Abstract
AMPA receptors (AMPARs) are fundamental elements in excitatory synaptic transmission and synaptic plasticity in the CNS. Long term potentiation (LTP), a form of synaptic plasticity which contributes to learning and memory formation, relies on the accumulation of AMPARs at the postsynapse. This phenomenon requires the coordinated recruitment of different elements in the AMPAR complex. Based on recent research reviewed herein, we propose an updated AMPAR trafficking and LTP model which incorporates both extracellular as well as intracellular mechanisms.
Keywords: AMPA receptor, Long-term potentiation, LTP, AMPAR amino-terminal domain, TARP, Synaptic plasticity
Introduction
It is a pleasure and honor to contribute to this special issue of Neuropharmacology that celebrates the landmark paper by Jeff Watkins and Dick Evans (Watkins and Evans, 1981). In reviewing the history of glutamatergic synaptic transmission this paper is a testament to the extraordinary power of pharmacology. Remarkably the cloning of the ionotropic glutamate receptors beautifully confirmed their pharmacological classification. AMPA receptors (AMPARs), the subject of this review, are expressed throughout the central nervous system, being present on virtually all central neurons. AMPARs mediate the vast majority of fast excitatory transmission in the brain and changes in the synaptic content of AMPARs underlie multiple forms of synaptic plasticity. Among these forms of synaptic plasticity, long-term potentiation (LTP) of excitatory synaptic transmission constitutes one of the most important molecular and cellular mechanisms underlying learning and memory formation (Martin et al., 2000; Nicoll, 2017). The mechanisms controlling (sub)synaptic AMPAR content directly determine synaptic strength, underlie LTP and several other forms of synaptic plasticity and are therefore tightly regulated.
Ever since its discovery (Bliss and Lomo, 1973; Lomo, 1966), multiple forms of LTP have been reported to exist throughout the CNS and the animal lifespan (Box 1). There is general agreement that NMDA receptor (NMDAR)-dependent LTP, the most widespread and functionally relevant form of LTP, relies on the synaptic incorporation of AMPARs (Kauer et al., 1988; Muller et al., 1988). This review focuses exclusively on the mechanisms of NMDAR-dependent LTP (Box 1). Admittedly, we do not list all the relevant work on AMPAR trafficking and LTP. Rather, we focus on some of the recent findings regarding the role played by different elements in the AMPAR complex which call for an updated subunit composition-dependent synaptic AMPAR trafficking model. Although it is likely that the molecular logic governing AMPAR trafficking discussed herein applies to multiple synapses, circuits and brain regions, we will discuss findings focusing on the hippocampal synapses formed between CA3 Schaffer collaterals and CA1 pyramidal neurons, one of the most paradigmatic excitatory synapses in the mammalian brain.
Box 1. Simplifying the experimental LTP problem.
To the outsider, the LTP literature seems extremely complex, verging on off putting. One of the problems is that the field has never explicitly settled on a precise definition for this phenomenon and it is clear that fundamentally distinct forms can exist at different synapses, e.g., hippocampal CA1 LTP and hippocampal mossy fiber LTP (Nicoll and Malenka, 1995; Nicoll and Schmitz, 2005). Most research on LTP comes from studies on the excitatory synapses onto CA1 hippocampal pyramidal cells. Given the possibility that differences exist at different synapses in the brain, it would seem prudent to focus one’s attention on the CA1 excitatory synapse where most of our knowledge already exists. Furthermore, it is generally agreed that it is the unique properties of the NMDAR, which mediates CA1 LTP, that make LTP such a compelling model of learning and memory (Martin et al., 2000; Nicoll, 2017). However, even at the CA1 synapse, multiple forms of NMDAR LTP have been postulated that depend on both the frequency and pattern of stimulation (e.g., 100 versus 200 Hz, theta burst stimulation etc.), the stimulus strength, the age of the animal, and the time after induction (Nicoll, 2017).
What strategies can be used in an attempt to simplify the problem? Part of the confusion in the study of LTP is the failure to appreciate that there are two separate questions regarding LTP. The first question is What controls the activation of the NMDAR? and the second question is What happens after activation of the NMDAR? It is well established that there are only two requirements for the induction of LTP: glutamate binding to the NMDAR and membrane depolarization. Most studies have used various forms of tetanic stimulation to cause the depolarization of the postsynaptic membrane. However, the effectiveness of the tetanus in depolarizing the neuron is influenced by a large number of variables. For instance, altering the level of GABAergic inhibition will have a profound effect on the degree to which a tetanus will depolarize the postsynaptic neuron and therefore contribute to NMDAR activation. In fact, any manipulation that affects the level of depolarization (e.g., postsynaptic excitability, number of synapses activated, presynaptic transmitter release, etc.) and therefore the degree of NMDAR activation will all affect LTP, but this has nothing to do with the central mechanisms underlying LTP. This confusion may well explain the long list of proteins postulated to mediate LTP (Sanes and Lichtman, 1999). To determine the essential requirements for LTP, the important question is What happens after NMDAR activation. Thus, in order to study LTP in a controlled fashion, one must be able to precisely control the depolarization. This is accomplished by a “pairing” protocol. Specifically, one uses cesium in the recording pipette to block potassium channels in the postsynaptic neuron, so the neuron can be held at a given membrane potential (e.g., 0 mV) during synaptic stimulation. If a manipulation blocks LTP, there are only two possible explanations. If it blocks NMDAR activation it tells us nothing new about LTP, but if the NMDAR is spared, the manipulation has identified a critical component of LTP.
AMPAR composition
AMPARs are assembled as heteromers comprising pore forming subunits (GluA1-GluA4) and auxiliary subunits. In most cases, the pore forming core is arranged as a dimer of edited GluA2 (GluA2 (R)), which makes the receptor calcium-impermeable and resistant to blockade by polyamines (but see (Bowie, 2012) for exceptions), and two other subunits (Traynelis et al., 2010). Selective knockout of endogenous AMPAR subunits coupled with electrophysiological characterization of the resulting AMPAR mediated EPSC revealed that, in pyramidal neurons in the mature hippocampal CA1 region, AMPARs are formed by two GluA2 (R) subunits and 2 either GluA1 (80%) or GluA3 (20%) subunits. Hence, approximately 80% of the receptors are GluA1/GluA2 heteromers and the remaining ones are GluA2/GluA3 heteromers (Lu et al., 2009). Clearly, the subunit composition of AMPARs depends on cell type, among other factors. For instance, interneurons, depending on subtype, express different levels of GluA1-4 (Angulo et al., 1997; Geiger et al., 1995). Recent cryo-EM structural data from native AMPARs emphasize this heterogeneity with the identification of a sizable pool of heterotrimeric receptors containing 2 GluA2 (R) subunits in positions B and D, and GluA1 and GluA3 subunits occupying the A and C positions, respectively (Zhao et al., 2019).
The AMPAR subunits have four structural subdivisions: an intracellular carboxy-terminal domain (CTD), a transmembrane domain (TM), an extracellular ligand binding domain (LBD) and, lastly, the amino-terminal domain (ATD). While the LBD and the TM, involved in glutamate binding and channel pore formation, respectively, show high sequence identity among AMPAR subunits and are highly evolutionarily conserved, the ATD and the CTD are highly sequence diverse (Garcia--Nafria et al., 2016). The CTD is the most sequence divergent part of the receptor and has been extensively studied in the past decades for its role in subunit selective AMPAR trafficking (Henley and Wilkinson, 2016; Huganir and Nicoll, 2013). The ATD comprises about 50% of the AMPAR protein and is crucial for receptor assembly: it guides subunit dimerization and is also involved in receptor oligomerization (Traynelis et al., 2010). In contrast to the NMDAR, where the critical roles played by the ATD in receptor function are well established, a role for the AMPAR ATD has remained elusive until recently, when its crucial function regulating receptor synaptic docking has emerged.
AMPAR synaptic clustering. General trafficking rules
AMPAR trafficking can be divided into 3 main steps (Opazo and Choquet, 2011). First, the biogenesis and oligomerization of the receptor complex, which includes assembly of the pore-forming subunits and the auxiliary subunits, e.g., TARPs and cornichons, and occurs in the endoplasmic reticulum (Schwenk and Fakler, 2020) and the constitutive exocytosis of the receptor, which maintains the pool of surface membrane receptors available to synapses. Second, the lateral diffusion of surface receptors to the synapse. The third step is the synaptic capture of laterally diffusing AMPARs by synaptic “AMPAR slots”, the nature of which is likely to entail interactions with synaptic scaffolding proteins, as discussed later in this review, and whose abundance or availability can be strongly influenced by changes in activity (Huganir and Nicoll, 2013; Opazo et al., 2012). Hence, surface AMPARs are localized in two different pools: extrasynaptic and synaptic. The extrasynaptic pool of receptors represents a sizable pool. For instance, nucleated patches of somatic membrane, which lack excitatory synapses, generate nAs of AMPAR current (Zamanillo et al., 1999) and dendritic outside out patch recordings (Andrasfalvy and Magee, 2004) have demonstrated the existence of a pool of dendritic extrasynaptic AMPARs. This reservoir of mobile AMPARs can laterally diffuse to the synapse to support activity-dependent increases in the postsynaptic AMPAR complement, such as with LTP induction (Granger et al., 2013; Lledo et al., 1998; Makino and Malinow, 2009; Patterson et al., 2010; Penn et al., 2017).
Synaptic AMPAR complexes are positioned strategically in the PSD via interactions with a variety of synaptic scaffolding proteins and possibly trans-synaptic adhesion proteins and other synaptic organizing elements. Given the relatively low affinity of AMPARs for glutamate it is critical that these receptors be positioned immediately across from the presynaptic active zone to maximize their responsiveness to presynaptic glutamate (Lisman and Raghavachari, 2006). In fact, the emerging model predicts that the density of receptors in the area directly opposed to the active zone release site, rather than the total number of receptors in the PSD, determines postsynaptic responses (Biederer et al., 2017).
Synapse size is directly correlated to its strength (Holler et al., 2021) and the number of receptors (Chen et al., 2015; Matsuzaki et al., 2001; Takumi et al., 1999). This provides a link between structural and physiological long-term potentiation (Bosch et al., 2014; Matsuzaki et al., 2004; Meyer et al., 2014). There is growing evidence that the PSD is not homogeneous but instead is comprised by several AMPAR enriched nanodomains (Hruska et al., 2018; MacGillavry et al., 2013; Nair et al., 2013) precisely aligned with presynaptic release sites in independent nanocolumns (Tang et al., 2016). These trans-synaptic nanocolumns might act as functional units which scale up or down in response to (and supporting) plasticity (Chen et al., 2018). Therefore, to understand synaptic plasticity it is essential to unveil the mechanisms whereby AMPARs are recruited and clustered within nanodomains at the synapse, which might involve the concerted action of several AMPAR domains, and multiple associated proteins inside and out of the cell.
Role of the GluA1 CTD in AMPAR synaptic clustering and LTP
Early studies using GluA1 and GluA2 knockout mice suggested a differential requirement of these two subunits for LTP. Whereas GluA1 is essential (Zamanillo et al., 1999) GluA2 is not (Jia et al., 1996). Based on the findings that the CTDs display the highest subunit divergence and are the least structured domains of the AMPARs, and that they are embedded in the protein matrix of the PSD, it was natural to focus on these domains as the substrate for subunit specific control of AMPAR trafficking and LTP. Two lines of research were pursued. The first focused on the role of PDZ domain interactions and the second focused on the role of phosphorylation.
A possible role of PDZ binding interactions playing a role in AMPAR function was first suggested by the finding that the PDZ binding motif (PBM) on the C-terminus of GluA1 bound to the MAGUK SAP97 (Leonard et al., 1998). Hayashi et al. reported that mutations in the PBM blocked LTP (Hayashi et al., 2000). However, it was later found that a knock in mouse lacking the PBM of GluA1 had normal LTP (Kim et al., 2005). More recent studies showed that the GluA1 PBM is not essential for AMPAR synaptic clustering (Granger et al., 2013; Kerr and Blanpied, 2012; Watson et al., 2020). Furthermore, deletion of the entire CTD does not impede LTP (Diaz-Alonso et al., 2020; Granger et al., 2013). Thus, a role for the PBM or for that matter the entire CTD of GluA1, as discussed below, remains unclear.
Different types of posttranslational modifications occurring at the GluA1 CTD, which may determine its trafficking behavior, have been identified. Phosphorylation is, undoubtedly, the most exhaustively characterized and a large body of literature has emerged on identifying kinases and their phosphorylation of the GluA1 CTD (Diering and Huganir, 2018; Roche et al., 1996; Shepherd and Huganir, 2007).
The possible role of AMPAR phosphorylation in determining the trafficking of the receptor grew out of the realization that kinases, and in particular Ca2+/calmodulin-dependent protein kinase II (CaMKII), play a critical role in LTP (Herring and Nicoll, 2016). CaMKII is the most abundant protein in the PSD, the alpha and beta isoforms combined representing ~8.5% of the protein content in the PSD (Sheng and Hoogenraad, 2007). CaMKII plays an essential role in excitatory synaptic transmission and plasticity. It supports both AMPAR and NMDAR basal synaptic transmission (Incontro et al., 2018) and LTP (Silva et al., 1992). LTP and CaMKII potentiate synaptic transmission by selectively enhancing AMPAR mediated synaptic transmission (Lledo et al., 1995; Pettit et al., 1994) and CaMKII occludes LTP (Ehrlich and Malinow, 2004; Poncer et al., 2002; Stein et al., 2003). Finally, it has recently been reported that brief activation of a photoactivatable CaMKII initiates LTP (Shibata et al., 2021). However, the specific mechanisms through which CaMKII mediates LTP are still a matter of debate (Nicoll, 2017).
CaMKII phosphorylates the GluA1 CTD (Barria et al., 1997a; Mammen et al., 1997), and this phosphorylation is increased during LTP (Barria et al., 1997b). Other phosphorylation sites in the GluA1 CTD mediated by different kinases have also been identified and a role in LTP was proposed (Esteban et al., 2003; Hayashi et al., 2000). However, whether GluA1 phosphorylation plays a necessary role in LTP is unclear, given several factors. Among these, i) Single point mutations in the best characterized phosphorylated residues, serine 831 and serine 845, show no impact on LTP in knock-in studies (Lee et al., 2010). The simultaneous mutation of these residues results in impaired LTP in adult, but not young mice (Lee et al., 2003). In overexpression studies, mutation of serine 845, but not serine 831, blocked LTP (Esteban et al., 2003). ii) Whether the phosphorylation levels of these residues in vivo are consistent with an essential role of these modifications in synaptic plasticity is controversial. A study found that less than 1% of GluA1 is phosphorylated at serine 831 and less than 0.1% at serine 845, which, given the number of receptors per synapse means that only between 0 and 1 AMPAR are phosphorylated at any given time per synapse on average (Hosokawa et al., 2015). In contrast, another study found that 15–20% of GluA1 is phosphorylated at either of these residues in basal conditions, and that stimulation can increase this proportion to up to 50% (Diering et al., 2016). iii) LTP can occur in the absence of the GluA1 CTD (Diaz-Alonso et al., 2020; Granger et al., 2013; Liu et al., 2020). A recent study challenged this notion using knock-in mouse lines in which the GluA1 and GluA2 CTDs were swapped. GluA1/A2 CTD mice showed impaired LTP (Zhou et al., 2018), although a subsequent study revealed that this effect depends on the induction protocol and the age of the mice employed (Box 1) (Liu et al., 2020). Surprisingly, these mice also show severely impaired hidden platform learning and memory in the Morris water maze, in striking contrast with GluA1 KO mice (Zamanillo et al., 1999) and with knock-in mice in which the GluA1 CTD is truncated (Diaz-Alonso et al., 2020). These findings suggest that perhaps the grafting the GluA2 CTD fundamentally changes GluA1 trafficking or function.
In summary, the phosphorylation of residues in the GluA1 CTD happens in vivo, but it is not essential for LTP. However, it could well play a modulatory role in some synaptic plasticity mechanisms in a synapse type, induction protocol and age dependent fashion (Box 1).
Role of the ATD in AMPAR synaptic clustering and LTP
Although the ATD comprises almost half of the AMPAR subunit polypeptide, its function is far from fully understood. During receptor biogenesis, in the ER, the ATD guides the formation of dimers (Ayalon and Stern-Bach, 2001; Clayton et al., 2009; Jin et al., 2009) and then the oligomerization, preferentially forming GluA2(R) containing, heteromeric receptors (Rossmann et al., 2011). However, the fact that the heterologous expression of GluA1 receptors lacking the ATD appear to function normally, clearly indicates that it is not required for basic AMPAR function (Diaz-Alonso et al., 2017; Tomita et al., 2007). What might its role be?
An initial awareness of the crucial role of the ATD in synaptic trafficking came from the reevaluation of the long-standing subunit specific, receptor-centric AMPAR trafficking model (Shi et al., 2001). This model, which has dominated the field during the last 20 years, predicts that GluA1-containing AMPARs are excluded from the synapse in the absence of a strong synaptic stimulation. The experiments leading to the proposal of this model relied on the overexpression of an ATD-tagged GluA1 subunit. The original results showed that GFP-GluA1 is excluded from the synapse in basal conditions, hence baseline AMPAR mediated EPSCs were interpreted to be mediated by GluA2/A3 containing AMPARs. Upon induction of LTP or expression of a constitutively active CaMKII mutant, AMPAR-mediated EPSCs showed synaptic rectification in GFP-GluA1 expressing cells (Hayashi et al., 2000; Shi et al., 2001), which indicates that GluA2(R)-lacking AMPARs (presumably homomeric overexpressed GFP-GluA1 receptors) are recruited to the synapse. To note, LTP in adult WT cells does not change rectification, i. e., does not involve the insertion of endogenous calcium permeable homomeric GluA1 receptors (Adesnik and Nicoll, 2007; Hayashi et al., 2000).
Hence, the receptor-centric model assumed that GluA1-containing AMPAR do not contribute to basal synaptic transmission and are only recruited during LTP expression. However, this notion was challenged in experiments where WT GluA1 was overexpressed in CA1 pyramidal neurons in hippocampal slice cultures. Baseline AMPAR mediated synaptic currents show strong rectification, indicating the effective insertion of overexpressed homomeric GluA1 receptors (Diaz-Alonso et al., 2017; Granger et al., 2013; Watson et al., 2017), but see (Nabavi et al., 2014), hence the ability of GluA1 containing receptors to participate in constitutive synaptic trafficking. Further corroborating this notion, chronic activity blockade with TTX does not prevent synaptic insertion of GluA1 (Diaz-Alonso et al., 2017; Watson, 2020). In contrast, cells overexpressing GFP-tagged GluA1 do not show rectification (Diaz-Alonso et al., 2017; Hayashi et al., 2000; Watson, 2020). Therefore, the ATD GFP tag fundamentally changes the synaptic trafficking behavior of the GluA1 subunit. GluA2 trafficking does not seem to be affected to the same degree as GluA1 by the presence of a GFP tag. We did not observe a significant difference in the trafficking of WT and GFP-tagged GluA2 (Diaz-Alonso et al., 2017), in agreement with (Nabavi et al., 2014). Watson et al. also report significant rectification in GFP-GluA2(Q) expressing cells, indicating that this modified subunit is readily expressed at the synapse. However, the increase in EPSC size observed with overexpression of WT GluA2 is absent (Watson et al., 2017).
Interestingly, in the original experiments, the exclusion of GFP-tagged GluA1 from synapses is not observed in dissociated neuron cultures (Shi et al., 1999), suggesting that perhaps the less complex extracellular environment does not pose a significant steric hindrance to synaptic docking of ATD-tagged receptors and further indicating that extracellular interactions involving the AMPAR ATD and some yet to be identified synaptic cleft moieties are key to the docking of AMPARs.
Reassuringly, the recent results with the GFP-AMPAR subunit fully recapitulate previous results. Both expression of constitutively active CaMKII and induction of LTP were able to promote synaptic insertion of GFP-GluA1 (Diaz-Alonso et al., 2017; Hayashi et al., 2000). How the signaling cascade triggered by CaMKII is able to circumvent the trafficking restriction imposed by the GFP tag remains to be elucidated. As further discussed later, AMPAR trafficking and synaptic anchoring have two main requisites. First, an intact extracellular ATD. Second, interactions between TARP auxiliary subunits and scaffolding proteins in the PSD (Chen et al., 2000; Watson, 2020; Zeng et al., 2019). CaMKII-dependent AMPAR trafficking is more likely to directly involve the second, although indirect action over the ATD, via a transmembrane or secreted factor, for instance, is also plausible.
Altogether, these observations suggest that the GFP tag can interfere with AMPAR ATD-dependent synaptic clustering, and constitute a cautionary note in experiments relying on extracellular GFP or similar tags to image AMPAR trafficking. The AMPAR extracellular region projects far into the synaptic cleft, at least halfway towards the presynaptic membrane (Greger et al., 2017). Therefore, it can participate in molecular interactions with extracellular elements, thereby contributing to the trans-synaptic clustering of AMPARs (Fig. 1). Indeed, although receptor function, assembly and surface delivery are intact in the absence of the ATD (Diaz-Alonso et al., 2017; Watson et al., 2017), ATD-lacking AMPAR subunit enrichment in dendritic spines is partially impaired compared to full length subunits. Furthermore, their localization in nanoclusters within the PSD appears more diffuse, in particular that of GluA1 (Watson, 2020). Finally, the truncation of the ATD leads to a severe impairment in GluA1 subunit synaptic trafficking and LTP (Diaz-Alonso et al., 2017; Jiang et al., 2021; Watson et al., 2017). In stark contrast, ATD-lacking GluA2 can rescue constitutive AMPAR trafficking and LTP (Diaz-Alonso et al., 2017; Jiang et al., 2021). A chimeric GluA1/A2ATD construct can rescue constitutive transmission in an AMPAR null background, but can’t support LTP (Jiang et al., 2021), further pointing to the ATD as a critical domain controlling GluA1, but not GluA2 trafficking. Given the dominance of the GluA1 ATD in heteromeric AMPAR trafficking (Diaz-Alonso et al., 2017), we hypothesize that the GluA1 ATD is essential for AMPAR trafficking and LTP in physiological conditions (Figs. 1 and 3).
Fig. 1.

Synaptic clustering mechanisms involving the extracellular AMPAR ATD.
AMPAR ATDs are essential for AMPAR mediated synaptic transmission. The existence and identity of extracellular AMPAR “slots” is yet to be demonstrated, but multiple lines of evidence point to that possibility, including the necessary role played by this domain in synaptic trafficking and LTP. Several elements of the synaptic cleft, which can potentially modulate AMPAR synaptic clustering by interacting with the ATD, are shown, such as: i) trans-synaptic adhesion proteins, including synaptic cell adhesion molecules (CAMs), neuroligin/neurexin, LRRTM/neurexin, etc.; ii) the extracellular matrix (ECM); iii) neuronal or glial-derived secreted factors, such as pentraxins, glypicans, etc. and iv) pre- and postsynaptic AMPAR ATD-interacting membrane-associated proteins spanning into the synaptic cleft.
Fig. 3.
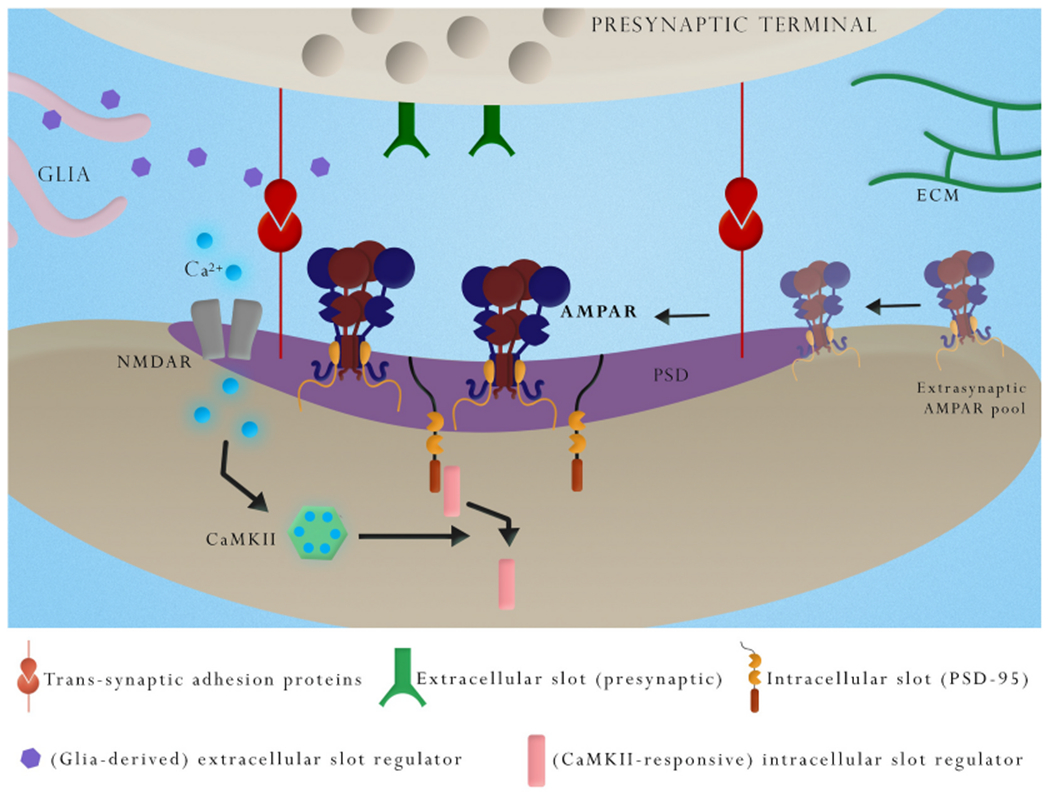
A simplified model for AMPAR accumulation during LTP mediated by increased intra- and extracellular slot availability.
Postsynaptic rise in Ca2+ upon NMDAR activation and subsequent CaMKII activity triggers LTP. Of the many targets of CaMKII identified over the past decades, the essential components mediating LTP are yet to be fully discerned. Ultimately, the stabilization of laterally diffusing AMPARs involves the concerted interactions between elements of the AMPAR complex such as the ATD and TARPs with extracellular and intracellular slots. The availability of extracellular slots might be regulated by pre-, post- or peri-synaptic ATD-interacting protein(s), trans-synaptic adhesion proteins, including synaptic cell adhesion molecules (CAMs), neuronal or glial-derived secreted proteins and extracellular matrix (ECM) elements. Availability of extracellular slots may be regulated by synaptic activity. Increased availability of intracellular AMPAR slots can also occur in an activity-regulated fashion. An attractive hypothetic mechanism involves the detachment of “masking” PDZ-binding proteins from PSD-95 by the action of CaMKII, thereby exposing TARP binding sites.
Why is the GluA1 ATD particularly important for AMPAR synaptic docking? There are several possibilities. First, an affinity reason: the AMPAR ATDs are highly sequence-divergent, with very little amino acid conservation in the surfaces exposed to the cleft. Hence, interactions with extracellular elements are potentially subunit-specific (Garcia--Nafria et al., 2016). Second, a structural reason: the ATD of subunits in positions A and C, typically occupied by GluA1 and/or GluA3, are more exposed to protein-protein interactions in the synaptic cleft compared to GluA2 subunits, almost exclusively found in positions B and D (Zhao et al., 2019). In summary, AMPAR synaptic clustering depends, perhaps, on subunit-specific and activity-regulated interactions with extracellular moieties. The nature of these “extracellular AMPAR slots” (Choquet, 2018; Diering and Huganir, 2018) is not yet fully clear and could involve, for instance, proteins anchored to the pre- or postsynapse, trans-synaptic adhesion proteins or proteins secreted into the cleft by neurons and glia (Fig. 1). There is some evidence for such interactions. Neuronal pentraxins are a family with 3 members, the secreted glycoproteins NP1/NPTX1 and NP2/NPTX2 (also referred to as neuronal activity-regulated pentraxin (Narp), and Neuronal pentraxin receptor NPR/NPTXR. NP1 can stabilize AMPAR at the synapse by interacting with the ATD. This mechanism seems to be particularly important for GluA4 containing AMPA receptors, such as those expressed by parvalbumin positive interneurons in the hippocampus (Pelkey et al., 2016; Sia et al., 2007). Glypicans, secreted by astrocytes, stimulate synaptic AMPAR clustering by promoting NP1 release from the presynaptic terminal (Farhy-Tselnicker et al., 2017). Recently, Suzuki et al. designed a synthetic synaptic organizer molecule consisting of a combination of structural elements from NP1 and cerebellin which holds promise for the treatment of a variety of excitatory synaptic dysfunctions (Suzuki et al., 2020). NP2 is an immediate-early gene (Tsui et al., 1996) and early studies already showed that it may play a role in synaptic plasticity by modulating AMPAR trafficking (O’Brien et al., 1999). NPR is able to cluster the secreted pentraxins at the synapse and has recently been shown to stimulate synaptogenesis presynaptically (Lee et al., 2017). Triple NP1/NP2/NPR knockout mice display severely impaired synaptogenesis onto GluA4 overexpressing cultured astrocytes (Sia et al., 2007). However, AMPAR clustering in cultured hippocampal neurons shows no substantial defect in triple KO vs WT (Bjartmar et al., 2006).
Unbiased proteomics-based approaches may lead to the discovery of more ATD binding proteins relevant to AMPAR docking and synaptic plasticity. A recent analysis of GluA1 ATD interacting proteins in the rat brain found that the cell adhesion molecule neuroplastin 65 can interact with the GluA1 ATD. This study shows that this interaction occurs in cis, i.e., in the postsynaptic membrane, and that it is necessary for GluA1 synaptic trafficking, whereas it does not affect that of the GluA2 subunit (Jiang et al., 2021).
The extracellular matrix regulates various forms of synaptic plasticity (Dityatev et al., 2010) and may participate in the dynamic control of AMPAR mobility, by imposing a more or less restrictive steric impediment to receptor lateral diffusion (Frischknecht et al., 2009), by modulating the availability of ATD interacting elements in the cleft or by directly interacting with the ATD (Fig. 1). Brevican, a key component of perineuronal nets which wrap parvalbumin positive interneurons, regulates GluA1 synaptic clustering and synaptic plasticity (Favuzzi et al., 2017). Whether the extracellular matrix controls synaptic AMPAR clustering via similar mechanisms in other cell types has not been yet elucidated. Other proteins shown to interact with GluA1 extracellularly, hence potentially involving the ATD, are Neuropilin-2/PlexinA3 (Wang et al., 2017) and LRRTM2 (de Wit et al., 2009). The GluA2 ATD has also been shown to participate in synaptic targeting via N-cadherin interaction (Saglietti et al., 2007).
Other important roles for the AMPAR ATD are emerging. Recently, the ATD has emerged as a receptor region involved in allosteric modulation, previously attributed mainly to the LBD (Jin et al., 2005; Lee et al., 2019). It has been proposed that the ATD contributes to AMPAR/TARP interaction in a subunit-composition dependent fashion (Cais et al., 2014) and that this interaction might participate in the TARP mediated control of AMPAR gating. The non-psychoactive cannabinoid cannabidiol (CBD) can bind to the GluA1 and GluA2 ATD and reduce evoked AMPAR EPSC size and mini EPSC amplitude and frequency (Yu et al., 2020). CBD is currently being used in combination with antiepileptic drugs in some forms of epilepsy such as Dravet syndrome and Lennox–Gastaut syndrome (Devinsky et al., 2017, 2018; Thiele et al., 2018). Part of its anti-seizure actions might be mediated by an ATD-dependent AMPAR allosteric inhibition (Yu et al., 2020).
Role of TARP/MAGUK interactions in AMPAR synaptic clustering and LTP
Native AMPARs at excitatory synapses exist tightly associated to auxiliary subunits, together forming AMPAR complexes. These auxiliary subunits modulate assembly, trafficking, gating and pharmacology (Greger et al., 2017; Jackson and Nicoll, 2011; Schwenk et al., 2012; Straub and Tomita, 2012). Some of the best characterized AMPAR auxiliary subunits are TARPs, cornichons, CKAMPs and GSG1L (Chen and Gouaux, 2019; Jacobi and von Engelhardt, 2021; Kamalova and Nakagawa, 2021; Schwenk et al., 2014; Schwenk et al., 2012). TARPs are the most widely expressed AMPAR auxiliary proteins and play an essential role in AMPAR function (Chen et al., 2000; Jackson and Nicoll, 2011; Tomita et al., 2003). Recent advances in single-particle cryo-electron microscopy have allowed the elucidation of the interactions, stoichiometry and functional implications of TARP/AMPAR complexes (Herguedas et al., 2019; Twomey et al., 2016; Zhao et al., 2019) (Fig. 2).
Fig. 2.

Synaptic clustering mechanisms involving multiple TARP C-tail domains.
A; diagram showing highly conserved and functionally relevant domains in the TARP cytoplasmic tail involved in the interaction with MAGUKs, based on TARP-γ-2, including a series of serine, arginine and hydrophobic amino acids, and a PDZ-binding motif. B; cartoon showing the recently proposed simplified TARP-MAGUK binding mechanism, which involves, in addition to the well documented PBM-PDZ 2 interactions, strong ionic interactions between a series of positively charged arginine residues in the TARP c-tail and negative charges on the surface of the PDZ 1 domain opposite to the binding pocket (Zeng et al., 2019). C; Functional consequences of TARP mutations that impact its binding to the PSD scaffolding protein PSD-95. Top, diagram illustrating simultaneous patch clamp recording from a transfected cell (green) and a neighboring control cell (black). Bottom, summary bar graph showing that removing the PBM (Δ4), replacing the arginines with alanine (R/A), replacing the serines with aspartate (S/D) and replacing the hydrophobic residues ϕ123 with serine (ϕ/S), all decreased AMPAR mediated EPSCs in transfected vs control cells. These physiological results parallel the effects of these mutations on the binding of TARP to PSD-95. *, p < 0.05; **, p < 0.01 and ***, p < 0.001 versus control condition. #, p < 0.05 versus GluA1-γ-8_Δ4 condition. Image partially reproduced from Zeng et al. (2019).
The membrane-associated guanylyl kinase (MAGUK) family of scaffolding proteins plays an essential role in ionotropic glutamate receptor localization. Simultaneous knockdown of PSD-93, PSD-95, and SAP102 results in severely reduced AMPAR and NMDAR-mediated EPSCs (Levy et al., 2015). PSD-95 is the key postsynaptic organizer and plays a fundamental role in clustering AMPARs at the synapse. Overexpression of PSD-95 results in a robust and specific enhancement of AMPAR-mediated EPSCs (El-Husseini et al., 2000; Schnell et al., 2002), which mimics and occludes LTP (Ehrlich and Malinow, 2004; Stein et al., 2003), and PSD-95 loss of function results in decreased AMPAR EPSCs (Beique et al., 2006; Elias et al., 2006). TARP-PSD-95 interaction stabilizes AMPAR complexes at the PSD (Bats et al., 2007; Schnell et al., 2002). These interactions, which involve PDZ domains in PSD-95 and PBMs in TARPs (Fig. 2), constitute the most plausible “AMPAR slots” in the PSD and their availability regulates access of AMPAR to the PSD both in basal synaptic transmission and during LTP (Figs. 2 and 3). One study found that the PBM in TARP γ-8 is critical for constitutive synaptic transmission but not for LTP using a Δ-PBM TARP γ–8 knock-in mouse (Sumioka et al., 2011). However, other TARPs expressed in CA1 neurons might influence this finding. In order to study the contribution of different protein domains in a particular TARP to AMPAR synaptic clustering without the confounding presence of other TARPs, endogenous AMPARs were replaced by AMPAR-TARP tethered constructs in an AMPAR null background (Shi et al., 2009). Tethering virtually excludes the possibility of endogenous TARPs binding to the expressed AMPARs (Fig. 2). In these experimental conditions, the TARP C-terminal PBM is essential for baseline AMPAR transmission and LTP (Sheng et al., 2018; Zeng et al., 2019), in agreement with previous data (Bats et al., 2007; Chen et al., 2000; Schnell et al., 2002). However, surprisingly, isothermal titration calorimetry (ITC) measurements revealed that the isolated TARP PBM and MAGUKs interact with low affinity, insufficient to explain the physiological relevance of this interaction, whereas PSD-95 interacts with the entire TARP C-tail with an affinity almost 2 orders of magnitude higher, sufficient to drive spontaneous condensation via liquid-liquid phase transition with other PSD proteins (Zeng et al., 2019). This work also identified other motifs in the TARP C-tail which make a substantial contribution to MAGUK binding and AMPAR synaptic clustering, including a stretch of arginine- and serine-rich sequences and a small number of aromatic residue-containing hydrophobic sites. Mutation of these motifs results in impaired AMPAR trafficking and (at least in the case of the arginine residues) LTP (Fig. 2 (Zeng et al., 2019),). Thus, a multivalent binding model was proposed in which the TARP PBM binds to PDZ2 of PSD-95 and the more proximal region of the CTD of TARPs binds to PDZ1.
Therapeutic targeting of AMPAR complexes
Given its central role in excitatory synaptic transmission throughout the CNS, altered AMPAR trafficking and function can have devastating consequences and result in psychiatric and neurological conditions as well as cognitive dysfunction (Henley and Wilkinson, 2016). Therefore, AMPARs are highly sought-after drug targets for therapies. However, AMPAR subunits expression is widespread, consistent with their essential role in mediating glutamatergic synaptic transmission. Hence, although there are pharmacological tools directly acting on AMPAR subunits currently used in the clinic, such as perampanel for epilepsy, their blockade, not unexpectantly, has several associated side effects, such as dizziness and ataxia (Zwart et al., 2014).
TARPs, on the other hand, have redundant functions (Menuz et al., 2008), but relatively non-overlapping expression domains, with TARP γ–8 highly enriched in the hippocampus and cerebral cortex and TARP γ–2 in the cerebellum, for example (Fukaya et al., 2005; Schwenk et al., 2014; Tomita et al., 2003). Hence, TARPs are promising targets for region-specific AMPAR modulation, since drugs can be designed to affect specifically the pool of AMPARs associated with a particular TARP. For instance, LY3130481, a drug which targets specifically forebrain TARP γ–8 associated AMPARs shows promising results as an antiepileptic drug in preclinical studies without the motor side effects associated with AMPAR antagonists (Kato et al., 2016; Maher et al., 2016).
Concluding remarks
In conclusion, synaptic clustering of AMPARs is a fundamental process directly determining synaptic strength and is central to LTP and other synaptic plasticity phenomena. Recent evidence discussed in this review points to mechanisms involving the AMPAR ATD and the AMPAR auxiliary proteins TARPs as the essential orchestrators of this process. This body of research supports an updated slot model for synaptic AMPAR trafficking which considers both intracellular as well as extracellular interactions, which in concert precisely direct and hold AMPARs at the synapse. Numerous questions remain to be elucidated regarding the trans-synaptic positioning of AMPARs, the coordination of intracellular and extracellular mechanisms and how these phenomena respond to synaptic plasticity. Answering these questions will expand our understanding of the molecular neurobiology of learning and memory and other brain functions. Furthermore, it will provide insights potentially relevant to efforts towards the efficient targeting of AMPAR in multiple disease states.
Acknowledgements
We thank Gerardo Sandoval for his help with figures. This work was supported by grants R01MH070957 and R01MH117139 (to RAN), and K99/R00 MH118425 and institutional setup funds from the University of California, Irvine (to JD-A).
References
- Adesnik H, Nicoll RA, 2007. Conservation of glutamate receptor 2-containing AMPA receptors during long-term potentiation. J. Neurosci. : the official journal of the Society for Neuroscience 27, 4598–4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrasfalvy BK, Magee JC, 2004. Changes in AMPA receptor currents following LTP induction on rat CA1 pyramidal neurones. J. Physiol 559, 543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angulo MC, Lambolez B, Audinat E, Hestrin S, Rossier J, 1997. Subunit composition, kinetic, and permeation properties of AMPA receptors in single neocortical nonpyramidal cells. J. Neurosci. : the official journal of the Society for Neuroscience 17, 6685–6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayalon G, Stern-Bach Y, 2001. Functional assembly of AMPA and kainate receptors is mediated by several discrete protein-protein interactions. Neuron 31, 103–113. [DOI] [PubMed] [Google Scholar]
- Barria A, Derkach V, Soderling T, 1997a. Identification of the Ca2+/calmodulin-dependent protein kinase II regulatory phosphorylation site in the alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate-type glutamate receptor. J. Biol. Chem 272, 32727–32730. [DOI] [PubMed] [Google Scholar]
- Barria A, Muller D, Derkach V, Griffith LC, Soderling TR, 1997b. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science 276, 2042–2045. [DOI] [PubMed] [Google Scholar]
- Bats C, Groc L, Choquet D, 2007. The interaction between Stargazin and PSD-95 regulates AMPA receptor surface trafficking. Neuron 53, 719–734. [DOI] [PubMed] [Google Scholar]
- Beique JC, Lin DT, Kang MG, Aizawa H, Takamiya K, Huganir RL, 2006. Synapse-specific regulation of AMPA receptor function by PSD-95. Proc. Natl. Acad. Sci. U. S. A 103, 19535–19540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederer T, Kaeser PS, Blanpied TA, 2017. Transcellular nanoalignment of synaptic function. Neuron 96, 680–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjartmar L, Huberman AD, Ullian EM, Renteria RC, Liu X, Xu W, Prezioso J, Susman MW, Stellwagen D, Stokes CC, et al. , 2006. Neuronal pentraxins mediate synaptic refinement in the developing visual system. J. Neurosci. : the official journal of the Society for Neuroscience 26, 6269–6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Lomo T, 1973. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol 232, 331–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch M, Castro J, Saneyoshi T, Matsuno H, Sur M, Hayashi Y, 2014. Structural and molecular remodeling of dendritic spine substructures during long-term potentiation. Neuron 82, 444–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie D, 2012. Redefining the classification of AMPA-selective ionotropic glutamate receptors. J. Physiol 590, 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cais O, Herguedas B, Krol K, Cull-Candy SG, Farrant M, Greger IH, 2014. Mapping the interaction sites between AMPA receptors and TARPs reveals a role for the receptor N-terminal domain in channel gating. Cell Rep. 9, 728–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Tang AH, Blanpied TA, 2018. Subsynaptic spatial organization as a regulator of synaptic strength and plasticity. Curr. Opin. Neurobiol 51, 147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, Wenthold RJ, Bredt DS, Nicoll RA, 2000. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature 408, 936–943. [DOI] [PubMed] [Google Scholar]
- Chen S, Gouaux E, 2019. Structure and mechanism of AMPA receptor - auxiliary protein complexes. Curr. Opin. Struct. Biol 54, 104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Levy JM, Hou A, Winters C, Azzam R, Sousa AA, Leapman RD, Nicoll RA, Reese TS, 2015. PSD-95 family MAGUKs are essential for anchoring AMPA and NMDA receptor complexes at the postsynaptic density. Proc. Natl. Acad. Sci. U. S. A 112, E6983–E6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choquet D, 2018. Linking nanoscale dynamics of AMPA receptor organization to plasticity of excitatory synapses and learning. J. Neurosci.: the official journal of the Society for Neuroscience 38, 9318–9329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton A, Siebold C, Gilbert RJ, Sutton GC, Harlos K, McIlhinney RA, Jones EY, Aricescu AR, 2009. Crystal structure of the GluR2 amino-terminal domain provides insights into the architecture and assembly of ionotropic glutamate receptors. J. Mol. Biol 392, 1125–1132. [DOI] [PubMed] [Google Scholar]
- de Wit J, Sylwestrak E, O’Sullivan ML, Otto S, Tiglio K, Savas JN, Yates JR 3rd, Comoletti D, Taylor P, Ghosh A, 2009. LRRTM2 interacts with Neurexin1 and regulates excitatory synapse formation. Neuron 64, 799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Cross JH, Laux L, Marsh E, Miller I, Nabbout R, Scheffer IE, Thiele EA, Wright S, Cannabidiol in Dravet Syndrome Study, G., 2017. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N. Engl. J. Med 376, 2011–2020. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Patel AD, Cross JH, Villanueva V, Wirrell EC, Privitera M, Greenwood SM, Roberts C, Checketts D, VanLandingham KE, et al. , 2018. Effect of cannabidiol on drop seizures in the lennox-gastaut syndrome. N. Engl. J. Med 378, 1888–1897. [DOI] [PubMed] [Google Scholar]
- Diaz-Alonso J, Morishita W, Incontro S, Simms J, Holtzman J, Gill M, Mucke L, Malenka RC, Nicoll RA, 2020. Long-term potentiation is independent of the C-tail of the GluA1 AMPA receptor subunit. eLife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Alonso J, Sun YJ, Granger AJ, Levy JM, Blankenship SM, Nicoll RA, 2017. Subunit-specific role for the amino-terminal domain of AMPA receptors in synaptic targeting. Proc. Natl. Acad. Sci. U. S. A 114, 7136–7141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diering GH, Heo S, Hussain NK, Liu B, Huganir RL, 2016. Extensive phosphorylation of AMPA receptors in neurons. Proc. Natl. Acad. Sci. U. S. A 113, E4920–E4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diering GH, Huganir RL, 2018. The AMPA receptor code of synaptic plasticity. Neuron 100, 314–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dityatev A, Schachner M, Sonderegger P, 2010. The dual role of the extracellular matrix in synaptic plasticity and homeostasis. Nat. Rev. Neurosci 11, 735–746. [DOI] [PubMed] [Google Scholar]
- Ehrlich I, Malinow R, 2004. Postsynaptic density 95 controls AMPA receptor incorporation during long-term potentiation and experience-driven synaptic plasticity. J. Neurosci. : the official journal of the Society for Neuroscience 24, 916–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Husseini AE, Sehnell E, Chetkovich DM, Nicoll RA, Bredt DS, 2000. PSD-95 involvement in maturation of excitatory synapses. Science 290, 1364–1368. [PubMed] [Google Scholar]
- Elias GM, Funke L, Stein V, Grant SG, Bredt DS, Nicoll RA, 2006. Synapse-specific and developmentally regulated targeting of AMPA receptors by a family of MAGUK scaffolding proteins. Neuron 52, 307–320. [DOI] [PubMed] [Google Scholar]
- Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R, 2003. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat. Neurosci 6, 136–143. [DOI] [PubMed] [Google Scholar]
- Farhy-Tselnicker I, van Casteren ACM, Lee A, Chang VT, Aricescu AR, Allen NJ, 2017. Astrocyte-secreted glypican 4 regulates release of neuronal pentraxin 1 from axons to induce functional synapse formation. Neuron 96, 428–445 e413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favuzzi E, Marques-Smith A, Deogracias R, Winterflood CM, Sanchez-Aguilera A, Mantoan L, Maeso P, Fernandes C, Ewers H, Rico B, 2017. Activity-dependent gating of parvalbumin interneuron function by the peri neuronal net protein brevican. Neuron 95, 639–655 e610. [DOI] [PubMed] [Google Scholar]
- Frischknecht R, Heine M, Perrais D, Seidenbecher CI, Choquet D, Gundelfinger ED, 2009. Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity. Nat. Neurosci 12, 897–904. [DOI] [PubMed] [Google Scholar]
- Fukaya M, Yamazaki M, Sakimura K, Watanabe M, 2005. Spatial diversity in gene expression for VDCCgamma subunit family in developing and adult mouse brains. Neurosci. Res 53, 376–383. [DOI] [PubMed] [Google Scholar]
- Garcia-Nafria J, Herguedas B, Watson JF, Greger IH, 2016. The dynamic AMPA receptor extracellular region: a platform for synaptic protein interactions. J. Physiol 594, 5449–5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger JR, Melcher T, Koh DS, Sakmann B, Seeburg PH, Jonas P, Monyer H, 1995. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron 15, 193–204. [DOI] [PubMed] [Google Scholar]
- Granger AJ, Shi Y, Lu W, Cerpas M, Nicoll RA, 2013. LTP requires a reserve pool of glutamate receptors independent of subunit type. Nature 493, 495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greger IH, Watson JF, Cull-Candy SG, 2017. Structural and functional architecture of AMPA-type glutamate receptors and their auxiliary proteins. Neuron 94, 713–730. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R, 2000. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science 287, 2262–2267. [DOI] [PubMed] [Google Scholar]
- Henley JM, Wilkinson KA, 2016. Synaptic AMPA receptor composition in development, plasticity and disease. Nat. Rev. Neurosci 17, 337–350. [DOI] [PubMed] [Google Scholar]
- Herguedas B, Watson JF, Ho H, Cais O, Garcia-Nafria J, Greger IH, 2019. Architecture of the heteromeric GluA1/2 AMPA receptor in complex with the auxiliary subunit TARP garnma8. Science 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring BE, Nicoll RA, 2016. Long-term potentiation: from CaMKII to AMPA receptor trafficking. Annu. Rev. Physiol 78, 351–365. [DOI] [PubMed] [Google Scholar]
- Holler S, Kostinger G, Martin KAC, Schuhknecht GFP, Stratford KJ, 2021. Structure and function of a neocortical synapse. Nature. [DOI] [PubMed] [Google Scholar]
- Hosokawa T, Mitsushima D, Kaneko R, Hayashi Y, 2015. Stoichiometry and phosphoisotypes of hippocampal AMPA-type glutamate receptor phosphorylation. Neuron 85, 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruska M, Henderson N, Le Marchand SJ, Jafri H, Dalva MB, 2018. Synaptic nanomodules underlie the organization and plasticity of spine synapses. Nat. Neurosci 21, 671–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huganir RL, Nicoll RA, 2013. AMPARs and synaptic plasticity: the last 25 years. Neuron 80, 704–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incontro S, Diaz-Alonso J, Iafrati J, Vieira M, Asensio CS, Sohal VS, Roche KW, Bender KJ, Nicoll RA, 2018. The CaMKII/NMDA receptor complex controls hippocampal synaptic transmission by kinase-dependent and independent mechanisms. Nat. Commun 9, 2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AC, Nicoll RA, 2011. The expanding social network of ionotropic glutamate receptors: TARPs and other transmembrane auxiliary subunits. Neuron 70, 178–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobi E, von Engelhardt J, 2021. Modulation of information processing by AMPA receptor auxiliary subunits. J. Physiol 599, 471–483. [DOI] [PubMed] [Google Scholar]
- Jia Z, Agopyan N, Miu P, Xiong Z, Henderson J, Gerlai R, Taverna FA, Velumian A, MacDonald J, Carlen P, et al. , 1996. Enhanced LTP in mice deficient in the AMPA receptor GluR2. Neuron 17, 945–956. [DOI] [PubMed] [Google Scholar]
- Jiang CH, Wei M, Zhang C, Shi YS, 2021. The amino-terminal domain of GluA1 mediates LTP maintenance via interaction with neuroplastin-65. Proc. Natl. Acad. Sci. U. S. A 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R, Clark S, Weeks AM, Dudman JT, Gouaux E, Partin KM, 2005. Mechanism of positive allosteric modulators acting on AMPA receptors. J. Neurosci.: the official journal of the Society for Neuroscience 25, 9027–9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R, Singh SK, Gu S, Furukawa H, Sobolevsky AI, Zhou J, Jin Y, Gouaux E, 2009. Crystal structure and association behaviour of the GluR2 ami no-terminal domain. EMBO J. 28, 1812–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamalova A, Nakagawa T, 2021. AMPA receptor structure and auxiliary subunits. J. Physiol 599, 453–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato AS, Burris KD, Gardinier KM, Gernert DL, Porter WJ, Reel J, Ding C, Tu Y, Schober DA, Lee MR, et al. , 2016. Forebrain-selective AMPA-receptor antagonism guided by TARP gamma-8 as an antiepileptic mechanism. Nat. Med 22, 1496–1501. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC, Nicoll RA, 1988. A persistent postsynaptic modification mediates long-term potentiation in the hippocampus. Neuron 1, 911–917. [DOI] [PubMed] [Google Scholar]
- Kerr JM, Blanpied TA, 2012. Subsynaptic AMPA receptor distribution is acutely regulated by actin-driven reorganization of the postsynaptic density. J. Neurosci. : the official journal of the Society for Neuroscience 32, 658–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Takamiya K, Petralia RS, Sattler R, Yu S, Zhou W, Kalb R, Wenthold R, Huganir R, 2005. Persistent hippocampal CA1 LTP in mice lacking the C-terminal PDZ ligand of GluR1. Nat. Neurosci 8, 985–987. [DOI] [PubMed] [Google Scholar]
- Lee HK, Takamiya K, Han JS, Man H, Kim CH, Rumbaugh G, Yu S, Ding L, He C, Petralia RS, et al. , 2003. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell 112, 631–643. [DOI] [PubMed] [Google Scholar]
- Lee HK, Takamiya K, He K, Song L, Huganir RL, 2010. Specific roles of AMPA receptor subunit GluR1 (GluA1) phosphorylation sites in regulating synaptic plasticity in the CA1 region of hippocampus. J. Neurophysiol 103, 479–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Krieger J, Herguedas B, Garcia-Nafria J, Dutta A, Shaikh SA, Greger IH, Bahar I, 2019. Druggability simulations and X-ray crystallography reveal a ligand-binding site in the GluA3 AMPA receptor N-terminal domain. Structure 27, 241–252 e243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Wei M, Zhang C, Maxeiner S, Pak C, Calado Botelho S, Trotter J, Sterky FH, Sudhof TC, 2017. Presynaptic neuronal pentraxin receptor organizes excitatory and inhibitory synapses. J. Neurosci. : the official journal of the Society for Neuroscience 37, 1062–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard AS, Davare MA, Horne MC, Garner CC, Hell JW, 1998. SAP97 is associated with the alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR1 subunit. J. Biol. Chem 273, 19518–19524. [DOI] [PubMed] [Google Scholar]
- Levy JM, Chen X, Reese TS, Nicoll RA, 2015. Synaptic consolidation normalizes AMPAR quantal size following MAGUK loss. Neuron 87, 534–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Raghavachari S, 2006. A unified model of the presynaptic and postsynaptic changes during LTP at CA1 synapses. Sci. STKE, 2006, re11. [DOI] [PubMed] [Google Scholar]
- Liu A, Ji H, Ren Q, Meng Y, Zhang H, Collingride G, Xie W, Jia Z, 2020. The requirement of the C-terminal domain of GluA1 in different forms of long-term potentiation in the Hippocampus is age-dependent. Front. Synaptic Neurosci 12, 588785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lledo PM, Hjelmstad GO, Mukherji S, Soderling TR, Malenka RC, Nicoll RA, 1995. Calcium/calmodulin-dependent kinase II and long-term potentiation enhance synaptic transmission by the same mechanism. Proc. Natl. Acad. Sci. U. S. A 92, 11175–11179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lledo PM, Zhang X, Sudhof TC, Malenka RC, Nicoll RA, 1998. Postsynaptic membrane fusion and long-term potentiation. Science 279, 399–403. [DOI] [PubMed] [Google Scholar]
- Lomo T, 1966. Frequency potentiation of excitatory synaptic activity in the dentate area of the hippocampal formation. Acta Physiol. Scand 68, 128. [Google Scholar]
- Lu W, Shi Y, Jackson AC, Bjorgan K, During MJ, Sprengel R, Seeburg PH, Nicoll RA, 2009. Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron 62, 254–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGillavry HD, Song Y, Raghavachari S, Blanpied TA, 2013. Nanoscale scaffolding domains within the postsynaptic density concentrate synaptic AMPA receptors. Neuron 78, 615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher MP, Wu N, Ravula S, Ameriks MK, Savall BM, Liu C, Lord B, Wyatt RM, Matta JA, Dugovic C, et al. , 2016. Discovery and characterization of AMPA receptor modulators selective for TARP-gamma8. J. Pharmacol. Exp. Therapeut 357, 394–414. [DOI] [PubMed] [Google Scholar]
- Makino H, Malinow R, 2009. AMPA receptor incorporation into synapses during LTP: the role of lateral movement and exocytosis. Neuron 64, 381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammen AL, Kameyama K, Roche KW, Huganir RL, 1997. Phosphorylation of the alpha-amino-3-hydroxy-5-methylisoxazole4-propionic acid receptor GluR1 subunit by calcium/calmodulin-dependent kinase II. J. Biol. Chem 272, 32528–32533. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG, 2000. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu. Rev. Neurosci 23, 649–711. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Ellis-Davies GC, Nemoto T, Miyashita Y, lino M, Kasai H, 2001. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat. Neurosci 4, 1086–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H, 2004. Structural basis of long-term potentiation in single dendritic spines. Nature 429, 761–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menuz K, O’Brien JL, Karmizadegan S, Bredt DS, Nicoll RA, 2008. TARP redundancy is critical for maintaining AMPA receptor function. J. Neurosci. : the official journal of the Society for Neuroscience 28, 8740–8746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D, Bonhoeffer T, Scheuss V, 2014. Balance and stability of synaptic structures during synaptic plasticity. Neuron 82, 430–443. [DOI] [PubMed] [Google Scholar]
- Muller D, Joly M, Lynch G, 1988. Contributions of quisqualate and NMDA receptors to the induction and expression of LTP. Science 242, 1694–1697. [DOI] [PubMed] [Google Scholar]
- Nabavi S, Fox R, Alfonso S, Aow J, Malinow R, 2014. GluA1 trafficking and metabotropic NMDA: addressing results from other laboratories inconsistent with ours. Phil. Trans. Roy. Soc. Lond. B Biol. Sci 369, 20130145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair D, Hosy E, Petersen JD, Constals A, Giannone G, Choquet D, Sibarita JB, 2013. Super-resolution imaging reveals that AMPA receptors inside synapses are dynamically organized in nanodomains regulated by PSD95. J. Neurosci. : the official journal of the Society for Neuroscience 33, 13204–13224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, 2017. A brief history of long-term potentiation. Neuron 93, 281–290. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Malenka RC, 1995. Contrasting properties of two forms of long-term potentiation in the hippocampus. Nature 377, 115–118. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Schmitz D, 2005. Synaptic plasticity at hippocampal mossy fibre synapses. Nat. Rev. Neurosci 6, 863–876. [DOI] [PubMed] [Google Scholar]
- O’Brien RJ, Xu D, Petralia RS, Steward O, Huganir RL, Worley P, 1999. Synaptic clustering of AMPA receptors by the extracellular immediate-early gene product Narp. Neuron 23, 309–323. [DOI] [PubMed] [Google Scholar]
- Opazo P, Choquet D, 2011. A three-step model for the synaptic recruitment of AMPA receptors. Molecular and cellular neurosciences 46, 1–8. [DOI] [PubMed] [Google Scholar]
- Opazo P, Sainlos M, Choquet D, 2012. Regulation of AMPA receptor surface diffusion by PSD-95 slots. Curr. Opin. Neurobiol 22, 453–460. [DOI] [PubMed] [Google Scholar]
- Patterson MA, Szatmari EM, Yasuda R, 2010. AMPA receptors are exocytosed in stimulated spines and adjacent dendrites in a Ras-ERK-dependent manner during long-term potentiation. Proc. Natl. Acad. Sci. U. S. A 107, 15951–15956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkey KA, Barksdale E, Craig MT, Yuan X, Sukumaran M, Vargish GA, Mitchell RM, Wyeth MS, Petralia RS, Chittajallu R, et al. , 2016. Pentraxins coordinate excitatory synapse maturation and circuit integration of parvalbumin interneurons. Neuron 90, 661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn AC, Zhang CL, Georges F, Royer L, Breillat C, Hosy E, Petersen JD, Humeau Y, Choquet D, 2017. Hippocampal LTP and contextual learning require surface diffusion of AMPA receptors. Nature 549, 384–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit DL, Perlman S, Malinow R, 1994. Potentiated transmission and prevention of further LTP by increased CaMKII activity in postsynaptic hippocampal slice neurons. Science 266, 1881–1885. [DOI] [PubMed] [Google Scholar]
- Poncer JC, Esteban JA, Malinow R, 2002. Multiple mechanisms for the potentiation of AMPA receptor-mediated transmission by alpha-Ca2+/calmodulin-dependent protein kinase II. J. Neurosci.: the official journal of the Society for Neuroscience 22, 4406–4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche KW, O’Brien RJ, Mammen AL, Bernhardt J, Huganir RL, 1996. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron 16, 1179–1188. [DOI] [PubMed] [Google Scholar]
- Rossmann M, Sukumaran M, Penn AC, Veprintsev DB, Babu MM, Greger IH, 2011. Subunit-selective N-terminal domain associations organize the formation of AMPA receptor heteromers. EMBO J. 30, 959–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saglietti L, Dequidt C, Kamieniarz K, Rousset MC, Valnegri P, Thoumine O, Beretta F, Fagni L, Choquet D, Sala C, et al. , 2007. Extracellular interactions between GluR2 and N-cadherin in spine regulation. Neuron 54, 461–477. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW, 1999. Can molecules explain long-term potentiation? Nat. Neurosci 2, 597–604. [DOI] [PubMed] [Google Scholar]
- Schnell E, Sizemore M, Karimzadegan S, Chen L, Bredt DS, Nicoll RA, 2002. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc. Natl. Acad. Sci. U. S. A 99, 13902–13907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenk J, Baehrens D, Haupt A, Bildl W, Boudkkazi S, Roeper J, Fakler B, Schulte U, 2014. Regional diversity and developmental dynamics of the AMPA-receptor proteome in the mammalian brain. Neuron 84, 41–54. [DOI] [PubMed] [Google Scholar]
- Schwenk J, Fakler B, 2020. Building of AMPA-type glutamate receptors in the endoplasmic reticulum and its implication for excitatory neurotransmission. J. Physiol [DOI] [PubMed] [Google Scholar]
- Schwenk J, Harmel N, Brechet A, Zolles G, Berkefeld H, Muller CS, Bildl W, Baehrens D, Huber B, Kulik A, et al. , 2012. High-resolution proteomics unravel architecture and molecular diversity of native AMPA receptor complexes. Neuron 74, 621–633. [DOI] [PubMed] [Google Scholar]
- Sheng M, Hoogenraad CC, 2007. The postsynaptic architecture of excitatory synapses: a more quantitative view. Annu. Rev. Biochem 76, 823–847. [DOI] [PubMed] [Google Scholar]
- Sheng N, Bemben MA, Diaz-Alonso J, Tao W, Shi YS, Nicoll RA, 2018. LTP requires postsynaptic PDZ-domain interactions with glutamate receptor/auxiliary protein complexes. Proc. Natl. Acad. Sci. U. S. A 115, 3948–3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JD, Huganir RL, 2007. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu. Rev. Cell Dev. Biol 23, 613–643. [DOI] [PubMed] [Google Scholar]
- Shi S, Hayashi Y, Esteban JA, Malinow R, 2001. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell 105, 331–343. [DOI] [PubMed] [Google Scholar]
- Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R, 1999. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science 284, 1811–1816. [DOI] [PubMed] [Google Scholar]
- Shi Y, Lu W, Milstein AD, Nicoll RA, 2009. The stoichiometry of AMPA receptors and TARPs varies by neuronal cell type. Neuron 62, 633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata ACE, Ueda HH, Eto K, Onda M, Sato A, Ohba T, Nabekura J, Murakoshi H, 2021. Photoactivatable CaMKII induces synaptic plasticity in single synapses. Nat. Commun 12, 751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sia GM, Beique JC, Rumbaugh G, Cho R, Worley PF, Huganir RL, 2007. Interaction of the N-terminal domain of the AMPA receptor GluR4 subunit with the neuronal pentraxin NP1 mediates GluR4 synaptic recruitment. Neuron 55, 87–102. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Stevens CF, Tonegawa S, Wang Y, 1992. Deficient hippocampal long-term potentiation in alpha-calcium-calmodulin kinase II mutant mice. Science 257, 201–206. [DOI] [PubMed] [Google Scholar]
- Stein V, House DR, Bredt DS, Nicoll RA, 2003. Postsynaptic density-95 mimics and occludes hippocampal long-term potentiation and enhances long-term depression. J. Neurosci. : the official journal of the Society for Neuroscience 23, 5503–5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub C, Tomita S, 2012. The regulation of glutamate receptor trafficking and function by TARPs and other transmembrane auxiliary subunits. Curr. Opin. Neurobiol 22, 488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumioka A, Brown TE, Kato AS, Bredt DS, Kauer JA, Tomita S, 2011. PDZ binding of TARPgamma-8 controls synaptic transmission but not synaptic plasticity. Nat. Neurosci 14, 1410–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Elegheert J, Song I, Sasakura H, Senkov O, Matsuda K, Kakegawa W, Clayton AJ, Chang VT, Ferrer-Ferrer M, et al. , 2020. A synthetic synaptic organizer protein restores glutamatergic neuronal circuits. Science 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takumi Y, Ramirez-Leon V, Laake P, Rinvik E, Ottersen OP, 1999. Different modes of expression of AMPA and NMDA receptors in hippocampal synapses. Nat. Neurosci 2, 618–624. [DOI] [PubMed] [Google Scholar]
- Tang AH, Chen H, Li TP, Metzbower SR, MacGillavry HD, Blanpied TA, 2016. A trans-synaptic nanocolumn aligns neurotransmitter release to receptors. Nature 536, 210–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele EA, Marsh ED, French JA, Mazurkiewicz-Beldzinska M, Benbadis SR, Joshi C, Lyons PD, Taylor A, Roberts C, Sommerville K, Group GS, 2018. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 391, 1085–1096. [DOI] [PubMed] [Google Scholar]
- Tomita S, Chen L, Kawasaki Y, Petralia RS, Wenthold RJ, Nicoll RA, Bredt DS, 2003. Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins. J. Cell Biol 161, 805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S, Shenoy A, Fukata Y, Nicoll RA, Bredt DS, 2007. Stargazin interacts functionally with the AMPA receptor glutamate-binding module. Neuropharmacology 52, 87–91. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R, 2010. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol. Rev 62, 405–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui CC, Copeland NG, Gilbert DJ, Jenkins NA, Barnes C, Worley PF, 1996. Narp, a novel member of the pentraxin family, promotes neurite outgrowth and is dynamically regulated by neuronal activity. J. Neurosci. : the official journal of the Society for Neuroscience 16, 2463–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twomey EC, Yelshanskaya MV, Grassucci RA, Frank J, Sobolevsky AI, 2016. Elucidation of AMPA receptor-stargazin complexes by cryo-electron microscopy. Science 353, 83–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Chiu SL, Koropouli E, Hong I, Mitchell S, Easwaran TP, Hamilton NR, Gustina AS, Zhu Q, Ginty DD, et al. , 2017. Neuropilin-2/PlexinA3 receptors associate with GluA1 and mediate Sema3F-dependent homeostatic scaling in cortical neurons. Neuron 96, 1084–1098 e1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins JC, Evans RH, 1981. Excitatory amino acid transmitters. Annu. Rev. Pharmacol. Toxicol 21, 165–204. [DOI] [PubMed] [Google Scholar]
- Watson JF, Ho H, Greger IH, 2017. Synaptic transmission and plasticity require AMPA receptor anchoring via its N-terminal domain. eLife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson JF, Pinggera A, Ho H, Greger IH, 2020. AMPA receptor anchoring at CA1 synapses is determined by an interplay of N- terminal domain and TARP γ8 interactions. bioRxiv preprint, 10.1101/2020.07.09.196154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Yang Z, Jin B, Qin X, Zhu X, Sun J, Huo L, Wang R, Shi Y, Jia Z, et al. , 2020. Cannabidiol inhibits febrile seizure by modulating AMPA receptor kinetics through its interaction with the N-terminal domain of GluA1/GluA2. Pharmacol. Res 161, 105128. [DOI] [PubMed] [Google Scholar]
- Zamanillo D, Sprengel R, Hvalby O, Jensen V, Burnashev N, Rozov A, Kaiser KM, Koster HJ, Borchardt T, Worley P, et al. , 1999. Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science 284, 1805–1811. [DOI] [PubMed] [Google Scholar]
- Zeng M, Diaz-Alonso J, Ye F, Chen X, Xu J, Ji Z, Nicoll RA, Zhang M, 2019. Phase separation-mediated TARP/MAGUK complex condensation and AMPA receptor synaptic transmission. Neuron 104, 529–543 e526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Chen S, Swensen AC, Qian WJ, Gouaux E, 2019. Architecture and subunit arrangement of native AMPA receptors elucidated by cryo-EM. Science 364, 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Liu A, Xia S, Leung C, Qi J, Meng Y, Xie W, Park P, Collingridge GL, Jia Z, 2018. The C-terminal tails of endogenous GluA1 and GluA2 differentially contribute to hippocampal synaptic plasticity and learning. Nat. Neurosci 21, 50–62. [DOI] [PubMed] [Google Scholar]
- Zwart R, Sher E, Ping X, Jin X, Sims JR Jr, Chappell AS, Gleason SD, Hahn PJ, Gardinier K, Gernert DL, et al. , 2014. Perampanel, an antagonist of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors, for the treatment of epilepsy: studies in human epileptic brain and nonepileptic brain and in rodent models. J. Pharmacol. Exp. Therapeut 351, 124–133. [DOI] [PubMed] [Google Scholar]


