In the phase 3 IMbrave150 trial, atezolizumab plus bevacizumab improved PFS and OS in patients with previously untreated, unresect-able HCC.1 A retrospective, multi-center analysis by the Korean Cancer Study Group evaluated real-world data to identify prognostic factors among patients with advanced HCC treated with first-line atezolizumab plus beva-cizumab.2
The trial enrolled 121 patients with a Child-Pugh score of A5 (74.4%) or A6 (25.6%) and BCLC stage B (20.7%) or C (79.3%) disease.2 Their median age was 61 years, and most patients (84%) were male. Macrovas-cular invasion was reported in 37.2% of patients, and 70.2% of patients had extrahepatic spread. The cause of HCC was hepatitis B in 76.9% and hepatitis C in 5%. Prior treatment included TACE in 57.9% of patients, radiotherapy in 37.2%, surgery in 31.4%, and radiofrequency ablation in 14.9%.
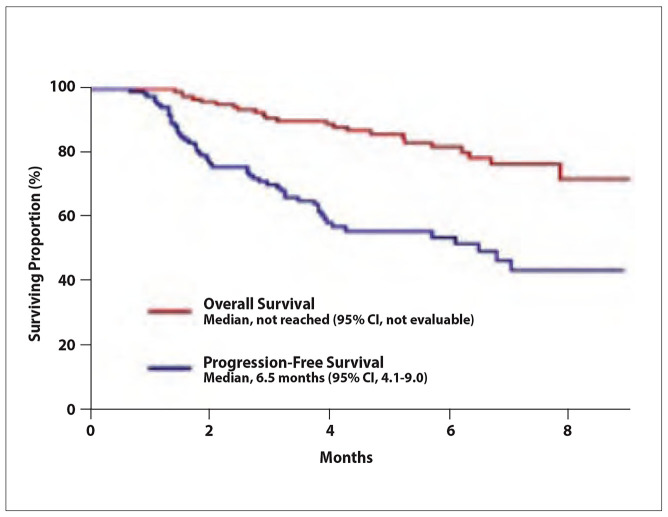
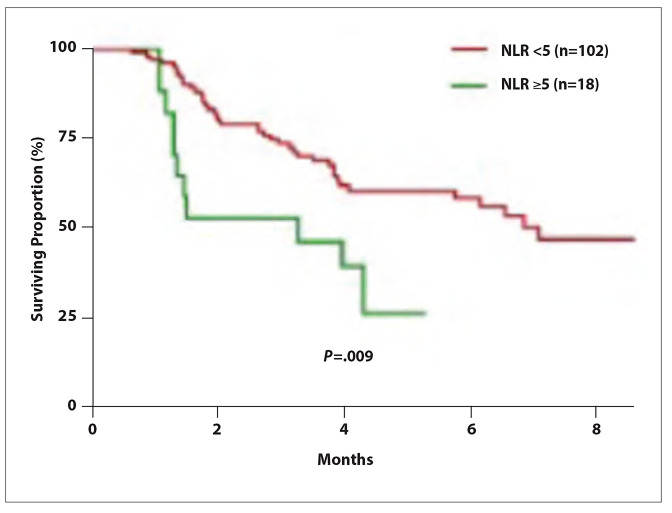
The ORR was 24.0%, with a CR rate of 1.7%.2 The median OS was not reached (95% CI, not evaluable; Figure 4), and the median PFS was 6.5 months (95% CI, 4.1-9.0). Based on multivariate analysis, the PFS and OS were significantly better in patients with a neutrophil-to-lymphocyte ratio of less than 5 (Figure 5). The hazard ratio for the neutrophil-to-lymphocyte ratio (>5 vs <5) was 2.23 (95% CI, 1.12-4.45; P=.023) for PFS and 4.68 (95% CI, 1.87-11.73; P<.001) for OS. The median PFS was superior in patients who achieved a CR or PR vs those with stable or progressive disease (P<.001), as well as in those with an increase in alpha-fetoprotein vs those with a decrease (P=.002).
Figure 4.
Overall and progression-free survival in a retrospective, multicenter analysis of patients with advanced hepatocellular carcinoma treated with first-line atezolizumab plus bevacizumab. Adapted from Cheon J et al. ESMO abstract 955P. Ann Oncol. 2021;32(suppl 5).2
Figure 5.
Median progression-free survival according to the NLR in a retrospective, multicenter analysis of patients with advanced hepatocellular carcinoma treated with first-line atezolizumab plus bevacizumab. NLR, neutrophil-to-lymphocyte ratio. Adapted from Cheon J et al. ESMO abstract 955P. Ann Oncol. 2021;32(suppl 5).2
A grade 3/4 AE was reported in 28.9%. The most common grade 3/4 AEs were elevated aspartate amino-transferase in 10.7%, hypertension in 6.6%, and thrombocytopenia in 4.9%.
The study investigators concluded that the results of their real-world analysis were similar to those reported in the IMbrave150 trial.1,2 They noted that careful assessment of treatment response is needed in patients with an elevated neutrophil-to-lymphocyte ratio, who had lower survival outcomes in this analysis.
References
- 1.Finn RS, Qin S, Ikeda M et al. IMbrave150 Investigators. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 2.Cheon J, Yoo C, Hong JY et al. Prognostic factor analysis of atezolizumab-bevacizumab in unresectable hepatocellular carcinoma: Korean Cancer Study Group (KCSG) study [ESMO abstract 955P]. Ann Oncol. 2021;32(suppl 5) [Google Scholar]