Abstract
Purpose:
Large-scale genetics education appropriate for general practice providers is a growing priority. We describe the content and impact of a mandatory system-wide program implemented at Sanford Health.
Methods:
The Imagenetics Initiative at Sanford Health developed a 2-year genetics education program with quarterly web-based modules that were mandatory for all physicians and advanced practice providers. Scores of 0–5 were calculated for each module based on the number of objectives that participants reported as fulfilled. In addition, participants completed surveys prior to starting and after finishing the education program that included a 7-item measure scored 7–28 of perceived preparedness to practice genetics.
Results:
Between 2,252 and 2,822 Sanford Health employees completed each of the 8 quarterly education modules. Ratings were highest for the module about using genomics to improve patient management (mean score: 4.3) and lowest for the module about different types of genetic tests and specialists. Mean perceived preparedness scores increased from 15.7 at pre-education to 19.1 at post-education (p<0.001).
Conclusion:
Web-based genetics education was highly effective in increasing healthcare providers’ confidence about using genetics. Both comfort with personal knowledge and confidence regarding access to the system’s Genomic Medicine experts increased significantly. Results demonstrate how scalable approaches can improve provider preparedness.
Keywords: Pharmacogenomic Testing, Genetic Counseling, Decision Support Systems, Clinical, Genetic Testing, Primary Health Care
INTRODUCTION
The role of genetic testing in all aspects of medicine continues to increase rapidly,1 but the number of genetic specialists is inadequate to meet current demands.2 To realize the potential of genomic medicine, health care providers (HCPs) of all specialties, including those not trained in genetics, must be prepared to receive and act on genetic information.3 Nongenetic HCPs, however, consistently report poor knowledge and low confidence about using genetic test results in the care of their patients, particularly those in primary care.4–9 Moreover, educational efforts often neglect the needs of HCPs such as nurses and advanced practice providers.10,11 It is imperative for health systems to develop scalable strategies to engage, educate and empower nongenetics HCPs in large numbers to use genetic information.10,12,13
The majority of studies about genomics education for nongenetic HCPs is encouraging, showing improvements in knowledge, self-efficacy, and attitudes.14 Only a few of these data were derived from larger studies and over longer periods. Data from 143 general practitioners who completed a two-year program showed substantial improvement in knowledge.15 Similarly, one-year program that included eight or more hours of teaching, supplemented by written materials, showed improved knowledge and attitudes towards genetic services in 121 primary care providers.16 Pilot test data from a hybrid program of web-based modules and face-to-face lectures also showed improvements in knowledge.17 Whether such improvements would be observed in larger, more sustained efforts that include non-volunteers is unclear.
Here, we summarize a mandatory two-year provider education program implemented at Sanford Health. We summarize feedback regarding how well modules increased participants’ self-reported knowledge, competence, and performance. We describe how effectively the modules met their stated objectives, and we identify factors that influenced ratings. The goal of this report is to offer guidance to health systems developing genetic education programs appropriate to the needs of providers who are not genetic specialists.
MATERIALS AND METHODS
Overview
Sanford Health serves more than 2 million patients through more than 2,500 HCPs. In 2014, Sanford Health launched the Imagenetics (internal medicine + genetics) Initiative to accelerate the integration of genetics into patient care system-wide.18 A goal of the Imagenetics Initiative is to increase HCPs’ preparedness to manage genetic findings, with an emphasis on the needs of general practice providers.
A multimodal education plan was launched in 2017. Between 2017 and 2019, the quarterly educational program that all physicians and advanced practice providers (APPs) are required to complete included eight novel computer-based training modules. The modules included a combination of text, case vignettes, embedded videos, infographics, and recorded lectures released quarterly. Medical residents and fellows were not required to complete the modules. Modules were also available to other Sanford Health healthcare providers and administrators. The overarching goals of the modules were to: 1) increase awareness and baseline knowledge of genomic medicine, 2) increase comfort using genomic medicine in routine clinical care, and 3) increase understanding of when and how to access genetics specialists within the Sanford Health system.
Development and Administration of Educational Modules
Existing provider education programs were considered, but could not be obtained and adapted to the needs of Sanford Health due to licensing restrictions. Thus module content was internally developed. The scope and sequence of learning objectives were established by an expert leadership panel of subject-matter experts, including medical geneticists, clinical pharmacists with training in PGx, laboratory directors, and genetic counselors. Learning objectives were refined by smaller groups. The format of each web-based presentation was determined based on the content and varied from voiceover lectures to interactive modules that provided access to external resources. Committees drafted content for approval from Imagenetics clinical leadership. Approved content was then sent to an internal learning, education, and development team for implementation. Content, including module-specific objectives, was also sent to the continuing medical education (CME) office for review, and providers could earn CME credits for completing each of six of the eight modules. Two modules addressing the genetics of drug response and genetic screening using the Sanford Chip were not granted CME credit. The CME-granting committee felt that because these modules focused on specific tests offered from the Sanford Medical Genetics Laboratory they were not free of commercial bias and therefore did not qualify for CME credit.
Modules were distributed through an internal education portal used regularly for all mandatory provider educational programs, and which tracked the amount of time individuals spent on each module. Genetics education targeted to internists existed in the educational portal during the period of interest, although it was not promoted during the time period of interest. Modules are available in the educational portal for providers to review as often as desired. Participation in each module was tied to compensation, with providers given three months to complete each successive module.
Content of Modules
The content and objectives of each module is summarized in Table 1. The first series of four modules provided an overview of genomic medicine. The first module served as a foundation to help individuals better understand genomic medicine and how it can impact clinical practice. Content included how to recognize “red flags” for genetic disorders and an overview of how genes may affect responses to medications. The second module aimed to help individuals recognize genomic applications of precision medicine and the potential transformative effect on patient care. Components included traditional applications, including analyses of family histories of disease. The module also addressed emerging applications, such as genomic risk assessments for common diseases, and provided an overview of the Sanford Chip Program. The third module focused on PGx and drug metabolism in the context of prodrug versus active drug. Content included where to find guidelines for PGx testing and the use of clinical decision support tools. In the final module of the first year, participants learned the difference between somatic and germline genetic testing. Various types of genetic testing options and their clinical applications were addressed, as were the roles of genetics professionals such as genetic counselors.
Table 1.
Content and objectives of individual modules
| Module and Dates | Minutes to Complete, Median (IQR) | CME Credit | Overview | Learning Objectives |
|---|---|---|---|---|
|
| ||||
| Series 1 (2017–2018): Genomic medicine in the clinical setting | ||||
| Module 1a,d: What is genomic medicine? Jul-Sept 2017 |
26 (38) | Yes | Foundational material to better understand genomic medicine and how it can impact clinical practice | • Describe genomic medicine. • Interpret a pedigree. • Differentiate between genotype and phenotype. • Determine when to refer a patient for a genetic medicine consult. • Describe PGx and its benefits. |
| Module 2b,d: Current applications of genomic medicine Oct-Dec 2017 |
21 (29) | Yes | Descriptions of genomic applications of precision medicine, including preemptive genomic screening. | • Recognize genomic applications of precision medicine. • Classify the components of genomic medicine. • Describe the Sanford Chip and its clinical utility. • Recognize the strengths and limitations of preemptive precision medicine. |
| Module 3b,e: The genetics of drug response Jan-Mar 2018 |
14 (20) | No | Clinical utility and application of PGx testing and the basics of drug metabolism | • Define PGx metabolizer types in the context of prodrug versus active drugs. • Identify the scientific organizations that create the guidelines for clinical application of PGx. • Recognize the components of the PGx test and utilize decision support tools. • Order PGx testing and apply results. |
| Module 4b,e: Different types of genetic tests and specialists Apr-Jun 2018 |
22 (33) | Yes | Examples of genetic variation and types of tests used to identify each, along with types of genetic specialists available to offer support | • Differentiate between somatic and germline variation. • Summarize the different types of genetic testing. • Recognize the clinical application for each type of genetic testing. • Examine the clinical relevance of the genetic counseling process. • Distinguish the difference between genetic professionals. |
| Series 2 (2018–2019): Clinical applications of genomic medicine | ||||
| Module 5c,f: PGx in patient care Jul-Sept 2018 |
25 (30) | Yes | PGx principles review and common case examples showcasing available clinical decision support tools (recorded video lecture) | • Apply the principles of PGx to patient care. • Recognize cases in which PGx testing is appropriate. • Discuss the advantages and disadvantages of current approaches to PGx testing. • Recognize the components of PGx reports and utilize decision support tools. • Identify clinical resources related to PGx testing. |
| Module 6c,d: The spectrum of genetic variants Oct-Dec 2018 |
16 (16) | Yes | Comparison of the genetics of mendelian and common diseases with an introduction to the identification and analysis of single nucleotide polymorphisms (SNPs) (recorded video lecture) | • Summarize past efforts and current opportunities related to precision medicine. • Outline the spectrum of genetic changes, or variants, between mendelian inheritance and common disease. • Characterize SNPs. • Appreciate the design and clinical utility of genome-wide association studies (GWAS). • Assess the clinical application of polygenic risk scores (PRS) to modify patients’ clinical risk categories for more precise screening and treatment. |
| Module 7b,f: Genetic screening and the Sanford Chip Jan-Mar 2019 |
8 (12) | No | High-level overview of Imagenetics with a focus on the return of results workflow for Sanford’s precision prevention tool, the Sanford Chip | • Describe the three main initiatives of Sanford Imagenetics with an emphasis on the Sanford Chip. • Delineate the Sanford Chip workflow for return of results. • Apply Sanford Chip results to clinical practice through case examples. |
| Module 8b,f: Using genomics to improve management Apr-Jun 2019 |
13 (15) | Yes | Case examples outlining how a genetic diagnosis improves patient outcomes and a brief description of Sanford’s rare disease registry | • Describe how a genetic diagnosis can aid patient care. • Evaluate cases in which referring a patient for a genetic medicine consult may be valuable. • Apply PGx testing results to medical management. • Discuss the value that the Coordination of Rare Diseases at Sanford (CoRDS) provides to patients, families, and researchers. |
Combination of recorded lecture and interactive format
Recorded lecture format
Interactive format
Genetic principles content
Combination of general principles and “how-to” content
“how-to” content
The second series of four educational modules addressed more specific clinical applications. The first module of the second year focused primarily on the clinical application of PGx testing. Through a recorded lecture format, participants learned when to order PGx testing and the pros and cons of current approaches. The module also reviewed components of a PGx report, and expanded upon how to utilize available decision support resources. The second module was also a recorded lecture, and addressed the association between different types of genetic variants, mendelian inheritance, and common disease. The module also provided details about single nucleotide polymorphisms, how they are identified, and how they may be used to estimate polygenic risk for common diseases. The third module addressed the Sanford Chip Program, an elective genetic test that provides preemptive PGx testing and screens individuals for medically actionable genetic predispositions. Using case studies, the module provided examples of the application of the Sanford Chip to patient care, as well as workflow for return of results to providers and patients. The final module explained how a genetic diagnosis could improve patient care in a series of case studies. Content reviewed how to identify situations where a consult with a genetic specialist is warranted and how PGx findings could influence medical management. The module concluded with a discussion of the rare disease registry at Sanford Health.
Measures of Effectiveness
Outcomes data were analyzed from all available anonymous surveys administered to educational program participants via SurveyMonkey for quality improvement purposes. Perceptions about preparedness, access to genetic specialists, and utility were queried rather than more objective measures, such as genetic knowledge scales, to minimize participant burden and because the content of Year 2 modules had not been finalized at the time the program was launched.
Assessments of Individual Modules
Following the completion of each of the 8 educational modules, participants provided yes/no responses to the following statements: 1) “The content of this activity matched my current (or potential) scope of practice;” 2) “This activity increased my knowledge (knowing what to do);” 3) “This activity increased my competence (knowing how to do something);” and 4) “This activity improved my performance (ones actual behavior in practice).” All modules except the last also include a fifth statement, “Were your personal objectives successfully achieved?”
Pre- and Post- Education Assessment
Prior to starting the first educational module and after completing the final educational module, surveys included the following measures.
Perceived Preparedness
Individuals were asked to rate how prepared they felt about genetics in medicine in a set of seven items: 1) I feel well-informed about knowledge of genetics; 2) I feel well-informed about genetic testing in general; 3) I feel comfortable ordering a genetic test to genetic conditions in my specialty; 4) I feel comfortable ordering a genetic test for disease susceptibility (e.g., BRCA1/BRCA2 testing for risk of breast and ovarian cancer); 5) I feel comfortable ordering a pharmacogenetic test to predict risk of adverse events or likelihood of response (e.g., CYP2C9/VKORC1 and warfarin therapy). 6) I have access to genetics expertise when I have a question related to a patient. 7) I feel that my medical training adequately prepared me to appropriately order and use genetics tests. Response options of “strongly disagree”, “disagree”, “agree,” and “strongly agree” were scored 1–4 and summed to create a summary score of 7–28 with higher scores indicated stronger feelings of perceived preparedness. Scale items were novel, but demonstrated strong internal consistency (Cronbach’s alpha of 0.91 at pre-education and 0.93 at post-education).
Perceived Access to Genetic Specialists
Individuals were asked separate yes/no questions about whether they had a geneticist or genetic counselor to whom they could refer patients.
Perceived Utility
Individuals were asked how useful they thought pharmacogenetic results would be for managing their patients’ health, with response options of “very useful,” “somewhat useful,” “not very useful,” and “not at all useful.”
Open-Ended Items
Respondents provided open-ended feedback to the following two questions on both the pre- and post-education questionnaires: “How do you feel about genetic testing becoming part of routine clinical care?” and “What additional information would be helpful to increase your comfort with using genetics in clinical care?”
Respondent Characteristics
Respondents self-reported their gender, age in 10-year increments, role (Physician, APP, Nursing, Pharmacy, other), specialty, and, if applicable, years out of residency, training (U.S. or non-U.S. medical school), and residency training setting (university-based, hospital-based, other).
Data Analysis
Analyses of pre- and post-education surveys were limited to data from physicians and APPs because these providers were the target audience and required to complete the modules. Module-specific ratings included all survey completers because physicians and APPs could not be distinguished from individuals with other roles. To avoid instances where outcomes were provided long after completion of education modules, data were analyzed from surveys we could match to records confirming module completion within 14 days. We also omitted 462 respondents from analyses for pre-education surveys who reported that they had already completed required Sanford training modules.
Chi-squared and Wilcoxon rank sum tests were used to compare the characteristics of respondents of the pre-education and post-education assessments. Linear and logistic regression models were used to compare pre- and post-education survey data, as appropriate to the distributions of responses. Covariates included gender, age, and role. Generalized linear models with logit links and binomial distributions were also used to compare module-specific evaluation data by topic, although no respondent characteristics were included in these statistical models because the surveys were anonymous. We also analyzed module-specific education descriptively by content and format. The content of individual modules was classified using frameworks proposed for genetic literacy19,20 as primarily principles knowledge (underlying theoretical principles of genetics and medical genetics), “how-to” knowledge (practical knowledge concerning the proper use of genetic testing) or both. The format of individual modules was classified as interactive, recorded presentation or both.
Open-ended data were classified using approaches developed for coding qualitative data.21 First, one study team member (KDC) proposed an initial codebook based on review of responses. Two study team members (LG and CP) then coded each response set independently. In instances where interrater reliability metrics were suboptimal, codebooks were revised and data were re-coded until agreement was strong (Cohen’s κ>0.8). Final differences in coding were reconciled by a single study team member (LG).
Available-case analyses were conducted using R version 4.0.3. The study was deemed exempt from human subjects research by the Sanford Research Institutional Review Board.
RESULTS
Response Rates and Provider Characteristics
Between 2,252 and 2,822 individuals completed each module, and between 1,263 and 2,377 individuals completed each post-module assessment (Supplementary Table 1). Average response rates to module-specific surveys decreased from 95.3% following completion of the first module to 50.9% following completion of the final module. The median time required to complete each module ranged from 8 to 26 minutes (Table 1), with participants spending an average of 221 minutes (st dev: 151 minutes) overall.
Pre- and post-education assessments had comparable completion percentages from physicians and APPs. At pre-education, 1,719 of 2,102 physicians and APPs (81.8%) completed the pre-education survey, and 1,263 of 2,482 physicians and APPs completed the post-education survey (50.9%). Characteristics of physicians and APPs who completed the pre-education and post-education surveys are summarized in Table 2. Compared to respondents of the pre-education survey, respondents analyzed from the post-education survey were more likely to be APPs (38.8% vs 43.1%, respectively, p=0.019), were older (58.2% over the age of 40 vs 65.1%, respectively, p<0.001), and were more likely to report receiving genetics education in medical school or during residency (52.4% vs 64.1%, respectively, p<0.001). Compared to physicians who completed the pre-education survey, physicians who completed the post-education survey tended to be more experienced (51.7% at least 10 years out of residency vs 64.9%, respectively, p<0.001).
Table 2.
Characteristics of pre-education and post-education survey respondents.a
| Characteristic | Pre-education Survey n (%) |
Post-education Survey n (%) |
p |
|---|---|---|---|
|
| |||
| Role | 0.019 | ||
| Physician | 1052 (61.2%) | 719 (56.9%) | |
| APP | 667 (38.8%) | 544 (43.1%) | |
| Age | <0.001 | ||
| <30 | 97 (5.6%) | 54 (4.3%) | |
| 30–39 | 618 (36.0%) | 385 (30.5%) | |
| 40–49 | 428 (24.9%) | 343 (27.2%) | |
| 50–59 | 304 (17.7%) | 254 (20.1%) | |
| 60–69 | 225 (13.1%) | 197 (15.6%) | |
| 70+ | 38 (2.2%) | 24 (1.9%) | |
| Missing age | 9 (0.5%) | 6 (0.5%) | |
| Primary Specialtyb | |||
| Family Medicine | 383 (22.3%) | 274 (21.7%) | |
| Internal Medicine | 137 (8.0%) | 90 (7.1%) | |
| OB/Gyn | 76 (4.4%) | 43 (3.4%) | |
| Pediatrics/Pediatric Sub-Specialties | 156 (9.1%) | 99 (7.8%) | |
| Years out of residencyc | <0.001 | ||
| <5 years | 289 (27.5%) | 113 (15.7%) | |
| 5–9 years | 208 (19.8%) | 130 (18.1%) | |
| 10–14 years | 136 (12.9%) | 99 (13.8%) | |
| 15–19 years | 131 (12.5%) | 87 (12.1%) | |
| 20+ years | 266 (25.3%) | 263 (36.6%) | |
| Out of residency missing | 22 (2.1%) | 27 (3.8%) | |
| Genetics education in medical school or residency | 900 (52.4%) | 810 (64.1%) | <0.001 |
Evaluations of specific modules did not collect information about respondent characteristics.
At pre-education, respondents could endorse multiple primary specialties and write in others. At post-education, respondents could choose a single response option and write in others. Additional specialties reported are summarized in Appendix 2.
Data for these items were collected only from physicians.
Post-Module Assessments
The majority of individuals who completed assessments judged the modules’ formats to be satisfactory. Between 77.1% and 92.6% of respondents reported that the format of each module was appropriate, and when queried in Year 2, over 96% of participants said that the length of each module was appropriate (Supplementary Table 3). When improvements to the format of modules were suggested, respondents asked for case-based presentations more often than other changes.
Assessments of whether individual modules met goals are summarized in Table 3. The percentages of respondents who reported that modules increased their knowledge, increased their competence, and improved their performance were lowest for modules that addressed different types of genetic tests and specialists and current applications of genomic medicine. In contrast, the percentages of respondents who reported that the same module goals (knowledge, competence, performance) were achieved were highest for modules about using genomics to improve management, the module about genetic screening and the Sanford Chip, and the module about PGx in patient care.
Table 3.
Percentage of respondents who reported that educational modules achieved goals.
| Module | Increased knowledge | Increased competence | Improved performance | Matched scope of practice | Personal objectives met |
|---|---|---|---|---|---|
|
| |||||
| 1: What is genomic medicine? (n=1914–2043) | 86.5% | 76.1% | 63.6% | 88.1% | 91.8% |
| 2: Current applications of genomic medicine (n=1687–1960) | 87.3% | 76.4% | 60.9% | 83.1% | 86.1% |
| 3: The genetics of drug response (n=1635–2049) | 90.3% | 82.1% | 65.7% | 85.0% | 89.6% |
| 4: Different types of genetic tests and specialists (n=1224–1292) | 84.1% | 71.5% | 54.7% | 74.7% | 79.9% |
| 5: PGx in patient care (n=1471–1699) | 93.0% | 87.9% | 73.4% | 79.7% | 87.0% |
| 6: The spectrum of genetic variants (n=1445–1643) | 89.1% | 76.4% | 65.5% | 74.1% | 88.1% |
| 7: Genetic screening and the Sanford Chip (n=2063–2370) | 92.5% | 87.5% | 73.2% | 79.4% | 90.5% |
| 8: Using genomics to improve management (n=1211–1233) | 93.3% | 86.9% | 74.3% | 80.8% | a |
Item was not included in the module-specific survey.
Analyses suggested that respondents preferred modules that focused on practical, “how-to” knowledge. For the three modules where content was primarily “how-to” knowledge, 92.9% of respondents reported increased knowledge, 87.4% reported increased competence, and 73.6% reported improved performance (Supplementary Table 4). In contrast, across the other five modules, 87.5% reported increased knowledge, 76.7% reported increased competence, and 62.3 reported improved performance (all p<0.001). Interestingly, analyses showed little difference in respondents’ ratings of modules that used primarily either an interactive or a recorded format (Supplementary Table 5).
Pre- and Post-Education Assessments
Mean scores on our 7–28 scale of perceived preparedness increased from 15.7 at pre-education to 19.2 at post-education (diff=3.5, 95%CI: 3.2 to 3.8, p<0.001). Regression analyses are summarized in Table 4. Changes were particularly large among APPs, where mean scores increased from 14.2 to 18.8 (diff=4.6, 95%CI: 4.1 to 5.1, p<0.001). Mean scores among physicians increased from 16.7 to 19.4 (diff=2.7, 95%CI: 2.3 to 3.1, p<0.001), in contrast. Perceived preparedness scores varied by age across time points, with an average decrease of 0.3 points on the scale per 10-year increase in age (95%CI: −0.4 to −0.2, p<0.001). We also observed lower scores at both time points among older respondents.
Table 4.
Summary of linear regression analyses of perceived preparedness scores (7–28 scale). Model estimates represent the difference in scale scores compared to the reference group.
| Estimate | Std Error | p | |
|---|---|---|---|
|
| |||
| Intercept | 17.44 | 0.39 | <0.001 |
| Male gender (ref: female) | −0.26 | 0.18 | 0.156 |
| Age in years (ref: <30) | <0.001 | ||
| 30–39 | −0.28 | 0.37 | |
| 40–49 | −0.64 | 0.38 | |
| 50–59 | −0.98 | 0.39 | |
| 60–69 | −1.15 | 0.41 | |
| 70+ | −0.52 | 0.64 | |
| APP role (ref: physician) | −2.50 | 0.23 | <0.001 |
| Change among physicians, post minus pre | 2.67 | 0.20 | <0.001 |
| Change among APPs post minus pre | 1.96 | 0.32 | <0.001 |
Increases on all items on all items of the perceived preparedness scale were observed from pre-education to post-education (Figure 1). The largest increases were observed on the percentage of participants who reported feeling well-informed about their knowledge of genetics (36% agreed at pre-education vs 81% agreed at post-education) and the percentage of participants who reported feeling comfortable ordering PGx testing (19% agreed at pre-education vs 60% agreed at post-education). At pre-education, respondents were least likely to report feeling comfortable ordering PGx testing (19% agreed) while at post-education, respondents were least likely to report that their medical training adequately prepared them to order and use genetic tests (55% agreed). At both time points, respondents were most likely to report that they had access to genetics expertise when they had questions about a patient (59% agreed at pre-education, 86% agreed at post-education).
Figure 1.
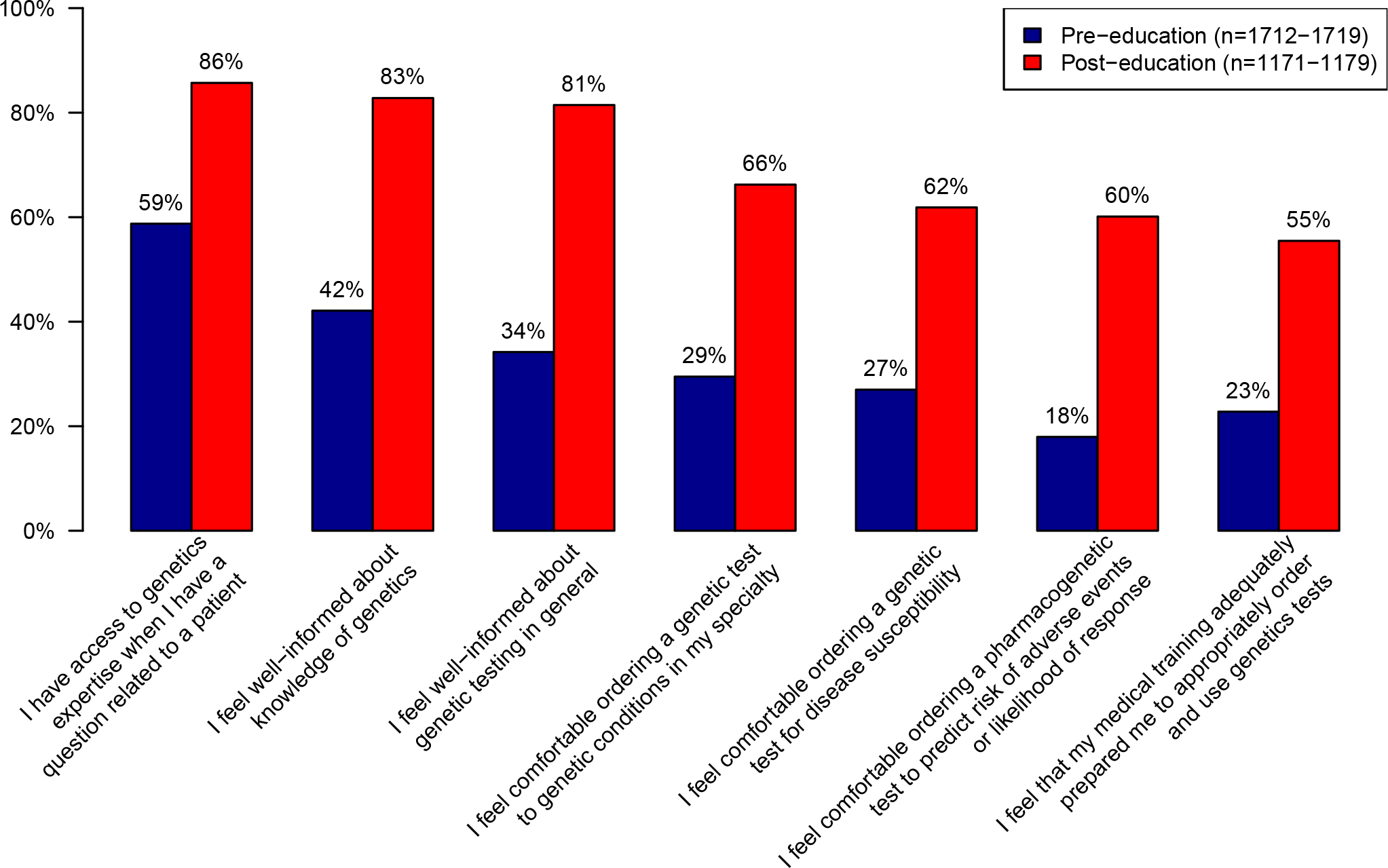
Percentage of physicians and advanced practice providers who agreed or strongly agreed with each item of the perceived preparedness scale. Percentages were estimated using logistic regression equations, with adjustment for role, age and gender.
We also observed an increase in the proportion of physicians and APPs who reported having access to a geneticist or genetic counselor to whom they could refer patients when asked as a yes or no question. 64.9% of physicians and APPs reported access to one or both of these specialists at pre-education, compared to 82.7% at post-education (p<0.001). Increases were also observed in the perceived utility of PGx testing. At pre-education, 22.2% of respondents reported that PGx results would be “very useful” for managing their patients’ health, compared to 37.8% of respondents at post-education (p<0.001).
Analyses of open-ended items also showed a lower likelihood of addressing further education as helpful in the post-education survey than in the pre-education survey. The odds that respondents’ written responses about what would increase their comfort with genetics addressed education decreased by 45.6% after education than before (p=0.003). Interestingly, the odds that respondents’ written responses were about the need for more experience or practice were 4.95 times higher at post-education than pre-education (p<0.001).
DISCUSSION
The Sanford Health experience is one of the first examples of a sustained, mandatory genetics education program at a major health system. Over 2,000 providers completed the program over a two-year period, and the program yielded significant improvement in provider preparedness, including large increases in provider confidence, awareness of help, and perceived utility of genetic testing. A large majority of individuals reported that the modules increased their knowledge and competence, and that their personal objectives were met. Findings from this program demonstrate how committed health systems can effectively provide genetics education to their health care providers as part of a comprehensive plan to implement genomic medicine.
Importantly, while substantial investment was necessary to create the educational modules, program completion did not appear to be a burden. Well over 90% of individuals reported that the length of each of the modules were appropriate, when that question was included in module assessments. Efforts to educate providers about genetics have varied greatly in their time demands, ranging from half-day courses to multi-day seminars, to monthly meetings over a year.22–26 Such efforts often are difficult for providers to accommodate in their schedules, however, motivating organizations such as the Jackson Laboratory, the Centers for Disease Control and Prevention, and the International Society of Nurses in Genetics to develop and curate web-based provider education programs.27–29 The Sanford Health program has taken these efforts a step further by providing web-based programs that individuals can complete at their own pace, but have been tailored to the services and health care provider support infrastructure developed by Sanford’s Imagenetics Initiative.
One of the key goals of Sanford’s genomics educational program was to make providers who may have little experience with genetics more comfortable with population preemptive genetic screening. Modules focused on the use of genetic information in the care of both healthy and sick patients, as well as benefits and limitations of genetic testing. The percentage of individuals who reported that modules increased their competence and performance was more than 10% higher when modules emphasized “how-to” knowledge or case examples rather than genetic principles. In particular, the module about genetic screening and the Sanford Chip was among the highest-rated even though it did not qualify for CME credit. Findings from our work add weight to calls for greater use of theoretical frameworks and educational theory to inform program development by demonstrating how content may affect responses to genetic education programs.14,30–32 While it is important to include principles knowledge, doing so in a manner that also includes “how-to” content may yield the best results, especially if it is linked to a high-profile program that could affect the practice of most participants.19,20
One factor that may have increased the effectiveness of the genetics education program was a significant effort to increase provider support for and awareness of the role for genetics in medicine prior to the launch of the formal educational program. Experts generally agree that education alone may be insufficient to ensure the appropriate use of genetic testing.33,34 Efforts at Sanford Health included infrastructure development for the integration of genetic information into the electronic medical record and development of automated clinical decision support that would provide point-of-care guidance to providers. Educational modules that addressed “how-to” content leveraged the content of the existing infrastructure. In a parallel effort, Sanford Health increased the number of genetic counselors in its system and embedded them in all internal medicine clinics. The combination of the “human” resource with support in the electronic medical record (EMR) may have enhanced educational efforts by making providers more aware that help for responding to genetic information was readily available.35,36
Notably, the genetic education program overlapped with the launch of the Sanford Chip in 2018, a flagship genetic testing program in primary care settings that offers pharmacogenomic testing and optional genetic risk information. Efforts to make providers and patients aware of this new elective service likely had a significant impact on provider awareness for the role of genetics in medicine and increased the salience of the genetics education program and increased engagement with the content. Importantly, other studies suggest that providers may be unwilling to engage with genetic information if they feel inadequately prepared or supported.37–39 The development of an environment to manage genetic information and the launch of provider education in genetics prior to offering the Sanford Chip may be a reason that over 11,000 patients have participated in the program to date.
One limitation of our study was the use of self-assessments of genomic readiness. Preliminary analyses of data from 2018 showed that providers altered medication choices or patient monitoring in 45% of encounters where potential drug-gene interactions were identified, including 59% of encounters involving clopidogrel. These rates are higher than those observed in related clinical trials,40 and much higher than a 10% concordance rate with clinical decision support recommendations observed at Sanford Health overall. Future work will refine these analyses, as well as examine more objective measures of provider knowledge and behavior. Limitations also include the analysis of anonymous data that did not allow comparisons of pre- and post-education responses for specific individuals. It is possible that individuals with more positive attitudes about genomics were more likely to complete the post-education survey. The program was implemented in a single healthcare system and results may not generalize well to others, particularly systems lacking the bioinformatics infrastructure and clinical decision support to complement the education.
Despite the significant efforts described here, our analyses still suggest that providers felt additional education would be helpful. This, along with the perception that genetics has promise for the future, demonstrates the need to supplement system-wide educational efforts. Additional programs that Sanford Health has implemented include developing brief educational PowerPoint presentations that are available on demand. These presentations address topics such as the meaning of uninformative findings and how to use Genomic Indicators, tools in the Epic EMR system which document genomic findings as discrete fields that can trigger automated decision support.
Nevertheless, our work demonstrates that health systems can effectively deliver provider-directed genetic education at scale. The modules summarized here have been combined into a single module, which is updated as needed to ensure content is current with evolving best practice recommendations. All new physicians and APP hires complete this single module during orientation. We intend to provide ongoing education to build upon this existing foundation and respond to the rapid speed at which genetics is impacting medicine.
Supplementary Material
ACKNOWLEDGMENTS
The following people contributed to the development of the educational modules: Aissa Aifaoui, Jordan Baye, Megan Bell, Laura Davis-Keppen, Kristen DeBerg, Catherine Hajek, Allison Hutchinson, Patricia Crotwell Leiferman, Amanda Massmann, Lisa Mullineaux, Natasha Petry, Dylan Platt, April Schultz, and D. Isum Ward. This work was funded by the Sanford Health System. KDC was supported by NIH grants K01-HG009173 and R01-HD090019, and RCG and CLBZ were also supported by NIH grant R01-HL143295.
Footnotes
Code Availability
Code will be made available at request. Inquires can be directed to the corresponding author.
Ethics Declaration
The study was deemed exempt from human subjects research by the Sanford Research Institutional Review Board.
Data Availability Statement
Data will be made available at request. Inquiries can be directed to the corresponding author.
REFERENCES
- 1.Phillips KA, Deverka PA, Hooker GW, Douglas MP. Genetic test availability and spending: where are we now? Where are we going? Health Aff (Millwood). 2018;37(5):710–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Educational Kurnat-Thoma E. and ethical considerations for genetic test implementation within health care systems. Netw Syst Med. 2020;3(1):58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dragojlovic N, Borle K, Kopac N, et al. The composition and capacity of the clinical genetics workforce in high-income countries: a scoping review. Genet Med. 2020;22(9):1437–1449. [DOI] [PubMed] [Google Scholar]
- 4.Nippert I, Harris HJ, Julian-Reynier C, et al. Confidence of primary care physicians in their ability to carry out basic medical genetic tasks-a European survey in five countries- Part 1. J Community Genet. 2011;2(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mainous AG 3rd, Johnson SP, Chirina S, Baker R. Academic family physicians’ perception of genetic testing and integration into practice: a CERA study. Fam Med. 2013;45(4):257–262. [PubMed] [Google Scholar]
- 6.Carroll JC, Makuwaza T, Manca DP, et al. Primary care providers’ experiences with and perceptions of personalized genomic medicine. Can Fam Physician. 2016;62(10):e626–e635. [PMC free article] [PubMed] [Google Scholar]
- 7.Harding B, Webber C, Ruhland L, et al. Primary care providers’ lived experiences of genetics in practice. J Community Genet. 2019;10(1):85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mikat-Stevens NA, Larson IA, Tarini BA. Primary-care providers’ perceived barriers to integration of genetics services: a systematic review of the literature. Genet Med. 2015;17(3):169–176. [DOI] [PubMed] [Google Scholar]
- 9.Scheuner MT, Sieverding P, Shekelle PG. Delivery of genomic medicine for common chronic adult diseases: a systematic review. JAMA. 2008;299(11):1320–1334. [DOI] [PubMed] [Google Scholar]
- 10.Campion M, Goldgar C, Hopkin RJ, Prows CA, Dasgupta S. Genomic education for the next generation of health-care providers. Genet Med. 2019;21(11):2422–2430. [DOI] [PubMed] [Google Scholar]
- 11.Crellin E, McClaren B, Nisselle A, Best S, Gaff C, Metcalfe S. Preparing medical specialists to practice genomic medicine: education an essential part of a broader strategy. Front Genet 2019;10(789). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Secretary’s Advisory Committee on Genetics H, and Society,. Genetics Education and Training of Health Care Professionals, Public Health Providers, and Consumers. 2011.
- 13.Rubanovich CK, Cheung C, Mandel J, Bloss CS. Physician preparedness for big genomic data: a review of genomic medicine education initiatives in the United States. Hum Mol Genet. 2018;27(R2):R250–r258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talwar D, Tseng TS, Foster M, Xu L, Chen LS. Genetics/genomics education for nongenetic health professionals: a systematic literature review. Genet Med. 2017;19(7):725–732. [DOI] [PubMed] [Google Scholar]
- 15.Clyman JC, Nazir F, Tarolli S, Black E, Lombardi RQ, Higgins JJ. The impact of a genetics education program on physicians’ knowledge and genetic counseling referral patterns. Med Teach. 2007;29(6):e143–150. [DOI] [PubMed] [Google Scholar]
- 16.Kolb SE, Aguilar MC, Dinenberg M, Kaye CI. Genetics education for primary care providers in community health settings. J Community Health. 1999;24(1):45–59. [DOI] [PubMed] [Google Scholar]
- 17.Wallen GR, Cusack G, Parada S, Miller-Davis C, Cartledge T, Yates J. Evaluating a hybrid web-based basic genetics course for health professionals. Nurse Educ Today. 2011;31(6):638–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christensen KD, Bell M, Zawatsky CLB, et al. Precision population medicine in primary care: the Sanford Chip experience. Front Genet 2021;12(274). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smerecnik CMR, Mesters I, de Vries NK, de Vries H. Educating the general public about multifactorial genetic disease: applying a theory-based framework to understand current public knowledge. Genet Med. 2008;10(4):251–258. [DOI] [PubMed] [Google Scholar]
- 20.Smerecnik CMR, Mesters I, de Vries NK, de Vries H. Applying a theory-based framework to understand public knowledge of genetic risk factors: a case for the distinction between how-to knowledge and principles knowledge. Public Health Genomics. 2011;14(4–5):259–270. [DOI] [PubMed] [Google Scholar]
- 21.Strauss A, Corbin J. Basics of Qualitative Research. Techniques and Procedures for Developing Grounded Theory. Second ed. Thousand Oaks: SAGE Publications; 1998. [Google Scholar]
- 22.Manolio TA, Chisholm RL, Ozenberger B, et al. Implementing genomic medicine in the clinic: the future is here. Genet Med. 2013;15(4):258–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demmer LA, Waggoner DJ. Professional medical education and genomics. Annu Rev Genomics Hum Genet. 2014;15:507–516. [DOI] [PubMed] [Google Scholar]
- 24.Carroll JC, Wilson BJ, Allanson J, et al. GenetiKit: a randomized controlled trial to enhance delivery of genetics services by family physicians. Fam Pract. 2011;28(6):615–623. [DOI] [PubMed] [Google Scholar]
- 25.Carroll JC, Rideout AL, Wilson BJ, et al. Genetic education for primary care providers: improving attitudes, knowledge, and confidence. Can Fam Physician. 2009;55(12):e92–99. [PMC free article] [PubMed] [Google Scholar]
- 26.Blazer KR, Christie C, Uman G, Weitzel JN. Impact of web-based case conferencing on cancer genetics training outcomes for community-based clinicians. J Cancer Educ. 2012;27(2):217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laboratory J. Clinical and Continuing Education. https://www.jax.org/education-and-learning/clinical-and-continuing-education#. Published 2021. Accessed Jan 10, 2021.
- 28.Centers for Disease Control and Prevention. Training Programs and Courses. https://www.cdc.gov/genomics/training/index.htm. Published 2021. Accessed Jan 10, 2021.
- 29.International Society of Nurses in Genetics. Online Resources for Health Professionals. https://www.isong.org/page-1325075. Published 2021. Accessed Jan 10, 2021.
- 30.Guttmacher AE, Porteous ME, McInerney JD. Educating health-care professionals about genetics and genomics. Nat Rev Genet. 2007;8(2):151–157. [DOI] [PubMed] [Google Scholar]
- 31.Burke W, Emery J. Genetics education for primary-care providers. Nat Rev Genet. 2002;3(7):561–566. [DOI] [PubMed] [Google Scholar]
- 32.Reed EK, Johansen Taber KA, Ingram Nissen T, et al. What works in genomics education: outcomes of an evidenced-based instructional model for community-based physicians. Genet Med. 2016;18(7):737–745. [DOI] [PubMed] [Google Scholar]
- 33.Gaff CL, MW I, MF S, et al. Preparing for genomic medicine: a real world demonstration of health system change. NPJ Genom Med. 2017;2:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bennett CL, Burke SE, Burton H, Farndon PA. A toolkit for incorporating genetics into mainstream medical services: Learning from service development pilots in England. BMC Health Serv Res. 2010;10:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sasaki N, Yamaguchi N, Okumura A, et al. Factors affecting the use of clinical practice guidelines by hospital physicians: the interplay of IT infrastructure and physician attitudes. Implement Sci. 2020;15(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trinidad SB, Fryer-Edwards K, Crest A, Kyler P, Lloyd-Puryear MA, Burke W. Educational needs in genetic medicine: primary care perspectives. Community Genet. 2008;11(3):160–165. [DOI] [PubMed] [Google Scholar]
- 37.Pet DB, Holm IA, Williams JL, et al. Physicians’ perspectives on receiving unsolicited genomic results. Genet Med. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christensen KD, Bernhardt BA, Jarvik GP, et al. Anticipated responses of early adopter genetic specialists and nongenetic specialists to unsolicited genomic secondary findings. Genet Med. 2018;20(10):1186–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peterson JF, Field JR, Shi Y, et al. Attitudes of clinicians following large-scale pharmacogenomics implementation. Pharmacogenomics J. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tuteja S, Glick H, Matthai W, et al. Prospective CYP2C19 genotyping to guide antiplatelet therapy following percutaneous coronary intervention: a pragmatic randomized clinical trial. Circ Genom Precis Med. 2020;13(1):e002640. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available at request. Inquiries can be directed to the corresponding author.


