Abstract
Background:
Cardiogenic shock (CS) complicating acute myocardial infarction (MI) is associated with high mortality. In the absence of data to support coronary revascularization beyond the infarct artery and selection of circulatory support devices or medications, clinical practice may vary substantially.
Methods:
We distributed a survey to interventional cardiologists and cardiothoracic surgeons through relevant professional societies to determine contemporary coronary revascularization and circulatory support strategies for MI with CS and multi-vessel coronary artery disease (CAD).
Results:
A total of 143 participants completed the survey between 1/2019 and 8/2019. Overall, 55.2% of participants reported that standard approach to coronary revascularization was single vessel PCI of the infarct related artery (IRA) with staged PCI of non-culprit lesions. Single vessel PCI of the IRA only (28.0%), emergency multi-vessel PCI (11.9%), and coronary artery bypass grafting (CABG) (4.9%) were standard approaches at some centers. A plurality of survey respondents (46.9%) believed initial PCI with staged CABG for multi-vessel CAD would be associated with the most favorable outcomes. A minority of respondents believed PCI-only strategies (23.1%) and CABG alone (6.3%) provided optimal care, and 23.1% were unsure of the best strategy. After PCI for CS, Impella (76.9%), intra-aortic balloon pump (IABP) (12.8%), and extra-corporeal membrane oxygenation (ECMO) (7.7%) were preferred. After CABG, IABP (34.3%), Impella (32.2%), and ECMO (28%) were preferred.
Conclusions:
This survey indicates substantial heterogeneity in clinical care in CS. There is evidence of provider uncertainty and clinical equipoise regarding the optimal management of patients with MI, multi-vessel CAD, and CS.
Keywords: Cardiogenic shock, coronary artery bypass grafting, myocardial infarction, percutaneous coronary intervention, mortality, multi-vessel coronary artery disease
Short Abstract:
We sought to determine contemporary practice patterns of coronary revascularization and circulatory support in patients with MI, multi-vessel coronary artery disease (CAD), and cardiogenic shock. A survey was distributed to interventional cardiologists and cardiothoracic surgeons through relevant professional societies. Survey respondents identified substantial heterogeneity in clinical care and evidence of provider uncertainty and clinical equipoise regarding the optimal management of patients with MI, multi-vessel CAD, and CS.
Background:
Cardiogenic shock (CS) complicating acute myocardial infarction (MI) is associated with high mortality.1 Emergency revascularization of the infarct-related coronary artery (IRA) for CS improves survival,2 but the 35-45% 30-day mortality rate associated with this approach has persisted for decades despite advances in revascularization techniques, pharmacology, and mechanical circulatory support (MCS).3 Beyond emergency infarct-artery revascularization, no interventions have been proven to reduce mortality in CS. In patients with MI, CS, and multivessel coronary artery disease (CAD), emergent multi-vessel percutaneous coronary intervention (PCI) was associated with a greater risk of 30-day death or severe renal failure compared to infarct-artery PCI only.4 Intra-aortic balloon counter-pulsation did not reduce 30-day mortality in a large randomized trial of patients with MI complicated by CS.5 Other MCS strategies have not been tested in adequately powered clinical trials.6 In the absence of robust data to support the performance of revascularization beyond the infarct artery in patients with multivessel CAD, the selection of circulatory support devices or medications, clinical practice may vary substantially. Current procedure based registries do not allow for an assessment of contemporary treatment patterns of shock because they do not adequately capture a true denominator. We sought to determine contemporary practice patterns with regard to coronary revascularization, medical therapies, and circulatory support strategies in patients with MI, multi-vessel CAD, and CS.
Methods:
We distributed a digital survey (http://is.gd/CABG_SHOCK, Supplemental Figure 1) to interventional and critical care cardiologists and cardiothoracic surgeons directly (to relevant faculty of the 2019 Society for Cardiovascular Angiography and Interventions Scientific Sessions) and through relevant United States professional societies (American College of Cardiology and Society of Thoracic Surgeons). Responses were collected from 1/2019 through 8/2019. Categorical data are presented as number and percentages and were compared using chisquare analysis and the Fisher’s exact test when appropriate. Statistical analysis was performed using SPSS version 26 (IBM, Armonk, NY). Two-sided P-values <0.05 were considered to be statistically significant. The study was approved by the New York University School of Medicine Institutional Review Board. No extramural funding was used to support this work. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
Results:
A total of 143 participants representing 120 institutions completed the survey, including 78 interventional and critical care cardiologists and 65 cardiothoracic surgeons. The digital survey response rate was 3.0%. Characteristics of survey respondents are shown in Table 1. A majority of participants were located in the United States or Canada (80.4%) and many worked at academic medical centers (46.2%) or university-affiliated hospitals (22.4%). Participants frequently represented high-volume institutions that cared for >100 ST-segment elevation MI patients each year (59.4%) and >10 patients with MI with multivessel CAD complicated by CS each year (79.7%), and most had established programs in advanced heart failure (68.5%) and MCS (63.6%).
Table 1.
Characteristics of survey participants and their institutions
| Characteristics of Survey Participants | n (%) |
|---|---|
| Specialty Training | |
| Interventional Cardiology | 76 (53.1%) |
| Critical Care Cardiology | 2 (1.4%) |
| Cardiothoracic Surgery | 65 (45.5%) |
| Years in Specialty Practice | |
| 1-5 Years | 26 (18.2%) |
| 6-10 Years | 27 (18.9%) |
| 11-20 Years | 38 (26.6%) |
| 21-30 Years | 37 (25.9%) |
| >30 Years | 15 (10.5%) |
| Practice Setting | |
| Academic Medical Center | 67 (46.9%) |
| University Affiliate | 32 (22.4%) |
| Private Hospital | 30 (21.0%) |
| Public Hospital | 14 (9.8%) |
| Hospital Location | |
| United States of America | 113 (79.0%) |
| Canada | 2 (1.4%) |
| Other † | 28 (19.6%) |
| Hospital Annual STEMI Volume * | |
| <50 | 19 (13.3%) |
| 51-100 | 31 (21.7%) |
| 101-200 | 37 (25.9%) |
| 201-300 | 20 (14%) |
| 300+ | 28 (19.6%) |
| Unknown / Not Reported | 8 (5.6%) |
| Annual Volume of MI Complicated by Cardiogenic Shock * | |
| <10 | 12 (8.4%) |
| 11-25 | 53 (37.1%) |
| 26-50 | 47 (32.9%) |
| 51-100 | 17 (11.9%) |
| 100+ | 8 (5.6%) |
| Unknown / Not Reported | 6 (4.2%) |
| Annual Volume of MI Complicated by Cardiogenic Shock with Multivessel CAD * | |
| <10 | 29 (20.3%) |
| 11-25 | 59 (41.3%) |
| 26-50 | 28 (19.6%) |
| 51-100 | 12 (8.4%) |
| 100+ | 4 (2.8%) |
| Unknown / Not Reported | 11 (4.9%) |
As per survey respondents. MI volumes may be estimates.
International survey respondents represent the following 18 countries: Argentina, Australia, Austria, Brazil, Chile, China, Colombia, Egypt, Germany, Greece, Indonesia, Iran, Italy, Mexico, Poland, Russia, Thailand, and the United Kingdom.
Overall, 55.2% of participants reported that the institutional standard approach to coronary revascularization for MI, CS, and multi-vessel CAD was single vessel PCI of the infarct related artery (IRA) with staged PCI of non-culprit severe disease. The remaining participants reported single vessel PCI of the infarct-related artery only (28.0%), multi-vessel PCI of all lesions during the index revascularization procedure (11.9%), and multivessel CABG (4.9%) as the standard institutional approaches to revascularization for MI, CS, and multi-vessel CAD (Figure 1). However, 46.9% of participants believed that initial PCI with staged CABG would be associated with the most favorable outcomes in patients MI, CS and multivessel CAD. In contrast, 23.1% indicated PCI-only strategies would provide the greatest benefit, 6.3% indicated that surgical revascularization with CABG only would be optimal, and 23.1% were unsure of which revascularization strategy would yield the greatest benefit. Responses varied by cardiovascular specialty training (p<0.001), as shown in Figure 1. Participant responses stratified by practice setting and years of specialty practice are shown in Supplemental Table 1.
Figure 1:
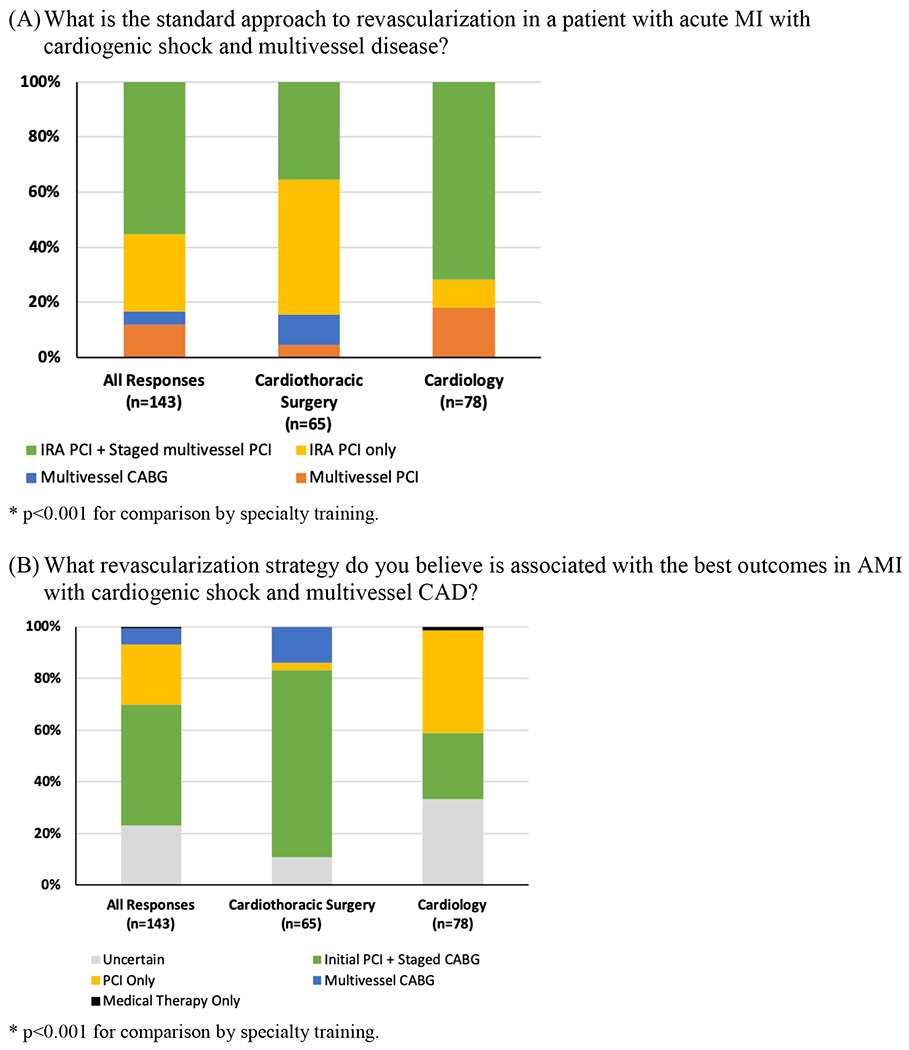
Participant responses to the questions: “What is the standard approach to revascularization in a patient with acute MI with cardiogenic shock and multivessel disease?” (Panel A) and “What revascularization strategy do you believe is associated with the best outcomes in AMI with cardiogenic shock and multivessel CAD?” (Panel B).
Among cardiologists who completed the survey, common MCS therapies used after PCI in MI with multivessel CAD and CS included the Impella percutaneous left ventricular assist device (VAD) (85.9% of respondents), intra-aortic balloon pump (IABP) (55.1%), ECMO (43.6%), and surgical VADs (21.8%). However, 76.9% of cardiologists preferred Impella, 12.8% preferred IABP, and 7.7% preferred ECMO for MCS after PCI for CS. Survey respondents reported that patients with residual cardiogenic shock after PCI were also commonly treated with norepinephrine (75.6%), dobutamine (41.0%), dopamine (37.2%), vasopressin (34.6%), and epinephrine (23.1%).
Based on responses from all survey participants, including cardiologists and cardiothoracic surgeons, MCS therapy after CABG for MI, multivessel CAD, and CS frequently included ECMO (62.2%), Impella (51.7%), and surgical VAD (20.3%). However, the preferred MCS strategy after CABG was IABP in 34.3%, Impella in 32.2%, and ECMO in 28%. Participants reported that patients with residual cardiogenic shock after CABG were also commonly treated with norepinephrine (75.5%), epinephrine (67.8%), vasopressin (50.3%), milrinone (50.3%) and dobutamine (49.0%) at their centers. Approaches to circulatory support after PCI and CABG in MI with multivessel CAD complicated by CS are shown in Table 2.
Table 2.
Circulatory support after PCI and CABG in MI with multivessel CAD complicated by cardiogenic shock *
| Circulatory Support in MI with Multivessel CAD Complicated by Cardiogenic Shock | CABG (n=143) | PCI (n=78)* | p-value |
|---|---|---|---|
| In patients with MI, cardiogenic shock, and multivessel disease undergoing revascularization by PCI or CABG, what is the preferred method of mechanical circulatory support at your center? | |||
| IABP | 49 (34.3%) | 10 (12.8%) | <0.001 |
| Impella | 46 (32.2%) | 60 (76.9%) | |
| ECMO | 40 (28%) | 6 (7.7%) | |
| Surgical VAD | 3 (2.1%) | 0 (0%) | |
| Other | 2 (1.4%) | 0 (0%) | |
| No Response | 3 (2.1%) | 2 (2.6%) | |
| Is there a minimum lactate threshold for which you would consider placement of a mechanical circulatory support device (other than IABP)? | |||
| Yes | 30 (21%) | 20 (25.6%) | |
| 2-4 mmol/L | 10 (33.3%) | 8 (40.0%) | 0.22 † |
| 4-10 mmol/L | 17 (56.7%) | 10 (50.0%) | |
| 10-20 mmol/L | 2 (6.7%) | 0 (0%) | |
| Unknown | 1 (3.3%) | 2 (10.0%) | |
| No | 108 (75.5%) | 58 (74.4%) | |
| Unknown | 5 (3.5%) | 0 (0%) | |
| In patients with residual cardiogenic shock after PCI or CABG, which of the following interventions do you commonly use for circulatory / hemodynamic support? | |||
| Norepinephrine | 108 (75.5%) | 59 (75.6%) | 0.89 |
| Epinephrine | 97 (67.8%) | 18 (23.1%) | <0.001 |
| Vasopressin | 72 (50.3%) | 27 (34.6%) | 0.035 |
| Milrinone | 72 (50.3%) | 18 (23.1%) | <0.001 |
| Dobutamine | 70 (49.0%) | 32 (41%) | 0.32 |
| Dopamine | 34 (23.8%) | 29 (37.2%) | 0.051 |
| Phenylephrine | 33 (23.1%) | 17 (21.8%) | 0.99 |
| Does your center have an algorithm for the stepwise escalation of hemodynamic / circulatory support post-PCI or post-CABG? | |||
| Yes | 23 (16.1%) | 29 (37.2%) | |
| No | 115 (80.4%) | 48 (61.5%) | 0.0012 |
| Unknown | 5 (3.5%) | 1 (1.3%) | |
Cardiology participants only
Discussion:
These data demonstrate heterogeneity in the contemporary approach to coronary revascularization, MCS use, and pharmacology among patients with MI, multivessel CAD and CS. Although most survey respondents indicated that PCI-based coronary revascularization strategies represented the current standard of practice, cardiothoracic surgeons were more likely than cardiologists to believe in superiority of CABG-based strategies for MI with CS and multivessel CAD. Among cardiothoracic surgeons who favored surgical revascularization for CS, most indicated that acute IRA PCI followed by CABG would yield the most favorable outcomes.
Approaches to MCS and pharmacology varied based on the preferred mode of coronary revascularization; most respondents reported ECMO and epinephrine were frequently selected for use after CABG, while cardiologists reported Impella and norepinephrine use for CS after PCI. In light of this heterogeneity, trials to establish optimal approaches to CS care are urgently needed. Ongoing randomized trials evaluating Impella (Danish-German cardiogenic shock trial [DanGer Shock, NCT01633502], planned enrollment: 360) and ECMO (Extracorporeal Life Support in Cardiogenic Shock trial [ECLS-SHOCK, NCT03637205], planned enrollment: 420; Testing the Value of Novel Strategy and Its Cost Efficacy in Order to Improve the Poor Outcomes in Cardiogenic Shock, [EUROSHOCK, NCT03813134], planned enrollment: 428) in patients with MI and CS will provide key insights into the role for MCS in CS.7 Still, prospective studies evaluating optimal pharmacology for MI with CS are lacking and the optimal approach to coronary revascularization remains uncertain. Outcomes associated with acute PCI of the infarct-artery with balloon angioplasty to restore coronary flow followed by urgent multivessel surgical revascularization with CABG versus initial infarct-artery only PCI with or without staged non-culprit PCI should be evaluated in a prospective manner, preferably in a randomized control trial.
Limitations:
There are a few limitations of this survey-based study. First, institutional practice patterns were based solely on survey respondents and were not independently verified. A greater proportion of cardiologists (94%) practiced in North America compared to cardiothoracic surgeons (63%) who completed the survey. Survey questions regarding common MCS and vasoactive therapies did not incorporate heterogeneity in clinical presentations of cardiogenic shock or define specific clinical scenarios. We did not query survey respondents regarding therapies that are not approved by the United States Food and Drug Administration (e.g. levosimendan). Combinations of MCS and vasoactive pharmacology were not evaluated. The survey included relatively few centers without heart failure or mechanical circulatory support programs and consequently, and the results of this survey are unlikely to reflect practice patterns at small, community hospitals. Invitations to complete the survey were distributed by email through some, but not all, relevant professional societies. Nevertheless, these data represent contemporary management strategies and perspectives on the optimal treatment of MI with multivessel CAD and CS from 120 hospitals worldwide.
Conclusion:
In conclusion, data from our survey indicate substantial heterogeneity in clinical care and provider uncertainty regarding the optimal management of patients with MI, multivessel CAD, and CS that suggest equipoise for future clinical trials.
Supplementary Material
Sponsor / Funding:
Dr. Smilowitz is supported in part by an NYU CTSA grant, UL1 TR001445 and KL2 TR001446, from the National Center for Advancing Translational Sciences, National Institutes of Health.
Disclosures:
Dr. Bangalore is on the advisory board for Abbott Vascular, Biotronik, Meril, Pfizer, and Amgen and has received research grants from Abbott Vascular. The other authors report no relationships that could be construed as a conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.van Diepen S, Katz JN, Albert NM, et al. Contemporary Management of Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation. 2017;136(16):e232–e268. [DOI] [PubMed] [Google Scholar]
- 2.Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med. 1999;341(9):625–634. [DOI] [PubMed] [Google Scholar]
- 3.Wayangankar SA, Bangalore S, McCoy LA, et al. Temporal Trends and Outcomes of Patients Undergoing Percutaneous Coronary Interventions for Cardiogenic Shock in the Setting of Acute Myocardial Infarction: A Report From the CathPCI Registry. JACC Cardiovasc Interv. 2016;9(4):341–351. [DOI] [PubMed] [Google Scholar]
- 4.Thiele H, Akin I, Sandri M, et al. PCI Strategies in Patients with Acute Myocardial Infarction and Cardiogenic Shock. N Engl J Med. 2017;377(25):2419–2432. [DOI] [PubMed] [Google Scholar]
- 5.Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367(14):1287–1296. [DOI] [PubMed] [Google Scholar]
- 6.Ouweneel DM, Eriksen E, Sjauw KD, et al. Percutaneous Mechanical Circulatory Support Versus Intra-Aortic Balloon Pump in Cardiogenic Shock After Acute Myocardial Infarction. J Am Coll Cardiol. 2017;69(3):278–287. [DOI] [PubMed] [Google Scholar]
- 7.Udesen NJ, Moller JE, Lindholm MG, et al. Rationale and design of DanGer shock: Danish-German cardiogenic shock trial. Am Heart J. 2019;214:60–68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


