097/00004872-200304000-00013. [DOI] [PubMed] [Google Scholar]
- 478.Kalkman DN, Brouwer TF, Vehmeijer JT, et al. J curve in patients randomly assigned to different systolic blood pressure targets: an experimental approach to an observational paradigm. Circulation. 2017;136:2220–2229. doi: 10.1161/CIRCULATIONAHA.117.030342. [DOI] [PubMed] [Google Scholar]
- 479.Bundy JD, Li C, Stuchlik P, et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol. 2017;2:775–781. doi: 10.1001/jamacardio.2017.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.O'Donnell MJ, Chin SL, Rangarajan S, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet. 2016;388:761–775. doi: 10.1016/S0140-6736(16)30506-2. [DOI] [PubMed] [Google Scholar]
- 481.Fischer U, Cooney MT, Bull LM, et al. Acute post-stroke blood pressure relative to premorbid levels in intracerebral haemorrhage versus major ischaemic stroke: a population-based study. Lancet Neurol. 2014;13:374–384. doi: 10.1016/S1474-4422(14)70031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Prehospital transdermal glyceryl trinitrate in patients with ultra-acute presumed stroke (RIGHT-2): an ambulance-based, randomised, sham-controlled, blinded, phase 3 trial. Lancet. 2019;393:1009–1020. doi: 10.1016/S0140-6736(19)30194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Ishitsuka K, Kamouchi M, Hata J, et al. High blood pressure after acute ischemic stroke is associated with poor clinical outcomes: Fukuoka Stroke Registry. Hypertension. 2014;63:54–60. doi: 10.1161/HYPERTENSIONAHA.113.02189. [DOI] [PubMed] [Google Scholar]
- 484.Robinson TG, Potter JF, Ford GA, et al. Effects of antihypertensive treatment after acute stroke in the Continue or Stop Post-Stroke Antihypertensives Collaborative Study (COSSACS): a prospective, randomised, open, blinded-endpoint trial. Lancet Neurol. 2010;9:767–775. doi: 10.1016/S1474-4422(10)70163-0. [DOI] [PubMed] [Google Scholar]
- 485.Sandset EC, Bath PM, Boysen G, et al. The angiotensin-receptor blocker candesartan for treatment of acute stroke (SCAST): a randomised, placebo-controlled, double-blind trial. Lancet. 2011;377:741–750. doi: 10.1016/S0140-6736(11)60104-9. [DOI] [PubMed] [Google Scholar]
- 486.He J, Zhang Y, Xu T, et al. Effects of immediate blood pressure reduction on death and major disability in patients with acute ischemic stroke: the CATIS randomized clinical trial. JAMA. 2014;311:479–489. doi: 10.1001/jama.2013.282543. [DOI] [PubMed] [Google Scholar]
- 487.Efficacy of nitric oxide, with or without continuing antihypertensive treatment, for management of high blood pressure in acute stroke (ENOS): a partial-factorial randomised controlled trial. Lancet. 2015;385:617–628. doi: 10.1016/S0140-6736(14)61121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Yuan F, Yang F, Zhao J, et al. Controlling Hypertension After Severe Cerebrovascular Event (CHASE): a randomized, multicenter, controlled study. Int J Stroke. 2021;16:456–465. doi: 10.1177/1747493020932784. [DOI] [PubMed] [Google Scholar]
- 489.Nasi LA, Martins SCO, Gus M, et al. Early manipulation of arterial blood pressure in acute ischemic stroke (MAPAS): results of a randomized controlled trial. Neurocrit Care. 2019;30:372–379. doi: 10.1007/s12028-018-0642-5. [DOI] [PubMed] [Google Scholar]
- 490.Sandset EC, Murray GD, Bath PM, et al. Relation between change in blood pressure in acute stroke and risk of early adverse events and poor outcome. Stroke. 2012;43:2108–2114. doi: 10.1161/STROKEAHA.111.647362. [DOI] [PubMed] [Google Scholar]
- 491.Hornslien AG, Sandset EC, Igland J, et al. Effects of candesartan in acute stroke on vascular events during long-term follow-up: results from the Scandinavian Candesartan Acute Stroke Trial (SCAST). Int J Stroke. 2015;10:830–835. doi: 10.1111/ijs.12477. [DOI] [PubMed] [Google Scholar]
- 492.He WJ, Zhong C, Xu T, et al. Early antihypertensive treatment and clinical outcomes in acute ischemic stroke: subgroup analysis by baseline blood pressure. J Hypertens. 2018;36:1372–1381. doi: 10.1097/HJH.0000000000001690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Bu X, Li C, Zhang Y, et al. Early blood pressure reduction in acute ischemic stroke with various severities: a subgroup analysis of the CATIS trial. Cerebrovasc Dis. 2016;42:186–195. doi: 10.1159/000444722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Lee M, Ovbiagele B, Hong KS, et al. Effect of blood pressure lowering in early ischemic stroke: meta-analysis. Stroke. 2015;46:1883–1889. doi: 10.1161/STROKEAHA.115.009552. [DOI] [PubMed] [Google Scholar]
- 495.Liu S, Li C, Li T, et al. Effects of early hypertension control after ischaemic stroke on the outcome: a meta-analysis. Cerebrovasc Dis. 2015;40:270–278. doi: 10.1159/000441097. [DOI] [PubMed] [Google Scholar]
- 496.Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–110. doi: 10.1161/STR.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 497.An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med. 1993;329:673–682. doi: 10.1056/NEJM199309023291001. [DOI] [PubMed] [Google Scholar]
- 498.Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 499.Tsivgoulis G, Frey JL, Flaster M, et al. Pre-tissue plasminogen activator blood pressure levels and risk of symptomatic intracerebral hemorrhage. Stroke. 2009;40:3631–3634. doi: 10.1161/STROKEAHA.109.564096. [DOI] [PubMed] [Google Scholar]
- 500.Teng RSY, Tan BYQ, Miny S, et al. Effect of pretreatment blood pressure on outcomes in thrombolysed acute ischemic stroke patients: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis. 2019;28:906–919. doi: 10.1016/j.jstrokecerebrovasdis.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 501.Ahmed N, Wahlgren N, Brainin M, et al. Relationship of blood pressure, antihypertensive therapy, and outcome in ischemic stroke treated with intravenous thrombolysis: retrospective analysis from Safe Implementation of Thrombolysis in Stroke-International Stroke Thrombolysis Register (SITS-ISTR). Stroke. 2009;40:2442–2449. doi: 10.1161/STROKEAHA.109.548602. [DOI] [PubMed] [Google Scholar]
- 502.Malhotra K, Ahmed N, Filippatou A, et al. Association of elevated blood pressure levels with outcomes in acute ischemic stroke patients treated with intravenous thrombolysis: a systematic review and meta-analysis. J Stroke. 2019;21:78–90. doi: 10.5853/jos.2018.02369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Anderson CS, Huang Y, Lindley RI, et al. Intensive blood pressure reduction with intravenous thrombolysis therapy for acute ischaemic stroke (ENCHANTED): an international, randomised, open-label, blinded-endpoint, phase 3 trial. Lancet. 2019;393:877–888. doi: 10.1016/S0140-6736(19)30038-8. [DOI] [PubMed] [Google Scholar]
- 504.CP C, HM C, CH L, et al. 2020 Taiwan Stroke Society Guideline on the blood pressure control for treatment and prevention of ischemic stroke. Formosan Stroke Journal. 2021;2:169–205. [Google Scholar]
- 505.Kellert L, Sykora M, Gumbinger C, et al. Blood pressure variability after intravenous thrombolysis in acute stroke does not predict intracerebral hemorrhage but poor outcome. Cerebrovasc Dis. 2012;33:135–140. doi: 10.1159/000334186. [DOI] [PubMed] [Google Scholar]
- 506.Liu K, Yan S, Zhang S, et al. Systolic blood pressure variability is associated with severe hemorrhagic transformation in the early stage after thrombolysis. Transl Stroke Res. 2016;7:186–191. doi: 10.1007/s12975-016-0458-6. [DOI] [PubMed] [Google Scholar]
- 507.Nogueira RG, Liebeskind DS, Sung G, et al. Predictors of good clinical outcomes, mortality, and successful revascularization in patients with acute ischemic stroke undergoing thrombectomy: pooled analysis of the Mechanical Embolus Removal in Cerebral Ischemia (MERCI) and Multi MERCI Trials. Stroke. 2009;40:3777–3783. doi: 10.1161/STROKEAHA.109.561431. [DOI] [PubMed] [Google Scholar]
- 508.Mulder M, Ergezen S, Lingsma HF, et al. Baseline blood pressure effect on the benefit and safety of intra-arterial treatment in MR CLEAN (Multicenter Randomized Clinical Trial of Endovascular Treatment of Acute Ischemic Stroke in the Netherlands). Stroke. 2017;48:1869–1876. doi: 10.1161/STROKEAHA.116.016225. [DOI] [PubMed] [Google Scholar]
- 509.Petersen NH, Ortega-Gutierrez S, Wang A, et al. Decreases in blood pressure during thrombectomy are associated with larger infarct volumes and worse functional outcome. Stroke. 2019;50:1797–1804. doi: 10.1161/STROKEAHA.118.024286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Löwhagen Hendén P, Rentzos A, Karlsson JE, et al. Hypotension during endovascular treatment of ischemic stroke is a risk factor for poor neurological outcome. Stroke. 2015;46:2678–2680. doi: 10.1161/STROKEAHA.115.009808. [DOI] [PubMed] [Google Scholar]
- 511.Whalin MK, Halenda KM, Haussen DC, et al. Even small decreases in blood pressure during conscious sedation affect clinical outcome after stroke thrombectomy: an analysis of hemodynamic thresholds. AJNR Am J Neuroradiol. 2017;38:294–298. doi: 10.3174/ajnr.A4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Maïer B, Fahed R, Khoury N, et al. Association of blood pressure during thrombectomy for acute ischemic stroke with functional outcome: a systematic review. Stroke. 2019;50:2805–2812. doi: 10.1161/STROKEAHA.119.024915. [DOI] [PubMed] [Google Scholar]
- 513.Rasmussen M, Schönenberger S, Hendèn PL, et al. Blood pressure thresholds and neurologic outcomes after endovascular therapy for acute ischemic stroke: an analysis of individual patient data from 3 randomized clinical trials. JAMA Neurol. 2020;77:622–631. doi: 10.1001/jamaneurol.2019.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Goyal N, Tsivgoulis G, Pandhi A, et al. Blood pressure levels post mechanical thrombectomy and outcomes in large vessel occlusion strokes. Neurology. 2017;89:540–547. doi: 10.1212/WNL.0000000000004184. [DOI] [PubMed] [Google Scholar]
- 515.Mistry EA, Sucharew H, Mistry AM, et al. Blood pressure after endovascular therapy for ischemic stroke (BEST): a multicenter prospective cohort study. Stroke. 2019;50:3449–3455. doi: 10.1161/STROKEAHA.119.026889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Cernik D, Sanak D, Divisova P, et al. Impact of blood pressure levels within first 24 hours after mechanical thrombectomy on clinical outcome in acute ischemic stroke patients. J Neurointerv Surg. 2019;11:735–739. doi: 10.1136/neurintsurg-2018-014548. [DOI] [PubMed] [Google Scholar]
- 517.Anadani M, Arthur AS, Tsivgoulis G, et al. Blood pressure goals and clinical outcomes after successful endovascular therapy: a multicenter study. Ann Neurol. 2020;87:830–839. doi: 10.1002/ana.25716. [DOI] [PubMed] [Google Scholar]
- 518.Chu HJ, Lin CH, Chen CH, et al. Effect of blood pressure parameters on functional independence in patients with acute ischemic stroke in the first 6 hours after endovascular thrombectomy. J Neurointerv Surg. 2020;12:937–941. doi: 10.1136/neurintsurg-2019-015412. [DOI] [PubMed] [Google Scholar]
- 519.Chang JY, Jeon SB, Lee JH, et al. The Relationship between blood pressure variability, recanalization degree, and clinical outcome in large vessel occlusive stroke after an intra-arterial thrombectomy. Cerebrovasc Dis. 2018;46:279–286. doi: 10.1159/000495300. [DOI] [PubMed] [Google Scholar]
- 520.Bennett AE, Wilder MJ, McNally JS, et al. Increased blood pressure variability after endovascular thrombectomy for acute stroke is associated with worse clinical outcome. J Neurointerv Surg. 2018;10:823–827. doi: 10.1136/neurintsurg-2017-013473. [DOI] [PubMed] [Google Scholar]
- 521.Mistry EA, Mehta T, Mistry A, et al. Blood pressure variability and neurologic outcome after endovascular thrombectomy: a secondary analysis of the BEST study. Stroke. 2020;51:511–518. doi: 10.1161/STROKEAHA.119.027549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Liu M, Wu B, Wang WZ, et al. Stroke in China: epidemiology, prevention, and management strategies. Lancet Neurol. 2007;6:456–464. doi: 10.1016/S1474-4422(07)70004-2. [DOI] [PubMed] [Google Scholar]
- 523.Hemphill JC, 3rd, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:2032–2060. doi: 10.1161/STR.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 524.Anderson CS, Heeley E, Huang Y, et al. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med. 2013;368:2355–2365. doi: 10.1056/NEJMoa1214609. [DOI] [PubMed] [Google Scholar]
- 525.Butcher KS, Jeerakathil T, Hill M, et al. The intracerebral hemorrhage acutely decreasing arterial pressure trial. Stroke. 2013;44:620–626. doi: 10.1161/STROKEAHA.111.000188. [DOI] [PubMed] [Google Scholar]
- 526.Gould B, McCourt R, Gioia LC, et al. Acute blood pressure reduction in patients with intracerebral hemorrhage does not result in borderzone region hypoperfusion. Stroke. 2014;45:2894–2899. doi: 10.1161/STROKEAHA.114.005614. [DOI] [PubMed] [Google Scholar]
- 527.Anderson CS, Huang Y, Wang JG, et al. Intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT): a randomised pilot trial. Lancet Neurol. 2008;7:391–399. doi: 10.1016/S1474-4422(08)70069-3. [DOI] [PubMed] [Google Scholar]
- 528.Qureshi AI, Palesch YY, Barsan WG, et al. Intensive blood-pressure lowering in patients with acute cerebral hemorrhage. N Engl J Med. 2016;375:1033–1043. doi: 10.1056/NEJMoa1603460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Qureshi AI, Foster LD, Lobanova I, et al. Intensive blood pressure lowering in patients with moderate to severe grade acute cerebral hemorrhage: post hoc analysis of antihypertensive treatment of acute cerebral hemorrhage (ATACH)-2 trial. Cerebrovasc Dis. 2020;49:244–252. doi: 10.1159/000506358. [DOI] [PubMed] [Google Scholar]
- 530.Wang X, Arima H, Heeley E, et al. Magnitude of blood pressure reduction and clinical outcomes in acute intracerebral hemorrhage: intensive blood pressure reduction in acute cerebral hemorrhage trial study. Hypertension. 2015;65:1026–1032. doi: 10.1161/HYPERTENSIONAHA.114.05044. [DOI] [PubMed] [Google Scholar]
- 531.Carcel C, Wang X, Sato S, et al. Degree and timing of intensive blood pressure lowering on hematoma growth in intracerebral hemorrhage: intensive blood pressure reduction in acute cerebral hemorrhage trial-2 results. Stroke. 2016;47:1651–1653. doi: 10.1161/STROKEAHA.116.013326. [DOI] [PubMed] [Google Scholar]
- 532.Yamaguchi Y, Koga M, Sato S, et al. Early achievement of blood pressure lowering and hematoma growth in acute intracerebral hemorrhage: stroke acute management with urgent risk-factor assessment and improvement-intracerebral hemorrhage study. Cerebrovasc Dis. 2018;46:118–124. doi: 10.1159/000492728. [DOI] [PubMed] [Google Scholar]
- 533.Moullaali TJ, Wang X, Martin RH, et al. Blood pressure control and clinical outcomes in acute intracerebral haemorrhage: a preplanned pooled analysis of individual participant data. Lancet Neurol. 2019;18:857–864. doi: 10.1016/S1474-4422(19)30196-6. [DOI] [PubMed] [Google Scholar]
- 534.Connolly ES, Jr., Rabinstein AA, Carhuapoma JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke. 2012;43:1711–1737. doi: 10.1161/STR.0b013e3182587839. [DOI] [PubMed] [Google Scholar]
- 535.Ohkuma H, Tsurutani H, Suzuki S. Incidence and significance of early aneurysmal rebleeding before neurosurgical or neurological management. Stroke. 2001;32:1176–1180. doi: 10.1161/01.str.32.5.1176. [DOI] [PubMed] [Google Scholar]
- 536.Hall A, O'Kane R. The management of hypertension in pre-aneurysmal treatment subarachnoid hemorrhage patients. World Neurosurg. 2019;125:469–474. doi: 10.1016/j.wneu.2019.02.041. [DOI] [PubMed] [Google Scholar]
- 537.Silverman A, Kodali S, Strander S, et al. Deviation from personalized blood pressure targets is associated with worse outcome after subarachnoid hemorrhage. Stroke. 2019;50:2729–2737. doi: 10.1161/STROKEAHA.119.026282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Lee M, Wu YL, Ovbiagele B. Trends in incident and recurrent rates of first-ever ischemic stroke in Taiwan between 2000 and 2011. J Stroke. 2016;18:60–65. doi: 10.5853/jos.2015.01326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Arima H, Tzourio C, Butcher K, et al. Prior events predict cerebrovascular and coronary outcomes in the PROGRESS trial. Stroke. 2006;37:1497–1502. doi: 10.1161/01.STR.0000221212.36860.c9. [DOI] [PubMed] [Google Scholar]
- 540.Hanger HC, Wilkinson TJ, Fayez-Iskander N, et al. The risk of recurrent stroke after intracerebral haemorrhage. J Neurol Neurosurg Psychiatry. 2007;78:836–840. doi: 10.1136/jnnp.2006.106500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Liu L, Wang Z, Gong L, et al. Blood pressure reduction for the secondary prevention of stroke: a Chinese trial and a systematic review of the literature. Hypertens Res. 2009;32:1032–1040. doi: 10.1038/hr.2009.139. [DOI] [PubMed] [Google Scholar]
- 542.Schrader J, Lüders S, Kulschewski A, et al. Morbidity and mortality after stroke, eprosartan compared with nitrendipine for secondary prevention: principal results of a prospective randomized controlled study (MOSES). Stroke. 2005;36:1218–1226. doi: 10.1161/01.STR.0000166048.35740.a9. [DOI] [PubMed] [Google Scholar]
- 543.Yusuf S, Diener HC, Sacco RL, et al. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med. 2008;359:1225–1237. doi: 10.1056/NEJMoa0804593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Benavente OR, Coffey CS, Conwit R, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382:507–515. doi: 10.1016/S0140-6736(13)60852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Mant J, McManus RJ, Roalfe A, et al. Different systolic blood pressure targets for people with history of stroke or transient ischaemic attack: PAST-BP (Prevention After Stroke--Blood Pressure) randomised controlled trial. BMJ. 2016;352:i708. doi: 10.1136/bmj.i708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Kitagawa K, Yamamoto Y, Arima H, et al. Effect of standard vs intensive blood pressure control on the risk of recurrent stroke: a randomized clinical trial and meta-analysis. JAMA Neurol. 2019;76:1309–1318. doi: 10.1001/jamaneurol.2019.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Chapman N, Huxley R, Anderson C, et al. Effects of a perindopril-based blood pressure-lowering regimen on the risk of recurrent stroke according to stroke subtype and medical history: the PROGRESS trial. Stroke. 2004;35:116–121. doi: 10.1161/01.STR.0000106480.76217.6F. [DOI] [PubMed] [Google Scholar]
- 548.Arima H, Anderson C, Omae T, et al. Effects of blood pressure lowering on major vascular events among patients with isolated diastolic hypertension: the perindopril protection against recurrent stroke study (PROGRESS) trial. Stroke. 2011;42:2339–2341. doi: 10.1161/STROKEAHA.110.606764. [DOI] [PubMed] [Google Scholar]
- 549.Arima H, Chalmers J, Woodward M, et al. Lower target blood pressures are safe and effective for the prevention of recurrent stroke: the PROGRESS trial. J Hypertens. 2006;24:1201–1208. doi: 10.1097/01.hjh.0000226212.34055.86. [DOI] [PubMed] [Google Scholar]
- 550.Arima H, Anderson C, Omae T, et al. Perindopril-based blood pressure lowering reduces major vascular events in Asian and Western participants with cerebrovascular disease: the PROGRESS trial. J Hypertens. 2010;28:395–400. doi: 10.1097/HJH.0b013e328333b009. [DOI] [PubMed] [Google Scholar]
- 551.Peralta CA, McClure LA, Scherzer R, et al. Effect of intensive versus usual blood pressure control on kidney function among individuals with prior lacunar stroke: a post hoc analysis of the secondary prevention of small subcortical strokes (SPS3) randomized trial. Circulation. 2016;133:584–591. doi: 10.1161/CIRCULATIONAHA.115.019657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Katsanos AH, Filippatou A, Manios E, et al. Blood pressure reduction and secondary stroke prevention: a systematic review and metaregression analysis of randomized clinical trials. Hypertension. 2017;69:171–179. doi: 10.1161/HYPERTENSIONAHA.116.08485. [DOI] [PubMed] [Google Scholar]
- 553.Bath PM, Martin RH, Palesch Y, et al. Effect of telmisartan on functional outcome, recurrence, and blood pressure in patients with acute mild ischemic stroke: a PRoFESS subgroup analysis. Stroke. 2009;40:3541–3546. doi: 10.1161/STROKEAHA.109.555623. [DOI] [PubMed] [Google Scholar]
- 554.Weber MA. Managing the patient at risk for a second stroke. J Hypertens Suppl. 2005;23:S41–S47. doi: 10.1097/01.hjh.0000165627.64686.cc. [DOI] [PubMed] [Google Scholar]
- 555.Wang WT, You LK, Chiang CE, et al. Comparative effectiveness of blood pressure-lowering drugs in patients who have already suffered from stroke: traditional and Bayesian network meta-analysis of randomized trials. Medicine (Baltimore) 2016;95:e3302. doi: 10.1097/MD.0000000000003302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Xie W, Zheng F, Evangelou E, et al. Blood pressure-lowering drugs and secondary prevention of cardiovascular disease: systematic review and meta-analysis. J Hypertens. 2018;36:1256–1265. doi: 10.1097/HJH.0000000000001720. [DOI] [PubMed] [Google Scholar]
- 557.Turan TN, Cotsonis G, Lynn MJ, et al. Relationship between blood pressure and stroke recurrence in patients with intracranial arterial stenosis. Circulation. 2007;115:2969–2975. doi: 10.1161/CIRCULATIONAHA.106.622464. [DOI] [PubMed] [Google Scholar]
- 558.Turan TN, Nizam A, Lynn MJ, et al. Relationship between risk factor control and vascular events in the SAMMPRIS trial. Neurology. 2017;88:379–385. doi: 10.1212/WNL.0000000000003534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Amin-Hanjani S, Turan TN, Du X, et al. Higher stroke risk with lower blood pressure in hemodynamic vertebrobasilar disease: analysis from the VERiTAS study. J Stroke Cerebrovasc Dis. 2017;26:403–410. doi: 10.1016/j.jstrokecerebrovasdis.2016.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Park JM, Kim BJ, Kwon SU, et al. Intensive blood pressure control may not be safe in subacute ischemic stroke by intracranial atherosclerosis: a result of randomized trial. J Hypertens. 2018;36:1936–1941. doi: 10.1097/HJH.0000000000001784. [DOI] [PubMed] [Google Scholar]
- 561.Rothwell PM, Howard SC, Spence JD. Relationship between blood pressure and stroke risk in patients with symptomatic carotid occlusive disease. Stroke. 2003;34:2583–2590. doi: 10.1161/01.STR.0000094424.38761.56. [DOI] [PubMed] [Google Scholar]
- 562.Powers WJ, Clarke WR, Grubb RL, Jr., et al. Lower stroke risk with lower blood pressure in hemodynamic cerebral ischemia. Neurology. 2014;82:1027–1032. doi: 10.1212/WNL.0000000000000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Ikeme JC, Pergola PE, Scherzer R, et al. Cerebral white matter hyperintensities, kidney function decline, and recurrent stroke after intensive blood pressure lowering: results from the secondary prevention of small subcortical strokes (SPS 3) trial. J Am Heart Assoc. 2019;8:e010091. doi: 10.1161/JAHA.118.010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.White WB, Wakefield DB, Moscufo N, et al. Effects of intensive versus standard ambulatory blood pressure control on cerebrovascular outcomes in older people (INFINITY). Circulation. 2019;140:1626–1635. doi: 10.1161/CIRCULATIONAHA.119.041603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Croall ID, Tozer DJ, Moynihan B, et al. Effect of standard vs intensive blood pressure control on cerebral blood flow in small vessel disease: The PRESERVE randomized clinical trial. JAMA Neurol. 2018;75:720–727. doi: 10.1001/jamaneurol.2017.5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Townsend RR, Taler SJ. Management of hypertension in chronic kidney disease. Nat Rev Nephrol. 2015;11:555–563. doi: 10.1038/nrneph.2015.114. [DOI] [PubMed] [Google Scholar]
- 567.Ku E, Lee BJ, Wei J, et al. Hypertension in CKD: core curriculum 2019. Am J Kidney Dis. 2019;74:120–131. doi: 10.1053/j.ajkd.2018.12.044. [DOI] [PubMed] [Google Scholar]
- 568.Ahmad FS, Chan C, Rosenman MB, et al. Validity of cardiovascular data from electronic sources: the multi-ethnic study of atherosclerosis and HealthLNK. Circulation. 2017;136:1207–1216. doi: 10.1161/CIRCULATIONAHA.117.027436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Ruggenenti P, Perna A, Loriga G, et al. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet. 2005;365:939–946. doi: 10.1016/S0140-6736(05)71082-5. [DOI] [PubMed] [Google Scholar]
- 570.Wright JT, Jr., Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421–2431. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 571.Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med. 1994;330:877–884. doi: 10.1056/NEJM199403313301301. [DOI] [PubMed] [Google Scholar]
- 572.Reboldi G, Gentile G, Angeli F, et al. Effects of intensive blood pressure reduction on myocardial infarction and stroke in diabetes: a meta-analysis in 73,913 patients. J Hypertens. 2011;29:1253–1269. doi: 10.1097/HJH.0b013e3283469976. [DOI] [PubMed] [Google Scholar]
- 573.Karmali KN, Lloyd-Jones DM, van der Leeuw J, et al. Blood pressure-lowering treatment strategies based on cardiovascular risk versus blood pressure: a meta-analysis of individual participant data. PLoS Med. 2018;15:e1002538. doi: 10.1371/journal.pmed.1002538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Ferreira JP, Gregson J, Böhm M, et al. Blood pressure reduction and anti-hypertensive treatment choice: a post-hoc analysis of the SPRINT trial. Clin Cardiol. 2021;44:665–674. doi: 10.1002/clc.23591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Hou FF, Zhang X, Zhang GH, et al. Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med. 2006;354:131–140. doi: 10.1056/NEJMoa053107. [DOI] [PubMed] [Google Scholar]
- 576.Agrawal V, Marinescu V, Agarwal M, et al. Cardiovascular implications of proteinuria: an indicator of chronic kidney disease. Nat Rev Cardiol. 2009;6:301–311. doi: 10.1038/nrcardio.2009.11. [DOI] [PubMed] [Google Scholar]
- 577.Chang AR, Kramer H, Wei G, et al. Effects of intensive blood pressure control in patients with and without albuminuria: post hoc analyses from SPRINT. Clin J Am Soc Nephrol. 2020;15:1121–1128. doi: 10.2215/CJN.12371019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2018;138:e426–e483. doi: 10.1161/CIR.0000000000000597. [DOI] [PubMed] [Google Scholar]
- 579.Rocco MV, Flanigan MJ, Beaver S, et al. Report from the 1995 core indicators for peritoneal dialysis study group. Am J Kidney Dis. 1997;30:165–173. doi: 10.1016/s0272-6386(97)90049-4. [DOI] [PubMed] [Google Scholar]
- 580.Rahman M, Dixit A, Donley V, et al. Factors associated with inadequate blood pressure control in hypertensive hemodialysis patients. Am J Kidney Dis. 1999;33:498–506. doi: 10.1016/s0272-6386(99)70187-3. [DOI] [PubMed] [Google Scholar]
- 581.Sankaranarayanan N, Santos SF, Peixoto AJ. Blood pressure measurement in dialysis patients. Adv Chronic Kidney Dis. 2004;11:134–142. doi: 10.1053/j.arrt.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 582.Agarwal R, Metiku T, Tegegne GG, et al. Diagnosing hypertension by intradialytic blood pressure recordings. Clin J Am Soc Nephrol. 2008;3:1364–1372. doi: 10.2215/CJN.01510308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.Bansal N, McCulloch CE, Rahman M, et al. Blood pressure and risk of all-cause mortality in advanced chronic kidney disease and hemodialysis: the chronic renal insufficiency cohort study. Hypertension. 2015;65:93–100. doi: 10.1161/HYPERTENSIONAHA.114.04334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Schömig M, Eisenhardt A, Ritz E. Controversy on optimal blood pressure on haemodialysis: normotensive blood pressure values are essential for survival. Nephrol Dial Transplant. 2001;16:469–474. doi: 10.1093/ndt/16.3.469. [DOI] [PubMed] [Google Scholar]
- 585.Kalantar-Zadeh K, Kilpatrick RD, McAllister CJ, et al. Reverse epidemiology of hypertension and cardiovascular death in the hemodialysis population: the 58th annual fall conference and scientific sessions. Hypertension. 2005;45:811–817. doi: 10.1161/01.HYP.0000154895.18269.67. [DOI] [PubMed] [Google Scholar]
- 586.Workgroup KD. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45:S1–S153. [PubMed] [Google Scholar]
- 587.Clase CM, Barzilay J, Gao P, et al. Acute change in glomerular filtration rate with inhibition of the renin-angiotensin system does not predict subsequent renal and cardiovascular outcomes. Kidney Int. 2017;91:683–690. doi: 10.1016/j.kint.2016.09.038. [DOI] [PubMed] [Google Scholar]
- 588.Bakris GL, Sarafidis PA, Weir MR, et al. Renal outcomes with different fixed-dose combination therapies in patients with hypertension at high risk for cardiovascular events (ACCOMPLISH): a prespecified secondary analysis of a randomised controlled trial. Lancet. 2010;375:1173–1181. doi: 10.1016/S0140-6736(09)62100-0. [DOI] [PubMed] [Google Scholar]
- 589.Haider AW, Larson MG, Franklin SS, et al. Systolic blood pressure, diastolic blood pressure, and pulse pressure as predictors of risk for congestive heart failure in the Framingham Heart Study. Ann Intern Med. 2003;138:10–16. doi: 10.7326/0003-4819-138-1-200301070-00006. [DOI] [PubMed] [Google Scholar]
- 590.Rodriguez CJ, Swett K, Agarwal SK, et al. Systolic blood pressure levels among adults with hypertension and incident cardiovascular events: the atherosclerosis risk in communities study. JAMA Intern Med. 2014;174:1252–1261. doi: 10.1001/jamainternmed.2014.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 591.Butler J, Kalogeropoulos AP, Georgiopoulou VV, et al. Systolic blood pressure and incident heart failure in the elderly. The Cardiovascular Health Study and the Health, Ageing and Body Composition Study. Heart. 2011;97:1304–1311. doi: 10.1136/hrt.2011.225482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592.Chien KL, Sung FC, Hsu HC, et al. Left ventricular mass and correlated atherosclerotic risk factors in young adolescents: report from Chin-Shan community cardiovascular study in Taiwan. Atherosclerosis. 2001;155:431–437. doi: 10.1016/s0021-9150(00)00579-7. [DOI] [PubMed] [Google Scholar]
- 593.Psaty BM, Smith NL, Siscovick DS, et al. Health outcomes associated with antihypertensive therapies used as first-line agents. A systematic review and meta-analysis. JAMA. 1997;277:739–745. [PubMed] [Google Scholar]
- 594.Arguedas JA, Leiva V, Wright JM. Blood pressure targets in adults with hypertension. Cochrane Database Syst Rev. 2020;12:CD004349. doi: 10.1002/14651858.CD004349.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 7. Effects of more vs. less intensive blood pressure lowering and different achieved blood pressure levels - updated overview and meta-analyses of randomized trials. J Hypertens. 2016;34:613–622. doi: 10.1097/HJH.0000000000000881. [DOI] [PubMed] [Google Scholar]
- 596.Tsimploulis A, Lam PH, Arundel C, et al. Systolic blood pressure and outcomes in patients with heart failure with preserved ejection fraction. JAMA Cardiol. 2018;3:288–297. doi: 10.1001/jamacardio.2017.5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597.Böhm M, Young R, Jhund PS, et al. Systolic blood pressure,cardiovascular outcomes and efficacy and safety of sacubitril/valsartan (LCZ696) in patients with chronic heart failure and reduced ejection fraction: results from PARADIGM-HF. Eur Heart J. 2017;38:1132–1143. doi: 10.1093/eurheartj/ehw570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.Selvaraj S, Claggett BL, Böhm M, et al. Systolic blood pressure in heart failure with preserved ejection fraction treated with sacubitril/valsartan. J Am Coll Cardiol. 2020;75:1644–1656. doi: 10.1016/j.jacc.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599.Lee SE, Lee HY, Cho HJ, et al. Reverse J-curve relationship between on-treatment blood pressure and mortality in patients with heart failure. JACC Heart Fail. 2017;5:810–819. doi: 10.1016/j.jchf.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 600.Arundel C, Lam PH, Gill GS, et al. Systolic blood pressure and outcomes in patients with heart failure with reduced ejection fraction. J Am Coll Cardiol. 2019;73:3054–3063. doi: 10.1016/j.jacc.2019.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.Ariesen MJ, Claus SP, Rinkel GJ, et al. Risk factors for intracerebral hemorrhage in the general population: a systematic review. Stroke. 2003;34:2060–2065. doi: 10.1161/01.STR.0000080678.09344.8D. [DOI] [PubMed] [Google Scholar]
- 602.Toyoda K, Yasaka M, Uchiyama S, et al. Blood pressure levels and bleeding events during antithrombotic therapy: the Bleeding with Antithrombotic Therapy (BAT) Study. Stroke. 2010;41:1440–1444. doi: 10.1161/STROKEAHA.110.580506. [DOI] [PubMed] [Google Scholar]
- 603.Arima H, Anderson C, Omae T, et al. Effects of blood pressure lowering on intracranial and extracranial bleeding in patients on antithrombotic therapy: the PROGRESS trial. Stroke. 2012;43:1675–1677. doi: 10.1161/STROKEAHA.112.651448. [DOI] [PubMed] [Google Scholar]
- 604.Chao TF, Liu CJ, Tuan TC, et al. Comparisons of CHADS2 and CHA2DS2-VASc scores for stroke risk stratification in atrial fibrillation: which scoring system should be used for Asians? Heart Rhythm. 2016;13:46–53. doi: 10.1016/j.hrthm.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 605.Chao TF, Liu CJ, Tuan TC, et al. Lifetime risks,projected numbers, and adverse outcomes in Asian patients with atrial fibrillation: a report from the Taiwan Nationwide AF Cohort Study. Chest. 2018;153:453–466. doi: 10.1016/j.chest.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 606.Hankey GJ, Stevens SR, Piccini JP, et al. Intracranial hemorrhage among patients with atrial fibrillation anticoagulated with warfarin or rivaroxaban: the rivaroxaban once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation. Stroke. 2014;45:1304–1312. doi: 10.1161/STROKEAHA.113.004506. [DOI] [PubMed] [Google Scholar]
- 607.Park S, Bergmark BA, Shi M, et al. Edoxaban versus warfarin stratified by average blood pressure in 19 679 patients with atrial fibrillation and a history of hypertension in the ENGAGE AF-TIMI 48 trial. Hypertension. 2019;74:597–605. doi: 10.1161/HYPERTENSIONAHA.119.13138. [DOI] [PubMed] [Google Scholar]
- 608.Böhm M, Brueckmann M, Eikelboom JW, et al. Cardiovascular outcomes,bleeding risk, and achieved blood pressure in patients on long-term anticoagulation with the thrombin antagonist dabigatran or warfarin: data from the RE-LY trial. Eur Heart J. 2020;41:2848–2859. doi: 10.1093/eurheartj/ehaa247. [DOI] [PubMed] [Google Scholar]
- 609.Chiang CE, Wang KL, Lip GY. Stroke prevention in atrial fibrillation: an Asian perspective. Thromb Haemost. 2014;111:789–797. doi: 10.1160/TH13-11-0948. [DOI] [PubMed] [Google Scholar]
- 610.Chao TF, Chen SA, Ruff CT, et al. Clinical outcomes,edoxaban concentration, and anti-factor Xa activity of Asian patients with atrial fibrillation compared with non-Asians in the ENGAGE AF-TIMI 48 trial. Eur Heart J. 2019;40:1518–1527. doi: 10.1093/eurheartj/ehy807. [DOI] [PubMed] [Google Scholar]
- 611.Kodani E, Atarashi H, Inoue H, et al. Impact of blood pressure control on thromboembolism and major hemorrhage in patients with nonvalvular atrial fibrillation: a subanalysis of the J-RHYTHM registry. J Am Heart Assoc. 2016;5:e004075. doi: 10.1161/JAHA.116.004075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 612.Li YH, Hsieh IC, Ueng KC, et al. Antithrombotic treatment of stable coronary artery disease. Acta Cardiol Sin. 2021;37:574–579. doi: 10.6515/ACS.202111_37(6).20210513A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613.Zhang Y, Zhang X, Liu L, et al. Is a systolic blood pressure target < 140 mmHg indicated in all hypertensives? Subgroup analyses of findings from the randomized FEVER trial. Eur Heart J. 2011;32:1500–1508. doi: 10.1093/eurheartj/ehr039. [DOI] [PubMed] [Google Scholar]
- 614.Franklin SS, Jacobs MJ, Wong ND, et al. Predominance of isolated systolic hypertension among middle-aged and elderly US hypertensives: analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension. 2001;37:869–874. doi: 10.1161/01.hyp.37.3.869. [DOI] [PubMed] [Google Scholar]
- 615.Rapsomaniki E, Timmis A, George J, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet. 2014;383:1899–1911. doi: 10.1016/S0140-6736(14)60685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 616.Group SCR. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA. 1991;265:3255–3264. [PubMed] [Google Scholar]
- 617.Staessen JA, Fagard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet. 1997;350:757–764. doi: 10.1016/s0140-6736(97)05381-6. [DOI] [PubMed] [Google Scholar]
- 618.Liu L, Wang JG, Gong L, et al. Comparison of active treatment and placebo in older Chinese patients with isolated systolic hypertension. J Hypertens. 1998;16:1823–1829. doi: 10.1097/00004872-199816120-00016. [DOI] [PubMed] [Google Scholar]
- 619.Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. New England Journal of Medicine. 2008;358:1887–1898. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 620.Group JS. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res. 2008;31:2115–2127. doi: 10.1291/hypres.31.2115. [DOI] [PubMed] [Google Scholar]
- 621.Ogihara T, Saruta T, Rakugi H, et al. Target blood pressure for treatment of isolated systolic hypertension in the elderly: valsartan in elderly isolated systolic hypertension study. Hypertension. 2010;56:196–202. doi: 10.1161/HYPERTENSIONAHA.109.146035. [DOI] [PubMed] [Google Scholar]
- 622.Lewis CE, Fine LJ, Beddhu S, et al. Final report of a trial of intensive versus standard blood-pressure control. N Engl J Med. 2021;384:1921–1930. doi: 10.1056/NEJMoa1901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 623.Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥ 75 years: a randomized clinical trial. JAMA. 2016;315:2673–2682. doi: 10.1001/jama.2016.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 624.Juraschek SP, Taylor AA, Wright JT, Jr., et al. Orthostatic hypotension, cardiovascular outcomes, and adverse events: results from SPRINT. Hypertension. 2020;75:660–667. doi: 10.1161/HYPERTENSIONAHA.119.14309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 625.Pajewski NM, Berlowitz DR, Bress AP, et al. Intensive vs standard blood pressure control in adults 80 years or older: a secondary analysis of the systolic blood pressure intervention trial. J Am Geriatr Soc. 2020;68:496–504. doi: 10.1111/jgs.16272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626.Ramirez LA, Sullivan JC. Sex differences in hypertension: where we have been and where we are going. Am J Hypertens. 2018;31:1247–1254. doi: 10.1093/ajh/hpy148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 627.Wenger NK, Arnold A, Bairey Merz CN, et al. Hypertension across a woman’s life cycle. J Am Coll Cardiol. 2018;71:1797–1813. doi: 10.1016/j.jacc.2018.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628.Song JJ, Ma Z, Wang J, et al. Gender differences in hypertension. J Cardiovasc Transl Res. 2020;13:47–54. doi: 10.1007/s12265-019-09888-z. [DOI] [PubMed] [Google Scholar]
- 629.Su TC, Hwang LC, You SL, et al. Ethnic variation in hypertension prevalence of women in Taiwan. J Hum Hypertens. 2009;23:160–167. doi: 10.1038/jhh.2008.120. [DOI] [PubMed] [Google Scholar]
- 630.Chen PC, Sung FC, Su TC, et al. Two-year change in body mass index and subsequent risk of hypertension among men and women in a Taiwan community. J Hypertens. 2009;27:1370–1376. doi: 10.1097/HJH.0b013e32832af6d4. [DOI] [PubMed] [Google Scholar]
- 631.Tadic M, Cuspidi C, Grassi G, et al. Gender-specific therapeutic approach in arterial hypertension - challenges ahead. Pharmacol Res. 2019;141:181–188. doi: 10.1016/j.phrs.2018.12.021. [DOI] [PubMed] [Google Scholar]
- 632.Newson L. Menopause and cardiovascular disease. Post Reprod Health. 2018;24:44–49. doi: 10.1177/2053369117749675. [DOI] [PubMed] [Google Scholar]
- 633.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2019;139:e1082–e1143. doi: 10.1161/CIR.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 634.Wenger NK. Female-friendly focus: 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease. Clin Cardiol. 2019;42:706–709. doi: 10.1002/clc.23218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 635.Brown MA, Magee LA, Kenny LC, et al. Hypertensive disorders of pregnancy: isshp classification, diagnosis, and management recommendations for international practice. Hypertension. 2018;72:24–43. doi: 10.1161/HYPERTENSIONAHA.117.10803. [DOI] [PubMed] [Google Scholar]
- 636.Os I, Franco V, Kjeldsen SE, et al. Effects of losartan in women with hypertension and left ventricular hypertrophy: results from the Losartan Intervention for Endpoint Reduction in Hypertension Study. Hypertension. 2008;51:1103–1108. doi: 10.1161/HYPERTENSIONAHA.107.105296. [DOI] [PubMed] [Google Scholar]
- 637.Wing LM, Reid CM, Ryan P, et al. A comparison of outcomes with angiotensin-converting--enzyme inhibitors and diuretics for hypertension in the elderly. N Engl J Med. 2003;348:583–592. doi: 10.1056/NEJMoa021716. [DOI] [PubMed] [Google Scholar]
- 638.Ambrosius WT, Sink KM, Foy CG, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials. 2014;11:532–546. doi: 10.1177/1740774514537404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 639.Rydberg DM, Mejyr S, Loikas D, et al. Sex differences in spontaneous reports on adverse drug events for common antihypertensive drugs. Eur J Clin Pharmacol. 2018;74:1165–1173. doi: 10.1007/s00228-018-2480-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640.Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, et al. 2018 ESC guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39:3165–3241. doi: 10.1093/eurheartj/ehy340. [DOI] [PubMed] [Google Scholar]
- 641.Gestational hypertension and preeclampsia: ACOG practice bulletin, number 222. Obstet Gynecol. 2020;135:e237–e260. doi: 10.1097/AOG.0000000000003891. [DOI] [PubMed] [Google Scholar]
- 642.Gestational hypertension and preeclampsia: ACOG practice bulletin summary, number 222. Obstet Gynecol. 2020;135:1492–1495. doi: 10.1097/AOG.0000000000003892. [DOI] [PubMed] [Google Scholar]
- 643.Tita AT, Szychowski JM, Boggess K, et al. Treatment for mild chronic hypertension during pregnancy. N Engl J Med. 2022 doi: 10.1056/NEJMc2207889. [DOI] [PubMed] [Google Scholar]
- 644.Lane-Cordova AD, Khan SS, Grobman WA, et al. Long-term cardiovascular risks associated with adverse pregnancy outcomes: JACC review topic of the week. J Am Coll Cardiol. 2019;73:2106–2116. doi: 10.1016/j.jacc.2018.12.092. [DOI] [PubMed] [Google Scholar]
- 645.Søndergaard MM, Hlatky MA, Stefanick ML, et al. Association of adverse pregnancy outcomes with risk of atherosclerotic cardiovascular disease in postmenopausal women. JAMA Cardiol. 2020;5:1390–1398. doi: 10.1001/jamacardio.2020.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 646.Parikh NI, Gonzalez JM, Anderson CAM, et al. Adverse pregnancy outcomes and cardiovascular disease risk: unique opportunities for cardiovascular disease prevention in women: a scientific statement from the American Heart Association. Circulation. 2021;143:e902–e916. doi: 10.1161/CIR.0000000000000961. [DOI] [PubMed] [Google Scholar]
- 647.Huang CC, Huang CC, Lin SY, et al. Association between hypertensive pregnancy disorders and future risk of stroke in Taiwan: a nationwide population-based retrospective case-control study. BMC Pregnancy Childbirth. 2020;20:217. doi: 10.1186/s12884-020-02898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 648.Hwu LJ, Sung FC, Mou CH, et al. Risk of subsequent hypertension and diabetes in women with hypertension during pregnancy and gestational diabetes. Mayo Clin Proc. 2016;91:1158–1165. doi: 10.1016/j.mayocp.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 649.Kuo YL, Chan TF, Wu CY, et al. Preeclampsia-eclampsia and future cardiovascular risk among women in Taiwan. Taiwan J Obstet Gynecol. 2018;57:364–369. doi: 10.1016/j.tjog.2018.04.035. [DOI] [PubMed] [Google Scholar]
- 650.Chen KH, Chen LR. Provoking factors for postpartum chronic hypertension in women with preceding gestational hypertension/preeclampsia: a longitudinal cohort study of 22,798 pregnancies. Int J Med Sci. 2020;17:543–548. doi: 10.7150/ijms.39432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 651.Chasan-Taber L, Willett WC, Manson JE, et al. Prospective study of oral contraceptives and hypertension among women in the United States. Circulation. 1996;94:483–489. doi: 10.1161/01.cir.94.3.483. [DOI] [PubMed] [Google Scholar]
- 652.Dong W, Colhoun HM, Poulter NR. Blood pressure in women using oral contraceptives: results from the Health Survey for England 1994. J Hypertens. 1997;15:1063–1068. doi: 10.1097/00004872-199715100-00003. [DOI] [PubMed] [Google Scholar]
- 653.Gillum LA, Mamidipudi SK, Johnston SC. Ischemic stroke risk with oral contraceptives: a meta-analysis. JAMA. 2000;284:72–78. doi: 10.1001/jama.284.1.72. [DOI] [PubMed] [Google Scholar]
- 654.World Health Organization. Medical eligibility criteria for contraceptive use. http://apps.who.int/iris/bitstream/10665/42907/1/9241562668.pdf. Geneva: World Health Organization; 2004. [Google Scholar]
- 655.Lubianca JN, Moreira LB, Gus M, et al. Stopping oral contraceptives: an effective blood pressure-lowering intervention in women with hypertension. J Hum Hypertens. 2005;19:451–455. doi: 10.1038/sj.jhh.1001841. [DOI] [PubMed] [Google Scholar]
- 656.Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women--2011 update: a guideline from the American Heart Association. J Am Coll Cardiol. 2011;57:1404–1423. doi: 10.1016/j.jacc.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 657.Issa Z, Seely EW, Rahme M, et al. Effects of hormone therapy on blood pressure. Menopause. 2015;22:456–468. doi: 10.1097/GME.0000000000000322. [DOI] [PubMed] [Google Scholar]
- 658.Anagnostis P, Theocharis P, Lallas K, et al. Early menopause is associated with increased risk of arterial hypertension: a systematic review and meta-analysis. Maturitas. 2020;135:74–79. doi: 10.1016/j.maturitas.2020.03.006. [DOI] [PubMed] [Google Scholar]
- 659.Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53–e90. doi: 10.1161/HYP.0000000000000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 660.Smith SM, Gurka MJ, Calhoun DA, et al. Optimal systolic blood pressure target in resistant and non-resistant hypertension: a pooled analysis of patient-level data from SPRINT and ACCORD. Am J Med. 2018;131:1463–1472. doi: 10.1016/j.amjmed.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 661.Acelajado MC, Hughes ZH, Oparil S, et al. Treatment of resistant and refractory hypertension. Circ Res. 2019;124:1061–1070. doi: 10.1161/CIRCRESAHA.118.312156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 662.Noubiap JJ, Nansseu JR, Nyaga UF, et al. Global prevalence of resistant hypertension: a meta-analysis of data from 3.2 million patients. Heart. 2019;105:98–105. doi: 10.1136/heartjnl-2018-313599. [DOI] [PubMed] [Google Scholar]
- 663.Smith SM, Gurka MJ, Winterstein AG, et al. Incidence, prevalence, and predictors of treatment-resistant hypertension with intensive blood pressure lowering. J Clin Hypertens (Greenwich) 2019;21:825–834. doi: 10.1111/jch.13550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 664.An J, Sim JJ, Calhoun DA, et al. Apparent treatment-resistant hypertension: characteristics and prevalence in a real-world environment of an integrated health system. J Hypertens. 2020;38:1603–1611. doi: 10.1097/HJH.0000000000002419. [DOI] [PubMed] [Google Scholar]
- 665.Sim JJ, Bhandari SK, Shi J, et al. Comparative risk of renal, cardiovascular, and mortality outcomes in controlled, uncontrolled resistant, and nonresistant hypertension. Kidney Int. 2015;88:622–632. doi: 10.1038/ki.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 666.Chia R, Pandey A, Vongpatanasin W. Resistant hypertension-defining the scope of the problem. Prog Cardiovasc Dis. 2020;63:46–50. doi: 10.1016/j.pcad.2019.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 667.Berra E, Azizi M, Capron A, et al. Evaluation of adherence should become an integral part of assessment of patients with apparently treatment-resistant hypertension. Hypertension. 2016;68:297–306. doi: 10.1161/HYPERTENSIONAHA.116.07464. [DOI] [PubMed] [Google Scholar]
- 668.Tomaszewski M, White C, Patel P, et al. High rates of non-adherence to antihypertensive treatment revealed by high-performance liquid chromatography-tandem mass spectrometry (HP LC-MS/MS) urine analysis. Heart. 2014;100:855–861. doi: 10.1136/heartjnl-2013-305063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 669.Gupta P, Patel P, Štrauch B, et al. Biochemical screening for nonadherence is associated with blood pressure reduction and improvement in adherence. Hypertension. 2017;70:1042–1048. doi: 10.1161/HYPERTENSIONAHA.117.09631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 670.Yaxley JP, Thambar SV. Resistant hypertension: an approach to management in primary care. J Family Med Prim Care. 2015;4:193–199. doi: 10.4103/2249-4863.154630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 671.Bădilă E, Japie C, Weiss E, et al. The road to better management in resistant hypertension-diagnostic and therapeutic insights. Pharmaceutics. 2021;13:714. doi: 10.3390/pharmaceutics13050714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 672.Collins AJ, Pitt B, Reaven N, et al. Association of serum potassium with all-cause mortality in patients with and without heart failure, chronic kidney disease, and/or diabetes. Am J Nephrol. 2017;46:213–221. doi: 10.1159/000479802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 673.Agarwal R, Rossignol P, Romero A, et al. Patiromer versus placebo to enable spironolactone use in patients with resistant hypertension and chronic kidney disease (AMBER): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2019;394:1540–1550. doi: 10.1016/S0140-6736(19)32135-X. [DOI] [PubMed] [Google Scholar]
- 674.Anderson SL, Marrs JC. Sacubitril/valsartan: evaluation of safety and efficacy as an antihypertensive treatment. Drugs Context. 2018;7:212542. doi: 10.7573/dic.212542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 675.Wang JG, Yukisada K, Sibulo A Jr, et al. Efficacy and safety of sacubitril/valsartan (LCZ696) add-on to amlodipine in Asian patients with systolic hypertension uncontrolled with amlodipine monotherapy. J Hypertens. 2017;35:877–885. doi: 10.1097/HJH.0000000000001219. [DOI] [PubMed] [Google Scholar]
- 676.Blumenthal JA, Sherwood A, Smith PJ, et al. Lifestyle modification for resistant hypertension: The TRIUMPH randomized clinical trial. Am Heart J. 2015;170:986–994. doi: 10.1016/j.ahj.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 677.Bursztyn M. Resistant hypertension and healthy lifestyle: impact on prognosis. Hypertension. 2014;64:459–460. doi: 10.1161/HYPERTENSIONAHA.114.03687. [DOI] [PubMed] [Google Scholar]
- 678.Ozemek C, Tiwari S, Sabbahi A, et al. Impact of therapeutic lifestyle changes in resistant hypertension. Prog Cardiovasc Dis. 2020;63:4–9. doi: 10.1016/j.pcad.2019.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 679.Grassi G, Ram VS. Evidence for a critical role of the sympathetic nervous system in hypertension. J Am Soc Hypertens. 2016;10:457–466. doi: 10.1016/j.jash.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 680.Fisher JP, Fadel PJ. Therapeutic strategies for targeting excessive central sympathetic activation in human hypertension. Exp Physiol. 2010;95:572–580. doi: 10.1113/expphysiol.2009.047332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 681.Azizi M, Sapoval M, Gosse P, et al. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet. 2015;385:1957–1965. doi: 10.1016/S0140-6736(14)61942-5. [DOI] [PubMed] [Google Scholar]
- 682.Kandzari DE, Böhm M, Mahfoud F, et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. 2018;391:2346–2355. doi: 10.1016/S0140-6736(18)30951-6. [DOI] [PubMed] [Google Scholar]
- 683.Azizi M, Sanghvi K, Saxena M, et al. Ultrasound renal denervation for hypertension resistant to a triple medication pill (RADIANCE-HTN TRIO): a randomised, multicentre, single-blind, sham-controlled trial. Lancet. 2021;397:2476–2486. doi: 10.1016/S0140-6736(21)00788-1. [DOI] [PubMed] [Google Scholar]
- 684.Kandzari DE, Mahfoud F, Bhatt DL, et al. Confounding factors in renal denervation trials: revisiting old and identifying new challenges in trial design of device therapies for hypertension. Hypertension. 2020;76:1410–1417. doi: 10.1161/HYPERTENSIONAHA.120.15745. [DOI] [PubMed] [Google Scholar]