Ozanimod is an oral immunomodulatory agent that acts as a selective sphingosine-1-phosphate (S1P) receptor agonist. The multicenter, double-blind phase 3 True North trial evaluated ozanimod as induction and maintenance therapy in patients with moderately to severely active ulcerative colitis.1,2 During the 10-week induction period, patients in cohort 1 were randomly assigned in a 2:1 ratio to receive daily ozanimod hydrochloride (1 mg, equivalent to 0.92 mg of ozanimod) or placebo. Patients in cohort 2 received open-label ozanimod hydrochloride (1 mg). After 10 weeks, patients who demonstrated a clinical response to ozanimod were randomly assigned in a double-blind manner to receive ozanimod or placebo during the 42 weeks of the maintenance period. The primary end-point was the proportion of patients with clinical remission, based on the 3-component Mayo score.3
To assess induction therapy, the True North trial randomly assigned 429 patients to ozanimod and 216 to placebo in cohort 1. In cohort 2, open-label ozanimod was administered to 367 patients. The maintenance period of the trial included 457 patients. The study demonstrated a significant increase in the incidence of clinical remission with ozanimod vs placebo, during both induction (18.4% vs 6.0%; P<.001) and maintenance (37.0% vs 18.5%; P<.001).1 Treatment with ozanimod also yielded a greater proportion of patients with a clinical response compared with placebo, during both induction (47.8% vs 25.9%; P<.001) and maintenance (60.0% vs 41.0%; P<.001).
Britta Siegmund, MD, presented an analysis of the True North trial that evaluated the rapidity of symptomatic response and remission among patients who received ozanimod during the 10-week induction period.2 A symptomatic response was defined as a decrease in the adapted partial Mayo score of at least 1 point and at least 30% from baseline, as well as a decrease of at least 1 point from baseline in the rectal bleeding subscore or an absolute rectal bleeding subscore of 1 or less. Symptomatic remission was defined as a rectal bleeding subscore of 0 and a stool frequency subscore of 1 point or less, as well as a decrease from baseline of 1 or more points.
The patients’ baseline characteristics were generally well balanced among the 3 cohorts. The patients’ mean age was 42 years, and their mean body mass index (BMI) was 25 to 26. The mean total Mayo score was approximately 9±1.5, and the mean partial Mayo score was approximately 6.1±1.2. Across the 3 cohorts, the proportion of patients with a rectal bleeding subscore of 2 or 3 ranged from 92% to 96%, and the proportion of patients with a stool frequency subscore of 2 or 3 ranged from 42% to 47%. Prior use of anti-tumor necrosis factor (TNF) agents was reported in 30% of patients in cohort 1 and 42% in cohort 2.
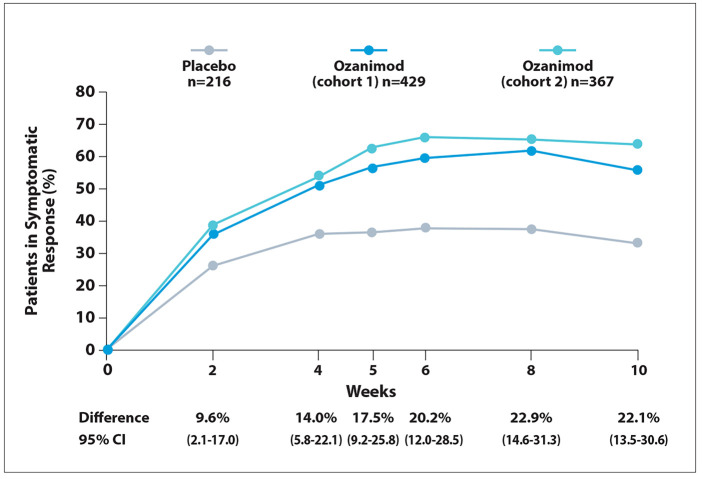
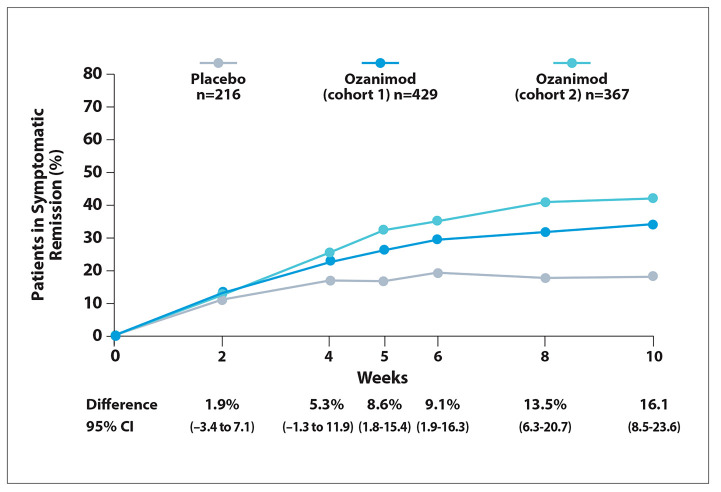
A first symptomatic response to treatment with ozanimod vs placebo was evident after 2 weeks of induction therapy, in both the overall study population (difference, 9.6%; Figure 1) and among patients without prior exposure to anti-TNF therapy (difference, 9.4%). Among patients with prior anti-TNF treatment, the first response to induction therapy was observed at 4 weeks (difference, 15.8%). Symptomatic remission was observed with ozanimod vs placebo at week 5 in the overall study population (difference, 8.6%; Figure 2), at week 4 in patients without prior exposure to anti-TNF therapy (difference, 9.4%), and at week 8 in patients with prior exposure to anti-TNF therapy (difference, 11.7%).
Figure 1.
Symptomatic response in patients with moderately to severely active ulcerative colitis during induction treatment with ozanimod in the phase 3 True North trial. Adapted from Siegmund B et al. ECCO abstract DOP43. J Crohns Colitis. 2022;16(suppl 1).2
Figure 2.
Symptomatic remission in patients with moderately to severely active ulcerative colitis during induction treatment with ozanimod in the phase 3 True North trial. Adapted from Siegmund B et al. ECCO abstract DOP43. J Crohns Colitis. 2022;16(suppl 1).2
The investigators concluded that treatment with ozanimod improved initiation of treatment. Symptomatic symptomatic response compared with remission was seen with ozanimod as placebo as early as 2 weeks after the early as 5 weeks after treatment began.
For patients who were naive to TNF inhibitors, ozanimod led to a significant improvement in symptomatic response in as early as 2 weeks. This duration increased to 4 weeks among patients previously treated with TNF inhibitors. For symptomatic remission, ozanimod was associated with significant improvement as early as 4 weeks for patients naive to TNF inhibitors and as early as 8 weeks for those previously treated with TNF inhibitors.
References
- 1.Sandborn WJ, Feagan BG, D’Haens G et al. True North Study Group. Ozanimod as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2021;385(14):1280–1291. doi: 10.1056/NEJMoa2033617. [DOI] [PubMed] [Google Scholar]
- 2.Siegmund B, Axelrad J, Osterman MT et al. Rapidity of ozanimod-induced symptomatic response and remission in patients with moderately to severely active ulcerative colitis: results from the induction period of True North [ECCO abstract DOP43]. J Crohns Colitis. 2022;16(suppl 1) [PMC free article] [PubMed] [Google Scholar]
- 3.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317(26):1625–1629. doi: 10.1056/NEJM198712243172603. [DOI] [PubMed] [Google Scholar]