The randomized, double-blind phase 2b QUASAR Induction Study 1 evaluated the safety and efficacy of12 weeks of guselkumab in patients with moderately to severely active ulcerative colitis.1 The trial enrolled patients previously treated with conventional or advanced therapy that was intolerable or inadequate. Their Mayo rectal bleeding score was 1 or higher at baseline and their Mayo endoscopy subscore was at least 2, based on central review. The patients were randomly assigned to receive placebo, guselkumab at 200 mg every 4 weeks, or guselkumab at 400 mg every 4 weeks. The primary endpoint was the clinical response at week 12.
Among the entire study population of 313 patients, the median age was 41.6±14.40 years, and 59.1% were male. The mean duration of ulcerative colitis was 7.55±6.79 years. The mean Mayo score was 9.2±1.32, and the mean modified Mayo score was 7.0±1.0. Seventy percent of patients had a modified Mayo score of 7, 8, or 9, and 70% of patients had an endoscopy subscore of 3, indicating severe disease. Medications in use at baseline included oral aminosalicylates (77.3%), oral corticosteroids (39.6%), and immuno-suppressants (21.7%), and 23.3% of patients were intolerant to 2 or more classes of advanced therapy.
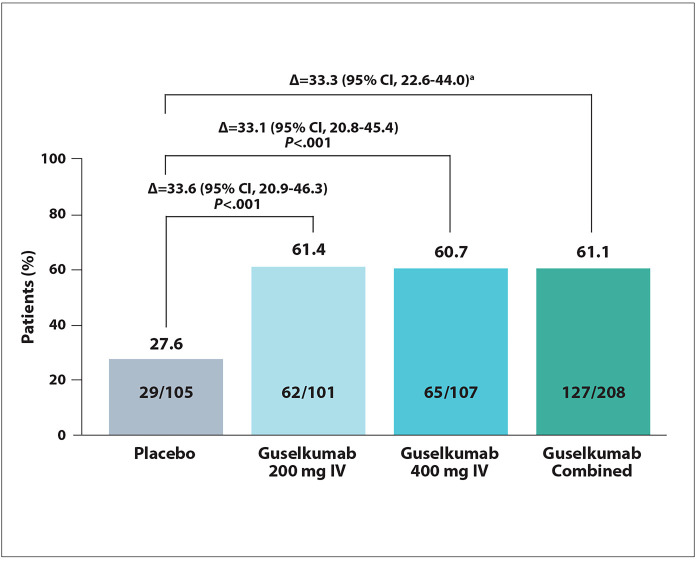
Nine patients (2.9%) discontinued treatment, most commonly owing to study withdrawal (1.0%) or worsening ulcerative colitis (1.0%). Results with the 2 doses of guselkumab were generally comparable. At week 12 of induction, the proportion of patients with a clinical response was 27.6% with placebo, 61.4% with the lower dose of guselkumab, and 60.7% with the higher dose of guselkumab (P<.001 for both; Figure 11). Rates of clinical remission were 9.5% with placebo vs 25.7% with the lower dose of guselkumab and 25.2% with the higher dose of guselkumab (P<.05 for both). The rates of symptomatic remission were 50.5% with the lower dose of guselkumab, 47.7% with the higher dose, and 20% with placebo (P<.001 for both). The rate of endoscopic improvement was 30.7% (P<.05 vs placebo), 30.8% (P<.001 vs placebo), and 12.4%, respectively. The rate of histo-endoscopic improvement at week 12 was 19.8% (P<.05 vs placebo) with the lower dose of guselkumab, 27.1% (P<.001 vs placebo) with the higher dose, and 8.6% with placebo. The rate of endoscopic normalization was also significantly worse with placebo (6.7%) compared with the lower dose of guselkumab (19.8%; P<.05 vs placebo). The comparison did not reach statistical significance with the higher dose of guselkumab (14.0%; P>.05 vs placebo).
Figure 11.
Clinical response at week 12 in the phase 2b QUASAR study, which evaluated induction therapy with guselkumab in patients with moderately to severely active ulcerative colitis. a Nominal P value <.001. IV, intravenous. Adapted from Dignass A et al. ECCO abstract OP32. J Crohns Colitis. 2022;16(suppl 1).1
Safety results were generally consistent with observations from previous studies in approved indications. The rate of serious AEs was 1% in the guselkumab arms vs 5.7% in the placebo arm. AEs required treatment discontinuation in 0.5% vs 1.9%, respectively. The rate of infection was 10.6% vs 11.4%, with serious infections occurring in 0% vs 1.9%. No deaths occurred during the study.
References
- 1.Dignass A, Rubin DT, Bressler B et al. The efficacy and safety of guselkumab induction therapy in patients with moderately to severely active ulcerative colitis: phase 2b QUASAR study results through week 12 [ECCO abstract OP32]. J Crohns Colitis. 2022;16(suppl 1) [PMC free article] [PubMed] [Google Scholar]