Abstract
Strains of Escherichia coli O157 isolated from patients with clinical cases of food-borne illness and other sources exhibited wide differences in resistance to high hydrostatic pressure. The most pressure-resistant strains were also more resistant to mild heat than other strains. Strain C9490, a representative pressure-resistant strain, was also more resistant to acid, oxidative, and osmotic stresses than the pressure-sensitive strain NCTC 12079. Most of these differences in resistance were observed only in stationary-phase cells, the only exception being acid resistance, where differences were also apparent in the exponential phase. Membrane damage in pressure-treated cells was revealed by increased uptake of the fluorescent dyes ethidium bromide and propidium iodide. When strains were exposed to the same pressure for different lengths of time, the pressure-sensitive strains took up stain sooner than the more resistant strain, which suggested that the differences in resistance may be related to susceptibility to membrane damage. Our results emphasize the importance of including stress-resistant strains of E. coli O157 when the efficacy of a novel or mild food preservation treatment is tested.
High hydrostatic pressure is viewed as one of the more promising nonthermal methods for inactivating microbes in food (10, 11, 15, 16). Although there is active research into methods for inactivating spores (for example, pressure cycling combined with mild heat [14, 25, 30]), it is likely that the initial applications of pressure processing will be aimed at replacing thermal pasteurization as a means of killing vegetative microbes. In this context, Escherichia coli O157 is clearly a major concern because it has a low infective dose, causes severe illness, and has been associated with a wide range of foods (2). Any pressure process must therefore be capable of inactivating this organism.
Studies of the pressure resistance of E. coli O157 and Listeria monocytogenes have shown that there are appreciable differences between the most pressure-sensitive and most pressure-resistant types (26). It has also been possible to isolate pressure-resistant mutants of E. coli by using repeated cycles of pressure treatment and outgrowth (13). Strains of Salmonella enteritidis PT4 have been divided into two groups on the basis of their resistance to mild heat, drying, and other environmental stresses (19). The most resistant strains were more virulent in mice and more invasive in chickens than other strains (18). If there are generally robust strains of E. coli O157, they would obviously pose problems for the development of safe food-processing treatments, and this possibility is particularly important when attempts are made to reduce processing to a minimum in order to preserve the fresh attributes of a food commodity.
In this work we examined the variation in the pressure resistance of natural isolates of E. coli O157 and investigated whether pressure-resistant strains were resistant to other forms of stress. Our results revealed that there are wide differences in pressure resistance among strains and that the most pressure-resistant strains are also more resistant to other adverse treatments than other strains are. Preliminary results suggested that differences in pressure resistance among strains may be related to differences in susceptibility to membrane damage.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The following E. coli O157:H7 strains were kindly provided by M. Doyle, University of Georgia, Griffin: C9490 (a clinical isolate from the Jack-in-the-Box western states hamburger patty outbreak of 1993); 30-2C4 (a clinical isolate from an outbreak associated with dry cured salami); and W2-2 (a poultry isolate). The other E. coli O157 strains used included NCTC 12079 and H1071 (a clinical isolate from M. Patterson, Queen’s University, Belfast, United Kingdom). Strain NCTC 8003 (serotype 0124) also was tested. Cultures were prepared by inoculating 10 ml of tryptone soy broth (TSB) (Oxoid catalog no. CM129) with a loopful of growth from tryptone soy agar (Oxoid catalog no. CM131) and incubating the resulting culture with shaking at 37°C for 18 h. Cells in the stationary phase of growth were prepared by subculturing a loopful of this culture in 100 ml of fresh TSB and incubating the preparation for 18 h under the same conditions. To obtain exponential-phase cells, 100 μl of the stationary-phase culture was inoculated into 100 ml of fresh medium and incubated for 3 h, which resulted in an optical density at 680 nm of 0.2.
Viable counts.
Cell suspensions were serially diluted in Maximum Recovery Diluent (MRD) (Oxoid catalog no. CM733) and plated onto tryptone soy agar containing 0.3% yeast extract. Colonies were counted after the plates were incubated at 37°C for 48 h. Most of the survival curves were based on mean values obtained from two to five independent experiments; the only exceptions were the survival curves shown in Fig. 3, which were based on values obtained from single representative experiments performed to illustrate the multiphasic inactivation behavior. The error bars on the figures indicate the mean standard deviations for the curves. The pressure inactivation curves were nonlinear, so the curve-fitting procedure of Baranyi et al. (4) was used to allow comparisons between curves on the basis of the time necessary to reduce the numbers by a specific amount.
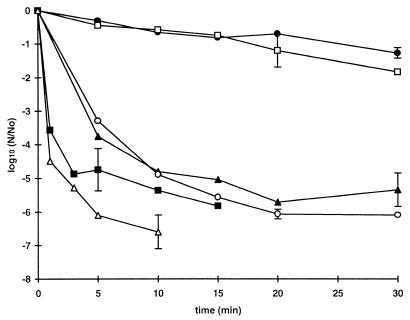
FIG. 3.
Acid resistance of E. coli O157 at 37°C. Strains C9490 (● and ○) and NCTC 12079 (▴ and ▵) were grown to the stationary phase in TSB and diluted 1:100 in TSB acidified to pH 2.5 with HCl without an additive (solid symbols) or supplemented with 50 mM undissociated acetic acid (open symbols). The curves show the data obtained in single experiments.
Pressure treatment.
Cells were centrifuged at 3,000 × g for 20 min at 4°C, and the pellets were resuspended in phosphate-buffered saline (PBS) (pH 7.0) to give viable counts of about 109 CFU · ml−1. Cell suspensions (2 ml) were placed in sterile plastic pouches (4 by 5 cm) that were heat sealed and kept on ice before pressurization. Samples were pressure treated in a 300-ml pressure vessel (model S-FL-850-9-W; Stanstead Fluid Power, Stanstead, United Kingdom). The pressure-transmitting fluid used was ethanol-castor oil (80:20). Stationary-phase cells were treated at a pressure of 500 MPa, and exponential-phase cells were treated at a pressure of 200 MPa for different lengths of time. The increase in the temperature of the pressurization fluid caused by adiabatic heating was monitored with a thermocouple. The maximum temperature reached during pressurization at 500 MPa was approximately 45°C for 80 s. At the end of each time interval pouches were removed from the unit and placed on ice before viable counts were determined.
Heat resistance.
Stationary-phase cultures were diluted 1:100 in TSB, and 1-ml portions were sealed in glass ampoules and kept on ice. The ampoules were heated by fully submerging them in a stirred water bath at 52 or 57°C. After the contents of the ampoules had reached the desired temperature, the ampoules were withdrawn at intervals and kept on ice before viable counts were determined.
Resistance to a low pH.
TSB was acidified with HCl to pH 2.5 for stationary-phase cells or to pH 3.0 for exponential-phase cells, as determined at 37°C. When indicated below, enough acetic acid was added to achieve a concentration of undissociated acid of 50 mM. The acidified broth was filter sterilized. Cells were added to a final concentration of 107 CFU · ml−1, and the preparation was incubated at 37°C. Samples (1 ml) were withdrawn at intervals and transferred into 9 ml of MRD containing 100 mM HEPES buffer (pH 7.0). Subsequent serial dilutions were made in MRD.
Resistance to oxidative stress.
Cells were centrifuged, washed in PBS, added to 10 ml of 50 mM H2O2 at a final concentration of 107 CFU · ml−1, and incubated at 37°C. Samples (1 ml) were removed at intervals and transferred into 9 ml of MRD containing 100 μg of catalase (catalog no. C40; Sigma Chemical Co.) per ml to neutralize the H2O2 before the samples were serially diluted with MRD.
Resistance to high osmotic pressure.
Cells were added to TSB containing 20% NaCl to a final concentration of 107 CFU · ml−1 and incubated at 37°C. Samples were removed daily to determine viable counts.
Staining cells with EB and PI.
Propidium iodide (PI) and ethidium bromide (EB) were added to cell suspensions to final concentrations of 2.9 and 100 μM, respectively. After incubation for 10 min, the samples were centrifuged and washed twice in PBS. Fluorescence was measured with a fluorometer (model LS-5B; Perkin-Elmer); the excitation wavelength was set at 493 nm for EB and at 495 nm for PI, and the emission wavelengths were set at 610 and 615 nm, respectively. The slit width was 10 nm. Fluorescence data for cell suspensions were normalized against optical density at 680 nm and expressed as percentages of the value obtained for cells permeabilized by heating at 80°C for 5 min. Fluorescence values obtained for untreated cells were subtracted from all experimental values.
Membrane fatty acid composition.
Cells were grown to the stationary phase in TSB at 37°C and then centrifuged and washed twice in PBS. Samples were saponified and methylated by using the method of Miller and Berger (24) and then were examined with a Hewlett-Packard model HP 6890 gas chromatograph fitted with a capillary column (25 m by 0.32 mm; SGE 054119). The carrier gas was ultrahigh-purity hydrogen delivered at a constant flow rate. The initial temperature was 40°C, and the temperature was increased at a rate of 10°C/min to 150°C and then at a rate of 4°C/min to 280°C. The results were analyzed by using HP Chemstation software. Fatty acids were identified by comparing them with a standard mixture of fatty acid methyl esters (bacterial acid methyl ester mix; Supelco Inc.).
RESULTS
Resistance to hydrostatic pressure.
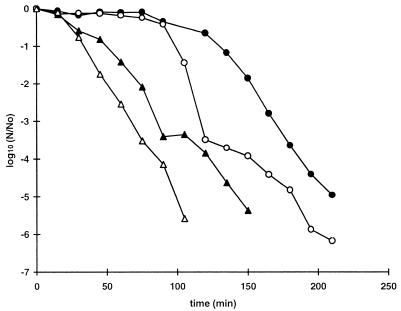
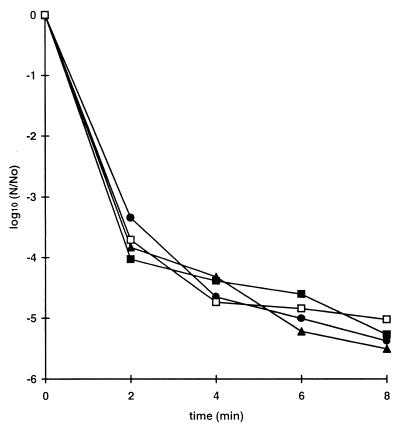
The survival curves for six strains of E. coli were compared at a pressure of 500 MPa by using cells taken from the stationary phase of growth (Fig. 1). Two strains, C9490 and 30-2C4, were consistently much more pressure resistant than the other strains. Strains H1071 and NCTC 8003 (a non-O157 strain) were the most sensitive strains tested. The inactivation kinetics of the pressure-sensitive strains were nonlinear when they were plotted on a semilogarithmic scale, and it was therefore not possible to represent pressure resistance in terms of decimal reduction times (D values; the D value is the treatment time required to reduce viable numbers by a factor of 10, based on exponential inactivation kinetics). The times needed to reduce viable numbers by 3 log10 units differed by more than 30-fold when the most pressure-resistant strains and the most pressure-sensitive strains were compared (<1 min versus >30 min).
FIG. 1.
Pressure resistance of E. coli strains. Cells were grown to the stationary phase in TSB, harvested by centrifugation, resuspended in PBS (pH 7) (109 CFU · ml−1), and then treated at a pressure of 500 MPa. Each curve shows the average values obtained from at least two independent experiments. The mean standard deviation, shown for each curve, varied between 0.14 and 0.6. The strains used were E. coli O157 C9490 (●), 30-2C4 (□), NCTC 12079 (▴), W2-2 (○), and H1071 (■) and E. coli O124 NCTC 8003 (▵).
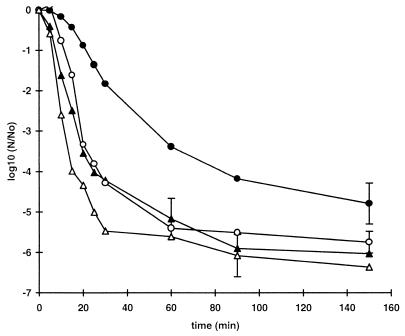
Resistance to heat.
Thermal inactivation curves for stationary-phase cells at 52 and 57°C are shown in Fig. 2. The two most pressure-resistant strains, strains C9490 and 30-2C4, were substantially more heat resistant than the other strains. The differences in heat resistance among the more sensitive strains were small, but it was apparent that the most pressure-sensitive strain, strain NCTC 8003, was not the most heat-sensitive strain. Within the sensitive group, therefore, the correlation between heat resistance and pressure resistance was not absolute. Strains C9490 and NCTC 12079 were chosen to represent the sensitive and resistant types, respectively, during further investigations.
FIG. 2.
Heat resistance of E. coli strains. Cells grown to the stationary phase in TSB were diluted 1:100 in fresh TSB (107 CFU · ml−1) and heated at 52°C (A) or 57°C (B). Each curve shows the average values obtained from at least two independent experiments. The mean standard deviation, shown for each curve, varied between 0.15 and 0.7. The strains used were E. coli O157 C9490 (●), 30-2C4 (□), NCTC 12079 (▴), W2-2 (○), and H1071 (■) and E. coli O124 NCTC 8003 (▵).
Acid resistance.
The acid resistance of strain C9490 and the acid resistance of strain NCTC 12079 were compared in broth acidified to pH 2.5 with HCl alone or in the presence of 50 mM undissociated acetic acid (Fig. 3). Inactivation of both strains was more rapid in the presence of acetic acid than in its absence, but strain C9490 was more acid resistant than strain NCTC 12079 irrespective of the acidulant. The survival curves for both strains had an initial shoulder region, but the strain C9490 shoulder was about 10 times longer than the strain NCTC 12079 shoulder. This difference largely accounted for the difference in resistance, since the subsequent rates of inactivation were similar. In two or possibly three of the four curves shown in Fig. 3 there is a discontinuity at the point where viable numbers decreased by about 3.5 log10 units. At this point the curves appear to have a second shoulder region, which suggests that there was a resistant subpopulation (the effect was very slight for strain NCTC 12079 in broth containing acetic acid but nevertheless occurred at a similar point on the curve).
Resistance to hydrogen peroxide.
We compared the resistance of the two strains to oxidative stress by challenging each strain with 50 mM hydrogen peroxide. Strain C9490 was more resistant than NCTC 12079, and the times needed to reduce viable numbers by 3 log10 units were 8 and 25 min, respectively (data not shown).
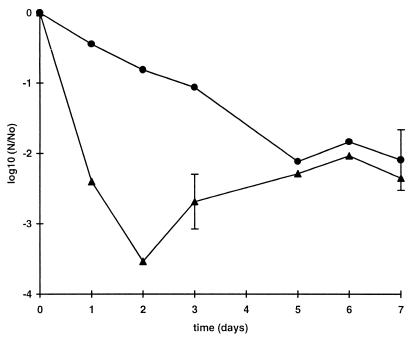
Resistance to osmotic stress.
The survival curves for strains C9490 and NCTC 12079 in the presence of 20% sodium chloride at 37°C are shown in Fig. 4. The initial rate of inactivation was much greater for strain NCTC 12079 than for strain C9490, but after 2 days the viable counts of NCTC 12079 increased until they approximately equalled those of the more resistant strain, strain C9490. This behavior suggested that the plate counts obtained for strain NCTC 12079 during the first 5 days underestimated the true viable counts; however, attempts to increase the recovery of this strain by including betaine in the diluent used for viable counting or by preparing the dilutions with 10% NaCl to decrease the osmotic downshock were unsuccessful (data not shown). Microscopic examination provided no evidence that the effect was caused by clumping of the cells.
FIG. 4.
Resistance of E. coli O157 to high osmotic pressure. Strains C9490 (●) and NCTC 12079 (▴) were grown to the stationary phase in TSB and then diluted in TSB containing 20% sodium chloride at 37°C (107 CFU · ml−1). Each curve shows the average values obtained from at least two independent experiments. The mean standard deviations shown for each curve were around 0.4 in both cases.
Stress resistance of exponential-phase cells of E. coli O157.
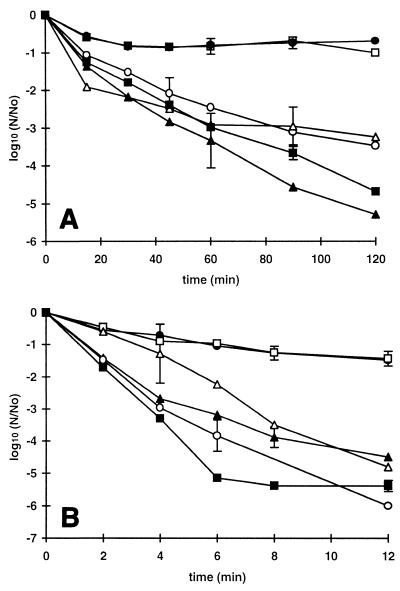
Two strains that were pressure resistant when they were tested in the stationary phase (C9490 and 30-2C4) and two strains that were more pressure sensitive (NCTC 12079 and H1071) were reexamined by using exponential-phase cells. The test pressure used was lower than the pressure used for stationary-phase cells (200 instead of 500 MPa) to allow for the greater sensitivity of exponential-phase cells. There were no differences in the pressure resistance characteristics of exponential-phase cells of these strains (Fig. 5). There were also negligible differences in resistance to osmotic or oxidative stress (data not shown). Strain C9490 was more acid resistant than NCTC 12079 in broth acidified with HCl alone, but the difference largely disappeared in the presence of acetic acid (Fig. 6). The shapes of the inactivation curves for exponential-phase cells exposed to acid were different from the shapes obtained with stationary-phase cells; the former curves were characterized by a short shoulder, followed by a rapid rate of inactivation and a long tail region.
FIG. 5.
Pressure resistance of exponential-phase E. coli strains. Cells from an overnight culture were diluted 1:1,000 in fresh TSB, grown to a density of 108 CFU · ml−1, harvested by centrifugation, resuspended in PBS (pH 7.0), and treated at a pressure of 200 MPa. Each curve shows the average values obtained from at least two independent experiments. The strains used were E. coli O157 C9490 (●), 30-2C4 (□), NCTC 12079 (▴), and H1071 (■).
FIG. 6.
Acid resistance of exponential-phase E. coli O157 strains at 37°C. Strains C9490 (● and ○) and NCTC 12079 (▴ and ▵) were grown to the stationary phase, diluted in fresh TSB (106 CFU · ml−1), grown to a concentration of 108 CFU · ml−1, and then inoculated (1:10) into TSB acidified to pH 3 with HCl without an additive (solid symbols) or supplemented with 50 mM undissociated acetic acid (open symbols). Each curve shows the average values obtained from at least two independent experiments. The mean standard deviation shown for each curve varied between 0.3 and 0.5.
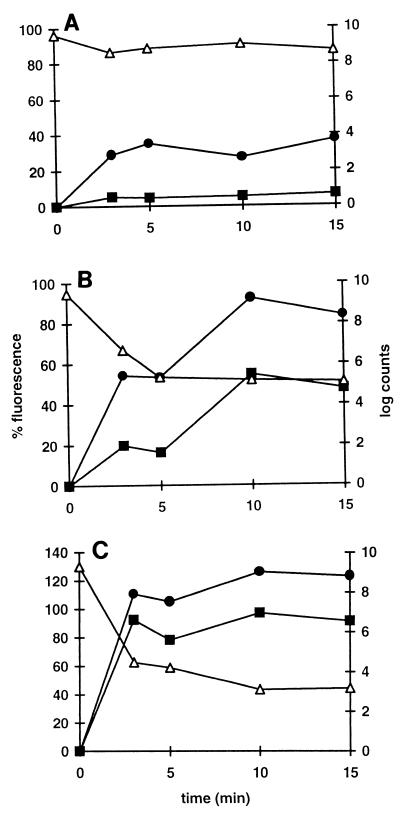
Pressure-induced changes in membrane integrity.
Staining of cells with the fluorescent dyes EB and PI was used as an index of membrane damage in pressure-treated cells (Fig. 7). The pressure-resistant strain C9490 exhibited little loss of viability or uptake of PI within 15 min at a pressure of 500 MPa, whereas the viability of strain H1071 decreased 1,000-fold within 3 min and PI staining was almost maximal after this. Strain NCTC 12079 exhibited an intermediate response. Pressure-treated cells of all strains took up EB more readily than they took up PI, but the pattern of responses was the same.
FIG. 7.
Uptake of fluorescent dyes by pressure-treated E. coli cells. EB (●) and PI (■) were added to stationary-phase cells of strains C9490 (A), NCTC 12079 (B), and H1071 (C) treated at a pressure of 500 MPa in PBS for different lengths of time. Viable counts (▵) were determined at each measurement time. Fluorescence was expressed as a percentage of the value obtained with cells heated at 80°C for 5 min, which was assumed to be 100%. The curves show the data obtained in single experiments.
Membrane fatty acid composition.
The differences in the membrane fatty acid profiles of strains C9490, 12079, and H1071 were small (Table 1). Strain C9490 contained about one-half as much β-hydroxymyristic acid (14:0-OH), which is a component of lipopolysaccharide, as the other two strains and slightly higher proportions of palmitic acid (16:0) and total cyclopropane fatty acid; however, there was no obvious relationship between pressure resistance and membrane composition in the three strains.
TABLE 1.
Membrane fatty acids of E. coli O157 strains
| Fatty acid type | % of total area ina:
|
||
|---|---|---|---|
| Strain C9490 | Strain NCTC 12079 | Strain H1071 | |
| 12:0 | 5.2 | 7.8 | 7.2 |
| 14:0 | 17.6 | 17.3 | 13.9 |
| 14:0-OH | 9.3 | 20.6 | 17.6 |
| 16:0 | 38.8 | 31 | 34.2 |
| 17 cyclopropane | 19.5 | 16.3 | 17.8 |
| 19 cyclopropane | 8.8 | 4.9 | 6.1 |
| Total cyclopropane | 28.2 | 21.2 | 23.8 |
Data are the averages of four values. The standard deviations varied between 0.1 and 1%.
DISCUSSION
The differences in pressure resistance among strains of E. coli O157 reported here are in agreement with the results of Patterson et al. (26) but are even more pronounced. Patterson et al. (26) examined three strains of E. coli O157, and NCTC 12079 was the most pressure resistant of these strains. However we found in this study that two strains isolated from salami and hamburger patties associated with large E. coli food-poisoning outbreaks are substantially more pressure resistant than NCTC 12079. The most sensitive strain examined by Patterson et al. (26) was H1071. We also found that this strain was sensitive, although strain NCTC 8003 was slightly more sensitive. We also screened a number of other O157 strains whose levels of pressure resistance fall between the extremes presented in this paper (data not shown).
The two most pressure-resistant strains examined, strains C9490 and 30-2C4, were also the strains that were most resistant to heating at 52 and 57°C. There is a rough correlation between heat resistance and pressure resistance in food-borne bacteria, and gram-positive organisms tend to be more resistant to both heat and pressure than gram-negative organisms (3, 9, 12, 28); however, there are many exceptions to this general trend. Some strains of E. coli are appreciably more pressure resistant than some strains of L. monocytogenes (26); and Salmonella senftenberg 775W, which was five times more heat resistant than Salmonella typhimurium ATCC 7136 (based on their relative D values at 57.5°C), was actually more sensitive to hydrostatic pressure (23). Two of three pressure-resistant mutants of E. coli K-12 isolated by successive cycles of pressure treatment and outgrowth of survivors were more heat resistant than the parent organism was, but the heat resistance of a third mutant was unchanged (13). Hauben et al. (13) concluded that increased barotolerance probably arose from an accumulation of multiple mutations and was not necessarily related to altered thermotolerance. Both pressure and heat are known to affect many components of the bacterial cell, including nucleic acids, membranes, and ribosomes, and the critical lethal events have yet to be identified (5, 10, 17).
Strain C9490 was also more resistant to acid, osmotic, and oxidative stresses than the more pressure-sensitive strain NCTC 12079. Apart from resistance to acetic acid, these differences were apparent only in stationary-phase cells and may therefore involve the rpoS gene, but other loci could also be involved and genetic studies will be needed to clarify the situation. The acid resistance of strain C9490 reported here is in agreement with observations of Buchanan and Edelson (8). These authors noted that strain C9490 did not mount an acid tolerance response and suggested that this organism may be analogous to constitutively acid-tolerant mutants of S. typhimurium (22). The pH of TSB at the time when stationary-phase cells were harvested was approximately 6.5; thus, the differences in acid resistance were probably not related to a specific acid tolerance response. The enhanced acid resistance of stationary-phase cells of strain C9490 was manifest as a long shoulder on survival curves, followed by a more rapid decrease in viable numbers. This behavior is consistent with a loss of membrane integrity or a collapse of homeostatic or repair systems after a critical period. The multiphasic inactivation kinetics of stationary-phase cells and the presence of tails on survival curves of exponential-phase cells strongly suggest that there is physiological heterogeneity within populations with regard to acid resistance. The existence of resistant subpopulations has important implications for the safety of acid foods, and the causes of this phenomenon need to be clarified.
Strain C9490 appeared to be more resistant to osmotic stress than strain NCTC 12079, but the results are somewhat difficult to interpret because the viable counts of strain NCTC 12079 initially decreased but then apparently increased until they equalled those of strain C9490. Similar apparent losses and recoveries of viability were described previously for osmotically stressed cells of L. monocytogenes and E. coli (6, 27). In both of these cases, reducing the osmotic downshock associated with viable count procedures by increasing the osmolarity of the diluent increased the recovery of viable cells. We were not able to increase recovery by raising the osmolarity of the diluent, but our results nevertheless indicate that strain NCTC 12079 is susceptible to a form of osmotically induced sublethal injury which does not occur in strain C9490. Sublethal injury may be an important factor in explaining strain differences in stress resistance generally, and the susceptibilities of different strains to secondary stresses (oxidative stress, osmotic stress, etc.) encountered during enumeration procedures may shed light on this. These factors should also be important in determining whether injured cells survive in processed foods.
Differences in the extents of staining by PI and EB after pressure treatment suggest that resistance to pressure may be related to the relative susceptibilities of strains to membrane damage. During pressure treatment, cells could be stained with EB before they could be stained with PI. EB can enter cells with intact membranes but is rapidly pumped out by an efflux system (20). Uptake of this dye can therefore result either from physical membrane damage or from failure of the efflux system. Uptake of EB thus appears to be a more sensitive indicator of perturbation of membrane function than uptake of PI, but it does not necessarily reflect permanent damage to the membrane. A correlation between membrane permeability to PI and cell death has been found for Lactobacillus plantarum cells pressure treated for different times and at different pressures (29).
In E. coli the cyclopropane fatty acid content of the membrane lipids increases during the stationary phase and also during the acid tolerance response in exponential-phase cells (7, 31). This fatty acid may therefore contribute to a general increase in stress resistance; however, there were no significant differences in the membrane fatty acids of the strains of E. coli O157 examined, apart from an apparently lower β-hydroxymyristic acid content in strain C9490. Examination of four resistant mutants of E. coli isolated by Hauben et al. (13) also revealed no differences in membrane fatty acid composition. The differences in susceptibility to pressure-induced membrane damage reported here must therefore be related to some other property of the membrane, possibly a property associated with the protein component. Loss of membrane-bound ATPase activity and loss of the ability to maintain a transmembrane pH gradient have been shown to occur during inactivation of L. plantarum by high pressure (32). Our results support the notion that the cell membrane is an important target of pressure damage to the cell and also imply that differences in membrane properties may be an important factor in determining the stress resistance of different E. coli strains; however, the nature of the lethal effect and the role of membrane structure in determining resistance to pressure and possibly to other stresses must be clarified.
Strain C9490 was associated with a very large outbreak of food-borne illness, and its success as a pathogen may perhaps be related to its resistance to a wide range of environmental stresses. Concern has been expressed that milder food preservation regimens may provide conditions that allow the emergence of resistant strains which are better adapted to survive in lightly processed food (1). An understanding of selective pressures that lead to the emergence of strains that are resistant to multiple stresses and the genetic basis of strain variation is therefore important in avoiding preservation regimens that may promote the spread of food-borne illness.
Recent work has shown that the lethal effects of high pressure are much enhanced by other preservative factors, such as a low pH, moderate temperatures (temperatures up to 45°C), and the presence of bacteriocins (21). Inactivation of resistant strains by mild processes may depend on the use of combined “multiple-hurdle” systems of food preservation. An immediate practical consequence of this work is that any cocktail of E. coli O157 organisms used to test the efficacy of pressure treatments or other processes should include stress-resistant strains, such as C9490 and 30-2C4.
ACKNOWLEDGMENT
We are grateful to the Ministry of Agriculture Fisheries and Food, London, United Kingdom, for financial support of this work.
REFERENCES
- 1.Archer D L. Preservation microbiology and safety: evidence that stress enhances virulence and triggers adaptive mutations. Trends Food Sci Technol. 1996;7:91–95. [Google Scholar]
- 2.Armstrong G L, Hollingsworth J, Morris J G. Emerging foodborne pathogens—Escherichia coli O157-H7 as a model of entry of a new pathogen into the food-supply of the developed world. Epidemiol Rev. 1996;18:29–51. doi: 10.1093/oxfordjournals.epirev.a017914. [DOI] [PubMed] [Google Scholar]
- 3.Arroyo G, Sanz P D, Préstamo G. Effect of high pressure on the reduction of microbial populations in vegetables. J Appl Microbiol. 1997;82:735–742. doi: 10.1046/j.1365-2672.1997.00149.x. [DOI] [PubMed] [Google Scholar]
- 4.Baranyi J, Jones A, Walker C, Kaloti A, Robinson T P, Mackey B M. A combined model for growth and subsequent thermal inactivation of Brochothrix thermosphacta. Appl Environ Microbiol. 1996;62:1029–1035. doi: 10.1128/aem.62.3.1029-1035.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartlett D H. Microbial life at high pressures. Sci Prog. 1992;76:479–496. [PubMed] [Google Scholar]
- 6.Brocklehurst T F, Mitchell G A, Pleass W, Smith A C. The effect of step changes in sucrose concentration on the growth of Salmonella typhimurium LT2. J Appl Bacteriol. 1995;78:495–500. doi: 10.1111/j.1365-2672.1995.tb03091.x. [DOI] [PubMed] [Google Scholar]
- 7.Brown J L, Ross T, McMeekin T A, Nichols P D. Acid habituation of Escherichia coli and the potential role of cyclopropane fatty acids in low pH tolerance. Int J Food Microbiol. 1997;37:163–173. doi: 10.1016/s0168-1605(97)00068-8. [DOI] [PubMed] [Google Scholar]
- 8.Buchanan R L, Edelson S G. Culturing enterohemorrhagic Escherichia coli in the presence and absence of glucose as a simple means of evaluating the acid tolerance of stationary-phase cells. Appl Environ Microbiol. 1996;62:4009–4013. doi: 10.1128/aem.62.11.4009-4013.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlez A, Rosec J-P, Richard N, Cheftel J-C. High pressure inactivation of Citrobacter freundii, Pseudomonas fluorescens and Listeria innocua in inoculated minced beef muscle. Lebensm-Wiss Technol. 1993;26:357–363. [Google Scholar]
- 10.Cheftel J-C. Hautes pressions, inactivation microbienne et conservation des aliments. C R Acad Agric Fr. 1995;81:13–38. [Google Scholar]
- 11.Earnshaw R G, Appleyard J, Hurst R M. Understanding physical inactivation processes: combined preservation opportunities using heat, ultrasound and pressure. Int J Food Microbiol. 1995;28:197–219. doi: 10.1016/0168-1605(95)00057-7. [DOI] [PubMed] [Google Scholar]
- 12.Earnshaw R G. High pressure microbial inactivation kinetics. In: Ledward D A, Johnston D E, Earnshaw R G, Hasting A P M, editors. High pressure processing of foods. Nottingham, United Kingdom: Nottingham University Press; 1995. pp. 37–46. [Google Scholar]
- 13.Hauben K J A, Bartlett D H, Soontjens C C F, Cornelis K, Wuytack E Y, Michiels C W. Escherichia coli mutants resistant to inactivation by high hydrostatic pressure. Appl Environ Microbiol. 1997;63:945–950. doi: 10.1128/aem.63.3.945-950.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayakawa I, Kanno T, Yoshiyama K, Fujio Y. Oscillatory compared with continuous high pressure sterilization on Bacillus stearothermophilus spores. J Food Sci. 1997;59:164–167. [Google Scholar]
- 15.Hayashi R. Utilization of pressure in addition to temperature in food science and technology. In: Balny C, Hayashi R, Heremans K, Masson P, editors. High pressure and biotechnology. London, United Kingdom: Colloque INSERM/John Libbey Eurotext Ltd.; 1992. pp. 185–193. [Google Scholar]
- 16.Hoover D. Minimally processed fruits and vegetables: reducing microbial load by nonthermal physical treatments. Food Technol. 1997;51:66–71. [Google Scholar]
- 17.Hoover D G, Metrick C, Papineau A M, Farkas D, Knorr D. Biological effects of high hydrostatic pressure on food microorganisms. Food Technol. 1989;43:99–107. [Google Scholar]
- 18.Humphrey T J, Williams A, McAlpine K, Lever M S, Guard-Petter J, Cox J M. Isolates of Salmonella enterica Enteritidis PT4 with enhanced heat and acid tolerance are more virulent in mice and more invasive in chickens. Epidemiol Infect. 1996;177:79–88. doi: 10.1017/s0950268800001151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Humphrey T J, Slater E, McAlpine K, Rowbury R J, Gilbert R J. Salmonella enteritidis phage type PT4 isolates more tolerant of heat, acid, or hydrogen peroxide also survive longer on surfaces. Appl Environ Microbiol. 1995;61:3161–3164. doi: 10.1128/aem.61.8.3161-3164.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jernaes M W, Steen H B. Staining of Escherichia coli for flow cytometry: influx and efflux of ethidium bromide. Cytometry. 1994;17:302–309. doi: 10.1002/cyto.990170405. [DOI] [PubMed] [Google Scholar]
- 21.Kalchayanand N, Sikes A, Dunne C P, Ray B. Factors influencing death and injury of foodborne pathogens by hydrostatic pressure-pasteurisation. Food Microbiol. 1998;15:207–214. [Google Scholar]
- 22.Lee I S, Lin J, Hall H K, Bearson B, Foster J W. The stationary-phase sigma factor ςS (RpoS) is required for sustained acid tolerance response in virulent Salmonella typhimurium. Mol Microbiol. 1995;17:155–167. doi: 10.1111/j.1365-2958.1995.mmi_17010155.x. [DOI] [PubMed] [Google Scholar]
- 23.Metrick C, Hoover D G, Farkas D F. Effects of high hydrostatic pressure on heat-resistant and heat-sensitive strains of Salmonella. J Food Sci. 1989;54:1547–1549. [Google Scholar]
- 24.Miller L, Berger T. Bacterial identification by gas chromatography of whole cell fatty acids. Hewlett-Packard gas chromatography application note 228–41. Palo Alto, Calif: Hewlett-Packard Co.; 1985. [Google Scholar]
- 25.Mills G, Earnshaw R, Patterson M F. Effects of high hydrostatic pressure on Clostridium botulinum. Lett Appl Microbiol. 1988;26:227–230. doi: 10.1046/j.1472-765x.1998.00329.x. [DOI] [PubMed] [Google Scholar]
- 26.Patterson M F, Quinn M, Simpson R, Gilmour A. Sensitivity of vegetative pathogens to high hydrostatic pressure treatment in phosphate-buffered saline and foods. J Food Prot. 1995;58:524–529. doi: 10.4315/0362-028X-58.5.524. [DOI] [PubMed] [Google Scholar]
- 27.Robinson T P, Ocio M J, Lyn F, Kaloti A, Mackey B M. The use of ethidium bromide to assess a novel injury/recovery phenomenon in Listeria monocytogenes in inhibitory NaCl conditions. Lett Appl Microbiol. 1997;25:367–370. doi: 10.1046/j.1472-765x.1997.00244.x. [DOI] [PubMed] [Google Scholar]
- 28.Shigehisa T, Ohmori T, Saito A, Taji S, Hayashi R. Effects of high hydrostatic pressure on characteristics of pork slurries and inactivation of microorganisms associated with meat products. Int J Food Microbiol. 1991;12:207–216. doi: 10.1016/0168-1605(91)90071-v. [DOI] [PubMed] [Google Scholar]
- 29.Smelt J P P M, Rijke A G F, Hayhurst A. Possible mechanism of high pressure inactivation of microorganisms. High Pressure Res. 1994;12:199–203. [Google Scholar]
- 30.Sojka B, Ludwig H. Effect of rapid pressure changes on the inactivation of Bacillus subtilis spores. Pharm Ind. 1997;59:436–438. [Google Scholar]
- 31.Wang A-Y, Cronan J E. The growth phase-dependent synthesis of cyclopropane fatty acids in Escherichia coli is the result of an RpoS(KatF)-dependent promoter plus enzyme instability. Mol Microbiol. 1994;11:1009–1017. doi: 10.1111/j.1365-2958.1994.tb00379.x. [DOI] [PubMed] [Google Scholar]
- 32.Wouters P C, Glaasker E, Smelt J P P M. Effects of high pressure on inactivation kinetics and events related to proton efflux in Lactobacillus plantarum. Appl Environ Microbiol. 1998;64:509–514. doi: 10.1128/aem.64.2.509-514.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]