Abstract
The Wnt/frizzled signaling pathway is one of the major regulators of endothelial biology, controlling key cellular activities. Many secreted Wnt ligands have been identified and can initiate diverse signaling via binding to a complex set of Frizzled (Fzd) transmembrane receptors and coreceptors. Roughly, Wnt signaling is subdivided into two pathways: the canonical Wnt/β-catenin signaling pathway whose main downstream effector is the transcriptional coactivator β-catenin, and the noncanonical Wnt signaling pathway, which is subdivided into the Wnt/Ca2+ pathway and the planar cell polarity pathway. Here, we will focus on its cross talk with other angiogenic pathways and on its role in blood-retinal- and blood-brain-barrier formation and its maintenance in a differentiated state. We will unravel how retinal vascular pathologies and neurovascular degenerative diseases result from disruption of the Wnt pathway related to vascular instability, and highlight current research into therapeutic options.
The Wnt signaling pathway is one of the main regulators of endothelial biology. In mammals, 19 different Wnt secreted ligands have been identified to date (Nusse and Clevers 2017); they can initiate distinct signaling upon binding at the cell surface to a combination of multiple transmembrane receptors of the Frizzled (Fzd) family (10 in humans and mice), and low-density lipoprotein receptor-related proteins 5/6 (LRP5/6) coreceptors (Gordon and Nusse 2006). Wnt proteins are highly hydrophobic and posttranslationally modified with lipids by the membrane-bound-O-acyltransferase porcupine (PORCN), restricting their diffusion in the aqueous environment primarily toward neighboring cells (Willert et al. 2003; Takada et al. 2006). Studies of their crystal structures show that its lipid tail is required for Wnt interaction within the Fzd extracellular cysteine-rich domain (Janda et al. 2012). Wnt secretion relies on the conserved multipass transmembrane and putative sorting receptor Wntless (Wls, also known as Evi/ Sprinter), which shuttles Wnt proteins from the Golgi to the plasma membrane (Bänziger et al. 2006).
On a molecular level, two types of Wnt signaling pathways have been described: the canonical Wnt/β-catenin signaling pathway, which functions to stabilize and induce nuclear translocation of β-catenin, and the noncanonical Wnt signaling pathway, apparently β-catenin independent, which is subdivided into the Wnt/Ca2+ pathway and the planar cell polarity (PCP) pathway (Gordon and Nusse 2006; Rao and Kühl 2010). Although the Wnt pathways have been separated into two branches for simplicity, the canonical and noncanonical pathways overlap on several levels. It has been shown that the noncanonical Wnt signaling may inhibit the canonical Wnt pathway in receptor- and ligand-dependent manners or reciprocally (Grumolato et al. 2010). The cytoplasmic scaffold protein Dishevelled (Dvl) is then activated and acts as a hub for the transduction of distinct Wnt pathways though different domains (Beitia et al. 2021).
Previous studies have shown that endothelial cells (ECs) express high levels of distinct Fzd receptors, LRP5/6, and Wnt factors (Goodwin et al. 2006). However, the mechanisms underlie the variety of outcomes triggered by Wnt in vascular homeostasis is still under debate. The canonical β-catenin-mediated pathway is the most well-characterized, whereas mechanisms of the downstream noncanonical pathways in vascular development and stabilization are just beginning to be elucidated.
In this paper, we have included a limited discussion on the role of Wnt/Fzd signaling in EC in the development of the blood-retina and blood-brain-barriers (BRB and BBB, respectively) and its involvement in the pathogenesis of retinal and neurodegenerative diseases.
Wnt SIGNALING PATHWAYS
Canonical Wnt Signaling
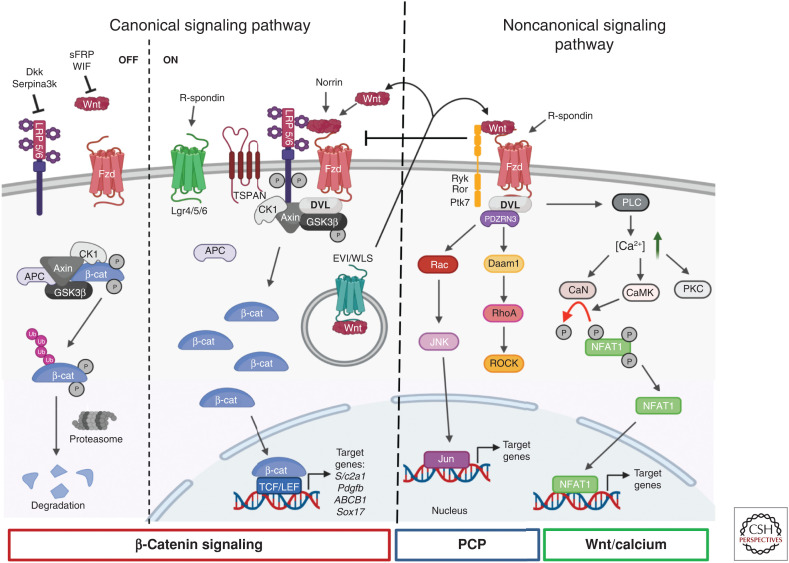
Wnt canonical signaling has been reported to be required for the complex regulation of new vessel development, mainly for angiogenesis of the BRB and BBB differentiation and maturation (Liebner et al. 2008; Daneman et al. 2009). β-Catenin is reported as the central player of the canonical Wnt pathway. In the presence of Wnt ligands, Wnt/Fzd forms a ternary complex with the coreceptor LRP5/6, which in turn triggers LRP5/6 phosphorylation by casein kinase 1γ (CK1γ). This signaling cascade results in the formation of large signalosomes with the recruitment of Dvl and Axin proteins (Davidson et al. 2005; Zeng et al. 2005), which are endocytosed (Bilić et al. 2007; Schwarz-Romond et al. 2007). This mechanism leads to disruption of the destruction complex, halting degradation of the Wnt signaling transducer β-catenin in the cytoplasm and ultimately induces its translocation to the nucleus. β-Catenin is then associated with the lymphoid enhancer factor (Lef)/T-cell factor (TCF) transcription factors and other cofactors, resulting in the expression of target genes, such as Cldn5 (coding for claudin-5) (Liebner et al. 2008), Slc2a1 (coding for GLUT1) (Daneman et al. 2009), Pdgfb (coding for platelet-derived growth factor B [PDGF-B]) (Reis et al. 2012), and ABCB1 (coding for the efflux transporters of the blood-brain-barrier P-glycoprotein [P-gp]) expression (Lim et al. 2008; Kania et al. 2011). Without Wnt stimulation, cytoplasmic β-catenin is targeted to a complex with the glycogen synthase kinase 3β (GSK3β), CK1γ, and the tumor suppressor adenomatosis polyposis coli (APC) complex linked to the scaffolding protein Axin that induces phosphorylation of β-catenin by GSK3β and its rapid proteasomal degradation (Sakanaka et al. 1998). Target genes are repressed in the absence of nuclear β-catenin (Fig. 1; Cavallo et al. 1998).
Figure 1.
Schematic representation of the canonical and noncanonical Wnt signaling pathways. (Left) Canonical signaling pathway or Wnt/β-catenin signaling pathway. In the OFF state, Wnt ligands do not bind to Frizzled (Fzd) receptors. Thus, cytoplasmic β-catenin binds to its destruction complex formed by glycogen synthase kinase 3β (GSK3β), Axin, adenomatous polyposis coli (APC), and casein kinase 1 (CK1), which induces β-catenin phosphorylation (P), thereby targeting it for degradation by the ubiquitin (Ub) proteasome system. Inhibitors of Wnt ligands (secreted Frizzled-related protein [sFRP] or Wnt inhibitory factor [WIF]) or of coreceptors low-density lipoprotein (LDL) receptor-related protein (LRP5/6) (Dickkopf [Dkk] and serine protease inhibitor [Serpina3K]) contribute to blocking of Wnt signaling. In the ON state, Wnt ligands bind to the receptor complex consisting of Fzd and LRP5/6, which then recruits the Dishevelled (DVL) protein to the plasma membrane. The β-catenin destruction complex is recruited to the membrane, which prevents phosphorylation and degradation of β-catenin. This protein can then accumulate in the cytoplasm and translocate to the nucleus to form a complex with the transcription factors T-cell factor (TCF)/lymphoid enhancer factor (LEF) and stimulate the transcription of Wnt/β-catenin target genes. Wnt secretion is a fine process controlled by the evenness interrupted (EVI)/Wntless (WLS) protein. Other coreceptors (tetraspanin [TSPAN] and leucine-rich repeat containing [Lgr4/5/6]) and ligands (Norrin and R-spondin) can activate or enhance the Wnt signaling pathways. (Right) Noncanonical signaling pathways. Activation of these pathways involve binding of Wnt ligands to the receptor complex formed by Fzd and other key coreceptors (related to tyrosine [Y] kinase [Ryk], receptor tyrosine kinase-like orphan receptor [Ror], protein tyrosine kinase 7 [Ptk7]). Depending on Wnt/Fzd interactions and coreceptors, distinct downstream effectors are involved to transduce alternative β-catenin-independent signaling pathways, namely, the planar cell polarity (PCP) signaling and Wnt/Ca2+ signaling pathways. Activation of the Wnt/PCP pathway leads to the formation of a DVL/Dishevelled-associated activator of morphogenesis 1 (Daam1) complex, which activates the small GTPase RhoA and subsequently Rho-associated protein kinase (ROCK) to regulate actin cytoskeleton. The PCP pathway can also activate the small GTPase Ras-related C3 botulinum toxin substrate (Rac) and then c-Jun amino-terminal kinase (JNK), which then phosphorylates and activates Jun and induces transcription of Jun target genes. The Wnt/calcium pathway involves activation of downstream phospholipase C (PLC), which induces increased release of intracellular Ca2+ levels and then activates protein kinase C (PKC) and Ca2+/calmodulin-dependent protein kinase (CaMK). This last protein can now dephosphorylate the transcription factor nuclear factor of activated T cells (NFAT) leading to its nuclear import and induction of the transcription of its target genes. The calcium pathway can also promote NFAT dephosphorylation and activation through the calcineurin (CaN) axis. R-spondin ligands (more specifically R-spondin3) can also modulate and activate the Wnt/calcium pathway. Created with BioRender.com. (β-cat) β-Catenin, (Ca2+) calcium.
Evidence that several inhibitors are present during angiogenesis has further increased the complexity of this pathway. Secreted Fzd-related proteins (sFRP members) (Dufourcq et al. 2008) and Wnt inhibitory factor 1 (WIF-1) (Ramachandran et al. 2012) can directly bind and sequester Wnt ligands, and thereby block Wnt signaling output. For example, sFRP1 (FzdA) expression impairs EC proliferation as a result of reduced expression of cyclin E and cdk2 activity and subsequently reduces neovascularization in a model of mouse hindlimb ischemia (Ezan 2004). Binding of SFRP2 to Fzd receptors on the surface of tumor ECs activates downstream Wnt signaling, enhancing angiogenesis (van Loon et al. 2021). In contrast to sFRPs, another class of inhibitors, the Dickkopf (DKK) protein family, which includes members of DKK1, 2, 3, and 4, inhibits Wnt signaling by preventing the interaction of Wnts with the coreceptors LRP5/6 (Niehrs 2006) and have been shown to be involved in development or tumoral angiogenic events (Pendás-Franco et al. 2008; Min et al. 2011; Choi et al. 2017). For the reader, other β-catenin-independent Wnt/Lrp6 signaling routes have emerged that add a high level of complexity for Wnt signaling (Acebron and Niehrs 2016). Their roles in vascular biology are still unknown.
The Wnt Noncanonical Pathway
As alluded to above, depending on Wnt/Fzd interaction and coreceptors, distinct Wnt downstream effectors are involved with transducing alternative β-catenin-independent Wnt signaling pathways, such as the Wnt/Ca2+ and PCP signaling (Fig. 1). Several groups have reported that noncanonical Wnt signaling regulates EC proliferation and vascular network formation (Masckauchán et al. 2006; Cheng et al. 2008; Descamps et al. 2012). Deletion of the Wnt secretion factor Evi (Evi-ECKO) in mouse ECs, uncovered the role of noncanonical Wnt proteins secreted by EC in vascular network stabilization. Evi-ECKO mice showed reduced retinal and tumoral vascularization. This effect was rescued by noncanonical Wnt5a treatment (Korn et al. 2014).
In the Wnt/Ca2+cascade, the binding of Wnts to Fzd receptors leads to activation of DVL and downstream phospholipase C (PLC), which allow the release of intracellular Ca2+ and activation of Ca2+-sensitive enzymes, such as Ca2+/calmodulin-dependent protein kinase (CaMKII) and protein kinase C (PKC). CaMKII activation dephosphorylates the transcription factor nuclear factor of activated T cells (NFAT) leading to its nuclear import. sFRP2 was reported to activate Wnt/ Ca2+ signaling in ECs to induce angiogenesis (Courtwright et al. 2009). Stefaner et al. (2011, 2013) have shown that macrophages secrete non-noncanonical Wnt proteins to control endothelial sFlt1/VEGFR1 expression through an NFAT-calcineurin axis, to limit retinal vessel branching and wound healing. Another study showed that the Wnt signaling enhancer R-spondin3 (RSPO3), a secreted protein, highly expressed in endothelium, maintains vascular stability during blood retinal development and vascular remodeling via the activation of Wnt/ Ca2+ pathway in ECs (Scholz et al. 2016).
ECs are characterized by distinct forms of polarity, creating morphological and functional asymmetries that are a prerequisite for their functions. At the tissue level, ECs form a planar polarized monolayer, with a directional cell alignment. It was proposed that this polar tissue pattern along a plane axis (orthogonal to the apico/basal axis) was mediated to some extent by the noncanonical Wnt/PCP pathway. At the cellular level, ECs possess an apicobasal polarity that has been extensively studied in the context of endothelial lumen formation (Iruela-Arispe and Davis 2009; Xu and Cleaver 2011; Lammert and Axnick 2012; Pelton et al. 2014). At the molecular level, both forms of polarity appeared to be intertwined.
First observed and dissected in model organisms as yeast, Caenorhabditis elegans, and Drosophila melanogaster, where it regulates the orientation of hair on wings and facets in eye, PCP is now known to be involved in mammals in a variety of tissue in regulating gastrulation, sensory cell orientation (Montcouquiol et al. 2003; Wang and Nathans 2007), alveologenesis (Zhang et al. 2020), and in cellular processes such as asymmetric cell division and collective cell migration (Wallingford 2012). Fzd receptors and intracellular Dvl are necessary components. A requirement for Wnt as upstream regulators is still controversial (Ewen-Campen et al. 2020; Yu et al. 2020). A key feature of planar polarity, as reported in Drosophila studies, is the asymmetrical localization of PCP core proteins (Simons and Mlodzik 2008). The core PCP proteins are conserved in vertebrate species and include Fzd3/6, Celsr1-3 cadherins, Vangl1/2, Prickle1/2, DVL1-3, and Ankrd6. Their localization in an asymmetric manner, at or near the cell membrane, is thought to create the polarization of cells. These proteins at the membrane would favor interaction with neighboring cells to spread polarization across tissues. Finally, the PCP pathway activates, via the hub DVL, PCP downstream effector proteins, including DAAM1 and small GTPases such as RhoA, and Rac1 to activate Jun amino-terminal kinase (Wu and Mlodzik 2009). The effector proteins control cytoskeletal rearrangements but are not asymmetrically localized within the cell.
In EC, the identification of molecular PCP effectors is still highly challenging. This is partly due to the nature of this signaling pathway, which typically contributes to tissue organization, and to the limited set of suitable tools to study PCP signaling dynamics. Genetic studies have started to add some pieces to this puzzle, specifically related to the PCP effect on vascular formation. Loss of Wnt5a leads to impaired blood vessel development (Cirone et al. 2008), whereas Wnt5a activation induces the noncanonical Par3/PKC complex and thus reinforces brain EC tight junctions (Artus et al. 2014). The Scribble polarity protein was shown to be required for endothelial-directed migration (Michaelis et al. 2013). In lymphatic ECs, asymmetric localization of CELSR1 and VANGL2, PCP core proteins, was reported during lymphatic valve formation controlling the stabilization of endothelial adherent junctions (Tatin et al. 2013). However, mechanisms by which PCP signaling elicits context-specific processes is still unclear. The study of PCP is hampered by a lack of clarity and consensus on how to (1) define adequate noncanonical EC properties, (2) analyze the regulation of the effectors, and (3) measure signaling in vascular cells. It appears crucial to identify and assess the activity of downstream signaling events in ECs to understand the role of PCP signaling in tissue formation and maintenance. Using genetic screening, our group has identified an actor in the PCP pathway in ECs, the E3 ubiquitin ligase Pdzrn3, and demonstrated its role in vessel network formation (Sewduth et al. 2014). Proteomic screening has shown that Pdzrn3 is an effector in Wnt5a/ROR signal transduction (Konopelski Snavely et al. 2021).
ROLE OF THE Wnt/PCP SIGNALING IN ECs TO ORCHESTRATE VASCULAR DEVELOPMENT
In this work, we review studies in which Wnt proteins were shown to provide polarity cues at the level of the endothelium during angiogenesis. This research has benefited from an increasing body of genetic tools, sophisticated imaging technology, and computational modeling of blood flow.
Laminar shear flow was shown to drive EC elongation and planar polarization with an asymmetric organization of organelles against the flow axis (Rogers et al. 1985; Tzima et al. 2005). Robust analyzes demonstrate that EC dynamically sense the blood flow, migrating against the direction of the blood flow to form a stable vascular network. Consequently, vessel segments under low flow constraint are unstable and regress (Franco et al. 2015). Wnt5a and Wnt11 ligands have been reported to participate functionally in the control of the flow-induced EC polarization response (Franco et al. 2016). In this context, noncanonical Wnt signaling modulates the threshold for flow-dependent EC polarization, inducing premature vessel regression, and leading to a decrease in vessel density (Franco et al. 2016).
Noncanonical Wnt ligands coordinate polarized EC behaviors during vascular morphogenesis (Carvalho et al. 2019). Cell polarization entails highly orchestrated intracellular molecular reorganization events mediated by key regulators of polarity. Wnt5a signaling may activate the Rho family small GTPases Cdc42 reinforcing the link between actin cytoskeleton and intercellular junction proteins during angiogenic collective cell migration (Carvalho et al. 2019). Mouse mutants of PCP signaling effectors, such as the ubiquitin ligase Pdzrn3 (Sewduth et al. 2014) and the polarity protein PAR3 (Hikita et al. 2018), display similar vascular defects with a destabilization of the polarized mouse retinal vascular network. EC polarization may be achieved by the integration of the Par3/aPKCζ polarity protein complex–induced signaling and cytoskeleton rearrangement in ECs (Sewduth et al. 2014, 2017; Hikita et al. 2018).
CROSS TALK BETWEEN Wnt AND OTHER ANGIOGENIC PATHWAYS
Only a few studies have examined the interplay between the Wnt signaling and other key pathways involved in angiogenesis. One of the most crucial regulators of vascular development is the vascular endothelial growth factor (VEGF) signaling pathway, which includes VEGF ligands (VEGFA, B, C, and D) and receptors (VEGFR1-3) (Ferrara 2009). In EC, VEGF is a key initiator of angiogenesis and sprouting, and a fine regulator of tip cells selection through activation of VEGFR2 (Ruhrberg et al. 2002; Gerhardt et al. 2003; Jakobsson et al. 2010).
An interesting link between the VEGF and Wnt pathways has been described in vitro by Skurk and coworkers. The authors propose that the VEGF/PI3-kinase/Akt signaling is downstream of β-catenin and contributes to the pro-angiogenic actions of β-catenin on ECs (Skurk et al. 2005). Subsequently, two studies demonstrated the role of R-spondin (RSPO), a family of secreted proteins that activate Wnt/β-catenin signaling, in regulating developmental vasculogenesis and angiogenesis through the regulation of VEGF (Kazanskaya et al. 2008; Gore et al. 2011). In fact, RSPO3 was identified as a key regulator of VEGF expression via the activation of Wnt/β-catenin signaling in mouse placenta and in a Xenopus model (Kazanskaya et al. 2008). In addition, the RSPO3/Wnt signaling pathway has been shown to promote angiogenesis via VEGFC and VEGFR3 in zebrafish (Gore et al. 2011).
In central nervous system (CNS) microvessels, impaired endothelial β-catenin signaling following β-catenin genetic depletion or overexpression of Axin1, a member of the β-catenin protein degradation complex, led to a decreased expression of VEGFR2 and VEGFR3 (Martowicz et al. 2019). Interestingly, the β-catenin-dependent regulation of VEGFR2 and 3 was tissue-specific and loss of β-catenin did not affect the level of VEGF receptors in lung tissues. Since the transcription factor SOX17 has been identified as a β-catenin target in EC, a positive inducer of Wnt/β-catenin signaling and a promoter of both angiogenesis and VEGFR2 expression in the CNS (Corada et al. 2013, 2019; Zhou et al. 2015), the authors proposed a model where β-catenin drives VEGFR2 expression via the up-regulation of Sox17 (Martowicz et al. 2019). However, how β-catenin precisely regulates VEGFR2 levels during angiogenesis in the CNS is still poorly understood and could involve multiple partners. DKK1 was reported to act via the VEGFR1 and SDF-1 signaling pathway, independent of the Wnt canonical signaling pathway, to regulate angiogenesis (Choi et al. 2017). Additional studies are needed to identify the possible tissue-specific molecular mechanism by which β-catenin drives endothelial VEGFR2/R3 expression to gain a better understanding of the cross talk between these two pathways in this context.
The Notch signaling pathway is an evolutionary highly conserved signaling machinery, which has emerged as an essential regulator of multiple steps involved in vascular development and angiogenesis including endothelial sprouting, tip versus stalk cells selection, and arterial specification (Roca and Adams 2007; Mack and Iruela-Arispe 2018). This pathway involves five ligands, namely, Delta-like (Dll)1, Dll3, Dll4, Jagged1, and Jagged2 that interact with four Notch transmembrane receptors (Notch1–Notch4). Activation of the receptors leads to the proteolytic cleavage of the Notch intracellular domain (NICD) and its translocation to the nucleus where it can regulate gene expression through cooperation with recombination signal-binding protein for immunoglobulin κ J (RBPJ) (Guruharsha et al. 2012).
Different studies have demonstrated an interplay between the Notch and the Wnt/β-catenin signaling pathways during vascular morphogenesis. Phng et al. reported that the Notch-regulated ankyrin repeat protein (Nrarp), induced by Notch signaling, acts downstream of this pathway to regulate vascular density by controlling stalk cell proliferation and stabilization of new endothelial connections in mouse retina and zebrafish. Nrarp was found to be a negative regulator of Notch signaling by destabilizing NICD, and an activator of the Wnt/β-catenin signaling by binding to lymphoid enhancer factor 1 (Lef1) (Phng et al. 2009). Moreover, in a mouse model overexpressing β-catenin specifically in ECs, treatment with DAPT, an inhibitor of Notch signaling, was able to partially rescue embryonic vascular defects (Corada et al. 2010). It was shown that in ECs, the transcriptional complex formed by β-catenin/NICD/RBPJ induced by the angiopoietin1/Tie2 axis, drove the expression of Dll4 to enhance Notch signaling and promote vascular quiescence (Zhang et al. 2011). Moreover, the endothelial transcription factor ERG has been shown to directly interact with the β-catenin/NICD/RBPJ complex, to control the fine balance between Dll4 and Jagged1 expression in vitro and in mouse retina to maintain vascular homeostasis (Shah et al. 2017). Similarly, our group reported that mice with EC-specific deletion of Fzd7 displayed reduced β-catenin and Notch signaling. Notably, impairment of the Notch signaling was rescued in vitro and in vivo by activation of β-catenin signaling (Peghaire et al. 2016).
Wnt SIGNALING AND EYE VASCULATURE
Wnt Signaling in the Development of Eye Vasculature
The discovery of a genetic link between Wnt signaling and human retinal vascular defects has fostered studies on the role of Wnt signaling in retinal angiogenesis in mice (Xu et al. 2004). Over time, the vascular system of the mouse retina has been used as a robust model for analyzing the molecular and cellular mechanisms regulating angiogenesis (Stahl et al. 2010).
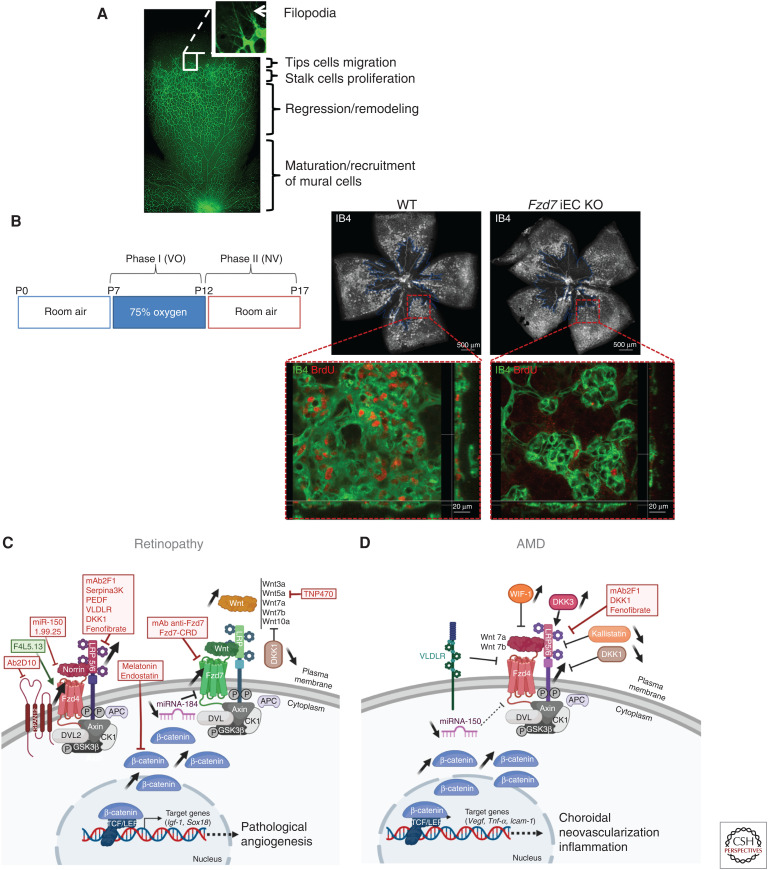
At birth, the mouse retina is avascular, then vascular networks are formed, following a hierarchical pattern under the control of major angiogenic factor such as VEGF (Gerhardt et al. 2003). Deposition of a basement membrane, recruitment of mural cells and dynamic vessel regression contribute to the shaping and maintenance of a functional BRB (Fig. 2A; Korn and Augustin 2015).
Figure 2.
Role of Wnt signaling in vascular eye pathology. (A) Representative image of a whole flat-mounted retina at P7 from WT mouse with vessels stained with isolectin (IB4, green). Unlike in humans, mouse intraretinal vasculature occurs postnatally to be essentially mature at about 3 weeks postnatal. With a highly regulated patterning in the development of eye vasculature, mice retina provides a useful experimental tool to deciphering angiogenic mechanisms such as vessel sprouting corresponding to tip cells migration and stalk cells proliferation, vessel regression, maturation, and remodeling during postnatal period. (B, left) Experimental studies of retinopathy of prematurity (ROP) and proliferative diabetic retinopathy (DR) are enabled by the oxygen-induced retinopathy (OIR) model, a well-established animal model of ischemia-induced retinal neovascularization (NV). Neonatal mice are exposed to 75% oxygen from postnatal day 7 (P7) to P12, corresponding to vasobliterative phase (phase I, VO) inducing a central avascular zone (delimited by blue line, image on the right) and returned to room air from P12 to P17 to induce maximum pathologic NV at P17 (phase II). (Right) Representative images showed areas of VO (indicated by blue line) in P17 OIR retinas from WT mice and from Fzd7 iEC-KO mice. Screenshot of retinal quadrants focus on proliferation (BrdU incorporation, red) in preretinal tufts (IB4 staining, green) at P17 from WT and Fzd7 iEC-KO OIR mice. Note that Fzd7 endothelial deletion limits EC proliferation in pathological neovessels. (Panel B from Bats et al. 2020; reprinted, with permission, from John Wiley and Sons © 2019.) Acquired vascular eye disorders include retinopathies (C) and age-related macular degeneration (AMD) (D) in which the involvement of Wnt signaling is fully recognized. (C) Dysregulation of Wnt signaling, with an emphasis on the canonical Wnt/β-catenin pathway, has been widely studied in retinopathies for its implication in the development of pathological angiogenesis. Fzd4, LRP5/6, and β-catenin are up-regulated either in retinas from diabetic patients or in animal models of retinopathies. Plasma and vitreous fluid levels of DKK1, a Wnt inhibitor, are lower in patients with DR compared to controls. Experimental genetic loss of Lrp5, Dvl2, or Fzd4 significantly attenuates NV in OIR. Increased expression of Wnt3a, Wnt5a, Wnt7a, Wnt7b, and Wnt10a but not Norrin is associated with pathologic angiogenesis in retinopathies. The suggested protective effect of Norrin against OIR is mediated by the induction of insulin-like growth factor (IGF-1), a very potent angiogenic molecule. A negative regulator of Wnt/β-catenin signaling, miR-184, is down-regulated in OIR, while the expression of Fzd7, its downstream target, is induced during the NV phase. Fzd7 endothelial deletion exerts a specific inhibitory effect on pathological angiogenesis after OIR by limiting endothelial cell (EC) proliferation in pathological neovessels. (D) Alteration of Wnt signaling is also associated with abnormal choroidal neovascularization (CNV) and inflammation in AMD. CNV initiation involves activation of Wnt/β-catenin pathway, which in turn stimulates vascular endothelial growth factor (VEGF) to mediate angiogenesis. AMD patients exhibit high levels of phospho-LRP6 and β-catenin, especially in the endothelium of choroidal tissue, low plasma levels of the endogenous Wnt inhibitors, kallistatin or DKK1, and, on the contrary, high levels of other Wnt modulators, WIF-1 and DKK3, in the aqueous humor. In mouse, Wnt7a and Wnt7b deletion decrease severity of laser injury-induced CNV. Deficiency for VLDLR, a negative regulator of Wnt pathway, leads to abnormal intraretinal and choroidal NV and inflammation. Finally, laser-induced CNV is promoted by miR-150 deficiency, a negative regulator of Fzd4 expression. Antiangiogenic strategies targeting Wnt signaling are of real interest in the treatment of retinopathies (C) and AMD (D). Inhibitors and inducers of the Wnt pathway, already tested in angiogenic models of retinopathy and AMD, are shown in red and green, respectively. Their therapeutic potential is detailed in the Therapies for Vascular Diseases section. Created with BioRender.com.
Consistent with human disease, mice deficient for Ndp (or Norrie Disease Protein gene encoding Norrin), Fzd4, or Tspan12 show major defects in retinal vascularization, including loss of BRB integrity, a delayed regression of hyaloid vasculature and absence of the secondary and tertiary plexus (Richter et al. 1998; Ye et al. 2009; Wang et al. 2012; Lai et al. 2017). LRP-5-null mice display vascular defects with a persistent hyaloid vasculature, similar to the Norrin and Frizzled4 mutants (Kato et al. 2002). These studies highlight the importance of Norrin ligand-induced signaling through the FZD4/LRP5/TSPAN12 receptor complex, for the development and maintenance of retinal vasculature (Xu et al. 2004; Junge et al. 2009; Ye et al. 2010; Chen et al. 2011). Stabilization of β-catenin by genetic approaches, or promoted by the ETS transcription factor ERG, rescues these phenotypes (Zhou et al. 2014b; Birdsey et al. 2015). Molecular analyses identified claudin-5 and plasmalemma vesicle-associated protein (PLVAP) as downstream targets of Norrin/FZD4/LRP5 signaling (Chen et al. 2012; Wang et al. 2012; Zhang et al. 2017). Finally, the major facilitator superfamily domain-containing protein 2 (MSFD2A), a membrane transport protein, was recently identified as a transcriptional target of the Norrin/LRP5/β-catenin pathway in governing endothelial transcytosis and inner BRB integrity (Wang et al. 2020).
A number of reports highlight the molecular complexity of Wnt signaling in the regulation of ocular angiogenesis (Liu et al. 2003; Lobov et al. 2005; Yi et al. 2007; Liu and Nathans 2008; Franco et al. 2016; Bats et al. 2020). In the developing eye, hyaloid vessel regression is tightly regulated by Fzd5 and the macrophage-secreted Wnt7b (Lobov et al. 2005; Liu and Nathans 2008). Our group demonstrated that endothelial Fzd7 drives postnatal retinal angiogenesis via the activation of Dvl/β-catenin signaling (Peghaire et al. 2016). Disruption of noncanonical Wnt5a and Wnt11 ligands or the endothelial ubiquitin ligase PDZRN3, was associated with premature vessel regression in the mouse retina (Sewduth et al. 2014; Franco et al. 2016). A similar phenotype was observed in murine retinal vessels deficient for the Wnt secretion factor Evi, or for endothelial RSPO3, an enhancer of Wnt/Ca2+ signaling (Korn et al. 2014; Scholz et al. 2016; Carvalho et al. 2019). Additionally, the noncanonical Wnt5a/ROR2/PCP signaling contributes to the regulation of retinal vessel sprouting by coordinating the collective polarity of ECs, a process closely related to cell migration (Korn et al. 2014; Franco et al. 2016; Carvalho et al. 2019).
Wnt Signaling in Human and Murine Retinal Vascular Diseases
In humans, Norrie disease is an X-linked recessive disorder, due to mutations in the NDP gene, VI, which causes persistent hyaloid vessels, incomplete retinal vascularization, and pathologic neovessel proliferation, often resulting in blindness from birth (Berger et al. 1992).
Familial exudative vitreoretinopathy (FEVR) is another inherited blinding disorder that shares phenotypic characteristics with Norrie disease (Gilmour 2015). While half of all FEVR pathogenic mutations are found within FZD4, LRP5, TSPAN12, and NDP, others affect genes functionally related to the Wnt/β-catenin signaling: CTNNB1, ILK, KIF11, JAG1, and DLG1 (Xiao et al. 2019; Jia and Ma 2021; Zhang et al. 2021).
Osteoporosis-pseudoglioma syndrome (OPPG) is an autosomal recessive disorder caused by inactivating mutations in LRP5, and also leads to persistent primary vitreous vasculature (Gong et al. 2001).
Coats disease is a single-eye condition, predominantly affecting men, that is characterized by microaneurysms, retinal detachments, and lipid exudates (Sen et al. 2019), as a direct consequence of Wnt pathway alterations in NDP, FZD4, and RCBTB1 (Black et al. 1999; Robitaille et al. 2011; Wu et al. 2016).
Ischemic retinopathies, including retinopathy of prematurity (ROP) and diabetic retinopathy (DR), are ocular disorders characterized by an initial phase of ischemia, followed by a second phase of abnormal neovascularization that may culminate into retinal detachment and blindness (Rivera et al. 2017). In humans, mutations on NDP, FZD4, and LRP5 genes have been identified as risk factors for developing severe ROP (Drenser 2016). Association between the activation of Fzd4/LRP5-6/β-catenin pathway and pathological angiogenesis has been widely documented in both human DR and experimental models of retinopathy (Wang et al. 2019b). Patients with DR also exhibit low plasma and vitreous fluid levels of the Wnt inhibitor DKK1 (Qiu et al. 2014). In mice, genetic loss of Lrp5, Dvl2, or Fzd4 significantly attenuates neovascularization in oxygen-induced retinopathy (OIR) (Chen et al. 2011; Ngo et al. 2016). Inactivation of endothelial Wnt ligands secretion does not impact the vasobliterative phase of OIR (Franco et al. 2016), further suggesting that Wnt signaling contributes preferentially to the neovascular process of the disease. Unlike many Wnt ligands, Norrin is not up-regulated during retinopathy (Chen et al. 2011; Franco et al. 2016; Lee et al. 2017). Its protective effect against OIR may be mediated by the induction of insulin-like growth factor (IGF-1), a highly potent angiogenic molecule (Zeilbeck et al. 2016).
A negative regulator of Wnt/β-catenin signaling, miR-184, is down-regulated in OIR, whereas Fzd7, its downstream target, is induced during neovascularization (Takahashi et al. 2015). Similarly, Fzd7 endothelial deletion exerts a specific inhibitory effect on ischemia-induced retinal pathological neovascularization and EC proliferation (Fig. 2B; Bats et al. 2020).
Altered Wnt signaling is also associated with the exudative form of age-related macular degeneration (AMD). The development of abnormal VEGF-mediated choroidal neovascularization (CNV), under the activation of canonical Wnt pathway, leads to deterioration in central vision (Zhou et al. 2010). First associated with experimental laser-induced CNV (Hu et al. 2013), high levels of phospho-LRP6 and β-catenin were also identified in the endothelium of human choroidal tissues from AMD patients (Tuo et al. 2015; Lin et al. 2018). Dysregulation of circulating levels of Wnt modulators such as kallistatin, DKK1/3 and WIF-1, correlates with the severity of CNV in AMD patients (Park et al. 2014; Tuo et al. 2015; Qiu et al. 2017). Mice deficient for Wnt7a and Wnt7b exhibit decreased CNV severity (Lin et al. 2018), whereas genetic deletion of Vldlr, a negative regulator of the Wnt pathway, recapitulates key features of wet AMD (Chen et al. 2007; Hu et al. 2008). Finally, recent studies have identified a novel causal link between dysregulation of the Wnt pathway, circadian rhythms, and extensive metabolic reprogramming in exudative AMD (Vallée et al. 2020). Altered Wnt signaling in retinopathies and AMD is shown in Figure 2C.
Wnt AND BLOOD-BRAIN-BARRIER (BBB) VASCULATURE
The BBB structure creates a physical interface between blood and brain tissue (Bundgaard and Abbott 2008), contributing to the maintenance of a safe and specific microenvironment for proper synaptic functioning and neuronal connectivity (Zhao et al. 2015). BBB dysfunction and damage are increasingly recognized as potential contributors to the pathogenesis of a number of neurodegenerative diseases and of age-related cognitive decline including Alzheimer's disease (The Alzheimer's Disease Neuroimaging Initiative et al. 2016; Nation et al. 2019; Sweeney et al. 2019).
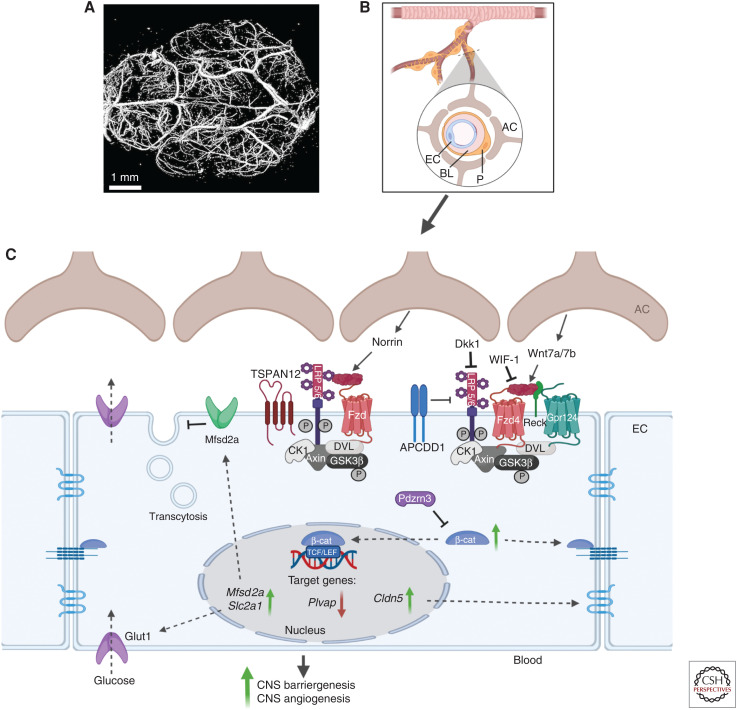
The BBB is characterized by specialized brain microvascular ECs that display unique biological characteristics linked to their barrier function, namely, specialized tight junctions (Huber et al. 2001; Wolburg and Lippoldt 2002), a lack of fenestration, selective nutrient and efflux transporters, low rates of transcytosis (Ben-Zvi et al. 2014; Andreone et al. 2017; Yang et al. 2020), and low expression of leukocyte adhesion protein. The interactions between ECs, mural cells, and glial cells, forming the neurovascular unit (NVU) in the CNS (Abbott et al. 2006), are crucial for the formation and maintenance of the BBB (Armulik et al. 2010; Guérit et al. 2021; Heithoff et al. 2021) and the control of blood flow (Mishra et al. 2016).
The canonical Wnt/β-catenin signaling has a major role during development postnatal brain angiogenesis and barriergenesis (Liebner et al. 2008; Ye et al. 2009; Wang et al. 2012, 2018; Chang et al. 2017). Consistent with this concept, EC-specific deletion of Ctnnb1 (gene-encoding β-catenin) leads to embryonic lethality as a result of hemorrhages in the CNS (Daneman et al. 2009). Activation of this pathway requires interaction of the ligands Wnt7a/b to Fzd4 receptors (Liebner et al. 2008; Wang et al. 2018) with the coreceptors LRP5/6 (Zhou et al. 2014b), in concert with the Wnt7-binding glycosylphosphatidylinositol-anchored glycoprotein Reck (Chandana et al. 2010; Vanhollebeke et al. 2015; Eubelen et al. 2018) and the adhesion G-protein-coupled receptor 124 (encoded by Gpr124) (Kuhnert et al. 2010; Anderson et al. 2011; Cullen et al. 2011; Eubelen et al. 2018). The receptor complex Fzd4/LRP5/6/Reck/Gpr124 may confer a functional specificity for transducing the Wnt canonical pathway in brain ECs, forming a “Wnt-decoding module” (Eubelen et al. 2018; Vallon et al. 2018; Cho et al. 2019). In addition to Wnt7a/b, Norrin ligand is also involved in Wnt canonical signal induction in brain ECs (Zhou et al. 2014b; Wang et al. 2018). Norrin requires the presence of the coreceptor Tspan-12 (Junge et al. 2009). Both ligands, Wnt7a/b and Norrin, are produced by the neuroepithelium of the developing CNS, concomitant with vessel formation (Stenman et al. 2008; Ye et al. 2011). Their expression is restricted to specific CNS regions, implying local regulation of Wnt pathways. As a result, their combined depletion during development induces a severe leakage throughout the CNS (Zhou et al. 2014b; Wang et al. 2018).
The canonical Wnt/β-catenin signaling plays an essential role in the final differentiation step of brain EC. Its activation up-regulates the expression of genes required to maintain EC barrier features such as claudin 5 and Slc2a1 (Glut1) extracellular matrix, whereas Pvlap, a component of fenestrated ECs, is repressed (Liebner et al. 2008; Daneman et al. 2009; Zhou et al. 2014b; Jensen et al. 2019). In the adult, understanding the role of endothelial canonical Wnt/β-catenin signaling in CNS under homeostatic and pathologic conditions remains an active field of research. Different groups report that active Wnt-β-catenin signaling is detected in the adult cerebrovasculature (Zhou et al. 2014b) and is required for maintaining BBB integrity (Tran et al. 2016). Mouse mutants with a conditional deletion of Wls in astrocytes (to repress Wnt ligand secretion), display edema and increased vascular tracer leakage in adult brain as well as low Wnt pathway activity in brain EC (Guérit et al. 2020; Wang et al. 2020). It is important to note that the regulation of the Wnt/β-catenin signaling pathway has not been determined in the CNS vasculature. However, Wnt modulators such as APCDD1, are expressed throughout the CNS vasculature (Daneman et al. 2010) and have been involved in the maintenance of the retinal barrier (Mazzoni et al. 2017). Wnt antagonists such as Wif1 and Dkk1 were shown to disrupt the endothelial BBB phenotype in cerebral tumors (Fig. 3; Phoenix et al. 2016).
Figure 3.
The Wnt signaling pathways in central nervous system (CNS) blood vessels. (A) Mouse brain vasculature was imaged with a high-resolution micro-computed tomography (CT) imaging system (Bruker MicroCT). Scale bar, 1 mm. (B) The blood-brain-barrier (BBB) creates a physical interface between blood and brain tissue. The interactions between endothelial cells (ECs), pericytes (Ps), and astrocytes (ACs) form the neurovascular unit in the CNS and are crucial for the formation and maintenance of the BBB. The BBB is characterized by microvascular ECs, which display unique properties linked to their barrier function, including specialized tight junctions, a lack of fenestration, a selective nutrient and efflux transporters system, and a low rate of transcytosis. (C) The canonical Wnt/β-catenin signaling has a major role during CNS barriergenesis and angiogenesis. Activation of the pathway involves the Norrin ligand produced by astrocytes and the downstream activation of a receptor complex formed by Fzd, LRP5/6, and Tetraspanin12. Alternatively, Wnt ligands (Wnt7a/b) secreted by astrocytes can also bind to the receptor complex consisting of Fzd and LRP5/6, which then recruits the DVL protein to the plasma membrane and induces β-catenin stabilization and its accumulation in the cytoplasm. The ubiquitin ligase PDZRN3 can block this process. Other receptors and coreceptors (probable G-protein-coupled receptor 124 [Gpr124] and reversion-inducing cysteine-rich protein with kazal motifs [Reck]) can enhance this Wnt-dependent signaling pathway, while the Wnt modulator adenomatosis polyposis coli down-regulated 1 protein (APCDD1) can block it. Inhibitors of Wnt ligands (Wnt inhibitory factor 1 [WIF-1]) or of coreceptors LRP5/6 (Dickkopf [Dkk1]) also contribute to the blocking of Wnt signaling. Stabilized β-catenin can then either contribute to the adherens junction at the plasma membrane or translocate to the nucleus and stimulate the transcription of target genes such as claudin 5 (Cldn5), which then contributes to the formation of tight junctions. Wnt/β-catenin signaling also increases the expression of solute carrier family 2 member 1 (Slc2a1), which is a specialized glucose transporter encoding for Glut1 protein and induces the expression of major facilitator superfamily domain-containing protein 2A (Msfd2a), which inhibits transcytosis. Created with BioRender.com. (BL) Basal lamina, (Plvap) plasmalemma vesicle-associated protein.
There is increasing evidence that the Wnt canonical pathway is essential to regulating the plasticity of brain ECs. For example, activation of the canonical Wnt pathway in choroid plexus ECs (capillaries that do not present BBB properties) partially promotes the acquisition of the EC barrier phenotype (Benz et al. 2019; Wang et al. 2019a). However, activation of Wnt canonical signaling did not appear to be sufficient to maintain the brain EC differentiated phenotype (Sabbagh and Nathans 2020). These data suggest that the maintenance of BBB properties may require activation of additional extrinsic environmental factors and signaling pathways other than Wnt canonical signaling. This concept is supported by a study reporting that activation of Wnt canonical signaling was not sufficient to induce a BBB-specific transcriptional profile in peripheral lung and liver EC (Munji et al. 2019).
While the canonical Wnt signaling pathway's role during BBB formation has been thoroughly investigated, its role in the pathophysiology of vascular cognitive impairment (VCI) remains to be fully elucidated. Dysregulation of Wnt/β-catenin signaling has been linked to vascular disorders in the CNS such as multiple sclerosis (Lengfeld et al. 2017), Alzheimer disease (Liu et al. 2014), and Huntington disease (Lim et al. 2017). Recently, He et al. evaluated the potential effect of Fzd7 in BBB protection after intracerebral hemorrhage. Frizzled-7 activation attenuates BBB permeability and neurological deficits after intracerebral hemorrhage through Dvl/β-catenin/WNT1-inducible signaling pathway protein 1 (WISP) pathway (He et al. 2021). We have proposed that a reactivation of the Wnt/PCP signals in brain EC during pathological ischemic events, such as stroke, would be deleterious in mouse models (Sewduth et al. 2017). Overexpressing Pdzrn3 in EC exacerbates BBB hyperpermeability and accelerates cognitive decline and is correlated with decreased claudin5 expression in brain vessels under chronic cerebral hypoperfusion, whereas depletion of Pdzrn3 is protective for BBB breakdown (Gueniot et al. 2021). Thus, there is great interest in delineating the signaling pathways that regulate the physiological functions of the BBB, and gaining insights into the pathological conditions causing loss or breakdown of the BBB (Fig. 3).
THERAPIES FOR VASCULAR DISEASES
A multiplicity of studies has recently highlighted the real potential to modulate different component of the Wnt/signaling pathways to treat vascular diseases, particularly ocular vascular disorders (Foulquier et al. 2018; Wang et al. 2019b). For the purpose of this review focusing on the Wnt/signaling pathways in ECs, only studies showing evidence of therapies’ efficacy in ECs or in angiogenesis models, have been selected and shown in Table 1 and Figure 2.
Table 1.
Modulators of Wnt signaling pathways as potential innovative therapies for vascular diseases
| Name | Type of compound/molecule | Target | Tested in | Effects on endothelial cells (ECs) and/or angiogenesis | References |
|---|---|---|---|---|---|
| F4L5.13 | Tetravalent antibody | Induces Fzd4 and LRP5 proximity | In vitro: in cultured EC In vivo: in postnatal vascularization of mouse retina and in OIR mouse models |
Triggers β-catenin signaling; promotes EC barrier function in vitro; restores retinal angiogenesis and barrier function in vivo; normalizes NV in OIR models | Chidiac et al. 2021 |
| mAbFzd7 | Monoclonal antibody | Specifically recognizes Fzd7 extracellular domain to block Fzd7 signaling | In vitro: in cultured EC In vivo: in OIR mouse models |
Normalizes pathological NV in OIR models | Bats et al. 2020 |
| Fzd7CRD | Soluble Fzd7 receptor | Traps Wnt molecules to block Fzd7 signaling | In vitro: in cultured EC In vivo: in OIR mouse models |
Normalizes pathological NV in OIR models | Bats et al. 2020 |
| 1.99.25 | Monoclonal antibody | Specifically recognizes Fzd4-CRD | In vitro: in cultured EC In vivo: in postnatal vascularization of mouse retina and in OIR mouse models; in Vldlr−/− KO mouse |
Antagonizes Norrin- and WNT3A-induced β-catenin signaling; disrupts physiological angiogenesis and induces loss of barrier function; inhibits pathological NV in OIR models; inhibits physiological and retinal pathological angiogenesis in Vldlr−/− KO mouse | Paes et al. 2011 |
| miR-184 | Liposome-based nanoparticles containing a microRNA-184 mimic | Decreases Fzd7 expression and LRP6 phosphorylation | In vivo: in OIR mouse models | Inhibits Wnt/β-catenin signaling in the retina in OIR mice models | Takahashi et al. 2015 |
| miR-150 | MicroRNA-150 mimics | Down-regulates Fzd4 expression | In vitro: in cultured EC In vivo: in OIR mouse models; in CNV mouse models (models of AMD) |
Suppresses EC proliferation, migration, and tube formation in vitro; suppresses pathological angiogenesis in OIR models | Liu et al. 2015 |
| Mab2F1 | Murine monoclonal antibody | Specifically recognizes E1E2 domain of LRP6 | In vitro: in cultured EC In vivo: in rat models of OIR and in streptozotocin-induced diabetes models (models of DR); in CNV (mouse and rat) models |
Inhibits β-catenin signaling at the Wnt receptor level; inhibits migration and tube formation in vitro; reduces retinal pathological NV in OIR models; reduces retinal vascular leakage and inflammation in OIR and DR models and in Vldlr KO mouse; decreases vascular leakage from CNV lesions and reduces the neovascular area in laser-induced CNV models | Lee et al. 2012; Hu et al. 2013 |
| H1L1 | Humanized monoclonal antibody | Specifically recognizes E1E2 domain of LRP6 |
In vivo: in alkali burn-induced corneal NV rat models | Reduces inflammation and glial stress in corneal NV models | Qiu et al. 2018 |
| Ab 2D10 | Antibody | Interacts with human and mouse Tspan12 antigen; blocks interaction between Tspan12 and Fzd4 | In vitro: in cultured EC In vivo: in postnatal vascularization of mouse retina; in OIR and Vldlr−/− KO mouse |
Blocks β-catenin signaling; inhibits migration and tube formation in vitro; delays physiological angiogenesis; attenuates retinal pathological NV in retinal vasoproliferative disease | Bucher et al. 2017 |
| Dkk1 | Recombinant protein | Binds to LRP5/6 | In vivo: in Vldlr−/− KO mouse; in OIR mouse models; in streptozotocin-induced DR rat models | Inhibits Wnt/β-catenin signaling and decreases VEGF in Vldlr−/− KO mouse; reduces retinal avascular area and neovascular tufts in OIR mouse models; ameliorates retinal inflammation, vascular leakage, and retinal NV in DR models | Chen et al. 2007, 2009; Tokunaga et al. 2013 |
| Norrin | Recombinant protein | Activates Wnt/β-catenin signaling | In vitro: in cultured EC In vivo: in OIR models in mouse with ectopic overexpression of Norrin (β B1-Norrin and Rpe65-Norrin); in Ndpy/− mice; in streptozotocin-induced DR rat models |
Increases proliferation, migration, and tube formation in vitro; Norrin mutant mice displayed increased physiological angiogenesis and decreased avascular area and pathological NV in OIR models; restores normal retinal angiogenesis in Ndpy/− mice; restores tight junction complex organization and blood-retinal-barrier (BRB) properties | Ohlmann et al. 2005, 2010; Tokunaga et al. 2013; Zeilbeck et al. 2016; Díaz-Coránguez et al. 2020 |
| VLDLR | Nanoparticles containing a plasmid expressing the VLN extracellular domain | Inhibits phosphorylation of LRP6, β-catenin accumulation, and Wnt/ β-catenin signaling | In vitro: in cultured EC In vivo: in OIR models, alkali burn-induced corneal NV models, and Vldlr−/− mouse models |
Inhibits proliferation, migration, and tube formation in vitro; inhibits abnormal neovascularization (NV) in Vldlr−/− mice, in OIR, and corneal NV mouse models | Wang et al. 2015 |
| Curcumin | Organic compound | Attenuates LRP6 phosphorylation, and inhibits β-catenin nuclear accumulation | In vitro: in cultured EC In vivo: in a suture-induced corneal NV model (Lrp5−/− mice) |
Attenuates proliferation and disrupts tube formation in vitro; inhibits corneal NV |
Zhang et al. 2017 |
| PEDF | Recombinant protein | Binds to LRP6 at the E1E2 domain; blocks Wnt ligand-induced LRP6-Frizzled receptor dimerization | In vitro: in cultured EC In vivo: in OIR mouse models |
Blocks Wnt/ β-catenin signaling; inhibits migration and increases apoptosis in vitro; reduces ischemia-induced retinal NV in vivo | Longeras et al. 2012 |
| PEDF-34 | Amino terminal 34 PEDF peptide | Binds to LRP6 at the E1E2 domain; blocks Wnt ligand-induced LRP6-Frizzled receptor dimerization | In vivo: in OIR mouse models | Inhibits endothelial progenitor cells mobilization from bone marrow into blood in vascular retinal NV | Park et al. 2011 |
| SERPINA3K | Adenoviral vectors encoding SERPINA3K | Binds to LRP6 at the extracellular domain | In vivo: in OIR models; in rat streptozotocin-induced DR models; in rat suture-induced corneal NV models | Blocks LRP6 dimerization with Fzd receptor in vitro; reduces NV, inflammation, and vascular leakage in OIR models; displays antifibrinogenic activity; suppresses corneal NV and inflammation | Zhang et al. 2009, 2010a,b; Zhou et al. 2014a |
| Lithium | Chemical compound | Inhibits GSK3β; blocks β-catenin degradation | In vitro: in cultured EC In vivo: in Lrp5−/− KO mouse; in postnatal vascularization of mouse retina |
Activates β-catenin signaling; restores tube formation in vitro in LRP5-deficient EC; normalizes deeper layer vessels and ameliorates pathologic glomeruloid vessels in Lrp5−/− mice; rescues physiological angiogenesis in mice deleted for Fzd7 in EC | Peghaire et al. 2016; Wang et al. 2016 |
| XAV-939 | Chemical compound | Tankyrase 1/2 inhibitor that promotes axin2 stabilization | In vitro: in cultured EC | Promotes β-catenin degradation; inhibits β-catenin signaling; modest effect on BRB properties | Huang et al. 2009 |
| Melatonin | Chemical compound | Inhibits Wnt/ β-catenin signaling | In vitro: in cultured EC in streptozotocin-induced DR rat models | Inhibits high glucose-induced proliferation, migration, invasion, tube formation, and inflammation in vitro; alleviates BRB disruption | Yan et al. 2021 |
| TNP470 | Chemical compound | A noncanonical Wnt5a inhibitor | In vitro: in cultured EC In vivo: in postnatal vascularization of mouse retina; in OIR mouse models |
Blocks noncanonical Wnt signaling by targeting methionine aminopeptidase-2; increases vessel regression accompanied by decreased EC proliferation in retinal postnatal angiogenesis; decreases pathological NV in OIR models | Korn et al. 2014 |
(OIR) Oxygen-induced retinopathy, (DR) diabetic retinopathy, (AMD) age-related macular degeneration, (CNV) choroidal neovascularization.
CONCLUSION
Taken together, the data point toward a central role of Wnt/β-catenin signaling during vascular development and homeostasis, where Wnt signaling is a hub integrating other crucial angiogenic intertwined pathways. With the advent of single-cell RNA-sequencing technology and the huge source of available transcriptomic data, one of the next exciting challenges will be to identify the precise combination of Wnt/Fzd partners that interact during physiological or pathological angiogenesis to identify new cell- or tissue-specific pathways.
ACKNOWLEDGMENTS
This work was funded by grants overseen by the French National Agency (ANR) as part of the “Investissements d'Avenir” Program ANR-18-RHUS-002 and as part of the “ERA-CVD” Program ANR-20-ECVD-0002-01 (ANR ENRICH).
Footnotes
Editors: Diane R. Bielenberg and Patricia A. D'Amore
Additional Perspectives on Angiogenesis: Biology and Pathology available at www.perspectivesinmedicine.org
REFERENCES
- Abbott NJ, Rönnbäck L, Hansson E. 2006. Astrocyte-endothelial interactions at the blood–brain barrier. Nat Rev Neurosci 7: 41–53. 10.1038/nrn1824 [DOI] [PubMed] [Google Scholar]
- Acebron SP, Niehrs C. 2016. β-Catenin-independent roles of Wnt/LRP6 signaling. Trends Cell Biol 26: 956–967. 10.1016/j.tcb.2016.07.009 [DOI] [PubMed] [Google Scholar]
- Anderson KD, Pan L, Yang XM, Hughes VC, Walls JR, Dominguez MG, Simmons MV, Burfeind P, Xue Y, Wei Y, et al. 2011. Angiogenic sprouting into neural tissue requires Gpr124, an orphan G protein-coupled receptor. Proc Natl Acad Sci 108: 2807–2812. 10.1073/pnas.1019761108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreone BJ, Chow BW, Tata A, Lacoste B, Ben-Zvi A, Bullock K, Deik AA, Ginty DD, Clish CB, Gu C. 2017. Blood–brain barrier permeability is regulated by lipid transport-dependent suppression of caveolae-mediated transcytosis. Neuron 94: 581–594.e5. 10.1016/j.neuron.2017.03.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, et al. 2010. Pericytes regulate the blood–brain barrier. Nature 468: 557–561. 10.1038/nature09522 [DOI] [PubMed] [Google Scholar]
- Artus C, Glacial F, Ganeshamoorthy K, Ziegler N, Godet M, Guilbert T, Liebner S, Couraud PO. 2014. The Wnt/planar cell polarity signaling pathway contributes to the integrity of tight junctions in brain endothelial cells. J Cereb Blood Flow Metab 34: 433–440. 10.1038/jcbfm.2013.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bänziger C, Soldini D, Schütt C, Zipperlen P, Hausmann G, Basler K. 2006. Wntless, a conserved membrane protein dedicated to the secretion of wnt proteins from signaling cells. Cell 125: 509–522. 10.1016/j.cell.2006.02.049 [DOI] [PubMed] [Google Scholar]
- Bats ML, Bougaran P, Peghaire C, Gueniot F, Abelanet A, Chan H, Séguy C, Jeanningros S, Jaspard-Vinassa B, Couffinhal T, et al. 2020. Therapies targeting Frizzled-7/β-catenin pathway prevent the development of pathological angiogenesis in an ischemic retinopathy model. FASEB J 34: 1288–1303. 10.1096/fj.201901886R [DOI] [PubMed] [Google Scholar]
- Beitia GJ, Rutherford TJ, Freund SMV, Pelham HR, Bienz M, Gammons MV. 2021. Regulation of Dishevelled DEP domain swapping by conserved phosphorylation sites. Proc Natl Acad Sci 118: e2103258118. 10.1073/pnas.2103258118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz F, Wichitnaowarat V, Lehmann M, Germano RF, Mihova D, Macas J, Adams RH, Taketo MM, Plate KH, Guérit S, et al. 2019. Low wnt/β-catenin signaling determines leaky vessels in the subfornical organ and affects water homeostasis in mice. eLife 8: e43818. 10.7554/eLife.43818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Zvi A, Lacoste B, Kur E, Andreone BJ, Mayshar Y, Yan H, Gu C. 2014. Mfsd2a is critical for the formation and function of the blood–brain barrier. Nature 509: 507–511. 10.1038/nature13324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger W, Pol D, Warburg M, Gal A, Bleeker-Wagemakers L, De Silva H, Meindl A, Meitinger T, Cremers F, Ropers HH. 1992. Mutations in the candidate gene for Norrie disease. Hum Mol Genet 1: 461–465. 10.1093/hmg/1.7.461 [DOI] [PubMed] [Google Scholar]
- Bilić J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M, Niehrs C. 2007. Wnt induces LRP6 signalosomes and promotes Dishevelled-dependent LRP6 phosphorylation. Science 316: 1619–1622. 10.1126/science.1137065 [DOI] [PubMed] [Google Scholar]
- Birdsey GM, Shah AV, Dufton N, Reynolds LE, Osuna Almagro L, Yang Y, Aspalter IM, Khan ST, Mason JC, Dejana E, et al. 2015. The endothelial transcription factor ERG promotes vascular stability and growth through Wnt/β-catenin signaling. Dev Cell 32: 82–96. 10.1016/j.devcel.2014.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black GC, Perveen R, Bonshek R, Cahill M, Clayton-Smith J, Lloyd IC, McLeod D. 1999. Coats’ disease of the retina (unilateral retinal telangiectasis) caused by somatic mutation in the NDP gene: a role for norrin in retinal angiogenesis. Hum Mol Genet 8: 2031–2035. 10.1093/hmg/8.11.2031 [DOI] [PubMed] [Google Scholar]
- Bucher F, Zhang D, Aguilar E, Sakimoto S, Diaz-Aguilar S, Rosenfeld M, Zha Z, Zhang H, Friedlander M, Yea K. 2017. Antibody-mediated inhibition of Tspan12 ameliorates vasoproliferative retinopathy through suppression of β-catenin signaling. Circulation 136: 180–195. [DOI] [PubMed] [Google Scholar]
- Bundgaard M, Abbott NJ. 2008. All vertebrates started out with a glial blood–brain barrier 4–500 million years ago. Glia 56: 699–708. 10.1002/glia.20642 [DOI] [PubMed] [Google Scholar]
- Carvalho JR, Fortunato IC, Fonseca CG, Pezzarossa A, Barbacena P, Dominguez-Cejudo MA, Vasconcelos FF, Santos NC, Carvalho FA, Franco CA. 2019. Non-canonical Wnt signaling regulates junctional mechanocoupling during angiogenic collective cell migration. eLife 8: e45853. 10.7554/eLife.45853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallo RA, Cox RT, Moline MM, Roose J, Polevoy GA, Clevers H, Peifer M, Bejsovec A. 1998. Drosophila Tcf and Groucho interact to repress Wingless signalling activity. Nature 395: 604–608. 10.1038/26982 [DOI] [PubMed] [Google Scholar]
- Chandana EP, Maeda Y, Ueda A, Kiyonari H, Oshima N, Yamamoto M, Kondo S, Oh J, Takahashi R, Yoshida Y, et al. 2010. Involvement of the Reck tumor suppressor protein in maternal and embryonic vascular remodeling in mice. BMC Dev Biol 10: 84. 10.1186/1471-213X-10-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Mancuso MR, Maier C, Liang X, Yuki K, Yang L, Kwong JW, Wang J, Rao V, Vallon M, et al. 2017. Gpr124 is essential for blood–brain barrier integrity in central nervous system disease. Nat Med 23: 450–460. 10.1038/nm.4309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Hu Y, Lu K, Flannery JG, Ma J. 2007. Very low density lipoprotein receptor, a negative regulator of the wnt signaling pathway and choroidal neovascularization. J Biol Chem 282: 34420–34428. 10.1074/jbc.M611289200 [DOI] [PubMed] [Google Scholar]
- Chen Y, Hu Y, Zhou T, Zhou KK, Mott R, Wu M, Boulton M, Lyons TJ, Gao G, Ma J. 2009. Activation of the Wnt pathway plays a pathogenic role in diabetic retinopathy in humans and animal models. Am J Pathol 175: 2676–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Stahl A, Krah NM, Seaward MR, Dennison RJ, Sapieha P, Hua J, Hatton CJ, Juan AM, Aderman CM, et al. 2011. Wnt signaling mediates pathological vascular growth in proliferative retinopathy. Circulation 124: 1871–1881. 10.1161/CIRCULATIONAHA.111.040337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Stahl A, Krah NM, Seaward MR, Joyal JS, Juan AM, Hatton CJ, Aderman CM, Dennison RJ, Willett KL, et al. 2012. Retinal expression of Wnt-pathway mediated genes in low-density lipoprotein receptor-related protein 5 (Lrp5) knockout mice. PLoS ONE 7: e30203. 10.1371/journal.pone.0030203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Yeh J, Fan TP, Smith SK, Charnock-Jones DS. 2008. Wnt5a-mediated non-canonical Wnt signalling regulates human endothelial cell proliferation and migration. Biochem Biophys Res Commun 365: 285–290. 10.1016/j.bbrc.2007.10.166 [DOI] [PubMed] [Google Scholar]
- Chidiac R, Abedin M, Macleod G, Yang A, PE Thibeault, LL Blazer, JJ Adams, Zhang L, Roehrich H, H-N Jo, et al. 2021. A Norrin/Wnt surrogate antibody stimulates endothelial cell barrier function and rescues retinopathy. EMBO Mol Med 13: e13977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho C, Wang Y, Smallwood PM, Williams J, Nathans J. 2019. Molecular determinants in Frizzled, Reck, and Wnt7a for ligand-specific signaling in neurovascular development. eLife 8: e47300. 10.7554/eLife.47300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Kim H, Lee HG, Kim BK, Park JY, Kim DY, Ahn SH, Han KH, Kim SU. 2017. Dickkopf-1 induces angiogenesis via VEGF receptor 2 regulation independent of the Wnt signaling pathway. Oncotarget 8: 58974–58984. 10.18632/oncotarget.19769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirone P, Lin S, Griesbach HL, Zhang Y, Slusarski DC, Crews CM. 2008. A role for planar cell polarity signaling in angiogenesis. Angiogenesis 11: 347–360. 10.1007/s10456-008-9116-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corada M, Nyqvist D, Orsenigo F, Caprini A, Giampietro C, Taketo MM, Iruela-Arispe ML, Adams RH, Dejana E. 2010. The Wnt/β-catenin pathway modulates vascular remodeling and specification by upregulating Dll4/Notch signaling. Dev Cell 18: 938–949. 10.1016/j.devcel.2010.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corada M, Orsenigo F, Morini MF, Pitulescu ME, Bhat G, Nyqvist D, Breviario F, Conti V, Briot A, Iruela-Arispe ML, et al. 2013. Sox17 is indispensable for acquisition and maintenance of arterial identity. Nat Commun 4: 2609. 10.1038/ncomms3609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corada M, Orsenigo F, Bhat GP, Conze LL, Breviario F, Cunha SI, Claesson-Welsh L, Beznoussenko GV, Mironov AA, Bacigaluppi M, et al. 2019. Fine-tuning of Sox17 and canonical wnt coordinates the permeability properties of the blood–brain barrier. Circ Res 124: 511–525. 10.1161/CIRCRESAHA.118.313316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtwright A, Siamakpour-Reihani S, Arbiser JL, Banet N, Hilliard E, Fried L, Livasy C, Ketelsen D, Nepal DB, Perou CM, et al. 2009. Secreted Frizzle-related protein 2 stimulates angiogenesis via a calcineurin/NFAT signaling pathway. Cancer Res 69: 4621–4628. 10.1158/0008-5472.CAN-08-3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen M, Elzarrad MK, Seaman S, Zudaire E, Stevens J, Yang MY, Li X, Chaudhary A, Xu L, Hilton MB, et al. 2011. GPR124, an orphan G protein-coupled receptor, is required for CNS-specific vascularization and establishment of the blood–brain barrier. Proc Natl Acad Sci 108: 5759–5764. 10.1073/pnas.1017192108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. 2009. Wnt/β-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci 106: 641–646. 10.1073/pnas.0805165106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Agalliu D, Cahoy JD, Kaushal A, Barres BA. 2010. The mouse blood–brain barrier transcriptome: a new resource for understanding the development and function of brain endothelial cells. PLoS ONE 5: e13741. 10.1371/journal.pone.0013741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson G, Wu W, Shen J, Bilic J, Fenger U, Stannek P, Glinka A, Niehrs C. 2005. Casein kinase 1 γ couples Wnt receptor activation to cytoplasmic signal transduction. Nature 438: 867–872. 10.1038/nature04170 [DOI] [PubMed] [Google Scholar]
- Descamps B, Sewduth R, Ferreira Tojais N, Jaspard B, Reynaud A, Sohet F, Lacolley P, Allières C, Lamazière J-MD, Moreau C, et al. 2012. Frizzled 4 regulates arterial network organization through noncanonical wnt/planar cell polarity signaling. Circ Res 110: 47–58. 10.1161/CIRCRESAHA.111.250936 [DOI] [PubMed] [Google Scholar]
- Díaz-Coránguez M, Lin C-M, Liebner S, Antonetti DA. 2020. Norrin restores blood-retinal barrier properties after vascular endothelial growth factor-induced permeability. J Biol Chem 295: 4647–4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenser KA. 2016. Wnt signaling pathway in retinal vascularization. Eye Brain 8: 141–146. 10.2147/EB.S94452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufourcq P, Descamps B, Tojais NF, Leroux L, Oses P, Daret D, Moreau C, Lamazière JMD, Couffinhal T, Duplàa C. 2008. Secreted Frizzled-related protein-1 enhances mesenchymal stem cell function in angiogenesis and contributes to neovessel maturation. Stem Cells 26: 2991–3001. 10.1634/stemcells.2008-0372 [DOI] [PubMed] [Google Scholar]
- Eubelen M, Bostaille N, Cabochette P, Gauquier A, Tebabi P, Dumitru AC, Koehler M, Gut P, Alsteens D, Stainier DYR, et al. 2018. A molecular mechanism for Wnt ligand-specific signaling. Science 361: eaat1178. 10.1126/science.aat1178 [DOI] [PubMed] [Google Scholar]
- Ewen-Campen B, Comyn T, Vogt E, Perrimon N. 2020. No evidence that wnt ligands are required for planar cell polarity in Drosophila. Cell Rep 32: 108121. 10.1016/j.celrep.2020.108121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezan J. 2004. Frza/sFRP-1, a secreted antagonist of the Wnt-Frizzled pathway, controls vascular cell proliferation in vitro and in vivo. Cardiovasc Res 63: 731–738. 10.1016/j.cardiores.2004.05.006 [DOI] [PubMed] [Google Scholar]
- Ferrara N. 2009. Vascular endothelial growth factor. Arterioscler Thromb Vasc Biol 29: 789–791. 10.1161/ATVBAHA.108.179663 [DOI] [PubMed] [Google Scholar]
- Foulquier S, Daskalopoulos EP, Lluri G, Hermans KCM, Deb A, Blankesteijn WM. 2018. WNT signaling in cardiac and vascular disease. Pharmacol Rev 70: 68–141. 10.1124/pr.117.013896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco CA, Jones ML, Bernabeu MO, Geudens I, Mathivet T, Rosa A, Lopes FM, Lima AP, Ragab A, Collins RT, et al. 2015. Dynamic endothelial cell rearrangements drive developmental vessel regression. PLoS Biol 13: e1002125. 10.1371/journal.pbio.1002125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco CA, Jones ML, Bernabeu MO, Vion AC, Barbacena P, Fan J, Mathivet T, Fonseca CG, Ragab A, Yamaguchi TP, et al. 2016. Non-canonical Wnt signalling modulates the endothelial shear stress flow sensor in vascular remodelling. eLife 5: e07727. 10.7554/eLife.07727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, et al. 2003. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol 161: 1163–1177. 10.1083/jcb.200302047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour DF. 2015. Familial exudative vitreoretinopathy and related retinopathies. Eye (Lond) 29: 1–14. 10.1038/eye.2014.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, et al. 2001. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 107: 513–523. 10.1016/S0092-8674(01)00571-2 [DOI] [PubMed] [Google Scholar]
- Goodwin AM, Sullivan KM, D'Amore PA. 2006. Cultured endothelial cells display endogenous activation of the canonical Wnt signaling pathway and express multiple ligands, receptors, and secreted modulators of Wnt signaling. Dev Dyn 235: 3110–3120. 10.1002/dvdy.20939 [DOI] [PubMed] [Google Scholar]
- Gordon MD, Nusse R. 2006. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem 281: 22429–22433. 10.1074/jbc.R600015200 [DOI] [PubMed] [Google Scholar]
- Gore AV, Swift MR, Cha YR, Lo B, McKinney MC, Li W, Castranova D, Davis A, Mukouyama Y, Weinstein BM. 2011. Rspo1/Wnt signaling promotes angiogenesis via Vegfc/Vegfr3. Development 138: 4875–4886. 10.1242/dev.068460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumolato L, Liu G, Mong P, Mudbhary R, Biswas R, Arroyave R, Vijayakumar S, Economides AN, Aaronson SA. 2010. Canonical and noncanonical Wnts use a common mechanism to activate completely unrelated coreceptors. Genes Dev 24: 2517–2530. 10.1101/gad.1957710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueniot F, Rubin S, Bougaran P, Abelanet A, Morel J, Bontempi B, Proust C, Dufourcq P, Couffinhal T, Duplàa C. 2021. Targeting Pdzrn3 maintains adult blood–brain barrier and central nervous system homeostasis. J Cereb Blood Flow Metab. 10.1177/0271678X211048981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guérit S, Fidan E, Macas J, Czupalla CJ, Figueiredo R, Vijikumar A, Yalcin BH, Thom S, Winter P, Gerhardt H, et al. 2020. Astrocyte-derived Wnt growth factors are required for endothelial blood–brain barrier maintenance. Prog Neurobiol 199: 101937. [DOI] [PubMed] [Google Scholar]
- Guérit S, Fidan E, Macas J, Czupalla CJ, Figueiredo R, Vijikumar A, Yalcin BH, Thom S, Winter P, Gerhardt H, et al. 2021. Astrocyte-derived Wnt growth factors are required for endothelial blood–brain barrier maintenance. Prog Neurobiol 199: 101937. 10.1016/j.pneurobio.2020.101937 [DOI] [PubMed] [Google Scholar]
- Guruharsha KG, Kankel MW, Artavanis-Tsakonas S. 2012. The Notch signalling system: recent insights into the complexity of a conserved pathway. Nat Rev Genet 13: 654–666. 10.1038/nrg3272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Lu Q, Sherchan P, Huang L, Hu X, Zhang JH, Dai H, Tang J. 2021. Activation of Frizzled-7 attenuates blood–brain barrier disruption through Dvl/β-catenin/WISP1 signaling pathway after intracerebral hemorrhage in mice. Fluids Barriers CNS 18: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heithoff BP, George KK, Phares AN, Zuidhoek IA, Munoz-Ballester C, Robel S. 2021. Astrocytes are necessary for blood–brain barrier maintenance in the adult mouse brain. Glia 69: 436–472. 10.1002/glia.23908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikita T, Mirzapourshafiyi F, Barbacena P, Riddell M, Pasha A, Li M, Kawamura T, Brandes RP, Hirose T, Ohno S, et al. 2018. PAR-3 controls endothelial planar polarity and vascular inflammation under laminar flow. EMBO Rep 19: e45253. 10.15252/embr.201745253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Jiang A, Liang J, Meng H, Chang B, Gao H, Qiao X. 2008. Expression of VLDLR in the retina and evolution of subretinal neovascularization in the knockout mouse model's retinal angiomatous proliferation. Invest Ophthalmol Vis Sci 49: 407–415. 10.1167/iovs.07-0870 [DOI] [PubMed] [Google Scholar]
- Hu Y, Chen Y, Lin M, Lee K, Mott RA, Ma J. 2013. Pathogenic role of the Wnt signaling pathway activation in laser-induced choroidal neovascularization. Invest Ophthalmol Vis Sci 54: 141–154. 10.1167/iovs.12-10281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S-MA, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner S, et al. 2009. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 461: 614–620. [DOI] [PubMed] [Google Scholar]
- Huber JD, Egleton RD, Davis TP. 2001. Molecular physiology and pathophysiology of tight junctions in the blood–brain barrier. Trends Neurosci 24: 719–725. 10.1016/S0166-2236(00)02004-X [DOI] [PubMed] [Google Scholar]
- Iruela-Arispe ML, Davis GE. 2009. Cellular and molecular mechanisms of vascular lumen formation. Dev Cell 16: 222–231. 10.1016/j.devcel.2009.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson L, Franco CA, Bentley K, Collins RT, Ponsioen B, Aspalter IM, Rosewell I, Busse M, Thurston G, Medvinsky A, et al. 2010. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat Cell Biol 12: 943–953. 10.1038/ncb2103 [DOI] [PubMed] [Google Scholar]
- Janda CY, Waghray D, Levin AM, Thomas C, Garcia KC. 2012. Structural basis of wnt recognition by Frizzled. Science 337: 59–64. 10.1126/science.1222879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen LD, Hot B, Ramsköld D, Germano RFV, Yokota C, Giatrellis S, Lauschke VM, Hubmacher D, Li MX, Hupe M, et al. 2019. Disruption of the extracellular matrix progressively impairs central nervous system vascular maturation downstream of β-catenin signaling. Arterioscler Thromb Vasc Biol 39: 1432–1447. 10.1161/ATVBAHA.119.312388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L-Y, Ma K. 2021. Novel Norrie disease gene mutations in Chinese patients with familial exudative vitreoretinopathy. BMC Ophthalmol 21: 84. 10.1186/s12886-021-01852-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junge HJ, Yang S, Burton JB, Paes K, Shu X, French DM, Costa M, Rice DS, Ye W. 2009. TSPAN12 regulates retinal vascular development by promoting Norrin- but not wnt-induced FZD4/β-catenin signaling. Cell 139: 299–311. 10.1016/j.cell.2009.07.048 [DOI] [PubMed] [Google Scholar]
- Kania KD, Wijesuriya HC, Hladky SB, Barrand MA. 2011. β Amyloid effects on expression of multidrug efflux transporters in brain endothelial cells. Brain Res 1418: 1–11. 10.1016/j.brainres.2011.08.044 [DOI] [PubMed] [Google Scholar]
- Kato M, Patel MS, Levasseur R, Lobov I, Chang BHJ, Glass DA, Hartmann C, Li L, Hwang TH, Brayton CF, et al. 2002. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol 157: 303–314. 10.1083/jcb.200201089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazanskaya O, Ohkawara B, Heroult M, Wu W, Maltry N, Augustin HG, Niehrs C. 2008. The Wnt signaling regulator R-spondin 3 promotes angioblast and vascular development. Development 135: 3655–3664. 10.1242/dev.027284 [DOI] [PubMed] [Google Scholar]
- Konopelski Snavely SE, Susman MW, Kunz RC, Tan J, Srinivasan S, Cohen MD, Okada K, Lamb H, Choi SS, Karuna EP, et al. 2021. Proteomic analysis identifies the E3 ubiquitin ligase Pdzrn3 as a regulatory target of Wnt5a-Ror signaling. Proc Natl Acad Sci 118: e2104944118. 10.1073/pnas.2104944118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn C, Augustin HG. 2015. Mechanisms of vessel pruning and regression. Dev Cell 34: 5–17. 10.1016/j.devcel.2015.06.004 [DOI] [PubMed] [Google Scholar]
- Korn C, Scholz B, Hu J, Srivastava K, Wojtarowicz J, Arnsperger T, Adams RH, Boutros M, Augustin HG, Augustin I. 2014. Endothelial cell-derived non-canonical Wnt ligands control vascular pruning in angiogenesis. Development 141: 1757–1766. 10.1242/dev.104422 [DOI] [PubMed] [Google Scholar]
- Kuhnert F, Mancuso MR, Shamloo A, Wang HT, Choksi V, Florek M, Su H, Fruttiger M, Young WL, Heilshorn SC, et al. 2010. Essential regulation of CNS angiogenesis by the orphan G protein-coupled receptor GPR124. Science 330: 985–989. 10.1126/science.1196554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai MB, Zhang C, Shi J, Johnson V, Khandan L, McVey J, Klymkowsky MW, Chen Z, Junge HJ. 2017. TSPAN12 is a Norrin co-receptor that amplifies Frizzled4 ligand selectivity and signaling. Cell Rep 19: 2809–2822. 10.1016/j.celrep.2017.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammert E, Axnick J. 2012. Vascular lumen formation. Cold Spring Harb Perspect Med 2: a006619. 10.1101/cshperspect.a006619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Hu Y, Ding L, Chen Y, Takahashi Y, Mott R, Ma J-X. 2012. Therapeutic potential of a monoclonal antibody blocking the Wnt pathway in diabetic retinopathy. Diabetes 61: 2948–2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Elaskandrany M, Lau LF, Lazzaro D, Grant MB, Chaqour B. 2017. Interplay between CCN1 and Wnt5a in endothelial cells and pericytes determines the angiogenic outcome in a model of ischemic retinopathy. Sci Rep 7: 1405. 10.1038/s41598-017-01585-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengfeld JE, Lutz SE, Smith JR, Diaconu C, Scott C, Kofman SB, Choi C, Walsh CM, Raine CS, Agalliu I, et al. 2017. Endothelial Wnt/β-catenin signaling reduces immune cell infiltration in multiple sclerosis. Proc Natl Acad Sci 114: E1168–E1177. 10.1073/pnas.1609905114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, et al. 2008. Wnt/β-catenin signaling controls development of the blood–brain barrier. J Cell Biol 183: 409–417. 10.1083/jcb.200806024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JC, Kania KD, Wijesuriya H, Chawla S, Sethi JK, Pulaski L, Romero IA, Couraud PO, Weksler BB, Hladky SB, et al. 2008. Activation of β-catenin signalling by GSK-3 inhibition increases p-glycoprotein expression in brain endothelial cells. J Neurochem 106: 1855–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim RG, Quan C, Reyes-Ortiz AM, Lutz SE, Kedaigle AJ, Gipson TA, Wu J, Vatine GD, Stocksdale J, Casale MS, et al. 2017. Huntington's disease iPSC-derived brain microvascular endothelial cells reveal wnt-mediated angiogenic and blood–brain barrier deficits. Cell Rep 19: 1365–1377. 10.1016/j.celrep.2017.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JB, Sene A, Wiley LA, Santeford A, Nudleman E, Nakamura R, Lin JB, Moolani HV, Apte RS. 2018. WNT7A/B promote choroidal neovascularization. Exp Eye Res 174: 107–112. 10.1016/j.exer.2018.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Nathans J. 2008. An essential role for frizzled 5 in mammalian ocular development. Development 135: 3567–3576. 10.1242/dev.028076 [DOI] [PubMed] [Google Scholar]
- Liu H, Mohamed O, Dufort D, Wallace VA. 2003. Characterization of Wnt signaling components and activation of the Wnt canonical pathway in the murine retina. Dev Dyn 227: 323–334. 10.1002/dvdy.10315 [DOI] [PubMed] [Google Scholar]
- Liu L, Wan W, Xia S, Kalionis B, Li Y. 2014. Dysfunctional Wnt/β-catenin signaling contributes to blood–brain barrier breakdown in Alzheimer's disease. Neurochem Int 75: 19–25. 10.1016/j.neuint.2014.05.004 [DOI] [PubMed] [Google Scholar]
- Liu C-H, Sun Y, Li J, Gong Y, Tian KT, Evans LP, Morss PC, Fredrick TW, Saba NJ, Chen J. 2015. Endothelial microRNA-150 is an intrinsic suppressor of pathologic ocular neovascularization. Proc Natl Acad Sci 112: 12163–12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobov IB, Rao S, Carroll TJ, Vallance JE, Ito M, Ondr JK, Kurup S, Glass DA, Patel MS, Shu W, et al. 2005. WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature. Nature 437: 417–421. 10.1038/nature03928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longeras R, Farjo K, Ihnat M, Ma J-X. 2012. A PEDF-derived peptide inhibits retinal neovascularization and blocks mobilization of bone marrow-derived endothelial progenitor cells. Exp Diabetes Res 2012: 518426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack JJ, Iruela-Arispe ML. 2018. NOTCH regulation of the endothelial cell phenotype. Curr Opin Hematol 25: 212–218. 10.1097/MOH.0000000000000425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martowicz A, Trusohamn M, Jensen N, Wisniewska-Kruk J, Corada M, Ning FC, Kele J, Dejana E, Nyqvist D. 2019. Endothelial β-catenin signaling supports postnatal brain and retinal angiogenesis by promoting sprouting, tip cell formation, and VEGFR (vascular endothelial growth factor receptor) 2 expression. Arterioscler Thromb Vasc Biol 39: 2273–2288. 10.1161/ATVBAHA.119.312749 [DOI] [PubMed] [Google Scholar]
- Masckauchán TNH, Agalliu D, Vorontchikhina M, Ahn A, Parmalee NL, Li CM, Khoo A, Tycko B, Brown AMC, Kitajewski J. 2006. Wnt5a signaling induces proliferation and survival of endothelial cells in vitro and expression of MMP-1 and tie-2. Mol Biol Cell 17: 5163–5172. 10.1091/mbc.e06-04-0320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni J, Smith JR, Shahriar S, Cutforth T, Ceja B, Agalliu D. 2017. The wnt inhibitor Apcdd1 coordinates vascular remodeling and barrier maturation of retinal blood vessels. Neuron 96: 1055–1069.e6. 10.1016/j.neuron.2017.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis UR, Chavakis E, Kruse C, Jungblut B, Kaluza D, Wandzioch K, Manavski Y, Heide H, Santoni MJ, Potente M, et al. 2013. The polarity protein Scrib is essential for directed endothelial cell migration. Circ Res 112: 924–934. 10.1161/CIRCRESAHA.112.300592 [DOI] [PubMed] [Google Scholar]
- Min JK, Park H, Choi HJ, Kim Y, Pyun BJ, Agrawal V, Song BW, Jeon J, Maeng YS, Rho SS, et al. 2011. The WNT antagonist Dickkopf2 promotes angiogenesis in rodent and human endothelial cells. J Clin Invest 121: 1882–1893. 10.1172/JCI42556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A, Reynolds JP, Chen Y, Gourine AV, Rusakov DA, Attwell D. 2016. Astrocytes mediate neurovascular signaling to capillary pericytes but not to arterioles. Nat Neurosci 19: 1619–1627. 10.1038/nn.4428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montcouquiol M, Rachel RA, Lanford PJ, Copeland NG, Jenkins NA, Kelley MW. 2003. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature 423: 173–177. 10.1038/nature01618 [DOI] [PubMed] [Google Scholar]
- Munji RN, Soung AL, Weiner GA, Sohet F, Semple BD, Trivedi A, Gimlin K, Kotoda M, Korai M, Aydin S, et al. 2019. Profiling the mouse brain endothelial transcriptome in health and disease models reveals a core blood–brain barrier dysfunction module. Nat Neurosci 22: 1892–1902. 10.1038/s41593-019-0497-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nation DA, Sweeney MD, Montagne A, Sagare AP, D'Orazio LM, Pachicano M, Sepehrband F, Nelson AR, Buennagel DP, Harrington MG, et al. 2019. Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med 25: 270–276. 10.1038/s41591-018-0297-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo MH, Borowska-Fielding J, Heathcote G, Nejat S, Kelly ME, McMaster CR, Robitaille JM. 2016. Fzd4 haploinsufficiency delays retinal revascularization in the mouse model of oxygen induced retinopathy. PLoS ONE 11: e0158320. 10.1371/journal.pone.0158320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehrs C. 2006. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene 25: 7469–7481. 10.1038/sj.onc.1210054 [DOI] [PubMed] [Google Scholar]
- Nusse R, Clevers H. 2017. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell 169: 985–999. 10.1016/j.cell.2017.05.016 [DOI] [PubMed] [Google Scholar]
- Ohlmann A, Scholz M, Goldwich A, Chauhan BK, Hudl K, Ohlmann AV, Zrenner E, Berger W, Cvekl A, Seeliger MW, et al. 2005. Ectopic Norrin induces growth of ocular capillaries and restores normal retinal angiogenesis in Norrie disease mutant mice. J Neurosci 25: 1701–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlmann A, Seitz R, Braunger B, Seitz D, Bösl MR, Tamm ER. 2010. Norrin promotes vascular regrowth after oxygen-induced retinal vessel loss and suppresses retinopathy in mice. J Neurosci 30: 183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paes KT, Wang E, Henze K, Vogel P, Read R, Suwanichkul A, Kirkpatrick LL, Potter D, Newhouse MM, Rice DS. 2011. Frizzled 4 Is required for retinal angiogenesis and maintenance of the blood-retina barrier. Invest Ophthalmol Vis Sci 52: 6452–6461. [DOI] [PubMed] [Google Scholar]
- Park K, Lee K, Zhang B, Zhou T, He X, Gao G, Murray AR, Ma J-X. 2011. Identification of a novel inhibitor of the canonical Wnt pathway. Mol Cell Biol 31: 3038–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KH, Choi AJ, Yoon J, Lim D, Woo SJ, Park SJ, Kim HC, Chung H. 2014. Wnt modulators in the aqueous humor are associated with outer retinal damage severity in patients with neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci 55: 5522–5530. 10.1167/iovs.14-14566 [DOI] [PubMed] [Google Scholar]
- Peghaire C, Bats ML, Sewduth R, Jeanningros S, Jaspard B, Couffinhal T, Duplàa C, Dufourcq P. 2016. Fzd7 (Frizzled-7) expressed by endothelial cells controls blood vessel formation through Wnt/β-catenin canonical signaling. Arterioscler Thromb Vasc Biol 36: 2369–2380. 10.1161/ATVBAHA.116.307926 [DOI] [PubMed] [Google Scholar]
- Pelton JC, Wright CE, Leitges M, Bautch VL. 2014. Multiple endothelial cells constitute the tip of developing blood vessels and polarize to promote lumen formation. Development 141: 4121–4126. 10.1242/dev.110296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendás-Franco N, García JM, Peña C, Valle N, Pálmer HG, Heinäniemi M, Carlberg C, Jiménez B, Bonilla F, Muñoz A, et al. 2008. DICKKOPF-4 is induced by TCF/β-catenin and upregulated in human colon cancer, promotes tumour cell invasion and angiogenesis and is repressed by 1α,25-dihydroxyvitamin D3. Oncogene 27: 4467–4477. 10.1038/onc.2008.88 [DOI] [PubMed] [Google Scholar]
- Phng LK, Potente M, Leslie JD, Babbage J, Nyqvist D, Lobov I, Ondr JK, Rao S, Lang RA, Thurston G, et al. 2009. Nrarp coordinates endothelial notch and wnt signaling to control vessel density in angiogenesis. Dev Cell 16: 70–82. 10.1016/j.devcel.2008.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phoenix TN, Patmore DM, Boop S, Boulos N, Jacus MO, Patel YT, Roussel MF, Finkelstein D, Goumnerova L, Perreault S, et al. 2016. Medulloblastoma genotype dictates blood brain barrier phenotype. Cancer Cell 29: 508–522. 10.1016/j.ccell.2016.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu F, He J, Zhou Y, Bai X, Wu G, Wang X, Liu Z, Chen Y, Ma JX, Liu Z. 2014. Plasma and vitreous fluid levels of Dickkopf-1 in patients with diabetic retinopathy. Eye (Lond) 28: 402–409. 10.1038/eye.2013.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu F, Liu Z, Zhou Y, He J, Gong S, Bai X, Zeng Y, Liu Z, Ma JX. 2017. Decreased circulating levels of Dickkopf-1 in patients with exudative age-related macular degeneration. Sci Rep 7: 1263. 10.1038/s41598-017-01119-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu F, Shin Y, Chen D, Cheng R, Chen Q, Zhou K, Larrick JW, Mendelson AR, Ma J-X. 2018. Anti-angiogenic effect of a humanized antibody blocking the Wnt/β-catenin signaling pathway. Microvasc Res 119: 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran I, Thavathiru E, Ramalingam S, Natarajan G, Mills WK, Benbrook DM, Zuna R, Lightfoot S, Reis A, Anant S, et al. 2012. Wnt inhibitory factor 1 induces apoptosis and inhibits cervical cancer growth, invasion and angiogenesis in vivo. Oncogene 31: 2725–2737. 10.1038/onc.2011.455 [DOI] [PubMed] [Google Scholar]
- Rao TP, Kühl M. 2010. An updated overview on wnt signaling pathways: a prelude for more. Circ Res 106: 1798–1806. 10.1161/CIRCRESAHA.110.219840 [DOI] [PubMed] [Google Scholar]
- Reis M, Czupalla CJ, Ziegler N, Devraj K, Zinke J, Seidel S, Heck R, Thom S, Macas J, Bockamp E, et al. 2012. Endothelial Wnt/β-catenin signaling inhibits glioma angiogenesis and normalizes tumor blood vessels by inducing PDGF-B expression. J Exp Med 209: 1611–1627. 10.1084/jem.20111580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter M, Gottanka J, May CA, Welge-Lüssen U, Berger W, Lütjen-Drecoll E. 1998. Retinal vasculature changes in Norrie disease mice. Invest Ophthalmol Vis Sci 39: 2450–2457. [PubMed] [Google Scholar]
- Rivera JC, Dabouz R, Noueihed B, Omri S, Tahiri H, Chemtob S. 2017. Ischemic retinopathies: oxidative stress and inflammation. Oxid Med Cell Longev 2017: 3940241. 10.1155/2017/3940241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille JM, Zheng B, Wallace K, Beis MJ, Tatlidil C, Yang J, Sheidow TG, Siebert L, Levin AV, Lam WC, et al. 2011. The role of Frizzled-4 mutations in familial exudative vitreoretinopathy and Coats disease. Br J Ophthalmol 95: 574–579. 10.1136/bjo.2010.190116 [DOI] [PubMed] [Google Scholar]
- Roca C, Adams RH. 2007. Regulation of vascular morphogenesis by Notch signaling. Genes Dev 21: 2511–2524. 10.1101/gad.1589207 [DOI] [PubMed] [Google Scholar]
- Rogers KA, McKee NH, Kalnins VI. 1985. Preferential orientation of centrioles toward the heart in endothelial cells of major blood vessels is reestablished after reversal of a segment. Proc Natl Acad Sci 82: 3272–3276. 10.1073/pnas.82.10.3272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhrberg C, Gerhardt H, Golding M, Watson R, Ioannidou S, Fujisawa H, Betsholtz C, Shima DT. 2002. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev 16: 2684–2698. 10.1101/gad.242002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbagh MF, Nathans J. 2020. A genome-wide view of the de-differentiation of central nervous system endothelial cells in culture. eLife 9: e51276. 10.7554/eLife.51276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakanaka C, Weiss JB, Williams LT. 1998. Bridging of β-catenin and glycogen synthase kinase-3β by Axin and inhibition of β-catenin-mediated transcription. Proc Natl Acad Sci 95: 3020–3023. 10.1073/pnas.95.6.3020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz B, Korn C, Wojtarowicz J, Mogler C, Augustin I, Boutros M, Niehrs C, Augustin HG. 2016. Endothelial RSPO3 controls vascular stability and pruning through non-canonical WNT/Ca2+/NFAT signaling. Dev Cell 36: 79–93. 10.1016/j.devcel.2015.12.015 [DOI] [PubMed] [Google Scholar]
- Schwarz-Romond T, Fiedler M, Shibata N, Butler PJG, Kikuchi A, Higuchi Y, Bienz M. 2007. The DIX domain of Dishevelled confers Wnt signaling by dynamic polymerization. Nat Struct Mol Biol 14: 484–492. 10.1038/nsmb1247 [DOI] [PubMed] [Google Scholar]
- Sen M, Shields CL, Honavar SG, Shields JA. 2019. Coats disease: an overview of classification, management and outcomes. Indian J Ophthalmol 67: 763–771. 10.4103/ijo.IJO_841_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewduth RN, Jaspard-Vinassa B, Peghaire C, Guillabert A, Franzl N, Larrieu-Lahargue F, Moreau C, Fruttiger M, Dufourcq P, Couffinhal T, et al. 2014. The ubiquitin ligase PDZRN3 is required for vascular morphogenesis through Wnt/planar cell polarity signalling. Nat Commun 5: 4832. 10.1038/ncomms5832 [DOI] [PubMed] [Google Scholar]
- Sewduth RN, Kovacic H, Jaspard-Vinassa B, Jecko V, Wavasseur T, Fritsch N, Pernot M, Jeaningros S, Roux E, Dufourcq P, et al. 2017. PDZRN3 destabilizes endothelial cell–cell junctions through a PKCζ-containing polarity complex to increase vascular permeability. Sci Signal 10: eaag3209. 10.1126/scisignal.aag3209 [DOI] [PubMed] [Google Scholar]
- Shah AV, Birdsey GM, Peghaire C, Pitulescu ME, Dufton NP, Yang Y, Weinberg I, Osuna Almagro L, Payne L, Mason JC, et al. 2017. The endothelial transcription factor ERG mediates Angiopoietin-1-dependent control of Notch signalling and vascular stability. Nat Commun 8: 16002. 10.1038/ncomms16002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M, Mlodzik M. 2008. Planar cell polarity signaling: from fly development to human disease. Annu Rev Genet 42: 517–540. 10.1146/annurev.genet.42.110807.091432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurk C, Maatz H, Rocnik E, Bialik A, Force T, Walsh K. 2005. Glycogen-synthase kinase3β/β-catenin axis promotes angiogenesis through activation of vascular endothelial growth factor signaling in endothelial cells. Circ Res 96: 308–318. 10.1161/01.RES.0000156273.30274.f7 [DOI] [PubMed] [Google Scholar]
- Stahl A, Connor KM, Sapieha P, Chen J, Dennison RJ, Krah NM, Seaward MR, Willett KL, Aderman CM, Guerin KI, et al. 2010. The mouse retina as an angiogenesis model. Invest Ophthalmol Vis Sci 51: 2813–2826. 10.1167/iovs.10-5176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefater JA III, Lewkowich I, Rao S, Mariggi G, Carpenter AC, Burr AR, Fan J, Ajima R, Molkentin JD, Williams BO, et al. 2011. Regulation of angiogenesis by a non-canonical Wnt–Flt1 pathway in myeloid cells. Nature 474: 511–515. 10.1038/nature10085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefater JA, Rao S, Bezold K, Aplin AC, Nicosia RF, Pollard JW, Ferrara N, Lang RA. 2013. Macrophage Wnt-Calcineurin-Flt1 signaling regulates mouse wound angiogenesis and repair. Blood 121: 2574–2578. 10.1182/blood-2012-06-434621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP. 2008. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science 322: 1247–1250. 10.1126/science.1164594 [DOI] [PubMed] [Google Scholar]
- Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV. 2019. Blood–brain barrier: from physiology to disease and back. Physiol Rev 99: 21–78. 10.1152/physrev.00050.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada R, Satomi Y, Kurata T, Ueno N, Norioka S, Kondoh H, Takao T, Takada S. 2006. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev Cell 11: 791–801. 10.1016/j.devcel.2006.10.003 [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Chen Q, Rajala RVS, Ma JX. 2015. MicroRNA-184 modulates canonical Wnt signaling through the regulation of frizzled-7 expression in the retina with ischemia-induced neovascularization. FEBS Lett 589: 1143–1149. 10.1016/j.febslet.2015.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatin F, Taddei A, Weston A, Fuchs E, Devenport D, Tissir F, Makinen T. 2013. Planar cell polarity protein Celsr1 regulates endothelial adherens junctions and directed cell rearrangements during valve morphogenesis. Dev Cell 26: 31–44. 10.1016/j.devcel.2013.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Alzheimer's Disease Neuroimaging Initiative; Iturria-Medina Y, Sotero RC, Toussaint PJ, Mateos-Pérez JM, Evans AC. 2016. Early role of vascular dysregulation on late-onset Alzheimer's disease based on multifactorial data-driven analysis. Nat Commun 7: 11. 10.1038/ncomms11934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga CC, Chen Y-H, Dailey W, Cheng M, Drenser KA. 2013. Retinal vascular rescue of oxygen-induced retinopathy in mice by Norrin. Invest Ophthalmol Vis Sci 54: 222–229. [DOI] [PubMed] [Google Scholar]
- Tran KA, Zhang X, Predescu D, Huang X, Machado RF, Göthert JR, Malik AB, Valyi-Nagy T, Zhao YY. 2016. Endothelial β-catenin signaling is required for maintaining adult blood–brain barrier integrity and central nervous system homeostasis. Circulation 133: 177–186. 10.1161/CIRCULATIONAHA.115.015982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuo J, Wang Y, Cheng R, Li Y, Chen M, Qiu F, Qian H, Shen D, Penalva R, Xu H, et al. 2015. Wnt signaling in age-related macular degeneration: human macular tissue and mouse model. J Transl Med 13: 330. 10.1186/s12967-015-0683-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. 2005. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437: 426–431. 10.1038/nature03952 [DOI] [PubMed] [Google Scholar]
- Vallée A, Lecarpentier Y, Vallée R, Guillevin R, Vallée JN. 2020. Circadian rhythms in exudative age-related macular degeneration: the key role of the canonical WNT/β-catenin pathway. Int J Mol Sci 21: 820. 10.3390/ijms21030820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallon M, Yuki K, Nguyen TD, Chang J, Yuan J, Siepe D, Miao Y, Essler M, Noda M, Garcia KC, et al. 2018. A RECK-WNT7 receptor-ligand interaction enables isoform-specific regulation of wnt bioavailability. Cell Rep 25: 339–349.e9. 10.1016/j.celrep.2018.09.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhollebeke B, Stone OA, Bostaille N, Cho C, Zhou Y, Maquet E, Gauquier A, Cabochette P, Fukuhara S, Mochizuki N, et al. 2015. Tip cell-specific requirement for an atypical Gpr124- and Reck-dependent Wnt/β-catenin pathway during brain angiogenesis. eLife 4: e06489. 10.7554/eLife.06489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon K, Huijbers EJM, Griffioen AW. 2021. Secreted frizzled-related protein 2: a key player in noncanonical Wnt signaling and tumor angiogenesis. Cancer Metastasis Rev 40: 191–203. 10.1007/s10555-020-09941-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallingford JB. 2012. Planar cell polarity and the developmental control of cell behavior in vertebrate embryos. Annu Rev Cell Dev Biol 28: 627–653. 10.1146/annurev-cellbio-092910-154208 [DOI] [PubMed] [Google Scholar]
- Wang Y, Nathans J. 2007. Tissue/planar cell polarity in vertebrates: new insights and new questions. Development 134: 647–658. 10.1242/dev.02772 [DOI] [PubMed] [Google Scholar]
- Wang Y, Rattner A, Zhou Y, Williams J, Smallwood PM, Nathans J. 2012. Norrin/Frizzled4 signaling in retinal vascular development and blood brain barrier plasticity. Cell 151: 1332–1344. 10.1016/j.cell.2012.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Cheng R, Lee K, Tyagi P, Ding L, Kompella UB, Chen J, Xu X, Ma J-X. 2015. Nanoparticle-mediated expression of a Wnt pathway inhibitor ameliorates ocular neovascularization. Arterioscler Thromb Vasc Biol 35: 855–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Liu C-H, Sun Y, Gong Y, Favazza TL, Morss PC, Saba NJ, Fredrick TW, He X, Akula JD, et al. 2016. Pharmacologic activation of Wnt signaling by lithium normalizes retinal vasculature in a murine model of familial exudative vitreoretinopathy. Am J Pathol 186: 2588–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Cho C, Williams J, Smallwood PM, Zhang C, Junge HJ, Nathans J. 2018. Interplay of the Norrin and Wnt7a/Wnt7b signaling systems in blood–brain barrier and blood–retina barrier development and maintenance. Proc Natl Acad Sci 115: E11827–E11836. 10.1073/pnas.1813217115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Sabbagh MF, Gu X, Rattner A, Williams J, Nathans J. 2019a. β-Catenin signaling regulates barrier-specific gene expression in circumventricular organ and ocular vasculatures. eLife 8: e43257. 10.7554/eLife.43257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Liu CH, Huang S, Chen J. 2019b. Wnt signaling in vascular eye diseases. Prog Retin Eye Res 70: 110–133. 10.1016/j.preteyeres.2018.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Liu CH, Huang S, Fu Z, Tomita Y, Britton WR, Cho SS, Chen CT, Sun Y, Ma J, et al. 2020. Wnt signaling activates MFSD2A to suppress vascular endothelial transcytosis and maintain blood–retinal barrier. Sci Adv 6: eaba7457. 10.1126/sciadv.aba7457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, Nusse R. 2003. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423: 448–452. 10.1038/nature01611 [DOI] [PubMed] [Google Scholar]
- Wolburg H, Lippoldt A. 2002. Tight junctions of the blood–brain barrier: development, composition and regulation. Vascul Pharmacol 38: 323–337. 10.1016/S1537-1891(02)00200-8 [DOI] [PubMed] [Google Scholar]
- Wu J, Mlodzik M. 2009. A quest for the mechanism regulating global planar cell polarity of tissues. Trends Cell Biol 19: 295–305. 10.1016/j.tcb.2009.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JH, Liu JH, Ko YC, Wang CT, Chung YC, Chu KC, Liu TT, Chao HM, Jiang YJ, Chen SJ, et al. 2016. Haploinsufficiency of RCBTB1 is associated with coats disease and familial exudative vitreoretinopathy. Hum Mol Genet 25: 1637–1647. 10.1093/hmg/ddw041 [DOI] [PubMed] [Google Scholar]
- Xiao H, Tong Y, Zhu Y, Peng M. 2019. Familial exudative vitreoretinopathy-related disease-causing genes and Norrin/β-catenin signal pathway: structure, function, and mutation spectrums. J Ophthalmol 2019: 5782536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Cleaver O. 2011. Tubulogenesis during blood vessel formation. Semin Cell Dev Biol 22: 993–1004. 10.1016/j.semcdb.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Wang Y, Dabdoub A, Smallwood PM, Williams J, Woods C, Kelley MW, Jiang L, Tasman W, Zhang K, et al. 2004. Vascular development in the retina and inner ear. Cell 116: 883–895. 10.1016/S0092-8674(04)00216-8 [DOI] [PubMed] [Google Scholar]
- Yan M, Wang H, Gu Y, Li X, Tao L, Lu P. 2021. Melatonin exerts protective effects on diabetic retinopathy via inhibition of Wnt/β-catenin pathway as revealed by quantitative proteomics. Exp Eye Res 205: 108521. [DOI] [PubMed] [Google Scholar]
- Yang AC, Stevens MY, Chen MB, Lee DP, Stähli D, Gate D, Contrepois K, Chen W, Iram T, Zhang L, et al. 2020. Physiological blood–brain transport is impaired with age by a shift in transcytosis. Nature 583: 425–430. 10.1038/s41586-020-2453-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Wang Y, Cahill H, Yu M, Badea TC, Smallwood PM, Peachey NS, Nathans J. 2009. Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell 139: 285–298. 10.1016/j.cell.2009.07.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Wang Y, Nathans J. 2010. The Norrin/Frizzled4 signaling pathway in retinal vascular development and disease. Trends Mol Med 16: 417–425. 10.1016/j.molmed.2010.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Smallwood P, Nathans J. 2011. Expression of the Norrie disease gene (Ndp) in developing and adult mouse eye, ear, and brain. Gene Expr Patterns 11: 151–155. 10.1016/j.gep.2010.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi H, Nakamura REI, Mohamed O, Dufort D, Hackam AS. 2007. Characterization of Wnt signaling during photoreceptor degeneration. Invest Ophthalmol Vis Sci 48: 5733–5741. 10.1167/iovs.07-0097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JJS, Maugarny-Calès A, Pelletier S, Alexandre C, Bellaiche Y, Vincent JP, McGough IJ. 2020. Frizzled-dependent planar cell polarity without secreted wnt ligands. Dev Cell 54: 583–592.e5. 10.1016/j.devcel.2020.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeilbeck LF, Müller BB, Leopold SA, Senturk B, Langmann T, Tamm ER, Ohlmann A. 2016. Norrin mediates angiogenic properties via the induction of insulin-like growth factor-1. Exp Eye Res 145: 317–326. 10.1016/j.exer.2015.12.001 [DOI] [PubMed] [Google Scholar]
- Zeng X, Tamai K, Doble B, Li S, Huang H, Habas R, Okamura H, Woodgett J, He X. 2005. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature 438: 873–877. 10.1038/nature04185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Hu Y, Ma J. 2009. Anti-inflammatory and antioxidant effects of SERPINA3K in the retina. Invest Ophthalmol Vis Sci 50: 3943–3952. [DOI] [PubMed] [Google Scholar]
- Zhang B, Abreu JG, Zhou K, Chen Y, Hu Y, Zhou T, He X, Ma J. 2010a. Blocking the Wnt pathway, a unifying mechanism for an angiogenic inhibitor in the serine proteinase inhibitor family. Proc Natl Acad Sci 107: 6900–6905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Zhou KK, Ma J. 2010b. Inhibition of connective tissue growth factor overexpression in diabetic retinopathy by SERPINA3K via blocking the WNT/β-catenin pathway. Diabetes 59: 1809–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Fukuhara S, Sako K, Takenouchi T, Kitani H, Kume T, Koh GY, Mochizuki N. 2011. Angiopoietin-1/Tie2 signal augments basal Notch signal controlling vascular quiescence by inducing Delta-like 4 expression through AKT-mediated activation of β-catenin. J Biol Chem 286: 8055–8066. 10.1074/jbc.M110.192641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Lai MB, Khandan L, Lee LA, Chen Z, Junge HJ. 2017. Norrin-induced Frizzled4 endocytosis and endo-lysosomal trafficking control retinal angiogenesis and barrier function. Nat Commun 8: 16050. 10.1038/ncomms16050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Yao E, Lin C, Chou YT, Wong J, Li J, Wolters PJ, Chuang PT. 2020. A mammalian Wnt5a–Ror2–Vangl2 axis controls the cytoskeleton and confers cellular properties required for alveologenesis. eLife 9: e53688. 10.7554/eLife.53688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li X, Liu W, Zhang X, Huang L, Li S, Yang M, Zhao P, Yang J, Fei P, et al. 2021. Whole-exome sequencing identified DLG1 as a candidate gene for familial exudative vitreoretinopathy. Genet Test Mol Biomarkers 25: 309–316. 10.1089/gtmb.2021.0013 [DOI] [PubMed] [Google Scholar]
- Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV. 2015. Establishment and dysfunction of the blood–brain barrier. Cell 163: 1064–1078. 10.1016/j.cell.2015.10.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Hu Y, Chen Y, Zhou KK, Zhang B, Gao G, Ma J. 2010. The pathogenic role of the canonical Wnt pathway in age-related macular degeneration. Invest Ophthalmol Vis Sci 51: 4371–4379. 10.1167/iovs.09-4278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Chen L, Huang C-H, Lin Z, Zong R, Zhu C, Pan F, Ma J-X, Liu Z-G, Zhou Y. 2014a. Serine proteinase inhibitor SERPINA3K suppresses corneal neovascularization via inhibiting Wnt signaling and VEGF. Invest Ophthalmol Vis Sci 10.1167/jovs.14-14023 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Wang Y, Tischfield M, Williams J, Smallwood PM, Rattner A, Taketo MM, Nathans J. 2014b. Canonical WNT signaling components in vascular development and barrier formation. J Clin Invest 124: 3825–3846. 10.1172/JCI76431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Williams J, Smallwood PM, Nathans J. 2015. Sox7, Sox17, and Sox18 cooperatively regulate vascular development in the mouse retina. PLoS ONE 10: e0143650. 10.1371/journal.pone.0143650 [DOI] [PMC free article] [PubMed] [Google Scholar]