Summary
Background
The main objective of the present study was to analyze both clinical characteristics and evolution during hospitalization of a cohort of patients admitted for COVID-19 pneumonia who were not vaccinated, or with a complete or incomplete vaccination schedule.
Methods
This COVID-19 specialized single-center cohort study of 1888 COVID-19 patients hospitalized at the “Enfermera Isabel Zendal” Emergencies Hospital (HEEIZ), Madrid (Spain) was performed between July 1 and September 30, 2021. It compared the results of 1327 hospitalized unvaccinated patients to 209 hospitalized fully vaccinated and 352 hospitalized partially vaccinated patients. The four different COVID-19 vaccines authorized in Spain during the time-period studied were: BNT162b2 (Pfizer); ChAdOx1 nCoV-19 (AstraZeneca), mRNA-1273 (Moderna); Ad26.COV2.S (Janssen).
Findings
Hospitalized patients’ median age was 41 years (IQR 33–50) for the unvaccinated and 61 years (IQR 53–67) for the fully vaccinated ones. The main comorbidities were obesity, hypertension and diabetes mellitus. 20% of unvaccinated patients (266) required noninvasive respiratory care, as did 14% (51) of partially and 14% (30) of fully vaccinated; 6% (78) of the unvaccinated patients also needed invasive respiratory care, as did 5% (16) of partially and 11 (5%) fully vaccinated.
Interpretation
Fully vaccinated patients were 84% (95% CI: 82–86%) less likely to be admitted to hospital, and protection rose for those aged <50 years. Once hospitalized, vaccinated patients displayed more protection against requiring respiratory care than unvaccinated ones, despite being older and having more comorbidities. No differences appeared for the four studied COVID-19 vaccines and complying with vaccination recommendations proved relevant.
Funding
The research was funded by the “Plan Propio de Investigación” Program of the Castilla-La Mancha University /European Regional Development Fund (2021-GRIN-31,039).
Keywords: COVID-19, Vaccines, Vaccinated patients, Pfizer, BNT162b2, AstraZeneca, Azd1222, Moderna, mRNA-1273, Janssen, Ad26.COV2.S, Invasive respiratory care, Non-invasive respiratory care
Research in context.
Evidence before this study
We searched PubMed and Web of Science until December 10, 2021, for articles documenting the clinical characteristics of hospitalized vaccinated patients infected by SARS-CoV-2. We used keywords (“SARS-CoV-2″ OR “COVID-19″) AND (“vaccinated hospitalized” OR “hospitalized patients”). We applied no language or time restrictions. We found that COVID-19 vaccines were extremely efficient against infection by SARS-CoV-2 for those who were: admitted to hospital, to an ICU, went to A&E, or were urgently or clinically attended. We found not a single published work with such a complete sample about the clinical characteristics of hospitalized vaccinated patients infected by SARS-CoV-2 compared to unvaccinated patients in the same hospital and also included noninvasive and invasive respiratory care.
Added value of this study
We describe the epidemiological, clinical, laboratory and invasive/noninvasive respiratory care characteristics of the 1888 patients admitted to “Enfermera Isabel Zendal” Emergencies Hospital (Madrid, Spain), of whom 1327 (70%) were unvaccinated patients and 561 (30%) were vaccinated when hospitalized with at least one dose. Of the 561, 209 (13%) were fully vaccinated. The hospitalized patients’ median age was 41 years (IQR 33.0–50.0) for the unvaccinated group (IQR 34.0–54.0), 43 years for the partially vaccinated patients and 61 years (IQR 53.0–67.0) for those fully vaccinated. Fully vaccinated patients were older and had more comorbidities than unvaccinated patients, which affected all comorbidity types (heart disease, pneumophathy, diabetes, hypertension, chronic kidney disease, cancer, obesity); 266 (20% of 1327) unvaccinated patients required noninvasive respiratory care, as did 51 (14% of 352) partially vaccinated and 30 (14% of 209) fully vaccinated patients; 78 (6% of 1327) of the unvaccinated patients needed invasive respiratory care, compared to 16 (4% of 352) of the partially vaccinated and 11 (5% of 209) of the fully vaccinated patients.
Implications of all the available evidence
Vaccines prevent hospitalizations in all the age ranges. Once hospitalized, the vaccinated patients were protected versus the unvaccinated patients in relation to respiratory care despite being older and presenting more comorbidities than those unvaccinated. The clinical differences of the patients did not depend on the four inoculated vaccines: BNT162b2 (Pfizer), ChAdOx1 nCoV-19 (AstraZeneca), mRNA-1273 (Moderna); Ad26.COV2.S (Janssen).
Alt-text: Unlabelled box
Introduction
In December 2019, a series of pneumonia cases of unknown cause emerged in Wuhan, Hubei (China) with similar clinical presentation to viral pneumonia.1 On March 11, 2020, the World Health Organization announced that the disease called COVID-19, and produced by SARS-CoV-2, was a world pandemic because it had quickly spread among humans.2 The clinical COVID-19 spectrum is wide, and infection is characterized by a process that goes from mild asymptomatic disease to severe systemic symptoms that affect mainly lungs and the gastrointestinal tract, and may eventually lead to multi-organ dysfunction.3,4 Fortunately, different very efficient vaccines have been developed against the most serious consequences of infection by SARS-CoV-2, such as those who need to go to A&E, hospitalization and are admitted to an intensive care unit (ICU).5, 6, 7, 8, 9, 10, 11 Nevertheless, very little is known about the clinical characteristics of hospitalized vaccinated patients, including those who need noninvasive and/or invasive respiratory care.
Spain is one of the most advanced countries in vaccination percentages, an important fact that has successfully overcome its fifth COVID-19 wave (July-September 2021). In this context, it is worth studying in-depth outbreak management in Madrid, Spain's capital where the “Enfermera Isabel Zendal” Emergencies Hospital (HEEIZ) was opened as the first specialized COVID-19 hospital on December 1, 2020. During many periods, it has hospitalized more than 20% of all the patients admitted to hospitals in the Community of Madrid (CM), which indicates its representativeness of hospitalized patients.
Our objective is to describe the epidemiological, clinical and laboratory characteristics of patients confirmed with infection by SARS-CoV-2, and to compare clinical characteristics between hospitalized vaccinated and hospitalized unvaccinated patients. We offer details of the 1888 patients hospitalized at HEEIZ, which include 561 patients who had received in July, August and September 2021 at least one of the four vaccines authorized in the UE; BNT162b2 (Pfizer); ChAdOx1 nCoV-19 (AstraZeneca), mRNA-1273 (Moderna); Ad26.COV2.S (Janssen). We compared them to the 1327 unvaccinated patients hospitalized in the same months. As far as we know, this is the first study to compare the clinical and laboratory characteristics of a large sample of vaccinated patients and another sample of unvaccinated patients hospitalized in the same monographic hospital and on the same treatment according to their clinical status. The possibility of receiving noninvasive and invasive respiratory care is stressed.
Methods
Specialized COVID-19 “Enfermera Isabel Zendal” Emergencies Hospital (HEEIZ)
This monographic healthcare center has supported the hospital network of the Madrid Health Service (MHS) and has attended more than 8000 patients since it opened. It receives those patients from other public/private hospitals in the CM, all of them with confirmed diagnosis of infection by SARS-CoV-2 and secondary pneumonia. It comprises a conventional hospitalization area, an intermediate respiratory care unit for noninvasive respiratory care and an ICU for invasive respiratory care.
Study design and patients
This single-center observational cohort study included 1888 patients hospitalized consecutively at COVID-19 specialized HEEIZ for pneumonia caused by SARS-CoV-2 between July 1 and September 30, 2021. The study included those with infection by SARS-CoV-2 confirmed by either polymerase chain reaction (PCR) or antigen testing in nose/throat exude, and pneumonia by chest X-ray or CT scan, who voluntarily accepted being transferred to HEEIZ from emergency departments within the hospital network of the Madrid Health Service (MHS), Spain. Treatments suggested by WHO12 were followed for all hospitalized patients. Exclusion criteria for admission at HEEIZ were patients aged <18 years, dependent, had cognitive impairment, on cancer treatment with chemotherapy or radiotherapy, transplanted or on dialysis.
Ethics
In Spain, vaccination has been always voluntary, and there has not had contraindication to vaccination among the comorbidities recorded. Due to the pandemic situation and as it is a specialized COVID-19 hospital, all informed consent was verbal after the development of a validated protocol. This study was approved by the Ethics Committee of Research with Medication from the University La Paz Hospital.
Data collection
The epidemiological, clinical and laboratory data were prospectively collected, and electronic clinical records were prepared. A protected anonymized database was used in compliance with EU data protection laws. All the data were verified by two researchers (JT and PL). A third researcher (JGR) analyzed any differences in the interpretations of the two principal investigators. Vaccine status was recorded with the administered vaccine type and dates of doses; (BNT162b2 (Pfizer); ChAdOx1 nCoV-19 (AstraZeneca); mRNA-1273 (Moderna; Ad26.COV2.S (Janssen). Comorbidities were grouped as cardiopathy (ischemic cardiopathy, heart failure, arrhythmia), pneumopathy (obstructive sleep apnea, chronic obstructive pulmonary disease, asthma, interstitial lung disease), diabetes, hypertension, obesity (defined as body mass index (BMI) >30), chronic kidney disease and cancer (including solid and hematological organ tumors). The analytical variables considered predictors of poor prognosis in former studies were recorded:13 lymphocyte and platelet counts, C-reactive protein, procalcitonin, lactate dehydrogenase, ferritin and D-dimer.
Procedures
Complete blood count, coagulation and serum biochemical tests were done upon admission to HEEIZ. The individuals with hypoxemia, defined as <94% peripheral oxygen saturation (SpO2) or arterial oxygen pressure (pO2) <60 mmHg, received low-flow oxygen delivery by nasal prongs. Those requiring >50% fraction of inspired oxygen (FiO2) by masks with a venturi system or mask with a reservoir to maintain SpO2 >94% and/or respiratory rate (RR) >24 breaths per minute were transferred to a specific unit to be monitored. Then noninvasive respiratory care commenced with oxygenotherapy by high-flow nasal cannula (HFNC), continuous positive airway pressure (CPAP) or bilevel ventilation. The patients who clinically deteriorated, understood as worse mechanical breathing (tachypnea with accessory muscles and/or thoracoabdominal disassociation), 90–94% SpO2 despite optimizing noninvasive therapy or whose clinical progress was the same after 48 h in the unit, went from mechanic ventilation to invasive respiratory care by orotracheal intubation.
Definitions
Unvaccinated patients were those who had not received any vaccine dose before being hospitalized. Vaccinated patients had received at least one dose. Fully vaccinated patients had completed the vaccination program recommended by the Spanish Ministry of Health with two doses for Pfizer, AstraZeneca and Moderna, or one dose for Janssen, with more than 14 days between the last dose and hospitalization. Partially vaccinated patients were those with one dose of Pfizer, AstraZeneca or Moderna, or that had received two doses of those three vaccines or one dose of Janssen but 14 days had not elapsed between their last dose and being hospitalized.
Statistical analysis
The statistical analysis was carried out with SPSS, version 24.0 (SPSS Inc., Chicago, IL, US) and the R Software (R-4.1.2, R Foundation, Viena, Austria). The continuous and categorical variables were presented as the median (IQR) and n (%), respectively. To study vaccination status according to age two different sets of age ranges were used. First set: patients were divided in two age ranges: ≥ and <50 years of age; second set: patients were classified in eight different age ranges (<20, 20-29, 30-39, 40-49, 50-59, 60-69, 70-79 and ≥80 years old).
Univariate analysis: Mann-Whitney U, χ2 or Fisher's exact test was used to compare differences between the vaccinated and unvaccinated patients according to requirements. Statistical significance was set at p < 0·05.
Multivariate analysis: Three binary logistic regressions (backward stepwise modeling) were performed to check for confounders. Dependent variables were non-invasive respiratory therapy (yes/no), invasive respiratory therapy (yes/no), and exitus (yes/no). Categorical covariates considered were vaccinated status (unvaccinated, partially vaccinated, and fully vaccinated), age (<50,≥50), sex (male and female) and comorbidities (yes/no). In addition, we included the following quantitative covariates: lymphocytes, platelets, C-reactive protein, lactate dehydrogenase, ferritin and D-dimer. Quantitative variables were transformed into dichotomous (0: normal range, 1: high) or polytomous (0: normal range, 1: low, 2: high) when appropriate. Dichotomous: C-reactive protein [normal range: 0–5 mg/L], d-dimer [normal range: 0–500 ng/mL] and lactate dehydrogenase [normal range: 100–190 IU/L). Polytomous: lymphocytes [normal range: 1·1–4·5 × 10e3/µL], platelets [normal range: 150–370×10e3/µL] and ferritin [normal range: 22–322 ng/mL]. Odds Ratio (OR) and 95% confidence interval (CI) were calculated.
Relative Risk (RR). Protective effect of vaccination against either hospitalization or non-invasive and invasive respiratory treatments was analyzed by calculating the RR and 95% CI for vaccinated vs. unvaccinated patients compared to the target population of CM (see Figure 3) by age. This was possible because both internal hospital data and data from CM were available by age group.
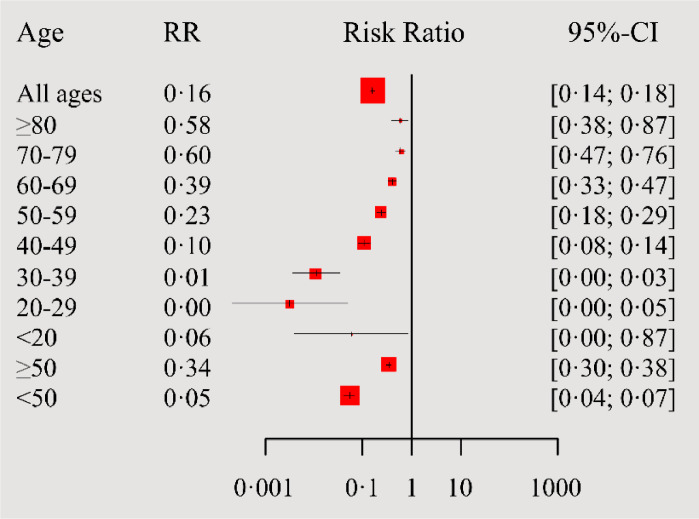
Figure 3.
Forest plot showing the association between age and risk of hospitalization due to COVID-19 for fully vaccinated vs. unvaccinated patients compared to the target population of the Community of Madrid; RR, relative risk; CI, confidence interval.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
A total of 1888 consecutive hospitalizations of patients with PCR-confirmed SARS-CoV-2 infection and pneumonia, aged 18–96 years, were analyzed. These 1888 were all the hospitalizations that took place at the COVID-19-specialized HEEIZ in Madrid, Spain over the 3-month period of the study (July 1-September 30, 2021, which corresponds to the fifth COVID-19 pandemic wave in Spain). HEEIZ is a COVID-19-specialized hospital which serves the entire population (≈6·7 million residents) of the Community of Madrid (CM). As detailed in supplementary Fig. S1, from July to September 1888 hospitalizations corresponded to more than 20% of all COVID-19 hospitalizations throughout CM; i.e., HEEIZ treated above 20% of all COVID-19 hospitalizations during the period studied making our study sample representative the population of CM and Spain (Fig. S1).
COVID-19 vaccination status and hospitalization
Unvaccinated patients composed 70% of the patients hospitalized during the 3-month period (Table 1), while only 30% of the hospitalized patients had received at least one dose of one of the four vaccines approved in Spain during the study period; 11% of the hospitalized patients were fully vaccinated with the corresponding vaccine 15 days before admission; 19% did not meet this criterion and were considered partially vaccinated (Table 1).
Table 1.
Patients’ demographic characteristics according to vaccination status.
| All patients (N = 1888) | Unvaccinated (N = 1327) | Partially Vaccinated (N = 352) | Fully vaccinated (N = 209) | Partially vaccinated p value † | Fully vaccinated p value † | |
|---|---|---|---|---|---|---|
| Characteristic | ||||||
| Age, years | 43·0 (34·0–54·0) | 41·0 (33·0–50·0) | 43·0 (34·0–54·0) | 61·0 (53·0–67·0) | 0·002 | <0·0001 |
| Age group | ||||||
| <50 yr | 1273 (67%) | 986 (74%) | 245 (70%) | 42 (20%) | 0·08 | <0·0001 |
| ≥50 yr | 615 (33%) | 341 (26%) | 107 (30%) | 167 (80%) | 0·08 | <0·0001 |
| <20 yr | 13 (1%) | 11 (1%) | 2 (1%) | 0 (0%) | 0·99 | 0·38 |
| 20–29 yr | 252 (13%) | 211 (16%) | 41 (12%) | 0 (0%) | 0·05 | <0·0001 |
| 30–39 yr | 455 (24%) | 363 (27%) | 89 (25%) | 3 (1%) | 0·44 | <0·0001 |
| 40–49 yr | 553 (29%) | 401 (30%) | 113 (32%) | 39 (19%) | 0·50 | 0·0006 |
| 50–59 yr | 298 (16%) | 196 (15%) | 46 (13%) | 56 (27%) | 0·42 | <0·0001 |
| 60–69 yr | 245 (13%) | 117 (9%) | 55 (16%) | 73 (35%) | 0·0002 | <0·0001 |
| 70–79 yr | 53 (3%) | 21 (2%) | 3 (1%) | 29 (14%) | 0·30 | <0·0001 |
| ≥80 yr | 19 (1%) | 7 (0·5%) | 3 (1%) | 9 (4%) | 0·48 | <0·0001 |
| Sex | ||||||
| Men | 1086 (57·5%) | 755 (57%) | 210 (60%) | 121 (58%) | 0·35 | 0·79 |
| Women | 802 (42·5%) | 572 (43%) | 142 (40) | 88 (42%) | ||
| COVID-19 vaccine | ||||||
| BNT162b2 (Pfizer) | 297 (53%) | 240 (68%) | 57 (27%) | |||
| mRNA-1273 (Moderna) | 55 (10%) | 53 (15%) | 2 (1%) | |||
| Ad26.COV2.S (Jansen) | 104 (19%) | 12 (3%) | 92 (44%) | |||
| ChAdOx1 nCoV-19 (Astra-Zeneca) | 105 (19%) | 47 (13%) | 58 (28%) | |||
Data are the median (IQR) or the number of cases (n) and percentage (%) calculated as [n/N]*100, where N is the total number of patients in the corresponding group. † p values were calculated by comparing the unvaccinated group and the corresponding vaccinated group (partially or fully vaccinated patients) with the χ2 test, Fisher`s exact test or Mann-Whitney U test. yr = years of age.
Regarding vaccine type, of all the vaccinated patients, 63% had received mRNA vaccines, and 53% and 10% received the BNT162b2 (Pfizer) or mRNA-1273 (Moderna) vaccine, respectively; 19% had Ad26.COV2.S (Jansen) and 19% received ChAdOx1 nCoV-19 (Astra-Zeneca). See Table 1.
Sex, age and vaccination status
Gender was not shown significant differences between unvaccinated and partial/fully vaccinated patients (Table 1). One of the largest differences between vaccinated and unvaccinated patients was age. Fully vaccinated patients were significantly older than unvaccinated ones (Table 1, Figure 1). The median age for fully vaccinated patients was 61 years (IQR, 53–67) and only 41 years (IQR, 33–50) for the unvaccinated group.
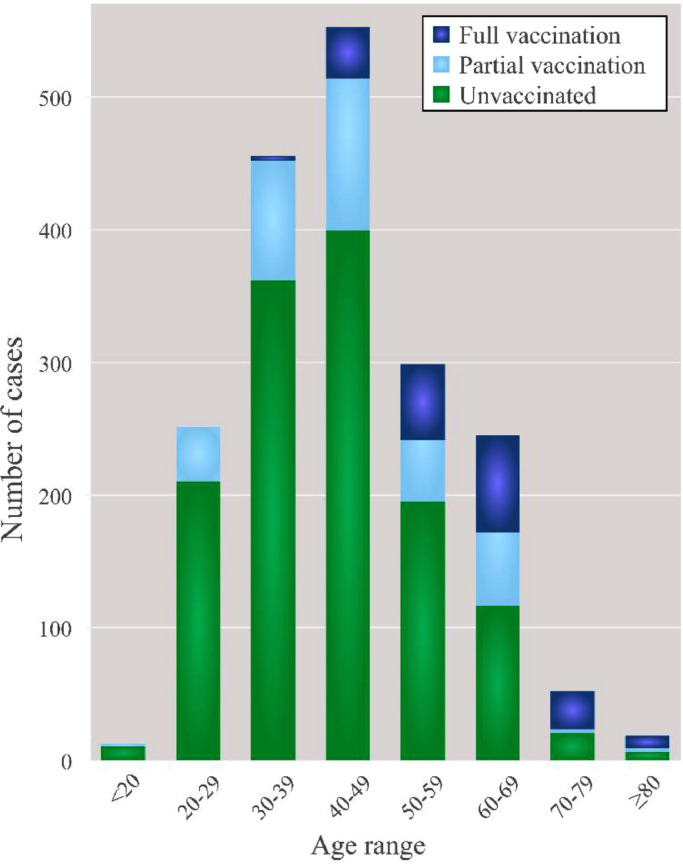
Figure 1.
Age distribution of the patients with laboratory- and chest Rx-confirmed COVID-19 according to vaccination status. Number of hospital admissions during the fifth wave of the COVID-19 in Spain at “Enfermera Isabel Zendal” Emergencies Hospital (HEEIZ) considering vaccination status.
First, we analyzed the vaccination status of patients younger or older than 50 years of age. As detailed in Table 1, most hospitalized patients were aged <50 years (1273; 67%), and within them, only 23% (287) were vaccinated (either partially or fully) while 986 (77%) were unvaccinated. On the contrary, hospitalized patients ≥50 years of age (615, 33%) included only 26% of all the unvaccinated patients but up to 80% of all the hospitalized fully vaccinated individuals (Table 1).
We also analyzed the vaccination status of all hospitalizations at HEEIZ considering narrower age ranges (Table 1, Figure 1; age ranges: <20, 20–29, 30–39, 40–49, 50–59, 60–69, 70–79 and ≥80 years old). The number of hospitalizations (cases) according to vaccination status was different for all the studied age ranges (Figure 1; Table 1). Only 1% (3 cases) of all full vaccinated hospitalized patients were <40 years while most of them (35%; 73 cases) were aged 60–69 years (Table 1). Conversely, the number of unvaccinated cases admitted at HEEIZ was always larger for all age ranges below 60 years compared to the combined number of partial+fully vaccinated cases (Figure 1).
Collectively, these results show that most HEEIZ hospitalizations during the fifth Spanish COVID-19 wave were unvaccinated patients. Hospitalized vaccinated cases were mainly older than 50 years, which suggests marked vaccination protection against hospitalization for all age ranges studied (18–96), which is more robust for patients aged <50 years.
These results were confirmed by a temporal (month by month) analysis of vaccination status of COVID-19 patients hospitalized at HEEIZ according to the age ranges accepted to vaccinate during the fifth wave period (Figure 2). Vaccination status of the entire target population served by HEEIZ was also included in Figure 2 for comparative purposes (vaccination status of hospitalized patients at HEEIZ vs. vaccination levels of target population in CM, according to age).
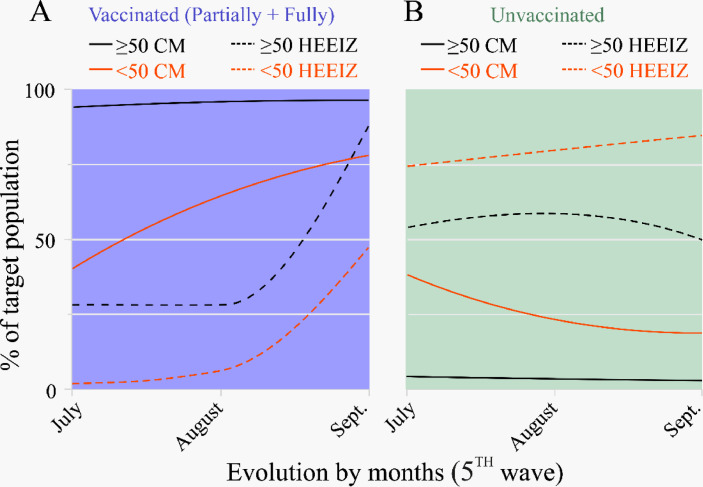
Figure 2.
Vaccination percentage evolution in the target population classified per age range (<50 and >50) in the Community of Madrid at HEEIZ during the fifth COVID-19 wave in Spain (July 1 to September 30, 2021). Plots showing the percentages of those vaccinated with at least one dose (A) and the unvaccinated population (B). CM, Community of Madrid; “Enfermera Isabel Zendal” Emergencies Hospital (HEEIZ).
According to official Spanish government data14 during the fifth COVID-19 wave vaccination levels of target population ≥50 years old in CM obtained high percentages showing a slight increase from 94% in July to 96% in September (Table S1; Figure 2A, black solid line). Vaccinated target population in CM in the <50 age range raised from 40% to 78% (Table S1; Figure 2A red solid line). However, when analyzing vaccinated hospitalized cases at HEEIZ, much lower percentages were found for both age ranges (≥ and <50 years) during the 3-month period (Figure 2A; dashed lines) although admissions increased from July to September (28% to 88% for ≥50 years; 2% to 47% for <50 years; Figure 2A, black and red dashed line, respectively).
Conversely, the percentages of the unvaccinated older (≥50) and younger (<50) populations in CM remained below those of the corresponding population hospitalized at HEEIZ during the fifth wave (Figure 2B). The percentage of the younger unvaccinated (<50 years) hospitalized patients rose from 74% to 84% between July and September (Figure 2B).
Basically, the percentage of vaccinated people in CM was much higher than the vaccinated patients at HEEIZ (Figure 2A), with many more unvaccinated patients at HEEIZ than in the unvaccinated target population in CM (Figure 2B) for all age ranges (Table S1, Fig. S2). In both cases, this was evidenced more for >50 years, which confirmed the greater likelihood of young unvaccinated or much older vaccinated people being hospitalized (Fig. S2).
Without considering age groups, we found that fully vaccinated cases were 84% (95% CI: 82–86%) less likely to be hospitalized, which increased and denoted much better protection (95%; 95% CI: 93–96%) for those <50 years, and slightly lowered to 66% (95% CI: 62–70%) for those >50 years (Figure 3). When we evaluated the RR per ten-years age groups, significant protection against hospitalization, which decreased with increasing age, was found for the vaccinated cases in all groups. RR and 95% CI for all age ranges are included in Figure 3.
Laboratory findings and comorbidities
Regarding the laboratory data (Table 2), all the patients had abnormally high levels of C-reactive protein, ferritin, D-dimer, procalcitonin, platelets, lymphocytes and lactate dehydrogenase. However, the partially vaccinated patients had higher C-reactive protein values (p = 0·017) median 37·05 mg/L [IQR16·2·0–81·5]) and significantly lower values for ferritin (p = 0·01; median 433·0 ng/mL [IQR 195·0–932·0] and lactate dehydrogenase (p < 0·0001; median 282·0 units/L [IQR 232·0–352·5]). The same significant changes were found for the fully vaccinated patients (Table 2; p < 0·0001 for C-reactive protein, ferritin and lactate dehydrogenase).
Table 2.
Laboratory findings and comorbidities of the hospitalized patients according to their vaccination status.
| All patients (N = 1888) | Unvaccinated (N = 1327) | Partially vaccinated (N = 352) | Fully vaccinated (N = 209) | Partially vaccinated p value † | Full vaccinated p value † | |
|---|---|---|---|---|---|---|
| Comorbidities | ||||||
| Any comorbidity | 705 (37·5%) | 434 (33%) | 139 (40%) | 132 (63·5%) | 0·017 | <0·0001 |
| No comorbidities | 1177 (62·5%) | 889 (67%) | 212 (60%) | 76 (36·5%) | 0·017 | <0·0001 |
| 1 Comorbidity | 447 (24%) | 306 (23%) | 84 (24%) | 57 (27%) | 0·75 | 0·18 |
| 2 Comorbidities | 157 (8%) | 83 (6%) | 38(11%) | 36 (17%) | 0·003 | <0·0001 |
| More than 2 | 101 (6%) | 45 (3%) | 17 (5%) | 39 (19%) | 0·20 | <0·0001 |
| Obesity | 330 (17·5%) | 229 (17%) | 54 (15%) | 47 (22%) | 0·399 | 0·069 |
| Hypertension | 289 (15%) | 144 (11%) | 59 (17%) | 86 (41%) | 0·002 | <0·0001 |
| Pneumophathy | 155 (8%) | 91 (7%) | 28 (8%) | 36 (17%) | 0·472 | <0·0001 |
| Diabetes mellitus | 186 (10%) | 92 (7%) | 50 (14%) | 44 (21%) | <0·0001 | <0·0001 |
| Heart disease | 78 (4%) | 37 (3%) | 14 (4%) | 27 (13%) | 0·246 | <0·0001 |
| Cancer | 39 (2%) | 23 (2%) | 6 (2%) | 10 (5%) | 0·972 | 0·009 |
| Chronic kidney disease | 31 (2%) | 13 (1%) | 5 (1%) | 13 (6%) | 0·474 | <0·0001 |
| Laboratory findings | ||||||
| Lymphocytes (units/µL) | 1190·0 (850·0–1650·0) | 1180·0 (840·0–1617·5) | 1240·0 (900·0–1725·0) | 1180·0 (850·0–1785·0) | 0·046 | 0·622 |
| Platelets (units/mL) | 237·0 (184·0–314·0) | 233·0 (180·0–313·0) | 241·0 (189·0–314·5) | 253·0 (195·25–325·25) | 0·298 | 0·038 |
| C-reactive protein (mg/L) | 34·5 (15·0–75·6) | 31·8 (14·1–68·7) | 37·05 (16·2·0–81·5) | 48·9 (21·7–102·9) | 0·017 | <0·0001 |
| Lactate dehydrog. (units/L) | 304·0 (248·0–374·0) | 313·0 (258·0–386·0) | 282·0 (232·0–352·5) | 269·0 (218·5–330·5) | <0·0001 | <0·0001 |
| Ferritin (ng/mL) | 490·0 (222·0–1028·7) | 527·0 (237·2–1083·5) | 433·0 (195·0–932·0) | 367·0 (182·0–731·0) | 0·010 | <0·0001 |
| D-dimer (ug/mL) | 400·0 (280·0–605·0) | 410·0 (280·0–600·0) | 370·0 (270·0–560·0) | 410·0 (270·0–695·0) | 0·053 | 0·659 |
| Procalcitonin (ng/mL) | 0·09 (0·05–0·17) | 0·08 (0·05–0·16) | 0·09 (0·06–0·16) | 0·11 (0·06–0·35) | 0·572 | 0·135 |
Data are the median (IQR) or the number of cases (n) and percentage (%) calculated as [n/N]*100, where N is the total number of patients in the corresponding group. †, p values were calculated by comparing the unvaccinated group and the corresponding vaccinated group (partially or fully vaccinated patients) with the χ2 test, Fisher`s exact test or Mann-Whitney U test. Lactate dehydrog., lactate dehydrogenase.
Patients’ clinical conditions and their comorbidities are listed in Table 2 according to their vaccination status. Only the comorbidities reported as the main risk factors for severe COVID-19 were studied. Comorbidities were present in 705 patients (37·5%), of whom 264 had two or more. The commonest comorbidities were obesity (330 [17% of 705]) and hypertension (289 [15% of 705]), followed, in decreasing order, by diabetes mellitus, pneumopathy, heart disease, cancer and chronic kidney disease (Table 2).
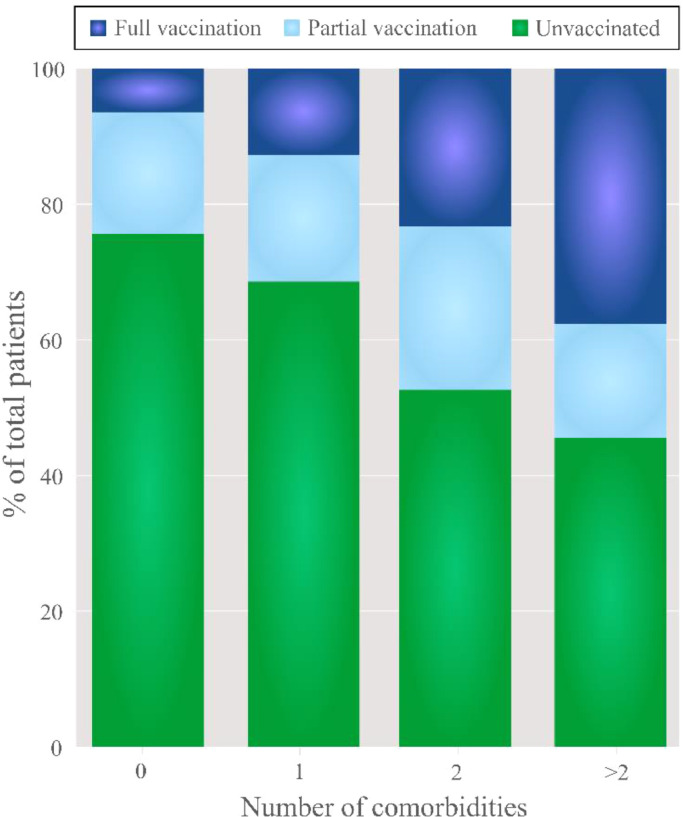
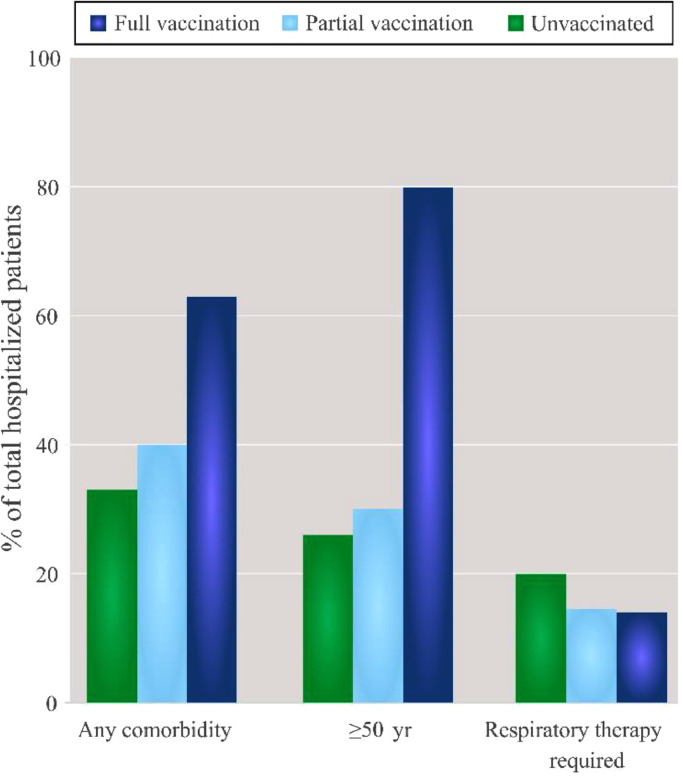
To evaluate whether the presence of one of those comorbidities or more could affect hospitalization, comorbid conditions were analyzed in relation to the vaccination status of the patients admitted to HEEIZ (Figure 4): 33% of the unvaccinated patients (434 of 1327 cases) with any (one, two or more) of the studied comorbidities (Table 2). Of the hospitalized patients with no comorbidities, 75·5% were unvaccinated and this percentage lowered to 49·6% when two comorbidities or more were present (Figure 4). Thus, as number of comorbidities increased percentage of fully vaccinated patients significantly increased in comparison to partially vaccinated or unvaccinated ones (p < 0.0001; Figure 4).
Figure 4.
Pipeline percentage graph of the admitted patients per vaccination status and number of comorbidities. As number of comorbidities increased percentage of fully vaccinated patients significantly increased in comparison to partially vaccinated or unvaccinated ones (p < 0.0001).
Taken together, our results showed that fully and partially vaccinated patients, albeit fewer in number, presented more comorbidities than the unvaccinated hospitalized patients at HEEIZ.
Respiratory treatment and outcome
As described in the Methods, HEEIZ is a COVID-19-specialized hospital exclusively built to monitor and treat patients positive for SARs-CoV2 infection (confirmed by PCR) and pneumonia (by chest X-ray). All the patients admitted to HEEIZ were treated following the same protocols. A specific clinical criterion (see details in the Methods) was approved at HEEIZ for respiratory therapy requirements and was applied to all the hospitalized patients included in this study during the fifth wave. According to that criterion, some hospitalized patients were treated first with noninvasive respiratory care. A subset of noninvasive treated patients showing clinical impairment according to the criterion was submitted to invasive respiratory treatment with orotracheal intubation.
According to Table 3, of the cohort of patients herein analyzed, 347 (18% of 1888) required noninvasive ventilation, of whom 105 also required invasive respiratory care (6% of 1888). Among patients who underwent noninvasive ventilation, 266 (20% of 1327) were unvaccinated, and significantly fewer were partially or fully vaccinated: 51 (14% of 561; p = 0·018) partially vaccinated; 30 (14% of 209; p = 0·05) fully vaccinated. The same tendency appeared for those patients who also required invasive ventilation: 78 (6% of 1327) unvaccinated; 27 (5% of 561) partially vaccinated; 11 (5% of 209) fully vaccinated.
Table 3.
Respiratory treatments of the hospitalized patients according to vaccination status, age and comorbidities.
| All patients (N = 1888) | Unvaccinated (N = 1327) | Partially vaccinated (N = 352) | Fully vaccinated (N = 209) | Partially vaccinated p value † | Full vaccinated p value † | |
|---|---|---|---|---|---|---|
| Respiratory therapy treatments | ||||||
| Non-invasive respiratory therapy | 347 (18%) | 266 (20%) | 51 (14%) | 30 (14%) | 0·018 | 0·05 |
| < 50 yr | 204 (59%) | 168 (63%) | 31 (39%) | 5 (17%) | 0·75 | <0·0001 |
| ≥ 50 yr | 143 (41%) | 98 (37%) | 20 (61%) | 25 (83%) | 0·75 | <0·0001 |
| No comorbidities | 173 (50%) | 139 (52%) | 20 (39%) | 14 (47%) | 0·09 | 0·56 |
| 1 comorbidity | 113 (33%) | 85 (32%) | 21 (41%) | 7 (23%) | 0·20 | 0·33 |
| 2 comorbidities | 30 (9%) | 21 (8%) | 6 (12%) | 3 (10%) | 0·41 | 0·72 |
| More than 2 | 29 (8%) | 21 (8%) | 4 (8%) | 6 (20%) | 0·99 | 0·04 |
| Days of non-invasive respiratory therapy | 5·0 (3·0–7·0) | 5·0 (3·0–7·0) | 5·0 (2·0–7·0) | 5·0 (4·0–8·0) | 0·782 | 0·248 |
| Invasive orotracheal intubation | 105 (6%) | 78 (6%) | 16 (5%) | 11 (5%) | 0·334 | 0·72 |
| <50 yr | 56 (53%) | 43 (55%) | 9 (69%) | 2 (18%) | 0·32 | 0·03 |
| ≥50 yr | 49 (47%) | 35 (45%) | 5 (31%) | 9 (82%) | 0·32 | 0·03 |
| No comorbidities | 47 (45%) | 36 (46%) | 7(44%) | 4 (36%) | 0·86 | 0·75 |
| 1 comorbidity | 41 (39%) | 29 (37%) | 8 (50%) | 4 (36%) | 0·34 | 0·99 |
| 2 comorbidities | 8 (8%) | 6 (8%) | 0 (0%) | 2 (18%) | 0·58 | 0·26 |
| More than 2 | 9 (9%) | 7 (9%) | 1 (6%) | 1 (9%) | 0·99 | 0·99 |
| Days of invasive orotracheal intubation | 7·0 (10·0–21·0) | 9·0 (7·0–20·0) | 12·0 (8·5–31·5) | 17·0 (9·0–22·0) | 0·228 | 0·082 |
Data are the median (IQR) or the number of cases (n) and percentage (%) calculated as [n/N]*100, where N is the total number of patients in the corresponding group. †, p values were calculated by comparing the unvaccinated group and the corresponding vaccinated group (partially vaccinated or fully vaccinated patients) with the χ2 test, Fisher`s exact test or Mann-Whitney U test yr, years.
When considering age, the fully vaccinated patients who required noninvasive and invasive therapy were significantly (noninvasive respiratory care, p < 0·0001; invasive respiratory care, p = 0·03) older (≥50 years old) than the unvaccinated who needed either treatment (Table 3).
Regarding comorbidities, the vaccinated patients were more comorbid not only globally, but also when comorbidities were individually studied, except for obesity (see Table 2). Interestingly, comorbidities came over as a key factor when they were considered to study HEEIZ patients’ respiratory care requirements. Table 3 shows that around 50% of the patients who needed noninvasive respiratory care were comorbid, but the fully vaccinated ones were even more comorbid (fully vaccinated with two comorbidities; p = 0·04). This likely indicates that vaccination protects against respiratory care requirement, which is less effective in very comorbid vaccinated patients. The percentages of the fully vaccinated patients with any comorbidity (one, two or three comorbidities) who needed subsequent invasive respiratory care were the same as the comorbid unvaccinated ones (Table 3). Once again, this suggests a protective effect of vaccination against requiring orotracheal intubation, even in comorbid patients.
In short, our results revealed that the vaccinated patients who needed noninvasive respiratory care were older and more comorbid than the unvaccinated ones, even though the unvaccinated cases were usually comorbid. Thus, our data indicate that fully vaccinated patients who need respiratory care are old and comorbid. What is equally important is that our results suggest that unvaccinated young people are at risk of requiring respiratory care despite their age, and those with comorbidities are at even higher risk.
To further estimate the protective effect of vaccination against non-invasive and invasive respiratory treatments for the HEEIZ hospitalized patients, we calculated the RR of vaccinated patients on noninvasive or invasive respiratory care compared to the complete target population in the CM, the district area that HEEIZ covers. As Fig. S3 illustrates, the vaccinated patients had 88% (95% CI: 92–94%) and 86% (95% CI: 75–92%) less chances of undergoing noninvasive or invasive respiratory care than the CM population, respectively. Chances were lower (96% (95% CI: 91–98%) and 94% (95% CI: 78–99%) less chances for noninvasive and invasive support, respectively) for the population aged <50 years.
Further multivariate analysis of the data by binary logistic regression (Supplementary Table S3) revealed that, in reference to non-invasive respiratory therapy, partially vaccinated cases were less likely to be in non-invasive respiratory therapy than unvaccinated patients (OR: 0·64, 95% CI 0·44–0·93, p = 0·019; Supplementary Table S3), and fully vaccinated were less likely to be in non-invasive respiratory therapy than unvaccinated (OR 0·47, 95% CI 0·29–0·97, p = 0·03). Regarding comorbidities, patients with comorbidities were more likely to be in non-invasive respiratory therapy than patients without them (OR: 2·11, 95% CI 1·60–2·77, p < 0·0001). Others variables that were significant for admission to non-invasive respiratory therapy were: Low Lymphocytes (OR: 4·52, 95% CI 3·39–6·03, p < 0·0001), high C-reactive protein (OR: 4·14, 95% CI 1·48–11·62, p = 0·007), high ferritine (OR: 2·00, 95% CI 1·44–2·77, p = 0·0001) and high D-dimer (OR: 1·36, 95% CI 1·03–1·79, p = 0·029).
In the same sense, in reference to invasive respiratory therapy patients with comorbidities were more likely to be in invasive respiratory therapy than patients without them (OR: 1·87, 95% CI 1·22–2·88, p = 0·004). Other variables that were significant for admission to invasive respiratory therapy were: Low Lymphocytes (OR: 5·406, 95% CI 3 14–9·31, p < 0·0001), high* lymphocytes (* only tree cases: OR: 29·476, 95% CI 2·09–416·170, p < 0·0001) and high ferritine (OR: 1·77, 95% CI 1·03–3·02, p = 0·038).
Exitus. Of the 1888 patients hospitalized at HEEIZ during the fifth COVID-19 pandemic wave, one main clinical outcome was obtained: only 13 died (0.7%). Three of them did not receive respiratory care due to the medical therapeutic ceiling in accordance with approved HEEIZ clinical protocols. Ten patients who were on both respiratory treatments (i.e., first noninvasive, then invasive) died. No differences were found between the vaccination status of these 10 patients and age or presence of none, one, two comorbidities or more (Table 4). Despite the few mortality data available, four of these 10 deaths presented no comorbidities and three were unvaccinated.
Table 4.
Demographic and clinical characteristics of the patients with a fatal outcome (exitus) who required invasive respiratory care.
| All patients (N = 105) | Unvaccinated (N = 78) | Partial Vaccinated (N = 16) | Fully vaccinated (N = 11) | Vaccinated p value † | Full vaccinated p value † | |
|---|---|---|---|---|---|---|
| Exitus post ICU | 10 (10%) | 6 (8%) | 2 (12·5%) | 2 (18%) | 0·68 | 0·30 |
| <50 yr | 1 (1%) | 1 (1%) | 0 (0%) | 0 (0%) | 0·99 | 0·99 |
| ≥50 yr | 9 (9%) | 5 (6%) | 2 (12·5%) | 2 (18%) | 0·67 | 0·99 |
| No Comorbidities | 4 (4%) | 3 (4%) | 0 (0%) | 1 (10%) | 0·99 | 0·28 |
| 1 Comorbidity | 4 (4%) | 2 (3%) | 2 (12·5%) | 0 (0%) | 0·20 | 0·99 |
| 2 Comorbidities | 1 (1%) | 0 (0%) | 0 (0%) | 1 (10%) | NA | 0·30 |
| More than 2 | 1 (1%) | 1 (1%) | 0 (0%) | 0 (0%) | 0·99 | 0·99 |
Data are the number of cases (n) and percentage (%) calculated as [n/N]*100, where N is the total number of patients in the corresponding group. †, p values were calculated by comparing the unvaccinated group and the corresponding vaccinated group (partially or fully vaccinated patients) with the χ2 test, Fisher`s exact test. yr, years.
In addition, multivariate analysis (supplementary Table S3) revealed that patients older than 49 years were more likely to die than younger patients (OR: 20·05, 95% CI 2·53–158·69, p = 0·004).
We checked if vaccine type could affect either the respiratory care type required by hospitalized patients or fatal outcome (i.e. exitus). No differences were observed (Table S2) despite the vaccine type being administered in a variable way according to age (Table S2).
In addition, we analyzed whether the number of days after complete vaccination could influence the need for noninvasive, invasive, or existing respiratory therapy. No significant differences were found in any case (p = 0.71, p = 0.87 and p = 0.28).
Together, our results showed that although the vaccinated hospitalized patients were older and more comorbid than the unvaccinated ones, they had fewer respiratory care requirements. This indicates the importance of vaccination during COVID-19 pandemic (Figure 5).
Figure 5.
Comparison of comorbidities, age and respiratory therapy requirement for the patients hospitalized at HEEIZ during the fifth COVID-19 pandemic wave in Spain.
Discussion
We present the epidemiological, clinical, laboratory, invasive and noninvasive respiratory care characteristics of the 1888 patients admitted to HEEIZ between July 1 and September 30, 2021. Of them, upon hospitalization 561 (30% of 1888) were vaccinated with at least one dose, 209 (11% of 1888) were fully vaccinated and 1327 (70% of 1888) were unvaccinated. These results were obtained during the fifth COVID-19 wave in Spain where, despite a large fully vaccinated population group, part of the Spanish population was still unvaccinated, especially young people.
There are reports of vaccines’ efficiency against hospitalization.8 We found that their degree of protection lowered with age from 95% for the <50 years to 66% for the over 50 s. However, we should bear in mind that those >50 years are fully vaccinated longer and, according to previous studies, the immune response tends to gradually diminish with time.15,16 Another aspect is that immunosenescence can affect vaccines’ effectiveness because naive T-lymphocytes lower in peripheral blood. Then there is the compromised differentiation of T CD4+ lymphocytes in functional subgroups or the reduction in memory B lymphocytes that act as a bridge between innate and adaptive immunity.17,18
The hospitalized fully vaccinated patients were generally much older (their median age was 61 years, IQR 53–67) and much more comorbid (p < 0·0001) in line with previous literature.19 Some confounders could result in a certain correlation between vaccines and respiratory care and could, therefore, underestimate vaccines’ protection against these therapies. This happens because the presence of variables causing both exposure (vaccine) and outcome (hospitalized or respiratory care) can lead to false associations.20 In our case, it seems clear that the more comorbidities and being older, the more likely patients are to need hospitalization, and the older and more comorbid patients are, the greater the likelihood of them being fully vaccinated. A binary logistic analysis was performed to reduce these confounders that showed that despite potential confounders such as comorbidities and age, vaccination continued to exert great protection to avoid non-invasive respiratory therapy among hospitalized patients. Therefore, protection due to vaccination would not only be when it comes to preventing hospitalization, but also to receiving respiratory support among patients hospitalized for COVID-19, who therefore present a serious condition. Present data acquires great value as it has been verified in a COVID-19-specialized single-center hospital with a large sample size.
This figure rose to 38% for those aged >50 years. It was noteworthy that all the patients who required >50% FiO2 received noninvasive respiratory care regardless of their age or comorbidities. The candidate patients for invasive respiratory care were selected according to the risk/benefit criterion and the resources management depending on how healthcare pressure varied, as an epidemiological study reports.21 Thus a percentage of those on noninvasive therapy were taken as a therapeutic ceiling. This, along with the presence of the aforementioned confounders, can explain why no differences were found for invasive support between vaccinated and unvaccinated patients. Apparently other factors also come into play when starting noninvasive respiratory care that can diminish vaccines’ protection. Specifically, our multivariate analysis showed that comorbidities were a key factor when requiring additional invasive respiratory therapy. Some studies have evaluated the role of hypoxia in lung inflammation and cytokines storm because it causes elevated hypoxia inducible factor-1-α (HIF-1 α) by alveolar epithelial cells. In serious cases, HIF-1 α can lead to the activation of macrophages and neutrophils, more inflammatory cytokines and, finally, to adult respiratory distress syndrome (ARDS) as a result of increased vascular permeability due to the destruction of the alveolo-interstitial-endothelial barrier.22,23
Despite IgG titers below 4·5% being demonstrated in diabetic patients versus non diabetic patients,24 and immunosuppressed patients, e.g. those with chronic kidney disease or cancer, or at higher risk of infection and showing worse COVID-19 evolution,25 our fully vaccinated group required less noninvasive respiratory care than the unvaccinated group despite presenting 21% vs. 7% of diabetes or 6% vs. 1% of chronic kidney disease versus the unvaccinated patients. This protective effect seemed to diminish with noninvasive respiratory care because no differences appeared in requiring noninvasive respiratory care between being fully vaccinated and unvaccinated, and comorbidities and age became determining factors for patients’ evolution despite vaccines.
Regarding exitus, 13 patients died (0·69% of the 1888 patients), of whom 10 received both invasive and noninvasive respiratory care, and 9 (90%) were >50 years. Four did not present comorbidities and three were unvaccinated. Despite the low number of exitus, logistic regression analysis, confirmed age (≥ 50) as a crucial factor.
Regarding laboratory findings, our results showed a higher C-reactive protein value in vaccinated vs. unvaccinated or partially vaccinated patients. C-reactive protein is produced and secreted primarily by the liver and is a major risk factor associated with age-related diseases, including cardiovascular disease, hypertension, kidney disease, diabetes mellitus, and cognitive decline. A possible explanation for the higher concentration of C-reactive protein in the vaccinated group could be related to the older age as well as the presence of greater cardiovascular and respiratory comorbidity. Both age and comorbidities have been identified as factors associated with higher baseline C-reactive protein values.26,27
Our patients ≥50 years old had significantly higher values (p < 0·0001) of C-reactive protein (mean 41·3 mg/l [IQR 19·1–88·0]) than those younger than 50 (median 31·6 mg/l [IQR 13·6–69·5]). In addition, significantly higher C-reactive protein values (p = 0·001) were detected in patients with diabetes (median 48·3 mg/l [IQR 20·8–93·3]) than in patients without diabetes (median 33·6 mg/l). [IQR 14·6–72·6]). Patients with arterial hypertension also had significantly higher values (p = 0·001) of C-reactive protein (mean 46·1 mg/l [IQR 19·4–89·4]) than patients with normal blood pressure values (mean 32·4 mg/l [IQR 14·6–72·3]). Obese patients had significantly higher (p = 0.002) C-reactive protein values (median 43·9 mg/l [IQR 18·8–84·3]) than non-obese patients (median 32·4 mg/l [IQR 14· 6–72·3]). Finally, patients with chronic kidney disease had significantly higher values (p = 0·007) of C-reactive protein (median 66·2 mg/l [IQR 26·8–146·2]) than patients without chronic kidney disease (median 34·1 mg/l [IQR 15·0–74·7]). In addition, 100% of the patients with chronic kidney disease (30) had values higher than those considered normal, this being a comorbidity clearly associated with increased values. In summary, the older the patients, the more comorbidities they present and the higher level of vaccination they present. This would justify higher C-reactive protein values in vaccinated patients. Finally, we analyzed whether the time between vaccination and admission might affect the fact of presenting normal or altered values of C-reactive protein. No significant differences were found (p = 0·64), suggesting that values of the C-reactive protein were motivated by the issues already described.
On the other hand, although the elevation of C-reactive protein is considered a biomarker of poor outcome risk, other risk biomarkers such as ferritin or lactate dehydrogenase showed a lower value in the vaccinated group compared to those partially vaccinated or unvaccinated. These results suggest less inflammation and cell destruction in vaccinated patients, which would be in line with their better evolution during admission.28 In addition, platelets were significantly higher in the vaccinated group, another parameter associated with better evolution during admission. We did not find differences in lymphocyte count, D-dimer and procalcitonin levels between groups. In summary, the isolated increase in C-reactive protein, without being accompanied by an increase in ferritin and lactate dehydrogenase or a significant decrease in lymphocyte and platelet counts, does not seem to be related to a worse evolution during admission in the groups studied.
We did not find any differences in endpoints according to vaccine's manufacturer or their technology (RNAm, nonRNAm). However, we observed differences between fully vaccinated and partially vaccinated patients, which are described in the Results, and evidence the need to follow the recommended vaccination guidelines and to use booster shots. We stress that the distribution of the different vaccine types was not homogeneous among age groups because it depended on the Spanish Ministry of Health's planning. This planning was amended after some side effects were noted for some age groups.
First, given the observational study design, the obtained evidence cannot be as robust as that acquired by clinical trials. Second, the interpretation of our results could be limited by sample size, especially when forming subgroups and separating into age groups, comorbidities, etc. In addition, previous history of COVID-19 was not collected for any group, which could reduce the confounding factor associated with the "natural" immunity that some patients may have achieved after SARS-CoV-2 infection. Third, lack of efficient antivirals and using high-dose corticosteroids could have also provided worse clinical results in some patients.13 Moreover, nonsignificant p-values do not necessarily rule out the difference between vaccinated and unvaccinated patients. Fourth, given the center's characteristics, patients aged <18, with cognitive impairment, were dependent on cancer treatment and needed chemotherapy or radiotherapy, were transplanted or on dialysis, were not transferred. Patients had to accept being transferred from their hospital. This could lead to a selection bias that must be considered when interpreting this work. However, as HEEIZ accepted approximately 20-40% of hospitalized patients in the CM, we believe that our sample is representative. Fifth, to interpret our results, it is worth noting that the inter-region mobility restrictions implemented in Spain to control COVID-19 became more flexible in May 2021. Restrictions in the number of people who could meet in restaurants or other catering establishments ceased on several dates in August depending on the region. Abolishing this latter restriction would influence SARS-CoV-2 infection and could explain the rise in hospitalizations at the end of the fifth wave for both vaccinated and unvaccinated patients.
Finally, the study was conducted during the fifth COVID-19 wave, which confers it special characteristics because the delta variant was predominant in Spain and Madrid,29 and vaccination distribution was heterogeneous and changing depending on age groups. Our study also has many strong points. First, it contributes further evidence found to date about vaccines’ effectiveness per age group. Second, as far as we know, this is the biggest cohort study to include vaccinated patients hospitalized for COVID-19 and records demographic, clinical and analytical variables, which gave a definitive result, which backs what other epidemiological studies have demonstrated for hospitalizations, admissions to ICUs or mortality. Finally, stratifying respiratory care into invasive and noninvasive allowed us to grade severity and plan hospital healthcare strategies.
Our main knowledge gaps, such as the time that vaccine's protection lasts, what could happen when new virus mutations like omicron occur, or the virus possibly escaping vaccine's protection in certain cases, must be covered by future studies.
Contributors
JT and PL had the idea for and designed the study and had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. JT, JGR, LJD, JNL and PL drafted the paper. AN contributed to critical revision of the report. JGR and AN contributed to the statistical analysis. JT, AC, MNC, IMG, LL, MLG, PPG, GSC, MSO and PL collected the data. All authors contributed to data acquisition, data analysis, or data interpretation, and reviewed and approved the final version.
JT, PL, JGR, LJD, JNL and ANL contributed equally.
Data sharing statement
The data presented in this study are available on request from the corresponding author
Declaration of interests
Jose Rafael Teran-Tinedo declares a Madrid Society of Pneumology Young Researchers Grant 2020; funds from Air Liquide Healthcare for registration in the National Congress of the Spanish Society of Pneumology and Thoracic Surgery 2021; funds from Bial for registration in the European Respiratory Congress 2021. Miguel Lorente-González is a Secondary researcher in NEPTUNO clinical trial to evaluate utility of plitidepsin in COVID-19 patients. PharmaMar payments were made to all the researchers. He also declares payment from Gilead Sciences for a session at his hospital about the use of remdesivir in COVID-19. Pedro Landete declares support for educational activities from Linde Healthcare, Bial, Boehringer Ingelheim, Air Liquide, GSK, FAES Farma and Novartis; payment from PharmaMar for expert investigation support; payment from Boehringer Ingelheim for registration for congress, travel and hotel; is on the PharmaMar Advisory board; funding from Phillips/Cardiva formedical writing at his institution. All other authors have nothing to declare.
Acknowledgments
Monica Sanchez Gioya, Gema Lizana Gonzalez, Carmen Lopez Camara Delgado, Julio Medina Gilabert, Silvia Herrero Martin, Elena andrea Muresanu, Cristina Becedillas Padrino and the Hospital Admission Service of the HEEIZ for collecting data.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101453.
Contributor Information
Alberto Najera, Email: Alberto.Najera@uclm.es.
Juan D Navarro-Lopez, Email: Juan.Navarro@uclm.es.
Lydia Jimenez-Diaz, Email: Lydia.Jimenez@uclm.es.
Pedro Landete, Email: pedro.landete@salud.madrid.org.
Appendix. Supplementary materials
REFERENCES
- 1.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mizukoshi A., Nakama C., Okumura J., Azuma K. Assessing the risk of COVID-19 from multiple pathways of exposure to SARS-CoV-2: modeling in health-care settings and effectiveness of nonpharmaceutical interventions. Environ Int. 2021;147 doi: 10.1016/j.envint.2020.106338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llorens S., Nava E., Muñoz-López M., Sánchez-Larsen Á., Segura T. Neurological Symptoms of COVID-19: the Zonulin hypothesis. Front Immunol. 2021;12:1344. doi: 10.3389/fimmu.2021.665300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 5.Chung H., He S., Nasreen S., et al. Effectiveness of BNT162b2 and mRNA-1273 covid-19 vaccines against symptomatic SARS-CoV-2 infection and severe COVID-19 outcomes in Ontario, Canada: test negative design study. Bmj Br Med J. 2021;374:n1943. doi: 10.1136/bmj.n1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dagan N., Barda N., Kepten E., et al. BNT162b2 mRNA COVID-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021 doi: 10.1056/NEJMoa2101765. published online 24 Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall V.J., Foulkes S., Saei A., et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet Lond Engl. 2021;397:1725–1735. doi: 10.1016/S0140-6736(21)00790-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson M.G., Stenehjem E., Grannis S., et al. Effectiveness of COVID-19 vaccines in ambulatory and inpatient care settings. N Engl J Med. 2021;385:1355–1371. doi: 10.1056/NEJMoa2110362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tenforde M.W., Olson S.M., Self W.H., et al. Effectiveness of Pfizer-BioNTech and moderna vaccines against COVID-19 among hospitalized adults aged >= 65 years - United States. Mmwr Morb Mortal Wkly Rep. 2021;70:674–679. doi: 10.15585/mmwr.mm7018e1. January-March 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mhawish H., Mady A., Alaklobi F., et al. Comparison of severity of immunized versus non-immunized COVID-19 patients admitted to ICU: a prospective observational study. Ann Med Surg. 2021;71 doi: 10.1016/j.amsu.2021.102951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desai A., Desai P., Mehta J., et al. Measuring the impact of a single dose of ChAdOx1 nCoV-19 (recombinant) coronavirus vaccine on hospital stay, ICU requirement, and mortality outcome in a tertiary care centre. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2021;113:282–287. doi: 10.1016/j.ijid.2021.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO. Therapeutics and COVID-19. https://www.who.int/teams/health-care-readiness/covid-19/therapeutics. Accessed 12 April 2022.
- 13.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ministerio de Sanidad. Estrategia de vacunación COVID-19 en España. https://www.sanidad.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/vacunaCovid19.htm. Accessed 1 April 2022.
- 15.Lau C.S., Phua S.K., Liang Y.L., Oh H.M.L., Aw T.C. Robust SARS-CoV-2 antibody responses in Asian COVID-naïve subjects 180 days after two doses of BNT162b2 mRNA COVID-19 vaccine. Vaccines. 2021;9:1241. doi: 10.3390/vaccines9111241. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levin E.G., Lustig Y., Cohen C., et al. Waning immune humoral response to BNT162b2 COVID-19 vaccine over 6 months. N Engl J Med. 2021;385 doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ciabattini A., Garagnani P., Santoro F., Rappuoli R., Franceschi C., Medaglini D. Shelter from the cytokine storm: pitfalls and prospects in the development of SARS-CoV-2 vaccines for an elderly population. Semin Immunopathol. 2020;42:619–634. doi: 10.1007/s00281-020-00821-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franceschi C., Bonafè M., Valensin S., et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 19.Brosh-Nissimov T., Orenbuch-Harroch E., Chowers M., et al. BNT162b2 vaccine breakthrough: clinical characteristics of 152 fully vaccinated hospitalized COVID-19 patients in Israel. Clin Microbiol Infect. 2021;27:1652–1657. doi: 10.1016/j.cmi.2021.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hernan M.A., Robins J.M. Chapman & Hall/CRC; Boca Raton: 2020. Causal Inference: What if. [Google Scholar]
- 21.Trentini F., Marziano V., Guzzetta G., et al. The pressure on healthcare system and intensive care utilization during the COVID-19 outbreak in the Lombardy region: a retrospective observational study on 43,538 hospitalized patients. Am J Epidemiol. 2022;191(1):137–146. doi: 10.1093/aje/kwab252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serebrovska Z.O., Chong E.Y., Serebrovska T.V., Tumanovska L.V., Xi L. Hypoxia, HIF-1α, and COVID-19: from pathogenic factors to potential therapeutic targets. Acta Pharmacol Sin. 2020;41:1539–1546. doi: 10.1038/s41401-020-00554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jahani M., Dokaneheifard S., Mansouri K. Hypoxia: a key feature of COVID-19 launching activation of HIF-1 and cytokine storm. J Inflamm Lond Engl. 2020;17:33. doi: 10.1186/s12950-020-00263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ali H., Alterki A., Sindhu S., et al. Robust antibody levels in both diabetic and non-diabetic individuals after BNT162b2 mRNA COVID-19 vaccination. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.752233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Fusco M., Moran M.M., Cane A., et al. Evaluation of COVID-19 vaccine breakthrough infections among immunocompromised patients fully vaccinated with BNT162b2. J Med Econ. 2021;24:1248–1260. doi: 10.1080/13696998.2021.2002063. [DOI] [PubMed] [Google Scholar]
- 26.Tang Y., Fung E., Xu A., Lan H.Y. C-reactive protein and ageing. Clin Exp Pharmacol Physiol. 2017;44(1):9–14. doi: 10.1111/1440-1681.12758. Suppl. [DOI] [PubMed] [Google Scholar]
- 27.Wener M.H., Daum P.R., McQuillan G.M. The influence of age, sex, and race on the upper reference limit of serum C-reactive protein concentration. J Rheumatol. 2000;27:2351–2359. [PubMed] [Google Scholar]
- 28.Mesas A.E., Cavero-Redondo I., Álvarez-Bueno C., et al. Predictors of in-hospital COVID-19 mortality: a comprehensive systematic review and meta-analysis exploring differences by age, sex and health conditions. PLoS One. 2020;15 doi: 10.1371/journal.pone.0241742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Información Oficial Coronavirus. Comunidad de madrid. 2020; published online Jan 27. https://www.comunidad.madrid/servicios/salud/coronavirus. Accessed 12 April 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.