Abstract
SET and MYND domain protein 2 (SMYD2) is a lysine methyltransferase that mediates histone H3 lysine 36 trimethylation (H3K36me3) and acts as a regulator of tumorgenesis and cystic growth. However, its role in renal fibrosis remains unknown. In this study, we found that SMYD2 was highly expressed in the murine kidney of renal fibrosis induced by unilateral ureteral obstruction, and primarily located in interstitial fibroblasts and renal tubular epithelial cells. Pharmacological inhibition of SMYD2 with AZ505, a highly selective inhibitor of SMYD2, protected against renal fibrosis and inhibited activation/proliferation of renal interstitial fibroblasts and conversion of epithelial cells to a profibrotic phenotype in this model. In cultured renal interstitial fibroblasts, treatment with AZ505 or silencing of SMYD2 by specific siRNA also inhibited serum- or TGF-β1-induced activation and proliferation of renal interstitial fibroblasts. Mechanistic studies showed that SMYD2 inhibition reduced phosphorylation of several profibrotic signaling molecules, including Smad3, extracellular signal-regulated kinase 1/2, AKT, signal transducer and activator of transcription-3 and nuclear factor-κB in both injured kidney and cultured renal fibroblasts. AZ505 was also effective in suppressing renal expression of Snail and Twist, two transcriptional factors that mediate renal partial epithelial-mesenchymal transition and fibrosis. Conversely, AZ505 treatment prevented downregulation of Smad7, a renoprotective factor in vivo and in vitro. These results indicate that SMYD2 plays a critical role in mediating conversion of epithelial cells to a profibrotic phenotype, renal fibroblast activation and renal fibrogenesis, and suggest that SMYD2 may be a potential target for the treatment of chronic fibrosis in kidney disease.
Keywords: proliferation, renal interstitial fibroblasts, Smad3, SMYD2, TGF-β1, unilateral ureteral obstruction
1 |. INTRODUCTION
Chronic kidney disease (CKD) is a common disease state, affecting 10%-13% of the US population. There are no available therapies to block progression to end stage renal disease (ESRD).1 In the past two decades, great effort has been made to understand the mechanisms by which CKD progresses to ESRD through increased renal fibrosis due to overexpression of extracellular matrix components (ECM).2 Research has also identified renal interstitial fibroblast activation and partial epithelial-mesenchymal transition (EMT) as the key cellular and molecular events in renal fibrogenesis.3,4 This has increased the focus on the development of drugs targeting EMT and renal interstitial fibroblast activation as possible treatments for CKD.
EMT is a process by which epithelial cells lose polarity and cell-cell adhesion and gain migratory and proliferative properties to become mesenchymal like cells.5 Recent studies have demonstrated that after injury, renal epithelial cells are unable to undergo a compete process of EMT, instead undergoing partial EMT through downregulation of E-cadherin and acquiring an ability to overproduce various growth factors/cytokines including transforming growth factor-β1 (TGF-β1) and platelet derived growth factor (PDGF).4,6,7 These growth factors/cytokines can stimulate renal fibroblasts to become myofibroblasts, a contractile/proliferative phenotype that expresses α-smooth muscle actin (α-SMA) and overproduces extracellular matrix (ECM) components such as collagen 1 and fibronectin.8 Multiple signaling pathways such as TGF-β/Smad3, extracellular signal-regulated protein kinases 1 and 2 (ERK1/2), AKT, signal transducer and activator of transcription 3 (STAT3), and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) have been shown to induce EMT and activate renal interstitial fibroblasts.8–12 Increased expression of various transcriptional factors, including snail and twist, and activation of some posttranslational modifiers such as lysine methyltransferases have also been reported to be involved in these processes.6,7,13
Increasing evidence suggests that lysine methyltransferase-mediated methylation of proteins plays an important role in epigenetic regulation of gene expression associated with many diseases, including renal fibrosis.14 Lysine methyltransferases are a group of enzymes that can transfer one, two, or three methyl groups to lysine residues of histone and non-histone proteins.14 Histone methylation can either repress or activate transcription, depending on the site of methylation. For example, the methylation of lysine 9 or 27 on histone H3 (H3K9me3, H3K27me3) in the promoter region of genes prevents expression of these genes, whereas methylation of histone residues H3K4, H3K36, and H3K79 is associated with active euchromatin transcription.15,16 The functional role of a histone methyltransferase is generally dependent on the histone residue it methylates. However, most histone methyltransferases can also induce methylation of some non-histone proteins.16 For example, SET and MYND domain-containing protein 2 (SMYD2) has been found to exert its biological functions by methylation of histone H3K36, H3K4, and non-histone proteins such as p53, Rb1, STAT3, and NF-κB.16–19 In addition, SMYD2 can also promote phosphorylation of ERK1/2 and AKT through downregulation of PTPN13, a protein tyrosine phosphatase, in cystic renal epithelial cells16–19 and triple-negative breast cancer cell lines.20
SMYD2 belongs to the SMYD family of lysine methyltransferases, which is composed of five members (SMYD1-5).21 These enzymes have various functions in development and cancer. SMYD1 activity is associated with cardiac morphogenesis and embryonic survival21; SMYD4 is a potential tumor suppressor, and SMYD5 is a negative regulator of inflammatory response genes.22,23 SMYD2 and SMYD3 have been extensively studied in hepatocellular, colorectal, and breast carcinomas and found to be associated with cancer cell proliferation.20,24,25 Recently, SMYD2 was also reported to be involved in the pathogenesis of autosomal dominant polycystic kidney disease (ADPKD).19 It is highly expressed in renal epithelial cells and tissue from Pkd1-knockout mice as well as in human ADPKD patients. However, to date, there have been no reports about a role for SMYD2 in chronic fibrotic disease.
In this study, we investigated the effect of SMYD2 inhibition on the development of renal fibrosis induced by unilateral urethral obstruction in mice and renal fibroblast activation in vitro. Our results show that SMYD2 was highly expressed in the mouse kidney after unilateral ureteral obstruction (UUO) and in cultured renal interstitial fibroblasts exposed to serum or TGF-β1. Inhibition of SMYD2 suppressed renal fibroblast activation and proliferation as well as accumulation of extracellular matrix proteins. Hence, we provide evidence that SMYD2 could be a critical mediator in renal fibrosis and a target for molecular therapy in patients with chronic fibrotic kidney disease.
2 |. MATERIALS AND METHODS
2.1 |. Chemicals and antibodies
Antibodies to collagen I (#SC-393573), Smad7(#SC-365846), Twist (#Sc-15393), and GAPDH (#SC-32233) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Phospho-histone H3(Ser10) antibody (#05-806) was purchased from Millipore (Billerica, MA). Fibronectin (#ab2413), H3K36me3 (#ab194677), phospho-Smad3 (#63403), and Smad3 (#Ab28379) antibodies were purchased from Abcam, Inc (Cambridge, MA). siRNA specific for rat SYMD2 (#S144314) was purchased from ThermoFisher (Waltham, MA). AZ505 (#1035227-43-0) was purchased from MedChemExpress (Monmouth, NJ). α-SMA (#A2547), vimentin (#V5255). α-Tubulin (#T5168) antibodies and all other chemicals were purchased from Sigma-Aldrich (St. Louis, MO). SMYD2 (#4251) and Phospho-STAT3 (#9145), STAT3 (#12640S), phospho-AKT (#9271S), AKT (#9272S), Snail1 (#3879S), histone H3 (#9715), and other antibodies used in this study were purchased from Cell Signaling Technology (Danvers, MA).
2.2 |. Cell culture and treatment
Rat renal interstitial fibroblasts (NRK-49F) were purchased from the ATCC (Manassas, VA) and cultured in DMEM with F12 containing 5% FBS and 0.5% penicillin and streptomycin in an atmosphere of 5% CO2 and 95% ambient air at 37°C. To determine the effect of AZ505, different doses of AZ505 were directly added to the subconfluent NRK-49F cells by incubation for the time as indicated in the figures. To determine the effect of SMYD2 inhibition on TGF-β1-induced activation and proliferation of renal fibroblasts, NRK-49F cells were starved for 24 hours with DMEM containing 0.5% FBS and then, exposed to 2 ng/mL TGF-β1 for the indicated time in the presence or absence of AZ505. To transfect siRNA into NRK-49F cells, cells were seed to 60%-70% confluence in serum-free medium and grown for 24 hours, and then then transfected with siRNA (100 pmol) specific for rat SMYD2 by Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. In parallel, scrambled siRNA (100 pmol) was used as a control for off-target changes in NRK-49F. Twenty-four hours after transfection, the medium was changed to DMEM containing 5% FBS or 2 ng/mL TGF-β1 and then cultured for an additional 24 hours before being harvested for analysis. Cell proliferation was assessed by the water-soluble tetrazolium (WST) assay using Cell Counting Kit-8 (CCK8) following the protocol provided by the manufacturer (Sigma-Aldrich, St. Louis, MO).
2.3 |. Animals and experimental design
The UUO model was established in male C57 black mice that weighed 20-25 g (The Jackson Laboratory, Bar Harbor, ME) as described in our previous studies.26 In brief, the abdominal cavity was exposed by a midline incision, and the left ureter was isolated and ligated. Sham operated mice were subjected to the initial surgery without ureter ligation. To examine the role of SMYD2 in renal fibrosis, AZ505 (10 mg/kg) in 50 μL DMSO was immediately administered by IP after surgery and then given daily for 6 days. The dose of AZ505 was selected according to a previous report.19 For the UUO alone group, mice received an equivalent amount of DMSO. For time course study, the animals were euthanized at day 1, 3, 7, 14 after UUO. Other groups of mice were sacrificed at day 7 after surgery. The kidneys were collected for protein analysis and histologic examination. Experimental procedures were performed according to the US Guidelines on the Care and Use of Laboratory Animals and approved by Tongji University School of Medicine.
2.4 |. Immunoblot analyses
The kidney tissue samples were homogenized with cell lysis buffer (Cell Signaling Technology) containing protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN). Cells were harvested in a cell lysis buffer mixed with a protease inhibitor cocktail. Proteins (25 μg) were separated by SDS-PAGE and transferred to nitrocellulose membranes. After incubation with 5% nonfat milk for 1 hour at room temperature, membranes were incubated with a primary antibody overnight at 4°C and then, appropriate horseradish peroxidase-conjugated secondary antibody for 1 hour at room temperature. Bound antibodies were visualized by chemiluminescence detection.
2.5 |. Histochemical and immunofluorescence staining
Immunofluorescence and Masson trichrome staining were carried out according to the procedure described in our previous studies.26 For assessment of renal fibrosis quantitatively, the collagen tissue area was measured using Image Pro-Plus Software (Media-Cybernetics, Silver Spring, MD) by drawing a line around the perimeter of the positive staining area, and the average ratio to each microscopic field was calculated and graphed. For immunofluorescence staining, rabbit anti-SMYD2, rabbit anti-H3K36me3, rabbit anti-phospho-H3(Ser10), and mouse anti-α-SMA antibodies were used. Human kidney samples were obtained from the Division of Shanghai East Hospital and approved by the Hospital Ethical Review Board.
2.6 |. Densitometry analyses
Semiquantitative analysis of different proteins was carried out using ImageJ software developed at the National Institutes of Health. The quantification is based on the intensity (density) of the band, which is calculated by the area and pixel value of the band. The quantification data are given as a ratio between target protein and loading control (housekeeping protein).
2.7 |. Statistical analyses
Data are presented as means ± SDs and were subjected to one-way ANOVA. Multiple means were compared using Tukey’s test, and differences between two groups were determined by t-test. P < .05 was considered statistically significant.
3 |. RESULTS
3.1 |. SYMD2 and its epigenetic marker H3K36me3 are upregulated in the kidney after UUO
As the first step toward understanding the role of SYMD2 in renal fibrosis, we examined the expression of SYMD2 and H3K36me3 at different times in a murine model of UUO. As indicated in Figure 1, A–C, SYMD2 and H3K36me3 were barely detected in the sham operated kidney. After UUO injury, expression of renal SYMD2 and H3K36me3 was detected on day 1 and then gradually increased over time, reaching the maximum level at day 14; similar levels of histone H3 were observed at various time points following UUO injury. Immunostaining indicated a basal level of SMYD2 in the cytosol of renal tubular cells in the sham kidney; UUO injured kidney demonstrated increased SMYD2 expression in both renal tubular cells and interstitial fibroblasts; expression of SMYD2 in renal interstitial fibroblasts was evident by co-expression of α-SMA and SMYD2 (Figure 1D). H3K36me3 was abundantly located in the nucleus of renal tubular cells in the sham kidney, and UUO injury further increased its expression in renal tubular cells and induced its expression in interstitial fibroblasts (Figure 1E). Notably, H3K36me3 was expressed in the nucleus of both renal tubular cells and interstitial fibroblasts. Co-staining of SMYD2 and H3K36me3 further indicated that their expression in the cytosol and nucleus, respectively, in these two cell types (Figure S1). Increased expression of SMYD2 and H3K36me3 in the kidney after UUO injury suggests that SMYD2 activation may be involved in the development of renal fibrosis.
FIGURE 1.
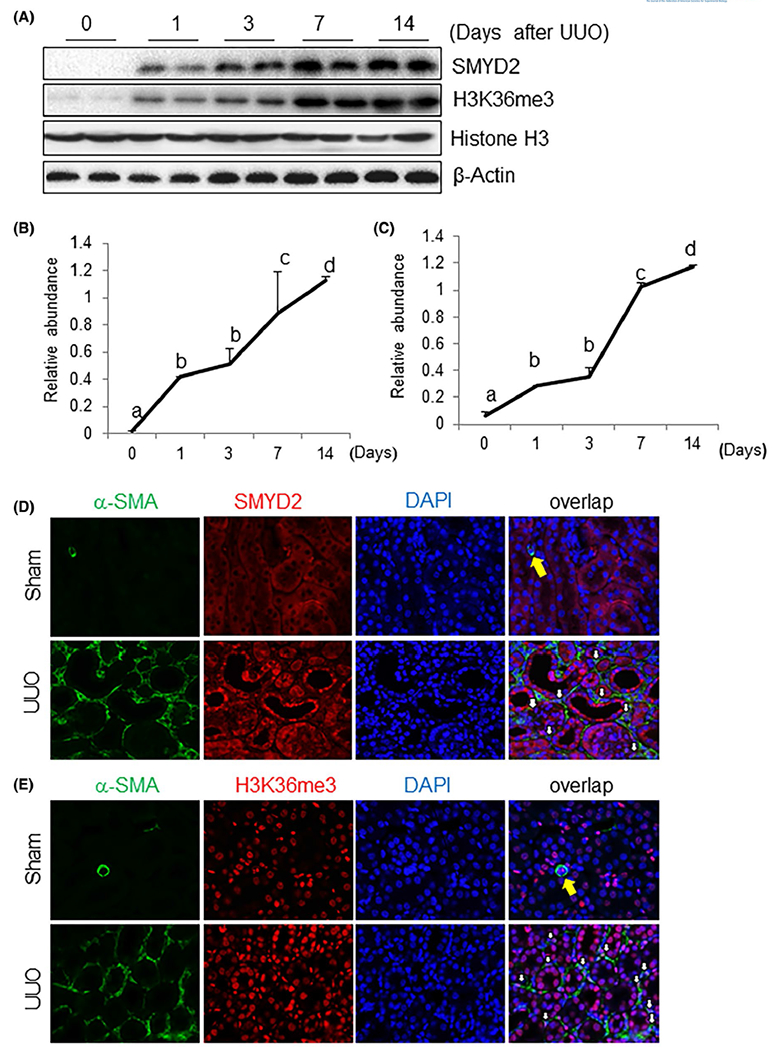
Time dependent SMYD2 expression and H3K36 trimethylation in the kidney after obstructed kidneys. A, Kidneys were collected at different time points as indicated from sham-operated or obstructed kidneys of mice and the prepared tissue lysates were subjected to immunoblot analysis with antibodies against SMYD2, H3K36me3 or β-actin (A). The levels of SMYD2, H3K36me3, and β-actin were quantified by densitometry; SMYD2 and H3K36me3 levels were normalized to β-actin and Histone H3, respectively (B, C). Values are the means ± SDs (n = 6). Bars with different letters (a-d) are significantly different from one another (P < .05). D, E, Photomicrographs illustrate co-staining of α-SMA and SMYD2 (D) or H3K36me3 (E) in the tissue section of the obstructed kidney (magnification ×600). DAPI: 4’,6-diamidino-2-phenylindole. The yellow arrow indicates small blood vessels, and white arrows indicate SMYD2 or H3K36me3-positive myofibroblasts
3.2 |. Targeting SMYD2 using AZ505 protects against renal fibrosis development
To assess the role of SMYD2 in the development of renal fibrosis, we examined the effect of AZ505 on UUO-induced renal fibrosis in mice. AZ505 is a potent and highly selective inhibitor of SMYD2,27 which acts though binding to the SMYD2 substrate binding groove,27 AZ505 has 100-fold selectivity for SMYD2 over 24 other protein or DNA methyltransferases, including related family members SMYD3, SUVH420H1, and SUV420H2.28 At day 7 after ureteral ureter ligation with or without administration of AZ505, kidneys were collected and then subjected to Masson trichrome staining and immunoblot analysis of ECM protein expression. Masson trichrome staining showed that ECM proteins were extensively deposited within the interstitium of UUO injured kidneys (Figure 2A). Semiquantitative analysis of Masson trichrome-positive areas revealed about a four-fold increase in deposition of ECM components in the obstructed kidney compared with control kidneys, whereas administration of AZ505 significantly reduced ECM deposition by 80% (Figure 2B). To confirm the antifibrotic effect of AZ505, we further examined the effect of AZ505 on the expression of α-SMA, a hallmark of myofibroblasts, and deposition of fibronectin and collagen type 1 in obstructed kidneys. Immunoblot analysis of whole-kidney tissue lysate indicated that administration of AZ505 reduced expressions of SMYD2 and H3K36me3 to the basal levels. AZ505 also significantly decreased the levels of α-SMA, fibronectin, and collagen 1 (Figure 2C–G). Taken together, these data indicate that SMYD2 activation is critically involved in the activation of renal interstitial fibroblasts and pathogenesis of renal fibrosis and that pharmacological inhibition of SMYD2 can protect the kidney from fibrogenesis.
FIGURE 2.
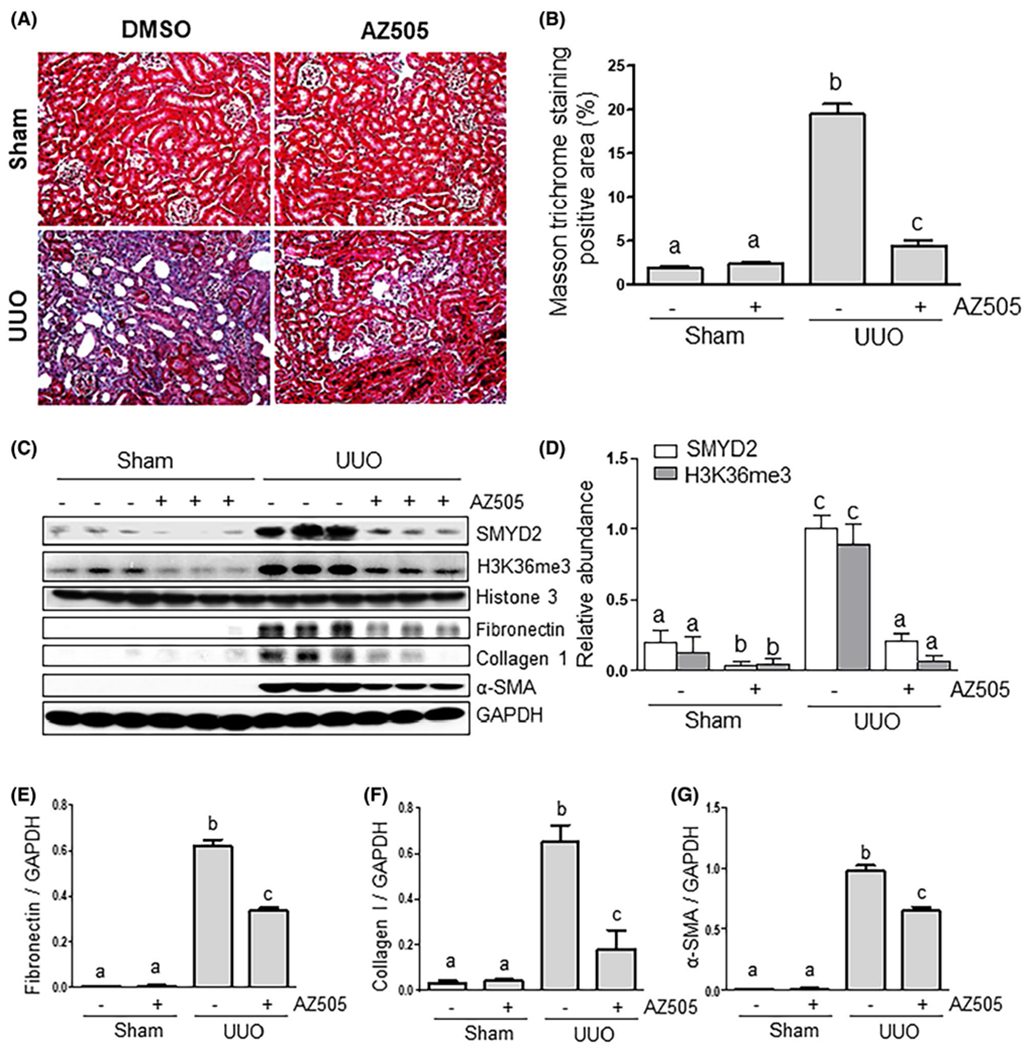
AZ505 attenuates development of renal fibrosis and deposition of ECM in obstructed kidneys. A, Photomicrographs illustrating Masson trichrome staining of kidney tissue (magnification ×200). B, The Masson trichrome-positive tubulointerstitial area (blue in A) relative to the whole area from ten random cortical fields was analyzed. Data are represented as the means ± SDs (n = 6). C, Kidney tissue lysates were subjected to immunoblot analysis with antibodies against SMYD2, H3K36me3, Histone H3, α-SMA, collagen 1, fibronectin, or GAPDH (C). Expression levels of SMYD2, H3K36me3, fibronectin, collagen 1, α-SMA, or GAPDH were quantified by densitometry, and the levels of SMYD2 (D), fibronectin (E), collagen I (F), and α-SMA (G) were normalized with GAPDH. H3K36me3 levels were normalized with Histone H3 (D). Values are the means ± SDs (n = 6). Means with different letters (a-c) are significantly different from one another (P < .05)
3.3 |. SYMD2 mediates serum- and TGF-β1-induced activation of renal fibroblasts and expression of ECM proteins in culture
To demonstrate the direct role of SYMD2 in the activation of renal interstitial fibroblasts and expression of ECM proteins, we examined the effect of SMYD2 inhibition with AZ505 and its specific siRNA on the expression of α-SMA, fibronectin, and collagen 1 in cultured rat renal interstitial fibroblasts (NRK-49F) in response to TGF-β1, a potent profibrotic factor, or 5% FBS, a mixture of growth factors. TGF-β1 exposure resulted in an increase in the expression of α-SMA, fibronectin, and collagen 1, indicative of induction of renal fibroblast activation; treatment with AZ505 dose-dependently suppressed their expression with the maximum inhibition at 25 μM (Figure 3A,C). Both SMYD2 and H3K36me3 were expressed in serum starved-NRK-49F cells; TGF-β1 increased their expression levels. AZ505 treatment suppressed SYMD2 and H3K36me3 expression in a dose dependent manner (Figure 3B,D). Similarly, AZ505 also dose-dependently and time-dependently suppressed serum-induced expression of α-SMA, fibronectin and collagen 1 as well as that of SMYD2 and H3K36me3 in NRK-49F cells (Figures S2A–F and S3A–F). In addition, AZ505 was also effective in suppressing TGF-β1-induced stress fiber formation as detected by immunostaining of α-SMA in this cell type (Figure S2G). Thus, these data suggest that SYMD2 is an important mediator of renal fibroblast activation.
FIGURE 3.
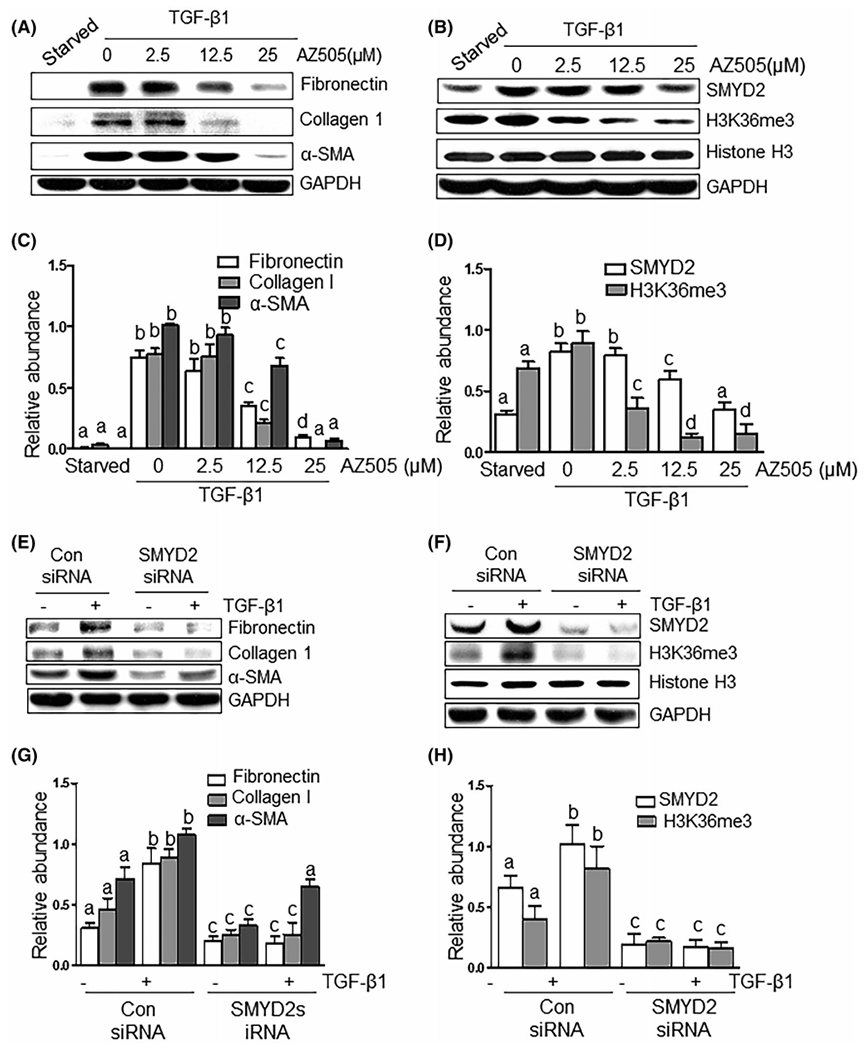
Blocking SMYD2 with AZ505 inhibits TGF-β1-induced activation of renal interstitial fibroblasts. Starved NRK-49F cells were cultured in the DMEM with TGFβ1 (2 ng/mL) in the presence or absence of AZ505 at the doses as indicated for 36 hours (A-D) or Serum–starved NRK-49F cells were transfected with siRNA targeting SMYD2 or scrambled siRNA and then, incubated in 2 ng/mL TGFβ1 for an additional 24 hours (E-H). Cell lysates were prepared for immunoblot analysis with antibodies against fibronectin, collagen 1, α-SMA, SMYD2, H3K36me3, Histone H3, or GAPDH (A, B, E, F). The levels of fibronectin, collagen I and α-SMA (C, G), as well as SMYD2 (D, H) were quantified by densitometry and normalized with GAPDH. H3K36me3 levels were normalized with Histone H3 (D, H). Values are the means ± SDs of at least three independent experiments. Bars with different letters (a-c) for each molecule are significantly different from one another (P < .05)
To validate the role of SYMD2 in mediating activation of renal interstitial fibroblasts, we also examined the effect of siRNA-mediated SYMD2 silencing on the expression of α-SMA, fibronectin, and collagen 1 in NRK-49F cells exposed to either TGF-β1 or serum. Similar to the inhibitory effect of AZ505 on the expression of these proteins, transfection of cells with SYMD2 siRNA largely suppressed their expression in either TGF-β1 or serum-treated cells (Figure 3E,G; Figure S4A,C). Notably, SYMD2 siRNA was effective in the downregulation of SYMD2 levels and suppression of H3K36me3 (Figure 3F,H; Figure S4B,D). This evidence further supports the case for involvement of SYMD2 in mediating activation of renal interstitial fibroblasts.
3.4 |. Administration of AZ505 inhibits proliferation of renal interstitial fibroblasts in vivo and in vitro
Given that proliferation of renal interstitial fibroblasts accelerates progression of renal fibrosis,29 we further examined the effect of SMYD2 inhibition on the proliferation of renal interstitial fibroblasts in the kidney after UUO injury and in cultured NRK49F cells. Immunofluorescence analysis showed that a population of interstitial fibroblasts was co-stained by α-SMA and proliferating cell nuclear antigen (PCNA) was increased in the injured kidney, indicative of proliferating myofibroblasts; they were decreased after AZ505 treatment (Figure 4A,B). Immunoblot analysis also demonstrated that expression of PCNA was increased in the kidney after UUO injury and reduced with administration of AZ505 (Figure 4C,D). It should be noted that some tubular cells also exhibited PCNA-positive staining, indicating that a portion of tubular cells also enter a proliferative stage after injury (Figure 4A). To validate that proliferation of renal interstitial fibroblasts is regulated by SMYD2, we examined the effect of AZ505 on the proliferation of renal interstitial fibroblasts in vitro. AZ505 treatment suppressed serum and TGF-βl-stimulated proliferation of NRK-49F cells, respectively, as measured by the WST-8 assay (Figure S5A,B). This was confirmed by immunoblot analysis showing that AZ505 was effective in suppressing the expression of proliferation markers, PCNA and cyclin D1, and increased that of p21, a potent cyclin-dependent kinase inhibitor, in NRK-49F cells cultured with 5% FBS (Figure 4E,G). Similarly, AZ505 inhibited TGF-β1-induced expression of PCNA and cyclin D1 and increased expression of p21 in a dose dependent manner (Figure 4F,H). Therefore, SMYD2 activation is required for promoting renal fibroblast activation and proliferation.
FIGURE 4.
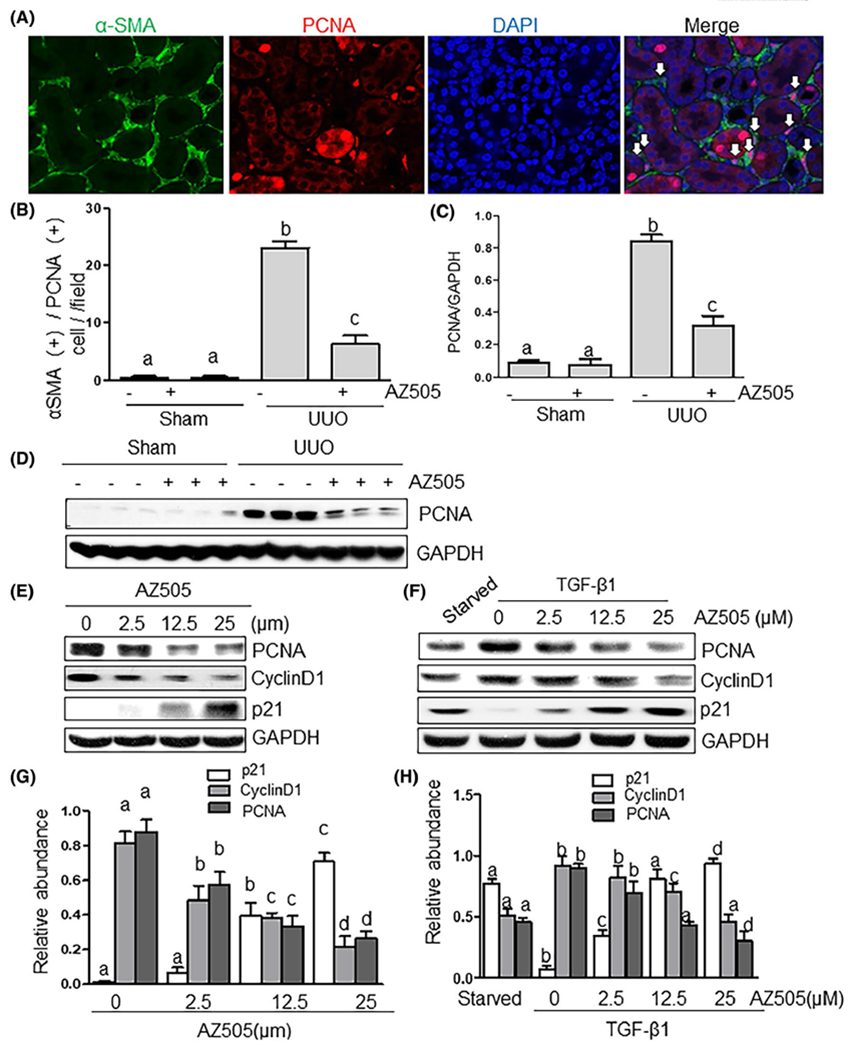
AZ505 inhibits cell proliferation in the kidney after UUO injury and in the culture of renal interstitial fibroblasts. A, Photomicrographs illustrating the kidney tissue stained with DAPI and antibodies against α-SMA and proliferating cell nuclear antigen (PCNA) (magnification ×200). B, The α-SMA (+)/PCNA (+) cells in the interstitium were calculated in ten high-power fields and expressed as means ± SDs. White arrows indicate PCNA-positive myofibroblasts. Kidney tissue lysates were subjected to immunoblot analysis with antibodies against PCNA or GAPDH (D). Expression levels of PCNA were quantified by densitometry and normalized with GAPDH (C). NRK-49F cells were cultured in the DMEM with 5% FBS (E, G) or 2 ng/mL TGFβ1 (F, H) in the presence or absence of AZ505 at the doses as indicated for 36 hours. Cell lysates were subjected to immunoblot analysis with antibodies against PCNA, cyclin D1, p21, or GAPDH (E, F). Expression levels of PCNA, cyclin D1, p21, or GAPDH were quantified by densitometry, and the levels of PCNA, cyclin D1, p21 were normalized with GAPDH (G, H). Values are the means ± SDs of at least three independent experiments. Means with different letters (a-d) are significantly different from one another (P < .05)
3.5 |. AZ505 inhibits epithelial cell arrest at G2/M phase and expression of transcriptional factors Snail and Twist in the kidney after UUO injury
Recent studies have revealed that injured renal epithelial cells are arrested at G2/M of the cell cycle and that partial EMT is a profibrotic process that occurs in these cells, producing multiple profibrotic growth factors/cytokines, such as TGF-β1, leading to activation and proliferation of renal fibroblasts.6,7 We hypothesized that SMYD2 might play a role in this process after injury and explored the effect of AZ505 on G2/M arrest and partial EMT. G2/M arrest is characterized by expression of phosphorylated histone H3 at serine 10 (p-H3er10) and partial EMT, which is characterized by re-expression of vimentin and driven by upregulated transcriptional factors Snail and Twist.6,7 Immunofluorescence staining showed an increase in the number of renal tubular cells with expression of p-H3er10 in the kidney after UUO injury, and AZ505 treatment largely reduced p-Histone H3 positive cells (Figure 5A,B). Immunoblot analysis indicated a minimal amount of p-H3er10 in the kidney of sham-operated animals while its expression was increased after UUO injury. Treatment with AZ505 blocked expression of p-H3er10 in the injured kidney (Figure 5C,D). In addition, we demonstrated that AZ505 reduced UUO-induced increased expression of Snail and Twist as well as Vimentin (Figure 5C,E–G). These results provide strong evidence that SMYD2 activation promotes conversion of renal epithelial cells to a profibrotic phenotype upon injury.
FIGURE 5.

AZ505 inhibits conversion of epithelial cells to a profibrotic phenotype in the kidney after UUO injury. Photomicrographs illustrating the kidney tissue stained with DAPI and an antibody against phospho-histone H3 serine 10 (p-H3ser10) (magnification ×200) (A). Tubular cells with positive staining of H3pSer10 were calculated in ten high-power fields and expressed as means ± SDs (B). Kidney tissue lysates were subjected to immunoblot analysis with antibodies against p-H3ser10, Snail, Twist, Vimentin and GAPDH. C, Expression levels of p-H3ser10 (D), Snail (E), Twist (F), Vimentin (G) were quantified by densitometry and normalized with and GAPDH. Values are the means ± SDs of at least three independent experiments. Means with different letters (a-c) are significantly different from one another (P < .05)
3.6 |. AZ505 inhibits activation of the TGF-β/Smad3 signaling in the kidney after UUO injury and cultured renal interstitial fibroblasts
To further demonstrate the mechanism of SMYD2 -mediated pro-fibrotic cellular responses, we examined the effect of SMYD2 inhibition on phosphorylation of Smad3, a critical mediator in TGF-β signaling, and expression of Smad7, a renoprotective molecule,30 in the UUO injured kidney and NRK-49F exposed to TGF-β1. As indicated in Figure 6A,B, UUO injury induced Smad-3 phosphorylation, while treatment with AZ505 reduced Smad-3 phosphorylation to the basal level. Similarly, AZ505 effectively suppressed TGF-β1-induced Smad-3 phosphorylation in cultured NRK-49F cells (Figure 6E,F). Both UUO injury and TGF-β1 led to a decrease in the expression of Smad7; this was partially restored by AZ505 treatment (Figure 6A,D,E,H). It should be noted that UUO injury and TGF-β1 also increased expression of Smad3, but AZ505 did not alter its expression (Figure 6A,C,E,G). Thus, SMYD2 activation is essential for the activation of Smad3 and downregulation of Smad7 in the kidney after injury and in the culture renal fibroblasts with TGF-β1 stimulation.
FIGURE 6.
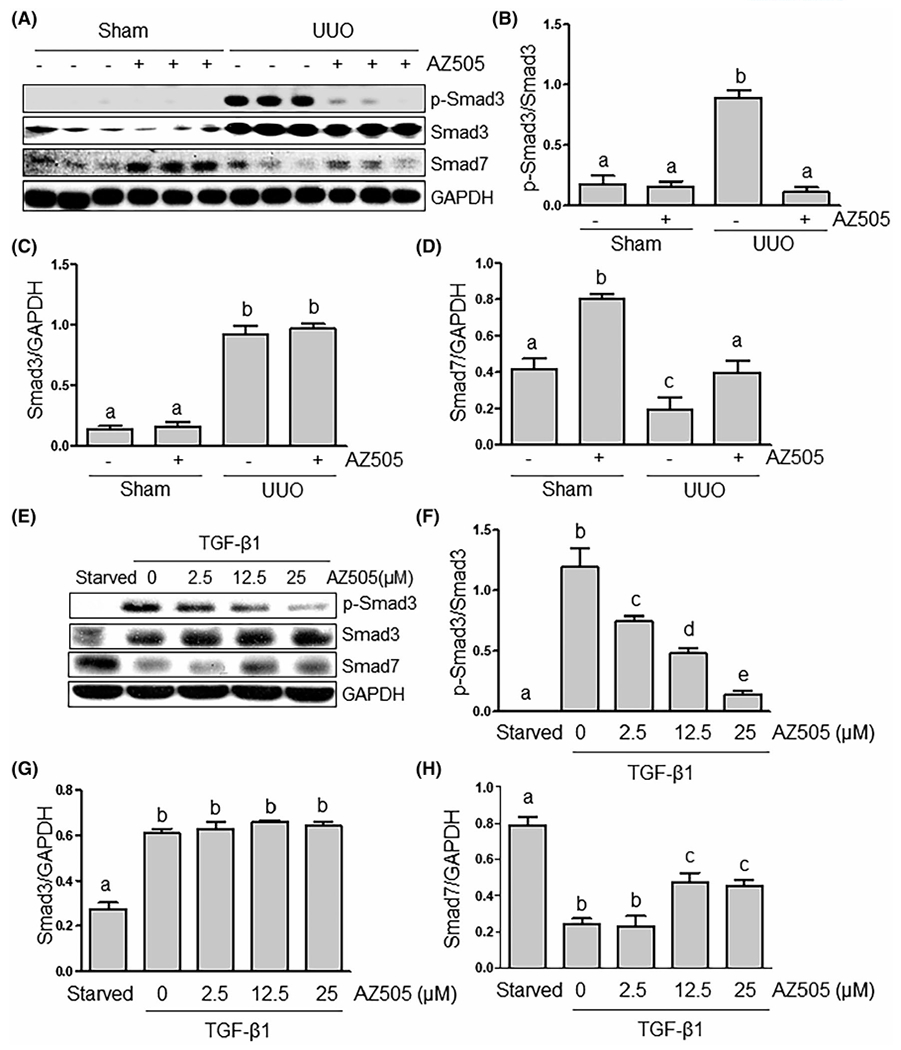
AZ505 inhibits Smad3 phosphorylation and restores Smad-7 expression in the kidney after UUO injury and in the culture of renal interstitial fibroblasts exposed to TGFβ1. Kidney tissue (A-D) or cell lysates (E-G) were subjected to immunoblot analysis with antibodies against Phospho-Smad3, Smad3, Smad7, or GAPDH (A, E). Expression levels of Phospho-Smad3 (B, F), Smad3 (C, G), Smad7 (D, H), or GAPDH were quantified by densitometry, and normalized with GAPDH. Values are the means ± SDs of at least three independent experiments. Means with different letters (a-e) are significantly different from one another (P < .05)
3.7 |. AZ505 inhibits activation of multiple signaling pathways associated with renal fibrosis in the kidney after UUO injury and in cultured renal interstitial fibroblasts
Our previous studies and studies by other groups have shown that development of renal fibrosis is associated with activation of multiple signaling pathways, including ERK1/2, AKT, STAT3, and NF-kB.8–12 It is possible that SMYD2 may mediate activation of these profibrotic signaling pathways. To test this hypothesis, we examined the effect of AZ505 on the phosphorylation of these signaling molecules in the kidney after UUO and in cultured renal interstitial fibroblasts. UUO injury led to increased phosphorylation of ERK1/2, AKT, STAT3, and NF-kB; AZ505 treatment suppressed their phosphorylation to the basal levels (Figure 7A–F). Similarly, AZ505 treatment inhibited phosphorylation of ERK1/2 and AKT in the cultured NRK-49F cells exposed to serum (Figure S6A–C), and of STAT3 and NF-kB in cultured NRK-49F cells exposed to either serum (Figure S7A–C) or TGF-β1 (Figure S8A–C). Collectively, these data demonstrate that SMYD2 is involved in the activation of multiple profibrotic signaling pathways.
FIGURE 7.

AZ505 inhibits phosphorylation of AKT, ERK1/2, STAT3, and NF-kB in the kidney after UUO injury. Kidney tissues were subjected to immunoblot analysis with antibodies against phospho-ERK1/2, ERK1/2, phospho-AKT, AKT (A-C), phopho-STAT3, STAT3, phospho-NF-κB, NF-κB (D-F), or GAPDH (A, D). Expression levels of all those proteins were quantified by densitometry and phospho-ERK1/2 (B), phospho-AKT (C), phospho-STAT3 (E), and phospho-NF-κB (F) were normalized with total ERK1/2, AKT, STAT3, and NF-κB, respectively. Values are the means ± SDs of at least three independent experiments. Means with different letters (a-c) are significantly different from one another (P < .05)
4 |. DISCUSSION
SMYD2 was initially identified as an oncogene that is highly expressed in various types of human cancers, mediating cancer cell proliferation and migration.31 Recently, Li et al, reported that pharmacological and genetic inhibition of SMYD2 also alleviates cyst growth in ADPKD,19 suggesting its involvement in the pathogenesis of polycystic kidney disease. To date, functional characterization of SMYD2 in renal fibrosis remains unexplored. In this study, we examined the effect of SMYD2 inhibition on renal fibroblast activation and development of fibrosis. We provide evidence that SMYD2 can mediate renal fibrogenesis by promoting transition of renal epithelial cells to a profibrotic phenotype and activation of renal interstitial fibroblasts. Thus, we demonstrate here for the first time that SMYD2 exhibits a crucial profibrotic role and is a potential therapeutic target in chronic fibrotic kidney disease.
SMYD2 protein is significantly upregulated and activated in the kidney after chronic injury. In the murine model of renal fibrosis induced by UUO, SMYD2 and its epigenetic mark, H3K36me3, were highly expressed in the kidney, and located in both renal epithelial cells and renal interstitial fibroblasts. This indicates that SMYD2 activation may be associated with the development and progression of renal fibrosis. In support of this hypothesis, we demonstrated that administration of AZ505, at the concentration that blocked expression of SMYD2 and H3K36me3, reduced expression of α-SMA, a hallmark of activated fibroblasts, and of fibronectin and collagen 1, two ECM proteins, in the kidney after UUO injury. Furthermore, inhibition of SMYD2 with AZ505 or its specific siRNA significantly suppressed the expression of those proteins in cultured renal interstitial fibroblasts exposed to serum containing a mixture of growth factors and to TGF-β1, a potent cytokine that induces activation of interstitial fibroblasts. As such, we identify SMYD2 as an important mediator in the activation of renal fibroblasts and development of renal fibrosis. Nevertheless, since our data showed that H3K36me3 was expressed more abundantly than SMYD2 in sham-operated kidneys, it is possible that some H3K36 methyltransferases are constitutively activated and mediate H3K36 trimethylation under physiological conditions. In mammalian cells, several histone methyltransferases, such as SMYD3, ASH1L, and SETD2, have been reported to be able to trimethylate H3K36.21 Additional studies are required to identify the histone methyltransferases that induce H3K36 trimethylation in normal kidneys.
SMYD2-mediated renal fibrosis is associated with transition of renal epithelial cells to a profibrotic phenotype, a process analogous to partial EMT. Unlike complete EMT, partial EMT is a state in which cells remain attached to the basement membrane but express markers of both epithelial and mesenchymal cells.6,7 Following severe renal injury, remaining tubular cells often undergo defective repair characterized by partial EMT, G2/M arrest and production of an excessive amount of profibrotic cytokines/growth factors, thereby promoting interstitial fibrosis.32 Since fibrotic injury to the kidney leads to increased expression of SMYD2 in renal epithelial cells, SMYD2 may contribute to renal fibrogenesis by regulating profibrotic behaviors of this cell type. In this study, we found that inhibition of SMYD2 with AZ505 completely blocked expression of vimentin, a marker of epithelial dedifferentiation, and of phospho-histone H3 at serine 10, a marker of the G2/M arrest of cell cycle,32 favoring the role of SMYD2 in mediating regulation of partial EMT. This idea is also supported by our results showing that SMYD2 inhibition effectively suppressed expression of Snail1 and Twist, two key transcriptional repressors that drive partial EMT, the prerequisite for epithelial cell G2/M arrest.6 Based on these data, we propose that SMYD2 activation may initiate a machinery that leads to the conversion of renal epithelial cells to a profibrotic phenotype that subsequently produces and releases growth factors/cytokines to promote activation of renal interstitial fibroblasts. Given the reported importance of Snail1 and Twists in mediating the partial EMT of renal epithelial cells and the role of protein methylation in regulating stability and function of transcriptional factors,3,4 SYMD2-mediated expression of Snail1 and Twists may be essential for triggering transition of renal epithelial cells to a profibrotic phenotype.
Although our results suggest that SMYD2 contributes to renal fibroblast activation and TGF-β1 expression in the injured kidney, it remains unclear about the mechanism(s) by which TGF-β1 released from the tubular cells to regulate renal interstitial fibroblasts following injury. So far, there is no direct evidence indicating that TGF-β1 and other profibrotic factors released from renal tubular cells can cross the basal membrane and get into the interstitium despite it has been reported that UUO injury can cause a broken, discontinuous tubular basement membrane.33 Further studies are needed to address this issue.
SMYD2 may contribute to renal fibrogenesis through activation of renal fibroblasts directly as well. This hypothesis is supported by our data showing that (1) SMYD2 was highly expressed in renal interstitial fibroblasts in the kidney after UUO injury and in cultured renal fibroblasts exposed to serum or TGF-β1, (2) pharmacological inhibition of SMYD2 reduced expression of SMYD2, α-SMA, and ECM proteins in the kidney after UUO injury, and (3) treatment with AZ505 or knockdown of SMYD2 with its specific siRNA inhibited activation and proliferation of renal interstitial fibroblasts in vitro. As our immunostaining analysis indicates that SMYD2 is mainly localized in the cytoplasm, we assumed that SMYD2 might primarily exert its regulatory role through activation/regulation of some non-histone proteins key to renal fibrogenesis. In this study, we found that administration of AZ505 effectively inhibited phosphorylation of Smad3 and increased expression of Smad7 (an antagonist of Smad3) in vitro and in vivo, suggesting that SMYD2 is functionally linked to the TGF-β/Smad3 signaling pathway to induce its activation. Given that Smad3 is a key signaling molecule that is phosphorylated in the cytosol and mediates renal fibroblast activation and proliferation, inactivation of this pathway by targeting Smad7 may be an important mechanism by which inhibition of SMYD2 suppresses renal fibrosis. The mechanism by which SMYD2 regulates Smad7 expression needs additional investigations.
Our data suggest that SMYD2 also mediates activation of other intracellular signaling pathways associated with renal fibrosis. Previous studies have demonstrated that in addition to TGF-β/Smad3, other signaling pathways such as STAT3, NF-kB, ERK1/2, and AKT, are activated and involved in cellular events, including survival, proliferation, and inflammation during the course of renal fibrosis.8–12,34–38 Our current studies showed that SMYD2 inhibition blocked phosphorylation of all these signaling molecules in vivo and in vitro, supporting the importance of these pathways in transducing SMYD2 activation to renal fibrogenesis. The mechanism by which SMYD2 controls phosphorylation and activation of intracellular signaling molecules remains incompletely understood but is being explored. Li et al, has recently identified STAT3 and the p65 subunit of NF-κB as non-histone substrates of SMYD2 and suggested that SMYD2 can induce STAT3 methylation at lysine 685 and NF-κB p65 at lysine 310 and partially at lysine 221.19 They have also shown that SMYD2 can target PTPN 13 (protein tyrosine phosphatase nonreceptor type 13) and reduce its expression. PTPN13 can dephosphorylate multiple intracellular signaling molecules including ERK1/2 and AKT.19 Thus, it is anticipated that SMYD2 may mediate activation of some intracellular signaling molecules through a direct mechanism associated lysine methylation or an indirect mechanism involving suppression of PTPN13 or other protein phosphatases. In this context, Nakakido et al reported that SMYD2 can also induce methylation of PTEN, another protein phosphatase, and reduce its ability to suppress the phosphatidylinositol 3-kinase (PI3K)-AKT signaling pathway.39 Further investigation is required to elucidate how SMYD2 regulates methylation of these molecules to alter their activation and functions.
SMYD2 is a key mediator in driving the progression of cell cycle and proliferation of renal fibroblasts. This is clearly indicated by our in vivo observations that inhibition of SMYD2 by AZ505 reduced the number of renal interstitial fibroblasts and expression of PCNA in cultured NRK-49F cells exposed to serum and TGF-β1. In particular, AZ505 was also shown to dose-dependently inhibit expression of cyclin D1, a protein required for progression through the G1 phase of the cell cycle.40 As cyclin D1 is a regulatory subunit of p21, a potent cyclin-dependent kinase inhibitor that can bind to and inhibit the activity of CDK4/6 complexes,41,42 an increase of p21 expression will result in the repression of cell cycle. Indeed, we observed that inactivation of SMYD2 significantly upregulated p21, suggesting that SMYD2 may promote cell cycle progression by interfering with the molecular events leading to p21 expression. In this regard, it has been reported that SMYD2 can methylate p53 on Lys 370 and that this methylation leads to repression of its activity.17 As p21 is also a key mediator of p53-dependent cell cycle arrest, SMYD2-mediated p53 methylation and inactivation may decrease expression of p21 expression, thereby increasing activation of CDK4 and CDK6 and expression of cyclinD1, promoting cell cycle progression. In addition to p53, SMYD2 may also regulate cell cycle progression through retinoblastoma tumor suppressor 1 (RB1). A recent study shows that SMYD2 can induce transcriptional corepressor 1 (RB1) methylation on Lys K860, and that SMYD2-dependent RB1 methylation accelerates cell cycle progression through increasing its phosphorylation and enhancing E2F transcriptional activity.18 Therefore, directly altering expression/activation of cell cycle regulators by methylation may be one of the molecular mechanisms by which SMYD2 mediates cell proliferation.
SMYD2 has been considered an attractive target for disease treatments. It is overexpressed in various human malignancies, such as esophageal squamous cell carcinoma,43 bladder carcinoma,40 and pediatric acute lymphoblastic leukemia,36 indicating that SMYD2 acts as a cancer-promoting factor and therapeutic target. AZ505 is the first potent and selective SMYD2 inhibitor, and other inhibitors such as LLY-507 and (S)-4, are being developed.27,28 It has been reported that blocking SMYD2 with AZ505 was effective in slowing cyst growth, inhibiting tumorgenesis, and increasing protective proinflammatory response in murine models.19,20,37,38 With application of AZ505 and other SMYD2 inhibitors, it is expected that functional roles for SMYD2 in different diseases will become clearer. Given that SMYD2 is minimally expressed in normal kidneys and upregulated in the fibrotic kidney, and administration of AZ505 effectively attenuated renal fibrosis in a preclinical model, it may be a promising target for therapeutic treatment to suppress progression of CKD. Additionally, it has been reported that long-term use of AZ505 at 10 mg/kg for 6 months can delay renal cyst formation without affecting body weights of mice,19 suggesting that AZ505 may be a safe inhibitor in kidney disease.
In summary, we demonstrate that SMYD2 is highly expressed in the chronically injured kidney and that pharmacological inhibition with AZ505 alleviates renal fibrosis by blocking several key cellular and molecular events, including epithelial arrest at the G2/M phase of cell cycle, activation and proliferation of renal interstitial fibroblasts, and phosphorylation and/or expression of several intracellular signaling pathway and transcriptional factors. Although other animal models are needed to demonstrate the universal role of SMYD2 in mediating the pathology of CKD induced by diverse etiologies or other fibrotic diseases, our results provide evidence for its critical involvement in renal fibrosis and lay the foundation for those investigations and clinical translational studies in the future.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China grants (81670623 and 81830021 to SZ), National key R&D Program of China (2018YFA0108802 to SZ), and US National Institutes of Health (2R01DK08506505A1 to SZ).
Funding information
National Natural Science Foundation of China, Grant/Award Number: 81670623 and 81830021; National key R&D Program of China, Grant/Award Number: 2018YFA0108802; US National Institutes of Health, Grant/Award Number: 2R01DK08506505A1
Abbreviations:
- ADPKD
autosomal dominant polycystic kidney disease
- CKD
chronic kidney disease
- ECM
extracellular matrix components
- EMT
epithelial-mesenchymal transition
- ERK1/2
extracellular signal-regulated kinase 1/2 (ERK1/2)
- ESRD
end-stage of renal disease
- H3K36me3
histone H3 lysine 36 trimethylation
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- PCNA
proliferating cell nuclear antigen
- PDGF
platelet-derived growth factor
- RB1
transcriptional corepressor 1
- SMYD2
SET and MYND domain protein 2
- STAT3
signal transducer and activator of transcription-3
- TGF-β1
transforming growth factor-β1
- UUO
unilateral ureteral obstruction
Footnotes
DISCLOSURES
The authors have no financial conflicts of interest.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the Supporting Information section.
REFERENCES
- 1.Stenvinkel P, Painer J, Kuro OM, et al. Novel treatment strategies for chronic kidney disease: insights from the animal kingdom. Nat Rev Nephrol. 2018;14:265–284. [DOI] [PubMed] [Google Scholar]
- 2.Zoccali C, Vanholder R, Massy ZA, et al. The systemic nature of CKD. Nat Rev Nephrol. 2017;13:344–358. [DOI] [PubMed] [Google Scholar]
- 3.Tang PM, Nikolic-Paterson DJ, Lan HY. Macrophages: versatile players in renal inflammation and fibrosis. Nat Rev Nephrol. 2019;15:144–158. [DOI] [PubMed] [Google Scholar]
- 4.Lovisa S, Zeisberg M, Kalluri R. Partial epithelial-to-mesenchymal transition and other new mechanisms of kidney fibrosis. Trends Endocrinol Metab. 2016;27:681–695. [DOI] [PubMed] [Google Scholar]
- 5.Santos F, Moreira C, Nobrega-Pereira S, Bernardes de Jesus B. New insights into the role of epithelial(-)mesenchymal transition during aging. Int J Mol Sci. 2019;20:891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lovisa S, LeBleu VS, Tampe B, et al. Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis. Nat Med. 2015;21:998–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grande MT, Sanchez-Laorden B, Lopez-Blau C, et al. Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat Med. 2015;21:989–997. [DOI] [PubMed] [Google Scholar]
- 8.Liu BC, Tang TT, Lv LL, Lan HY. Renal tubule injury: a driving force toward chronic kidney disease. Kidney Int. 2018;93:568–579. [DOI] [PubMed] [Google Scholar]
- 9.Pang M, Ma L, Gong R, et al. A novel STAT3 inhibitor, S3I–201, attenuates renal interstitial fibroblast activation and interstitial fibrosis in obstructive nephropathy. Kidney Int. 2010;78:257–268. [DOI] [PubMed] [Google Scholar]
- 10.Andrikopoulos P, Kieswich J, Pacheco S, et al. The MEK inhibitor trametinib ameliorates kidney fibrosis by suppressing ERK1/2 and mTORC1 signaling. J Am Soc Nephrol. 2019;30:33–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lan A, Zhang J, Xiao Z, Peng X, Qi Y, Du J. Akt2 is involved in loss of epithelial cells and renal fibrosis following unilateral ureteral obstruction. PLoS One. 2014;9:e105451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamada S, Asai T, Kuwabara N, et al. Molecular mechanisms and therapeutic strategies of chronic renal injury: the role of nuclear factor kappaB activation in the development of renal fibrosis. J Pharmacol Sci. 2006;100:17–21. [DOI] [PubMed] [Google Scholar]
- 13.Wanner N, Bechtel-Walz W. Epigenetics of kidney disease. Cell Tissue Res. 2017;369:75–92. [DOI] [PubMed] [Google Scholar]
- 14.Zhou X, Zang X, Ponnusamy M, et al. Enhancer of zeste homolog 2 inhibition attenuates renal fibrosis by maintaining Smad7 and phosphatase and tensin homolog expression. J Am Soc Nephrol J. 2016;27:2092–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi Y. Histone lysine demethylases: emerging roles in development, physiology and disease. Nat Rev Genet. 2007;8:829–833. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Huang Y, Shi X. Emerging roles of lysine methylation on non-histone proteins. Cell Mol Life Sci. 2015;72:4257–4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang J, Perez-Burgos L, Placek BJ, et al. Repression of p53 activity by Smyd2-mediated methylation. Nature. 2006;444:629–632. [DOI] [PubMed] [Google Scholar]
- 18.Cho HS, Hayami S, Toyokawa G, et al. RB1 methylation by SMYD2 enhances cell cycle progression through an increase of RB1 phosphorylation. Neoplasia. 2012;14:476–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li LX, Fan LX, Zhou JX, et al. Lysine methyltransferase SMYD2 promotes cyst growth in autosomal dominant polycystic kidney disease. J Clin Invest. 2017;127:2751–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li LX, Zhou JX, Calvet JP, Godwin AK, Jensen RA, Li X. Lysine methyltransferase SMYD2 promotes triple negative breast cancer progression. Cell Death Dis. 2018;9:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tracy C, Warren JS, Szulik M, et al. The Smyd family of methyltransferases: role in cardiac and skeletal muscle physiology and pathology. Curr Opin Physiol. 2018;1:140–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu L, Zhu YT, Qi C, Zhu YJ. Identification of Smyd4 as a potential tumor suppressor gene involved in breast cancer development. Cancer Res. 2009;69:4067–4072. [DOI] [PubMed] [Google Scholar]
- 23.Stender JD, Pascual G, Liu W, et al. Control of proinflammatory gene programs by regulated trimethylation and demethylation of histone H4K20. Mol Cell. 2012;48:28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li B, Pan R, Zhou C, et al. SMYD3 promoter hypomethylation is associated with the risk of colorectal cancer. Future Oncol. 2018;14:1825–1834. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Xie BH, Lin WH, et al. Amplification of SMYD3 promotes tumorigenicity and intrahepatic metastasis of hepatocellular carcinoma via upregulation of CDK2 and MMP2. Oncogene. 2019;38(25):4948–4961. [DOI] [PubMed] [Google Scholar]
- 26.Liu N, Tolbert E, Pang M, Ponnusamy M, Yan H, Zhuang S. Suramin inhibits renal fibrosis in chronic kidney disease. J Am Soc Nephrol. 2011;22:1064–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferguson AD, Larsen NA, Howard T, et al. Structural basis of substrate methylation and inhibition of SMYD2. Structure. 2011; 19(9):1262–1273. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen H, Allali-Hassani A, Antonysamy S, et al. LLY-507, a cell-active, potent, and selective inhibitor of protein-lysine methyltrans-ferase SMYD2. J Biol Chem. 2015;290:13641–13653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chevalier RL, Forbes MS, Thornhill BA. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int. 2009;75:1145–1152. [DOI] [PubMed] [Google Scholar]
- 30.Chung AC, Dong Y, Yang W, Zhong X, Li R, Lan HY. Smad7 suppresses renal fibrosis via altering expression of TGF-beta/Smad3-regulated microRNAs. Mol Ther. 2013;21:388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yi X, Jiang XJ, Fang ZM. Histone methyltransferase SMYD2: ubiquitous regulator of disease. Clin Epigenetics. 2019;11:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med. 2010;16:535–543, 531p following 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang J, Shultz RW, Mars WM, et al. Disruption of tissue-type plasminogen activator gene in mice reduces renal interstitial fibrosis in obstructive nephropathy. J Clin Invest. 2002;110:1525–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pace J, Paladugu P, Das B, He JC, Mallipattu SK. Targeting STAT3 signaling in kidney disease. Am J Physiol Renal Physiol. 2019;316:F1151 –F1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang H, Sun SC. NF-kappaB in inflammation and renal diseases. Cell Biosci. 2015;5:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakamoto LH, Andrade RV, Felipe MS, Motoyama AB, Pittella Silva F. SMYD2 is highly expressed in pediatric acute lymphoblastic leukemia and constitutes a bad prognostic factor. Leuk Res. 2014;38:496–502. [DOI] [PubMed] [Google Scholar]
- 37.Yan L, Ding B, Liu H, et al. Inhibition of SMYD2 suppresses tumor progression by down-regulating microRNA-125b and attenuates multi-drug resistance in renal cell carcinoma. Theranostics. 2019;9:8377–8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parmar N, Chandrakar P, Kar S. Leishmania donovani subverts host immune response by epigenetic reprogramming of macrophage M(Lipopolysaccharides + IFN-γ)/M(IL-10) polarization. J Immunol. 2020:204:2762–2778. [DOI] [PubMed] [Google Scholar]
- 39.Nakakido M, Deng Z, Suzuki T, Dohmae N, Nakamura Y, Hamamoto R. Dysregulation of AKT pathway by SMYD2-mediated lysine methylation on PTEN. Neoplasia. 2015;17:367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qie S, Diehl JA. Cyclin D1, cancer progression, and opportunities in cancer treatment. J Mol Med. 2016;94:1313–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paternot S, Bockstaele L, Bisteau X, Kooken H, Coulonval K, Roger PP. Rb inactivation in cell cycle and cancer: the puzzle of highly regulated activating phosphorylation of CDK4 versus constitutively active CDK-activating kinase. Cell Cycle. 2010;9:689–699. [DOI] [PubMed] [Google Scholar]
- 42.El-Deiry WS. p21(WAF1) mediates cell-cycle inhibition, relevant to cancer suppression and therapy. Cancer Res. 2016;76:5189–5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Komatsu S, Imoto I, Tsuda H, et al. Overexpression of SMYD2 relates to tumor cell proliferation and malignant outcome of esophageal squamous cell carcinoma. Carcinogenesis. 2009;30:1139–1146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


