Abstract
Purpose:
Enteral feeding intolerance (EFI) is a frequent problem in the intensive care unit (ICU), but current prokinetic agents have uncertain efficacy and safety profiles. The current study compared the efficacy and safety of ulimorelin, a ghrelin agonist, with metoclopramide in the treatment of EFI.
Methods:
One hundred twenty ICU patients were randomized 1:1 to ulimorelin or metoclopramide for 5 days. EFI was diagnosed by a gastric residual volume (GRV) ≥ 500 ml. A volume-based feeding protocol was employed, and enteral formulas were standardized. The primary end point was the percentage daily protein prescription (%DPP) received by patients over 5 days of treatment. Secondary end points included feeding success, defined as 80% DPP; gastric emptying, assessed by paracetamol absorption; incidences of recurrent intolerance (GRV ≥ 500 ml); vomiting or regurgitation; aspiration, defined by positive tracheal aspirates for pepsin; and pulmonary infection.
Results:
One hundred twenty patients were randomized and received the study drug (ulimorelin 62, metoclopramide 58). Mean APACHE II and SOFA scores were 21.6 and 8.6, and 63.3% of patients had medical reasons for ICU admission. Ulimorelin and metoclopramide resulted in comparable %DPPs over 5 days of treatment (median [Q1, Q3]: 82.9% [38.4%, 100.2%] and 82.3% [65.6%, 100.2%], respectively, p = 0.49). Five-day rates of feeding success were 67.7% and 70.6% when terminations unrelated to feeding were excluded, and there were no differences in any secondary outcomes or adverse events between the two groups.
Conclusions:
Both prokinetic agents achieved similar rates of feeding success, and no safety differences between the two treatment groups were observed.
Keywords: Ulimorelin, Metoclopramide, PROMOTE, Enteral feeding intolerance, Volume-based feeding, Gastric residual volume
Introduction
Enteral feeding intolerance (EFI), defined as the inability to deliver adequate enteral nutrition to critically ill patients due to delayed gastric emptying in the absence of mechanical obstruction, is a frequent problem in critically ill patients. Observational studies and meta-analyses have revealed that > 30% of critically ill patients experience intolerance to enteral feeding and that these patients have longer intensive care unit (ICU) stays and higher mortality compared with the tolerant population [1, 2].
EFI is most commonly diagnosed by the presence of high gastric residual volume (GRV). Elevated GRV has been shown to predict delayed gastric emptying and portend worse ICU prognosis [3, 4]. A GRV of 500 ml is the recommended threshold for EFI diagnosis in US and European critical care and nutrition society guidelines [5–7].
Prokinetic agents are recommended to promote GI motility and facilitate enteral feedings in patients with EFI [8, 9]. Metoclopramide, the most commonly used agent, has been associated with delirium, agitation, QT prolongation and sudden cardiac death [10]. Erythromycin has been associated with drug-drug interactions, QT prolongation and super-infection with multiple drug-resistant organisms [9]. While the synergy of these drugs has been reported, both agents may rapidly lose effectiveness [11], and neither is approved by regulatory agencies for EFI treatment.
Ghrelin is a naturally occurring peptide and a potent stimulator of growth hormone (GH) secretion and gastric emptying [12, 13]. It possesses anticatabolic and anti-inflammatory properties that could be beneficial in critical illness [14–18]. Ulimorelin, an intravenous, selective synthetic ghrelin agonist, has been studied in over 900 subjects in clinical trials without effects on the central nervous system (CNS) or QT interval [19–21].
The purpose of this trial was to evaluate the efficacy and safety of ulimorelin in critically ill patients with EFI. The hypothesis was that ulimorelin would be superior to metoclopramide in improving nutritional intake with enhanced safety. Because metoclopramide represents the standard of care for EFI treatment at most institutions [9], the study was designed to compare the efficacy and safety of ulimorelin with metoclopramide in this condition.
Methods
Study design
This was a randomized, double-blind, comparator-controlled study conducted at 20 sites in the USA, Canada, Spain and The Netherlands between October 2016 and March 2018. The Institutional Review Boards (IRBs)/Independent Ethics Committees (ECs) approved the study protocol at all participating sites. Informed consent was obtained from the patient’s legally authorized representative or proxy, as required by national laws, respective regulations and IRB/ECs. Translations were provided for non-English-speaking patients. Whenever possible, written informed consent was sought from the patient as soon as he/she became capable of comprehending the scope of the study. All study procedures were consistent with Good Clinical Practice Guidelines and followed the Declaration of Helsinki for protection of human subjects.
This study was overseen by an independent data monitoring committee. The trial was registered in ClinicalTrials.gov (NCT02784392) and EUDRACT (2016–000723-94).
Study population
Patients were eligible for study participation if they were in the ICU, intubated and mechanically ventilated and intolerant to gastric tube feedings. EFI was defined as a GRV ≥ 500 ml on one or more measurements. Patients were required to have a ≥ 12-Fr feeding tube and expected to remain intubated, mechanically ventilated and receiving gastric tube feedings for ≥ 48 h. Patients were excluded from the study for use of prokinetic medications within 48 h; weight > 150 kg; pregnancy; suspicion of active bowel obstruction, perforation or leakage; esophageal or gastric surgery prior to or during the current hospitalization; a history of gastroparesis; Child’s C cirrhosis; QT interval by Fridericia’s formula (QTcF) > 450 ms on a 12-lead ECG or rapid deterioration. When 50% of patients had enrolled, the QTcF restriction was liberalized to 480 ms.
Study intervention
Patients were randomized 1:1 to ulimorelin (600 μg/kg) or metoclopramide (10 mg) administered as a 50-ml IV infusion over 30 min every 8 h for 5 days.
Ulimorelin was supplied as a sterile solution of ulimorelin·HCl monohydrate (2 mg/ml) in water; metoclopramide injection (5 mg/ml) was supplied as a sterile solution in single-use vials. The dose of metoclopramide was reduced 50% in patients with creatinine clearance ≤ 40 ml/min by the Cockcroft-Gault formula and by 75% for patients whose clearance was ≤ 10 ml/min or who were on dialysis or continuous renal replacement techniques [22].
The randomization schedule was generated with SAS software (SAS Institute Inc., Cary, NC, USA) using a blocked randomization scheme and a block size of 4. Study medications were prepared by an unblinded pharmacist to ensure correct treatment assignment. Standard operating procedures assured that all other operational personnel remained blinded to treatment assignment.
Study procedures
The daily protein prescription (DPP) and daily caloric prescription (DCP) were set based on the clinical condition of the patient and to achieve no less than 1.2 g protein/kg body weight and 25 kcal/kg per 24-h period based on estimated dry weight of the patient at ICU admission. Feedings in obese patients (BMI ≥ 30) were adjusted by adding 25% of the differences between estimated dry weight and ideal body weight (IBW) to the DPP and DCP, using a standard formula for IBW [23]. Oral protein supplements were permitted in obese patients, but they were not included in the assessment of the primary end point.
A volume-based feeding protocol was employed to compensate for enteral nutrition missed because of interruptions to feeding for procedures or other interventions [24] (Supplementary Fig. 1). To minimize the effects of different enteral formula contents on gastric emptying, formulas were restricted to products containing 125–133 kcal, 6.2–7.0 g protein and 3.4–4.9 g fat per 100 ml. Blood glucose was maintained to a target glucose < 180 mg/dl (10 mmol/l). Endotracheal aspirates were obtained each day, autolyzed and analyzed for pepsin by LC–MS/MS (LGC Ltd., London, UK). Non-nutritional calories from propofol were recorded.
Gastric emptying was assessed by the maximal concentration (Cmax) of paracetamol (1 g) absorption at screening and day 4 [25]. Serum levels of GH, IGF-1, CRP, IL-6, IL-10 and electrocardiograms (ECGs) were obtained daily, and peak GH, IGF-1 and ulimorelin plasma concentrations were measured at the end of drug infusions.
Patients discontinued the study drug if they were discharged from the ICU, discontinued tube feedings, developed a severe or serious adverse event that was deemed related to the study drug, or treatment failure, defined as the need for another prokinetic medication, small bowel feedings or total parenteral nutrition. When the study drug or feeding protocol was terminated early, all safety and laboratory procedures were continued through study completion.
Outcomes
Efficacy end points
The primary efficacy end point was the median daily average percentage of DPP received through enteral nutrition over the 5 days of treatment with the study drug. The secondary efficacy end point was the median daily average percentage of DCP. Other efficacy end points included feeding success, defined as receiving 80% of DPP; recurrence of intolerance (GRV ≥ 500 ml); vomiting and regurgitation; gastric emptying; episodes of pepsin in tracheal aspirates; ventilator-free days and 30-day mortality. Patients were assessed for pulmonary infection up to 3 days following the final dose of study drug, employing a previously described method [26].
Safety end points
Safety end points included AEs, ECGs, laboratory data and 30-day mortality. Patients were followed for AEs from the signing of informed consent through 3 days following the final dose of study drug.
Statistical methods
Study population
The primary analysis population was a modified intention-to-treat (mITT) population, defined as patients who were randomized and received at least one dose of study drug.
Continuous end points were analyzed by paired or unpaired t-test for Gaussian distributions and by the Wilcoxon signed rank or Mann-Whitney test for non-parametric distribution, with the Gaussian distribution of continuous variables assessed by the Shapiro-Wilk test. Categorical end points were analyzed by Fisher’s exact test. Site effects were evaluated using a logistic mixed effect model with treatment group and site as fixed and random effects. Normal gastric emptying was defined as paracetamol Cmax 8100 ng/ml [25]. Durations of ICU and hospital stays were analyzed according to the Kaplan-Meier method considering discharge of ICU or hospital as the event. Patients who died prior to discharge from the ICU or hospital were censored at the time of death. The log-rank test was used to compare the duration of ICU stay between treatment groups. Associations among elevated GRV, pepsin in tracheal aspirates and the incidence of pulmonary infection were evaluated by non-parametric regression using Spearman regression coefficients (rho). Analyses were performed with SAS 9.4, and α ≤ 0.05 (two-sided) was used for statistical significance.
Patients who discontinued tube feeding because of resumption of oral intake were assumed to have reached 100% of DPP on all days from the point that oral intake commenced. Conversely, patients who otherwise discontinued enteral feedings or died prior to day 5 were assumed to have received 0% of DPP on the days from the point that enteral nutrition ceased. In situations where the primary or secondary end point was missing for reasons where imputation of 0% or 100% was not reasonable, multiple imputations were performed by a data review committee on blinded data using non-missing days to impute the missing days. Sensitivity analyses were performed by imputing the missing values following the distribution of the other arm and by excluding patients with missing data.
Sample size
A total of 120 evaluable patients (60 in the ulimorelin arm and 60 in the metoclopramide arm) were estimated to provide 80% power and a two-sided α of 0.05 to demonstrate the superiority of ulimorelin versus metoclopramide with respect to the primary end point. This assumed a within-group standard deviation (SD) of 25%, a 15% increase in DPP between the ulimorelin and metoclopramide treatment arms, and 15% of patients included in the mITT analysis despite receiving nil to minimal enteral feedings because of death, surgical procedures or disruptions unrelated to intolerance.
Results
Patient demographics and disposition
The CONSORT diagram is shown in Supplementary Fig. 2. Four hundred patients were screened, 122 randomized and 120 dosed. Baseline demographics were comparable between the two treatment groups (Table 1).
Table 1.
Study population: baseline demographics
| Ulimorelin (n = 62) | Metoclopramide (n = 58) | Total (n = 120) | |
|---|---|---|---|
|
| |||
| Demographics | |||
| Age, years, mean (SD) | 59.4 (13.5) | 57.3 (17.3) | 58.4 (15.4) |
| Female, n (%) | 15 (24.2) | 18 (31.0) | 33 (27.5) |
| Male, n (%) | 47 (75.8) | 40 (69.0) | 87 (72.5) |
| Dry body weight at ICU admission, kg, mean (SD) | 85.5 (16.4) | 82.4 (16.9) | 84.0 (16.6) |
| Body mass index (BMI), kg/m2, mean (SD) | 28.8 (5.7) | 27.5 (4.9) | 28.2 (5.4) |
| Days in ICU before 1st dose study drug, median [Q1, Q3] | 4.6 [3.0, 6.9] | 5.5 [3.5, 7.7] | 4.9 [3.3, 7.6] |
| Reasons for ICU admission | |||
| Medical, n (%) | 40 (64.5) | 36 (62.1) | 76 (63.3) |
| Respiratory | 20 (32.3) | 15 (25.9) | 35 (29.2) |
| Neurologic | 9 (14.5) | 9 (15.5) | 18 (15.0) |
| Trauma | 5 (8.1) | 4 (6.9) | 9 (7.5) |
| Vascular | 5 (8.1) | 0 (0.0) | 5 (4.2) |
| Sepsis | 1 (1.6) | 4 (6.9) | 5 (4.2) |
| Gastrointestinal | 0 (0.0) | 1 (1.7) | 1 (0.8) |
| Metabolic | 0 (0.0) | 1 (1.7) | 1 (0.8) |
| Other | 1 (1.6) | 2 (3.4) | 3 (2.5) |
| Surgical, n (%) | 22 (35.4) | 22 (37.9) | 44 (36.7) |
| Trauma | 6 (9.7) | 9 (15.5) | 15 (12.5) |
| Neurologic | 7 (11.3) | 2 (3.4) | 9 (7.5) |
| Gastrointestinal | 4 (6.5) | 1 (1.7) | 8 (4.2) |
| Respiratory | 1 (1.6) | 4 (6.9) | 5 (4.2) |
| Vascular | 1 (1.6) | 2 (3.4) | 3 (2.5) |
| Other | 3 (6.4) | 4 (6.8) | 7 (5.8) |
| Risk scores, mean (SD) | |||
| APACHE II | 22.8 (9.6) | 20.7 (7.3) | 21.8 (8.6) |
| SOFA | 8.8 (3.9) | 8.3 (4.1) | 8.6 (4.0) |
| NUTRIC score | 4.9 (2.3) | 4.4 (2.2) | 4.7 (2.3) |
| Concomitant medications, n (%) | |||
| Vasopressors | 29 (46.8) | 25 (43.1) | 54 (45.0) |
| Opiates | 10 (16.1) | 12 (20.7) | 22 (18.3) |
| Sedatives | 14 (22.5) | 18 (31.0) | 32 (26.7) |
| Paralytic agents | 9 (14.5) | 4 (6.5) | 13 (10.8) |
| Propofol | 11 (17.7) | 5 (8.6) | 16 (13.3) |
| Nutritional targets, median [Q1, Q3] | |||
| Daily protein prescription, g/kg/day | 1.3 [1.2, 1.3] | 1.3 [1.2, 1.3] | 1.3 [1.2, 1.3] |
| Daily caloric prescription, kcal/kg/day | 25.0 [25.0, 25.1] | 25.0 [25.0, 25.8] | 25.0 [25.0, 25.2] |
SD, standard deviation; Q1, Q3, first and third quartiles
Differences between groups were not statistically significant (Wilcoxon signed rank test)
Of the patients, 74.2% completed 5 days of therapy or advanced to oral feedings; 25.8% terminated feedings before the completion of the 5-day treatment period. Most early terminations of treatment were for reasons unrelated to feeding; only 10.0% of patients terminated for treatment failure (Supplementary Table 1).
Efficacy
The primary end point, the daily average %DPP received through enteral nutrition on efficacy phase days 1 through 5, was not different between groups. The median [Q1, Q3] %DPPs achieved across the 5 days of treatment, days 1–5, were 82.9% [38.4%, 100.2%] and 82.3% [65.6%, 100.2%] for the ulimorelin and metoclopramide treatment groups, respectively (p = 0.49), with no differences on any of the individual 5 treatment days (Fig. 1). Median calories from propofol were 24 kcal/day in both treatment groups, and only eight patients received protein supplements. The daily average percentage of DCP received through enteral nutrition co-varied exactly with the primary end point.
Fig. 1.
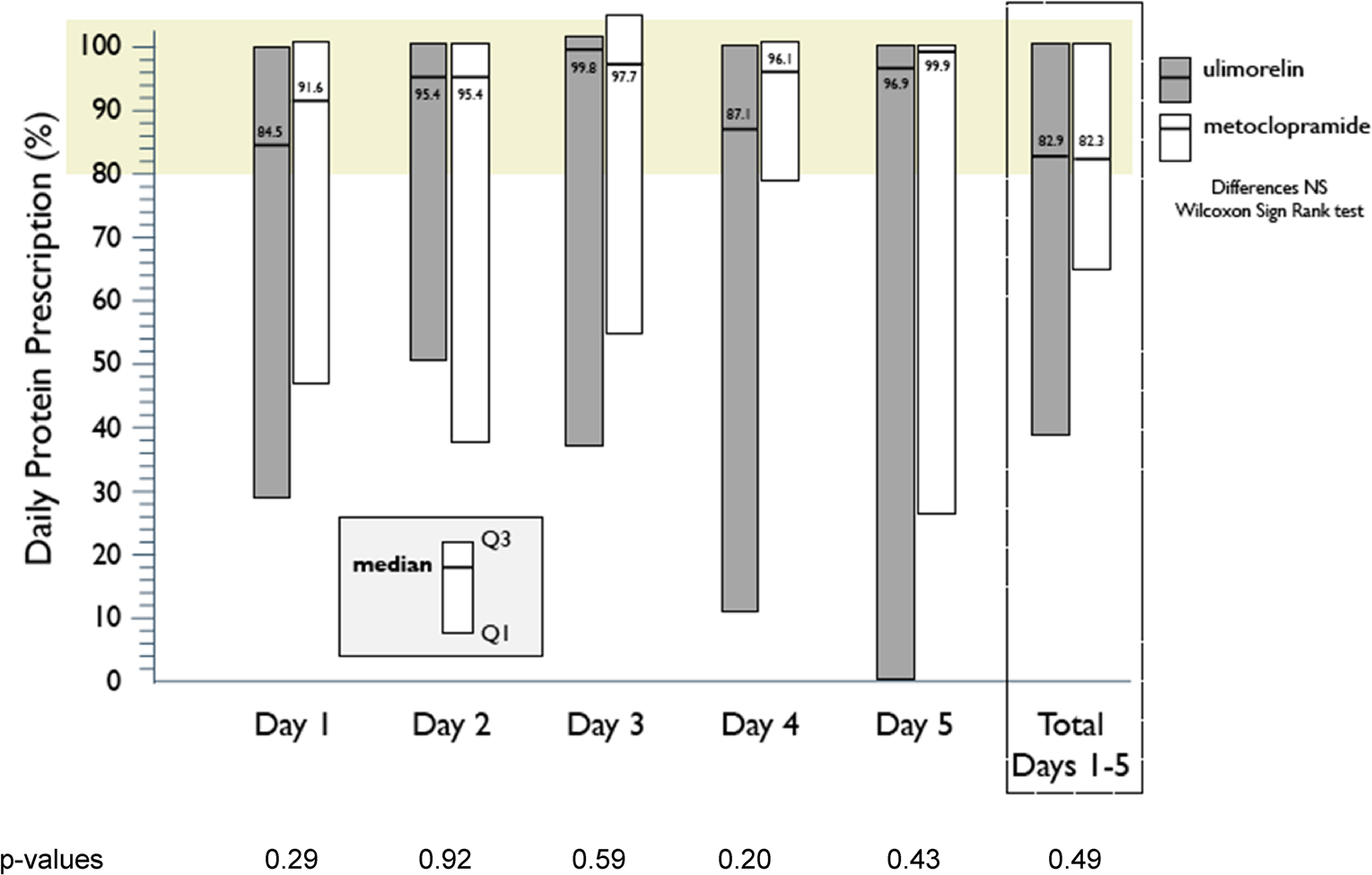
Primary end point of the trial, the % daily protein prescription achieved through enteral nutrition on days 1 through 5 and on individual study days. The results are expressed as median [1st quartile, 3rd quartile]. High median %DPP rates are noted on each of the study days commencing with day 1. Median percentages for days 1–5 and the totals for the 5 days include all patients dosed (ulimorelin 62, metoclopramide 58), imputing values for patients with missing data, as discussed in “Methods.” No significant differences were noted between treatment groups over the 5 days of study drug administration or any of the study days (Wilcoxon signed rank test). The shaded area represents ≥ 80% DPP achieved
Of the ulimorelin and metoclopramide patients, 51.6% and 55.2% achieved feeding success over the 5 days of treatment (Fig. 2a). Feeding success rates increased to 67.7% and 70.6% for ulimorelin and metoclopramide, respectively, when terminations for reasons unrelated to feeding were excluded. Although rates of feeding success improved with each study day, this was attributed, in part, to patient withdrawals.
Fig. 2.
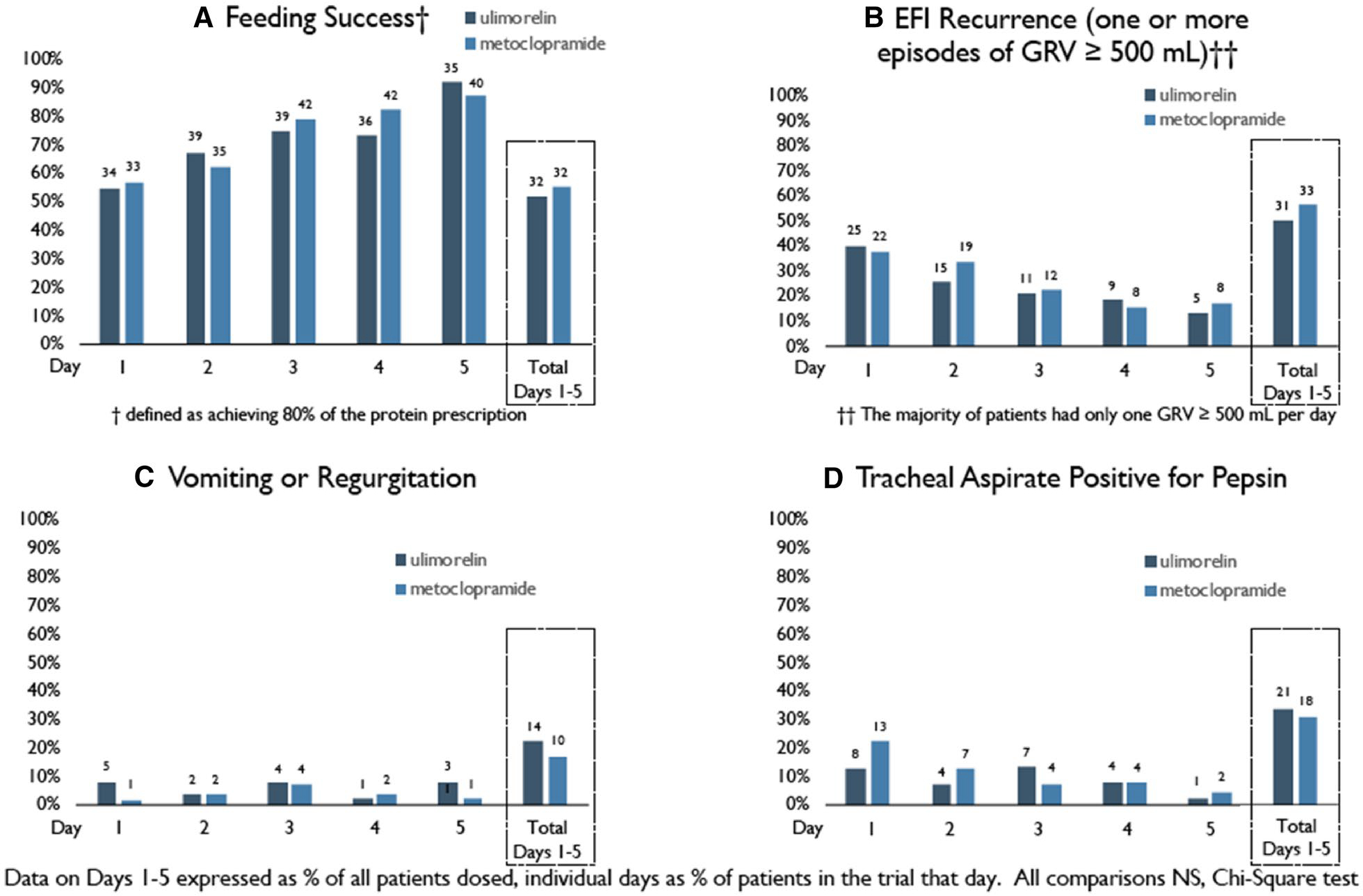
Rates of a feeding success, b episodes of recurrent EFI (GRV ≥ 500 ml), c vomiting or regurgitation and d a positive tracheal aspirate for pepsin on each of the study days, days 1–5, and over the 5 days of the study. Totals, days 1–5, represent the percentages of patients who achieved feeding success over the 5 study days or the total numbers of patients who experienced one or more episodes of EFI recurrence, vomiting or regurgitation, or a positive tracheal aspirate for pepsin on any of the 5 study days, as the percentage of patients dosed. The proportions on individual days are expressed as the percentages of subjects remaining in the trial each day [n = 62, 58, 52, 49 and 38 (ulimorelin) and 58, 56, 53, 51 and 46 (metoclopramide), days 1 through 5, respectively]. None of the differences between treatment groups were statistically significant (chi-squared test). While the daily proportions of patients with feeding success increased, in part because of the declining number of patients remaining in the trial, daily declines are evident in the percentages of patients who experienced EFI recurrence or a positive tracheal aspirate for pepsin
Of the ulimorelin and metoclopramide patients, 50.0% and 56.9% had one or more episodes of EFI recurrence during the trial, but rates declined progressively; by day 5, only 13.2% and 17.4% of patients continued to have EFI episodes (Fig. 2b). Twenty-two percent and 17.2% experienced one or more episodes of regurgitation during the 5 days of feeding (Fig. 2c), but < 8.1% of patients experienced an episode on any single day; 33.9% and 31.0% of patients had a positive tracheal aspirate over the 5 days of treatment, but rates declined steadily over the treatment duration (Fig. 2d).
Of the ulimorelin-treated patients 81.3% and of metoclopramide-treated patients 83.3% had delayed GE by Cmax at baseline; mean (SD) increases in Cmax on day 4 were 98.2% (335.5) and 76.7% (121.2) for ulimorelin vs. metoclopramide, respectively (p = 0.77). Target ulimorelin plasma concentrations were achieved in 98% of patients.
Ulimorelin and metoclopramide patients experienced similar ventilator-free days, ICU length of stay, hospital length of stay and incidences of ICU-acquired pneumonia (Supplementary Table 2). On linear regression, no associations were noted between the number of episodes of elevated GRV and the incidence of a positive tracheal aspirate for pepsin (p = 0.12, r2 = 0.009). Likewise, no association was noted between the occurrence of an elevated GRV or positive tracheal aspirate for pepsin and the incidence of pulmonary infection (Table 2). Changes in GH, IGF-1 and inflammatory markers are shown in Supplementary Table 3.
Table 2.
Associations among episodes of intolerance (GRV ≥ 500 ml), aspiration and pulmonary infection
|
(A) Relationship between episodes of intolerance and the incidence of pulmonary infections
| |||||||
| Pulmonary infection |
Patients with GRV ≥ 500 ml, by number of episodes experienced
|
ρ | p value | ||||
| 0 (n = 56) | 1 (n = 14) | 2 (n = 11) | 3 (n = 11) | ≥ 4 (n = 28) | |||
|
| |||||||
| ICU-acquired pneumonia, n (%) | 10 (17.9) | 4 (28.6) | 2 (18.2) | 1 (9.1) | 7 (25.0) | 0.05 | 0.48 |
| Lower respiratory tract infection (excluding pneumonia), n (%) | 11 (19.6) | 4 (28.6) | 3 (27.3) | 1 (9.1) | 9 (32.1) | 0.08 | 0.28 |
|
| |||||||
|
(B) Relationship between positive tracheal aspirates for pepsin and pulmonary infection
| |||||||
| Pulmonary infection |
Patients with tracheal aspirates positive for pepsin, by number of positive aspirates
|
ρ | p value | ||||
|
0 (n = 75)
n (%) |
1 (n = 20)
n (%) |
2 (n = 10)
n (%) |
3 or more (n = 9)
n (%) |
||||
|
| |||||||
| ICU-acquired pneumonia | 19 (25.3) | 2 (10.0) | 1 (10.0) | 1 (11.1) | 0.17 | 0.067 | |
| Lower respiratory tract infection (excluding pneumonia) | 21 (28.0) | 2 (10.0) | 3 (30.0) | 1 (11.1) | 0.13 | 0.17 | |
p value from the Spearman correlation coefficient
(A) Relationship between GRV elevation (≥ 500 ml) and the incidence of pulmonary infection, expressed as the number patients and the number of episodes of GRV elevation experienced by that patient over the 5-day treatment period post-randomization; (B) relationship between positive tracheal aspirates for pepsin and incidence of pulmonary infection, expressed as the number patients and number of aspirates positive for pepsin in that patient over the 5-day treatment period post-randomization. Definitions of ICU-acquired pneumonia and lower respiratory infection were per Heyland [33]. For these analyses, the two treatment groups were combined. Differences between patient groups were not statistically significant by chi-squared test
Safety
The proportions of AEs and SAEs were similar between the two treatment groups. A greater proportion of ulimorelin-treated patients experienced an SAE or an adverse event leading to death, while a greater proportion of metoclopramide-treated patients experienced an AE leading to study drug discontinuation (Table 3). None of these differences were statistically significant.
Table 3.
Summary of adverse events (AEs)
| Ulimorelin (n = 62) n (%) |
Metoclopramide (n = 58) n (%) |
Total (n = 120) n (%) |
|
|---|---|---|---|
|
| |||
| AEs, total | 48 (77.4) | 47 (81.0) | 95 (79.2) |
| Severe AEs | 19 (30.6) | 13 (22.4) | 32 (26.7) |
| Serious adverse events (SAEs) | 3 (4.8) | 3 (5.2) | 6 (5.0) |
| AEs leading to study drug discontinuationa | 3 (4.8) | 6 (10.3) | 9 (7.5) |
| Death due to an adverse eventb | 3 (4.8) | 1 (1.7) | 4 (3.3) |
| 30-day mortality | 19 (30.6) | 15 (25.9) | 34 (28.3) |
| AEs occurring in ≥ 5% of patientsc | |||
| CNS | |||
| Agitation | 7 (11.3) | 3 (5.5) | 10 (8.3) |
| Anxiety | 1 (1.6) | 3 (5.2) | 4 (3.3) |
| Delirium | 9 (14.5) | 6 (10.3) | 15 (12.5) |
| Cardiovascular | |||
| Atrial fibrillation | 1 (1.6) | 8 (13.8)† | 9 (7.5) |
| Supraventricular tachycardia | 2 (3.2) | 3 (5.2) | 5 (4.2) |
| Hypertension | 3 (4.8) | 4 (6.9) | 7 (5.8) |
| QT interval prolongationd | 2 (3.2) | 2 (3.4) | 4 (3.3) |
| Ventricular fibrillationd | 0 (0.0) | 1 (1.7) | 1 (0.8) |
| Ventricular tachycardia | 2 (3.2) | 3 (5.2) | 5 (4.1) |
| Respiratory | |||
| Pneumonia | 3 (4.8) | 6 (10.3) | 9 (7.5) |
| Prolonged mechanical ventilation | 6 (9.7) | 7 (12.1) | 13 (10.8) |
| Metabolic | |||
| Hyperglycemia | 8 (12.9) | 8 (13.8) | 16 (13.8) |
| Hypophosphatemia | 4 (6.5) | 1 (1.7) | 5 (4.3) |
| Other | |||
| Acute kidney injury | 7 (11.3) | 3 (5.2) | 10 (8.3) |
| Anemia | 6 (9.7) | 5 (8.6) | 11 (9.2) |
| Constipation | 2 (3.2) | 0 (6.9) | 2 (1.7) |
| Edema | 5 (8.1) | 5 (8.6) | 10 (8.3) |
| Elevated AST | 1 (1.6) | 3 (5.2) | 4 (3.3) |
| Fever | 4 (6.5) | 3 (5.2) | 7 (5.8) |
AST, aspartate amino transferase
p = 0.014; other differences were not statistically significant (chi-squared test)
AEs leading to study drug discontinuation: ulimorelin: failed stem cell bone marrow transplant, increased intracranial pressure and delirium; metoclopramide: diarrhea, paracetamol overdose, QT interval prolongation, delirium, atrial fibrillation and gastrointestinal bleeding
AEs leading to death, including the 5-day period of study drug treatment and the 3-day period following the final dose of study drug: ulimorelin; failed stem cell transplantation, progressive pulmonary disease and increased intracranial pressure; metoclopramide: progressive pulmonary disease
AEs occurring in ≥ 5% of patients in either treatment arm
incidences of QT prolongation and ventricular fibrillation are shown although they did not reach this reporting threshold
The incidences of delirium, agitation and anxiety were similar between treatment groups. Mean glucose levels were significantly higher in ulimorelin vs. metoclopramide patients [mean (SD) 9.12 (1.82) vs. 8.31 (1.97) mmol/l, p = 0.009]. Two patients in each of the treatment arms experienced AEs of QT interval prolongation. ECG parameters, including QTcF intervals, were similar between treatment groups: there were no significant differences in the incidences of ventricular tachycardia or ventricular fibrillation. Atrial fibrillation (AF) was observed in higher incidence in metoclopramide (13.8%) vs. ulimorelin (1.6%) (p = 0.014), resulting in discontinuation in one metoclopramide patient.
Thirty-day mortality was 30.6% in the ulimorelin group and 25.9% in the metoclopramide group (p = 0.69), with no differences in underlying causes of death.
Discussion
This was the largest prospective randomized clinical trial to date on the treatment of enteral feeding intolerance. No differences were noted in the primary end point of the trial, the percentage of the daily protein prescription (DPP) over 5 days of treatment. Although > 50% of patients experienced one or more recurrent EFI episode post initiation of treatment, the incidence of EFI declined with each study day, and the majority of patients achieved feeding success.
The primary efficacy end point was based on protein delivery because protein has been determined to one of the most important nutritional factors impacting ICU outcome [27, 28]. The protein composition of the enteral formulas used in the study was maintained within a narrow range so that the primary end point would reflect the volume of formula delivered and gastric emptying.
The safety profiles of ulimorelin and metoclopramide were comparable. The incidences of delirium and agitation, which have been associated with metoclopramide administration, were no different between treatment groups. Unlike metoclopramide, ulimorelin has not been associated with these side effects and does not penetrate the CNS [21, 29]. Likewise, no differences in ECG intervals or ventricular arrhythmias were observed. Of interest, metoclopramide was associated with a significantly higher number of episodes of AF compared with ulimorelin, but this incidence (13.8%) was comparable to its reported incidence in other ICU trials (10.5%) [30]. Ghrelin agonists have been shown to enhance vagal tone [31], which could have suppressed AF events.
Metoclopramide is ordinarily administered as a slow 10-ml syringe push over 2–3 min [22], but in the current study, metoclopramide was administered as a 50-ml infusion over 30 min. This modification was made to avoid the need for administrations of the two blinded study medications by syringe pushes and infusions. It has been suggested that the cardiotoxicity of metoclopramide results from inadvertently rapid injection [32], and it is possible that the cardiotoxicity of metoclopramide was minimized by slower infusion. The exclusion of patients with significant QT prolongation, instituted at the request of one of the regulatory agencies, may have also masked cardiotoxic drug effects.
Although this study did not demonstrate efficacy of either agent compared with placebo, the GH, gastric emptying and blood glucose data suggested that ulimorelin had a relevant biologic effect. An unresolved question was whether the prokinetic effects of ulimorelin and metoclopramide were meaningful or whether EFI was merely a self-limited condition, as suggested by the declining rates of EFI recurrence over the 5 days of the study. Nguyen compared metoclopramide with erythromycin over 7 days of treatment using a Kaplan-Meier survival analysis of patients not experiencing another episode intolerance [11]. The GRV used to define EFI in this trial was 250 ml, and patients were censored after the first episode of EFI recurrence. Only 16% of metoclopramide and 31% of erythromycin patients met feeding goals through day 7. However, the current trial demonstrates that high success rates can be achieved when a GRV of 500 ml is employed and patients continue to be fed after EFI recurs. While a GRV of 250 ml has been associated with delayed GE, it has not been shown to have clinical relevance [33].
In the current trial, feeding success rates were high, and the rates of vomiting or regurgitation, aspiration and pulmonary infection were low compared with other ICU studies [34, 35]. Furthermore, no association could be shown among the incidences of GRV elevation, aspiration and pulmonary infection. These findings suggest that a definition of EFI more stringent than a single episode of 500 ml might be needed in clinical practice. These findings also suggest that volume-based feeding could be safe in EFI when a prokinetic agent is employed. This conclusion should be confirmed in other studies, and no extrapolations should be made to other causes of intolerance, such as paralytic ileus, bowel distension, Ogilvie syndrome, bowel ischemia or severe diarrhea.
The strengths of this study were that treatments were blinded and randomized, a standard feeding protocol was used with the standard for formula composition, and a standard definition of EFI (500 ml) was employed that conformed to recent guidelines. Patients were also followed for 5 days irrespective of whether EFI was again encountered, a procedure that showed that patients can be fed to goal despite EFI recurrence. The weakness of the study was that it was comparator, not placebo, controlled, a design implemented when it was determined that investigators would not enroll patients into a placebo-controlled study and/or that ethics committees would not approve one.
Conclusion
In this randomized controlled trial of critically ill patients with EFI, we were unable to observe differences between ulimorelin and metoclopramide in the median percentages of target nutrition achieved or the proportions of patients with feeding success, and the safety profiles of ulimorelin and metoclopramide were generally comparable, with low rates of vomiting, regurgitation and aspiration.
Supplementary Material
Take-home message.
In this randomized controlled trial of ulimorelin, a ghrelin agonist, and metoclopramide in critically ill patients with enteral feeding intolerance (EFI), no differences in feeding outcomes or adverse events were observed. Both groups achieved high rates of feeding success with low rates of vomiting and aspiration.
Acknowledgements
This study was funded by Lyric Pharmaceuticals, South San Francisco, CA, USA. The authors wish to thank the following investigators who dedicated their time and effort to the PROMOTE trial: Lead Study Investigator: Daren K Heyland, Queens University, Kingston, ON, Canada. USA: David Evans, Wexner Medical Center, The Ohio State University, Columbus, OH (Lead Investigator, US); Sarah Jolley, Louisiana State University Health Science Center-New Orleans, New Orleans, LA; Ronald Raines, Rockies Regional Medical Center, Colorado Springs, CO; Kenneth Krell, Eastern Idaho Regional Medical Center, Idaho Falls, ID. Spain: Teodoro Grau-Carmona, Hospital Universitario Doce de Octubre, Madrid (Lead Investigator); Lluis Serviá-Goixart, Hospital Arnau de Vilanova, Lleida; Sonia Perez-Quesada, Hospital General de Alicante, Alicante; Jose Ignacio Herrero-Meseguer, Hospital Universitario de Bellvitge, Barcelona; Enrique Calvo-Herranz, Hospital Universitario de Getafe, Getafe; Carol Lorencio, Hospital Universitario Josep Trueta, Girona; Amparo Peredes, Hospital Qurónsalud Sur, Alcorcón; Juan Carlos Yébenes-Reyes, Hospital de Mataró, Mataró; Miguel Angel Garcia-Martinez, Hospital de Torrevieja, Torrevieja; Manuel Cervera, Hospital Dr. Peset, Valencia; Maria Luisa Bordejé, Hospital Universitari Germans Trias i Pujol, Badalona; Juan Franscisco Fernadez-Ortega, Hospital Universitario Carlos Haya, Malaga; Inmaculada Fernández-González, Hospital Quiron San Camilo, Madrid. The Netherlands: Arthur van Zanten (Lead Investigator), Gelderse Vallei Hospital, Ede; Albertus Beishuizen, Medisch Spectrum Twente, Enschede; Jeroen Schouten, Canisius Wilhelmina Ziekenhuis, Nijmegen; Oscar Hoiting, Canisius Wilhelmina Ziekenhuis, Nijmegen. Canada: Tom Stelfox, University of Alberta, Calgary; Juan Posadas, University of Alberta, Calgary.
Footnotes
Electronic supplementary material
The online version of this article (https://doi.org/10.1007/s00134-019-05593-2) contains supplementary material, which is available to authorized users.
Conflicts of interest
Dr. Heyland served as a consultant to Lyric Pharmaceuticals in the design and execution of this trial. Dr. James, Dr. Harris and Mr. Brown were employees of Lyric Pharmaceuticals. Dr. Gonzalez and Mr. Perez were employees of Pivotal SL, the clinical research organization that conducted the trial on behalf of Lyric.
Compliance with ethical standards
Ethical approval
The Institutional Review Boards (IRBs)/Independent Ethics Committees (ECs) approved the study protocol at all participating sites.
Access to the full trial protocol and data
The full trial protocol and trial data supporting this publication can be obtained by contacting the corresponding author.
References
- 1.Gungabissoon U, Hacquoil K, Bains C, Irizarry M, Dukes G et al. (2014) Prevalence, risk factors, clinical consequences, and treatment of enteral feed intolerance during critical illness. JPEN 39:441–448 [DOI] [PubMed] [Google Scholar]
- 2.Reintam Blaser A, Starkopf J, Kirismägi Ü, Deane AM (2014) Definition, prevalence, and outcome of feeding intolerance in intensive care: a systematic review and meta-analysis. Acta Anaesthesiol Scand 58:914–922 [DOI] [PubMed] [Google Scholar]
- 3.Chapman MJ, Besanko LK, Burgstad CM, Fraser RJ, Bellon M et al. (2011) Gastric emptying of a liquid nutrient meal in the critically ill: relationship between scintigraphic and carbon breath test measurement. Gut 60:1336–1343 [DOI] [PubMed] [Google Scholar]
- 4.Elke G, Feibinger TW, Heyland DK (2015) Gastric residual volume in critically ill patients: a dead marker or still alive? Nutr Clin Pract 30:59–71 [DOI] [PubMed] [Google Scholar]
- 5.McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR et al. (2016) Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and Society for Parenteral and Enteral Nutrition (ASPEN). JPEN 40:159–211 [DOI] [PubMed] [Google Scholar]
- 6.Reintam Blaser A, Startkopf J, Alhazzani W, Berger MM, Casaer MP et al. (2017) Early enteral nutrition in critically ill patients: ESICM clinical practice guidelines. Intensive Care Med 43:380–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC, Casaer MP, Hiesmayr M, Mayer K, Montejo JC, Pichard C, Preiser JC, van Zanten ARH, Oczkowski S, Szczeklik W, Bischoff SC (2019) ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr 38:48–79. 10.1016/j.clnu.2018.08.037 [DOI] [PubMed] [Google Scholar]
- 8.Ukleja A (2010) Altered GI motility in critically ill patients: current understanding of pathophysiology, clinical impact, and diagnostic approach. Nutr Clin Pract 25:16–25 [DOI] [PubMed] [Google Scholar]
- 9.Heyland DK, Dhaliwal R, Drover JW, Gramlich L, Dodek P (2003) Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. JPEN 27:355–373 [DOI] [PubMed] [Google Scholar]
- 10.European Medicines Agency (2013) European Medicines Agency recommends changes to the use of metoclopramide, 26 July 2013, EMA/44303/2013 [Google Scholar]
- 11.Nguyen NQ, Chapman NJ, Fraser RJ, Bryant LK, Holloway RH (2007) Erythromycin is more effective than metoclopramide in the treatment of feeding intolerance in critical illness. Crit Care Med 35:483–489 [DOI] [PubMed] [Google Scholar]
- 12.Dass NB, Munonyara M, Bassil AK, Hervieu GJ, Osbourne S et al. (2003) Growth hormone secretagogue receptors in rat and human gastrointestinal tract and the effects of ghrelin. Neuroscience 120:443–453 [DOI] [PubMed] [Google Scholar]
- 13.Camilleri M, Papathanasopolous A, Odunsi S (2009) Actions and therapeutic pathways of ghrelin for gastrointestinal disorders. Nat Rev Gastroenterol Hepatol 6:342–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nass R, Gaylinn BD, Thorner MO (2011) The tole of ghrelin in GH secretion and GH disorders. Mol Cell Endocrinol 340:10–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagaya N, Itoh T, Murakami S, Oya H, Uematsu M et al. (2005) Treatment of cachexia with ghrelin in patients with COPD. Chest 126:1187–1193 [DOI] [PubMed] [Google Scholar]
- 16.Temel JS, Abernethy AP, Currow DC, Friend J, Duus EM et al. (2016) Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomized, double-blind, phase 3 trials. Lancet 17:519–531 [DOI] [PubMed] [Google Scholar]
- 17.Jacob A, Wu R, Zhou M, Coppa GF, Wang P (2010) Mechanism of the inhibitory effect of ghrelin in sepsis. Hepat Med Evid Res 2:33–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deane A, Chapman MJ, Fraser RJL, Horowitz M (2010) Bench-to-bedside review: the gut as an endocrine organ in the critically ill. Crit Care 14:228–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Venkova K, Fraser G, Greenwood-van Hoveyda HR, Meervald B (2007) Prokinetic effects of a new ghrelin receptor agonist TZP-101 in a rat model of post-operative ileus. Dig Dis Sci 52:2241–2248 [DOI] [PubMed] [Google Scholar]
- 20.Ejskaer N, Dimcevski G, Wo J, Helstrom PM, Gormsen LC et al. (2010) Safety and efficacy of ghrelin agonist TZP-101 in relieving symptoms in patients with diabetic gastroparesis: a randomized, placebo-controlled study. Neurogastroenterol Motil 22:1069–1078 [DOI] [PubMed] [Google Scholar]
- 21.Shaw M, Pediconi C, McVey D, Mondou E, Quinn J et al. (2013) Safety and efficacy of ulimorelin administered postoperatively to accelerate recovery of gastrointestinal motility following partial bowel resection: results of two randomized, placebo-controlled phase 3 trials. Dis Col Rectum 56:888–897 [DOI] [PubMed] [Google Scholar]
- 22.Global RxPh. Metoclopramide. http://www.globalrph.com/renal/metoclopramide/. Accessed 6 Dec 2018
- 23.Peterson CM, Thomas DM, Blackburn GL, Heymsfield SB (2016) Universal equation for estimating ideal body weight and body weight at any BMM. Am J Clin Nutr 103:1197–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heyland DK, Cahill NE, Dhaliwal R, Wang M, Day AG et al. (2010) Enhanced protein-energy provision via the enteral route in critically ill patients: a single center feasibility trial of the PEP uP protocol. Crit Care 14:R78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.James J, Doll W, Harris S (2018) Method to assess gastric emptying in the fed state in enterally tube fed patients: comparison of the paracetamol absorption test to scintigraphy. Crit Care 22(Suppl 1):13529793515 [Google Scholar]
- 26.Heyland DK, Cook D, Dodek P, Muscedere J, Day A (2006) A randomized trial of diagnostic techniques for ventilator-associated pneumonia. N Engl J Med 355:2619–2630 [DOI] [PubMed] [Google Scholar]
- 27.Heyland DK (2015) Should we PERMIT systemic underfeeding in all intensive care unit patients? Integrating the results of the PERMIT study in our clinical practice guidelines. JPEN 40:156–168 [DOI] [PubMed] [Google Scholar]
- 28.Nicolo M, Heyland DK, Chittams J, Sammarco T, Compher C (2016) Clinical outcomes related to protein delivery in a critically ill population: a multicenter, multinational observation study. JPEN 40:45–51 [DOI] [PubMed] [Google Scholar]
- 29.Pusovit RV, Callaghan B, Kosari S, Rivera LR, Thomas H et al. (2014) The mechanism of enhanced defecation caused by the ghrelin receptor agonist, ulimorelin. Neurogastroenterol Motil 26:264–271 [DOI] [PubMed] [Google Scholar]
- 30.Kanji S, Williamson DR, Mohammadzadeh Yaghchi B, Albert M, McIntyre L (2012) Epidemiology and management of atrial fibrillation in medical and noncardiac surgical adult intensive care unit patients. J Crit Care 27:326–332 [DOI] [PubMed] [Google Scholar]
- 31.Avau B, Carbone F, Tack J, DePoortere I (2013) Ghrelin signaling in the gut, its properties, and therapeutic potential. Neurogastroenterol Motil 25:720–732 [DOI] [PubMed] [Google Scholar]
- 32.Rumore MM (2012) Cardiovascular adverse effects of metoclopramide: review of literature. IJCRI 3:1–10 [Google Scholar]
- 33.Montejo JC, Miñarmbres E, Bordejé L, Mesejo A, Acosta J et al. (2010) Gastric residual volume during enteral nutrition in ICU patients: the REGANE study. Intensive Care Med 36:1386–1393 [DOI] [PubMed] [Google Scholar]
- 34.Reigner J, Mercier E, Le Gouge A, Boulain T, Desachy A et al. (2013) Effect of not monitoring residual gastric volume on risk of ventilator-associated pneumonia in adults receiving mechanical ventilation and early enteral feeding: a randomized controlled trial. JAMA 209:249–256 [DOI] [PubMed] [Google Scholar]
- 35.Reigner J, Boisramé-Helms J, Brisard L, Lascarrou J-B, Ait Hassan A et al. (2018) Enteral versus parenteral early nutrition in ventilated adults with shock: a randomized, controlled, multicenter, open-label, parallel-group study (NUTRIREA-2). Lancet 391:133–143 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


