Abstract
Background:
This study explored trends in utilization of marginal donors for orthotopic heart transplantation (OHT) in the United States.
Methods:
Using the United Network for Organ Sharing database, adults (≥18 years) undergoing OHT between 2009 and 2019 were identified. Marginal donors were defined as having ≥2 of the following: age ≥50 years, ejection fraction less than 50%, ischemic time greater than 240 min, donor‐to‐recipient body mass index ratio less than 0.8, or donor inotrope use. Kaplan–Meier analysis was utilized to model survival with multivariable Cox regression analysis used for risk‐adjustment.
Results:
A total of 23,580 recipients underwent OHT with 4896 (20.76%) receiving organs from marginal donors. The use of marginal donors decreased from 25.6% in 2009 to 16.0% in 2017 but accounted for 24.7% of OHTs in 2019. This recent increase in marginal donor use was largely attributable to increased use of donors with ischemic time greater than 240 min, whereas other marginal donor criteria remained stable. Among 140 centers, median marginal donor use was 20.07% (interquartile range, 14.17%–26.51%). An increasing proportion of marginal donors was not associated with increased center‐level OHT volume (R2 < 0.001, p = .833). Marginal donor use was associated with reduced 1‐ (88.75% vs. 91.87%) and 5‐year survival (76.73% vs. 80.08%, p < .001). Following adjustment, marginal donor use remained a significant predictor of post‐OHT mortality (hazard ratio, 1.17; p < .001).
Conclusion:
Marginal donors account for approximately 20% of OHTs performed in the United States. Despite a reduction in utilization over the past decade, the 2018 allocation change has resulted in a significant increase in use, largely attributable to longer ischemic times.
Keywords: cardiovascular pathology, transplant
1 |. INTRODUCTION
Limited donor availability for orthotopic heart transplantation (OHT) has resulted in reexamination of historical contraindications for heart transplant donors. This includes recent incorporation of the use of hepatitis C positive donors,1,2 older donors,3 and donors with borderline left ventricular dysfunction.4 The use of extended criteria and marginal donors has expanded the donor pool for end‐stage heart failure patients with evidence of favorable outcomes in selected candidate populations. Prior studies have demonstrated comparable post-OHT survival in recipients receiving organs with reduced ejection fraction compared to those with normal left ventricular function.4,5 Furthermore, midterm mortality utilizing organs from older donors appears favorable.6–8 These findings are particularly important given that many of these end-stage heart failure patients may not have survived while waitlisted had they awaited an optimal donor. Prior work has explored the outcomes of marginal donor use in relation to OHT volume, finding that higher volume centers displayed superior outcomes with marginal donors compared to lower volume centers.9 We sought to explore temporal trends in the use of marginal donors for OHT in the United States.
2 |. METHODS
2.1 |. Study cohort
Utilizing the United Network for Organ Sharing (UNOS) and Scientific Registry of Transplant Recipients databases, we explored adult patients (≥18 years) undergoing OHT between January 1, 2009 and December 31, 2019. Patients undergoing multiorgan transplants (e.g., heart-lung, heart-kidney, etc.) or redo heart transplantation were excluded.
Marginal donors were defined as donors having two or more of the following characteristics: donor age ≥50 years, ischemic time greater than 240 min, donor-to-recipient body mass index (BMI) ratio less than 0.8, donor left ventricular ejection fraction less than 50%, and donor inotrope use.
2.2 |. Statistical analysis
Continuous data are reported as mean (standard deviation) for Gaussian data and median (interquartile range [IQR]) for non-Gaussian data. Categorical data are reported as number (percentage). χ2 testing was utilized for comparison of categorical data. Pairwise comparison was performed utilizing Student’s t test or Mann–Whitney U test as appropriate for continuous data. Linear regression was utilized to correlate total OHT volume at each center with the percentage of marginal donors used at each center. Multivariable Cox regression analysis with all recipient baseline characteristics was utilized to determine variables independently associated with post-OHT survival. Kaplan–Meier analysis was utilized to model one-year and five-year survival, stratified by the use of marginal donors. Log rank testing was employed to compare survival curves. All hypothesis testing was two-sided. Statistical analyses were performed using the Stata 16 software package (2017, Stata Statistical Software: Release 16; StataCorp) and GraphPad Prism (Version 8; GraphPad Software). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Individual consent for this retrospective analysis was waived. This study was approved by the Institutional Review Board at the University of Pittsburgh.
3 |. RESULTS
3.1 |. Baseline characteristics
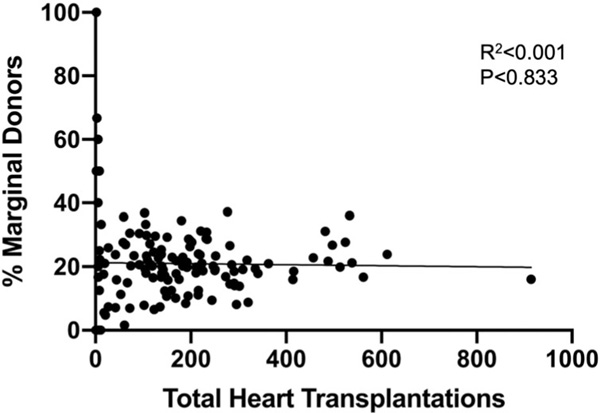
A total of 23,580 patients underwent OHT during the study period with 4896 (20.76%) receiving organs from marginal donors (Table 1). Recipients undergoing OHT using marginal donors were significantly older (54.19 vs. 53.38 years, p < .001), had a higher BMI (28.71 vs. 27.14 kg/m2, p < .001), and were more likely to have diabetes (29.68 vs. 25.80%, p < .001). The use of a left ventricular assist device (LVAD) was lower in the marginal donor group (42.71 vs. 44.30%, p = .013) while there were no significant differences in the use of an intra-aortic balloon pump (9.01 vs. 8.42%, p = .190). There were also no differences in the use of extracorporeal membrane oxygenation between the groups (1.14 vs. 1.23%, p = .619). Mean donor age among nonmarginal donors was 31.22 years compared to 35.63 years in the marginal donor group (p < .001). Total ischemic time was significantly greater in the marginal donor group (226.68 vs. 180.89 min, p < .001). The use of marginal donors also varied based on UNOS region (p < .001). Marginal donor utilization did not increase with increasing center volume (R2 < 0.001, p = .833) (Figure 1).
TABLE 1.
Baseline characteristics of patients undergoing orthotopic heart transplantation, stratified by the use of marginal donors
| Overall, N = 23,580 | Non-marginal donors, N = 18,684 | Marginal donors, N = 4896 | p Value | |
|---|---|---|---|---|
| Recipient characteristics | ||||
| Age (years) | 53.55 (12.63) | 53.38 (12.72) | 54.19 (12.24) | <.001 |
| Sex, no. (%) | <.001 | |||
| - Female | 6 232 26.43) | 4827 (25.83) | 1405 (28.70) | |
| - Male | 17,348 (73.57) | 13,857 (74.17) | 3491 (71.30) | |
| Race/ethnicity, no. (%) | .958 | |||
| - White | 15,567 (66.02) | 12,341 (66.05) | 3226 (65.89) | |
| - Black | 5009 (21.24) | 3965 (21.22) | 1,044 (21.32) | |
| - Hispanic | 1946 (8.25) | 1535 (8.22) | 411 (8.39) | |
| - Other | 1058 (4.49) | 843 (4.51) | 215 (4.39) | |
| Body mass index (kg/m2) | 27.46 (5.06) | 27.14 (4.94) | 28.71 (5.31) | <.001 |
| Diagnosis, no. %) | .154 | |||
| - Nonischemic cardiomyopathy | 12,249 (51.95) | 9782 (52.35) | 2467 (50.39) | |
| - Ischemic cardiomyopathy | 8171 34.65) | 6399 (34.25) | 1772 (36.19) | |
| - Congenital | 726 (3.08) | 580 (3.10) | 146 (2.98) | |
| - Restrictive | 826 (3.50) | 642 (3.44) | 184 (3.76) | |
| - Valvular | 326 (1.38) | 259 (1.39) | 67 (1.37) | |
| - Hypertrophic cardiomyopathy | 622 (2.64) | 502 (2.69) | 120 (2.45) | |
| - Other/unknown | 660 (2.80) | 520 (2.78) | 140 (2.86) | |
| Diabetes, no. %) | 6274 (26.61) | 4821 (25.80) | 1453 (29.68) | <.001 |
| Prior malignancy, no. (%) | 1967 (8.36) | 1540 (8.25) | 427 (8.74) | .463 |
| Steroid use, no. %) | 1526 (6.55) | 1212 (6.54) | 314 (6.59) | .994 |
| Creatinine (mg/dl) | 1.24 (0.61) | 1.24 (0.62) | 1.27 (0.55) | .001 |
| Total bilirubin ( mg/dl) | 0.70 0.50–1.10) | 0.70 (0.50–1.10) | 0.70 (0.50–1.10) | .393 |
| Pulmonary capillary wedge pressure (mmHg) | 17.76 (8.70) | 17.71 (8.72) | 17.93 (8.59) | .148 |
| Pulmonary artery systolic pressure (mmHg) | 40.03 (13.93) | 39.91 (13.93) | 40.48 (13.92) | .013 |
| Left ventricular assist device, no. (%) | 10,368 (43.97) | 8,277 (44.30) | 2091 (42.71) | .046 |
| Intra-aortic balloon pump, no. (%) | 2014 (8.54) | 1573 (8.42) | 441 (9.01) | .190 |
| Extracorporeal membrane oxygenation, no. (%) | 273 (1.16) | 213 (1.14) | 60 (1.23) | .619 |
| Inotrope use, no. ( %) | 8498 (36.04) | 6810 (36.45) | 1688 (34.48) | .011 |
| Functional status | .010 | |||
| - Independent | 3253 (14.52) | 2645 (14.83) | 608 (13.32) | |
| - Partially independent | 10,737 (47.93) | 8474 (47.51) | 2263 (49.56) | |
| - Fully dependent | 8412 (37.55) | 6717 (37.66) | 1695 (37.12) | |
| Donor characteristics | ||||
| Age (years) | 32.13 (11.31) | 31.22 (10.36) | 35.63 (13.82) | <.001 |
| Sex, no. (%) | .019 | |||
| - Female | 7011 (29.73) | 5622 (30.09) | 1389 (28.37) | |
| - Male | 16,569 (70.27) | 13,062 (69.91) | 3507 (71.63) | |
| Race/ethnicity, no. (%) | <.001 | |||
| - White | 15,185 (64.40) | 11,911 (63.75) | 3274 (66.87) | |
| - Black | 3843 (16.30) | 3048 (16.31) | 795 (16.24) | |
| - Hispanic | 3907 (16.57) | 3224 (17.26) | 683 (13.95) | |
| - Other | 645 (2.74) | 501 (2.68) | 144 (2.94) | |
| Body mass index (kg/m2) | 27.47 (5.99) | 27.91 (5.99) | 25.77 (5.68) | <.001 |
| Mechanism of death | <.001 | |||
| - Trauma | 11,357 (48.17) | 9008 (48.23) | 2349 (47.98) | |
| - Cerebrovascular | 4930 (20.91) | 3675 (19.67) | 1255 (25.63) | |
| - Drug overdose | 3001 (12.73) | 2506 (13.42) | 495 (10.11) | |
| - Other | 4287 (18.18) | 3490 (18.68) | 797 (16.28) | |
| Ejection fraction | 61.64 (6.73) | 61.78 (6.51) | 61.13 (7.50) | <.001 |
| Inotrope use | 10,020 (42.74) | 6018 (32.40) | 4002 (82.24) | <.001 |
| Transplant characteristics | ||||
| Ischemic time (min) | 190.16 (62.64) | 180.89 (56.95) | 226.68 (70.25) | <.001 |
| UNOS region, no. (%) | <.001 | |||
| − 1 | 1077 | 828 (76.88) | 249 (23.12) | |
| − 2 | 2902 | 2160 (74.43) | 742 (25.57) | |
| − 3 | 2764 | 2140 (77.42) | 624 (22.58) | |
| − 4 | 2566 | 2163 (84.29) | 403 (15.71) | |
| − 5 | 3563 | 2924 (82.07) | 639 (17.93) | |
| − 6 | 757 | 558 (73.71) | 199 (26.29) | |
| − 7 | 2100 | 1604 (76.38) | 496 (23.62) | |
| − 8 | 1535 | 1306 (85.08) | 229 (14.92) | |
| − 9 | 1517 | 1201 (79.17) | 316 (20.83) | |
| − 10 | 1898 | 1522 (80.19) | 376 (19.81) | |
| − 11 | 2901 | 2278 (78.52) | 623 (21.48) | |
FIGURE 1.
Scatter plot of total heart transplantations during the study period compared to the percentage of marginal donors used at each center. Linear regression results are shown in the inset
3.2 |. Survival analysis
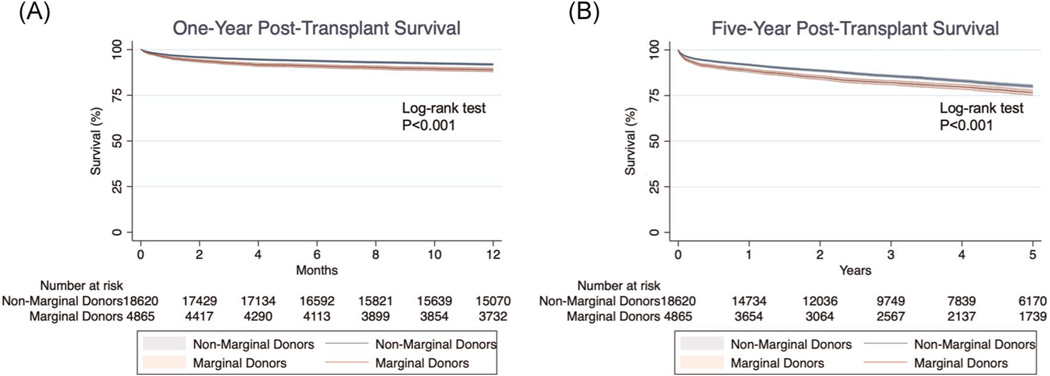
In a multivariable analysis of all-cause mortality following OHT, the use of a marginal donor was significantly associated with death (hazard ratio [HR], 1.18; 95% confidence interval [CI], 1.10–1.27, p < .001) (Table 2). Additionally, recipient diabetes (HR, 1.24; 95% CI, 1.16–1.33), increasing creatinine (HR, 1.10; 95% CI, 1.07–1.13), increasing total bilirubin (HR, 1.06; 95% CI, 1.04–1.07), and the use of a LVAD (HR, 1.15; 95% CI, 1.09–1.25) were associated with post-OHT mortality. One-year post-OHT survival was significantly greater among patients receiving organs from non-marginal donors (p < .001) (Figure 2A). Survival at 1-year was 91.87% with non-marginal donors and 88.75% with marginal donors. At five years, survival remained significantly different between the two groups (p < .001) (Figure 2B). Survival at 5 years was 80.08% with non-marginal donors and 76.73% with marginal donors.
TABLE 2.
Multivariable Cox regression analysis of mortality following orthotopic heart transplantation
| Hazard ratio | 95% Confidence interval | p Value | |
|---|---|---|---|
| Marginal donor | 1.18 | 1.10−1.27 | <.001 |
| Recipient age | 1.00 | 1.00−1.01 | .002 |
| Race/ethnicity | |||
| - White | Reference | Reference | Reference |
| - Black | 1.23 | 1.14−1.33 | <.001 |
| - Hispanic | 0.99 | 0.88−1.12 | .971 |
| - Other | 0.86 | 0.72−1.01 | .071 |
| Diagnosis, no. (%) | |||
| - Nonischemic cardiomyopathy | Reference | Reference | Reference |
| - Ischemic cardiomyopathy | 1.33 | 1.24−1.43 | <.001 |
| - Congenital | 1.52 | 1.24−1.85 | <.001 |
| - Restrictive | 1.31 | 1.10−1.56 | .002 |
| - Valvular | 0.89 | 0.67−1.18 | .424 |
| - Hypertrophic cardiomyopathy | 0.89 | 0.70−1.13 | .349 |
| - Other/unknown | 0.96 | 0.77−1.19 | .694 |
| Diabetes | 1.24 | 1.16−1.33 | <.001 |
| Creatinine level | 1.10 | 1.07−1.13 | <.001 |
| Total bilirubin | 1.06 | 1.04−1.07 | <.001 |
| Pulmonary capillary wedge pressure | 0.99 | 0.99−0.99 | .021 |
| Pulmonary artery systolic pressure | 1.00 | 1.00−1.01 | .027 |
| Left ventricular assist device | 1.17 | 1.09−1.25 | <.001 |
| Functional status | |||
| - Independent | Reference | Reference | Reference |
| - Partially independent | 1.13 | 1.03–1.24 | .011 |
| - Fully dependent | 1.29 | 1.17–1.42 | <.001 |
FIGURE 2.
Kaplan–Meier analysis of 1-year (A) and 5-year (B) mortality following orthotopic heart transplantation, stratified by the use of marginal donors
3.3 |. Center-level analysis of marginal donor use
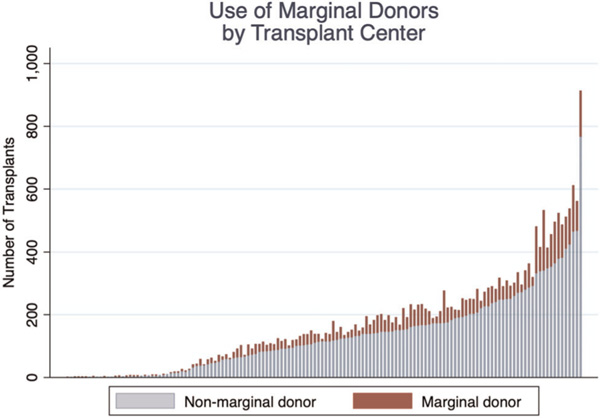
Among the 140 transplant centers that performed at least one OHT during the study period, a mean of 168.4 ± 156.7 total transplants were performed at each center (median, 139; IQR, 42–234). The mean percentage of use of marginal donors across the study period for each center was 20.99 ± 13.70% (median, 20.07%; IQR, 14.17%–26.51%). The use of non-marginal and marginal donors at each transplant center is shown (Figure 3).
FIGURE 3.
Total number of heart transplants during the study period at each center demonstrating the use of non-marginal and marginal donors
3.4 |. Trends in marginal donor use
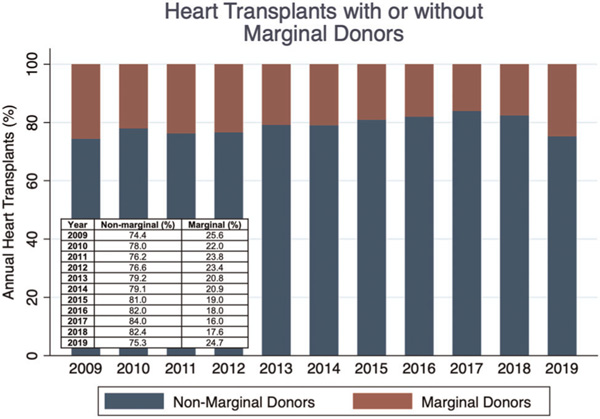
The use of marginal donors varied significantly during the study period (p < .001) (Figure 4). In 2009, 25.6% of donors were considered marginal while in 2017, 16.0% of donors were marginal. The proportion of donors considered marginal was 20.30% in OHTs occurring before the 2018 UNOS allocation policy change and 23.99% following this change (p < .001). In contrast, the proportion of marginal donors was 24.7% in 2019. More specifically, the proportion of donors with an ischemic time greater than 240 min increased (p < .001, Figure S1) and the use of donors requiring inotropes decreased over time then remained stable from 2016 to 2019 (p < .001, Figure S2). There were no significant trends observed in the use of donors with age greater than 50 years (p = .092, Figure S3), BMI ratio less than 0.8 (p = .183, Figure S4), and donor ejection fraction less than 50% (p = .335, Figure S5).
FIGURE 4.
Use of marginal donors during each year of the study period for orthotopic heart transplantation
4 |. CONCLUSION
OHT can extend life expectancy in patients with end-stage heart failure, however, the limited availability of donor organs may result in waitlist mortality in many candidates. Recent work has focused on expanding the donor pool through reconsideration of donor selection criteria with promising results demonstrated with the use of marginal donors. Herein, we found that the use of a marginal donor varied significantly between 2009 and 2019 with an apparent increase in marginal donor use in the last year of the study period. In particular, the proportion of donors with an ischemic time greater than 4 h increased significantly in 2019. Additionally, the use of marginal donors at transplant centers across the United States varied with a median of 20% of donors being considered marginal at each center. Though we found that marginal donor use was independently associated with post-OHT mortality, transplant with marginal donors may still extend life in candidates who otherwise might not have survived to receive an organ from an optimal donor. These findings elucidate trends in the use of marginal donors with implications for guiding organ allocation and improving understanding of OHT outcomes.
Though we found that the use of a marginal donor was independently associated with post-OHT mortality, prior studies have demonstrated relatively favorable outcomes with these donors.10,11 Donor age in particular has been a focus of study. In an analysis of factors contributing to 10-year survival following OHT, recipients who were alive at 10 years had received organs from significantly younger donors.12 Others have demonstrated no differences in overall mortality utilizing donors ≥50 years.6,8 Nevertheless, older donor age may be associated with an increased risk of coronary allograft vasculopathy and thus the use of these donors may perhaps be better suited for only selected populations of OHT candidates.6,13 Evolving definitions of a marginal or extended criteria donor may explain some variability in survival results reported for these donors. Ultimately, the decision to consider a marginal donor should be individualized, weighing the risks of transplant-related adverse outcomes with the potential for waitlist mortality in OHT candidates.
Multiple studies have focused on the relationship between OHT center volume and recipient outcomes with one study demonstrating that higher volume centers displayed superior outcomes with marginal donors.14 More generally, improved graft survival has been linked to higher OHT center volume.15 Here, we found that the use of marginal donors approximated 20% though the IQR (14%–26%) suggests considerable variation based on the center. There are multiple factors, aside from center volume, that may account for the variability in marginal donor use. Regional heterogeneity in organ availability and waitlist times may contribute to some of the observed distribution.16,17 This is also supported by our finding of differences in regional use of marginal donors. It is difficult to account for all factors that may contribute to varying practice patterns, though it is also important to ensure that allocation policies allow for equitable organ distribution across all geographic territories.
We also noted distinct trends in the individual donor characteristics used to define a marginal donor. The use of donors with an ejection fraction less than 50% remained very low and stable at around 1.5% of transplants during the study period. Similarly, donor age ≥50 years did not vary significantly. Multiple studies have demonstrated that, in carefully selected recipients, either older donor age3,6 or decreased ejection fraction4,5 can result in favorable recipient outcomes. Despite these data, it does not appear that donor selection has shifted to reflect these results. During the same time period, the use of donors with longer ischemic times increased while the use of donors requiring inotropes decreased over time although has remained stable over the last few years. While there is data correlating longer donor ischemic time with inferior outcomes, we observed that the proportion of donors with ischemic times greater than 4 h was amongst the highest in 2019. This is likely related to the recent UNOS allocation policy change in 2018. As broader organ sharing and reducing geographic disparities was a major goal of the new policy, it seems that this may have been achieved in that longer ischemic times are evident in this past year.18
Indeed, the increased proportion of donors with an ischemic time greater than 240 h in 2019 is consistent with prior data demonstrating longer donor-to-recipient hospital distances following the policy change.18 Studies have also shown that waitlist mortality appears to have improved since the policy change though post-OHT survival may be worse.18 While these mortality outcomes may be related to the clinical condition of recipients at the time of transplant, it is plausible that the prioritization of patients requiring intra-aortic balloon pump and extracorporeal membrane oxygen support, for example, may have shifted the criteria for donor selection. Thus, to ensure that these critically ill patients are transplanted, expediency may prevail in donor selection rather than awaiting an optimal donor.
This study has several limitations. Due to the retrospective nature of the study design, we were unable to account for decision-making for the use of a marginal donor. Thus, we were only able to report on observed center-level or regional variations in marginal donor use without necessarily accounting for the granular factors that may influence such decisions. We also acknowledge that there is some subjectivity and variability in defining a marginal donor.
In conclusion we report temporal trends in marginal donor use with an apparent increase in donor ischemic time, which appears to be driving the increased use of marginal donors in 2019. Despite evidence suggesting that the use of older donors and donors with borderline ejection fraction may yield comparable recipient outcomes, we did not find any significant trend in their usage. Though marginal donor use may be associated with inferior survival, it is important to consider survival outcomes in critically ill patients who remain on the waitlist and are subject to clinical deterioration and death. Further work will be necessary to understand the implications of marginal donor use to expand the donor pool. Importantly, this study highlights national trends and the critical role of organ allocation policy in ensuring equitable sharing of organs.
Supplementary Material
Footnotes
DISCLOSURES
Dr. Arman Kilic serves on a Medical Advisory Board for Medtronic, Inc. All other authors have nothing to disclose with regard to commercial support.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from UNOS. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the Organ Procurement and Transplantation Network at https://optn.transplant.hrsa.gov/data/request-data/.
REFERENCES
- 1.Moayedi Y, Fan CPS, Gulamhusein AF, et al. Current use of hearts from hepatitis C viremic donors. Circ Heart Fail. 2018;11(12): e005276. 10.1161/CIRCHEARTFAILURE.118.005276 [DOI] [PubMed] [Google Scholar]
- 2.Kilic A, Hickey G, Mathier M, et al. Outcomes of adult heart transplantation using hepatitis C–positive donors. J Am Heart Assoc. 2020;9(2). 10.1161/JAHA.119.014495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prieto D, Correia P, Baptista M, Antunes MJ. Outcome after heart transplantation from older donor age: expanding the donor pool. Eur J Cardio-thoracic Surg. 2015;47(4):672–678. 10.1093/ejcts/ezu257 [DOI] [PubMed] [Google Scholar]
- 4.Sibona A, Khush KK, Oyoyo UE, et al. Long-term transplant outcomes of donor hearts with left ventricular dysfunction. J Thorac Cardiovasc Surg. 2019;157(5):1865–1875. 10.1016/j.jtcvs.2018.07.115 [DOI] [PubMed] [Google Scholar]
- 5.Chen CW, Sprys MH, Gaffey AC, et al. Low ejection fraction in donor hearts is not directly associated with increased recipient mortality. J Hear Lung Transplant. 2017;36(6):611–615. 10.1016/j.healun.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 6.Roig E, Almenar L, Crespo-Leiro M, et al. Heart transplantation using allografts from older donors: multicenter study results. J Hear Lung Transplant. 2015;34(6):790–796. 10.1016/j.healun.2014.10.006 [DOI] [PubMed] [Google Scholar]
- 7.Bruschi G, Colombo T, Oliva F, et al. Orthotopic heart transplantation with donors greater than or equal to 60 years of age: a singlecenter experience. Eur J Cardio-thoracic Surg. 2011;40(1):55–61. 10.1016/j.ejcts.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 8.Shudo Y, Guenther SP, Lingala B, et al. Relation of length of survival after orthotopic heart transplantation to age of the donor. Am J Cardiol. 2020;131:54–59. 10.1016/j.amjcard.2020.06.036 [DOI] [PubMed] [Google Scholar]
- 9.Kilic A, Weiss ES, Allen JG, et al. Should orthotopic heart transplantation using marginal donors be limited to higher volume centers? Ann Thorac Surg. 2012;94(3):695–702. 10.1016/j.athoracsur.2012.03.069 [DOI] [PubMed] [Google Scholar]
- 10.Forni A, Luciani GB, Chiominto B, Pizzuti M, Mazzucco A, Faggian G. Results with expanded donor acceptance criteria in heart transplantation. Transplant Proc. 2011;43(4):953–959. 10.1016/j.transproceed.2011.01.117 [DOI] [PubMed] [Google Scholar]
- 11.Galeone A, Lebreton G, Coutance G, et al. A single-center long-term experience with marginal donor utilization for heart transplantation. Clin Transplant. 2020;34. 10.1111/ctr.14057 [DOI] [PubMed] [Google Scholar]
- 12.Kilic A, Weiss ES, George TJ, et al. What predicts long-term survival after heart transplantation? An analysis of 9,400 ten-year survivors. Ann Thorac Surg. 2012;93(3):699–704. 10.1016/j.athoracsur.2011.09.037 [DOI] [PubMed] [Google Scholar]
- 13.Nagji AS, Hranjec T, Swenson BR, et al. Donor Age is associated with chronic allograft vasculopathy after adult heart transplantation: implications for donor allocation. Ann Thorac Surg. 2010;90(1): 168–175. 10.1016/j.athoracsur.2010.03.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kilic A, Weiss ES, Allen JG, et al. Should orthotopic heart transplantation using marginal donors be limited to higher volume centers? Ann Thorac Surg. 2012;94(3):695–702. 10.1016/j.athoracsur.2012.03.069 [DOI] [PubMed] [Google Scholar]
- 15.Russo MJ, Iribarne A, Easterwood R, et al. Post-heart transplant survival is inferior at low-volume centers across all risk strata. Circulation. 2010;122(11 SUPPL. 1). 10.1161/CIRCULATIONAHA.109.926659 [DOI] [PubMed] [Google Scholar]
- 16.Parker WF, Anderson AS, Hedeker D, et al. Geographic variation in the treatment of U.S. Adult Heart Transplant candidates. J Am Coll Cardiol. 2018;71(16):1715–1725. 10.1016/j.jacc.2018.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen VP, Givens RC, Cheng RK, et al. Effect of regional competition on heart transplant waiting list outcomes. J Hear Lung Transplant. 2016;35(8):986–994. 10.1016/j.healun.2016.03.017 [DOI] [PubMed] [Google Scholar]
- 18.Kilic A, Hickey G, Mathier MA, et al. Outcomes of the First 1300 Adult Heart Transplants in the United States after the allocation policy change. Circulation. 2020;141:1662–1664. 10.1161/CIRCULATIONAHA.119.045354 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from UNOS. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the Organ Procurement and Transplantation Network at https://optn.transplant.hrsa.gov/data/request-data/.