Abstract
Background
Sacrococcygeal pilonidal sinus disease is a common debilitating condition that predominantly affects young adults, with a profound impact on their activities of daily living. The condition is treated surgically, and in some cases the wound in the natal cleft is left open to heal by itself. Many dressings and topical agents are available to aid healing of these wounds.
Objectives
To assess the effects of dressings and topical agents for the management of open wounds following surgical treatment for sacrococcygeal pilonidal sinus in any care setting.
Search methods
In March 2021, we searched the Cochrane Wounds Specialised Register, CENTRAL, MEDLINE, Embase and EBSCO CINAHL Plus. We also searched clinical trials registries for ongoing and unpublished studies, and we scanned reference lists of included studies, reviews, meta‐analyses and health technology reports to identify additional studies. There were no restrictions with respect to language, date of publication or study setting.
Selection criteria
We included parallel‐group randomised controlled trials (RCTs) only. We included studies with participants who had undergone any type of sacrococcygeal pilonidal sinus disease surgery and were left with an open wound.
Data collection and analysis
We used the standard methodological procedures expected by Cochrane. We used GRADE to assess the certainty of the evidence for each outcome.
Main results
We included 11 RCTs comprising 932 participants. Two studies compared topical negative pressure wound therapy (TNPWT) with conventional open wound healing, two studies compared platelet‐rich plasma with sterile absorbent gauze, and the other seven studies compared various dressings and topical agents. All studies were at high risk of bias in at least one domain, whilst one study was judged to be at low risk of bias in all but one domain. All studies were conducted in secondary care. Mean participant ages were between 20 and 30 years, and nearly 80% of participants were male. No studies provided data on quality of life, cost‐effectiveness, pain at first dressing change or proportion of wounds healed at 6 or 12 months, and very few adverse effects were recorded in any study.
It is unclear whether TNPWT reduces time to wound healing compared with conventional open wound healing (comparison 1), as the certainty of evidence is very low. The two studies provided conflicting results, with one study showing benefit (mean difference (MD) −24.01 days, 95% confidence interval (CI) −35.65 to −12.37; 19 participants), whilst the other reported no difference. It is also unclear whether TNPWT has any effect on the proportion of wounds healed by 30 days (risk ratio (RR) 3.60, 95% CI 0.49 to 26.54; 19 participants, 1 study; very low‐certainty evidence). Limited data were available for our secondary outcomes time to return to normal daily activities and recurrence rate; we do not know whether TNPWT has any effect on these outcomes.
Lietofix cream may increase the proportion of wounds that heal by 30 days compared with an iodine dressing (comparison 4; RR 8.06, 95% CI 1.05 to 61.68; 205 participants, 1 study; low‐certainty evidence). The study did not provide data on time to wound healing.
We do not know whether hydrogel dressings reduce time to wound healing compared with wound cleaning with 10% povidone iodine (comparison 5; MD −24.54 days, 95% CI −47.72 to −1.36; 31 participants, 1 study; very low‐certainty evidence). The study did not provide data on the proportion of wounds healed. It is unclear whether hydrogel dressings have any effect on adverse effects as the certainty of the evidence is very low.
Platelet‐rich plasma may reduce time to wound healing compared with sterile absorbent gauze (comparison 6; MD −19.63 days, 95% CI −34.69 to −4.57; 210 participants, 2 studies; low‐certainty evidence). No studies provided data on the proportion of wounds healed. Platelet‐rich plasma may reduce time to return to normal daily activities (MD −15.49, 95% CI −28.95 to −2.02; 210 participants, 2 studies; low‐certainty evidence).
Zinc oxide mesh may make little or no difference to time to wound healing compared with placebo (comparison 2; median 54 days in the zinc oxide mesh group versus 62 days in the placebo mesh group; low‐certainty evidence). We do not know whether zinc oxide mesh has an effect on the proportion of wounds healed by 30 days as the certainty of the evidence is very low (RR 2.35, 95% CI 0.49 to 11.23).
It is unclear whether gentamicin‐impregnated collagen sponge reduces time to wound healing compared with no dressing (comparison 7; MD −1.40 days, 95% CI −5.05 to 2.25; 50 participants, 1 study; very low‐certainty evidence). The study did not provide data on the proportion of wounds healed.
Dialkylcarbamoyl chloride (DACC)‐coated dressings may make little or no difference to time to wound healing compared with alginate dressings (comparison 8; median 69 (95% CI 62 to 72) days in the DACC group versus 71 (95% CI 69 to 85) days in the alginate group; 1 study, 246 participants; low‐certainty evidence).
One study compared a polyurethane foam hydrophilic dressing with an alginate dressing (comparison 3) whilst another study compared a hydrocolloid dressing with an iodine dressing (comparison 9). It is unclear whether either intervention has any effect on time to wound healing as the certainty of evidence is very low.
Authors' conclusions
At present, the evidence that any of the dressings or topical agents contained in this review have a benefit on time to wound healing, the proportion of wounds that heal at a specific time point or on any of the secondary outcomes of our review ranges from low certainty to very low certainty. There is low‐certainty evidence on the benefit on wound healing of platelet‐rich plasma from two studies and of Lietofix cream and hydrogel dressings from single studies. Further studies are required to investigate these interventions further.
Plain language summary
How effective are dressings and topical agents in the management of wounds after surgical treatment for pilonidal sinus of the buttocks?
Key messages
‐ Platelet‐rich plasma (part of the participant's own blood that promotes tissue regeneration) may reduce time to wound healing compared with sterile gauze ‐ Lietofix skin repair cream may help wounds to heal by 30 days compared with a dressing with iodine (which helps to reduce bacteria in the wound) ‐ It is not clear whether hydrogel dressings (designed to keep the wound moist) reduce time to wound healing compared with wound cleaning with iodine
What is pilonidal sinus disease of the buttocks?
Pilonidal sinus disease of the buttocks is a common painful condition that mainly affects young adults.
It occurs in the natal cleft (the groove between the buttocks). It begins as infected or inflamed hair follicles. A vacuum effect, created by the motion of the buttocks, may draw more hairs down into the inflamed area. Symptoms can be very painful and sometimes last for a long time.
How is pilonidal sinus of the buttocks treated?
The condition is often treated surgically, by cutting out the inflamed area containing the hair and debris, and in some cases the wounds are not closed by stitches but left open to heal naturally. A lot of dressings and topical agents (creams or lotions) are available to help these wounds heal.
What did we want to find out?
We wanted to see which dressings and topical agents are better for treating open wounds after surgical treatment for pilonidal sinus of the buttocks.
For each intervention we looked at:
‐ how long it took wounds to heal; ‐ the number of wounds healed after 30 days, 6 months and 1 year; ‐ whether the wounds came back; ‐ how long it took people who had been treated to return to normal daily activities; ‐ quality of life; ‐ value for money; ‐ pain during the first dressing change; ‐ harmful effects (for example surgical site infection or allergic reaction) after treatment.
What did we do?
We included participants of any age and either sex who had been treated in any care setting. We searched for studies where:
‐ participants had been treated for pilonidal sinus disease of the buttocks and were left with an open wound; ‐ different dressings and topical agents were compared to see how effective they were for helping wounds to heal.
What did we find?
We included 11 studies with a combined total of 932 participants. Two studies compared topical negative pressure wound therapy (which applies controlled suction to the surface of the wound) with simple wound dressings. Two studies compared platelet‐rich plasma with sterile absorbent gauze. The other seven studies compared various dressings and topical agents. All the studies took place in hospitals.
‐ No studies provided data on quality of life, value for money or pain at the first dressing change. ‐ We do not know if topical negative pressure wound therapy helps wounds to heal faster than simple wound dressings. ‐ Lietofix skin repair cream may help wounds to heal by 30 days. ‐ We do not know if hydrogel dressings help wounds to heal faster or protect better against surgical site infection compared with wound cleaning with 10% povidone iodine. ‐ Platelet‐rich plasma may reduce the time to wound healing compared with sterile absorbent gauze. ‐ Compared with placebo mesh, mesh with zinc oxide (which is thought to have healing properties) may have little or no effect on whether wounds heal by 30 days, and it is unclear if it reduces the time to wound healing. ‐ We do not know if collagen sponge soaked in antibiotic has any effect on the time to wound healing compared with no dressing. ‐ Dressings coated with dialkylcarbamoyl chloride (a substance that bacteria sticks to) may make little to no difference to wound healing time compared with alginate dressings (derived from seaweed).
What are the limitations of the evidence?
We are not very confident in the evidence because there were only one or two studies in each comparison and most of the studies were very small. It is also possible that people in the studies were aware of what treatment they were getting.
How up to date is this evidence?
The evidence in this review is up to date to March 2021.
Summary of findings
Background
Description of the condition
Pilonidal sinus disease is a common, debilitating condition, first described in the literature nearly 200 years ago (Mayo 1833). The etymology of the word 'pilonidal' is from the Latin words 'pilus' and 'nidus', with the literal translation being 'nest of hair'. The condition predominantly affects young adults and is more common in men, obese individuals and those with a sedentary occupation (Søndenaa 1995a). The natal cleft (the recess between the buttocks) is by far the most common site for pilonidal sinus formation, but it can also occur in other areas of the body, such as the umbilicus (Meher 2016), or the web spaces of the fingers (Stern 2004). Pilonidal sinus disease occurring in the natal cleft is often termed 'sacrococcygeal pilonidal sinus disease', referring to its anatomical location between the sacrum and the coccyx. The disease begins as a folliculitis (infection and inflammation of hair follicles), leading to blockage of the follicle. A vacuum effect, created by the motion of the buttocks, may draw further hairs down into the pits (Bendewald 2007). Sacrococcygeal pilonidal sinus disease has an estimated incidence of 26 cases per 100,000 (calculated using secondary care data from Norway; Søndenaa 1995a). It has a range of clinical presentations, from acute abscesses to a painful chronic condition.
An acute pilonidal abscess is managed by incision and drainage, as is the case for abscesses of other aetiologies. However, many people may present with a chronic pilonidal sinus (a longstanding, intermittently discharging sinus) either without ever having experienced an acute abscess, or after they have had an acute abscess drained. These people may go on to have one of many possible elective surgical procedures to treat the underlying pilonidal sinus. These operations can range from minimally invasive procedures (Lund 2017; Tien 2018; Sian 2018), to wide excision of the diseased tissue. After excision, a variety of techniques have been described to deal with the wound (Al‐Khamis 2010). It may be packed and left open to heal by secondary intention (i.e. the wound is left unstitched), meaning that the wound edges are not brought together, and the defect is healed by the growth of new granulation tissue. Alternatively, it can be closed primarily (where the wound edges are brought together), using either a simple sutured closure or a more complex operation involving the use of tissue flaps (Al‐Khamis 2010). A previous Cochrane Review concluded that there was no evidence of a clear benefit for either off‐midline primary closure or leaving wounds open to heal by secondary intention over each other; hence both techniques are routinely employed (Al‐Khamis 2010). It is also estimated that up to 20% of complications from wounds closed primarily are cases of dehiscence (wound breakdown), which creates a new open wound that then has to heal by secondary intention (Onder 2012).
Management of an open wound can have a profound physical and psychological impact on the affected person (McCaughan 2018), leading to the inability to carry out their normal activities of daily living (Stewart 2012). They may also require multiple visits to healthcare professionals for dressing changes, and the healing process may take months (Chetter 2017).
Description of the intervention
The objective of managing an open wound following pilonidal sinus surgery is to promote rapid healing by secondary intention, control excess exudate (the fluid that leaks from the wound) and minimise the risk of pilonidal sinus recurrence. The location of a wound after surgery for sacrococcygeal pilonidal sinus disease is the natal cleft, which is close to the anus. For this reason, care must be taken to prevent faecal contamination of the wound and possible subsequent infection (Harris 2012). A range of dressings and topical agents are available for managing open cavity wounds. Those recommended for treating pilonidal sinus wounds include:
alginates (a highly absorbent fibre derived from brown seaweed; BNF 2019a);
hydrocolloids (waterproof dressings intended to promote a moist wound healing environment whilst providing a barrier to bacteria; Fowler 2012);
topical antimicrobials (dressings containing ingredients such as honey, silver or iodine to reduce the bacterial load in a wound; BNF 2019b);
foam dressings (dressings made from a polyurethane foam designed to absorb exudate and cushion the wound; BNF 2019c); and
hydrogels (gel‐based dressings designed to absorb exudate whilst also maintaining a moist wound environment; Jones 2005).
How the intervention might work
Normal wound healing is a complex process that occurs in three main phases: inflammation, proliferation and remodelling. A variety of problems can disrupt normal, orderly wound healing, resulting in the development of chronic, non‐healing wounds. Progression from the inflammatory stage may be prevented if the wound develops a chronic deep infection or a bacterial biofilm, or contains a foreign body or area of necrotic tissue. This can initiate a process of chronic inflammation. Excessive tension on wound edges and repeated lateral pressure forcing the wound apart can prevent proper progress of the proliferative and remodelling phases. Poor circulation can compromise all three stages of acute wound healing. Interventions to promote wound healing therefore seek to prevent or resolve these issues (Han 2017).
Pioneering work on wounds made experimentally in pigs demonstrated that wounds maintained in a moist environment healed more effectively than those allowed to scab over (Winter 1962). It has since been shown that retaining a limited amount of exudate on the wound allows for autolytic debridement, supporting the inflammatory phase of wound healing (Han 2017). Today, all advanced wound‐dressing products help to create a moist wound environment to facilitate healing.
The anatomical location of sacrococcygeal pilonidal wounds results in a risk of faecal contamination, leading to a high bacterial load within the wound (Søndenaa 1995b). Furthermore, the area has relatively poor blood supply, and is subjected to tension and lateral pressure when the person sits. The ideal dressing for use in a sacrococcygeal pilonidal wound would therefore need to absorb excess exudate, fill any cavities, prevent contamination to reduce the bacterial load on the wound bed, encourage blood supply and maintain a moist environment (Harris 2012).
Alginate dressings are considered to be highly absorptive, and are intended to remove excess slough and exudate from the wound (Dabiri 2016). Foams have a similar mechanism of action, and may also reduce trauma to the wound during dressing changes (Han 2017). Antimicrobial solutions and dressings, such as silver and polyhexamethylene biguanide (PHMB), may reduce bacterial load in the wound (Collier 2017; Schultz 2017), and there is some evidence for their effectiveness against biofilms (Percival 2008).
Negative pressure wound therapy (NPWT) has multiple possible mechanisms of action, including maintaining a moist environment whilst removing excess exudate, optimising blood flow, applying traction to wound edges and maintaining a seal to prevent bacterial contamination of the wound. There is some evidence of its effectiveness in improving healing in chronic wounds (Venturi 2005).
The interventions detailed above are examples of possible wound treatments. Any one, or a combination of possible treatments, may help to mitigate the challenges of healing open sacrococcygeal pilonidal sinus wounds. It may be that advanced wound products are no more effective than simple dressings.
Why it is important to do this review
Sacrococcygeal pilonidal sinus disease is a common condition, which has already been the subject of two Cochrane Reviews within the Cochrane Wounds Group (Al‐Khamis 2010; Lund 2017). A range of products and non‐surgical techniques is available to manage open wounds left after surgery for sacrococcygeal pilonidal sinus disease, and some of these options have been assessed in randomised controlled trials. However, there is no current consensus on the optimal management of these wounds. To date, there has not been a systematic review of the evidence regarding the most effective means of achieving healing of open pilonidal sinus wounds after surgery.
Objectives
To assess the effects of dressings and topical agents for the management of open wounds following surgical treatment for sacrococcygeal pilonidal sinus in any care setting.
Methods
Criteria for considering studies for this review
Types of studies
We only included parallel‐group randomised controlled trials (RCTs) of individuals. We would also have included cluster‐randomised trials and unpublished studies if we had identified any. We excluded cross‐over studies, as the intervention period covers the entire healing process, with no possibility of a washout period between interventions. Our search had no date or language limitations.
Types of participants
We included studies in which all participants had undergone surgical treatment for sacrococcygeal pilonidal sinus disease that left an open wound in the natal cleft. The wounds managed with topical agents or dressing could be those deliberately left open to heal by secondary intention, or those that had broken down after primary closure. We included all ages and both sexes of participant in this review, and we did not restrict studies by care setting of wound management (primary or secondary care).
If we had identified studies containing a mixture of participants (some with wounds from other types of surgery) and the data were not presented separately for the different groups, we would have contacted the corresponding authors to attempt to acquire the data specific to wounds from sacrococcygeal pilonidal sinus disease surgery. If we had identified studies involving a mixture of wounds from sacrococcygeal pilonidal sinus disease surgery (some healing by primary intention and others by secondary intention) and the data for the respective participants were not presented separately, we would have contacted the corresponding authors to attempt to obtain separate datasets. If we had been unable to obtain separate data for pilonidal sinus healing by secondary intention in either case, then these mixed population studies would have been excluded.
Types of interventions
The interventions of interest were any topical agent or dressing applied to either a wound deliberately left open to heal by secondary intention, or a wound that had broken down after primary closure, compared with any other topical agent or dressing. We classified the interventions according to the categories outlined in the relevant section of the British National Formulary BNF 2019a. We also included studies that compared any topical agent or dressing with no intervention, although we expected these to be rare. We did not include studies with co‐interventions, unless both groups had received these.
Types of outcome measures
Primary outcomes
The primary outcome for this review is complete wound healing. We regarded the following measures as providing the most relevant and rigorous measures of this outcome.
Time to wound healing (time in days until wound has healed), assessed clinically by researchers or a clinical team, using a validated wound healing score. Where possible, we aimed to present time‐to‐event data as hazard ratios (HRs) with 95% confidence intervals (CIs). Mean time‐to‐healing data were only included where we were certain that all wounds had healed.
Proportion of wounds healed (number of wounds healed/not healed) during short (30‐day), medium (6‐month) and long‐term (1‐year) follow‐up, assessed clinically by researchers or a clinical team
Secondary outcomes
Recurrence rate (number of wounds that recurred at the same site as the original wound/number of wounds that did not recur), reported during the longest follow‐up in the study and assessed clinically by researchers or a clinical team
Time (in days) to return to normal daily activities, as described during study follow‐up. Where possible, we aimed to present data as time‐to‐event (HR).
Quality of life, measured using validated scales such as the 36‐Item Short Form Survey (SF‐36; Ware 1992), EuroQol‐5 Dimension (EQ‐5D; EuroQol 1990) or the Cardiff Wound Impact Schedule (Price 2004) during study follow‐up
Cost‐effectiveness, assessed using the quality‐adjusted life year (QALY) for the primary outcomes
Pain, measured using a validated scale such as a visual analogue scale (VAS) during the first dressing change
Adverse effects (surgical site infection or allergic reaction) during study follow‐up, reported as the number of participants in each group with an adverse effect, assessed clinically by researchers or a clinical team
Search methods for identification of studies
Electronic searches
We searched the following electronic databases to identify reports of relevant clinical studies.
The Cochrane Wounds Specialised Register (searched 3 March 2021)
The Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 2) in the Cochrane Library (searched 3 March 2021)
Ovid MEDLINE including In‐Process & Other Non‐Indexed Citations (1946 to 3 March 2021)
Ovid Embase (1974 to 3 March 2021)
EBSCO CINAHL Plus (Cumulative Index to Nursing and Allied Health Literature; 1937 to 3 March 2021)
The search strategies for the Cochrane Wounds Specialised Register, CENTRAL, Ovid MEDLINE, Ovid Embase and EBSCO CINAHL Plus can be found in Appendix 1. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version, 2008 revision (Lefebvre 2021). We combined the Ovid Embase search with an adapted version of the Cochrane Centralised Search Project filter for identifying RCTs in Ovid Embase developed by the UK Cochrane Centre (Lefebvre 2021). We combined the CINAHL Plus search with the trial filter developed by Glanville et al. (Glanville 2019). There were no restrictions with respect to language, date of publication or study setting.
We also searched the following clinical trials registries.
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 3 March 2021)
World Health Organization (WHO) International Clinical Trials Registry Platform (trialsearch.who.int); searched 3 March 2021)
Search strategies for clinical trial registries can be found in Appendix 1.
Searching other resources
We searched the reference lists of identified studies to identify other potentially relevant studies. We used Google Scholar to identify potentially relevant studies that cited studies included from the electronic searches.
We also searched the last three years' conference proceedings of the Association of Surgeons of Great Britain and Ireland, the Association of Coloproctology of Great Britain and Ireland, European Society of Coloproctology, the European Wound Management Association, Wounds UK and the Journal of Wound Care conference.
Data collection and analysis
We carried out data collection and analysis according to the methods stated in the published protocol (Herrod 2019), which were based on the Cochrane Handbook for Systematic Reviews of Interventions (Li 2021). Changes from the protocol or previous published versions of the review are documented in Differences between protocol and review.
Selection of studies
Two authors (PH and TM) independently screened the titles and abstracts using Rayyan systematic review management software (Ouzzani 2016), resolving any disagreement by consulting a third author (PJH) until reaching consensus. Two authors (PJH and EH) then independently screened potentially relevant full texts against the inclusion criteria, resolving any disagreement by consulting a third author (BD). We identified any duplicate publications at this stage using author name, study date and details of the intervention. We obtained all publications for studies that had multiple references. Whilst we only included the study once in the review, we obtained all publications to maximise the amount of extracted data. If we had found studies that satisfied our inclusion criteria but did not report any relevant outcomes, we would have contacted the authors to enquire about the missing data.
Data extraction and management
Two review authors (PJH and EH) independently collected and extracted study data into an electronic database. The authors compared the extracted data, resolving any disagreements by consensus and consultation with a third author (BD) when required, prior to transferring the data into Review Manager 5 (RevMan 5; Review Manager 2020). Where relevant data were missing from studies, we attempted to contact the study authors to obtain this.
We extracted the following data.
Country in which the study took place
Publication status of study
Source of funding
Care setting
Study design
Number of participants randomised to each study arm
Study inclusion and exclusion criteria
Participant baseline characteristics (including age, sex, BMI)
Details of operation performed
Details of treatment regimen
Details of any co‐interventions
Duration of follow‐up
Primary and secondary outcomes of the studies (with definitions)
Outcome data for primary and secondary outcomes
Number of withdrawals per group, with reasons
Had we identified studies with more than two intervention arms, each would have been used in the relevant comparisons. Intervention arms that were similar in nature would have been combined into one group as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021).
Assessment of risk of bias in included studies
Two review authors (PJH and EH) assessed risk of bias independently, resolving any disagreement by consensus and involving a third author when required. We used the Cochrane tool for assessing risk of bias (RoB1; Higgins 2017), described in Appendix 2. We assessed random sequence generation, allocation concealment, blinding of participants, blinding of personnel and outcome assessors, incomplete outcome data, selective outcome reporting and other sources of bias. We accepted that blinding in these studies would be difficult and expected many to be at high risk for this domain. We then assigned each study either low, high or unclear risk of bias using the criteria of the Cochrane tool. To assess selective outcome reporting, we searched clinical trials databases for the original study registration or MEDLINE for a pre‐published protocol, and then compared this with the published study. We presented risk of bias data in a summary table (Figure 1) and a risk of bias graph (Figure 2). We decided that a significant imbalance in participant characteristics at baseline constituted a high risk of 'other' bias and reported this accordingly. If we had identified any cluster‐randomised trials, we would have also considered the risk of bias in terms of recruitment bias, baseline imbalance, loss of clusters, incorrect analysis and comparability with individually randomised trials (Higgins 2021), as described in Appendix 3).
1.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
Measures of treatment effect
For the primary outcome of time to wound healing, we planned to report time‐to‐event data as hazard ratios (HRs). For studies reporting time‐to‐event data without hazard ratios, we planned to estimate these using other reported outcomes (Tierney 2007), however this was not possible. We presented dichotomous outcomes as risk ratios (RRs) with 95% confidence intervals (CIs). For continuous outcome data, we presented mean differences (MDs) with 95% CIs. We only used a mean time‐to‐healing measure where it was clear that all wounds had healed. If different scales had been used, we would have presented results as standardised mean differences (SMDs). We planned to analyse continuous outcomes dependent on baseline risk (pain) using meta‐regression, presenting reductions from a meta‐regression equation (Doleman 2018), but we found no such data.
Unit of analysis issues
We expected nearly all studies to be parallel‐group RCTs, where each participant would be the unit of analysis. Since participants only have one natal cleft and all recognised operations for sacrococcygeal pilonidal sinus disease form only one wound, we did not anticipate any unit of analysis issues.
We planned to analyse any cluster‐randomised trials using appropriate methods that take account of unit of analysis issues (Donner 2002). Had we found any studies of this type, we would have included the data in the analysis using methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021). We would have used the intra‐cluster correlation coefficient to calculate an effective sample size if the primary study had conducted an inappropriate analysis, and we would have directly entered effect estimates if the primary study had derived these using appropriate methods (e.g. multilevel models). We would have used the generic inverse variance method for the meta‐analysis of cluster‐randomised trials.
Dealing with missing data
Where we identified studies with missing data, we first attempted to contact the corresponding author to obtain them. Where we did not receive a response, we planned to extract data from published graphs using WebPlotDigitizer, however this was not possible. Where the publications did not report standard deviations, we attempted to estimate these from other included studies in the review or from other reported measures of variance such as the interquartile range. Where studies reported medians rather than means, we reported this data separately in a narrative synthesis, but excluded them from the meta‐analysis. Had there been any participants with data missing for dichotomous outcomes, we would have assumed they had not suffered the event (best‐case scenario). In addition, we would have conducted a sensitivity analysis assuming participants with missing data had suffered the event (worst‐case scenario). Where data that were required to calculate time‐to‐event outcomes were missing, and study authors could not provide additional data, we presented data as mean differences instead.
Assessment of heterogeneity
We assessed clinical heterogeneity during the data extraction process using information on study populations, nature of the interventions and types of wounds. Where we found substantial clinical heterogeneity, we discussed the studies in a narrative review without pooling them for meta‐analysis. We assessed statistical heterogeneity using the I2 measure (Higgins 2003), interpreting the resulting values as described in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2021).
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity
We expected heterogeneity between participants whose wound was deliberately left open compared with those whose wound had broken down after primary closure. We planned to investigate this heterogeneity using subgroup analysis.
Assessment of reporting biases
For any analysis including 10 or more studies, we planned to assess publication bias qualitatively using funnel plots and quantitatively using Egger’s regression test (Egger 1997). For continuous outcomes dependent on baseline risk (pain), we planned to use a novel test based on meta‐regression residuals and inverse sample size (Doleman 2020). We would have regarded P < 0.1 as evidence of small study effects and therefore possible publication bias.
Data synthesis
We grouped dressings and topical agents for synthesis using the classification described within the British National Formulary wound management products section (BNF 2019d). We combined studies in random‐effects meta‐analyses where possible. We presented effect estimates and precision using forest plots. We calculated pooled relative risks and mean differences as appropriate. All pooled outcomes are presented with 95% CI. We combined hazard ratios and continuous outcomes using generic inverse variance if the studies only reported effect estimates and did not provide enough information to enter raw data. Where studies reported hazard ratios and continuous outcomes for the same outcome, we analysed and reported these separately. We aggregated results using a DerSimonian and Laird random‐effects model for all analyses as we anticipated an element of clinical heterogeneity and therefore different underlying effects to estimate. We used Review Manager 2020 to aggregate study data and had planned to use Stata to conduct Egger's linear regression test (Egger 1997). We did not conduct a network meta‐analysis of interventions.
Subgroup analysis and investigation of heterogeneity
We planned to perform a subgroup analysis to explore differences in the primary and secondary outcomes between participants whose wound was deliberately left open and those whose wound had dehisced after primary closure, but none of the included studies made this distinction.
Sensitivity analysis
We planned to repeat meta‐analyses removing studies we considered to be at high risk of bias in any domain. In addition, we planned to conduct a sensitivity analysis assuming the worst‐case scenario, meaning participants with missing follow‐up data had either suffered an event (if negative, for example recurrence) or did not achieve a desired outcome (if positive, for example proportion of wounds healed).
Summary of findings and assessment of the certainty of the evidence
We presented the main results of the review in summary of findings tables (Deeks 2021). The summary of findings tables also included an overall grading of the evidence related to each of the main outcomes using the GRADE approach (Schünemann 2013). The primary and secondary outcomes included in the summary of findings tables are
time to wound healing;
proportion of wounds healed;
cost effectiveness;
pain;
adverse effects.
We downgraded the certainty of evidence from high to moderate, low, or very low if concerns existed in any of the five domains. Two review authors independently downgraded the evidence, reaching agreement by consensus. We carried out GRADE assessment on all outcomes in the review. Characteristics of the evidence that could result in downgrading include:
limitations in the design and implementation of available studies, suggesting a high likelihood of bias (e.g. high risk in blinding);
indirectness of evidence (indirect population, intervention, control, or outcomes);
unexplained heterogeneity (I2 > 50%), or inconsistency of results not explained through subgroup analyses;
imprecision of results (wide confidence intervals);
evidence of publication bias (P < 0.1 on Egger’s linear regression test and visual evidence on funnel plot).
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies.
Results of the search
We identified 191 studies from searches of the electronic databases and 58 studies from trial registry searches (Figure 3). We did not identify any further studies from reference lists, conference abstracts or from searches of studies citing included studies on Google Scholar. After removing duplicates, we screened 194 records, excluding 178 and reviewing the full text of the remaining 16. We considered 11 randomised controlled trials to be eligible for inclusion in the final review (Agren 2005; Banasiewicz 2013; Berry 1996; Biter 2014; Giannini 2019; Gohar 2020; Kayaoglu 2006; Mohammadi 2017; Ozbalci 2014; Romain 2020; Viciano 2000).
3.
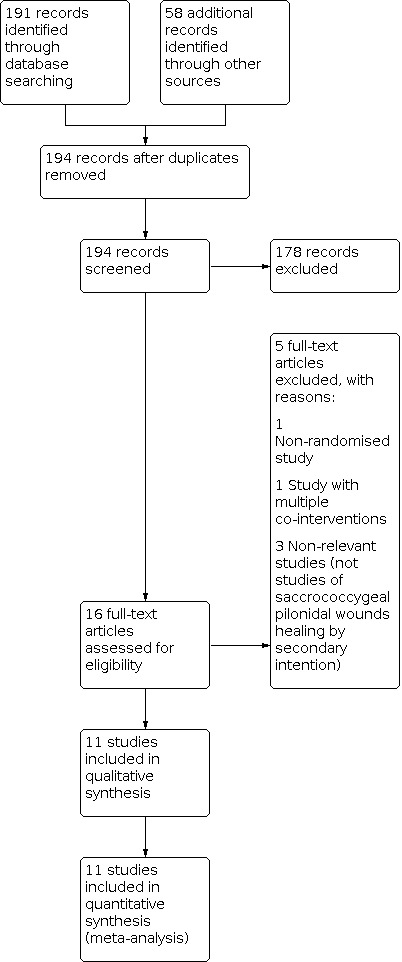
Study flow diagram
Included studies
We included 11 RCTs comprising 932 participants (Agren 2005; Banasiewicz 2013; Berry 1996; Biter 2014; Giannini 2019; Gohar 2020; Kayaoglu 2006; Mohammadi 2017; Ozbalci 2014; Romain 2020; Viciano 2000). All studies were published as full manuscripts, and had been conducted in secondary care in European and Middle Eastern countries. Ten studies were published in English and one was only available in Turkish (Characteristics of included studies). We translated this study with Google Translate. The earliest study was published in 1996 and the latest in 2020. The interventions and comparators used in our included studies are displayed in Table 8.
1. Summary of interventions and comparators in included studies.
| Study | Intervention | Comparator |
| Agren 2005 | Zinc oxide mesh | Placebo mesh |
| Banasiewicz 2013 | Topical negative pressure wound therapy | Conventional absorbent dressing |
| Berry 1996 | Polyurethane foam hydrophilic dressing | Calcium sodium alginate dressing |
| Biter 2014 | Topical negative pressure wound therapy | Silicone dressing |
| Giannini 2019 | Lietofix cream | Iodoform dressing |
| Gohar 2020 | Platelet‐rich plasma | Absorbent sterile cotton gauze |
| Kayaoglu 2006 | Hydrogel dressing | Wound cleaning with 10% povidone iodine |
| Mohammadi 2017 | Platelet‐rich plasma | Absorbent sterile cotton gauze |
| Ozbalci 2014 | Gentamicin‐impregnated collagen sponge | No intervention |
| Romain 2020 | Dialkylcarbamoyl chloride‐coated dressing | Alginate dressing |
| Viciano 2000 | Hydrocolloid dressing | Iodine dressing |
Participants and surgery
The mean age of participants in all included studies was between 20 and 30 years, in keeping with the previously described peak incidence of sacrococcygeal pilonidal sinus disease (Søndenaa 1995a). Most participants (78%) were male, which was to be expected as male sex is a risk factor for the disease (Søndenaa 1995a). The surgery described in all included studies consisted of excisional surgery to remove the pilonidal sinus tissue, and all wounds were left open to heal by secondary intention. Six studies used intraoperative injection of methylene blue into the sinus to aid recognition of tracts. Eight included studies described a limit to the depth of their surgical resection, with two studies specifying an excision down to bone if required, whilst the other six reported excision to the level of the presacral fascia. The wounds in one study were marsupialised, whilst in the other 10 studies no intervention to the wound margins was described. Seven studies reported follow‐up until wounds in all participants had healed, whilst four studies provided follow‐up to a designated time point, ranging from 60 days to 6 months.
Interventions
Two studies (Banasiewicz 2013; Biter 2014) compared topical negative pressure wound therapy dressings with other dressings, whilst the remaining nine studies compared different passive dressings. Intervention dressings included a topical zinc oxide mesh, a polyurethane foam hydrophilic dressing, Lietofix cream, platelet‐rich plasma, a hydrogel dressing, Gentamicin‐impregnated collagen sponge, a dialkylcarbamoyl chloride‐coated dressing and a hydrocolloid dressing.
Comparators
The comparators used in the included studies were wide‐ranging, with one study leaving the wound open with no dressing, whilst the other 10 studies used a comparator dressing or topical agent. One of these 10 studies used "wound cleaning with 10% povidone iodine", and the other nine described the comparator dressings as "conventional absorbent dressing", "silicone dressing", "placebo mesh", "iodoform dressing", "absorbent sterile cotton gauze", "alginate dressing" and "iodine dressing".
Excluded studies
We excluded five studies (see Characteristics of excluded studies). Cherkasov 2016 was a non‐randomised study. Panahi 2015 studied chronic wounds but did not include any sacrococcygeal pilonidal wounds. Rao 2010 was an RCT, but it compared wounds closed primarily with those left to heal by secondary intention, rather than comparing dressings or topical agents. Yetim 2010 was an RCT comparing different techniques for primary closure of sacrococcygeal pilonidal wounds. Sadati 2019 was an RCT comparing dressings used in the open healing of sacrococcygeal pilonidal wounds; however, the use of different co‐interventions in two of the study treatment arms invalidated between‐group comparisons (the treatment regimen in one group consisted of a combination of a hydrogel and hydrocolloid, switching to an alginate and hydrocolloid from the second week after surgery, whilst another group used a combination of a hydrogel and a vaseline gauze and the third group underwent daily wound cleaning and packing with sterile gauze).
We did not identify any ongoing studies or studies awaiting classification.
Risk of bias in included studies
The risk of bias in the included studies is displayed in Figure 1 and Figure 2.
Allocation
We judged five studies to be at low risk of bias for random sequence generation as they used computer‐generated randomisation (Agren 2005; Biter 2014; Giannini 2019; Mohammadi 2017; Romain 2020). However, the other six studies did not provide sufficient information to judge their risk of bias and so we considered them to be at unclear risk of bias in this domain (Banasiewicz 2013; Berry 1996; Gohar 2020 Kayaoglu 2006; Ozbalci 2014; Viciano 2000). For allocation concealment, seven studies provided insufficient information and we therefore judged them to be at unclear risk of bias (Banasiewicz 2013; Berry 1996; Biter 2014; Gohar 2020; Kayaoglu 2006; Ozbalci 2014; Viciano 2000). Three studies used a random permuted block allocation, with the recruiting investigators blinded to the centralised allocation, so we judged them to be at low risk of bias (Agren 2005; Mohammadi 2017; Romain 2020). We judged one study to be at high risk of bias due to lack of allocation concealment because the recruiting investigators received the whole randomisation list by email (Giannini 2019).
Blinding
We considered that only one study was at low risk of both performance bias and detection bias, as the placebo mesh it used was indistinguishable from the intervention mesh, and outcome assessment was performed by blinded investigators (Agren 2005). The remaining 10 studies were all judged to be at high risk of performance bias because the treatment regimens between intervention and control groups varied substantially, and no attempt was made to blind participants (Banasiewicz 2013; Berry 1996; Biter 2014; Giannini 2019; Gohar 2020; Kayaoglu 2006; Mohammadi 2017; Ozbalci 2014; Romain 2020; Viciano 2000). We judged two studies to be at low risk of detection bias (Agren 2005; Giannini 2019), as both described outcome assessment by a blinded investigator. We judged one study to be at unclear risk of detection bias because it failed to clarify whether the investigators had been blinded to outcome assessment or only to treatment allocation (Mohammadi 2017). We judged the remaining eight studies to be at high risk of detection bias as there was no blinding of outcome assessment (Banasiewicz 2013; Berry 1996; Biter 2014; Gohar 2020; Kayaoglu 2006; Ozbalci 2014; Romain 2020; Viciano 2000).
Incomplete outcome data
We judged one study to be at unclear risk of attrition bias because the information it provided regarding study dropouts was insufficient to make a judgement (Viciano 2000). We considered six studies to be at low risk of bias in this domain, as they either had a very low dropout rate or had complete outcome data (Agren 2005; Banasiewicz 2013; Biter 2014; Giannini 2019; Mohammadi 2017; Ozbalci 2014). We judged four studies to be at high risk of bias as they had dropout rates exceeding 10% (Berry 1996; Gohar 2020; Kayaoglu 2006; Romain 2020). We considered 10% to be an acceptable cutoff point as dropouts were likely to be related to negative study events (e.g. admission to hospital with a wound complication).
Selective reporting
We judged nine studies to be at unclear risk of bias as we were unable to identify either a prospective trial registration or a protocol (Banasiewicz 2013; Berry 1996; Biter 2014; Giannini 2019; Gohar 2020; Kayaoglu 2006; Ozbalci 2014; Romain 2020; Viciano 2000). One study had a prospective trial registration and so was judged to be at low risk of reporting bias (Agren 2005). Another study was considered to be at high risk of bias because the publication and the prospective trial registration mentioned different primary outcomes (Mohammadi 2017).
Other potential sources of bias
One study provided insufficient descriptions of the methodology and participants to judge the risk of bias due to other factors, so we judged it to be at unclear risk of bias in this domain (Viciano 2000). We judged five studies to be at low risk of bias due to other factors as they had groups with similar baseline characteristics and declared no industry funding (Banasiewicz 2013; Gohar 2020; Kayaoglu 2006; Mohammadi 2017; Romain 2020). We judged five studies to be at high risk of bias in this domain because of several imbalances in baseline characteristics between the study groups (Agren 2005; Berry 1996; Biter 2014; Giannini 2019; Ozbalci 2014).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7
Summary of findings 1. Topical negative pressure wound therapy versus conventional open wound healing.
| Topical negative pressure wound therapy compared with conventional open wound healing for open wounds after surgery for sacrococcygeal pilonidal sinus disease | ||||||
|
Patient or population: adults with open wounds after surgery for sacrococcygeal pilonidal sinus disease Settings: secondary care Intervention: topical negative pressure wound therapy Comparison: conventional open wound healing | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Risk with conventional open wound healing | Risk with topical negative pressure wound therapy | |||||
| Time to wound healing (days) | Mean time to wound healing was 59.11 days | Mean time to wound healing was 35.10 days | MD −24.01 (−35.65 to −12.37) days | 19 (1 study) |
⊕⊝⊝⊝ Very lowa | Unpublished data. One other study (49 participants) reported no difference in median time to wound healing (84 days vs 93 days, P = 0.44). |
| Proportion of wounds healed at 30 days | 111 per 1000 |
289 per 1000 (57 fewer to 2838 more) |
RR 3.60 (0.49 to 26.54) |
19 (1 study) |
⊕⊝⊝⊝ Verylowb | Unpublished data |
| Proportion of wounds healed at 6 months | N/A | |||||
| Proportion of wounds healed at 12 months | N/A |
|||||
| Cost‐effectiveness (assessed using quality‐adjusted life years) | N/A |
|||||
| Pain at first postoperative dressing change (measured using a validated scale such as a visual analogue scale) | N/A |
|||||
| Adverse effects (surgical site infection or allergic reaction) | 0 | 0 | Not estimable | 68 (2 studies) | Not estimable | No adverse effects were reported in either study |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded 3 levels to very low due to concerns over risk of bias (1 level), imprecision (1 level) and inconsistency (1 level). bDowngraded 3 levels to very low due to concerns over risk of bias (1 level) and imprecision (2 levels).
Summary of findings 2. Zinc oxide mesh versus placebo mesh.
| Zinc oxide mesh compared with placebo mesh for open wounds after surgery for sacrococcygeal pilonidal sinus disease | ||||||
|
Patient or population: adults with open wounds after surgery for sacrococcygeal pilonidal sinus disease Settings: secondary care Intervention: zinc oxide mesh Comparison: placebo mesh | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo mesh | Zinc oxide mesh | |||||
|
Time to wound healing (days) |
Median time for complete wound healing was 54 (interquartile range 42‐71) days in the zinc oxide mesh group and 62 (interquartile range 55‐82) days in the placebo mesh group; P = 0.32 |
⊕⊕⊝⊝ Lowa | Zinc oxide mesh may make little or no difference to time to wound healing. The certainty of evidence is low. | |||
|
Proportion of wounds healed at 30 days |
65 per 1000 | 153 per 1000 (32 to 730) | RR 2.35 (0.49 to 11.23) | 64 (1 study) | ⊕⊝⊝⊝ Verylowb | It is unclear whether Zinc oxide has an effect on the proportion of wounds healed at 30 days. The certainty of the evidence is very low. |
| Proportion of wounds healed at 6 months | N/A |
|||||
| Proportion of wounds healed at 12 months | N/A |
|||||
|
Cost‐effectiveness (assessed using quality‐adjusted life years) |
N/A |
|||||
| Pain at first postoperative dressing change (measured using a validated scale such as a visual analogue scale) | N/A |
|||||
| Adverse effects (surgical site infection or allergic reaction) | 0 | 0 | Not estimable | 64 (1 study) | Not estimable | No adverse effects were reported in the study |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded 2 levels to low due to concerns over risk of bias (1 level) and imprecision (1 level) bDowngraded 3 levels to very low due to concerns over risk of bias (1 level) and imprecision (2 levels).
Summary of findings 3. Lietofix cream versus iodoform dressing.
| Lietofix cream compared with iodoform dressing for open wounds after surgery for sacrococcygeal pilonidal sinus disease | ||||||
|
Patient or population: adults with open wounds after surgery for sacrococcygeal pilonidal sinus disease Settings: secondary care Intervention: Lietofix cream Comparison: iodoform dressing | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Iodoform dressing | Lietofix cream | |||||
|
Time to wound healing (days) |
N/A |
Outcome not reported | ||||
|
Proportion of wounds healed at 30 days |
12 per 1000 | 97 per 1000 (13 to 740) | RR 8.06 (1.05 to 61.68) | 205 (1 study) | ⊕⊕⊝⊝ Lowa | Lietofix cream may increase the proportion of wounds healed at 30 days. The certainty of the evidence is low. |
| Proportion of wounds healed at 6 months | N/A |
|||||
| Proportion of wounds healed at 12 months | N/A |
|||||
| Cost‐effectiveness (assessed using quality‐adjusted life years) | N/A |
|||||
| Pain at first postoperative dressing change (measured using a validated scale such as a visual analogue scale) | N/A |
|||||
| Adverse effects (surgical site infection or allergic reaction) | 0 | 0 | Not estimable | 205 (1 study) | Not estimable | No adverse effects were reported in the study |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded 2 levels to low due to concerns over risk of bias (1 level) and imprecision (1 level).
Summary of findings 4. Hydrogel dressing versus wound cleaning with 10% povidone iodine.
| Hydrogel dressing compared with wound cleaning with 10% povidone iodine for open wounds after surgery for sacrococcygeal pilonidal sinus disease | ||||||
|
Patient or population: adults with open wounds after surgery for sacrococcygeal pilonidal sinus disease Settings: secondary care Intervention: hydrogel dressing Comparison: wound cleaning with 10% povidone iodine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Wound cleaning with 10% povidone iodine | Hydrogel dressing | |||||
|
Time to wound healing (days) |
Mean time to wound healing was 64.73 days | Mean time to wound healing was 40.19 days | MD −24.54 (−47.72 to −1.36) days | 31 (1 study) | ⊕⊝⊝⊝ Verylowa | The certainty of the evidence is very low. |
|
Proportion of wounds healed at 30 days |
N/A |
|||||
| Proportion of wounds healed at 6 months | N/A |
|||||
| Proportion of wounds healed at 12 months | N/A |
|||||
| Cost‐effectiveness (assessed using quality‐adjusted life years) | N/A |
|||||
| Pain at first postoperative dressing change (measured using a validated scale such as a visual analogue scale) | N/A |
|||||
|
Adverse effects (surgical site infection or allergic reaction) |
63 per 1000 | 134 per 1000 (14 to 1000) | RR 2.13 (0.22 to 21.17) | 31 (1 study) | ⊕⊝⊝⊝ Verylowb | 3 surgical site infections reported in study. Data is for surgical site infection only |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference RR: risk ratio | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded 3 levels to low due to concerns over risk of bias (1 level) and imprecision (2 levels). bDowngraded 3 levels to very low due to concerns over risk of bias (1 level) and imprecision (2 levels).
Summary of findings 5. Platelet‐rich plasma gel versus absorbent sterile cotton gauze.
| Platelet‐rich plasma gel compared with absorbent sterile cotton gauze for open wounds after surgery for sacrococcygeal pilonidal sinus disease | ||||||
|
Patient or population: adults with open wounds after surgery for sacrococcygeal pilonidal sinus disease Settings: secondary care Intervention: platelet‐rich plasma gel Comparison: absorbent sterile cotton gauze | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Absorbent sterile cotton gauze | Platelet‐rich plasma gel | |||||
|
Time to wound healing (days) |
Mean time to wound healing was 59 days | Mean time to wound healing was 39 days | MD −19.63 (34.69 to 4.57) days | 210 (2 studies) | ⊕⊕⊝⊝ Lowa |
Platelet‐rich plasma may reduce the time to wound healing. The certainty of the evidence is low. |
|
Proportion of wounds healed at 30 days |
N/A |
|||||
| Proportion of wounds healed at 6 months | N/A |
|||||
| Proportion of wounds healed at 12 months | N/A |
|||||
| Cost‐effectiveness (assessed using quality‐adjusted life years) | N/A |
|||||
| Pain at first postoperative dressing change (measured using a validated scale such as a visual analogue scale) | N/A |
|||||
|
Adverse effects (surgical site infection or allergic reaction) |
0 | 0 | N/A | N/A | N/A | N/A |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded 2 levels to low due to concerns over risk of bias (1 level) and imprecision (1 level)
Summary of findings 6. Gentamicin‐impregnated collagen sponge versus no dressing.
| Gentamicin‐impregnated collagen sponge compared with no dressing for open wounds after surgery for sacrococcygeal pilonidal sinus disease | ||||||
|
Patient or population: adults with open wounds after surgery for sacrococcygeal pilonidal sinus disease Settings: secondary care Intervention: gentamicin‐impregnated collagen sponge Comparison: no dressing | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No dressing | Gentamicin‐impregnated collagen sponge | |||||
|
Time to wound healing (days) |
Mean time to wound healing was 29.6 days | Mean time to wound healing was 28.2 days | MD −1.40 (−5.05 to 2.25) days | 50 (1 study) | ⊕⊝⊝⊝ Verylow | Gentamicin‐impregnated collagen sponge may have little to no effect on time to wound healing. The certainty of the evidence is very low. |
| Proportion of wounds healed at 30 days | N/A |
|||||
| Proportion of wounds healed at 6 months | N/A |
|||||
| Proportion of wounds healed at 12 months | N/A |
|||||
| Cost‐effectiveness (assessed using quality‐adjusted life years) | N/A |
|||||
| Pain at first postoperative dressing change (measured using a validated scale such as a visual analogue scale) | N/A |
|||||
|
Adverse effects (surgical site infection or allergic reaction) |
0 | 0 | N/A | N/A | N/A | N/A |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded 3 levels to very low due to concerns over risk of bias (1 level) and imprecision (2 levels).
Summary of findings 7. Dialkylcarbamoyl chloride (DACC)‐coated dressing versus alginate dressing.
| Dialkylcarbamoyl chloride (DACC)‐coated dressing compared with alginate dressing for open wounds after surgery for sacrococcygeal pilonidal sinus disease | ||||||
|
Patient or population: adults with open wounds after surgery for sacrococcygeal pilonidal sinus disease Settings: secondary care Intervention: dialkylcarbamoyl chloride‐coated dressing Comparison: alginate dressing | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Alginate dressing | DACC‐coated dressing | |||||
|
Time to wound healing (days) |
Median time for complete wound healing was 69 (95% CI 62 to 72) days in the DACC group and 71 (95% CI 69 to 85) days in the alginate group. | 246 (1 study) | ⊕⊕⊝⊝ Lowa | DACC‐coated dressings may make little or no difference to time to wound healing. The certainty of evidence is low. | ||
|
Proportion of wounds healed at 25 days |
17 per 1000 | 9 per 1000 (1 to 94) | RR 0.51 (0.05 to 5.53) | 246 (1 study) | ⊕⊝⊝⊝ Very lowb | Data not reported at 30 days, but was available at 25 days |
| Proportion of wounds healed at 6 months | N/A |
|||||
| Proportion of wounds healed at 12 months | N/A |
|||||
| Cost‐effectiveness (assessed using quality‐adjusted life years) | N/A |
|||||
| Pain at first postoperative dressing change (measured using a validated scale such as a visual analogue scale) | N/A |
|||||
|
Adverse effects (surgical site infection or allergic reaction) |
0 | 0 | N/A | N/A | N/A | N/A |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded 2 levels to low due to concerns over risk of bias (1 level) and imprecision (1 level). bDowngraded 3 levels to very low due to concerns over risk of bias (1 level) and imprecision (2 levels).
We describe below the results of our comparisons between various dressings and topical agents. Unfortunately, we were unable to carry out either of our pre‐specified sensitivity or subgroup analyses due to the small number of studies for every comparison.
Comparison 1. Topical negative pressure wound therapy (TNPWT) versus conventional open wound healing (2 RCTs, 68 participants)
Primary outcomes
Time to wound healing (68 participants)
It is unclear whether TNPWT reduces the time to wound healing compared with conventional open wound healing. Two studies evaluated the use of TNPWT therapy compared with conventional open wound healing (Banasiewicz 2013, Biter 2014); however, one of them did not report this outcome in their publication (Banasiewicz 2013), and the other reported this data narratively (as medians). The authors of Banasiewicz 2013 provided their data upon request, and we found that the two studies provided conflicting results: Banasiewicz 2013 observed a reduction in time to wound healing (MD −24.01 days, 95% CI −35.65 to −12.37; Analysis 1.1), whilst Biter 2014 reported no difference in time to wound healing (median 84 versus 93 days; P = 0.44). We downgraded the certainty of evidence to very low due to concerns over risk of bias (one level), imprecision (one level) and inconsistency (one level).
1.1. Analysis.

Comparison 1: Topical negative pressure wound therapy versus conventional open wound healing therapy, Outcome 1: Time to wound healing (days)
Proportion of wounds healed (19 participants)
This outcome was not reported in either study, but the authors of Banasiewicz 2013 provided the relevant data upon request. It is unclear whether TNPWT affects the proportion of wounds healed at 30 days compared with conventional open wound healing (RR 3.60, 95% CI 0.49 to 26.54; Analysis 1.2). We downgraded the certainty of evidence to very low due to concerns over risk of bias (one level) and imprecision (two levels). The authors of Banasiewicz 2013 informed us that all wounds in both groups were healed at 6 months and at 12 months.
1.2. Analysis.

Comparison 1: Topical negative pressure wound therapy versus conventional open wound healing therapy, Outcome 2: Proportion of wounds healed at 30 days
Secondary outcomes
Recurrence rate (49 participants)
One study reported this outcome as the number of participants who had recurrent disease at six months (Biter 2014). It is unclear whether TNPWT affects the recurrence rate following wound healing compared with open wound healing (RR 3.13, 95% CI 0.35 to 28.00; Analysis 1.4). We downgraded the certainty of evidence to very low due to concerns over risk of bias (one level) and imprecision (two levels).
1.4. Analysis.

Comparison 1: Topical negative pressure wound therapy versus conventional open wound healing therapy, Outcome 4: Recurrence
Time to return to normal daily activities (68 participants)
It is unclear whether TNPWT has any effect on the time to return to normal daily activities. Two studies reported this outcome (Banasiewicz 2013; Biter 2014); however, the results of Biter 2014 are presented in a narrative synthesis with medians. The two studies provide conflicting results: Banasiewicz 2013 observed a reduction in time to return to normal daily activities (MD −8.60 days, 95% CI −13.40 to −3.80; Analysis 1.3), whilst Biter 2014 showed no difference in the median time to return to normal daily activities (27 days in the TNPWT group versus 29 days in the conventional open wound healing group; P = 0.92). We downgraded the certainty of evidence to very low due to concerns over risk of bias (one level), imprecision (one level) and inconsistency (one level).
1.3. Analysis.

Comparison 1: Topical negative pressure wound therapy versus conventional open wound healing therapy, Outcome 3: Time to return to normal activities (days)
Neither study reported data for quality of life, cost‐effectiveness, pain during the first dressing change or adverse effects.
Comparison 2. Zinc oxide mesh versus placebo mesh (1 RCT, 64 participants)
Primary outcomes
Time to wound healing (64 participants)
Zinc oxide mesh may make little or no difference to the time to wound healing. The one included study reported this outcome (Agren 2005), however only as median values (median 54 days in the zinc oxide mesh group versus 62 days in the placebo mesh group; P = 0.32). We downgraded the certainty of evidence to low due to concerns over risk of bias (one level) and imprecision (one level).
Proportion of wounds healed (64 participants)
It is unclear whether zinc oxide mesh improves the proportion of wounds healed at 30 days. This outcome was reported in the included study (Agren 2005), but only data at 30 days postoperatively were provided. The single study's small sample size led to imprecision in the effect estimate (5/33 in the zinc oxide mesh group versus 2/31 in the placebo mesh group; RR 2.35, 95% CI 0.49 to 11.23; Analysis 2.1). We downgraded the certainty of evidence to very low due to concerns over risk of bias (one level) and imprecision (two levels). No data were reported for the six‐month or one‐year time points.
2.1. Analysis.

Comparison 2: Zinc oxide mesh versus placebo mesh, Outcome 1: Proportion of wounds healed at 30 days
Secondary outcomes
The study did not report data for any of our secondary outcomes.
Comparison 3. Allevyn polyurethane foam hydrophilic dressing versus Kaltostat alginate dressing (1 RCT, 20 participants)
Primary outcomes
Time to wound healing (20 participants)
It is unclear whether polyurethane foam hydrophilic dressings reduce the time to wound healing. One included study reported this outcome (Berry 1996), but with ranges as a measure of variance, and no hypothesis testing. Mean time to wound healing was 57 days in the polyurethane foam hydrophilic dressing group compared with 66 days in the alginate dressing group. We were unable to calculate a mean difference with 95% CI for this comparison owing to the lack of any acceptable measure of variance. Standard deviations could not be imputed as there were no comparable studies. We downgraded the certainty of evidence to very low due to imprecision (two levels: no acceptable measure of variance) and concerns over risk of bias (one level).
Proportion of wounds healed
This outcome was not reported in the included study.
Secondary outcomes
The study did not report data for any of our secondary outcomes.
In view of the paucity of relevant data, we have not produced a summary of findings table for this comparison.
Comparison 4. Lietofix cream versus iodine dressing (1 RCT, 205 participants)
Primary outcomes
Time to wound healing
The one included study did not report this outcome (Giannini 2019).
Proportion of wounds healed (205 participants)
Lietofix cream may increase the number of wounds healed at 30 days compared with iodoform dressings. The included study reported the proportion of wounds healed at 30 days postoperatively (10/103 participants versus 1/83 participants; RR 8.06, 95% CI 1.05 to 61.68; Analysis 3.1). We downgraded the certainty of evidence to low due to concerns over risk of bias (one level) and imprecision (one level). No data were reported for the six‐month or one‐year time points.
3.1. Analysis.

Comparison 3: Lietofix cream versus iodoform dressing, Outcome 1: Proportion of wounds healed at 30 days
Secondary outcomes
The study did not report data for any of our secondary outcomes.
Comparison 5. Hydrogel versus wound cleaning with 10% povidone iodine (1 RCT, 31 participants)
Primary outcomes
Time to wound healing (31 participants)
It is unclear whether hydrogel dressings reduce the time to wound healing compared with wound cleaning with 10% povidone iodine. One included study reported this outcome (Kayaoglu 2006), with a mean difference of −24.54 days (95% CI −47.72 to −1.36; Analysis 4.1). We downgraded the certainty of evidence to very low due to concerns over risk of bias (one level) and imprecision (two levels).
4.1. Analysis.

Comparison 4: Hydrogel dressing versus wound cleaning with 10% povidone iodine, Outcome 1: Time to wound healing
Proportion of wounds healed
This outcome was not reported in the included study.
Secondary outcomes
Adverse effects (31 participants)
Surgical site infections were reported in the included study. It is unclear whether hydrogel dressings have any effect on surgical site infection (RR 2.13, 95% CI 0.22 to 21.17; Analysis 4.2). We downgraded the certainty of evidence to very low due to concerns over risk of bias (one level) and imprecision (two levels).
4.2. Analysis.

Comparison 4: Hydrogel dressing versus wound cleaning with 10% povidone iodine, Outcome 2: Surgical site infection
The study did not report data for recurrence rate, time to return to normal daily activities, quality of life, cost‐effectiveness or pain during the first dressing change.
Comparison 6. Platelet‐rich plasma gel versus absorbent sterile cotton gauze (2 RCTs, 210 participants)
Primary outcomes
Time to wound healing (210 participants)
Platelet‐rich plasma may reduce the time to wound healing compared with absorbent sterile cotton gauze. Both Gohar 2020 and Mohammadi 2017 reported this outcome (MD −19.63 days, 95% CI −34.69 to −4.57; Analysis 5.1). We downgraded the certainty of the evidence to low due to concerns over risk of bias (one level) and imprecision (one level).
5.1. Analysis.

Comparison 5: Platelet‐rich plasma versus absorbent sterile cotton gauze, Outcome 1: Time to wound healing (days)
Proportion of wounds healed
This outcome was not reported in either of the included studies.
Secondary outcomes
Time to return to normal daily activities (210 participants)
Platelet‐rich plasma gel may reduce the time taken to return to normal daily activities compared with absorbent sterile cotton gauze. This outcome was reported in both included studies (MD −15.49, 95% CI −28.95 to −2.02; Analysis 5.2). We downgraded the certainty of evidence to low due to concerns over risk of bias (one level) and imprecision (one level).
5.2. Analysis.

Comparison 5: Platelet‐rich plasma versus absorbent sterile cotton gauze, Outcome 2: Time to return to normal daily activities (days)
Neither study reported data for recurrence rate, quality of life, cost‐effectiveness, pain during the first dressing change or adverse effects.
Comparison 7: Gentamicin‐impregnated collagen sponge versus no dressing (1 RCT, 50 participants)
Primary outcomes
Time to wound healing (50 participants)
It is unclear whether gentamicin‐impregnated collagen sponge reduces the time to wound healing. One included study reported this outcome (Ozbalci 2014), with a mean difference of −1.40 days (95% CI −5.05 to 2.25; Analysis 6.1). We downgraded the certainty of evidence to very low due to concerns over risk of bias (one level) and imprecision (two levels).
6.1. Analysis.

Comparison 6: Gentamicin‐impregnated collagen sponge versus no dressing, Outcome 1: Time to wound healing (days)
Proportion of wounds healed
This outcome was not reported in the included study.
Secondary outcomes
The study did not report data for any of our secondary outcomes.
Comparison 8. Dialkylcarbamoyl chloride (DACC)‐coated dressing versus alginate dressing (1 RCT, 246 participants)
Primary outcomes
Time to wound healing (246 participants)
DACC‐coated dressings may make little or no difference to the time to wound healing compared with alginate dressings. One included study reported this outcome (Romain 2020), however only as medians with 95% CIs. The median time for complete wound healing was 69 (95% CI 62 to 72) days in the DACC group and 71 (95% CI 69 to 85) days in the alginate group. We downgraded the certainty of evidence to low due to concerns over risk of bias (one level) and imprecision (one level).
Proportion of wounds healed (246 participants)
This outcome was reported in the included study, however not for the 30‐day, 6‐month or 1‐year time points specified in the review protocol. It is unclear whether DACC‐coated dressings have any effect on the proportion of wounds healed compared with alginate dressing at 25 days, the time point for which data are provided in the study (RR 0.51, 95% CI 0.05 to 5.53; Analysis 7.1). We downgraded the certainty of evidence to very low due to concerns over risk of bias (one level) and imprecision (two levels).
7.1. Analysis.

Comparison 7: Dialkylcarbamoyl chloride (DACC)‐coated dressing versus alginate dressing, Outcome 1: Proportion of wounds healed
Secondary outcomes
Time to return to normal daily activities
This outcome was reported in the included study, but only as a proportion of participants who had returned to their normal activities at 100 days.
The study did not provide data for recurrence rate, quality of life, cost‐effectiveness, pain during the first dressing change or adverse effects.
Comparison 9. Hydrocolloid dressing versus iodine dressing (1 RCT, 38 participants)
Primary outcomes
Time to wound healing (38 participants)
It is unclear whether hydrocolloid dressings reduce the time to wound healing compared with iodine dressings. The one included study reported this outcome (Viciano 2000), but only as medians with no measure of variance except the range (median 65 days in the hydrocolloid dressing group versus 68 days in the gauze and povidone iodine dressing group). We downgraded the certainty of the evidence to very low due to imprecision (two levels: no acceptable measure of variance) and concerns over risk of bias (one level).
Proportion of wounds healed
This outcome was not reported in the included study.
Secondary outcomes
The study did not report data for any of our secondary outcomes.
In view of the paucity of relevant data, we have not produced a summary of findings table for this comparison.
Discussion
Summary of main results
Our review included 11 RCTs (932 participants) comparing different dressings and topical agents for the management of open wounds after surgical treatment of sacrococcygeal pilonidal sinus disease. Two studies compared TNPWT with conventional open wound healing, two studies compared platelet‐rich plasma with sterile absorbent gauze, and seven studies compared a variety of other dressings and topical agents (Characteristics of included studies).
All included studies were at high risk of bias in at least one domain and 10 studies were at high risk of bias in two or more domains (Figure 1; Figure 2).
It is unclear whether TNPWT reduces the time to wound healing or the proportion of wounds healed compared with conventional open wound healing, because we considered the certainty of the evidence to be very low. The two studies disagreed on the secondary outcome of time to return to normal daily activities: one found that TNPWT reduced this time by eight days on average, while the other found no difference between the two treatments. Unfortunately, reporting differences precluded meta‐analysis and the certainty of the evidence for this outcome was very low.
The low‐certainty evidence from one study suggests that Lietofix cream may increase the proportion of wounds healed by 30 days compared with an iodoform dressing.
The low‐certainty evidence from two studies suggests that the application of platelet‐rich plasma may reduce the time to wound healing compared with sterile absorbent gauze for open wounds after surgical treatment of sacrococcygeal pilonidal sinus disease. Platelet‐rich plasma may also reduce the time to return to normal daily activities.
Six other studies, each at high risk of bias in at least one domain, compared eight different classes of dressings and topical agents for the management of open wounds after surgical treatment of sacrococcygeal pilonidal sinus disease. As each study assessed a different dressing or topical agent, no meta‐analysis was possible (Effects of interventions).
We are not sure whether zinc oxide mesh, hydrogel dressings, polyurethane foam hydrophilic dressings, gentamicin‐impregnated collagen sponge, Dialkylcarbamoyl chloride‐coated dressings or hydrocolloid dressings have any beneficial effect on our primary or secondary outcomes, compared with comparator dressings or no dressing, because all evidence was either low or very low certainty.
Overall completeness and applicability of evidence
As we were unable to obtain all the information we required from the published papers of the 11 included RCTs, we contacted several authors for further data. We are unaware of any eligible studies not included in this review. All included studies were carried out in either Europe, Egypt, Turkey or Iran in secondary care institutions over the last 25 years. Nonetheless, some studies utilised comparator dressing methods that are seldom used in modern practice (e.g. no dressing at all, or a cotton gauze dressing). In addition, none of the 'advanced' wound therapies were compared with each other. The mean age of the participants in the included studies was 30 years or below, in keeping with pilonidal sinus disease predominantly affecting young adults. Very few studies reported complete data for the outcomes that we considered. Where possible, we contacted corresponding authors to obtain missing data, and some of them replied. As with other areas of research into the treatment of pilonidal sinus disease, the evidence is almost uniformly of low quality (Brown 2019). As this condition is common and may have a substantial impact on the education, employment or social interactions of the people it affects, higher‐quality studies are needed to better inform treatment decisions.
Quality of the evidence
The overall quality of the evidence for all comparisons was low or very low. Reasons for downgrading included concerns over the risk of bias in the included studies, and imprecision due to the data for most comparisons coming from single studies, often with low numbers of participants (Characteristics of included studies). For some comparisons, we downgraded the certainty of evidence by two levels for imprecision because the confidence intervals were wide and included both substantial benefit and substantial harm.
Potential biases in the review process
Two authors independently conducted abstract screening, full‐text reviews, assessments for risk of bias and GRADE, and resolved disagreements by discussion with a third author, as per our protocol, to which we strictly adhered. Nevertheless, some decisions for judging risk of bias may be subjective, as some of the study methodology was poorly described in the studies. None of the review authors were involved in any of the included trials. In addition, most of the review authors practice clinically in this field and may have pre‐held views about certain dressings, which may have caused unconscious bias.
Agreements and disagreements with other studies or reviews
We are unaware of any other systematic reviews comparing the use of dressings and topical agents for the management of open wounds after surgical treatment for sacrococcygeal pilonidal sinus disease.
Authors' conclusions
Implications for practice.
At present, we are uncertain whether any of the dressings or topical agents assessed in the included studies have a beneficial effect on time to wound healing, the proportion of wounds healed at a specific time point, or any of the secondary endpoints of our review. We found low‐ or very low‐certainty evidence, mostly from single studies, that interventions such as topical negative pressure wound therapy, Lietofix cream, hydrogel dressings and platelet‐rich plasma gel may improve wound healing. Further studies are required to investigate these interventions further.
Implications for research.
Future randomised controlled trials should be adequately powered and compare dressings and topical agents that are commonly used for the management of open wounds after surgery for sacrococcygeal pilonidal sinus disease. It is crucial to adequately report relevant endpoints such as the time to wound healing and the proportion of wounds healed within certain time frames. Due to the low‐certainty evidence, which was mostly from single studies for each comparison, further randomised controlled trials are required to answer our review's objective. Future studies should also attempt to blind researchers and participants from interventions to help improve the certainty of evidence (Agren 2005).
History
Protocol first published: Issue 9, 2019
Acknowledgements
The authors would like to thank peer reviewers Chunhu Shi, Janet Wale and Sharon Van Wicklin for providing feedback on both the protocol and the review. They would also like to thank the copy editors, Andrea Takeda (protocol) and Julia M Turner (review).
Appendices
Appendix 1. Search strategies
Cochrane Wounds Specialised Register
1 MESH DESCRIPTOR Pilonidal Sinus EXPLODE ALL AND INREGISTER
2 pilonidal AND INREGISTER
3 #1 OR #2 AND INREGISTER
4 MESH DESCRIPTOR Postoperative Care EXPLODE ALL AND INREGISTER
5 MESH DESCRIPTOR Alginates EXPLODE ALL AND INREGISTER
6 MESH DESCRIPTOR Hydrogel, Polyethylene Glycol Dimethacrylate EXPLODE ALL AND INREGISTER
7 MESH DESCRIPTOR Honey EXPLODE ALL AND INREGISTER
8 MESH DESCRIPTOR Silver EXPLODE ALL AND INREGISTER
9 MESH DESCRIPTOR Silver Sulfadiazine EXPLODE ALL AND INREGISTER
10 MESH DESCRIPTOR Silicones EXPLODE ALL AND INREGISTER
11 MESH DESCRIPTOR Polyurethanes EXPLODE ALL AND INREGISTER
12 (dressing* or pad or pads or gauze or tulle or film or bead or foam* or non‐adherent or (non next adherent) or hydrocolloid* ) AND INREGISTER
13 (sodium next hyaluronate) or alginat* or hydrogel* or silver* or honey* or matrix or iodine* or (protease next modulat*) AND INREGISTER
14 (capillary next action) AND INREGISTER
15 (silicon* or polymer* or polyurethane* or hydropolymer* or carboxymethylcellulose or carboxymethyl‐cellulose or NaCMC) AND INREGISTER
16 (gel next forming) or gel‐forming AND INREGISTER
17 (wound near2 pack*) AND INREGISTER
18 ((odour or odor) near3 absorb*) AND INREGISTER
19 #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 AND INREGISTER
20 MESH DESCRIPTOR Anti‐Infective Agents, Local EXPLODE ALL AND INREGISTER
21 MESH DESCRIPTOR Administration, Topical EXPLODE ALL AND INREGISTER
22 MESH DESCRIPTOR Anti‐Bacterial Agents EXPLODE ALL AND INREGISTER
23 MESH DESCRIPTOR Metronidazole EXPLODE ALL AND INREGISTER
24 MESH DESCRIPTOR Iodophors EXPLODE ALL AND INREGISTER
25 MESH DESCRIPTOR Collagenases EXPLODE ALL AND INREGISTER
26 MESH DESCRIPTOR Zinc Oxide EXPLODE ALL AND INREGISTER
27 #22 OR #23 OR #24 OR #25 OR #26 AND INREGISTER
28 #21 AND #27 AND INREGISTER
29 ((topical or applicat*) near2 (metronidazole or antibiotic* or antimicrobial* or antibacterial* or iodine or collagen* or zinc or phenol)) AND INREGISTER
30 (iodosorb or actiformcool or aquaflo or flamazine or silvadene) AND INREGISTER 47
31 MESH DESCRIPTOR Biguanides EXPLODE ALL AND INREGISTER
32 ((polyhexamethylene next biguanide) or PHMB or polyhexanide or prontosan) AND INREGISTER
33 antiseptic* AND INREGISTER
34 MESH DESCRIPTOR Ointments EXPLODE ALL AND INREGISTER
35 (ointment* or lotion* or cream* or powder* or gel or gels) AND INREGISTER
36 (topical next (agent* or preparation* or therap* or treatment*)) AND INREGISTER
37 MESH DESCRIPTOR Platelet‐Rich Plasma EXPLODE ALL AND INREGISTER
38 (platelet* next plasma) AND INREGISTER
39 PRP AND INREGISTER
40 MESH DESCRIPTOR Negative‐Pressure Wound Therapy EXPLODE ALL AND INREGISTER
41 (negative pressure or TNP or NPWT) AND INREGISTER
42 ((vacuum next assist*) or (vacuum next therap*) or VAC) AND INREGISTER
43 #19 OR #20 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 AND INREGISTER
44 #3 AND #43 AND INREGISTER
The Cochrane Central Register of Controlled Clinical Trials (CENTRAL)
#1 MeSH descriptor: [Pilonidal Sinus] explode all trees
#2 pilonidal:ti,ab,kw
#3 #1 or #2
#4 MeSH descriptor: [Postoperative Care] explode all trees
#5 MeSH descriptor: [Alginates] explode all trees
#6 MeSH descriptor: [Hydrogel, Polyethylene Glycol Dimethacrylate] explode all trees
#7 MeSH descriptor: [Honey] explode all trees
#8 MeSH descriptor: [Silver] explode all trees
#9 MeSH descriptor: [Silver Sulfadiazine] explode all trees
#10 MeSH descriptor: [Silicones] explode all trees
#11 MeSH descriptor: [Polyurethanes] explode all trees
#12 (dressing* or pad or pads or gauze or tulle or film or bead or foam* or non‐adherent or (non next adherent) or hydrocolloid* or (sodium next hyaluronate) or alginat* or hydrogel* or silver* or honey* or matrix or iodine* or (protease next modulat*) or (capillary next action) or silicon* or polymer* or polyurethane* or hydropolymer* or carboxymethylcellulose or carboxymethyl‐cellulose or NaCMC or (gel next forming) or gel‐forming):ti,ab,kw
#13 (wound near/2 pack*):ti,ab,kw
#14 ((odour or odor) near/3 absorb*):ti,ab,kw
#15 #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14
#16 MeSH descriptor: [Anti‐Infective Agents, Local] explode all trees
#17 MeSH descriptor: [Administration, Topical] explode all trees
#18 MeSH descriptor: [Anti‐Bacterial Agents] explode all trees
#19 MeSH descriptor: [Metronidazole] explode all trees
#20 MeSH descriptor: [Iodophors] explode all trees
#21 MeSH descriptor: [Collagenases] explode all trees
#22 MeSH descriptor: [Zinc Oxide] explode all trees
#23 #18 or #19 or #20 or #21 or #22
#24 #17 and #23
#25 ((topical or applicat*) near/2 (metronidazole or antibiotic* or antimicrobial* or antibacterial* or iodine or collagen* or zinc or phenol)):ti,ab,kw
#26 (iodosorb or actiformcool or aquaflo or flamazine or silvadene):ti,ab,kw
#27 MeSH descriptor: [Biguanides] explode all trees
#28 ((polyhexamethylene adj biguanide) or PHMB or polyhexanide or prontosan):ti,ab,kw
#29 antiseptic*:ti,ab,kw
#30 MeSH descriptor: [Ointments] explode all trees
#31 (ointment* or lotion* or cream* or powder* or gel or gels):ti,ab,kw
#32 (topical next (agent* or preparation* or therap* or treatment*)):ti,ab,kw
#33 MeSH descriptor: [Platelet‐Rich Plasma] explode all trees
#34 (platelet* next plasma):ti,ab,kw
#35 PRP:ti,ab,kw
#36 MeSH descriptor: [Negative‐Pressure Wound Therapy] explode all trees
#37 (negative pressure or TNP or NPWT):ti,ab,kw
#38 ((vacuum next assist*) or (vacuum next therap*) or VAC):ti,ab,kw
#39 #15 or #16 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38
#40 #3 and #39 in Trials
The Cochrane Central Register of Controlled Clinical Trials (CENTRAL) via Cochrane Register of Studies
1 MESH DESCRIPTOR Pilonidal Sinus EXPLODE ALL AND CENTRAL:TARGET
2 pilonidal AND CENTRAL:TARGET
3 #1 OR #2 AND CENTRAL:TARGET
4 MESH DESCRIPTOR Postoperative Care EXPLODE ALL AND CENTRAL:TARGET
5 MESH DESCRIPTOR Alginates EXPLODE ALL AND CENTRAL:TARGET
6 MESH DESCRIPTOR Hydrogel, Polyethylene Glycol Dimethacrylate EXPLODE ALL AND CENTRAL:TARGET
7 MESH DESCRIPTOR Honey EXPLODE ALL AND CENTRAL:TARGET
8 MESH DESCRIPTOR Silver EXPLODE ALL AND CENTRAL:TARGET
9 MESH DESCRIPTOR Silver Sulfadiazine EXPLODE ALL AND CENTRAL:TARGET
10 MESH DESCRIPTOR Silicones EXPLODE ALL AND CENTRAL:TARGET
11 MESH DESCRIPTOR Polyurethanes EXPLODE ALL AND CENTRAL:TARGET
12 (dressing* or pad or pads or gauze or tulle or film or bead or foam* or non‐adherent or (non next adherent) or hydrocolloid* ) AND CENTRAL:TARGET
13 (sodium next hyaluronate) or alginat* or hydrogel* or silver* or honey* or matrix or iodine* or (protease next modulat*) AND CENTRAL:TARGET
14 (capillary next action) AND CENTRAL:TARGET
15 (silicon* or polymer* or polyurethane* or hydropolymer* or carboxymethylcellulose or carboxymethyl‐cellulose or NaCMC) AND CENTRAL:TARGET
16 (gel next forming) or gel‐forming AND CENTRAL:TARGET
17 (wound near2 pack*) AND CENTRAL:TARGET
18 ((odour or odor) near3 absorb*) AND CENTRAL:TARGET
19 #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 AND CENTRAL:TARGET
20 MESH DESCRIPTOR Anti‐Infective Agents, Local EXPLODE ALL AND CENTRAL:TARGET
21 MESH DESCRIPTOR Administration, Topical EXPLODE ALL AND CENTRAL:TARGET
22 MESH DESCRIPTOR Anti‐Bacterial Agents EXPLODE ALL AND CENTRAL:TARGET
23 MESH DESCRIPTOR Metronidazole EXPLODE ALL AND CENTRAL:TARGET
24 MESH DESCRIPTOR Iodophors EXPLODE ALL AND CENTRAL:TARGET
25 MESH DESCRIPTOR Collagenases EXPLODE ALL AND CENTRAL:TARGET
26 MESH DESCRIPTOR Zinc Oxide EXPLODE ALL AND CENTRAL:TARGET
27 #22 OR #23 OR #24 OR #25 OR #26 AND CENTRAL:TARGET
28 #21 AND #27 AND CENTRAL:TARGET
29 ((topical or applicat*) near2 (metronidazole or antibiotic* or antimicrobial* or antibacterial* or iodine or collagen* or zinc or phenol)) AND CENTRAL:TARGET
30 (iodosorb or actiformcool or aquaflo or flamazine or silvadene) AND CENTRAL:TARGET
31 MESH DESCRIPTOR Biguanides EXPLODE ALL AND CENTRAL:TARGET
32 ((polyhexamethylene next biguanide) or PHMB or polyhexanide or prontosan) AND CENTRAL:TARGET
33 antiseptic* AND CENTRAL:TARGET
34 MESH DESCRIPTOR Ointments EXPLODE ALL AND CENTRAL:TARGET
35 (ointment* or lotion* or cream* or powder* or gel or gels) AND CENTRAL:TARGET
36 (topical next (agent* or preparation* or therap* or treatment*)) AND CENTRAL:TARGET
37 MESH DESCRIPTOR Platelet‐Rich Plasma EXPLODE ALL AND CENTRAL:TARGET
38 (platelet* next plasma) AND CENTRAL:TARGET
39 PRP AND CENTRAL:TARGET
40 MESH DESCRIPTOR Negative‐Pressure Wound Therapy EXPLODE ALL AND CENTRAL:TARGET
41 (negative pressure or TNP or NPWT) AND CENTRAL:TARGET
42 ((vacuum next assist*) or (vacuum next therap*) or VAC) AND CENTRAL:TARGET
43 #19 OR #20 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 AND CENTRAL:TARGET
44 #3 AND #43 AND CENTRAL:TARGET
45 (NCT0* or ACTRN* or ChiCTR* or DRKS* or EUCTR* or eudract* or IRCT* or ISRCTN* or JapicCTI* or JPRN* or NTR0* or NTR1* or NTR2* or NTR3* or NTR4* or NTR5* or NTR6* or NTR7* or NTR8* or NTR9* or SRCTN* or UMIN0*):AU AND CENTRAL:TARGET
46 http*:SO AND CENTRAL:TARGET
47 #45 OR #46 AND CENTRAL:TARGET
48 #44 AND #47
Ovid MEDLINE
1 exp Pilonidal Sinus/
2 pilonidal.ti,ab.
3 1 or 2
4 exp Postoperative care/
5 exp ALGINATES/
6 exp Hydrogels/
7 exp Honey/
8 exp Silver/
9 exp Silver Sulfadiazine/
10 exp Silicones/
11 exp Polyurethanes/
12 (dressing* or pad or pads or gauze or tulle or film or bead or foam* or non‐adherent or (non adj adherent) or hydrocolloid* or (sodium adj hyaluronate) or alginat* or hydrogel* or silver* or honey* or matrix or iodine* or (protease adj modulat*) or (capillary adj action) or silicon* or polymer* or polyurethane* or hydropolymer* or carboxymethylcellulose or carboxymethyl‐cellulose or NaCMC or (gel adj forming) or gel‐forming).ab,ti.
13 (wound adj2 pack*).ti,ab.
14 ((odour or odor) adj3 absorb*).ab,ti.
15 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14
16 exp Anti‐Infective Agents, Local/
17 exp Administration, Topical/
18 exp Anti‐Bacterial Agents/
19 exp METRONIDAZOLE/
20 exp IODOPHORS/
21 exp Collagenases/
22 exp Zinc Oxide/
23 18 or 19 or 20 or 21 or 22
24 17 and 23
25 ((topical or applicat*) adj2 (metronidazole or antibiotic* or antimicrobial* or antibacterial* or iodine or collagen* or zinc or phenol)).ab,ti.
26 (iodosorb or actiformcool or aquaflo or flamazine or silvadene).ab,ti.
27 exp biguanides/
28 ((polyhexamethylene adj biguanide) or PHMB or polyhexanide or prontosan).ti,ab.
29 antiseptic*.ti,ab.
30 exp OINTMENTS/
31 (ointment* or lotion* or cream* or powder* or gel or gels).ab,ti.
32 (topical adj (agent* or preparation* or therap* or treatment*)).ab,ti.
33 exp Platelet‐rich Plasma/
34 (platelet* adj plasma).ti,ab.
35 PRP.ti,ab.
36 exp Negative‐Pressure Wound Therapy/
37 (negative pressure or TNP or NPWT).ab,ti.
38 ((vacuum adj assist*) or (vacuum adj therap*) or VAC).ab,ti.
39 15 or 16 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38
40 3 and 39
41 randomized controlled trial.pt.
42 controlled clinical trial.pt.
43 randomi?ed.ab.
44 placebo.ab.
45 clinical trials as topic.sh.
46 randomly.ab.
47 trial.ti.
48 or/41‐47
49 exp animals/ not humans.sh.
50 48 not 49
51 40 and 50
Ovid Embase
1 exp pilonidal sinus/
2 pilonidal.ti,ab.
3 1 or 2
4 exp Postoperative care/
5 exp alginic acid/
6 exp hydrogel/
7 exp honey/
8 exp silver/
9 exp sulfadiazine silver/
10 exp silicone/
11 exp polyurethan/
12 (dressing* or pad or pads or gauze or tulle or film or bead or foam* or non‐adherent or (non adj adherent) or hydrocolloid* or (sodium adj hyaluronate) or alginat* or hydrogel* or silver* or honey* or matrix or iodine* or (protease adj modulat*) or (capillary adj action) or silicon* or polymer* or polyurethane* or hydropolymer* or carboxymethylcellulose or carboxymethyl‐cellulose or NaCMC or (gel adj forming) or gel‐forming).ti,ab.
13 (wound adj2 pack*).ti,ab. 181
14 ((odour or odor) adj3 absorb*).ti,ab.
15 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14
16 exp Anti‐Infective Agents, Local/
17 exp topical drug administration/
18 exp Anti‐Bacterial Agents/
19 exp metronidazole/
20 exp iodophor/
21 exp collagenase/
22 exp zinc oxide/
23 18 or 19 or 20 or 21 or 22
24 17 and 23
25 ((topical or applicat*) adj2 (metronidazole or antibiotic* or antimicrobial* or antibacterial* or iodine or collagen* or zinc or phenol)).ti,ab.
26 (iodosorb or actiformcool or aquaflo or flamazine or silvadene).ti,ab.
27 exp biguanide/
28 ((polyhexamethylene adj biguanide) or PHMB or polyhexanide or prontosan).ti,ab.
29 antiseptic*.ti,ab.
30 exp ointment/
31 (ointment* or lotion* or cream* or powder* or gel or gels).ti,ab.
32 (topical adj (agent* or preparation* or therap* or treatment*)).ti,ab.
33 exp thrombocyte rich plasma/
34 (platelet* adj plasma).ti,ab.
35 PRP.ti,ab.
36 exp vacuum assisted closure/
37 (negative pressure or TNP or NPWT).ti,ab.
38 ((vacuum adj assist*) or (vacuum adj therap*) or VAC).ti,ab.
39 15 or 16 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38
40 3 and 39
41 Randomized controlled trial/
42 Controlled clinical study/
43 Random$.ti,ab.
44 randomisation/
45 intermethod comparison/
46 placebo.ti,ab.
47 (compare or compared or comparison).ti.
48 ((evaluated or evaluate or evaluating or assessed or assess) and (compare orcomparedor comparing or comparison)).ab.
49 (open adj label).ti,ab.
50 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab.
51 double blind procedure/
52 parallel group$1.ti,ab.
53 (crossover or cross over).ti,ab.
54 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 orintervention$1 or patient$1 or subject$1 or participant$1)).ti,ab.
55 (assigned or allocated).ti,ab.
56 (controlled adj7 (study or design or trial)).ti,ab.
57 (volunteer or volunteers).ti,ab.
58 trial.ti.
59 or/41‐58
60 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.)
61 59 not 60
62 40 and 61
EBSCO CINAHL Plus
S62 S38 AND S61
S61 S60 NOT S59
S60 S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53
S59 S57 NOT S58
S58 MH (human)
S57 S54 OR S55 OR S56
S56 TI (animal model*)
S55 MH (animal studies)
S54 MH animals+
S53 AB (cluster W3 RCT)
S52 MH (crossover design) OR MH (comparative studies)
S51 AB (control W5 group)
S50 PT (randomized controlled trial)
S49 MH (placebos)
S48 MH (sample size) AND AB (assigned OR allocated OR control)
S47 TI (trial)
S46 AB (random*)
S45 TI (randomised OR randomized)
S44 MH cluster sample
S43 MH pretest‐posttest design
S42 MH random assignment
S41 MH single‐blind studies
S40 MH double‐blind studies
S39 MH randomized controlled trials
S38 S3 AND S37
S37 S15 OR S16 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36
S36 TI ( ((vacuum N1 assist*) or (vacuum N1 therap*) or VAC) ) OR AB ( ((vacuum N1 assist*) or (vacuum N1 therap*) or VAC) )
S35 TI ( (negative pressure or TNP or NPWT) ) OR AB ( (negative pressure or TNP or NPWT) )
S34 (MH "Negative Pressure Wound Therapy")
S33 TI PRP OR AB PRP
S32 TI (platelet* N1 plasma) OR AB (platelet* N1 plasma)
S31 (MH "Platelet‐Rich Plasma+")
S30 TI ( (topical N1 (agent* or preparation* or therap* or treatment*)) ) OR AB ( (topical N1 (agent* or preparation* or therap* or treatment*)) )
S29 TI ( (ointment* or lotion* or cream* or powder* or gel or gels) ) OR AB ( (ointment* or lotion* or cream* or powder* or gel or gels) )
S28 (MH "Ointments")
S27 TI antiseptic* OR AB antiseptic*
S26 TI ( ((polyhexamethylene N1 biguanide) or PHMB or polyhexanide or prontosan) ) OR AB ( ((polyhexamethylene N1 biguanide) or PHMB or polyhexanide or prontosan) )
S25 TI biguanide* OR AB biguanide*
S24 TI ( (iodosorb or actiformcool or aquaflo or flamazine or silvadene) ) OR AB ( (iodosorb or actiformcool or aquaflo or flamazine or silvadene) )
S23 TI ( ((topical or applicat*) N2 (metronidazole or antibiotic* or antimicrobial* or antibacterial* or iodine or collagen* or phenytoin or zinc or phenol)) ) OR AB ( ((topical or applicat*) N2 (metronidazole or antibiotic* or antimicrobial* or antibacterial* or iodine or collagen* or phenytoin or zinc or phenol)) )
S22 S16 AND S21
S21 S17 OR S18 OR S19 OR S20
S20 (MH "Zinc Oxide")
S19 (MH "Iodophors+")
S18 (MH "Metronidazole")
S17 (MH "Antibiotics+")
S16 (MH "Administration, Topical+")
S15 (MH "Antiinfective Agents, Local+")
S14 S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13
S13 TI ((odour or odor) N3 absorb*) ) OR AB ( ((odour or odor) N3 absorb*)
S12 TI (wound N2 pack*) OR AB (wound N2 pack*)
S11 TI ( (dressing* or pad or pads or gauze or tulle or film or bead or foam* or non‐adherent or (non N1 adherent) or hydrocolloid* or (sodium N1 hyaluronate) or alginate* or hydrogel* or silver* or honey* or matrix or iodine* or (protease N1 modulat*) or (capillary N1 action) or silicon* or polymer* or polyurethane* or hydropolymer* or carboxymethylcellulose or carboxymethyl‐cellulose or NaCMC or (gel N1 forming) or gel‐forming) ) OR AB ( (dressing* or pad or pads or gauze or tulle or film or bead or foam* or non‐adherent or (non N1 adherent) or hydrocolloid* or (sodium N1 hyaluronate) or alginate* or hydrogel* or silver* or honey* or matrix or iodine* or (protease N1 modulat*) or (capillary N1 action) or charcoal or silicon* or polymer* or polyurethane* or hydropolymer* or carboxymethylcellulose or carboxymethyl‐cellulose or NaCMC or (gel N1 forming) or gel‐forming) )
S10 (MH "Polyurethanes")
S9 (MH "Silicones+")
S8 (MH "Silver Sulfadiazine")
S7 (MH "Silver")
S6 (MH "Honey")
S5 (MH "Alginates")
S4 (MH "Postoperative Care+")
S3 S1 OR S2
S2 TI pilonidal OR AB pilonidal
S1 (MH "Pilonidal Cyst")
US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov)
dressing OR pad OR gauze OR tulle OR film OR bead OR foam OR hydrocolloid OR alginate OR hydrogel OR silver OR honey OR iodine OR silicone OR polyurethane OR gel OR topical OR plasma OR anti bacterial | Pilonidal Sinus
dressing OR pad OR gauze OR tulle OR film OR bead OR foam OR hydrocolloid OR alginate OR hydrogel OR silver OR honey OR iodine OR silicone OR polyurethane OR gel OR topical OR plasma OR anti bacterial | Pilonidal
World Health Organization International Clinical Trials Registry Platform
pilonidal [Condition] Dressing or pad or gauze or tulle or film or bead or foam or hydrocolloid or alginate or hydrogel or silver or honey or iodine or silicone or polyurethane or gel or topical or plasma or anti bacterial [Intervention]
Appendix 2. Risk of bias assessment (individually randomised controlled trials)
1. Was the allocation sequence randomly generated?
Low risk of bias
The investigators describe a random component in the sequence generation process such as:
referring to a random number table;
using a computer random number generator;
coin tossing;
shuffling cards or envelopes;
throwing dice;
drawing of lots.
High risk of bias
The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example:
sequence generated by odd or even date of birth;
sequence generated by some rule based on date (or day) of admission;
sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process provided to permit a judgement of low or high risk of bias.
2. Was the treatment allocation adequately concealed?
Low risk of bias
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation:
central allocation (including telephone, web‐based and pharmacy‐controlled randomisation);
sequentially‐numbered drug containers of identical appearance;
sequentially‐numbered, opaque, sealed envelopes.
High risk of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on:
use of an open random allocation schedule (e.g. a list of random numbers);
assignment envelopes without appropriate safeguards (e.g. envelopes were unsealed, non‐opaque, or not sequentially numbered);
alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear
Insufficient information provided to permit a judgement of low or high risk of bias. This is usually the case if the method of concealment is not described, or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
3. Blinding ‐ was knowledge of the allocated interventions adequately prevented during the study?
Low risk of bias
Any one of the following:
no blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding;
blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken; or
either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others was unlikely to introduce bias.
High risk of bias
Any one of the following:
no blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding;
blinding of key study participants and personnel attempted, but likely that the blinding could have been broken;
either participants or some key study personnel were not blinded, and the non‐blinding of others was likely to introduce bias; or
unclear.
Either of the following:
insufficient information to permit judgement of low or high risk of bias; or
the study did not address this outcome.
4. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following:
no missing outcome data;
reasons for missing outcome data are unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias);
missing outcome data are balanced in numbers across intervention groups, with similar reasons for missing data across groups;
for dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk is not enough to have a clinically relevant impact on the intervention effect estimate;
for continuous outcome data, a plausible effect size (difference in means or standardised difference in means) among missing outcomes is not enough to have a clinically relevant impact on the observed effect size; or
missing data have been imputed using appropriate methods.
High risk of bias
Any one of the following:
reasons for missing outcome data are likely to be related to the true outcome, with either an imbalance in numbers or reasons for missing data across intervention groups;
for dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk is enough to induce clinically relevant bias in the intervention effect estimate;
for continuous outcome data, a plausible effect size (difference in means or standardised difference in means) among missing outcomes is enough to induce a clinically relevant bias in the observed effect size;
'as‐treated' analysis done with a substantial departure of the intervention received from that assigned at randomisation; or
potentially inappropriate application of simple imputation.
Unclear
Either of the following:
insufficient reporting of attrition/exclusions to permit a judgement of low or high risk of bias (e.g. number randomised not stated, no reasons for missing data provided); or
the study did not address this outcome.
5. Are reports of the study free of suggestion of selective outcome reporting?
Low risk of bias
Either of the following:
the study protocol is available and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way; or
the study protocol is not available, but it is clear that the published reports include all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon).
High risk of bias
Any one of the following:
not all of the study’s prespecified primary outcomes have been reported;
one or more primary outcomes were reported using measurements, analysis methods, or subsets of the data (e.g. subscales) that were not prespecified;
one or more reported primary outcomes were not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect);
one or more outcomes of interest in the review were reported incompletely so that they cannot be entered in a meta‐analysis; or
the study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information provided to permit a judgement of low or high risk of bias. It is likely that the majority of studies will fall into this category.
6. Other sources of potential bias
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important risk of bias. For example, the study:
had a potential source of bias related to the specific study design used; or
has been claimed to have been fraudulent; or
had some other problem.
Unclear
There may be a risk of bias, but there is either:
insufficient information to assess whether an important risk of bias exists; or
insufficient rationale or evidence that an identified problem will introduce bias.
Appendix 3. Risk of bias (cluster‐randomised controlled trials)
In cluster‐randomised trials, particular biases to consider include: recruitment bias; baseline imbalance; loss of clusters; incorrect analysis; and comparability with individually randomised trials.
Recruitment bias can occur when individuals are recruited to the trial after the clusters have been randomised, as the knowledge of whether each cluster is an ‘intervention’ or ‘control’ cluster could affect the types of participants recruited.
Cluster‐randomised trials often randomise all clusters at once, so lack of concealment of an allocation sequence should not usually be an issue. However, because small numbers of clusters are randomised, there is a possibility of chance baseline imbalance between the randomised groups, in terms of either the clusters or the individuals. Although not a form of bias as such, the risk of baseline differences can be reduced by using stratified or pair‐matched randomisation of clusters. Reporting of the baseline comparability of clusters, or statistical adjustment for baseline characteristics, can help reduce concern about the effects of baseline imbalance.
Occasionally, complete clusters are lost from a trial, and have to be omitted from the analysis. Just as for missing outcome data in individually randomised trials, this may lead to bias. In addition, missing outcomes for individuals within clusters may lead to a risk of bias in cluster‐randomised trials.
Many cluster‐randomised trials are analysed by incorrect statistical methods, not taking the clustering into account. Such analyses create a ‘unit of analysis error’ and produce over‐precise results (the standard error of the estimated intervention effect is too small) and P values that are too small. They do not lead to biased estimates of effect. However, if they remain uncorrected, they will receive too much weight in a meta‐analysis.
In a meta‐analysis including both cluster‐ and individually randomised trials, or including cluster‐randomised trials with different types of clusters, possible differences between the intervention effects being estimated need to be considered. For example, in a vaccine trial of infectious diseases, a vaccine applied to all individuals in a community would be expected to be more effective than if the vaccine was applied to only half of the people. Another example is provided by a Cochrane review of hip protectors (Hahn 2005). The cluster trials showed a large positive effect, whereas individually randomised trials did not show any clear benefit. One possibility is that there was a ‘herd effect’ in the cluster‐randomised trials (which were often performed in nursing homes, where compliance with using the protectors may have been enhanced). In general, such ‘contamination’ would lead to underestimates of effect. Thus, if an intervention effect is still demonstrated despite contamination in those trials that were not cluster‐randomised, a confident conclusion about the presence of an effect can be drawn. However, the size of the effect is likely to be underestimated. Contamination and ‘herd effects’ may be different for different types of cluster.
Data and analyses
Comparison 1. Topical negative pressure wound therapy versus conventional open wound healing therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Time to wound healing (days) | 1 | 19 | Mean Difference (IV, Random, 95% CI) | ‐24.01 [‐35.65, ‐12.37] |
| 1.2 Proportion of wounds healed at 30 days | 1 | 19 | Risk Ratio (M‐H, Random, 95% CI) | 3.60 [0.49, 26.54] |
| 1.3 Time to return to normal activities (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.4 Recurrence | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.12 [0.35, 28.00] |
Comparison 2. Zinc oxide mesh versus placebo mesh.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Proportion of wounds healed at 30 days | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
Comparison 3. Lietofix cream versus iodoform dressing.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Proportion of wounds healed at 30 days | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
Comparison 4. Hydrogel dressing versus wound cleaning with 10% povidone iodine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Time to wound healing | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.2 Surgical site infection | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
Comparison 5. Platelet‐rich plasma versus absorbent sterile cotton gauze.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Time to wound healing (days) | 2 | 210 | Mean Difference (IV, Random, 95% CI) | ‐19.63 [‐34.69, ‐4.57] |
| 5.2 Time to return to normal daily activities (days) | 2 | 210 | Mean Difference (IV, Random, 95% CI) | ‐15.49 [‐28.95, ‐2.02] |
Comparison 6. Gentamicin‐impregnated collagen sponge versus no dressing.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Time to wound healing (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
Comparison 7. Dialkylcarbamoyl chloride (DACC)‐coated dressing versus alginate dressing.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Proportion of wounds healed | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agren 2005.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT Sample size: 64 Country: Denmark Setting: secondary care Date conducted: February 2002‐May 2004 Surgical technique: wide excision down to pre‐sacral fascia after methylene blue injection Duration of follow‐up: 90 days |
|
| Participants |
Inclusion criteria Adults aged ≥18 First surgical intervention for sacrococcygeal pilonidal sinus disease Either abscess or chronic disease included Exclusion criteria Zinc hypersensitivity Unable to consent Pregnancy or lactating Baseline characteristics of intervention group Median age (IQR): 26 (22‐31) years Sex (no. of males/females): 27/6 Median BMI (IQR): 25 (23‐29) kg/m2 Baseline characteristics of control group Median age (IQR): 25 (21‐32) years Sex (no. of males/females) 26/5 Median BMI (IQR): 27 (24.0‐29) kg/m2 |
|
| Interventions |
Intervention group Topical zinc oxide mesh Hydrofiber dressing 33 participants randomised, 3 withdrawals (reasons not stated) Control group Placebo mesh Hydrofiber dressing 31 participants randomised, 2 withdrawals (reasons not stated) |
|
| Outcomes |
Primary outcome Time to complete wound healing Secondary outcomes Need for post‐operative antibiotics Reoperation Pain (at day 7 post‐operatively) Adverse effects |
|
| Notes | Funding: Danish Medical Research Council and The Pharmacy Foundation Authors contacted: we did not contact the study authors for further information. Publication status: published |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The patient allocation sequence was computer‐generated 1: 1 in variable block sizes of four or six stratified for centre" |
| Allocation concealment (selection bias) | Low risk | "Allocation concealment was performed using centrally packaged, consecutively numbered, identical packages containing zinc oxide or placebo meshes. The investigators were asked to use the next available number when a new patient entered the trial." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The zinc and placebo meshes were manufactured in Class 100,000 facilities, sterile, and indistinguishable in colour, texture, and smell." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The wound was evaluated clinically with respect to complete wound closure by assessors blinded to treatment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | < 10% dropout, equally spaced between groups, ITT analysis |
| Selective reporting (reporting bias) | Low risk | ISRCTN35311675 and main results reported |
| Other bias | High risk | Double the rate of smokers in intervention group compared with the placebo group, may have impacted on wound healing |
Banasiewicz 2013.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT Sample size: 19 Country: Poland Setting: secondary care Date conducted: "The study was conducted in 2012" Surgical technique: wide excision after methylene blue injection Duration of follow‐up: until all wounds healed |
|
| Participants |
Inclusion criteria All ages Only chronic disease (acute abscess excluded) Primary or recurrent disease included Exclusion criteria None stated Baseline characteristics of intervention group Mean age (SEM): 24 (4) years Sex (no. of males/females): 10/0 BMI: not stated Baseline characteristics of control group Mean age (SEM): 23 (4) years Sex (no. of males/females): 9/0 BMI: not stated |
|
| Interventions |
Intervention group TNPWT: KCI VAC freedom system 10 participants randomised, 0 withdrawals Control group Conventional absorbent dressing 9 participants randomised, 0 withdrawals |
|
| Outcomes |
Primary outcome Number of days the participant was under the care of the outpatient department Secondary outcomes Number of days until resumption of normal daily activity Pain (at several time points) Total number of visits to the outpatient department |
|
| Notes | Funding: not specified Authors contacted: we contacted the study authors for further information and received further data, which is included in this review. Publication status: published |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of method of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No description to allow categorising of potential risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding undertaken |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment undertaken |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete outcome data presented |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | Balanced groups, no other sources of potential bias detected |
Berry 1996.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT Sample size: 20 Country: UK Setting: secondary care Date conducted: not reported Surgical technique: "standard sacrococcygeal pilonidal sinus excision wounds" Duration of follow‐up: until all wounds healed |
|
| Participants |
Inclusion criteria None stated Exclusion criteria None stated Baseline characteristics of intervention group Mean age: 27 years Sex (no. of males/females): 7/3 BMI: not stated Baseline characteristics of control group Mean age: 28 years Sex (no. of males/females): 8/2 BMI: not stated |
|
| Interventions |
Intervention group Polyurethane foam hydrophilic dressing (Allevyn cavity wound dressing) Polyurethane foam sheet dressing 10 participants randomised, 3 withdrawals (1 due to discomfort from wound biopsies, 1 due to recurrent wound infection, 1 due to need for further surgery) Control group Calcium sodium alginate dressing (Kaltostat) Polyurethane foam sheet dressing 10 participants randomised, 3 withdrawals (1 due to discomfort from wound biopsies, 2 due to recurrent wound infection) |
|
| Outcomes | Clinician rating of ease of use Clinician rating of dressing performance Wound histology Time to wound healing |
|
| Notes | Funding: industry funded (Smith and Nephew) Authors contacted: we did not contact the study authors for further information. Publication status: published |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of method of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No description to allow categorising of potential risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding undertaken |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment undertaken |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 30% dropout rate, introduction states ITT analysis but unclear if this was actually performed on the results |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | High risk | Several imbalances in group characteristics at baseline (participant weight and wound size) |
Biter 2014.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT Sample size: 49 Country: Netherlands Setting: secondary care Date conducted: October 2009‐May 2012 Surgical technique: wide excision down to bone if required after methylene blue injection Duration of follow‐up: 6 months |
|
| Participants |
Inclusion criteria Symptomatic sacrococcygeal pilonidal sinus; 1st attempt at excisional surgery Exclusion criteria Age < 16 Previous excisional surgery Wound < 3 cm from anus Baseline characteristics of intervention group Mean age: 23 years Sex (no. of males/females): 18/6 BMI: not stated Baseline characteristics of control group Median age: 20 years Sex (no. of males/females): 23/2 BMI: not stated |
|
| Interventions |
Intervention group TNPWT 24 participants randomised, 6 withdrawals (2 technically unable to fit TNPWT device, 2 due to pain, 1 due to smell, 1 due to "practical considerations") Control group Silicone dressing 25 participants randomised, 2 withdrawals (reason not provided) |
|
| Outcomes |
Primary outcome Time to complete wound healing Secondary outcomes Wound size ratio at day 14 Pain at day 14 Time to resume usual daily activities Recurrence |
|
| Notes | Funding: not specified Authors contacted: we contacted the study authors for further information but received no response. Publication status: published |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated randomisation" |
| Allocation concealment (selection bias) | Unclear risk | No description to allow categorising of potential risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding undertaken |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment undertaken |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout rate |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | High risk | Control group older and far more male participants |
Giannini 2019.
| Study characteristics | ||
| Methods | Study design: parallel‐RCT Sample size: 205 Country: Italy Setting: secondary care Date conducted: April 2016‐December 2017 Surgical technique: wide excision down to bone if required Duration of follow‐up: 60 days |
|
| Participants |
Inclusion criteria Chronic sacrococcygeal pilonidal sinus; 1st attempt at excisional surgery Exclusion criteria Cancer, HIV, diabetes, pregnancy, Crohn's disease, liver disease, allergy to Lietofix, recurrent disease, steroids, previous radiotherapy to sacrococcygeal area, wound >15 cm long Baseline characteristics of intervention group Mean age: 24 years Sex (no. of males/females): 62/41 BMI: not stated Baseline characteristics of control group Mean age: 26 years Sex (no. of males/females): 61/22 BMI: not stated |
|
| Interventions |
Intervention group Lietofix cream 103 participants randomised, 5 withdrawals (reasons not provided) Control group Iodoform dressing 83 participants randomised, 2 withdrawals (reasons not provided) |
|
| Outcomes | Pain at multiple time points Grade of healing at multiple time points Time to wound healing |
|
| Notes | Funding: not specified Authors contacted: we contacted the study authors for further information and received a response, but the information we required was not available. Publication status: published |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The study population was randomised into two arms (group A and group B) using a dedicated computer program" |
| Allocation concealment (selection bias) | High risk | "Received the randomisation list by e‐mail" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding undertaken |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "At each follow‐up, an independent observer, blinded to the assigned treatment, recorded patients’ symptoms" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low numbers of dropouts |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | High risk | More male participants in the control group |
Gohar 2020.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT Sample size: 100 Country: Egypt Setting: secondary care Date conducted: December 2018‐December 2019 Surgical technique: wide excision down to presacral fascia after methylene blue injection Duration of follow‐up: until all wounds healed |
|
| Participants |
Inclusion criteria All people with chronic pilonidal sinus disease including recurrent cases Exclusion criteria Acute abscess, diabetes mellitus, anaemia, concurrent use of anticoagulant, platelet count < 105/uL, wound cavity > 35 cc Baseline characteristics of intervention group Mean age: 25 years Sex (no. of males/females): 40/10 BMI: not stated Baseline characteristics of control group Mean age: 26 years Sex (no. of males/females): 43/7 BMI: not stated |
|
| Interventions |
Intervention group PRP 60 participants randomised, 10 withdrawals (7 excluded by study team as met exclusion criteria of wound volume > 35 cc, 2 excluded due to "change of the operative decision", 1 lost to follow‐up) Control group Absorbent sterile cotton gauze 60 participants randomised, 10 withdrawals (8 excluded by study team as met exclusion criteria of wound volume > 35 cc, 2 lost to follow‐up) |
|
| Outcomes | Wound volume at various time points Time to wound healing Pain at various time points Time to return to work Surgical site infections Duration of use of analgesia |
|
| Notes | Funding: not specified Authors contacted: we did not contact the study authors for further information. Publication status: published |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of method of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No description to allow categorising of potential risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | > 10% dropouts in both arms |
| Selective reporting (reporting bias) | Unclear risk | Retrospective trial registration NCT04430413. |
| Other bias | Low risk | Balanced groups, no other sources of potential bias detected |
Kayaoglu 2006.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT Sample size: 31 Country: Turkey Setting: secondary care Date conducted: January 2003‐October 2005 Surgical technique: not described Duration of follow‐up: until all wounds healed |
|
| Participants |
Inclusion criteria Not described Exclusion criteria Recurrent disease Cases in which the infection continued despite the antibiotic treatment Diabetes mellitus People with conditions that lead to delay in wound healing, such as the use of immunosuppressive drugs. Baseline characteristics of intervention group Mean age: 28 years Sex (no. of males/females): 14/2 BMI: not stated Baseline characteristics of control group Mean age: 26 years Sex (no. of males/females): 14/1 BMI: not stated |
|
| Interventions |
Intervention group Hydrogel dressing Control group Wound cleaning with 10% povidone iodine Unclear how many participants were randomised to each group. 16 participants in intervention group included in the analysis (after an unspecified number of withdrawals) and 15 participants in the control group included (after an unspecified number of withdrawals) |
|
| Outcomes | Time to wound healing Cost Recurrence |
|
| Notes | Funding: not specified Authors contacted: we did not contact the study authors for further information. The article was published in Turkish and we translated it to English using Google Translate. Publication status: published |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The patients were randomly divided into two groups by the envelope method" |
| Allocation concealment (selection bias) | Unclear risk | No description to allow categorising of potential risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Significant numbers of dropouts (6 exclusions from 37). Allocation of dropouts not specified. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | Balanced groups, no other sources of potential bias detected |
Mohammadi 2017.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT Sample size: 110 Country: Iran Setting: secondary care Date conducted: June 2012‐September 2015 Surgical technique: wide excision down to level of presacral fascia Duration of follow‐up: until all wounds healed |
|
| Participants |
Inclusion criteria All people with pilonidal sinus disease scheduled for surgery Exclusion criteria Low platelet count Anaemia Cytotoxic chemotherapy or radiotherapy in the last 3 months Growth factor therapy Diabetes mellitus Coeliac disease Baseline characteristics of intervention group Mean age: 30 years Sex (no. of males/females): 54/1 Mean BMI: 25 kg/m2 Baseline characteristics of intervention group Mean age: 27 years Sex (no. of males/females): 52/3 Mean BMI: 25 kg/m2 |
|
| Interventions |
Intervention group PRP gel 55 participants randomised, 0 withdrawals Control group Absorbent sterile cotton gauze 55 participants randomised, 0 withdrawals |
|
| Outcomes | Time to wound healing Time to return to usual daily activities Duration of postoperative pain Antibiotic use Histology |
|
| Notes | Funding: university funded Authors contacted: we did not contact the study authors for further information. Publication status: published |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "They were then allocated into the control and PRP treatment parallel groups by random selection using randomly permuted blocks method (block size was two, but the investigators were blind)." |
| Allocation concealment (selection bias) | Low risk | "They were then allocated into the control and PRP treatment parallel groups by random selection using randomly permuted blocks method (block size was two, but the investigators were blind)." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding undertaken |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear. Early in the methods the phrase "the investigators were blind" is used, however it is unclear whether this pertains just to treatment allocation or also to outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "no individual of either group left the trial during the study." |
| Selective reporting (reporting bias) | High risk | IRCT2016020418842N11: QoL not reported but was primary outcome |
| Other bias | Low risk | Balanced groups, no other sources of potential bias detected |
Ozbalci 2014.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT Sample size: 50 Country: Turkey Setting: secondary care Date conducted: January 2011‐December 2012 Surgical technique: wide excision down to level of presacral fascia after methylene blue injection. Wound then marsupialised. Duration of follow‐up: until all wounds healed |
|
| Participants |
Inclusion criteria All patients Exclusion criteria Diabetes mellitus Baseline characteristics of intervention group Mean age: 26 years Sex (no.of males/females): 22/3 BMI: not stated Baseline characteristics of control group Mean age: 27 years Sex (no.of males/females): 18/7 BMI: not stated |
|
| Interventions |
Intervention group Gentamicin‐impregnated collagen sponge 25 participants randomised, 0 withdrawals Control group No intervention 25 participants randomised, 0 withdrawals |
|
| Outcomes | Wound size on day 7 and day 15 Time to complete wound healing Complications Infections Recurrence |
|
| Notes | Funding: not specified Authors contacted: we did not contact the study authors for further information. Publication status: published |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of method of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No description to allow categorising of potential risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding undertaken |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment undertaken |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | High risk | More male participants in the intervention group |
Romain 2020.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT Sample size: 238 Country: France Setting: secondary care Date conducted: December 2013‐September 2017 Surgical technique: wide excision down to level of presacral fascia after methylene blue injection Duration of follow‐up: 120 days (mean) |
|
| Participants |
Inclusion criteria Adults Exclusion criteria Current chemotherapy, uncontrolled hypertension, life expectancy < 12 months, acute cardiovascular disease, intolerance to one of the interventions, uncontrolled diabetes Active infection of pilonidal sinus. Baseline characteristics of intervention group Mean age: 26 years Sex (no. of males/females): 82/36 Mean BMI: 26 kg/m2 Baseline characteristics of control group Mean age: 26 years Sex (no. of males/females): 81/39 Mean BMI: 26 kg/m2 |
|
| Interventions |
Intervention group DACC‐coated dressing 120 participants randomised, 17 withdrawals (2 did not receive intervention, 9 lost to follow‐up, 6 discontinued intervention) Control group Alginate dressing 126 participants randomised, 29 withdrawals (6 did not receive intervention, 7 lost to follow‐up, 16 discontinued intervention) |
|
| Outcomes |
Primary outcome Wound healing at 75 days Secondary outcomes Wound healing at defined time points Pain at defined time points Time to return to usual activities |
|
| Notes | Funding: not specified Authors contacted: we did not contact the study authors for further information. Publication status: published |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomisation list was computer‐generated by an investigator with no clinical involvement in the trial" |
| Allocation concealment (selection bias) | Low risk | "The list used blocks of eight and was stratified by centre. After the surgeon had obtained the patient’s consent, they telephoned a contact at the clinical investigation centre of Strasbourg University Hospital who was independent of the recruitment process for allocation consignment." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No use of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment undertaking |
| Incomplete outcome data (attrition bias) All outcomes | High risk | >10% dropout in both arms |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available |
| Other bias | Low risk | No other sources of bias identified |
Viciano 2000.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT Sample size: 38 Country: Spain Setting: secondary care Date conducted: not recorded Surgical technique: wide excision down to level of presacral fascia Duration of follow‐up: until all wounds healed |
|
| Participants |
Inclusion criteria Adults Exclusion criteria Acute pilonidal abscess Baseline characteristics Baseline characteristics provided for all participants but not for each group Mean age: 24 years Sex (no. of males/females): 31/7 BMI: data not provided |
|
| Interventions |
Intervention group Hydrocolloid dressing 23 participants were randomised, 0 withdrawals Control group Iodine dressing 15 participants were randomised, 0 withdrawals |
|
| Outcomes | Pain at various time points Time to wound healing |
|
| Notes | Funding: not specified Authors contacted: we did not contact the study authors for further information Publication status: published |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information given on method of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information given on dropouts |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | Study and participants not described in sufficient detail to judge any other sources of bias |
BMI: body mass index; DACC: dialkylcarbamoyl chloride; IQR: interquartile range; ITT: intention to treat; PRP: platelet‐rich plasma; QoL: quality of life; RCT: randomised controlled trial; SEM: standard error of mean; TNPWT: topical negative pressure wound therapy; VAC: vacuum‐assisted closure
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cherkasov 2016 | Non‐randomised study |
| Panahi 2015 | Not relevant: not a study of sacrococcygeal pilonidal wounds |
| Rao 2010 | RCT comparing wound healing by primary wound closure versus healing by secondary intention |
| Sadati 2019 | Used different co‐interventions making comparison impossible: the treatment regimen in one group consisted of a combination of a hydrogel and hydrocolloid, switching to an alginate and hydrocolloid from the second week postoperatively. |
| Yetim 2010 | Study of wounds closed primarily with different techniques; not a study of the management of open wounds. |
RCT: randomised controlled trial
Differences between protocol and review
1) We have included updated methods used to analyse pain outcomes (Doleman 2018; Doleman 2020)
2) We decided that significant differences in baseline characteristics between groups constituted a high risk of "other" bias and judged this accordingly.
Contributions of authors
Philip J Herrod: conceived the review; designed the review; coordinated the review; extracted data; checked quality of data extraction; analysed or interpreted data; undertook quality assessment; checked quality assessment; performed statistical analysis; checked quality of statistical analysis; produced the first draft of the review; contributed to writing or editing the review; advised on the review; wrote to study authors/experts/companies; performed translations; approved final review prior to submission; is guarantor of the review.
Brett Doleman: conceived the review; designed the review; checked quality of data extraction; analysed or interpreted data; checked quality assessment; checked quality of statistical analysis; produced the first draft of the review; contributed to writing or editing the review; advised on the review; approved final review prior to submission.
Edward J Hardy: conceived the review; designed the review; extracted data; checked quality of data extraction; analysed or interpreted data; undertook quality assessment; checked quality assessment; produced the first draft of the review; contributed to writing or editing the review; advised on the review; approved final review prior to submission.
Paul Hardy: conceived the review; designed the review; extracted data; contributed to writing or editing the review; advised on the review; approved final review prior to submission.
Trevor Maloney: conceived the review; designed the review; extracted data; contributed to writing or editing the review; advised on the review; approved final review prior to submission.
John P Williams: conceived the review; designed the review; contributed to writing or editing the review; advised on the review; approved final review prior to submission.
Jon N Lund: conceived the review; designed the review; contributed to writing or editing the review; advised on the review; approved final review prior to submission.
Contributions of the editorial base
Gill Norman (Editor): edited the protocol and the review; advised on methodology, interpretation and content; approved the final version prior to submission. Gill Rizzello (Managing Editor): coordinated the editorial process; advised on content; edited the protocol and the review. Sophie Bishop (Information Specialist): designed the search strategy, edited the search methods section and ran the searches. Tom Patterson (Editorial Assistant): edited the reference sections and drafted the plain language summary.
Sources of support
Internal sources
No sources of support provided
External sources
-
National Institute for Health Research (NIHR), UK
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Wounds. The views and opinions expressed are those of the authors and not necessarily those of the NIHR, NHS or the Department of Health and Social Care.
Declarations of interest
Philip J Herrod: I work as a health professional. I received a two‐year research fellowship jointly awarded by the Royal College of Surgeons of England and the Dunhill Medical Trust in 2016. I have published several articles on the management of pilonidal sinus disease previously, however none are cited in this review. I have also recently co‐authored a chapter on pilonidal sinus disease for the next edition of the Oxford Textbook of Surgery
Brett Doleman: I have received a grant from the Association of Anaesthetists of Great Britain and Ireland (AAGBI) for a randomised controlled trial of preventative paracetamol and have previously undertaken a meta‐analysis of preventative paracetamol.
Edward J Hardy: none known.
Paul Hardy: I work as a health professional. I have carried out consultancy for Smith & Nephew. The funding for this was not received by me personally, and I did not benefit from this payment or have access to the funding.
Trevor Maloney: I work as a health professional. Conference fees and travel and accommodation expenses were paid on my behalf by Convatec PLC in 2017. This funding was not paid directly to me or my host institution.
John P Williams: I work as a health professional.
Jon N Lund: I work as a health professional.
New
References
References to studies included in this review
Agren 2005 {published data only}
- Agren MS, Ostenfeld U, Kallehave F, Gong Y, Raffn K, Crawford ME, et al.A randomized, double-blind, placebo-controlled multicenter trial evaluating topical zinc oxide for acute open wounds following pilonidal disease excision. Wound Repair and Regeneration 2006;14:526-35. [DOI] [PubMed] [Google Scholar]
Banasiewicz 2013 {published data only}
- Banasiewicz T, Bobkiewicz A, Borejsza-Wysocki M, Biczysko M, Ratajczak A, Malinger S, et al.Portable VAC therapy improve the results of the treatment of the pilonidal sinus – randomized prospective study. Polish Journal of Surgery 2013;85:371-6. [DOI] [PubMed] [Google Scholar]
Berry 1996 {published data only}
- Berry DP, Harding KG.Dressings for treating cavity wounds. Journal of Wound care 1996;5:10-3. [DOI] [PubMed] [Google Scholar]
Biter 2014 {published data only}
- Biter LU, Beck GM, Mannaerts GH, Stok MM, Van der Ham AC, Grotenhuis BA.The use of negative-pressure wound therapy in pilonidal sinus disease: a randomized controlled trial comparing negative-pressure wound therapy versus standard open wound care after surgical excision. Diseases of the Colon and Rectum 2014;57:1406-11. [DOI] [PubMed] [Google Scholar]
Giannini 2019 {published data only}
- Giannini I, Andreoli R, Bianchi FP, Cavallaro V, Corno F, Geccherle A, et al.Effectiveness of topical use of Lietofix® in wound healing after pilonidalis sinus excision: a multicenter study by the Italian Society of Colorectal Surgery (SICCR). Techniques in Coloproctology 2019;23:373-8. [DOI] [PubMed] [Google Scholar]
Gohar 2020 {published data only}
- Gohar MM, Ali RF, Ismail KA, Nosair NA.Assessment of the effect of platelet rich plasma on the healing of operated sacrococcygeal pilonidal sinus by lay-open technique: a randomized clinical trial. BMC Surgery 2020;20:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kayaoglu 2006 {published data only}
- Kayaoglu HA, Ozkan N, Ersoy OF, Celik A.The effect of hydrogel use on wound healing in pilonidal sinus patients undergoing surgical therapy by lay open technique [Sekonder iyileşmeye bırakılan pilonidal sinüs olgularında hidrojel kullanımının yara iyileşmesi üzerine etkisi]. Turkish Journal of Surgery 2006;22:26-9. [Google Scholar]
Mohammadi 2017 {published data only}
- Mohammadi M, Nasiri S, Mohammadi MH, Mohammadi AM, Nikbakht M, Panah MZ, et al.Evaluation of platelet-rich plasma gel potential in acceleration of wound healing duration in patients underwent pilonidal sinus surgery: a randomized controlled parallel clinical trial. Transfusion and Apheresis Science 2017;56:226-32. [DOI] [PubMed] [Google Scholar]
Ozbalci 2014 {published data only}
- Ozbalci GS, Tuncal S, Bayraktar K, Tasova V, Akkus MA.Is gentamicin-impregnated collagen sponge to be recommended in pilonidal sinus patient treated with marsupialization? A prospective randomized study. Annali Italiani di Chirurgia 2014;85:576-82. [PubMed] [Google Scholar]
Romain 2020 {published data only}
- Romain B, Mielcarek M, Delhorme JB, Meyer N, Brigand C, Rohr S.Dialkylcarbamoyl chloride-coated versus alginate dressings after pilonidal sinus excision: a randomized clinical trial (SORKYSA study). BJS Open 2020;4:225-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Viciano 2000 {published data only}
- Viciano V, Castera JE, Medrano J, Aguilo J, Torro J, Botella MG, et al.Effect of hydrocolloid dressings on healing by second intention after excision of pilonidal sinus. European Journal of Surgery 2000;166:229-32. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Cherkasov 2016 {published data only}
- Cherkasov MF, Galashokyan KM, Startsev YM, Cherkasov DM, Melikova SG.Comparative study of treatment methods of pilonidal sinus. New Armenian Medical Journal 2016;4:61-5. [Google Scholar]
Panahi 2015 {published data only}
- Panahi Y, Izadi M, Sayyadi N, Rezaee R, Jonaidi-Jafari N, Beiraghdar F, et al.Comparative trial of aloe vera/olive oil combination cream versus phenytoin cream in the treatment of chronic wounds. Journal of Wound Care 2015;24:459-65. [DOI] [PubMed] [Google Scholar]
Rao 2010 {published data only}
- Rao MM, Zawislak W, Kennedy R, Gilliand R.A prospective randomised study comparing two treatment modalities for chronic pilonidal sinus with a 5-year follow-up. International Journal of Colorectal Disease 2010;25:395-400. [DOI] [PubMed] [Google Scholar]
Sadati 2019 {published data only}
- Sadati L, Froozesh R, Beyrami A, Khaneghah ZN, Elahi SA, Asl MF, et al.A comparison of three dressing methods for pilonidal sinus surgery wound healing. Advances in Skin and Wound Care 2019;32:1-5. [DOI] [PubMed] [Google Scholar]
Yetim 2010 {published data only}
- Yetim I, Ozkan OV, Dervisoglu A, Erzurumlu K, Canbolant E.Effect of gentamicin-absorbed collagen in wound healing in pilonidal sinus surgery: a prospective randomized study. Journal of International Medical Research 2010;38:1029-33. [DOI] [PubMed] [Google Scholar]
Additional references
Al‐Khamis 2010
- Al-Khamis A, McCallum I, King PM, Bruce J.Healing by primary versus secondary intention after surgical treatment for pilonidal sinus. Cochrane Database of Systematic Reviews 2010, Issue 1. Art. No: CD006213. [DOI: 10.1002/14651858.CD006213.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bendewald 2007
- Bendewald FP, Cima RR.Pilonidal disease. Clinics in Colon and Rectal Surgery 2007;20(2):86-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
BNF 2019a
- British National Formulary.Alginate dressings. Available at: bnf.nice.org.uk/wound-management/alginate-dressings.html (accessed 19 February 2019).
BNF 2019b
- British National Formulary.Antimicrobial dressings. Available at: bnf.nice.org.uk/wound-management/antimicrobial-dressings.html (accessed 19 February 2019).
BNF 2019c
- British National Formulary.Foam dressings. Available at: bnf.nice.org.uk/wound-management/foam-dressings.html (accessed 19 February 2019).
BNF 2019d
- British National Formulary.Wound management. Available at: bnf.nice.org.uk/wound-management/ (accessed 08 July 2021).
Brown 2019
- Brown SR, Lund JN.The evidence base for pilonidal sinus surgery is the pits. Techniques in Coloproctology 2019;12:1173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chetter 2017
- Chetter IC, Oswald AV, Fletcher MJ, Dumville C, Cullum NA.A survey of patients with surgical wounds healing by secondary intention; an assessment of prevalence, aetiology, duration and management. Journal of Tissue Viability 2017;26(2):103-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Collier 2017
- Collier M, Hofer P.Taking wound cleansing seriously to minimise risk. Wounds UK 2017;13(1):58-64. [Google Scholar]
Dabiri 2016
- Dabiri G, Damstetter E, Phillips T.Choosing a wound dressing based on common wound characteristics. Advances in Wound Care 2016;5(1):32-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2021
- Deeks JJ, Higgins JP, Altman DG (editors).Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Doleman 2018
- Doleman B, Sutton AJ, Sherwin M, Lund JN, Williams JP.Baseline morphine consumption may explain between-study heterogeneity in meta-analyses of adjuvant analgesics and improve precision and accuracy of effect estimates. Anesthesia & Analgesia 2018;126:648-60. [DOI] [PubMed] [Google Scholar]
Doleman 2020
- Doleman B, Freeman SC, Lund JN, Williams JP, Sutton AJ.Funnel plots may show asymmetry in the absence of publication bias with continuous outcomes dependent on baseline risk: presentation of a new publication bias test. Research Synthesis Methods 2020;11:522-34. [DOI] [PubMed] [Google Scholar]
Donner 2002
- Donner A, Klar N.Issues in the meta-analysis of cluster randomized trials. Medical Statistics 2002;21(19):2971-80. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C.Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
EuroQol 1990
- EuroQol Group.EuroQol - a new facility for the measurement of health-related quality of life. Health Policy 1990;16(3):199-208. [DOI] [PubMed] [Google Scholar]
Fowler 2012
- Fowler A.Hydrocolloids in practice. Available at: www.wounds-uk.com/resources/details/hydrocolloids-in-practice (accessed 19 February 2019).
Glanville 2019
- Glanville J, Dooley G, Wisniewski S, Foxlee R, Noel-Storr A.Development of a search filter to identify reports of controlled clinical trials within CINAHL Plus. Health Information and Libraries Journal 2019;36(1):73-90. [DOI] [PubMed] [Google Scholar]
Hahn 2005
- Hahn S, Puffer S, Torgerson DJ, Watson J.Methodological bias in cluster randomised trials. BMC Medical Research Methodology 2005;5:10. [DOI: 10.1186/1471-2288-5-10] [DOI] [PMC free article] [PubMed] [Google Scholar]
Han 2017
- Han G, Ceilley R.Chronic wound healing: a review of current management and treatments. Advances in Therapy 2017;34(3):599-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harris 2012
- Harris CL, Holloway S.Development of an evidence-based protocol for care of pilonidal sinus wounds healing by secondary intent using a modified Reactive Delphi procedure. Part 2: methodology, analysis and results. International Wound Journal 2012;9(2):173-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG.Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA editor(s).Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from: www.training.cochrane.org/handbook.
Higgins 2021
- Higgins JP, Eldridge S, Li T (editors).Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Jones 2005
- Jones A, Vaughan D.Hydrogel dressings in the management of a variety of wound types: a review. Journal of Orthopaedic Nursing 2005;9:S1-11. [Google Scholar]
Lefebvre 2021
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al.Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook .
Li 2021
- Li T, Higgins JP, Deeks JJ (editors).Chapter 5: Collecting data. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Available from training.cochrane.org/handbook.
Lund 2017
- Lund J, Tou S, Doleman B, Williams JP.Fibrin glue for pilonidal sinus disease. Cochrane Database of Systematic Reviews 2017, Issue 1. Art. No: CD011923. [DOI: 10.1002/14651858.CD011923.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mayo 1833
- Mayo OH.Observations on Injuries and Diseases of the Rectum. London (UK): Burgess and Hill, 1833. [PMC free article] [PubMed] [Google Scholar]
McCaughan 2018
- McCaughan D, Sheard L, Cullum N, Dumville J, Chetter I.Patients’ perceptions and experiences of living with a surgical wound healing by secondary intention: a qualitative study. International Journal of Nursing Studies 2018;77:29-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meher 2016
- Meher S, Mishra TS, Sasmal PK, Sharma R, Rout B.Umbilical pilonidal sinus: a report of two cases and recent update of literature. Journal of Clinical and Diagnostic Research 2016;10(9):PD20-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Onder 2012
- Onder A, Girgin S, Kapan M, Toker M, Arikanoglu Z, Palanci Y, et al.Pilonidal sinus disease: risk factors for postoperative complications and recurrence. International Surgery 2012;97(3):224-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ouzzani 2016
- Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A.Rayyan-a web and mobile app for systematic reviews. Systematic Reviews 2016;5(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Percival 2008
- Percival SL, Bowler P, Woods EJ.Assessing the effect of an antimicrobial wound dressing on biofilms. Wound Repair and Regeneration 2008;16(1):52-7. [DOI] [PubMed] [Google Scholar]
Price 2004
- Price P, Harding K.Cardiff Wound Impact Schedule: the development of a condition-specific questionnaire to assess health-related quality of life in patients with chronic wounds of the lower limb. International Wound Journal 2004;1(1):10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5).Version 5.4.1. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Schultz 2017
- Schultz G, Bjarnsholt T, James GA, Leaper DJ, McBain AJ, Malone M, et al, Global Wound Biofilm Expert Panel.Consensus guidelines for the identification and treatment of biofilms in chronic nonhealing wounds. Wound Repair and Regeneration 2017;25(5):744-57. [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s).Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Sian 2018
- Sian TS, Herrod PJ, Blackwell JE, Hardy EJ, Lund JN.Fibrin glue is a quick and effective treatment for primary and recurrent pilonidal sinus disease. Techniques in Coloproctology 2018;22(10):779-84. [DOI] [PubMed] [Google Scholar]
Stata [Computer program]
- Stata Statistical Software.Version 15. College Station (TX): StataCorp, 2017. Available at www.stata.com.
Stern 2004
- Stern PJ, Goldfarb CA.Interdigital Pilonidal Sinus. New England Journal of Medicine 2004;350:e10. [DOI] [PubMed] [Google Scholar]
Stewart 2012
- Stewart AM, Baker JD, Elliott D.The psychological wellbeing of patients following excision of a pilonidal sinus. Journal of Wound Care 2012;21(12):595-600. [DOI] [PubMed] [Google Scholar]
Søndenaa 1995a
- Søndenaa K, Andersen E, Nesvik I, Søreide JA.Patient characteristics and symptoms in chronic pilonidal sinus disease. International Journal of Colorectal Disease 1995;10(1):39-42. [DOI] [PubMed] [Google Scholar]
Søndenaa 1995b
- Søndenaa K, Nesvik I, Andersen E, Natås O, Søreide JA.Bacteriology and complications of chronic pilonidal sinus treated with excision and primary suture. International Journal of Colorectal Disease 1995;10(3):161-6. [DOI] [PubMed] [Google Scholar]
Tien 2018
- Tien T, Athem R, Arulampalam T.Outcomes of endoscopic pilonidal sinus treatment (EPSiT): a systematic review. Techniques in Coloproctology 2018;22(5):325-31. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR.Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Venturi 2005
- Venturi ML, Attinger CE, Mesbahi AN, Hess CL, Graw KS.Mechanisms and clinical applications of the vacuum-assisted closure (VAC): a review. American Journal of Clinical Dermatology 2005;6(3):185-94. [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD.The MOS 36-item short-form health survey (SF-36). Conceptual framework and item selection. Med Care 1992;30(6):473-83. [PubMed] [Google Scholar]
WebPlotDigitizer [Computer program]
- WebPlotDigitizer: web based tool to extract data from plots, images, and maps.Version 4.1. Austin (TX): Ankit Rohatgi, 8 January 2018. Available from: automeris.io/WebPlotDigitizer.
Winter 1962
- Winter G.Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature 1962;193:293-4. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Herrod 2019
- Herrod PJ, Doleman B, Hardy EJ, Hardy P, Maloney T, WIlliams JP, et al.Dressings and topical agents for the management of open wounds after surgical treatment for sacrococcygeal pilonidal sinus. Cochrane Database of Systematic Reviews 2019, Issue 9. Art. No: CD013439. [DOI: 10.1002/14651858.CD013439] [DOI] [PMC free article] [PubMed] [Google Scholar]


