Abstract
Past research on the brain correlates of trait anger has been limited by small sample sizes, a focus on relatively few regions-of-interest, and poor test-retest reliability of functional brain measures. To address these limitations, we conducted a data-driven analysis of variability in connectome-wide functional connectivity in a sample of 1,048 young adult volunteers. Multi-dimensional matrix regression analysis showed that self-reported trait anger maps onto variability in the whole-brain functional connectivity patterns of three brain regions that serve action-related functions: bilateral supplementary motor area (SMA) and the right lateral frontal pole. We then demonstrate trait anger modulates the functional connectivity of these regions with canonical brain networks supporting somatomotor, affective, self-referential, and visual information processes. Our findings offer novel neuroimaging evidence for interpreting trait anger as a greater propensity to provoked action, supporting ongoing efforts to understand its utility as a potential transdiagnostic marker for disordered states characterized by aggressive behavior.
Keywords: Connectivity, fMRI, Trait anger, Amygdala, CWAS
Introduction
Trait anger refers to an individual’s dispositional tendency to more easily experience frustration, resulting in a decreased threshold for feeling angry in a wide range of situations (Spielberger, 1991). High trait anger has been broadly linked with higher reactive aggression and violent behavior (Bettencourt, Talley, Benjamin, & Valentine, 2006; Eckhardt, Jamison, & Watts, 2002). Such negative associations have prompted neuroimaging research into the brain correlates of high trait anger in effort to better understand its etiology and, subsequently, its possible modification or prevention.
Because of its key role in mediating defensive responses and facilitating acquisition of conditioned threat responses, the majority of existing functional neuroimaging research on trait anger has focused on the amygdala and the corticolimbic circuit (Rosell & Siever, 2016). For example, trait anger was positively correlated with amygdala activity to provocation in men (Repple et al., 2018), and facial expressions signaling interpersonal threat (i.e., angry faces) in men with high trait anxiety (Carré, Fisher, Manuck, & Hariri, 2012). A resting-state functional magnetic resonance imaging (rsfMRI) study found that trait anger was inversely correlated with the intrinsic functional connectivity between the amygdala and orbitofrontal cortex (Fulwiler, King, & Zhang, 2012). rsfMRI studies on disordered states characterized by high trait anger and reactive aggression, such as intermittent explosive disorder, have shown aberrant habenula-prefrontal connectivity patterns (Gan et al., 2019). Exaggerated resting-state amygdala-inferior frontal gyrus functional connectivity also has been observed in posttraumatic stress disorder following interpersonal anger induction (Gilam et al., 2017).
While these existing studies have helped outline possible brain correlates of trait anger, they have been limited in three ways. First, the majority of previous findings are derived from small sample sizes (<100), which is suboptimal for individual differences research using fMRI (Dubois & Adolphs, 2016). Second, as described above, prior studies have an a priori focus on discrete brain regions of interest (ROIs), which typically include the amygdala. Third, blood oxygen level dependent (BOLD) responses from task fMRI generally have poor psychometric properties to be considered as a reliable index of individual differences (Elliott et al., 2020).
Here, we attempted to overcome these prior limitations by conducting data-driven analyses of variability in connectome-wide functional connectivity patterns and trait anger in a large sample of young adult volunteers. First, we utilized of a dataset with substantially larger sample size (n = 1,048) than previous neuroimaging studies of trait anger. Second, we adopted a connectome-wide association study (CWAS) approach on our data (Shehzad et al., 2014). CWAS is particularly apt for the data-driven nature of the present investigation, as it enables the computation of multivariate connectivity patterns across the whole brain that correlates with trait anger, while making minimal assumptions about the data and not requiring a priori selection of networks. Third, we focused on assessing trait-like intrinsic functional architecture of brain networks, which are typically investigated using rsfMRI (Shehzad et al., 2009). Recently, we demonstrated that rsfMRI and task fMRI data could be combined to achieve more reliable estimations of intrinsic functional connectivity (GFC; general functional connectivity) by substantially increasing useable data points (Elliott et al., 2019).
Thus, through the use of CWAS, we sought to explore the neural correlates of trait anger with a reliable measure of functional connectivity across the whole brain. In order to supplement the data-driven approach, based on the existing literature emphasizing the role of the amygdala and the corticolimbic circuit (Rosell & Siever, 2016), we also aimed to test whether the functional connectivity patterns of the amygdala were associated with trait anger.
Methods
Participants
Neuroimaging and trait anger data were available from 1,048 young adult university students (621 women, age range 18-22 years, mean age = 19.68 years, mean years of education = 13.4 years) who voluntarily participated in the Duke Neurogenetics Study (DNS) between January 2010 and November 2016. Participants self-reported as being White or Caucasian (n = 506), Black or African American (n = 118), Asian (n = 314), American Indian or Alaska Native (n = 2), Multiracial (n = 76), and Other (n = 32), as well as Hispanic/Latino (n = 103). Structured clinical interview (Sheehan et al., 1998) was used to screen the participants for past or current DSM-IV (American Psychiatric Association, 1994) Axis I or select Axis II (borderline and antisocial personality) disorders. This procedure was included to facilitate comparisons with previous neuroimaging work using trait anger. The DNS was approved by the Duke University Medical Center Institutional Review Board and all participants provided written, informed consent prior to the study. To be eligible for the DNS, participants were required to be free of the following conditions: 1) medical diagnoses of cancer, stroke, head injury with loss of consciousness, untreated migraine headaches, diabetes requiring insulin treatment, chronic kidney, or liver disease; 2) use of psychotropic, glucocorticoid, or hypolipidemic medication; and 3) conditions affecting cerebral blood flow and metabolism (e.g., hypertension).
Self-Report Measures of Trait Anger
Trait anger was assessed using the trait version of the State-Trait Anger Expression Inventory (STAXI), a 10-item self-report questionnaire designed to assess individual differences in trait anger (Spielberger, 1991). Participants were instructed to read each of the 10 statements (e.g., When I get mad, I say nasty things) and rate how they generally feel on a 4-point Likert-like scale from almost never to almost always. STAXI Trait anger scores ranged from 10 to 40 (mean = 15.71 ± 4.25 standard deviation).
BOLD fMRI
Each participant was scanned using one of the two identical research-dedicated scanners at the Duke-UNC Brain Imaging and Analysis Center (see Supplementary Methods for acquisition parameters). For each participant, two back-to-back 4-minute 16-second (256 time points) rsfMRI scans were acquired. Participants were instructed to remain awake, with their eyes open during each resting-state scan. Participants also completed an emotional face-matching task (6:30 min, 195 time points), a card-guessing task (5:42 min, 171 time points), a working memory task (11:48 min, 354 time points), and a face-naming task (5:24 min, 162 time points). Detailed descriptions of these four tasks are provided in the Supplementary Methods.
BOLD fMRI data were then preprocessed following a standard protocol (see Supplementary Methods for further details). In order to compute GFC, identical time-series preprocessing steps were applied to both rsfMRI and task fMRI data with one exception. As task fMRI data include task-evoked coactivation that may drive functional connectivity, signal due to task structure was added as an additional nuisance covariate and removed from the time-series (Fair et al., 2007). GFC was computed by calculating functional connectivity after concatenating all rsfMRI and task fMRI scans to form a single time-series. Brain volumes exceeding 0.25-mm framewise displacement or 1.55 standardized DVARS were censored. All 1,048 participants had more than or equal to 185 time points left after censoring.
Connectome-wide Association Study
Following the preprocessing steps that included censoring and nuisance regressions to limit the influence of head motion and other artifacts, fMRI time-series data were extracted from the Power 264 atlas, which parcellated the brain into 264 regions (Power et al., 2011). BOLD data were averaged within 5 mm spheres surrounding each 264 coordinates in the parcellation. As noted elsewhere (Elliott et al., 2019), average time-series data were extracted independently from each scan session. This allowed the time-series from rsfMRI and task fMRI data to be flexibly concatenated and recombined. Extracted time-series data for each participant were then processed using a CWAS approach (Shehzad et al., 2014). CWAS utilizes multi-dimensional matrix regression (MDMR) to identify seed regions with whole-brain patterns of intrinsic functional connectivity that are associated with another variable. In brief, CWAS can be summarized into three steps. First, seed-based functional connectivity analysis is performed with a single ROI to generate a whole-brain functional connectivity map for each participant. Second, the average distance (1-Pearson correlation r) between each pair of participant’s functional connectivity maps is computed, resulting in a distance matrix encoding the multivariate similarity between each participant’s connectivity map. Finally, MDMR is used to generate a pseudo-F statistic quantifying the strength of the association between the variable of interest (e.g., trait anger) and the distance matrix created in the second step. These three steps are repeated for each of the 264 ROIs, resulting in a whole-brain map that represents the association between trait anger and whole-brain connectivity at each ROI. Age and sex were included as covariates, and 100,000 permutations were performed to generate p-values. To account for false positives across the 264 ROIs, a false discovery rate (FDR) correction was applied (Benjamini & Hochberg, 1995). Statistical significance threshold was set at q = 0.05. Follow-up seed-based connectivity analyses using 1) brain regions discovered through MDMR, and 2) a priori amygdala region-of-interests were performed. The latter analysis was performed as the Power 264 atlas does not cover the amygdala. Amygdala ROIs were defined using a high-resolution template generated from the 168 Human Connectome Project dataset (Tyszka & Pauli, 2016). For completeness, whole-brain functional connectivity analyses were performed separately for the basolateral (BL) and centromedial (CM) amygdala subregions in each hemisphere, yielding four amygdala ROIs (see Supplementary Methods for details).
Results
Multidimension Matrix Regression
MDMR identified three brain regions for which whole-brain functional connectivity patterns significantly varied as a function of trait anger (Figure 1). These regions were the left supplementary motor area (MNI −3, 2, 53; FDR-corrected p = 0.005), right supplementary motor area (MNI 7, 8, 51; FDR-corrected p = 0.008), and right lateral frontal pole (MNI 49, 35, −12; FDR-corrected p < 0.001).
Figure 1.
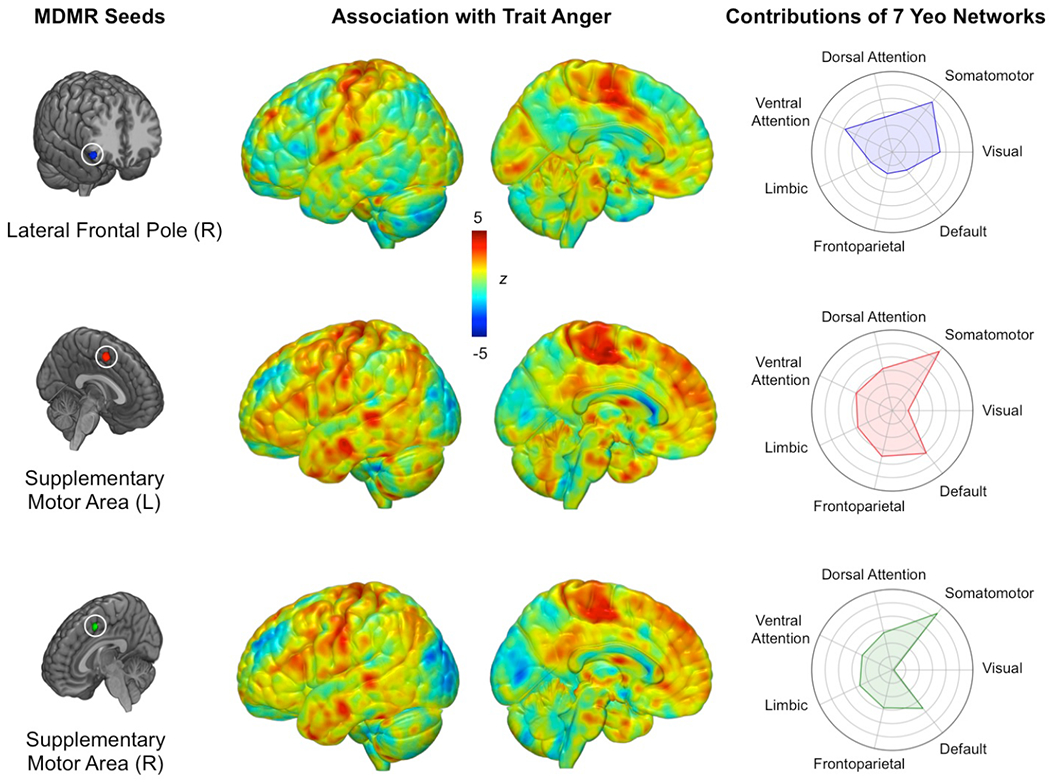
Data-driven MDMR analysis identified three regions with whole-brain functional connectivity patterns significantly associated with trait anger (left panel). Connectome-wide functional connectivity patterns for each MDMR-derived seed region as a function of trait anger (middle panel). Relative involvement of seven canonical functional networks to each connectivity pattern (right panel). Values farther toward the outer circle indicate greater functional connectivity to the corresponding network in high trait anger. Note that the brain maps in the middle panel were not thresholded thereby allowing for full visualization of all information relevant to the MDMR step, as they were performed post hoc to the family-wise error-controlled MDMR findings and thus do not represent independent statistical tests.
Follow-up Functional Connectivity Analyses
Follow-up seed-based connectivity analyses revealed that while showing some variability, the three MDMR-selected ROIs exhibited largely overlapping patterns of whole-brain functional connectivity as a function of trait anger that converged on several brain regions (pairwise correlations: lateral frontal pole map-left SMA map: r = 0.438; lateral frontal pole-right SMA map: r = 0.397; left SMA map-right SMA map: r = 0.936)(Figure 1). When mean functional connectivity estimates were calculated for each of seven canonical intrinsic networks (Yeo et al., 2011), high trait anger was associated with increased functional connectivity between all three MDMR-derived ROIs and the somatomotor network (SMN). Surveying each of the three MDMR-derived ROIs separately, the left and right supplementary motor areas showed nearly identical functional connectivity patterns: higher trait anger was associated with 1) increased functional connectivity with the default mode network (DMN) and 2) decreased functional connectivity with the visual network (VN). Conversely, the functional connectivity of the right lateral frontal pole did not exhibit such associations with either the DMN or the VN.
Amygdala Functional Connectivity Analyses
Of the four amygdala ROIs, the left CM did not yield significant associations. The remaining three amygdala ROIs exhibited variability in functional connectivity associated with trait anger across a network of cortical and subcortical brain regions. Converging results were observed across these three seed ROIs with minor differences (Figure 2). Generally, higher trait anger was associated with increased connectivity between these ROIs and the dorsomedial prefrontal cortex (dmPFC), dorsal anterior cingulate cortex, thalamus, caudate, putamen, nucleus accumbens, supplementary motor area, medial frontal pole, middle frontal gyrus, temporal pole, and brainstem (see Supplementary Tables for additional details). There were no brain regions exhibiting decreased functional connectivity with any amygdala ROIs as a function of trait anger. We note that the main findings remained consistent when analyzed separately for each sex. Similarly, STAXI scores did not show significant sex differences (t = −1.19, p = 0.234).
Figure 2.
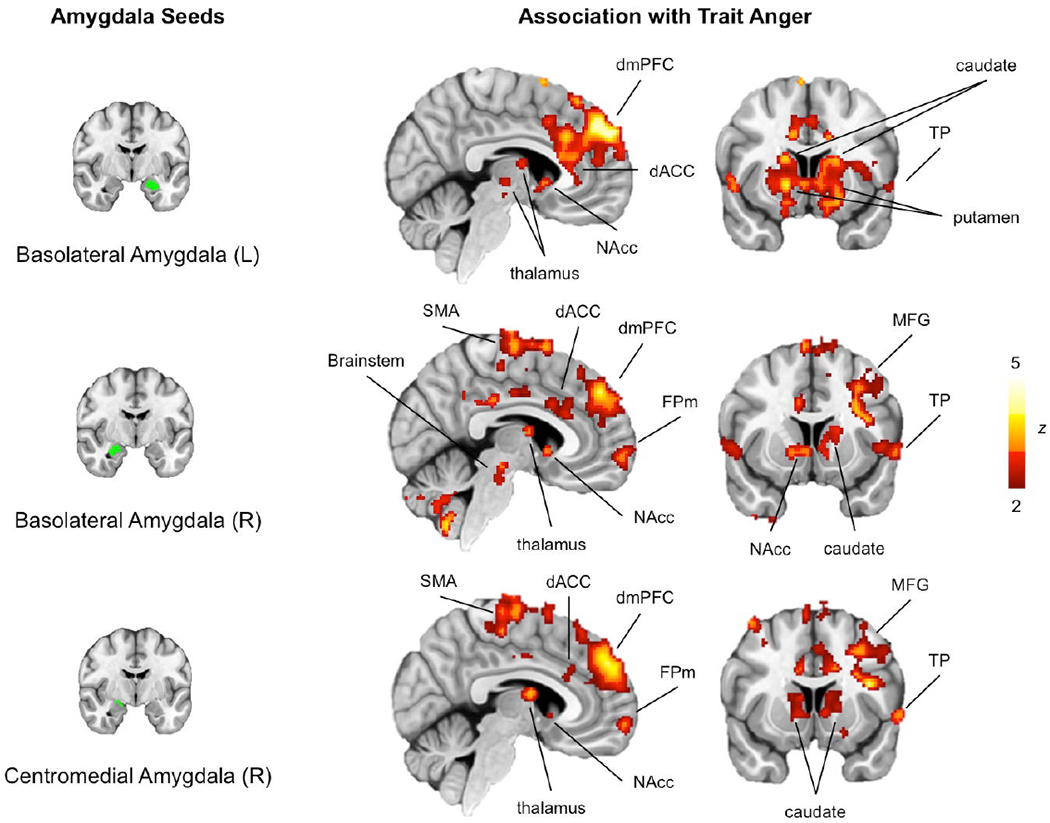
Seed-based analysis using amygdala ROIs (left panel) showed that high trait anger was associated with hyperconnectivity of the amygdala with a network of cortical and subcortical brain regions (right panel). Results shown here are visualized with a statistical threshold ofp < 0.05, corrected for multiple comparisons across the whole brain. No brain regions survived this threshold when the left centromedial amygdala was used as a seed region. Note: dACC (dorsal anterior cingulate cortex), dmPFC (dorsomedial prefrontal cortex), FPm (medial frontal pole), NAcc (nucleus accumbens), MFG (middle frontal gyrus), SMA (supplementary motor area), TP (temporal pole)
Discussion
By utilizing a data-driven approach on a reliable measure of the functional connectome in a large sample of 1,048 young adult volunteers, we provide novel evidence that trait anger is associated with variability in the functional connectivity patterns of multiple networks supporting somatomotor, affective, self-referential, and visual information processes. The connectome-wide data-driven MDMR analysis identified three brain regions, left and right supplementary motor area and right lateral frontal pole, with whole-brain functional connectivity patterns significantly associated with trait anger. Higher trait anger was associated with hyperconnectivity between all three of these regions and the somatomotor network. Further analyses revealed that, as a function of trait anger, the bilateral supplementary motor area but not the right lateral frontal pole showed hyperconnectivity with the default mode network and hypoconnectivity with the visual network. These findings expand upon previous work focused on associations between trait anger and variability within a corticolimbic circuit supporting threat learning to include action and action-planning brain regions and networks.
Specifically, all three brain regions identified through the MDMR analysis contribute to action and action-planning. The supplementary motor area is a well-known component of the motor cortex, functioning to prepare for voluntary action or movement (Cunnington et al., 2003). The lateral frontal pole is associated with the cognitive control of behavior (Orr, Smolker & Banich, 2015), which includes, of particular relevance to the current study, socioemotional approach-avoidance actions (Bramson et al., 2020). These findings are consistent with previously documented action-related aspects of trait anger, such as increased approach motivation (Harmon-Jones, 2003) and poor inhibitory control (Wilkowski & Robinson, 2008).
The involvement of action-related networks in the expression of trait anger was further reflected in the specific patterns of functional connectivity displayed by these MDMR-derived brain regions. The somatomotor network, which encompasses brain regions including the primary motor cortex (Brodmann area 4), premotor area (area 6), primary somatosensory cortex (areas 1, 2, 3), early somatosensory area (area 5L), and parts of the midcingulate sulcus, showed hyperconnectivity with all three seeds as a function of trait anger. One possible interpretation of this pattern associated with higher trait anger is that it may reflect a lowered threshold for action based on somatosensory information, as the three action-related brain regions became more synchronized with the SMN as a function of trait anger. Such speculation offers an interesting neural basis to an important characteristic of trait anger – that it reflects a greater propensity to provoked action. Future studies testing this idea would be able to contribute to an integrative account of anger (e.g., Wilkowski & Robinson, 2008), by elucidating the links that connect trait anger, neurocognitive processes and provoked action.
Some diverging patterns of connectivity across the three MDMR-derived brain regions were observed as well. The default mode network, best known for its integral role in self-projection and mental simulations (Buckner & DiNicola, 2019), showed hyperconnectivity with the bilateral supplementary motor area as a function of trait anger, while an opposite pattern was found for the visual network. Recently, it was reported that major functional networks form a distinct spatial organization that follows a macroscale gradient (Buckner & DiNicola, 2019; Margulies et al., 2016). Interestingly, the DMN is located at one extreme end of this gradient, maximally separated from both SMN and VN, which are situated at the opposite end (Margulies et al., 2016). This spatial gradient also reflects the orientation of the functional processes associated with each network, such that the DMN reflects processes oriented towards the self, whereas SMN and VN represent processes oriented towards the environment. From this perspective, trait anger may reflect the psychological manifestation of the changes in the distance between an internally oriented brain process (i.e., DMN activity) and externally oriented brain processes (i.e.. SMN, VN activity) along this gradient. In other words, trait anger could be thought of as action-readiness (supplementary motor area) becoming more influenced by self-referential and somatomotor information (hyperconnectivity with the DMN, SMN) while relying less on visual information (hypoconnectivity with the VN).
Our data-driven connectome-wide MDMR analysis was augmented by a seed-based analysis using the amygdala, which revealed a broad network of cortical and subcortical brain regions with altered functional connectivity as a function of trait anger. Previous investigations on the neural correlates of trait anger have typically focused on the amygdala (Repple et al., 2018; Carré et al., 2012) and its connectivity with the prefrontal cortices (Fulwiler et al., 2012) based on a priori theoretical and empirical work (LeDoux, 1996; Marsh & Blair, 2008). While other studies have individually reported a number of brain regions beyond the amygdala associated with trait anger, such as the thalamus (Alia-Klein et al., 2018), striatum (da Cunha-Bang et al., 2017), and anterior cingulate cortex (Repple et al., 2018), our analysis demonstrates that all of these neural sites reflect individual differences in trait anger with regards to the amygdala. Above and beyond the methodological differences (e.g., task vs. resting-state fMRI), it is likely that the large sample size we have employed for the present study secured sufficient statistical power to observe these associations that were previously undetected. Of particular interest from this final set of analyses is the hyperconnectivity between the amygdala and the dmPFC in high trait anger. While a direct evidence for trait anger is sparse, hyperconnectivity of the amygdala-dmPFC circuit is often reported to be associated with negative psychological and physical health outcomes, such as heightened and sustained anxiety (Vytal et al,, 2014), as well as enhanced inflammatory responses to stress (Muscatell et al,, 2015), Perhaps the amygdala-dmPFC hyperconnectivity observed in individuals with high trait anger may reflect their relative susceptibility to negative health outcomes, Overall, our findings converge on previous rsfMRI findings highlighting changes in amygdala functional connectivity patterns in trait anger or anger-related psychopathology (Fulwiler et al,, 2012; Gan et al,, 2019; Gilam et al,, 2017), Compared to prior studies of trait anger, the unique contributions of the present study include the identification of action-related brain regions.
The present study is not without limitations that can be addressed in future research. First, as the DNS sampled high functioning university students located in the US, the generalizability of the present findings to a broader population is yet to be confirmed. Future studies utilizing large-scale, population-representative datasets would be able to address this issue. Second, the assessment of trait anger relied on self-report, via a standardized questionnaire. Development of a more objective measurement of trait anger (e.g., behavioral observation) would be helpful in overcoming the limitations associated with self-report. Third, the current findings are derived from generally low levels of self-reported trait anger. Thus, we caution against making broader inferences from our data (e.g., to disordered states such as the intermittent explosive disorder). In addition, we did not observe strong evidence for the involvement of the limbic network with regards to the three MDMR-derived seed regions and trait anger. While this may be the case, it is noteworthy that out of the seven canonical functional networks, the limbic network displayed the lowest level of reliability in other large-scale fMRI datasets (Elliott et al., 2019). As such, we cannot rule of the possibility that our observations in the present study may have been affected by differences in reliability across the limbic network vs. other canonical functional networks. Lastly, recent work has demonstrated that task-induced brain states may be useful for drawing out inter-individual variability, by maximizing the ratio of within- to between-subject variability in functional connectivity patterns (Finn et al., 2017). While we opted to employ a commonly used strategy that regresses out signals due to task structure, future work could take advantage of such task-induced brain states by utilizing tasks that are relevant to trait anger (e.g., anger- or frustration-eliciting manipulations).
These limitations notwithstanding, our study design and analyses allowed us to address key limitations of prior studies, which includes small sample size, reliance on a priori ROIs, and poor psychometric properties of BOLD responses in task fMRI. Our large sample size enabled us to employ data-driven, exploratory approaches on the whole brain functional networks. The functional connectome was generated from data with good reliability and predictive utility, both of which are necessary psychometric properties for fMRI-based individual differences research (Elliott et al., 2020).
To summarize, the present study leveraged a large dataset and an unconstrained connectome-wide MDMR approach to highlight a collection of brain regions and canonical intrinsic networks associated with trait anger, many of which were previously undetected. Findings from our data-driven, exploratory analyses could help generate new hypotheses for future neuroimaging research of trait anger, as well as related constructs. These include emotional lability and irritability, both of which have implications for adversely affecting the developing brain and mental health (Bennett, Somandepalli, Roy & Di Martino, 2017; Dennis, Humphreys, King, Thompson & Gotlib, 2019). Future studies could be designed to utilize the present findings and examine the intrinsic functional networks in relevant psychiatric disorders such as the intermittent explosive disorder (Gan et al., 2019), in order to elucidate the utility of trait anger as a potential transdiagnostic marker.
Supplementary Material
Acknowledgements
We thank the Duke Neurogenetics Study participants and the staff of the Laboratory of NeuroGenetics.
Funding
The Duke Neurogenetics Study received support from Duke University as well as the National Institute on Drug Abuse under Grants R01DA033369 and R01DA031579. This work was further supported by National Institute on Aging under grant R01AG049789. M.L.E. was supported by the National Science Foundation Graduate Research Fellowship under Grant No. DGE-1644868 and the National Institute of Aging under grant F99AG068432-01. In addition, the Brain Imaging and Analysis Center received support from the Office of the Director, National Institutes of Health under Award S10OD021480.
Footnotes
Declaration of Conflicting Interests
The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
References
- Alia-Klein N, Preston-Campbell RN, Moeller SJ, Parvaz MA, Bachi K, Gan G,… Goldstein RZ (2018). Trait anger modulates neural activity in the fronto-parietal attention network. PLoS One, 13, e0194444. doi: 10.1371/journal.pone.0194444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (1994). Diagnostic and Statistical Manual of Mental Disorders (4th ed.). Washington, DC: Author [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological), 57, 289–300. doi: 10.im/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- Bennett RH, Somandepalli K, Roy AK, & Di Martino A (2017). The neural correlates of emotional lability in children with autism spectrum disorder. Brain Connectivity, 7, 281–288. doi: 10.1089/brain.2016.0472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettencourt BA, Talley A, Benjamin AJ, & Valentine J (2006). Personality and aggressive behavior under provoking and neutral conditions: A meta-analytic review. Psychological Bulletin, 132, 751– 777. doi: 10.1037/0033-2909.132.5.751 [DOI] [PubMed] [Google Scholar]
- Bramson B, Folloni D, Verhagen L, Hartogsveld B, Mars RB, Toni I,… & Roelofs K (2020). Human lateral frontal pole contributes to control over emotional approach–avoidance actions. Journal of Neuroscience, 40, 2925–2934. doi: 10.1523/JNEUROSCI.2048-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, & DiNicola LM (2019). The brain’s default network: updated anatomy, physiology and evolving insights. Nature Reviews Neuroscience, 20, 593–608. doi: 10.1038/s41583-019-0212-7 [DOI] [PubMed] [Google Scholar]
- Carré JM, Fisher PM, Manuck SB, & Hariri AR (2012). Interaction between trait anxiety and trait anger predict amygdala reactivity to angry facial expressions in men but not women. Social Cognitive and Affective Neuroscience, 7, 213–221. doi: 10.1093/scan/nsq101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnington R, Windischberger C, Deecke L, & Moser E (2003). The preparation and readiness for voluntary movement: a high-field event-related fMRI study of the Bereitschafts-BOLD response. Neuroimage, 20, 404–412. doi: 10.1016/s1053-8119(03)00291-x [DOI] [PubMed] [Google Scholar]
- da Cunha-Bang S, Fisher PM, Hjordt LV, Perfalk E, Persson Skibsted A, Bock C,… & Knudsen GM (2017). Violent offenders respond to provocations with high amygdala and striatal reactivity. Social Cognitive and Affective Neuroscience, 12, 802–810. doi: 10.1093/scan/nsx006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EL, Humphreys KL, King LS, Thompson PM, & Gotlib IH (2019). Irritability and brain volume in adolescents: cross-sectional and longitudinal associations. Social Cognitive and Affective Neuroscience, 14, 687–698. doi: 10.1093/scan/nsz053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J, & Adolphs R (2016). Building a science of individual differences from fMRI. Trends in Cognitive Sciences, 20, 425–443. doi: doi: 10.1016/j.tics.2016.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt CI, Jamison TR, & Watts K (2002). Anger experience and expression among male dating violence perpetrators during anger arousal. Journal of Interpersonal Violence, 17, 1102–1114. doi: 10.1177/088626002236662 [DOI] [Google Scholar]
- Elliott ML, Knodt AR, Cooke M, Kim MJ, Melzer T, Keenan R,… & Hariri AR (2019). General Functional Connectivity: shared features of resting-state and task fMRI drive reliable and heritable individual differences in functional brain networks. Neuroimage, 189, 516–532. doi: 10.1016/j.neuroimage.2019.01.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott ML, Knodt AR, Ireland D, Morris ML, Poulton R, Ramrakha S,… & Hariri AR (2020). What is the test-retest reliability of common task-functional MRI measures? New empirical evidence and a meta-analysis. Psychological Science, 31, 792–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NUF, Wenger KK,… & Petersen SE (2007). A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. Neuroimage, 35, 394–405. doi: 10.1016/j.neuroimage.2006.11.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn ES, Scheinost D, Finn DM, Shen X, Papademetris X, & Constable RT (2017). Can brain state be manipulated to emphasize individual differences in functional connectivity? Neuroimage, 160, 140–151. doi: 10.1016/j.neuroimage.2017.03.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulwiler CE, King JA, & Zhang N (2012). Amygdala-orbitofrontal resting-state functional connectivity is associated with trait anger. Neuroreport, 23, 606–610. doi: 10.1097/WNR.0b013e3283551cfc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan G, Zilverstand A, Parvaz MA, Preston-Campbell RN, d’Oleire Uquillas F, Moeller SJ,… N. Alia-Klein (2019). Habenula-prefrontal resting-state connectivity in reactive aggressive men – a pilot study. Neuropharmacology, 156, 107396. doi: 10.1016/j.neuropharm.2018.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilam G, Maron-Katz A, Kliper E, Lin T, Fruchter E, Shamir R,… Hendler T (2017). Tracing the neural carryover effects of interpersonal anger on resting-state fMRI in men and their relation to traumatic stress symptoms in a subsample of soldiers. Frontiers in Behavioral Neuroscience, 11, 252. doi: 10.3389/fnbeh.2017.00252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon-Jones E (2003). Anger and the behavioural approach system. Personality and Individual Differences, 35, 995–1005. doi: 10.1016/S0191-8869(02)00313-6 [DOI] [Google Scholar]
- LeDoux JE (1996). The Emotional Brain. New York, NY: Simon and Shuster. [Google Scholar]
- Margulies DS, Ghosh SS, Goulas A, Falkiewicz M, Huntenberg JM, Langs G,… Smallwood J (2016). Situating the default-mode network along a principal gradient of macroscale cortical organization. Proccedings of the National Academy of Sciences U.S.A, 113, 12574–12579. doi: 10.1073/pnas.1608282113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, & Blair RJ (2008). Deficits in facial affect recognition among antisocial populations: a meta-analysis. Neuroscience and Biobehavioral Reviews, 32, 454–465. doi: 10.1016/j.neubiorev.2007.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell KA, Dedovic K, Slavich GM, Jarcho MR, Breen EC, Bower JE,… & Eisenberger NI (2015). Greater amygdala activity and dorsomedial prefrontal—amygdala coupling are associated with enhanced inflammatory responses to stress. Brain, Behavior, and Immunity, 43, 46–53. doi: 10.1016/j.bbi.2014.06.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr JM, Smolker HR, & Banich MT (2015). Organization of the human frontal pole revealed by large-scale DTI-based connectivity: Implications for control of behavior. PLoS ONE, 10, e0124797. doi: 10.1371/journal.pone.0124797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, & Petersen SE (2011). Functional network organization of the human brain, Neuron, 72, 665–678. 10.1016/j.neuron.2011.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repple J, Habel U, Wagels L, Pawliczek CM, Schneider F, & Kohn N (2018). Sex differences in the neural corrleates of aggression. Brain Structure and Function, 223, 4415–4124. doi: 10.1007/s00429-018-1739-5 [DOI] [PubMed] [Google Scholar]
- Rosell DR, & Siever LJ (2016). The neurobiology of aggression and violence. CNS Spectrums, 20, 254–279. doi: 10.1017/S109285291500019X [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan HK, Amorim P, Janavs J, Weiller E,… Dunbar GC (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a Structured Diagnostic Psychiatric Interview for DSM—IV and ICD-10. Journal of Clinical Psychiatry, 59, 22–33. [PubMed] [Google Scholar]
- Shehzad Z, Kelly C, Reiss PT, Cameron Craddock R, Emerson JW, McMahon K,… Milham MP (2014). A multivariate distance-based analytic framework for connectome-wide association studies. Neuroimage, 93, 74–94. doi: 10.1016/j.neuroimage.2014.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehzad Z, Kelly AMC, Reiss PT, Gee DG, Gotimer K, Uddin LQ,… Milham MP (2009): The resting brain: Unconstrained yet reliable. Cereb Cortex 19, 2209–2229. doi: 10.1093/cercor/bhn256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD (1991). State-Trait Anger Expression Inventory. Odessa, FL: Psychological Assessment Resources Inc. [Google Scholar]
- Tyszka JM, & Pauli WM (2016). In vivo delineation of subdivisions of the human amygdaloid complex in a high-resolution group template. Human Brain Mapping, 37, 3979–3998. doi: 10.1002/hbm.23289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkowski BM, & Robinson MD (2008). The cognitive basis of trait anger and reactive aggression: An integrative analysis. Personality and Social Psychology Review, 12, 3–21. doi: 10.1177/1088868307309874 [DOI] [PubMed] [Google Scholar]
- Vytal KE, Overstreet C, Charney DR, Robinson OJ, & Grillon C (2014). Sustained anxiety increases amygdala—dorsomedial prefrontal coupling: a mechanism for maintaining an anxious state in healthy adults. Journal of Psychiatry & Neuroscience, 39, 321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M,… Buckner RL (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106, 1125–1165. doi: 10.1152/jn.000338.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


