Relapse after hematopoietic stem cell transplant (HSCT) remains the most common cause of mortality after HSCT. Previously, matching at the class 2 locus, HLA-DQ, has not been considered to be of major relevance to outcomes, but in this Plenary paper, Petersdorf et al demonstrate that specific heterodimers of HLA-DQ α and β chains are associated with higher rates of relapse and decreased disease-free survival. These findings may alter algorithms for HSCT donor selection and stimulate new investigations into the modulation of graft-versus-tumor activity.
Key Points
Cis- and trans-dimerization of HLA-DQα/DQβ chains produce molecules that influence the risk of relapse after transplantation.
The HLA-DQ heterodimer is a functional paradigm of the transplantation barrier.
Visual Abstract
Abstract
HLA-DQ heterodimers increase the susceptibility to autoimmune diseases, but their role in hematopoietic cell transplantation is unknown. We tested the hypothesis that outcome after HLA-matched and HLA-DQ–mismatched hematopoietic cell transplantation is influenced by HLA-DQ heterodimers. Heterodimers were defined in 5164 HLA-matched and 520 HLA-DQ–mismatched patients and their transplant donors according to well-established crystallographic criteria. Group 1 (G1) heterodimers are any DQA1*02/03/04/05/06α paired with any DQB1*02/03/04β. Group 2 (G2) heterodimers are DQA1*01α paired with any DQB1*05/06β. Multivariable models identified significantly higher relapse risk in G1G2 and G2G2 compared with G1G1 HLA-matched patients with malignant disease; risk increased with an increasing number of G2 molecules. In HLA-DQ–mismatched transplantation for malignant diseases, matching or mismatching for G2 increased relapse risk. G2 lowered disease-free survival after both HLA-matched and HLA-DQ–mismatched transplantation. A paradigm based on HLA-DQ heterodimers provides a functional definition of the hematopoietic cell transplantation barrier and a means to lower risks for future patients.
Introduction
In the late 1970s, a new series of antigens distinct from HLA-DR were identified on B cells through their novel serological reactivity patterns and ability to stimulate lymphocytes in vitro.1-3 The locus and its antigens became known as HLA-DQ.4 HLA-class II HLA-DQ, -DR, and -DP exhibit the strongest associations with autoimmunity of any marker in the human genome.5-8 In particular, the association of HLA-DQ with type 1 diabetes mellitus, celiac disease, and narcolepsy provides an advanced understanding of HLA-DQ in health and disease.9-11
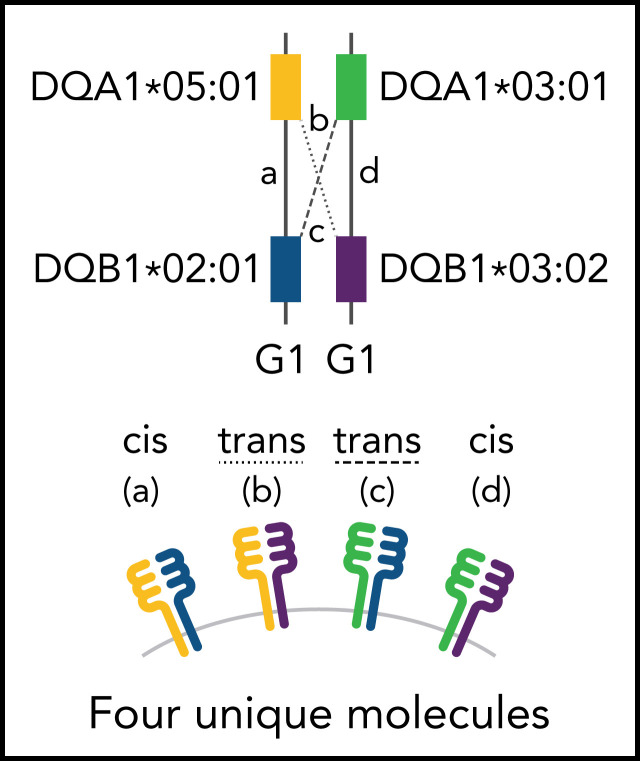
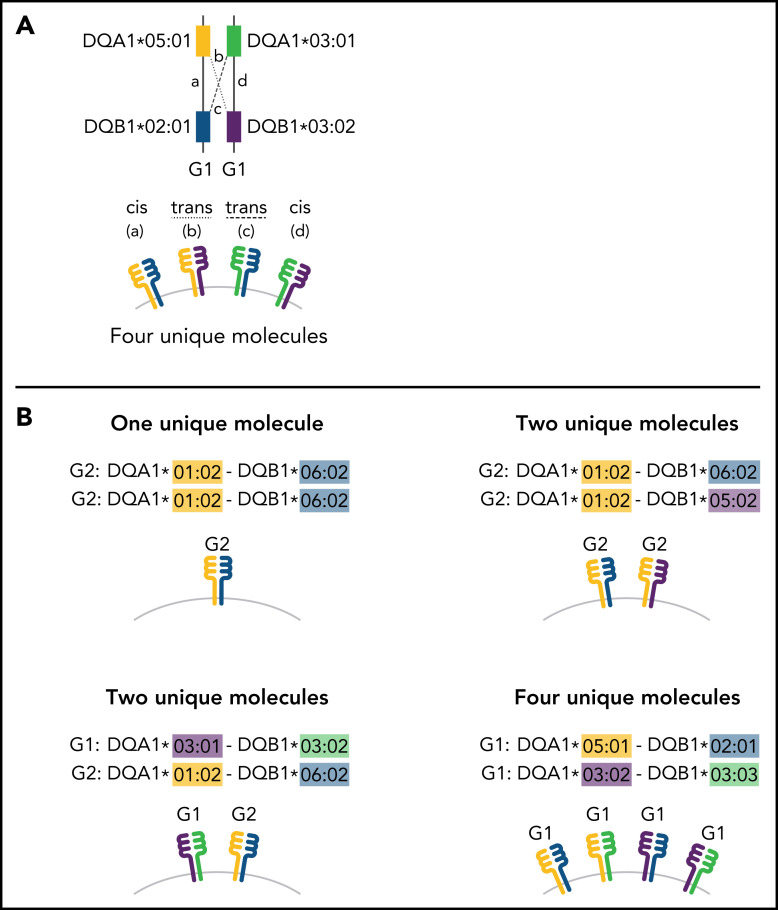
Much of what is known about class II function has come through an understanding of gene structure. Class II molecules are αβ heterodimers formed by pairing the α-chain protein product of HLA-DRA, -DQA1, or -DPA1 with the β-chain protein product of the companion HLA-DRB1, -DQB1, or -DPB1 gene coinherited on the same parental haplotype (cis).12 Sequence variation in the peptide-binding domain of class II molecules provides an armamentarium for host defense against foreign antigens or tissues, which is achieved differently for HLA-DR, -DQ, and -DP.13 Whereas HLA-DR and -DP variation is primarily defined by their β chains, both DQα and DQβ show extensive polymorphism.14 Additional HLA-DQ diversity is afforded by the trans-dimerization of the α chain from 1 parent with the β chain from the other parent (Figure 1).15 Successful HLA-DQ αβ trans-pairing is influenced by the stability of the heterodimer. The α chains from DQA1*02, 03, 04, 05, and 06 alleles form stable heterodimers with the β chains from DQB1*02, 03, and 04 alleles (group 1 [G1], molecules) as do DQA1*01α with DQB1*05β and 06β (group 2 [G2] molecules); however, structural constraints do not favor trans-dimerization of G1 DQα with G2 DQβ, or G2 DQα with G1 DQβ.16,17 In this way, some individuals may produce up to 4 unique HLA-DQ molecules, depending on the parental alleles.16 An increased diversity of molecules expands the HLA-DQ peptide repertoire and enhances T-cell reactivity both of which have profound consequences for susceptibility to type 1 diabetes and celiac disease.16-20
Figure 1.
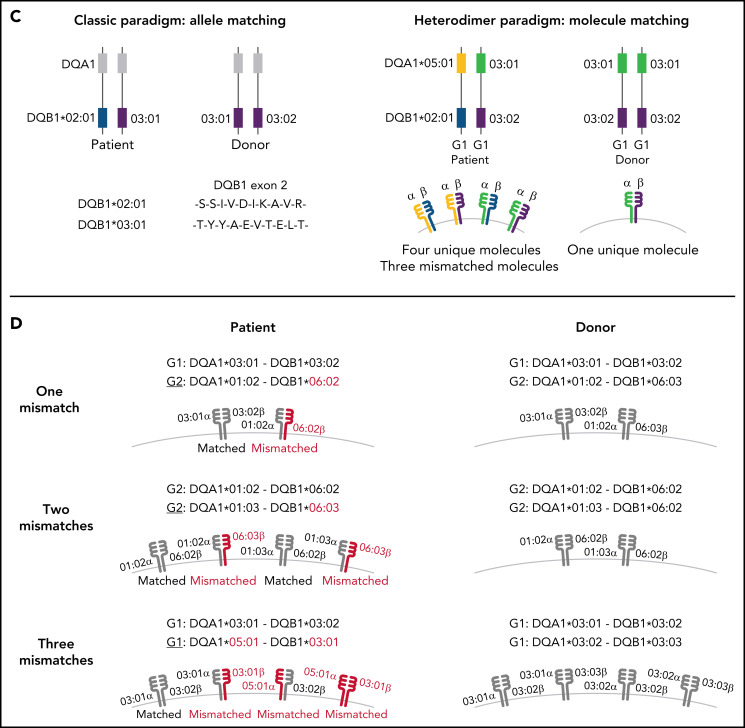
Schema for HLA-DQ heterodimers. (A) HLA-DQ molecules are heterodimers composed of an α-chain protein encoded by the HLA-DQA1 allele and a β-chain protein encoded by the HLA-DQB1 allele that are coinherited on the same parental haplotype (a and d cis heterodimers). Cis encoded parental DQA1-DQB1 haplotypes define the genotype of the individual: G1G1, G1G2, and G2G2. In addition to cis heterodimer molecules, trans-heterodimer molecules (b and c) are formed by the α-chain from 1 parent with the β chain of the other parent. Stable trans-dimerization occurs between G1α (DQA1*02/03/04/05/06) and G1β (DQB1*02/03/04) and between G2α (DQA1*01) and G2β (DQB1*05/06) but not between G1α and G2β or G2α and G1β. (B) Individuals may encode up to 4 unique HLA-DQ molecules depending on DQA1-DQB1 homozygosity and G1G1, G1G2, and G2G2 genotype. G1G1 and G2G2 individuals can form trans-dimers between the DQα from 1 haplotype with the DQβ of the opposing haplotype, generating up to 4 unique molecules. Trans-dimerization cannot occur between G1α and G2β or between G2α and G1β chains; hence, G1G2 individuals have 2 unique molecules each defined by the cis-encoded parental DQA1-DQB1 haplotypes. (C) The classic paradigm for HLA-DQB1 exon 2 allele matching (left) is based on donor compatibility for amino acid residues of the parentally inherited β chains. HLA-DQB1 exon 2 matching does not interrogate HLA-DQA1 (gray) and current donor selection criteria do not include HLA-DQA1. The heterodimer model (right) incorporates sequence information from the α-chain product of DQA1 and the β-chain product of DQB1. The heterodimer paradigm describes the total number of unique molecules, the number of mismatched molecules, and the specific α- and β-protein sequences of a given molecule. (D) Transplantation from donors with 1 HLA-DQB1 allele mismatch may result in 1, 2, or 3 mismatched HLA-DQ molecules in the patient depending on homozygosity of HLA-DQA1 and DQB1 alleles in the patient and the donor and G1G1, G1G2, and G2G2 genotype. The mismatch is denoted in red.
Class II antigens compose the hematopoietic cell transplantation barrier.21 Their role in graft-versus-host disease (GVHD) and disease recurrence (relapse) has been elucidated principally through the effects of donor mismatching for amino acid differences of the β chain encoded by exon 2 of HLA-DRB1 and -DPB1.22-26 Alleles in the patient that are absent in the transplant donor may stimulate unwanted alloresponses against patient tissues that lead to GVHD and/or desirable cytotoxicity against residual malignant cells that lowers relapse after transplantation.
For reasons that have remained elusive, a paradigm based on donor HLA-DQB1 exon 2 mismatching has not been clinically informative; furthermore, the role of HLA-DQA1 is undefined.22,25,26 We reasoned that if both HLA-DQα and -DQβ contribute to its peptide-binding repertoire, then a heterodimer model descriptive of novel molecules produced through trans-dimerization, may provide information on the role of HLA-DQ in transplantation. We determined HLA-DQ molecules in HLA-matched and HLA-DQ–mismatched transplants and examined risks associated with G1 and G2 molecules in a large international cohort of patients.
Patients and methods
Study population
Outcomes were assessed in 5684 patients who received a transplant from 1988 through 2016 from an unrelated donor for the treatment of a life-threatening blood disorder (Table 1, available on the Blood Web site). Data were contributed by members of the International Histocompatibility Working Group (IHWG) in Hematopoietic Cell Transplantation (supplemental Table 2, available on the Blood Web site). Protocols were approved by the institutional review boards of the National Institutes of Health Office for Human Research Protections and each participating IHWG center. The funding agencies had no role in study design, data collection and analysis, the decision to submit the manuscript for publication, or manuscript preparation.
Table 1.
HLA-DQ molecules in the study population
| Patient genotype | |
|---|---|
| Number of unique molecules in 5164 HLA-matched patients * | |
| Total number of G1 | |
| None | G2G2 (n = 900) |
| One | G1G1 (n = 292) |
| G1G2 (n = 2573) | |
| Two | G1G1 (n = 520) |
| Four | G1G1 (n = 879) |
| Total number of G2 | |
| None | G1G1 (n = 1691) |
| One | G2G2 (n = 240) |
| G1G2 (n = 2573) | |
| Two | G2G2 (n = 115) |
| Four | G2G2 (n = 545) |
| Number of mismatched molecules in 520 HLA-DQ–mismatched patients † | |
| One (n = 324) | G1: G1G1 (n = 32) |
| G1G2 (n = 148) | |
| G2: G1G2 (n = 123) | |
| G2G2 (n = 21) | |
| Two (n = 155) | G1: G1G1 (n = 128) |
| G2: G2G2 (n = 27) | |
| Three (n = 41) | G1: G1G1 (n = 22) |
| G2: G2G2 (n = 19) |
The total number of unique molecules in HLA-matched patients depends on the genotype, potential for trans-dimerization, and homozygosity of alleles.
The total number of mismatched molecules depends on the genotype, potential for trans-dimerization, and homozygosity of alleles. In HLA-DQ–mismatched transplants, the mismatched molecule is underlined.
HLA
Patient and donor HLA-A, -B, -C, -DRB1, -DQA1, -DQB1, and -DPB1 alleles were typed at high resolution.24 Because the heterodimer model describes unique molecules, each HLA-DQA1 and -DQB1 allele sequence was defined for exon 2 protein differences to establish G1 (DQ2; DQ3 [7, 8, 9]; DQ4) and G2 (DQ1 [5, 6]).27 A total of 5164 transplants were HLA-A, -B, -C, -DRB1, -DQA1, and -DQB1 matched; 520 were mismatched for 1 cis-encoded patient HLA-DQB1 allele in the graft-versus-host vector of incompatibility (the patient encoded 1 HLA-DQB1 allele that was not present in the donor and was matched for the second HLA-DQB1 allele). The goal of the current study was to define the role of the HLA-DQα/DQβ heterodimer in clinical outcome. Among the 520 HLA-DQB1–mismatched pairs, only 87 were HLA-DQA1–mismatched and precluded meaningful analysis of the significance of mismatching for HLA-DQA1, per se, in outcome.
The crystallographic-based algorithm for HLA-DQ G1 and G2 molecules is well established and validated and was applied to the allele typing to determine cis-encoded genotype (G1G1, G1G2, and G2G2), trans-dimers and unique molecules in each patient and donor (Figure 1).16,17,20 For HLA-matched patients and donors, the total number of unique G1 and G2 molecules was defined (Table 1). The total number of mismatched molecules that were not present in the donor was determined for the 520 HLA-DQ–mismatched transplants by comparing patient molecules to donor molecules (Table 1). The 324 pairs with 1 mismatched molecule were characterized for specific patient/donor combinations (supplemental Table 3).
Statistical analysis
We examined the association of HLA-DQ heterodimer genotype and mismatching with acute (grades 2-4 and 3-4) and chronic GVHD, relapse (studied in patients with malignant diagnoses), disease-free survival, and overall mortality. Logistic regression was used to assess associations with acute GVHD. For all other endpoints, Cox regression models were fit to compare the cause-specific hazards of failure between appropriate groups, and patients in whom failure did not occur by last contact were censored at that contact. Day 0 for all time-to-event outcomes was the day of transplantation. The point of failure of disease-free survival was the date of death or relapse. Models adjusted for patient age, donor age, source of cells, disease status, T-cell depletion, transplant type, use of total body irradiation, patient sex, donor sex, cytomegalovirus serologic status, HLA-DPB1 match status, and year of transplantation, as appropriate.24 Covariates with missing data were included in models by creating an additional category to reflect the missing value of the appropriate covariate. Individual patients were excluded from regression analysis if outcome data were missing for the particular endpoint. Two-sided P values from Cox and logistic regression models were obtained from the Wald test. The outcomes examined are highly correlated, minimizing the impact of multiple comparisons that result from the various outcomes. For this reason, no adjustments were made to the P values associated with the fitted regression model.
Results
HLA-matched transplantation
Parentally inherited (cis) HLA-DQA1 and -DQB1 alleles define the HLA-DQ genotype (G1G1, G1G2, and G2G2) of an individual (Figure 1A). HLA-A, -B, -C, -DRB1, -DQA1, and -DQB1-matched patients and donors have identical alleles and therefore have the same genotype. Among the 5164 HLA-matched transplants (supplemental Table 1), 1691 (33%) were G1G1, 2573 (50%) were G1G2, and 900 (17%) were G2G2. The total number of unique molecules ranged from 0 to 4 (Table 1). Relapse was studied in patients with a malignant disease. Compared with patients bearing G1G1, those with G1G2 and G2G2 each had a significantly increased risk of relapse and lower disease-free survival (Table 2), with similar outcome for other endpoints (supplemental Table 4). Because of the linkage of DR15 with DQ6 (G2), 41.8% of G1G2-, 65.4% of G2G2-, and 0% of G1G1-carrying patients were DR15+; however, the risks of relapse and disease-free survival correlated with G2 and not DR15 (supplemental Table 5). These results show that possession of G2 molecules negatively affects relapse after HLA-matched transplantation.
Table 2.
The role of HLA-DQ heterodimers in HLA-matched unrelated-donor transplantation
| Feature* | Population | Clinical endpoint | Genotype | Patients, n | Hazard ratio (95% CI; P value)† |
|---|---|---|---|---|---|
| HLA-DQ group genotype | HLA-matched patients | Relapse | G1G1 | 1597 | 1.0 |
| G1G2 | 2434 | 1.20 (1.08-1.34; .0009) | |||
| G2G2 | 848 | 1.22 (1.06-1.40; .006) | |||
| Disease-free survival | G1G1 | 1688 | 1.0 | ||
| G1G2 | 2566 | 1.08 (1.01-1.17; .03) | |||
| G2G2 | 897 | 1.11 (1.00-1.22; .04) | |||
| Dose effect of number of G1 molecules | HLA-matched patients | Relapse | No G1 | 848 | 1.0 |
| One G1 | 2702 | 0.97 (0.85-1.10; .65) | |||
| Two G1 | 495 | 0.82 (0.68-0.99; .04) | |||
| Four G1 | 834 | 0.85 (0.72-1.00; .05) | |||
| Disease-free survival | No G1 | 897 | 1.0 | ||
| One G1 | 2858 | 0.96 (0.88-1.05; .38) | |||
| Two G1 | 519 | 0.89 (0.78-1.01; .07) | |||
| Four G1 | 877 | 0.93 (0.83-1.04; .20) | |||
| Dose effect of number of G2 molecules | HLA-matched patients | Relapse | No G2 | 1597 | 1.0 |
| One G2 | 2661 | 1.19 (1.07-1.33; .001) | |||
| Two G2 | 107 | 1.53 (1.13-2.08; .006) | |||
| Four G2 | 514 | 1.25 (1.06-1.48; .008) | |||
| Disease-free survival | No G2 | 1688 | 1.0 | ||
| One G2 | 2806 | 1.07 (1.00-1.15; .06) | |||
| Two G2 | 115 | 1.24 (0.99-1.54; .06) | |||
| Four G2 | 542 | 1.12 (1.00-1.26; .05) |
n = 5164.
The most frequently observed G2 molecule in patients bearing G1G2 and G2G2 was DQA1*01:02P-DQB1*06:02P (0.38 haplotype frequency); those with G1G1 most frequently encoded DQA1*05:01P-DQB1*02:01P (28.8%).
Models adjusted for preparative regimen, diagnosis, cytomegalovirus serostatus, patient age, donor age, stem cell source, patient sex, donor sex, use of total body irradiation, use of T-cell depletion, presence of DPB1 mismatch, and year of transplantation (genotype model).
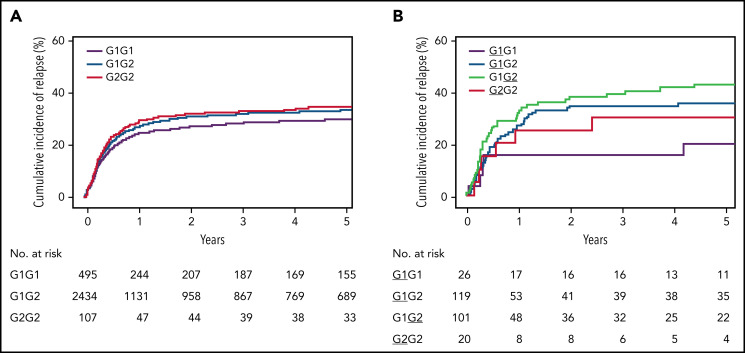
Patients with G1G2 have only 2 unique (cis) molecules, but those bearing G1G1 and G2G2 may have up to 4 unique molecules arising from trans-dimerization between the HLA-DQα and -DQβ parental chains (Figure 1B). When the total number of molecules is considered, patients with 2 or more molecules had higher risk of relapse but lower risk of chronic GVHD, suggesting that the number of molecules is clinically significant (supplemental Table 4). The effect of the number of G1 molecules was studied by examining patients with 1, 2, and 4 G1 molecules relative to no G1, taking the same approach for G2 (Table 1). As the number of G1 molecules increased, the risk of relapse decreased (Table 2). In contrast, as the number of G2 molecules increased, the risk of relapse increased and disease-free survival decreased, suggesting a biologically meaningful association between G2 molecules and clinical outcome. Among the 3208 patients with 2 unique HLA-DQ molecules, the risk of relapse correlated with the genotype (Figure 2).
Figure 2.
Cumulative incidence of relapse. (A) Probability of relapse in 3208 HLA-10/10-matched patients with 2 unique HLA-DQ molecules according to genotype. (B) Probability of relapse in 324 patients with 1 mismatched HLA-DQ molecule, according to patient genotype and mismatched molecule. The mismatches are underlined.
In summary, in this study, both the number and nature of HLA-DQ molecules influenced relapse after HLA-matched transplantation. Compared with G1 molecules, G2 molecules were associated with higher relapse and lower disease-free survival. The generation of trans-dimers in patients with G2G2 increased the total number of unique G2 molecules, which increased relapse risk. These results demonstrate that HLA-DQ molecules are classic disease-association markers in transplantation.
HLA-DQ–mismatched transplantation
The differential effects of G1 and G2 molecules in HLA-matched transplantation motivated examination of heterodimers in HLA-DQ–mismatched transplantation. We hypothesized that mismatching for G2 molecules confers different risks than mismatching for G1 molecules. Patient/donor pairs with a single HLA-DQB1 allele mismatch have at least 1 mismatched molecule, which is the molecule with the mismatched DQB1 allele; however, in G1G1- and G2G2-carrying patients, the trans-dimerization of the mismatched DQβ with the DQα from the opposing parental haplotype may give rise to additional mismatched molecules (Figure 1C-D). Hence, the evaluation of G1- and G2-associated risks requires knowledge of the number of mismatched molecules and outcome.
Among the 520 HLA-DQB1–mismatched patient/donor pairs, 324 had 2 unique HLA-DQ molecules, of which 1 was mismatched (Table 1; supplemental Table 3); the remaining 196 patients had 4 unique molecules and up to 3 mismatched molecules, depending on HLA-DQA1-DQB1 heterozygosity. Under the assumption that G1 and G2 mismatches confer similar risks, mismatching for 2 or more molecules was suggestive of higher risk of relapse and grades 2 to 4 acute GVHD and with lower disease-free survival relative to mismatching for only 1 molecule (supplemental Table 6A). The effect of G1 mismatching on transplant outcome was evaluated in patients with 1, 2, or 3 G1 mismatches relative to patients with no G1 mismatch, using the same approach for G2. The total number of G1 mismatches did not correlate with clinical outcome (supplemental Table 6B); however, among patients with G2 mismatches, 2 mismatches were associated with lower disease-free survival relative to no G2 mismatch (supplemental Table 6C). The results suggest that G2 mismatches carry risk and that mismatched molecules produced by trans-dimerization are functional.
Given the influence of the number of mismatched molecules on clinical outcome, the effects associated with G1 and G2 mismatching are best studied in transplants with the same total number of mismatches. To directly compare G1- and G2-associated risks, we evaluated the 324 patients with only 1 G1 mismatch or 1 G2 mismatch, to remove the effect of the number of mismatches (supplemental Table 3). Patients with G1G2 had higher relapse and lower disease-free survival than those with G1G1 (Table 3), with similar outcomes for other endpoints (supplemental Table 6D).
Table 3.
The role of HLA-DQ heterodimers in patients mismatched for 1 HLA-DQ molecule
| Feature | Population | Clinical endpoint | Genotype or mismatch* | Patients, n | Hazard ratio (95% CI; P value)† |
|---|---|---|---|---|---|
| Patient genotype | Patients with 1 HLA-DQ-mismatch | Relapse | G1G1 | 26 | 1.0 |
| G1G2 | 220 | 2.85 (1.14-7.12; .02) | |||
| G2G2 | 20 | 1.81 (0.53-6.11; .34) | |||
| Disease-free survival | G1G1 | 32 | 1.0 | ||
| G1G2 | 263 | 1.64 (1.00-2.69; .05) | |||
| G2G2 | 21 | 1.57 (0.76-3.23; .23) | |||
| Patient mismatch | Patients with 1 HLA-DQ-mismatch | Relapse | G1G1 | 26 | 1.0 |
| G1G2 | 119 | 2.48 (0.98-6.29; .06) | |||
| G1G2 | 101 | 3.25 (1.27-8.33; .01) | |||
| G2G2 | 20 | 1.76 (0.53-5.86; .36) | |||
| Disease-free survival | G1G1 | 32 | 1.0 | ||
| G1G2 | 142 | 1.50 (0.90-2.49; .12) | |||
| G1G2 | 121 | 1.69 (1.01-2.84; .05) | |||
| G2G2 | 21 | 1.43 (0.70-2.90; .33) |
The mismatched molecule is underlined.
Models adjusted for preparative regimen, diagnosis, patient age, donor age, and presence of DQA1 mismatch.
Patients with G1G2 had higher risks than those with G1G1; however, those with G1G2 were mismatched for either their G1 molecule (G1G2, mismatch underlined) or G2 molecule (G1G2) (Table 1). Among the 324 patients with 1 mismatched molecule, patients with G1G2 had higher risk of relapse and lower disease-free survival, and those with G1G2 had a higher relapse relative to patients with G1G1 (Figure 2; Table 3); similar outcomes were observed for other endpoints (supplemental Table 6E). These results suggest that patients carrying G1G2 do not tolerate a mismatch for their G2 molecule in particular; a matched G2 in these patients is also not optimal. These results are consistent with G2-associated relapse in HLA-matched transplants.
The donor’s mismatched molecule paralleled the patient’s mismatched molecule: 94% of patients with G1G1 had G1G1 donors; 91% of those with G1G2 had G1G2 donors; 81% of those with G1G2 had G1G2 donors; and 86% of those with G2G2 had G2G2 donors (supplemental Table 3). Hence, donor-associated risks paralleled those of the patient (supplemental Table 6F).
The most frequent G2 mismatches were associated with HLA-DRB1*15:01 (37.5%) and DRB1*13 (28.5%). The most common G1 mismatches were observed with HLA-DRB1*04 (50.6%) and DRB1*07:01 (30.6%). G1 mismatches were not different from one another, nor were G2 mismatches; however, the number of patients was limited (supplemental Table 6G-H).
In conclusion, matched and mismatched G2 molecules increase the risk of relapse after HLA-DQ–mismatched transplantation. The structural dichotomy of HLA-DQ heterodimers has functional significance and provides a schema for understanding the role of HLA-DQ in transplantation.
Discussion
Advances in histocompatibility over the past 50 years have contributed to the rapid growth of transplantation medicine. A highly sophisticated level of understanding of the extent and organization of HLA sequence variation has been accelerated in the genomic era,28,29 but the clinical significance of HLA variation in transplantation is currently much better understood for some loci than others. The current standard for the evaluation and selection of unrelated donors in support of hematopoietic cell transplantation includes assessment of the exons known to influence the sequence of the peptide-binding domain of HLA-A, -B, -C, and -DRB1 to lower the risks of posttransplant complications.22 However, when the classic paradigm of exon 2 allele mismatching is applied to class II genes, HLA-DQ mismatches consistently show a different risk profile than HLA-DR and HLA-DP, indicating that criteria based solely on HLA-DQB1 exon 2 are not clinically informative.22-26 We reasoned that the distinctive features of G1 and G2 HLA-DQ heterodimers provide a blueprint for understanding the role of HLA-DQ in transplantation, not only for the nature of the (mis)matched molecule, but also the number of unique molecules that can participate in the immune response. We tested these hypotheses in a large international clinical transplant experience that spanned almost 3 decades. Although specific transplant regimens change over time, the biological effects of G1 and G2 molecules are not expected to change, and inclusion of all available patients provides invaluable information on the role of HLA-DQ in transplant outcome.
Application of the heterodimer paradigm to HLA-matched transplants uncovered a strikingly higher risk of relapse in patients with 1 or 2 G2 molecules, compared with patients with none and was accompanied by lower disease-free survival. Furthermore, relapse was associated with G2, but not the HLA-DR15 linked to G2.30 The significance of these findings is twofold. G2 functions as a transplantation determinant in the absence of HLA-DQ mismatching and recapitulates the classic disease associations of this locus.9-11 A second feature is the dose effect. Relapse risk increases with an increasing number of G2 molecules, with an opposite effect of increasing G1 molecules. These observations fit a biological model in which G2 is a qualitatively different antigen from G1.
Application of the heterodimer model to HLA-DQ–mismatched transplants showed that the number and kind of mismatched molecule each play a key role in relapse after transplantation. Because outcome was associated with the total number of unique molecules, we studied G1- and G2-associated mismatch effects in patients with only 1 mismatched molecule to remove the effect of multiple mismatched molecules. G1G2 patients who were mismatched for their G2 molecule had substantially higher relapse risk, but those matched for their G2 also had inferior outcomes, independent of HLA-DP mismatching.23-25 The similarities of G2-associated risk among HLA-matched and HLA-DQ–mismatched patients reinforces the high-risk nature of G2 molecules and the consistency of those associations in 2 different clinical populations strengthens the functional significance of the HLA-DQ heterodimer. Several findings point to the presence of G2 molecules, more so than the absence of G1 molecules, as the biologically important factor: similarly high risks of relapse in patients with G1G2 or G2G2 compared with those with G1G1, a stronger dose effect and magnitude of G2-associated risks compared with G1, and a prominent effect of G2 mismatching on outcome.
The findings from the current study suggest that HLA-DQ is a classic transplantation antigen, but is one that follows a different paradigm than other class II antigens. A heterodimer model includes consideration of HLA-DQA1 to define total numbers of unique and/or mismatched molecules. The clinical significance of DQα mismatching on clinical outcome, per se, was not the goal of the current study. A more mature clinical experience is needed to isolate DQα-associated effects and functional residues. In addition, the direct ascertainment of intact intracellular RNA available for cis- and trans-heterodimers was not feasible in the current study population. Nonetheless, the use of HLA-DQA1 to define the number and kind of HLA-DQ molecules provides an approach for lowering risks for future patients.31 Although trans-dimers cannot be directly distinguished by serology or molecular methods, the structural definition of G1 and G2 heterodimers can be readily applied to predict trans-dimers for G1G1 and G2G2 individuals.16,17 Because these patients may encode up to 4 HLA-DQ molecules, they are likely to benefit from complete matching to lower the total number of heterodimer mismatches with their donors. For G2+ patients, particularly G1G2, donor matching for G2 may lower risks for the patient. In HLA-matched transplantation, G2+patients with malignant blood diseases may benefit from posttransplant therapies directed at lowering disease recurrence. These considerations may be readily introduced into practice for current patients, as HLA-DQA1 and -DQB1 assessment are routinely performed in the evaluation of patients and candidate donors before transplantation.
The lack of a strong association between G2 molecules and GVHD in both HLA-DQ–matched and –mismatched transplantation may provide insight into the opposing effects of relapse and GVHD.32 Because G2 molecules primarily affect relapse and not GVHD, a heterodimer model may permit dissection of GVHD from relapse. Future studies are warranted to validate the findings in independent transplant populations and to explore the precise mechanisms that underpin G2-associated relapse. In addition, organ involvement of acute and chronic GVHD was not available for the current study population and future investigation into whether G1 or G2 could impact organ-specific GVHD is warranted. Donor recognition of minor histocompatibility antigens may stimulate graft-versus-host recognition, but the presence of G2 molecules may still place the patient at higher risk for relapse. Given the complex series of events that begin with the production of α and β chains and culminate in stable cell-surface expression, possible mechanisms could include, but are not limited to, the relative expression of α and β chains; the assembly and transport of the HLA-DQ/invariant chain complex; the peptide repertoire; the peptide-binding region residues that distinguish G2 from G1 molecules, and the specific number, nature, and location of αβ residues that alter T-cell recognition of the peptide-HLA-DQ complex.20,33-39 Features of HLA-DQA1*01:02-DQB1*06:02, the same G2 molecule that predisposes to narcolepsy but is protective in type 1 diabetes, may offer additional insight.11,40 It is possible that as yet undefined variant(s) in strong positive linkage disequilibrium with G1 or G2 alleles could contribute to the observed effects and will require future fine-mapping studies of the HLA region in very large clinical populations. Finally, our results, together with those demonstrating the importance of HLA loss in relapse,41,42 support the relevance of HLA class II–restricted responses in immune control. Although HLA-DQ antigens have traditionally been considered to be low expression,43 potential differential expression of G1 and G2 molecules is an intriguing mechanism. The clinical effects associated with low-expression HLA-DQ mismatches may be amplified with additional mismatches elsewhere.44 The functional significance of multilocus disparity involving G1 and G2 is an important area of investigation for future studies. Lastly, G1- or G2-associated effects may include competition for heterodimer formation. The potential for G1G2 heterozygous individuals to differentially express particular heterodimers remains an intriguing mechanism.45
Despite the similarity of overall gene organization to other class II loci, HLA-DQ contributes unique clinical effects that are part and parcel of its structure. Trans-dimerization in HLA-matched and –mismatched transplantation is an important source of variation. G2 molecules are classic HLA antigens, the recognition of which is sufficient, per se, to trigger an immune response or lack thereof, without a specific requirement for mismatching. A paradigm that accommodates DQα and DQβ sequence variation and its influence on structure may provide a functional schema for understanding the role of HLA-DQ in transplantation.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
This work was supported by National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases grant AI069197 (E.W.P, T.A.G., P.S., C.M., and S.R.S); National Cancer Institute grants CA218285 (E.W.P, T.A.G., P.S., and C.M.), CA100019 (E.W.P., T.A.G., P.S., and C.M.), CA015704 (T.A.G. and P.S.), and 5U24CA076518 (M.H. and S.R.S.); National Heart, Lung, and Blood Institute grant HL069294 (M.H. and S.R.S.); and US Office of Naval Research grants N00014-20-1-2705 and N00014-20-1-2832 (M.H. and S.R.S.).
Footnotes
Requests for data may be made to the corresponding author (epetersd@fredhutch.org).
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: E.W.P. designed the study and drafted the manuscript; P.S. and T.A.G. performed the statistical analyses; and all authors assembled the data and critically reviewed, edited, and approved the final version of the manuscript.
Conflict-of-interest disclosure: E.S. is listed as an inventor on patent applications filed by the University Medical Center Utrecht on the prediction of an alloimmune response against mismatched HLA. S.R.S. reports grants from Office of Naval Research, grants from NIH, during the conduct of the study. The remaining authors declare no competing financial interests.
Correspondence: Effie W. Petersdorf, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, Seattle, WA 98109; e-mail: epetersd@fredhutch.org.
REFERENCES
- 1.van Rood JJ, van Leeuwen A, Keuning JJ, van Oud Alblas AB. The serological recognition of the human MLC determinants using a modified cytotoxicity technique. Tissue Antigens. 1975;5(2):73-79. [DOI] [PubMed] [Google Scholar]
- 2.Bodmer JG. Ia Serology. In: Bodmer WF, Batchelor JR, Bodmer, JG, eds. Histocompatibility Testing 1977. Copenhagen, Denmark: Munksgaard, 1978:351-357. [Google Scholar]
- 3.Duquesnoy RJ, Marrari M, Annen K. Identification of an HLA-DR-associated system of B-cell alloantigens. Transplant Proc. 1979;11(4):1757-1760. [PubMed] [Google Scholar]
- 4.Terasaki PI, Park MS, Bernoco D, et al. Overview of the 1980 International Histocompatibility Workshop. In: Terasaki PI, ed. Histocompatibility Testing 1980. Los Angeles, CA: UCLA Tissue Typing Laboratory, 1980. [Google Scholar]
- 5.McDevitt HO, Bodmer WF. HL-A, immune-response genes, and disease. Lancet. 1974;303(7869):1269-1275. [DOI] [PubMed] [Google Scholar]
- 6.Thorsby E. Invited anniversary review: HLA associated diseases. Hum Immunol. 1997;53(1):1-11. [DOI] [PubMed] [Google Scholar]
- 7.Hill AVS. The immunogenetics of human infectious diseases. Annu Rev Immunol. 1998;16(1):593-617. [DOI] [PubMed] [Google Scholar]
- 8.Shiina T, Hosomichi K, Inoko H, Kulski JK. The HLA genomic loci map: expression, interaction, diversity and disease. J Hum Genet. 2009;54(1):15-39. [DOI] [PubMed] [Google Scholar]
- 9.Erlich H, Valdes AM, Noble J, et al. ; Type 1 Diabetes Genetics Consortium . HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the type 1 diabetes genetics consortium families. Diabetes. 2008;57(4):1084-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sollid LM. The roles of MHC class II genes and post-translational modification in celiac disease. Immunogenetics. 2017;69(8-9):605-616. [DOI] [PubMed] [Google Scholar]
- 11.Chabas D, Taheri S, Renier C, Mignot E. The genetics of narcolepsy. Annu Rev Genomics Hum Genet. 2003;4(1): 459-483. [DOI] [PubMed] [Google Scholar]
- 12.Brown JH, Jardetzky TS, Gorga JC, et al. Three-dimensional structure of the human class II histocompatibility antigen HLA-DR1. Nature. 1993;364(6432):33-39. [DOI] [PubMed] [Google Scholar]
- 13.Parham P. HLA, anthropology, and transplantation. Transplant Proc. 1993; 25(1 Pt 1):159-161. [PubMed] [Google Scholar]
- 14.The Immuno Polymorphism Database (IPD)-ImMunoGeneTics (IMGT)/HLA Database . Available at: https://www.ebi.ac.uk/ipd/imgt/hla/. Accessed 5 February 2022.
- 15.Charron DJ, Lotteau V, Turmel P. Hybrid HLA-DC antigens provide molecular evidence for gene trans-complementation. Nature. 1984;312(5990):157-159. [DOI] [PubMed] [Google Scholar]
- 16.Kwok WW, Nepom GT. Structural and functional constraints on HLA class II dimers implicated in susceptibility to insulin dependent diabetes mellitus. Baillieres Clin Endocrinol Metab. 1991;5(3):375-393. [DOI] [PubMed] [Google Scholar]
- 17.Tollefsen S, Hotta K, Chen X, et al. Structural and functional studies of trans-encoded HLA-DQ2.3 (DQA1*03:01/DQB1*02:01) protein molecule. J Biol Chem. 2012;287 (17):13611-13619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kooy-Winkelaar Y, van Lummel M, Moustakas AK, et al. Gluten-specific T cells cross-react between HLA-DQ8 and the HLA-DQ2α/DQ8β transdimer. J Immunol. 2011;187(10):5123-5129. [DOI] [PubMed] [Google Scholar]
- 19.van Lummel M, van Veelen PA, Zaldumbide A, et al. Type 1 diabetes-associated HLA-DQ8 transdimer accommodates a unique peptide repertoire. J Biol Chem. 2012; 287(12):9514-9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chow IT, Gates TJ, Papadopoulos GK, et al. Discriminative T cell recognition of cross-reactive islet-antigens is associated with HLA-DQ8 transdimer-mediated autoimmune diabetes. Sci Adv. 2019;5(8):eaaw9336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bach FH, van Rood JJ. The major histocompatibility complex--genetics and biology (first of three parts). N Engl J Med. 1976;295(15):806-813. [DOI] [PubMed] [Google Scholar]
- 22.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110(13):4576-4583. [DOI] [PubMed] [Google Scholar]
- 23.Fleischhauer K, Shaw BE, Gooley T, et al. ; International Histocompatibility Working Group in Hematopoietic Cell Transplantation . Effect of T-cell-epitope matching at HLA-DPB1 in recipients of unrelated-donor haemopoietic-cell transplantation: a retrospective study. Lancet Oncol. 2012;13(4):366-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petersdorf EW, Carrington M, O’hUigin C, et al. ; International Histocompatibility Working Group in Hematopoietic Cell Transplantation . Role of HLA-B exon 1 in graft-versus-host disease after unrelated haemopoietic cell transplantation: a retrospective cohort study. Lancet Haematol. 2020;7(1):e50-e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fürst D, Müller C, Vucinic V, et al. High-resolution HLA matching in hematopoietic stem cell transplantation: a retrospective collaborative analysis [published correction appears in Blood. 2014;123(11):1768]. Blood. 2013;122(18):3220-3229. [DOI] [PubMed] [Google Scholar]
- 26.Little A-M, Akbarzad-Yousefi A, Anand A, et al. BSHI guideline: HLA matching and donor selection for haematopoietic progenitor cell transplantation. Int J Immunogenet. 2021;48(2):75-109. [DOI] [PubMed] [Google Scholar]
- 27.Robinson J, Halliwell JA, Hayhurst JD, Flicek P, Parham P, Marsh SGE. The IPD and IMGT/HLA database: allele variant databases. Nucleic Acids Res. 2015;43(database issue):D423-D431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raymond CK, Kas A, Paddock M, et al. Ancient haplotypes of the HLA Class II region. Genome Res. 2005;15(9):1250-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trowsdale J, Knight JC. Major histocompatibility complex genomics and human disease. Annu Rev Genomics Hum Genet. 2013;14(1):301-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Battiwalla M, Ellis K, Li P, et al. HLA DR15 antigen status does not impact graft-versus-host disease or survival in HLA-matched sibling transplantation for hematologic malignancies. Biol Blood Marrow Transplant. 2012;18(8):1302-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt AH, Sauter J, Baier DM, et al. Immunogenetics in stem cell donor registry work: The DKMS example (part 2). Int J Immunogenet. 2020;47(2):139-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horowitz MM, Gale RP, Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75(3):555-562. [PubMed] [Google Scholar]
- 33.Yamamoto F, Suzuki S, Mizutani A, et al. Capturing differential allele-level expression and genotypes of all classical HLA loci and haplotypes by a new capture RNA-Seq method. Front Immunol. 2020;11:941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Unanue ER, Turk V, Neefjes J. Variations in MHC class II antigen processing and presentation in health and disease. Annu Rev Immunol. 2016;34(1):265-297. [DOI] [PubMed] [Google Scholar]
- 35.van Lith M, McEwen-Smith RM, Benham AM. HLA-DP, HLA-DQ, and HLA-DR have different requirements for invariant chain and HLA-DM. J Biol Chem. 2010;285(52):40800-40808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silva DG, Socha L, Correcha M, Petrovsky N. Elevated lymphocyte expression of CLIP is associated with type 1 diabetes and may be a useful marker of autoimmune susceptibility. Ann N Y Acad Sci. 2004;1037(1):65-68. [DOI] [PubMed] [Google Scholar]
- 37.Busch R, De Riva A, Hadjinicolaou AV, Jiang W, Hou T, Mellins ED. On the perils of poor editing: regulation of peptide loading by HLA-DQ and H2-A molecules associated with celiac disease and type 1 diabetes. Expert Rev Mol Med. 2012;14:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siebold C, Hansen BE, Wyer JR, et al. Crystal structure of HLA-DQ0602 that protects against type 1 diabetes and confers strong susceptibility to narcolepsy. Proc Natl Acad Sci USA. 2004;101(7):1999-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou Z, Jensen PE. Structural characteristics of HLA-DQ that may impact DM editing and susceptibility to type-1 diabetes. Front Immunol. 2013;4:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Lummel M, Buis DTP, Ringeling C, et al. Epitope stealing as a mechanism of dominant protection by HLA-DQ6 in type 1 diabetes. Diabetes. 2019;68(4):787-795. [DOI] [PubMed] [Google Scholar]
- 41.Christopher MJ, Petti AA, Rettig MP, et al. Immune escape of relapsed AML cells after allogeneic transplantation. N Engl J Med. 2018;379(24):2330-2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toffalori C, Zito L, Gambacorta V, et al. Immune signature drives leukemia escape and relapse after hematopoietic cell transplantation. Nat Med. 2019;25(4):603-611. [DOI] [PubMed] [Google Scholar]
- 43.Alonso MC, Navarrete C, Solana R, Torres A, Pena J, Festenstein H. Differential expression of HLA-DR and HLA-DQ antigens on normal cells of the myelomonocytic lineage. Tissue Antigens. 1985;26(5):310-317. [DOI] [PubMed] [Google Scholar]
- 44.Fernández-Viña MA, Klein JP, Haagenson M, et al. Multiple mismatches at the low expression HLA loci DP, DQ, and DRB3/4/5 associate with adverse outcomes in hematopoietic stem cell transplantation. Blood. 2013;121(22):4603-4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ollila HM, Fernández-Viña M, Mignot E. HLA-DQ allele competition in narcolepsy: a comment on Tafti et al. DQB1 locus alone explains most of the risk and protection in narcolepsy with cataplexy in Europe. Sleep (Basel). 2015;38(1):147-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.